- 1Health and Rehabilitation College, Chengdu University of Traditional Chinese Medicine, Chengdu, Sichuan, China
- 2School of Preclinical Medicine, Chengdu University, Chengdu, China
Chronic rhinosinusitis (CRS), a common clinical condition characterized by persistent mucosal inflammation and tissue remodeling, has a complex pathogenesis that is intricately linked to innate and adaptive immunity. A number of studies have demonstrated that a variety of immune cells and cytokines that play a vital role in mediating inflammation in CRS are also involved in remodeling of the nasal mucosa and the cells as well as different cytokines involved in remodeling in CRS are also able to exert some influence on inflammation, even though the exact relationship between inflammation and remodeling in CRS has not yet been fully elucidated. In this review, the potential role of immune cells and cytokines in regulating inflammation and remodeling of CRS mucosa has been described, starting with the immune cells and cytokines that act together in inflammation and remodeling. The goal is to aid researchers in understanding intimate connection between inflammation and remodeling of CRS and to offer novel ideas for future research.
1 Introduction
Chronic rhinosinusitis (CRS) is described as a chronic inflammatory disease of the mucosa of the nasal cavity and sinuses which can lead to 12 consecutive weeks of clinical symptoms such as nasal runny nose, nasal congestion, facial swelling, decreased sense of smell, headache and dizziness. In addition, signs of disease can be diagnosed by endoscopy or associated CT scan changes (1, 2).The pathology of CRS is commonly characterized by persistent mucosal inflammation and tissue remodeling (3). The immune response in CRS can be divided into three main inflammatory endotypes based on a distinctive characteristic spectrum which is composed of various inflammatory mediators, immune cells, and physiological functions (4–6). The three immunological subtypes display the following characteristics: Type 1 inflammation is primarily mediated by 1 helper T lymphocytes (TH1), type 1 innate lymphoid cells (ILC1), natural killer (NK) cells, and neutrophils, with a priority expression of interferon-γ (IFN-γ), tumor necrosis factor-α(TNF-α), and IL-12. Type 2 inflammation is predominantly driven by TH2, ILC2, eosinophils, macrophages, and B cells, and is associated with interleukin-4 (IL-4), IL-5 and IL-13. Lastly, Type 3 inflammation involves neutrophils, TH17 as well as ILC3 and is characterized by elevated levels of IL-17 and IL-22. The terms Th1, Th2 and Th17 inflammation have been also used to describe these three endotypes. The remodeling features of CRS include goblet cells proliferation, basement membrane thickening (BMT), subepithelial edema and fibrosis, subepithelial glandular hyperplasia, collagen deposition, epithelial-mesenchymal transition (EMT),and osteitis (7–9). The typical remodeling related factors that can regulate these features are matrix metalloproteinase (MMP), tissue inhibitor of metalloproteinases (TIMP), transforming growth factor-β (TGF-β), vascular endothelial growth factor (VEGF), platelet-derived factor (PDGF), bone morphogenetic protein (BMP) and osteopontin factors (10–12). Interestingly, at the same time, it has been reported that the two most common phenotypes, chronic rhinosinusitis with nasal polyps (CRSwNP) as well as chronic rhinosinusitis without nasal polyps(CRSsNP), possess different immunomodulatory mechanisms and remodeling characteristics (2, 13). However, the potential link between inflammation and remodeling in CRS is not well understood as for a very long time, remodeling characteristics in CRS were thought to be secondary processes resulting from the protracted inflammatory process (14). But, the findings reported in early CRS studies that increased fibroblast and collagen deposition in the nasal cavity can occurs prior to local inflammation has opened new areas of investigation (15).
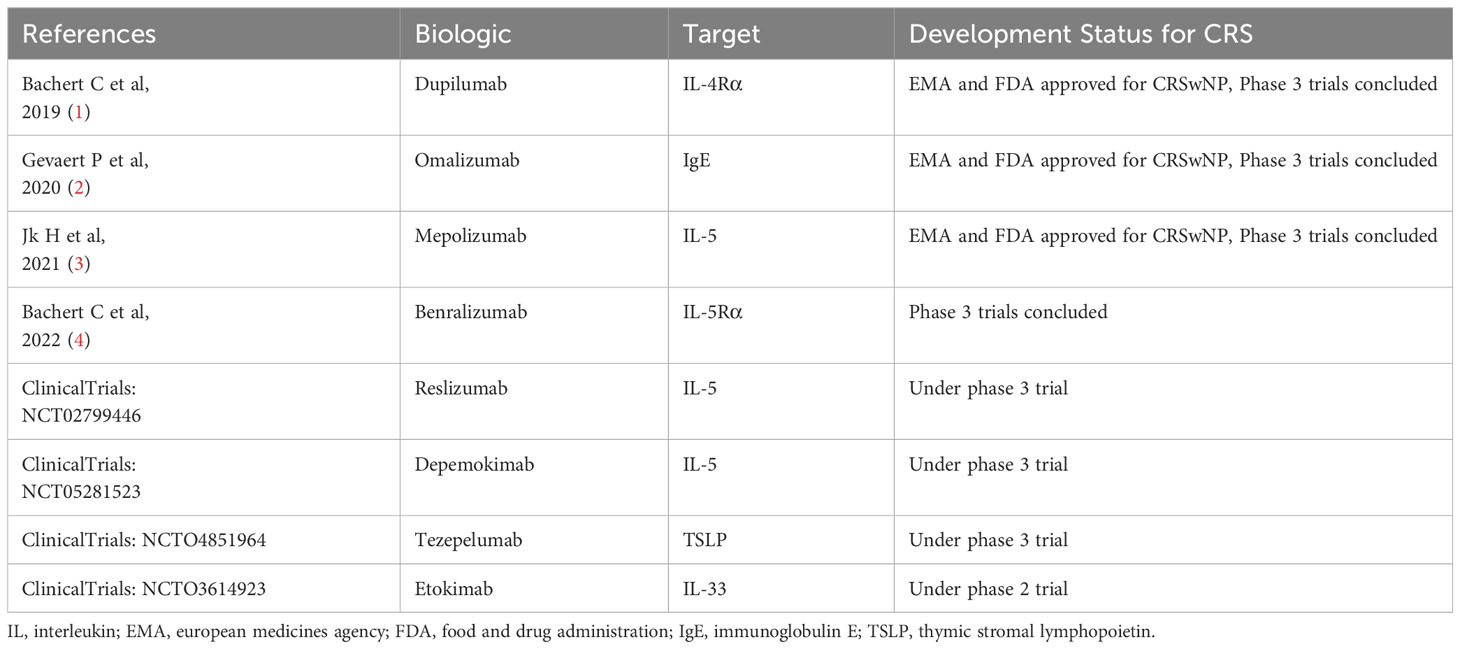
The pathogenesis of CRS is complex as it is a chronic disease that requires long-term maintenance treatment to control the condition (16). In recent years, control has been proposed as a more subtle outcome indicator by different experts, which refers to the maintenance of disease performance at an acceptable level (17). At present, the dominant methods of control include pharmacological treatments, followed by surgical intervention. However, with advent of refractory CRSwNP that persists or recurs despite long-term pharmacological and surgical treatment and acute exacerbations of chronic rhinosinusitis (AECRS) that can cause transient worsening of symptom intensity in patients with CRS, the traditional methods of medication and surgery do not often provide ideal results. Additionally, inspired by significant advances in asthma treatment, biologics have become the focus of CRS treatment in recent years (Table 1). In fact, some biologics have shown remarkable improvements in the severity of refractory CRSwNP and AECRS on both the subjective and objective indicators (18, 19). These biologics, primarily focusing on cytokines, cytokine receptors or antibody bindings, appear to provide a new perspective that the different cytokines, immune cells and remodeling factors related to CRS could potentially be key in breaking the prevailing belief that remodeling and inflammation do not interact during disease progression. Thus, based on these recent findings in the field, the current article reviews the potential roles of cytokines, immune cells and remodeling factors in the inflammation and remodeling mechanisms of CRS. The goal is to further explore the possible relationship between inflammation and remodeling, thus laying a theoretical foundation for the formulation of more refined and individualized CRS treatment strategies in the future.
2 Important cytokines involved in CRS remodeling and inflammation
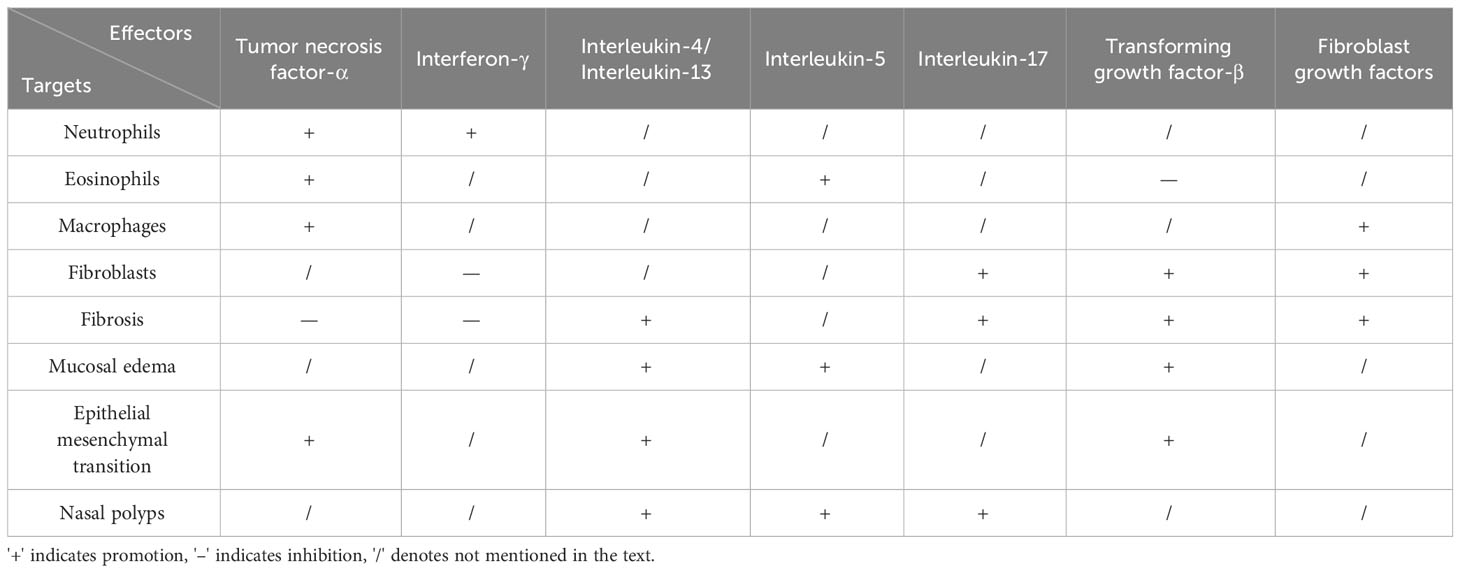
It has been established that cytokine expression and regulation serve as the most crucial factors in the pathological mechanisms of CRS that have been identified after years of research development. The TH1, TH2, TH17 and remodeling-associated cytokines that can participate in CRS inflammation and remodeling (Table 2), as well as the pathways of cytokine action, have been discussed in the sections that follow.
2.1 Impact of cytokines of the TH1 on remodeling and inflammation
The TH1 cytokines involved in CRS are mainly TNF-α and IFN-γ which are produced by Th1 cells, cytotoxic T cells, NK cells as well as ILC1 and prior studies have reported that both TNF-α and IFN-γ play a substantial role in the development of inflammation and remodeling pathology, where the inflammatory response primarily driven by TNF-α and IFN-γ is even considered to be the main characteristic of TH1 inflammation (20).
It has been found that multiple pathways can be used by TNF-α to have an impact on inflammation and remodeling. TNF-α is frequently regarded as a key pro-inflammatory factor in the pathogenesis of CRS due to the fact that nasal polyp tissues have been observed to express markedly high levels of TNF-α (21). In fact, previous studies have revealed that TNF-α can directly harm the nasal mucosal epithelium by causing mononuclear-macrophages formation, triggering their migration, and increasing their cytotoxicity. It can also activate the T lymphocytes, stimulate the production of immunoglobulin by B lymphocytes (22), and increase neutrophil levels, by promoting eosinophil survival, and enhancing their cytotoxic effects (23). In CRSsNP, which constitutes up to more than two-thirds of CRS cases (24), fibrosis has been identified as the primary remodeling feature (25). This condition is typically characterized by excessive fibroblast proliferation and deposition of the collagen-rich extracellular matrix (26). TNF-α, on the other hand, has been demonstrated to exert significant antifibrotic properties in preclinical models of TNF receptor-deficient mice (27), and in experiments with TNF-α blockers alone (28). EMT is a process that can effectively convert polar epithelial cells into cells with a mesenchymal phenotype (29) and it facilitates a pervasive inflammatory damage repair and remodeling process that takes place in mucosal and skin barriers. It is well-known that TNF-α acts as a key signal for EMT induction in the tumor environment (30), and it has the ability to promote accelerated EMT through a variety of mechanistic pathways to increase the invasiveness of cancer cells (31, 32). Additionally, increased TNF-α expression in CRS can lead to increased EMT, which could be potentially related to the development of subepithelial fibrous tissue as well as thickening of the basement membrane and thickening of nasal sinus mucosal tissue on sinus CT images (29).
IFN-γ is a key cytokine that predominantly aids in host defense againstTh1 inflammation caused by intracellular pathogens (33), but it has also been reported to display strong antagonistic effects in a number of fibrotic disease models. IFN-γ plays a complex role in regulation of inflammation in CRS. On the one hand, it can promote inflammation by causing neutrophil oxidation, phagocytosis, as well as chemotaxis, disrupting tight junction proteins, and inducing apoptosis in nasal mucosal epithelial cells (34). On the other hand, it can protect by preventing the development of more pathogenic T cell phenotypes (Th2 and Th17). The impact of IFN-γ on remodeling is reflected in its prominent antiproliferative and antifibrotic effects (35) and IFN-γ has been found to reduce fibrosis in liver (36) and kidney (37) fibrosis models by inhibiting TGF-β activity, or can also inhibit fibroblast activation and proliferation to exert anti-fibrotic effects (37) and suppress collagen synthesis (38). Therefore, it is highly likely that the elevated levels of IFN-γ in CRS can also affect the remodeling of the nasal mucosa’s fibrosis.
2.2 The impact of TH2 cytokines on remodeling and inflammation
Eosinophil and mast cell infiltration, goblet cells proliferation, elevated immunoglobulin(Ig) E levels, and the production of cytokines like IL-4, IL-5, and IL-13 by ILC2, T cells2, and Th2 cells have all been identified as the characteristics of the TH2 immune response in CRS (39). It has been reported that different CRS phenotypes have TH2 immune responses, and medium CRSwNP can have up to 80% of them (40). There are even several guidelines published for the management of differentiated CRS with type 2 and non-type 2 immune responses. The TH2 immune response can be found in the different TH2 inflammatory factors such as IL-4, IL-5, and IL-13 which have been extensively studied in CRS due to their association with more severe symptoms, higher recurrence rates, and more complex concomitant symptoms. They can be identified as biomarkers in the peripheral blood and nasal mucosal tissue of CRS patients, and have also been shown to play a significant role in the pathological mechanisms of both inflammation and remodeling in CRS (41).
A pair of closely related cytokines, IL-4 and IL-13, share the IL-4R/signal transduction and activator of transcription 6 (STAT6) signaling pathway for mediating their actions (42). Inflammation and remodeling in response to CRS also reveal similarities between IL-4 and IL-13 pathological manifestations. For instance, IL-4 can exert immunomodulatory effects on B cells, T cells, mast cells, as well as macrophages, and serves as an autocrine growth factor for helper T cells that can influence CRS inflammation. In CRS, IL-4 can stimulate B cells to differentiate into the plasma cells and produce IgE that can bind to the mast cells and cause the release of a number of inflammatory mediators, particularly eosinophil chemokines, which ultimately can damages sinus mucosal tissues and promote the development of nasal polyps (43). Moreover, IL-4 can induce the expression of vascular cell adhesion factors by endothelial cells, and it can also facilitate the binding of monocytes, lymphocytes, as well as eosinophils to the vascular endothelial adhesion, which ultimately can augment the overall inflammatory response (44). IL-13, on the other hand, is a pleiotropic cytokine that is primarily produced by activated Th2 cells, and prior studies have shown that the nasal mucosal tissues from CRS patients have significantly elevated levels of IL-13 (45). IL-13 is involved in the nasal mucosa’s defense mechanism against the respiratory viral and bacterial infections and it can activate B cells to regulate IgE production and epithelial remodeling (46, 47). It can also act synergistically with other proinflammatory cytokines. As a result of the discovery that IL-4 and IL-13 play a critical role in the inflammatory process that can lead CRS, several biological agents that target the IL-4/IL-13 pathway have been discovered for use in CRS. Although IL-4 and IL-13 can influence CRS remodeling in remarkably identical fashion, IL-13 generally has been reported to have a greater impact. It is believed that IL-13 is a crucial component in pro-fibrosis because numerous studies have indicated that it can either directly or indirectly promote fibrosis (48, 49). It had been previously also hypothesized that IL-4 could be responsible for the pro-fibrotic effect of IL-13 (50). However, it has been found that IL-13 can still exert its fibrosis-inducing effect when the traditional IL-4R/STAT6-mediated signaling pathway has been blocked, i.e., IL-13 can induce fibrosis by activating additional signaling mechanisms through its own receptors (51). In addition, through activating its downstream signaling pathways, IL-13 can also stimulate TGF-β expression, which can enhance fibroblast activation and collagen deposition, thereby causing inflammatory edema and thickening of the basement membrane (52). Moreover, IL-13 and IL-4 can work in conjunction to suppress the tissue fibrinogen activator expression, activate coagulation factor XIIIa, and negatively modulate eosinophil levels. These events can result in increased fibrin deposition and cross-linking, tight tetrameric complex formation, and worsened edema remodeling in rhinitis (53).
As IL-5 has been identified a crucial component in eosinophil proliferation, chemotaxis, differentiation, activation, and survival (54), it appears that IL-5 can primarily affect inflammation and remodeling through eosinophils during the pathological development of CRS. For example, in CRS, activated IL-5 can promote a large number of eosinophils to migrate into the mucosa and strengthen their adhesion, thus promoting nasal mucosal inflammation, which has been established as the tissue basis of nasal polyps (55). The expression of IL-5, on the other hand, was reported to be significantly increased in CRS, both in the nasal mucosal tissue and serum, and it was closely correlated with both the subjective and objective measures of the severity of the disease (56). Eosinophils play a vital part in how IL-5 can affect remodeling as well, and hence the topic is discussed in more detail under eosinophils below.
In conclusion, TH2 cytokines IL-4, IL-5, and IL-13 not only contribute to CRS inflammation by rupturing the epithelial barrier, mediating the cilia dysfunction and mucus production, mediating altered nasal mucosal macrophage function, reducing innate immune function in the nasal cavity stimulating the development of mucosal edema and pseudocysts (57), but can also display strong pro-fibrotic properties. Overall, they possess potent pro-fibrotic properties in the context of CRS remodeling, which are intimately linked to the onset of fibrosis (58).
2.3 The impact of TH17 cytokines on remodeling and inflammation
ILC3 and Th17 cells can all produce two main cytokines of the TH17 immune response, IL-17, and IL-22. An important characteristic of the TH17immune response is inflammation that is mainly driven by IL-17 and IL-22 cytokines (59). A number of related to CRS have also shown that IL-17 and IL-22 can have a significant impact on the inflammatory process, but their influence on CRS remodeling is currently unknown. IL-17 is a general term for the various cytokines like IL-17A, B, C, D, E, and F (60), among which abnormal expression of IL-17A has been strongly associated with chronic inflammation and autoimmune diseases (61)and it has been reported to play a significant role in promoting nasal polyp formation (60). Thus, by influencing the pro-inflammatory response of inflammatory cells in the nasal mucosa, high expression of the IL-17 protein found in CRS patients could effectively contribute to the pathogenesis of nasal polyps (60). Additionally, since IL-17 was discovered to be significantly pro-fibrotic in skin (62), liver (63), lung (64), intestine (65), kidney (66), and heart (67)models of fibrosis, IL-17 is also identified to function as a significant pro-fibrotic factor. For instance, in asthma model mice, anti-IL-17 treatment was found to decrease the lung inflammation, edema, oxidative stress, and extracellular matrix remodeling (68). It could also induce collagen production in myofibroblasts and modulate the expression of MMP-3, MMP-9, and TIMP1 (69, 70). Therefore, it is implied that IL-17 may also be crucial for the remodeling of the nasal mucosa in CRS.
A member of the IL-10 cytokine family, IL-22 has been reported to be crucial for mucosal intrinsic immunity in the respiratory, digestive, and skin tracts, because it can aid in anti-microbial defense, the protection and repair of the tissue damage, and acute phase responses (71). Interestingly, prior studies on IL-22 in CRS, however, are still unclear, and the inconsistent results of IL-22 content measurements in CRS could be a significant impediment. This could be attributed to the fact that nasal polyps themselves have more pathological types and can be affected by various factors, such as the site of sampling and the application of hormonal drugs at the time of the study, as well as the different ethnicities of the study subjects (72). However, in terms of influencing the remodeling, researchers have hypothesized that its function is similar to that of IL-10; however, in contrast to the hazily predicted remodeling effects, inflammatory effects of IL22 are relatively evident in CRS, where it was discovered to exhibit a favorable effect on the expression of different adhesion molecules and chemokines. Thus, through affecting these important functions, it can attract diverse inflammatory cells, which in turn can intensify the Th2-type immune response and ultimately induce pathological effects (73).
2.4 The impact of remodeling-related cytokines on remodeling and inflammation
Normally considered to be typical cytokines affecting CRS remodeling, TGF-β, fibroblast growth factors (FGF) and VEGF have been found to have a profound impact on the pathological process of CRS inflammation through prior research.
TGF-β refers to TGF-β1 when not otherwise specified because TGF-β1 has an overwhelming predominance of up to 80% and is the most abundant member of TGF-β family. Given that TGF-β1 is the most fibrogenic factor (74), it has been proposed that the differences in TGF-β levels between CRSwNP and CRSsNP could contribute to the different remodeling characteristics of CRS tissue remodeling (75). Moreover, When it comes to its inflammatory impact on CRS, TGF-β1 has been reported to have both pro- and anti-inflammatory effects. For example, studies have elegantly shown that TGF-β1 can promote fibrosis through a variety of pathways as CRS pathology develops.
The number of fibroblasts is directly correlated with the expression of TGF-β1, which has been found to induce fibroblast proliferation and differentiation into the myofibroblasts (76). Additionally, TGF-β1 can regulate the balance between TIMP and MMP, mediate collagen release and extracellular matrix synthesis (77). Furthermore, TGF-β is the most effective inducer of EMT (78) and can contribute significantly to EMT through modulating Smad signaling (79). In conclusion, TGF-β can mediate EMT and induces extracellular matrix (ECM) protein expression in mesenchymal cells by regulating the stromal MMP and TIMP expression, thus leading to increased stromal permeability, ECM degradation, albumin deposition, and nasal mucosal edema. It can thus actively participate in the tissue remodeling of the sinus mucosa in CRS. TGF-β1, meanwhile, also plays a dual role in the inflammatory immune response in CRS. On the one hand, TGF-β1 can increase inflammation by triggering the differentiation of T lymphocytes into inflammatory Th17 (80), but on the other hand, it can suppress the production of various inflammatory cytokines and mediators (e.g. IL-1, IL-8, TNF-α) (81), thus suppressing the release of mediators from eosinophils and increasing their apoptosis rate (82). It can also significantly inhibit the proliferation and cytokine production of naive T cells as well as Th1 as well as Th2 clones (83), and promote the differentiation of T regulatory cell (84) by exerting diverse anti-inflammatory effects.
FGF is a crucial cytokine involved in the various developmental processes like cell proliferation, differentiation, and migration as well as in injury and tissue remodeling (85). FGF can promote fibrosis when it is involved in CRS remodeling by transforming the epithelial cells into fibroblast-like cells (86), whereas it was discovered that significantly more basic FGF-2 was found in the nasal secretions of CRS patients (87), which can attract leukocytes to secrete the different inflammatory mediators in acute and chronic inflammatory conditions (88). FGF-2 can also stimulate the development of inflammatory cells and improve their infiltration into tissues, including the macrophages and T lymphocytes (89). Additionally, research findings using FGF receptor inhibitors to block FGF signaling have revealed that it can cause inflammatory reactions that involve TNF-α (90).
An important factor regulating the tissue angiogenesis, tissue proliferation remodeling, and increasing vascular permeability is VEGF, which is a mitogenic peptide that is specific to endothelial cells (91–94). At the beginning of CRS, the nasal mucosal tissue is already neovascularized and as the proliferating tissue grows, the rate of VEGF positive expression increases substantially (95). Consequently, it is also believed that VEGF can control both the vascular growth and inflammation during CRS.
3 Important immune and effector cells involved in CRS remodeling and inflammation
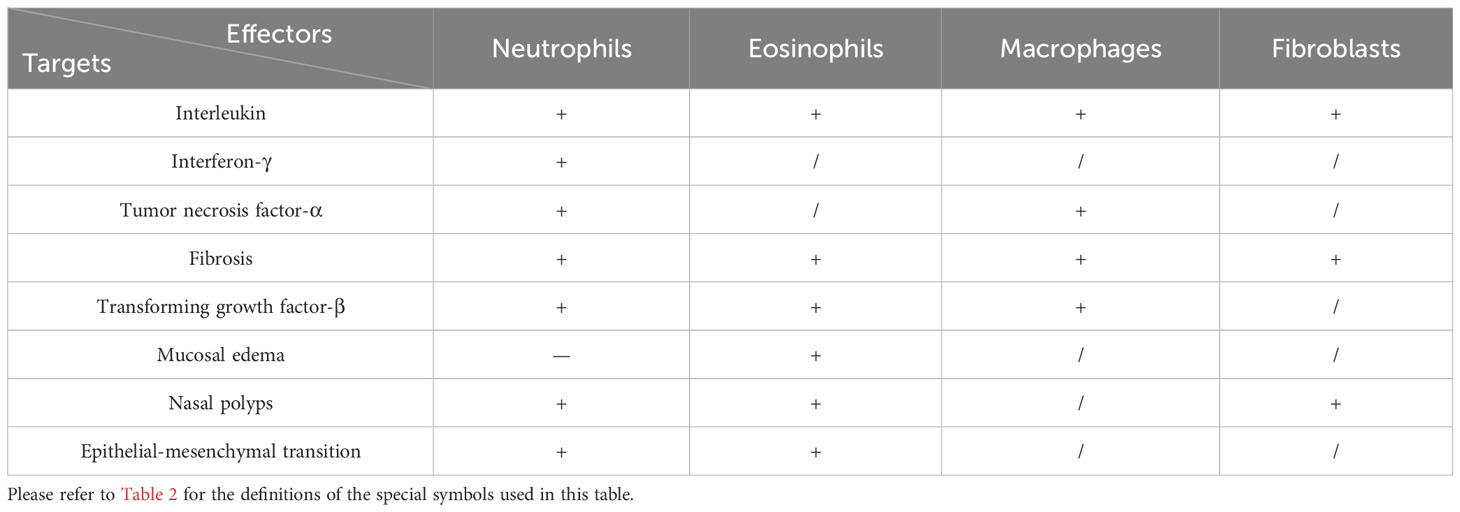
The pathological mechanisms of CRS inflammation and remodeling are complicated due to the impact of the main immune and effector cells involved in this condition (Table 3). This can be attributed to the fact that these cells are able to exert differential effects on them at various stages of the disease development and because they also constitute a part of interconnected pathological networks that can sometimes have completely divergent effects on one another depending on the situation.
3.1 Neutrophils
Neutrophils have been reported to play a significant role in the development of all forms of CRS pathology, both in terms of inflammation as well as remodeling, and their importance cannot be underestimated. Neutrophils are the primary effector cells of the intrinsic immune system and they exhibit a variety of synergistic functions that work together to eliminate pathogens (96). On the contrary, it was discovered that chronic inflammatory diseases associated with the respiratory system have persistent neutrophil infiltration, and the level of infiltration was positively correlated with the degree of inflammation and disease (97). Neutrophils can contribute to the immune response by secreting various cytokines, such as IL-36 and IL-33, IL-1, IL-6, IL-8, IFN-γ and TNF-α, to upregulate the inflammatory response, which is one of the major event in CRS (98). Additionally, CRSsNPs that clearly demonstrate the neutrophil infiltration, in addition to neutrophils that may contribute to nasal polypogenesis have been identified in non-eosinophilic CRSwNPs (99).
Neutrophils can display a variety of effects on CRS remodeling, including fibrosis, edema, EMT and cause an imbalance between MMP and TIMP. A number of studies have reported a positive correlation between fibrosis and neutrophils in CRS, probably because neutrophils are the primary source of TGF-β2-positive cells that can positively correlate with the myofibroblast number and fibronectin expression levels (100), i.e., the number of neutrophils which can positively correlate with the expression of pro-fibrotic factors, thus suggesting that neutrophils likely promote CRS via TGF-β2 in tissue fibrosis. The quantity of the neutrophils was also discovered to be negatively correlated with the level of edema (100). Neutrophil elastase, a serine protease released from the neutrophils has been found to affect goblet cells proliferation and mucin overproduction in patients with CRS (101, 102), EMT is a phenomenon involved in tissue remodeling that can eventually result in the local pools of fibroblasts, abnormal extracellular matrix deposition and the formation of nasal polyps (9). Whereas patients with neutrophil-dominated CRS can promote EMT via the IFN-γ pathway (103, 104); In addition, hypoxia-inducible factor-1α, which can induce EMT (105) has also been positively correlated with the number of neutrophils (100) and one of the primary mechanisms of pathological tissue remodeling in CRS is the imbalance between MMP and TIMP (7), where MMP-9 mainly degrades the gelatin, proteoglycan and elastin, a key factor in remodeling, whereas there is an association found between MMP-9 and neutrophils (106), which is an important source of MMP-9 (107).
3.2 Eosinophils
Eosinophils, which are a crucial part of leukocytes and like other granulocytes, are derived from the hematopoietic stem cells in the bone marrow can release granular materials that can damage tissue and advance inflammation (108). According to the prior reports, eosinophils have been implicated in a variety of biological functions (109), including mediating inflammation or inducing immunity. Moreover, studies have found that eosinophilia is positively correlated with the severity of CRS, thus indicating that more the number of eosinophils, greater is the severity of the disease (110). This is in addition to the pro-inflammatory role of eosinophils in releasing IL-4, IL-5, and IL-13 cytokines. Similarly, tissue eosinophilia has been significantly linked to the worsening of symptoms, lower quality of life, and substantially higher risk of recurrence in patients with CRSwNP after endoscopic nasal surgery (ESS) (111, 112). Hence, for these reasons, eosinophils are regarded as potential biomarkers for more severe and refractory diseases (113).
Eosinophils exhibit an extraordinarily strong positive correlation with various remodeling-related factors during the progression of CRS pathology, in addition to their capacity to release different pro-fibrotic and pro-angiogenic substances like TGF-β1, FGF-9, and VEGF. Interestingly, researchers have speculated that there exists a very close link between eosinophils and remodeling because sinus mucosal remodeling is more pronounced in CRS patients with co-morbid asthma and higher eosinophil load (14, 114, 115). The ability of eosinophil products to induce submucosal edema and epithelial damage has been highlighted in particular by the positive correlation between eosinophil cationic protein levels and edema (116). Additionally, BMT has been positively correlated with tissue eosinophil infiltration (117), but both edema and collagen deposition in the basement membrane in CRS have been positively correlated with eosinophil (100, 118), where increased albumin deposition mainly caused by eosinophil-driven inflammation (119). The ability of eosinophils to promote fibrosis may be attributed to TGF-β, whose main source is eosinophils (120), which can induce fibroblast proliferation and differentiation into the myofibroblasts, as well as possesses capacity to produce IL-11 and IL-17, which have significant pro-fibrotic effects (121). Furthermore, it has been reported that eosinophils and fibronectin levels can be significantly correlated in nasal polyps of CRS patients (122). Moreover, a stromal cell protein called periostein has been linked to extracellular matrix buildup and fibrosis in the tissue remodeling (123, 124). Interestingly, expression of this protein in the sinus tissues has been positively correlated with eosinophil infiltration (125). Eosinophil infiltration and EMT in CRS have also been linked, and it was found that eosinophils play a significant role in promoting EMT process (9). In addition, eosinophils can effectively contribute to the secretion of MMP-9 from the nasal mucosal epithelium and have been positively correlated with it (126, 127). Eosinophils are crucial for osteitis bone remodeling as observed by the positive correlation between eosinophilic inflammation and upregulated growth differentiation factors as well as exostein glycosyltransferases in osteitis of CRS patients (128).
Eosinophils undoubtedly play a significant role in regulating both mucosal inflammation and remodeling in CRS, but consistent remodeling was also observed in nasal polyps in Western and Asian populations in comparison to Asian patients with different inflammatory features and generally lower eosinophil levels (7), thereby presumably indicating that eosinophils may not be the primary factors influencing remodeling (129).
3.3 Macrophages
Macrophages have been recognized to play a key role in the development and resolution of inflammation (130). After phagocytosing cellular debris, invaders, neutrophils, and other apoptotic cells that appear after the tissue injury (131, 132), local tissue macrophages and recruited monocytes quickly transform from M1-type macrophages, which secrete a wide variety of proinflammatory cytokines such as IL-1, IL-6, TNF-α, IL-17A and IL-12 (133, 134), to M2-type macrophages with an increased capacity for anti-inflammatory response. M2-type macrophages can contribute to the remodeling by secreting diverse cytokines like TGF-β1, IL-6, and IL-13 to promote the fibroblast survival, proliferation, myofibroblast activation, collagen production, and increased transcription of pro-fibrotic genes (135–138), as well as by secreting factors like IL-10 and TGF-β1 which can exhibit anti-inflammatory effects (139). Moreover, both M1 and M2 macrophage numbers have been reported to increase during the pathological progression of CRS, with an especially large increase in M2 macrophage numbers in the later stages (140). This finding suggests that macrophages also exhibit a pattern from M1 to M2 types, or from pro-inflammatory to anti-inflammatory, during this process (141).
Given the intricate relationship between the reported pleiotropic effects of macrophages on inflammation and tissue remodeling in CRS, it has been hypothesized that the development of nasal polyps could be closely linked to the tissue remodeling caused by M2 macrophage polarization (142). It has been established that because macrophages can serve as a significant cellular source of different chemokines like eosinophil chemokines in the nasal mucosa (143), Th2 cytokines have been positively correlated with increased macrophage numbers during the pro-inflammatory developmental phase of CRS (140) and can directly or indirectly induce the production of Th2 cytokines thereby regulating the immune environment. In addition, M2 macrophages in CRS can significantly alter the vascular permeability (77), regulate associated inflammatory factors and control coagulation mechanisms (144), which can result in tissue remodeling in CRS. In conclusion, research has shown that monocytes-macrophages, depending on their polarization within the tissue as well as the type and stage of the disease, can play a complex but significant role in the regulation of inflammation, proliferation, and fibrosis (58).
3.4 T cells and innate lymphoid cells
T cells play multifaceted roles in regulating various physiological processes, which include recruiting effector cells, neutralizing infected cells, aiding B cells in immunoglobulin production and functioning as potential memory cells in innate immune system (145). Predominantly, T cells can be categorized into CD4+ T helper cells and CD8+ cytotoxic T cells. CD4+ T cells can further differentiate into five primary subsets: Th1, Th2, Th17, follicular helper T cells, and T regulatory cell (146). Th1 once activated by phagocytosed microbes along with support from ILC1s, can release IFN-γ, TNF-α, and TNF-β. These cytokines can then aid in the phagocytosis of the microbiome by activating macrophages and promoting antigen presentation, in addition to stimulating IgG production by B cells, neutrophils, and inducing local tissue inflammation (147). Th2 are primarily activated by the different parasites, which can trigger eosinophils, mast cells, ILC2s and enhance IgE production (148). Th2 can secrete IL-4, IL-5, and IL-13, thus contributing to the activation of eosinophils, mucus production and macrophage stimulation (149), thereby potentially leading to the production of several growth factors that can initiate tissue repair mechanisms. The Th17 subpopulation can activate neutrophils and monocytes to stimulate the secretion of IL-17A, IL-17F, and IL-22 (2). Most of T cells accomplish their objectives by producing a wide array of cytokines, which have been discussed in detail above in part two of this article.
Innate lymphoid cells (ILCs) are a subpopulation of innate immune cells that can produce a variety of cytokines that are compatible with the Th subpopulation of adaptive immune cells. These cytokines have been found to be crucial for the coordination of innate and adaptive immune responses. ILCs are also referred to as intrinsic immune cells, which are a subset of lymphocytes that are distinct from T and B cells and are primarily found in the mucosal barrier tissues. ILCs play a vital role in inflammatory diseases of the respiratory system by promoting lymphoid organ formation, enhancing immune responses and maintaining mucosal integrity (150). Moreover, based on the transcription factors and the cytokines produced, ILCs have been divided into three subtypes, ILC1s, ILC2s, and ILC3s (151), and these three cell subtypes produce high levels of the similar cytokines as TH cells, such as ILC1 secretes IFN-γ, ILC2 produces IL-5 and IL-13, and ILC3 secretes IL-17 as well as IL-22 (152), and thus play an important role in the pathogenesis of CRS.
3.5 Fibroblasts and goblet cells
The pathological development of CRS involves the epithelium, which can serve as the primary barrier by defending the host from external physical, chemical, and immune stimuli (153). Fibroblasts and goblet cells have been identified as significant contributors to the epithelium in this process. It has been reported that in comparison to CRSwNP, which exhibits more edema and less fibrosis, remodeling of CRSsNP can display a marked feature of BMT, fibrosis, and goblet cells proliferation (154). The contribution of fibroblasts and goblet cells to remodeling is undeniable, but more studies are needed to fully understand how inflammation can potentially modulate these cells.
Extracellular matrix that is rich in collagen is typically deposited concomitant with excessive fibroblast proliferation is considered as a symptom of fibrosis (26), and fibroblasts function as the primary effector cells that can promote fibrosis. For example, in organ related diseases like those of the lung, liver, and kidney, anti-fibrosis has been a hot topic of research. The complex mechanism of inflammation and remodeling of the nasal mucosa by fibroblasts in CRS has been thought to play an important role in the etiology and persistence of nasal polyps (155). However, because the proportion of fibroblasts in nasal polyps is significantly higher than that in the healthy nasal mucosa, in addition to promoting fibrosis, they can also release eochemokine, which plays an important role in stimulating eosinophil recruitment in nasal polyps (156), and also can mediate the release pro-inflammatory IL-6 and IL-8 cytokines (155). Therefore, it has been speculated that they may serve as the key source of inflammatory mediators (157).
The proliferation of goblet cells, a type of nasal mucosal epithelial cell whose primary function is to secrete plenty of mucus, can increase the secretion of mucin, the primary component of the mucus in the nasal membrane (158). Goblet cells are presence in abundance to secrete mucin in excess and hypersecretion of mucin is associated with pathological changes in CRS (159). In addition, increased mucus volume and viscosity arising as a result of hypersecretion by goblet cells can cause mucociliary dysfunction (160), mucus retention, and aggravation of inflammation (161). In addition, numerous proinflammatory cytokines have been demonstrated to control the goblet cells metaplasia and excessive mucin secretion (162), thereby creating a vicious cycle that can exacerbate the inflammatory response of CRS. As a result, some researchers have concluded that important pathogenic mechanisms of CRS include the proliferation and metaplasia of glandular cells and goblet cells as well as the promotion of sinusitis mucin expression (163).
4 Discussion
CRS is a highly heterogeneous disease with a relatively high prevalence. Its pathogenesis involves several factors, including microbial infection, immune dysfunction, sinus anatomy and concomitant diseases. The primary strategies employed for treating CRS are pharmacological drugs and surgical treatments, but given the complex heterogeneity of CRS, formulating universally applicable treatment modalities poses a significant challenge. All these factors inevitably lead us back to the core of CRS pathophysiology-inflammation and remodeling. In recent years, there has been growing interest in the area, yet surprisingly few reviews have explored this burgeoning field. Recently, Wang et al. (164) stratified CRS endotypes by amalgamating inflammatory biomarkers with typical remodeling factors, identified five clusters and subsequently classified them into endotypes: non-type 2 inflammation (clusters1 and 2) and type 2 inflammation (clusters 3, 4, and 5). However, like most of the previous studies in this field, this research has not delved deeply into the complex relationship reported between inflammation and remodeling. Moreover, relationship between inflammation and remodeling has been drawn from experiences related to asthma and other upper respiratory diseases, but there is no consensus in the academic community on whether CRS follows identical mechanisms and whether its internal remodeling is reversible. Additionally, while pharmacological drugs that are effective against CRS inflammation have been identified, their role in remodeling is not clearly defined. Therefore, this dearth of comprehensive reviews presents an opportunity for our article to fill a significant research gap and provide a fresh, novel perspective on inflammation and remodeling in CRS.
We have explored the complex roles of cytokines, immune cells and remodeling factors in CRS inflammation and remodeling in the present study, which led us to recognize that in the pathological setting of CRS, inflammation as well as remodeling are closely interrelated, and that they promote each other to form positive feedback thereby exacerbating the pathology. It is therefore reasonable to assume that inflammation and remodeling in CRS are in a dynamic equilibrium that is clearly interrelated. Although the interactions between immune cells and cytokines and their roles in different environments can make it difficult to comprehend this complex relationship, a clear understanding of their relationship as an important feature of CRS is of key importance for our in-depth interpretation of the pathological mechanisms of CRS. In addition, precision medicine which has been developed for the inflammatory and molecular features of CRS subtypes has been regarded as an important avenue for CRS research. The various biologics developed for targeting specific immune cells or cytokines have opened up the possibility of individualized and targeted therapy for CRS, leading the way for future research, although their general use is limited by high cost and insufficient evidence. Therefore, in future studies, one should utilize advanced technologies such as multi-omics analysis, single-cell RNA sequencing, and artificial intelligence analysis to completely elucidate of the role of different inflammatory cells, cytokines, and remodeling-associated factors on inflammation and remodeling, and thus promote the effectiveness and precision of the treatment.
5 Limitations
First, given the limited data available related to the relationship between inflammation and remodeling, the majority of literature covered in our study is relatively outdated, and may not completely represent current research findings and perspectives. Second, a large body of the evidence presented has not undergone meta-analysis, a type of aggregate statistical analysis that can facilitate the extraction and reanalysis of data from the multiple studies to obtain a more profound and conclusive understanding. In the absence of meta-analysis, the validity, consistency and reliability of the provided evidence may be a little hard to determine. Thus, although our study furnishes a plethora of evidence, the lack of meta-analysis might limit its overall utility. Finally, the sheer breadth of topics covered in our study could impose limitations on the depth and scope of the discussion. The detailed content segmentation might aid in better understanding and investigating a particular topic, but excessive subdivision could also render the research fragmented and disjointed, thereby potentially impinging on the integrity and coherence of the theory. Overall, these limitations could affect the reliability and validity of our study. Future directions in inflammation and remodeling research should aim to provide high-quality randomized controlled trials, which can lead to the creation of robust evidence-based guidelines to assist the clinicians.
6 Conclusions
Our preliminary research has elegantly unveiled the intricate interplay between inflammation and remodeling in CRS, where typical inflammatory cells and cytokines, along with the several remodeling-associated factors, exhibit a complex pattern of mutual enhancement or suppression, thus posing significant challenges for precision medicine. This necessitates more research related to different advanced technologies such as multi-omics analysis, single-cell RNA sequencing, and artificial intelligence analytics which can effectively aid to deepen our understanding of the relationship between inflammation and remodeling. This enhanced knowledge could provide novel strategies both for the prevention and personalized treatment of CRS.
Author contributions
HL, TZ and XG selected the topic. XG and YF thought through idea and frame of the article. ZH, HF and XG collected the related studies. XG wrote the first draft of the manuscript. YW and YH created the tables. HL, TZ and YF proofed the text and tables. HL, TZ and YF revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This research was supported by the Natural Science Foundation of China (81674037) and the Sichuan Science and Technology Program (2022YFS0421).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
CRS, chronic rhinosinusitis; CRSwNP, chronic sinusitis with nasal polyps; CRSsN, chronicsinusitis without nasal polyps; BMT, basementmembrane thickening; EMT, epithelial-mesenchymal transition; TH, helper T lymphocytes; ILCs, innate lymphoid cells; NK, natural killer cells; IFN-γ, interferon-γ; IL, interleukin; TNF-α, tumor necrosis factor-α; MMP, matrix metalloproteinase; TIMP, tissue inhibitor of metalloproteinases; TGF-β, transforming growth factor-β; VEGF, vascular endothelial growth factor; PDGF, platelet-derived factor; BMP, bone morphogenetic protein; IgE, immunoglobulin E; FGF, fibroblast growth factors; ECM, extracellular matrix.
References
1. Bachert C, Pawankar R, Zhang L, Bunnag C, Fokkens WJ, Hamilos DL, et al. ICON: chronic rhinosinusitis. World Allergy Organ J (2014) 7:25. doi: 10.1186/1939-4551-7-25
2. Fokkens WJ, Lund VJ, Hopkins C, Hellings P, Kern R, Reitsma S, et al. European position paper on rhinosinusitis and nasal polyps 2020. Rhinology (2020) 58:1–464. doi: 10.4193/Rhin20.401
3. Alt JA, Lee WY, Davis BM, Savage JR, Kennedy TP, Prestwich GD, et al. A synthetic glycosaminoglycan reduces sinonasal inflammation in a murine model of chronic rhinosinusitis. PloS One (2018) 13:e0204709. doi: 10.1371/journal.pone.0204709
4. Staudacher AG, Peters AT, Kato A, Stevens WW. Use of endotypes, phenotypes, and inflammatory markers to guide treatment decisions in chronic rhinosinusitis. Ann Allergy Asthma Immunol (2020) 124:318–25. doi: 10.1016/j.anai.2020.01.013
5. Klingler AI, Stevens WW, Tan BK, Peters AT, Poposki JA, Grammer LC, et al. Mechanisms and biomarkers of inflammatory endotypes in chronic rhinosinusitis without nasal polyps. J Allergy Clin Immunol (2021) 147:1306–17. doi: 10.1016/j.jaci.2020.11.037
6. Bachert C, Marple B, Hosemann W, Cavaliere C, Wen W, Zhang N. Endotypes of chronic rhinosinusitis with nasal polyps: Pathology and possible therapeutic implications. J Allergy Clin Immunology: In Pract (2020) 8:1514–9. doi: 10.1016/j.jaip.2020.03.007
7. Van Bruaene N, Bachert C. Tissue remodeling in chronic rhinosinusitis. Curr Opin Allergy Clin Immunol (2011) 11:8–11. doi: 10.1097/ACI.0b013e32834233ef
8. Samitas K, Carter A, Kariyawasam HH, Xanthou G. Upper and lower airway remodelling mechanisms in asthma, allergic rhinitis and chronic rhinosinusitis: The one airway concept revisited. Allergy (2018) 73:993–1002. doi: 10.1111/all.13373
9. Wang M, Sun Y, Li C, Qu J, Zhou B. Eosinophils correlate with epithelial-mesenchymal transition in chronic rhinosinusitis with nasal polyps. ORL (2022) 84:70–80. doi: 10.1159/000516847
10. Li X, Huang J, Chen X, Lai X, Huang Z, Li Y, et al. IL-19 induced by IL-13/IL-17A in the nasal epithelium of patients with chronic rhinosinusitis upregulates MMP-9 expression via ERK/NF-κB signaling pathway. Clin Trans Allergy (2021) 11:e12003. doi: 10.1002/clt2.12003
11. Shimizu S, Gabazza EC, Ogawa T, Tojima I, Hoshi E, Kouzaki H, et al. Role of thrombin in chronic rhinosinusitis–associated tissue remodeling. Am J Rhinol Allergy (2011) 25:7–11. doi: 10.2500/ajra.2011.25.3535
12. Hamilton DW. Functional role of periostin in development and wound repair: implications for connective tissue disease. J Cell Commun Signal (2008) 2:9–17. doi: 10.1007/s12079-008-0023-5
13. Orlandi RR, Kingdom TT, Hwang PH, Smith TL, Alt JA, Baroody FM, et al. International consensus statement on allergy and rhinology: Rhinosinusitis: international consensus on rhinosinusitis. Int Forum Allergy Rhinology (2016) 6:S22–S209. doi: 10.1002/alr.21695
14. Bassiouni A, Chen PG, Wormald P-J. Mucosal remodeling and reversibility in chronic rhinosinusitis. Curr Opin Allergy Clin Immunol (2013) 13:4–12. doi: 10.1097/ACI.0b013e32835ad09e
15. Van Bruaene N CP-N, Van Crombruggen K, De Ruyck N, Holtappels G, Van Cauwenberge P, Gevaert P, et al. Inflammation and remodelling patterns in early stage chronic rhinosinusitis. Clin Exp Allergy (2012) 42:883–90. doi: 10.1111/j.1365-2222.2011.03898.x
16. Tai J, Han M, Kim TH. Therapeutic strategies of biologics in chronic rhinosinusitis: Current options and future targets. Int J Mol Sci (2022) 23:5523. doi: 10.3390/ijms23105523
17. Sedaghat AR, Phillips KM. Chronic rhinosinusitis disease control: a review of the history and the evidence. Expert Rev Clin Immunol (2023) 19:903–910. doi: 10.1080/1744666X.2023.2229027
18. Patel GB, Kudlaty EA, Guo A, Yeh C, Kim MS, Price CPE, et al. Impact of type 2 targeting biologics on acute exacerbations of chronic rhinosinusitis. Allergy Asthma Proc (2021) 42:417–24. doi: 10.2500/aap.2021.42.210058
19. Haloob N, KaraMali K, Hopkins C. The role of biologics in the treatment of chronic rhinosinusitis. BioDrugs (2023) 37:477–87. doi: 10.1007/s40259-023-00602-9
20. Zhu L, Li D, Xu S. Expression and clinical value of related inflammatory factors, VEGF and COX-2 in sinusitis serum. Chinese Journal of Laboratory Diagnosis (2022) 26:17–19. doi: 10.6040/j.issn.1673-3770.0.2021.561
21. Fen QIN, Xiao-song. Expression WU. and significance of inflammatory mediators in nasal mucosa of patients with different phenotypes of nasal polyps and chronic rhinosinusitis. Hainan Med J (2018) 29:2678–81. doi: 10.3969/j.issn.1007-4287.2022.01.006
22. Min ZHANG, Jianting WANG, Dawei WU, Qian SONG. Progress of mucosal remodeling in chronic rhinosinusitis. Review of mucosa remodeling in chronic rhinosinusitis (2016) 40:75–9. doi: 10.3760/cma.j.issn.1673-4106.2016.02.003
23. Chen DONG, Bo YUE, Yaling BO, Runfen LUO, Yanqun ZHANG. Efficacy of clarithromycin combined with Myrtol Standardized Enteric Coated Soft Capsule and Mometasone Furoate Nasal Spray on chronic rhinosinusitis. J Chin Pract Diagnosis Ther (2020) 34:520–3. doi: 10.13507/j.issn.1674-3474.2020.05.026
24. Lee S-N, Yoon J-H. The role of proprotein convertases in upper airway remodeling. Mol Cells (2022) 45:353–61. doi: 10.14348/molcells.2022.0019
25. Van Bruaene N, Derycke L, Perez-Novo CA, Gevaert P, Holtappels G, De Ruyck N, et al. TGF-β signaling and collagen deposition in chronic rhinosinusitis. J Allergy Clin Immunol (2009) 124:253–259.e2. doi: 10.1016/j.jaci.2009.04.013
26. Wynn T. Cellular and molecular mechanisms of fibrosis. J Pathol (2008) 214:199–210. doi: 10.1002/path.2277
27. Liu JY, Brass DM, Hoyle GW, Brody AR. TNF-alpha receptor knockout mice are protected from the fibroproliferative effects of inhaled asbestos fibers. Am J Pathol (1998) 153:1839–47. doi: 10.1016/s0002-9440(10)65698-2
28. Bahcecioglu IH, Koca SS, Poyrazoglu OK, Yalniz M, Ozercan IH, Ustundag B, et al. Hepatoprotective effect of infliximab, an anti-TNF-alpha agent, on carbon tetrachloride-induced hepatic fibrosis. Inflammation (2008) 31:215–21. doi: 10.1007/s10753-008-9067-1
29. Gao YB, Zhang Y, Zhang L. [Advance in epithelial-mesenchymal transition in chronic rhinosinusitis]. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi (2019) 54:231–6. doi: 10.3760/cma.j.issn.1673-0860.2019.03.015
30. Wu K, Bonavida B. The activated NF-kappaB-Snail-RKIP circuitry in cancer regulates both the metastatic cascade and resistance to apoptosis by cytotoxic drugs. Crit Rev Immunol (2009) 29:241–54. doi: 10.1615/critrevimmunol.v29.i3.40
31. Chua HL, Bhat-Nakshatri P, Clare SE, Morimiya A, Badve S, Nakshatri H. NF-kappaB represses E-cadherin expression and enhances epithelial to mesenchymal transition of mammary epithelial cells: potential involvement of ZEB-1 and ZEB-2. Oncogene (2007) 26:711–24. doi: 10.1038/sj.onc.1209808
32. Chuang M-J, Sun K-H, Tang S-J, Deng M-W, Wu Y-H, Sung J-S, et al. Tumor-derived tumor necrosis factor-alpha promotes progression and epithelial-mesenchymal transition in renal cell carcinoma cells. Cancer Sci (2008) 99:905–13. doi: 10.1111/j.1349-7006.2008.00756.x
33. Xiao-Mei ZHANG, Chun-Yan LIU, Zong-Hong SHAO. Progression in regulatory mechanism of helper T cell subsets. Chin J Immunol (2020) 36:3045–54. doi: 10.3969/j.issn.1000-484X.2020.24.020
34. Cao P-P, Wang Z-C, Schleimer RP, Liu Z. Pathophysiologic mechanisms of chronic rhinosinusitis and their roles in emerging disease endotypes. Ann Allergy Asthma Immunol (2019) 122:33–40. doi: 10.1016/j.anai.2018.10.014
35. Poosti F, Bansal R, Yazdani S, Prakash J, Post E, Klok P, et al. Selective delivery of IFN-γ to renal interstitial myofibroblasts: a novel strategy for the treatment of renal fibrosis. FASEB J (2015) 29:1029–42. doi: 10.1096/fj.14-258459
36. Baroni GS, D’Ambrosio L, Curto P, Casini A, Mancini R, Jezequel AM, et al. Interferon gamma decreases hepatic stellate cell activation and extracellular matrix deposition in rat liver fibrosis. Hepatology (1996) 23:1189–99. doi: 10.1002/hep.510230538
37. Oldroyd SD, Thomas GL, Gabbiani G, El Nahas AM. Interferon-gamma inhibits experimental renal fibrosis. Kidney Int (1999) 56:2116–27. doi: 10.1046/j.1523-1755.1999.00775.x
38. Forbes MS, Thornhill BA, Minor JJ, Gordon KA, Galarreta CI, Chevalier RL. Fight-or-flight: murine unilateral ureteral obstruction causes extensive proximal tubular degeneration, collecting duct dilatation, and minimal fibrosis. Am J Physiology-Renal Physiol (2012) 303:F120–9. doi: 10.1152/ajprenal.00110.2012
39. Van Crombruggen K, Zhang N, Gevaert P, Tomassen P, Bachert C. Pathogenesis of chronic rhinosinusitis: Inflammation. J Allergy Clin Immunol (2011) 128:728–32. doi: 10.1016/j.jaci.2011.07.049
40. Bachert C, Zhang N, Cavaliere C, Weiping W, Gevaert E, Krysko O. Biologics for chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol (2020) 145:725–39. doi: 10.1016/j.jaci.2020.01.020
41. Chen XH, Zhang GH. [Research advances in endotypes and precision medicine of chronic rhinosinusitis]. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi (2022) 57:783–8. doi: 10.3760/cma.j.cn115330-20210813-00547
42. Zurawski SM, Vega F, Huyghe B, Zurawski G. Receptors for interleukin-13 and interleukin-4 are complex and share a novel component that functions in signal transduction. EMBO J (1993) 12:2663–70. doi: 10.1002/j.1460-2075.1993.tb05927.x
43. Jing ZHANG, Dongli ZHANG, WANG M. Inflammatory mechanisms of T cells in chronic sinusitis. Chin J Otorhinolaryngology-Skull Base Surg (2022) 28:106–10. doi: 10.11798/j.issn.1007-1520.202221381
44. Merlo LMF, DuHadaway JB, Montgomery JD, Peng W-D, Murray PJ, Prendergast GC, et al. Differential roles of IDO1 and IDO2 in T and B cell inflammatory immune responses. Front Immunol (2020) 11:1861. doi: 10.3389/fimmu.2020.01861
45. Yu ZHANG, Xiangdong WANG, Hong WANG, Erzhong FAN, Ying LI, Xicheng SONG, et al. Study on expression of IL-13 and correlation between IL-13 and MUC5AC in eosinophilic chronic rhinosinusitis with nasal polyps. Chin Arch Otolaryngology-Head Neck Surg (2017) 24:371–4. doi: 10.16066/j.1672-7002.2017.07.013
46. Cho GS, Moon B-J, Lee B-J, Gong C-H, Kim NH, Kim Y-S, et al. High rates of detection of respiratory viruses in the nasal washes and mucosae of patients with chronic rhinosinusitis. J Clin Microbiol (2013) 51:979–84. doi: 10.1128/JCM.02806-12
47. Lan F, Zhang N, Gevaert E, Zhang L, Bachert C. Viruses and bacteria in Th2-biased allergic airway disease. Allergy (2016) 71:1381–92. doi: 10.1111/all.12934
48. Lee CG, Homer RJ, Zhu Z, Lanone S, Wang X, Koteliansky V, et al. Interleukin-13 induces tissue fibrosis by selectively stimulating and activating transforming growth factor β1. J Exp Med (2001) 194:809–22. doi: 10.1084/jem.194.6.809
49. Oriente A, Fedarko NS, Pacocha SE, Huang SK, Lichtenstein LM, Essayan DM. Interleukin-13 modulates collagen homeostasis in human skin and keloid fibroblasts. J Pharmacol Exp Ther (2000) 292:988–94.
50. Fertin C, Nicolas JF, Gillery P, Kalis B, Banchereau J, Maquart FX. Interleukin-4 stimulates collagen synthesis by normal and scleroderma fibroblasts in dermal equivalents. Cell Mol Biol (1991) 37:823–9.
51. Fichtner-, Strober W, Kawakami K, Puri RK, Kitani A. IL-13 signaling through the IL-13alpha2 receptor is involved in induction of TGF-beta1 production and fibrosis. Nat Med (2006) 12:99–106. doi: 10.1038/nm1332
52. Xiuxing L, Ping H, Junping X, Lixia X. Role of IL-13 in allergic diseases. Chin J Cell Mol Immunol (2017) 33:996–1000. doi: 10.13423/j.cnki.cjcmi.008205
53. Kim DW, Cho SH. Emerging endotypes of chronic rhinosinusitis and its application to precision medicine. Allergy Asthma Immunol Res (2017) 9:299. doi: 10.4168/aair.2017.9.4.299
54. Kim H, Ellis AK, Fischer D, Noseworthy M, Olivenstein R, Chapman KR, et al. Asthma biomarkers in the age of biologics. Allergy Asthma Clin Immunol (2017) 13:48. doi: 10.1186/s13223-017-0219-4
55. Fujieda S, Imoto Y, Kato Y, Ninomiya T, Tokunaga T, Tsutsumiuchi T, et al. Eosinophilic chronic rhinosinusitis. Allergology Int (2019) 68:403–12. doi: 10.1016/j.alit.2019.07.002
56. Yusi T. Expression and clinical significance of IL-5, IL-17 in chronic rhinosinusitis with nasal polyps. Zunyi Med Univ. doi: 10.27680/d.cnki.gzyyc.2021.000440
57. Jie C, Yiyou MAO, Zhuo C, Min L, Suang LI, Jiangyi L, et al. Mechanism and treatment progress of type II inflammation in chronic rhinosinusitis with nasal polyps. Research progress on the role of type II inflammation in chronic rhinosinusitis with polyps (2020) 55:993–7. doi: 10.3760/cma.j.cn115330-20200813-00671
58. Mack M. Inflammation and fibrosis. Matrix Biol (2018) 68–69:106–21. doi: 10.1016/j.matbio.2017.11.010
59. Kato A, Peters AT, Stevens WW, Schleimer RP, Tan BK, Kern RC. Endotypes of chronic rhinosinusitis: Relationships to disease phenotypes, pathogenesis, clinical findings, and treatment approaches. Allergy (2022) 77:812–26. doi: 10.1111/all.15074
60. Yang W, Zhenzhen T, Dongfang YAO, Jieen LI. Progress of TH17 cells in chronic rhinosinusitis. J Clin Otorhinolaryngology Head Neck Surg (2016) 30:161–6. doi: 10.13201/j.issn.1001-1781.2016.02.023
61. Gaffen SL. Recent advances in the IL-17 cytokine family. Curr Opin Immunol (2011) 23:613–9. doi: 10.1016/j.coi.2011.07.006
62. Okamoto Y, Hasegawa M, Matsushita T, Hamaguchi Y, Huu DL, Iwakura Y, et al. Potential roles of interleukin-17A in the development of skin fibrosis in mice. Arthritis Rheum (2012) 64:3726–35. doi: 10.1002/art.34643
63. Tan Z, Qian X, Jiang R, Liu Q, Wang Y, Chen C, et al. IL-17A plays a critical role in the pathogenesis of liver fibrosis through hepatic stellate cell activation. JI (2013) 191:1835–44. doi: 10.4049/jimmunol.1203013
64. Wang T, Liu Y, Zou J-F, Cheng Z-S. Interleukin-17 induces human alveolar epithelial to mesenchymal cell transition via the TGF-β1 mediated Smad2/3 and ERK1/2 activation. PloS One (2017) 12:e0183972. doi: 10.1371/journal.pone.0183972
65. Paul J, Singh AK, Kathania M, Elviche TL, Zeng M, Basrur V, et al. IL-17-driven intestinal fibrosis is inhibited by Itch-mediated ubiquitination of HIC-5. Mucosal Immunol (2018) 11:427–36. doi: 10.1038/mi.2017.53
66. Peng X, Xiao Z, Zhang J, Li Y, Dong Y, Du J. IL-17A produced by both γδ T and Th17 cells promotes renal fibrosis via RANTES-mediated leukocyte infiltration after renal obstruction. J Pathol (2015) 235:79–89. doi: 10.1002/path.4430
67. Li Y, Wu Y, Zhang C, Li P, Cui W, Hao J, et al. γδT Cell-derived interleukin-17A via an interleukin-1β-dependent mechanism mediates cardiac injury and fibrosis in hypertension. Hypertension (2014) 64:305–14. doi: 10.1161/HYPERTENSIONAHA.113.02604
68. Camargo L do N, Righetti RF, Aristóteles LR de CRB, dos Santos TM, de Souza FCR, Fukuzaki S, et al. Effects of anti-IL-17 on inflammation, remodeling, and oxidative stress in an experimental model of asthma exacerbated by LPS. Front Immunol (2018) 8. doi: 10.3389/fimmu.2017.01835
69. Bamba S, Andoh A, Yasui H, Araki Y, Bamba T, Fujiyama Y. Matrix metalloproteinase-3 secretion from human colonic subepithelial myofibroblasts: role of interleukin-17. J Gastroenterol (2003) 38:548–54. doi: 10.1007/s00535-002-1101-8
70. Gao Y. Exploring The Correlation between Neutrophil, IL-17A and chronic rhinosinusitis. Univ Electronic Sci Technol China (2021). doi: 10.27005/d.cnki.gdzku.2021.003979
71. Dudakov JA, Hanash AM, van den Brink MRM. Interleukin-22: immunobiology and pathology. Annu Rev Immunol (2015) 33:747–85. doi: 10.1146/annurev-immunol-032414-112123
72. Li XIAO, Lu BAI, Mingyuan LIU, Peng XIE, Tong WANG, Danping XIONG. The expresssion of IL-22 in nasal polyps tissues. J Clin Otorhinolaryngology Head Neck Surg (2017) 31:1749–52. doi: 10.13201/j.issn.1001-1781.2017.22.011
73. An-qi ZHANG, Peng-long SONG, Ling-lin JIANG, Jian-nan ZHAO, Lei ZHANG. Expression of IL-25,IL-17RB,IL-4,IL-5,and IL-13 inchronic rhinosinusitis with nasal polyps and its significance. J Harbin Med Univ (2021) 55:513–616. doi: 10.3969/j.issn.1000-1905.2021.06.010
74. Kariyawasam HH, Gane SB. Allergen-induced asthma, chronic rhinosinusitis and transforming growth factor-β superfamily signaling: mechanisms and functional consequences. Expert Rev Clin Immunol (2019) 15:1155–70. doi: 10.1080/1744666X.2020.1672538
75. Vanbruaene N. T cell regulation in chronic paranasal sinus disease. J Allergy Clin Immunol (2008) 121:S72–2. doi: 10.1016/j.jaci.2007.12.286
76. Serpero L, Petecchia L, Sabatini F, Giuliani M, Silvestri M, Di Blasi P, et al. The effect of transforming growth factor (TGF)-β1 and (TGF)-β2 on nasal polyp fibroblast activities involved upper airway remodeling: Modulation by fluticasone propionate. Immunol Lett (2006) 105:61–7. doi: 10.1016/j.imlet.2006.01.003
77. Meng J, Zhou P, Liu Y, Liu F, Yi X, Liu S, et al. The development of nasal polyp disease involves early nasal mucosal inflammation and remodelling. PloS One (2013) 8:e82373. doi: 10.1371/journal.pone.0082373
78. Ijaz T, Pazdrak K, Kalita M, Konig R, Choudhary S, Tian B, et al. Systems biology approaches to understanding Epithelial Mesenchymal Transition (EMT) in mucosal remodeling and signaling in asthma. World Allergy Organ J (2014) 7:13. doi: 10.1186/1939-4551-7-13
79. Biernacka A, Dobaczewski M, Frangogiannis NG. TGF-β signaling in fibrosis. Growth Factors (2011) 29:196–202. doi: 10.3109/08977194.2011.595714
80. O’Garra A, Stockinger B, Veldhoen M. Differentiation of human T(H)-17 cells does require TGF-beta! Nat Immunol (2008) 9:588–90. doi: 10.1038/ni0608-588
81. Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J Clin Invest (1998) 101:890–8. doi: 10.1172/JCI1112
82. Alam R, Forsythe P, Stafford S, Fukuda Y. Transforming growth factor beta abrogates the effects of hematopoietins on eosinophils and induces their apoptosis. J Exp Med (1994) 179:1041–5. doi: 10.1084/jem.179.3.1041
83. Sillett HK, Cruickshank SM, Southgate J, Trejdosiewicz LK. Transforming growth factor-beta promotes “death by neglect” in post-activated human T cells. Immunology (2001) 102:310–6. doi: 10.1046/j.1365-2567.2001.01185.x
84. Zhao S, Wang C. Regulatory T cells and asthma. J Zhejiang Univ Sci B (2018) 19:663–73. doi: 10.1631/jzus.B1700346
85. Teven CM, Farina EM, Rivas J, Reid RR. Fibroblast growth factor (FGF) signaling in development and skeletal diseases. Genes Dis (2014) 1:199–213. doi: 10.1016/j.gendis.2014.09.005
86. Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest (2009) 119:1420–8. doi: 10.1172/JCI39104
87. Sansoni ER, Sautter NB, Mace JC, Smith TL, Yawn JR, Lawrence LA, et al. Vitamin D 3 as a novel regulator of basic fibroblast growth factor in chronic rhinosinusitis with nasal polyposis: VD3 and bFGF in CRS with polyposis. Int Forum Allergy Rhinology (2015) 5:191–6. doi: 10.1002/alr.21474
88. Zittermann SI, Issekutz AC. Basic fibroblast growth factor (bFGF, FGF-2) potentiates leukocyte recruitment to inflammation by enhancing endothelial adhesion molecule expression. Am J Pathol (2006) 168:835–46. doi: 10.2353/ajpath.2006.050479
89. Tsunoda S, Sakurai H, Saito Y, Ueno Y, Koizumi K, Saiki I. Massive T-Lymphocyte Infiltration into the Host Stroma Is Essential for Fibroblast Growth Factor-2-Promoted Growth and Metastasis of Mammary Tumors via Neovascular Stability. Am J Pathol (2009) 174:671–83. doi: 10.2353/ajpath.2009.080471
90. Wang C, Li Y, Li H, Zhang Y, Ying Z, Wang X, et al. Disruption of FGF signaling ameliorates inflammatory response in hepatic stellate cells. Front Cell Dev Biol (2020) 8. doi: 10.3389/fcell.2020.00601
91. Lee KS, Kim SR, Park SJ, Min KH, Lee KY, Choe YH, et al. Mast cells can mediate vascular permeability through regulation of the PI3K–HIF-1α–VEGF axis. Am J Respir Crit Care Med (2008) 178:787–97. doi: 10.1164/rccm.200801-008OC
92. Rabquer BJ, Koch AE. Angiogenesis and vasculopathy in systemic sclerosis: evolving concepts. Curr Rheumatol Rep (2012) 14:56–63. doi: 10.1007/s11926-011-0219-1
93. Kirton JP, Xu Q. Endothelial precursors in vascular repair. Microvascular Res (2010) 79:193–9. doi: 10.1016/j.mvr.2010.02.009
94. Yamauchi H, Cristofanilli M, Nakamura S, Hortobagyi GN, Ueno NT. Molecular targets for treatment of inflammatory breast cancer. Nat Rev Clin Oncol (2009) 6:387–94. doi: 10.1038/nrclinonc.2009.73
95. Liming ZHU, Denghui LI, Song. Expression XU. Expression and clinical value of related inflammatory factors, VEGF and COX-2 in sinusitis serum. Chin J Lab Diagnosis (2022) 26:17–9. doi: 10.3969/j.issn.1007-4287.2022.01.006
96. Liew PX, Kubes P. The neutrophil’s role during health and disease. Physiol Rev (2019) 99:1223–48. doi: 10.1152/physrev.00012.2018
97. Castanheira FVS, Kubes P. Neutrophils and NETs in modulating acute and chronic inflammation. Blood (2019) 133:2178–85. doi: 10.1182/blood-2018-11-844530
98. Suzuki H, Takahashi Y, Wataya H, Ikeda K, Nakabayashi S, Shimomura A, et al. Mechanism of neutrophil recruitment induced by IL-8 in chronic sinusitis. J Allergy Clin Immunol (1996) 98:659–70. doi: 10.1016/S0091-6749(96)70100-8
99. Wang X, Zhang N, Bo M, Holtappels G, Zheng M, Lou H, et al. Diversity of T H cytokine profiles in patients with chronic rhinosinusitis: A multicenter study in Europe, Asia, and Oceania. J Allergy Clin Immunol (2016) 138:1344–53. doi: 10.1016/j.jaci.2016.05.041
100. Shi L-L, Xiong P, Zhang L, Cao P-P, Liao B, Lu X, et al. Features of airway remodeling in different types of Chinese chronic rhinosinusitis are associated with inflammation patterns. Allergy (2013) 68:101–9. doi: 10.1111/all.12064
101. Luo Q, Zhang Z, Liu D, Feng K, Jin X, Zhang J. Human neutrophil elastase induces MUC5AC overexpression in chronic rhinosinusitis through tumour necrosis factor-α converting enzyme. Acta Oto-Laryngologica (2016) 136:641–8. doi: 10.3109/00016489.2016.1144145
102. Yan D, Ye Y, Zhang J, Zhao J, Yu J, Luo Q. Human neutrophil elastase induces MUC5AC overexpression in chronic rhinosinusitis through miR-146a. Am J Rhinol Allergy (2020) 34:59–69. doi: 10.1177/1945892419871798
103. Ryu G, Mo J-H, Shin H-W. Epithelial-to-mesenchymal transition in neutrophilic chronic rhinosinusitis. Curr Opin Allergy Clin Immunol (2021) 21:30–7. doi: 10.1097/ACI.0000000000000701
104. Lee M, Kim DW, Khalmuratova R, Shin S-H, Kim Y-M, Han DH, et al. The IFN-γ–p38, ERK kinase axis exacerbates neutrophilic chronic rhinosinusitis by inducing the epithelial-to-mesenchymal transition. Mucosal Immunol (2019) 12:601–11. doi: 10.1038/s41385-019-0149-1
105. Shin H-W, Cho K, Kim DW, Han DH, Khalmuratova R, Kim S-W, et al. Hypoxia-inducible factor 1 mediates nasal polypogenesis by inducing epithelial-to-mesenchymal transition. Am J Respir Crit Care Med (2012) 185:944–54. doi: 10.1164/rccm.201109-1706OC
106. Huvenne W, van Bruaene N, Zhang N, van Zele T, Patou J, Gevaert P, et al. Chronic rhinosinusitis with and without nasal polyps: What is the difference? Curr Allergy Asthma Rep (2009) 9:213–20. doi: 10.1007/s11882-009-0031-4
107. Pawankar R, Nonaka M. Inflammatory mechanisms and remodeling in chronic rhinosinusitis and nasal polyps. Curr Allergy Asthma Rep (2007) 7:202–8. doi: 10.1007/s11882-007-0073-4
108. Quanxin LI, Zhongmei HE, Feng ZHANG, Linglong LI, Hong WAN, Dehong MAO. Research progress on the mechanism of granulocyte-macrophage colony-stimulating factor in chronic rhinosinusitis. J Modern Med Health (2021) 37:961–3. doi: 10.3969/j.issn.1009-5519.2021.06.018
109. Kanda A, Yasutaka Y, Bui DV, Suzuki K, Sawada S, Kobayashi Y, et al. Multiple biological aspects of eosinophils in host defense, eosinophil-associated diseases, immunoregulation, and homeostasis: Is their role beneficial, detrimental, regulator, or bystander? Biol Pharm Bull (2020) 43:20–30. doi: 10.1248/bpb.b19-00892
110. Sharbel D, Li M, Unsal AA, Tadros SY, Lee J, Biddinger P, et al. Use of mucosal eosinophil count as a guide in the management of chronic rhinosinusitis. Int Forum Allergy Rhinol (2020) 10:474–80. doi: 10.1002/alr.22517
111. Snidvongs K, Lam M, Sacks R, Earls P, Kalish L, Phillips PS, et al. Structured histopathology profiling of chronic rhinosinusitis in routine practice. Int Forum Allergy Rhinology (2012) 2:376–85. doi: 10.1002/alr.21032
112. Soler ZM, Sauer D, Mace J, Smith TL. Impact of mucosal eosinophilia and nasal polyposis on quality-of-life outcomes after sinus surgery. Otolaryngol Head Neck Surg (2010) 142:64–71. doi: 10.1016/j.otohns.2009.10.005
113. Barham HP, Osborn JL, Snidvongs K, Mrad N, Sacks R, Harvey RJ. Remodeling changes of the upper airway with chronic rhinosinusitis: Remodeling of upper airway in CRS. Int Forum Allergy Rhinology (2015) 5:565–72. doi: 10.1002/alr.21546
114. Haruna S, Nakanishi M, Otori N, Moriyama H. Histopathological features of nasal polyps with asthma association: an immunohistochemical study. Am J Rhinology (2004) 18:165–72. doi: 10.1177/194589240401800307
115. Ardehali MM, AMali A, Bakhshaee M, Madani Z, Amiri M. The comparison of histopathological characteristics of polyps in asthmatic and nonasthmatic patients. Otolaryngol Head Neck Surg (2009) 140:748–51. doi: 10.1016/j.otohns.2009.01.027
116. Shi L, Liu Z. Remodeling Features. In: Zhang L, Bachert C, editors. Chronic Rhinosinusitis. Singapore: Springer Nature Singapore (2022). p. 81–7. doi: 10.1007/978-981-16-0784-4_10
117. Saitoh T, Kusunoki T, Yao T, Kawano K, Kojima Y, Miyahara K, et al. Relationship between epithelial damage or basement membrane thickness and eosinophilic infiltration in nasal polyps with chronic rhinosinusitis. Rhinology (2009) 47:275–9. doi: 10.4193/Rhin08.109
118. Tanaka H, Komai M, Nagao K, Ishizaki M, Kajiwara D, Takatsu K, et al. Role of interleukin-5 and eosinophils in allergen-induced airway remodeling in mice. Am J Respir Cell Mol Biol (2004) 31:62–8. doi: 10.1165/rcmb.2003-0305OC
119. Bachert C, Gevaert P, Holtappels G, Cuvelier C, Van Cauwenberge P. Nasal polyposis: from cytokines to growth. Am J Rhinology (2000) 14:279–90. doi: 10.2500/105065800781329573
120. Eisma RJ, Allen JS, Lafreniere D, Leonard G, Kreutzer DL. Eosinophil expression of transforming growth factor-beta and its receptors in nasal polyposis: Role of the cytokines in this disease process. Am J Otolaryngol (1997) 18:405–11. doi: 10.1016/S0196-0709(97)90062-4
121. Molet SM, Hamid QA, Hamilos DL. IL-11 and IL-17 expression in nasal polyps: Relationship to collagen deposition and suppression by intranasal fluticasone propionate. Laryngoscope (2010) 113:1803–12. doi: 10.1097/00005537-200310000-00027
122. Takabayashi T, Tanaka Y, Susuki D, Yoshida K, Tomita K, Sakashita M, et al. Increased expression of L-plastin in nasal polyp of patients with nonsteroidal anti-inflammatory drug-exacerbated respiratory disease. Allergy (2018) 74:1307–16. doi: 10.1111/all.13677
123. Ko D-Y, Shin J-M, Um J-Y, Kang B, Park I-H, Lee H-M. Rapamycin inhibits transforming growth factor beta 1 induced myofibroblast differentiation via the phosphorylated-phosphatidylinositol 3-kinase mamMalian target of rapamycin signal pathways in nasal polyp–derived fibroblasts. Am J Rhinol Allergy (2016) 30:e211–7. doi: 10.2500/ajra.2016.30.4389
124. Cho J-S, Kang J-H, Shin J-M, Park I-H, Lee H-M. Inhibitory effect of delphinidin on extracellular matrix production via the MAPK/NF-κB pathway in nasal polyp-derived fibroblasts. Allergy Asthma Immunol Res (2015) 7:276. doi: 10.4168/aair.2015.7.3.276
125. Yang H-W, Park J-H, Shin J-M, Lee H-M. Glucocorticoids ameliorate periostin-induced tissue remodeling in chronic rhinosinusitis with nasal polyps. Clin Exp Allergy (2018). doi: 10.1111/cea.13267
126. Ohno I, Ohtani H, Nitta Y, Suzuki J, Hoshi H, Honma M, et al. Eosinophils as a source of matrix metalloproteinase-9 in asthmatic airway inflammation. Am J Respir Cell Mol Biol (1997) 16:212–9. doi: 10.1165/ajrcmb.16.3.9070604
127. Schwingshackl A, Duszyk M, Brown N, Moqbel R. Human eosinophils release matrix metalloproteinase-9 on stimulation with TNF-α☆☆☆★. J Allergy Clin Immunol (1999) 104:983–90. doi: 10.1016/S0091-6749(99)70079-5
128. Günel C, Bleier BS, Bozkurt G, Eliyatkin N. Microarray analysis of the genes associated with osteitis in chronic rhinosinusitis: Genes Associated With Osteitis in CRS. Laryngoscope (2017) 127:E85–90. doi: 10.1002/lary.26414
129. Baraldo S, Turato G, Bazzan E, Ballarin A, Damin M, Balestro E, et al. Noneosinophilic asthma in children: relation with airway remodelling. Eur Respir J (2011) 38:575–83. doi: 10.1183/09031936.00168210
130. Kim KK, Sheppard D, Chapman HA. TGF-β1 signaling and tissue fibrosis. Cold Spring Harb Perspect Biol (2018) 10:a022293. doi: 10.1101/cshperspect.a022293
131. Pearson AM. Scavenger receptors in innate immunity. Curr Opin Immunol (1996) 8:20–8. doi: 10.1016/S0952-7915(96)80100-2
132. Gordon S. Phagocytosis: an immunobiologic process. Immunity (2016) 44:463–75. doi: 10.1016/j.immuni.2016.02.026
133. Murray PJ. Macrophage polarization. Annu Rev Physiol (2017) 79:541–66. doi: 10.1146/annurev-physiol-022516-034339
134. Ryu G, Bae J-S, Kim JH, Kim EH, Lyu L, Chung Y-J, et al. Role of IL-17A in chronic rhinosinusitis with nasal polyp. Allergy Asthma Immunol Res (2020) 12:507. doi: 10.4168/aair.2020.12.3.507
135. Ploeger DT, Hosper NA, Schipper M, Koerts JA, de Rond S, Bank RA. Cell plasticity in wound healing: paracrine factors of M1/ M2 polarized macrophages influence the phenotypical state of dermal fibroblasts. Cell Commun Signal (2013) 11:29. doi: 10.1186/1478-811X-11-29
136. Mewhort HEM, Lipon BD, Svystonyuk DA, Teng G, Guzzardi DG, Silva C, et al. Monocytes increase human cardiac myofibroblast-mediated extracellular matrix remodeling through TGF-β1. Am J Physiology-Heart Circulatory Physiol (2016) 310:H716–24. doi: 10.1152/ajpheart.00309.2015
137. Lanone S, Zheng T, Zhu Z, Liu W, Lee CG, Ma B, et al. Overlapping and enzyme-specific contributions of matrix metalloproteinases-9 and -12 in IL-13–induced inflammation and remodeling. J Clin Invest (2002) 110:463–74. doi: 10.1172/JCI0214136
138. Zhu Z, Ding J, Ma Z, Iwashina T, Tredget EE. Alternatively activated macrophages derived from THP-1 cells promote the fibrogenic activities of human dermal fibroblasts: M2 macrophages promote fibrogenic activities of fibroblasts. Wound Rep Reg (2017) 25:377–88. doi: 10.1111/wrr.12532
139. Vannella KM, Wynn TA. Mechanisms of organ injury and repair by macrophages. Annu Rev Physiol (2017) 79:593–617. doi: 10.1146/annurev-physiol-022516-034356
140. Krysko O, Holtappels G, Zhang N, Kubica M, Deswarte K, Derycke L, et al. Alternatively activated macrophages and impaired phagocytosis of S. aureus in chronic rhinosinusitis: Phagocytosis and macrophage phenotype in CRS. Allergy (2011) 66:396–403. doi: 10.1111/j.1398-9995.2010.02498.x
141. Lucas T, Waisman A, Ranjan R, Roes J, Krieg T, Müller W, et al. Differential roles of macrophages in diverse phases of skin repair. JI (2010) 184:3964–77. doi: 10.4049/jimmunol.0903356
142. Honda E, Park A-M, Yoshida K, Tabuchi M, Munakata H. Myofibroblasts: biochemical and proteomic approaches to fibrosis. Tohoku J Exp Med (2013) 230:67–73. doi: 10.1620/tjem.230.67
143. Minshall EM, Cameron L, Lavigne F, Leung DYM, Hamilos D, Garcia-Zepada EA, et al. Eotaxin mRNA and protein expression in chronic sinusitis and allergen-induced nasal responses in seasonal allergic rhinitis. Am J Respir Cell Mol Biol (1997) 17:683–90. doi: 10.1165/ajrcmb.17.6.2865
144. Takabayashi T, Kato A, Peters AT, Hulse KE, Suh LA, Carter R, et al. Increased expression of factor XIII-A in patients with chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol (2013) 132:584–592.e4. doi: 10.1016/j.jaci.2013.02.003
145. Liu Z, Chen J, Cheng L, Li H, Liu S, Lou H, et al. Chinese society of allergy and Chinese society of otorhinolaryngology-head and neck surgery guideline for chronic rhinosinusitis. Allergy Asthma Immunol Res (2020) 12:176–237. doi: 10.4168/aair.2020.12.2.176
146. Abbas AK, Lichtman AH, Pillai S. Basic Immunology: Functions and Disorders of the Immune System, in: 6e: Sae-E-Book (2019). India: Elsevier: New Delhi. Available at: https://sc.panda321.com/extdomains/books.google.com/books/about/Basic_Immunology_E_Book.html?hl=zh-CN&id=bSuFDwAAQBAJ (Accessed 2, 2023).
147. Sallusto F. Heterogeneity of human CD4(+) T cells against microbes. Annu Rev Immunol (2016) 34:317–34. doi: 10.1146/annurev-immunol-032414-112056
148. O’Shea JJ, Paul WE. Mechanisms underlying lineage commitment and plasticity of helper CD4+ T cells. Science (2010) 327:1098–102. doi: 10.1126/science.1178334
149. Henry EK, Inclan-Rico JM, Siracusa MC. Type 2 cytokine responses: regulating immunity to helminth parasites and allergic inflammation. Curr Pharmacol Rep (2017) 3:346–59. doi: 10.1007/s40495-017-0114-1
150. Vivier E, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G, et al. Innate lymphoid cells: 10 years on. Cell (2018) 174:1054–66. doi: 10.1016/j.cell.2018.07.017
151. Yunxia HE, Baohui CHENG, Hailiang ZHAO. Role of type 2 innate lymphoid cells in inflammatory diseases of Respiratory system. Med Recapitulate (2021) 27:422–7. doi: 10.3969/j.issn.1006-2084.2021.03.002
152. Dietz CP, Luong A. Innate lymphoid cells: The innate counterpart to T helper cells. Rhinosinusitis Nasal Polyposis (2016) 79:58–68. doi: 10.1159/000445130
153. Hellings PW, Steelant B. Epithelial barriers in allergy and asthma. J Allergy Clin Immunol (2020) 145:1499–509. doi: 10.1016/j.jaci.2020.04.010
154. Lee K, Tai J, Lee SH, Kim TH. Advances in the knowledge of the underlying airway remodeling mechanisms in chronic rhinosinusitis based on the endotypes: A review. IJMS (2021) 22:910. doi: 10.3390/ijms22020910
155. Ball SL, Mann DA, Wilson JA, Fisher AJ. The role of the fibroblast in inflammatory upper airway conditions. Am J Pathol (2016) 186:225–33. doi: 10.1016/j.ajpath.2015.09.020
156. Nonaka M, Pawankar R, Saji F, Yagi T. Eotaxin synthesis by nasal polyp fibroblasts. Acta Otolaryngol (1999) 119:816–20. doi: 10.1080/00016489950180478
157. Nonaka M, Ogihara N, Fukumoto A, Sakanushi A, Kusama K, Pawankar R, et al. Nasal polyp fibroblasts produce MIP-3alpha in response to toll-like receptor ligands and cytokine stimulation. Rhinology (2010) 48:41–6. doi: 10.4193/Rhin09.019
158. Sun G, Li D, Guo S, Zhang D, Cui S. The role of neutrophil in the pathogenesis of chronic rhinosinusitis. J Clin Otorhinolaryngology Head Neck Surg (2019) 33:789–92. doi: 10.13201/j.issn.1001-1781.2019.08.028
159. Tong J, Gu Q. Expression and clinical significance of mucin gene in chronic rhinosinusitis. Curr Allergy Asthma Rep (2020) 20:63. doi: 10.1007/s11882-020-00958-w
160. Tipirneni KE, Zhang S, Cho D-Y, Grayson J, Skinner DF, Mackey C, et al. Submucosal gland mucus strand velocity is decreased in chronic rhinosinusitis: Submucosal gland mucus strand velocity in CRS. Int Forum Allergy Rhinol (2018) 8:509–12. doi: 10.1002/alr.22065
161. Song KS, Lee T-J, Kim K, Chung KC, Yoon J-H. cAMP-responding element-binding protein and c-Ets1 interact in the regulation of ATP-dependent MUC5AC gene expression. J Biol Chem (2008) 283:26869–78. doi: 10.1074/jbc.M802507200
162. Wu S, Li H, Yu L, Wang N, Li X, Chen W. IL-1β upregulates muc5ac expression via NF-κB-induced HIF-1α in asthma. Immunol Lett (2017) 192:20–6. doi: 10.1016/j.imlet.2017.10.006
163. Bachert C, Zhang L, Gevaert P. Current and future treatment options for adult chronic rhinosinusitis: Focus on nasal polyposis. J Allergy Clin Immunol (2015) 136:1431–40. doi: 10.1016/j.jaci.2015.10.010
Keywords: CRS, remodeling, inflammation, TGF-β, TNF-α, neutrophils
Citation: Gong X, Han Z, Fan H, Wu Y, He Y, Fu Y, Zhu T and Li H (2023) The interplay of inflammation and remodeling in the pathogenesis of chronic rhinosinusitis: current understanding and future directions. Front. Immunol. 14:1238673. doi: 10.3389/fimmu.2023.1238673
Received: 12 June 2023; Accepted: 28 August 2023;
Published: 12 September 2023.
Edited by:
Susetta Finotto, University Hospital Erlangen, GermanyReviewed by:
Xiangdong Wang, Beijing Institute of Otolaryngology, ChinaYusei Ohshima, University of Fukui, Japan
Copyright © 2023 Gong, Han, Fan, Wu, He, Fu, Zhu and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hui Li, dHRsaWh1aUAxNjMuY29t; Tianmin Zhu, dGlhbm1pbnpodUBjZHV0Y20uZWR1LmNu
 Xinru Gong
Xinru Gong Zhoutong Han1
Zhoutong Han1 Yuqi Wu
Yuqi Wu Yuanqiong He
Yuanqiong He