- 1Shanghai Public Health Clinical Center, Shanghai Institute of Infectious Disease and Biosecurity, Fudan University, Shanghai, China
- 2TB Center, Shanghai Emerging and Re-emerging Infectious Disease Institute, Fudan University, Shanghai, China
Tuberculosis (TB), also known as the “White Plague”, is caused by Mycobacterium tuberculosis (Mtb). Before the COVID-19 epidemic, TB had the highest mortality rate of any single infectious disease. Vaccination is considered one of the most effective strategies for controlling TB. Despite the limitations of the Bacille Calmette-Guérin (BCG) vaccine in terms of protection against TB among adults, it is currently the only licensed TB vaccine. Recently, with the evolution of bioinformatics and structural biology techniques to screen and optimize protective antigens of Mtb, the tremendous potential of protein subunit vaccines is being exploited. Multistage subunit vaccines obtained by fusing immunodominant antigens from different stages of TB infection are being used both to prevent and to treat TB. Additionally, the development of novel adjuvants is compensating for weaknesses of immunogenicity, which is conducive to the flourishing of subunit vaccines. With advances in the development of animal models, preclinical vaccine protection assessments are becoming increasingly accurate. This review summarizes progress in the research of protein subunit TB vaccines during the past decades to facilitate the further optimization of protein subunit vaccines that may eradicate TB.
1 Introduction
Tuberculosis (TB), caused by Mycobacterium tuberculosis (Mtb), has afflicted humans for thousands of years. Mtb is highly contagious and colonizes the respiratory tract through airborne droplets (1). TB remains a serious threat to public health, with 10.6 million new cases and 1.6 million deaths reported worldwide in 2021 (2). In addition, enormous challenges for TB prevention and treatment are posed by the emergence of multidrug-resistant TB (MDR-TB) (3), the lack of effective methods for the differential diagnosis of latent TB infection (LTBI) (4), immune disorder caused by co-infection with HIV (5).
The development of the Bacille Calmette- Guérin (BCG) vaccine was a major milestone in the history of global TB control. Even though it has been more than 100 years since BCG was developed, BCG is still the only licensed TB vaccine worldwide. However, its protective efficiency is still controversial due to its limited immune protection in adults (6). Therefore, a more effective TB vaccine that protects against different stages of the disease’s development is urgently needed.
The main TB vaccine candidates include live attenuated vaccines, inactivated vaccines, recombinant viral vector vaccines, and protein subunit vaccines. Since both whole-cell-based and virus-based vaccines pose potential risks to human health, protein-subunit vaccines consisting of protective antigens may be safer and more attractive (7). However, the biggest concern with protein subunit vaccines is inadequate immunogenicity, therefore, optimizing the vaccine composition to trigger a potent and long-lasting immune response is crucial. Novel vaccine adjuvants are powerful tools that may overcome the immunogenicity limitations of protein subunit vaccines.
In this review, we focus on the advances in antigen optimization, adjuvant selection, clinical trials, animal models, and vaccination strategies of protein subunit vaccines, which may foretell the future of TB vaccine research and development.
2 Protein epitope optimization strategy
The complex genetic composition, multiple immune evasion strategies, and the lack of rigorous immune markers make the identification of key protective epitopes against Mtb a major challenge. Several methods have been used to predict the optimal epitopes for vaccine design.
Although CD4+ T cells are necessary to protect against TB, they may not be sufficient to obtain a completely protective immune response (8). Many researchers have focused on identifying antigens that stimulate the CD8+ T-cell-mediated responses that also play a protective role against TB and latent TB infection (LTBI) (9). Additionally, a growing number of studies have shown that antibodies produced by B cells contribute to the fight against TB (10). Therefore, vaccines that induce combined CD4+, CD8+ T-, and B-cell immune responses may be the most effective. Bioinformatics tools enable the rapid analysis of the entire genome and proteome of pathogens to predict potentially protective T- or B-cell epitopes and the character of their specific binding to major histocompatibility complex (MHC) molecules (11).
The structure of an antigen determines the specificity, affinity, and accessibility of the binding sites to MHC or antibody, which affects the potency of the immune response (12). Therefore, antigen geometry can be another critical factor in vaccine design (13). “Reverse vaccinology” was proposed 20 years ago, based on the availability of genome sequence information to design vaccines. With the development and application of immunology, proteomics, systems biology, and structural biology, we have entered the era of “Reverse vaccinology 2.0”, in which the structural features of antigens and antibodies are used to guide the design of recombinant vaccine antigens. Developments in X-ray crystallography, electron microscopy, and computational biology have all contributed (14). Currently, AlphaFold2 is the most advanced protein 3D structure prediction tool (15). By predicting and analyzing the higher configuration of the 3D antigen structure, the linear epitopes for T-cell receptors and the conformational epitopes for B-cell receptors can be comprehensively optimized to improve vaccine protective efficiency (16).
Combining bioinformatics, structural information, and the AlphaFold2 prediction model to obtain the structural basis underlying protective immune responses to key epitopes is now a popular design strategy to get efficient, long-term, and broad-spectrum responses with multi-epitope TB protein subunit vaccine candidates (14–17).
3 Protective antigens of Mtb
The composition of Mtb is complex, and many components exhibit immunogenicity. According to different characteristics and associated growth states, Mtb antigens are mainly divided into the following types:
3.1 Antigens on the cell wall and capsule
The cell wall and capsule of Mtb contain a large number of glycolipids, lipoproteins, and glycoproteins such as cord factor, phthiocerol dimycocerosates, phosphatidylethanolamine, diacyl trehaloses, lipoarabinomannan, phosphatidyl-myoinositol mannosides, and heparin-binding adhesin, etc. (18, 19) They can activate immune responses and serve as candidate antigens or adjuvants for TB vaccines.
3.2 Secretory antigens
Mtb can secrete numerous proteins, some of which can inhibit or induce the host immune response by promoting immune escape or activating immune signaling pathways, respectively. Most of the candidate proteins for existing TB vaccines are based on those found as secreted antigens during logarithmic growth of Mtb, such as Ag85A/B, ESAT-6, CFP10, TB10.4, MPT64, and PPE18 (20). The secretory antigens are ideal candidate antigens for the recombinant protein subunit vaccine because of their strong immunogenicity and ease of heterologous expression and amplification.
3.3 Dormancy phase antigens
The antigens modulated under the DosR regulon are the main proteins involved in the dormant survival process of Mtb. A total of 48 structural proteins are known to be involved in aerobic respiration and carbon monoxide inhibition; representative genes include HspX, Rv2623, Rv2660c, etc. (21) Members of the durable hypoxia response (EHR) regulon are structural genes induced after exposure to hypoxia. EHR proteins are presumed to be involved in the adaptation and survival of bacteria during a long-term bacteriostatic process (22). Members of the DosR and EHR regulons are considered promising antigens to be incorporated into protein subunit vaccines for treating LTBI (23).
3.4 Resuscitation phase antigens
Resuscitation promotion factors (Rpfs) are involved in the resuscitation and reactivation of dormant Mtb infection and induce specific humoral and cellular immune responses in individuals with LTBI (24). There are 5 Rpf-like proteins (RpfA, RpfB, RpfC, RpfD, and RpfE) with partially overlapping functions in Mtb. Rpfs, especially RpfB, can trigger a memory T-cell response and has been hypothesized to be an essential antigenic target controlling bacterial activation. Rpfs can be used as candidate antigens for protein subunit vaccines against LTBI infection (25).
3.5 BCG regions of difference (RD) antigens
BCG strains have structures similar to Mtb, but 16 genomic region of difference (RD) antigens are deficient in BCG compared to Mtb (26). The RD1 gene products contain a variety of potential virulence factors, such as ESAT-6 and CFP10 (26). They play multiple roles in Mtb progression and pathogenicity, and are considered suitable candidates for use in treatment and diagnosis (27). The poor protective effect of BCG may be related to the loss of a large number of genes encoding protective antigens. Therefore, RD antigens should be emphasized in constructing recombinant protein subunit vaccines.
4 Adjuvants
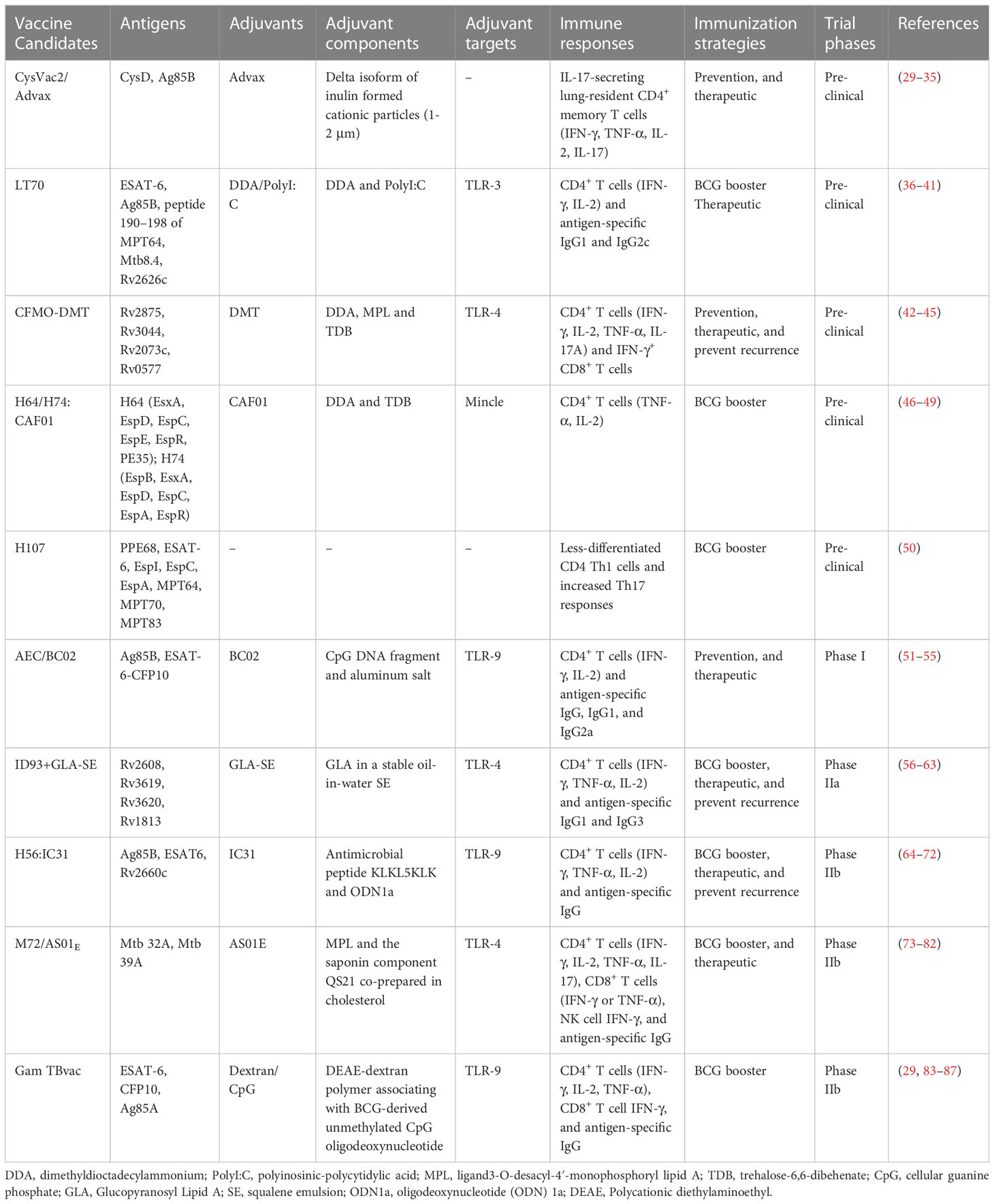
With the limitations in immunogenicity and bioavailability, excellent adjuvants are critical for protein subunit vaccines. Alum has been the only licensed adjuvant in human vaccines for several decades. However, it has been considered unsuitable for vaccines against intracellular pathogens such as Mtb due to its insufficient ability to induce Th1 cellular immunity and CD8+ cytotoxic responses. TB-specific adjuvants that induce a strong immune response in the lungs but minimize the corresponding tissue damage are ideal. In order to meet the needs of TB vaccine development, a workshop entitled “Vaccine Adjuvants for Advancing the treatment of Mycobacterium tuberculosis” was held in July 2020, and factors correlates of protective immunity, targeting specific immune cells, immune evasion mechanisms, and animal models were identified as four research areas critical to the development of optimal TB vaccine adjuvants (28). In recent years, a variety of novel adjuvants have been developed, and most available protein subunit vaccine adjuvants are based on Toll-like receptor (TLR) agonists and use liposomes and emulsions as delivery vehicles as shown in Table 1. In addition, nanoparticle-based adjuvants have received extensive attention in recent years, and various novel nanoadjuvants have been used in some of these vaccines.
4.1 CAF01
CAF01 comprises cationic surfactant lipid-based liposomes dimethyldioctadecylammonium (DDA) and glycolipid immunomodulator trehalose-6,6-dibehenate (TDB). DDA is a potent adjuvant capable of eliciting cellular and humoral immune responses (46). TDB is a synthetic analog of mycobacterial cord factor that is located in the cell wall of mycobacteria and has intrinsic immunostimulatory properties that activate Mincle (47). TDB incorporated with DDA creates a stable liposome by forming hydrogen bonds between the liposome membrane and the surrounding water. CAF01 has been shown to generate a Th1/Th17 polarization response via Mincle-dependent IL-1 production and subsequent MyD88 signaling (48).
4.2 AS01 and DMT
AS01 is a liposome-based adjuvant that consists of the 3-O-desacyl-4′-monophosphoryl lipid A (MPL) and the saponin QS-21 (Quillaja saponaria extract), co-prepared in the presence of cholesterol (73). MPL acts as a TLR4 agonist, stimulates NF-κB transcriptional activity, and induces a Th1 response. QS-21 can enhance the antigen presentation ability of antigen-presenting cells (APCs) and activate/differentiate T cells towards Th1 immune responses. DMT is a combination of the MPL, DDA, and TDB that provides more potent and longer-lasting protective efficacy, including antigen-specific CD4+ Th1 response, IFN-γ+ CD8+ CTL response, and limited humoral response (42).
4.3 GLA-SE
GLA-SE is a mixture of the TLR4 agonist glucopyranosyl lipid A (GLA) and squalene emulsion (SE) (56). GLA is a synthetic lipopolysaccharide (LPS) derivative that maintains vigorous immunostimulatory activity and has low toxicity (57). SE is able to increase the secretion of proinflammatory cytokines such as IL-6, IL-12, and TNF (58). Both GLA and SE alone can promote IgG2 response, while the combination of GLA-SE can induce considerable Th1 response (56).
4.4 IC31
IC31 comprises the synthetic, positively charged antimicrobial peptide KLKL5KLK and oligodeoxynucleotide 1a (ODN1a) (64). ODN1a is an immune stimulatory molecule that promotes Th1-biased immune responses through the TLR9/MyD88 pathway. KLK can act as an immune stimulator that aids transfer into cells in the absence of cell membrane permeability, allowing more efficient functioning of intracellular TLRs (65). KLK induces a Th2 immune response when used alone and a stronger Th1 and Th2 immunity when combined with ODNla (66, 67).
4.5 Dextran/CpG
Dextran/CpG is a novel adjuvant developed with diethyl aminoethyl (DEAE)-dextran and CpG ODN (83). CpG is a TLR9 agonist with the ability to promote Th1 immune responses (secretion of IFN-γ, TNF-α, and IL-12 cytokines), opsonizing antibodies (IgG2a), and potent CD8+ T cell responses (84). Dextran interacts with DC-SIGN family receptors, mannose receptors, and langerin, all triggering innate immunity that promotes inflammation. Furthermore, Dextran/CpG adjuvant enhances activation of lymph node-resident APCs, thus enhancing T-cell priming (29, 83).
4.6 Advax
Advax is a novel cationic adjuvant based on the Delta inulin isoform and has a diameter of about 1-2 μm (30). Advax-based adjuvants have been shown to promote protective immunity against several pathogens in various animal species (31, 32). The potent chemotactic effect induced by Advax enables leukocyte recruitment to the site of inoculation and elicits a broad range of immune responses, including humoral response, Th1, Th2, and Th17 T-cell responses (31).
4.7 BC02
BC02 consists of BCG-derived unmethylated CpG DNA fragments and aluminum salts (Al(OH)3) (51). CpG tends to induce Th1-type immune responses, while alum skews the response to promote the Th2 response to secrete IL-4 and IL-5 cytokines and produce IgG1 and IgE-type antibodies (52). BC02 induces robust Th1 and Th2 responses with acceptable safety (51).
4.8 DDA/poly(I:C)
DDA/poly(I:C) is composed of cationic liposome vector DDA and polyriboinosinic acid–polyribocytidylic acid, poly(I:C). Poly(I:C) mimics viral dsRNA and is a promising immune stimulator candidate for vaccines against intracellular pathogens. Poly(I:C) signaling primarily depends on TLR3 and melanoma differentiation-associated gene-5 (MDA-5) (36). Moreover, poly(I:C) induces strong Th1-skewed immune responses, with enhanced IFN-γ, IL-6, IL-12p70 as well as high antigen-specific IgG antibody (37, 38).
4.9 Nanoadjuvants
With the development of nanotechnology and the increasing understanding of immune responses to metals, different types of inorganic nanoadjuvants have been developed, including manganese (88), iron (89), silicon (90), magnesium (91), and gold-based adjuvants (92), etc. The commonly used polymers are poly-lactic-co-glycolic acid (PLGA), which can be constructed into nano- or larger particles to improve immune response efficiency (93). Compared with the traditional adjuvants, the novel inorganic nanoadjuvants can better activate both humoral and cellular immunity, induce a more balanced Th1/Th2 immune response and improve the safety and effectiveness of vaccines (94). Inorganic nanoadjuvants have been used in vaccines for various diseases, such as coronavirus (95), cancer (91, 96), and pertussis (97). Nanoadjuvants for TB vaccines are also being developed to enhance the immune response and extend the duration of protection (98, 99).
5 Pre-clinical and clinical trials
Pre-clinical and clinical trials are always needed to evaluate the safety and efficacy of novel vaccine candidates. We summarize the significant progression of protein subunit vaccines in recent trials.
5.1 Pre-clinical phases
5.1.1 CysVac2/Advax
CysD is an important protein in the sulfur assimilation pathway of Mtb that is up-regulated during LTBI (33). CysVac2, which consists of CysD and the acute phase antigen Ag85B, is an effective prophylactic and therapeutic vaccine, particularly effective in controlling an advanced infection (34). Notably, administration of CysVac2 to mice previously infected with TB significantly reduced bacterial load and immunopathological damage in the lungs compared to mice vaccinated with BCG (33). CysVac2 with Advax elicited multifunctional CD4+ T cells with enhanced secretion of IFN-γ, TNF, and IL-2. Moreover, CysVac2/Advax induced the accumulation of lung-resident memory T cells expressing IL-17 and RORγT before and after the Mtb aerosol challenge (35). Thus, CysVac2/Advax was shown to be a suitable vaccine candidate for the control of TB pulmonary infection.
5.1.2 LT70
LT70 is a multistage protein subunit vaccine composed of antigens prominent at different metabolic stages of the Mtb life cycle, including ESAT-6, Ag85B, peptide 190-198 of MPT64, proliferative phase antigen Mtb8.4 and latency-associated antigen Rv2626c, with DDA/Poly(I:C) as an adjuvant (39). In a murine model, LT70 induced robust antigen-specific humoral (secretion of IgG1 and IgG2c antibodies) and Th1 cell immunity response (IFN-γ, IL-2) with immune protection against Mtb infection superior to that provided by BCG. When used as a booster vaccine, it enhanced the protective effect of BCG by reducing the bacterial load in the lungs of mice (39). Another study showed that LT70 had a significant therapeutic effect on LTBI in mice (40). In addition, prolonged LT70 inoculation intervals (0-4-12w) produced stronger protective effects and tended to induce long-term central memory T cells (TCM, stronger IL-2 secretion capacity) rather than effector memory T cells (TEM, stronger IFN-γ secretion capacity) (41).
5.1.3 CFMO-DMT
CMFO is a multistage subunit vaccine (containing Rv2875, Rv3044, Rv2073c, and Rv0577) administered subcutaneously adjuvanted with DMT (43, 44). CMFO-DMT could induce the immune response of IFN-γ+ or IL-2+ CD4+ T cells and IFN-γ+ CD8+ TEM cells in spleen more effectively than BCG (43–45). CMFO-DMT prevented Mtb reactivation by eliminating the bacterial load from the lung and spleen in LTBI mice (43), suggesting CMFO-DMT is a promising adult TB vaccine candidate for preventive and therapeutic purposes.
5.1.4 H64/H74/H107
H64 (EsxA, EspD, EspC, EspE, EspR, and PE35), H74 (EspB, EsxA, EspD, EspC, EspA, and EspR), and H107 (PPE68, ESAT-6, EspI, EspC, EspA, MPT64, MPT70, and MPT83) are protein subunit vaccines composed of Mtb–specific antigens (49, 50). H64, and H74 showed comparable protection to H65 (consisting of antigens also present in BCG) in mice and guinea pigs. However, when used as a BCG booster vaccine, H65-induced highly differentiated CD4+ T cells that did not contribute to the protective effect of BCG, while H64 and H74 induced less differentiated and versatile CD4+ T cells (secreting TNF-α alone or TNF-α and IL-2 in combination) with a protective effect against Mtb pulmonary infection (49). H107 vaccination also significantly increased the clonal diversity of the BCG-induced CD4+ T cell repertoire, including Th17-responsive and poorly differentiated memory CD4+ Th1 cells (50). Therefore, protein subunit vaccines containing Mtb-specific antigens may have more potential to serve as booster vaccines in BCG-primed populations.
5.2 Phase I clinical trials
5.2.1 AEC/BC02
AEC/BC02 is a vaccine candidate for LTBI consisting of Ag85B and the fusion protein ESAT-6-CFP10 with adjuvant BC02 (53). Preclinical studies have shown that AEC/BC02 can induce long-term antigen-specific cellular immune responses in mice. In addition, AEC/BC02 reduced the risk of the Koch phenomenon in a guinea pig LTBI model (51, 54). In a murine LTBI model, after AEC/BC02 therapy, the bacterial load in the spleen and lung was significantly reduced. Furthermore, AEC/BC02 induced a significant Th1 response with antigen-specific release of IFN-γ, IL-2, and IgG (IgG1, and IgG2) (55). A phase Ib clinical trial evaluating the safety and immunogenicity of AEC/BC02 in healthy adults has been completed (NCT04239313), and volunteers are currently being recruited for phase II trials.
5.3 Phase II clinical trials
5.3.1 ID93+GLA-SE
ID93+GLA-SE comprises four Mtb antigens (Rv2608, Rv3619, Rv3620, and Rv1813) with GLA-SE as an adjuvant (59). In mice and guinea pigs, ID93+GLA-SE protected against Mtb virulent strain H37Rv and multidrug-resistant strain TN5904 (60). ID93+GLA-SE combined with the first-line anti-TB drugs rifampicin and isoniazid showed therapeutic efficacy in Mtb-infected mice and nonhuman primate (NHP) models (61). ID93+GLA-SE was found to provide long-lasting protection by inducing antigen-specific IgG1 and IgG3 and multifunctional CD4+ T cell responses with enhanced IFN-γ, TNF, and IL-2 secretion in a phase I trial (59, 62). A phase IIa trial showed that ID93+GLA-SE enhanced therapeutic efficacy and reduced disease recurrence by inducing robust cellular and humoral immune responses (63). Phase IIb trials that are aimed at preventing TB recurrence are currently in preparation.
5.3.2 H56:IC31
H56:IC31 is formed by Ag85B, ESAT-6, and Rv2660c protein fusion with adjuvant IC31. Due to the presence of the latency-associated protein Rv2660c, a protective effect of H56 in the murine LTBI model was expected and this was observed (68). In NHP aerosol challenge models, H56:1C31 limited the development of advanced infection and LTBI (69). In a phase I trial, the vaccine induced antigen-specific IgG and CD4+ T cell responses (IFN-γ, TNF-α, IL-2) (70). In a phase I/IIa clinical trial, variations in the dose and time of H56:IC31 inoculation were studied. Two to three vaccination doses were optimal with acceptable safety and tolerability (71). Phase IIb trials of H56:IC31 to reduce TB recurrence in HIV-negative patients receiving anti-TB chemotherapy are ongoing (NCT03512249) (72).
5.3.3 M72/AS01E
M72/AS01E is composed of two immunogenic Mtb fusion proteins (Mtb32A and Mtb39A) with AS01E as an adjuvant. M72/AS01E protected against Mtb invasion after aerosol infection when administered intramuscularly to C57BL/6 mice and guinea pigs (74). When used as a BCG booster vaccine, M72/AS01E provided long-term protection and improved guinea pig and NHP survival post Mtb infection (75, 76). The vaccine was protective against TB in adults in a phase II trial, but the trial was suspended because local reactions were observed in some vaccinated individuals (77). The safety and immunogenicity of M72/AS01E were evaluated in HIV-negative adolescents in TB-endemic areas. The results showed that M72/AS01E was safe and could induce M72/AS01E -specific IgG antibody, CD4+ (IFN-γ, TNF-α, IL-2 and/or IL-17), CD8+ (IFN-γ, TNF-α) T-cells and antigen-dependent NK cell IFN-γ production (78). Another phase II trial, in India, showed elevated cellular and humoral responses by M72/AS01E in both HIV-negative and HIV-positive individuals that persisted for 3 years with no safety concerns (79). Subsequently, a Phase II clinical trial showed that M72/AS01E provided 54% protection against progression to active pulmonary TB in LTBI adults, without significant adverse effects (80). In a randomized placebo-controlled phase IIb study, M72/AS01E protected adults against active TB by 49.7% for at least 3 years without serious safety concerns (81). However, it is doubtful that the excellent protection of M72/AS01E is mainly based on data from a single population, and large-scale long-term trials in a wider population are needed (82).
5.3.4 GamTBvac
The GamTBvac vaccine combines TB antigens ESAT-6, CFP10, and Ag85A with a novel adjuvant, dextran/CpG. GamTBvac showed significant immunogenicity and protection in Mtb-infected mice and guinea pigs when used as a BCG booster vaccine (85). GamTBvac was found to be immunogenic and safe in a phase I trial in BCG-vaccinated, uninfected healthy people (86). A completed phase IIa trial showed that GamTBvac was safe and had considerable immunogenicity in inducing CD4+ T cells expressing Th1 cytokines (IFN-γ, IL-2, and TNF-α), CD8+ T cells secreting IFN-γ, and IgG responses (87). Phase III trials to evaluate the vaccine’s protective efficacy against TB in large populations are currently enrolling volunteers (NCT04975737).
6 Animal models
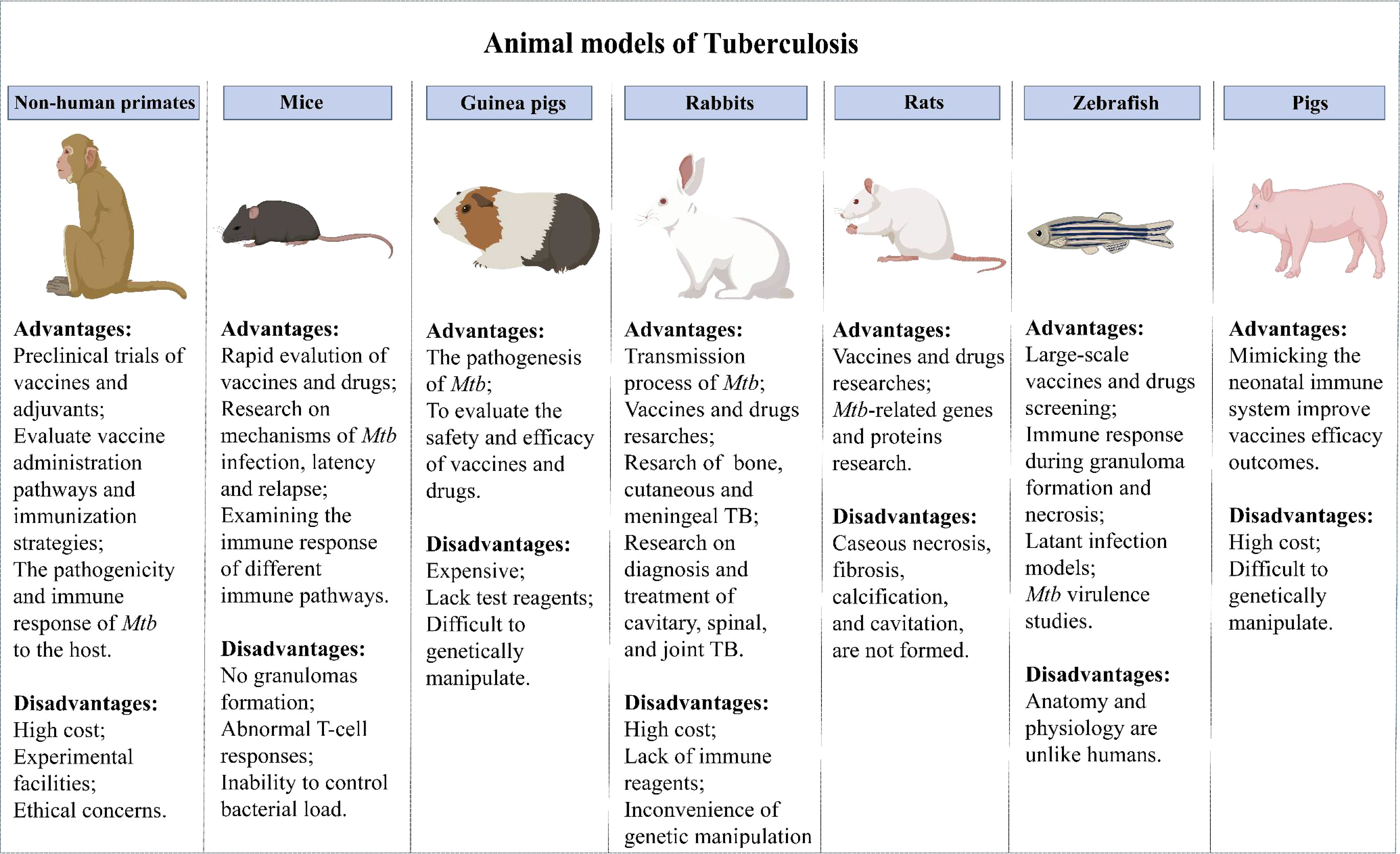
Evaluating vaccine safety and protection in animal models is obligatory before a vaccine enters clinical trials. The development of animal models of TB has advanced the understanding of host responses to Mtb infection and accelerated the development of TB vaccines. Currently, many animal models are used for TB vaccine evaluation (Figure 1).
6.1 Mice
Mice provide the most widely used models due to the advantages of relatively low price, short experiment cycle, mature immunological evaluation indicators, abundant commercial reagents and genetically modified inbred strains (100). The mouse strains most commonly used in evaluating the immune efficacy of TB vaccines are BALB/c and C57BL/6, which are sensitive to TB vaccines immunization routes (101). However, the immune response induced by TB vaccine was different in BALB/c and C57BL/6 mice. M Carmen Garcia-Pelayo et al. found that although BCG present equally protective in BALB/c and C57BL/6 mice, it was display more enhanced Th1 and Th17 response in BALB/c mice than C57BL/6 mice (102). In another study, ChAdOx1.PPE15 as a booster vaccine for BCG improved the efficacy of BCG in C57BL/6 mice, but not in BALB/c mice (103). The susceptibility to TB and the protective responses to the vaccines vary according to the route of infection and immunization. Subcutaneous immunization is the most classic immunization method for TB vaccine, but mucosal immunization has received extensive attention in the pathogenic bacteria infected by mucosal route. A multrivalent chimpanzee adenovirus vectored vaccine developed by Sam Afkhami et al. showed strong protection against both replicating and dormant Mtb through mucosal immunization (104). Previous research by Claudio Counoupas et al. have shown that intratracheal instillation of CysVac2/Advax protected mice more effectively than the intramuscular vaccine (35).
However, despite a high genetic similarity between mice and humans, significant differences in clinical immune responses between mice and humans have stalled clinical trials of many novel vaccines that had previously shown considerable efficacy in murine models. To overcome this problem, humanized mouse models have been extensively studied in recent years. Humanized mice have a reshaped immune system, making the immune responses more like those of humans. They have been widely used in studies of epitopes and epitope-based TB protein subunit vaccine development (105). Although the use of humanized murine models has enabled many advances in TB vaccine research, deficiencies in the models such as the inability to establish LTBI and granulomas (100), abnormal T-cell responses, and the inability to control bacterial load have limited their use.
6.2 NHPs
NHPs can better represent the human immune responses for assessment of the safety and efficacy of TB vaccines and adjuvants due to the close genetic and pathophysiological similarities between NHPs and humans. Rhesus macaques (RM) and cynomolgus macaques (CM) are the most commonly used primate models for TB vaccine research. It is well known that there are differences between macaque species in their ability to control disease progression, with RM showing higher rates of progression and higher levels of bacterial burden compared to CM (106). RM are often used in vaccine evaluation studies because the results of infection are more uniform than CM, while RM are often used in drug evaluation studies because they are better able to control the disease (107). NHPs provide essential insights into host-pathogen interactions during TB infection by simulating the pathogenesis of TB in humans, including the occurrence of LTBI and granuloma formation (108). NHPs can be used to evaluate the immune effect of different vaccine administration pathways and immunization strategies (75, 109).
The use of the NHP models has brought some breakthroughs in TB vaccine development in recent years. First of all, the preclinical evaluation of novel vaccines by the NHP model has facilitated the transformation of vaccines to prevent and therapy Mtb infection (110–112). The ultra-low dose aerosol-infected NHP model better simulates the course of human infection with TB and can accurately evaluate the vaccine immune efficacy (113). Moreover, using NHP makes it possible to study the interactions of cells within lung granulomas, which cannot be done in human samples. Laura Hunter et al. used infection in RM and CM models to determine the basic composition of granulomas induced after infection with the Mtb Erdman strain, as well as the spatial distribution of immune cells in granulomas in RM and CM and changes over time (114). This informs research into TB vaccines and treatments, and may provide novel immunotherapy strategies against TB. Furthermore, the development of body scanning technology, particularly the combination of PET and CT scans, has made it possible to quantitatively evaluate the protective efficacy of TB vaccines in NHP models (115–117). This strategy allows vaccine evaluation in less time and at a lower cost. However, the high cost of the animals and experimental facilities, as well as the limited quantity available, have hindered their widespread application.
6.3 Guinea pigs
Guinea pigs are also a commonly used animal model in the study of TB. Guinea pigs are more susceptible to Mtb than mice and can form classical granulomas similar to humans (118). Therefore, they are suited to studies of the pathogenesis of TB and the assessment of vaccines and drugs (119). Guinea pigs have also been used to study the response of Ag-specific T cells to mycobacterium lipids and lipopeptide-rich Ag preparations (120). Diabetes can fuel TB epidemics, and T2D co-infection with TB has been modeled in guinea pigs in recent years and used to test novel therapy approaches (121–123). However, guinea pigs are more expensive, lack test reagents, and are more difficult to genetically manipulate than mice. Adjuvant subunit vaccines tend to be less protective in guinea pigs than in mice, resulting in few successful trials of adjuvants in guinea pig models (60, 124). The cause of the limited protective immunity provided by adjuvants in guinea pig models awaits clarification, and more tools and reagents are needed for guinea pig models.
6.4 Pigs
The immunity to Mtb infection in neonates is markedly distinct from that in adults. Innate and adaptive immune responses in infants cannot be inferred from adult human or animal models (125). Due to their high similarity to humans in terms of anatomy, genetics, and immune response, pigs are widely used in numerous studies (126, 127). The isolated and sterile state of the porcine fetus during pregnancy is conducive to the study of the interaction between the immature immune system and microorganisms and to determine the changes in the immune structure and function during fetus development (128). Surprisingly, pigs can undergo pathological changes to Mtb infection including caseous necrosis, liquefaction, and cavitation and mimic the immune response of vaccination BCG in humans (129). Mimicking the human neonatal immune system in pigs could improve our understanding of the infant immune response to TB. More neonatal and early-life animal models are needed to advance the development of anti-TB vaccines and drugs for neonates.
6.5 Other animal models
Other animal models, such as rabbits, rats, and zebrafish, have also been used in Mtb vaccine evaluation. Depending on the characteristics of each model, they have been used in different ways. Rabbits are usually infected with Mtb by aerosol route (130), and susceptibility to Mtb varies among different populations (131). Most rabbits available today are highly resistant to infection with Mtb (As Lurie’s - susceptible breed have become extinct), but highly susceptible to infection with the closely related Mycobacterium bovis. They can form granulomas, liquefaction, and cavities similar to the events found in humans, making them suitable for the study of processes leading to transmission of Mtb as well as for vaccine and drug research. In addition, the rabbit model has been used in studies of cavitary, spinal, joint, cutaneous, and meningeal TB (132, 133). However, due to the high cost, lack of immune reagents, and the inconvenience of genetic manipulation, the utility of the rabbit model is limited.
The types of rats commonly used in TB studies are American cotton rats, Wistar rats and diabetic rat strains. Several studies have found that rats exhibit delayed hypersensitivity to Mtb infection (134). Mtb infected rats can form well-organized granulomas, including epithelioid cells, multinucleated giant cells and foam macrophages, etc., which provide a common research object for the study of host control of Mtb and the establishment of latent infection (135). Rats are suitable for TB-related gene and protein research and have the advantages of low cost and simple blood collection, befitting vaccine and drug research (135). Yet, pathological changes in human lungs, such as caseous necrosis, fibrosis, calcification, and cavitation, are not formed in rats.
Recently, zebrafish have attracted increased attention as an animal model for TB. Zebrafish infected with M. marinum can form a typical granulomatous structure, which provides an excellent model for scientists to further study the mechanism of granulomatous formation (136, 137). Moreover, zebrafish have the advantages of visual monitoring, convenient genetic manipulation, fast reproduction, and low cost, they are now widely used for bacterial virulence studies and large-scale vaccine and drug screening. The immune responses during granuloma formation and necrosis can be well monitored, making zebrafish one of the best choices for studying latent TB infection (138, 139). Nevertheless, anatomical and physiological differences between zebrafish and humans impede the application of zebrafish models for vaccine development.
6.6 Ultra-low dose infection models
TB is characteristically caused by respiratory infection when the smallest aerosol droplets containing only 1 or 2 colonies reach the alveolar spaces (1). Hence, the high-dose challenge that has been typically used in animal models might have contributed to discrepant results between pre-clinical and clinical trials. To better simulate the natural human infection process, ultra-low dose infection models have been developed. Infection of conventional mice with 1-3 CFU Mtb produced granulomas with well-defined boundaries similar to human granulomas (140). In addition, the ultra-low dose aerosol-infected NHP model more closely mimicked the process of human natural TB infection. It is being used as a precise and sensitive system to assess the effectiveness of TB vaccines (113).
7 Vaccination strategies of protein subunit vaccines
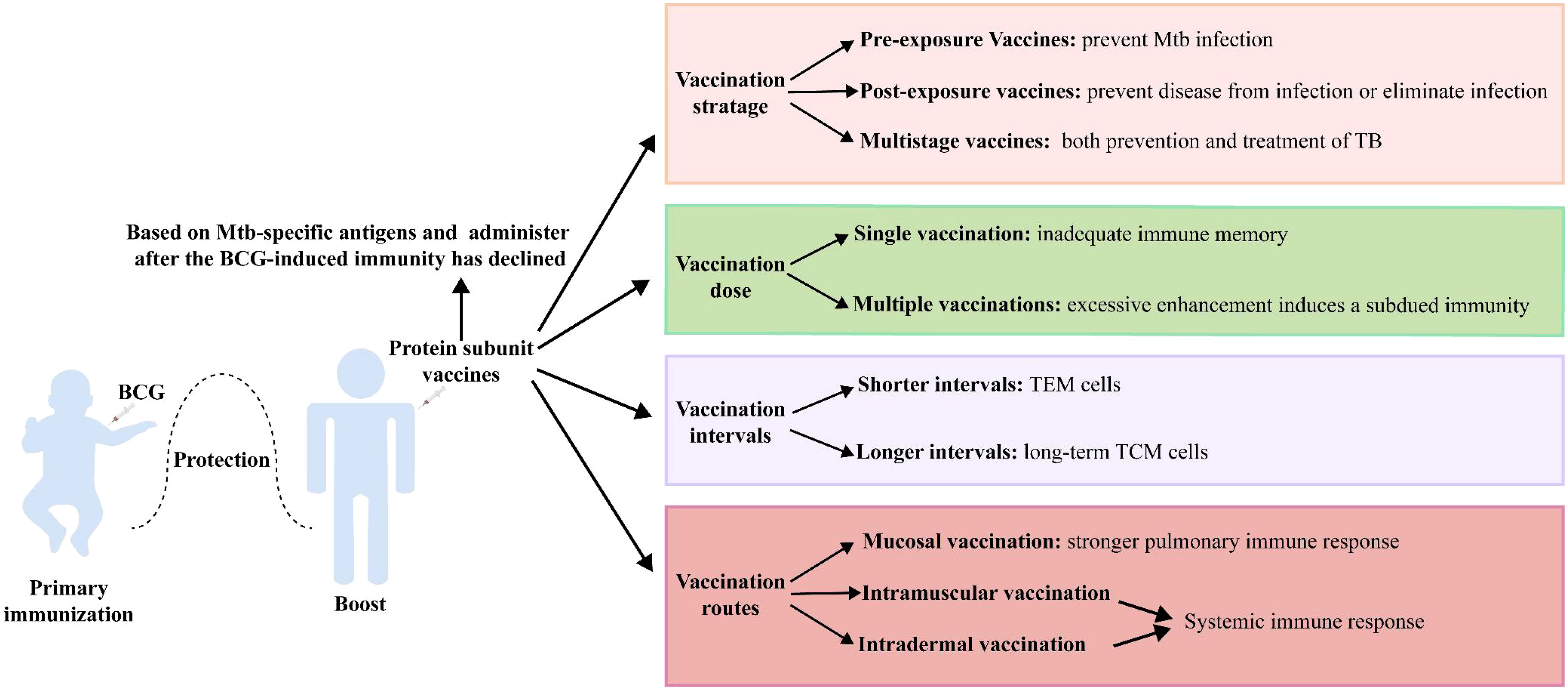
Vaccination strategies are critical to the effectiveness of protein subunit vaccines (Figure 2). Mtb metabolism is profoundly influenced by the different pathophysiological states in different stages of infection. Although current TB vaccines mainly consist of early secreted antigens of Mtb and are used for prevention of infection, they are less than ideal in controlling LTBI and active TB. Protein subunit vaccines currently in clinical trials have jumped out of this framework, with M72/AS01E prevents latent infected people exposed to Mtb from developing active pulmonary TB disease, and ID93+GLA-SE and H56:IC31 showing promise in the treatment of people with active TB infection. Even more promising is the fact that several multistage protein subunit vaccines comprised of Mtb antigens expressed in early growth, dormancy, and resuscitation phases for both prevention and treatment of TB infection have entered pre-clinical (CysVac2, LT70, and CMFO) and clinical trials (H56 and ID93) (35, 39, 43, 63, 71).
Protein subunit vaccines are often used as booster vaccines after BCG priming, and when the antigen in the booster vaccine is shared with BCG, its boosting effect is impaired. BCG vaccination induces highly differentiated CD4+ Th1 cells, and the functional plasticity of these cells is limited. Moreover, BCG-generated immunity impedes the subsequent induction of additional protective T cells with memory and lung homing potential by the booster vaccine (141). Therefore, the development of protein subunit vaccine candidates based on Mtb-specific antigens (such as H64, H74, and H107) may circumvent this dilemma (49, 50).
Preclinical and clinical trials have shown that some protein subunit vaccines (H56, LT70, CFMO) can elicit more robust protection than BCG when used alone, suggesting that such a vaccine could use as an alternative to BCG (39, 43, 68). However, BCG has definite efficacy against childhood TB and is almost universally given to infants as soon as they are born, so that replacement with an alternative vaccine presents ethical and practical challenges. Consequently, a protein subunit vaccine is more likely to use initially as a booster vaccine. Tests have shown that the protective effect of a BCG-booster vaccine is more pronounced when the immune response to BCG is attenuated (49, 142). One explanation for this could be that reduced levels of BCG-induced immunity open the opportunity for protein subunit vaccines to initiate less differentiated T-cell responses. Therefore, it seems more reasonable to administer the protein subunit vaccine after the BCG-induced immunity has declined (49).
The dose and time of boosting with a protein subunit vaccine are also pivotal factors affecting the effect. Multiple vaccinations are usually required to obtain a substantial immune memory with protein subunit vaccines. However, excessive enhancement induces the production of Tregs, leading to a subdued protective effect of the vaccine (143). Moreover, the interval between vaccinations may impact the type of immunological memory. The strategy with protein subunit vaccines is usually a 2- or 3-week booster regimen, which elicits more TEM cells. A booster regimen with longer intervals of 4 weeks appeared to favor the generation of long-term TCM cells (41).
Finally, different vaccination routes exert a significant influence on efficacy. Mtb is transmitted through the respiratory tract, and the protective effect of specific B-cell and strong central memory CD4+ and CD8+ T-cell responses activated by respiratory mucosal vaccination against Mtb infection should be an important consideration (144, 145). Zhang Y et al. found that Ag85A-Mtb32 in adenoviral vectored TB vaccine was more likely to induce systemic immune response through subcutaneous and muscular inoculation, while oral and nasal mucosal immune pathways induced stronger pulmonary immune response (105). Moreover, trained immunity was more strongly induced by submucosal BCG or MTBVAC vaccination than by standard intradermal vaccination (146). A variety of immunostimulatory adjuvants (e.g., bacterial toxins, TLR ligands, and cytokines) and nanoparticle adjuvants (e.g., virus-like particles, liposomes, and protoplasts) have been used in mucosal vaccines to enhance the immune responses (147).
8 Conclusion
Vaccines are powerful weapons for people to prevent and treatment many diseases. The sudden outbreak of the COVID-19 has pushed the development of vaccinology to a climax, and also provided valuable guidance for the development of TB vaccines. The BCG vaccine is undoubtedly one of the most potent weapons that humankind has acquired in the struggle against TB, but its limited protective effect is not sufficient to win the war. Based on the existing WHO-recommended immunization strategy for TB vaccines, protein subunit TB vaccines for specific populations (BCG-immunized, LTBI, and HIV-infected, etc.) have great potential for development and utilization. By far, multiple protein subunit TB vaccines have entered clinical or preclinical trials and have broken the barrier that BCG can only be used for pre-infection prevention. And even some vaccines have shown surprising protection in post-exposure prophylaxis in people with LTBI and in the treatment of people with active TB infection. Rapidly evolved bioinformatics and structural informatics technologies represent a large reservoir to filter out plentiful numbers of Mtb-protective antigens. Training immunity has been proposed in recent years and has received extensive attention in the field of TB. Trained immune cells are able to produce a rapid and effective protective response against Mtb attacks. Therefore, the activation of trained immunity should be considered in the development of vaccines and adjuvants. With the participation of various novel adjuvants, as well as the continuous optimization of animal models and vaccination strategies, effective protein subunit vaccines can be expected in the future to help achieve the grand goal of TB eradication.
Author contributions
XF, and ZH contributed to the conception and revised of this manuscript. ZY and JX drafted and revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
The work was supported by the grants from National Key Research and Development Program of China (2022YFC2302900, 2021YFC2301503), National Natural and Science Foundation of China (82171815, 82171739), Shanghai Hygiene and Health Outstanding Leader Project (2022XD060), Shanghai Science and Technology Commission (20Y11903400).
Acknowledgments
We thank Dr. Douglas B. Lowrie for his critical comments and suggestions to improve the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Donald PR, Diacon AH, Lange C, Demers AM, von Groote-Bidlingmaier F, Nardell E. Droplets, dust and guinea pigs: an historical review of tuberculosis transmission research, 1878-1940. Int J Tuberc Lung Dis (2018) 22(9):972–82. doi: 10.5588/ijtld.18.0173
2. WHO. Global Tuberculosis Report 2022. Geneva: World Health Organization (2022). Licence: CC BY-NC-SA 3.0 IGO.
3. Allue-Guardia A, Garcia JI, Torrelles JB. Evolution of drug-resistant mycobacterium tuberculosis strains and their adaptation to the human lung environment. Front Microbiol (2021) 12:612675. doi: 10.3389/fmicb.2021.612675
4. Gong W, Wu X. Differential diagnosis of latent tuberculosis infection and active tuberculosis: A key to a successful tuberculosis control strategy. Front Microbiol (2021) 12:745592. doi: 10.3389/fmicb.2021.745592
5. Bell LCK, Noursadeghi M. Pathogenesis of hiv-1 and mycobacterium tuberculosis co-infection. Nat Rev Microbiol (2018) 16(2):80–90. doi: 10.1038/nrmicro.2017.128
6. Lange C, Aaby P, Behr MA, Donald PR, Kaufmann SHE, Netea MG, et al. 100 years of mycobacterium bovis bacille calmette-guerin. Lancet Infect Dis (2022) 22(1):e2–e12. doi: 10.1016/S1473-3099(21)00403-5
7. Khademi F, Derakhshan M, Yousefi-Avarvand A, Tafaghodi M, Soleimanpour S. Multi-stage subunit vaccines against mycobacterium tuberculosis: an alternative to the bcg vaccine or a bcg-prime boost? Expert Rev Vaccines (2018) 17(1):31–44. doi: 10.1080/14760584.2018.1406309
8. Schrager LK, Vekemens J, Drager N, Lewinsohn DM, Olesen OF. The status of tuberculosis vaccine development. Lancet Infect Dis (2020) 20(3):e28–37. doi: 10.1016/s1473-3099(19)30625-5
9. Lewinsohn DA, Swarbrick GM, Park B, Cansler ME, Null MD, Toren KG, et al. Comprehensive definition of human immunodominant cd8 antigens in tuberculosis. NPJ Vaccines (2017) 2:8. doi: 10.1038/s41541-017-0008-6
10. Irvine EB, O’Neil A, Darrah PA, Shin S, Choudhary A, Li W, et al. Robust igm responses following intravenous vaccination with bacille calmette-guerin associate with prevention of mycobacterium tuberculosis infection in macaques. Nat Immunol (2021) 22(12):1515–23. doi: 10.1038/s41590-021-01066-1
11. Gong W, Pan C, Cheng P, Wang J, Zhao G, Wu X. Peptide-based vaccines for tuberculosis. Front Immunol (2022) 13:830497. doi: 10.3389/fimmu.2022.830497
12. Williams WV, Weiner DB, Kieber-Emmons T, Greene MI. Antibody geometry and form: three-dimensional relationships between anti-idiotypic antibodies and external antigens. Trends Biotechnol (1990) 8(9):256–63. doi: 10.1016/0167-7799(90)90188-4
13. Hoyos D, Greenbaum BD. Perfecting antigen prediction. J Exp Med (2022) 219(9):e20220846. doi: 10.1084/jem.20220846
14. Rappuoli R, Bottomley MJ, D’Oro U, Finco O, De Gregorio E. Reverse vaccinology 2.0: human immunology instructs vaccine antigen design. J Exp Med (2016) 213(4):469–81. doi: 10.1084/jem.20151960
15. Jumper J, Evans R, Pritzel A, Green T, Figurnov M, Ronneberger O, et al. Highly accurate protein structure prediction with alphafold. Nature (2021) 596(7873):583–9. doi: 10.1038/s41586-021-03819-2
16. Zeng D, Xin J, Yang K, Guo S, Wang Q, Gao Y, et al. A hemagglutinin stem vaccine designed rationally by alphafold2 confers broad protection against influenza B infection. Viruses (2022) 14(6):1305. doi: 10.3390/v14061305
17. Monterrubio-Lopez GP, Gonzalez YMJA, Ribas-Aparicio RM. Identification of novel potential vaccine candidates against tuberculosis based on reverse vaccinology. BioMed Res Int (2015) 2015:483150. doi: 10.1155/2015/483150
18. Correia-Neves M, Sundling C, Cooper A, Kallenius G. LipoarabinOmannan in active and passive protection against tuberculosis. Front Immunol (2019) 10:1968. doi: 10.3389/fimmu.2019.01968
19. Wolfe LM, Mahaffey SB, Kruh NA, Dobos KM. Proteomic definition of the cell wall of mycobacterium tuberculosis. J Proteome Res (2010) 9(11):5816–26. doi: 10.1021/pr1005873
20. Pal R, Bisht MK, Mukhopadhyay S. Secretory proteins of mycobacterium tuberculosis and their roles in modulation of host immune responses: focus on therapeutic targets. FEBS J (2022) 289(14):4146–71. doi: 10.1111/febs.16369
21. Boon C, Dick T. How mycobacterium tuberculosis goes to sleep: the dormancy survival regulator dosr a decade later. Future Microbiol (2012) 7(4):513–8. doi: 10.2217/fmb.12.14
22. Rustad TR, Harrell MI, Liao R, Sherman DR. The enduring hypoxic response of mycobacterium tuberculosis. PloS One (2008) 3(1):e1502. doi: 10.1371/journal.pone.0001502
23. Murphy DJ, Brown JR. Novel drug target strategies against mycobacterium tuberculosis. Curr Opin Microbiol (2008) 11(5):422–7. doi: 10.1016/j.mib.2008.08.001
24. Serra-Vidal MM, Latorre I, Franken KL, Diaz J, de Souza-Galvao ML, Casas I, et al. Immunogenicity of 60 novel latency-related antigens of mycobacterium tuberculosis. Front Microbiol (2014) 5:517. doi: 10.3389/fmicb.2014.00517
25. ROmano M, Aryan E, Korf H, Bruffaerts N, Franken CL, Ottenhoff TH, et al. Potential of mycobacterium tuberculosis resuscitation-promoting factors as antigens in novel tuberculosis sub-unit vaccines. Microbes Infect (2012) 14(1):86–95. doi: 10.1016/j.micinf.2011.08.011
26. Lewis KN, Liao R, Guinn KM, Hickey MJ, Smith S, Behr MA, et al. Deletion of rd1 from mycobacterium tuberculosis mimics bacille calmette-guerin attenuation. J Infect Dis (2003) 187(1):117–23. doi: 10.1086/345862
27. Daugelat S, Kowall J, Mattow J, Bumann D, Winter R, Hurwitz R, et al. The rd1 proteins of mycobacterium tuberculosis: expression in mycobacterium smegmatis and biochemical characterization. Microbes Infect (2003) 5(12):1082–95. doi: 10.1016/s1286-4579(03)00205-3
28. Enriquez AB, Izzo A, Miller SM, Stewart EL, Mahon RN, Frank DJ, et al. Advancing adjuvants for mycobacterium tuberculosis therapeutics. Front Immunol (2021) 12:740117. doi: 10.3389/fimmu.2021.740117
29. Xu X, Jin Z, Liu Y, Gong H, Sun Q, Zhang W, et al. Carbohydrate-based adjuvants activate tumor-specific th1 and cd8(+) T-cell responses and reduce the immunosuppressive activity of mdscs. Cancer Lett (2019) 440-441:94–105. doi: 10.1016/j.canlet.2018.10.013
30. Quan DH, Counoupas C, Nagalingam G, Pinto R, Petrovsky N, Britton WJ, et al. Advax adjuvant formulations promote protective immunity against aerosol mycobacterium tuberculosis in the absence of deleterious inflammation and reactogenicity. Vaccine (2021) 39(14):1990–6. doi: 10.1016/j.vaccine.2021.02.041
31. Hayashi M, Aoshi T, Haseda Y, Kobiyama K, Wijaya E, Nakatsu N, et al. Advax, a delta inulin microparticle, potentiates in-built adjuvant property of co-administered vaccines. EBioMedicine (2017) 15:127–36. doi: 10.1016/j.ebiom.2016.11.015
32. Tomar J, Patil HP, Bracho G, Tonnis WF, Frijlink HW, Petrovsky N, et al. Advax augments B and T cell responses upon influenza vaccination via the respiratory tract and enables complete protection of mice against lethal influenza virus challenge. J Control Release (2018) 288:199–211. doi: 10.1016/j.jconrel.2018.09.006
33. Counoupas C, Pinto R, Nagalingam G, Hill-Cawthorne GA, Feng CG, Britton WJ, et al. Mycobacterium tuberculosis components expressed during chronic infection of the lung contribute to long-term control of pulmonary tuberculosis in mice. NPJ Vaccines (2016) 1:16012. doi: 10.1038/npjvaccines.2016.12
34. Counoupas C, Pinto R, Nagalingam G, Britton WJ, Petrovsky N, Triccas JA. Delta inulin-based adjuvants promote the generation of polyfunctional cd4(+) T cell responses and protection against mycobacterium tuberculosis infection. Sci Rep (2017) 7(1):8582. doi: 10.1038/s41598-017-09119-y
35. Counoupas C, Ferrell KC, Ashhurst A, Bhattacharyya ND, Nagalingam G, Stewart EL, et al. Mucosal delivery of a multistage subunit vaccine promotes development of lung-resident memory T cells and affords interleukin-17-dependent protection against pulmonary tuberculosis. NPJ Vaccines (2020) 5(1):105. doi: 10.1038/s41541-020-00255-7
36. Hafner AM, Corthésy B. Merkle HP. Particulate formulations for the delivery of poly(I:C) as vaccine adjuvant. Adv Drug Delivery Rev (2013) 65(10):1386–99. doi: 10.1016/j.addr.2013.05.013
37. Tewari K, Flynn BJ, Boscardin SB, Kastenmueller K, Salazar AM, Anderson CA, et al. Poly(I:C) is an effective adjuvant for antibody and multi-functional cd4+ T cell responses to plasmodium falciparum circumsporozoite protein (Csp) and Adec-csp in non human primates. Vaccine (2010) 28(45):7256–66. doi: 10.1016/j.vaccine.2010.08.098
38. Stahl-Hennig C, Eisenblätter M, Jasny E, Rzehak T, Tenner-Racz K, Trumpfheller C, et al. Synthetic double-stranded rnas are adjuvants for the induction of T helper 1 and humoral immune responses to human papillomavirus in rhesus macaques. PloS Pathog (2009) 5(4):e1000373. doi: 10.1371/journal.ppat.1000373
39. Liu X, Peng J, Hu L, Luo Y, Niu H, Bai C, et al. A multistage mycobacterium tuberculosis subunit vaccine lt70 including latency antigen rv2626c induces long-term protection against tuberculosis. Hum Vaccin Immunother (2016) 12(7):1670–7. doi: 10.1080/21645515.2016.1141159
40. Li F, Kang H, Li J, Zhang D, Zhang Y, Dannenberg AM Jr., et al. Subunit vaccines consisting of antigens from dormant and replicating bacteria show promising therapeutic effect against mycobacterium bovis bcg latent infection. Scand J Immunol (2017) 85(6):425–32. doi: 10.1111/sji.12556
41. Bai C, He J, Niu H, Hu L, Luo Y, Liu X, et al. Prolonged intervals during mycobacterium tuberculosis subunit vaccine boosting contributes to eliciting immunity mediated by central memory-like T cells. Tuberculosis (Edinb) (2018) 110:104–11. doi: 10.1016/j.tube.2018.04.006
42. Tian M, Zhou Z, Tan S, Fan X, Li L, Ullah N. Formulation in dda-mpla-tdb liposome enhances the immunogenicity and protective efficacy of a DNA vaccine against mycobacterium tuberculosis infection. Front Immunol (2018) 9:310. doi: 10.3389/fimmu.2018.00310
43. Ma J, Teng X, Wang X, Fan X, Wu Y, Tian M, et al. A multistage subunit vaccine effectively protects mice against primary progressive tuberculosis, latency and reactivation. EBioMedicine (2017) 22:143–54. doi: 10.1016/j.ebiom.2017.07.005
44. Ullah N, Hao L, Wu Y, Zhang Y, Lei Q, Banga Ndzouboukou JL, et al. Differential immunogenicity and protective efficacy elicited by mto- and dmt-adjuvanted cmfo subunit vaccines against mycobacterium tuberculosis infection. J Immunol Res (2020) 2020:2083793. doi: 10.1155/2020/2083793
45. Hao L, Wu Y, Zhang Y, Zhou Z, Lei Q, Ullah N, et al. Combinational prr agonists in liposomal adjuvant enhances immunogenicity and protective efficacy in a tuberculosis subunit vaccine. Front Immunol (2020) 11:575504. doi: 10.3389/fimmu.2020.575504
46. Hilgers LA, Snippe H. Dda as an immunological adjuvant. Res Immunol (1992) 143(5):494–503. doi: 10.1016/0923-2494(92)80060-x. discussion 74-6.
47. Ishikawa E, Ishikawa T, Morita YS, Toyonaga K, Yamada H, Takeuchi O, et al. Direct recognition of the mycobacterial glycolipid, trehalose dimycolate, by C-type lectin mincle. J Exp Med (2009) 206(13):2879–88. doi: 10.1084/jem.20091750
48. van Dissel JT, Joosten SA, Hoff ST, Soonawala D, Prins C, Hokey DA, et al. A novel liposomal adjuvant system, caf01, promotes long-lived mycobacterium tuberculosis-specific T-cell responses in human. Vaccine (2014) 32(52):7098–107. doi: 10.1016/j.vaccine.2014.10.036
49. Aagaard C, Knudsen NPH, Sohn I, Izzo AA, Kim H, Kristiansen EH, et al. Immunization with mycobacterium tuberculosis-specific antigens bypasses T cell differentiation from prior bacillus calmette-guérin vaccination and improves protection in mice. J Immunol (2020) 205(8):2146–55. doi: 10.4049/jimmunol.2000563
50. Woodworth JS, Clemmensen HS, Battey H, Dijkman K, Lindenstrøm T, Laureano RS, et al. A mycobacterium tuberculosis-specific subunit vaccine that provides synergistic immunity upon co-administration with bacillus calmette-guérin. Nat Commun (2021) 12(1):6658. doi: 10.1038/s41467-021-26934-0
51. Lu JB, Chen BW, Wang GZ, Fu LL, Shen XB, Su C, et al. Recombinant tuberculosis vaccine aec/bc02 induces antigen-specific cellular responses in mice and protects Guinea pigs in a model of latent infection. J Microbiol Immunol Infect (2015) 48(6):597–603. doi: 10.1016/j.jmii.2014.03.005
52. Bomford R. Will adjuvants be needed for vaccines of the future? Dev Biol Stand (1998) 92:13–7. doi: 10.1038/s41423-021-00669-w
53. Chen L, Xu M, Wang ZY, Chen BW, Du WX, Su C, et al. The development and preliminary evaluation of a new mycobacterium tuberculosis vaccine comprising ag85b, hspx and cfp-10:Esat-6 fusion protein with cpg DNA and aluminum hydroxide adjuvants. FEMS Immunol Med Microbiol (2010) 59(1):42–52. doi: 10.1111/j.1574-695X.2010.00660.x
54. Lu JB, Cheng BW, Deng HQ, Su C, Shen XB, Du WX, et al. Analysis of koch phenomenon of mycobacterium tuberculosis-infected Guinea pigs vaccinated with recombinant tuberculosis vaccine aec/bc02. Zhonghua Jie He He Hu Xi Za Zhi (2016) 39(7):524–8. doi: 10.3760/cma.j.issn.1001-0939.2016.07.007
55. Lu J, Guo X, Wang C, Du W, Shen X, Su C, et al. Therapeutic effect of subunit vaccine aec/bc02 on mycobacterium tuberculosis post-chemotherapy relapse using a latent infection murine model. Vaccines (Basel) (2022) 10(5):825. doi: 10.3390/vaccines10050825
56. Dubois Cauwelaert N, Desbien AL, Hudson TE, Pine SO, Reed SG, Coler RN, et al. The tlr4 agonist vaccine adjuvant, gla-se, requires canonical and atypical mechanisms of action for th1 induction. PloS One (2016) 11(1):e0146372. doi: 10.1371/journal.pone.0146372
57. Coler RN, Bertholet S, Moutaftsi M, Guderian JA, Windish HP, Baldwin SL, et al. Development and characterization of synthetic glucopyranosyl lipid adjuvant system as a vaccine adjuvant. PloS One (2011) 6(1):e16333. doi: 10.1371/journal.pone.0016333
58. Kim EH, Woodruff MC, Grigoryan L, Maier B, Lee SH, Mandal P, et al. Squalene Emulsion-Based Vaccine Adjuvants Stimulate Cd8 T Cell, but Not Antibody Responses, through a Ripk3-Dependent Pathway. Elife (2020) 9:e52687. doi: 10.7554/eLife.52687
59. Penn-Nicholson A, Tameris M, Smit E, Day TA, Musvosvi M, Jayashankar L, et al. Safety and immunogenicity of the novel tuberculosis vaccine id93 + Gla-se in bcg-vaccinated healthy adults in South Africa: A randomised, double-blind, placebo-controlled phase 1 trial. Lancet Respir Med (2018) 6(4):287–98. doi: 10.1016/s2213-2600(18)30077-8
60. Baldwin SL, Bertholet S, Reese VA, Ching LK, Reed SG, Coler RN. The importance of adjuvant formulation in the development of a tuberculosis vaccine. J Immunol (2012) 188(5):2189–97. doi: 10.4049/jimmunol.1102696
61. Coler RN, Bertholet S, Pine SO, Orr MT, Reese V, Windish HP, et al. Therapeutic immunization against mycobacterium tuberculosis is an effective adjunct to antibiotic treatment. J Infect Dis (2013) 207(8):1242–52. doi: 10.1093/infdis/jis425
62. Coler RN, Day TA, Ellis R, Piazza FM, Beckmann AM, Vergara J, et al. The tlr-4 agonist adjuvant, gla-se, improves magnitude and quality of immune responses elicited by the id93 tuberculosis vaccine: first-in-human trial. NPJ Vaccines (2018) 3:34. doi: 10.1038/s41541-018-0057-5
63. Day TA, Penn-Nicholson A, Luabeya AKK, Fiore-Gartland A, Du Plessis N, Loxton AG, et al. Safety and immunogenicity of the adjunct therapeutic vaccine id93 + Gla-se in adults who have completed treatment for tuberculosis: A randomised, double-blind, placebo-controlled, phase 2a trial. Lancet Respir Med (2021) 9(4):373–86. doi: 10.1016/s2213-2600(20)30319-2
64. Khademi F, Taheri RA, Momtazi-Borojeni AA, Farnoosh G, Johnston TP, Sahebkar A. Potential of cationic liposomes as adjuvants/delivery systems for tuberculosis subunit vaccines. Rev Physiol Biochem Pharmacol (2018) 175:47–69. doi: 10.1007/112_2018_9
65. Fritz JH, Brunner S, Birnstiel ML, Buschle M, Gabain A, Mattner F, et al. The artificial antimicrobial peptide klklllllklk induces predominantly a th2-type immune response to co-injected antigens. Vaccine (2004) 22(25-26):3274–84. doi: 10.1016/j.vaccine.2004.03.007
66. Aichinger MC, Ginzler M, Weghuber J, Zimmermann L, Riedl K, Schütz G, et al. Adjuvating the adjuvant: facilitated delivery of an immunomodulatory oligonucleotide to tlr9 by a cationic antimicrobial peptide in dendritic cells. Vaccine (2011) 29(3):426–36. doi: 10.1016/j.vaccine.2010.11.003
67. Schellack C, Prinz K, Egyed A, Fritz JH, Wittmann B, Ginzler M, et al. Ic31, a novel adjuvant signaling via tlr9, induces potent cellular and humoral immune responses. Vaccine (2006) 24(26):5461–72. doi: 10.1016/j.vaccine.2006.03.071
68. Aagaard C, Hoang T, Dietrich J, Cardona PJ, Izzo A, Dolganov G, et al. A Multistage Tuberculosis Vaccine That Confers Efficient Protection before and after Exposure. Nat Med (2011) 17(2):189–94. doi: 10.1038/nm.2285
69. Lin PL, Dietrich J, Tan E, Abalos RM, Burgos J, Bigbee C, et al. The multistage vaccine H56 boosts the effects of bcg to protect cynomolgus macaques against active tuberculosis and reactivation of latent mycobacterium tuberculosis infection. J Clin Invest (2012) 122(1):303–14. doi: 10.1172/jci46252
70. Luabeya AK, Kagina BM, Tameris MD, Geldenhuys H, Hoff ST, Shi Z, et al. First-in-human trial of the post-exposure tuberculosis vaccine H56:Ic31 in mycobacterium tuberculosis infected and non-infected healthy adults. Vaccine (2015) 33(33):4130–40. doi: 10.1016/j.vaccine.2015.06.051
71. Suliman S, Luabeya AKK, Geldenhuys H, Tameris M, Hoff ST, Shi Z, et al. Dose optimization of H56:Ic31 vaccine for tuberculosis-endemic populations. A double-blind, placebo-controlled, dose-selection trial. Am J Respir Crit Care Med (2019) 199(2):220–31. doi: 10.1164/rccm.201802-0366OC
72. Li J, Zhao A, Tang J, Wang G, Shi Y, Zhan L, et al. Tuberculosis vaccine development: from classic to clinical candidates. Eur J Clin Microbiol Infect Dis (2020) 39(8):1405–25. doi: 10.1007/s10096-020-03843-6
73. Didierlaurent AM, Laupèze B, Di Pasquale A, Hergli N, Collignon C, Garçon N. Adjuvant system as01: helping to overcome the challenges of modern vaccines. Expert Rev Vaccines (2017) 16(1):55–63. doi: 10.1080/14760584.2016.1213632
74. Skeiky YA, Alderson MR, Ovendale PJ, Guderian JA, Brandt L, Dillon DC, et al. Differential immune responses and protective efficacy induced by components of a tuberculosis polyprotein vaccine, mtb72f, delivered as naked DNA or recombinant protein. J Immunol (2004) 172(12):7618–28. doi: 10.4049/jimmunol.172.12.7618
75. Reed SG, Coler RN, Dalemans W, Tan EV, DeLa Cruz EC, Basaraba RJ, et al. Defined tuberculosis vaccine, mtb72f/as02a, evidence of protection in cynomolgus monkeys. Proc Natl Acad Sci U.S.A. (2009) 106(7):2301–6. doi: 10.1073/pnas.0712077106
76. Brandt L, Skeiky YA, Alderson MR, Lobet Y, Dalemans W, Turner OC, et al. The protective effect of the mycobacterium bovis bcg vaccine is increased by coadministration with the mycobacterium tuberculosis 72-kilodalton fusion polyprotein mtb72f in M. Tuberculosis-infected Guinea pigs. Infect Immun (2004) 72(11):6622–32. doi: 10.1128/iai.72.11.6622-6632.2004
77. Gillard P, Yang PC, Danilovits M, Su WJ, Cheng SL, Pehme L, et al. Safety and immunogenicity of the M72/as01e candidate tuberculosis vaccine in adults with tuberculosis: A phase ii randomised study. Tuberculosis (Edinb) (2016) 100:118–27. doi: 10.1016/j.tube.2016.07.005
78. Penn-Nicholson A, Geldenhuys H, Burny W, van der Most R, Day CL, Jongert E, et al. Safety and immunogenicity of candidate vaccine M72/as01e in adolescents in a tb endemic setting. Vaccine (2015) 33(32):4025–34. doi: 10.1016/j.vaccine.2015.05.088
79. Kumarasamy N, Poongulali S, Beulah FE, Akite EJ, Ayuk LN, Bollaerts A, et al. Long-term safety and immunogenicity of the M72/as01e candidate tuberculosis vaccine in hiv-positive and -negative Indian adults: results from a phase ii randomized controlled trial. Med (Baltimore) (2018) 97(45):e13120. doi: 10.1097/md.0000000000013120
80. Van Der Meeren O, Hatherill M, Nduba V, Wilkinson RJ, Muyoyeta M, Van Brakel E, et al. Phase 2b controlled trial of M72/as01(E) vaccine to prevent tuberculosis. N Engl J Med (2018) 379(17):1621–34. doi: 10.1056/NEJMoa1803484
81. Tait DR, Hatherill M, van der Meeren O, Ginsberg AM, Van Brakel E, Salaun B, et al. Final analysis of a trial of M72/as01(E) vaccine to prevent tuberculosis. N Engl J Med (2019) 381(25):2429–39. doi: 10.1056/NEJMoa1909953
82. Ottenhoff THM. A trial of M72/as01e vaccine to prevent tuberculosis. N Engl J Med (2020) 382(16):1576–7. doi: 10.1056/NEJMc2001364
83. Zhang W, An M, Xi J, Liu H. Targeting cpg adjuvant to lymph node via dextran conjugate enhances antitumor immunotherapy. Bioconjug Chem (2017) 28(7):1993–2000. doi: 10.1021/acs.bioconjchem.7b00313
84. Brazolot Millan CL, Weeratna R, Krieg AM, Siegrist CA, Davis HL. Cpg DNA can induce strong th1 humoral and cell-mediated immune responses against hepatitis B surface antigen in young mice. Proc Natl Acad Sci U.S.A. (1998) 95(26):15553–8. doi: 10.1073/pnas.95.26.15553
85. Tkachuk AP, Gushchin VA, Potapov VD, Demidenko AV, Lunin VG, Gintsburg AL. Multi-subunit bcg booster vaccine gamtbvac: assessment of immunogenicity and protective efficacy in murine and Guinea pig tb models. PloS One (2017) 12(4):e0176784. doi: 10.1371/journal.pone.0176784
86. Vasina DV, Kleymenov DA, Manuylov VA, Mazunina EP, Koptev EY, Tukhovskaya EA, et al. First-in-human trials of gamtbvac, a recombinant subunit tuberculosis vaccine candidate: safety and immunogenicity assessment. Vaccines (Basel) (2019) 7(4):166. doi: 10.3390/vaccines7040166
87. Tkachuk AP, Bykonia EN, Popova LI, Kleymenov DA, Semashko MA, Chulanov VP, et al. Safety and immunogenicity of the gamtbvac, the recombinant subunit tuberculosis vaccine candidate: A phase ii, multi-center, double-blind, randomized, placebo-controlled study. Vaccines (2020) 8(4):652. doi: 10.3390/vaccines8040652
88. Zhang R, Wang C, Guan Y, Wei X, Sha M, Yi M, et al. Manganese salts function as potent adjuvants. Cell Mol Immunol (2021) 18(5):1222–34. doi: 10.1038/s41423-021-00669-w
89. Zhao Y, Zhao X, Cheng Y, Guo X, Yuan W. Iron oxide nanoparticles-based vaccine delivery for cancer treatment. Mol Pharm (2018) 15(5):1791–9. doi: 10.1021/acs.molpharmaceut.7b01103
90. Kim J, Li WA, Choi Y, Lewin SA, Verbeke CS, Dranoff G, et al. Injectable, spontaneously assembling, inorganic scaffolds modulate immune cells in vivo and increase vaccine efficacy. Nat Biotechnol (2015) 33(1):64–72. doi: 10.1038/nbt.3071
91. Dai H, Huang Y, Guo J, Li L, Ke Y, Cen L, et al. Engineering a hemomap nanovaccine for inducing immune responses against melanoma. ACS Appl Mater Interfaces (2022) 14(47):52634–42. doi: 10.1021/acsami.2c14379
92. Zhou Q, Zhang Y, Du J, Li Y, Zhou Y, Fu Q, et al. Different-sized gold nanoparticle activator/antigen increases dendritic cells accumulation in liver-draining lymph nodes and cd8+ T cell responses. ACS Nano (2016) 10(2):2678–92. doi: 10.1021/acsnano.5b07716
93. Danhier F, Ansorena E, Silva JM, Coco R, Le Breton A, Préat V. Plga-based nanoparticles: an overview of biomedical applications. J Control Release (2012) 161(2):505–22. doi: 10.1016/j.jconrel.2012.01.043
94. Li X, Wang X, Ito A. Tailoring inorganic nanoadjuvants towards next-generation vaccines. Chem Soc Rev (2018) 47(13):4954–80. doi: 10.1039/c8cs00028j
95. Rodrigues KA, Rodriguez-Aponte SA, Dalvie NC, Lee JH, Abraham W, Carnathan DG, et al. Phosphate-mediated coanchoring of rbd immunogens and molecular adjuvants to alum potentiates humoral immunity against Sars-Cov-2. Sci Adv (2021) 7(50):eabj6538. doi: 10.1126/sciadv.abj6538
96. Chen A, Wu L, Luo Y, Lu S, Wang Y, Zhou Z, et al. Deep tumor penetrating gold nano-adjuvant for nir-ii-triggered in situ tumor vaccination. Small (2022) 18(20):e2200993. doi: 10.1002/smll.202200993
97. Li D, Xu M, Li G, Zheng Y, Zhang Y, Xia D, et al. Mg/al-ldh as a nano-adjuvant for pertussis vaccine: A evaluation compared with aluminum hydroxide adjuvant. Nanotechnology (2022) 33(23):10. doi: 10.1088/1361-6528/ac56f3
98. Yousefi Avarvand A, Meshkat Z, Khademi F, Tafaghodi M. Immunogenicity of hspx/esxs fusion protein of mycobacterium tuberculosis along with iscomatrix and pluscom nano-adjuvants after subcutaneous administration in animal model. Microb Pathog (2021) 154:104842. doi: 10.1016/j.micpath.2021.104842
99. Qian K, Shan L, Shang S, Li T, Wang S, Wei M, et al. Manganese enhances macrophage defense against mycobacterium tuberculosis via the sting-tnf signaling pathway. Int Immunopharmacol (2022) 113(Pt B):109471. doi: 10.1016/j.intimp.2022.109471
100. Gong W, Liang Y, Wu X. Animal models of tuberculosis vaccine research: an important component in the fight against tuberculosis. BioMed Res Int (2020) 2020:4263079. doi: 10.1155/2020/4263079
101. Mata E, Tarancon R, Guerrero C, Moreo E, Moreau F, Uranga S, et al. Pulmonary bcg induces lung-resident macrophage activation and confers long-term protection against tuberculosis. Sci Immunol (2021) 6(63):eabc2934. doi: 10.1126/sciimmunol.abc2934
102. Garcia-Pelayo MC, Bachy VS, Kaveh DA, Hogarth PJ. Balb/C mice display more enhanced bcg vaccine induced th1 and th17 response than C57bl/6 mice but have equivalent protection. Tuberculosis (Edinb) (2015) 95(1):48–53. doi: 10.1016/j.tube.2014.10.012
103. Stylianou E, Harrington-Kandt R, Beglov J, Bull N, Pinpathomrat N, Swarbrick GM, et al. Identification and evaluation of novel protective antigens for the development of a candidate tuberculosis subunit vaccine. Infect Immun (2018) 86(7):e00014–18. doi: 10.1128/iai.00014-18
104. Afkhami S, D’Agostino MR, Vaseghi-Shanjani M, Lepard M, Yang JX, Lai R, et al. Intranasal multivalent adenoviral-vectored vaccine protects against replicating and dormant M.Tb in conventional and humanized mice. NPJ Vaccines (2023) 8(1):25. doi: 10.1038/s41541-023-00623-z
105. Gong W, Liang Y, Mi J, Jia Z, Xue Y, Wang J, et al. Peptides-based vaccine mp3rt induced protective immunity against mycobacterium tuberculosis infection in a humanized mouse model. Front Immunol (2021) 12:666290. doi: 10.3389/fimmu.2021.666290
106. Maiello P, DiFazio RM, Cadena AM, Rodgers MA, Lin PL, Scanga CA, et al. Rhesus macaques are more susceptible to progressive tuberculosis than cynomolgus macaques: A quantitative comparison. Infect Immun (2018) 86(2):e00505–17. doi: 10.1128/iai.00505-17
107. Peña JC, Ho WZ. Non-human primate models of tuberculosis. Microbiol Spectr (2016) 4(4):10. doi: 10.1128/microbiolspec.TBTB2-0007-2016
108. Luciw PA, Oslund KL, Yang XW, Adamson L, Ravindran R, Canfield DR, et al. Stereological analysis of bacterial load and lung lesions in nonhuman primates (Rhesus macaques) experimentally infected with mycobacterium tuberculosis. Am J Physiol Lung Cell Mol Physiol (2011) 301(5):L731–8. doi: 10.1152/ajplung.00120.2011
109. Darrah PA, Zeppa JJ, Maiello P, Hackney JA, Wadsworth MH 2nd, Hughes TK, et al. Prevention of tuberculosis in macaques after intravenous bcg immunization. Nature (2020) 577(7788):95–102. doi: 10.1038/s41586-019-1817-8
110. Rivera-Hernandez T, Carnathan DG, Moyle PM, Toth I, West NP, Young PR, et al. The contribution of non-human primate models to the development of human vaccines. Discovery Med (2014) 18(101):313–22.
111. Verreck FA, Vervenne RA, Kondova I, van Kralingen KW, Remarque EJ, Braskamp G, et al. Mva.85a boosting of bcg and an attenuated, phop deficient M. Tuberculosis vaccine both show protective efficacy against tuberculosis in rhesus macaques. PloS One (2009) 4(4):e5264. doi: 10.1371/journal.pone.0005264
112. Rahman S, Magalhaes I, Rahman J, Ahmed RK, Sizemore DR, Scanga CA, et al. Prime-boost vaccination with rbcg/rad35 enhances cd8+ Cytolytic T-cell responses in lesions from mycobacterium tuberculosis-infected primates. Mol Med (2012) 18(1):647–58. doi: 10.2119/molmed.2011.00222
113. White AD, Sarfas C, Sibley LS, Gullick J, Clark S, Rayner E, et al. Protective efficacy of inhaled bcg vaccination against ultra-low dose aerosol M. Tuberculosis challenge in rhesus macaques. Pharmaceutics (2020) 12(5):394. doi: 10.3390/pharmaceutics12050394
114. Hunter L, Hingley-Wilson S, Stewart GR, Sharpe SA, Salguero FJ. Dynamics of macrophage, T and B cell infiltration within pulmonary granulomas induced by mycobacterium tuberculosis in two non-human primate models of aerosol infection. Front Immunol (2021) 12:776913. doi: 10.3389/fimmu.2021.776913
115. Coleman MT, Maiello P, Tomko J, Frye LJ, Fillmore D, Janssen C, et al. Early changes by (18)Fluorodeoxyglucose positron emission tomography coregistered with computed tomography predict outcome after mycobacterium tuberculosis infection in cynomolgus macaques. Infect Immun (2014) 82(6):2400–4. doi: 10.1128/iai.01599-13
116. Laddy DJ, Bonavia A, Hanekom WA, Kaushal D, Williams A, Roederer M, et al. Toward tuberculosis vaccine development: recommendations for nonhuman primate study design. Infect Immun (2018) 86(2):e00776–17. doi: 10.1128/iai.00776-17
117. White AG, Maiello P, Coleman MT, Tomko JA, Frye LJ, Scanga CA, et al. Analysis of 18fdg pet/ct imaging as a tool for studying mycobacterium tuberculosis infection and treatment in non-human primates. J Vis Exp (2017) (127):56375. doi: 10.3791/56375
119. Clark S, Hall Y, Williams A. Animal models of tuberculosis: Guinea pigs. Cold Spring Harb Perspect Med (2014) 5(5):a018572. doi: 10.1101/cshperspect.a018572
120. Kaufmann E, Spohr C, Battenfeld S, De Paepe D, Holzhauser T, Balks E, et al. Bcg vaccination induces robust cd4+ T cell responses to mycobacterium tuberculosis complex-specific lipopeptides in Guinea pigs. J Immunol (2016) 196(6):2723–32. doi: 10.4049/jimmunol.1502307
121. Podell BK, Ackart DF, Obregon-Henao A, Eck SP, Henao-Tamayo M, Richardson M, et al. Increased severity of tuberculosis in Guinea pigs with type 2 diabetes: A model of diabetes-tuberculosis comorbidity. Am J Pathol (2014) 184(4):1104–18. doi: 10.1016/j.ajpath.2013.12.015
122. Podell BK, Ackart DF, Richardson MA, DiLisio JE, Pulford B, Basaraba RJ. A model of type 2 diabetes in the Guinea pig using sequential diet-induced glucose intolerance and streptozotocin treatment. Dis Model Mech (2017) 10(2):151–62. doi: 10.1242/dmm.025593
123. Dooley KE, Chaisson RE. Tuberculosis and diabetes mellitus: convergence of two epidemics. Lancet Infect Dis (2009) 9(12):737–46. doi: 10.1016/s1473-3099(09)70282-8
124. Vipond J, Clark SO, Hatch GJ, Vipond R, Marie Agger E, Tree JA, et al. Re-formulation of selected DNA vaccine candidates and their evaluation as protein vaccines using a Guinea pig aerosol infection model of tuberculosis. Tuberculosis (Edinb) (2006) 86(3-4):218–24. doi: 10.1016/j.tube.2006.01.014
125. Saso A, Kampmann B. Vaccine responses in newborns. Semin Immunopathol (2017) 39(6):627–42. doi: 10.1007/s00281-017-0654-9
126. Sinkora M, Butler JE. The ontogeny of the porcine immune system. Dev Comp Immunol (2009) 33(3):273–83. doi: 10.1016/j.dci.2008.07.011
127. Rubic-Schneider T, Christen B, Brees D, Kammüller M. Minipigs in translational immunosafety sciences: A perspective. Toxicol Pathol (2016) 44(3):315–24. doi: 10.1177/0192623315621628
128. Tlaskalova-Hogenova H, Mandel L, Trebichavsky I, Kovaru F, Barot R, Sterzl J. Development of immune responses in early pig ontogeny. Vet Immunol Immunopathol (1994) 43(1-3):135–42. doi: 10.1016/0165-2427(94)90129-5
129. Ramos L, Obregon-Henao A, Henao-Tamayo M, Bowen R, Izzo A, Lunney JK, et al. Minipigs as a neonatal animal model for tuberculosis vaccine efficacy testing. Vet Immunol Immunopathol (2019) 215:109884. doi: 10.1016/j.vetimm.2019.109884
130. Manabe YC, Kesavan AK, Lopez-Molina J, Hatem CL, Brooks M, Fujiwara R, et al. The aerosol rabbit model of tb latency, reactivation and immune reconstitution inflammatory syndrome. Tuberculosis (Edinb) (2008) 88(3):187–96. doi: 10.1016/j.tube.2007.10.006
131. Lurie MB, Zappasodi P, Tickner C. On the nature of genetic resistance to tuberculosis in the light of the host-parasite relationships in natively resistant and susceptible rabbits. Am Rev Tuberc (1955) 72(3):297–329. doi: 10.1164/artpd.1955.72.3.297
132. Tsenova L, Ellison E, Harbacheuski R, Moreira AL, Kurepina N, Reed MB, et al. Virulence of selected mycobacterium tuberculosis clinical isolates in the rabbit model of meningitis is dependent on phenolic glycolipid produced by the bacilli. J Infect Dis (2005) 192(1):98–106. doi: 10.1086/430614
133. Sun H, Ma X, Zhang G, Luo Y, Tang K, Lin X, et al. Effects of immunomodulators on liquefaction and ulceration in the rabbit skin model of tuberculosis. Tuberculosis (Edinb) (2012) 92(4):345–50. doi: 10.1016/j.tube.2012.03.005
134. Gray DF. The relative natural resistance of rats and mice to experimental pulmonary tuberculosis. J Hyg (Lond) (1961) 59(4):471–7. doi: 10.1017/s0022172400039164
135. Singhal A, Aliouat el M, Hervé M, Mathys V, Kiass M, Creusy C, et al. Experimental tuberculosis in the wistar rat: A model for protective immunity and control of infection. PloS One (2011) 6(4):e18632. doi: 10.1371/journal.pone.0018632
136. Ramakrishnan L. The zebrafish guide to tuberculosis immunity and treatment. Cold Spring Harb Symp Quant Biol (2013) 78:179–92. doi: 10.1101/sqb.2013.78.023283
137. Volkman HE, Pozos TC, Zheng J, Davis JM, Rawls JF, Ramakrishnan L. Tuberculous granuloma induction via interaction of a bacterial secreted protein with host epithelium. Science (2010) 327(5964):466–9. doi: 10.1126/science.1179663
138. Bouz G, Al Hasawi N. The zebrafish model of tuberculosis - no lungs needed. Crit Rev Microbiol (2018) 44(6):779–92. doi: 10.1080/1040841x.2018.1523132
139. van Leeuwen LM, van der Sar AM, Bitter W. Animal models of tuberculosis: zebrafish. Cold Spring Harb Perspect Med (2014) 5(3):a018580. doi: 10.1101/cshperspect.a018580
140. Plumlee CR, Duffy FJ, Gern BH, Delahaye JL, Cohen SB, Stoltzfus CR, et al. Ultra-low dose aerosol infection of mice with mycobacterium tuberculosis more closely models human tuberculosis. Cell Host Microbe (2021) 29(1):68–82.e5. doi: 10.1016/j.chom.2020.10.003
141. Zhu J, Paul WE. Heterogeneity and plasticity of T helper cells. Cell Res (2010) 20(1):4–12. doi: 10.1038/cr.2009.138
142. ROmano M, D’Souza S, Adnet PY, Laali R, Jurion F, Palfliet K, et al. Priming but Not Boosting with Plasmid DNA Encoding Mycolyl-Transferase Ag85a from Mycobacterium Tuberculosis Increases the Survival Time of Mycobacterium Bovis Bcg Vaccinated Mice against Low Dose Intravenous Challenge with M. Tuberculosis H37rv. Vaccine (2006) 24(16):3353–64. doi: 10.1016/j.vaccine.2005.12.066
143. Luo Y, Jiang W, Da Z, Wang B, Hu L, Zhang Y, et al. Subunit vaccine candidate amm down-regulated the regulatory T cells and enhanced the protective immunity of bcg on a suitable schedule. Scand J Immunol (2012) 75(3):293–300. doi: 10.1111/j.1365-3083.2011.02666.x
144. Chai Q, Lu Z, Liu CH. Host defense mechanisms against mycobacterium tuberculosis. Cell Mol Life Sci (2020) 77(10):1859–78. doi: 10.1007/s00018-019-03353-5
145. Khan A, Sayedahmed EE, Singh VK, Mishra A, Dorta-Estremera S, Nookala S, et al. A recombinant bovine adenoviral mucosal vaccine expressing mycobacterial antigen-85b generates robust protection against tuberculosis in mice. Cell Rep Med (2021) 2(8):100372. doi: 10.1016/j.xcrm.2021.100372
146. Vierboom MPM, Dijkman K, Sombroek CC, Hofman SO, Boot C, Vervenne RAW, et al. Stronger induction of trained immunity by mucosal bcg or mtbvac vaccination compared to standard intradermal vaccination. Cell Rep Med (2021) 2(1):100185. doi: 10.1016/j.xcrm.2020.100185
Keywords: tuberculosis, protein subunit vaccines, antigen epitopes, adjuvants, clinical trials, animal models
Citation: Zhang Y, Xu J-c, Hu Z-d and Fan X-y (2023) Advances in protein subunit vaccines against tuberculosis. Front. Immunol. 14:1238586. doi: 10.3389/fimmu.2023.1238586
Received: 12 June 2023; Accepted: 25 July 2023;
Published: 15 August 2023.
Edited by:
Subash Babu, International Centers for Excellence in Research (ICER), IndiaReviewed by:
Joanna Kirman, University of Otago, New ZealandAndre Bafica, Federal University of Santa Catarina, Brazil
Copyright © 2023 Zhang, Xu, Hu and Fan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiao-yong Fan, xyfan008@fudan.edu.cn; Zhi-dong Hu, huzhidong@fudan.edu.cn
 Ying Zhang
Ying Zhang Jin-chuan Xu
Jin-chuan Xu Zhi-dong Hu
Zhi-dong Hu Xiao-yong Fan
Xiao-yong Fan