
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol., 21 August 2023
Sec. Cancer Immunity and Immunotherapy
Volume 14 - 2023 | https://doi.org/10.3389/fimmu.2023.1229823
 John Marsiglio1
John Marsiglio1 Jordan P. McPherson2
Jordan P. McPherson2 Magdalena Kovacsovics-Bankowski2
Magdalena Kovacsovics-Bankowski2 Joanne Jeter2
Joanne Jeter2 Christos Vaklavas2
Christos Vaklavas2 Umang Swami2
Umang Swami2 Douglas Grossmann3
Douglas Grossmann3 Alyssa Erickson-Wayman2
Alyssa Erickson-Wayman2 Heloisa P. Soares2
Heloisa P. Soares2 Katie Kerrigan2
Katie Kerrigan2 Berit Gibson2
Berit Gibson2 Jennifer Anne Doherty2
Jennifer Anne Doherty2 John Hyngstrom2
John Hyngstrom2 Sheetal Hardikar4
Sheetal Hardikar4 Siwen Hu-Lieskovan2*
Siwen Hu-Lieskovan2*Background: Type 1 diabetes mellitus (T1DM) is a rare, but serious immune-related adverse event (irAE) of immune checkpoint inhibitors (ICIs). Our goal was to characterize treatment outcomes associated with ICI-induced T1DM through analysis of clinical, immunological and proteomic data.
Methods: This was a single-center case series of patients with solid tumors who received ICIs and subsequently had a new diagnosis of T1DM. ICD codes and C-peptide levels were used to identify patients for chart review to confirm ICI-induced T1DM. Baseline blood specimens were studied for proteomic and immunophenotypic changes.
Results: Between 2011 and 2023, 18 of 3744 patients treated at Huntsman Cancer Institute with ICIs were confirmed to have ICI-induced T1DM (0.48%). Eleven of the 18 patients received anti-PD1 monotherapy, 4 received anti-PD1 plus chemotherapy or targeted therapy, and 3 received ipilimumab plus nivolumab. The mean time to onset was 218 days (range 22-418 days). Patients had sudden elevated serum glucose within 2-3 weeks prior to diagnosis. Sixteen (89%) presented with diabetic ketoacidosis. Three of 12 patients had positive T1DM-associated autoantibodies. All patients with T1DM became insulin-dependent through follow-up. At median follow-up of 21.9 months (range 8.4-82.4), no patients in the melanoma group had progressed or died from disease. In the melanoma group, best responses were 2 complete response and 2 partial response while on active treatment; none in the adjuvant group had disease recurrence. Proteomic analysis of baseline blood suggested low inflammatory (IL-6, OSMR) markers and high metabolic (GLO1, DXCR) markers in ICI-induced T1DM cohort.
Conclusions: Our case series demonstrates rapid onset and irreversibility of ICI-induced T1DM. Melanoma patients with ICI-induced T1DM display excellent clinical response and survival. Limited proteomic data also suggested a unique proteomic profile. Our study helps clinicians to understand the unique clinical presentation and long-term outcomes of this rare irAE for best clinical management.
The use of immune checkpoint inhibitors (ICIs) has been increasingly prevalent in cancer treatment. ICIs target immune checkpoint molecules that regulate immune cell activation, such as cytotoxic T lymphocyte associated antigen-4 (CTLA-4) and programmed cell death-1 (PD-1). The binding of CD80/86 to CTLA-4 and PD-1/PD-L1 normally inactivates T-cells and blocks pro-apoptotic pathways, eventually leading to effector T cell exhaustion (1). By overexpressing these ligands, cancer cells block T cell activation and evade the immune system. ICIs block these interactions and allow for immune overactivation to take place. Unfortunately, this same mechanism also blocks the normal negative signaling of T cells, leading to autoimmune-like toxicities known as immune-related adverse events (irAEs) (1).
irAEs can affect nearly every organ system and be life-threatening. While most irAEs are reversible with steroid treatment, some are irreversible. Endocrinopathies account for 4-30% of reported irAEs and are usually permanent (1). The most common immune-mediated endocrinopathy is hypothyroidism, which is present in about 10% of patients treated with anti-PD-1 monotherapy, but easily treatable (1). In contrast, ICI-induced type 1 diabetes mellitus (T1DM) is rare, but associated with significant long-term morbidity.
The mechanism of ICI-induced T1DM is not fully understood. Both autoimmune and ICI-induced T1DM are thought to be caused when autoreactive CD4+ T cells, CD8+ T cells, and B lymphocytes destroy the insulin-producing beta cells in the islets of Langerhans. ICI-induced T1DM is only sometimes associated with positive T1DM-associated autoantibodies, suggesting a unique pathophysiology (2). Common islet cell protein targets of these autoantibodies include glutamic acid decarboxylase (GAD), insulin (IA), insulinoma associated antigen-2 (IAA), zinc transporter 8 (ZnT8), and islet cells (ICA). Given the serious and irreversible nature of ICI-induced T1DM, it is critical to identify biomarkers to predict risk of this rare complication. While genetic associations with human leukocyte antigens (HLA) have been documented in autoimmune T1DM, HLAs specific for irAEs are an exciting new field (3, 4). Predictive biomarkers of ICI-induced T1DM have also been proposed but are not yet fully understood (5).
Despite several published cohorts and case reports of ICI-induced T1DM, impact on treatment outcomes remains unclear given the small number of patients reported thus far (2, 6–12). About half of the patients in these cohorts were treated for melanoma. Nearly all received anti-PD1 or PD-L1 therapy for active treatment in stage IV disease as well as adjuvant therapy in other stages. The clinical course in these cohorts is well described. On average, the time to T1DM diagnosis is approximately 23 weeks, 60% of patients present in diabetic ketoacidosis, 40% of patients have positive autoantibodies, and 80% of patients have low or undetectable c-peptide (2, 6–12). However, treatment response data is reported for only a few studies, with variable response rates. Four cohorts reported positive treatment response data (7–10). These cohorts showed objective response rates (ORR) of up to 80-70% in patients with melanoma although results varied (7–10). These data suggest greater radiographic clinical response in melanoma patients with ICI-induced T1DM compared to historical controls (reported ORR 30-50%). To better characterize clinical outcomes, we reviewed all cases of ICI-induced T1DM at our institution. Here we report clinical and proteomic data for this large cohort of patients after long-term follow-up.
This was a single-center case series of adult patients with solid tumors at Huntsman Cancer Institute at the University of Utah Health. Electronic medical records were searched for patients who received ICIs (ipilimumab, pembrolizumab, nivolumab, cemiplimab, atezolizumab, durvalumab and avelumab) during an 12-year period (2011 to 2023). To identify T1DM diagnoses after ICI therapy, the enterprise data warehouse (EDW) at the University of Utah used ICD codes for T1DM (ICD-10-CM E10 and ICD-9-CM 250.01) or obtained C-peptide levels measured after starting ICI therapy. A chart review of these cases was then conducted to confirm diagnosis of ICI-induced T1DM.
Chart review confirmed ICI-induced T1DM cases if they met the following inclusion criteria: sustained insulin dependence with a low or undetectable C-peptide level through longitudinal follow-up after high dose steroids were stopped if they were started. Patients were excluded due to the following reasons: 1) pre-existing T1DM diagnosis, 2) C-peptide drawn for other reasons in non-diabetic patient, 3) non-insulin-dependent worsening of preexisting T2DM, 4) new T2DM diagnosis, 5) worsened hyperglycemia related to steroid use and 6) insufficient data.
Blood chemistry and complete blood count prior to ICI therapy, at six weeks prior to T1DM development, and three weeks prior to T1DM development were available for 11 of 18 patients. Lab data was analyzed by two-tailed, two-sample t-tests for significant changes from baseline to investigate possible association with the development of T1DM.
Through an IRB-approved protocol, peripheral blood was collected from patients with melanoma prior to starting immunotherapy (baseline levels). Plasma was then isolated and stored at –80C. Baseline plasma from 2 patients who later developed ICI induced T1DM and 5 patients who did not have any irAE were available for study and sent for a multiplexed Proximity Extension Assay (PEA), developed by Olink Proteomics (Uppsala, Sweden). This assay was used to measure protein abundance directly in the plasma obtained from patients. In brief, the PEA is a dual recognition assay, where each protein is recognized by oligonucleotide-labeled antibody probes, then amplified and quantified by PCR. The baseline abundance levels of 1472 plasma proteins were analyzed using 4 pre-defined PEA-panels (Olink® Multiplex Inflammation, Oncology, Cardiometabolic and Neurology). The results were analyzed using the Olink® proteomics program that can be found at https://olink.com/products-services/data-analysis-products/olink-statistical-analysis-app/.
Between April 14, 2011, and April 1, 2023, 75 of 3744 patients who received ICIs at HCI had a T1DM ICD-10 diagnosis or C-peptide levels measured after the first cycle (Figure 1). Of these, 23 (35%) were identified as having type II diabetes without use of insulin, and five (7%) had C-peptide levels not indicative of insulin dependence. Seven (9%) had a documented T1DM diagnosis prior to ICI treatment, ten (13%) developed non-insulin dependent T2DM during treatment, seven (9%) had worsening of T2DM during ICI treatment leading to a nonpermanent insulin requirement, and two patients (3%) did not have sufficient data to confirm a T1DM diagnosis outside of their listed diagnosis. Eighteen patients were confirmed to have ICI-induced T1DM with a prevalence of 0.48% and are summarized in Table 1. There were eleven males and seven females. The mean age at the time of treatment was 61 years (range 32-75). All patients were diagnosed between 2012-2022. None of these patients had a history of diabetes prior to starting ICIs (Figure 2).
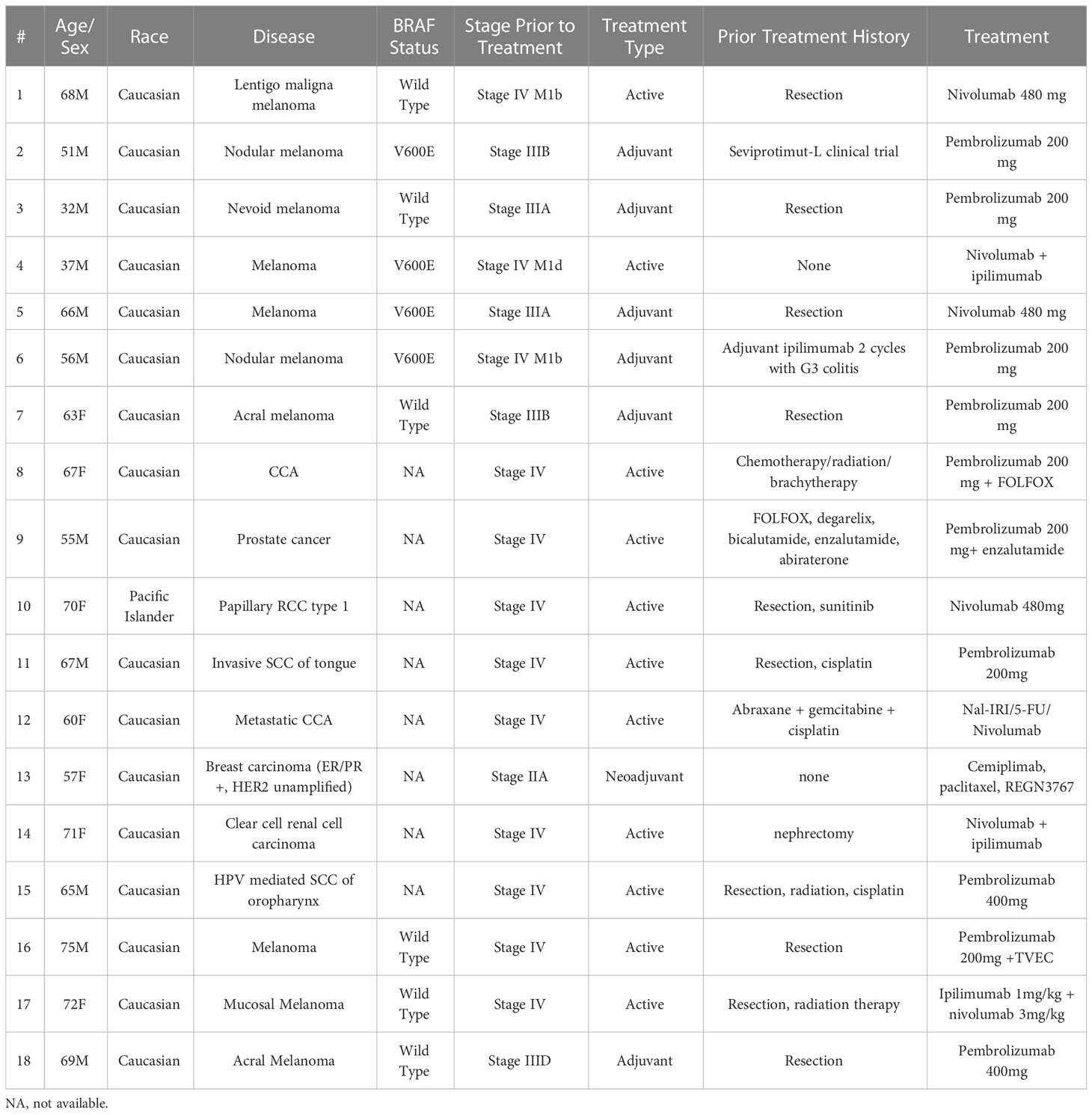
Table 1 Baseline clinical characteristics of patients prior to development of immune checkpoint inhibitor-induced diabetes.
Malignancies included 10 melanomas (1 lentigo maligna, 2 nodular, 1 nevoid, 2 acral, 1 mucosal and 3 unspecified), 2 cholangiocarcinomas, 1 prostate cancer, 1 papillary renal cell carcinoma, 1 clear cell renal carcinoma, 2 head and neck squamous cell carcinomas, and 1 invasive breast ductal carcinoma (Table 1). In the melanoma group, 4 of 10 patients had a BRAF V600E mutation, 2 were stage IIIA, 2 were stage IIIB, 1 was stage IIID and 5 were stage IV at the time of initiating ICIs. Of the patients with other malignancies, 7 were stage IV, while one was stage IIA on neoadjuvant therapy. Treatment history prior to ICIs varied widely among the patients. Six melanoma patients had prior surgery, one prior surgery and radiation, and 5 of 6 started ICIs as adjuvant therapy. The sixth had recurrence and subsequently started therapy for unresectable disease. One patient was previously treated with ipilimumab that was later stopped, 1 was on a seviprotimul-L clinical trial, and 1 had no prior treatment. Of the non-melanoma patients, prior treatments were more diverse, as shown in Table 2. Two had prior chemotherapy/radiation, 3 had prior chemotherapy, 1 had radiation therapy and 2 were treated with kinase inhibitors. Only 3 of the non-melanoma patients had prior surgical resections. Ten of 16 patients received anti-PD1 monotherapy (8 pembrolizumab, 3 nivolumab), 2 received pembrolizumab plus chemotherapy (FOLFOX) or enzalutamide, 1 received nivolumab plus chemotherapy (nanoliposomal irinotecan/fluorouracil), 1 received cemiplimab, anti-LAG-3 plus chemotherapy (REGN3767, an anti-lymphocyte activation gene 3 protein, LAG-3 + paclitaxel), 1 received pembrolizumab and TVEC and 3 received ipilimumab plus nivolumab. Of the melanoma patients, 4 received active treatment, while the other 6 received adjuvant treatment. In the other cancers group, 7 received active treatment, while 1 received neoadjuvant treatment.
Sixteen patients (88%) had diabetic ketoacidosis at presentation (Table 3). Mean hemoglobin A1c on presented was 8.8 (range 6-11.4%), and glucose was 633 mg/dl (range 341-1021). The mean time to T1DM onset was 218 days (median 205, range 23-418 days) from ICI initiation. Median number of cycles completed prior to diagnosis was 6 (range 1-18). Interestingly onset of hyperglycemia was rapid and severe in some patients (Figure 3). Compared to baseline, glucose was usually significantly elevated at 3 weeks prior to diagnosis but not significantly different 6 weeks prior. Fifteen of 16 DKA patients had an elevated anion gap (mean 19, range 15-27). Serum bicarbonate was low in these patients (mean 13.6 mEq/l, range 5-24mEq/l). Urinary ketones were positive on all 11 of the patients checked. C-peptide was checked at this time in 12 patients; it was undetectable in 4 patients, low in 5, and normal in 3. Lipase levels were checked in 3 patients and elevated in one who had prior grade 3 pancreatitis as an irAE. Only 2 patients did not present with DKA, but both had elevated hemoglobin A1c (8.4 and 9.2) as well as elevated blood glucose (376 and 760) consistent with the rest of our cohort (Table 3). One of these 2 patients had an undetectable C-peptide, while the other had a low level when tested. All patients required insulin therapy through the duration of follow-up.
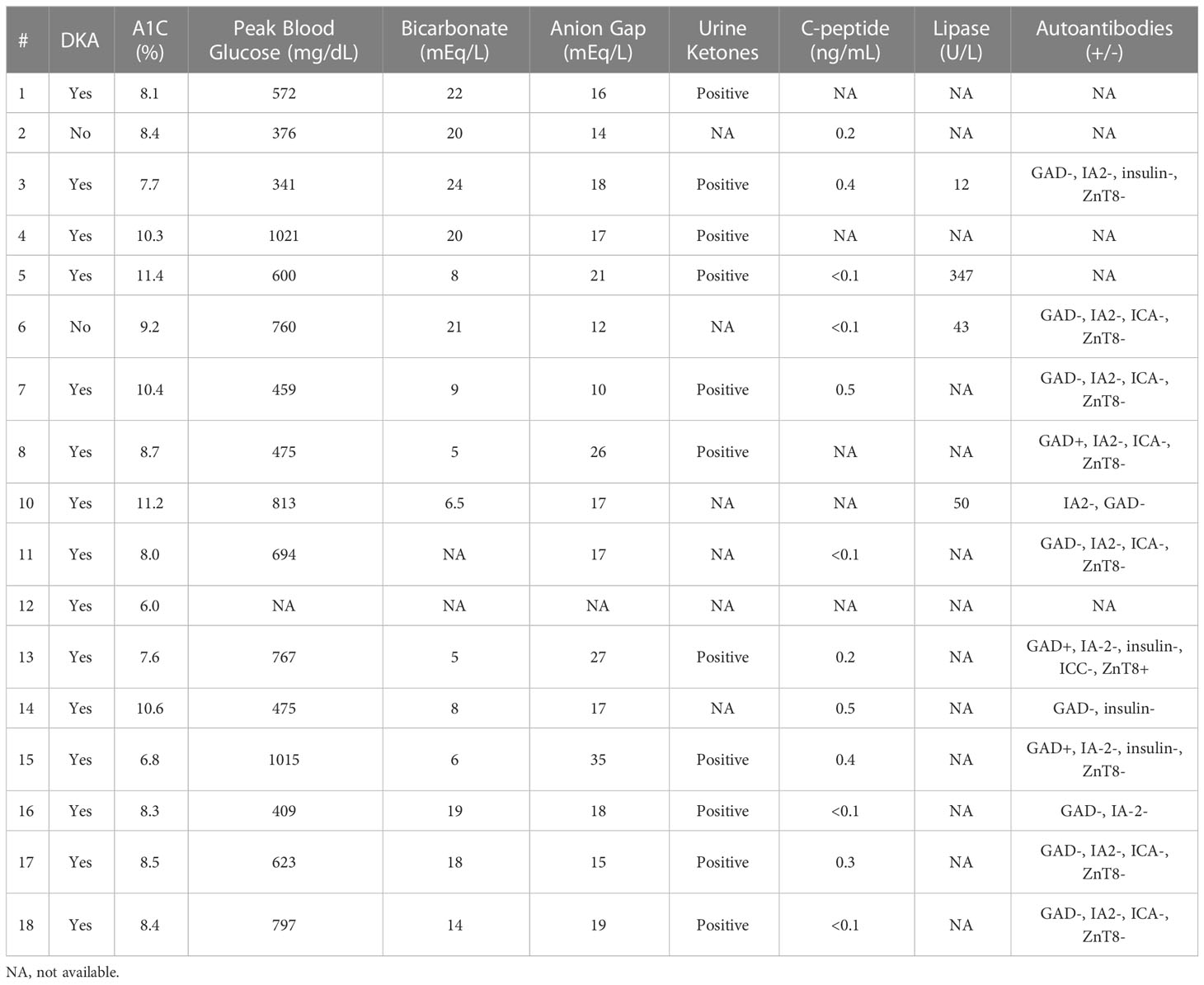
Table 3 Clinical presentation at time of initial identification of immune checkpoint inhibitor-induced type 1 diabetetps.
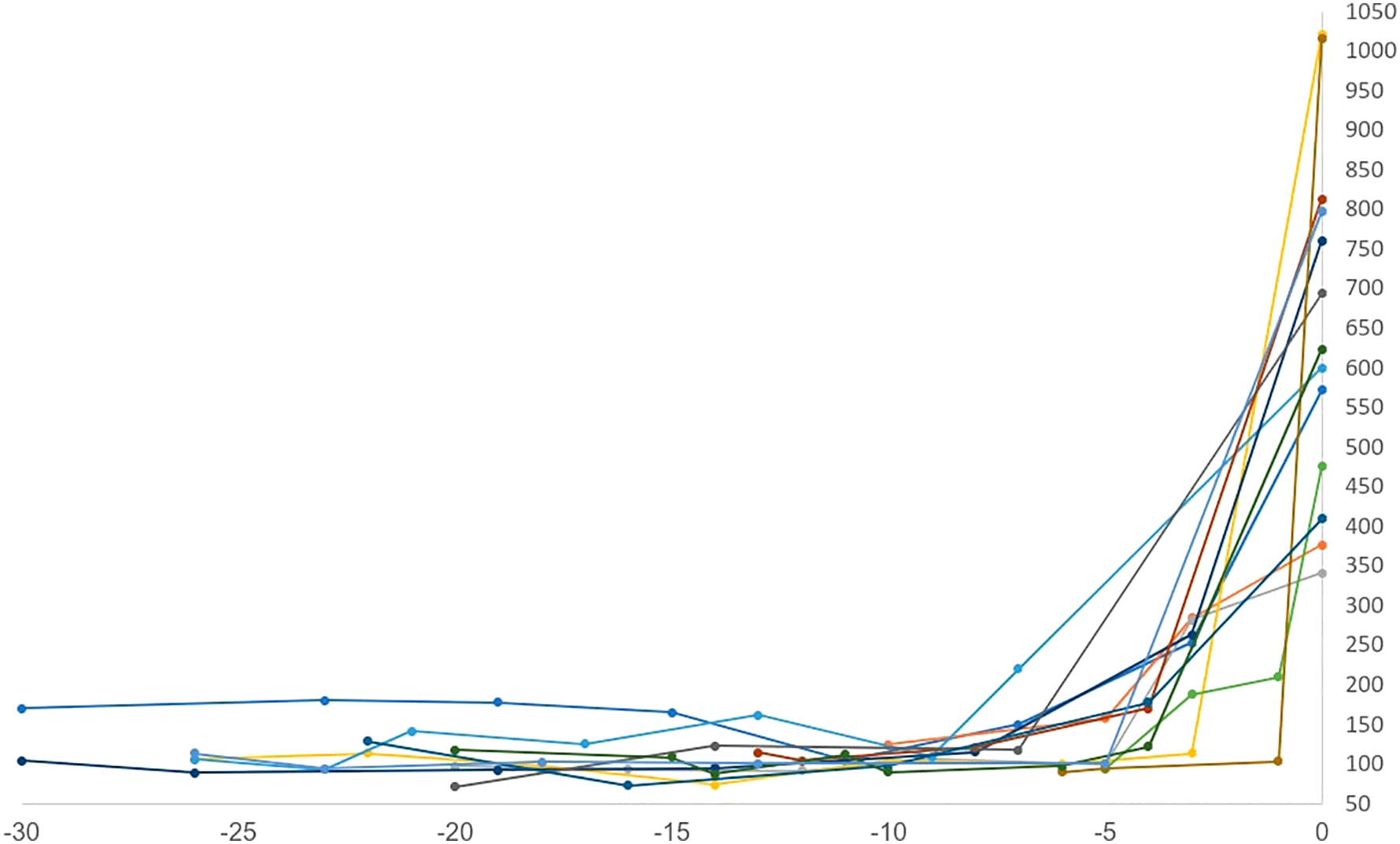
Figure 3 Time to onset (weeks) vs random serum glucose (mg/dl). Onset of hyperglycemia was rapid and severe for some patients.
Autoantibodies were tested in a total of 12 patients as shown in Table 2. Tested autoantibodies included anti-glutamic acid decarboxylase (anti-GAD), anti-insulin (IA), anti-insulinoma associated antigen-2 (IAA), anti-zinc transporter 8 (ZnT8), and anti-islet cells (ICA). Two had elevated anti-GAD, and another had both elevated anti-GAD and anti-ZnT8. Autoantibody status prior to T1DM diagnosis was not available for all patients. All patients presented in DKA.
Eight of the 18 patients experienced other endocrine irAEs. Seven of them experienced hypothyroidism requiring replacement medication, with 1 patient initially experiencing hyperthyroidism requiring medication (grade 2). One experienced grade 2 adrenal insufficiency. Eleven patients experienced a wide range of other irAEs that are shown in Table 2. Most of the experienced irAEs were grade 1 or 2, and common including liver enzyme elevations, fatigue, and arthralgias. Severe irAEs (grade 3 or greater) occurred in three of those 10 patients, 1 had grade 3 colitis, 1 had grade 3 pancreatitis, and 1 had a grade 3 rash. All required steroids and inpatient treatment. Of these 3 patients, 2 stopped their ICIs due to these other severe irAEs while 1 patient continued pembrolizumab after grade 3 colitis resolved. In total, ICIs were continued after the diagnosis of T1DM in six of the 18 patients, with most stopping due to ICI-induced T1DM.
At median follow-up of 21.9 months (range 8.4-82.4 months), no patients in the melanoma group had progressive disease or died from their cancer. One patient on active treatment for stage IV disease died as a likely complication of his T1DM, but had no evidence of cancer progression prior to this unfortunate event. For the 4 patients who received active treatment, the best responses were 2 complete response (CR) and 2 partial response (PR). The 6 patients who received adjuvant therapy had no evidence of disease at follow-up (median follow-up 35.9 months). Median progression-free survival and overall survival were not reached in these patients. Seven of 8 patients with other cancers received active treatment, and 1 received neoadjuvant treatment. In the active treatment group, the best responses were 1 PR, 2 stable disease (SD) and 4 with progression of disease. The patient who received neoadjuvant therapy had a complete pathologic response. At data cut off, 3 were deceased, 3 had progression of disease and 1 did not. Median PFS and OS were 2.7 months and 18.6 months, respectively.
PEA analysis of the 2 melanoma patients who developed ICI-induced T1DM versus 5 controls who did not develop any irAE was illustrated in the heat map (Figure 2). This is at the baseline prior to starting immunotherapy to show differences between the cohorts. Red illustrates higher expression while blue illustrates relatively lower expression in patients who developed ICI induced T1DM. While not statistically significant given the small sample size, there were visualized differences between groups. Lower levels of inflammatory pathway proteins (IL-6 and OSMR (oncostatin M regulator), present in IL-6 signaling) were found in the patients that developed ICI-induced T1DM. Furthermore, proteins related to metabolism were found to be increased in the ICI-induced T1DM patients. These included GLO1 (glyoxalase I), which is involved in the pyruvate pathway and glutathione reduction, and DXCR (dicarbonyl and L-xylulose reductase), which catalyzes diacetyl reductase and L-xylulose reductase reactions that play a role in glucose metabolism. ICI-induced T1DM patients also saw increased levels of certain proteins involved in cell signaling and communication. These included NPTN (neuroplastin), an Ig family transporter protein involved in cell-to-cell interactions, and ENPP5 (ectonucleotide pyrophosphatase/phosphodiesterase family member 5), a transmembrane glycoprotein that functions in neuronal cell communication.
To our knowledge, this is the largest reported case series describing oncologic outcomes, clinical attributes, and proteomic data from patients that developed ICI-induced T1DM at a single institution. While melanoma patients had excellent outcomes (100% ORR), in other solid tumors, outcomes were mixed, with most patients having progression of disease. All patients received anti-PD-1 therapy (nivolumab, cemiplimab or pembrolizumab) either as sole immunotherapy or, for 3 patients, as nivolumab in combination with ipilimumab. This is consistent with prior literature documenting anti-PD-1 therapy as opposed to anti-CTLA-4 (ipilimumab) therapy having a stronger predilection for disease (13). The mechanism underlying this observation is complex, but prior animal studies have shown that the PD-1/PD-L1 axis is more important to the self-tolerance of pancreatic beta cells than CTLA-4, given the low rates of ICI-induced T1DM from ipilimumab (14).
Our case series had important clinical implications. Patients began to show significantly elevated glucose levels 3 weeks prior to T1DM onset compared to pre-ICI baseline (Figure 3). This is a unique finding not reported in prior studies of predictive biomarkers, or prior case series (2, 5–12). Of note, glucose levels were not significantly elevated 6 weeks prior to presentation. This finding, taken in conjunction with some patients presenting in DKA with A1c <6.5%, suggests that the progression of pancreatic beta cell destruction and resulting glucose intolerance is very rapid, with development of the disease in a short period of time, in the span of days to weeks. Any significant increase in glucose on routine testing should prompt further evaluation or, at minimum, increased monitoring for ICI-induced T1DM. Early diagnosis is the only way to prevent further complications such as DKA. Through duration of follow-up, all patients required insulin therapy, highlighting the known irreversibility of this irAE (15). Interestingly, despite a lack of contraindication to resuming therapy, most patients did not receive further ICI therapy after developing T1DM. It is important for clinicians to recognize that ICI-induced T1DM should not preclude patients from further ICI therapy if indicated.
There are several proposed distinct subtypes of ICI-induced T1DM (16). Fulminant T1DM, characterized by rapid onset of T1DM presenting with DKA, usually presents with normal or near normal A1c (16). Two of our patients with A1c <7 may represent this subtype. Another proposed subtype is a phenotype like “decompensated” type 2 diabetes, which presents with a high A1c and detectable C-peptide, but new insulin dependency with or without prior history of type 2 diabetes. Five of our patients presenting with high A1c and low C-peptide levels were consistent with this group. One would expect positive autoantibody prevalence reflecting the proposed mechanism, with a more frequent prevalence in the fulminant variety, and less prevalent in a phenotype like type 2 diabetic decompensation. Another proposed cause is secondary to prior ICI induced pancreatitis, which is possible in our patient with an elevated lipase. Few patients had a lipase level checked, and it would be helpful in future workup. In our case series, only 3 of the 10 tested patients had positive autoantibodies which is similar prevalence to other published cohorts (30% vs 40-50%). It might be explained that most of the subtypes we observed were more like decompensated T2DM rather than a fulminant T1DM which could be present in slightly higher abundance in other studies (2, 6–12). Prior cohorts have suggested some correlation between the number of positive autoantibodies and shortened time from treatment to onset of T1DM (10). The three patients in our cohort did not support any clear conclusions, having time to onset comparable to our average.
Thirteen of 18 patients (72%) experienced at least 1 other irAE, with most experiencing multiple irAEs. Endocrinopathies are relatively common in patients with ICI-induced T1DM. One systematic review found ICI-induced thyroid dysfunction in 24% of cases (2). Eight in our cohort (44%) had other endocrine irAEs, comparable to published literature. It is not clear if prior severe irAEs are a predictor of future severe irAEs (T1DM). Only three of 18 patients had developed a severe (grade 3 or higher) irAE prior to their diagnosis of T1DM. Severe irAEs have been previously cited in one large case series as a poor prognostic factor, as patients are not often able to resume their ICI therapy (17). In our cohort, this was not the case, as none of these patients had progression of disease.
While limited by small sample size, our PEA analysis suggested several interesting relative differences. Decreased levels of proteins involved in inflammatory pathways such as IL-6, OSMR, and AMBP in patients who developed ICI-induced T1DM could help support the excellent response. IL-6 and OSMR play a role in tumor proliferation by activating transcription factors. Therapies targeting these pathways have been shown to be inhibitory to tumor growth (18). The IL-6 signaling pathway has also been described as promoting self-tolerance of pancreatic beta cells in autoimmune T1DM, although this is not well understood (19). Increased expression of proteins in metabolic pathways like GLO1 and DXCR were seen in T1DM patients without clear implications. GLO1 has been shown in several preclinical models to promote tumor cell proliferation, and one study showed GLO1 upregulating PD-L1 expression, suggesting positive treatment response when it is targeted (20, 21). Finally, increased levels of cell signaling proteins such as NPTN and ENPP5 were also noted. ENPP5 has been cited as a biomarker for increased insulin resistance in non-insulin dependent diabetes (22). NPTN is described as an important factor in T-cell activation, helping explain excellent response (23). This interpretation is entirely speculative and subject to confounding factors between patients but could be a clue for future larger studies with subject matching that is required for validation.
Despite ICI-induced T1DM, patients with melanoma displayed excellent clinical response and survival. No patient in this group experienced either progression of disease in the active treatment group or recurrence in the adjuvant group. In comparison, the other malignancies group only had two on active therapy that did not progress. Prior case series note a favorable response in melanoma compared to around 50% response rates to anti-PD-1 therapy in melanoma in general (24). More specifically immunotherapy trials involving patients with advanced (stage III or IV), unresectable disease noted a PFS over a comparable time period in the 40-50% range (25, 26). While patients with lower recurrence stage IIIA or IIIB disease need longer follow up times to determine clinical outcome, the 5 patients with metastatic disease all responded and none have relapsed with a mean follow up time of 39 months. Reasons for this excellent clinical response are difficult to determine although proteomic data may help guide future research. While there is a known association between severe irAEs and good clinical outcomes, in many instances this may be due to immortal time bias (27).
While our search criteria were broad, and our prevalence falls within the expected range, it is possible some patients with ICI-induced T1DM were not included. There was a patient identified during our EDW based screening who could not be included due to lack of documentation of lab testing needed to validate the diagnosis as ICI-induced T1DM. Other patients could have been missed by our search entirely, as our institution is a referral center for one of the country’s largest geographic catchments, and many patients were more likely to be hospitalized acutely closer to home. This also highlights the necessity of further diagnostic testing when patients present with possible irAEs or suspicious history. Further diagnostic testing of great interest would be HLA typing in these patients, which was not available in our study. Our case series is biased heavily towards melanoma, and it is difficult to see trends with other malignancies. While the response in melanoma patients was excellent, it is limited by the small size of the cohort. The follow up time for our patients with stage IIIA or IIIB melanoma may not be long enough to see a difference given the long PFS known for these stages.
As a point of future study, germline, HLA, and immunophenotypic data could be helpful in providing insight and predictive value in determining which patients are at higher risk of ICI-induced T1DM. Clinicians should be aware of the rapid onset and irreversibility of ICI-induced T1DM, with evidence of elevated blood glucose 3 weeks prior to diagnosis. Positive treatment outcomes in patients with melanoma who develop ICI-induced T1DM may help guide treatment considerations. Larger studies are needed to elucidate strategies to identify high-risk patients and improve treatment outcomes for this rare irAE.
The original contributions presented in the study are included in the article/supplementary materials. Further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by 00138167. The patients/participants provided their written informed consent to participate in this study.
JM and JPM identified cases. JM and SH-L verified and analyzed patient clinical data. SH-L and MK analyzed proteomic data. JM and JPM drafted the manuscript. All authors contributed to the article and approved the submitted version.
This research is partially supported by the Huntsman Cancer Institute at the University of Utah Cancer Center Support Grant P30CA042014. The biostatistical resources used were partially funded by the NIH Shared Instrumentation Grant 1S10OD021644-01A1.
We would like to thank Anne Lyons medical informationist who helped in our search as well as our patients for participating in this study.
SH-L have been scientific advisor/consultant for: Amgen, Astellas, BMS, Genmab, Merck, Nektar, Neon Therapeutics, Novartis, Regeneron, Vaccinex, Xencor, and have done contracted research through affiliated institutions with: Astellas, BioAtla, BMS, Boehringer Ingelheim, Checkmate, Dragonfly, F Star, Genentech, Kite Pharma, Merck, Neon Therapeutics, OncoC4, Pfizer, Plexxikon, Vaccinex, Vedanta, Xencor.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
irAE, immune-related adverse event; ICI, immune checkpoint inhibitor; T1DM, type 1 diabetes mellitus; CTLA-4, cytotoxic T lymphocyte associated antigen-4; PD-1, programmed cell death-1; GAD, glutamic acid decarboxylase; IA, insulin; IAA, insulinoma associated antigen-2; ZnT8, zinc transporter 8; ICA, islet cells; ORR, objective response rate; PEA, Proximity Extension Assay; EDW, enterprise data warehouse; CBC, complete blood count; BMP, basic metabolic panel; PCR, polymerase chain reaction; NPX, normalized protein expression; DKA, diabetic ketoacidosis; CCA, cholangiocarcinoma; TVEC, talimogene laherparepvec; PFS, progression free survival; OFS, overall survival; OSMR, oncostatin M regulator; IL-6, interleukin-6; AMBP, alpha-1-microglobulin; GLO1, glyoxalase 1; DXCR, dicarbonyl and L-xylulose reductase; NPTN, neuroplastin; ENPP5, Ectonucleotide pyrophosphatase/phosphodiesterase family member 5; TNFSF10, tumor necrosis factor superfamily member 10.
1. Wright JJ, Powers AC, Johnson DB. Endocrine toxicities of immune checkpoint inhibitors. Nat Rev Endocrinol (2021) 17(7):389–99. doi: 10.1038/s41574-021-00484-3
2. de Filette JMK, Pen JJ, Decoster L, Vissers T, Bravenboer B, van der Auwera BJ, et al. Immune checkpoint inhibitors and type 1 diabetes mellitus: a case report and systematic review. Eur J Endocrinol (2019) 181(3):363–74. doi: 10.1530/EJE-19-0291
3. Hosokawa Y, Hanafusa T, Imagawa A. Pathogenesis of fulminant type 1 diabetes: Genes, viruses and the immune mechanism, and usefulness of patient-derived induced pluripotent stem cells for future research. J Diabetes Investig (2019) 10(5):1158–64. doi: 10.1111/jdi.13091
4. Akturk HK, Couts KL, Baschal EE, Karakus KE, Van Gulick RJ, Turner JA, et al. Analysis of human leukocyte antigen DR alleles, immune-related adverse events, and survival associated with immune checkpoint inhibitor use among patients with advanced Malignant melanoma. JAMA Netw Open (2022) 5(12):e2246400. doi: 10.1001/jamanetworkopen.2022.46400
5. Inaba H, Kaido Y, Ito S, Hirobata T, Inoue G, Sugita T, et al. Human leukocyte antigens and biomarkers in type 1 diabetes mellitus induced by immune-checkpoint inhibitors. Endocrinol Metab (Seoul) (2022) 37(1):84–95. doi: 10.3803/EnM.2021.1282
6. Yun K, Daniels G, Gold K, Mccowen K, Patel SP. Rapid onset type 1 diabetes with anti-PD-1 directed therapy. Oncotarget (2020) 11(28):2740–6. doi: 10.18632/oncotarget.27665
7. Muniz TP, Araujo DV, Savage KJ, Cheng T, Saha M, Song X, et al. CANDIED: A pan-canadian cohort of immune checkpoint inhibitor-induced insulin-dependent diabetes mellitus. Cancers (Basel) (2021) 14(1):89. doi: 10.3390/cancers14010089
8. Byun DJ, Braunstein R, Flynn J, Zheng J, Lefkowitz RA, Kanbour S, et al. Immune checkpoint inhibitor-associated diabetes: A single-institution experience. Diabetes Care (2020) 43(12):3106–9. doi: 10.2337/dc20-0609
9. Stamatouli AM, Quandt Z, Perdigoto AL, Clark PL, Kluger H, Weiss SA, et al. Collateral damage: insulin-dependent diabetes induced with checkpoint inhibitors. Diabetes (2018) 67(8):1471–80. doi: 10.2337/dbi18-0002
10. Tsang VHM, McGrath RT, Clifton-Bligh RJ, Scolyer RA, Jakrot V, Guminski AD, et al. Checkpoint inhibitor-associated autoimmune diabetes is distinct from type 1 diabetes. J Clin Endocrinol Metab (2019) 104(11):5499–506. doi: 10.1210/jc.2019-00423
11. Kotwal A, Haddox C, Block M, Kudva YC. Immune checkpoint inhibitors: an emerging cause of insulin-dependent diabetes. BMJ Open Diabetes Res Care (2019) 7(1):e000591. doi: 10.1136/bmjdrc-2018-000591
12. Wright JJ, Salem JE, Johnson DB, Lebrun-Vignes B, Stamatouli A, Thomas JW, et al. Increased reporting of immune checkpoint inhibitor-associated diabetes. Diabetes Care (2018) 41(12):e150–1. doi: 10.2337/dc18-1465
13. Clotman K, Janssens K, Specenier P, Weets I, De Block CEM. Programmed cell death-1 (PD-1) inhibitor induced type 1 diabetes mellitus: mini-review. J Clin Endocrinol Metab (2018) 103(9):3144–54. doi: 10.1210/jc.2018-00728
14. Quandt Z, Young A, Anderson M. Immune checkpoint inhibitor diabetes mellitus: a novel form of autoimmune diabetes. Clin Exp Immunol (2020) 200(2):131–40. doi: 10.1111/cei.13424
15. Aleksova J, Lau PK, Soldatos G, McArthur G, Sørgjerd EP, Thorsby PM, et al. Glucocorticoids did not reverse type 1 diabetes mellitus secondary to pembrolizumab in a patient with metastatic melanoma Presence of anti-GAD in a non-diabetic population of adults; time dynamics and clinical influence: results from the HUNT study. BMJ Case Rep BMJ Open Diabetes Res Care (2016) 20163(1):bcr2016217454:e000076. doi: 10.1136/bcr-2016-21745410.1136/bmjdrc-2014-000076
16. Marchand L, Disse E, Dalle S, Reffet S, Vouillarmet J, Fabien N, et al. The multifaceted nature of diabetes mellitus induced by checkpoint inhibitors. Acta Diabetol (2019) 56(12):1239–45. doi: 10.1007/s00592-019-01402-w
17. Matsuoka H, Hayashi T, Takigami K, Imaizumi K, Shiroki R, Ohmiya N, et al. Correlation between immune-related adverse events and prognosis in patients with various cancers treated with anti PD-1 antibody. BMC Cancer (2020) 20(1):656. doi: 10.1186/s12885-020-07142-3
18. Geethadevi A, Nair A, Parashar D, Ku Z, Xiong W, Deng H, et al. Oncostatin M receptor-targeted antibodies suppress STAT3 signaling and inhibit ovarian cancer growth. Cancer Res (2021) 81(20):5336–52. doi: 10.1158/0008-5472.CAN-21-0483
19. Rajendran S, Anquetil F, Quesada-Masachs E, Graef M, Gonzalez N, McArdle S, et al. IL-6 is present in beta and alpha cells in human pancreatic islets: Expression is reduced in subjects with type 1 diabetes. Clin Immunol (2020) 211:108320. doi: 10.1016/j.clim.2019.108320
20. Jandova J, Wondrak GT. Genomic GLO1 deletion modulates TXNIP expression, glucose metabolism, and redox homeostasis while accelerating human A375 Malignant melanoma tumor growth. Redox Biol (2021) 39:101838. doi: 10.1016/j.redox.2020.101838
21. Antognelli C, Mandarano M, Prosperi E, Sidoni A, Talesa VN. Glyoxalase-1-dependent methylglyoxal depletion sustains PD-L1 expression in metastatic prostate cancer cells: A novel mechanism in cancer immunosurveillance escape and a potential novel target to overcome PD-L1 blockade resistance. Cancers (Basel) (2021) 13(12):2965. doi: 10.3390/cancers13122965
22. Pujar MK, Vastrad B, Vastrad C. Integrative analyses of genes associated with subcutaneous insulin resistance. Biomolecules (2019) 9(2):37. doi: 10.3390/biom9020037
23. Korthals M, Langnaese K, Smalla KH, Kähne T, Herrera-Molina R, Handschuh J, et al. A complex of Neuroplastin and Plasma Membrane Ca2+ ATPase controls T cell activation. Sci Rep (2017) 7(1):8358. doi: 10.1038/s41598-017-08519-4
24. Das S, Johnson DB. Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. J Immunother Cancer (2019) 7(1):306. doi: 10.1186/s40425-019-0805-8
25. Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Rutkowski P, Lao CD, et al. Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med (2019) 381(16):1535–46. doi: 10.1056/NEJMoa1910836
26. Wolchok JD, Chiarion-Sileni V, Gonzalez R, Grob JJ, Rutkowski P, Lao CD, et al. Long-term outcomes with nivolumab plus ipilimumab or nivolumab alone versus ipilimumab in patients with advanced melanoma. J Clin Oncol (2022) 40(2):127–37. doi: 10.1200/JCO.21.02229
Keywords: immune checkpoint inhibitors, immunotherapy, immune-related adverse events, type 1 diabetes, proteomics
Citation: Marsiglio J, McPherson JP, Kovacsovics-Bankowski M, Jeter J, Vaklavas C, Swami U, Grossmann D, Erickson-Wayman A, Soares HP, Kerrigan K, Gibson B, Doherty JA, Hyngstrom J, Hardikar S and Hu-Lieskovan S (2023) A single center case series of immune checkpoint inhibitor-induced type 1 diabetes mellitus, patterns of disease onset and long-term clinical outcome. Front. Immunol. 14:1229823. doi: 10.3389/fimmu.2023.1229823
Received: 27 May 2023; Accepted: 01 August 2023;
Published: 21 August 2023.
Edited by:
Halis Kaan Akturk, University of Colorado Anschutz Medical Campus, United StatesReviewed by:
Jonathan Chee, University of Western Australia, AustraliaCopyright © 2023 Marsiglio, McPherson, Kovacsovics-Bankowski, Jeter, Vaklavas, Swami, Grossmann, Erickson-Wayman, Soares, Kerrigan, Gibson, Doherty, Hyngstrom, Hardikar and Hu-Lieskovan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Siwen Hu-Lieskovan, c2l3ZW4uaHUtbGllc2tvdmFuQGhjaS51dGFoLmVkdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.