
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Immunol. , 06 October 2023
Sec. Cancer Immunity and Immunotherapy
Volume 14 - 2023 | https://doi.org/10.3389/fimmu.2023.1226993
This article is part of the Research Topic Reducing Adverse Effects of Cancer Immunotherapy View all 15 articles
Immune checkpoint inhibitor (ICI) is an up-to-date therapy for cancer with a promising efficacy, but it may cause unique immune-related adverse events (irAEs). Although irAEs could affect any organ, irAEs-induced whole urinary tract expansion was rarely reported. Herein, we reported a 27-year-old male patient with thymic carcinoma who received the treatment of tislelizumab, paclitaxel albumin and carboplatin. He was hospitalized for severe bellyache and lumbago after 6 courses of treatment. Antibiotic and antispasmodic treatment did not relieve his symptoms. The imaging examinations reported whole urinary tract expansion and cystitis. Therefore, we proposed that the patient’s pain was caused by tislelizumab-induced ureteritis/cystitis. After the discontinuation of tislelizumab and the administration of methylprednisolone, his symptoms were markedly alleviated. Herein, we reported a rare case of ICI-induced ureteritis/cystitis in the treatment of thymic cancer and reviewed other cases of immunotherapy-related cystitis and tislelizumab-related adverse events, which will provide a reference for the diagnosis and treatment of ICI-related irAEs.
Immune checkpoint inhibitor (ICI) is an emerging immunotherapy for cancers. However, since ICI will activate immune responses, it may cause unique immune-related adverse events (irAEs). irAEs can affect different organs and reduce the survival benefit of immunotherapy if untreated (1). In some cases, irAEs will endanger the lives of patients (2, 3).
Tislelizumab (BGB-A317) is a humanized anti-programmed death receptor 1 (PD-1) monoclonal antibody. In clinical studies, tislelizumab has shown promising anti-tumor activity in various solid tumors (4). In these studies, tislelizumab-related adverse events are briefly recorded (5), but few adverse events related to the urinary system are reported. In other case reports, tislelizumab is suggested to induce various immune-related adverse events (2, 3, 6–15). A previous case report indicated that tislizumab could induce ureteritis and cystitis in patients with esophageal cancer (15). However, the chief complaint of the patient in that case report differed from that in our case. Moreover, another PD-1 inhibitor, sintilimab, was reported to cause cystitis and ureteritis (16). Our patient was hospitalized for bellyache with paroxysmal lumbago. He had no obvious symptom of frequent urination, urgency, and pain in urination. The positron emission tomography/computed tomography (PET/CT) and magnetic resonance urography (MRU) scans revealed an expanded whole urinary tract, which is rarely reported. Therefore, this is the first report of ICI-induced ureteritis and cystitis during the treatment of thymic cancer. In addition, we reviewed several cases of immune-induced cystitis (15–25), in which the main manifestations of patients were frequent urination, dysuria, pain on urination, nocturia or incontinence. Hence, our report would provide a reference for the diagnosis and treatment of patients who received ICI and complained of bellyache.
A 27-year-old male patient was admitted to Zhejiang Hospital due to chest pain. He had a history of fatty liver and kidney stones with no history of smoking and drinking. He did not have a medical history of hypertension, diabetes, kidney disease, or hepatitis. His father had hepatitis B, and no family members had a tumor history. The CT showed anterior superior mediastinal and liver mass on March 30, 2022. Pathological results of liver puncture indicated poorly differentiated carcinoma with necrosis. Then the patient was admitted to our hospital on April 9, 2022. The immunohistochemical examination of the liver mass indicated CK (Pan) (+), CD5 (+), CgA (-), SYN (-), P63 (scattered cells+), CD117 (+), GLUT-1 (+), Muc-1 (+), CD3 (-), P40 (scattered cells+), CD56 (-), CK20 (-), Ki67(+≈90%) (Appendix Figure S1), which suggested that it was liver metastasis of thymic carcinoma. The expression of PD-L1 is positive in 70% of tumor cells (clone 22C3, Dako, Glostrup, Denmark). Also, we sequenced the 520 pan-cancer genes in formalin fixation and paraffin embedding specimens (Appendix Table S1). The tumor mutation burden (TMB) was 10.2 Muts/Mb, which was higher than 99% thymic carcinoma. The ratio of mutation at microsatellite site was 1.65% (2/121), which indicated microsatellite stability (MSS). PET/CT showed that the size of the tumor in anterior superior mediastinum was 4.1 cm × 3.5 cm, and standard uptake value (SUV) max was 16.6. The boundaries between the tumor and adjacent superior vena cava, pericardium and mediastinal pleura were not clear (Figure 1A). The tumor had liver (Figure 1B), lymph nodes and bone metastasis. Based on these results, the tumor was staged as pTxN1M1. His performance score (PS) was 1 (PS ranged from 0 to 6, and the lower value indicated better physical condition). The patient began to receive chemo-immunotherapy on April 14, 2022. He was administered with paclitaxel albumin (CSPC, OUYI, Pharmaceutical Co, Ltd) (200 mg at Day 1, Day 8), carboplatin (Bristol-Myers Squibb S.r.l., 0.3 g at Day 1, Day 8) and tislelizumab (Baize’an, BeiGene Ltd., Beijing, China, 200mg at Day 1) for 6 courses. Chest CT and liver MRI showed significant reduction of the tumor and the treatment reached partial response (PR). On August 19, the patient developed bellyache, which was day 7 since the last treatment course. The bellyache last for 2 days, with significant pain on the left side and paroxysmal lumbago and no gross hematuria. The symptom could relieve on itself.
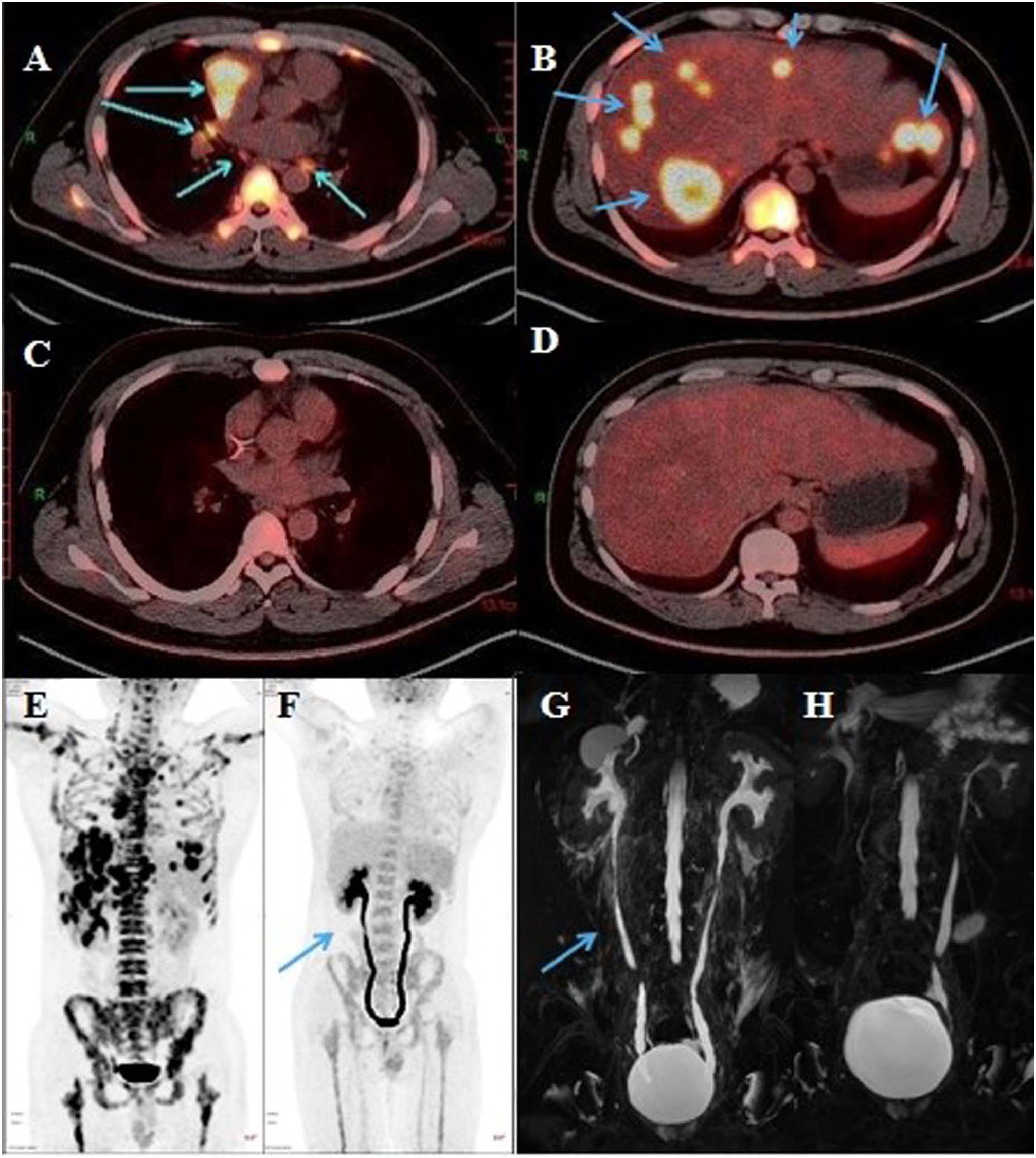
Figure 1 Representative radiological images of the patient. (A) Mediastinum tumor and (B) hepatic metastatic lesion on PET/CT (April 11, 2022). (C) Mediastinum tumor and (D) hepatic metastatic lesion on PET/CT (August 24, 2022). (E) PET/CT (April 11, 2022). (F) Bilateral ureters were slightly dilated on PET/CT (blue arrow, August 24, 2022). (G) Bilateral ureteral wall was slightly thickened and whole ureteral was full expanded on urinary MRU (blue arrow, August 31, 2022. (H) Right ureter expansion was improved, and ureteral exudation was absorbed on urinary MRU (September 22, 2022).
The patient had percussion pain (+) in renal area. Urinalysis showed red blood cells (+++) and white blood cells (++). His white blood cells in routine blood examination was 16.76 (normal 3.5-9.5)×10^9/L. B-ultrasound examination in bladder was normal and that in abdomen showed bilateral kidney stones. The level of glutamic pyruvic transaminase (GPT), serum amylase and serum creatinine was normal. Therefore, we proposed that the patient had a urinary tract infection due to the urinary calculi, and we administered levofloxacin (0.5 g) and phloroglucinol (80 mg) for 3 days. However, his pain did not subside. Initially, the patient had a breakthrough pain once a day, with the Numerical Rating Scale (NRS) score of 7-8. Later, the frequency of pain increased to 3 times a day and the pain was obvious when urinating, based on which we conducted urine culture, but failed to identify any pathogen. PET/CT scan showed that the mass at the right anterior mediastinal was smaller than before (2.3 cm × 2.2 cm vs 4.1 cm × 3.5 cm), and the metabolism was reduced (SUVmax 4.5 vs 16.6). There was no significant increase of 18F-Fluorodeoxyglucose (FDG) fluorodeoxyglucose (FDG) metabolism in metastatic tumor at lymph nodes and liver (Figures 1C, D). Meanwhile, PET/CT revealed, compared to the previous images (Figure 1E), poor bladder filling, slightly thickened bladder wall, slightly enlarged left kidney, increased FDG metabolism in bilateral renal parenchyma, dilated bilateral ureters with smooth excretion, and no obvious ureteral calculus (Figure 1F). Consistent with previous findings, MRU scan showed that bilateral ureteral wall was slightly thickened and whole ureteral fully expanded (Figure 1G). Cystoscopy indicated cystitis (Appendix Figure S2). Based on these results, a multi-disciplinary treatment (MDT) meeting was organized. The cause of pain excluded the urinary infection, tumor metastasis and nephrolithiasis, and the pain was most probably caused by tislelizumab-induced ureteritis/cystitis. The patient was administered with methylprednisolone intravenously, 1 mg/kg once daily and tislelizumab was discontinued. The patient’s persistent bellyache and lumbago were basically relieved, the intensity of paroxysmal pain reduced, and the pain after urination improved after 3 days of treatment. After he was discharged on September 8, Methylprednisolone (12 mg) was administered orally once daily. During the follow-up, the patient had slightly poor sleep but had no obvious adverse events such as drug allergy, gastric bleeding, and edema, etc. The patient’s pain did not recur and the dose of methylprednisolone was decreased gradually. On September 22, the MRU indicated that the dilation of left kidney and right ureter was improved compared to those on August 31, and the around ureteral exudation was absorbed. (Figure 1H). Methylprednisolone was finally discontinued about 40 days after treatment. Because the patient’s pain was caused by ICI rather than chemotherapy drugs, the patient received paclitaxel albumin and platinum again on September 22, 2022. No similar pain or urinary symptoms occurred during the follow-up. The timeline of treatment course was summarized in Figure 2.
The structure of tislelizumab has been modified to maximally block the binding of PD-1 to programmed death ligand 1 (PD-L1) (4). The binding of Fcγ receptors (FcγRs) will impair the anti-tumor activity of anti-PD-1 antibody (26). Several Fc-hinge regions of tislelizumab has been muted to minimize its binding to FcγRs. Up to now, tislelizumab has been approved for the treatment of various tumors, including classical Hodgkin’s lymphoma, urothelium cancer, lung adenocarcinoma, non-squamous cancer, liver cancer, esophageal squamous cancer, nasopharyngeal cancer, advanced colorectal cancer, and solid tumors with microsatellite instability-high (MSI-H) or mismatch repair protein deficiency (dMMR) MSI-H or dMMR in China. However, adverse events may occur during tislelizumab treatment. Existing clinical studies suggested that adverse effects of tislelizumab included anemia, leukopenia, thrombocytopenia, nausea, increased aspartate transaminase (AST), neutropenia, fatigue, decreased appetite, vomiting, musculoskeletal pain, constipation, hypoproteinemia and rash (4, 5). To better understand the adverse events of tislelizumab, we searched for available case reports and reviewed tislelizumab-associated adverse events (Table 1). Although irAEs can affect any organ, tislelizumab-related irAEs in the urinary system are rarely reported. A previous case report indicated that tislizumab could induce ureteritis and cystitis in patients with esophageal cancer (15). However, the chief complaint of the patient in that case report differed from that in our case.
Thymic carcinomas are rare malignancies. For unresectable or metastatic thymic carcinomas, chemotherapy is the standard treatment. ICIs are new drugs with promising efficacies in cancers. A phase 2 clinical trial of pembrolizumab (27), an anti-PD-1 antibody, IN 40 patients with thymic carcinoma showed that the overall response rate (ORR) was 22.5% and the median progression-free survival (mPFS) was 4.2 months. Moreover, those with high PD-L1 expression benefit more from pembrolizumab treatment. In our case, PD-L1 was 70% positive in patient’s tumor cells with a high TMB. A study showed that patients with high TMB and PD-L1 expression had a high rate of durable clinical benefit from ICIs treatment (28). Therefore, our patient is likely to benefit from ICI. However, due to the financial issue, the patient chose another ICI, tislelizumab, for subsequent treatment.
Here, we report a case of tislelizumab-induced ureteritis and cystitis. Up until now, there is no standard for the diagnosis of immune-related cystitis, where cystoscopic biopsy may help. In our case, we suspected that patient’s bellyache was caused by kidney stones since abdominal B-ultrasound showed bilateral kidney stones. However, no stone was found on urinary CT, which might be due to the small size of the stone that was not shown on CT. Since small kidney stones rarely caused such severe and long-term pain, we further performed PET/CT and found no tumor metastasis of the urinary system. Nevertheless, ureteral expansion was identified by PET/CT and MRU, and cystoscopy suggested cystitis. After excluding kidney stones, tumor invasion, and urinary tract infection, we considered that the patient’s pain was caused by immune-related ureteritis and cystitis. After the steroid administration, the pain was markedly alleviated, and the following MRU suggested that ureteral expansion was relieved. Therefore, we confirmed the diagnosis that the patient’s pain and ureteral dilatation were caused by tislelizumab-induced ureteritis and cystitis.
irAEs are toxicities caused by non-specific activation of the immune system and can affect almost any organ (29). However, the exact mechanism of irAEs is not clear, which may involve the activation of various inflammatory cells, such as Th17 and other types of cells (29). Other studies indicated that irAEs might occur because of impaired immune tolerance and molecular mimicry (21). Studies had found that PD-L1 was expressed in bladder tissue in patients with severe bladder inflammation. Therefore, it is speculated that PD-1/PD-L1 mAb-induced cytotoxic T-cell activation may simultaneously target at cancer and normal urothelial cells (15). A meta-analysis showed that the incidence of irAEs significantly increased when ICI was combined with chemotherapy (21).
In published clinical trials and case reports, there is rarely report on tislelizumab-induced ureteritis and cystitis. Therefore, we referred to previous case reports on autoimmune cystitis caused by more than tislelizumab (Table 2). Among these cases, 50% (7/14) patients received nivolumab, 14% (2/14) patients received pembrolizumab, 21% (3/14) patients received sintilimab, 7% (1/14) patient received atezolizumab, and 7% (1/14) patient took tislelizumab. Moreover, there is no clear timing for the onset of immune-related cystitis during ICIs treatment. The main manifestations of patients in these case reports were frequent urination, dysuria, urination pain, nocturia, incontinence, or diarrhea. Only two of them had low back pain, and one received tislelizuma (16) and another one received sintilimab (15). As a comparison, the manifestation of our patient was bellyache, which was different from that in these immune-induced cystitis cases. More importantly, the ureter of this patient was expanded. Such cases with irAEs in urinary system and expanded ureter are rarely reported. A case of irAEs induced by tislelizumab exhibited different manifestation. Therefore, this is the first report of autoimmune ureteritis/cystitis in the treatment of thymic cancer. Our case will provide a reference for the diagnosis and treatment of ICI-induced ureteritis and cystitis manifested by bellyache.
This study had some limitations. Firstly, we did not give timely steroids treatment. Since the patient had a history of kidney stone, our primary thought of the patient’s pain was consequence of kidney stones. When conventional treatment of antibiotics and antispasmodic treatment failed to relive the pain, additional examination of PET/CT and MRU indicated the ureter expansion and cystoscopy indicated cystitis. Based on these results, we considered the pain was caused by tislelizumab-induced ureteritis/cystitis. Secondly, we did not perform cystoscopy after his pain was alleviated because the patient refused. Otherwise, we cloud better observe the changes in the inner wall of the bladder after discontinuing tislelizumab. Thirdly, the diagnosis of cystitis was made based on cystoscopy examination revealing the inflammatory reaction of the inner wall of the bladder, and we did not perform pathological examination.
In conclusion, we reported a rare case of tislelizumab-induced ureteritis/cystitis mainly presented with severe pain and ureteral expansion. This case reminds us of the potential risk of urinary system during ICI treatment. Since tislelizumab is currently used in various malignancies, our case will provide a reference for the diagnosis and treatment of ICI-related irAEs.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
The studies involving humans were approved by Ethics Committee of Zhejiang Provincial People’s Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual for the publication of any potentially identifiable images or data included in this article. Written informed consent was obtained from the participant/patient(s) for the publication of this case report.
QZ designed the case report and drafted the manuscript. ZQ analyzed the patient data and revised the manuscript. PY proposed the concept of this case report. QW and JQ administered the whole course of diagnosis and treatment in this patient. YC analyzed the patient data, and provided significant contributions to the analysis of the patient data. All authors contributed to the article and approved the submitted version.
We thank the patient for providing his information for this case report. We acknowledge TopEdit LLC for the linguistic editing and proofreading during the preparation of this manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1226993/full#supplementary-material
Supplementary Figure 1 | Histopathology and immunohistochemistry of the liver biopsy specimens of this patient in April 2022. (A) HE staining revealed poorly differentiated carcinomas (20×), IHC staining showed that liver cells were positive for (B) CK(Pan) (20×), (C) CD5 (20×), (D) CD117 (20×), (E) GLUT-1 (20×), (F) Muc-1 (20×).
Supplementary Figure 2 | Cystoscope scan (September 1st, 2022).
1. Ramos-Casals M, Brahmer JR, Callahan MK, Flores-Chávez A, Keegan N, Khamashta MA, et al. Immune-related adverse events of checkpoint inhibitors. Nat Rev Dis Primers (2020) 6(1):38. doi: 10.1038/s41572-020-0160-6
2. Fu C, Wang G, Yang W. Vascular thrombosis and anti-pd-1 therapy: A series of cases. Cancer Manage Res (2021) 13:8849–53. doi: 10.2147/cmar.s338023
3. Deng C, Yang M, Jiang H, Wang R, Yang Z, Sun H, et al. Immune-related multiple-organs injuries following ici treatment with tislelizumab in an advanced non-small cell lung cancer patient: A case report. Front Oncol (2021) 11:664809. doi: 10.3389/fonc.2021.664809
4. Zhang L, Geng Z, Hao B, Geng Q. Tislelizumab: A modified anti-tumor programmed death receptor 1 antibody. Cancer Control J Moffitt Cancer Center (2022) 29:10732748221111296. doi: 10.1177/10732748221111296
5. Lu S, Wang J, Yu Y, Yu X, Hu Y, Ai X, et al. Tislelizumab plus chemotherapy as first-line treatment for locally advanced or metastatic nonsquamous nsclc (Rationale 304): A randomized phase 3 trial. J Thorac Oncol (2021) 16(9):1512–22. doi: 10.1016/j.jtho.2021.05.005
6. Kerkemeyer K, Lai F, Mar A. Lichen planus pemphigoides during therapy with tislelizumab and sitravatinib in a patient with metastatic lung cancer. Australas J Dermatol (2020) 61(2):180–2. doi: 10.1111/ajd.13214
7. Ni J, Zhang X, Zhang L. Opportunistic bowel infection after corticosteroid dosage tapering in a stage iv lung cancer patient with tislelizumab-related colitis. Thorac Cancer (2020) 11(6):1699–702. doi: 10.1111/1759-7714.13401
8. Chen M, Zhang L, Zhong W, Zheng K, Ye W, Wang M. Case report: thsd7a-positive membranous nephropathy caused by tislelizumab in a lung cancer patient. Front Immunol (2021) 12:619147. doi: 10.3389/fimmu.2021.619147
9. Hu X, Wei Y, Shuai X. Case report: glucocorticoid effect observation in a ureteral urothelial cancer patient with ici-associated myocarditis and multiple organ injuries. Front Immunol (2021) 12:799077. doi: 10.3389/fimmu.2021.799077
10. Zhang Y, Zhang M, Xie J, Wu W, Lu J. Pemphigus herpetiformis-type drug reaction caused by programmed cell death protein-1 inhibitor treatment. Clinical Cosmetic Investigational Dermatol (2021) 14:1125–9. doi: 10.2147/ccid.s330354
11. Baek J. Adrenal insufficiency development during chemotherapy plus anti-programmed death receptor-1 monoclonal antibody (Tislelizumab) therapy in patients with advanced gastric cancer: case series. J Yeungnam Med Sci (2022) 39(1):62–6. doi: 10.12701/yujm.2021.00934
12. Zhou Y, Ma B, Li T, Gao Q, Zhao L. Immune-related pneumonitis requiring low-dose prednisone maintenance in one patient with durable complete response. J Oncol Pharm Pract (2023) 29(4):986–91. doi: 10.1177/10781552221127699
13. Wang S, Peng D, Zhu H, Min W, Xue M, Wu R, et al. Acetylcholine receptor binding antibody-associated myasthenia gravis, myocarditis, and rhabdomyolysis induced by tislelizumab in a patient with colon cancer: A case report and literature review. Front Oncol (2022) 12:1053370. doi: 10.3389/fonc.2022.1053370
14. Zhu C, Zhao Y, Yu F, Huang W, Wu W, He J, et al. Tumor flare reaction in a classic hodgkin lymphoma patient treated with brentuximab vedotin and tislelizumab: A case report. Front Immunol (2021) 12:756583. doi: 10.3389/fimmu.2021.756583
15. Li J, Yu YF, Qi XW, Du Y, Li CQ. Immune-related ureteritis and cystitis induced by immune checkpoint inhibitors: case report and literature review. Front Immunol (2022) 13:1051577. doi: 10.3389/fimmu.2022.1051577
16. Tu L, Ye Y, Tang X, Liang Z, You Q, Zhou J, et al. Case report: A case of sintilimab-induced cystitis/ureteritis and review of sintilimab-related adverse events. Front Oncol (2021) 11:757069. doi: 10.3389/fonc.2021.757069
17. Ozaki K, Takahashi H, Murakami Y, Kiyoku H, Kanayama H. A case of cystitis after administration of nivolumab. Int Cancer Conf J (2017) 6(4):164–6. doi: 10.1007/s13691-017-0298-6
18. Shimatani K, Yoshimoto T, Doi Y, Sonoda T, Yamamoto S, Kanematsu A. Two cases of nonbacterial cystitis associated with nivolumab, the anti-programmed-death-receptor-1 inhibitor. Urol Case Rep (2018) 17:97–9. doi: 10.1016/j.eucr.2017.12.006
19. Ueki Y, Matsuki M, Kubo T, Morita R, Hirohashi Y, Sato S, et al. Non-bacterial cystitis with increased expression of programmed death-ligand 1 in the urothelium: an unusual immune-related adverse event during treatment with pembrolizumab for lung adenocarcinoma. IJU Case Rep (2020) 3(6):266–9. doi: 10.1002/iju5.12211
20. Yajima S, Nakanishi Y, Matsumoto S, Tanabe K, Sugano M, Masuda H. Improvement of urinary symptoms after bladder biopsy: A case of pathologically proven allergy-related cystitis during administration of nivolumab. IJU Case Rep (2021) 4(4):213–5. doi: 10.1002/iju5.12286
21. Hong Y, Feng Y, Sun H, Zhang B, Wu H, Zhu Q, et al. Tislelizumab uniquely binds to the cc' Loop of pd-1 with slow-dissociated rate and complete pd-L1 blockage. FEBS Open Bio (2021) 11(3):782–92. doi: 10.1002/2211-5463.13102
22. Wang Z, Zhu L, Huang Y, Peng L. Successful treatment of immune-related cystitis by Chai-Ling-Tang (Sairei-to) in a gastric carcinoma patient: case report and literature review. Explore (New York NY) (2023) 19(3):458–62. doi: 10.1016/j.explore.2022.04.002
23. Obayashi A, Hamada-Nishimoto M, Fujimoto Y, Yoshimoto Y, Takahara S. Non-bacterial cystitis with increased expression of programmed cell death ligand 1 in the urothelium: an unusual immune-related adverse event after atezolizumab administration for metastatic breast cancer. Cureus (2022) 14(5):e25486. doi: 10.7759/cureus.25486
24. He X, Tu R, Zeng S, He Z, Liu S, Fang Y. Non-bacterial cystitis secondary to pembrolizumab: A case report and review of the literature. Curr Problems Cancer (2022) 46(4):100863. doi: 10.1016/j.currproblcancer.2022.100863
25. Fukunaga H, Sumii K, Kawamura S, Okuno M, Taguchi I, Kawabata G. A case of steroid-resistant cystitis as an immune-related adverse event during treatment with nivolumab for lung cancer, which was successfully treated with infliximab. IJU Case Rep (2022) 5(6):521–3. doi: 10.1002/iju5.12532
26. Zhang T, Song X, Xu L, Ma J, Zhang Y, Gong W, et al. The binding of an anti-pd-1 antibody to fcγrι Has a profound impact on its biological functions. Cancer Immunol Immunother CII (2018) 67(7):1079–90. doi: 10.1007/s00262-018-2160-x
27. Giaccone G, Kim C, Thompson J, McGuire C, Kallakury B, Chahine J, et al. Pembrolizumab in patients with thymic carcinoma: A single-arm, single-centre, phase 2 study. Lancet Oncol (2018) 19(3):347–55. doi: 10.1016/s1470-2045(18)30062-7
28. Rizvi H, Sanchez-Vega F, La K, Chatila W, Jonsson P, Halpenny D, et al. Molecular determinants of response to anti-programmed cell death (Pd)-1 and anti-programmed death-ligand 1 (Pd-L1) blockade in patients with non-small-cell lung cancer profiled with targeted next-generation sequencing. J Clin Oncol (2018) 36(7):633–41. doi: 10.1200/jco.2017.75.3384
29. Puzanov I, Diab A, Abdallah K, Bingham CO 3rd, Brogdon C, Dadu R, et al. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (Sitc) toxicity management working group. J Immunother Cancer (2017) 5(1):95. doi: 10.1186/s40425-017-0300-z
Keywords: immune-related adverse events, tislelizumab, cystitis, ureteritis, case report
Citation: Zhou Q, Qin Z, Yan P, Wang Q, Qu J and Chen Y (2023) Immune-related adverse events with severe pain and ureteral expansion as the main manifestations: a case report of tislelizumab-induced ureteritis/cystitis and review of the literature. Front. Immunol. 14:1226993. doi: 10.3389/fimmu.2023.1226993
Received: 22 May 2023; Accepted: 25 September 2023;
Published: 06 October 2023.
Edited by:
Daniele Maria-Ferreira, Pelé Pequeno Príncipe Research Institute, BrazilReviewed by:
Qijin Shu, Zhejiang Chinese Medical University, ChinaCopyright © 2023 Zhou, Qin, Yan, Wang, Qu and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yun Chen, aGxxbTE5ODZAMTYzLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.