- Department of Biotechnological and Applied Clinical Sciences, University of L’Aquila, L’Aquila, Italy
Background: The idea of psoriatic disease continuum has been progressively prompted based on the advances of the knowledge about the pathogenic steps underpinning the occurrence of psoriasis (PSO) and psoriatic arthritis (PSA). To evaluate biomolecules (inflammatory cytokines, inflammatory chemokines, cell adhesion and cellular mediators) in naïve patients with PSO, PSA with PSO, and PSA sine PSO. To stratify the results considering the presence of psoriatic nail involvement, extensive skin disease and obesity evaluating all involved patients.
Methods: By multiplex technology, 20 serum biomolecules were assessed with the inclusion of pro-inflammatory cytokines (GM-CSF, IFN-γ, IL-1α, IL-1β, IL-6, IL-8, IL-12p70, IL-17A, IL-23, TNF), anti-inflammatory cytokines (IFN-α, IL-4, IL-10, IL-13), inflammatory chemokines (IP-10, MCP-1, MIP-1α, MIP-1β), cell adhesion and cellular mediators (ICAM-1, E-selectin, P-selectin). The assessment of possible statistical differences between the means of the three groups was performed by One-Way ANOVA. In addition, by non-parametric T-tests, we stratified the results according to selected clinical characteristics (psoriatic nail involvement, PASI ≥ 10, BMI ≥ 30).
Results: In 80 assessed naïve patients, patients with PSO showed significant increases of E-selectin (p=0.021) and IL-8 (0.041) than other groups. In patients with PSA with PSO, significant higher levels of ICAM-1 were observed (p=0.009) than other groups. We did not observe further differences comparing pro-inflammatory and anti-inflammatory cytokines, inflammatory chemokines, and cell adhesion and cellular mediators in patients with PSO, PSA with PSO, and PSA sine PSO. Patients with psoriatic onychopathy showed significant increased levels of ICAM-1 (p=0.010) and IP-10 (0.030) than others. In patients with PASI ≥ 10, significantly enhanced values of IL-8 (p=0.004), TNF (p=0.013), E-selectin (p=0.004), MIP-1α (p=0.003), and MIP-1β (p=0.039). In patients with BMI ≥ 30, significantly higher levels of E-selectin were pointed out (p=0.035) than others.
Conclusion: Our findings may suggest that a similar cytokine profile may characterize naïve patients with PSO, PSA with PSO, and PSA sine PSO, reinforcing the concept of psoriatic disease continuum. However, some differences may be also shown, underlying possible pathogenic differences and leading to the clinical heterogeneity of these patients.
Introduction
The idea of psoriatic disease continuum has been progressively prompted based on the advances of the knowledge about the pathogenic steps underpinning the occurrence of psoriasis (PSO) and psoriatic arthritis (PSA) (1). PSO is an inflammatory skin disease characterized by erythematous and scaly papules and plaques, located predominantly on the elbows, knees, and scalp (2, 3). Among patients with PSO, musculoskeletal inflammatory manifestations, involving peripheral joints, entheses, and axial skeleton, may be observed due to the occurrence of PSA (2). The latter may also be recognized in patients without a clinically evident PSO but with a suggestive family history; this is named PSA sine PSO (2).
Concerning the pathogenesis of psoriatic disease continuum, a complex and multidimensional model has been recently proposed linking the inflammatory mechanisms underlying both skin and joint manifestations (1–3). On a predisposing genetic background, a combination of environmental triggering factors (smoking, biomechanical stress, infections, or obesity), and an aberrant immune response may induce the development of a chronic inflammatory process (3). The resulting dysregulation of the innate and adaptive arms of the immune system may be also stimulated by cutaneous tissue, microbiome, and/or the entheses (3). Given the inflammatory pathogenesis of PSO and PSA, the assessment of cytokines may have the potential to accurately reflect the ongoing cellular processes and signaling pathways (4). Cytokines comprises interleukins (ILs), chemokines, interferons, and tumor necrosis factors. All these mediators have many pleiotropic effects which are strongly associated with the pathogenesis of PSA and PSO (5). Thus, a specific cytokine profile could better identify specific subtypes of patients in the context of psoriatic disease and, in particular, referring to a potential cytokine phenotype at increased risk of disease transition from PSO to PSA.
In this study, we aimed at evaluating the cytokine profile in patients with PSO, PsA with PSO, and PsA sine PSO, to identify possible differences or similarity in these groups of the same disease spectrum. We also assessed these biomolecules considering the presence of psoriatic nail involvement, the extension of skin disease, and the presence of obesity evaluating all involved patients
Patients and methods
Consecutive patients were assessed among those attending the Dermatologic and/or Rheumatologic Clinics of the University of L’Aquila, L’Aquila, Italy and fulfilling classification criteria for PSO and/or PSA (6, 7). The treatment with systemic immunomodulating therapies for PSO and/or PSA was considered as a criterion of exclusion. The study was built on the assessment and comparison of three patient categories: i. PSO group, patients with evident skin involvement but without any musculoskeletal manifestation; ii. PSA with PSO group, patients with evident skin involvement and inflammatory musculoskeletal manifestations; iii. PSA sine PSO group, patients without an evident skin involvement but with inflammatory musculoskeletal manifestations. The cytokine profile of these patients was compared according to these three groups. In addition, patients were stratified considering the presence of psoriatic nail involvement, the extension of skin disease, and the presence of obesity. Severity and extension of skin involvement was evaluated by Psoriasis Area and Severity Index (PASI) and the nail involvement by Nail Area and Severity Index (NAPSI) (8, 9). Furthermore, obese patients were defined as those with body mass index (BMI) ≥ 30.
Written informed consents for all involved patients were collected allowing the use of retrieved findings for scientific purposes. The study protocol was submitted to the Local Ethics Committee (Comitato Etico Azienda Sanitaria Locale 1 Avezzano/Sulmona/L’Aquila, L’Aquila, Italy; protocol number 0204194/22) and the Internal Review Board of the University of L’Aquila (protocol number Internal Review Board University of L’Aquila 01/2022).
Following the current procedures for clinical samples, collected peripheral blood samples from patients were processed and analyzed. Collection tubes were rotated at 20°C for 30’ and centrifuged at 2000g for 15’, to spin down blood cells in order to separate the serum from cellular components. Patient sera were stored at −80°C into Eppendorf tubes to the execution of the analysis. The assessment was performed at a laboratory service provider equipped with the technology of Luminex (19 Plex) for biomarkers assay service (Labospace srl - Milano IT). The sera biomolecules were assessed by multiplex technology following the instructions of the manufacturer (Inflammation 20-Plex Human ProcartaPlex™ Panel, EPX200-12185-901). Twenty biomolecules were assessed with the inclusion of inflammatory cytokines (GM-CSF, IFN-γ, IL-1α, IL-1β, IL-6, IL-8, IL-12p70, IL-17A, IL-23, TNF, IFN-α, IL-4, IL-10, IL-13), inflammatory chemokines (IP-10, MCP-1, MIP-1α, MIP-1β), cell adhesion and cellular mediators (ICAM-1, E-selectin, P-Selectin). The interpretation of results was performed by using a dedicated software.
Firstly, a descriptive statistic was made of patient clinical manifestations. Given their distribution, continuous variables were presented as mean/standard deviation (SD) or median/interquartile range (IQR), as appropriate. One-Way ANOVA test was used to compare the groups, considering the main categories and the selected clinical features (psoriatic nail involvement, PASI ≥ 10, BMI ≥ 30). After that, possible correlations were estimated among biomolecules, NAPSI, PASI, and BMI by Spearman’s rank correlation coefficient. Two-sided P values < 0.05 were considered as being statistically significant. Prism – GraphPad version 8.0 was used for all analyses.
Results
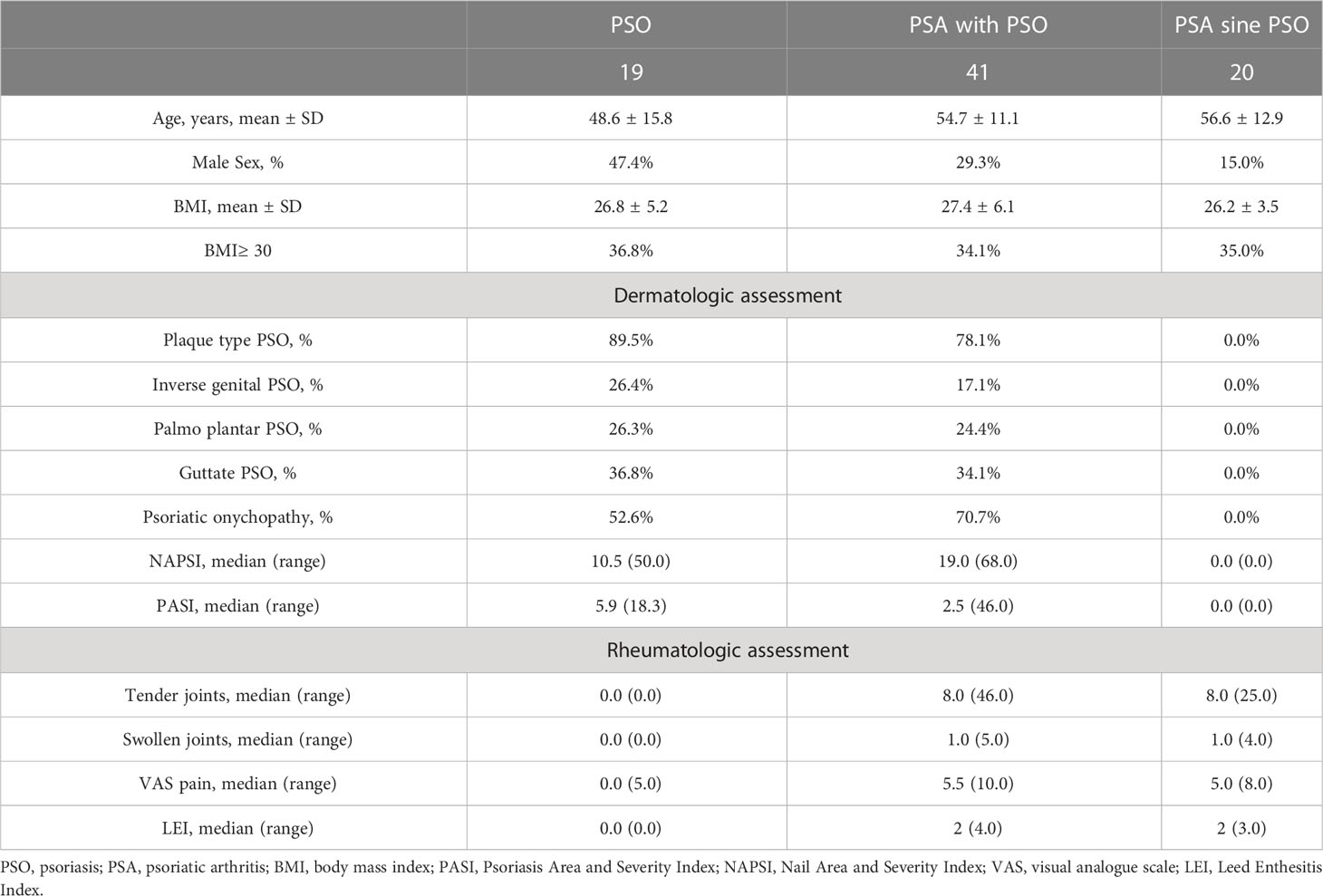
In this evaluation, 80 consecutive naïve patients were assessed, before the administration of systemic immunomodulating therapies, as reported in Table 1. In the PSO group, 19 patients were comprised (age: 48.6 ± 15.8 years, male sex 47.4%, BMI: 26.8 ± 5.2). Psoriatic onychopathy was observed in 52.6% of the assessed patients with PSO, NAPSI resulted to be median 10.5 (50.0) and PASI was median 5.9 (18.3). No tender or swollen joints were recorded in these patients. Forty-one PSA with PSO patients were assessed (age: 54.7 ± 11.1, male sex: 29.3%, BMI: 27.4 ± 6.0). In this group, 70.7% of patients showed psoriatic onychopathy, NAPSI resulted to be 19.0 (68.0) and PASI 2.5 (46.0). These patients affected by PSA with PSO were also characterized by tender [8.0 (46.0)] and swollen joints [1.0 (5.0)]. In addition, 20 patients with PSA sine PSO were evaluated (age: 56.6 ± 12.9, male sex: 15.0%, BMI: 26.2 ± 3.5). These patients did not show skin involvement but tender [8.0 (25.0)] and swollen joints [1.0 (4.0)].
Comparing the cytokine profile, some significant differences were observed in the three groups, as shown in Figure 1. Patients with PSO were characterized by a significant increase of levels of E-selectin (p=0.021) than patients with PSA with PSO or patients with PSA sine PSO. Similarly, levels of IL-8 (0.041) were significantly increased in patients with PSO in respect to others. In the PSA with PSO group, significant enhanced levels of ICAM-1 were detected (p=0.009) than patients with PSO and patients with PSA sine PSO. No additional significant differences were retrieved comparing other inflammatory cytokines, inflammatory chemokines, and cell adhesion and cellular mediators in patients with PSO, PSA with PSO, and PSA sine PSO. These results are summarized in Figure 2. The assessment of P-selectin and IFN-γ did not produce any result, these biomolecules appeared to be not expressed.
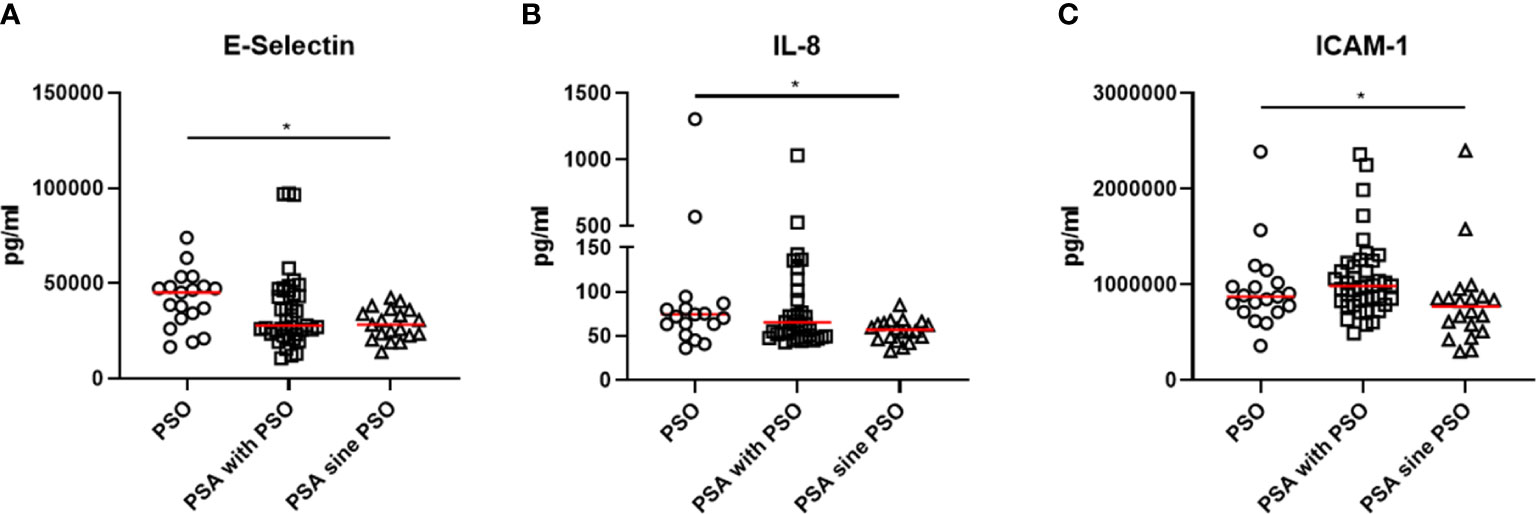
Figure 1 (A, B) increased levels of E-selectin and IL-8 were retrieved in patients with PSO than patients with PSA with PSO or patients with PSA sine PSO. (C) significant higher levels of ICAM-1 characterized patients with PSA than patients with PSO and patients with PSA sine PSO. *: p < 0.05. IL, interleukin, PSO, psoriasis; PSA, psoriatic arthritis; ICAM-1, Intercellular Adhesion Molecule 1.
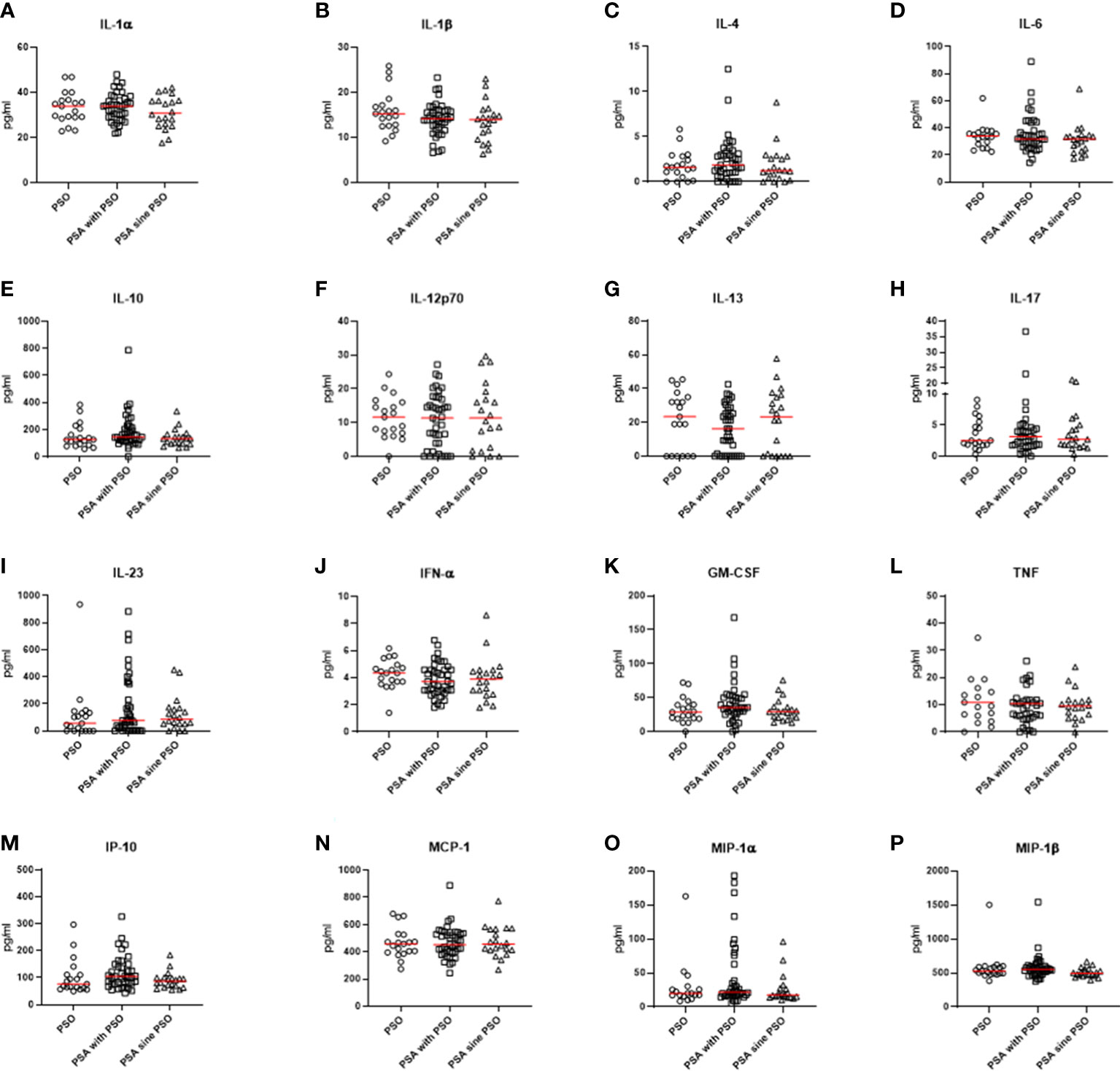
Figure 2 (A-P) No significant differences were observed comparing pro-inflammatory and anti-inflammatory cytokines, inflammatory chemokines, and cell adhesion and cellular mediators in patients with PSO, PSA with PSO, and PSA sine PSO. PSO, psoriasis; PSA, psoriatic arthritis; IL, interleukin; IFN-α, interferon α; GM-CSF, granulocyte-macrophage colony-stimulating factor; TNF, tumor necrosis factor; IP-10, interferon gamma-induced protein 10; MCP-1, Monocyte Chemoattractant Protein-1; MIP, Macrophage Inflammatory Proteins.
After that, our results were stratified according to selected clinical characteristics (i.e., psoriatic onychopathy, PASI ≥ 10, BMI ≥ 30) considering all involved patients, as shown in Figure 3. Patients characterized by psoriatic onychopathy showed significant enhanced values of ICAM-1 (p=0.010) and IP-10 (p=0.030) than others. Assessing possible associations among these results, a positive correlation between IL-8 and NAPSI was observed (R=0.233, p=0.046). Similarly, NAPSI directly correlated with ICAM-1 (R=0.235, p=0.045), and with MIP-1α (R=0.319, p=0.006). Furthermore, the results were analyzed based on the extension of skin involvement. In patients with PASI ≥ 10, significantly increased values of IL-8 (p=0.004) and TNF (p=0.013) were observed than others. Additionally, these patients were distinguished by significantly enhanced values of E-selectin (p=0.004), MIP-1α (p=0.003), and MIP-1β (p=0.039). Possible correlations between values of assessed biomolecules and PASI were also exploited. Direct correlations were also observed analyzing PASI and IL-8 (R=0.360, p=0.002) and PASI and TNF (R=0.238, p=0.042). Moreover, PASI positively correlated with E-selectin (R=0.649, p<0.001), MIP-1α (R=0.355, p=0.002), and MIP-1β (R=0.244, p=0.037), respectively. Finally, our results were assessed considering the presence of obesity. In patients with BMI ≥ 30, significantly higher levels of E-selectin were pointed out (p=0.035) than others. In addition, a monotonic effect between BMI and E-selectin was reported (R=0.339, p=0.003).
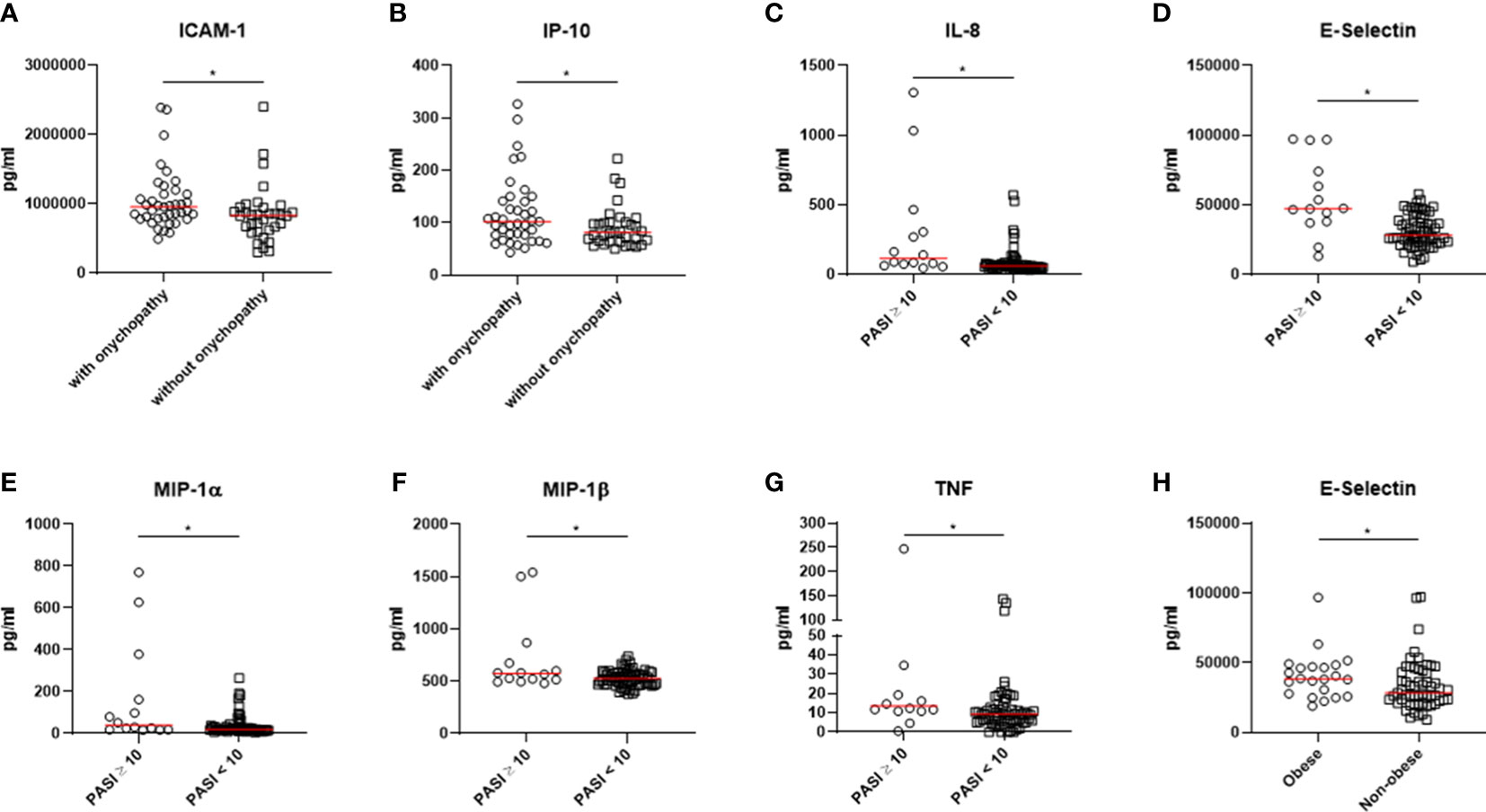
Figure 3 (A, B) Patients characterized by psoriatic onychopathy showed significant higher levels of ICAM-1 and IP-10. (C-G) Higher values of IL-8, TNF, E-selectin, MIP-1α, and MIP-1β were observed in patients with PASI ≥ 10. (H) Patients with BMI ≥ 30 were characterized by significantly higher levels of E-selectin. *: p < 0.05. PSO, psoriasis; PSA, psoriatic arthritis; ICAM-1, Intercellular Adhesion Molecule 1; IP-10, interferon gamma-induced protein 10; IL, interleukin; MIP, Macrophage Inflammatory Proteins; PASI, Psoriasis Area and Severity Index; BMI, body mass index.
Discussion
Comparing naïve patients with PSO, PSA with PSO, and PSA sine PSO, our data suggested a similar cytokine profile according to a psoriatic disease continuum. No differences were retrieved about IL-23/IL-17-pathway in these patients. The prominent role of this inflammatory axis has been proposed in this context of psoriatic disease (10). More specifically, IL-23 is a cytokine member of IL-12 superfamily, showing both immunoregulatory activity and effector functions (11). IL-23 may induce the production of IL-17 and TNF which contribute to both synovial inflammation and skin lesions (3). The clinical relevance of the inhibition of the IL-23/IL-17-axis and TNF has been clearly pointed out in patients with PSO and PSA (3, 11, 12). Furthermore, no additional significant differences were retrieved in the assessment of further inflammatory biomolecules, including IL-1β, IL-6, IL-10, and IL-13. Taking together these results, the cytokine profile of the group of PSA sine PSO did not significantly differ than those with evident PSO, suggesting these patients as an extreme of the psoriatic disease continuum. In addition, these results may provide a further rationale behind the clinical findings concerning the similar subclinical abnormalities of nails and musculoskeletal features observed in these patients, which may reinforce the concept of the psoriatic disease continuum (13–15). In addition, although further confirmatory studies are needed, these results may highlight possible mechanistic biomarkers which could better identify non-responder patients to the treatment since these biomolecules may more closely reflect the manipulated pathogenic pathways (4, 5)
Assessing our data, we found some differences in biomolecules in these groups of patients. Higher levels of IL-8 characterized patients with PSO than those with PSA. IL-8 is also known as “neutrophil chemotactic factor” and has two main functions, primarily inducing the chemotaxis of neutrophils and stimulating their phagocytotic activity (16). It has been suggested as responsible for the exacerbation of PSO, enhancing expression of IL-17 in keratinocytes and favoring the development of skin lesions (17). In fact, IL-8 correlated with the extension of skin involvement in our cohort of patients. In addition, in our study, levels of E-selectin were higher in PSO group in respect to PSA. This molecule, also identified as CD62 antigen-like family member E (CD62E), is a selectin cell adhesion mediator which is found on activated endothelial cells activated (18). In this context, previous evidence showed high levels of E-selectin in patients with PSO relating to the extension of skin involvement (18, 19). The presence of E-selectin on the surface of endothelial cells may lead to the interaction of between these cells and circulating leukocytes. Thus, a trans-endothelial migration of leukocytes from the blood to the epidermis may be induced, contributing to the inflammation of the psoriatic lesions (18–20). Furthermore, we showed higher values of ICAM-1 in PSA with PSO group than others. This molecule is codified as a cell surface glycoprotein. It is generally found on both endothelial cells and immune cells and contributes to the migration of leukocytes toward the tissue inflammatory infiltrate. The overexpression of ICAM-1 has been previously documented in synovial fluids collected from patients with PSA (21). In addition, the inhibition of the interaction between LFA-1 and ICAM-1 may induce the occurrence of arthritis in patients with PSO, possibly altering the balance of leucocyte extravasation (22). Finally, considering that IL-8, E-selectin, and ICAM-1 are all involved in angiogenesis, it is also possible to speculate that the hyperactivation of angiogenetic pathways could be implicated in patients with PSO in respect to those sine PSO. In fact, the crucial role of angiogenesis in PSO has been already suggested (23), but its role in PSA sine PSO should be better evaluated.
After these evaluations among main patient groups, we stratified our results according to relevant clinical features in the context of psoriatic disease (i.e., presence of psoriatic nail involvement, PASI ≥ 10, BMI ≥ 30) considering the whole cohort. Patients characterized by psoriatic onychopathy showed significant higher levels of ICAM-1 and IP-10. The latter, also named CXCL10, has been related to the occurrence of development of joint manifestations in patients with PSO, which are associated with psoriatic nail involvement (24–26). However, additional data are needed to fully elucidate the pathogenic involvement IP-10 in the development of psoriatic onychopathy and possibly arthritis. Moreover, the severity of psoriatic onychopathy correlated with IL-8, ICAM-1, and with MIP-1α, suggesting a possible more specific involvement of these mediators in the occurrence of such clinical manifestation. In addition, in patients with PASI ≥ 10, we observed significantly higher values of IL-8, TNF, E-selectin, MIP-1α, and MIP-1β than others. In previous studies, the expression of these biomolecules was associated with the severity of skin involvement in PSO (27, 28). Moreover, in patients with BMI ≥ 30, significantly higher levels of E-selectin were pointed out than others. This finding may parallel the available literature since the endothelial dysfunction due to expression of E-selectin is to be considered a consequence of the metabolic alterations of obesity (29, 30).
Overall, our results may point out some pathogenic differences according to relevant clinical features, psoriatic nail involvement, extensive skin involvement, and obesity, in the transition from PSO to PSA (15). Nevertheless, the available evidence suggests that additional clinical variables may be associated with transition from skin involvement to synovio-entheseal disease in psoriatic disease continuum, such as first-degree relative with PSA, joint biomechanical stress, and microbiome-related event (15). However, additional studies are warranted to clarify further clinical differences in this context. The relevance of sex-related differences in patients with PSA has been recently highlighted (31), but their influence in inducing the transition from PSO to PSA should be fully investigated. Also, the possible impact of racial differences should be taken into account to design more studies in regard to this topic considering that some differences were shown according to such features (32, 33). In addition, given that both intestinal microbiome perturbations and obesity are linked with development of PSA (15, 34), the role of diet and associated weight loss may be carefully evaluated in patients with PSO who are considered at high risk to develop PSA (34). Finally, specific designed prospective studies are also advocated to entirely evaluate the potential impact of systemic therapies, which are administered for the skin involvement, in attenuating the occurrence of PSA in patients with PSO (34). In fact, the use of biologic agents in patients with plaque PSO appeared to delay or reduce the risk of incident PsA (35).
Some limitations may be recognized in our study; our findings would be cautiously interpretated. Despite the assessment of naïve patients, the somewhat small number of assessed patients and the single center design may impair the generalization of the results. In fact, our study may be underpowered to detect some differences between PSO and PSA, considering the heterogeneous clinical courses and possible fluctuation of disease severity over time. In addition, the serum cytokine profile could not fully mirror the histopathology of the affected tissues by psoriatic disease, suggesting the need of specifically designed evaluations on both skin as well as synovium of these patients. Therefore, the hypothesis-generating nature of our study should be recognized and future confirmatory works are warranted to fully elucidate these issues and their clinical consequences.
In conclusion, a similar cytokine profile was observed in naïve patients with PSO, PSA with PSO, and PSA sine PSO, reinforcing the idea of psoriatic disease according to a pathogenic continuum. Our data also suggested some differences which may underly possible pathogenic diversities leading to the clinical heterogeneity of these patients. Further data are needed to entirely clarify these issues and their clinical consequences in improving the management of such patients of the psoriatic disease continuum.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by Comitato Etico Azienda Sanitaria Locale 1 Avezzano/Sulmona/L’Aquila, L’Aquila, Italy. The patients/participants provided their written informed consent to participate in this study.
Author contributions
All authors made substantial contributions to the conception or design of the work, the acquisition and interpretation of data. All authors contributed to the critical review and revision of the manuscript and approved the final version. All the authors agreed to be accountable for all aspects of the work.
Funding
This work has been supported by “Bando per il finanziamento dei progetti anno 2022 (RIA 2022)” from Department of Biotechnological and Applied Clinical Sciences, University of L’Aquila, L’Aquila, Italy.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Perez-Chada LM, Haberman RH, Chandran V, Rosen CF, Ritchlin C, Eder L, et al. Consensus terminology for preclinical phases of psoriatic arthritis for use in research studies: results from a Delphi consensus study. Nat Rev Rheumatol (2021) 17:238–43. doi: 10.1038/s41584-021-00578-2
2. Frischknecht L, Vecellio M, Selmi C. The role of epigenetics and immunological imbalance in the etiopathogenesis of psoriasis and psoriatic arthritis. Ther Adv Musculoskelet Dis (2019) 11:1759720X1988650. doi: 10.1177/1759720X19886505
3. Azuaga AB, Ramírez J, Cañete JD. Psoriatic arthritis: pathogenesis and targeted therapies. Int J Mol Sci (2023) 24:4901. doi: 10.3390/ijms24054901
4. Pouw J, Leijten E, Radstake T, Boes M. Emerging molecular biomarkers for predicting therapy response in psoriatic arthritis: a review of literature. Clin Immunol (2020) 211:108318. doi: 10.1016/j.clim.2019.108318
5. Robinson WH, Lindstrom TM, Cheung RK, Sokolove J. Mechanistic biomarkers for clinical decision making in rheumatic diseases. Nat Rev Rheumatol (2013) 9:267–76. doi: 10.1038/nrrheum.2013.14
6. Taylor W, Gladman D, Helliwell P, Marchesoni A, Mease P, Mielants H. Classification criteria for psoriatic arthritis: development of new criteria from a large international study. Arthritis Rheum (2006) 54:2665–73. doi: 10.1002/art.21972
7. Raychaudhuri SK, Maverakis E, Raychaudhuri SP. Diagnosis and classification of psoriasis. Autoimmun Rev (2014) 13:490–5. doi: 10.1016/j.autrev.2014.01.008
8. Fredriksson T, Pettersson U. Severe psoriasis – oral therapy with a new retinoid. Dermatology (1978) 157:238–44. doi: 10.1159/000250839
9. Rich P, Scher RK. Nail psoriasis severity index: a useful tool for evaluation of nail psoriasis. J Am Acad Dermatol (2003) 49:206–12. doi: 10.1067/S0190-9622(03)00910-1
10. Sundanum S, Orr C, Veale D. Targeted therapies in psoriatic arthritis–an update. Int J Mol Sci (2023) 24:6384. doi: 10.3390/ijms24076384
11. Schett G, Rahman P, Ritchlin C, McInnes IB, Elewaut D, Scher JU. Psoriatic arthritis from a mechanistic perspective. Nat Rev Rheumatol (2022) 18:311–25. doi: 10.1038/s41584-022-00776-6
12. Hutton J, Mease P, Jadon D. Horizon scan: state-of-the-art therapeutics for psoriatic arthritis. Best Pract Res Clin Rheumatol (2022), 36(4):101809. doi: 10.1016/j.berh.2022.101809
13. Ruscitti P, Esposito M, Gianneramo C, Di Cola I, De Berardinis A, Martinese A, et al. Nail and enthesis assessment in patients with psoriatic disease by high frequency ultrasonography: findings from a single-centre cross-sectional study. Radiol Med (2022) 127:1400–6. doi: 10.1007/s11547-022-01568-4
14. Lubrano E, Scriffignano S, Perrotta FM. Psoriatic arthritis, psoriatic disease, or psoriatic syndrome? J Rheumatol (2019) 46:1428–30. doi: 10.3899/jrheum.190054
15. Scher JU, Ogdie A, Merola JF, Ritchlin C. Preventing psoriatic arthritis: focusing on patients with psoriasis at increased risk of transition. Nat Rev Rheumatol (2019) 15:153–66. doi: 10.1038/s41584-019-0175-0
16. Cambier S, Gouwy M, Proost P. The chemokines CXCL8 and CXCL12: molecular and functional properties, role in disease and efforts towards pharmacological intervention. Cell Mol Immunol (2023) 20:217–51. doi: 10.1038/s41423-023-00974-6
17. Liu C-T, Yen J-HJ, Brown DA, Song Y-C, Chu M-Y, Hung Y-H, et al. Targeting Nrf2 with 3 H-1,2-dithiole-3-thione to moderate OXPHOS-driven oxidative stress attenuates IL-17A-induced psoriasis. Biomed Pharmacother (2023) 159:114294. doi: 10.1016/j.biopha.2023.114294
18. Szepietowski J, Wasik F, Bielicka E, Nockowski P, Noworolska A. Soluble E-selectin serum levels correlate with disease activity in psoriatic patients. Clin Exp Dermatol (1999) 24:33–6. doi: 10.1046/j.1365-2230.1999.00401.x
19. Czech W, Schöpf E, Kapp A. Soluble E-selectin in sera of patients with atopic dermatitis and psoriasis–correlation with disease activity. Br J Dermatol (1996) 134:17–21.
20. Dowlatshahi EA, van der Voort EAM, Arends LR, Nijsten T. Markers of systemic inflammation in psoriasis: a systematic review and meta-analysis. Br J Dermatol (2013) 169:266–82. doi: 10.1111/bjd.12355
21. Caso F, Saviano A, Tasso M, Raucci F, Marigliano N, Passavanti S, et al. Analysis of rheumatoid- vs psoriatic arthritis synovial fluid reveals differential macrophage (CCR2) and T helper subsets (STAT3/4 and FOXP3) activation. Autoimmun Rev (2022) 21:103207. doi: 10.1016/j.autrev.2022.103207
22. Viguier M, Richette P, Aubin F, Beylot-Barry M, Lahfa M, Bedane C, et al. Onset of psoriatic arthritis in patients treated with efalizumab for moderate to severe psoriasis. Arthritis Rheum (2008) 58:1796–802. doi: 10.1002/art.23507
23. Yamamoto T. Angiogenic and inflammatory properties of psoriatic arthritis. ISRN Dermatol (2013) 2013:1–7. doi: 10.1155/2013/630620
24. Abji F, Pollock RA, Liang K, Chandran V, Gladman DD. Brief report: CXCL10 is a possible biomarker for the development of psoriatic arthritis among patients with psoriasis. Arthritis Rheumatol (2016) 68:2911–6. doi: 10.1002/art.39800
25. Abji F, Lee K-A, Pollock ra, Machhar R, Cook RJ, Chandran V, et al. Declining levels of serum chemokine (C-X-C motif) ligand 10 over time are associated with new onset of psoriatic arthritis in patients with psoriasis: a new biomarker? Br J Dermatol (2020) 183:920–7. doi: 10.1111/bjd.18940
26. Ramessur R, Corbett M, Marshall D, Acencio ML, Barbosa IA, Dand N, et al. Biomarkers of disease progression in people with psoriasis: a scoping review. Br J Dermatol (2022) 187:481–93. doi: 10.1111/bjd.21627
27. Dai Y-J, Li Y-Y, Zeng H-M, Liang X-A, Xie Z-J, Zheng Z-A, et al. Effect of pharmacological intervention on MIP-1α, MIP-1β and MCP-1 expression in patients with psoriasis vulgaris. Asian Pac J Trop Med (2014) 7:582–4. doi: 10.1016/S1995-7645(14)60098-5
28. Purzycka-Bohdan D, Nedoszytko B, Zabłotna M, Gleń J, Szczerkowska-Dobosz A, Nowicki RJ. Chemokine profile in psoriasis patients in correlation with disease severity and pruritus. Int J Mol Sci (2022) 23:13330. doi: 10.3390/ijms232113330
29. Abulnaja KO, Kannan K, Mohammed K Al-Manzlawi A, Kumosani TA, Qari M, Moselhy SS. Sensitivity, specificity of biochemical markers for early prediction of endothelial dysfunction in atherosclerotic obese subjects. Afr Health Sci (2022) 22:286–94. doi: 10.4314/ahs.v22i2.32
30. Liu M, Wang P, Xie P, Xu X, He L, Chen X, et al. Expression of ICAM-1 and E-selectin in different metabolic obesity phenotypes: discrepancy for endothelial dysfunction. J Endocrinol Invest (2023). doi: 10.1007/s40618-023-02094-4
31. Tarannum S, Leung YY, Johnson SR, Widdifield J, Strand V, Rochon P, et al. Sex- and gender-related differences in psoriatic arthritis. Nat Rev Rheumatol (2022) 18(9):513–26. doi: 10.1038/s41584-022-00810-7
32. Ogdie A, Matthias W, Thielen RJ, Chin D, Saffore CD. Racial differences in prevalence and treatment for psoriatic arthritis and ankylosing spondylitis by insurance coverage in the USA. Rheumatol Ther (2021) 8(4):1725–39. doi: 10.1007/s40744-021-00370-4
33. Ross Y, Jaleel S, Magrey M. Racial disparities in comorbidities of patients with psoriatic arthritis. Rheumatol Int (2023) 43(8):1525–9. doi: 10.1007/s00296-023-05322-5
34. Zabotti A, De Marco G, Gossec L, Baraliakos X, Aletaha D, Iagnocco A, et al. EULAR points to consider for the definition of clinical and imaging features suspicious for progression from psoriasis to psoriatic arthritis. Ann Rheum Dis (2023), ard–2023-224148. doi: 10.1136/ard-2023-224148
Keywords: psoriatic disease, psoriasis, psoriatic arthritis, mechanistic biomarkers, biomarkers
Citation: Ruscitti P, Esposito M, Di Cola I, Pellegrini C, De Berardinis A, Mastrangelo M, Gianneramo C, Barile A, Fargnoli MC and Cipriani P (2023) Cytokine profile characterization of naïve patients with psoriasis and psoriatic arthritis: implications for a pathogenic disease continuum. Front. Immunol. 14:1229516. doi: 10.3389/fimmu.2023.1229516
Received: 26 May 2023; Accepted: 26 June 2023;
Published: 13 July 2023.
Edited by:
Juan Bautista De Sanctis, Palacký University Olomouc, CzechiaReviewed by:
Ana Maria Saenz, Central University of Venezuela, VenezuelaThusanth Thuraisingam, McGill University, Canada
Copyright © 2023 Ruscitti, Esposito, Di Cola, Pellegrini, De Berardinis, Mastrangelo, Gianneramo, Barile, Fargnoli and Cipriani. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Piero Ruscitti, cGllcm8ucnVzY2l0dGlAdW5pdmFxLml0
†These authors share first authorship
‡These authors share last authorship
 Piero Ruscitti
Piero Ruscitti Maria Esposito
Maria Esposito Ilenia Di Cola
Ilenia Di Cola Cristina Pellegrini
Cristina Pellegrini Andrea De Berardinis
Andrea De Berardinis Antonio Barile
Antonio Barile