
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Immunol., 11 July 2023
Sec. Mucosal Immunity
Volume 14 - 2023 | https://doi.org/10.3389/fimmu.2023.1225639
Background: Endometriosis is a chronic disease affecting 6–10% of women of reproductive age. It is an important cause of infertility and chronic pelvic pain with poorly understood aetiology. CD8+ T (CD8 T) cells were shown to be linked to infertility and chronic pain and play a significant role in lesion clearance in other pathologies, yet their function in endometriosis is unknown. We systematically evaluated the literature on the CD8 T in peripheral blood and endometriosis-associated tissues to determine the current understanding of their pathophysiological and clinical relevance in the disease and associated conditions (e.g. infertility and pelvic pain).
Methods: Four databases were searched (MEDLINE, EMBASE, Web of Science, CINAHL), from database inception until September 2022, for papers written in the English language with database-specific relevant terms/free-text terms from two categories: CD8 T cells and endometriosis. We included peer-reviewed papers investigating CD8 T cells in peripheral blood and endometriosis-associated tissues of patients with surgically confirmed endometriosis between menarche and menopause, and animal models with oestrous cycles. Studies enrolling participants with other gynaecological pathologies (except uterine fibroids and tubal factor infertility used as controls), cancer, immune diseases, or taking immune or hormonal therapy were excluded.
Results: 28 published case-control studies and gene set analyses investigating CD8 T cells in endometriosis were included. Data consistently indicate that CD8 T cells are enriched in endometriotic lesions in comparison to eutopic endometrium, with no differences in peripheral blood CD8 T populations between patients and healthy controls. Evidence on CD8 T cells in peritoneal fluid and eutopic endometrium is conflicting. CD8 T cell cytotoxicity was increased in the menstrual effluent of patients, and genomic analyses have shown a clear trend of enriched CD8 T effector memory cells in the eutopic endometrium of patients.
Conclusion: Literature on CD8 T cells in endometriosis-associated tissues is inconsistent. Increased CD8 T levels are found in endometriotic lesions, however, their activation potential is understudied in all relevant tissues. Future research should focus on identifying clinically relevant phenotypes to support the development of non-invasive diagnostic and treatment strategies.
Systematic Review Registration: PROSPERO identifier CRD42021233304
Endometriosis is a common gynaecological disease defined as the presence of endometrium-like tissue outside the uterus (1, 2). It affects one in ten females of reproductive age, translating to approximately 190 million worldwide (3). Although the exact pathophysiology of the disease is still unclear, the oldest and most widely accepted explanation is the retrograde menstruation theory proposing that endometrial cells travel through the uterine tubes into the abdominal cavity, adhere to the peritoneum and invade the mesothelium and deeper layers (4). Retrograde menstruation is a physiological process occurring in most women, but only in some do these cells persist and cause inflammation, with potential pelvic pain and infertility. Therefore, additional factors have been suggested to play a role, including altered systemic and local immunity, resulting in a disruption in the removal of endometrial cells from the abdominal cavity (1).
Endometrial mucosa is populated by various immune cells (Figure 1) which not only provide immunity against pathogens but also help facilitate embryo implantation and pregnancy (5). T cells are the most abundant leukocytes comprising 40–60% in the proliferative phase, decreasing to <10% in the late secretory phase due to the accumulation of natural killer (NK) cells post ovulation (5, 6). Of all T cell populations, approximately 60% are CD8+ T (CD8 T) cells and are found scattered throughout the endometrium either as single cells surrounded by stromal cells, as intraepithelial cells adjacent to the luminal or glandular epithelium or as a part of large “lymphoid aggregates (LAs)” (7).
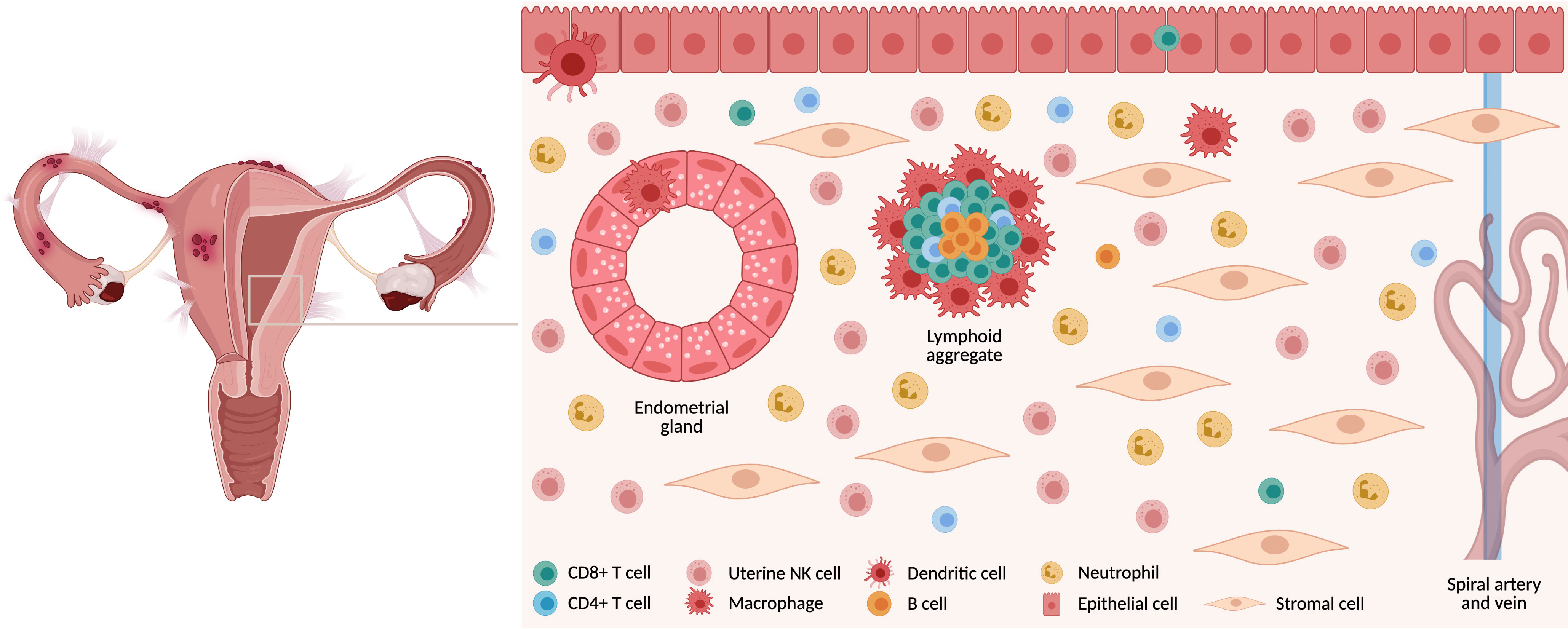
Figure 1 Immune cells in endometrial mucosa. The endometrium is comprised of a variety of cell types. Luminal and glandular epithelium are surrounded by stromal cells, which are interspersed with a variety of immune cells. These are either found as single cells or in lymphoid aggregates. Created with BioRender.com.
Based on their distinct thymic developmental pathways, mucosal CD8 T cells can be divided into two subsets (8, 9). The “unconventional” types such as T-cell receptor (TCR) γδ+ T cells, natural killer T (NKT) cells and mucosal-associated invariant T (MAIT) cells resemble innate immune cells and express TCRαβ/TCRγδ and typically CD8αα homodimers, while the “conventional” CD8 T cells express TCRαβ along with CD8αβ. The latter are further differentiated by multiple cell surface markers based on their function and the phases of the typical adaptive immune response as shown in Figure 2 (10). During the initial activation and expansion, naïve CD8 T cells (TN) that circulate between the peripheral blood and secondary lymphoid organs recognize antigens presented via major histocompatibility complex (MHC) class I on antigen-presenting cells, start expanding and develop into effector CD8 T cells (TE) (11). They leave secondary lymphoid organs, secrete pro-inflammatory cytokines, such as IFN-γ and TNF-α and kill the antigen-positive cells with cytolytic molecules, such as perforins and granzymes. Most of these CD8 T cells die by apoptosis during the contraction phase. In the last phase, surviving cells develop into several forms of memory CD8 T subtypes, such as central (TCM), effector (TEM) and tissue-resident (TRM) memory subsets (12). TCM and TEM are collectively referred to as circulating memory CD8 T cells predominantly found in blood, lymph nodes and secondary lymphoid organs. Conversely, TRM remain in tissue even without constant antigen stimulus and provide a rapid response to previously exposed pathogens without requiring co-stimulatory signals (13). Mucosal CD8 T cells usually acquire a tissue-resident memory phenotype, widely but not exclusively determined by CD69 and CD103 markers (14, 15).
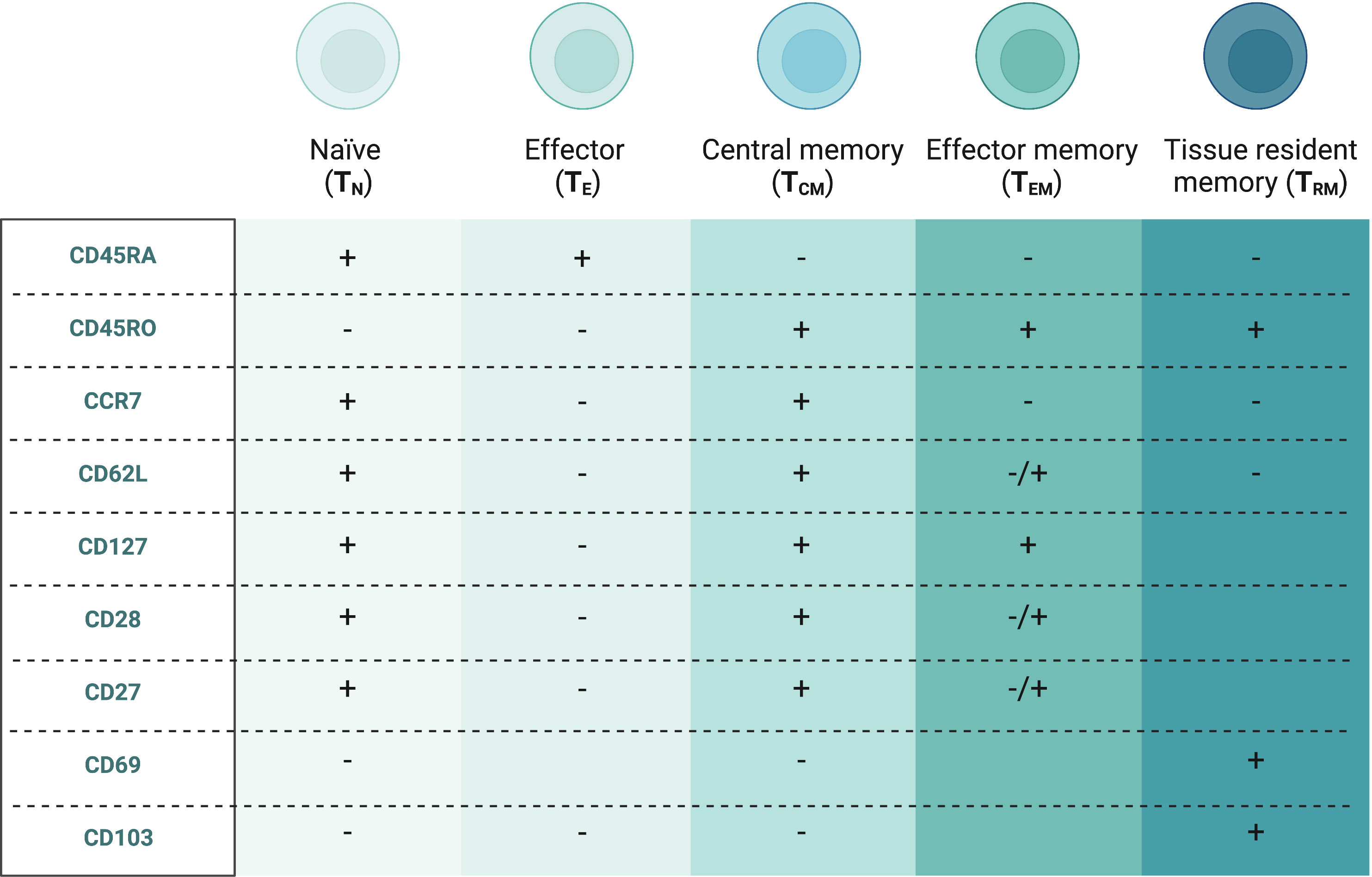
Figure 2 Conventional CD8+ T cell subsets in peripheral blood and endometriosis-associated tissues (10). Created with BioRender.com.
In contrast to the mucosal tissue in the eutopic and ectopic endometrium, the peritoneal fluid mainly derives as an ovarian exudate induced by enhanced vascular permeability, but small amounts have been found in healthy men (16, 17). Although the peritoneal fluid immune milieu strongly depends on the hormonal milieu, macrophages remain the most prevalent population of peritoneal immune cells throughout the uterine cycle in women with endometriosis (55%), followed by T cells (20%) (18). Similar to the eutopic endometrium, the peritoneal fluid CD4/CD8 ratio is in favour of CD8 T cells with increased relative frequencies of this population, when compared to the peripheral blood (19).
Despite being the major T cell population in both the human endometrium and peritoneal fluid, data regarding CD8 T cells in endometriosis remain conflicting. While there have been recent publications of systematic reviews focusing on CD4+ T cells (20) and regulatory CD4+ T cells (21), there has been no systematic assessment of the data on CD8 T cells in this highly prevalent disease. Importantly, our review aims to provide the rationale for the potential role of the CD8 T population in the pathophysiology of endometriosis and associated conditions (e.g. pelvic pain, subfertility, miscarriage) as well as for its predisposition to disease recurrence.
This systematic review was registered in the international prospective database of systematic reviews - PROSPERO in January 2021 (PROSPERO ID Number: CRD42021233304) (22).
The methodology followed the standardised PRISMA statement guidelines for systematic reviews (23). Multiple searches were conducted from January 2021 through December 2022. Medline database (Ovid MEDLINE® Epub Ahead of Print, In-Process & Other Non-Indexed Citations, Ovid MEDLINE® Daily and Ovid MEDLINE®; 1946 to present), Embase (Ovid; 1974 to present), The Web of Science (Clarivate; 1900 to present) and CINAHL (HDAS; 1937 to present) were searched for the relevant terms from two categories: CD8 T cells and endometriosis. The following keywords were used to search text words and Medical Subject Headings (MeSH-terms): CD8, CD8+, cytotoxic T or CTL; natural killer T or NKT; mucosal-associated invariant T or MAIT; intraepithelial lymphocyte* or intra-epithelial lymphocyte*; endometriosis; endometriotic; endometrioma. Results were limited to the English language with no publication period restrictions. Additionally, relevant papers that reviewers had been aware of were also included.
Duplicates were removed and all studies were screened for eligibility using the adapted population, intervention, comparator, and outcomes (PICO) framework (Table 1) (24). Non-peer-reviewed studies such as editorial letters, expert opinions and conference abstracts were excluded. For the first round of screening, AK and JHS independently screened the titles, abstracts, and keywords, applying eligibility criteria. If the abstract did not clearly indicate whether a study met the initial inclusion criteria, the entire article was assessed. In the second round, full records of the selected articles were retrieved and screened, and articles not complying with eligibility criteria were excluded. AK and JHS assessed papers independently through stages 1 and 2 then compared decisions and discussed discrepancies. In this instance, IG and CMB were consulted and final decisions were reached. The screening was undertaken using Rayyan QCRI (25).
The following data were extracted from each study by AK: first author’s last name, publication year, study design, study period, sample size (patients with endometriosis and controls), participant demographics and baseline characteristics, outcomes related to CD8 T cells (e. g. concentration, activation and proliferation status, cytokine production, an association between CD8 T cells and disease stage, treatment response, recurrence rate after surgery, pregnancy rate, live birth rate, and miscarriage rate). The data were extracted into an Excel spreadsheet and were checked by JHS.
Quality assessment of included studies was conducted independently by AK and JHS using the Newcastle-Ottawa Scale (Selection, Comparability, Exposure/Outcome) (26). Each paper was graded and defined accordingly as “good”, “fair”, or “poor” quality. Any disagreements or uncertainties were resolved with IG and CMB.
Initially, we identified 517 papers of which 28 met all the inclusion criteria and were included in the narrative synthesis (Figure 3). A detailed overview of their key characteristics is presented in Table 2. In summary, most studies were conducted in Asia (11), followed by Europe (8), North America (4) and South America (3), and were conducted in a university hospital setting (19).
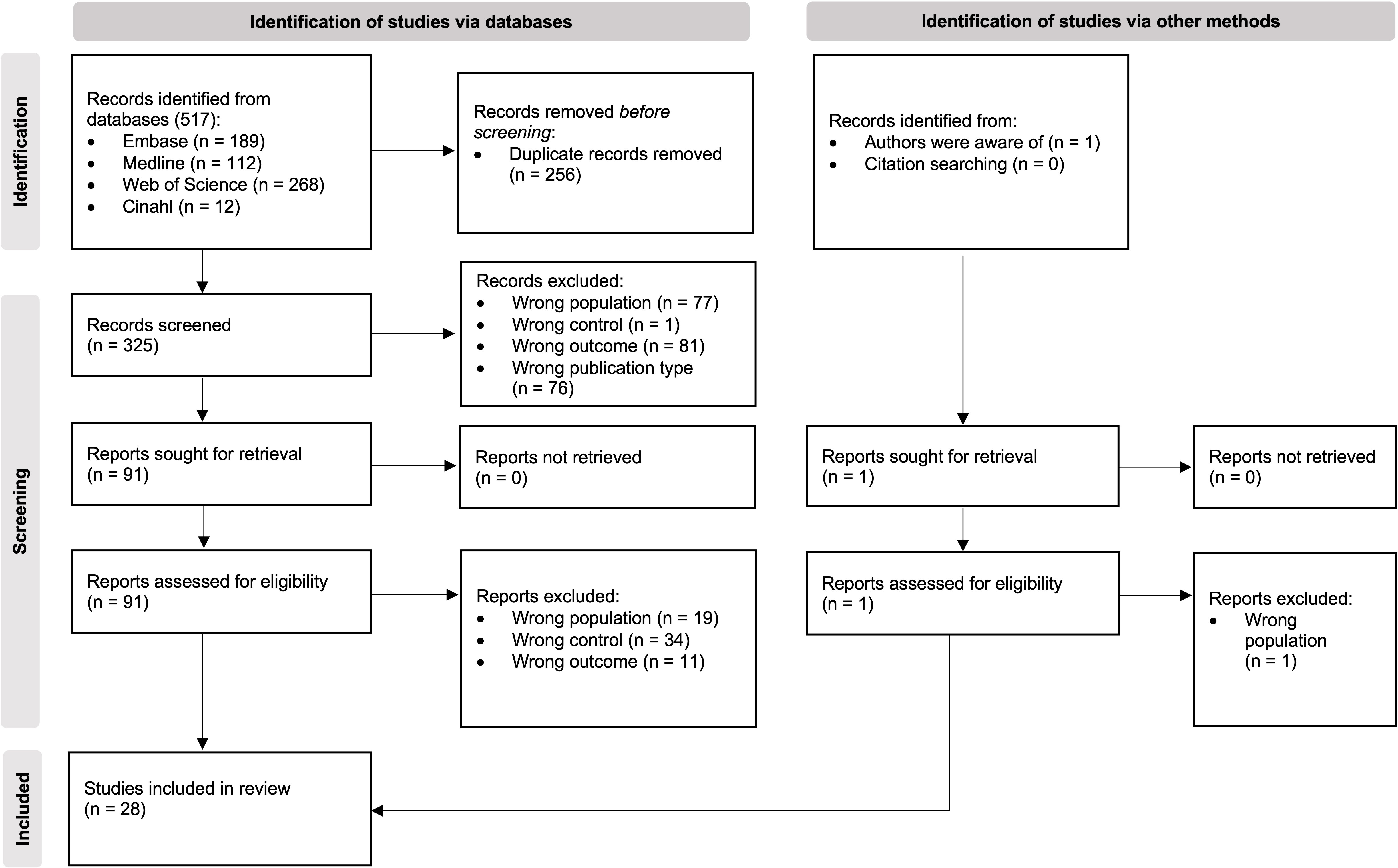
Figure 3 PRISMA flow diagram of study selection (23).
Quality assessment was carried out for each of the included studies using the Newcastle-Ottawa Scale. Most of the papers were scored as “good” or “fair” (Table 2). Overall, all papers scored in the definition of cases and controls and the ascertainment of exposure. Only 2 papers reported on the non-response rate showing a potential risk of bias in the sample selection in most of the studies. One paper was graded as “low” quality due to the low score in the comparability category. One paper could not be graded as it was an animal study performed on baboons.
Recent bioinformatic analyses looking at several CD8 T cell subtypes found changes in TN, TCM, and TEM cell scores between the eutopic endometrium of patients and controls (Table 3). However, 5 out of 9 papers reported no difference in the concentration of the total CD8 T cell population between patients with endometriosis and healthy controls.
Using gene set enrichment analysis (GSEA) on data from eutopic endometrial biopsies taken during the proliferative phase with CIBERSORT, Zhong et al. found no difference in CD8 T cell fraction between patients with severe endometriosis and controls (2). Nevertheless, in a similar analysis, investigators found increased CD8 TEM cell gene enrichment scores in women with revised American Society for Reproductive Medicine (rASRM) stage I/II as well as stage III/IV disease (p = 0.000 and p = 0.027, respectively) and decreased CD8 TN and TCM scores (p = 0.001 and p = 0.000; p = 0.035 and p = 0.782) when compared to controls (27). Another GSEA by a different group shows an increased CD8 T cell score in endometriosis versus controls when analysed using CIBERSORT (0.1659 ± 0.0968 vs 0.1086 ± 0.0939, respectively; p = 0.0033), MCP-counter (p = 0.00018) and ImmuneCellAI (p = 7.2e-8) (28). Comparing different subsets with ImmuneCellAI, decreased CD8 TN cells (p = 7.6e-5), TCM (p = 0.00076), and TEM cells (p = 1.1e-6) were present in the endometrium of women with endometriosis when compared with controls. In a second analysis (GSE120103), performed with MCP-counter, increased CD8 T cell scores were revealed in endometrium of infertile versus fertile women with endometriosis (p = 0.0019, AUC (area under the receiver operating characteristic curve (ROC)) = 0.722). Immunohistochemical validation of their transcriptomic data confirmed increased proportions of CD8+ cells among all leukocytes in the eutopic endometrium of endometriosis patients versus controls in both the proliferative and early secretory phases (0.2292 ± 0.0591 vs 0.1790 ± 0.0562, p = 0.0132, AUC = 0.727) (28).
Similarly, increased quantity of CD8+ cells per mm2 was detected in severe endometriosis versus controls, of note samples were collected in the proliferative phase (0.019 ± 0.004 vs 0.007 ± 0.002 cell/mm2, respectively; p < 0.01) (29). Focusing on the eutopic endometrial tissue in the secretory phase, single-cell RNA sequencing analysis did not reveal any difference in the expression of the CD8A gene between endometriosis and controls (30). Again, no difference was found in the microscopic analysis of the secretory phase tissue from infertile women with endometriosis and fertile controls (31). In samples equally distributed across the uterine cycle, no difference was found in either glandular or intraepithelial CD8+ cell count in either of the uterine cycle phases between endometriosis and controls (32), or between endometriosis and controls with laparoscopically proven tubal factor infertility resulting from prior pelvic inflammatory disease (PID) (33).
Finally, in the findings from Bunis et al., the endometrial CD8 TEM signature had higher gene enrichment scores across all menstrual phases in endometriosis patients when compared to the control (34). Additionally, they were significantly increased in the mid-secretory phase when compared to the preceding phases in both disease and control (NES = 1 for unstratified samples). The activation potential of eutopic endometrial CD8 T cells was decreased in the endometriosis patients when compared to controls and genes with functions known to be relevant to phagocytosis and complement activation were stimulated in the eutopic endometrium of patients. No papers were found assessing the proliferative and cytotoxic capacities of eutopic endometrial CD8 T cells in endometriosis.
All 5 studies researching ectopic endometrium reported an increased proportion of CD8 T cells in endometriotic lesions when compared to matched eutopic endometrium and/or healthy eutopic endometrium (Table 4). Additionally, 2 studies also investigated their activation and cytotoxic properties.
Zhong et al. performed transcriptome meta-analysis with CIBERSORT on 24 ectopic, 82 eutopic and 68 healthy endometrial samples in the proliferative phase (GSE120103, GSE51981, GSE25628, GSE37837, GSE7846, GSE6364, GSE7305) (2). They described an increased CD8 T cell fraction among all infiltrating immune cells in the endometriosis lesions when compared to the eutopic endometrium of endometriosis patients (p = 0.085) and endometrium of controls (p = 0.033). Similarly, analyzing scRNA-seq data of proliferative phase timed samples, a significantly higher percentage of CD8 TN cells was reported in endometriotic lesions when compared to matched eutopic endometrium and normal endometrium (43.48% vs 0.91% and 0.33%, respectively), and a lower proportion of TE subtypes (35). In addition, the authors describe decreased cytotoxic T cell populations in lesions, however, data was not shown. Interestingly, a smaller immunohistochemistry study on proliferative phase samples confirmed increased CD8+ cell counts in ectopic endometrium when compared to the control endometrium (0.019 ± 0.002 vs 0.007 ± 0.002 per mm2; p < 0.01), but not in ectopic versus matched eutopic endometrium (29).
Investigating samples in the secretory phase of the menstrual cycle, genes associated with several immune cell surface markers, including CD8A, were upregulated in ectopic tissues compared with eutopic endometrium of patients as well as control endometrium (p < 0.05) (30). In the same study, they also found increased expression of immune genes for T cell co-stimulation (e. g. CD27 and CD28) as well as less specific HLA genes associated with T cell activation, such as HLA-DPA1, HLA-DPB1, HLA-DQA1, HLA-DRA, and HLA-DMA (p < 0.05). On the other hand, a different IHC study found no difference in the CD8+ cell count between the glandular epithelium of ectopic lesions and matched eutopic endometrium in the proliferative and late secretory phase but confirmed an increased count in the early secretory phase (p = 0.0002) (32).
Of the 9 studies researching CD8 T cell ratios in the peritoneal fluid, 3 found no difference between endometriosis patients and controls, 1 paper noted no difference in CD8 T cell fraction among lymphocytes but increased proportions among all peritoneal cells in patients with endometriosis and 2 found increased concentrations in the disease (Table 5). Similarly, as shown, no consensus was reached on the activation status of the peritoneal CD8 T cells: 3 studies found no difference, 2 reported decreased and 1 increased activation in endometriosis patients.
The proportion of CD8 T cells among total mononuclear cells, between patients with rASRM stage I/II endometriosis and fertile controls are unchanged in the proliferative and secretory phases as well as when uncontrolled for the cycle phase (36). Similarly, some authors found comparable rates of CD8 T cells among mononuclear cells in women with and without the disease in the proliferative phase (37) and others reported no difference in the percentage of CD8+ cells among all lymphocytes in the periovulatory phase between endometriosis patients and healthy controls (38). While Deng et al. identified no difference in the proportion of CD8+ cells among lymphocytes in the proliferative phase, they found increased numbers of CD8+ cells among all peritoneal fluid cells in samples from patients with endometriosis (p = 0.0032) (39).
Increased proportions of specific CD8 T cell subsets were reported in studies studying baboons and MAIT cells. In baboons, a significant increase in the proportion of peritoneal CD8 T cells was detected in the group with long-term spontaneous endometriosis, when compared to other groups with no disease, induced disease, and recent spontaneous endometriosis (74.3 ± 6% vs 67.1 ± 8.3% vs 65.8 ± 8% vs 54.8 ± 21.9%, respectively; p = 0.01) (40). In humans among MAIT cells defined as CD3+CD161+Vα7.2+ enhanced CD8+ MAIT cell subsets were found in the disease compared to control (6.52 ± 1.05 vs 2.77 ± 0.42, respectively; p = 0.0454) (41). In the same study, peritoneal fluid CD8+ MAIT cells from endometriosis patients displayed higher levels of the activation marker CD38, when compared to non-endometriosis participants (2.98 ± 0.53 vs 0.36 ± 0.09, respectively; p = 0.0071). The difference was more pronounced in the group with stages I/II than with stages III/IV when compared to controls.
On the other hand, Gallinelli et al. showed decreased proportions of CD3+CD8+HLA-DR+ cells in the endometriosis versus the control group (42.8 ± 6.8 vs 57.1 ± 7.6, respectively; p < 0.02) (42). Additionally, they found a significantly higher CD8/CD4 ratio in the disease (2.8 ± 0.5 vs 2.1 ± 0.4, respectively; p < 0.05). Nevertheless, 2 publications report no difference in activation potential between endometriosis and non-endometriosis groups (38, 43). In a study from Ho et al., they found no differences between stage I/II and III/IV endometriosis and controls in any of the identified activated phenotypes, including HLA-DR+, CD25+, CD28+, and CD69+ CD8 T cells (43).
Schmitz et al. investigated the variance in immune cells between menstrual effluent of patients with endometriosis and healthy controls (44). They found no statistically significant differences in either the proportion of CD8 T cells or the CD4/CD8 ratio. However, a decreased proportion of perforin-high CD8 T cells was present in the menstrual effluent of patients versus controls (4.0 ± 4.8 vs 11.2 ± 10.4, respectively; p = 0.029). In the study of Shih et al. four distinct CD8 T cell clusters were differentially enriched between patients with surgically confirmed endometriosis and community controls, although the differential markers for those four clusters have not been explored (45).
As presented in Table 6, data from 10 papers looking at the general CD8 T cell population in peripheral blood indicate no difference in ratios amongst the immune compartment between patients with endometriosis and controls, but one study on different subtypes revealed potential changes in the levels of TCM and TEM in the disease (46). Additionally, there seem to be no differences in their activation status between patients and controls and their proliferative capacity has not been assessed.
A study comparing leukocyte subsets in peripheral blood samples that were equally distributed across the menstrual cycle reported no differences in either CD8+ or CD8 T cell proportions between patients with endometriosis and healthy controls neither within the unadjusted model nor the model adjusted for age, smoking, oral contraception, parity, and history of an acute infection (CD8+: 16.4 ± 6.6 vs 16.7 ± 5.7, respectively; CD3+CD8+: 14.3 ± 5.3 vs 13.8 ± 5.7) (47). Focusing on the proliferative phase of the cycle, the median percentages of CD8 T cells among CD3+ cells and CD4/CD8 ratio in the peripheral blood between patients and healthy controls were unchanged (37) (44). Additionally, no difference was revealed in perforin-high CD8 T cells (9.0 ± 6.6 vs 12.9 ± 17.3, respectively) (44) or in the proportions of CD8 T cells expressing activation markers, such as HLA-DR, CD25, CD28 and CD69 in different disease stages when compared to controls (48). The only study investigating peripheral blood CD8 T cells solely in the secretory phase found no difference in either concentration or activation status (38).
A significant number of papers did not consider the menstrual cycle phase in their analysis. Investigators identified comparable findings with no difference in CD8 T cell proportions among leukocytes between endometriosis and control group (29.3 ± 5.5 vs 30.2 ± 4.9, respectively) and no difference in CD4/CD8 ratio (1.4 (0.73–2.7) vs 1.3 (0.93–4.5), respectively) (49). Interestingly, no difference was observed even when they compared CD8 T rates and CD4/CD8 ratio between different disease stages. Similarly, no significant changes were observed in the percentages of CD8 T cells among total mononuclear cells (36) and no variations revealed in CD8 T cell rates and CD4/CD8 ratio among CD3+ cells in endometriosis regardless of the stage (50). However, the latter group found lower expression of TNF-alpha and IFN-gamma in CD8 T cells in the endometriosis group when compared with the control group (17.4 ± 1.6 vs. 25.5 ± 2.0, respectively; p = 0.0045). Focusing on the cytotoxic activity of peripheral blood mononuclear cells, authors found no difference in CD8+CD28+ T cell ratios between endometriosis patients and controls (15.16 ± 3.26 vs 11.89 ± 2.65, respectively; p = 0.45) (51). Finally, the study on baboons discovered no differences in the CD8 T cell ratio between baboons with spontaneous endometriosis and those with the induced disease as well as between animals with the disease and healthy subjects (40).
In addition to these studies which largely compare bulk CD8 T cell ratios, some papers report on CD8 expression within other subpopulations of T cells, such as iNKT (52) and MAIT (41), or subsets of the global CD8 T repertoire such as TCM and TEM subtypes (46). CD8 expression on iNKT cells in combination with the regulatory cytokines IL-6, IL-17 and IL-10 were unaltered between health and endometriosis controls (52). Although Li et al. did not identify differences in the overall CD8+ MAIT population between endometriosis patients and controls (the majority of MAIT cells are CD8+), they found an increased frequency of CD8+ cells among MAIT subtypes in the peripheral blood of patients as well as controls when compared to global CD8 T cells (p = 0.003 and p = 0.0002, respectively) (41). Importantly, the only study looking into distinct CD8 T subtypes found increased levels of TCM (CD3+CD8+CD45RA-CCR7+; p < 0.05) and decreased levels of terminally differentiated TEM subtypes (CD3+CD8+CD28−; p = 0.006) (46).
Endometriosis-associated immune alterations are complex (53). Our systematic review aimed to provide a rationale for further research into the CD8 T cells in endometriosis and associated conditions by summarizing and critically appraising the currently available data. Due to the observational study design of the included papers, this systematic review does not provide evidence for the direct involvement of the CD8 T cells in the development of endometriosis and associated disorders. Nevertheless, its findings highlight significant gaps in the literature that must be addressed in future research to determine the pathophysiological significance, non-invasive diagnostic potential and targeted treatment possibilities for this immune population. Out of 28 papers included in this systematic review, 14 could provide high-quality findings, which show that CD8 T cell levels are higher in endometriotic lesions than in the eutopic endometrium of patients as well as healthy controls, with no changes in peripheral blood levels between patients and healthy controls. However, the evidence for changes to CD8 T in peritoneal fluid and eutopic endometrium is debatable. Although older studies mostly found no difference in eutopic endometrial CD8 T cell concentrations, recent genomic analyses of distinct subtypes revealed a strong tendency of enriched CD8 T effector memory cells in the eutopic endometrium of patients with the disease. In one study investigating CD8 T cells in menstrual effluent, cytotoxicity was increased in patients while no difference was found in concentration and CD4/CD8 ratio.
Numerous review articles have been published describing CD8 T cells in endometriosis (53–66). However, none of them has comprehensively investigated the literature on this immune cell population in the relevant tissues, including peripheral blood and endometriosis-associated tissues, such as eutopic endometrium, ectopic lesions, peritoneal fluid, and menstrual effluent. Our review is the first to systematically summarize adequate quality evidence of CD8 T cells in endometriosis and was based on a comprehensive search strategy and strict inclusion criteria to ensure better comparability and reliability. For example, we have excluded studies investigating participants on hormonal contraception and menopausal women since it is known that oestrogen and progesterone significantly impact the CD8 T cell milieu and might undermine the accuracy of the findings (67, 68). Additionally, we have excluded papers recruiting individuals with certain gynaecological pathologies, which could also have a significant impact on the relevant outcomes, such as adenomyosis, acute pelvic inflammatory disease, hydro/pyo/hematosalpinx, idiopathic infertility, and immune diseases (69–72).
Despite the strict inclusion criteria, one of the weaknesses of this systematic review is that it is based on observational studies with small sample sizes which did not consistently account for confounders, so bias cannot be ruled out. While it is widely accepted that the menstrual cycle phase influences CD8 T cell counts and activation status in endometriosis-associated tissue, one of the included studies also found a clear correlation between the levels of several blood leukocytes and age, parity, previous use of oral contraceptives, smoking, and recent history of acute infection, but no other studies accounted for this (5, 47). With surgery being the gold diagnostic standard, it was expected that this review would suffer from the heterogeneity of the control population, with 8 studies having recruited control participants with various gynaecological conditions, such as uterine fibroids, ovarian cysts, and tubal factor infertility, and 4 of them including “healthy” controls without having undergone surgery to rule out potential pathological conditions. Additionally, some participants might have undergone several diagnostic and therapeutic surgeries before being enrolled in the study, which might have impacted the outcomes as significant immune changes are associated with the postoperative period (73).
Imaging techniques have recently been recognized as a reliable diagnostic alternative to invasive procedures but they still have significant limitations for the detection of early disease (74). Therefore, the development of novel non-invasive biomarkers is urgently needed to provide alternatives to surgery for diagnosis, and to reduce the prolonged time to diagnosis that is widely recognised for this condition (75). Furthermore, biomarkers are a dynamic and effective tool to understand the range of the disease and provide a means for a consistent disease and risk factor assessment, which could help us learn more about the underlying aetiology of the disease (76). Regarding the pathophysiology, none of the studies included in this review were designed to elucidate whether an altered CD8 T population could be the cause or the consequence of endometriosis. The closest is the study by D’Hooghe et al. in which they investigated peripheral blood and peritoneal fluid of baboons with spontaneous and induced disease compared to healthy controls (40). While no difference was found in blood, an increased CD8 T cell ratio was found in peritoneal fluid of long-term spontaneous endometriosis when compared to animals with the recent spontaneous disease, induced disease and healthy controls, which could point out to the altered immune environment being a consequence rather than a cause of the disease. No conclusions can be drawn from human studies, which did not find any differences in the CD8 T cells between different disease stages and healthy controls.
The eutopic endometrium is an important mucosal barrier and the location of embryo implantation. Not only is immune dysregulation in eutopic endometrium associated with subfertility it may also play a role in lesion establishment (77, 78). Therefore, along with other immune cell populations, CD8 T cells are an important consideration in endometriosis. Although the data regarding eutopic endometrial CD8 T cells in endometriosis altogether are controversial, our systematic review gives an overview not only of the whole CD8 T cell population, but of alterations in other CD8 T cell subtypes in the disease. CD8 TN and TCM cell gene enrichment scores were found to be decreased while TEM cells seem to be increased (27, 28, 34). There is increasing evidence demonstrating an association between endometriosis and autoimmunity and CD8 TEM cells are likely an important player in the pathogenesis of autoimmune diseases due to their effectiveness and durability (72). Nevertheless, evidence is not uniform in other autoimmune disease where for example, CD8 TEM cells are increased in the cerebrospinal fluid of patients with multiple sclerosis. However the opposite was found in the gut of patients with inflammatory bowel disease (79, 80). More research is certainly needed to ascertain their role in the pathogenesis of endometriosis (81).
TRM are thought to be the most prevalent CD8 T subpopulation in mucosal tissues but our understanding of this subtype in the human endometrium is poor (12). TRM in the gut are widely researched and there is strong evidence they are implicated in the pathogenesis of inflammatory bowel disease (IBD) and other intestinal immunopathologies such as coeliac disease (13, 82). For example, authors investigating TRM in IBD show considerably lower levels of CD103+ TRM in patients with flares versus patients with endoscopic remission and healthy controls, which suggests their contribution to tissue homeostasis and immune regulation (83). Higher levels of CD69+CD103+ TRM were associated with active disease in patients with vitiligo, and importantly, these cells can attract cytotoxic effector CD8 T cells from the blood, which is critical for disease persistence (84). In other gynaecological pathologies, authors revealed a modified eutopic endometrial CD8 TRM phenotype in recurrent miscarriage with considerably lower expression of tissue residency marker CD69 (85).
In adenomyosis, CD8 T cell subsets are increased not only in the eutopic, but also in the ectopic endometrium of patients compared to healthy controls (86). Similar seems to be true for endometriosis, although there are discrepancies in the results between the lesions and matched eutopic endometrium. However, we emphasize that studies reporting no difference in CD8 T cells between eutopic and ectopic endometrium did not investigate specific phenotypes (2, 29). Although the potential roles of CD8 T cells in lesions are not fully defined, some parallels may be drawn from cancer research, where CD8 T cells are regarded as the most potent effectors in the anti-cancer immune response and form the basis of some current cancer immunotherapies (87). For example, increased intraepithelial CD8 T cell count in the tumour microenvironment is associated with prolonged survival in colorectal, ovarian and endometrial cancer (88–90) and the intraepithelial subpopulation of CD8 T cells was found to be increased in ectopic lesions in the early secretory phase when compared to matched eutopic and healthy endometrium (32). However, no studies were conducted comparing intraepithelial CD8 T cells between lesions of patients with different endometriosis stages and between those who benefited from the surgery or relapsed, which would give us valuable insights into the prognostic role of this population.
It has been suggested that endometriotic lesions ought to be investigated along with peritoneal fluid, since this dynamic environment strongly influences their pathophysiology (91). Nevertheless, to date, studies have examined lesions and peritoneal fluid in isolation. Similar to eutopic endometrium, data on peritoneal CD8 T cells are contradictory and no definitive conclusions can be made about the role of the CD8 T cell population in this regard. On the other hand, macrophages are a well-researched population in this setting as they are the most abundant peritoneal immune cell population, representing around 50% of peritoneal cavity leukocytes (92). They are required for lesion growth, development, vascularization, and innervation, and are even associated with pain symptoms. In endometriosis, an increased ratio of altered peritoneal macrophages has been indicated, and since they are involved in antigen presentation to CD8 T cells, this points to potentially related dysfunctions within the CD8 T cell compartment. Similarly, changes were found in regulatory T cells (Tregs) in endometriosis, with findings showing an increased number of activated Tregs in the peritoneal fluid of patients with endometriosis (93). Like macrophages, Tregs are involved in CD8 T cell activation and play an important role in the generation of high-avidity initial responses and effective memory (94).
One aim of this review was to gain a better understanding of the activation characteristics of the CD8 T cells in endometriosis. Unfortunately, no functional analyses have been performed to date on either eutopic or ectopic endometrial CD8 T cells, despite clear evidence of endometriosis-related dysfunctions in key regulators of CD8 T function, such as programmed cell death protein 1 (PD-1), programmed death-ligand 1 (PD-L1) and CD4+ T regulatory cells (29, 53, 95). Functional analyses of CD8 T cells in peritoneal fluid demonstrate inconsistent findings, where data mostly report no difference or decreased activation potential of peritoneal CD8 T cells with only one study reporting an increase in activated CD8+ MAIT cells. There is some evidence of upregulated activation marker CD69 expression on peritoneal fluid T cells in endometriosis patients, although it is important to emphasize that the investigated cohort had been taking hormonal treatment hence this study was not included in our systematic review (19).
Studies on immune cells in the female genital tract are challenging given the invasive nature of collecting mucosal and peritoneal samples (96). As previously mentioned, most research depends on biopsies and hysterectomies from women with underlying pathologies and samples from healthy women are limited. Menstrual effluent contains cells of endometrial origin and could be a valuable non-invasive source of endometrial immune cells, but has so far been overlooked in endometriosis research (44). Authors found decreased expression of perforin in CD8 T cells of patients when compared with controls and argued that perforin-mediated cytotoxicity may play a crucial role in the establishment of the lesions, drawing from research on CD8 T cells in susceptibility and elimination of cancer cells. Importantly, this could provide some answers to why only a few women develop endometriosis while retrograde menstruation occurs in most females of reproductive age (97). However, further investigation of menstrual effluent is required to understand the processes responsible for endometrial cell attachment and development outside of the endometrium, especially given the key differences in immune milieu between eutopic and ectopic endometrium and peritoneal fluid which we have confirmed in our review.
The ease of sampling makes peripheral blood one of the most studied tissues in endometriosis leading to valuable insights into the systemic nature of endometriosis and its association with autoimmune diseases, including abnormalities in the T cell population (72, 93, 98). Peripheral blood immune cells are influenced by sex hormones, with higher CD8 T cell numbers in males, but greater activation, proliferation and cytotoxic capacity in females (99). Although longitudinal studies investigating CD8 T in the peripheral blood at different menstrual phases are limited, modifications in Treg cells (100) and Th1/Th2 ratios (101) indicate that it is important to consider the menstrual cycle when investigating CD8 T population in the peripheral blood. However, this was not the case in some of the included studies. While most of our data does not suggest discrepancies in the peripheral CD8 T cells, it is important to highlight that most papers did not describe CD8 T cell subpopulations. They were shown to be significantly changed in patients in one of the recent analyses with increased central memory and decreased effector memory subtypes in the disease (46). Interestingly, several authors confirmed a decreased frequency of peripheral effector memory CD8 T cells in patients with multiple sclerosis at the onset of the disease and throughout its clinical course, which underlines its importance in the initiation of the disease rather than being its consequence (102). Additionally, there is evidence of changes in innate immune cells in the peripheral blood of patients with endometriosis, such as monocytes, which are important for antigen presentation and CD8 T cell activation (103). Reduced numbers of classical and intermediate monocytes were found in the peripheral blood of endometriosis patients, while the levels of plasmacytoid dendritic cells and non-classical monocytes were increased. Nevertheless, to better understand the systemic role of CD8 T cells in endometriosis, detailed phenotypic studies are needed, which will address specific subtypes and their activation characteristics found to be changed in the peripheral blood of other immune diseases (84).
Although the pathophysiology of endometriosis-associated subfertility remains uncertain, several findings support altered endometrial immune receptivity as one of the possible causes (77, 104). The exact nature of CD8 T in eutopic endometrium is poorly understood, but the suggested hormonal influence on LA formation and CD8 T cell cytotoxicity underline the importance of optimal regulation during implantation and pregnancy (5, 68). As noted in our review, authors found significantly increased CD8 T cell scores in infertile women with endometriosis when compared to fertile women as well as in infertile versus fertile women with or without endometriosis (28). Despite the limited sample size of this GSEA analysis, it reveals possible changes in CD8 T cells in patients with endometriosis-associated infertility and urges for more investigation into the phenotypic characterisation of the population in this related disorder. It is likely that different CD8 T phenotypes could contribute to subfertility and pregnancy complications as their findings correspond to the results of several authors, who confirmed altered endometrial CD8 T characteristics in endometrial biopsies obtained during the window of implantation from patients with recurrent pregnancy loss, pre-eclampsia, and preterm labour (85, 105–107).
By combining cutting-edge technologies, such as single-cell RNA sequencing, mass cytometry and spectral flow cytometry with established high-resolution, high-plex and multi-omic spatial biology solutions, such as spatial transcriptomics and multiplex immunostaining, we could spatially verify immune phenotypes, their functional capacities, and cell-cell interactions to ascertain the physiological and pathophysiological function of endometrial CD8 T cells. Importantly, structures similar to LA and intraepithelial lymphocytes are known to play a role in intestinal inflammatory disorders therefore a deeper understanding of endometrial LA and intraepithelial CD8 T subsets may finally unravel their involvement in endometrial pathologies and infertility. For example, Tan et al. undertook image mass cytometry in addition to single-cell transcriptomic characterization of ectopic and eutopic endometrium, providing a cell atlas of the endometriosis microenvironment (108). This is a promising direction for future research and the analysis of a larger cohort, including those not taking hormonal contraceptives, will enable us to draw conclusions on the disease pathophysiology and contribute critical information for future diagnostics and therapies. Importantly, further research should also provide more detailed insights into menstrual effluent, as well as functional and cytotoxic properties of the population in relevant tissues with consistent considerations for the phase of the uterine cycle and parity.
Understanding of CD8 T cells in endometriosis is limited, with significant discrepancies in current data in endometriosis-associated tissues. We have identified relevant gaps in the literature, such as deficient phenotypic and functional analyses in all relevant tissues. In the future, a detailed pathophysiological characterization could lead to the discovery of non-invasive diagnostic biomarkers as well as successful drug repurposing and the development of targeted treatments to alter the immunological niche for better lesion clearing as well as endometrial homeostasis, vital for pregnancy success.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
AK contributed to all phases of the study, including its design, data collection, screening, analysis, and interpretation, as well as the paper’s drafting. Two rounds of screening, data extraction and grading were simultaneously handled by JHS, who also participated in data interpretation and synthesis. IG and CMB assisted with the screening process and interpretation of the results. All authors contributed to the article and approved the submitted version.
This study was funded by the Nuffield Department of Women’s and Reproductive Health, University of Oxford and supported from the Academy of Medical Science award [SBF007\100078], Medical Research Council award [MR/W028255/1] and British Society for Immunology Career Enhancing Grant.
CMB reports grants from Bayer Healthcare and MDNA Life Sciences receives consultancy fees for Theramex, ObsEva, Myovant and Gedeon Richter.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1225639/full#supplementary-material
1. Zondervan KT, Becker CM, Koga K, Missmer SA, Taylor RN, Viganò P. Endometriosis. Nat Rev Dis Primers. (2018) 4(1):9. doi: 10.1038/s41572-018-0008-5
2. Zhong Q, Yang F, Chen X, Li J, Zhong C, Chen S. Patterns of immune infiltration in endometriosis and their relationship to r-AFS stages. Front Genet (2021) 12:631715. doi: 10.3389/fgene.2021.631715
3. Zondervan KT, Longo DL, Becker CM, Missmer SA. Endometriosis. N Engl J Med (2020) 382(13):1244–56. doi: 10.1056/NEJMra1810764
4. Sampson JA. Heterotopic or misplaced endometrial tissue. Am J Obstet Gynecol. (1925) 10(5):649–64. doi: 10.1016/s0002-9378(25)90629-1
5. Wira CR, Rodriguez-Garcia M, Patel MV. The role of sex hormones in immune protection of the female reproductive tract. Nat Rev Immunol (2015) 15(4):217–30. doi: 10.1038/nri3819
6. Flynn L, Byrne B, Carton J, O’Farrelly C, Kelehan P, O’Herlihy C. Menstrual cycle dependent fluctuations in NK and T-lymphocyte subsets from non-pregnant human endometrium. Am J Reprod Immunol (2000) 43(4):209–17. doi: 10.1111/j.8755-8920.2000.430405.x
7. Yeaman GR, Guyre PM, Fanger MW, Collins JE, White HD, Rathbun W, et al. Unique CD8+ T cell-rich lymphoid aggregates in human uterine endometrium. J Leukoc Biol (1997) 61(4):427–35. doi: 10.1002/jlb.61.4.427
8. Pellicci DG, Koay H-F, Berzins SP. Thymic development of unconventional T cells: how NKT cells, MAIT cells and γδ T cells emerge. Nat Rev Immunol (2020) 20(12):756–70. doi: 10.1038/s41577-020-0345-y
9. Ma H, Tao W, Zhu S. T Lymphocytes in the intestinal mucosa: defense and tolerance. Cell Mol Immunol (2019) 16(3):216–24. doi: 10.1038/s41423-019-0208-2
10. Kieffer TEC, Laskewitz A, Scherjon SA, Faas MM, Prins JR. Memory T cells in pregnancy. Front Immunol (2019) 10:625. doi: 10.3389/fimmu.2019.00625
11. Harty JT, Badovinac VP. Shaping and reshaping CD8+ T-cell memory. Nat Rev Immunol (2008) 8(2):107–19. doi: 10.1038/nri2251
12. Kok L, Masopust D, Schumacher TN. The precursors of CD8+ tissue resident memory T cells: from lymphoid organs to infected tissues. Nat Rev Immunol (2022) 22(5):283–93. doi: 10.1038/s41577-021-00590-3
13. Lyu Y, Zhou Y, Shen J. An overview of tissue-resident memory T cells in the intestine: from physiological functions to pathological mechanisms. Front Immunol (2022) 13:912393. doi: 10.3389/fimmu.2022.912393
14. Yenyuwadee S, Sanchez-Trincado Lopez JL, Shah R, Rosato PC, Boussiotis VA. The evolving role of tissue-resident memory T cells in infections and cancer. Sci Adv (2022) 8(33):eabo5871. doi: 10.1126/sciadv.abo5871
15. Steinert Elizabeth M, Schenkel Jason M, Fraser Kathryn A, Beura Lalit K, Manlove Luke S, Igyártó Botond Z, et al. Quantifying memory CD8 T cells reveals regionalization of immunosurveillance. Cell. (2015) 161(4):737–49. doi: 10.1016/j.cell.2015.03.031
16. Koninckx PR, Kennedy SH, Barlow DH. Endometriotic disease: the role of peritoneal fluid. Hum Reprod Update. (1998) 4(5):741–51. doi: 10.1093/humupd/4.5.741
17. Yoshikawa T, Hayashi N, Maeda E, Matsuda I, Sasaki H, Ohtsu H, et al. Peritoneal fluid accumulation in healthy men and postmenopausal women: evaluation on pelvic MRI. Am J Roentgenol. (2013) 200(6):1181–5. doi: 10.2214/ajr.12.9645
18. Zou G, Wang J, Xu X, Xu P, Zhu L, Yu Q, et al. Cell subtypes and immune dysfunction in peritoneal fluid of endometriosis revealed by single-cell RNA-sequencing. Cell Biosci (2021) 11(1):98. doi: 10.1186/s13578-021-00613-5
19. Guo M, Bafligil C, Tapmeier T, Hubbard C, Manek S, Shang C, et al. Mass cytometry analysis reveals a distinct immune environment in peritoneal fluid in endometriosis: a characterisation study. BMC Med (2020) 18(1):3. doi: 10.1186/s12916-019-1470-y
20. Yang H, Zhuang Y. The deviations of CD4 + T cells during peripheral blood and peritoneal fluid of endometriosis: a systematic review and meta-analysis. Arch Gynecol Obstet (2023). doi: 10.1007/s00404-023-06964-3
21. De Barros IBL, Malvezzi H, Gueuvoghlanian-Silva BY, Piccinato CA, Rizzo LV, Podgaec S. “What do we know about regulatory T cells and endometriosis? a systematic review”. J Reprod Immunol (2017) 120:48–55. doi: 10.1016/j.jri.2017.04.003
22. Kisovar A, Southcombe JH, Granne I, Becker CM. The role of CD8+ T cells in endometriosis: a systematic review. PROSPERO (2021), CRD42021233304.
23. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Br Med J (2021) 372:n71. doi: 10.1136/bmj.n71
24. Richardson WS, Wilson MC, Nishikawa J, Hayward RS. The well-built clinical question: a key to evidence-based decisions. ACP J Club. (1995) 123(3):A12–3. doi: 10.7326/ACPJC-1995-123-3-A12
25. Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan–a web and mobile app for systematic reviews. Syst Rev (2016) 5(1):210. doi: 10.1186/s13643-016-0384-4
26. Wells G SB, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses (2020). Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
27. Poli-Neto OB, Carlos D, Favaretto A, Rosa-E-Silva JC, Meola J, Tiezzi D. Eutopic endometrium from women with endometriosis and chlamydial endometritis share immunological cell types and DNA repair imbalance: a transcriptome meta-analytical perspective. J Reprod Immunol (2021) 145:103307. doi: 10.1016/j.jri.2021.103307
28. Wu X-G, Chen J-J, Zhou H-L, Wu Y, Lin F, Shi J, et al. Identification and validation of the signatures of infiltrating immune cells in the eutopic endometrium endometria of women with endometriosis. Front Immunol (2021) 12:671201. doi: 10.3389/fimmu.2021.671201
29. Wu L, Lv C, Su Y, Li C, Zhang H, Zhao X, et al. Expression of programmed death-1 (PD-1) and its ligand PD-L1 is upregulated in endometriosis and promoted by 17beta-estradiol. Gynecol Endocrinol (2019) 35(3):251–6. doi: 10.1080/09513590.2018.1519787
30. Ahn SH, Khalaj K, Young SL, Lessey BA, Koti M, Tayade C. Immune-inflammation gene signatures in endometriosis patients. Fertil Steril. (2016) 106(6):1420–31. doi: 10.1016/j.fertnstert.2016.07.005
31. Klentzeris LD, Bulmer JN, Liu DT, Morrison L. Endometrial leukocyte subpopulations in women with endometriosis. Eur J Obstet Gynecol Reprod Biol (1995) 63(1):41–7. doi: 10.1016/0301-2115(95)02222-s
32. Bulmer JN, Jones RK, Searle RF. Intraepithelial leukocytes in endometriosis and adenomyosis: comparison of eutopic and ectopic endometrium with normal endometrium. Hum Reprod (1998) 13(1O):2910–5. doi: 10.1093/humrep/13.10.2910
33. Mettler L, Jurgensen A, Volkov NI, Kulakov V, Parwaresch MR. Lmmuno histochemical profile of endometrium in patients with genital endometriosis. Diagn Ther Endosc. (1997) 3(3):127–45. doi: 10.1155/DTE.3.127
34. Bunis DG, Wang W, Vallve-Juanico J, Houshdaran S, Sen S, Ben Soltane I, et al. Whole-tissue deconvolution and scRNAseq analysis identify altered endometrial cellular compositions and functionality associated with endometriosis. Front Immunol (2021) 12:788315. doi: 10.3389/fimmu.2021.788315
35. Ma J, Zhang L, Zhan H, Mo Y, Ren Z, Shao A, et al. Single-cell transcriptomic analysis of endometriosis provides insights into fibroblast fates and immune cell heterogeneity. Cell Biosci (2021) 11(1):125. doi: 10.1186/s13578-021-00637-x
36. Opsahl MS, Hayslip CC, Klein TA, Cunningham DS. Characterization of peripheral-blood and peritoneal-fluid mononuclear cell subsets in fertile and infertile women. Gynecol Obstet. (1994) 37(3):176–9. doi: 10.1159/000292553
37. Chen H, Qin S, Lei A, Li X, Gao Q, Dong J, et al. Expansion of monocytic myeloid-derived suppressor cells in endometriosis patients: a pilot study. Int Immunopharmacol. (2017) 47:150–8. doi: 10.1016/j.intimp.2017.03.026
38. Mier-Cabrera J, Jimenez-Zamudio L, Garcia-Latorre E, Cruz-Orozco O, Hernandez-Guerrero C. Quantitative and qualitative peritoneal immune profiles, T-cell apoptosis and oxidative stress-associated characteristics in women with minimal and mild endometriosis. BJOG: Int J Obstet Gynaecol. (2011) 118(1):6–16. doi: 10.1111/j.1471-0528.2010.02777.x
39. Deng C, Zhang L, Yosef HK, Yang Y, Jiang J, Yu L, et al. Single-cell raman trapping analysis revealed immunometabolism changes in peritoneal fluid in endometriosis. Scand J Immunol (2022) 95:1–16. doi: 10.1111/sji.13093
40. D’Hooghe TM, Hill JA, Oosterlynck DJ, Koninckx PR, Bambra CS. Effect of endometriosis on white blood cell subpopulations in peripheral blood and peritoneal fluid of baboons. Hum Reprod (1996) 11(8):1736–40. doi: 10.1093/oxfordjournals.humrep.a019478
41. Li CH, Lu ZM, Bi KH, Wang KX, Xu YP, Guo PP, et al. CD4(+)/CD8(+) mucosa-associated invariant T cells foster the development of endometriosis: a pilot study. Reprod Biol Endocrinol (2019) 17(1):78. doi: 10.1186/s12958-019-0524-5
42. Gallinelli A, Chiossi G, Giannella L, Marsella T, Genazzani AD, Volpe A. Different concentrations of interleukins in the peritoneal fluid of women with endometriosis: relationships with lymphocyte subsets. Gynecol Endocrinol (2004) 18(3):144–51. doi: 10.1080/09513590310001653044
43. Ho HN, Wu MY, Chao KH, Chen CD, Chen SU, Yang YS. Peritoneal interleukin-10 increases with decrease in activated CD4+ T lymphocytes in women with endometriosis. Hum Reprod (1997) 12(11):2528–33. doi: 10.1093/humrep/12.11.2528
44. Schmitz T, Hoffmann V, Olliges E, Bobinger A, Popovici R, Nosner E, et al. Reduced frequency of perforin-positive CD8+ T cells in menstrual effluent of endometriosis patients. J Reprod Immunol (2021) 148:103424. doi: 10.1016/j.jri.2021.103424
45. Shih AJ, Adelson RP, Vashistha H, Khalili H, Nayyar A, Puran R, et al. Single-cell analysis of menstrual endometrial tissues defines phenotypes associated with endometriosis. BMC Med (2022) 20(1):315. doi: 10.1186/s12916-022-02500-3
46. Liu Z-Q, Lu M-Y, Liu B. Circulating CD56+ NKG2D+ NK cells and postoperative fertility in ovarian endometrioma. Sci Rep (2020) 10(1):18598. doi: 10.1038/s41598-020-75570-z
47. Gagne D, Rivard M, Page M, Shazand K, Hugo P, Gosselin D. Blood leukocyte subsets are modulated in patients with endometriosis. Fertil Steril. (2003) 80(1):43–53. doi: 10.1016/s0015-0282(03)00552-1
48. Ho HN, Chao KH, Chen HF, Wu MY, Yang YS, Lee TY. Peritoneal natural-killer cytotoxicity and CD25(+)CD3(+) lymphocyte subpopulation are decreased in women with stage III-IV endometriosis. Hum Reprod (1995) 10(10):2671–5. doi: 10.1093/oxfordjournals.humrep.a135765
49. Sobstyl M, Niedzwiedzka-Rystwej P, Grywalska E, Korona-Glowniak I, Sobstyl A, Bednarek W, et al. Toll-like receptor 2 expression as a new hallmark of advanced endometriosis. Cells. (2020) 9(8):1–18. doi: 10.3390/cells9081813
50. Gmyrek GB, Sieradzka U, Goluda M, Gabrys M, Sozanski R, Jerzak M, et al. Flow cytometric evaluation of intracellular cytokine synthesis in peripheral mononuclear cells of women with endometriosis. Immunol Invest. (2008) 37(1):43–61. doi: 10.1080/08820130701554962
51. Muharam R, Bustami A, Mansur IG, Jacoeb TZ, Giustiniani J, Schlavon V, et al. Cytotoxic activity of peripheral blood mononuclear cells in patients with endometriosis: a cross-sectional study. Int J Reprod Biomed (2022) 20(8):691–700. doi: 10.18502/ijrm.v20i8.11758
52. Correa FJS, Andres MP, Rocha TP, Carvalho AEZ, Aloia TPA, Corpa MVN, et al. Invariant natural killer T-cells and their subtypes may play a role in the pathogenesis of endometriosis. Clinics. (2022) 77:100032. doi: 10.1016/j.clinsp.2022.100032
53. Vallve-Juanico J, Houshdaran S, Giudice LC. The endometrial immune environment of women with endometriosis. Hum Reprod Update. (2019) 25(5):565–92. doi: 10.1093/humupd/dmz018
54. Izumi G, Koga K, Takamura M, Makabe T, Satake E, Takeuchi A, et al. Involvement of immune cells in the pathogenesis of endometriosis. J Obstet Gynaecol Res (2018) 44(2):191–8. doi: 10.1111/jog.13559
55. Kralickova M, Fiala L, Losan P, Tomes P, Vetvicka V. Altered immunity in endometriosis: what came first? Immunol Invest (2018) 47(6):569–82. doi: 10.1080/08820139.2018.1467926
56. Berkkanoglu M, Arici A. Immunology and endometriosis. Am J Reprod Immunol (2003) 50(1):48–59. doi: 10.1034/j.1600-0897.2003.00042.x
57. Bianco B, Andre GM, Vilarino FL, Peluso C, Mafra FA, Christofolini DM, et al. The possible role of genetic variants in autoimmune-related genes in the development of endometriosis. Hum Immunol (2012) 73(3):306–15. doi: 10.1016/j.humimm.2011.12.009
58. Garcia-Gomez E, Vazquez-Martinez ER, Reyes-Mayoral C, Cruz-Orozco OP, Camacho-Arroyo I, Cerbon M. Regulation of inflammation pathways and inflammasome by sex steroid hormones in endometriosis. Front Endocrinol (2020) 10:935. doi: 10.3389/fendo.2019.00935
59. Lagana AS, Triolo O, Salmeri FM, Granese R, Palmara VI, Frangez HB, et al. Natural killer T cell subsets in eutopic and ectopic endometrium: a fresh look to a busy corner. Arch Gynecol Obstet. (2016) 293(5):941–9. doi: 10.1007/s00404-015-4004-7
60. Osuga Y, Koga K, Hirota Y, Hirata T, Yoshino O, Taketani Y. Lymphocytes in endometriosis. Am J Reprod Immunol (2011) 65(1):1–10. doi: 10.1111/j.1600-0897.2010.00887.x
61. Parkin KL, Fazleabas AT. Uterine leukocyte function and dysfunction: a hypothesis on the impact of endometriosis. Am J Reprod Immunol (2016) 75(3):411–7. doi: 10.1111/aji.12487
62. Riccio LDC, Santulli P, Marcellin L, Abrao MS, Batteux F, Chapron C. Immunology of endometriosis. Best Pract Res Clin Obstet Gynaecol. (2018) 50:39–49. doi: 10.1016/j.bpobgyn.2018.01.010
63. Symons LK, Miller JE, Kay VR, Marks RM, Liblik K, Koti M, et al. The immunopathophysiology of endometriosis. Trends Mol Med (2018) 24(9):748–62. doi: 10.1016/j.molmed.2018.07.004
64. Vinatier D, Dufour P, Oosterlynck D. Immunological aspects of endometriosis. Hum Reprod Update. (1996) 2(5):371–84. doi: 10.1093/humupd/2.5.371
65. Wu MY, Ho HN. The role of cytokines in endometriosis. Am J Reprod Immunol (2003) 49(5):285–96. doi: 10.1034/j.1600-0897.2003.01207.x
66. Zhang T, De Carolis C, Man GCW, Wang CC. The link between immunity, autoimmunity and endometriosis: a literature update. Autoimmun Rev (2018) 17(10):945–55. doi: 10.1016/j.autrev.2018.03.017
67. Tibbetts TA, Conneely OM, O’Malley BW. Progesterone via its receptor antagonizes the pro-inflammatory activity of estrogen in the mouse uterus. Biol Reprod (1999) 60(5):1158–65. doi: 10.1095/biolreprod60.5.1158
68. Shen Z, Rodriguez-Garcia M, Patel MV, Wira CR. Direct and indirect endocrine-mediated suppression of human endometrial CD8+T cell cytotoxicity. Sci Rep (2021) 11(1):1773. doi: 10.1038/s41598-021-81380-8
69. Bourdon M, Santulli P, Jeljeli M, Vannuccini S, Marcellin L, Doridot L, et al. Immunological changes associated with adenomyosis: a systematic review. Hum Reprod Update. (2021) 27(1):108–29. doi: 10.1093/humupd/dmaa038
70. Rigby CH, Aljassim F, Powell SG, Wyatt JNR, Hill CJ, Hapangama DK. The immune cell profile of human fallopian tubes in health and benign pathology: a systematic review. J Reprod Immunol (2022) 152. doi: 10.1016/j.jri.2022.103646
71. Deroux A, Dumestre-Perard C, Dunand-Faure C, Bouillet L, Hoffmann P. Female infertility and serum auto-antibodies: a systematic review. Clin Rev Allergy Immunol (2017) 53(1):78–86. doi: 10.1007/s12016-016-8586-z
72. Shigesi N, Kvaskoff M, Kirtley S, Feng Q, Fang H, Knight JC, et al. The association between endometriosis and autoimmune diseases: a systematic review and meta-analysis. Hum Reprod Update. (2019) 25(4):486–503. doi: 10.1093/humupd/dmz014
73. Ni Choileain N, Redmond HP. Cell response to surgery. Arch Surg (2006) 141(11):1132–40. doi: 10.1001/archsurg.141.11.1132
74. Becker CM, Bokor A, Heikinheimo O, Horne A, Jansen F, Kiesel L, et al. ESHRE guideline: endometriosis. Hum Reprod Open (2022) 2022(2):1–26. doi: 10.1093/hropen/hoac009
75. Surrey E, Soliman AM, Trenz H, Blauer-Peterson C, Sluis A. Impact of endometriosis diagnostic delays on healthcare resource utilization and costs. Adv Ther (2020) 37(3):1087–99. doi: 10.1007/s12325-019-01215-x
76. Mayer B, Heinzel A, Lukas A, Perco P. Predictive biomarkers for linking disease pathology and drug effect. Curr Pharm Des (2017) 23(1):29–54. doi: 10.2174/1381612822666161006153639
77. Lessey BA, Kim JJ. Endometrial receptivity in the eutopic endometrium of women with endometriosis: it is affected, and let me show you why. Fertil Steril. (2017) 108(1):19–27. doi: 10.1016/j.fertnstert.2017.05.031
78. de Ziegler D, Frydman RF. Recurrent pregnancy losses, a lasting cause of infertility. Fertil Steril. (2021) 115(3):531–2. doi: 10.1016/j.fertnstert.2020.12.004
79. Bhargava P, Calabresi PA. Novel therapies for memory cells in autoimmune diseases. Clin Exp Immunol (2015) 180(3):353–60. doi: 10.1111/cei.12602
80. Smids C, Horjus Talabur Horje CS, Drylewicz J, Roosenboom B, Groenen MJM, van Koolwijk E, et al. Intestinal T cell profiling in inflammatory bowel disease: linking T cell subsets to disease activity and disease course. J Crohns Colitis. (2018) 12(4):465–75. doi: 10.1093/ecco-jcc/jjx160
81. Devarajan P, Chen Z. Autoimmune effector memory T cells: the bad and the good. Immunol Res (2013) 57(1-3):12–22. doi: 10.1007/s12026-013-8448-1
82. Casalegno Garduño R, Däbritz J. New insights on CD8+ T cells in inflammatory bowel disease and therapeutic approaches. Front Immunol (2021) 12:738762. doi: 10.3389/fimmu.2021.738762
83. Roosenboom B, Wahab PJ, Smids C, Groenen MJM, van Koolwijk E, van Lochem EG, et al. Intestinal CD103+CD4+ and CD103+CD8+ T-cell subsets in the gut of inflammatory bowel disease patients at diagnosis and during follow-up. Inflammation Bowel Dis (2019) 25(9):1497–509. doi: 10.1093/ibd/izz049
84. Collier JL, Weiss SA, Pauken KE, Sen DR, Sharpe AH. Not-so-opposite ends of the spectrum: CD8+ T cell dysfunction across chronic infection, cancer and autoimmunity. Nat Immunol (2021) 22(7):809–19. doi: 10.1038/s41590-021-00949-7
85. Southcombe JH, Mounce G, McGee K, Elghajiji A, Brosens J, Quenby S, et al. An altered endometrial CD8 tissue resident memory T cell population in recurrent miscarriage. Sci Rep (2017) 7(1):41335. doi: 10.1038/srep41335
86. Scheerer C, Bauer P, Chiantera V, Sehouli J, Kaufmann A, Mechsner S. Characterization of endometriosis-associated immune cell infiltrates (EMaICI). Arch Gynecol Obstet. (2016) 294(3):657–64. doi: 10.1007/s00404-016-4142-6
87. Raskov H, Orhan A, Christensen JP, Gögenur I. Cytotoxic CD8+ T cells in cancer and cancer immunotherapy. Br J Cancer. (2021) 124(2):359–67. doi: 10.1038/s41416-020-01048-4
88. Chiba T, Ohtani H, Mizoi T, Naito Y, Sato E, Nagura H, et al. Intraepithelial CD8+ T-cell-count becomes a prognostic factor after a longer follow-up period in human colorectal carcinoma: possible association with suppression of micrometastasis. Br J Cancer. (2004) 91(9):1711–7. doi: 10.1038/sj.bjc.6602201
89. Workel HH, Komdeur FL, Wouters MCA, Plat A, Klip HG, Eggink FA, et al. CD103 defines intraepithelial CD8+ PD1+ tumour-infiltrating lymphocytes of prognostic significance in endometrial adenocarcinoma. Eur J Cancer. (2016) 60:1–11. doi: 10.1016/j.ejca.2016.02.026
90. Stumpf M, Hasenburg A, Riener MO, Jütting U, Wang C, Shen Y, et al. Intraepithelial CD8-positive T lymphocytes predict survival for patients with serous stage III ovarian carcinomas: relevance of clonal selection of T lymphocytes. Br J Cancer. (2009) 101(9):1513–21. doi: 10.1038/sj.bjc.6605274
91. Bricou A, Batt RE, Chapron C. Peritoneal fluid flow influences anatomical distribution of endometriotic lesions: why Sampson seems to be right. Eur J Obstet Gynecol Reprod Biol (2008) 138(2):127–34. doi: 10.1016/j.ejogrb.2008.01.014
92. Hogg C, Horne AW, Greaves E. Endometriosis-associated macrophages: origin, phenotype, and function. Front Endocrinol (2020) 11:7. doi: 10.3389/fendo.2020.00007
93. Szukiewicz D. Epigenetic regulation and T-cell responses in endometriosis – something other than autoimmunity. Front Immunol (2022) 13:943839. doi: 10.3389/fimmu.2022.943839
94. Pace L, Tempez A, Arnold-Schrauf C, Lemaitre F, Bousso P, Fetler L, et al. Regulatory T cells increase the avidity of primary CD8+ T cell responses and promote memory. Science. (2012) 338(6106):532–6. doi: 10.1126/science.1227049
95. Chen S, Zhang J, Huang C, Lu W, Liang Y, Wan X. Expression of the T regulatory cell transcription factor FoxP3 in peri-implantation phase endometrium in infertile women with endometriosis. Reprod Biol Endocrinol (2012) 10(1):34. doi: 10.1186/1477-7827-10-34
96. Sabbaj S, Hel Z, Richter HE, Mestecky J, Goepfert PA. Menstrual blood as a potential source of endometrial derived CD3+ T cells. PloS One (2011) 6(12):e28894. doi: 10.1371/journal.pone.0028894
97. Sasson IE, Taylor HS. Stem cells and the pathogenesis of endometriosis. Ann N Y Acad Sci (2008) 1127(1):106–15. doi: 10.1196/annals.1434.014
98. Taylor HS, Kotlyar AM, Flores VA. Endometriosis is a chronic systemic disease: clinical challenges and novel innovations. Lancet. (2021) 397(10276):839–52. doi: 10.1016/s0140-6736(21)00389-5
99. Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol (2016) 16(10):626–38. doi: 10.1038/nri.2016.90
100. Okimura H, Tanaka Y, Fujii M, Shimura K, Maeda E, Ito F, et al. Changes in the proportion of regulatory T cell subpopulations during menstrual cycle and early pregnancy. Am J Reprod Immunol (2022) 88(6):e13636. doi: 10.1111/aji.13636
101. Lorenz TK, Heiman JR, Demas GE. Sexual activity modulates shifts in TH1/TH2 cytokine profile across the menstrual cycle: an observational study. Fertil Steril. (2015) 104(6):1513–21. doi: 10.1016/j.fertnstert.2015.09.001
102. Pender MP, Csurhes PA, Pfluger CM, Burrows SR. Deficiency of CD8+ effector memory T cells is an early and persistent feature of multiple sclerosis. Mult Scler J (2014) 20(14):1825–32. doi: 10.1177/1352458514536252
103. Vallvé-Juanico J, George AF, Sen S, Thomas R, Shin M-G, Kushnoor D, et al. Deep immunophenotyping reveals endometriosis is marked by dysregulation of the mononuclear phagocytic system in endometrium and peripheral blood. BMC Med (2022) 20(1):158. doi: 10.1186/s12916-022-02359-4
104. de Ziegler D, Borghese B, Chapron C. Endometriosis and infertility: pathophysiology and management. Lancet. (2010) 376(9742):730–8. doi: 10.1016/s0140-6736(10)60490-4
105. Guo C, Cai P, Jin L, Sha Q, Yu Q, Zhang W, et al. Single-cell profiling of the human decidual immune microenvironment in patients with recurrent pregnancy loss. Cell Discovery (2021) 7(1):1. doi: 10.1038/s41421-020-00236-z
106. Kieffer TEC, Laskewitz A, Vledder A, Scherjon SA, Faas MM, Prins JR. Decidual memory T-cell subsets and memory T-cell stimulatory cytokines in early- and late-onset preeclampsia. Am J Reprod Immunol (2020) 84(4):e13293. doi: 10.1111/aji.13293
107. Arenas-Hernandez M, Romero R, Xu Y, Panaitescu B, Garcia-Flores V, Miller D, et al. Effector and activated T cells induce preterm labor and birth that is prevented by treatment with progesterone. J Immun (2019) 202(9):2585–608. doi: 10.4049/jimmunol.1801350
Keywords: CD8, T cell, endometriosis, endometrium, peritoneal fluid
Citation: Kisovar A, Becker CM, Granne I and Southcombe JH (2023) The role of CD8+ T cells in endometriosis: a systematic review. Front. Immunol. 14:1225639. doi: 10.3389/fimmu.2023.1225639
Received: 19 May 2023; Accepted: 26 June 2023;
Published: 11 July 2023.
Edited by:
Christoph Mueller, University of Bern, SwitzerlandCopyright © 2023 Kisovar, Becker, Granne and Southcombe. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jennifer H. Southcombe, amVuLnNvdXRoY29tYmVAd3JoLm94LmFjLnVr
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.