
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Immunol., 31 August 2023
Sec. Cancer Immunity and Immunotherapy
Volume 14 - 2023 | https://doi.org/10.3389/fimmu.2023.1235884
This article is part of the Research TopicUnderstanding bladder tumor microenvironment to optimize immunotherapyView all 5 articles
Treatment with neoadjuvant cisplatin-based chemotherapy followed by radical cystectomy is the default treatment for muscle-invasive bladder cancer (BC). However, with the encouraging results of immune checkpoint inhibitiors (ICI) directed against PD-1/PD-L1 and CTLA-4 in recent years, the treatment landscape of BC is rapidly changing. In addition, it is becoming clear that the effect of ICI is highly dependent on the interaction between tumor cells and the tumor immune micro-environment (TIME). Different immune cells are involved in an anti-tumor response in BC. Cytotoxic CD8+ T-cells are the main effector cells, aided by other immune cells including other T-cells, B-cells and pro-inflammatory macrophages. As part of the ongoing anti-tumor immune response, lymphocytes aggregate in clusters called tertiary lymphoid structures (TLS). Tumor mutational burden (TMB) and infiltration of immune cells into the tumor are both important factors for establishing an anti-tumor immune response. In contrast, transforming growth factor beta (TGF-β) signaling in cancer-associated fibroblasts (CAFs) prevents infiltration of lymphocytes and potentially has an immunosuppressive effect. In conclusion, the effect of ICI seems to be reliant on a combination of tumor-intrinsic and TIME-related parameters. More research is needed to fully understand the underlying biological mechanisms to further improve patient care.
Urothelial carcinoma of the bladder, more commonly described as bladder cancer (BC), is the 10th most common cancer worldwide (1, 2). In approximately 25% of BC cases, patients present with muscle-invasive disease, characterized by invasion of tumor cells into the muscularis propria layer of the urothelium. Standard treatment for muscle-invasive bladder cancer (MIBC) consists of radical cystectomy (RC) including removal of the locoregional lymph nodes (3–5). However, overall survival (OS) and recurrence-free survival (RFS) after surgery alone are still poor (6, 7).
Pre-treating patients with neoadjuvant cisplatin-based chemotherapy (NAC) leads to a pathological complete response (pCR) rate of 20-40% after RC (8). OS is improved by 5-8% compared to patients treated with a direct cystectomy with most benefit for patients that have a pCR after NAC (9).
Treatment with NAC has been the default treatment for MIBC for many years. However, this might change given the encouraging clinical efficacy of immune checkpoint inhibitors (ICI). It is becoming apparent that treatment with ICI is not a one size fits all treatment, but its efficacy is instead highly dependent on characteristics of the tumor and the tumor immune micro-environment (TIME). Here, we will review the research on ICI in MIBC and its reciprocal effects on the TIME.
The physiological role of immune checkpoints is to maintain self-tolerance and regulate the extent and duration of inflammatory processes. However, these pathways are used by tumors in an attempt to escape from an inevitable immune response (10, 11). The mechanism of action of ICI is complex and dependent on pre-existing factors in the TIME such as the abundance and activation state of CD8+ T-cells, the presence of other immune cells and local cytokine signaling. In addition, treatment with ICI directly influences and changes the TIME, resulting in a delicate interplay. The best-known immune checkpoints are cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) and programmed cell death protein 1 (PD-1) together with its ligands programmed death-ligand 1 and 2 (PD-L1 and PD-L2). However, more immune checkpoints are currently being investigated, including TIGIT, TIM3 and LAG3 (12).
CTLA-4 is expressed by T-cells, where it competes with CD28 for binding with CD80 or CD86, expressed primarily by antigen-presenting cells. Binding with CD28 elicits a signaling cascade eventually leading to T-cell activation (11, 13). Instead, binding of CTLA-4 to CD80 or CD86 results in an inhibitory response. By blocking the CTLA-4 receptor with monoclonal antibodies such as ipilimumab and tremelimumab, this inhibitory response can be negated, allowing for binding of CD80 or CD86 with CD28 to co-stimulate T-cell activation (11, 14). Ipilimumab and tremelimumab have been used in numerous clinical trials as monotherapy and in combination with other ICI. Both ipilimumab and tremelimumab have been approved by the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) for clinical use, however neither drug have been approved as a standard treatment option for urothelial cancer specifically.
PD-1 is expressed by T-cells during initial antigen-mediated activation (15). By engaging its ligands PD-L1 and PD-L2, it functions to counter the activating signals after antigen stimulation, contributing to self-tolerance under physiological conditions (16–18). The PD-1 pathway is commonly exploited by tumor cells in order to evade the immune system. By inhibiting this pathway with monoclonal antibodies, an effective immune-mediated anti-tumor response can be mounted (19, 20). Multiple monoclonal inhibitory antibodies have been developed that target either PD-1 (nivolumab, pembrolizumab, cemiplimab and dostarlimab) or PD-L1 (durvalumab, avelumab and atezolizumab). These antibodies have been tested elaborately in multiple cancer types, including urothelial cancer, and are all approved by the FDA and EMA for clinical use.
Standard first-line therapy for metastatic urothelial cancer (mUC) is cisplatin-based combination chemotherapy (21, 22). Carboplatin can be considered as an alternative for patients ineligible for cisplatin (21, 22). Following the success in other cancer types, such as melanoma, renal cell carcinoma and non-small cell lung cancer (NSCLC), ICI were tested in multiple trials in platinum-refractory mUC (Table 1) (23, 24). An improved OS was observed for pembrolizumab compared to chemotherapy in platinum-refractory mUC in the KEYNOTE-045 trial (23). These results led to the approval of pembrolizumab for the treatment of platinum-refractory mUC. The IMvigor211 trial explored atezolizumab as second-line therapy in mUC and showed improved OS for the intention-to-treat (ITT) population. However, this trial did not meet its primary endpoint of improved OS in patients with tumors with high PD-L1 expression (24).
Encouraged by these first results of ICI, a number of phase 3 trials were initiated to investigate the effect of first-line treatment with anti-PD-1/PD-L1 compared to standard platinum-based chemotherapy (Table 1). These included the KEYNOTE-361 (pembrolizumab), DANUBE (durvalumab) and the IMvigor130 (atezolizumab) trials. Unfortunately, no meaningful improvement in clinical benefit for anti-PD-1/PD-L1 monotherapy versus standard first-line platinum-based chemotherapy treatment was observed (25, 26, 42). Both the KEYNOTE-361 and IMvigor130 trials also tested the effect of combined chemotherapy and ICI in this setting. Comparable to treatment with ICI monotherapy, the combination treatment did not confer any benefit over standard treatment with chemotherapy in the ITT analysis (25, 27). The results of another phase 3 trial (CheckMate-901) are still pending. In this trial the investigators assessed the effect of standard cisplatin-based chemotherapy together with nivolumab. A 2023 press release reported a statistically significant improvement in progression-free survival (PFS) and OS for cisplatin/gemcitabine plus nivolumab, in comparison with cisplatin/gemcitabine alone. Interestingly, a subgroup analysis of the IMvigor130 study suggested that the combination of cisplatin-based chemotherapy with atezolizumab had better synergy than the carboplatin-based combination (27), potentially explaining this phenomenon. The full results of the CheckMate-901 trial are pending and are required to better understand the discrepancies between these results and the results from the other first-line trials with ICI.
The DANUBE (durvalumab plus tremelimumab) and CheckMate-901 (ipilimumab plus nivolumab) trials also evaluated the combination of PD-1/PD-L1 inhibition with CTLA-4 inhibition in mUC (26, 42). The DANUBE trial did not reach its primary endpoint(s). However, a numerical difference can be observed in favor of combined treatment with durvalumab plus tremelimumab compared to durvalumab monotherapy, especially in the PD-L1+ population (26). The formal results of the CheckMate-901 trial are still pending, however, the trial failed to meet one of its (co)primary endpoints of improved OS for ipilimumab plus nivolumab in patients with tumors with high PD-L1 expression, according to a 2022 press release by Bristol Myers Squibb.
In defining the optimal treatment setting, some recent trials suggest there is a place for ICI in the adjuvant setting in MIBC (Table 1). Two phase 3 trials have explored the efficacy and clinical benefit of adjuvant nivolumab (CheckMate-274) and atezolizumab (IMvigor010) in patients with residual muscle-invasive disease after RC (28, 29). Only the trial with nivolumab met its primary endpoint of improved RFS in the ITT population, as well as in the PD-L1high population (28). However, as PD-1/PD-L1 inhibition is approved as second-line treatment for mUC, it is possible that the group that did not receive adjuvant nivolumab will eventually benefit from checkpoint blockade in a later disease stage, as the disease would still be naïve to exposure to checkpoint inhibition. Therefore, OS data are needed to fully assess clinical benefit.
Interestingly, clinical benefit in the CheckMate-274 trial was most prominent in patients that were previously treated with NAC. A potentially similar phenomenon has also been observed in the phase 3 JAVELIN Bladder 100 trial, where patients were treated with avelumab as maintenance therapy after platinum-based treatment in the metastatic setting (30). Patients treated with avelumab had an improved OS compared to the group receiving best supportive care (30). In addition to trials investigating the feasibility in the adjuvant or metastatic setting or as maintenance therapy, a number of phase 1/2 trials investigated ICI in the neoadjuvant setting, primarily to offer an alternative for patients who refused cisplatin or were ineligible (Table 1). Preoperative pembrolizumab was investigated in the PURE-01 trial (31, 43). Patients with cT2-4N0 BC were treated with three cycles of pembrolizumab, followed by RC. A pCR rate of 38.5% was observed (31, 43). In the ABACUS trial, preoperative atezolizumab was investigated in cT2-4aN0 BC patients (32). This trial yielded a response rate of 31% (32).
Following up on the success of these trials, two neoadjuvant trials were initiated using a combination of CTLA-4 inhibition and PD-1/PD-L1 inhibition (33, 34). In the NABUCCO trial, 24 patients with locally advanced BC were treated with a combination of ipilimumab plus nivolumab followed by radical surgery (34). Here, a pCR was observed in 46% of patients. In addition, 58% of patients had no remaining residual muscle-invasive disease after surgery (ypT0/Tis/Ta/TaN0) (34).
The group of Gao and colleagues conducted a trial with two cycles of tremelimumab plus durvalumab in locally advanced BC. A pCR was observed in 38% of patients that underwent surgery, which is comparable to platinum-based chemotherapy regimens in locally advanced disease (8, 33).
In addition to the results observed in the NABUCCO trial, encouraging results with preoperative ipilimumab plus nivolumab have been observed in multiple other tumor types (44–48). These studies suggested a lower dose of ipilimumab may be sufficient in the non-metastatic setting. To find the optimal dose of ipilimumab and nivolumab in locally advanced BC, patients in cohort 2 of the NABUCCO trial were randomized to receive either two cycles of 3 mg/kg ipilimumab plus 1 mg/kg of nivolumab or two cycles of 1 mg/kg ipilimumab plus 3 mg/kg of nivolumab in both arms followed by one cycle of 3 mg/kg nivolumab and RC (35). A pCR was observed in 43% of patients treated with ipilimumab 3 mg/kg in combination with nivolumab, similar to the results from cohort 1 of the NABUCCO trial. In contrast, a pCR was observed in only 7% of patients treated with ipilimumab 1 mg/kg in combination with nivolumab (35).
Similarly, in the CheckMate-032 trial, patients with advanced BC were treated with either nivolumab monotherapy, or in combination with ipilimumab with different dose combinations (36, 49). While this trial was not properly powered to detect a difference in OS, a higher objective response rate was observed for patients treated with plus ipilimumab 3 mg/kg plus nivolumab 1 mg/kg (38.0%) compared to patients treated with ipilimumab 1 mg/kg plus nivolumab 3 mg/kg (26.9%) or nivolumab monotherapy (25.6%) (49).
Taken together, the data in BC suggests that a high dose of CTLA-4 blockade in combination with PD-1/PD-L1 blockade yields better clinical responses compared to a low dose of CTLA-4 blockade. For the locally advanced setting, this could be an alternative treatment especially for cisplatin-ineligible patients.
Across different tumor types, there are certain aspects that impact the general immunogenicity of tumors and the general efficacy of ICI. Tumor mutational burden (TMB) is a metric to indicate the average number of mutations in the DNA of tumor cells compared to healthy cells. Only a small fraction of these mutations are ‘driver’ mutations, while the majority are ‘passenger’ mutations with no direct function in tumor development or progression (50, 51). Potentially, these ‘passenger mutations’ generate aberrant proteins which can be detected by the immune system as neoantigens, triggering an immune response directed against the tumor (52, 53). TMB is a surrogate measure of neoantigen load, which allows it to serve as a predictive biomarker for general immunogenicity and tendency of tumors to respond to ICI (54–56). TMB varies per individual tumor. However, different tumor types have a different average TMB. Melanoma and other skin cancers typically have the highest TMB. Although not as high as melanoma, average TMB in BC is relatively high, similar to NSCLC (57, 58).
TMB has been investigated in a number of BC trials mentioned earlier. In the preoperative setting, it was positively associated with response in the PURE-01 trial (pembrolizumab), and numerically higher in responders compared to non-responders in the ABACUS trial (atezolizumab), NABUCCO trial (ipilimumab plus nivolumab) and in the preoperative trial with tremelimumab and durvalumab (31–34). In addition, changes in the TMB after treatment were assessed in the PURE-01 trial (pembrolizumab) in fourteen patients for which paired tissue samples were available (≥ypT2). Interestingly, TMB was significantly lower compared with the baseline TMB after treatment with pembrolizumab (31).
In addition to an increased TMB, some specific genomic alterations also impact tumor behavior, prognosis and response to ICI. Recently, it was shown that loss of the Y-chromosome is associated with poor prognosis in male BC patients and was related to intratumoral CD8+ T-cell dysfunction and exhaustion. Interestingly, patients with loss of the Y-chromosome exhibited an increased response to PD-1 inhibition in both mice and BC patients (59).
Multiple immune cell subsets are implied to play a role in the TIME. Cytotoxic CD8+ T-cells have been established as one of the major players in the TIME in BC as well as in most other tumor types. Within the CD8+ T-cells, different subsets have been observed with varying degrees of tumor-reactivity. CD8+ T-cells expressing the combination of CD103 (integrin αE) and CD39 (an ectonucleotidase) are enriched for tumor-reactive cells in multiple different tumor types. These cells also efficiently kill autologous tumor cells in a major histocompatibility complex (MHC) class I-dependent manner (60). Specifically in BC, it has been shown that patients with tumors with high infiltration of CD8+CD103+ tissue-resident memory T-cells are more likely to benefit from ICI and adjuvant chemotherapy (61).
Based on the abundance of CD8+ T-cells and other immune cells and their spatial organization in relation to the tumor, distinct immune phenotypes can be defined (62, 63).
Immune-Inflamed tumors are considered to be immunologically ‘hot’ tumors and are characterized by an abundancy of immune cells invading the tumor and the surrounding stroma. Apart from CD8+ T-cells, these include other T-cells, B-cells and pro-inflammatory macrophages. In addition, these tumors are characterized by a type I Interferon gamma (IFN-γ) signature (63).
Tumors that are populated with immune cells but with relatively few cytotoxic T-cells inside the core of the tumor are commonly referred to as tumors with an immune-excluded phenotype (62, 63). It is currently not completely understood if these cytotoxic cells are insufficiently stimulated to infiltrate the tumor, or whether tumor infiltration is physically being prevented by interfering fibroblasts or stromal cells or due to other pro-tumorigenic cells (64). It has been suggested that tumor-associated macrophages along the tumor margins prevent cytotoxic lymphocytes from tumor core infiltration (65).
Tumors with very few immune cells are referred to as immunologically ‘cold’ tumors or having an immune-desert phenotype. These tumors are characterized by a low number of lymphocytes and a high macrophage-to-lymphocyte ratio (66).
Multiple studies have shown that greater infiltration of CD8+ T-cells is related to a more favorable clinical outcome and a better response to ICI in multiple disease stages in BC (32, 67–71).
In the ABACUS trial mentioned above, a relatively high proportion of immune-inflamed (73%) tumors was found based on the abundance and spatial organization of CD8+ T-cells. However, the immune-inflamed phenotype did not correlate with response (32). A CD8/GZMB co-staining was performed to further enrich for tumors with high anti-tumor reactivity. Indeed, the percentage of tumors with an immune-inflamed phenotype that contained CD8+GZMB+ cells was higher in responders versus patients that relapsed (87% and 30%, respectively) (32). In addition, a significant increase in infiltrating CD8+ T-cells was observed based on immunohistochemistry when comparing post-treatment tissue to pre-treatment tissue (32).
In the PURE-01 trial mentioned above, the effect of ICI on the TIME was assessed by comparing pre- and post- treatment samples from patients with residual disease after treatment with preoperative pembrolizumab (72). These findings were compared to patients that were treated with a direct RC, or with NAC followed by RC. It was found that patients with residual tumor after treatment with pembrolizumab and cystectomy showed a high rate of stroma-rich calls with a decreased tumor purity and increased stromal content (72). In addition, these tumors also expressed luminal markers, distinguishing them from the untreated and tumors that did not respond to NAC. This would suggest that luminal tumors may have an intrinsic resistance to treatment with ICI or that treatment with ICI may select for, or induce, a luminal phenotype (72, 73)
In another study, immune phenotypes were classified based on CD8+ T-cell density in the tumor and stroma compartments in an untreated BC cohort (74). Immune-inflamed (42%) was the most common immune phenotype, whereas 32% and 26% of tumors were classified as immune-excluded and immune-desert phenotypes, respectively. Although tumors qualified as immune-desert showed a numerically high rate of recurrence (88%), no statistically significant correlation was found (74).
In contrast, in the NABUCCO trial (preoperative ipilimumab plus nivolumab) no correlation was observed between baseline CD8+ T-cell density and response to ipilimumab plus nivolumab. In addition, no significant difference was observed in IFN-γ signaling at baseline in responding tumors compared to non-responding tumors. This data suggests that the addition of anti-CTLA-4 to PD-1 blockade can induce a pCR in tumors irrespective of baseline immunity (34). In addition, the density of CD8+PD1+ T-cells in tumors from patients treated with ipilimumab and nivolumab was higher than that of patients treated with a direct cystectomy, regardless of response to ICI (74).
Current efforts to understand anti-tumor immunity are primarily focused on CD8+ T-cells. However, there is also a role for CD4+ T-cells in the interaction between the tumor and TIME. Regulatory CD4+ T-cells in the BC TIME are known for their role in inhibiting or dampening an ongoing immune response by producing anti-inflammatory cytokines like IL-10 and transforming growth factor beta (TGF-β), direct inhibition of dendritic cells and more (75, 76). Regulatory CD4+ T-cells in the BC TIME have been associated with adverse outcomes, similar to other tumor types (77). The exact underlying biological mechanism for this association remains unclear. However, one study found overexpression of sphingosine 1 phosphate receptor 1 (S1P1) in BC, promoting production of TGF-β and IL-10 in vitro and in vivo (78).
In addition to an immunosuppressive role, it was recently found that CD4+ T-cells also play an important role in anti-tumor immunity in BC (79). Based on data from single-cell RNA sequencing, multiple cytotoxic CD4+ T-cell states were identified based on the expression of granzyme B, granzyme K, perforin as well as other granule-associated proteins. These distinct populations were validated by flow cytometry and multiplex immunofluorescence tissue staining. In addition, it was found that cytotoxic CD4+ subsets in bladder tumors were clonally expanded, potentially resulting from recognition of bladder tumor antigens. Their functional importance was confirmed by their ability to kill autologous tumors ex vivo in a MHC class II-dependent manner. Overall, these findings highlight the importance of CD4+ T-cell heterogeneity and the relative balance between activation of cytotoxic CD4+ effector cells and inhibitory regulatory cells for killing autologous tumors (79).
Apart from lymphocytes, there is a prominent role for macrophages in the TIME. Macrophages are not a single cell population with a defined phenotype and biological activity but rather a diverse collection of cell types with a wide range of functional roles (80). Macrophages have been traditionally categorized as either M1 or M2 macrophages, characterized by different markers. M1 macrophages are considered anti-tumorigenic and express high levels of tumor necrosis factor alpha (TNFα), inducible nitric oxide synthase (iNOS) or MHC class II molecules. In contrast, M2 macrophages are considered pro-tumorigenic and express CD163, CD206 and high levels of arginase 1 (ARG1) and IL-10 (81). However, it is becoming clear that a broad spectrum of macrophage phenotypes exists, and using markers to delineate their functional role within the tumor is not straightforward (82). The majority of the work on macrophages has been done in other tumor types, but a few studies also investigated whether there is an association between macrophage abundance and polarization status (M1-like or M2-like) and prognosis and outcome in BC.
One study found that a high intratumoral density of M2 macrophages (based on expression of CD163) was associated with poor outcome in patients with BC (83). Another study by Sun and colleagues investigated macrophage polarization in relation to response to ICI treatment in the IMvigor210 trial (atezolizumab). It was found that patients with tumors with predominantly M1 macrophages were indeed more sensitive to PD-L1 blockade (84). Wang and collaborators found that a pro-tumorigenic inflammatory signature was correlated with poor outcome in the IMvigor210 (atezolizumab) and the CheckMate-275 (nivolumab) trials (85). One preclinical study used a conditional knockout BC mouse model which showed a heterogeneous response to treatment with PD-1 inhibition. Responding tumors showed a higher number of intratumoral macrophages (86).
While the exact mechanism still needs to be unraveled, the number of macrophages and their polarization status seems to be associated with the efficacy of ICI and thus may be relevant to predict which patients respond to treatment.
Similar to other tumor types, multiple other cell types likely also play a role in the TIME in BC. These include, but are not limited to: pericytes, dendritic cells, natural killer cells and eosinophils. While important, limited work has been done in BC specifically.
Tertiary lymphoid structures (TLS) are organized clusters of lymphocytes in chronically inflamed tissue (87). They resemble secondary lymphoid organs like lymph nodes and have similar functions such as mounting germinal center reactions and priming of antigen-specific T-cells (88, 89). The density of TLS in the TIME is associated with infiltration of adaptive immune cells and improved clinical outcome in multiple tumor types including in BC (90–92). However, it is currently unclear what the exact role is of TLS in the anti-tumor immune response and whether they are a prerequisite or rather a consequence of an anti-tumor immune response (91). It was proposed that TLS mature through different developmental stages with increasing proportions of activated lymphocytes throughout the maturation process (93). However, another study assessing different BC cohorts found that the proportions of activated B-cells, T-cells and progenitor-like CD8+ T-cells were similar when comparing maturation stages, and seemed to be more dependent on the number of TLS in the TIME (91).
Another study in BC made a distinction between superficial and deep TLS, based on their location relative to the bladder lumen. Superficial TLS were hypothesized to primarily play a role in the immune response against irritative chemicals and microbial pathogens present in the urine. Deep TLS presumably play a more prominent role in anti-tumor immunity. It was found that the density of CD4+ T-cells was higher in superficial TLS and the proportion of follicle-like, mature structures was higher in deep TLS (74).
Two trials that assessed combination ICI as a preoperative strategy in locally advanced BC investigated TLS at baseline as a predictive biomarker. One study testing preoperative tremelimumab plus durvalumab reported a higher density of pretreatment TLS in responders compared to non-responders (33). In addition, it was observed that a higher density of pretreatment TLS was associated with a longer OS and RFS.
In contrast, no difference in pretreatment TLS density was reported in responders compared to non-responders in patients with locally advanced BC treated with preoperative ipilimumab plus nivolumab in the NABUCCO trial (34). However, the density of TLS after treatment increased in responders, whereas the TLS density decreased in non-responders. In addition, it was found that regulatory T-cells in TLS decreased after treatment with ipilimumab plus nivolumab, showing that this treatment also influences the composition of TLS (34). In another recent study in BC, an association was found between TLS density and TMB as well as increased T-cell activation. Combining TLS density with TMB into a joint ‘TLSTMB’ score generated a novel prognostic biomarker that, in contrast to either TLS density of TMB alone, was independent from tumor stage and vascular invasion (91). The exact role of TLS remains to be elucidated. However, it is clear from multiple studies that TLS play a pivotal role in the anti-tumor immune response in BC.
TGF-β is a cytokine that is involved in multiple different pathways and interactions and is associated with poor clinical outcome in different tumor types (94–96). It is thought to have a pro-tumorigenic role in advanced cancers by promoting fibroblast activation, immunosuppression, angiogenesis, epithelial-to-mesenchymal transition, and metastasis (97). In a study by Mariathasan and colleagues, it was found that expression of TGF-β ligand 1 (TGFB1) and TGF-β receptor 2 (TGFBR2) were associated with non-response and reduced OS in patients with mUC who were treated with atezolizumab in the IMvigor210 trial (71). In addition, the authors speculated that TGF-β signaling in cancer-associated fibroblasts (CAFs) contributed to an immune-excluded TIME. To measure TGF-β signaling specifically in fibroblasts, a pan-fibroblast TGF-β response signature (F-TBRS) was created. Expression of this signature was particularly high in tumors with an inflamed or excluded phenotype, and low in tumors with an immune desert phenotype. In line with these findings, the F-TBRS was significantly associated with non-response in excluded tumors specifically (Figure 1) (71). In addition, it was confirmed in an EMT6 mouse mammary carcinoma model that combining PD-L1 blockade and a TGF-β inhibitor led to infiltration of lymphocytes into the tumor and an improved survival, whereas this was not observed upon monotherapy with either inhibitor (71).
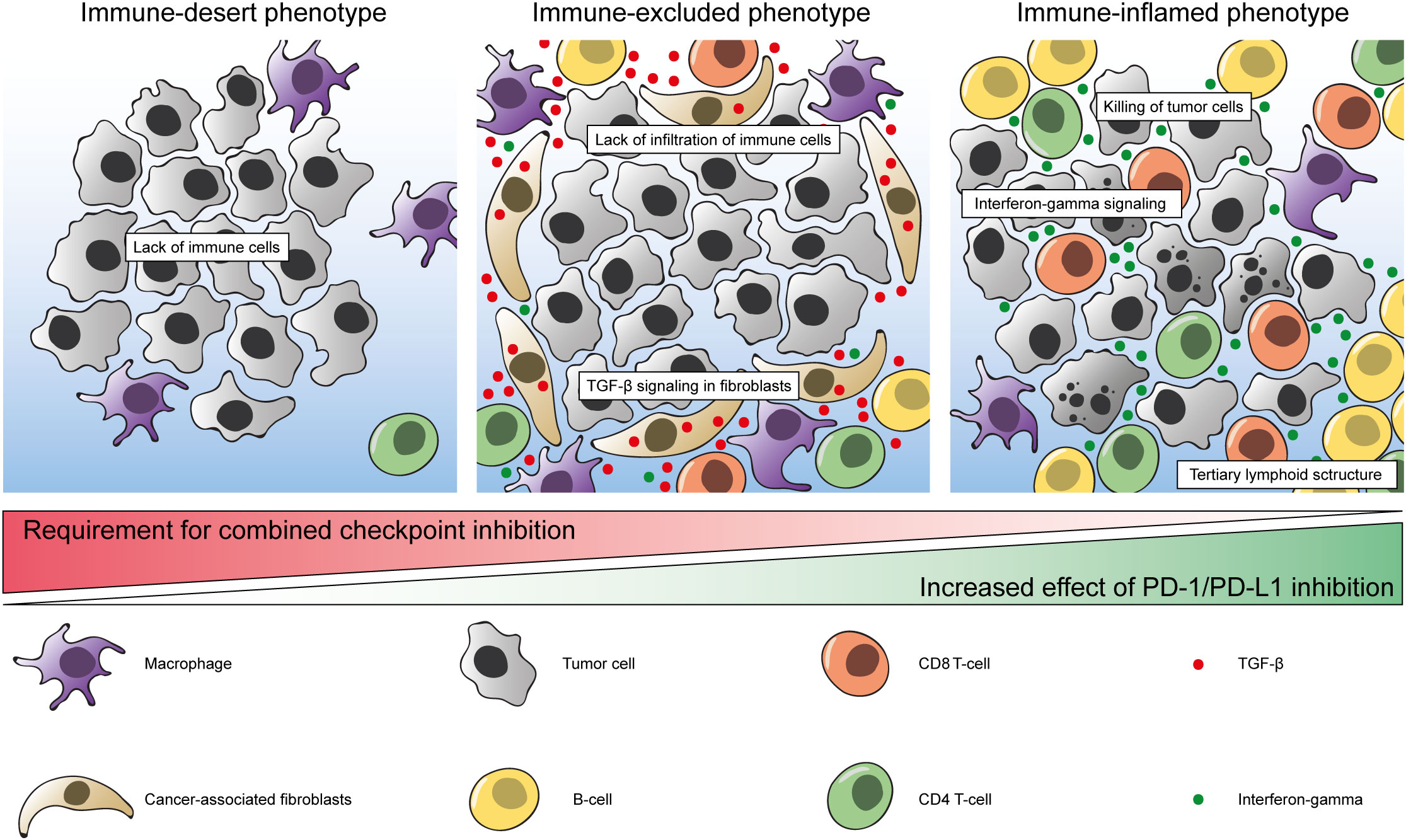
Figure 1 Model of immune phenotypes in the bladder tumor immune micro-environment. Left: immune-desert phenotype with limited amounts of immune cells. Middle: immune-excluded phenotype with stromal cells, cancer associated fibroblasts and TGF-β signaling preventing infiltration of CD8+ T-cells and other immune cells. Macrophages predominantly display an immunosuppressive (M2) phenotype. Right: immune-inflamed phenotype with extensive infiltration of CD8+ T-cells and other immune cells. Macrophages are primarily of the M1 phenotype. Bottom: Increased effect of PD-1/PD-L1 inhibition have been observed in tumors with an immune-inflamed phenotype. Addition of CTLA-4 inhibition might be required to mount an effective immune response in tumors without pre-existing immunity.
In the ABACUS trial, patients were treated with atezolizumab in the preoperative setting. Among the patients that relapsed, the patients with an immune-excluded bladder tumor had a numerically higher expression of the TGF-β response signature, which was not the case for patients that responded. However, no definitive conclusion can be drawn from this study due to lack of statistical power (32). In the NABUCCO trial (ipilimumab plus nivolumab), a significantly higher expression of the TGF-β response signature was observed in non-responders versus responders at baseline (34).
A number of TGF-β inhibitors have been developed for clinical use and are currently being investigated in early clinical trials (98). To our knowledge, there are currently two trials investigating TGF-β inhibitors in patients with mUC. In one trial, patients are treated with the oral TGF-β inhibitor vactosertib in combination with durvalumab (NCT04064190), for which results are pending. The other trial tested bintrafusp α (M7824), a bifunctional fusion protein composed of the extracellular domain of a TGF-β receptor fused to PD-L1 antibody (NCT04501094). This trial has been terminated due to low accrual.
In recent years, encouraging responses to ICI have been observed in BC patients. However, there is still a substantial subset of patients that does not respond to this treatment. This ICI resistance might be explained by various mechanisms, including tumor-intrinsic factors or factors related to the immune micro-environment (55, 56, 71). Combining ICI monotherapy with other drugs such as additional ICI or conventional chemotherapy, may alter the TIME to be more susceptible to respond to treatment.
Traditionally, chemotherapy has been regarded as immuno-suppressive, depleting immune cell subsets and leading to an increased rate of infections (99). However, it has been shown in numerous preclinical and clinical studies that treatment with chemotherapy can also have immunostimulatory effects (100, 101). One of the direct effects of chemotherapy is the induction of immunogenic cell death, a form of cell death that is being preceded by a cellular stress-response. Via complex intracellular pathways, phagocytosis of tumor cells (or portions thereof) by dendritic cells is facilitated (102). Processing of this cellular debris by dendritic cells eventually leads to presentation of neoantigens, as described earlier (52, 53).
In addition to the induction of immunogenic cell death, chemotherapy treatment also affects regulatory T-cells, tumor-associated macrophages and myeloid derived suppressor cells (103, 104). This phenomenon could be further exploited when used in conjunction with ICI. Indeed, a synergistic effect of concurrent chemotherapy and ICI has been observed in multiple tumor types, including in NSCLC and in triple-negative breast cancer (TNBC) (105–107).
In BC, one preclinical study assessed the effect of PD-L1 inhibition with or without platinum-based chemotherapy compared to platinum-based chemotherapy alone (108). The combination strategy was more effective in the MB49 subcutaneous model compared to either platinum-based chemotherapy or PD-L1 inhibition alone. Interestingly, PD-L1 inhibition monotherapy was more effective than the combination strategy in the MBT-2 subcutaneous model, suggesting that combined treatment results are model-dependent (108). Despite the positive results in other cancer types, no clinical benefit was observed when patients with mUC were treated with PD-1/PD-L1 inhibition or PD-1/PD-L1 inhibition in combination with standard platinum-based chemotherapy over chemotherapy alone in both the KEYNOTE-361 (pembrolizumab) and in the IMvigor130 (atezolizumab) trials (25, 27). However, a 2023 press release of the CheckMate-901 study reported a statistically significant improvement in PFS and OS for cisplatin/gemcitabine plus nivolumab, in comparison with cisplatin/gemcitabine alone. The full results are pending and are required to better understand the discrepancies between these trial results. Potentially, this might result in the first approved treatment strategy with concurrent chemotherapy and ICI in BC.
Theoretically, a sequential approach could be appealing, priming the TIME and allowing recovery of immune cell populations to subsequently further improve the anti-tumor response with ICI treatment (109). This sequential approach was studied in patients with TNBC, where patients were treated with different types of induction chemotherapy, followed by nivolumab (110). In metastatic BC, the JAVELIN Bladder 100 trial (avelumab) showed the efficacy of maintenance checkpoint inhibition after initial treatment with platinum-based chemotherapy (30). The improved results for adjuvant nivolumab in the subset of patients in the CheckMate-274 trial who received NAC similarly supports sequential treatment (28).
Given the accessibility of bladder tumors, the TIME could be modulated by local therapies to improve susceptibility to ICI treatment, without having to expose patients to systemic therapy. For example, intravesical instilments with Bacillus Calmette–Guerin (BCG) or chemotherapeutic agents such as epirubicin or mitomycin can be employed. BCG represents the first type of immunomodulatory treatment approved by the FDA (111). Despite its proven efficacy in reducing the chance of disease recurrence, its underlying biological mechanism is not fully understood. Generally, BCG is internalized primarily by cancer cells leading to cytokine production and the activation of CD4+ and CD8+ T-cells, leading to the killing of cancer cells (112, 113). Interestingly, one study showed that the efficacy of treatment with BCG is at least partly explained by PD-L1 expression, as the percentage of patients with PD-L1+ tumors at baseline was higher in patients that did not respond to treatment with BCG compared to patients that did respond (114). Indeed, systemic treatment with pembrolizumab in patients with BCG-unresponsive non-muscle invasive BC was tolerable and showed promising anti-tumor activity in a single-arm phase 2 trial, leading to the approval of pembrolizumab for BCG-unresponsive, high-risk non-muscle invasive BC (115).
Intravesical instilments with ICI could potentially evoke a local immune response with less systemic exposure. Two exploratory trials investigated whether intravesical instilments with pembrolizumab were feasible. In one trial, patients with BCG-unresponsive non-muscle invasive BC were treated with intravesical pembrolizumab. A significant increase in CD4+ T-cells and CD8+ T-cells was found in the urine after a single dose of pembrolizumab as well as an increase in infiltrating CD8+ T-cells in the tumor (116). Interestingly, even though treatment was only administered locally in the bladder, systemic immune-related adverse events were observed in some patients (116). In the PemBla trial, six patients were treated with increasing doses of intravesical installments of pembrolizumab after transurethral resection of the bladder, which was well tolerated. These two exploratory trials confirm prior pre-clinical observations in a mouse BC model (MBT-2), where intravesical PD-1 inhibition was used to treat localized BC, and showed a similar effect compared to systemic treatment with PD-1 inhibition and changes in the TIME including increased infiltration of CD8+ T-cells (117).
Enfortumab vedotin (EV) - an antibody-drug conjugate - is directed against nectin-4, a protein which is highly expressed in urothelial cancer cells and is linked to monomethyl auristatin E, an agent that disrupts microtubule formation (37, 118). Encouraging results have been observed when EV was used as monotherapy in pretreated mUC (Table 1) (38). In addition, it has also been observed that treatment with EV leads to hallmarks of immunogenic cell death leading to T-cell activation (118, 119). Combined with pembrolizumab, EV has shown promising results and this combination is currently under investigation in a phase 3 study (39–41).
We have come to understand that cancer cells rely heavily on their interaction with the surrounding TIME, especially in the context of ICI. It is becoming clear which cell types, pathways and processes are involved in anti-tumor immunity. Taken together, a combination of tumor-intrinsic and microenvironment-related parameters determine the success of therapies targeting immune checkpoints: i) A high TMB and a high rate of neoantigens resulting in aberrant proteins which can be recognized by immune cells to then mount an effective antitumor immune response ii) Pre-existing anti-tumor immunity with infiltrating cytotoxic T-cells, IFN-γ signaling and the formation of TLS; iii) Low expression of TGF-β in CAFs to prevent an immune-excluded immune phenotype (Figure 1) (71, 120). However, despite meeting all of these criteria, some tumors still do not respond well to ICI. This indicates that there are still some missing pieces in the puzzle of adequate immunological cancer treatment.
While there are many commonalities across different tumor types, there are also some features related to the TIME that have been found specifically in BC. These include the importance of TGF-β signaling and role of CD4+ T-cells and might explain some of the unique clinical findings observed in BC studies. For example, the improved clinical outcome when a high dose of CTLA-4 inhibition is used together with PD1/PD-L1 inhibition and the apparent lack of synergy when treating with a combination of ICI and systemic chemotherapy.
Recent findings highlight the rapidly changing treatment landscape of BC. We now understand that cancer cell characteristics are just part of the puzzle for effective cancer treatment. Targeted therapeutic strategies like ICI and antibody-drug conjugates such as EV are highly dependent on the interaction between cancer cells and the TIME. Ultimately, more research is needed to better understand the TIME.
JD and MH wrote the manuscript and created the illustrations. All authors contributed to the article and approved the submitted version.
MH received research support from Bristol-Myers Squibb, AstraZeneca, Roche and 4SC; consultancy fees from Bristol-Myers Squibb, Merck, Roche, AstraZeneca, Seagen, Pfizer and Janssen all paid to the Netherlands Cancer Institute.
The remaining author declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Powles T, Bellmunt J, Comperat E, De Santis M, Huddart R, Loriot Y, et al. Bladder cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol (2022) 33(3):244–58. doi: 10.1016/j.annonc.2021.11.012
2. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2018) 68(6):394–424. doi: 10.3322/caac.21492
3. Stein JP, Lieskovsky G, Cote R, Groshen S, Feng AC, Boyd S, et al. Radical cystectomy in the treatment of invasive bladder cancer: long-term results in 1,054 patients. J Clin Oncol (2001) 19(3):666–75. doi: 10.1200/JCO.2001.19.3.666
4. Dalbagni G, Genega E, Hashibe M, Zhang ZF, Russo P, Herr H, et al. Cystectomy for bladder cancer: a contemporary series. J Urol (2001) 165(4):1111–6. doi: 10.1016/S0022-5347(05)66440-3
5. Stein JP, Skinner DG. Radical cystectomy for invasive bladder cancer: long-term results of a standard procedure. World J Urol (2006) 24(3):296–304. doi: 10.1007/s00345-006-0061-7
6. Madersbacher S, Hochreiter W, Burkhard F, Thalmann GN, Danuser H, Markwalder R, et al. Radical cystectomy for bladder cancer today–a homogeneous series without neoadjuvant therapy. J Clin Oncol (2003) 21(4):690–6. doi: 10.1200/JCO.2003.05.101
7. Bruins HM, Huang GJ, Cai J, Skinner DG, Stein JP, Penson DF. Clinical outcomes and recurrence predictors of lymph node positive urothelial cancer after cystectomy. J Urol (2009) 182(5):2182–7. doi: 10.1016/j.juro.2009.07.017
8. Pfister C, Gravis G, Flechon A, Soulie M, Guy L, Laguerre B, et al. Randomized phase III trial of dose-dense methotrexate, vinblastine, doxorubicin, and cisplatin, or gemcitabine and cisplatin as perioperative chemotherapy for patients with muscle-invasive bladder cancer. Analysis of the GETUG/AFU V05 VESPER trial secondary endpoints: chemotherapy toxicity and pathological responses. Eur Urol (2021) 79(2):214–21. doi: 10.1016/j.eururo.2020.08.024
9. Advanced Bladder Cancer Meta-analysis C. Neoadjuvant chemotherapy in invasive bladder cancer: update of a systematic review and meta-analysis of individual patient data advanced bladder cancer (ABC) meta-analysis collaboration. Eur Urol (2005) 48(2):202–5. doi: 10.1016/j.eururo.2005.04.006
10. Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol (2008) 26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331
11. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer (2012) 12(4):252–64. doi: 10.1038/nrc3239
12. Walsh RJ, Sundar R, Lim JSJ. Immune checkpoint inhibitor combinations-current and emerging strategies. Br J Cancer (2023) 128(8):1415–7. doi: 10.1038/s41416-023-02181-6
13. Krummel MF, Allison JP. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J Exp Med (1995) 182(2):459–65. doi: 10.1084/jem.182.2.459
14. Linsley PS, Brady W, Urnes M, Grosmaire LS, Damle NK, Ledbetter JA. CTLA-4 is a second receptor for the B cell activation antigen B7. J Exp Med (1991) 174(3):561–9. doi: 10.1084/jem.174.3.561
15. Agata Y, Kawasaki A, Nishimura H, Ishida Y, Tsubata T, Yagita H, et al. Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. Int Immunol (1996) 8(5):765–72. doi: 10.1093/intimm/8.5.765
16. Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med (2000) 192(7):1027–34. doi: 10.1084/jem.192.7.1027
17. Latchman Y, Wood CR, Chernova T, Chaudhary D, Borde M, Chernova I, et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol (2001) 2(3):261–8. doi: 10.1038/85330
18. Nishimura H, Nose M, Hiai H, Minato N, Honjo T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity (1999) 11(2):141–51. doi: 10.1016/S1074-7613(00)80089-8
19. Iwai Y, Terawaki S, Honjo T. PD-1 blockade inhibits hematogenous spread of poorly immunogenic tumor cells by enhanced recruitment of effector T cells. Int Immunol (2005) 17(2):133–44. doi: 10.1093/intimm/dxh194
20. HIrano F, Kaneko K, Tamura H, Dong H, Wang S, Ichikawa M, et al. Blockade of B7-H1 and PD-1 by monoclonal antibodies potentiates cancer therapeutic immunity. Cancer Res (2005) 65(3):1089–96. doi: 10.1158/0008-5472.1089.65.3
21. von der Maase H, Hansen SW, Roberts JT, Dogliotti L, Oliver T, Moore MJ, et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol (2000) 18(17):3068–77. doi: 10.1200/JCO.2000.18.17.3068
22. De Santis M, Bellmunt J, Mead G, Kerst JM, Leahy M, Maroto P, et al. Randomized phase II/III trial assessing gemcitabine/carboplatin and methotrexate/carboplatin/vinblastine in patients with advanced urothelial cancer who are unfit for cisplatin-based chemotherapy: EORTC study 30986. J Clin Oncol (2012) 30(2):191–9. doi: 10.1200/JCO.2011.37.3571
23. Bellmunt J, de Wit R, Vaughn DJ, Fradet Y, Lee JL, Fong L, et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med (2017) 376(11):1015–26. doi: 10.1056/NEJMoa1613683
24. Powles T, Duran I, van der Heijden MS, Loriot Y, Vogelzang NJ, De Giorgi U, et al. Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): a multicentre, open-label, phase 3 randomised controlled trial. Lancet (2018) 391(10122):748–57. doi: 10.1016/S0140-6736(17)33297-X
25. Powles T, Csoszi T, Ozguroglu M, Matsubara N, Geczi L, Cheng SY, et al. Pembrolizumab alone or combined with chemotherapy versus chemotherapy as first-line therapy for advanced urothelial carcinoma (KEYNOTE-361): a randomised, open-label, phase 3 trial. Lancet Oncol (2021) 22(7):931–45. doi: 10.1016/S1470-2045(21)00152-2
26. Powles T, van der Heijden MS, Castellano D, Galsky MD, Loriot Y, Petrylak DP, et al. Durvalumab alone and durvalumab plus tremelimumab versus chemotherapy in previously untreated patients with unresectable, locally advanced or metastatic urothelial carcinoma (DANUBE): a randomised, open-label, multicentre, phase 3 trial. Lancet Oncol (2020) 21(12):1574–88. doi: 10.1016/S1470-2045(20)30541-6
27. Galsky MD, Arija JAA, Bamias A, Davis ID, De Santis M, Kikuchi E, et al. Atezolizumab with or without chemotherapy in metastatic urothelial cancer (IMvigor130): a multicentre, randomised, placebo-controlled phase 3 trial. Lancet (2020) 395(10236):1547–57. doi: 10.1016/S0140-6736(20)30230-0
28. Bajorin DF, Witjes JA, Gschwend JE, Schenker M, Valderrama BP, Tomita Y, et al. Adjuvant nivolumab versus placebo in muscle-invasive urothelial carcinoma. N Engl J Med (2021) 384(22):2102–14. doi: 10.1056/NEJMoa2034442
29. Bellmunt J, Hussain M, Gschwend JE, Albers P, Oudard S, Castellano D, et al. Adjuvant atezolizumab versus observation in muscle-invasive urothelial carcinoma (IMvigor010): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol (2021) 22(4):525–37. doi: 10.1016/S1470-2045(21)00004-8
30. Powles T, Park SH, Voog E, Caserta C, Valderrama BP, Gurney H, et al. Avelumab maintenance therapy for advanced or metastatic urothelial carcinoma. N Engl J Med (2020) 383(13):1218–30. doi: 10.1056/NEJMoa2002788
31. Necchi A, Anichini A, Raggi D, Briganti A, Massa S, Luciano R, et al. Pembrolizumab as neoadjuvant therapy before radical cystectomy in patients with muscle-invasive urothelial bladder carcinoma (PURE-01): an open-label, single-arm, phase II study. J Clin Oncol (2018) 36(34):3353–60. doi: 10.1200/JCO.18.01148
32. Powles T, Kockx M, Rodriguez-Vida A, Duran I, Crabb SJ, van der Heijden MS, et al. Clinical efficacy and biomarker analysis of neoadjuvant atezolizumab in operable urothelial carcinoma in the ABACUS trial. Nat Med (2019) 25(11):1706–14. doi: 10.1038/s41591-019-0628-7
33. Gao J, Navai N, Alhalabi O, Siefker-Radtke A, Campbell MT, Tidwell RS, et al. Neoadjuvant PD-L1 plus CTLA-4 blockade in patients with cisplatin-ineligible operable high-risk urothelial carcinoma. Nat Med (2020) 26(12):1845–51. doi: 10.1038/s41591-020-1086-y
34. van Dijk N, Gil-Jimenez A, Silina K, Hendricksen K, Smit LA, de Feijter JM, et al. Preoperative ipilimumab plus nivolumab in locoregionally advanced urothelial cancer: the NABUCCO trial. Nat Med (2020) 26(12):1839–44. doi: 10.1038/s41591-020-1085-z
35. van Dorp J, Pipinikas C, Suelmann BBM, Mehra N, van Dijk N, Marsico G, et al. High- or low-dose preoperative ipilimumab plus nivolumab in stage III urothelial cancer: the phase 1B NABUCCO trial. Nat Med (2023) 29:588–92. doi: 10.1038/s41591-023-02500-7
36. Sharma P, Callahan MK, Bono P, Kim J, Spiliopoulou P, Calvo E, et al. Nivolumab monotherapy in recurrent metastatic urothelial carcinoma (CheckMate 032): a multicentre, open-label, two-stage, multi-arm, phase 1/2 trial. Lancet Oncol (2016) 17(11):1590–8. doi: 10.1016/S1470-2045(16)30496-X
37. Rosenberg J, Sridhar SS, Zhang J, Smith D, Ruether D, Flaig TW, et al. EV-101: a phase I study of single-agent enfortumab vedotin in patients with nectin-4-positive solid tumors, including metastatic urothelial carcinoma. J Clin Oncol (2020) 38(10):1041–9. doi: 10.1200/JCO.19.02044
38. Powles T, Rosenberg JE, Sonpavde GP, Loriot Y, Duran I, Lee JL, et al. Enfortumab vedotin in previously treated advanced urothelial carcinoma. N Engl J Med (2021) 384(12):1125–35. doi: 10.1056/NEJMoa2035807
39. Hoimes CJ, Flaig TW, Milowsky MI, Friedlander TW, Bilen MA, Gupta S, et al. Enfortumab vedotin plus pembrolizumab in previously untreated advanced urothelial cancer. J Clin Oncol (2023) 41(1):22–31. doi: 10.1200/JCO.22.01643
40. O'Donnell PH, Rosenberg JE, Hoimes CJ, Petrylak DP, Milowsky MI, McKay RR, et al. Enfortumab vedotin (EV) alone or in combination with pembrolizumab (P) in previously untreated cisplatin-ineligible patients with locally advanced or metastatic urothelial cancer (la/mUC): Subgroup analyses of confirmed objective response rate (cORR) from EV-103 cohort K. J Clin Oncol (2023) 41(6_suppl):499. doi: 10.1200/JCO.2023.41.6_suppl.499
41. Heijden MSVD, Gupta S, Galsky MD, Derleth CL, Lee S, Kataria RS, et al. Study EV-302: A two-arm, open-label, randomized controlled phase 3 study of enfortumab vedotin in combination with pembrolizumab versus chemotherapy in previously untreated advanced urothelial carcinoma (aUC) (trial in progress). J Clin Oncol (2022) 40(6_suppl):TPS589–TPS. doi: 10.1200/JCO.2022.40.6_suppl.TPS589
42. Galsky MD, Powles T, Li S, Hennicken D, Sonpavde G. A phase 3, open-label, randomized study of nivolumab plus ipilimumab or standard of care (SOC) versus SOC alone in patients (pts) with previously untreated unresectable or metastatic urothelial carcinoma (mUC; CheckMate 901). J Clin Oncol (2018) 36(6_suppl):TPS539–TPS. doi: 10.1200/JCO.2018.36.6_suppl.TPS539
43. Bandini M, Gibb EA, Gallina A, Raggi D, Marandino L, Bianchi M, et al. Does the administration of preoperative pembrolizumab lead to sustained remission post-cystectomy? First survival outcomes from the PURE-01 study(☆). Ann Oncol (2020) 31(12):1755–63. doi: 10.1016/j.annonc.2020.09.011
44. Amaria RN, Reddy SM, Tawbi HA, Davies MA, Ross MI, Glitza IC, et al. Neoadjuvant immune checkpoint blockade in high-risk resectable melanoma. Nat Med (2018) 24(11):1649–54. doi: 10.1038/s41591-018-0197-1
45. Blank CU, Rozeman EA, Fanchi LF, Sikorska K, van de Wiel B, Kvistborg P, et al. Neoadjuvant versus adjuvant ipilimumab plus nivolumab in macroscopic stage III melanoma. Nat Med (2018) 24(11):1655–61. doi: 10.1038/s41591-018-0198-0
46. Chalabi M, Fanchi LF, Dijkstra KK, Van den Berg JG, Aalbers AG, Sikorska K, et al. Neoadjuvant immunotherapy leads to pathological responses in MMR-proficient and MMR-deficient early-stage colon cancers. Nat Med (2020) 26(4):566–76. doi: 10.1038/s41591-020-0805-8
47. Vos JL, Elbers JBW, Krijgsman O, Traets JJH, Qiao X, van der Leun AM, et al. Neoadjuvant immunotherapy with nivolumab and ipilimumab induces major pathological responses in patients with head and neck squamous cell carcinoma. Nat Commun (2021) 12(1):7348. doi: 10.1038/s41467-021-26472-9
48. Cascone T, William WN Jr., Weissferdt A, Leung CH, Lin HY, Pataer A, et al. Neoadjuvant nivolumab or nivolumab plus ipilimumab in operable non-small cell lung cancer: the phase 2 randomized NEOSTAR trial. Nat Med (2021) 27(3):504–14. doi: 10.1038/s41591-020-01224-2
49. Janjigian YY, Bendell J, Calvo E, Kim JW, Ascierto PA, Sharma P, et al. CheckMate-032 study: efficacy and safety of nivolumab and nivolumab plus ipilimumab in patients with metastatic esophagogastric cancer. J Clin Oncol (2018) 36(28):2836–44. doi: 10.1200/JCO.2017.76.6212
50. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell (2011) 144(5):646–74. doi: 10.1016/j.cell.2011.02.013
51. McFarland CD, Yaglom JA, Wojtkowiak JW, Scott JG, Morse DL, Sherman MY, et al. The damaging effect of passenger mutations on cancer progression. Cancer Res (2017) 77(18):4763–72. doi: 10.1158/0008-5472.CAN-15-3283-T
52. Ott PA, Hu Z, Keskin DB, Shukla SA, Sun J, Bozym DJ, et al. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature (2017) 547(7662):217–21. doi: 10.1038/nature22991
53. Keskin DB, Anandappa AJ, Sun J, Tirosh I, Mathewson ND, Li S, et al. Neoantigen vaccine generates intratumoral T cell responses in phase Ib glioblastoma trial. Nature (2019) 565(7738):234–9. doi: 10.1038/s41586-018-0792-9
54. Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med (2015) 372(26):2509–20. doi: 10.1056/NEJMoa1500596
55. Samstein RM, Lee CH, Shoushtari AN, Hellmann MD, Shen R, Janjigian YY, et al. Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat Genet (2019) 51(2):202–6. doi: 10.1038/s41588-018-0312-8
56. Goodman AM, Kato S, Bazhenova L, Patel SP, Frampton GM, Miller V, et al. Tumor mutational burden as an independent predictor of response to immunotherapy in diverse cancers. Mol Cancer Ther (2017) 16(11):2598–608. doi: 10.1158/1535-7163.MCT-17-0386
57. Chalmers ZR, Connelly CF, Fabrizio D, Gay L, Ali SM, Ennis R, et al. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med (2017) 9(1):34. doi: 10.1186/s13073-017-0424-2
58. Alexandrov LB, Nik-Zainal S, Wedge DC, Aparicio SA, Behjati S, Biankin AV, et al. Signatures of mutational processes in human cancer. Nature (2013) 500(7463):415–21. doi: 10.1038/nature12477
59. Abdel-Hafiz HA, Schafer JM, Chen X, Xiao T, Gauntner TD, Li Z, et al. Y chromosome loss in cancer drives growth by evasion of adaptive immunity. Nature (2023) 384:1125–1135. doi: 10.1038/s41586-023-06234-x
60. Duhen T, Duhen R, Montler R, Moses J, Moudgil T, de MIranda NF, et al. Co-expression of CD39 and CD103 identifies tumor-reactive CD8 T cells in human solid tumors. Nat Commun (2018) 9(1):2724. doi: 10.1038/s41467-018-05072-0
61. Jin K, Yu Y, Zeng H, Liu Z, You R, Zhang H, et al. CD103(+)CD8(+) tissue-resident memory T cell infiltration predicts clinical outcome and adjuvant therapeutic benefit in muscle-invasive bladder cancer. Br J Cancer (2022) 126(11):1581–8. doi: 10.1038/s41416-022-01725-6
62. Binnewies M, Roberts EW, Kersten K, Chan V, Fearon DF, Merad M, et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat Med (2018) 24(5):541–50. doi: 10.1038/s41591-018-0014-x
63. Gajewski TF, Corrales L, Williams J, Horton B, Sivan A, Spranger S. Cancer immunotherapy targets based on understanding the T cell-inflamed versus non-T cell-inflamed tumor microenvironment. Adv Exp Med Biol (2017) 1036:19–31. doi: 10.1007/978-3-319-67577-0_2
64. Spranger S. Mechanisms of tumor escape in the context of the T-cell-inflamed and the non-T-cell-inflamed tumor microenvironment. Int Immunol (2016) 28(8):383–91. doi: 10.1093/intimm/dxw014
65. Beatty GL, Winograd R, Evans RA, Long KB, Luque SL, Lee JW, et al. Exclusion of T cells from pancreatic carcinomas in mice is regulated by ly6C(low) F4/80(+) extratumoral macrophages. Gastroenterology (2015) 149(1):201–10. doi: 10.1053/j.gastro.2015.04.010
66. Perez-Romero K, Rodriguez RM, Amedei A, Barcelo-Coblijn G, Lopez DH. Immune landscape in tumor microenvironment: implications for biomarker development and immunotherapy. Int J Mol Sci (2020) 21(15):5521. doi: 10.3390/ijms21155521
67. Faraj SF, Munari E, Guner G, Taube J, Anders R, Hicks J, et al. Assessment of tumoral PD-L1 expression and intratumoral CD8+ T cells in urothelial carcinoma. Urology (2015) 85(3):703 e1–6. doi: 10.1016/j.urology.2014.10.020
68. Krpina K, Babarovic E, Jonjic N. Correlation of tumor-infiltrating lymphocytes with bladder cancer recurrence in patients with solitary low-grade urothelial carcinoma. Virchows Arch (2015) 467(4):443–8. doi: 10.1007/s00428-015-1808-6
69. Sharma P, Shen Y, Wen S, Yamada S, Jungbluth AA, Gnjatic S, et al. CD8 tumor-infiltrating lymphocytes are predictive of survival in muscle-invasive urothelial carcinoma. Proc Natl Acad Sci U S A (2007) 104(10):3967–72. doi: 10.1073/pnas.0611618104
70. Rizvi H, Sanchez-Vega F, La K, Chatila W, Jonsson P, Halpenny D, et al. Molecular determinants of response to anti-programmed cell death (PD)-1 and anti-programmed death-ligand 1 (PD-L1) blockade in patients with non-small-cell lung cancer profiled with targeted next-generation sequencing. J Clin Oncol (2018) 36(7):633–41. doi: 10.1200/JCO.2017.75.3384
71. Mariathasan S, Turley SJ, Nickles D, Castiglioni A, Yuen K, Wang Y, et al. TGFbeta attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature (2018) 554(7693):544–8. doi: 10.1038/nature25501
72. Necchi A, de Jong JJ, Raggi D, Briganti A, Marandino L, Gallina A, et al. Molecular characterization of residual bladder cancer after neoadjuvant pembrolizumab. Eur Urol (2021) 80(2):149–59. doi: 10.1016/j.eururo.2021.03.014
73. Kamoun A, de Reynies A, Allory Y, Sjodahl G, Robertson AG, Seiler R, et al. A consensus molecular classification of muscle-invasive bladder cancer. Eur Urol (2020) 77(4):420–33. doi: 10.1016/j.eururo.2019.09.006
74. van Dijk N, Gil-Jimenez A, Silina K, van Montfoort ML, Einerhand S, Jonkman L, et al. The tumor immune landscape and architecture of tertiary lymphoid structures in urothelial cancer. Front Immunol (2021) 12:793964. doi: 10.3389/fimmu.2021.793964
75. Li MO, Wan YY, Flavell RA. T cell-produced transforming growth factor-beta1 controls T cell tolerance and regulates Th1- and Th17-cell differentiation. Immunity (2007) 26(5):579–91. doi: 10.1016/j.immuni.2007.03.014
76. Asseman C, Mauze S, Leach MW, Coffman RL, Powrie F. An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. J Exp Med (1999) 190(7):995–1004. doi: 10.1084/jem.190.7.995
77. Baras AS, Drake C, Liu JJ, Gandhi N, Kates M, Hoque MO, et al. The ratio of CD8 to Treg tumor-infiltrating lymphocytes is associated with response to cisplatin-based neoadjuvant chemotherapy in patients with muscle invasive urothelial carcinoma of the bladder. Oncoimmunology (2016) 5(5):e1134412. doi: 10.1080/2162402X.2015.1134412
78. Liu YN, Zhang H, Zhang L, Cai TT, Huang DJ, He J, et al. Sphingosine 1 phosphate receptor-1 (S1P1) promotes tumor-associated regulatory T cell expansion: leading to poor survival in bladder cancer. Cell Death Dis (2019) 10(2):50. doi: 10.1038/s41419-018-1298-y
79. Oh DY, Kwek SS, Raju SS, Li T, McCarthy E, Chow E, et al. Intratumoral CD4(+) T cells mediate anti-tumor cytotoxicity in human bladder cancer. Cell (2020) 181(7):1612–25 e13. doi: 10.1016/j.cell.2020.05.017
80. DeNardo DG, Ruffell B. Macrophages as regulators of tumour immunity and immunotherapy. Nat Rev Immunol (2019) 19(6):369–82. doi: 10.1038/s41577-019-0127-6
81. Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol (2002) 23(11):549–55. doi: 10.1016/S1471-4906(02)02302-5
82. Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity (2014) 41(1):14–20. doi: 10.1016/j.immuni.2014.06.008
83. Koll FJ, Banek S, Kluth L, Kollermann J, Bankov K, Chun FK, et al. Tumor-associated macrophages and Tregs influence and represent immune cell infiltration of muscle-invasive bladder cancer and predict prognosis. J Transl Med (2023) 21(1):124. doi: 10.1186/s12967-023-03949-3
84. Sun M, Zeng H, Jin K, Liu Z, Hu B, Liu C, et al. Infiltration and polarization of tumor-associated macrophages predict prognosis and therapeutic benefit in muscle-invasive bladder cancer. Cancer Immunol Immunother (2022) 71(6):1497–506. doi: 10.1007/s00262-021-03098-w
85. Wang L, Sfakianos JP, Beaumont KG, Akturk G, Horowitz A, Sebra RP, et al. Myeloid cell-associated resistance to PD-1/PD-L1 blockade in urothelial cancer revealed through bulk and single-cell RNA sequencing. Clin Cancer Res (2021) 27(15):4287–300. doi: 10.1158/1078-0432.CCR-20-4574
86. Xu D, Wang L, Wieczorek K, Zhang Y, Wang Z, Wang J, et al. Single-cell analyses of a novel mouse urothelial carcinoma model reveal a role of tumor-associated macrophages in response to anti-PD-1 therapy. Cancers (Basel) (2022) 14(10):2511. doi: 10.3390/cancers14102511
87. Luo S, Zhu R, Yu T, Fan H, Hu Y, Mohanta SK, et al. Chronic inflammation: a common promoter in tertiary lymphoid organ neogenesis. Front Immunol (2019) 10:2938. doi: 10.3389/fimmu.2019.02938
88. Carragher DM, Rangel-Moreno J, Randall TD. Ectopic lymphoid tissues and local immunity. Semin Immunol (2008) 20(1):26–42. doi: 10.1016/j.smim.2007.12.004
89. Jones GW, Jones SA. Ectopic lymphoid follicles: inducible centres for generating antigen-specific immune responses within tissues. Immunology (2016) 147(2):141–51. doi: 10.1111/imm.12554
90. Sarma KP. The role of lymphoid reaction in bladder cancer. J Urol (1970) 104(6):843–9. doi: 10.1016/S0022-5347(17)61849-4
91. Pagliarulo F, Cheng PF, Brugger L, van Dijk N, van den Heijden M, Levesque MP, et al. Molecular, immunological, and clinical features associated with lymphoid neogenesis in muscle invasive bladder cancer. Front Immunol (2021) 12:793992. doi: 10.3389/fimmu.2021.793992
92. Pfannstiel C, Strissel PL, Chiappinelli KB, Sikic D, Wach S, Wirtz RM, et al. The tumor immune microenvironment drives a prognostic relevance that correlates with bladder cancer subtypes. Cancer Immunol Res (2019) 7(6):923–38. doi: 10.1158/2326-6066.CIR-18-0758
93. Silina K, Soltermann A, Attar FM, Casanova R, Uckeley ZM, Thut H, et al. Germinal centers determine the prognostic relevance of tertiary lymphoid structures and are impaired by corticosteroids in lung squamous cell carcinoma. Cancer Res (2018) 78(5):1308–20. doi: 10.1158/0008-5472.CAN-17-1987
94. Lin RL, Zhao LJ. Mechanistic basis and clinical relevance of the role of transforming growth factor-beta in cancer. Cancer Biol Med (2015) 12(4):385–93. doi: 10.7497/j.issn.2095-3941.2015.0015
96. Calon A, Lonardo E, Berenguer-Llergo A, Espinet E, Hernando-Momblona X, Iglesias M, et al. Stromal gene expression defines poor-prognosis subtypes in colorectal cancer. Nat Genet (2015) 47(4):320–9. doi: 10.1038/ng.3225
97. Flavell RA, Sanjabi S, Wrzesinski SH, Licona-Limon P. The polarization of immune cells in the tumour environment by TGFbeta. Nat Rev Immunol (2010) 10(8):554–67. doi: 10.1038/nri2808
98. Benjamin DJ, Lyou Y. Advances in immunotherapy and the TGF-beta resistance pathway in metastatic bladder cancer. Cancers (Basel) (2021) 13(22):5724. doi: 10.3390/cancers13225724
99. Vento S, Cainelli F. Infections in patients with cancer undergoing chemotherapy: aetiology, prevention, and treatment. Lancet Oncol (2003) 4(10):595–604. doi: 10.1016/S1470-2045(03)01218-X
100. Bracci L, Schiavoni G, Sistigu A, Belardelli F. Immune-based mechanisms of cytotoxic chemotherapy: implications for the design of novel and rationale-based combined treatments against cancer. Cell Death Differ (2014) 21(1):15–25. doi: 10.1038/cdd.2013.67
101. Sakai H, Kokura S, Ishikawa T, Tsuchiya R, Okajima M, Matsuyama T, et al. Effects of anticancer agents on cell viability, proliferative activity and cytokine production of peripheral blood mononuclear cells. J Clin Biochem Nutr (2013) 52(1):64–71. doi: 10.3164/jcbn.12-60
102. Galluzzi L, Vitale I, Warren S, Adjemian S, Agostinis P, Martinez AB, et al. Consensus guidelines for the definition, detection and interpretation of immunogenic cell death. J Immunother Cancer (2020) 8(1):1. doi: 10.1136/jitc-2019-000337corr1
103. Wang Z, Till B, Gao Q. Chemotherapeutic agent-mediated elimination of myeloid-derived suppressor cells. Oncoimmunology (2017) 6(7):e1331807. doi: 10.1080/2162402X.2017.1331807
104. Roselli M, Cereda V, di Bari MG, Formica V, Spila A, Jochems C, et al. Effects of conventional therapeutic interventions on the number and function of regulatory T cells. Oncoimmunology (2013) 2(10):e27025. doi: 10.4161/onci.27025
105. Gandhi L, Rodriguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med (2018) 378(22):2078–92. doi: 10.1056/NEJMoa1801005
106. Paz-Ares L, Ciuleanu TE, Cobo M, Schenker M, Zurawski B, Menezes J, et al. First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in patients with non-small-cell lung cancer (CheckMate 9LA): an international, randomised, open-label, phase 3 trial. Lancet Oncol (2021) 22(2):198–211. doi: 10.1016/S1470-2045(20)30641-0
107. Cortes J, Rugo HS, Cescon DW, Im SA, Yusof MM, Gallardo C, et al. Pembrolizumab plus chemotherapy in advanced triple-negative breast cancer. N Engl J Med (2022) 387(3):217–26. doi: 10.1056/NEJMoa2202809
108. Grasselly C, Denis M, Bourguignon A, Talhi N, Mathe D, Tourette A, et al. The antitumor activity of combinations of cytotoxic chemotherapy and immune checkpoint inhibitors is model-dependent. Front Immunol (2018) 9:2100. doi: 10.3389/fimmu.2018.02100
109. Pfirschke C, Engblom C, Rickelt S, Cortez-Retamozo V, Garris C, Pucci F, et al. Immunogenic chemotherapy sensitizes tumors to checkpoint blockade therapy. Immunity (2016) 44(2):343–54. doi: 10.1016/j.immuni.2015.11.024
110. Voorwerk L, Slagter M, Horlings HM, Sikorska K, van de Vijver KK, de Maaker M, et al. Immune induction strategies in metastatic triple-negative breast cancer to enhance the sensitivity to PD-1 blockade: the TONIC trial. Nat Med (2019) 25(6):920–8. doi: 10.1038/s41591-019-0432-4
111. Morales A, Eidinger D, Bruce AW. Intracavitary Bacillus Calmette-Guerin in the treatment of superficial bladder tumors. J Urol (1976) 116(2):180–3. doi: 10.1016/S0022-5347(17)58737-6
112. Bisiaux A, Thiounn N, Timsit MO, Eladaoui A, Chang HH, Mapes J, et al. Molecular analyte profiling of the early events and tissue conditioning following intravesical bacillus calmette-guerin therapy in patients with superficial bladder cancer. J Urol (2009) 181(4):1571–80. doi: 10.1016/j.juro.2008.11.124
113. Ratliff TL, Ritchey JK, Yuan JJ, Andriole GL, Catalona WJ. T-cell subsets required for intravesical BCG immunotherapy for bladder cancer. J Urol (1993) 150(3):1018–23. doi: 10.1016/S0022-5347(17)35678-1
114. Kates M, Matoso A, Choi W, Baras AS, Daniels MJ, Lombardo K, et al. Adaptive immune resistance to intravesical BCG in non-muscle invasive bladder cancer: implications for prospective BCG-unresponsive trials. Clin Cancer Res (2020) 26(4):882–91. doi: 10.1158/1078-0432.CCR-19-1920
115. Balar AV, Kamat AM, Kulkarni GS, Uchio EM, Boormans JL, Roumiguie M, et al. Pembrolizumab monotherapy for the treatment of high-risk non-muscle-invasive bladder cancer unresponsive to BCG (KEYNOTE-057): an open-label, single-arm, multicentre, phase 2 study. Lancet Oncol (2021) 22(7):919–30. doi: 10.1016/S1470-2045(21)00147-9
116. Meghani K, Cooley LF, Choy B, Kocherginsky M, Swaminathan S, Munir SS, et al. First-in-human intravesical delivery of pembrolizumab identifies immune activation in bladder cancer unresponsive to Bacillus calmette-guerin. Eur Urol (2022) 82(6):602–10. doi: 10.1016/j.eururo.2022.08.004
117. Kirschner AN, Wang J, Rajkumar-Calkins A, Neuzil KE, Chang SS. Intravesical anti-PD-1 immune checkpoint inhibition treats urothelial bladder cancer in a mouse model. J Urol (2021) 205(5):1336–43. doi: 10.1097/JU.0000000000001576
118. Challita-Eid PM, Satpayev D, Yang P, An Z, Morrison K, Shostak Y, et al. Enfortumab vedotin antibody-drug conjugate targeting nectin-4 is a highly potent therapeutic agent in multiple preclinical cancer models. Cancer Res (2016) 76(10):3003–13. doi: 10.1158/0008-5472.CAN-15-1313
119. Krysko DV, Garg AD, Kaczmarek A, Krysko O, Agostinis P, Vandenabeele P. Immunogenic cell death and DAMPs in cancer therapy. Nat Rev Cancer (2012) 12(12):860–75. doi: 10.1038/nrc3380
Keywords: bladder cancer, immune checkpoint inhibition, immune micro-environment, tertiary lymphoid structure, PD-1, PD-L1, CTLA-4
Citation: van Dorp J and van der Heijden MS (2023) The bladder cancer immune micro-environment in the context of response to immune checkpoint inhibition. Front. Immunol. 14:1235884. doi: 10.3389/fimmu.2023.1235884
Received: 06 June 2023; Accepted: 21 August 2023;
Published: 31 August 2023.
Edited by:
Derré Laurent, Centre Hospitalier Universitaire Vaudois (CHUV), SwitzerlandReviewed by:
Shun Wan, Lanzhou University Second Hospital, ChinaCopyright © 2023 van Dorp and van der Heijden. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michiel S. van der Heijden, bXMudmQuaGVpamRlbkBua2kubmw=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.