- 1Department of Otorhinolaryngology and Head & Neck Surgery, University Medical Center Ulm, Head & Neck Cancer Center of the Comprehensive Cancer Center Ulm, Ulm, Germany
- 2School of Medicine, Southeast University, Nanjing, China
- 3Department of Otorhinolaryngology, Head and Neck Surgery, Heidelberg University Hospital, Heidelberg, Germany
- 4Molecular Mechanisms of Head and Neck Tumors, German Cancer Research Center (DKFZ), Heidelberg, Germany
In the tumor milieu of head and neck squamous cell carcinoma (HNSCC), distinct B cell subpopulations are present, which exert either pro- or anti-tumor activities. Multiple factors, including hypoxia, cytokines, interactions with tumor cells, and other immune infiltrating lymphocytes (TILs), alter the equilibrium between the dual roles of B cells leading to cancerogenesis. Certain B cell subsets in the tumor microenvironment (TME) exhibit immunosuppressive function. These cells are known as regulatory B (Breg) cells. Breg cells suppress immune responses by secreting a series of immunosuppressive cytokines, including IL-10, IL-35, TGF-β, granzyme B, and adenosine or dampen effector TILs by intercellular contacts. Multiple Breg phenotypes have been discovered in human and mouse cancer models. However, when compartmentalized within a tertiary lymphoid structure (TLS), B cells predominantly play anti-tumor effects. A mature TLS contains a CD20+ B cell zone with several important types of B cells, including germinal-center like B cells, antibody-secreting plasma cells, and memory B cells. They kill tumor cells via antibody-dependent cytotoxicity and phagocytosis, and local complement activation effects. TLSs are also privileged sites for local T and B cell coordination and activation. Nonetheless, in some cases, TLSs may serve as a niche for hidden tumor cells and indicate a bad prognosis. Thus, TIL-B cells exhibit bidirectional immune-modulatory activity and are responsive to a variety of immunotherapies. In this review, we discuss the functional distinctions between immunosuppressive Breg cells and immunogenic effector B cells that mature within TLSs with the focus on tumors of HNSCC patients. Additionally, we review contemporary immunotherapies that aim to target TIL-B cells. For the development of innovative therapeutic approaches to complement T-cell-based immunotherapy, a full understanding of either effector B cells or Breg cells is necessary.
1 Introduction
Globally, head and neck squamous cell carcinoma (HNSCC) accounts for more than 870,000 new diagnoses, and 440,000 new deaths each year (1). The causes of HNSCC are either genetic alterations following environmental carcinogen exposures (i.e., smoking, alcohol), or through malignant transformation following human papillomavirus (HPV) infection (2). The majority of HPV-driven HNSCC are caused by HPV-16 infection in the oropharynx, which encompasses the base of the tongue and tonsil, whereas HNSCC driven by environmental carcinogens is more frequent in the oral cavity, hypopharynx or larynx. HNSCC patients with the anatomical site of larynx or without prior HPV infection show worse prognosis, and are more in need of multimodality treatments including both radiation and chemotherapy following surgical resection (3). Oppositely, HPV+ HNSCC patients have generally better prognosis compared to their HPV- counterparts, calling for treatment de-escalation for the purpose of an improved quality of life and functional outcome (4–6).
In the scenario of cancer immunity, an immunocompetent microenvironment with a high number of tumor-infiltrating lymphocytes (TILs) is often a good prognosticator. CD8+ cytotoxic T cells (CTLs) are primary anti-cancer cytotoxic effectors, which fight directly against tumor cells. However, the exorbitant tumor burden could also exaggerate CTL exhaustion, leading to the overexpression of a series of inhibitory receptors at the cell surface, which include programmed cell death protein 1 (PD-1), and cytotoxic T-lymphocyte associated protein 4 (CTLA-4) (7). Hence, multiple immune checkpoint blockade (ICB) reagents have been developed to reinvigorate CTL functions.
In contrast to T cells, the roles of TIL-B cells have not been fully elucidated yet. The heterogeneity, functional plasticity, and spatial variations of TIL-B cells make it even more challenging to sketch a comprehensive picture of B cell immunity in cancers. Generally, tertiary lymphoid structures (TLSs) serve as privileged sites for the aggregation of TILs and the co-activation of T and B cells. In normal physiological conditions, B cells fulfill pivotal roles in antigen processing and presentation. They can process and present antigenic peptides via both MHC class II to CD4+ T cells (8), and cross-presentation of peptide-MHC I complexes to CD8+ T cells (9–11). TIL-B cells localized within TLSs have been observed to harbor the necessary molecular machinery for effective antigen presentation to T cells (12–16).
Beyond this, TLSs house a diverse cast of effector B cell populations, including i.e. CD20+ germinal-center (GC) like follicular B cells, multi-valency antigen-presenting B cells, class-switched B cells, antibody-producing plasma cells, and memory B cells (17, 18). With their tremendous antibody producing capacity, plasma cells are considered as key anti-tumor effector TIL-B cells, leading the effects of complement-dependent cytotoxic, antibody-dependent cellular cytotoxicity (ADCC) and phagocytosis (ADCP). Meanwhile, the antigen-presenting B cells present antigens to effector T cells via major histocompatibility complex (MHC) molecules at the cell surface, which also aid in the anti-tumor immunity. Beside these aggregated anti-tumorigenic structures, multiple phenotypes of pro-tumorigenic B cells – collectively known as regulatory B (Breg) cells – are also found within the tumor microenvironment (TME) (19). They induce immune-suppressive cells like myeloid-derived suppressor cells (MDSCs) and regulatory T (Treg) cells, suppress effector TIL functions, and program the TME towards an immune-suppressive direction (20).
In this article, we will present a comprehensive picture of how phenotypically and functionally distinct TIL-B cells influence tumor growth and response to treatment, with a specific focus on HNSCC. We address the most important TIL-B cell populations, their functions, prognostic relevance, and related therapeutic approaches. We begin with the pro-tumorigenic Breg cells, from their phenotypes discovered yet in both murine and human cancers, the immune-suppressive mechanisms, to the relationships with clinicopathological features. Then we describe the anti-tumorigenic TIL-B cells, beginning with the fundamental concepts of TLS assembling, to the main effector TIL-B cells, their functions, and clinical relevance. We end by summarizing the current state-of-the-art of TIL-B-based immunotherapies, either by fostering TLS formation, or by eliminating the immune-suppressive functions of Breg cells.
2 The pro-tumor activity of Breg cells
Breg cells, a heterogenous population of B cells initially implicated in inhibiting delayed hypersensitivity reactions, are one of the primary immunosuppressive cell populations in numerous cancer types including HNSCC (21–23).
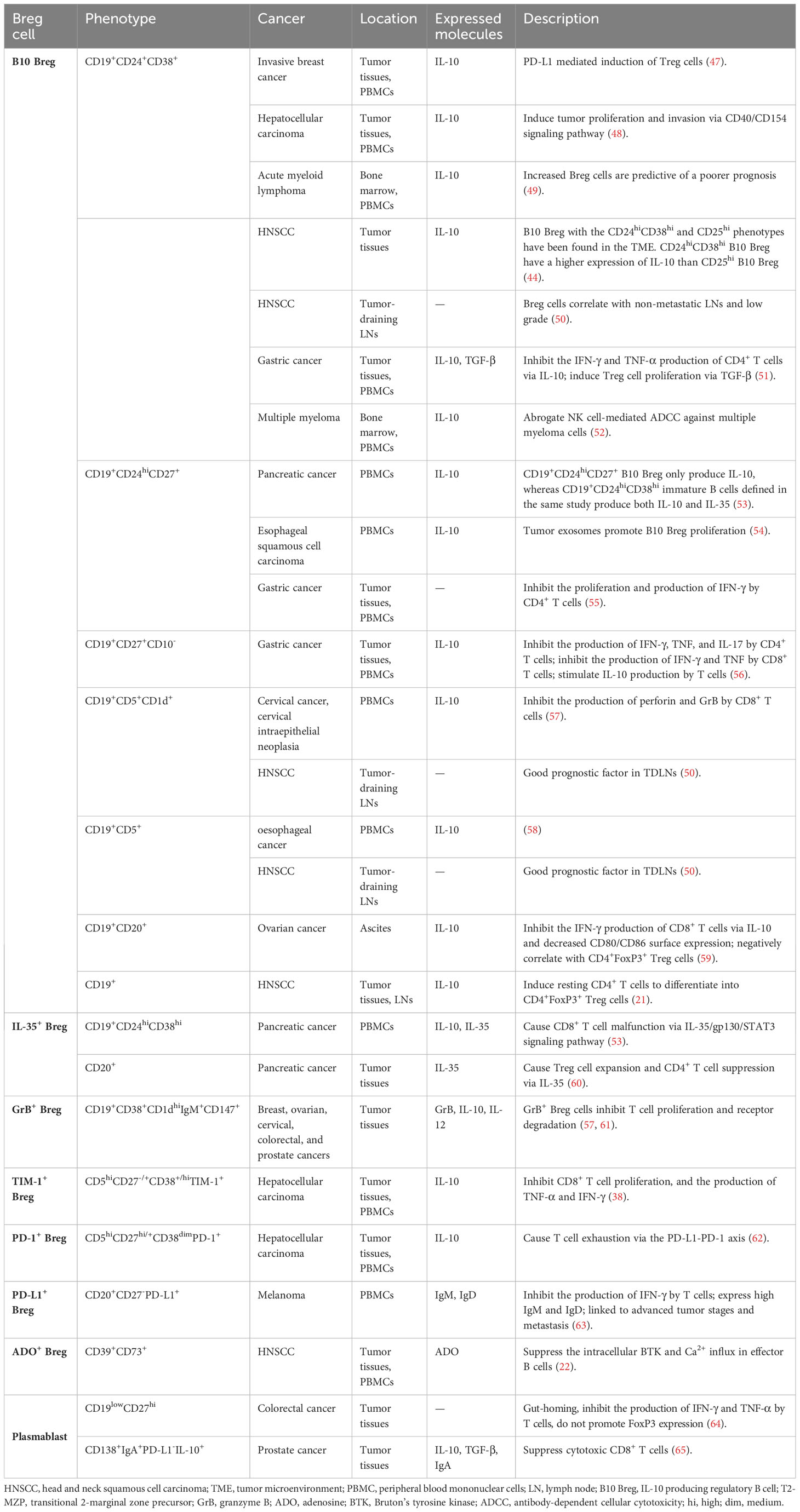
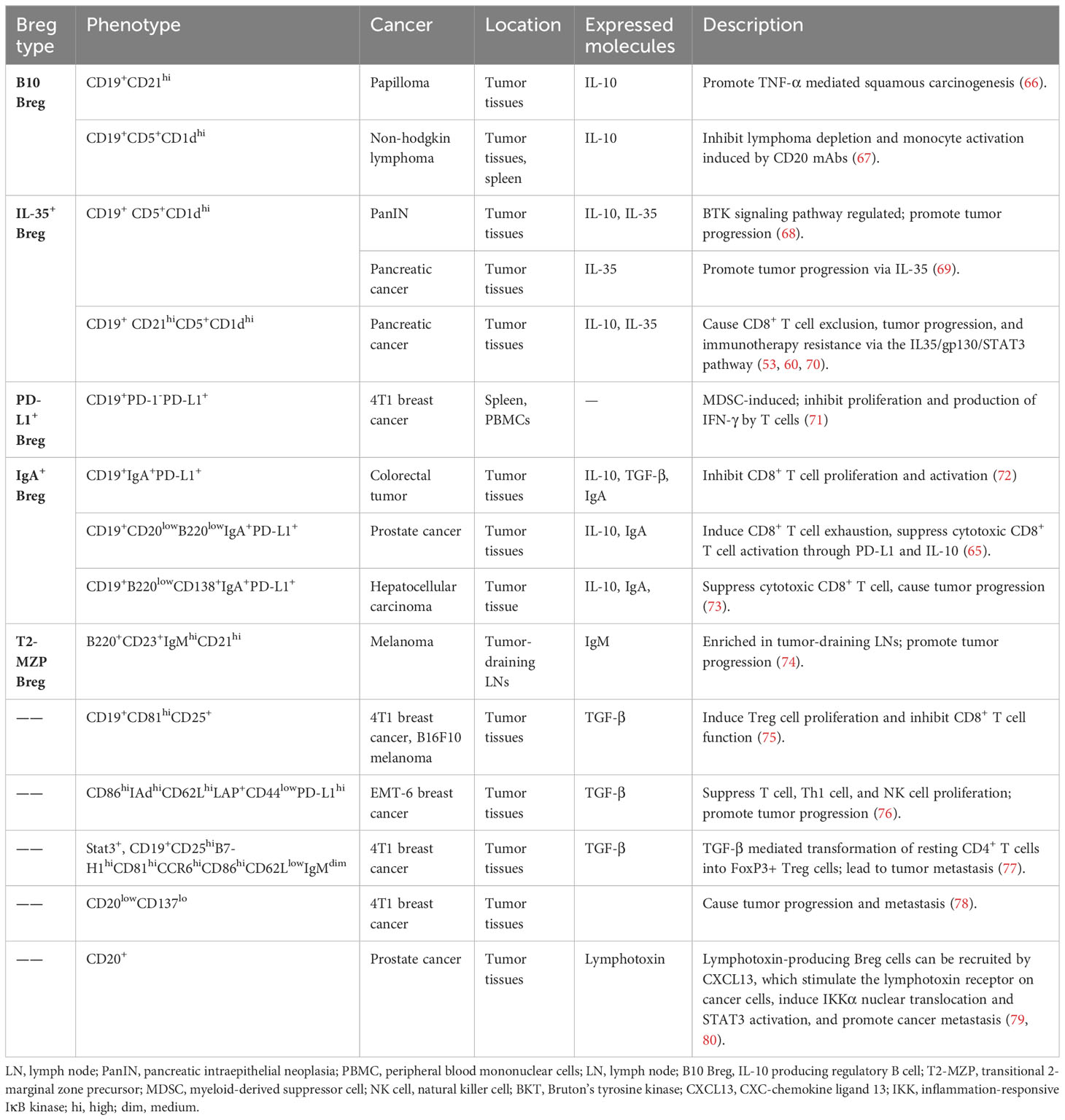
Breg cells were initially identified in autoimmune diseases, such as collagen-induced arthritis and systemic lupus erythematosus, for their ability to reduce inflammation through the action of interleukin (IL)-10 (24). In a mouse model of experimental arthritis, transitional 2-marginal zone precursors (T2-MZP) B cells (CD21hiCD23hiCD24hiCD1dhi) were found to possess regulatory capacities and produce IL-10 (25). Additionally, IL-10-producing B cells with regulatory properties were detected within the tumor microenvironment of solid tumors such as breast and ovarian cancer, collectively referred to as B10 Bregs (26). Their presence resulted in the suppression of anti-tumor immune responses and the promotion of tumor progression by inhibiting effector T cell functions. Subsequently, other types of Breg cells with diverse surface markers and the ability to secrete different immunosuppressive molecules were discovered in multiple types of human cancers. Ongoing studies are actively investigating the phenotypic markers, secreted molecules, and mechanisms of Breg cells within the tumor microenvironment. Figure 1 provides a schematic summary of the key milestones in the discovery of Breg cells.
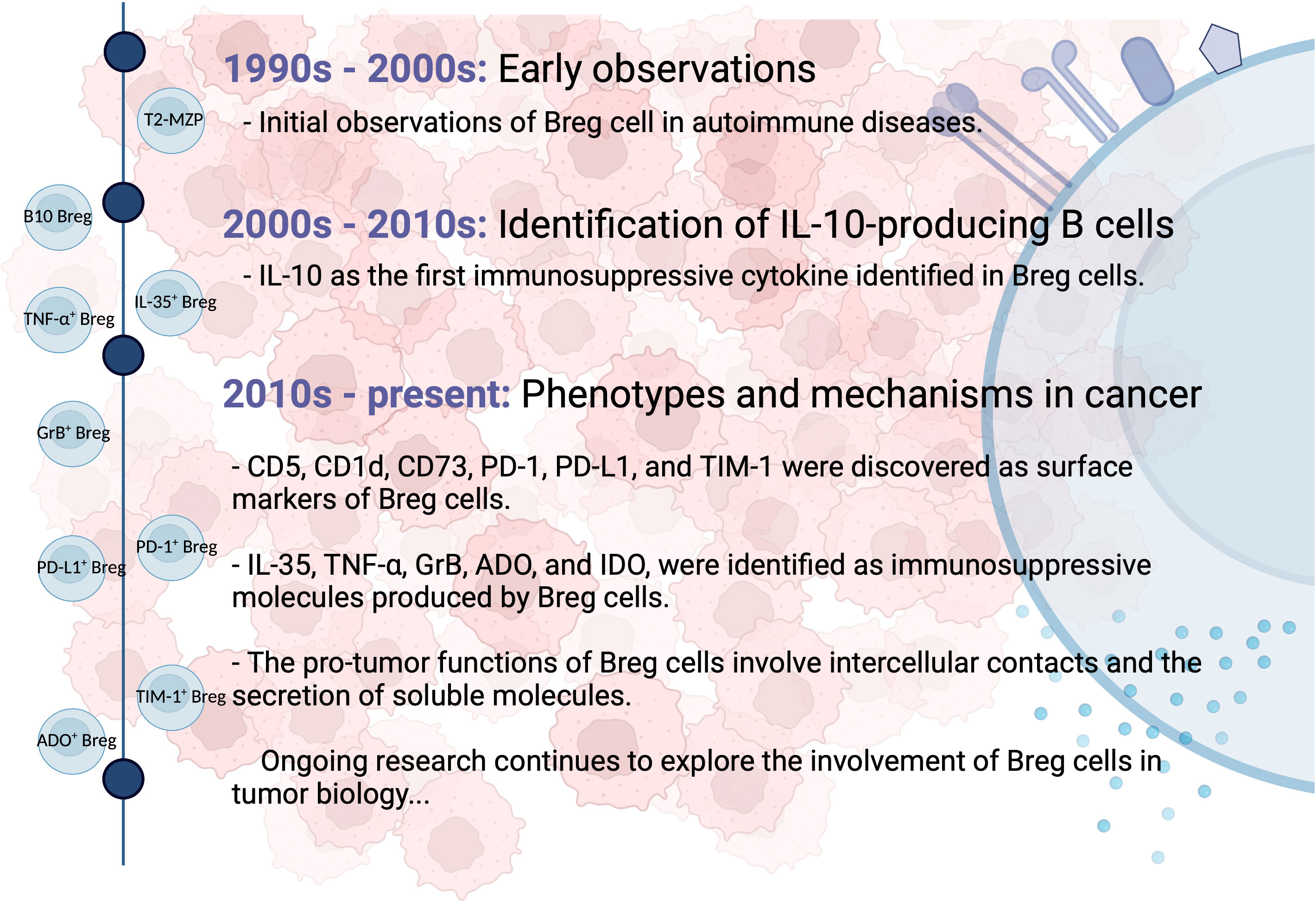
Figure 1 Milestones in the discovery of Breg cells. Breg cells were first identified in autoimmune diseases for their anti-inflammatory properties mediated by IL-10. They were later found in solid tumors, where they suppress anti-tumor immune responses. Multiple Breg cell phenotypes and secreted molecules have since been discovered. Ongoing studies investigate Breg cell characteristics within the tumor microenvironment.
Due to complex ontogeny and mode of activation, an agreement on the phenotypic and lineage trajectories of Breg cells is still lacking, as are the transcription factors that uniquely drive their development (27). The innovative strategy of employing single-cell RNA-sequencing to identify the Breg cell cluster is often unsuccessful (28, 29). Worth speculating that B cells acquire their regulatory capabilities during various stages of development and in response to certain environmental stimuli rather than Breg cells represent a distinct lineage (27, 30). Multiple local perturbations can induce Breg cells, which include hypoxia, acidosis, lipid metabolites and tumor exosomes (31–38), cytokines like IL-35, IL-21, IL-1β, IL-6 (39–42), Ca2+ influx (43), and activation of surface molecules including Toll-like receptors (TLRs), CD40 and B-cell receptors (BCRs) (40, 41, 44–46).
2.1 The immunosuppressive mechanisms of Breg cells
Breg cells promote carcinogenesis via several mechanisms. In this section, we first list the main discovered Breg phenotypes and functions of human and mouse cancer models separately in Tables 1 and 2. We then address the immunosuppressive mechanisms of several most important Breg cells, and also their association with the clinicopathological characteristics of HNSCC patients.
2.1.1 Autocrine and paracrine secretions
Breg cells are known for their role in dampening the immune response through the secretion of endogenous anti-inflammatory molecules, such as IL-10, IL-35, and transforming growth factor-beta (TGF-β), as depicted in Figure 2.
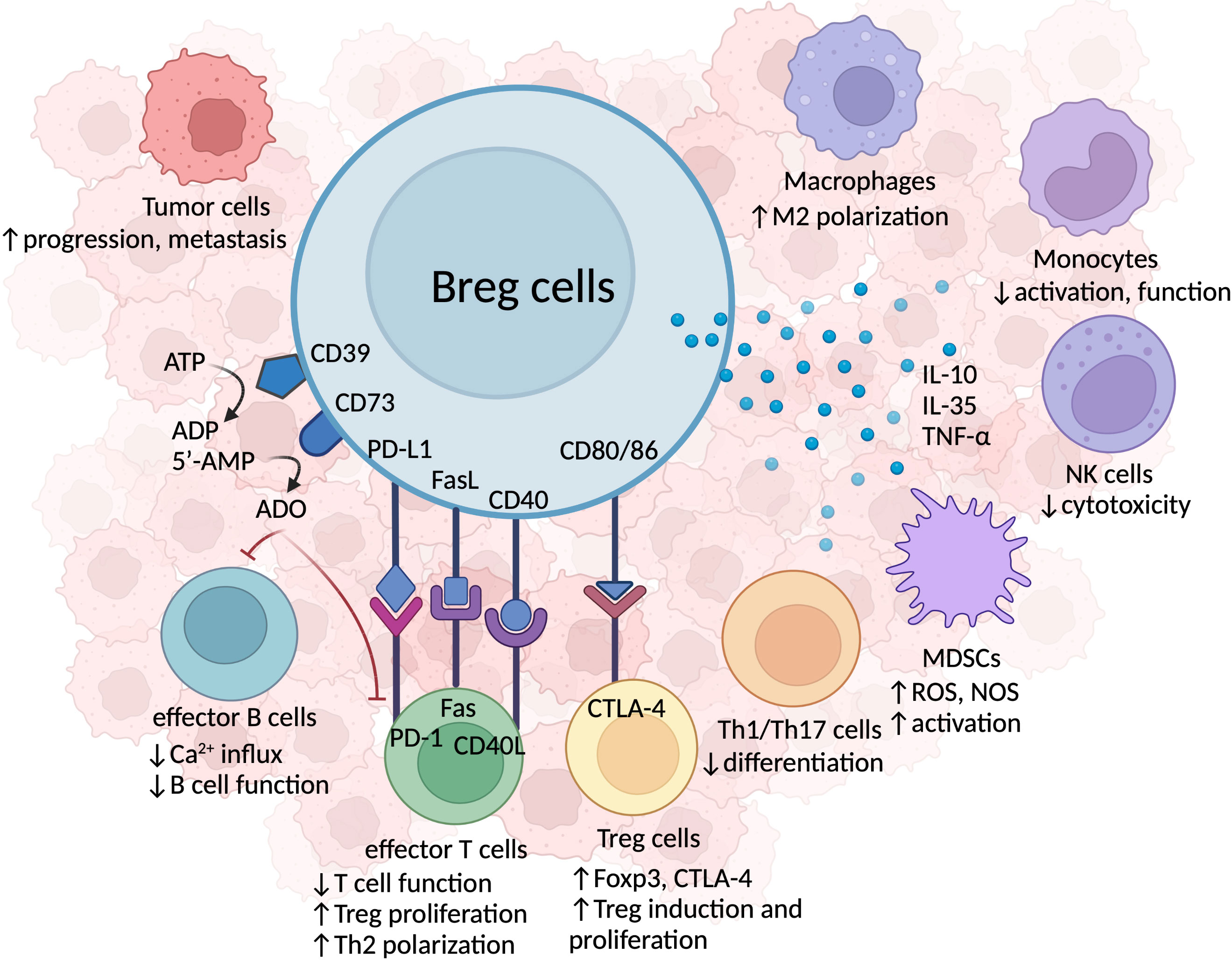
Figure 2 The immunosuppressive mechanisms of Breg cells in HNSCC. Breg cells secrete immunosuppressive cytokines, including IL-10, IL-35, and TNF-α, which inhibit anti-tumor immune activity, foster immunosuppressive TILs, and facilitate tumor progression and metastasis. The co-expression of CD39 and CD73 surface proteins permits Breg cells to hydrolyze ATP to adenosine (ADO), which acts on effector T and B cells, reduces Ca2+ influx, and results in the malfunction of effector immune cell. Breg cells also form cell-cell contacts with other TILs. Breg cells can induce anti-tumor T cell malfunction via PD-L1-PD-1 axis, or Fas/FasL binding. Breg cells and effector T cells interact via CD40/CD40L to promote Th1/Th2 cell polarization and Treg cell proliferation. Breg cells are also capable of forming cellular contacts with other immunosuppressive TILs, such as MDSCs and Treg cells. The CD80/CD86 marker expressed on Breg cells binds to CTLA-4 on Treg cells, inducing Treg cell proliferation.
In HNSCC patients, at least two kinds of IL-10-producing B10 Breg have been identified: CD19+CD24hiCD38hi and CD19+CD25hi B10 Bregs (23). Several immune-regulatory functions of B10 Bregs have been revealed: first, B10 Bregs inhibit CD4+ T cell differentiation into interferon (IFN)- γ and TNF-α producing helper T1 (Th1) cell and IL-17 producing helper T17 (Th17) cell (26). Second, B10 Bregs cause dendritic cells (DCs) to overexpress IL-4 and downregulate IL-12, disrupting the Th1/Th2 cell balance (81). Furthermore, B10 Bregs favorably influence the differentiation of tumor-associated macrophages (TAMs) into the M2 phenotype, and ultimately suppress effector T and natural killer (NK) cells (41, 63). B10 Bregs also dampen the activation and effector function of monocytes, and promote Treg cell development via IL-10-mediated suppressive pathways (26, 52, 67, 82–84).
Another anti-inflammatory cytokine, IL-35, promotes immunological tolerance by orchestrating the differentiation of conventional T cells to Treg cells, inducing effector T cell exhaustion, and upregulating anti-apoptotic and cell cycle genes which facilitate tumor cell growth (85, 86). Meanwhile, TGF-β converts naïve CD4+ T cells into Treg cells, limits effector T cell proliferation and function, and augments FoxP3 and CTLA-4 expression, hence facilitating tumor growth and metastasis (77, 87–89).
Aside from IL-10 and IL-35, unconventional Breg cells which produce other immunosuppressive molecules, including granzyme B (GrB), indoleamine 2,3-dioxygenase (IDO) and adenosine (ADO), are also detected. A unique subset of Breg cells expressing CD38, CD1d, IgM, and CD147 has been identified in various human malignancies, such as breast, ovarian, cervical, colorectal, and prostate cancers. These cells have been found to secrete regulatory molecules, including GrB, IDO, IL-12, and IL-10. Specifically, the secretion of GrB by the CD38+CD1dhiIgM+CD147+ Breg cells leads to the degradation of T-cell receptor (TCR) ζ-chain, resulting in the dampening of T cell responses (57, 61). The TIM-1-producing CD5hiCD24-CD38+/hi Breg cells found in hepatocellular carcinoma patients inhibit CD8+ T cell proliferation, and confine the production of TNF-α and IFN-γ (38). First discovered in HNSCC patients, the ADO-producing CD39+CD73+ Breg cells suppress the activity of effector B cells by inhibiting Bruton’s tyrosine kinase (BTK) phosphorylation and Ca2+ influx (22). These CD39+CD73+ Breg cells also deactivate T cells in healthy volunteers via the byproducts of ATP hydrolysis, AMP and ADO, resulting in immunological escape (90).
2.1.2 Intercellular interactions
Aside from producing a concoction of anti-inflammatory cytokines, Breg cells also program the immunosuppressive TME through extensive intercellular interactions with other TILs via ligand-receptor interactions including CTLA-4 — CD80/CD86, CD40 — CD40L, PD-1 — PD-L1, and Fas — FasL (Figure 2).
In conjunction with TGF-β, CTLA-4 — CD80/CD86 interaction enables Breg cells to form cell-to-cell contacts with Treg cells, hence boosting the expression of FoxP3 and CTLA-4 (87). The interaction between Breg cells and CD4+ T cells via CD40 and its ligand evokes Th1/Th2 cell polarization and Treg cell proliferation (21, 47, 51, 77, 91). Furthermore, Breg cells expressing either PD-1 or PD-L1 have been identified in cancerous tissues (62, 92). Through IL-10 signaling, PD-1hi Breg cells cause CTL malfunction, and promote cancer development (62). The activation of BCL6 by TLR4 is essential for the induction of PD-1hi Breg cells. Besides, PD-L1hi Breg cells inhibit BCL6 overexpression in CD4+CXCR5+PD-1+ follicular helper T (TFH) cells, reduce TFH proliferation, and hinder both the development of memory B cells, and the terminal differentiation of plasma cells (92). Intriguingly, multiple exhausted immune cells, including T cells, B cells, and NK cells, as well as senescence cells, have been found to have an increased expression of PD-1/PD-L1, allowing them to evade the immune surveillance of CTLs, and constantly release inflammatory cytokines (7, 93). These findings indicate that the PD-1/PD-L1-expressing Breg cells represent exhausted B cells that secrete immunosuppressive cytokines. Blocking the PD-L1-PD-1 axis, either by PD-1 blockade alone, or by concurrent blockade of IL-10 receptors and inhibitory PD-L1 receptors, have been shown to successfully reverse CTL dysfunction (93, 94).
Moreover, FasL+ Breg cells with the ability of promoting the death of Fas+ effector T cells and tumor cells have been identified (95). Since Treg cells highly express the anti-apoptotic gene c-FLIP, they are resistant to Fas-driven apoptosis. Therefore, the FasL expression by Breg cells is more likely to cause CTL elimination as opposed to Treg cells, resulting in an uneven CD8+ T cell/Treg cell ratio (96).
Breg cells also interact with other immunosuppressive TILs and reshape the local TME in an immunosuppressive direction. MDSCs are myeloid cells which expand under pathologic conditions, such as chronic inflammation, infection, cancer, autoimmune diseases and trauma, with potent immunosuppressive activity (97, 98). In the B-cell-deficient murine cancer model of 4T1 breast cancer and B16 melanoma, the inhibitory impact of MDSCs was greatly diminished, but adoptive transfer of tumor-evoked CD81hiCD25+CD20low4-1BBLlow Breg cells restored MDSC-mediated suppression of T cells, provoking tumor progression and metastasis (99). Coincidentally, the presence of MDSCs can confer immunosuppressive characteristics to B cells. B cells co-cultured with MDSCs for more than 24 hours were able to suppress T cell proliferation, increase IL-10 production, and decrease IFN-γ release in 4T1 breast cancer mice (71).
Last but not least, Breg cells directly promote cancer growth, invasion, and immune evasion (48, 74, 100). In the B-cell-deficient mouse model of Kras-expressing pancreatic intraepithelial neoplasia (PanIN), adoptive transfer of CD5+CD1dhi Breg cells rescued the tumor growth deficiency (69); Meanwhile, a study using a mouse model of melanoma showed that Breg cells with B220+CD23+IgMhiCD21hi T2-MZP phenotype specifically accumulated in tumor-draining lymph nodes (LNs) and promoted tumor growth in an IL-10-independent manner (25, 74).
2.2 The relation of Breg cells with clinicopathological features
While the correlation between various types of Breg cells, clinicopathological characteristics, and the prognosis of HNSCC patients remains unclear, some studies have shed light on these topics. In one study, the frequency of CD19+IL-10+ Breg cells was found to be highly associated with clinical stage, local and regional recurrence in a cohort of 46 patients with tongue squamous cell carcinoma (TSCC) (21). Moreover, higher levels of CD19+IL-10+ Breg cells and CD4+FoxP3+ Treg cells were correlated to reduced overall survival rates. Conversely, the presence of Breg cells in tumor-draining LNs appeared to be a positive prognostic indicator in HNSCC patients. In a study for non-sentinel LNs of 32 HNSCC patients, three distinct Breg phenotypes CD5+, CD5+CD1dhi, and CD24hiCD38hi were analyzed by flow cytometry (50). The frequency of CD24hiCD38hi Breg cells was significantly higher in patients with lower histological grades, while the frequency of CD5+ Breg cells decreased in advanced clinical stages. Considering the potential functional discrepancies involved in the tumor progression, it is possible that these Breg cell subsets perform certain immune priming functions before exerting their regulatory effects (7).
It’s also important to consider the proportion of Breg cells present within tumors. In the study on TSCC, Zhou et al. reported only 0.8% of IL-10+ Breg cells (21). In comparison, Lechner et al. reported a higher percentage of 2.4% IL-10+ Breg cells in HNSCC (23). Additionally, Hladíková et al. observed 2.7% of IL-10+ Breg cells in oropharyngeal cancers (101). Given that B cells are not the predominant cell type in the tumor milieu, the actual number of Breg cells is quite low. This raises the question of whether these relatively rare cells could have a significant biological impact.
Overall, amidst the diverse TILs within the TME, Breg cells discharge anti-inflammatory agents and showcase suppressive capacities to facilitate immune-regulating duties. They modulate the immune landscape of the tumor, although further research is required to thoroughly explore the relationship between Breg cells and the clinical-pathological attributes as well as the prognosis of patients with HNSCC.
3 Anti-tumor activity of effector B cells from TLSs
TLSs are non-encapsulated immunologically dense formations composed of lymphocytes and stromal cells, which develop in response to persistent inflammation or infection (102, 103). Their composition resembles that of secondary lymphoid organs (SLOs). Within human solid tumors, TLSs are typically found in the tumor nests and interstitium, generated as a result to persistent stimulation by tumor-associated inflammation. The aggregation of diverse TILs within a developed TLS endows it with the capacity to efficiently present tumor-associated antigens (TAAs), eliciting subsequent T and B cell effector activities.
In this section, we will initially delve into the mechanism underlying TLS formation. Subsequently, we will describe the major types of TIL-B cells located in the CD20+ B cell zone of TLSs, and elucidate their phenotypes, functions, and clinicopathologic significance, with a particular focus on HNSCC patients.
3.1 TLS formation
TLS development is a result of lymphatic neogenesis, which generates a local hub for antigen presentation and immunological activation (102). HPV+ HNSCC have a higher percentage of GC maturation and TLS formation within the tumor milieu (2, 104), most likely due to the presence of viral antigens throughout carcinogenesis. The episomal or integrated viral components of HPV+ tumors may prime for early-onset innate immune responses, and boost anti-tumor adaptive immunity (105, 106).
The recruitment of lymphoid tissue inducer (LTi) cells to the inflamed area initiates TLS neogenesis. CD4+ T cells, CD21+ follicular DCs, and stromal cells are capable of producing CXC-chemokine ligand (CXCL) 13 and IL-7, which aid in this process (107, 108) (Figure 3). Main LTi cells include IL-21 producing Th17 cells (109, 110), TIL-B cells (111), and M1-polarized TAMs (112). In virus infection, CD4+ Th cells partially polarized into IFN+IL-1+ TFH cells, which stimulate CXCL13 production and support ectopic GC formation (113, 114). Whereas in both breast cancer and nasopharyngeal carcinoma, CXCL13-producing CD4+ T cells with either PD-1hiT-bethiBCL6lowCXCR5- or PD-1hiCXCR5- phenotype prime TLS neogenesis (115, 116).
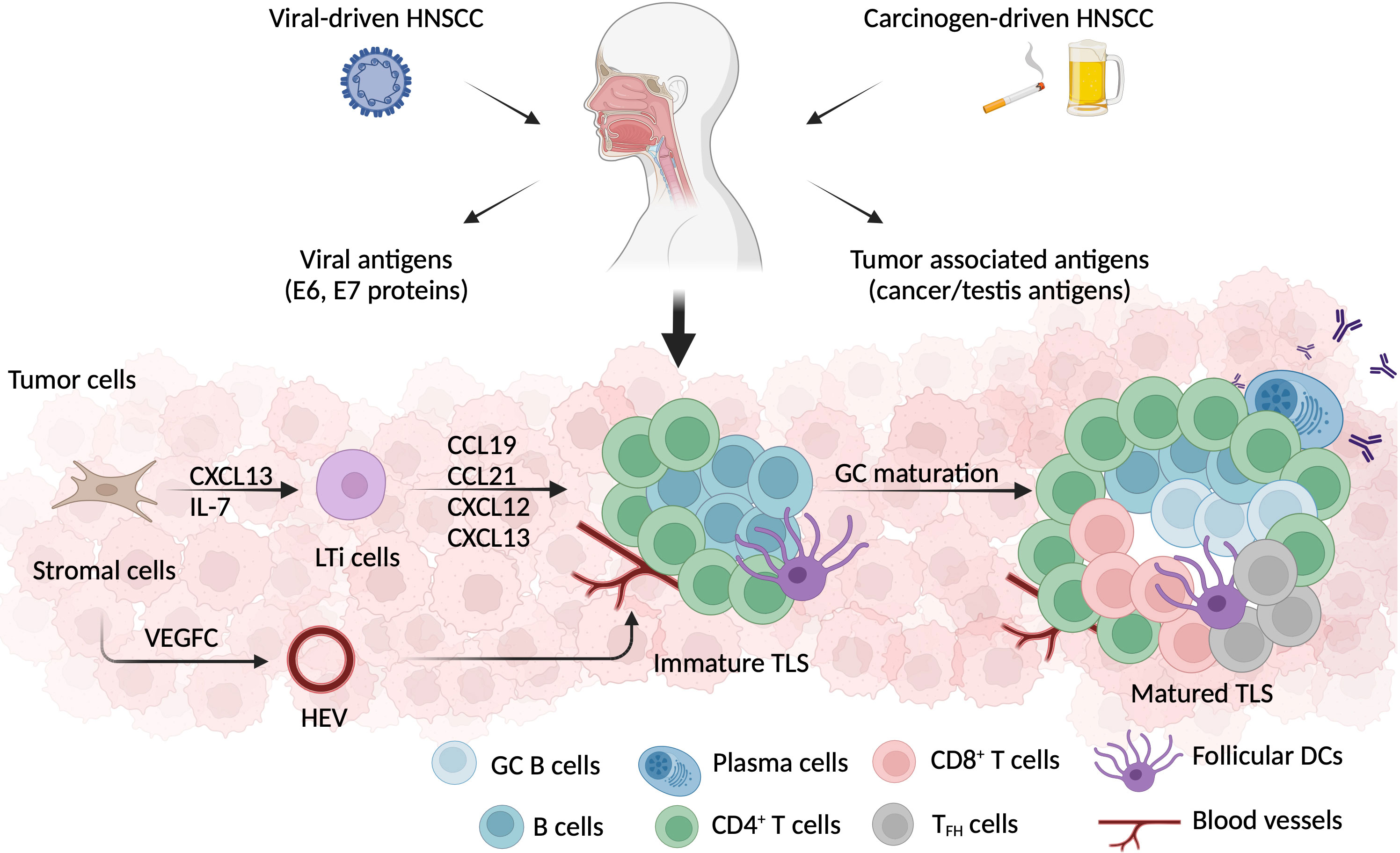
Figure 3 TLS formation in HNSCC. HNSCC can be induced by either viral infection, or environmental carcinogens, such as smoking or excessive alcohol consumption. Besides tumor associated antigens, the HPV+ HNSCC microenvironment also exposes E6 and E7 viral antigens. TLS, a crucial ectopic lymphoid cell formation, develops in the early stage of tumorigenesis. Local stromal cells that have been activated secret CXCL13 and attract lymphoid tissue inducer (LTi) cells. LTi cells produce a series of chemokines, including CCL19, CCL21, CXCL12, and CXCL13, which aid in the recruitment of LTi cells and lymphocytes. Additionally, stromal cells secrete VEGF-C, which promotes HEV development in the vicinity of TLSs. After germinal center (GC) maturation, a well-defined TLSs is formed with a CD3+ T cell zone composed of CD4+, CD8+, and TFH cells, and a CD20+ B cell zone comprising GC B cells, memory B cells, and plasma cells. Besides lymphocytes, follicular DCs, fibroblasts, and neovascular cells are also involved in the process of TLS formation.
After being recruited into the local region, LTi cells communicate with stromal cells via lymphotoxin (LT) α1β2 –LTβR interaction (117), allowing them to produce vascular endothelial growth factor (VEGF) C, to induce high endothelial venule (HEV) formation (118) (Figure 3). Then, HEVs serve as entrances for TILs to enter the TLS (119). LTi cells additionally secrete IL-17, which induces the production of CXCL12, CXCL13, CC-chemokine ligand (CCL) 19, and CCL21. This chemokine cocktail stimulates LTα1β2 expression on TILs, and reprograms them into mantle T or B cells (120, 121). The complete TLS neogenesis process is analogous to the formation of human SLOs. An entirely developed TLS, referred to as a secondary follicle-like TLS, encompasses an active GC zone populated by CD23+ B cells. On the contrary, an immature TLS (referred to as primary follicle-like TLS) consists of B cell clusters interlinked by FDC networks but lacks GCs (122).
A 12-chemokine gene expression signature (GES) associated with TLS development was identified in multiple solid tumors (103, 123–125). Among these, CCL19, CCL21, and CXCL13 stand out as crucial chemokines for TLS neogenesis. Later, a pan-cancer assessment of gene signatures from The Cancer Genome Atlas (TCGA) database indicated that this 12-chemokine GES is substantially related with TLS-associated cell types, including B lineage cells, T cells, and myeloid cells (103). The differential expression of the 12-chemokine GES in pan cancers demonstrates that TLS abundance is greatly reliant on cancer heterogeneity. Tumors situated in immunologically privileged sites (brain or eyes), or with severe fibrotic stroma (pancreatic cancer), typically have a low expression of the TLS signature, whereas HNSCC has a moderate to high expression (126). This result is consistent with several clinical studies (127–129). In a study of oral squamous cell carcinoma (OSCC) patients, 90.7% (68 of 75) of all cancer samples contained HEV invasion (128). CXCL12, CCL19, and CCL21 were significantly overexpressed in HEV+ cases, as were CD3+ T cells, CTLs, and CD20+ B cells (129). In another OSCC cohort, 26.8% (45 of 168) TLS+ patients were identified using immunohistochemical (IHC) staining (127).
TLSs can be detected in the stroma, invasive margin, and tumor core (130, 131). For HNSCC patients, TLSs are more prevalent in the invasive margin (23, 132). In a study of OSCC patients, intratumoral TLSs were observed in 33.8% (22 of 65) of tumor specimens, while peritumoral TLSs were observed in 75.4% (49 of 65) of tumor specimens (132).
3.2 Main effector B cells within TLSs
3.2.1 GC B cells
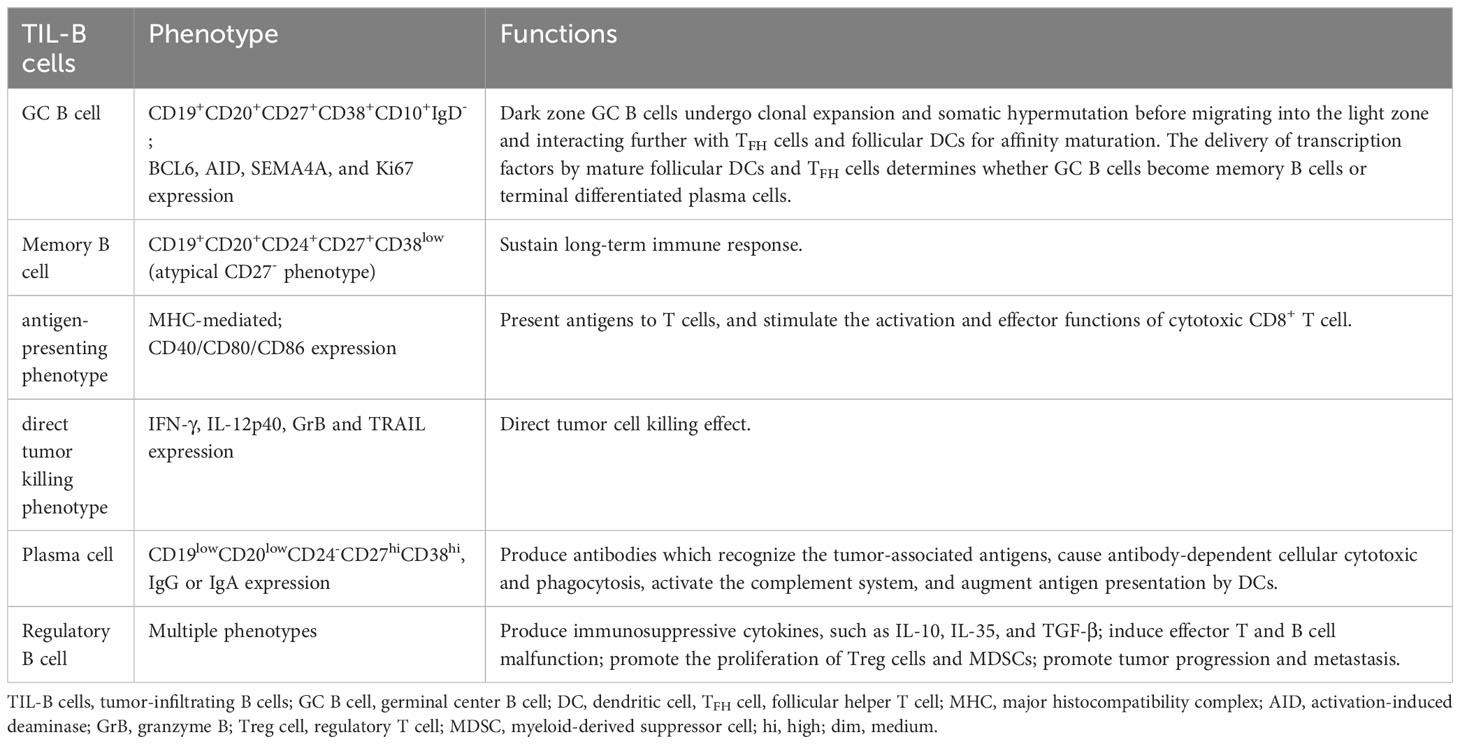
Increased numbers of TILs and TIL-B cells have long been noticed in the TME of HPV+ as compared to HPV- HNSCC (23, 133). However, instead of uncovering a T-cell-driven TIL signature that separates HPV+ from HPV- HNSCC, adjusting the number of TILs revealed a distinct set of B-cell associated genes which are highly expressed in HPV+ HNSCC, including BCL2, ADAM28, CD200, ICOSLG and SPIB (133). Among those, ICOSLG and SPIB are linked to activated B-cells within GCs (134, 135).
GC is the foundation of a matured lymphoid structure (136). It is the domain for B cells to undergo clonal expansion and receptor diversification, producing affinity-matured antibody-secreting plasma cells that effectively recognize the cognate antigens or memory B cells which sustain a durable humoral immunity. In SLO neogenesis, a GC is composed of a dark zone, where B cells undergo clonal expansion and somatic hypermutation (SHM), and a light zone, where B cells further interact with TFH cells for activation and affinity maturation (137). It was recently found that tumor-infiltrating GC B cells in HPV+ HNSCC have distinct waves of gene expression consistent with the dark zone, the light zone, and the transitional state, similar to SLOs (2). Moreover, comparing TLS+ and TLS- tumor tissues elucidated a B cell-related gene signature ranging from naïve B cells to terminally differentiated plasma cells, which further supports the concept of in situ B cell maturation within the TLSs (138).
GC B cells also express several important genes. For example, SEMA4A, a membrane-bound glycoprotein for T cell co-stimulation, was found in GC B cells of HNSCC patients (2, 139). The interactive ability of SMEA4a with endothelial and T cells facilitates TLS generation from unmatured immune aggregates (140, 141). Besides, AID, a critical enzyme for SHM and class-switch recombination of immunoglobulin genes, and BCL-6, the transcription factor involved in the late stage of B cell maturation, are also detectable in GC B cells and may be indicators of GC maturation (2, 136, 142).
The cellular compositions of a matured TLS are a core of lymphatic cells, follicular DCs, and fibroblasts, with surrounded neo-vessels for nutrition supply, and non-hematopoietic stromal cells to support the structure (143). T cells with effector memory phenotypes, CD4+ TFH cells, B cells with antigen-presenting function, antibody-producing plasma cells, and memory B cells are all generated within TLSs, and play a significant role in anti-tumor immunity (Figure 3).
3.2.2 Plasma cells
Plasma cells are one of the most important anti-tumor effector B cells, due to their ability to release antibodies against TAAs. Tumor-infiltrating plasma cells either aggregate in the interspace of TLSs, or disperse in the tumor stroma, forming cellular clusters (116, 136, 144, 145). Recent research by Meylan et al. demonstrates that, following terminal maturation within TLSs, IgG+ and IgA+ plasma cells could be distributed into the tumor milieu by a network of CXCL12+ fibroblasts (138).
The presence of plasma cells both positively correlates and reinforces the cytotoxic effect of CTLs (145). CXCL13-producing PD-1hiCD8+ T cells and bystander CD8+ T cells in close proximity to the CD20+ B cell zones of TLSs can detect a broad spectrum of cancer-irrelevant epitopes (146, 147). HPV, Epstein-Barr virus (EBV), human cytomegalovirus, and influenza virus are among the epitopes identified by bystander CD8+ T cells. Increased cytolytic activity in tumor patients with early virus infection suggests that bystander CD8+ T cells may be produced shortly after the B cell-dominated humoral immune response against viral antigens (148). In nearly all HPV+ HNSCC patients, antibodies against viral antigens were detected by serological analysis employing patient serum (149). For patients with high-grade cervical intraepithelial neoplasia, therapeutic vaccination against the E6 and E7 proteins of HPV-16 and HPV-18 promoted TLS neogenesis beneath the neoplasm (150). Clonally expanded T cells were discovered within lesions, which were possibly educated in TLSs. When TIL-B cells or plasma cells co-existed with T cells, the prognostic relevance was substantially higher, according to a meta-analysis of several forms of human cancer (151).
In contrast, it appears that the humoral immune responses in HPV- HNSCC are more diverse (152, 153). Lechner et al. discovered that antibody responses to TAAs were more frequently observed in HNSCC patients with advanced stages (UICC stage III/IV) or the HPV- status (154). Interestingly, only TAA-specific IgG1 antibodies produced by plasma cells can engage in complement activation, ADCC and ADCP (138, 155, 156), and improve the antigen presentation ability of DCs (157).
Non-tumor-specific, antigen-free IgG3 antibodies bind with high affinity to Fcγ receptors, thereby occupying the binding domains of TAMs and NK cells, consequently hindering the interaction with tumor cells as well as ADCC and antibody-mediated phagocytosis (158). In contrast, antigen-free IgG1 antibodies do not occupy the Fcγ receptors on TAMs and NK cells, allowing them to attach to tumor-associated IgG1 or IgG3. The allosteric increase of antigen-bound IgG1 also improve ADCC and phagocytosis (159, 160). Notably, in a pan-cancer study of TCGA database, the IgG3-1 switch is favorably associated with prognosis in patients with a high SHM rate, highlighting the significance of SHM in tumor immunology (161).
IgA is another antibody isotype within the TME, despite the non-mucosal nature of most cancers (65, 138). A high IgA level is related with an immunosuppressive milieu (65, 73, 103, 162). After encountering TGF-β-secreting Treg cells, plasma cells undergo class switch and produce IgA (103, 162). The IgA-producing plasma cells can also co-express IL-10 and PD-L1, which suppress CTL responses, induce effector T cell exhaustion, and accelerate tumor development (65, 73). Intriguingly, a protective humoral response of polyclonal IgA was revealed in ovarian cancer, since it binds to tumor-expressed polymeric IgA receptors (163).
Also identified intratumorally were IgM, IgE, and IgD deposits. IgM and IgD immunoglobulins are poorly expressed in the TME (164), and they are frequently associated with Breg cells (61, 63, 74). A high proportion of IgE or IgD is related with a poor prognosis in melanoma, but not in patients with other types of cancer (63, 136, 165).
3.2.3 Memory B cells
Memory B cells are capable of memorizing antigens and sustaining a lasting immune response. Generated from naive B cells, they constitute the majority of TIL-B cells. In the TME, atypical memory B cells with either antigen-presenting or direct tumor-killing phenotypes have been identified (12–16, 142, 151, 166, 167).
B cells could demonstrate adaptive antigen-presenting phenotypes, functioning effectively as antigen-presenting cells (APCs). Their interactions with T cells are particularly noteworthy, as they proficiently convey antigens and facilitate optimal T cell function (12–16, 122, 167). The competence of B cells as professional APCs is underpinned by their expression of peptide-loaded MHC molecules, costimulatory signals, and cytokine secretion (168). B cells can process and present antigenic peptides via both MHC class II to CD4+ T cells (8), and cross-presentation of peptide-MHC I complexes to CD8+ T cells (10, 11).
Exploring physiological contexts, marginal zone B cells characterized by B220+CD93+CD21+CD23-IgM+ expression have been observed to acquire dendritic cell functions through trogocytosis (169). This allows them to exhibit pMHC II-C3dg complexes on their cell membranes, thereby facilitating effective antigen presentation to T cells. Similarly, investigations into rheumatoid arthritis have revealed a CD21lowCD86+ memory-like (IgD-CD27+) B cell subset with elevated expression of MHC class I and II, suggestive of potent APC capabilities (170). Interestingly, this same B cell phenotype has been identified in TLSs across diverse tumor types, including HNSCC, emphasizing their potential role in antigen presentation to T cells within cancer (167). Additional studies of TIL-B cells have consistently demonstrated elevated levels of MHC class I and II expression, as well as the presence of key costimulatory molecules like CD40, CD80, CD86, and ICOSL, further supporting their enrichment within TLSs (12–16).
The functional prowess of TIL-B cells in inciting T cell effector responses is exemplified in ovarian and liver cancer, where atypical CD20+CD27-IgG+ memory B cells colocalizing with CD8+ T cells had the ability to present antigens, and the coexistence of both cells benefited prognosis (142, 151). Patients with lung cancer who had an abundance of intra-TLS B cells had an elevated CD4+ TCR clonality and a heightened T cell-dependent B cell response (171). Also in lung cancer, isolated TIL-B cells were found to induce CD4+ T cell expansion in response to tumor lysate or cancer-testis antigen (172). Intriguingly, CD4+ T cells exposed to activated (CD69+HLA-DR+CD27+CD21+) versus exhausted (CD69+HLA-DR+CD27-CD21-) TIL-B cells displayed a skew towards Th1 versus Treg phenotype. Another study with the HPV+ OPSCC demonstrated that, the interactions between CD20+ TIL-B cells and CD8+ T cells positively correlated with the abundance of HPV-specific CD8+ clones, suggesting potential roles of TIL-B cells in supporting CD8+ T cell responses (101).
Notably, naïve T cells are mainly situated within lymphoid aggregates like TLSs, compared to the tumor stromal. The study by de Chaisemartin et al. quantified that, TLS T cell zones contained 66% memory cells and 34% naïve T cells, whereas naïve T cells were rarely seen in the other areas of tumor . Engelhard et al. summarizes the necessary prerequisites for the recruitment of naïve T cells in the tumor bed (173, 174). Naïve T cells were previously not considered as present in the TME, as they express L-selectin and CCR7, which are attracted to lymphoid structures but not peripheral tissues. The successfully recruitment of naïve T cells in engineered or unmanipulated tumors are highly dependent on the tumor-associated blood vessels that express PNAd and CCL21 (175–177). The latter are expressed specifically on HEVs, which is an important vascular structure that support TLS formation. Moreover, compared to TLSlow tumors, TLShi cancers overexpress genes involved in T cell activation, chemotaxis, cytotoxicity, and Th1 cell skewing (119, 178–180), which further supports the hypothesis that TLSs are the initial site for T and B cells interaction and maturation.
In addition to the ability to deliver antigens, memory B cells with direct tumor-killing capabilities have been found in TLSs. Memory B cells in hepatocellular carcinoma (HCC) expressed large quantities of tumor-killing cytokines, including IFN-γ, IL-12p40, GrB, and TRAIL (166). The characteristics and roles of the predominant TIL-B cell groups observed in the tumor milieu are summarized in Table 3.
3.3 The relation of TLSs with clinicopathological features
The presence of TLS is associated with a higher tumor grade, clinical stage, and TILs infiltration (181–185). However, the percentage of HEV is higher in T1/T2 stage HNSCC patients (128, 129). At metastatic sites, the density of TLSs remains positively related to the number of TILs within tumor beds. The characteristics of effector T and B cells in metastatic cancers closely resemble those in primary tumors, encompassing traits such as effector T cell infiltration, CTL skewing, effector B cell clonal expansion, rearrangement of immunoglobulin genes, SHM, and isotype switching (164, 186–191).
A favorable impact of HEV/TLS density on survival was observed in several studies of HNSCC patients (127, 129, 132, 133, 192). HNSCC patients with enriched intratumoral HEV/TLS in the primary site had better overall survival and disease-free survival (129, 132). In patients with early-stage HNSCC, CD20+ TIL is a good prognostic factor (193). When analyzing the metastatic LNs of HNSCC patients, a high frequency of TIL-B cells was also associated with an improved disease-free survival (194).
However, few discordant results appeared in studies of other cancer entities. In HCC, TLSs located in the tumor-adjacent inflamed area were related with tumor progression (195, 196). In HER2- breast tumors, TLSs were related with lymphatic invasion, higher pathological nodal stage, and nodal involvement (182). Given that TLSs are locations where immune responses are initiated, yet not fully realized, they might not indicate the most reliable prognostic or predictive values. Alternatively, lympho-myeloid aggregates, such as plasma cell zones, have recently been proposed, calling for precise definitions and objective measurements in the near future (197). Meanwhile, in the research conducted by Noel et al, an active TLS associated with favorable prognosis is characterized by an increased proportion of functional Th1-oriented PD-1hiICOSint TFH TIL and a higher effector versus regulatory TIL ratio (198). Meanwhile, the CD25+CXCR5+GARP+FoxP3+ follicular regulatory T (TFR) cells prohibits the TFH TIL from faciliting the antibody production capacity of plasma cells, and a favorable functional TFH over TFR ratio would generate a Th1 microenvironment that governs active TLS maturation. Given the potential for certain TLSs to be halted in their developmental process due to immunosuppressive elements within the TME (199), and the likelihood of others regressing once their initiating antigens are eliminated (200), there is a pressing need to delve into a more all-encompassing understanding of the stimuli and mechanisms governing TLS formation and maturation.
In conclusion, TIL-B cells within TLSs are expected to be shielded from environmental challenges due to their unique spatial configuration. They interact directly with effector TILs, allowing them to mature into immunocompetent cells with anti-tumor effects. In an unmatured TLS, GC B cells first appear. Following clonal expansion and receptor diversification, memory B cells with different functional capacities, and matured plasma cells are present. These effector B cells efficiently produce antibodies, recognize TAAs, communicate with effector T cells, and maintain the anti-tumor immune response. Even if the link between TLSs and clinicopathologic characteristics of patients is still debatable, one cannot overlook the beneficial mechanisms of TLSs. Future analyses may also consider the location and proximity of TLSs, GC maturation, compositional zone liveness, and antibody isotypes produced by local plasma cells.
4 Targeting TIL-B cells for immunotherapy
Immunotherapies for cancer are currently predominated based on ICB treatment, which focuses mainly on reserving CTL effector functions. However, only a small subset of HNSCC patients may benefit from this approach, with only 20% of patients exhibiting an initial response to the PD-1 therapy (201–203). Moreover, some patients eventually develop acquired resistance to this therapy.
Given the limitations of single-agent ICB therapy, exploring TIL-B cells as possible targets for novel immunotherapy paradigms could be a valuable complement. The cellular and humoral immune resistance of cancer always work in tandem. Remarkably, individuals diagnosed with HNSCC who demonstrate either previous HPV infection (resulting in viral antigens) or a high tumor mutation burden (leading to increased TAAs) have shown enhanced responsiveness to ICB therapies (204, 205). Additionally, it has been observed that PD-L1, the ligand commonly expressed on APCs and tumor cells, is expressed in certain Breg cells, further highlighting the potential role of TIL-B cells in modulating the immune response in cancer.
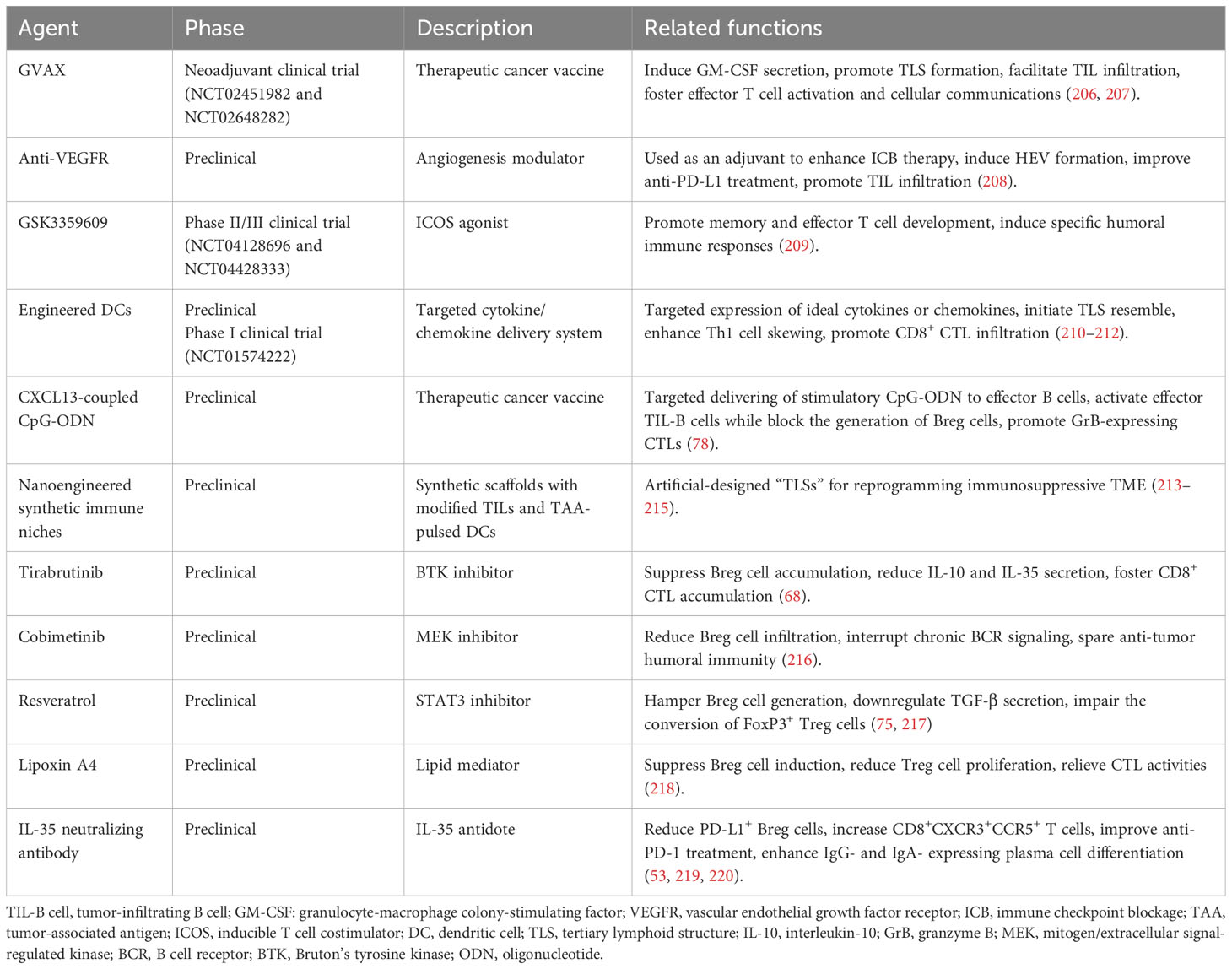
Despite the limited availability of B-cell-specific treatments for solid cancers, numerous immunotherapeutic agents have been shown to affect TIL-B cells and TLSs formation. In this section, we discuss the immunotherapeutic approaches surrounding TIL-B cells that either promote TLS neogenesis and functions or reduce the immunosuppressive potential of Breg cells. The immunotherapies mentioned in this section are summarized in Table 4.
4.1 Foster TLS formation
High amounts of TIL-B cells and TLSs are associated with better responses to ICB therapies (14–16, 148, 221, 222). In HPV+ HNSCC, patients who reacted to radiotherapy in conjunction with PD-1 antagonist showed increased GC formation, effector B cell generation, and enhanced IgG and IgM antibody responses (221). For non-small cell lung cancer patients, who responded to a neoadjuvant PD-1 antagonist, significant enrichment of TLSs was found in their tumor microdissections (223). The presence of pre-treatment PD-1hiCD8+ T cells within TLSs was also a predictor of anti-PD-1 response (147).
Several therapeutic cancer vaccines have demonstrated their efficacy. GVAX is a cancer vaccine that was genetically modified to induce the granulocyte-macrophage colony-stimulating factor (GM-CSF). Extensive TLS formation in patients with pancreatic cancer was found after GVAX treatment, and was associated with a good prognosis (NCT00727441) (206, 207). Subsequent gene expression analysis identified pathways regulating immune cell activation and communication. Treg cell suppression, Th17 cell activation, and elevated effector T cells to Tregs ratio were also noticed. Additionally, unmethylated cytosine-phosphate-guanine oligodeoxynucleotide (CpG ODN) is a type of tumor nanovaccines which utilized CpG nanoparticles as adjuvants (224). The water-soluable CpG can enter B cells and plasmacytoid DCs, triggering strong innate and adaptive immune response. When a CXCL13-coupled CpG ODN was applied in mice with 4T1 breast cancer metastasis, it successfully stimulated effector TIL-B cells via the CXCL13-CXC5R interaction, promoted GrB-expressing CTLs, without stimulating the CD20low Breg cells (78).
Besides, several combination therapies of ICBs and targeted agents have received satisfied results. ICBs and vascular targeting peptide combination therapy increased both the de novo formation of TLSs, and the effector T cell activation (225). A combined therapy of anti-VEGFR2 and anti-PD-L1, induced HEV formation, promoted cytolysis, and transformed the immunosuppressive tumor into an immune-active phenotype (208). Those findings point out that the pharmacological approaches to foster TLSs might serve as good supplements for ICB therapies, as they are likely to subvert the immune-resistant tumors to immunogenic types.
Studies have investigated the possible strategies to combine T cell-reinvigorated immunotherapies with a great harvest of TLSs. The inducible T cell costimulator (ICOS) is a co-stimulatory factor expressed on activated T cells, which plays an important role in cell-cell signaling, CTL formation, and Treg cell activation (209). Its ligand ICOSL is highly expressed in HPV+ HNSCC and is associated with activated TIL-B cells within GC (133). The effect of ICOS-agonist GSK3359609 in combination with anti-PD-1 pembrolizumab has been tested on HNSCC patients in the INDUCE-1 study. Anti-PD-1/PD-L1-naïve HNSCC patients who received the combined therapy (n = 34) had a significantly higher overall response rate and disease control rate compared to patients received GSK3359609 alone (n = 17) (226). Now the effect of GSK3359609 has been continuously studied in two phase II/III clinical trials (INDUCE-3 trial, NCT04128696 and INDUCE-4 trial, NCT04428333), comparing GSK3359609 plus pembrolizumab vs placebo plus pembrolizumab with and without the combination of 5-fluorouracil (5-FU)-platinum chemotherapy.
Additionally, the exploration of cell-based cytokine/chemokine delivery systems has provided valuable insights. Since endothelial cells express IL-36γ to sustain follicular B cell functions (227), engineered DCs were programmed to express both IL-36γ and the T cell-specific T box transcription factor (T-bet) and then delivered into a sarcoma mouse model (210, 211). This IL-36γ-dependent T-bet therapy enhanced both Th1 cell skewing and TLS neogenesis. Another compelling evidence comes from a phase I clinical trial (NCT01574222), where patients with advanced lung cancer received injections of CCL21-expressing engineered DCs. Evidently, this intervention triggered systemic anti-tumor responses, leading to heightened infiltration of CD8+ TILs (212).
Emerging technologies such as synthetic scaffolds have been developed with the purpose of cultivating modified LNs-derived cell lines along with tumor antigen-pulsed DCs in biocompatible scaffold materials (213, 214). This nanoengineered synthetic immune niches have the potential to function as a versatile platform for immune-reprogramming (215). Introducing a stromal cell line derived from LN-induced TLSs has demonstrated the enhancement of the anti-tumor immune response, leading to increased TILs in a mouse model of colon cancer (228).The previously mentioned 12-chemokine GES can be harnessed for the construction of ectopic designer TLSs (103, 123–125, 229).
The treatment methods related to the development of TLSs still have broad prospects. For example, methods could be explored to facilitate GC maturation, foster the entry of matured plasma cells into tumor tissue, and the secretion of tumor-associated antigens. Since SEMA4A is a marker for both early-stage and functional TLSs, promoting SEMA4A expression on TIL-B cells might serve as a potential therapeutic option (2, 140, 141). On the other hand, diet, CD20 antagonist rituximab, chemotherapy, and corticosteroids can eradicate the development of GCs, decrease TLSs density, and might impair the positive therapeutic impact (126, 230, 231).
4.2 Hamper Breg cells
Breg cells have been used as novel targets in cancer treatment because of their immunosuppressive and tumor-promoting activities. The presence of PD-L1 on some Breg cells also confirms their participation in ICB treatments (62, 63).
B-cell depletion therapies lacking more precise targeting, such as the CD20 antagonist rituximab, are scarcely applicable in solid cancers. It can enhance cancer progression and metastasis by evoking CD20low Breg cells (78, 232). Conversely, a more promising approach for Breg depletion therapy involves exploring alternative Breg-specific markers. For example, CD200 is a type I membrane-associated glycoprotein related to an immunoregulatory signaling pathway, which is detectable across multiple haemalogic malignancies and solid cancers (233). In HPV+ HNSCC patients, CD200+ expressing Breg cells were identified (133). However, Samalizumab, the anti-CD200 monoclonal antibody, was tested in haemalogic malignancies including B-cell chronic lymphocytic leukemia and multiple myeloma (NCT00648739), and solid cancers (NCT02987504) without satisfied outcome. Multiple adverse outcomes were reported in patients, including skin rashes, joint stiffness/pain, headaches, and blood disorders (234). Since CD200 is also widely expressed in normal cells of both haematopoietic and non-haematopoietic origin, one could speculate the potential toxicities (235). Therefore, opting for Breg cell depletion therapy must be used with caution.
In lieu of depleting Breg cells, inhibitors of MEK, BTK, and STAT3 have been reported to hinder Breg formation and promote anti-tumor immunity across various mouse tumor models. Tirabrutinib, a small molecule BTK inhibitor, effectively inhibits aberrant BCR signaling in B cell-related cancers. In PanIN-bearing mice, tirabrutinib was found to suppress CD5+CD1dhi Breg cells accumulation, reduce IL-10 and IL-35 secretion, increase CD8+IFN-γ+ CTLs, and attenuate PanIN growth (68). Mitogen-activated protein kinase (MAPK) kinase (MEK) inhibitor is a targeted therapeutic agent for tumors with BRAF or KRAS oncogene mutations (236, 237). Cobimetinib, a MEK inhibitor, decreases the number of T2-MZP Bregs, B10 Bregs, and TIM1+ Bregs in tumor-draining LNs of mice with colorectal cancer by interrupting chronic BCR signaling, while sparing the anti-tumor humoral immunity of functional B cells (216). While in 4T1 lung-metastatic breast adenocarcinoma, resveratrol, a phytoalexin and antioxidants, hampers Breg cell generation, downregulates TGF-β secretion by inactivating STAT3, and concurrently impairs the Breg cell-induced conversion of FoxP3+ Treg cells (75, 217). Nevertheless, cautious administration is required to mitigate potential non-targeted and wide-spread dysfunction of TIL-B cells.
Potential therapies for the prevention or conversion of Breg phenotypes are also available. In multiple murine cancer models, Lipoxin A4, a metabolite of arachidonic acid with anti-inflammatory characteristics, was found to selectively suppresses B10 Breg induction (218). This is accompanied with reduced Treg cells in the tumor tissues and draining LNs, while the proliferation, differentiation, and GC formation roles of effector B cells are reserved. Moreover, in mice with pancreatic cancer, neutralizing IL-35 reduces the frequency of PD-L1+ Breg cells, stimulates CD8+CXCR3+CCR5+ T cell production, and overcomes resistance to anti-PD-1 immunotherapy (53, 219). Also in pancreatic cancer, the B cell-specific deletion of IL-35 has been linked to enhanced plasma cell differentiation and the production of anti-tumor IgG and IgM antibodies (220).
Taken together, these data suggest that promoting TIL infiltration, activation, and differentiation, or promoting TLS neogenesis and function or manipulating Breg cells to inhibit cancer progression holds promise for immunotherapy of solid tumors, including HNSCC. On top of that, more studies are urgently needed for a comprehensive understanding of the factors that drive the assembly of TLSs, and the phenotypical and functional differences of Breg cells, in order to develop specifically-designed TIL-B cells targeting immunotherapeutic approaches.
5 Discussion
Largely overlooked in the past but now increasingly in the focus of recent research, TIL-B cells are gaining traction as key cellular players in the TME that can elicit either pro- or anti-tumor effects.
Scattered TIL-B cells in the TME are more likely to obtain a regulatory effect that inhibits the activation and function of effector T cells, hence dominating an immunosuppressive role. However, when TIL-B cells are spatially organized in an immune-privileged site, also known as TLSs, they develop anti-tumor capabilities due to their exceptional intercellular contacts with T cells and other effector TILs. TLS-matured effector B cells can either produce tumor-specific antibodies, thereby aiding in tumor recognition, ADCC, ADCP, and activation of the complement cascade, or deliver TAAs or viral antigens to CTLs and maintain long-term immunological memory.
As novel technologies continue to emerge, such as single-cell RNA-sequencing, spatial transcriptomics, and multiplex imaging, a greater understanding of the diverse subtypes of TIL-B cells, including their BCR clonality, spatial distribution, and cellular interactions, will be established. By expanding our knowledge of the fascinating role of TIL-B cells in the HNSCC tumor microenvironment, tailored therapeutic strategies can be designed for personalized clinical applications. Ultimately, the potential for manipulating TIL-B cells to enhance anti-tumor immune responses provides exciting opportunities for the future of cancer immunotherapy.
Author contributions
JB wrote the manuscript. CB, JH and AB reviewed and edited the manuscript. All authors contributed to the article and approved the submitted version.
Funding
Doctoral training of JB was supported by the China Scholarship Council (No.202106090002).
Acknowledgments
The authors thank all the reviewers who reviewed the manuscripts and provided precious comments to improve the integrity and clarity of the content, also to BioRender.com for producing very helpful and useful graphical illustrations.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Glossary
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J Clin (2021) 71:209–49. doi: 10.3322/caac.21660
2. Ruffin AT, Cillo AR, Tabib T, Liu A, Onkar S, Kunning SR, et al. B cell signatures and tertiary lymphoid structures contribute to outcome in head and neck squamous cell carcinoma. Nat Commun (2021) 12:3349. doi: 10.1038/s41467-021-23355-x
3. Lechner M, Liu J, Masterson L, Fenton TR. HPV-associated oropharyngeal cancer: epidemiology, molecular biology and clinical management. Nat Rev Clin Oncol (2022) 19:306–27. doi: 10.1038/s41571-022-00603-7
4. Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tân PF, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med (2010) 363:24–35. doi: 10.1056/NEJMoa0912217
5. Fakhry C, Zhang Q, Gillison ML, Nguyen-Tân PF, Rosenthal DI, Weber RS, et al. Validation of NRG oncology/RTOG-0129 risk groups for HPV-positive and HPV-negative oropharyngeal squamous cell cancer: Implications for risk-based therapeutic intensity trials. Cancer (2019) 125:2027–38. doi: 10.1002/cncr.32025
6. Yom SS, Torres-Saavedra P, Caudell JJ, Waldron JN, Gillison ML, Xia P, et al. Reduced-dose radiation therapy for HPV-associated oropharyngeal carcinoma (NRG oncology HN002). JCO (2021) 39:956–65. doi: 10.1200/JCO.20.03128
7. Roe K. NK-cell exhaustion, B-cell exhaustion and T-cell exhaustion—the differences and similarities. Immunology (2022) 166:155–68. doi: 10.1111/imm.13464
8. Hong S, Zhang Z, Liu H, Tian M, Zhu X, Zhang Z, et al. B cells are the dominant antigen-presenting cells that activate naive CD4+ T cells upon immunization with a virus-derived nanoparticle antigen. Immunity (2018) 49:695–708.e4. doi: 10.1016/j.immuni.2018.08.012
9. Heit A, Huster KM, Schmitz F, Schiemann M, Busch DH, Wagner H. CpG-DNA aided cross-priming by cross-presenting B cells. J Immunol (2004) 172:1501–7. doi: 10.4049/jimmunol.172.3.1501
10. Hon H, Oran A, Brocker T, Jacob J. B lymphocytes participate in cross-presentation of antigen following gene gun vaccination. J Immunol (2005) 174:5233–42. doi: 10.4049/jimmunol.174.9.5233
11. Mariño E, Tan B, Binge L, Mackay CR, Grey ST. B-cell cross-presentation of autologous antigen precipitates diabetes. Diabetes (2012) 61:2893–905. doi: 10.2337/db12-0006
12. Schlößer HA, Thelen M, Lechner A, Wennhold K, Garcia-Marquez MA, Rothschild SI, et al. B cells in esophago-gastric adenocarcinoma are highly differentiated, organize in tertiary lymphoid structures and produce tumor-specific antibodies. OncoImmunology (2019) 8:e1512458. doi: 10.1080/2162402X.2018.1512458
13. Yamakoshi Y, Tanaka H, Sakimura C, Deguchi S, Mori T, Tamura T, et al. Immunological potential of tertiary lymphoid structures surrounding the primary tumor in gastric cancer. Int J Oncol (2020) 57:171–82. doi: 10.3892/ijo.2020.5042
14. Petitprez F, de Reyniès A, Keung EZ, Chen TW-W, Sun C-M, Calderaro J, et al. B cells are associated with survival and immunotherapy response in sarcoma. Nature (2020) 577:556–60. doi: 10.1038/s41586-019-1906-8
15. Helmink BA, Reddy SM, Gao J, Zhang S, Basar R, Thakur R, et al. B cells and tertiary lymphoid structures promote immunotherapy response. Nature (2020) 577:549–55. doi: 10.1038/s41586-019-1922-8
16. Cabrita R, Lauss M, Sanna A, Donia M, Skaarup Larsen M, Mitra S, et al. Tertiary lymphoid structures improve immunotherapy and survival in melanoma. Nature (2020) 577:561–5. doi: 10.1038/s41586-019-1914-8
17. Laumont CM, Nelson BH. B cells in the tumor microenvironment: Multi-faceted organizers, regulators, and effectors of anti-tumor immunity. Cancer Cell (2023) 41:466–89. doi: 10.1016/j.ccell.2023.02.017
18. Paijens ST, Vledder A, de Bruyn M, Nijman HW. Tumor-infiltrating lymphocytes in the immunotherapy era. Cell Mol Immunol (2021) 18:842–59. doi: 10.1038/s41423-020-00565-9
19. Jansen K, Cevhertas L, Ma S, Satitsuksanoa P, Akdis M, van de Veen W. Regulatory B cells, A to Z. Allergy (2021) 76:2699–715. doi: 10.1111/all.14763
20. Michaud D, Steward CR, Mirlekar B, Pylayeva-Gupta Y. Regulatory B cells in cancer. Immunol Rev (2021) 299:74–92. doi: 10.1111/imr.12939
21. Zhou X, Su Y-X, Lao X-M, Liang Y-J, Liao G-Q. CD19+IL-10+ regulatory B cells affect survival of tongue squamous cell carcinoma patients and induce resting CD4+ T cells to CD4+Foxp3+ regulatory T cells. Oral Oncol (2016) 53:27–35. doi: 10.1016/j.oraloncology.2015.11.003
22. Jeske SS, Brand M, Ziebart A, Laban S, Doescher J, Greve J, et al. Adenosine-producing regulatory B cells in head and neck cancer. Cancer Immunol Immunother (2020) 69:1205–16. doi: 10.1007/s00262-020-02535-6
23. Lechner A, Schlößer HA, Thelen M, Wennhold K, Rothschild SI, Gilles R, et al. Tumor-associated B cells and humoral immune response in head and neck squamous cell carcinoma. OncoImmunology (2019) 8:1535293. doi: 10.1080/2162402X.2018.1535293
24. Catalán D, Mansilla MA, Ferrier A, Soto L, Oleinika K, Aguillón JC, et al. Immunosuppressive mechanisms of regulatory B cells. Front Immunol (2021) 12:611795. doi: 10.3389/fimmu.2021.611795
25. Evans JG, Chavez-Rueda KA, Eddaoudi A, Meyer-Bahlburg A, Rawlings DJ, Ehrenstein MR, et al. Novel suppressive function of transitional 2 B cells in experimental arthritis. J Immunol (2007) 178:7868–78. doi: 10.4049/jimmunol.178.12.7868
26. Iwata Y, Matsushita T, Horikawa M, DiLillo DJ, Yanaba K, Venturi GM, et al. Characterization of a rare IL-10–competent B-cell subset in humans that parallels mouse regulatory B10 cells. Blood (2011) 117:530–41. doi: 10.1182/blood-2010-07-294249
27. Oleinika K, Mauri C, Salama AD. Effector and regulatory B cells in immune-mediated kidney disease. Nat Rev Nephrol (2019) 15:11–26. doi: 10.1038/s41581-018-0074-7
28. Hollern DP, Xu N, Thennavan A, Glodowski C, Garcia-Recio S, Mott KR, et al. B cells and T follicular helper cells mediate response to checkpoint inhibitors in high mutation burden mouse models of breast cancer. Cell (2019) 179:1191–1206.e21. doi: 10.1016/j.cell.2019.10.028
29. Hu Q, Hong Y, Qi P, Lu G, Mai X, Xu S, et al. Atlas of breast cancer infiltrated B-lymphocytes revealed by paired single-cell RNA-sequencing and antigen receptor profiling. Nat Commun (2021) 12:2186. doi: 10.1038/s41467-021-22300-2
30. Dubois F, Limou S, Chesneau M, Degauque N, Brouard S, Danger R. Transcriptional meta-analysis of regulatory B cells. Eur J Immunol (2020) 50:1757–69. doi: 10.1002/eji.201948489
31. Wejksza K, Lee-Chang C, Bodogai M, Bonzo J, Gonzalez FJ, Lehrmann E, et al. Cancer-produced metabolites of 5-lipoxygenase induce tumor-evoked regulatory B cells via peroxisome proliferator–activated receptor α. JI (2013) 190:2575–84. doi: 10.4049/jimmunol.1201920
32. Caro-Maldonado A, Wang R, Nichols AG, Kuraoka M, Milasta S, Sun LD, et al. Metabolic reprogramming is required for antibody production that is suppressed in anergic but exaggerated in chronically BAFF-exposed B cells. JI (2014) 192:3626–36. doi: 10.4049/jimmunol.1302062
33. Shin DH, Lin H, Zheng H, Kim KS, Kim JY, Chun YS, et al. HIF-1α–mediated upregulation of TASK-2 K + Channels augments ca 2+ Signaling in mouse B cells under hypoxia. JI (2014) 193:4924–33. doi: 10.4049/jimmunol.1301829
34. Meng X, Grötsch B, Luo Y, Knaup KX, Wiesener MS, Chen X-X, et al. Hypoxia-inducible factor-1α is a critical transcription factor for IL-10-producing B cells in autoimmune disease. Nat Commun (2018) 9:251. doi: 10.1038/s41467-017-02683-x
35. Pimenta EM, De S, Weiss R, Feng D, Hall K, Kilic S, et al. IRF5 is a novel regulator of CXCL13 expression in breast cancer that regulates CXCR5 + B- and T-cell trafficking to tumor-conditioned media. Immunol Cell Biol (2015) 93:486–99. doi: 10.1038/icb.2014.110
36. Ricciardi M, Zanotto M, Malpeli G, Bassi G, Perbellini O, Chilosi M, et al. Epithelial-to-mesenchymal transition (EMT) induced by inflammatory priming elicits mesenchymal stromal cell-like immune-modulatory properties in cancer cells. Br J Cancer (2015) 112:1067–75. doi: 10.1038/bjc.2015.29
37. Somasundaram R, Zhang G, Fukunaga-Kalabis M, Perego M, Krepler C, Xu X, et al. Tumor-associated B-cells induce tumor heterogeneity and therapy resistance. Nat Commun (2017) 8:607. doi: 10.1038/s41467-017-00452-4
38. Ye L, Zhang Q, Cheng Y, Chen X, Wang G, Shi M, et al. Tumor-derived exosomal HMGB1 fosters hepatocellular carcinoma immune evasion by promoting TIM-1+ regulatory B cell expansion. J Immunother Cancer (2018) 6:145. doi: 10.1186/s40425-018-0451-6
39. Dambuza IM, He C, Choi JK, Yu C-R, Wang R, Mattapallil MJ, et al. IL-12p35 induces expansion of IL-10 and IL-35-expressing regulatory B cells and ameliorates autoimmune disease. Nat Commun (2017) 8:719. doi: 10.1038/s41467-017-00838-4
40. Yoshizaki A, Miyagaki T, DiLillo DJ, Matsushita T, Horikawa M, Kountikov EI, et al. Regulatory B cells control T-cell autoimmunity through IL-21-dependent cognate interactions. Nature (2012) 491:264–8. doi: 10.1038/nature11501
41. Rosser EC, Oleinika K, Tonon S, Doyle R, Bosma A, Carter NA, et al. Regulatory B cells are induced by gut microbiota–driven interleukin-1β and interleukin-6 production. Nat Med (2014) 20:1334–9. doi: 10.1038/nm.3680
42. Wang R-X, Yu C-R, Dambuza IM, Mahdi RM, Dolinska MB, Sergeev YV, et al. Interleukin-35 induces regulatory B cells that suppress autoimmune disease. Nat Med (2014) 20:633–41. doi: 10.1038/nm.3554
43. Matsumoto M, Fujii Y, Baba A, Hikida M, Kurosaki T, Baba Y. The calcium sensors STIM1 and STIM2 control B cell regulatory function through interleukin-10 production. Immunity (2011) 34:703–14. doi: 10.1016/j.immuni.2011.03.016
44. Menon M, Blair PA, Isenberg DA, Mauri C. A regulatory feedback between plasmacytoid dendritic cells and regulatory B cells is aberrant in systemic lupus erythematosus. Immunity (2016) 44:683–97. doi: 10.1016/j.immuni.2016.02.012
45. Fillatreau S, Sweenie CH, McGeachy MJ, Gray D, Anderton SM. B cells regulate autoimmunity by provision of IL-10. Nat Immunol (2002) 3:944–50. doi: 10.1038/ni833
46. Ran Z, Yue-Bei L, Qiu-Ming Z, Huan Y. Regulatory B cells and its role in central nervous system inflammatory demyelinating diseases. Front Immunol (2020) 11:1884. doi: 10.3389/fimmu.2020.01884
47. Guan H, Lan Y, Wan Y, Wang Q, Wang C, Xu L, et al. PD-L1 mediated the differentiation of tumor-infiltrating CD19 + B lymphocytes and T cells in Invasive breast cancer. OncoImmunology (2016) 5:e1075112. doi: 10.1080/2162402X.2015.1075112
48. Shao Y, Lo CM, Ling CC, Liu XB, Ng KT-P, Chu ACY, et al. Regulatory B cells accelerate hepatocellular carcinoma progression via CD40/CD154 signaling pathway. Cancer Lett (2014) 355:264–72. doi: 10.1016/j.canlet.2014.09.026
49. Lv Y, Wang H, Liu Z. The role of regulatory B cells in patients with acute myeloid leukemia. Med Sci Monit (2019) 25:3026–31. doi: 10.12659/MSM.915556
50. Norouzian M, Mehdipour F, Balouchi Anaraki S, Ashraf MJ, Khademi B, Ghaderi A. Atypical memory and regulatory B cell subsets in tumor draining lymph nodes of head and neck squamous cell carcinoma correlate with good prognostic factors. Head Neck Pathol (2020) 14:645–56. doi: 10.1007/s12105-019-01095-1
51. Wang W, Yuan X, Chen H, Xie G, Ma Y, Zheng Y, et al. CD19+CD24hiCD38hiBregs involved in downregulate helper T cells and upregulate regulatory T cells in gastric cancer. Oncotarget (2015) 6:33486–99. doi: 10.18632/oncotarget.5588
52. Zhang L, Tai Y-T, Ho M, Xing L, Chauhan D, Gang A, et al. Regulatory B cell-myeloma cell interaction confers immunosuppression and promotes their survival in the bone marrow milieu. Blood Cancer J (2017) 7:e547–7. doi: 10.1038/bcj.2017.24
53. Mirlekar B, Michaud D, Lee SJ, Kren NP, Harris C, Greene K, et al. B cell–derived IL35 drives STAT3-dependent CD8 + T-cell exclusion in pancreatic cancer. Cancer Immunol Res (2020) 8:292–308. doi: 10.1158/2326-6066.CIR-19-0349
54. Mao Y, Wang Y, Dong L, Zhang Q, Wang C, Zhang Y, et al. Circulating exosomes from esophageal squamous cell carcinoma mediate the generation of B10 and PD -1 high Breg cells. Cancer Sci (2019) 110:2700–10. doi: 10.1111/cas.14122
55. Murakami Y, Saito H, Shimizu S, Kono Y, Shishido Y, Miyatani K, et al. Increased regulatory B cells are involved in immune evasion in patients with gastric cancer. Sci Rep (2019) 9:13083. doi: 10.1038/s41598-019-49581-4
56. Hu H-T, Ai X, Lu M, Song Z, Li H. Characterization of intratumoral and circulating IL-10-producing B cells in gastric cancer. Exp Cell Res (2019) 384:111652. doi: 10.1016/j.yexcr.2019.111652
57. Chen Z, Zhu Y, Du R, Pang N, Zhang F, Dong D, et al. Role of regulatory B cells in the progression of cervical cancer. Mediators Inflammation (2019) 2019:1–8. doi: 10.1155/2019/6519427
58. Qian L, Bian G-R, Zhou Y, Wang Y, Hu J, Liu X, et al. Short communication Clinical significance of regulatory B cells in the peripheral blood of patients with oesophageal cancer. Cejoi (2015) 2:263–5. doi: 10.5114/ceji.2015.52840
59. Wei X, Jin Y, Tian Y, Zhang H, Wu J, Lu W, et al. Regulatory B cells contribute to the impaired antitumor immunity in ovarian cancer patients. Tumor Biol (2016) 37:6581–8. doi: 10.1007/s13277-015-4538-0
60. Mirlekar B, Michaud D, Searcy R, Greene K, Pylayeva-Gupta Y. IL35 hinders endogenous antitumor T-cell immunity and responsiveness to immunotherapy in pancreatic cancer. Cancer Immunol Res (2018) 6:1014–24. doi: 10.1158/2326-6066.CIR-17-0710
61. Lindner S, Dahlke K, Sontheimer K, Hagn M, Kaltenmeier C, Barth TFE, et al. Interleukin 21–induced granzyme B–expressing B cells infiltrate tumors and regulate T cells. Cancer Res (2013) 73:2468–79. doi: 10.1158/0008-5472.CAN-12-3450
62. Xiao X, Lao X-M, Chen M-M, Liu R-X, Wei Y, Ouyang F-Z, et al. PD-1hi identifies a novel regulatory B-cell population in human hepatoma that promotes disease progression. Cancer Discovery (2016) 6:546–59. doi: 10.1158/2159-8290.CD-15-1408
63. Wu H, Xia L, Jia D, Zou H, Jin G, Qian W, et al. PD-L1+ regulatory B cells act as a T cell suppressor in a PD-L1-dependent manner in melanoma patients with bone metastasis. Mol Immunol (2020) 119:83–91. doi: 10.1016/j.molimm.2020.01.008
64. Mao H, Pan F, Wu Z, Wang Z, Zhou Y, Zhang P, et al. Colorectal tumors are enriched with regulatory plasmablasts with capacity in suppressing T cell inflammation. Int Immunopharmacol (2017) 49:95–101. doi: 10.1016/j.intimp.2017.05.018
65. Shalapour S, Font-Burgada J, Di Caro G, Zhong Z, Sanchez-Lopez E, Dhar D, et al. Immunosuppressive plasma cells impede T-cell-dependent immunogenic chemotherapy. Nature (2015) 521:94–8. doi: 10.1038/nature14395
66. Schioppa T, Moore R, Thompson RG, Rosser EC, Kulbe H, Nedospasov S, et al. B regulatory cells and the tumor-promoting actions of TNF-α during squamous carcinogenesis. Proc Natl Acad Sci (2011) 108:10662–7. doi: 10.1073/pnas.1100994108
67. Horikawa M, Minard-Colin V, Matsushita T, Tedder TF. Regulatory B cell production of IL-10 inhibits lymphoma depletion during CD20 immunotherapy in mice. J Clin Invest (2011) 121:4268–80. doi: 10.1172/JCI59266
68. Das S, Bar-Sagi D. BTK signaling drives CD1dhiCD5+ regulatory B-cell differentiation to promote pancreatic carcinogenesis. Oncogene (2019) 38:3316–24. doi: 10.1038/s41388-018-0668-3
69. Pylayeva-Gupta Y, Das S, Handler JS, Hajdu CH, Coffre M, Koralov SB, et al. IL35-producing B cells promote the development of pancreatic neoplasia. Cancer Discovery (2016) 6:247–55. doi: 10.1158/2159-8290.CD-15-0843
70. Michaud D, Mirlekar B, Bischoff S, Cowley DO, Vignali DAA, Pylayeva-Gupta Y. Pancreatic cancer-associated inflammation drives dynamic regulation of p35 and Ebi3. Cytokine (2020) 125:154817. doi: 10.1016/j.cyto.2019.154817
71. Shen M, Wang J, Yu W, Zhang C, Liu M, Wang K, et al. A novel MDSC-induced PD-1–PD-L1+ B-cell subset in breast tumor microenvironment possesses immuno-suppressive properties. OncoImmunology (2018) 7:e1413520. doi: 10.1080/2162402X.2017.1413520
72. Liu R, Lu Z, Gu J, Liu J, Huang E, Liu X, et al. MicroRNAs 15A and 16–1 activate signaling pathways that mediate chemotaxis of immune regulatory B cells to colorectal tumors. Gastroenterology (2018) 154:637–651.e7. doi: 10.1053/j.gastro.2017.09.045
73. Shalapour S, Lin X-J, Bastian IN, Brain J, Burt AD, Aksenov AA, et al. Inflammation-induced IgA+ cells dismantle anti-liver cancer immunity. Nature (2017) 551:340–5. doi: 10.1038/nature24302
74. Ganti SN, Albershardt TC, Iritani BM, Ruddell A. Regulatory B cells preferentially accumulate in tumor-draining lymph nodes and promote tumor growth. Sci Rep (2015) 5:12255. doi: 10.1038/srep12255
75. Lee-Chang C, Bodogai M, Martin-Montalvo A, Wejksza K, Sanghvi M, Moaddel R, et al. Inhibition of breast cancer metastasis by resveratrol-mediated inactivation of tumor-evoked regulatory B cells. JI (2013) 191:4141–51. doi: 10.4049/jimmunol.1300606
76. Zhang Y, Morgan R, Chen C, Cai Y, Clark E, Khan WN, et al. Mammary-tumor-educated B cells acquire LAP/TGF-β and PD-L1 expression and suppress anti-tumor immune responses. INTIMM (2016) 28:423–33. doi: 10.1093/intimm/dxw007
77. Olkhanud PB, Damdinsuren B, Bodogai M, Gress RE, Sen R, Wejksza K, et al. Tumor-evoked regulatory B cells promote breast cancer metastasis by converting resting CD4 + T cells to T-regulatory cells. Cancer Res (2011) 71:3505–15. doi: 10.1158/0008-5472.CAN-10-4316
78. Bodogai M, Lee Chang C, Wejksza K, Lai J, Merino M, Wersto RP, et al. Anti-CD20 antibody promotes cancer escape via enrichment of tumor-evoked regulatory B cells expressing low levels of CD20 and CD137L. Cancer Res (2013) 73:2127–38. doi: 10.1158/0008-5472.CAN-12-4184
79. Ammirante M, Kuraishy AI, Shalapour S, Strasner A, Ramirez-Sanchez C, Zhang W, et al. An IKKα–E2F1–BMI1 cascade activated by infiltrating B cells controls prostate regeneration and tumor recurrence. Genes Dev (2013) 27:1435–40. doi: 10.1101/gad.220202.113
80. Ammirante M, Luo J-L, Grivennikov S, Nedospasov S, Karin M. B-cell-derived lymphotoxin promotes castration-resistant prostate cancer. Nature (2010) 464:302–5. doi: 10.1038/nature08782
81. Moulin V, Andris F, Thielemans K, Maliszewski C, Urbain J, Moser M. B lymphocytes regulate dendritic cell (DC) function in vivo: increased interleukin 12 production by DCs from B cell–defficient mice results in T helper cell type 1 deviation. J Exp Med (2000) 192(4):475–82. doi: 10.1084/jem.192.4.475
82. Rosser EC, Mauri C. Regulatory B cells: origin, phenotype, and function. Immunity (2015) 42:607–12. doi: 10.1016/j.immuni.2015.04.005
83. Madan R, Demircik F, Surianarayanan S, Allen JL, Divanovic S, Trompette A, et al. Nonredundant roles for B cell-derived IL-10 in immune counter-regulation. J Immunol (2009) 183:2312–20. doi: 10.4049/jimmunol.0900185
84. Inoue S, Leitner WW, Golding B, Scott D. Inhibitory effects of B cells on antitumor immunity. Cancer Res (2006) 66:7741–7. doi: 10.1158/0008-5472.CAN-05-3766
85. Choi JK, Egwuagu CE. Interleukin 35 regulatory B cells. J Mol Biol (2021) 433:166607. doi: 10.1016/j.jmb.2020.07.019
86. Egwuagu CE, Yu C-R, Sun L, Wang R. Interleukin 35: Critical regulator of immunity and lymphocyte-mediated diseases. Cytokine Growth Factor Rev (2015) 26:587–93. doi: 10.1016/j.cytogfr.2015.07.013
87. Kessel A, Haj T, Peri R, Snir A, Melamed D, Sabo E, et al. Human CD19+CD25high B regulatory cells suppress proliferation of CD4+ T cells and enhance Foxp3 and CTLA-4 expression in T-regulatory cells. Autoimmun Rev (2012) 11:670–7. doi: 10.1016/j.autrev.2011.11.018
88. Akdis M, Aab A, Altunbulakli C, Azkur K, Costa RA, Crameri R, et al. Interleukins (from IL-1 to IL-38), interferons, transforming growth factor β, and TNF-α: Receptors, functions, and roles in diseases. J Allergy Clin Immunol (2016) 138:984–1010. doi: 10.1016/j.jaci.2016.06.033
89. Butz H, Rácz K, Hunyady L, Patócs A. Crosstalk between TGF-β signaling and the microRNA machinery. Trends Pharmacol Sci (2012) 33:382–93. doi: 10.1016/j.tips.2012.04.003
90. Saze Z, Schuler PJ, Hong C-S, Cheng D, Jackson EK, Whiteside TL. Adenosine production by human B cells and B cell–mediated suppression of activated T cells. Blood (2013) 122:9–18. doi: 10.1182/blood-2013-02-482406
91. Podolsky MA, Bailey JT, Gunderson AJ, Oakes CJ, Breech K, Glick AB. Differentiated state of initiating tumor cells is key to distinctive immune responses seen in H-rasG12V–induced squamous tumors. Cancer Immunol Res (2017) 5:198–210. doi: 10.1158/2326-6066.CIR-16-0304
92. Khan AR, Hams E, Floudas A, Sparwasser T, Weaver CT, Fallon PG. PD-L1hi B cells are critical regulators of humoral immunity. Nat Commun (2015) 6:5997. doi: 10.1038/ncomms6997
93. Wang T-W, Johmura Y, Suzuki N, Omori S, Migita T, Yamaguchi K, et al. Blocking PD-L1–PD-1 improves senescence surveillance and ageing phenotypes. Nature (2022) 611:358–64. doi: 10.1038/s41586-022-05388-4
94. Wherry EJ, Kurachi M. Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol (2015) 15:486–99. doi: 10.1038/nri3862
95. Tao H, Lu L, Xia Y, Dai F, Wang Y, Bao Y, et al. Antitumor effector B cells directly kill tumor cells via the Fas/FasL pathway and are regulated by IL-10: Cellular immune response. Eur J Immunol (2015) 45:999–1009. doi: 10.1002/eji.201444625
96. Motz GT, Santoro SP, Wang L-P, Garrabrant T, Lastra RR, Hagemann IS, et al. Tumor endothelium FasL establishes a selective immune barrier promoting tolerance in tumors. Nat Med (2014) 20:607–15. doi: 10.1038/nm.3541
97. Gabrilovich DI. Myeloid-derived suppressor cells. Cancer Immunol Res (2017) 5:3–8. doi: 10.1158/2326-6066.CIR-16-0297
98. Veglia F, Sanseviero E, Gabrilovich DI. Myeloid-derived suppressor cells in the era of increasing myeloid cell diversity. Nat Rev Immunol (2021) 21:485–98. doi: 10.1038/s41577-020-00490-y
99. Bodogai M, Moritoh K, Lee-Chang C, Hollander CM, Sherman-Baust CA, Wersto RP, et al. Immunosuppressive and prometastatic functions of myeloid-derived suppressive cells rely upon education from tumor-associated B cells. Cancer Res (2015) 75:3456–65. doi: 10.1158/0008-5472.CAN-14-3077
100. Lee-Chang C, Rashidi A, Miska J, Zhang P, Pituch KC, Hou D, et al. Myeloid-Derived Suppressive Cells Promote B cell–Mediated Immunosuppression via Transfer of PD-L1 in Glioblastoma. Cancer Immunol Res (2019) 7:1928–43. doi: 10.1158/2326-6066.CIR-19-0240
101. Hladíková K, Koucký V, Bouček J, Laco J, Grega M, Hodek M, et al. Tumor-infiltrating B cells affect the progression of oropharyngeal squamous cell carcinoma via cell-to-cell interactions with CD8+ T cells. J Immunother Cancer (2019) 7:261. doi: 10.1186/s40425-019-0726-6
102. Pitzalis C, Jones GW, Bombardieri M, Jones SA. Ectopic lymphoid-like structures in infection, cancer and autoimmunity. Nat Rev Immunol (2014) 14:447–62. doi: 10.1038/nri3700
103. Sautès-Fridman C, Petitprez F, Calderaro J, Fridman WH. Tertiary lymphoid structures in the era of cancer immunotherapy. Nat Rev Cancer (2019) 19:307–25. doi: 10.1038/s41568-019-0144-6
104. Cillo AR, Kürten CHL, Tabib T, Qi Z, Onkar S, Wang T, et al. Immune landscape of viral- and carcinogen-driven head and neck cancer. Immunity (2020) 52:183–199.e9. doi: 10.1016/j.immuni.2019.11.014
105. Koneva LA, Zhang Y, Virani S, Hall PB, McHugh JB, Chepeha DB, et al. HPV integration in HNSCC correlates with survival outcomes, immune response signatures, and candidate drivers. Mol Cancer Res (2018) 16:90–102. doi: 10.1158/1541-7786.MCR-17-0153
106. Kobayashi K, Hisamatsu K, Suzui N, Hara A, Tomita H, Miyazaki T. A review of HPV-related head and neck cancer. JCM (2018) 7:241. doi: 10.3390/jcm7090241
107. Ukita M, Hamanishi J, Yoshitomi H, Yamanoi K, Takamatsu S, Ueda A, et al. CXCL13-producing CD4+ T cells accumulate in early phase of tertiary lymphoid structures in ovarian cancer. JCI Insight (2022) 7(12):e157215. doi: 10.1172/jci.insight.157215
108. Meier D, Bornmann C, Chappaz S, Schmutz S, Otten LA, Ceredig R, et al. Ectopic lymphoid-organ development occurs through interleukin 7-mediated enhanced survival of lymphoid-tissue-inducer cells. Immunity (2007) 26:643–54. doi: 10.1016/j.immuni.2007.04.009
109. Deteix C, Attuil-Audenis V, Duthey A, Patey N, McGregor B, Dubois V, et al. Intragraft th17 infiltrate promotes lymphoid neogenesis and hastens clinical chronic rejection. JI (2010) 184:5344–51. doi: 10.4049/jimmunol.0902999
110. Peters A, Pitcher LA, Sullivan JM, Mitsdoerffer M, Acton SE, Franz B, et al. Th17 cells induce ectopic lymphoid follicles in central nervous system tissue inflammation. Immunity (2011) 35:986–96. doi: 10.1016/j.immuni.2011.10.015
111. Lochner M, Ohnmacht C, Presley L, Bruhns P, Si-Tahar M, Sawa S, et al. Microbiota-induced tertiary lymphoid tissues aggravate inflammatory disease in the absence of RORγt and LTi cells. J Exp Med (2011) 208:125–34. doi: 10.1084/jem.20100052
112. Guedj K, Khallou-Laschet J, Clement M, Morvan M, Gaston A-T, Fornasa G, et al. M1 macrophages act as LTβR-independent lymphoid tissue inducer cells during atherosclerosis-related lymphoid neogenesis. Cardiovasc Res (2014) 101:434–43. doi: 10.1093/cvr/cvt263
113. Denton AE, Innocentin S, Carr EJ, Bradford BM, Lafouresse F, Mabbott NA, et al. Type I interferon induces CXCL13 to support ectopic germinal center formation. J Exp Med (2019) 216:621–37. doi: 10.1084/jem.20181216
114. Neyt K, GeurtsvanKessel CH, Deswarte K, Hammad H, Lambrecht BN. Early IL-1 Signaling Promotes iBALT Induction after Influenza Virus Infection. Front Immunol (2016) 7:312. doi: 10.3389/fimmu.2016.00312
115. Li J-P, Wu C-Y, Chen M-Y, Liu S-X, Yan S-M, Kang Y-F, et al. PD-1+CXCR5–CD4+ Th-CXCL13 cell subset drives B cells into tertiary lymphoid structures of nasopharyngeal carcinoma. J Immunother Cancer (2021) 9:e002101. doi: 10.1136/jitc-2020-002101
116. Gu-Trantien C, Migliori E, Buisseret L, de Wind A, Brohée S, Garaud S, et al. CXCL13-producing TFH cells link immune suppression and adaptive memory in human breast cancer. JCI Insight (2017) 2:e91487. doi: 10.1172/jci.insight.91487
117. Colbeck EJ, Ager A, Gallimore A, Jones GW. Tertiary lymphoid structures in cancer: drivers of antitumor immunity, immunosuppression, or bystander sentinels in disease? Front Immunol (2017) 8:1830. doi: 10.3389/fimmu.2017.01830
118. Furtado GC, Marinkovic T, Martin AP, Garin A, Hoch B, Hubner W, et al. Lymphotoxin beta receptor signaling is required for inflammatory lymphangiogenesis in the thyroid. Proc Natl Acad Sci U S A (2007) 104(12):5026–31. doi: 10.1073/pnas.0606697104
119. Martinet L, Garrido I, Filleron T, Le Guellec S, Bellard E, Fournie J-J, et al. Human solid tumors contain high endothelial venules: association with T- and B-lymphocyte infiltration and favorable prognosis in breast cancer. Cancer Res (2011) 71:5678–87. doi: 10.1158/0008-5472.CAN-11-0431
120. Fleige H, Ravens S, Moschovakis GL, Bölter J, Willenzon S, Sutter G, et al. IL-17–induced CXCL12 recruits B cells and induces follicle formation in BALT in the absence of differentiated FDCs. J Exp Med (2014) 211:643–51. doi: 10.1084/jem.20131737
121. Luther SA, Bidgol A, Hargreaves DC, Schmidt A, Xu Y, Paniyadi J, et al. Differing activities of homeostatic chemokines CCL19, CCL21, and CXCL12 in lymphocyte and dendritic cell recruitment and lymphoid neogenesis. J Immunol (2002) 169:424–33. doi: 10.4049/jimmunol.169.1.424
122. Kinker GS, Vitiello GAF, Ferreira WAS, Chaves AS, Cordeiro de Lima VC, da Silva Medina T. B cell orchestration of anti-tumor immune responses: A matter of cell localization and communication. Front Cell Dev Biol (2021) 9:678127. doi: 10.3389/fcell.2021.678127
123. Prabhakaran S, Rizk VT, Ma Z, Cheng C-H, Berglund AE, Coppola D, et al. Evaluation of invasive breast cancer samples using a 12-chemokine gene expression score: correlation with clinical outcomes. Breast Cancer Res (2017) 19:71. doi: 10.1186/s13058-017-0864-z
124. Coppola D, Nebozhyn M, Khalil F, Dai H, Yeatman T, Loboda A, et al. Unique ectopic lymph node-like structures present in human primary colorectal carcinoma are identified by immune gene array profiling. Am J Pathol (2011) 179:37–45. doi: 10.1016/j.ajpath.2011.03.007
125. Messina JL, Fenstermacher DA, Eschrich S, Qu X, Berglund AE, Lloyd MC, et al. 12-chemokine gene signature identifies lymph node-like structures in melanoma: potential for patient selection for immunotherapy? Sci Rep (2012) 2:765. doi: 10.1038/srep00765
126. Siliņa K, Soltermann A, Attar FM, Casanova R, Uckeley ZM, Thut H, et al. Germinal centers determine the prognostic relevance of tertiary lymphoid structures and are impaired by corticosteroids in lung squamous cell carcinoma. Cancer Res (2018) 78:1308–20. doi: 10.1158/0008-5472.CAN-17-1987
127. Li Q, Liu X, Wang D, Wang Y, Lu H, Wen S, et al. Prognostic value of tertiary lymphoid structure and tumour infiltrating lymphocytes in oral squamous cell carcinoma. Int J Oral Sci (2020) 12:24. doi: 10.1038/s41368-020-00092-3
128. Wirsing AM, Rikardsen OG, Steigen SE, Uhlin-Hansen L, Hadler-Olsen E. Presence of tumour high-endothelial venules is an independent positive prognostic factor and stratifies patients with advanced-stage oral squamous cell carcinoma. Tumor Biol (2016) 37:2449–59. doi: 10.1007/s13277-015-4036-4
129. Wirsing AM, Ervik IK, Seppola M, Uhlin-Hansen L, Steigen SE, Hadler-Olsen E. Presence of high-endothelial venules correlates with a favorable immune microenvironment in oral squamous cell carcinoma. Mod Pathol (2018) 31:910–22. doi: 10.1038/s41379-018-0019-5
130. Sautès-Fridman C, Lawand M, Giraldo NA, Kaplon H, Germain C, Fridman WH, et al. Tertiary lymphoid structures in cancers: prognostic value, regulation, and manipulation for therapeutic intervention. Front Immunol (2016) 7:407. doi: 10.3389/fimmu.2016.00407
131. Dieu-Nosjean M-C, Giraldo NA, Kaplon H, Germain C, Fridman WH, Sautès-Fridman C. Tertiary lymphoid structures, drivers of the anti-tumor responses in human cancers. Immunol Rev (2016) 271:260–75. doi: 10.1111/imr.12405
132. Li K, Guo Q, Zhang X, Dong X, Liu W, Zhang A, et al. Oral cancer-associated tertiary lymphoid structures: gene expression profile and prognostic value. Clin Exp Immunol (2020) 199:172–81. doi: 10.1111/cei.13389
133. for the SPARC Consortium, Wood O, Woo J, Seumois G, Savelyeva N, McCann KJ, et al. Gene expression analysis of TIL rich HPV-driven head and neck tumors reveals a distinct B-cell signature when compared to HPV independent tumors. Oncotarget (2016) 7:56781–97. doi: 10.18632/oncotarget.10788
134. Su GH, Ip HS, Cobb BS, Lu MM, Chen HM, Simon MC. The Ets protein Spi-B is expressed exclusively in B cells and T cells during development. J Exp Med (1996) 184:203–14. doi: 10.1084/jem.184.1.203
135. Liu D, Xu H, Shih C, Wan Z, Ma X, Ma W, et al. T–B-cell entanglement and ICOSL-driven feed-forward regulation of germinal centre reaction. Nature (2015) 517:214–8. doi: 10.1038/nature13803
136. Germain C, Gnjatic S, Tamzalit F, Knockaert S, Remark R, Goc J, et al. Presence of B cells in tertiary lymphoid structures is associated with a protective immunity in patients with lung cancer. Am J Respir Crit Care Med (2014) 189:832–44. doi: 10.1164/rccm.201309-1611OC
137. Gatto D, Brink R. The germinal center reaction. J Allergy Clin Immunol (2010) 126:898–907. doi: 10.1016/j.jaci.2010.09.007
138. Meylan M, Petitprez F, Becht E, Bougoüin A, Pupier G, Calvez A, et al. Tertiary lymphoid structures generate and propagate anti-tumor antibody-producing plasma cells in renal cell cancer. Immunity (2022) 55:527–541.e5. doi: 10.1016/j.immuni.2022.02.001
139. Lu N, Li Y, Zhang Z, Xing J, Sun Y, Yao S, Chen L. Human Semaphorin-4A drives Th2 responses by binding to receptor ILT-4. Nat Commun (2018) 9(1):742. doi: 10.1038/s41467-018-03128-9
140. Delgoffe GM, Woo S-R, Turnis ME, Gravano DM, Guy C, Overacre AE, et al. Stability and function of regulatory T cells is maintained by a neuropilin-1–semaphorin-4a axis. Nature (2013) 501:252–6. doi: 10.1038/nature12428
141. Van Der Zwaag B, Hellemons AJCGM, Leenders WPJ, Burbach JPH, Brunner HG, Padberg GW, et al. PLEXIN-D1, a novel plexin family member, is expressed in vascular endothelium and the central nervous system during mouse embryogenesis. Dev Dyn (2002) 225:336–43. doi: 10.1002/dvdy.10159
142. Nielsen JS, Sahota RA, Milne K, Kost SE, Nesslinger NJ, Watson PH, et al. CD20+tumor-infiltrating lymphocytes have an atypical CD27– memory phenotype and together with CD8+T cells promote favorable prognosis in ovarian cancer. Clin Cancer Res (2012) 18:3281–92. doi: 10.1158/1078-0432.CCR-12-0234
143. Buckley CD, Barone F, Nayar S, Bénézech C, Caamaño J. Stromal cells in chronic inflammation and tertiary lymphoid organ formation. Annu Rev Immunol (2015) 33:715–45. doi: 10.1146/annurev-immunol-032713-120252
144. Kim SS, Sumner WA, Miyauchi S, Cohen EEW, Califano JA, Sharabi AB. Role of B cells in responses to checkpoint blockade immunotherapy and overall survival of cancer patients. Clin Cancer Res (2021) 27:6075–82. doi: 10.1158/1078-0432.CCR-21-0697
145. Kroeger DR, Milne K, Nelson BH. Tumor-infiltrating plasma cells are associated with tertiary lymphoid structures, cytolytic T-cell responses, and superior prognosis in ovarian cancer. Clin Cancer Res (2016) 22:3005–15. doi: 10.1158/1078-0432.CCR-15-2762
146. Simoni Y, Becht E, Fehlings M, Loh CY, Koo S-L, Teng KWW, et al. Bystander CD8+ T cells are abundant and phenotypically distinct in human tumour infiltrates. Nature (2018) 557:575–9. doi: 10.1038/s41586-018-0130-2
147. Thommen DS, Koelzer VH, Herzig P, Roller A, Trefny M, Dimeloe S, et al. A transcriptionally and functionally distinct PD-1+ CD8+ T cell pool with predictive potential in non-small-cell lung cancer treated with PD-1 blockade. Nat Med (2018) 24:994–1004. doi: 10.1038/s41591-018-0057-z
148. Rooney MS, Shukla SA, Wu CJ, Getz G, Hacohen N. Molecular and genetic properties of tumors associated with local immune cytolytic activity. Cell (2015) 160:48–61. doi: 10.1016/j.cell.2014.12.033
149. Broglie MA, Jochum W, Michel A, Waterboer T, Foerbs D, Schoenegg R, et al. Evaluation of type-specific antibodies to high risk-human papillomavirus (HPV) proteins in patients with oropharyngeal cancer. Oral Oncol (2017) 70:43–50. doi: 10.1016/j.oraloncology.2017.05.010
150. Maldonado L, Teague JE, Morrow MP, Jotova I, Wu TC, Wang C, et al. Intramuscular therapeutic vaccination targeting HPV16 induces T cell responses that localize in mucosal lesions. Sci Transl Med (2014) 6(221):221ra13. doi: 10.1126/scitranslmed.3007323
151. Wouters MCA, Nelson BH. Prognostic significance of tumor-infiltrating B cells and plasma cells in human cancer. Clin Cancer Res (2018) 24:6125–35. doi: 10.1158/1078-0432.CCR-18-1481
152. Atanackovic D, Blum I, Cao Y, Wenzel S, Bartels K, Faltz C, et al. Expression of cancer-testis antigens as possible targets for antigen-specific immunotherapy in head and neck squamous cell carcinoma. Cancer Biol Ther (2006) 5:1218–25. doi: 10.4161/cbt.5.9.3174
153. Zamunér FT, Karia BTR, de Oliveira CZ, dos Santos CR, Carvalho AL, Vettore AL. A comprehensive expression analysis of cancer testis antigens in head and neck squamous cell carcinoma revels MAGEA3/6 as a marker for recurrence. Mol Cancer Ther (2015) 14:828–34. doi: 10.1158/1535-7163.MCT-14-0796
154. Lechner A, Schlößer HA, Thelen M, Wennhold K, Rothschild SI, Gilles R, et al. Tumor-associated B cells and humoral immune response in head and neck squamous cell carcinoma. OncoImmunology (2019) 8:1535293. doi: 10.1080/2162402X.2018.1535293
155. Gilbert AE, Karagiannis P, Dodev T, Koers A, Lacy K, Josephs DH, et al. Monitoring the systemic human memory B cell compartment of melanoma patients for anti-tumor igG antibodies. PloS One (2011) 6:e19330. doi: 10.1371/journal.pone.0019330
156. Boyerinas B, Jochems C, Fantini M, Heery CR, Gulley JL, Tsang KY, et al. Antibody-dependent cellular cytotoxicity activity of a novel anti–PD-L1 antibody avelumab (MSB0010718C) on human tumor cells. Cancer Immunol Res (2015) 3:1148–57. doi: 10.1158/2326-6066.CIR-15-0059
157. Carmi Y, Spitzer MH, Linde IL, Burt BM, Prestwood TR, Perlman N, et al. Allogeneic IgG combined with dendritic cell stimuli induce antitumour T-cell immunity. Nature (2015) 521:99–104. doi: 10.1038/nature14424
158. Bruhns P, Iannascoli B, England P, Mancardi DA, Fernandez N, Jorieux S, et al. Specificity and affinity of human Fcγ receptors and their polymorphic variants for human IgG subclasses. Blood (2009) 113:3716–25. doi: 10.1182/blood-2008-09-179754
159. Bowen A, Casadevall A. Revisiting the immunoglobulin intramolecular signaling hypothesis. Trends Immunol (2016) 37:721–3. doi: 10.1016/j.it.2016.08.014
160. Zhao J, Nussinov R, Ma B. Antigen binding allosterically promotes Fc receptor recognition. MAbs (2019) 11:58–74. doi: 10.1080/19420862.2018.1522178
161. Hu X, Zhang J, Wang J, Fu J, Li T, Zheng X, et al. Landscape of B cell immunity and related immune evasion in human cancers. Nat Genet (2019) 51:560–7. doi: 10.1038/s41588-018-0339-x
162. Stavnezer J, Kang J. The surprising discovery that TGFβ Specifically induces the igA class switch. J Immunol (2009) 182:5–7. doi: 10.4049/jimmunol.182.1.5
163. Biswas S, Mandal G, Payne KK, Anadon CM, Gatenbee CD, Chaurio RA, et al. IgA transcytosis and antigen recognition govern ovarian cancer immunity. Nature (2021) 591:464–70. doi: 10.1038/s41586-020-03144-0
164. Montfort A, Pearce O, Maniati E, Vincent BG, Bixby L, Böhm S, et al. A strong B-cell response is part of the immune landscape in human high-grade serous ovarian metastases. Clin Cancer Res (2017) 23:250–62. doi: 10.1158/1078-0432.CCR-16-0081
165. Bolotin DA, Poslavsky S, Davydov AN, Frenkel FE, Fanchi L, Zolotareva OI, et al. Antigen receptor repertoire profiling from RNA-seq data. Nat Biotechnol (2017) 35:908–11. doi: 10.1038/nbt.3979
166. Shi J-Y, Gao Q, Wang Z-C, Zhou J, Wang X-Y, Min Z-H, et al. Margin-infiltrating CD20(+) B cells display an atypical memory phenotype and correlate with favorable prognosis in hepatocellular carcinoma. Clin Cancer Res (2013) 19:5994–6005. doi: 10.1158/1078-0432.CCR-12-3497
167. Wennhold K, Thelen M, Lehmann J, Schran S, Preugszat E, Garcia-Marquez M, et al. CD86+ Antigen-presenting B cells are increased in cancer, localize in tertiary lymphoid structures, and induce specific T-cell responses. Cancer Immunol Res (2021) 9:1098–108. doi: 10.1158/2326-6066.CIR-20-0949
168. Kambayashi T, Laufer TM. Atypical MHC class II-expressing antigen-presenting cells: can anything replace a dendritic cell? Nat Rev Immunol (2014) 14:719–30. doi: 10.1038/nri3754
169. Schriek P, Ching AC, Moily NS, Moffat J, Beattie L, Steiner TM, et al. Marginal zone B cells acquire dendritic cell functions by trogocytosis. Science (2022) 375:eabf7470. doi: 10.1126/science.abf7470
170. Shimabukuro-Vornhagen A, García-Márquez M, Fischer RN, Iltgen-Breburda J, Fiedler A, Wennhold K, et al. Antigen-presenting human B cells are expanded in inflammatory conditions. J Leukoc Biol (2017) 101:577–87. doi: 10.1189/jlb.5A0416-182R
171. Zhu W, Germain C, Liu Z, Sebastian Y, Devi P, Knockaert S, et al. A high density of tertiary lymphoid structure B cells in lung tumors is associated with increased CD4C T cell receptor repertoire clonality. OncoImmunology (2015) 4:e1051922. doi: 10.1080/2162402X.2015.1051922
172. Bruno TC, Ebner PJ, Moore BL, Squalls OG, Waugh KA, Eruslanov EB, et al. Antigen-presenting intratumoral B cells affect CD4+ TIL phenotypes in non–small cell lung cancer patients. Cancer Immunol Res (2017) 5:898–907. doi: 10.1158/2326-6066.CIR-17-0075
173. Engelhard VH, Rodriguez AB, Mauldin IS, Woods AN, Peske JD, Slingluff CL. Immune cell infiltration and tertiary lymphoid structures as determinants of antitumor immunity. JI (2018) 200:432–42. doi: 10.4049/jimmunol.1701269
174. de Chaisemartin L, Goc J, Damotte D, Validire P, Magdeleinat P, Alifano M, et al. Characterization of chemokines and adhesion molecules associated with T cell presence in tertiary lymphoid structures in human lung cancer. Cancer Res (2011) 71:6391–9. doi: 10.1158/0008-5472.CAN-11-0952
175. Peske JD, Thompson ED, Gemta L, Baylis RA, Fu Y-X, Engelhard VH. Effector lymphocyte-induced lymph node-like vasculature enables naive T-cell entry into tumours and enhanced anti-tumour immunity. Nat Commun (2015) 6:7114. doi: 10.1038/ncomms8114
176. Yu P, Lee Y, Liu W, Chin RK, Wang J, Wang Y, et al. Priming of naive T cells inside tumors leads to eradication of established tumors. Nat Immunol (2004) 5:141–9. doi: 10.1038/ni1029
177. Thompson ED, Enriquez HL, Fu Y-X, Engelhard VH. Tumor masses support naive T cell infiltration, activation, and differentiation into effectors. J Exp Med (2010) 207:1791–804. doi: 10.1084/jem.20092454
178. Truxova I, Kasikova L, Hensler M, Skapa P, Laco J, Pecen L, et al. Mature dendritic cells correlate with favorable immune infiltrate and improved prognosis in ovarian carcinoma patients. J Immunother Cancer (2018) 6:139. doi: 10.1186/s40425-018-0446-3
179. Gu-Trantien C, Loi S, Garaud S, Equeter C, Libin M, de Wind A, et al. CD4+ follicular helper T cell infiltration predicts breast cancer survival. J Clin Invest (2013) 123:2873–92. doi: 10.1172/JCI67428
180. Hiraoka N, Ino Y, Yamazaki-Itoh R, Kanai Y, Kosuge T, Shimada K. Intratumoral tertiary lymphoid organ is a favourable prognosticator in patients with pancreatic cancer. Br J Cancer (2015) 112:1782–90. doi: 10.1038/bjc.2015.145
181. Lee HJ, Kim JY, Park IA, Song IH, Yu JH, Ahn J-H, et al. Prognostic significance of tumor-infiltrating lymphocytes and the tertiary lymphoid structures in HER2-positive breast cancer treated with adjuvant trastuzumab. Am J Clin Pathol (2015) 144:278–88. doi: 10.1309/AJCPIXUYDVZ0RZ3G
182. Liu X, Tsang JYS, Hlaing T, Hu J, Ni Y, Chan SK, et al. Distinct tertiary lymphoid structure associations and their prognostic relevance in HER2 positive and negative breast cancers. Oncol (2017) 22:1316–24. doi: 10.1634/theoncologist.2017-0029
183. Hill DG, Yu L, Gao H, Balic JJ, West A, Oshima H, et al. Hyperactive gp130/STAT3-driven gastric tumourigenesis promotes submucosal tertiary lymphoid structure development: gp130/STAT3 regulates TLSs in gastric cancer. Int J Cancer (2018) 143:167–78. doi: 10.1002/ijc.31298
184. Koti M, Xu AS, Ren KYM, Visram K, Ren R, Berman DM, et al. Tertiary lymphoid structures associate with tumour stage in urothelial bladder cancer. Bladder Cancer (2017) 3(4):259–67. doi: 10.3233/BLC-170120
185. Figenschau SL, Fismen S, Fenton KA, Fenton C, Mortensen ES. Tertiary lymphoid structures are associated with higher tumor grade in primary operable breast cancer patients. BMC Cancer (2015) 15:101. doi: 10.1186/s12885-015-1116-1
186. Meshcheryakova A, Tamandl D, Bajna E, Stift J, Mittlboeck M, Svoboda M, et al. B cells and ectopic follicular structures: novel players in anti-tumor programming with prognostic power for patients with metastatic colorectal cancer. PloS One (2014) 9:e99008. doi: 10.1371/journal.pone.0099008
187. Dieu-Nosjean M-C, Goc J, Giraldo NA, Sautès-Fridman C, Fridman WH. Tertiary lymphoid structures in cancer and beyond. Trends Immunol (2014) 35:571–80. doi: 10.1016/j.it.2014.09.006
188. Lee M, Heo S-H, Song IH, Rajayi H, Park HS, Park IA, et al. Presence of tertiary lymphoid structures determines the level of tumor-infiltrating lymphocytes in primary breast cancer and metastasis. Mod Pathol (2019) 32:70–80. doi: 10.1038/s41379-018-0113-8
189. Becht E, Giraldo NA, Germain C, de Reyniès A, Laurent-Puig P, Zucman-Rossi J, et al. Chapter Four - Immune Contexture, Immunoscore, and Malignant Cell Molecular Subgroups for Prognostic and Theranostic Classifications of Cancers. Adv Immunol (2016) 130:95–190. doi: 10.1016/bs.ai.2015.12.002
190. Schweiger T, Berghoff AS, Glogner C, Glueck O, Rajky O, Traxler D, et al. Tumor-infiltrating lymphocyte subsets and tertiary lymphoid structures in pulmonary metastases from colorectal cancer. Clin Exp Metastasis (2016) 33:727–39. doi: 10.1007/s10585-016-9813-y
191. Remark R, Alifano M, Cremer I, Lupo A, Dieu-Nosjean M-C, Riquet M, et al. Characteristics and clinical impacts of the immune environments in colorectal and renal cell carcinoma lung metastases: influence of tumor origin. Clin Cancer Res (2013) 19:4079–91. doi: 10.1158/1078-0432.CCR-12-3847
192. Ward MJ, Thirdborough SM, Mellows T, Riley C, Harris S, Suchak K, et al. Tumour-infiltrating lymphocytes predict for outcome in HPV-positive oropharyngeal cancer. Br J Cancer (2014) 110:489–500. doi: 10.1038/bjc.2013.639
193. Distel LV, Fickenscher R, Dietel K, Hung A, Iro H, Zenk J, et al. Tumour infiltrating lymphocytes in squamous cell carcinoma of the oro- and hypopharynx: Prognostic impact may depend on type of treatment and stage of disease. Oral Oncol (2009) 45:e167–74. doi: 10.1016/j.oraloncology.2009.05.640
194. Pretscher D, Distel LV, Grabenbauer GG, Wittlinger M, Buettner M, Niedobitek G. Distribution of immune cells in head and neck cancer: CD8+ T-cells and CD20+B-cells in metastatic lymph nodes are associated with favourable outcome in patients with oro- and hypopharyngeal carcinoma. BMC Cancer (2009) 9:292. doi: 10.1186/1471-2407-9-292
195. Finkin S, Yuan D, Stein I, Taniguchi K, Weber A, Unger K, et al. Ectopic lymphoid structures function as microniches for tumor progenitor cells in hepatocellular carcinoma. Nat Immunol (2015) 16:1235–44. doi: 10.1038/ni.3290
196. Fridman WH, Zitvogel L, Sautès–Fridman C, Kroemer G. The immune contexture in cancer prognosis and treatment. Nat Rev Clin Oncol (2017) 14:717–34. doi: 10.1038/nrclinonc.2017.101
197. Laumont CM, Banville AC, Gilardi M, Hollern DP, Nelson BH. Tumour-infiltrating B cells: immunological mechanisms, clinical impact and therapeutic opportunities. Nat Rev Cancer (2022) 22:414–30. doi: 10.1038/s41568-022-00466-1
198. Noël G, Fontsa ML, Garaud S, De Silva P, De Wind A, Van Den Eynden GG, et al. Functional Th1-oriented T follicular helper cells that infiltrate human breast cancer promote effective adaptive immunity. J Clin Invest (2021) 131:e139905. doi: 10.1172/JCI139905
199. Pagliarulo F, Cheng PF, Brugger L, Van Dijk N, Van Den Heijden M, Levesque MP, et al. Molecular, immunological, and clinical features associated with lymphoid neogenesis in muscle invasive bladder cancer. Front Immunol (2022) 12:793992. doi: 10.3389/fimmu.2021.793992
200. Nayar S, Campos J, Smith CG, Iannizzotto V, Gardner DH, Mourcin F, et al. Immunofibroblasts are pivotal drivers of tertiary lymphoid structure formation and local pathology. Proc Natl Acad Sci USA (2019) 116:13490–7. doi: 10.1073/pnas.1905301116
201. Wu X, Gu Z, Chen Y, Chen B, Chen W, Weng L, et al. Application of PD-1 blockade in cancer immunotherapy. Comput Struct Biotechnol J (2019) 17:661–74. doi: 10.1016/j.csbj.2019.03.006
202. Economopoulou P, Kotsantis I, Psyrri A. The promise of immunotherapy in head and neck squamous cell carcinoma: combinatorial immunotherapy approaches. ESMO Open (2016) 1:e000122. doi: 10.1136/esmoopen-2016-000122
203. Ferris RL, Blumenschein G, Fayette J, Guigay J, Colevas AD, Licitra L, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med (2016) 375:1856–67. doi: 10.1056/NEJMoa1602252
204. Hanna GJ, Lizotte P, Cavanaugh M, Kuo FC, Shivdasani P, Frieden A, et al. Frameshift events predict anti–PD-1/L1 response in head and neck cancer. JCI Insight (2018) 3:e98811. doi: 10.1172/jci.insight.98811
205. Ock C-Y, Hwang J-E, Keam B, Kim S-B, Shim J-J, Jang H-J, et al. Genomic landscape associated with potential response to anti-CTLA-4 treatment in cancers. Nat Commun (2017) 8:1050. doi: 10.1038/s41467-017-01018-0
206. Zheng L, Ding D, Edil BH, Judkins C, Durham JN, Thomas DL, et al. Vaccine-induced intratumoral lymphoid aggregates correlate with survival following treatment with a neoadjuvant and adjuvant vaccine in patients with resectable pancreatic adenocarcinoma. Clin Cancer Res (2021) 27:1278–86. doi: 10.1158/1078-0432.CCR-20-2974
207. Lutz ER, Wu AA, Bigelow E, Sharma R, Mo G, Soares K, et al. Immunotherapy converts nonimmunogenic pancreatic tumors into immunogenic foci of immune regulation. Cancer Immunol Res (2014) 2:616–31. doi: 10.1158/2326-6066.CIR-14-0027
208. Allen E, Jabouille A, Rivera LB, Lodewijckx I, Missiaen R, Steri V, et al. Combined antiangiogenic and anti–PD-L1 therapy stimulates tumor immunity through HEV formation. Sci Transl Med (2017) 9:eaak9679. doi: 10.1126/scitranslmed.aak9679
209. Amatore F, Gorvel L, Olive D. Inducible Co-Stimulator (ICOS) as a potential therapeutic target for anti-cancer therapy. Expert Opin Ther Targets (2018) 22:343–51. doi: 10.1080/14728222.2018.1444753
210. Weinstein AM, Chen L, Brzana EA, Patil PR, Taylor JL, Fabian KL, et al. Tbet and IL-36γ cooperate in therapeutic DC-mediated promotion of ectopic lymphoid organogenesis in the tumor microenvironment. OncoImmunology (2017) 6:e1322238. doi: 10.1080/2162402X.2017.1322238
211. Chen L, Taylor JL, Sabins NC, Lowe DB, Qu Y, You Z, et al. Extranodal induction of therapeutic immunity in the tumor microenvironment after intratumoral delivery of Tbet gene-modified dendritic cells. Cancer Gene Ther (2013) 20:469–77. doi: 10.1038/cgt.2013.42
212. Lee JM, Lee M-H, Garon E, Goldman JW, Salehi-Rad R, Baratelli FE, et al. Phase I trial of intratumoral injection of CCL21 gene–modified dendritic cells in lung cancer elicits tumor-specific immune responses and CD8+ T-cell infiltration. Clin Cancer Res (2017) 23:4556–68. doi: 10.1158/1078-0432.CCR-16-2821
213. Weiden J, Tel J, Figdor CG. Synthetic immune niches for cancer immunotherapy. Nat Rev Immunol (2018) 18:212–9. doi: 10.1038/nri.2017.89
214. Zhu G, Falahat R, Wang K, Mailloux A, Artzi N, Mulé JJ. Tumor-associated tertiary lymphoid structures: gene-expression profiling and their bioengineering. Front Immunol (2017) 8:767. doi: 10.3389/fimmu.2017.00767
215. Phuengkham H, Ren L, Shin IW, Lim YT. Nanoengineered immune niches for reprogramming the immunosuppressive tumor microenvironment and enhancing cancer immunotherapy. Adv Mater (2019) 31:1803322. doi: 10.1002/adma.201803322
216. Yarchoan M, Mohan AA, Dennison L, Vithayathil T, Ruggieri A, Lesinski GB, et al. MEK inhibition suppresses B regulatory cells and augments anti-tumor immunity. PloS One (2019) 14:e0224600. doi: 10.1371/journal.pone.0224600
217. Athar M, Back JH, Kopelovich L, Bickers DR, Kim AL. Multiple molecular targets of resveratrol: Anti-carcinogenic mechanisms. Arch Biochem Biophys (2009) 486:95–102. doi: 10.1016/j.abb.2009.01.018
218. Wang Z, Cheng Q, Tang K, Sun Y, Zhang K, Zhang Y, et al. Lipid mediator lipoxin A4 inhibits tumor growth by targeting IL-10-producing regulatory B (Breg) cells. Cancer Lett (2015) 364:118–24. doi: 10.1016/j.canlet.2015.04.030
219. Takahashi R, Macchini M, Sunagawa M, Jiang Z, Tanaka T, Valenti G, et al. Interleukin-1β-induced pancreatitis promotes pancreatic ductal adenocarcinoma via B lymphocyte–mediated immune suppression. Gut (2021) 70(2):330–41. doi: 10.1136/gutjnl-2019-319912
220. Mirlekar B, Wang Y, Li S, Zhou M, Entwistle S, De Buysscher T, et al. Balance between immunoregulatory B cells and plasma cells drives pancreatic tumor immunity. Cell Rep Med (2022) 3:100744. doi: 10.1016/j.xcrm.2022.100744
221. Kim SS, Shen S, Miyauchi S, Sanders PD, Franiak-Pietryga I, Mell L, et al. B cells improve overall survival in HPV-associated squamous cell carcinomas and are activated by radiation and PD-1 blockade. Clin Cancer Res (2020) 26:3345–59. doi: 10.1158/1078-0432.CCR-19-3211
222. Castino GF, Cortese N, Capretti G, Serio S, Di Caro G, Mineri R, et al. Spatial distribution of B cells predicts prognosis in human pancreatic adenocarcinoma. Oncoimmunology (2015) 5(4):e1085147. doi: 10.1080/2162402X.2015.1085147
223. Cottrell TR, Thompson ED, Forde PM, Stein JE, Duffield AS, Anagnostou V, et al. Pathologic features of response to neoadjuvant anti-PD-1 in resected non-small-cell lung carcinoma: a proposal for quantitative immune-related pathologic response criteria (irPRC). Ann Oncol (2018) 29:1853–60. doi: 10.1093/annonc/mdy218
224. Chen W, Jiang M, Yu W, Xu Z, Liu X, Jia Q, et al. CpG-based nanovaccines for cancer immunotherapy. Int J Nanomed (2021) 16:5281–99. doi: 10.2147/IJN.S317626
225. Johansson-Percival A, He B, Li Z-J, Kjellén A, Russell K, Li J, et al. De novo induction of intratumoral lymphoid structures and vessel normalization enhances immunotherapy in resistant tumors. Nat Immunol (2017) 18:1207–17. doi: 10.1038/ni.3836
226. Rischin D, Groenland SL, Lim AML, Martin-Liberal J, Moreno V, Trigo Perez JM, et al. Inducible T cell costimulatory (ICOS) receptor agonist, GSK3359609 (GSK609) alone and in combination with pembrolizumab (pembro): Preliminary results from INDUCE-1 expansion cohorts (EC) in head and neck squamous cell carcinoma (HNSCC). Ann Oncol (2019) 30:v454–5. doi: 10.1093/annonc/mdz252.011
227. Weinstein AM, Giraldo NA, Petitprez F, Julie C, Lacroix L, Peschaud F, et al. Association of IL-36γ with tertiary lymphoid structures and inflammatory immune infiltrates in human colorectal cancer. Cancer Immunol Immunother (2019) 68:109–20. doi: 10.1007/s00262-018-2259-0
228. Zhu G, Nemoto S, Mailloux AW, Perez-Villarroel P, Nakagawa R, Falahat R, et al. Induction of tertiary lymphoid structures with antitumor function by a lymph node-derived stromal cell line. Front Immunol (2018) 9:1609. doi: 10.3389/fimmu.2018.01609
229. Yagawa Y, Robertson-Tessi M, Zhou SL, Anderson ARA, Mulé JJ, Mailloux AW. Systematic screening of chemokines to identify candidates to model and create ectopic lymph node structures for cancer immunotherapy. Sci Rep (2017) 7:15996. doi: 10.1038/s41598-017-15924-2
230. Ryan ST, Zhang J, Burner DN, Liss M, Pittman E, Muldong M, et al. Neoadjuvant rituximab modulates the tumor immune environment in patients with high risk prostate cancer. J Transl Med (2020) 18:214. doi: 10.1186/s12967-020-02370-4
231. Liu L, Nishihara R, Qian ZR, Tabung FK, Nevo D, Zhang X, et al. Association between inflammatory diet pattern and risk of colorectal carcinoma subtypes classified by immune responses to tumor. Gastroenterology (2017) 153:1517–1530.e14. doi: 10.1053/j.gastro.2017.08.045
232. Affara NI, Ruffell B, Medler TR, Gunderson AJ, Johansson M, Bornstein S, et al. B cells regulate macrophage phenotype and response to chemotherapy in squamous carcinomas. Cancer Cell (2014) 25:809–21. doi: 10.1016/j.ccr.2014.04.026
233. Shao A, Owens DM. The immunoregulatory protein CD200 as a potentially lucrative yet elusive target for cancer therapy. Oncotarget (2023) 14:96–103. doi: 10.18632/oncotarget.28354
234. Mahadevan D, Lanasa MC, Farber C, Pandey M, Whelden M, Faas SJ, et al. Phase I study of samalizumab in chronic lymphocytic leukemia and multiple myeloma: blockade of the immune checkpoint CD200. J Immunother Cancer (2019) 7:227. doi: 10.1186/s40425-019-0710-1
235. Liu J-Q, Hu A, Zhu J, Yu J, Talebian F, Bai X-F. CD200-CD200R Pathway in the Regulation of Tumor Immune Microenvironment and Immunotherapy. In: Birbrair A, editor. Tumor Microenvironment, vol. 1223 . Cham: Springer International Publishing (2020). p. 155–65. doi: 10.1007/978-3-030-35582-1_8
236. Han J, Liu Y, Yang S, Wu X, Li H, Wang Q. MEK inhibitors for the treatment of non-small cell lung cancer. J Hematol Oncol (2021) 14:1. doi: 10.1186/s13045-020-01025-7
Keywords: head and neck cancer, tumor-infiltrating lymphocytes, regulatory B cells, tertiary lymphoid structures, tumor microenvironment, immunotherapy
Citation: Bao J, Betzler AC, Hess J and Brunner C (2023) Exploring the dual role of B cells in solid tumors: implications for head and neck squamous cell carcinoma. Front. Immunol. 14:1233085. doi: 10.3389/fimmu.2023.1233085
Received: 01 June 2023; Accepted: 06 September 2023;
Published: 05 October 2023.
Edited by:
Juan M. Zapata, Consejo Superior de Investigaciones Científicas (CSIC), SpainCopyright © 2023 Bao, Betzler, Hess and Brunner. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cornelia Brunner, Y29ybmVsaWEuYnJ1bm5lckB1bmlrbGluaWstdWxtLmRl
 Jiantong Bao
Jiantong Bao Annika C. Betzler
Annika C. Betzler Jochen Hess
Jochen Hess Cornelia Brunner
Cornelia Brunner