
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol., 17 August 2023
Sec. Autoimmune and Autoinflammatory Disorders : Autoimmune Disorders
Volume 14 - 2023 | https://doi.org/10.3389/fimmu.2023.1211790
This article is part of the Research TopicAdvances in understanding the pathogenesis of and designing therapies for connective tissue disease-associated interstitial lung diseaseView all 6 articles
 Rui Yu1,2†
Rui Yu1,2† Xiaomin Liu3†
Xiaomin Liu3† Xiaoyue Deng1,4
Xiaoyue Deng1,4 Siting Li1
Siting Li1 Yifei Wang1,2
Yifei Wang1,2 Yan Zhang3
Yan Zhang3 Dan Ke3
Dan Ke3 Rui Yan3
Rui Yan3 Qian Wang1
Qian Wang1 Xinping Tian1
Xinping Tian1 Mengtao Li1
Mengtao Li1 Xiaofeng Zeng1*
Xiaofeng Zeng1* Chaojun Hu1*
Chaojun Hu1*Background: Interstitial lung disease (ILD) is a relatively prevalent extra-articular manifestation of rheumatoid arthritis (RA) and contributes to significant morbidity and mortality. This study aimed to analyze the association between chitinase-3 like-protein-1(CHI3L1) and the presence of RA-ILD.
Methods: A total of 239 RA patients fulfilling the American Rheumatism Association (ACR) 1987 revised criteria were enrolled and subclassified as RA-ILD and RA-nILD based on the results of high-resolution computed tomography scans (HRCT) of the chest. The disease activity of RA was assessed by Disease Activity Score for 28 joints (DAS28) and categorized as high, moderate, low, and remission. Chemiluminescence immunoassays were applied to determine the serum levels of CHI3L1. Univariate analysis was performed and the receiver operating characteristics (ROC) curves were plotted to evaluate the correlation between RA-ILD and CHI3L1.
Results: Among the eligible RA patients studied, 60 (25.1%) patients were diagnosed with RA-ILD. Compared with RA-nILD, RA patients with ILD had significantly higher median age (median [IQR], 68.00 [62.00-71.75] vs 53.00 [40.00-63.00], p<0.001) and a higher proportion of males (21 (35.0%) vs 30 (16.8%), p=0.003). Notably, differences in DAS28 scores between the two groups were not observed. The serum level of CHI3L1 was significantly higher in RA-ILD patients (median [IQR], 69.69 [44.51-128.66] ng/ml vs 32.19 [21.63-56.99] ng/ml, p<0.001). Furthermore, the areas under the curve (AUC) of CHI3L1 attained 0.74 (95% confidence interval [CI], 0.68-0.81, p<0.001) in terms of identifying patients with RA-ILD from those without ILD. Similar trends were seen across the spectrum of disease activity based on DAS28-ESR.
Conclusion: Our findings of elevated serum CHI3L1 levels in RA-ILD patients suggest its possible role as a biomarker to detect RA-ILD noninvasively.
Rheumatoid arthritis (RA) is a prevalent chronic rheumatic disease that can cause progressive articular damage and extra-articular manifestations, including pulmonary involvement, vasculitis, rheumatoid nodules, and systemic comorbidities (1). Interstitial lung disease (ILD) is the most common pulmonary manifestation, occurring in 10% of patients with RA (2). ILD is strongly associated with poor prognosis and is the common cause of RA mortality, secondary only to cardiovascular diseases (3, 4). A recent study shows that the 1- and 5-year mortality rates of rheumatoid arthritis-associated ILD (RA-ILD) were 13.9% and 39.0%, while these rates were 3.8% and 18.2% in RA patients without ILD (RA-nILD) (5). Moreover, despite decreasing mortality rates of RA, there has been an increase in deaths from RA-ILD (2), thus prompting a great effort to achieve an earlier diagnosis and improve the prognosis.
High-resolution chest computed tomography (HRCT) is accepted as the gold-standard approach for the diagnosis of ILD (6). Although showing abnormalities in 19% of patients with RA (6), HRCT of the lungs is not performed for routine RA diagnoses in consideration of radiation exposure and high cost (7), which is not conducive to early detection of RA-ILD. Early recognition and monitoring are crucial to provide better treatment and improve the prognosis. Given the impressive advances in the development of molecular detection techniques, the discovery and validation of disease-specific biomarkers could be of great clinical importance (8).
Antibodies and lung epithelium-specific biomarkers play a crucial role in predicting RA-ILD (9). Rheumatoid factor (RF) and anti-citrullinated peptide antibodies (ACPA), two serologic biomarkers for RA included in the European League Against Rheumatism (EULAR)/American College of Rheumatology (ACR) 2010 criteria (10), have been demonstrated to act as biomarkers of RA-ILD (11, 12). Other antibodies have also been proven to have strong associations with RA-ILD, such as anti-malondialdehyde-acetaldehyde (anti-MAA) (13) and anti-carbamylated protein (anti-CarP) (14). In addition, inflammatory mechanisms may cause repetitive injuries to alveolar epithelial cells (AECs), such as the apoptosis of ACE type I (ACE I) and the hyperplastic of AEC type II (ACE II). The regenerating ACE II could produce and recruit a series of cytokines and growth factors, and release surfactant proteins (15), which have potential predictive value. Factors like Krebs von den Lungen-6 (KL-6) (16), matrix metalloproteinase (MMP)-7 (17), and C-X-C motif chemokine 10 (CXCL10) (17) have been demonstrated to aid RA-ILD identification.
Chitinase-3 like-protein-1 (CHI3L1) plays a vital role in tissue repair, inflammation, and remodeling responses. It is synthesized and secreted by a multitude of cells including macrophages, neutrophils, synoviocytes, chondrocytes, fibroblast-like cells, and tumor cells (18–21). CHI3L1 has been regarded as a promising biomarker for predicting and evaluating the severity of RA (21, 22). In addition, serum CHI3L1 levels are significantly higher in ILD patients (23). It plays a profibrotic role in lung fibrosis repair phase by augmenting alternative macrophage activation, fibroblast proliferation, and matrix deposition (24). However, the associations between CHI3L1 and RA-ILD remain unknown.
The purpose of this study is to evaluate whether CHI3L1 can identify the presence of RA-ILD in RA patients. Additionally, we will assess its diagnostic capacity for ILD in patients with different disease activities of RA.
We performed a large, single-center, retrospective observational study of 239 RA patients who received serum CHI3L1 test in the Department of Rheumatology of Peking Union Medical College Hospital (Beijing, China) from 2019 to 2021. Diagnosis of RA was based on the American Rheumatism Association (ACR) 1987 revised criteria (25) and ILD was evaluated through chest CT scans. Patients with other autoimmune diseases, other chronic lung diseases, liver damage, cancers, infections, or had a history of ILD before the diagnosis of RA were excluded. Patients were divided into two subgroups of RA-ILD (60, 25.1%) or RA-nILD (179, 74.9%) according to the complication of ILD. Articular disease activity was quantified and categorized by the disease activity score in 28 joints (DAS28) assessment (26). Baseline demographics were acquired from the medical records. This study was approved by the Medical Ethics Committee of Peking Union Medical College Hospital (PUMCH) (ethics number I-22PJ457). All participants provided written informed consent.
RA-ILD was diagnosed according to the presence of typical features in the lungs using HRCT. The features included irregular linear or reticular opacities, ground-glass opacities, consolidation, honeycombing, septal thickening, and traction bronchiectasis or bronchiolectasis according to the consensus for idiopathic interstitial pneumonia of the American Thoracic Society/European Respiratory Society (ATS/ERS) (27). Diagnoses were confirmed by expert radiologists in a blinded manner.
Two disease activity score (DAS) standards, DAS28-ESR (erythrocyte sedimentation rate) and DAS28-CRP (C reactive protein), were applied to categorize disease activity states of RA patients as high (DAS28>5.1), moderate (3.2<DAS28 ≤ 5.1), low (2.6<DAS28 ≤ 3.2) disease activity and remission (DAS28 ≤ 2.6) (26).
Serum levels of CHI3L1 were analyzed by a commercial test system, iFlash CLIA kits (YHLO Biotech Co., Shenzhen, China, Y-CLIA for short) that had been proved a suitable choice for the Chinese population in our previous studies (28). More specifically, Y-CLIA conducted paramagnetic particle chemiluminescent immunoassay using a fully automated iFlash 3000 Chemiluminescence Immunoassay Analyzer. Serum samples were obtained by separation from peripheral blood and stored at −80°C until use. No sample was exposed to more than one freeze-thaw cycle before analysis.
For the comparison of age, sex, DAS28-ESR score, DAS28-CRP score, and serum CHI3L1 levels between RA-ILD and RA-nILD, we used Chi-squared test and Mann-Whitney U test where appropriate. Kruskal-Wallis tests were used to compare the serum CHI3L1 levels within the groups categorized according to the disease activity of RA. Receiver operating characteristic (ROC) curves and areas under the curve (AUC) were generated to determine the efficacy of serum CHI3L1 levels in distinguishing individuals with RA-ILD from RA-nILD and determining the optimum cut-off level. All analyses were performed using SPSS, version 26.0. P value less than 0.050 was considered statistically significant.
The demographic, clinical, and laboratory features of the 239 RA patients are summarized in Table 1. Among all enrolled RA patients, 60 (25.1%) showed definite ILD features on chest HRCT. Compared with patients without ILD features, those with ILD were more likely to be older (median [IQR], 68.00 [62.00-71.75] vs 48.00 [37.00-57.00], p<0.001), have a higher proportion of males (21 (35.0%) vs 30 (16.8%), p=0.003), and have high titer positivity of RF or/and anti-CCP (55 [91.7%] vs 137 [76.5%]). Furthermore, the age of RA patients and CHI3L1 levels were moderately correlated, and the relationship was statistically significant revealed by Pearson Correlation Coefficient (r(237) = 0.323, p < 0.01). Different age groups were divided and patients older than 65 were more likely to have elevated CHI3L1 than 50-55 years old patients, as same as 60-65 and 30-40 years old patients (Supplementary Figure 1). However, there was no statistical difference between gender and CHI3L1 levels (Supplementary Figure 2). The presence of RA-ILD and DAS28 scores might have no relationship, neither DAS28-ESR (p=0.732) nor DAS28-CRP (p=0.202). In addition, the disease duration was similar in RA patients with and without ILD (median [IQR], 5.56 [1.06-18.31] vs 5.10 [1.68-10.16], p=0.548). Among the baseline therapies, patients with ILD were more likely to be treated with glucocorticoids (9 (90%) vs 85 (47.5%), p=0.010).
The CHI3L1 levels were significantly higher in the RA-ILD than in the RA-nILD group (Table 1; Figure 1A). Compared with RA-nILD (median [IQR], 32.19 [21.63-56.99] ng/mL), the serum CHI3L1 levels were increased in RA-ILD patients (median [IQR], 69.69 [44.51-128.66] ng/ml, p<0.001). In addition, statistically significant elevations of CHI3L1 were observed across the spectrum of disease activity based on DAS28-ESR, and in moderate and low disease activity groups based on DAS28-CRP (Table 1; Figures 1B, C).
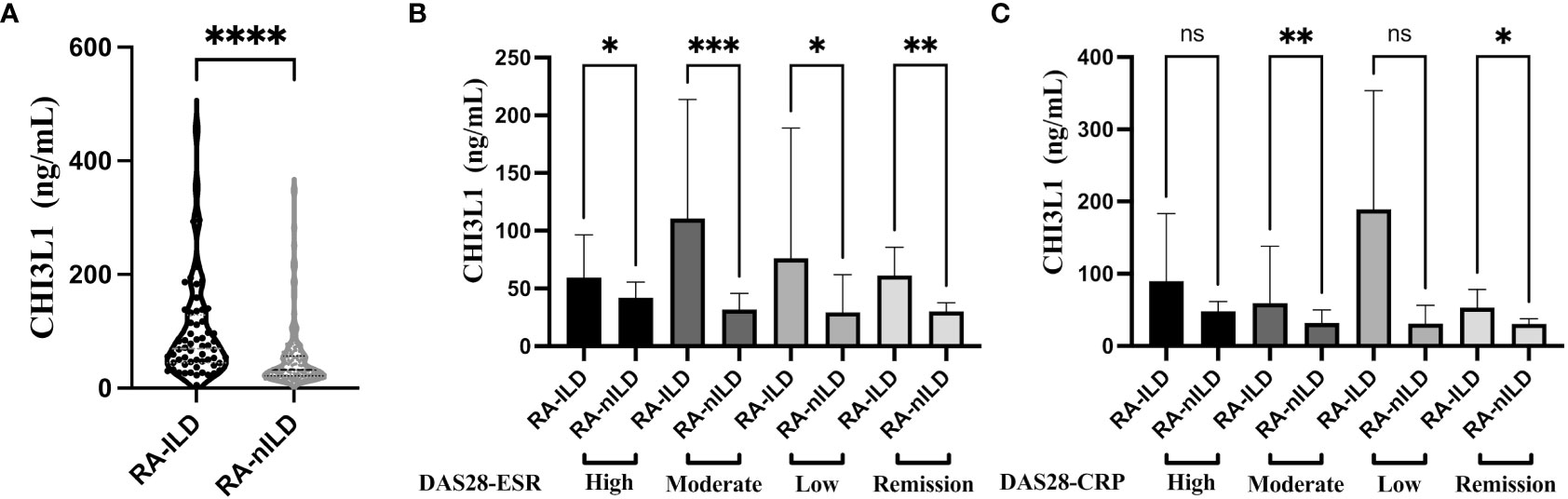
Figure 1 Serum level of CHI3L1 between RA-ILD and RA-nILD and among different disease activity subgroups. (A) RA patients with or without ILD. (B) RA patients with different activity states according to DAS28-ESR. (C) RA patients with different activity states according to DAS28-CRP. Violin plot depicts levels of CHI3L1 in individual serum samples from RA-ILD and RA-nILD. The dotted lines represent median and quartiles. The error bars show the median with 95% CI. P values were determined by Mann-Whitney U test (A) and Kruskal-Wallis test (B, C). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. "ns" means "no significance".
Further demonstrating the predictive potential of CHI3L1 as a biomarker for RA-ILD, ROC assessment revealed strong performance characteristics with AUC of 0.74 (95% CI, 0.68-0.81, p<0.001) (Figure 2A). The cross-validation of the ROC curve was performed for further confirmation (Supplementary Figure 3). A positive cut-off value was defined as ≥44.20 ng/mL according to the sensitivity (76.67%), specificity (62.57%), and Youden Index. CHI3L1 positivities were more frequent in RA-ILD patients (46 [76.67%] vs 70 [39.11%], p=0.006). Based on logistic regression analysis, however, only older age was found to be significantly associated with RA-ILD (p<0.001), while CHI3L1 levels (p=0.415) and male sex (p=0.074) were not.
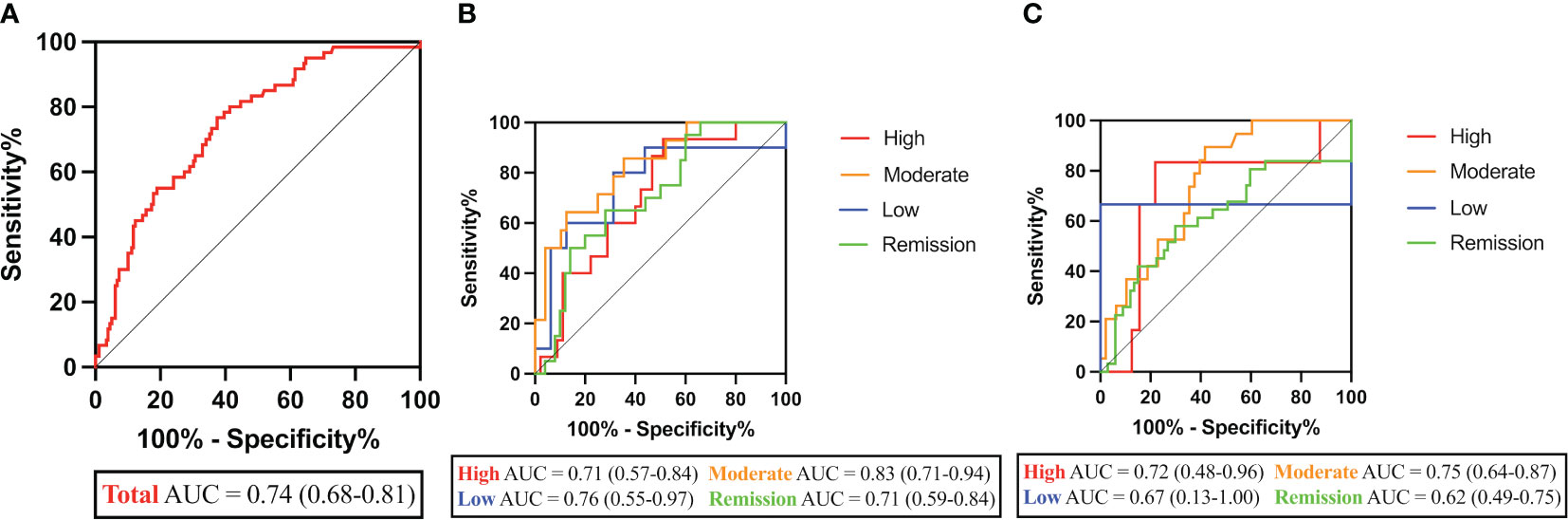
Figure 2 Performance characteristics of CHI3L1 levels in RA-ILD diagnosis. (A) ROC curves of CHI3L1 levels in RA-ILD and RA-nILD patients. (B) ROC curves of CHI3L1 levels in groups at different disease activity states based on DAS28-ESR. (C) ROC curves of CHI3L1 levels in groups at different disease activity states based on DAS28-CRP. AUC and 95% CI are shown in parentheses.
As shown in Figure 2B, the AUCs for CHI3L1 in the high/moderate/low/remission RA disease activity group based on DAS28-ESR were 0.71 (p=0.018), 0.83 (p<0.001), 0.76 (p=0.031), and 0.71 (p=0.006), respectively. However, there was no significant difference between the curves in high (p=0.093), low (p=0.355), and remission (p=0.062) disease activity groups based on DAS28-CRP (Figure 2C; Table 2).
To our knowledge, this is the first study to analyze the association between CHI3L1 and RA-ILD. We found that higher levels of serum CHI3L1 were measured among patients with radiographic evidence of ILD features compared with RA patients without lung involvement. Additionally, older age and male sex were associated with RA-ILD. In particular, CHI3L1 exhibited the valuable diagnostic ability to identify the presence of ILD in patients with different disease activities of RA.
Survival of RA has been improved in the last few decades, along with the changes in treatment paradigms, early intervention, and treat-to-target approaches (29). The declined mortality rates are mostly due to a decrease in cardiovascular mortality while deaths from ILD remain significant (30). Considering that respiratory symptoms are not always apparent in RA-ILD (31), early detection and management are crucial to improving the prognosis and survival. The relationship between CHI3L1 and RA or ILD has been addressed in different studies (21, 22, 24, 32), while no association between CHI3L1 and RA-ILD has been confirmed.
Consistent with multiple studies (33, 34), the association of RA-ILD with older age and male sex is demonstrated in this study. Older age is associated with RA-ILD suggesting that immunosenescence and telomere shortening may be linked with RA-ILD risk (35). Notably, it seems somewhat paradoxical that RA is more prevalent in females than males, with a female-to-male sex ratio ranging from 4:1 to less than 2:1 in younger and older patients respectively (36). The distinctions might be explained partly by different exposure to environmental risk factors like smoking (37), which is involved in chronic mucosal inflammation of lung and plays a positive role in the development of RA-ILD (38). Some studies have demonstrated that smoking might induce citrullination of peptide antigens in the lungs via peptidyl arginine deiminase-2 (PAD2) (39, 40). Moreover, increased PAD2 was observed in the broncho-alveolar lavage (BAL) fluid of smokers compared to non-smokers (41). In addition, higher level of ACPA has been found among RA patients with ILD (12). It seemed that smoking might partly explain the higher proportion of male RA-ILD patients. Furthermore, several studies suggest that gonadal hormones (36), genetic background (42–44), and other confounding factors (44) also contribute to RA-ILD. The role of aging and gender in RA-ILD remains to be clarified. Traditional disease-modifying antirheumatic drugs (DMARDs) like methotrexate are the basis of therapeutic intervention in most patients with RA, while the effects on the presence of RA-ILD remain controversial (45). Treating with methotrexate seemed not to affect the arise of ILD but glucocorticoids did in this study.
The disease activity of RA determined by DAS28 appears to have no influence on the presence of RA-ILD, indicating the lack of association between the disease activity of RA and RA-ILD. In contrast, a cross-sectional study identified mean DAS28 as an independent predictor for preclinical RA-ILD (odds ratio (OR)=2.0, 95% CI [1.2-3.4]) (46). However, the sample size of this study is smaller than ours, which might contribute to the variance. A previous prospective cohort study conducted in the UK with 1460 RA patients involved shared similar results with ours that the DAS28 score at baseline had no association with RA-ILD (hazard ratio (HR)=1.11, 95% CI [0.94-1.31]) (47). The difference among these studies may come from differences in patient population, exclusions of confounding factors, and disease definition.
Mann-Whitney U test and AUC analysis showed a robust relationship between CHI3L1 level and the presence of RA-ILD, suggesting the potential role of CHI3L1 as a biomarker and its immune response on RA-ILD development. So far, no studies have evaluated CHI3L1 levels in RA-ILD patients while CHI3L1 have been detected in RA (22) patients and ILD patients (32). Several serum biomarkers have been proposed for RA-ILD diagnosis, including antibodies (ie. RF and ACPA) (11, 12) and a range of cytokines and growth factors released by active specialized macrophages and stromal cells (ie. MMP-7, surface protein D, and KL-6) (16, 17). Given the similarities in clinical, radiographic, and genetic characteristics shared by RA-ILD and idiopathic pulmonary fibrosis (IPF), several studies have noted that the biomarkers might repurpose from one to the other (48). For instance, serum MMP-7 and ACPA levels which are significantly higher in IPF than controls are also elevated in RA-ILD compared to those with RA without ILD (17, 49–51). Interestingly, the serum levels of CHI3L1 could distinguish between IPF and controls (245.8 ± 180.2 ng/mL vs 116.0 ± 58.3 ng/mL, p<0.001) and predict survival (52). Considering the biological activities of CHI3L1 determined in RA and hepatic fibrosis such as stimulating the growth of fibroblasts (19), driving macrophage activation and differentiation (53), and regulating the ECM (54), it may have a significant impact on the potential pathobiology of both IPF and RA-ILD. In addition, the data from our study suggested that CHI3L1 was valuable for the diagnosis of RA-ILD in all spectrums of disease activity based on the DAS28-ESR score, and in moderate and low disease activity groups based on DAS28-CRP. It seems that applying DAS28-ESR as the scoring standard may increase the accuracy of RA-ILD assessment.
Our study has some limitations. First, patients’ smoking history and specific autoantibodies concentration are not included; thus, these confounders may introduce bias to our results. Cigarette smoking could increase the risk of RA-ILD with a dose effect relationship (55). Second, this is a single-center study focused on RA patients, which limits the representativeness of the results. In the future, large sample and multi-center studies are needed to evaluate the link between CHI3L1 and RA-ILD. Third, several studies have shown that the efficiency of biomarkers varies in different disease statuses of RA-ILD (17, 56), while our study did not assess the severity of ILD. Moreover, the inclusion of RF, ACPA, and other potential biomarkers determined by previous studies tends to improve the accuracy of the prediction model (16, 17, 56). Additionally, comparing and contrasting the diagnostic ability of CHI3L1 with validated indicators such as KL-6 and HRCT scores might help verify its potency as a biomarker. Finally, there were no hold-out test sets or external validation cohorts.
In conclusion, an association between CHI3L1 and RA-ILD was found. It poses the debate of whether CHI3L1 could contribute to the development of this devastating extra-articular manifestation. Future studies will help define the potential underlying pathological mechanism of this connection.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by Medical Ethics Committee of Peking Union Medical College Hospital (ethics number I-22PJ457). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
All authors were involved in the conception and design of this study. RYu, XL, XD, SL, and YW contributed to the performance of experiments. QW and XT assisted in the collection and preservation of blood samples. YZ, DK, and RYan collected and evaluated patient data. QW, XT, and ML assisted in the recruitment of patients. RYu and XL analyzed the data and wrote the first draft of the manuscript. XD, SL, and YW contributed to validation. YZ, DK, RYan, and ML assisted in supervision of general work. All authors contributed to manuscript revision, read, and approved the submitted version.
This study was supported by the National High Level Hospital Clinical Research Funding (2022-PUMCH-A-039, 2022-PUMCH-B-013), The National Key Research and Development Program of China (2019YFC0840603).
We would like to thank all researchers involved in this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1211790/full#supplementary-material
Supplementary Figure 1 | Serum level of CHI3L1 among different age groups. P values were determined by the Kruskal-Wallis test. **P < 0.01
Supplementary Figure 2 | Serum level of CHI3L1 between male and female patients. P values were determined by the Independent t-test. The dotted lines represent median and quartiles.
Supplementary Figure 3 | Cross-validation of ROC curves assessing the ability of CHI3L1 levels in RA-ILD diagnosis. The AUC of 100% training was 0.735, of 70% training was 0.692, and of K-Fold was 0.749.
1. Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet (2016) 388(10055):2023–38. doi: 10.1016/S0140-6736(16)30173-8
2. Olson AL, Swigris JJ, Sprunger DB, Fischer A, Fernandez-Perez ER, Solomon J, et al. Rheumatoid arthritis-interstitial lung disease-associated mortality. Am J Respir Crit Care Med (2011) 183(3):372–8. doi: 10.1164/rccm.201004-0622OC
3. Finckh A, Gilbert B, Hodkinson B, Bae SC, Thomas R, Deane KD, et al. Global epidemiology of rheumatoid arthritis. Nat Rev Rheumatol (2022) 18(10):591–602. doi: 10.1038/s41584-022-00827-y
4. Luppi F, Sebastiani M, Salvarani C, Bendstrup E, Manfredi A. Acute exacerbation of interstitial lung disease associated with rheumatic disease. Nat Rev Rheumatol (2022) 18(2):85–96. doi: 10.1038/s41584-021-00721-z
5. Hyldgaard C, Hilberg O, Pedersen AB, Ulrichsen SP, Lokke A, Bendstrup E, et al. A population-based cohort study of rheumatoid arthritis-associated interstitial lung disease: comorbidity and mortality. Ann Rheum Dis (2017) 76(10):1700–6. doi: 10.1136/annrheumdis-2017-211138
6. Dawson JK, Fewins HE, Desmond J, Lynch MP, Graham DR. Fibrosing alveolitis in patients with rheumatoid arthritis as assessed by high resolution computed tomography, chest radiography, and pulmonary function tests. Thorax (2001) 56(8):622–7. doi: 10.1136/thx.56.8.622
7. Picano E, Semelka R, Ravenel J, Matucci-Cerinic M. Rheumatological diseases and cancer: the hidden variable of radiation exposure. Ann Rheum Dis (2014) 73(12):2065–8. doi: 10.1136/annrheumdis-2014-206585
8. Broza YY, Zhou X, Yuan M, Qu D, Zheng Y, Vishinkin R, et al. Disease detection with molecular biomarkers: from chemistry of body fluids to nature-inspired chemical sensors. Chem Rev (2019) 119(22):11761–817. doi: 10.1021/acs.chemrev.9b00437
9. Bonella F, Costabel U. Biomarkers in connective tissue disease-associated interstitial lung disease. Semin Respir Crit Care Med (2014) 35(2):181–200. doi: 10.1055/s-0034-1371527
10. Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO 3rd, et al. Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum (2010) 62(9):2569–81. doi: 10.1002/art.27584
11. Yin Y, Liang D, Zhao L, Li Y, Liu W, Ren Y, et al. Anti-cyclic citrullinated Peptide antibody is associated with interstitial lung disease in patients with rheumatoid arthritis. PloS One (2014) 9(4):e92449. doi: 10.1371/journal.pone.0092449
12. Giles JT, Danoff SK, Sokolove J, Wagner CA, Winchester R, Pappas DA, et al. Association of fine specificity and repertoire expansion of anticitrullinated peptide antibodies with rheumatoid arthritis associated interstitial lung disease. Ann Rheum Dis (2014) 73(8):1487–94. doi: 10.1136/annrheumdis-2012-203160
13. England BR, Duryee MJ, Roul P, Mahajan TD, Singh N, Poole JA, et al. Malondialdehyde-acetaldehyde adducts and antibody responses in rheumatoid arthritis-associated interstitial lung disease. Arthritis Rheumatol (2019) 71(9):1483–93. doi: 10.1002/art.40900
14. Castellanos-Moreira R, Rodriguez-Garcia SC, Gomara MJ, Ruiz-Esquide V, Cuervo A, Casafont-Sole I, et al. Anti-carbamylated proteins antibody repertoire in rheumatoid arthritis: evidence of a new autoantibody linked to interstitial lung disease. Ann Rheum Dis (2020) 79(5):587–94. doi: 10.1136/annrheumdis-2019-216709
15. Lukacs NW, Hogaboam C, Chensue SW, Blease K, Kunkel SL. Type 1/type 2 cytokine paradigm and the progression of pulmonary fibrosis. Chest (2001) 120(1 Suppl):5S–8S. doi: 10.1378/chest.120.1_suppl.S5
16. Qin Y, Wang Y, Meng F, Feng M, Zhao X, Gao C, et al. Identification of biomarkers by machine learning classifiers to assist diagnose rheumatoid arthritis-associated interstitial lung disease. Arthritis Res Ther (2022) 24(1):115. doi: 10.1186/s13075-022-02800-2
17. Chen J, Doyle TJ, Liu Y, Aggarwal R, Wang X, Shi Y, et al. Biomarkers of rheumatoid arthritis-associated interstitial lung disease. Arthritis Rheumatol (2015) 67(1):28–38. doi: 10.1002/art.38904
18. Zhao T, Su Z, Li Y, Zhang X, You Q. Chitinase-3 like-protein-1 function and its role in diseases. Signal Transduct Target Ther (2020) 5(1):201. doi: 10.1038/s41392-020-00303-7
19. Recklies AD, White C, Ling H. The chitinase 3-like protein human cartilage glycoprotein 39 (HC-gp39) stimulates proliferation of human connective-tissue cells and activates both extracellular signal-regulated kinase- and protein kinase B-mediated signalling pathways. Biochem J (2002) 365(Pt 1):119–26. doi: 10.1042/bj20020075
20. Renkema GH, Boot RG, Au FL, Donker-Koopman WE, Strijland A, Muijsers AO, et al. Chitotriosidase, a chitinase, and the 39-kDa human cartilage glycoprotein, a chitin-binding lectin, are homologues of family 18 glycosyl hydrolases secreted by human macrophages. Eur J Biochem (1998) 251(1-2):504–9. doi: 10.1046/j.1432-1327.1998.2510504.x
21. Hakala BE, White C, Recklies AD. Human cartilage gp-39, a major secretory product of articular chondrocytes and synovial cells, is a mamMalian member of a chitinase protein family. J Biol Chem (1993) 268(34):25803–10. doi: 10.1016/S0021-9258(19)74461-5
22. Harvey S, Weisman M, O'Dell J, Scott T, Krusemeier M, Visor J, et al. Chondrex: new marker of joint disease. Clin Chem (1998) 44(3):509–16. doi: 10.1093/clinchem/44.3.509
23. Long X, He X, Ohshimo S, Griese M, Sarria R, Guzman J, et al. Serum YKL-40 as predictor of outcome in hypersensitivity pneumonitis. Eur Respir J (2017) 49(2):1501924. doi: 10.1183/13993003.01924-2015
24. Zhou Y, Peng H, Sun H, Peng X, Tang C, Gan Y, et al. Chitinase 3-like 1 suppresses injury and promotes fibroproliferative responses in MamMalian lung fibrosis. Sci Transl Med (2014) 6(240):240ra76. doi: 10.1126/scitranslmed.3007096
25. Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheumatol (1988) 31(3):315–24. doi: 10.1002/art.1780310302
26. van der Heijde DM, van 't Hof MA, van Riel PL, Theunisse LA, Lubberts EW, van Leeuwen MA, et al. Judging disease activity in clinical practice in rheumatoid arthritis: first step in the development of a disease activity score. Ann Rheum Dis (1990) 49(11):916–20. doi: 10.1136/ard.49.11.916
27. American Thoracic S, European Respiratory S. American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001. Am J Respir Crit Care Med (2002) 165(2):277–304. doi: 10.1164/ajrccm.165.2.ats01
28. Hu C, Li S, Xie Z, You H, Jiang H, Shi Y, et al. Comparison of different test systems for the detection of antiphospholipid antibodies in a Chinese cohort. Front Immunol (2021) 12:648881. doi: 10.3389/fimmu.2021.648881
29. Grigor C, Capell H, Stirling A, McMahon AD, Lock P, Vallance R, et al. Effect of a treatment strategy of tight control for rheumatoid arthritis (the TICORA study): a single-blind randomised controlled trial. Lancet (2004) 364(9430):263–9. doi: 10.1016/S0140-6736(04)16676-2
30. Provan SA, Lillegraven S, Sexton J, Angel K, Austad C, Haavardsholm EA, et al. Trends in all-cause and cardiovascular mortality in patients with incident rheumatoid arthritis: a 20-year follow-up matched case-cohort study. Rheumatol (Oxford) (2020) 59(3):505–12. doi: 10.1093/rheumatology/kez371
31. Nannini C, Medina-Velasquez YF, Achenbach SJ, Crowson CS, Ryu JH, Vassallo R, et al. Incidence and mortality of obstructive lung disease in rheumatoid arthritis: a population-based study. Arthritis Care Res (Hoboken) (2013) 65(8):1243–50. doi: 10.1002/acr.21986
32. Hozumi H, Fujisawa T, Enomoto N, Nakashima R, Enomoto Y, Suzuki Y, et al. Clinical utility of YKL-40 in polymyositis/dermatomyositis-associated interstitial lung disease. J Rheumatol (2017) 44(9):1394–401. doi: 10.3899/jrheum.170373
33. Kelly CA, Saravanan V, Nisar M, Arthanari S, Woodhead FA, Price-Forbes AN, et al. Rheumatoid arthritis-related interstitial lung disease: associations, prognostic factors and physiological and radiological characteristics–a large multicentre UK study. Rheumatol (Oxford) (2014) 53(9):1676–82. doi: 10.1093/rheumatology/keu165
34. Weyand CM, Schmidt D, Wagner U, Goronzy JJ. The influence of sex on the phenotype of rheumatoid arthritis. Arthritis Rheumatol (1998) 41(5):817–22. doi: 10.1002/1529-0131(199805)41:5<817::AID-ART7>3.0.CO;2-S
35. Juge PA, Borie R, Kannengiesser C, Gazal S, Revy P, Wemeau-Stervinou L, et al. Shared genetic predisposition in rheumatoid arthritis-interstitial lung disease and familial pulmonary fibrosis. Eur Respir J (2017) 49(5):1602314. doi: 10.1136/annrheumdis-2017-eular.5237
36. Alpizar-Rodriguez D, Pluchino N, Canny G, Gabay C, Finckh A. The role of female hormonal factors in the development of rheumatoid arthritis. Rheumatol (Oxford) (2017) 56(8):1254–63. doi: 10.1093/rheumatology/kew318
37. Baka Z, Buzas E, Nagy G. Rheumatoid arthritis and smoking: putting the pieces together. Arthritis Res Ther (2009) 11(4):238. doi: 10.1186/ar2751
38. Klareskog L, Stolt P, Lundberg K, Kallberg H, Bengtsson C, Grunewald J, et al. A new model for an etiology of rheumatoid arthritis: smoking may trigger HLA-DR (shared epitope)-restricted immune reactions to autoantigens modified by citrullination. Arthritis Rheumatol (2006) 54(1):38–46. doi: 10.1002/art.21575
39. Makrygiannakis D, Hermansson M, Ulfgren AK, Nicholas AP, Zendman AJ, Eklund A, et al. Smoking increases peptidylarginine deiminase 2 enzyme expression in human lungs and increases citrullination in BAL cells. Ann Rheum Dis (2008) 67(10):1488–92. doi: 10.1136/ard.2007.075192
40. Bongartz T, Cantaert T, Atkins SR, Harle P, Myers JL, Turesson C, et al. Citrullination in extra-articular manifestations of rheumatoid arthritis. Rheumatol (Oxford) (2007) 46(1):70–5. doi: 10.1093/rheumatology/kel202
41. Damgaard D, Friberg Bruun Nielsen M, Quisgaard Gaunsbaek M, Palarasah Y, Svane-Knudsen V, Nielsen CH. Smoking is associated with increased levels of extracellular peptidylarginine deiminase 2 (PAD2) in the lungs. Clin Exp Rheumatol (2015) 33(3):405–8.
42. Juge PA, Lee JS, Ebstein E, Furukawa H, Dobrinskikh E, Gazal S, et al. MUC5B promoter variant and rheumatoid arthritis with interstitial lung disease. N Engl J Med (2018) 379(23):2209–19. doi: 10.1056/NEJMoa1801562
43. Shirai Y, Honda S, Ikari K, Kanai M, Takeda Y, Kamatani Y, et al. Association of the RPA3-UMAD1 locus with interstitial lung diseases complicated with rheumatoid arthritis in Japanese. Ann Rheum Dis (2020) 79(10):1305–9. doi: 10.1136/annrheumdis-2020-217256
44. Akiyama M, Kaneko Y. Pathogenesis, clinical features, and treatment strategy for rheumatoid arthritis-associated interstitial lung disease. Autoimmun Rev (2022) 21(5):103056. doi: 10.1016/j.autrev.2022.103056
45. Kiely P, Busby AD, Nikiphorou E, Sullivan K, Walsh DA, Creamer P, et al. Is incident rheumatoid arthritis interstitial lung disease associated with methotrexate treatment? Results from a multivariate analysis in the ERAS and ERAN inception cohorts. BMJ Open (2019) 9(5):e028466. doi: 10.1136/bmjopen-2018-028466
46. Juge PA, Granger B, Debray MP, Ebstein E, Louis Sidney F, Kedra J, et al. POS0095 DEVELOPPING A SCORE TO PREDICT PRECLINICAL INTERSTITIAL LUNG DISEASE IN PATIENTS WITH RHEUMATOID ARTHRITIS – A CROSS-SECTIONAL STUDY FROM THE ESPOIR COHORT. Ann Rheumatic Dis (2021) 80(Suppl 1):258–. doi: 10.1136/annrheumdis-2021-eular.2817
47. Koduri G, Norton S, Young A, Cox N, Davies P, Devlin J, et al. Interstitial lung disease has a poor prognosis in rheumatoid arthritis: results from an inception cohort. Rheumatol (Oxford) (2010) 49(8):1483–9. doi: 10.1093/rheumatology/keq035
48. Matson S, Lee J, Eickelberg O. Two sides of the same coin? A review of the similarities and differences between idiopathic pulmonary fibrosis and rheumatoid arthritis-associated interstitial lung disease. Eur Respir J (2021) 57(5):2002533. doi: 10.1183/13993003.02533-2020
49. Bauer Y, White ES, de Bernard S, Cornelisse P, Leconte I, Morganti A, et al. MMP-7 is a predictive biomarker of disease progression in patients with idiopathic pulmonary fibrosis. ERJ Open Res (2017) 3(1):74–2016. doi: 10.1183/23120541.00074-2016
50. Solomon JJ, Matson S, Kelmenson LB, Chung JH, Hobbs SB, Rosas IO, et al. IgA antibodies directed against citrullinated protein antigens are elevated in patients with idiopathic pulmonary fibrosis. Chest (2020) 157(6):1513–21. doi: 10.1016/j.chest.2019.12.005
51. Luban S, Li ZG. Citrullinated peptide and its relevance to rheumatoid arthritis: an update. Int J Rheum Dis (2010) 13(4):284–7. doi: 10.1111/j.1756-185X.2010.01553.x
52. Furuhashi K, Suda T, Nakamura Y, Inui N, Hashimoto D, Miwa S, et al. Increased expression of YKL-40, a chitinase-like protein, in serum and lung of patients with idiopathic pulmonary fibrosis. Respir Med (2010) 104(8):1204–10. doi: 10.1016/j.rmed.2010.02.026
53. Rehli M, Niller HH, Ammon C, Langmann S, Schwarzfischer L, Andreesen R, et al. Transcriptional regulation of CHI3L1, a marker gene for late stages of macrophage differentiation. J Biol Chem (2003) 278(45):44058–67. doi: 10.1074/jbc.M306792200
54. Iwata T, Kuwajima M, Sukeno A, Ishimaru N, Hayashi Y, Wabitsch M, et al. YKL-40 secreted from adipose tissue inhibits degradation of type I collagen. Biochem Biophys Res Commun (2009) 388(3):511–6. doi: 10.1016/j.bbrc.2009.08.024
55. Saag KG, Kolluri S, Koehnke RK, Georgou TA, Rachow JW, Hunninghake GW, et al. Rheumatoid arthritis lung disease. Determinants of radiographic and physiologic abnorMalities. Arthritis Rheumatol (1996) 39(10):1711–9. doi: 10.1002/art.1780391014
Keywords: interstitial lung disease, rheumatoid arthritis, chitinase-3 like-protein-1, biomarkers, prediction
Citation: Yu R, Liu X, Deng X, Li S, Wang Y, Zhang Y, Ke D, Yan R, Wang Q, Tian X, Li M, Zeng X and Hu C (2023) Serum CHI3L1 as a biomarker of interstitial lung disease in rheumatoid arthritis. Front. Immunol. 14:1211790. doi: 10.3389/fimmu.2023.1211790
Received: 25 April 2023; Accepted: 31 July 2023;
Published: 17 August 2023.
Edited by:
Xiaoxia Zhu, Fudan University, ChinaReviewed by:
Jing Luo, Second Hospital of Shanxi Medical University, ChinaCopyright © 2023 Yu, Liu, Deng, Li, Wang, Zhang, Ke, Yan, Wang, Tian, Li, Zeng and Hu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chaojun Hu, aHVjaGFvanVuODE4QHFxLmNvbQ==; Xiaofeng Zeng, emVuZ3hmcHVtY0AxNjMuY29t
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.