
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Immunol. , 15 August 2023
Sec. Autoimmune and Autoinflammatory Disorders : Autoimmune Disorders
Volume 14 - 2023 | https://doi.org/10.3389/fimmu.2023.1238647
Psoriasis is a chronic inflammatory skin disease with a prevalence of 0.14% to 1.99%. The underlying pathology is mainly driven by the abnormal immune responses including activation of Th1, Th17, Th22 cells and secretion of cytokines. Patients with psoriasis are more likely to develop cardiovascular disease (CVD) which has been well recognized as a comorbidity of psoriasis. As mediators of hemostasis and thromboinflammation, platelets play an important part in CVD. However, less is known about their pathophysiological contribution to psoriasis and psoriasis-associated CVD. A comprehensive understanding of the role of platelet activation in psoriasis might pave the path for more accurate prediction of cardiovascular (CV) risk and provide new strategies for psoriasis management, which alleviates the increased CV burden associated with psoriasis. Here we review the available evidence about the biomarkers and mechanisms of platelet activation in psoriasis and the role of platelet activation in intriguing the common comorbidity, CVD. We further discussed the implications and efficacy of antiplatelet therapies in the treatment of psoriasis and prevention of psoriasis-associated CVD.
Psoriasis is a chronic immune-mediated inflammatory skin disease, with a prevalence in different populations varying from 0.14% to 1.99% (1). The classical lesion of psoriasis is a well-demarcated raised, red plaque with a white scaly surface, occurring on the elbows, knees, scalp, and even any skin surface of the body. According to WHO report, psoriasis is associated with substantial psychological and economic burden (2). Despite this, the molecular mechanisms underlying the pathogenesis of psoriasis are not fully understood. External stimuli and genetic factors co-contribute to the activation of dendritic cells (DCs) and thus initiate the inflammatory cascade. The increased expression of cytokines by DCs including interleukin (IL)-12 and IL-23 induce T cell activation and differentiation into T helper (Th) cells. A subsequent inflammation and keratinocyte proliferation are aggravated by secreted cytokines from Th1, Th17 and Th22 cells, which ultimately lead to psoriasis progression (3–6).
It is well established that psoriasis is associated with a number of comorbidities including psoriatic arthritis, psychological illnesses, inflammatory bowel diseases, and cardiovascular diseases (CVD) (7, 8). Data have shown that psoriasis patients, especially those with >10% body surface area affected or a long duration or candidates for systemic therapy or phototherapy, are at increased risk for CVD including stroke, myocardial infarction (MI), and cardiovascular (CV) death (9–12). In this context, the guideline issued by the American College of Cardiology (ACC)/American Heart Association (AHA) advocates psoriasis diagnosis into CV risk prediction, without specifying a psoriasis severity threshold (13).
Platelets are anucleate blood cells generated from megakaryocytes in the bone marrow. With mRNAs and platelet granules such as α-granules and dense granules inherited from megakaryocytes, and various glycoproteins (GPs) on cell membrane, platelets exert multiple functions in hemorrhagic, thrombotic, and inflammatory diseases (14, 15). An increasing body of evidence suggests that platelet hyperactivation is induced by the abnormal immune system in psoriasis patients, and excessive activation of platelets in return aggravates the inflammation, which results in a vicious cycle in the etiopathogenesis of psoriasis (16). On the other hand, platelet dysfunction is involved in the pathogenesis of CVD including atherosclerosis, coronary thrombosis, stroke, and MI (17, 18). A Gene Ontology (GO) enrichment analysis showed that platelet aggregation and platelet activation terms were in the top five biological process terms in the psoriasis group with CVD risk factors (19), highlighting the important role of platelets in psoriasis-associated CVD.
This review mainly focuses on the biomarkers, mechanisms, and effects of platelet activation in psoriasis and CVD. We discuss how activated platelets contribute to psoriasis progression and initiate psoriasis-associated CVD, thereby providing an outlook for antiplatelet therapies in psoriasis in the future.
Platelet volume has been proven to be associated with various diseases such as CVD, systemic lupus erythematosus, and Alzheimer’s disease. Recognized as a marker of proliferation, metabolism, and thrombopoiesis of megakaryocytes in bone marrow and platelet functions, platelet volume is typically reflected by mean platelet volume (MPV) and platelet distribution width (PDW).
MPV, representing platelet morphological changes, is a marker indicating the activation and functions of platelets. Several studies have found patients with psoriasis are usually accompanied by a higher level of MPV when compared with healthy controls (Table 1) (20–30). Most of these studies included all types of psoriasis patients (20–24, 28), while some focused on patients with psoriasis vulgaris or psoriatic arthritis and revealed a similar elevated MPV level in these patients (25–27, 29, 30). Though several studies showed negative or no correlation between MPV and the psoriasis area and severity index (PASI) scores (27–30), more studies demonstrated that MPV levels were positively correlated with PASI scores, suggesting that MPV is an indicator of disease severity (20–26). Additionally, MPV levels after biological therapies were drastically decreased, aligning well with the PASI scores (30). Interestingly, MPV levels are higher in patients who developed CVD than non-CVD controls, and the risk of death or MI is higher in those CVD patients with larger MPV, which makes MPV an important predictive factor of diagnosis and prognosis in CVD (48). Therefore, using MPV as a predictive marker for developing CVD in psoriatic patients would be of value to investigate.
PDW is another important biomarker representing heterogeneity of platelet volume and platelet activation. A higher PDW level means more platelets in different sizes in the peripheral blood. Usually, PDW is positively related to MPV. Studies revealed higher PDW levels in patients with psoriasis, compared to the healthy controls (Table 1) (24, 25, 27). Nevertheless, PDW showed no significant correlation with PASI scores in these studies, indicating that it was not associated with disease severity. Similar to MPV, PDW levels are also increased in atherosclerosis, coronary artery disease, and cerebrovascular disease, and can predict the occurrence of cardiac death and infarction recurrence in patients with acute MI (49, 50). Based on these findings, elevated levels of MPV and PDW, which represent platelet hyperactivation, are critical indicators in the development of CVD and conducive to predictive of CV risk in psoriasis patients.
P-selectin, also referred to as CD62P or granular membrane protein-140, is a GP found in the α-granules of platelets and the Weibel-Palade bodies of endothelial cells. The P-selectin in plasma, called soluble P-selectin (sP-selectin), is considered to originate from platelets only. Activated platelets trigger membrane surface expression of P-selectin and its secretion into the plasma (24), which makes P-selectin a marker of platelet activation. P-selectin has been shown to mediate the interaction between activated platelets, endothelial cells and neutrophils, monocytes, eosinophils, T lymphocyte subsets (51). This essential role contributes to the inflammatory infiltration in psoriasis patients. Most studies, except one study conducted by Kwiek et al. in 2017 (40), found that levels of P-selectin, especially sP-selectin, were higher in psoriasis patients than healthy controls (Table 1) (33–39). Besides, sP-selectin levels were reduced significantly after treatments including clobetasol propionate, calcipotriol, narrow-band ultraviolet B (NB-UVB) and biological drugs (34, 35, 37). Intriguingly, four of these studies showed a positive correlation between sP-selectin and PASI scores (24, 33–35), while the other three studies showed no correlation (36–38), suggesting a controversial relationship between sP-selectin and PASI scores, which may be related to the demographic characteristics or disease types (psoriasis vulgaris, psoriatic arthritis, pustular psoriasis, etc.) of patients and requires further studies. The raised sP-selectin in a wide variety of acute and chronic CVD has been confirmed previously (52). So P-selectin levels can show a certain relationship between CVD, psoriasis and platelet activation, and can serve as a special marker of irreversible platelet activation and an indicator for treatment efficacy in psoriasis and psoriasis-associated CVD.
It has been found that activated platelets secrete platelet-derived microparticles (PDMPs) in an exocytotic budding way, thus PDMP is supposed to be a biomarker of platelet activation. PDMPs are membrane vesicles that can carry various molecules such as cytokines and lipid mediators. Aside from the classical procoagulant property, PDMPs also have regulatory effects on immune function and intracellular communication. Benefited from the special structural characteristics, PDMPs can cross over from blood into the synovial fluid, lymph, and bone marrow (53). Levels of PDMPs were elevated in psoriasis patients (Table 1) (24, 37, 41–43), and a positive relationship between PDMP levels and PASI scores, as well as MPV, PDW, and P-selectin, were observed (24). These changes in PDMP levels demonstrate that psoriasis severity is closely related to platelet activation. Moreover, PDMP levels were reduced after treatment with anti-tumor necrosis factor-α (TNF-α) agents or topical therapies, such as 0.05% clobetasol propionate and 0.005% calcipotriol (37, 44). Nevertheless, not all biological therapies (e.g.: IL-12/13 blockages) exerted inhibitory effect on PDMP levels (42). These data showed different efficacy in decreasing PDMP levels of various treatments, which may attribute to the numerous subtypes of PDMPs in different sizes and carrying different components. On the other hand, elevated PDMP concentrations are also observed in various CV conditions such as ischemic stroke, coronary artery disease, and hypertension, attributed to its role in adhesion, inflammation, procoagulation, and lipid deposition. Together, monitoring PDMP levels may have beneficial clinical implications in screening and prevention of psoriasis and CVD (54).
Platelet factor 4 (PF4), also known as CXCL4, is a useful marker of platelet activation released from α-granules of activated platelets (55). PF4 is not only associated with platelet function, but also promotes recruitment and activation of different inflammatory cell types, and modulates the immune response. Significant higher PF4 levels were found in psoriasis patients when compared with healthy controls. Moreover, PF4 levels were correlated with PASI scores, and reduced after conventional therapies such as topical steroids (Table 1) (32). Additionally, PF4 was involved in the development and progression of atherosclerosis and other cardiovascular diseases (56, 57). Immunofluorescence studies by Gleissner et al. also revealed an increased expression of PF4 in human atherosclerotic plaques (58). In conclusion, PF4 is a proper marker of platelet activation associated with psoriasis and CVD, and elevated levels of PF4 suggests the essential role of platelet activation in these diseases, which still needs to be studied in depth.
Interferon (IFN) is one of the central factors in the pathogenesis of psoriasis inflammation. IFN-α and IFN-β initiate myeloid DCs (mDCs) activation and inflammatory cascade in psoriasis, while IFN-γ has been considered to play a role in determining disease severity and therapy evaluation (59). To investigate the association between IFN and activated platelet phenotype, Garshick et al. performed platelet RNA sequencing in psoriasis patients and healthy controls, and found IFN signaling was the top upregulated pathway in psoriasis platelets. Moreover, they demonstrated that expression levels of platelet IFN-induced transcripts (e.g.: IFIT1, IFIT3, IFIT2, IFIT5, IFITM3, OAS2, STAT1, etc.) correlated with PASI scores, circulating IFN-γ and IL-17A levels, and could be increased following stimulation with the combination of IFN-γ and IL-17A (Table 1) (45). Furthermore, study by Zou et al. suggested that some of these IFN-induced transcripts (STAT1, IFIT1, and IFIT3) were key genes involved in psoriasis development (60). Higher levels of STAT1 were confirmed in psoriatic skin and STAT1 phosphorylation was increased following IFN-α or IFN-γ stimulation (61–65). In addition, Huang’s study revealed elevated levels of OAS1, OAS2, and OAS3 in psoriatic skin and serum and these IFN-induced enzymes decreased after biological therapies (66). Although it is not known to what extent these elevated IFN-related transcripts levels in psoriatic skin and serum could be attributed to platelets, studies collectively suggest that the increased IFN-induced transcripts levels of platelet can represent platelet activation and probably make platelet an important factor in IFN-induced inflammation in psoriasis. Apart from the role in psoriasis pathogenesis, IFN-β is proved to inhibit angiogenesis and arteriogenesis, while IFN-γ can promote development of MI and stroke, the two main atherosclerotic diseases (67, 68). Both of them are highly expressed in atherosclerotic lesions, but whether platelet IFN-induced transcripts play an equally important role in CVD as in psoriasis remains to be explored.
Thrombospondin-1 (TSP-1) is a homotrimeric, multidomain glycoprotein that is released from α-granules of activated platelets, present in the extracellular matrix, and circulates in plasma. TSP-1 induces platelet activation and aggregation through several pathways including CD36-dependent inhibition of the cAMP/protein kinase A signaling cascade and/or blocking the antithrombotic activity of nitric oxide/cGMP signaling (69, 70). In accordance with its functions, overexpression of TSP-1 was found in a series of CVD such as MI, cardiac hypertrophy, heart failure, angiogenesis, and atherosclerosis (71). Intriguingly, keratinocytes from psoriatic skin exhibited a reduced level of TSP-1 (46), and TSP-1 expression in psoriasis patients was inversely correlated with disease activity, which was related to the dysregulated expression of TSP-1 in the immune cells and inhibition of Th17 differentiation (Table 1) (47). However, the role of TSP-1 as a biomarker of platelet activation in psoriasis and CVD and as a link between the two diseases is still inconclusive, and is deserved to explore.
Although not completely implicated in the pathogenesis of psoriasis, the impaired endothelial function in psoriasis progression has been proven. Previous studies investigated the endothelial dysfunction via measuring circulating biomarkers (asymmetrical dimethylarginine, oxidized LDL, and endothelial progenitor cells) and vascular markers (pulse wave velocity, flow-mediated dilation, nitroglycerine-induced vasodilation, and aortic stiffness parameters), and results appeared in line with the hypothesis that endothelial functions in psoriasis patients were significantly damaged (72, 73). It is well known that damaged endothelium can initiate the platelet activation cascade. In brief, at the sites of endothelial damage, platelets are activated and adhere to the subendothelial extracellular matrix, the main components of which are fibrillar collagens type I and III. With the help of GPIbα on platelet membrane, von Willebrand factor (vWF) and fibrillar collagens, fibrinogen bridging mediates aggregation of adjacent platelets (45, 74). Consequently, available data above suggest that endothelial damage in psoriasis initiate platelet activation and aggregation at the injured endothelium.
Psoriasis originates as a result of dysregulated interactions of innate and adaptive components of the immune system with resident cutaneous cell types. Plasmacytoid DCs (pDCs), keratinocytes, natural killer T cells, and macrophages produce cytokines such as TNF-α, IFN-α and IFN-β to activate mDCs. Subsequently, more cytokines (especially IL-12 and IL-23) are released to induce T cell activation and differentiation, followed by IL-17, IFN-γ, IL-6 and TNF-α secretion from Th cells (3, 4, 6). Interestingly, cytokines in this self-amplifying loop result in platelet activation. For example, Maione et al. found that platelets pre-treated with IL-17 exhibited an increased ADP-induced P-selectin expression and fibrinogen binding, representing excessive platelet activation and aggregation (75). Hot A et al. also revealed that supernatants of IL-17A-stimulated primary endothelial cells facilitated the aggregatory response in platelets (76). Similarly, IL-1β, IL-6 and IL-8 can exert a regulatory effect on platelet function (77–79). In addition, IL-9 is also increased in psoriasis patients, which not only promotes Th17-associated inflammation and angiogenesis in psoriasis, but also facilitates P-selectin expression and platelet activation via JAK2/STAT3 pathway in deep venous thrombosis (80, 81). Collectively, these observations indicate a strong positive effect of cytokines on platelet activation.
Platelet-activating factor (PAF) is a potent phospholipid-derived mediator with multiple biological effects on platelet aggregation, vasodilation, bronchoconstriction, host defense, inflammatory responses, and skin barrier function (82–84). PAF is produced by numerous cell types, such as platelets, keratinocytes, endothelial cells, and monocytes, the role of which has been implicated in several diseases including ischemic stroke, MI, and inflammatory diseases (85). Increased PAF levels were detected in plasma and scales of psoriasis patients (86, 87). In addition, the key factors (arachidonic acid [AA] and eicosanoids) and enzymes (phospholipase A2 [PLA2]) for PAF formation were also increased in psoriatic tissue (88). PAF regulates Th17 cells and IL-17 axis functions and contributes to chemotaxis, aggregation, and degranulation of peripheral polymorphonuclear leukocytes, thus enhancing inflammation in psoriasis (particularly, pustular psoriasis) (87, 89, 90). In accordance with these results, PAF receptor antagonist PCA-4248 was proved to lower leukocyte accumulation in the skin and block psoriasis progression (90). As a strong activator of platelets, PAF has been shown to induce shape change and release of granules contents in human platelets (91). Moreover, PAF mediated platelet activation and aggregation through activation of protein kinase C (PKC) pathway, mobilization of intracellular Ca2+, stimulation of the phosphatidylinositol cycle, and increased release of AA (92, 93). The higher levels of PAF in psoriatic skin, along with its regulatory effect on platelets in other diseases, suggest that PAF is also an important mediator of platelet activation in psoriasis.
Leukocyte migration is an essential step for both psoriasis and CVD, as it’s followed by recruitment of immune cells and inflammatory infiltration into lesional skin and blood vessels. Thus, microabscess formation and thromboinflammatory injury are representative manifestations of psoriasis and CVD, respectively.
Activated platelets are found to adhere to endothelial junctions and capture leukocytes via CD40-CD40 ligand (CD40L) cross-talk. CD40, belonging to the TNF receptor superfamily, is a co-stimulatory receptor which can recognize CD40L molecule. Typically, CD40L is translocated from intracellular region to platelet surface after platelet activation, followed by the interaction with CD40-expressing cells including immune cells and endothelial cells (94, 95). Denfeld et al. demonstrated that keratinocytes and endothelial cells in early and chronic psoriatic lesions exhibited higher CD40 levels. And CD40-CD40L cross-talk between platelets and keratinocytes could promote T cell and monocyte infiltration into the psoriatic plaques, via increasing intercellular cell adhesion molecule (ICAM)-1, Bcl-x, IL-8, CCL20, RANTES and MCP-1 levels (96). Similarly, circulating platelet-leukocyte aggregation, which leads to the development of both atherosclerosis initiation and plaque rupture, is also promoted by CD40L (particularly human platelet-bound or recombinant soluble CD40L) via eliciting the formation and tethering of net-like ultra-large vWF multimers on endothelium (97–99). In advanced atherosclerosis, foam cell formation and production of matrix-degrading metalloproteinase (MMPs) by activated macrophages are significantly induced by CD40-CD40L cross-talk and stromal cell derived factor 1 (SDF-1) following platelet activation, which will lead to plaque destabilization and rupture (100, 101).
P-selectin derived from activated platelets also contributes to leukocyte migration, which exhibits a synergistic effect with CD40-CD40L cross-talk. P-selectin glycoprotein ligand-1 (PSGL-1) is the major ligand for P-selectin and can be detected in almost all kinds of leukocytes. P-selectin-PSGL-1 binding is one of the factors which mediate the interaction between platelets and most leukocytes, including polymorphonuclear neutrophils (PMNs), monocytes/macrophages, and lymphocytes in psoriasis (102, 103). An earlier study by Ludwig et al. confirmed that activated platelets supported leukocyte rolling in skin microvasculature by forming leukocyte-platelet aggregates through P-selectin-PSGL-1 binding (104). A later study by Zuchtriegel et al. further explored the role of P-selectin-PSGL-1 cross-talk, and showed that platelets could work as “pathfinders” guiding these leukocytes to the sites of inflammation, which emphasized the critical role of P-selectin in platelets-guided leukocytes extravasation. Specifically, P-selectin-PSGL-1 binding regulated ERK1/2 MAPK pathway and induced the high-affinity conformation of surface-expressed β2 integrin in neutrophils (95). Upregulated β2 integrin, along with other adhesion molecules such as ICAM-1 and vascular cell adhesion molecule (VCAM)-1, further guides the extravasation of immune cells to the perivascular tissue (95). In CVD such as atherosclerosis, P-selectin-PSGL-1 cross-talk also mediates mechanical interaction between platelets and neutrophils (105). In this way, recruited neutrophils scans for activated platelets and fosters their migration to inflamed or injured vessel wall, resulting in thromboinflammatory injury (106).
Studies by Herster et al. have found that platelet depletion could prevent PMNs infiltration into psoriatic skin in the imiquimod-induced mouse model (107), confirming the hypothesis that platelets are involved in leukocyte infiltration in the pathogenesis of psoriasis. Also, the recruitment of neutrophils to thrombi have been well demonstrated in previous studies (108). In summary, platelet-leukocyte aggregation and leukocyte infiltration induced by activated platelets in both psoriasis and CVD indicate platelet activation might be the link between the two diseases (Figure 1i).
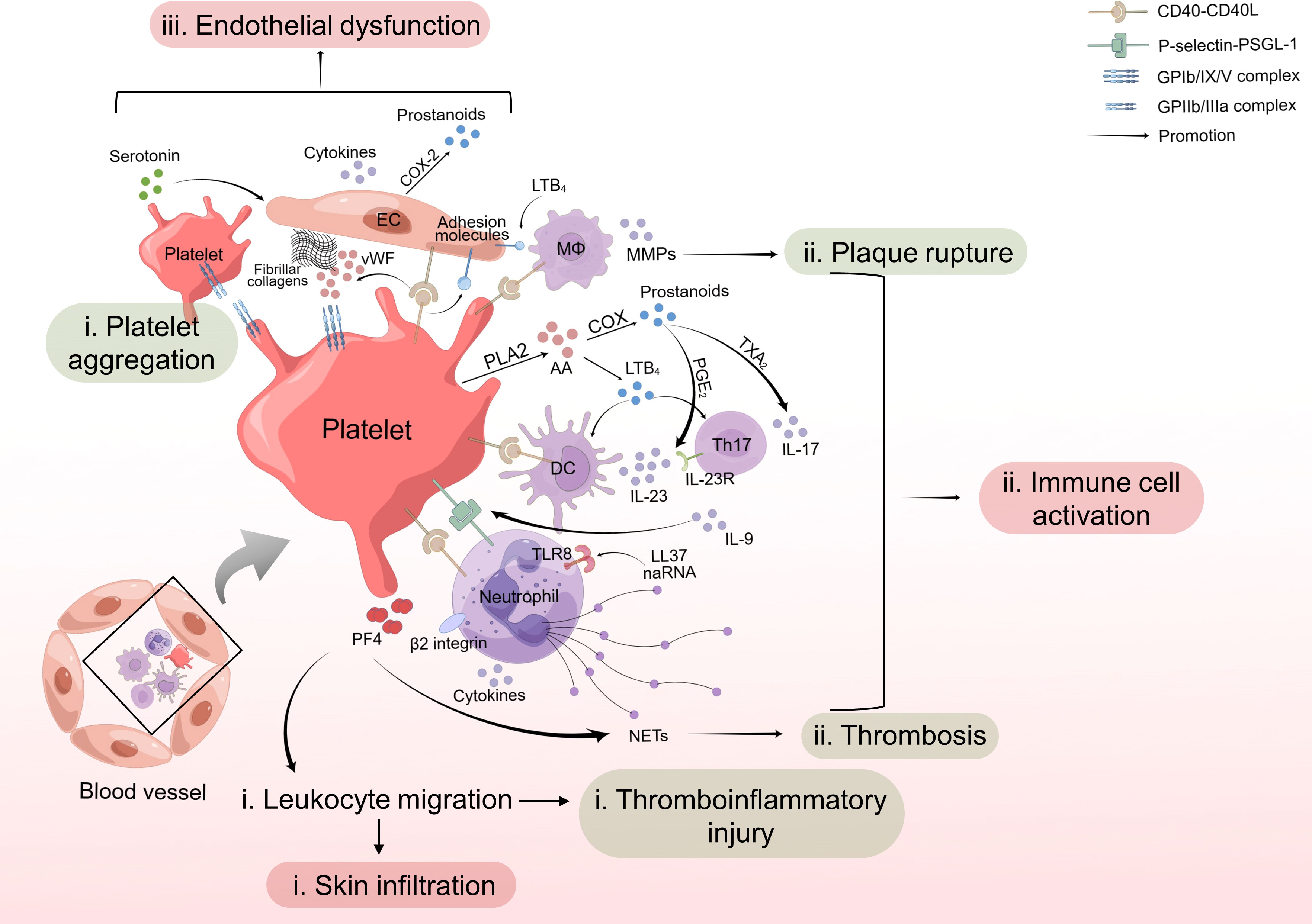
Figure 1 Role of platelet activation in psoriasis and cardiovascular disease. (i) Activated platelets interact with leukocytes through CD40-CD40L or P-selectin-PSGL-1 cross-talk and facilitate leukocyte migration, leading to skin infiltration and thromboinflammatory injury. (ii) Platelets contribute to activation of immune cells and increased release of NETs from PMNs, cytokines from DCs, Th17 cells and neutrophils, and MMPs from macrophages, further promoting thrombosis and plaque rupture. (iii) Platelet activation induces secretion of cytokines and adhesion molecules by endothelial cells, resulting in endothelial dysfunction. In summary, activated platelets work as promoters for psoriasis and psoriasis-associated CVD. CD40L, CD40 ligand; PSGL-1, P-selectin glycoprotein ligand-1; NET, neutrophil extracellular trap; PMN, polymorphonuclear neutrophil; DC, dendritic cell; EC, endothelial cell; MΦ, macrophage.
Researches on psoriasis and CVD have emphasized the role of abnormal immune cell activity in pathogenesis of the two diseases. Subsequent release of cytokines such as IL-17 following immune cell activation is regarded as a potential mechanistic link between psoriasis and CVD. The contribution of platelets to it has gradually been valued in the last decades.
Platelets are found by Gerdes et al. to enhance the differentiation of CD4+ T cells and the release of cytokines by Th1, Th17, and Treg subpopulations via both multiple chemokines and direct cell-cell contact (109). Later, Sanz-Martínez et al. further demonstrated that platelet activation in psoriasis stimulated the appearance of platelet-lymphocyte complexes and modulated the production of IL-17 (110). According to previous studies, AA and its metabolites, which can be produced from hyperactivated platelets with the activation of PLA2 and cyclooxygenase (COX), may contribute to platelet-induced immune cell activation (111–113). For example, 1) prostaglandin E2 (PGE2) facilitates the expansion of Th17 cells via up-regulated expression of IL-23 in DCs and IL-23 receptor subunit gene on Th17 cells (114, 115); 2) thromboxane A2 (TXA2) induces IL-17 secretion in psoriatic lesions (116); 3) leukotriene (LT)B4 promotes the recruitment of DCs and T cells and the production of cytokines including IL-23, IL-12 and IL-17, leading to intraepidermal microabscesses and hyperproliferation of epidermal cells (117, 118). However, there were no reports and literature of the relevant studies on AA metabolites directly derived from activated platelets in psoriasis. Although Vila et al. found an increased COX activity in platelets from psoriasis patients (119), suggesting a possible higher levels of AA metabolites released from psoriatic platelets, further studies are required to ascertain the effect of AA metabolites from these platelets, which has not yet been confirmed, in patients with psoriasis.
Neutrophil extracellular trap (NET) is another factor which plays an essential role in immune cell activation promoted by platelets. Hyperactivated PMNs secreting NETs and more cytokines, which promote keratinocytes proliferation and skin inflammation, have been observed in psoriasis patients in previous studies (120, 121). PAF was proved to mediate the promoting effect of activated platelets on NET formation in neutrophils in psoriasis patients (122). NET formation can also occur upon interaction of platelets with neutrophils via PF4 release in vasculitis (123, 124). Similarly in CVD, activated neutrophils produce NETs and consequently promote thrombosis via interactions with platelets (125). Low-density neutrophils co-localized with platelets induce increased NET formation and are strongly correlated with non-calcified coronary burden (122). In turn, NET-associated RNA (naRNA), along with the antimicrobial peptide LL37 that induces platelet aggregation and promotes thrombus formation (126, 127), triggers cytokine and NET release by PMNs through Toll-like receptor 8 (TLR8) signaling and aggravates inflammation in psoriasis and CVD (128).
In conclusion, platelet-intrigued dysfunction of T cells, DCs, neutrophils, macrophages or even more immune cells influences plaque instability of atherosclerosis, occurrence, and development of other CVD, and immune homeostasis of psoriasis, indicating that platelets work as a link between psoriasis and psoriasis-associated CVD through activating immune cells in different ways (Figure 1ii).
Endothelial dysfunction is one of the fundamental factors for both psoriasis and CVD. As shown above, impaired vascular health has been observed in psoriasis patients (72, 73) and platelets might be involved in the process of endothelial damage in psoriasis. While platelet-triggered endothelial damage, especially endothelial inflammation, also results in abnormal fluid homeostasis and the development of atherosclerosis and other CVD (129). Therefore, platelet may be the perfect link between inflammatory skin diseases and systemic vascular change (130, 131).
An In-vitro study revealed that platelets isolated from psoriasis patients, but not from healthy controls, preferentially adhered to human aortic endothelial cells (HAECs) and induced proinflammatory transcriptional changes including upregulation of IL-8, IL-1β and COX-2, mainly through the tubular connections between platelets and HAECs (45). Kotowicz et al. found expressions of adhesion molecules in endothelial cells, including ICAM-1, VCAM-1 and E-selectin, were significantly enhanced by stimulation with CD40L (132), which was usually located in the membrane of activated platelets and bound with CD40 on endothelial cells to function. On the other hand, Kim et al. confirmed that integrin was the one of the most important proteins in the psoriasis group with high CVD risk through GO and IPA analysis (19). Consistent with this, the β1 and β2 integrin-dependent affinity of monocyte/macrophage to ICAM-1 and VCAM-1 on endothelial cells is enhanced by platelet-induced LTB4 and accelerate the adhesion of monocyte/macrophage to endothelium in atherosclerotic plaque (133). Similar to monocytes/macrophages, neutrophils also accumulate at the dysfunctional endothelium after plaque rupture via integrins, which will amplify platelet aggregation and blood coagulation (127, 134).
Besides, as an important mediator derived from platelets, serotonin may initiate the formation of gaps between endothelial cells and aggravate vascular leakage in several systems, such as the cerebral ventricular system and the synovial vasculature in arthritis (135). Studies by Cloutier et al. demonstrated that platelet serotonin accumulated via the serotonin transporter in the inflamed joints increased vascular permeability and further mediating inflammatory infiltration in an inflammatory arthritis mouse model (135). Intriguingly, serotonin reuptake inhibitors were found to improve the physical symptoms of psoriasis (136), suggesting there might be similar effects of platelet serotonin on endothelial function in psoriasis.
Collectively, platelets facilitated endothelial inflammation, amplified vascular permeability and induced adhesion molecule expression, contributing to endothelial dysfunction, finally aggravating psoriasis and inducing a higher CV risk in psoriasis patients (Figure 1iii).
Aspirin, also known as acetylsalicylic acid, has been universally recognized as a classical antiplatelet medication, because of its inhibitory effect on COX-1. It is a very common therapy applied to the prevention and treatment of CVD, as it could attenuate platelet-induced NET formation, reduce platelet-leukocyte aggregation and subsequent immune cell activation (137, 138), which may also help to abrogate inflammatory infiltration in psoriasis and atherosclerosis. As for its role in psoriasis, previous studies suggested that aspirin was able to reduce risk of MI and stroke in patients with higher plasma C-reactive protein levels (139), and to ameliorate vascular inflammation in psoriasis (140). Moreover, low-dose aspirin has also been shown to reduce serum TXB2 (the stable metabolite of TXA2) and improve endothelial inflammation induced by hyperactive platelets in psoriasis, via inhibition of COX-1 (45). Although the efficacy of aspirin on improvement of psoriatic skin manifestations remains unclear, antiplatelet and anti-inflammatory properties of aspirin make it a potential therapeutic approach for psoriasis and psoriasis-associated CVD. Nevertheless, drug risks should be carefully evaluated when using aspirin in combination with methotrexate and cyclosporine because aspirin may increase the toxicity of the other two drugs (141).
It is well established that statins can drastically reduce CV risk because of their strong lipid-lowering effect. In addition, statins have been proven to attenuate platelet activation and aggregation in CVD (142). Various studies explored statin-specific molecular targets about platelets and their downstream effectors, including those involved in psoriasis pathogenesis which we have mentioned above. Firstly, statins (including simvastatin, atorvastatin and rosuvastatin, but not pravastatin) have been found to influence AA metabolism, via reducing platelet PLA2 phosphorylation and COX-1 activation, and to inhibit PG production as well as AA-induced platelet aggregation (143–146). Secondly, studies demonstrated that atorvastatin suppressed platelet CD40L expression and platelet activation with consequent reduction of leukocyte aggregation and MMP secretion (147, 148). Thirdly, expression of P-selectin and its major ligand, PSGL-1, were reduced by statin therapy (149), thus inhibiting the adhesion of activated platelets to leukocytes and extravasation of immune cells mediated by P-selectin-PSGL-1 interaction. However, it should be noted that different statins may have different effects on platelet activation according to their different molecular composition and variable impacts on signaling pathways, which calls for more research later on.
Considering the efficacy of statins in CVD treatment and the accumulation of evidence of accelerated CVD in psoriasis, earlier use of statins has been recommended in patients with psoriasis, particularly those with a ≥5% 10-year risk of atherosclerotic disease, by both the ACC/AHA guidelines and the American Academy of Dermatology/National Psoriasis Foundation (AAD/NPF) guidelines recently (9, 150). On the other hand, studies demonstrated that statins could significantly reduce PASI scores and improve disease severity in psoriasis (151, 152), indicating the nonnegligible role of statin therapy in both psoriasis and its comorbidity, CVD.
In addition, statins have been shown to increase the sensitivity of platelet cell membrane to aspirin treatment, which could consequently amplify the effect of aspirin on platelets (141). Psoriasis patients with higher CV risk usually need polydrug therapies, so the synergistic effect of aspirin and statins should be taken into account in disease management.
Apart from the conventional antiplatelet drugs, some therapies may have benefits beyond psoriasis in inhibiting platelet activation. Studies by Sanz-Martínez et al. demonstrated that psoriasis patients receiving anti-TNF-α therapy, one of the most common-used biological agents in psoriasis, showed a significantly lower risk of major adverse CV events compared to those receiving topical or oral and/or phototherapy agents (110). Decreased levels of platelet-lymphocyte complexes and PDMPs were found in responder group of patients (37, 44, 110), highlighting that anti-TNF-α therapy could reduce platelet activation and may mitigate CV risk in psoriasis.
However, the effects of other treatments for psoriasis on platelet activation are still inconclusive. For example, 1) secukinumab showed no effect on platelet/lymphocyte ratio (153); 2) anti-IL-12/23 treatment had no effect on PDMPs (42); 3) high concentrations of psoralen and ultraviolet A radiation (PUVA) promoted platelet aggregation in vitro, but caused no detectable abnormality in platelet function in vivo (154); 4) PUVA induced immune suppression in skin inflammation and apoptosis via activation of the PAF pathway (155), which led us to propose that PAF may also be involved in the effect of PUVA on platelet activation. Wu et al. found that patients with psoriasis who were treated with PUVA exhibited a higher CV risk than those treated with anti-TNF therapies (156), but no studies have been reported about effects of PUVA on CV risk in psoriasis patients compared with those who received no treatment. Prospective studies are still necessary to verify the effects of biological agents on more biomarkers of platelet activation such as MPV, PDW, and P-selectin in patients with psoriasis, and to further explore the role of PUVA in platelet activation.
Recent studies showed that natural products extracted from herbal medicines may have synergistic effects to alleviate psoriasis and platelet activation. For example, curcumin, frequently employed as an anti-inflammatory agent in conventional medicine, could be a complementary therapy of psoriasis which reduced serum levels of IL-22 and PASI scores (157, 158). Curcumin has also been proven to prevent platelet aggregation in atherosclerosis and lower platelet-leukocyte adhesion in cerebral microcirculation (159). Besides, several other natural products, including Grape seed proanthocyanidin extract, Thymol, Kaempferol, and Luteolin, also alleviate psoriasis via reducing the frequency and function of Th17 cells and increasing that of Treg cells (160), and simultaneously inhibit platelet aggregation, inflammatory cell and platelet adhesion, and thrombogenesis (161–164). However, research on the efficacy of natural products in psoriasis and psoriasis-associated CVD is still insufficient, awaiting further studies.
In general, given the important role of platelet activation in psoriasis and CVD, targeting platelets and platelet-derived mediators may be a potent novel strategy for psoriasis treatment in the future.
Platelets may be excessively activated via endothelial damage, numerous cytokines release and PAF production induced by abnormal immune system in psoriasis, and in turn overactivation of platelets may contribute to initiation and progression of immune responses in lesional skin and blood vessels, ultimately leading to psoriasis and its comorbidities such as atherosclerosis, ischemic heart disease, stroke, MI, and other CVD. Besides, biomarkers of platelet activation which have been well investigated showed a direct relationship between platelet activation and psoriasis. Based on this point, antiplatelet therapies including aspirin and statins might have positive efficacy on psoriasis and psoriasis-associated CVD. Although some encouraging data have been published, deeper studies are urgently warranted to establish the unambiguous role for platelet activation in the pathogenesis of psoriasis and psoriasis-associated CVD. Also, additional large and prospective studies are required to provide compelling evidence regarding good effects of antiplatelet therapies in psoriasis management.
ZJ reviewed the current literature, wrote the manuscript, and designed the figure. XJ reviewed the literature and drafted the manuscript. AC aided in revising the manuscript. WH devised the structure of the review and wrote the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by the financial support from the National Natural Science Foundation of China (Grant No. 31971054, Grant No. 82273533 and Grant No. 81874238), the Natural Science Foundation of Chongqing (Grant No. CSTB2022NSCQ-MSX1063) and the research foundation of the first affiliated hospital of Chongqing Medical University.
The authors gratefully thank Figdraw for providing basic material for the artwork.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Parisi R, Iskandar IYK, Kontopantelis E, Augustin M, Griffiths CEM, Ashcroft DM, et al. National, regional, and worldwide epidemiology of psoriasis: systematic analysis and modelling study. BMJ (2020) 369:m1590. doi: 10.1136/bmj.m1590
2. World Health Organization. Global Report on Psoriasis (2016). Available at: https://apps.who.int/iris/handle/10665/204417 (Accessed June 5, 2023).
3. Armstrong AW, Read C. Pathophysiology, clinical presentation, and treatment of psoriasis: A review. JAMA (2020) 323(19):1945–60. doi: 10.1001/jama.2020.4006
4. Greb JE, Goldminz AM, Elder JT, Lebwohl MG, Gladman DD, Wu JJ, et al. Psoriasis. Nat Rev Dis Primers (2016) 2:16082. doi: 10.1038/nrdp.2016.82
5. Griffiths CEM, Armstrong AW, Gudjonsson JE, Barker J. Psoriasis. Lancet (2021) 397(10281):1301–15. doi: 10.1016/S0140-6736(20)32549-6
6. Alwan W, Nestle FO. Pathogenesis and treatment of psoriasis: exploiting pathophysiological pathways for precision medicine. Clin Exp Rheumatol (2015) 33(5 Suppl 93):S2–6.
7. Garshick MS, Ward NL, Krueger JG, Berger JS. Cardiovascular risk in patients with psoriasis: JACC review topic of the week. J Am Coll Cardiol (2021) 77(13):1670–80. doi: 10.1016/j.jacc.2021.02.009
8. Yeung H, Takeshita J, Mehta NN, Kimmel SE, Ogdie A, Margolis DJ, et al. Psoriasis severity and the prevalence of major medical comorbidity: a population-based study. JAMA Dermatol (2013) 149(10):1173–9. doi: 10.1001/jamadermatol.2013.5015
9. Elmets CA, Leonardi CL, Davis DMR, Gelfand JM, Lichten J, Mehta NN, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with awareness and attention to comorbidities. J Am Acad Dermatol (2019) 80(4):1073–113. doi: 10.1016/j.jaad.2018.11.058
10. Samarasekera EJ, Neilson JM, Warren RB, Parnham J, Smith CH. Incidence of cardiovascular disease in individuals with psoriasis: a systematic review and meta-analysis. J Invest Dermatol (2013) 133(10):2340–6. doi: 10.1038/jid.2013.149
11. Lin HW, Wang KH, Lin HC, Lin HC. Increased risk of acute myocardial infarction in patients with psoriasis: a 5-year population-based study in Taiwan. J Am Acad Dermatol (2011) 64(3):495–501. doi: 10.1016/j.jaad.2010.01.050
12. Abuabara K, Azfar RS, Shin DB, Neimann AL, Troxel AB, Gelfand JM. Cause-specific mortality in patients with severe psoriasis: a population-based cohort study in the U. K Br J Dermatol (2010) 163(3):586–92. doi: 10.1111/j.1365-2133.2010.09941.x
13. Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: executive summary: A report of the American College of Cardiology/American heart association task force on clinical practice guidelines. J Am Coll Cardiol (2019) 74(10):1376–414. doi: 10.1016/j.jacc.2019.03.009
14. van der Meijden PEJ, Heemskerk JWM. Platelet biology and functions: new concepts and clinical perspectives. Nat Rev Cardiol (2019) 16(3):166–79. doi: 10.1038/s41569-018-0110-0
15. Liu X, Gorzelanny C, Schneider SW. Platelets in skin autoimmune diseases. Front Immunol (2019) 10:1453. doi: 10.3389/fimmu.2019.01453
16. Fan Z, Wang L, Jiang H, Lin Y, Wang Z. Platelet dysfunction and its role in the pathogenesis of psoriasis. Dermatology (2021) 237(1):56–65. doi: 10.1159/000505536
17. Benjamin EJ, Muntner P, Alonso A. American heart association council on epidemiology and prevention statistics committee and stroke statistics subcommittee, heart disease and stroke statistics-2019 update: a report from the American heart association. Circulation (2019) 139:e56–e528. doi: 10.1161/CIR.0000000000000659
18. Fuentes F, Palomo I, Fuentes E. Platelet oxidative stress as a novel target of cardiovascular risk in frail older people. Vascul Pharmacol (2017) 93-95:14–9. doi: 10.1016/j.vph.2017.07.003
19. Kim NY, Back JH, Shin JH, Ji MJ, Lee SJ, Park YE, et al. Quantitative proteomic analysis of human serum using tandem mass tags to predict cardiovascular risks in patients with psoriasis. Sci Rep (2023) 13(1):2869. doi: 10.1038/s41598-023-30103-2
20. Mir MM, Dogra D, Koul KK. Hemostatic and Coagulation Profile in Psoriasis: A Hospital-Based case control study. Indian J Dermatol (2022) 67(3):247–51. doi: 10.4103/ijd.IJD_630_20
21. Farag AG, Zytoon AA, Habib MS, Elnaidany NF, Ibrahem RA, Salman S, et al. Mean platelet volume: an immanent predictor of subclinical atherosclerosis in psoriatic patients compared with interleukin-1α and interleukin-6. Egypt Women Dermatol Soc (2018) 15:80–7. doi: 10.1097/01.EWX.0000532719.31339.7c
22. Mahrous EM. The relationship between platelet volume and risk of atherosclerosis in patients with psoriasis. Egypt J Dermatol Venerol (2018) 38(1):29. doi: 10.4103/ejdv.ejdv_36_17
23. Raghavan V, Radha RK, Rao RK, Kuberan A. A correlative study between platelet count, mean platelet volume and red cell distribution width with the disease severity index in psoriasis patients. J Clin Diagn Res (2017) 11(9):EC13–6. doi: 10.7860/JCDR/2017/31172.10639
24. Chandrashekar L, Rajappa M, Revathy G, Sundar I, Munisamy M, Ananthanarayanan PH, et al. Is enhanced platelet activation the missing link leading to increased cardiovascular risk in psoriasis? Clin Chim Acta (2015) 446:181–5. doi: 10.1016/j.cca.2015.04.023
25. Kim DS, Lee J, Kim SH, Kim SM, Lee MG. Mean platelet volume is elevated in patients with psoriasis vulgaris. Yonsei Med J (2015) 56(3):712–8. doi: 10.3349/ymj.2015.56.3.712
26. Kilic S, Resorlu H, Isik S, Oymak S, Akbal A, Hiz MM, et al. Association between mean platelet volume and disease severity in patients with psoriasis and psoriatic arthritis. Postepy Dermatol Alergol (2017) 34(2):126–30. doi: 10.5114/ada.2017.67076
27. Korkmaz S. Mean platelet volume and platelet distribution width levels in patients with mild psoriasis vulgaris with metabolic syndrome. Postepy Dermatol Alergol (2018) 35(4):367–71. doi: 10.5114/ada.2017.71285
28. Ozkur E, Seremet S, Afsar FS, Altunay IK, Calikoglu EE. Platelet count and mean platelet volume in psoriasis patients. Sisli Etfal Hastan Tip Bul (2020) 54(1):58–61. doi: 10.14744/SEMB.2018.69370
29. Unal M. Platelet mass index is increased in psoriasis. A possible link between psoriasis and atherosclerosis. Arch Med Sci Atheroscler Dis (2016) 1(1):e145–9. doi: 10.5114/amsad.2016.64444
30. Capo A, Di Nicola M, Auriemma M, Piaserico S, Cuccurullo C, Santilli F, et al. Mean platelet volume variation after biologic therapy in psoriasis and psoriatic arthritis. Eur J Dermatol (2014) 24(1):133–5. doi: 10.1684/ejd.2014.2269
31. Sirin MC, Korkmaz S, Erturan I, Filiz B, Aridogan BC, Cetin ES, et al. Evaluation of monocyte to HDL cholesterol ratio and other inflammatory markers in patients with psoriasis. Bras Dermatol (2020) 95(5):575–82. doi: 10.1016/j.abd.2020.02.008
32. Tamagawa-Mineoka R, Katoh N, Ueda E, Masuda K, Kishimoto S. Elevated platelet activation in patients with atopic dermatitis and psoriasis: increased plasma levels of beta-thromboglobulin and platelet factor 4. Allergol Int (2008) 57(4):391–6. doi: 10.2332/allergolint.O-08-537
33. Saleh HM, Attia EA, Onsy AM, Saad AA, Abd Ellah MM. Platelet activation: a link between psoriasis per se and subclinical atherosclerosis–a case-control study. Br J Dermatol (2013) 169(1):68–75. doi: 10.1111/bjd.12285
34. Garbaraviciene J, Diehl S, Varwig D, Bylaite M, Ackermann H, Ludwig RJ, et al. Platelet P-selectin reflects a state of cutaneous inflammation: possible application to monitor treatment efficacy in psoriasis. Exp Dermatol (2010) 19(8):736–41. doi: 10.1111/j.1600-0625.2010.01095.x
35. Fathy Agamia N. Evaluation of soluble P-selectin and leptin serum levels in sera of patients of psoriasis and their possible role in the increase in the cardiovascular risks in psoriatic patients. J Clin Exp Dermatol Res (2014) 05(01). doi: 10.4172/2155-9554.1000201
36. Ataseven A, Ataseven H, Ozturk P, Ozdemir M, Kesli R. Levels of serum soluble p-selectin and e-selectin in psoriatic patients. Ann Dermatol (2014) 26(2):275–7. doi: 10.5021/ad.2014.26.2.275
37. Tamagawa-Mineoka R, Katoh N, Kishimoto S. Platelet activation in patients with psoriasis: increased plasma levels of platelet-derived microparticles and soluble P-selectin. J Am Acad Dermatol (2010) 62(4):621–6. doi: 10.1016/j.jaad.2009.06.053
38. Long JW, Tao J, Pi XM, Wang YY, Tu YT. Effect of narrow-band UVB phototherapy on soluble cell adhesion molecules in patients with psoriasis vulgaris. J Int Med Res (2010) 38(4):1507–12. doi: 10.1177/147323001003800434
39. Visser MJE, Venter C, Roberts TJ, Tarr G, Pretorius E. Psoriatic disease is associated with systemic inflammation, endothelial activation, and altered haemostatic function. Sci Rep (2021) 11(1):13043. doi: 10.1038/s41598-021-90684-8
40. Kwiek B, Narbutt J, Sysa-Jedrzejowska A, Langner A, Lesiak A. Long-term treatment of chronic plaque psoriasis with biological drugs can control platelet activation: targeting the bridge between inflammation and atherothrombosis. Postepy Dermatol Alergol (2017) 34(2):131–7. doi: 10.5114/ada.2017.67077
41. Papadavid E, Diamanti K, Spathis A, Varoudi M, Andreadou I, Gravanis K, et al. Increased levels of circulating platelet-derived microparticles in psoriasis: Possible implications for the associated cardiovascular risk. World J Cardiol (2016) 8(11):667–75. doi: 10.4330/wjc.v8.i11.667
42. Ho JC, Lee CH, Lin SH. No significant reduction of circulating endothelial-derived and platelet-derived microparticles in patients with psoriasis successfully treated with anti-IL12/23. BioMed Res Int (2016) 2016:3242143. doi: 10.1155/2016/3242143
43. Takeshita J, Mohler ER, Krishnamoorthy P, Moore J, Rogers WT, Zhang L, et al. Endothelial cell-, platelet-, and monocyte/macrophage-derived microparticles are elevated in psoriasis beyond cardiometabolic risk factors. J Am Heart Assoc (2014) 3(1):e000507. doi: 10.1161/JAHA.113.000507
44. Pelletier F, Garnache-Ottou F, Biichle S, Vivot A, Humbert P, Saas P, et al. Effects of anti-TNF-alpha agents on circulating endothelial-derived and platelet-derived microparticles in psoriasis. Exp Dermatol (2014) 23(12):924–5. doi: 10.1111/exd.12551
45. Garshick MS, Tawil M, Barrett TJ, Salud-Gnilo CM, Eppler M, Lee A, et al. Activated platelets induce endothelial cell inflammatory response in psoriasis via COX-1. Arterioscler Thromb Vasc Biol (2020) 40(5):1340–51. doi: 10.1161/ATVBAHA.119.314008
46. Nickoloff BJ, Mitra RS, Varani J, Dixit VM, Polverini PJ. Aberrant production of interleukin-8 and thrombospondin-1 by psoriatic keratinocytes mediates angiogenesis. Am J Pathol (1994) 144(4):820–8.
47. Rodríguez-Jiménez P, Chicharro P, Llamas-Velasco M, Cibrian D, Trigo-Torres L, Vara A, et al. Thrombospondin-1/CD47 interaction regulates Th17 and treg differentiation in psoriasis. Front Immunol (2019) 10:1268. doi: 10.3389/fimmu.2019.01268
48. Sansanayudh N, Numthavaj P, Muntham D, Yamwong S, McEvoy M, Attia J, et al. Prognostic effect of mean platelet volume in patients with coronary artery disease. A systematic review and meta-analysis. Thromb Haemost (2015) 114(6):1299–309. doi: 10.1160/TH15-04-0280
49. Rechcinski T, Jasinska A, Forys J, Krzeminska-Pakula M, Wierzbowska-Drabik K, Plewka M, et al. Prognostic value of platelet indices after acute myocardial infarction treated with primary percutaneous coronary intervention. Cardiol J (2013) 20(5):491–8. doi: 10.5603/CJ.2013.0134
50. Li B, Lu J, Peng DZ, Zhang XY, You Z. Elevated platelet distribution width predicts poor prognosis in hilar cholangiocarcinoma. Med (Baltimore) (2020) 99(12):e19400. doi: 10.1097/MD.0000000000019400
51. Perkins LA, Anderson CJ, Novelli EM. Targeting P-selectin adhesion molecule in molecular imaging: P-selectin expression as a valuable imaging biomarker of inflammation in cardiovascular disease. J Nucl Med (2019) 60(12):1691–7. doi: 10.2967/jnumed.118.225169
52. Blann AD, Nadar SK, Lip GY. The adhesion molecule P-selectin and cardiovascular disease. Eur Heart J (2003) 24(24):2166–79. doi: 10.1016/j.ehj.2003.08.021
53. Puhm F, Boilard E, Machlus KR. Platelet extracellular vesicles: beyond the blood. Arterioscler Thromb Vasc Biol (2021) 41(1):87–96. doi: 10.1161/ATVBAHA.120.314644
54. Rosinska J, Lukasik M, Kozubski W. The impact of vascular disease treatment on platelet-derived microvesicles. Cardiovasc Drugs Ther (2017) 31(5-6):627–44. doi: 10.1007/s10557-017-6757-7
55. Kasperska-Zajac A, Brzoza Z, Rogala B. Platelet function in cutaneous diseases. Platelets (2008) 19(5):317–21. doi: 10.1080/09537100802082249
56. Bakogiannis C, Sachse M, Stamatelopoulos K, Stellos K. Platelet-derived chemokines in inflammation and atherosclerosis. Cytokine (2019) 122:154157. doi: 10.1016/j.cyto.2017.09.013
57. Kowalska MA, Rauova L, Poncz M. Role of the platelet chemokine platelet factor 4 (PF4) in hemostasis and thrombosis. Thromb Res (2010) 125(4):292–6. doi: 10.1016/j.thromres.2009.11.023
58. Gleissner CA, Shaked I, Erbel C, Böckler D, Katus HA, Ley K. CXCL4 downregulates the atheroprotective hemoglobin receptor CD163 in human macrophages. Circ Res (2010) 106(1):203–11. doi: 10.1161/CIRCRESAHA.109.199505
59. Abdallah MA, Abdel-Hamid MF, Kotb AM, Mabrouk EA. Serum interferon-gamma is a psoriasis severity and prognostic marker. Cutis (2009) 84(3):163–8.
60. Zou A, Jian Q. CXCL10 and its related key genes as potential biomarkers for psoriasis: Evidence from bioinformatics and real-time quantitative polymerase chain reaction. Med (Baltimore) (2021) 100(38):e27365. doi: 10.1097/MD.0000000000027365
61. Hald A, Andrés RM, Salskov-Iversen ML, Kjellerup RB, Iversen L, Johansen C. STAT1 expression and activation is increased in lesional psoriatic skin. Br J Dermatol (2013) 168(2):302–10. doi: 10.1111/bjd.12049
62. Du Y, Jiang S, Cheng L, Liu J. JAK/STAT and VEGF/PAK1 signaling as emerging targets for topical treatment of psoriasis: a pilot study. Int J Clin Exp Pathol (2020) 13(12):3111–19.
63. Choudhary S, Pradhan D, Khan NS, Singh H, Thomas G, Jain AK. Decoding psoriasis: integrated bioinformatics approach to understand hub genes and involved pathways. Curr Pharm Des (2020) 26(29):3619–30. doi: 10.2174/1381612826666200311130133
64. Zhou Z, Meng L, Cai Y, Yan W, Bai Y, Chen J. Exploration of the potential mechanism of the common differentially expressed genes in psoriasis and atopic dermatitis. BioMed Res Int (2022) 2022:1177299. doi: 10.1155/2022/1177299
65. Kim N, Lee S, Kang J, Choi YA, Jang YH, Jeong GS, et al. Cudraxanthone D ameliorates psoriasis-like skin inflammation in an imiquimod-induced mouse model via inhibiting the inflammatory signaling pathways. Molecules (2021) 26(19):6086. doi: 10.3390/molecules26196086
66. Huang YZ, Zheng YX, Zhou Y, Xu F, Cui YZ, Chen XY, et al. OAS1, OAS2, and OAS3 contribute to epidermal keratinocyte proliferation by regulating cell cycle and augmenting IFN-1-Induced jak1-Signal transducer and activator of transcription 1 phosphorylation in psoriasis. J Invest Dermatol (2022) 142(10):2635–45 e9. doi: 10.1016/j.jid.2022.02.018
67. Yildirim C, Nieuwenhuis S, Teunissen PF, Horrevoets AJ, van Royen N, van der Pouw Kraan TC. Interferon-Beta, a decisive factor in angiogenesis and arteriogenesis. J Interferon Cytokine Res (2015) 35(6):411–20. doi: 10.1089/jir.2014.0184
68. Elyasi A, Voloshyna I, Ahmed S, Kasselman LJ, Behbodikhah J, De Leon J, et al. The role of interferon-gamma in cardiovascular disease: an update. Inflammation Res (2020) 69(10):975–88. doi: 10.1007/s00011-020-01382-6
69. Isenberg JS, Romeo MJ, Yu C, Yu CK, Nghiem K, Monsale J, et al. Thrombospondin-1 stimulates platelet aggregation by blocking the antithrombotic activity of nitric oxide/cGMP signaling. Blood (2008) 111(2):613–23. doi: 10.1182/blood-2007-06-098392
70. Roberts W, Magwenzi S, Aburima A, Naseem KM. Thrombospondin-1 induces platelet activation through CD36-dependent inhibition of the cAMP/protein kinase A signaling cascade. Blood (2010) 116(20):4297–306. doi: 10.1182/blood-2010-01-265561
71. Zhang K, Li M, Yin L, Fu G, Liu Z. Role of thrombospondin−1 and thrombospondin−2 in cardiovascular diseases (Review). Int J Mol Med (2020) 45(5):1275–93. doi: 10.3892/ijmm.2020.4507
72. Garshick MS, Barrett TJ, Wechter T, Azarchi S, Scher JU, Neimann A, et al. Inflammasome signaling and impaired vascular health in psoriasis. Arterioscler Thromb Vasc Biol (2019) 39(4):787–98. doi: 10.1161/ATVBAHA.118.312246
73. Anyfanti P, Margouta A, Goulas K, Gavriilaki M, Lazaridou E, Patsatsi A, et al. Endothelial dysfunction in psoriasis: an updated review. Front Med (Lausanne) (2022) 9:864185. doi: 10.3389/fmed.2022.864185
74. Sang Y, Roest M, de Laat B, de Groot PG, Huskens D. Interplay between platelets and coagulation. Blood Rev (2021) 46:100733. doi: 10.1016/j.blre.2020.100733
75. Maione F, Cicala C, Liverani E, Mascolo N, Perretti M, D’Acquisto F. IL-17A increases ADP-induced platelet aggregation. Biochem Biophys Res Commun (2011) 408(4):658–62. doi: 10.1016/j.bbrc.2011.04.080
76. Hot A LV, Cazalis MA, Miossec P. Pathogenic role of IL-17 in endothelial dysfunction a link between rheumatoid arthritis and atherosclerosis. Ann Rheum Dis (2010) 69:44–5. doi: 10.1136/ard.2010.129635d
77. Lumadue JA, Lanzkron SM, Kennedy SD, Kuhl DT, Kickler TS. Cytokine induction of platelet activation. Am J Clin Pathol (1996) 106(6):795–8. doi: 10.1093/ajcp/106.6.795
78. Burstein SA, Peng J, Friese P, Wolf RF, Harrison P, Downs T, et al. Cytokine-induced alteration of platelet and hemostatic function. Stem Cells (1996) 14 Suppl 1:154–62. doi: 10.1002/stem.5530140720
79. Galgano L, Guidetti GF, Torti M, Canobbio I. The controversial role of LPS in platelet activation in vitro. Int J Mol Sci (2022) 23(18):10900. doi: 10.3390/ijms231810900
80. Singh TP, Schön MP, Wallbrecht K, Gruber-Wackernagel A, Wang XJ, Wolf P. Involvement of IL-9 in Th17-associated inflammation and angiogenesis of psoriasis. PloS One (2013) 8(1):e51752. doi: 10.1371/journal.pone.0051752
81. Feng Y, Yu M, Zhu F, Zhang S, Ding P, Wang M. IL-9 promotes the development of deep venous thrombosis by facilitating platelet function. Thromb Haemost (2018) 118(11):1885–94. doi: 10.1055/s-0038-1673614
82. Ashraf MA, Nookala V. Biochemistry of platelet activating factor. 2023 apr 10. In: statPearls [Internet]. Treasure Island (FL: StatPearls Publishing (2023).
83. Duan S, Wanke K, Wawrzyniak P, Meng Y, Kast JI, Ruckert B, et al. Platelet-activating factor decreases skin keratinocyte tight junction barrier integrity. J Allergy Clin Immunol (2016) 138(6):1725–8 e3. doi: 10.1016/j.jaci.2016.05.037
84. Krause K, Gimenez-Arnau A, Martinez-Escala E, Farre-Albadalejo M, Abajian M, Church MK, et al. Platelet-activating factor (PAF) induces wheal and flare skin reactions independent of mast cell degranulation. Allergy (2013) 68(2):256–8. doi: 10.1111/all.12083
85. Upton JEM, Grunebaum E, Sussman G, Vadas P. Platelet activating factor (PAF): A mediator of inflammation. Biofactors (2022) 48(6):1189–202. doi: 10.1002/biof.1883
86. Ramesha CS, Soter N, Pickett WC. Identification and quantitation of PAF from psoriatic scales. Agents Actions (1987) 21(3-4):382–3. doi: 10.1007/BF01966522
87. Izaki S, Yamamoto T, Goto Y, Ishimaru S, Yudate F, Kitamura K, et al. Platelet-activating factor and arachidonic acid metabolites in psoriatic inflammation. Br J Dermatol (1996) 134(6):1060–4. doi: 10.1111/j.1365-2133.1996.tb07943.x
88. Andersen S, Sjursen W, Laegreid A, Volden G, Johansen B. Elevated expression of human nonpancreatic phospholipase A2 in psoriatic tissue. Inflammation (1994) 18(1):1–12. doi: 10.1007/BF01534593
89. Braverman IM, Sibley J. Role of the microcirculation in the treatment and pathogenesis of psoriasis. J Invest Dermatol (1982) 78(1):12–7. doi: 10.1111/1523-1747.ep12497850
90. Singh TP, Huettner B, Koefeler H, Mayer G, Bambach I, Wallbrecht K, et al. Platelet-activating factor blockade inhibits the T-helper type 17 cell pathway and suppresses psoriasis-like skin disease in K5.hTGF-beta1 transgenic mice. Am J Pathol (2011) 178(2):699–708. doi: 10.1016/j.ajpath.2010.10.008
91. Lordan R, Tsoupras A, Zabetakis I. Platelet activation and prothrombotic mediators at the nexus of inflammation and atherosclerosis: Potential role of antiplatelet agents. Blood Rev (2021) 45:100694. doi: 10.1016/j.blre.2020.100694
92. Tsoupras A, Lordan R, Zabetakis I. Inflammation, not cholesterol, is a cause of chronic disease. Nutrients (2018) 10:604. doi: 10.3390/nu10050604
93. Rasheed H, Saeed SA. Involvement of thromboxane A2 and tyrosine kinase in the synergistic interaction of platelet activating factor and calcium ionophore A23187 in human platelet aggregation. Exp Mol Med (2004) 36(3):220–5. doi: 10.1038/emm.2004.30
94. Cognasse F, Duchez AC, Audoux E, Ebermeyer T, Arthaud CA, Prier A, et al. Platelets as key factors in inflammation: focus on CD40L/CD40. Front Immunol (2022) 13:825892. doi: 10.3389/fimmu.2022.825892
95. Zuchtriegel G, Uhl B, Puhr-Westerheide D, Pornbacher M, Lauber K, Krombach F, et al. Platelets guide leukocytes to their sites of extravasation. PloS Biol (2016) 14(5):e1002459. doi: 10.1371/journal.pbio.1002459
96. Peters AL, Stunz LL, Bishop GA. CD40 and autoimmunity: the dark side of a great activator. Semin Immunol (2009) 21(5):293–300. doi: 10.1016/j.smim.2009.05.012
97. Popa M, Tahir S, Elrod J, Kim SH, Leuschner F, Kessler T, et al. Role of CD40 and ADAMTS13 in von Willebrand factor-mediated endothelial cell-platelet-monocyte interaction. Proc Natl Acad Sci U.S.A. (2018) 115(24):E5556–E65. doi: 10.1073/pnas.1801366115
98. Moller K, Adolph O, Grunow J, Elrod J, Popa M, Ghosh S, et al. Mechanism and functional impact of CD40 ligand-induced von Willebrand factor release from endothelial cells. Thromb Haemost (2015) 113(5):1095–108. doi: 10.1160/TH14-04-0336
99. Gerdes N, Seijkens T, Lievens D, Kuijpers MJ, Winkels H, Projahn D, et al. Platelet CD40 exacerbates atherosclerosis by transcellular activation of endothelial cells and leukocytes. Arterioscler Thromb Vasc Biol (2016) 36(3):482–90. doi: 10.1161/ATVBAHA.115.307074
100. Yuan M, Fu H, Ren L, Wang H, Guo W. Soluble CD40 ligand promotes macrophage foam cell formation in the etiology of atherosclerosis. Cardiology (2015) 131(1):1–12. doi: 10.1159/000374105
101. Stellos K, Seizer P, Bigalke B, Daub K, Geisler T, Gawaz M. Platelet aggregates-induced human CD34+ progenitor cell proliferation and differentiation to macrophages and foam cells is mediated by stromal cell derived factor 1 in vitro. Semin Thromb Hemost (2010) 36(2):139–45. doi: 10.1055/s-0030-1251497
102. Morrell CN, Aggrey AA, Chapman LM, Modjeski KL. Emerging roles for platelets as immune and inflammatory cells. Blood (2014) 123:2759–67. doi: 10.1182/blood-2013-11-462432
103. Semple JW, Italiano JE Jr., Freedman J. Platelets and the immune continuum. Nat Rev Immunol (2011) 11(4):264–74. doi: 10.1038/nri2956
104. Ludwig RJ, Schultz JE, Boehncke WH, Podda M, Tandi C, Krombach F, et al. Activated, not resting, platelets increase leukocyte rolling in murine skin utilizing a distinct set of adhesion molecules. J Invest Dermatol (2004) 122(3):830–6. doi: 10.1111/j.0022-202X.2004.22318.x
105. Massberg S, Brand K, Gruner S, Page S, Muller E, Muller I, et al. A critical role of platelet adhesion in the initiation of atherosclerotic lesion formation. J Exp Med (2002) 196(7):887–96. doi: 10.1084/jem.20012044
106. Sreeramkumar V, Adrover JM, Ballesteros I, Cuartero MI, Rossaint J, Bilbao I, et al. Neutrophils scan for activated platelets to initiate inflammation. Science (2014) 346(6214):1234–8. doi: 10.1126/science.1256478
107. Herster F, Bittner Z, Codrea MC, Archer NK, Heister M, Loffler MW, et al. Platelets aggregate with neutrophils and promote skin pathology in psoriasis. Front Immunol (2019) 10:1867. doi: 10.3389/fimmu.2019.01867
108. Ghasemzadeh M, Hosseini E. Intravascular leukocyte migration through platelet thrombi: directing leukocytes to sites of vascular injury. Thromb Haemost (2015) 113(6):1224–35. doi: 10.1160/TH14-08-0662
109. Gerdes N, Zhu L, Ersoy M, Hermansson A, Hjemdahl P, Hu H, et al. Platelets regulate CD4(+) T-cell differentiation via multiple chemokines in humans. Thromb Haemost (2011) 106(2):353–62. doi: 10.1160/TH11-01-0020
110. Sanz-Martínez MT, Moga E, Sánchez Martínez MA, Zamora Atenza C, Vidal S, Juárez C, et al. High levels of platelet-lymphocyte complexes in patients with psoriasis are associated with a better response to anti-TNF-α Therapy. J Invest Dermatol (2020) 140(6):1176–83. doi: 10.1016/j.jid.2019.08.457
111. Paes AMA, Gaspar RS, Fuentes E, Wehinger S, Palomo I, Trostchansky A. Lipid metabolism and signaling in platelet function. Adv Exp Med Biol (2019) 1127:97–115. doi: 10.1007/978-3-030-11488-6_7
112. Ikai K. Psoriasis and the arachidonic acid cascade. J Dermatol Sci (1999) 21(3):135–46. doi: 10.1016/s0923-1811(99)00042-0
113. Liu CH, Liu J, Fan WH, Xu N, Fang X. Determination of serum lipids, TXB2 6-ketoPGF1α, and platelet aggregation function in psoriatic patients. Chin J Derm Venereol (1986) 3:7–9.
114. Schirmer C, Klein C, von Bergen M, Simon JC, Saalbach A. Human fibroblasts support the expansion of IL-17-producing T cells via up-regulation of IL-23 production by dendritic cells. Blood (2010) 116(10):1715–25. doi: 10.1182/blood-2010-01-263509
115. Lee J, Aoki T, Thumkeo D, Siriwach R, Yao C, Narumiya S. T cell-intrinsic prostaglandin E2-EP2/EP4 signaling is critical in pathogenic TH17 cell-driven inflammation. J Allergy Clin Immunol (2019) 143(2):631–43. doi: 10.1016/j.jaci.2018.05.036
116. Ueharaguchi Y, Honda T, Kusuba N, Hanakawa S, Adachi A, Sawada Y, et al. Thromboxane A2 facilitates IL-17A production from Vγ4+ γδ T cells and promotes psoriatic dermatitis in mice. J Allergy Clin Immunol (2018) 142(2):680–3.e2. doi: 10.1016/j.jaci.2018.01.054
117. Sawada Y, Honda T, Nakamizo S, Otsuka A, Ogawa N, Kobayashi Y, et al. Resolvin E1 attenuates murine psoriatic dermatitis. Sci Rep (2018) 8(1):11873. doi: 10.1038/s41598-018-30373-1
118. Hendriks AG, Keijsers RR, Seyger MM, van de Kerkhof PC, van Erp PE. Cutaneous application of leukotriene b4 as an in vivo model of psoriasis-like skin inflammation: an immunohistological study. Skin Pharmacol Physiol (2014) 27(3):120–6. doi: 10.1159/000354119
119. Vila L, Cullaré C, Solá J, Puig L, de Castellarnau C, de Moragas JM. Cyclooxygenase activity is increased in platelets from psoriatic patients. J Invest Dermatol (1991) 97(5):922–6. doi: 10.1111/1523-1747.ep12491695
120. Tecchio C, Micheletti A, Cassatella MA. Neutrophil-derived cytokines: facts beyond expression. Front Immunol (2014) 5:508. doi: 10.3389/fimmu.2014.00508
121. Ikeda S, Takahashi H, Suga Y, Eto H, Etoh T, Okuma K, et al. Therapeutic depletion of myeloid lineage leukocytes in patients with generalized pustular psoriasis indicates a major role for neutrophils in the immunopathogenesis of psoriasis. J Am Acad Dermatol (2013) 68(4):609–17. doi: 10.1016/j.jaad.2012.09.037
122. Teague HL, Varghese NJ, Tsoi LC, Dey AK, Garshick MS, Silverman JI, et al. Neutrophil subsets, platelets, and vascular disease in psoriasis. JACC Basic Transl Sci (2019) 4(1):1–14. doi: 10.1016/j.jacbts.2018.10.008
123. Michailidou D, Kuley R, Wang T, Hermanson P, Grayson PC, Cuthbertson D, et al. Neutrophil extracellular trap formation in anti-neutrophil cytoplasmic antibody-associated and large-vessel vasculitis. Clin Immunol (2023) 249:109274. doi: 10.1016/j.clim.2023.109274
124. Matsumoto K, Yasuoka H, Yoshimoto K, Suzuki K, Takeuchi T. Platelet CXCL4 mediates neutrophil extracellular traps formation in ANCA-associated vasculitis. Sci Rep (2021) 11(1):222. doi: 10.1038/s41598-020-80685-4
125. Franco AT, Corken A, Ware J. Platelets at the interface of thrombosis, inflammation, and cancer. Blood (2015) 126(5):582–8. doi: 10.1182/blood-2014-08-531582
126. Salamah MF, Ravishankar D, Kodji X, Moraes LA, Williams HF, Vallance TM, et al. The endogenous antimicrobial cathelicidin LL37 induces platelet activation and augments thrombus formation. Blood Adv (2018) 2(21):2973–85. doi: 10.1182/bloodadvances.2018021758
127. Pircher J, Czermak T, Ehrlich A, Eberle C, Gaitzsch E, Margraf A, et al. Cathelicidins prime platelets to mediate arterial thrombosis and tissue inflammation. Nat Commun (2018) 9(1):1523. doi: 10.1038/s41467-018-03925-2
128. Herster F, Bittner Z, Archer NK, Dickhofer S, Eisel D, Eigenbrod T, et al. Neutrophil extracellular trap-associated RNA and LL37 enable self-amplifying inflammation in psoriasis. Nat Commun (2020) 11(1):105. doi: 10.1038/s41467-019-13756-4
129. Medina-Leyte DJ, Zepeda-Garcia O, Dominguez-Perez M, Gonzalez-Garrido A, Villarreal-Molina T, Jacobo-Albavera L. Endothelial dysfunction, inflammation and coronary artery disease: potential biomarkers and promising therapeutical approaches. Int J Mol Sci (2021) 22(8):3850. doi: 10.3390/ijms22083850
130. O’Brien M, Montenont E, Hu L, Nardi MA, Valdes V, Merolla M, et al. Aspirin attenuates platelet activation and immune activation in HIV-1-infected subjects on antiretroviral therapy: a pilot study. J Acquir Immune Defic Syndr (2013) 63(3):280–8. doi: 10.1097/QAI.0b013e31828a292c
131. Nhek S, Clancy R, Lee KA, Allen NM, Barrett TJ, Marcantoni E, et al. Activated platelets induce endothelial cell activation via an interleukin-1beta pathway in systemic lupus erythematosus. Arterioscler Thromb Vasc Biol (2017) 37(4):707–16. doi: 10.1161/ATVBAHA.116.308126
132. Kotowicz K, Dixon GL, Klein NJ, Peters MJ, Callard RE. Biological function of CD40 on human endothelial cells: costimulation with CD40 ligand and interleukin-4 selectively induces expression of vascular cell adhesion molecule-1 and P-selectin resulting in preferential adhesion of lymphocytes. Immunology (2000) 100(4):441–8. doi: 10.1046/j.1365-2567.2000.00061.x
133. Friedrich EB, Tager AM, Liu E, Pettersson A, Owman C, Munn L, et al. Mechanisms of leukotriene B4—triggered monocyte adhesion. Arterioscler Thromb Vasc Biol (2003) 23(10):1761–7. doi: 10.1161/01.ATV.0000092941.77774.3C
134. Swystun LL, Liaw PC. The role of leukocytes in thrombosis. Blood (2016) 128(6):753–62. doi: 10.1182/blood-2016-05-718114
135. Cloutier N, Paré A, Farndale RW, Schumacher HR, Nigrovic PA, Lacroix S, et al. Platelets can enhance vascular permeability. Blood (2012) 120(6):1334–43. doi: 10.1182/blood-2012-02-413047
136. Martins AM, Ascenso A, Ribeiro HM, Marto J. Current and future therapies for psoriasis with a focus on serotonergic drugs. Mol Neurobiol (2020) 57(5):2391–419. doi: 10.1007/s12035-020-01889-3
137. Trelinski J, Tybura M, Smolewski P, Robak T, Chojnowski K. The influence of low-dose aspirin and hydroxyurea on platelet-leukocyte interactions in patients with essential thrombocythemia. Blood Coagul Fibrinolysis (2009) 20(8):646–51. doi: 10.1097/MBC.0b013e32832f6c5b
138. Carestia A, Kaufman T, Rivadeneyra L, Landoni VI, Pozner RG, Negrotto S, et al. Mediators and molecular pathways involved in the regulation of neutrophil extracellular trap formation mediated by activated platelets. J Leukoc Biol (2016) 99(1):153–62. doi: 10.1189/jlb.3A0415-161R
139. Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med (1997) 336(14):973–9. doi: 10.1056/NEJM199704033361401
140. Habets KL, Huizinga TW, Toes RE. Platelets and autoimmunity. Eur J Clin Invest (2013) 43(7):746–57. doi: 10.1111/eci.12101
141. Chi CC, Wu YW, Chao TH, Chen CC, Chen YJ, Cheng HM, et al. 2022 Taiwanese Dermatological Association (TDA), Taiwanese Association for Psoriasis and Skin Immunology (TAPSI), and Taiwan Society of cardiology (TSOC) joint consensus recommendations for the management of psoriatic disease with attention to cardiovascular comorbidities. J Formos Med Assoc (2023) 122(6):442–57. doi: 10.1016/j.jfma.2022.10.010
142. Nenna A, Nappi F, Lusini M, Satriano UM, Schiliro D, Spadaccio C, et al. Effect of statins on platelet activation and function: from molecular pathways to clinical effects. BioMed Res Int (2021) 2021:6661847. doi: 10.1155/2021/6661847
143. Zhao L, Liu D, Liu B, Hu H, Cui W. Effects of atorvastatin on ADP-, arachidonic acid-, collagen-, and epinephrine-induced platelet aggregation. J Int Med Res (2017) 45(1):82–8. doi: 10.1177/0300060516675681
144. Moscardo A, Valles J, Latorre A, Madrid I, Santos MT. Reduction of platelet cytosolic phospholipase A2 activity by atorvastatin and simvastatin: biochemical regulatory mechanisms. Thromb Res (2013) 131(4):e154–9. doi: 10.1016/j.thromres.2013.01.007
145. Puccetti L, Santilli F, Pasqui AL, Lattanzio S, Liani R, Ciani F, et al. Effects of atorvastatin and rosuvastatin on thromboxane-dependent platelet activation and oxidative stress in hypercholesterolemia. Atherosclerosis (2011) 214(1):122–8. doi: 10.1016/j.atherosclerosis.2010.10.006
146. Violi F, Carnevale R, Pastori D, Pignatelli P. Antioxidant and antiplatelet effects of atorvastatin by Nox2 inhibition. Trends Cardiovasc Med (2014) 24(4):142–8. doi: 10.1016/j.tcm.2013.09.006
147. Sanguigni V, Pignatelli P, Lenti L, Ferro D, Bellia A, Carnevale R, et al. Short-term treatment with atorvastatin reduces platelet CD40 ligand and thrombin generation in hypercholesterolemic patients. Circulation (2005) 111(4):412–9. doi: 10.1161/01.CIR.0000153810.81187.7D
148. Stach K, Nguyen XD, Lang S, Elmas E, Weiss C, Borggrefe M, et al. Simvastatin and atorvastatin attenuate VCAM-1 and uPAR expression on human endothelial cells and platelet surface expression of CD40 ligand. Cardiol J (2012) 19(1):20–8. doi: 10.5603/cj.2012.0005
149. Labiós M, Martínez M, Gabriel F, Guiral V, Martínez E, Aznar J. Effect of atorvastatin upon platelet activation in hypercholesterolemia, evaluated by flow cytometry. Thromb Res (2005) 115(4):263–70. doi: 10.1016/j.thromres
150. Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/aphA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: A report of the american college of cardiology/american heart association task force on clinical practice guidelines. Circulation (2019) 139(25):e1082–e143. doi: 10.1161/CIR.0000000000000625
151. Wang J, Zhang S, Xing M, Hong S, Liu L, Ding XJ, et al. Current evidence on the role of lipid lowering drugs in the treatment of psoriasis. Front Med (Lausanne) (2022) 9:900916. doi: 10.3389/fmed.2022.900916
152. Socha M, Pietrzak A, Grywalska E, Pietrzak D, Matosiuk D, Kicinski P, et al. The effect of statins on psoriasis severity: a meta-analysis of randomized clinical trials. Arch Med Sci (2020) 16(1):1–7. doi: 10.5114/aoms.2019.90343
153. An İ, Aksoy M, Ayhan E, Ozturk M. The effect of secukinumab treatment on inflammatory parameters in patients with psoriasis: A multicentre restrospective study. Int J Clin Pract (2021) 75(6):e14114. doi: 10.1111/ijcp.14114
154. Procaccini EM, Pandolfi G, Monfrecola G, Rotoli B. Effect of psoralen and ultraviolet A on platelet functioning: an in vitro and in vivo study. Photodermatol Photoimmunol Photomed (1992) 9(1):4–7.
155. Wolf P, Nghiem DX, Walterscheid JP, Byrne S, Matsumura Y, Matsumura Y, et al. Platelet-activating factor is crucial in psoralen and ultraviolet A-induced immune suppression, inflammation, and apoptosis. Am J Pathol (2006) 169(3):795–805. doi: 10.2353/ajpath.2006.060079
156. Wu JJ, Sundaram M, Cloutier M, Gauthier-Loiselle M, Guérin A, Singh R, et al. The risk of cardiovascular events in psoriasis patients treated with tumor necrosis factor-α inhibitors versus phototherapy: An observational cohort study. J Am Acad Dermatol (2018) 79(1):60–8. doi: 10.1016/j.jaad.2018.02.050
157. Bilia AR, Bergonzi MC, Isacchi B, Antiga E, Caproni M. Curcumin nanoparticles potentiate therapeutic effectiveness of acitrein in moderate-to-severe psoriasis patients and control serum cholesterol levels. J Pharm Pharmacol (2018) 70(7):919–28. doi: 10.1111/jphp
158. Antiga E, Bonciolini V, Volpi W, Del Bianco E, Caproni M. Oral curcumin (Meriva) is effective as an adjuvant treatment and is able to reduce IL-22 serum levels in patients with psoriasis vulgaris. BioMed Res Int (2015) 2015:283634. doi: 10.1155/2015/283634
159. Hussain Y, Abdullah, Khan F, Alsharif KF, Alzahrani KJ, Saso L, et al. Regulatory effects of curcumin on platelets: an update and future directions. Biomedicines (2022) 10(12):3180. doi: 10.3390/biomedicines10123180
160. Zhong L, Luo N, Zhong X, Xu T, Hao P. The immunoregulatory effects of natural products on psoriasis via its action on Th17 cells versus regulatory T cells balance. Int Immunopharmacol (2022) 110:109032. doi: 10.1016/j.intimp.2022.109032
161. Zhang Y, Shi H, Wang W, Ke Z, Xu P, Zhong Z, et al. Antithrombotic effect of grape seed proanthocyanidins extract in a rat model of deep vein thrombosis. J Vasc Surg (2011) 53(3):743–53. doi: 10.1016/j.jvs.2010.09.017
162. Adhar M, HadjKacem B, Périno-Issartier S, Ben Amor I, Feki A, Gargouri J, et al. Thymol-enriched extract from Thymus vulgaris L leaves: Green extraction processes and antiaggregant effects on human platelets. Bioorg Chem (2022) 125:105858. doi: 10.1016/j.bioorg.2022.105858
163. Choi JH, Park SE, Kim SJ, Kim S. Kaempferol inhibits thrombosis and platelet activation. Biochimie (2015) 115:177–86. doi: 10.1016/j.biochi.2015.06.001
Keywords: platelet, psoriasis, cardiovascular disease, leukocyte, endothelial cell, cytokines
Citation: Jiang Z, Jiang X, Chen A and He W (2023) Platelet activation: a promoter for psoriasis and its comorbidity, cardiovascular disease. Front. Immunol. 14:1238647. doi: 10.3389/fimmu.2023.1238647
Received: 12 June 2023; Accepted: 31 July 2023;
Published: 15 August 2023.
Edited by:
Marzia Caproni, University of Florence, ItalyReviewed by:
Cong Huang, Peking University, ChinaCopyright © 2023 Jiang, Jiang, Chen and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Aijun Chen, Y2FqaHhAYWxpeXVuLmNvbQ==; Wenyan He, d2VueWFuX2hlQGNxbXUuZWR1LmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.