
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Immunol., 21 September 2023
Sec. Cancer Immunity and Immunotherapy
Volume 14 - 2023 | https://doi.org/10.3389/fimmu.2023.1211399
This article is part of the Research TopicBiomarkers and Immunotherapy for Genitourinary TumorsView all 30 articles
Objective: Whether neutrophil-lymphocyte ratio (NLR) is an applicative predictor of poor prognosis in patients with hepatocellular carcinoma (HCC) remains controversial. In response to the current conflicting data, this meta-analysis was conducted to gain a comprehensive and systematic understanding of prognostic value of NLR in HCC.
Methods: Several English databases, including PubMed, EMBASE, and the Cochrane Library, with an update date of February 25, 2023, were systematically searched. We set the inclusion criteria to include randomized controlled trial (RCT) studies that reported the prognostic value of serum NLR levels in patients with HCC receiving treatment. Both the combined ratio (OR) and the diagnosis ratio (DOR) were used to assess the prognostic performance of NLR. Additionally, we completed the risk of bias assessment by Cochrane Risk of Bias Assessment Tool.
Results: This meta-analysis ultimately included 16 studies with a total of 4654 patients with HCC. The results showed that high baseline NLR was significantly associated with poor prognosis or recurrence of HCC. The sensitivity of 0.67 (95% confidence interval [CI]. 0.59-0.73); specificity of 0.723 (95% CI: 0.64-0.78) and DOR of 5.0 (95% CI: 4.0-7.0) were pooled estimated from patient-based analyses. Subsequently, the combined positive likelihood ratio (PLR) and negative likelihood ratio (NLHR) were calculated with the results of 2.4 (95% CI: 1.9-3.0) and 0.46 (95% CI: 0.39-0.56), respectively. In addition, area under the curve (AUC) of the summary receiver operating characteristic (SROC) reflecting prognostic accuracy was calculated to be 0.75 (95% CI: 0.71-0.78). The results of subgroup analysis suggested that high NLR was an effective predictive factor of poor prognosis in HCC in mainland China as well as in the northern region.
Conclusion: Our findings suggest that high baseline NLR is an excellent predictor of poor prognosis or relapse in patients with HCC, especially those from high-incidence East Asian populations.
Systematic review registration: https://www.crd.york.ac.uk/prospero/#recordDetails, identifier CRD42023440640.
Hepatocellular carcinoma (HCC) is a malignant tumor that seriously endangers human health, and its pathogenesis is still unclear (1, 2). According to global cancer statistics released in 2022, there were about 906,000 new cases of primary liver cancer and 830,000 deaths worldwide, with China accounting for 42.5% of these cases (3). Unfortunately, 70% to 80% of patients with HCC are in the middle to late stage when diagnosed and have lost the chance of surgical operation (4). Although surgical treatment, chemotherapy, local ablation, molecular targeted therapy and other treatments have been significantly improved, the average survival time of patients with HCC is still very short due to insidious symptoms, rapid development and aggressiveness in early stage (5–7). Therefore, there is an urgent need for sensitive prognostic predictors to help guide the development of treatment plans, improve prognosis, and prolong patient survival.
East Asia is a region with a high prevalence of viral hepatitis B and primary liver cancer (5). Most liver cancers occur after cirrhosis triggered by chronic inflammation, and the interaction between inflammation and tumor is particularly pronounced (8, 9). Systemic inflammation not only plays an important role in tumor development, but also helps to determine the prognosis of patients with HCC (10). The tumor microenvironment of HCC consists mainly of cellular components such as hepatocellular carcinoma cells and inflammatory cells, and non-cellular components such as secreted chemokines and inflammatory factors (11). Some studies have reported that tumor-associated neutrophils can accelerate the proliferation and inhibit the apoptosis of hepatocellular carcinoma cells, promote angiogenesis, and further induce the progression of HCC (12, 13). However, their specific regulation and mechanism of action in HCC are unclear (14). Therefore, whether neutrophil-lymphocyte ratio (NLR), a commonly used clinical index of inflammatory response, can be directly used to discriminate the prognosis of patients with HCC remains to be tested.
More recently, there is growing evidence that increased systemic inflammation in a wide range of cancers is associated with poor cancer-specific survival (15). Both elevated levels of both c-reactive protein (CRP) and NLR can be used to detect the presence of a systemic inflammatory response (16, 17). Although high levels of preoperative serum CRP have been reported to be associated with early recurrence of HCC and poorer survival after hepatectomy, in many hospitals CRP levels are not routinely tested and show non-specific changes after treatment (18). In addition, NLR has an advantage over CRP in terms of inflammatory mechanisms (19). Except for HCC, the expression level of NLRs is closely related to tumor progression, metastasis and prognosis. However, inconsistent data have been generated regarding the predictive power of NLRs for disease progression and overall survival (OS) in HCC (20, 21). Therefore, it is necessary to carry out a meta-analysis to provide a systematic and comprehensive understanding of the predictive value of NLR in HCC.
In this study, we aimed to evaluate the predictive value of high NLR in predicting prognosis and recurrence in patients with HCC. In addition, we considered several sub-analyses to determine the differences in predictive outcomes across countries or regions and dimensional divisions.
Several English-language databases were systematically searched, including PubMed, EMBASE and the Cochrane Library, from creation to 25 February 2023. The following search terms were used (Supplementary material 1): (“hepatocellular carcinoma” [Mesh], or “liver cancer” [Mesh], or “hepatoma” [Mesh], or “hepatic carcinoma” [Mesh]), and (“inflammatory markers” [Title/Abstract], OR “neutrophil-to-lymphocyte ratio” [Title/Abstract], OR “neutrophil lymphocyte ratio” [Title/Abstract], OR “neutrophil to lymphocyte ratio” [Title/Abstract]). The summary with full results section was included in the present study. The bibliographies of the retrieved articles were checked manually for additional references. This meta-analysis was conducted based on PRISMA statements (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) (22, 23). This present meta-analysis has been submitted to PROSPERO (ID 440640).
All citations are reviewed in order. Search for full text of potentially relevant articles by title or abstract, and two investigators (Chunhua Xu and Shan Lin) independently reviewed to identify eligible studies. Disagreements about eligibility were resolved through discussions with the arbitrator (Fenfang Wu). Studies that explicitly met the following inclusion criteria were considered for inclusion: (1) serum NLR levels were measured prior to formal treatment; (2) participants in human studies were ≥18 years old; (3) sample size was >20; (4) randomized controlled trials (RCTs) are observational; and (5) false positive (FP), sufficient true positive (TP), false negative (FN), as well as true negative (TN) data were provided to calculate the predictive power of NLR in patients with HCC. Studies meeting the following exclusion criteria were excluded. (1) lack of information on the prognostic accuracy of the control or experimental groups; (2) animal or in vitro studies only; (3) presence of duplicate data or insufficient information; and (4) article type of review, poster, commentary, editorial or supplemental question.
The data of each experiment were collected by Xu Chunhua and Lin Shan respectively. All disagreements or differences among the reviewers were discussed and evaluated by a third-party reviewer (Lailing Du) until a consensus was reached. Pre-specified data for each study included a request to record and recalculate the following variables: first author, country or region, publication year, study design, entry time, sample size (male), median age (years), area under the curve (AUC; 95% confidence interval [CI]), sensitivity, specificity, and baseline NLR cutoff value.
In this study, we applied the Cochrane Risk of Bias Assessment Tool with 2 independent reviewers (Chunhua Xu and Shan Lin) to assess the quality of the articles (24). The tool includes six domains: allocation concealment, random sequence generation, blinding, incomplete outcome data, outcome reporting options and other bias resources. All six domains were assessed as “risk of bias” and “applicability issues”, and each item was judged as “yes”, “no” or “unclear”.
Additionally, eligible studies were assessed using the Newcastle-Ottawa scale (NOS) (25). Estimates of study quality were based on comparability, selection and exposure by a star system of up to 9 stars. The quality of each trial was defined as 0-3 stars as poor, 4-6 stars as fair and 7-9 stars as good. Finally, the quality assessment of the NOS was based on previous studies with some modifications (26).
In this study, we used Stata version 12.0 (StataCorp, College Station, TX, USA) software for all statistical analyses of TN, TP, FP and FN rates for each study, as well as diagnostic odd ratio (DOR) sensitivity, specificity, positive likelihood ratio (PLR), as well as negative likelihood ratios (NLHR) were fully assessed. P-values <0.05 for the Q statistic as well as I2 values >50% for the I2 statistic were all considered to be statistically significant heterogeneity (27). When heterogeneity was high (I2 > 50%), a random effects model was applied (28). Hardy-Weinberg equilibrium (HWE) of each study in the control group was considered statistically significant by Pearson’s χ2 test with p- value <0.05 (29).
In addition, to evaluate the predictive performance of NLR in patients with HCC, we performed pooled receiver characteristics (SROC) curves and pooled sensitivity and specificity forest plots by assessing AUC as a summary metric (30). Then, subgroup analyses were also carried out on a geographic or regional basis. Finally, we detected possible publication bias using Begg’s and Egger’s tests, and considered statistically significant with a p-value < 0.05 (31, 32).
Initially, a search of an electronic database yielded 698 potentially relevant papers, but after culling the duplicates, 315 papers were eliminated. From the title and abstract, 112 studies were obviously irrelevant and were therefore excluded. After the remaining 271 papers were reviewed, 185 papers were rejected and 70 papers were rejected. In the end, 16 papers were selected. Through 16 literatures, including 4654 cases, the role of NLR in the prognosis of HCC was evaluated by using the Meta method. Figure 1 details the step-by-step screening procedure for the included trials.
All 16 papers are written in English. To assess the quality of the included trials, basic data were extracted, as shown in Table 1. Of the 16 trials, 15 were in East Asia and the remaining 1 was in West Africa (43), including 11 in mainland China (34, 37, 39–41, 44, 46–50), 1 in South Korea (38), 2 in Taiwan (33, 51), and 1 in Japan (36). All studies are from single-center clinical trials between 2012 and 2022. All the 16 observational studies included 4654 HCC patients, including 3986 in mainland China, 213 in Korea, 40 in Japan, 311 in Taiwan, and 104 in mainland China. Table 2 summarizes the predictive power of NLR in the prognosis of patients with HCC. The AUC is between 0.602 and 0.855, and the threshold is between 1.505 and 3.290. In addition, sensitivities and specificities were calculated or given for the included tests in the ranges 0.301 to 0.840 and 0.440 to 0.887, respectively.
In this study, all trials had detailed inclusion criteria and excluded patients. Second, the quality of each included study (all with a Cochrane score of 10 or above) was assessed using the Cochrane risk of bias tool (Cochrane). In addition, the overall quality of the included studies was average. Figure 2 shows the results of the Cochrane evaluation.
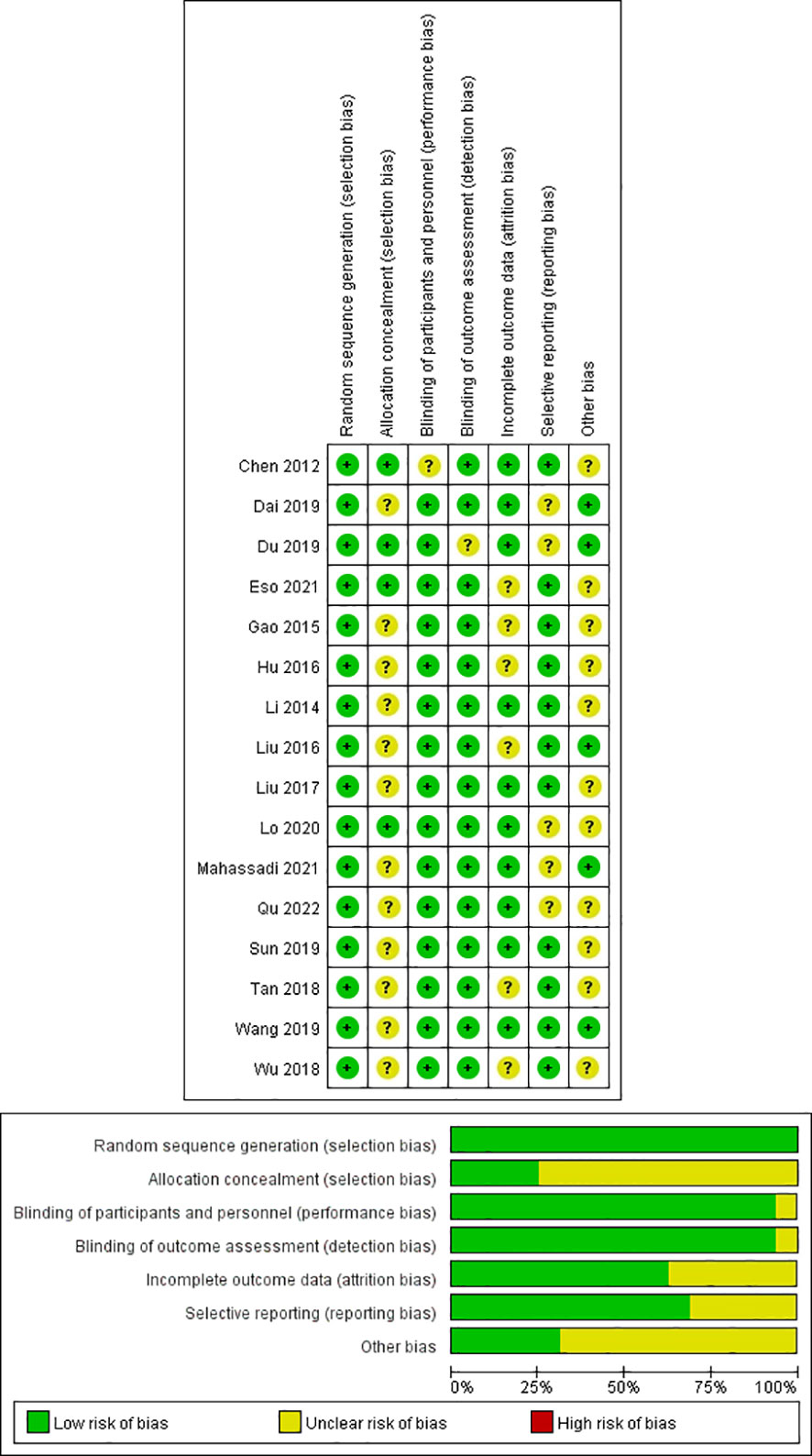
Figure 2 Quality assessment of included eligible studies by the Cochrane Risk of Bias Assessment Tool.
Moreover, the probability of publication bias was evaluated by Begg’s and Egger’s funnel plot, and the results were shown in Figures 3 and 4, respectively. It was suggested that there appears to be publication bias in the present study. Even though the overall risk of bias seemed low, the results do not appear to be significantly changed by studies that have not yet been published.
Sixteen data sets were extracted from 16 qualified literatures (Table 2), including AUC, 95% CI, optimal cut-off value of NLR, sensitivity, specificity, and TP, FP, FN, TN, etc. 16 studies examined the predictive value of NLR as a biomarker of prognosis in HCC patients with a total population of 4654. Table 2 summarizes the combined data from these trials. For the evaluation of the efficacy of NLR, it had a pooled sensitivity of 0.68 (95% CI: 0.58-0.77) (Figure 5A), specificity of 0.73 (95% CI: 0.61-0.82) (Figure 5B), and PLR of 2.5 (95% CI: 1.8-3.6), as well as NLHR of 0.43 (95% CI: 0.33-0.57). Subsequently, it had a pooled DOR of 6.347 (95% CI: 5.450-7.391) according to a random effects model.

Figure 5 Forest plot of sensitivity and specificity of high NLR predicting HCC prognosis. (A) Sensitivity. (B) Specificity.
In addition, forest plot of AUC of high NLR predicting HCC prognosis was carried out and shown in Figure 6. It was suggested that no “shoulder arm” pattern was seen in the SROC space, indicating the absence of a threshold effect. Furthermore, the AUC for the prognostic accuracy of SROC prediction was calculated as 0.76 (95% CI: 0.72-0.80). Moreover, the predictive value of NLR for prognosis of patients with HCC is summarized in Figure 7. Therefore, it is not difficult to conclude from the results that high baseline NLR is significantly associated with poor prognosis of HCC.
To further understand the differences in the predictive value of high NLR for recurrence and poor prognosis of HCC in populations from different countries (or regions) and in populations with different dimensional gradations, we performed a systematic subgroup analysis, and the results were shown in Table 3. There were significant differences in the prognostic value of NLR in patients with HCC according to the comparison of DOR and AUC. In the subgroup regional analysis, the DOR and AUC of NLRs in mainland China were higher than those in Korea (DOR, 5; AUC, 0.75 vs. DOR, 3; AUC, 0.64), and Côte d’Ivoire (DOR, 5; AUC, 0.75 vs. DOR, 4; AUC, 0.68), but significantly lower than those in Japan (DOR, 5; AUC, 0.75 vs. DOR, 12; AUC, 0.75), suggesting that the prognostic value of high NLR in mainland Chinese patients with HCC for HCC recurrence and poor prognosis value was superior to that of patients with HCC in Korea and Côte d’Ivoire, but inferior to Japan. In the subgroup latitude analysis, the prognostic value of high NLR for HCC recurrence and poor prognosis was better in patients with HCC at high latitudes than in patients with HCC at low latitudes (DOR, 7; AUC, 0.79 vs. DOR, 4; AUC, 0.71). Interestingly, combining the results of the above subgroup analyses, the prognostic predictive value for NLR was quite high in the high latitudes of East Asia, which may be related to the high incidence of HCC in this region.
Generally, the optimal cut-off value of NLR was determined by using receiver operating characteristic (ROC) curves. For the subgroup analysis of method of NLR cutoff identification (Table 3), the method of ROC showed DOR and AUC of 5(4–7) and 0.74(0.70-0.78), respectively. For the subgroup analysis of age, the group of median age ≤55 showed similar DOR and AUC to the group of median age >55 (DOR, 5(3–8); AUC, 0.74(0.70-0.78) vs. DOR, 5(4-8); AUC, 0.75(0.71-0.79)). Additionally, for the subgroup analysis of gender, the group of male >200 showed significantly higher DOR and AUC compared with the group of male ≤200 (DOR, 7(4-13); AUC, 0.79(0.75-0.82) vs. DOR, 4(3-5); AUC, 0.70(0.66-0.74)).
Each study included in the meta-analysis was removed each time to investigate the impact of a single data set on the combined OR. The results of the sensitivity analysis demonstrate the robustness of the findings in this study (data not shown).
In addition, possible explanations for heterogeneity were evaluated by meta-regression and subgroup analyses. The factor of high-latitude (I2 = 88.2%, P = 0.000) and mainland China (I2 = 87.8%, P ≤ 0.001) may be the main source of heterogeneity, while the factor of low-latitude (I2 = 18.0%, P = 0.287) may not be a source of heterogeneity in the predictive value of NLR. For subgroup analysis with heterogeneity, the random effects model was used to combine the effect size. For subgroup analyses without heterogeneity, fixed-effect models were used to combine effect sizes.
Factors affecting the prognosis of patients with primary HCC include tumor-related factors (such as the volume and load of the tumor) and patient-related factors (52). In addition, the inflammatory response plays an important role in the development of tumorigenesis, not only participating in the proliferation and metastasis of tumor cells, but also promoting the immune escape of tumors and affecting the responsiveness to treatment (53, 54). Here, in this meta-analysis of 16 studies comprising 4654 patients with HCC, the results showed that high baseline NLR was significantly associated with poor prognosis or recurrence of HCC, which suggested that NLR is an excellent predictor of poor prognosis or relapse in patients with HCC. In subgroups stratified by regions and latitude distribution, high NLR had better predictive value for poor prognosis of HCC in mainland China as well as in northern regions.
Systemic inflammatory responses are associated with the recurrence of certain tumors (55). The interaction of different types of immune cells leads to tumor immune escape and subsequently promotes tumor progression (56). Among the systemic inflammatory indicators, NLR and PLR are hot spots in the prognostic studies of various tumors (57). Neutrophils are involved in the progression of cancer in multiple stages and aspects (58). On the one hand, neutrophils directly promote tumor growth by secreting chemokines and cytokines and actively recruiting other tumor-supporting cells (59). On the other hand, tumor-associated neutrophils are involved in mediating the angiogenic switch and promoting tumor angiogenesis (60). At the same time, enzymes that degrade and modify the extracellular matrix are secreted to promote tumor cell invasion (61). In addition, lymphocytes reflect the body’s anti-tumor immunity, and changes in the ratio of the two are associated with an imbalance between the two types of cells, which reflects a disruption of the dynamic balance between the immune state and tumor inflammation (62).
In recent years, some simple indicators of inflammation have been used to predict tumor recurrence and metastasis with good practical value (63). NLR can be used to evaluate systemic inflammatory changes and can reflect the possible balance between preneutrophil neoplastic inflammation and lymphocyte-dependent antitumor immunity (64). Unfortunately, the specific mechanisms underlying the relationship between NLR and tumor recurrence and survival of tumor patients are not well understood, but the results of some basic studies may partially explain the mechanisms (65). One plausible explanation is that elevated neutrophils lead to the production of more inflammatory mediators, which in turn affect the tumor microenvironment and promote tumor recurrence and metastasis (66). Similarly, the role of neutrophils in tumourigenesis is to secrete high levels of vascular endothelial growth factor, IL-1 and IL-6, which in turn promote the production of tumor blood vessels, leading to tumor growth, development and metastasis (67). An increase in NLR indicates a relative increase in neutrophils or a relative decrease in lymphocytes, leading to a shift in the dynamic balance of inflammation towards tumor promotion. As suggested by the results of the present study, an increase in the NLR ratio favors the inflammatory response of the tumor, suggesting a propensity for malignancy to develop, proliferate and metastasize. Conversely, a weakening of the index reflects an increased antitumor function.
Recently, several studies showed that the NLR may be correlated with the prognosis of patients with HCC, but most of them were initially treated by liver transplantation (LT) (21, 45, 68). The findings obtained by Xiao et al. on 3094 patients showed that high NLR was associated with poor overall survival (OS) and disease free survival (DFS) in HCC initially treated by LT (21). Furthermore, Xu et al. conducted a study on 1936 patients and showed that elevated pretransplant NLR may be used as a new prognostic predictor after LT for HCC (68). Additionally, Sun et al. conducted a meta-analysis on 1687 patients and suggested that elevated preoperative NLR is associated with poor prognosis in HCC patients treated with LT (45). In the present study, the scope of the study was not limited to LT, which not only had a larger clinical sample size with 4654 patients, but also reflected the correlation between NLR and HCC prognosis more comprehensively.
Intriguingly, it is worth mentioning that there were other biomarkers not only inflammatory ones, such as APRI and CRP. It is reported that high APRI levels are associated with poor OS and DFS in the patients with HCC, and pretreatment APRI can be used as an independent prognostic factor, but it is necessary to incorporate other predictive prognostic systems to ensure accuracy (69). Furthermore, the significance of CRP has been demonstrated as a predictor of survival in HCC, but the current opinion on the prognostic role of CRP in HCC is still controversial (70). Therefore, even though it has been reported that APRI and CRP might be candidates as prognostic biomarkers in HCC, the clinical value of them in HCC were still inconsistent and debatable for many reasons, such as limited sample sizes. In this present study, an adequate sample size was included to evaluate the predictive value of high NLR in predicting prognosis and recurrence in patients with HCC, which could gain a comprehensive understanding of prognostic value of NLR in HCC.
There are some limitations in this study. First, the included studies were retrospective, and patients had different late treatment regimens, which may have had an impact on patient prognosis. Second, the levels of preoperative neutrophil and lymphocyte counts are susceptible to a variety of factors, such as hepatitis and the degree of cirrhosis. Furthermore, it was shown that there is low level of publication bias. Even though the overall risk of bias may be low, it can be avoided by including more published articles to inflate the sample size in the future. In addition, most of the original studies demonstrated an association between high baseline NLR and poor prognosis of HCC, which may be due to the ease of publication of positive results, ultimately making it difficult to find more controversial studies. Finally, the cutoff value for defining high NLR differed in studies, and it is essential that they be unified before high NLR can be utilized in clinical prognostication for HCC.
A high level of NLR is an excellent prognostic indicator for HCC and can be used to predict early relapse, late relapse and long-term survival of HCC. It can be an effective reference indicator for clinicians to judge the prognosis of patients and adjust the treatment in a timely manner. Even so, larger and better-designed investigations are needed to further fully elucidate the predictive value of NLR for the prognosis of HCC patients.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
CX and SL contributed to the conception and design. CX and FW contributed to the administrative support. CX and LD contributed to the provision of study materials or patients. SL and YD contributed to the collection and assembly of data. CX contributed to the data analysis and interpretation. All authors agree to the submission for publication.
This study was supported by the National Natural Science Foundation of China (no. 82100706, 82172107), the Scientific Research Start-Up Project for Talent Introduction of Zhejiang Shuren University (2022R065), Basic and Applied Basic Research Foundation of Guangdong Province (2021A1515011927, 2021A1515010918), Shenzhen Municipal Science and Technology Innovation Committee (JCYJ20210324135014040, JCYJ20220818103407016), and Longgang District Special Fund for Economic and Technological Development (LGKCYLWS2022007).
The authors thank the study participants in each of the individual studies for their involvement.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1211399/full#supplementary-material
1. Peng J, Lü M, Peng Y, Tang X. Global incidence of primary liver cancer by etiology among children, adolescents, and young adults. J Hepatol (2023) 79(2):e92–e94. doi: 10.1016/j.jhep.2023.02.019
2. Sethi G, Rath P, Chauhan A, Ranjan A, Choudhary R, Ramniwas S, et al. Apoptotic mechanisms of quercetin in liver cancer: recent trends and advancements. Pharmaceutics (2023) 15(2):712. doi: 10.3390/pharmaceutics15020712
3. Konyn P, Ahmed A, Kim D. Current epidemiology in hepatocellular carcinoma. Expert Rev Gastroenterol Hepatol (2021) 15(11):1295–307. doi: 10.1080/17474124.2021.1991792
4. Prasad D, Nguyen MH. Epidemiology, pathogenesis, diagnosis, surveillance, and management of hepatocellular carcinoma associated with vascular liver disease. Kaohsiung J Med Sci (2021) 37(5):355–60. doi: 10.1002/kjm2.12368
5. Zhang CH, Cheng Y, Zhang S, Fan J, Gao Q. Changing epidemiology of hepatocellular carcinoma in Asia. Liver Int (2022) 42(9):2029–41. doi: 10.1111/liv.15251
6. Tran NH. Shifting epidemiology of hepatocellular carcinoma in far eastern and southeast asian patients: explanations and implications. Curr Oncol Rep (2022) 24(2):187–93. doi: 10.1007/s11912-021-01160-5
7. Polychronidis G, Feng J, Murtha-Lemekhova A, Heger U, Mehrabi A, Hoffmann K. Factors influencing overall survival for patients with fibrolamellar hepatocellular carcinoma: analysis of the surveillance, epidemiology, and end results database. Int J Gen Med (2022) 15:393–406. doi: 10.2147/IJGM.S338066
8. Li Y, Zhao JF, Zhang J, Zhan GH, Li YK, Huang JT, et al. Inflammation and fibrosis in patients with non-cirrhotic hepatitis B virus-associated hepatocellular carcinoma: impact on prognosis after hepatectomy and mechanisms involved. Curr Oncol (2022) 30(1):196–218. doi: 10.3390/curroncol30010016
9. Huo TI, Liu PH, Ho SY. Are inflammation-based models feasible tools in predicting the outcome of patients with hepatocellular carcinoma? Liver Int (2020) 40(6):1498. doi: 10.1111/liv.14341
10. Liang Y, Zhang Z, Zhong D, Lai C, Dai Z, Zou H, et al. The prognostic significance of inflammation-immunity-nutrition score on postoperative survival and recurrence in hepatocellular carcinoma patients. Front Oncol (2022) 12:913731. doi: 10.3389/fonc.2022.913731
11. Mao JX, Teng F, Liu C, Yuan H, Dong JY, Fu H, et al. Immunometabolic inflammation and hepatocellular carcinoma. Hepatobil Pancreat Dis Int (2019) 18(3):298–300. doi: 10.1016/j.hbpd.2019.03.012
12. Arvanitakis K, Mitroulis I, Germanidis G. Tumor-associated neutrophils in hepatocellular carcinoma pathogenesis, prognosis, and therapy. Cancers (Basel) (2021) 13(12):2899. doi: 10.3390/cancers13122899
13. Geh D, Leslie J, Rumney R, Reeves HL, Bird TG, Mann DA. Neutrophils as potential therapeutic targets in hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol (2022) 19(4):257–73. doi: 10.1038/s41575-021-00568-5
14. Wang Y, Yao R, Zhang L, Xie X, Chen R, Ren Z. IDO and intra-tumoral neutrophils were independent prognostic factors for overall survival for hepatocellular carcinoma. J Clin Lab Anal (2019) 33(5):e22872. doi: 10.1002/jcla.22872
15. Ha F, Wang X, Han T, Jia K, Wang S, Song D. Value of preoperative systemic immune-inflammation index and albumin-bilirubin grade in patients with hepatocellular carcinoma undergoing transarterial embolization. Turk J Gastroenterol (2023) 34(4):413–20. doi: 10.5152/tjg.2023.22296
16. Minici R, Siciliano MA, Ammendola M, Santoro RC, Barbieri V, Ranieri G, et al. Prognostic role of neutrophil-to-lymphocyte ratio (NLR), lymphocyte-to-monocyte ratio (LMR), platelet-to-lymphocyte ratio (PLR) and Lymphocyte-to-C Reactive protein ratio (LCR) in patients with hepatocellular carcinoma (HCC) undergoing chemoembolizations (TACE) of the liver: the unexplored corner linking tumor microenvironment, biomarkers and interventional radiology. Cancers (Basel) (2022) 15(1):257. doi: 10.3390/cancers15010257
17. Oh BS, Jang JW, Kwon JH, You CR, Chung KW, Kay CS, et al. Prognostic value of C-reactive protein and neutrophil-to-lymphocyte ratio in patients with hepatocellular carcinoma. BMC Cancer (2013) 13:78. doi: 10.1186/1471-2407-13-78
18. Peng Q, Ren S, Lao X, Lu Y, Zhang X, Chen Z, et al. C-reactive protein genetic polymorphisms increase susceptibility to HBV-related hepatocellular carcinoma in a Chinese population. Tumour Biol (2014) 35(10):10169–76. doi: 10.1007/s13277-014-2334-x
19. Li SC, Xu Z, Deng YL, Wang YN, Jia YM. Higher neutrophil-lymphocyte ratio is associated with better prognosis of hepatocellular carcinoma. Med (Baltimore) (2020) 99(27):e20919. doi: 10.1097/MD.0000000000020919
20. Zeng F, Chen B, Zeng J, Wang Z, Xiao L, Deng G. Preoperative neutrophil-lymphocyte ratio predicts the risk of microvascular invasion in hepatocellular carcinoma: A meta-analysis. Int J Biol Markers (2019) 34(3):213–20. doi: 10.1177/1724600819874487
21. Xiao WK, Chen D, Li SQ, Fu SJ, Peng BG, Liang LJ. Prognostic significance of neutrophil-lymphocyte ratio in hepatocellular carcinoma: a meta-analysis. BMC Cancer (2014) 14:117. doi: 10.1186/1471-2407-14-117
22. Cacciamani GE, Chu TN, Sanford DI, Abreu A, Duddalwar V, Oberai A, et al. PRISMA AI reporting guidelines for systematic reviews and meta-analyses on AI in healthcare. Nat Med (2023) 29(1):14–5. doi: 10.1038/s41591-022-02139-w
23. Salameh JP, Bossuyt PM, McGrath TA, Thombs BD, Hyde CJ, Macaskill P, et al. Preferred reporting items for systematic review and meta-analysis of diagnostic test accuracy studies (PRISMA-DTA): explanation, elaboration, and checklist. BMJ (2020) 370:m2632. doi: 10.1136/bmj.m2632
24. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ (2011) 343:d5928. doi: 10.1136/bmj.d5928
25. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol (2010) 25(9):603–5. doi: 10.1007/s10654-010-9491-z
26. Qi C, Wang L, Duan G. Preoperative neutrophil-to-lymphocyte ratio (NLR) as a prognostic biomarker for patients with high-risk neuroblastoma. Asian J Surg (2023) 46(6):2474–5. doi: 10.1016/j.asjsur.2022.12.069
27. Wu H, Zhang H, Li P, Gao T, Lin J, Yang J, et al. Association between dietary carbohydrate intake and dietary glycemic index and risk of age-related cataract: a meta-analysis. Invest Ophthalmol Vis Sci (2014) 55(6):3660–8. doi: 10.1167/iovs.13-13695
28. Spineli LM, Pandis N. Meta-analysis: random-effects model. Am J Orthod Dentofacial Orthop (2020) 157(2):280–2. doi: 10.1016/j.ajodo.2019.10.007
29. Royo JL. Hardy weinberg equilibrium disturbances in case-control studies lead to non-conclusive results. Cell J (2021) 22(4):572–4. doi: 10.22074/cellj.2021.7195
30. Moi SH, Lee YC, Chuang LY, Yuan SF, Ou-Yang F, Hou MF, et al. Cumulative receiver operating characteristics for analyzing interaction between tissue visfatin and clinicopathologic factors in breast cancer progression. Cancer Cell Int (2018) 18:19. doi: 10.1186/s12935-018-0517-z
31. Xu C, Lin S, Mao L, Li Z. Neutrophil gelatinase-associated lipocalin as predictor of acute kidney injury requiring renal replacement therapy: A systematic review and meta-analysis. Front Med (Lausanne) (2022) 9:859318. doi: 10.3389/fmed.2022.859318
32. Lin Z, Pan I, Pan W. A practical problem with Egger regression in Mendelian randomization. PloS Genet (2022) 18(5):e1010166. doi: 10.1371/journal.pgen.1010166
33. Chen TM, Lin CC, Huang PT, Wen CF. Neutrophil-to-lymphocyte ratio associated with mortality in early hepatocellular carcinoma patients after radiofrequency ablation. J Gastroenterol Hepatol (2012) 27(3):553–61. doi: 10.1111/j.1440-1746.2011.06910.x
34. Dai T, Lin G, Deng M, Zhu S, Li H, Yao J, et al. The prognostic significance of neutrophil-to-lymphocyte ratio at different time points in patients with hepatocellular carcinoma receiving liver resection. Transl Cancer Res (2020) 9(2):441–57. doi: 10.21037/tcr.2019.11.29
35. Du Z, Dong J, Bi J, Bai R, Zhang J, Wu Z, et al. Correction: Predictive value of the preoperative neutrophil-to-lymphocyte ratio for the development of hepatocellular carcinoma in HBV-associated cirrhotic patients after splenectomy. PLoS One (2019) 14(4):e0215183.
36. Eso Y, Takeda H, Taura K, Takai A, Takahashi K, Seno H. Pretreatment neutrophil-to-lymphocyte ratio as a predictive marker of response to atezolizumab plus bevacizumab for hepatocellular carcinoma. Curr Oncol (2021) 28(5):4157–66. doi: 10.3390/curroncol28050352
37. Gao F, Li X, Geng M, Ye X, Liu H, Liu Y, et al. Pretreatment neutrophil-lymphocyte ratio: an independent predictor of survival in patients with hepatocellular carcinoma. Med (Baltimore) (2015) 94(11):e639. doi: 10.1097/MD.0000000000000639
38. Hu XG, Mao W, Park YK, Xu WG, Kim BW, Wang HJ. Blood Neutrophil-to-Lymphocyte Ratio Predicts Tumor Recurrence in Patients with Hepatocellular Carcinoma within Milan Criteria after Hepatectomy. Yonsei Med J (2016) 57(5):1115–23. doi: 10.3349/ymj.2016.57.5.1115
39. Li X, Han Z, Cheng Z, Yu J, Liu S, Yu X, et al. Preoperative neutrophil-to-lymphocyte ratio is a predictor of recurrence following thermal ablation for recurrent hepatocellular carcinoma: a retrospective analysis. PloS One (2014) 9(10):e110546. doi: 10.1371/journal.pone.0110546
40. Liu Y, Wang ZX, Cao Y, Zhang G, Chen WB, Jiang CP. Preoperative inflammation-based markers predict early and late recurrence of hepatocellular carcinoma after curative hepatectomy. Hepatobil Pancreat Dis Int (2016) 15(3):266–74. doi: 10.1016/s1499-3872(16)60094-2
41. Liu C, Jia BS, Zou BW, Du H, Yan LN, Yang JY, et al. Neutrophil-to-lymphocyte and aspartate-to-alanine aminotransferase ratios predict hepatocellular carcinoma prognosis after transarterial embolization. Med (Baltimore) (2017) 96(45):e8512. doi: 10.1097/MD.0000000000008512
42. Lo CH, Lee HL, Hsiang CW, Chiou JF, Lee MS, Chen SW, et al. Pretreatment neutrophil-to-lymphocyte ratio predicts survival and liver toxicity in patients with hepatocellular carcinoma treated with stereotactic ablative radiation therapy. Int J Radiat Oncol Biol Phys (2021) 109(2):474–484. doi: 10.1016/j.ijrobp.2020.09.001
43. Mahassadi AK, Anzouan-Kacou Kissi H, Attia AK. The prognostic values of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio at baseline in predicting the in-hospital mortality in black african patients with advanced hepatocellular carcinoma in palliative treatment: A comparative cohort study. Hepat Med (2021) 13:123–34. doi: 10.2147/HMER.S333980
44. Qu Z, Lu YJ, Feng JW, Chen YX, Shi LQ, Chen J, et al. Preoperative prognostic nutritional index and neutrophil-to-lymphocyte ratio predict survival outcomes of patients with hepatocellular carcinoma after curative resection. Front Oncol (2022) 11:823054. doi: 10.3389/fonc.2021.823054
45. Sun XD, Shi XJ, Chen YG, Wang CL, Ma Q, Lv GY. Elevated preoperative neutrophil-lymphocyte ratio is associated with poor prognosis in hepatocellular carcinoma patients treated with liver transplantation: A meta-analysis. Gastroenterol Res Pract (2016) 2016:4743808. doi: 10.1155/2016/4743808
46. Tan W, Sun W, Li X, Zhao L, Wang C, Zang A, et al. Preablation neutrophil-to-lymphocyte ratio as an independent prognostic factor in locally advanced hepatocellular carcinoma patients following radiofrequency ablation. J Cancer Res Ther (2018) 14(1):84–9. doi: 10.4103/jcrt.JCRT_835_17
47. Wu XL, Bi XY, Li ZY, Zhao H, Zhao JJ, Zhou JG, et al. [Correlation between postoperative neutrophil to lymphocyte ratio and recurrence and prognosis of hepatocellular carcinoma after radical liver resection]. Zhonghua Zhong Liu Za Zhi (2018) 40(5):365–71. doi: 10.3760/cma.j.issn.0253-3766.2018.05.009
48. Du Z, Dong J, Bi J, Bai R, Zhang J, Wu Z, et al. Predictive value of the preoperative neutrophil-to-lymphocyte ratio for the development of hepatocellular carcinoma in HBV-associated cirrhotic patients after splenectomy. PloS One (2018) 13(4):e0195336. doi: 10.1371/journal.pone.0195336
49. Sun S, Wang X, Chen J. Using pre-treatment neutrophil-to-lymphocyte ratio to predict the prognosis of young patients with hepatocellular carcinoma implemented minimally invasive treatment. J Adolesc Young Adult Oncol (2020) 9(1):85–9. doi: 10.1089/jayao.2019.0046
50. Wang D, Bai N, Hu X, OuYang XW, Yao L, Tao Y, et al. Preoperative inflammatory markers of NLR and PLR as indicators of poor prognosis in resectable HCC. PeerJ (2019) 7:e7132. doi: 10.7717/peerj.7132
51. Lo CH, Lee HL, Hsiang CW, Chiou JF, Lee MS, Chen SW, et al. Pretreatment neutrophil-to-lymphocyte ratio predicts survival and liver toxicity in patients with hepatocellular carcinoma treated with stereotactic ablative radiation therapy. Int J Radiat Oncol Biol Phys (2021) 109(2):474–84. doi: 10.1016/j.ijrobp.2020.09.001
52. Gao YX, Yang TW, Yin JM, Yang PX, Kou BX, Chai MY, et al. Progress and prospects of biomarkers in primary liver cancer (Review). Int J Oncol (2020) 57(1):54–66. doi: 10.3892/ijo.2020.5035
53. McClellan JL, Davis JM, Steiner JL, Day SD, Steck SE, Carmichael MD, et al. Intestinal inflammatory cytokine response in relation to tumorigenesis in the Apc(Min/+) mouse. Cytokine (2012) 57(1):113–9. doi: 10.1016/j.cyto.2011.09.027
54. Ragel BT, Jensen RL, Couldwell WT. Inflammatory response and meningioma tumorigenesis and the effect of cyclooxygenase-2 inhibitors. Neurosurg Focus (2007) 23(4):E7. doi: 10.3171/FOC-07/10/E7
55. Wang T, Zhang D, Tang D, Heng Y, Lu LM, Tao L. The role of systemic inflammatory response index (SIRI) and tumor-infiltrating lymphocytes (TILs) in the prognosis of patients with laryngeal squamous cell carcinoma. J Cancer Res Clin Oncol (2023). 149(9):5627–36. doi: 10.1007/s00432-022-04469-1
56. Ruiz-Ranz M, Lequerica-Fernández P, Rodríguez-Santamarta T, Suárez-Sánchez FJ, López-Pintor RM, García-Pedrero JM, et al. Prognostic implications of preoperative systemic inflammatory markers in oral squamous cell carcinoma, and correlations with the local immune tumor microenvironment. Front Immunol (2022) 13:941351. doi: 10.3389/fimmu.2022.941351
57. Lin ZQ, Ma C, Cao WZ, Ning Z, Tan G. Prognostic significance of NLR, PLR, LMR and tumor infiltrating T lymphocytes in patients undergoing surgical resection for hilar cholangiocarcinoma. Front Oncol (2022) 12:908907. doi: 10.3389/fonc.2022.908907
58. Gawiński C, Michalski W, Mróz A, Wyrwicz L. Correlation between lymphocyte-to-monocyte ratio (LMR), neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR) and tumor-infiltrating lymphocytes (TILs) in left-sided colorectal cancer patients. Biol (Basel) (2022) 11(3):385. doi: 10.3390/biology11030385
59. Hassan WA, ElBanna AK, Noufal N, El-Assmy M, Lotfy H, Ali RI. Significance of tumor-associated neutrophils, lymphocytes, and neutrophil-to-lymphocyte ratio in non-invasive and invasive bladder urothelial carcinoma. J Pathol Transl Med (2023) 57(2):88–94. doi: 10.4132/jptm.2022.11.06
60. Wang Y, Zhai J, Zhang T, Han S, Zhang Y, Yao X, et al. Tumor-associated neutrophils can predict lymph node metastasis in early gastric cancer. Front Oncol (2020) 10:570113. doi: 10.3389/fonc.2020.570113
61. Sun R, Xiong Y, Liu H, Gao C, Su L, Weng J, et al. Tumor-associated neutrophils suppress antitumor immunity of NK cells through the PD-L1/PD-1 axis. Transl Oncol (2020) 13(10):100825. doi: 10.1016/j.tranon.2020.100825
62. Kepp O, Liu P, Zitvogel L, Kroemer G. Tumor-infiltrating lymphocytes for melanoma immunotherapy. Oncoimmunology (2023) 12(1):2175506. doi: 10.1080/2162402X.2023.2175506
63. Yamamura K, Sugimoto H, Kanda M, Yamada S, Nomoto S, Nakayama G, et al. Comparison of inflammation-based prognostic scores as predictors of tumor recurrence in patients with hepatocellular carcinoma after curative resection. J Hepatobil Pancreat Sci (2014) 21(9):682–8. doi: 10.1002/jhbp.114
64. Cheng H, Luo G, Lu Y, Jin K, Guo M, Xu J, et al. The combination of systemic inflammation-based marker NLR and circulating regulatory T cells predicts the prognosis of resectable pancreatic cancer patients. Pancreatology (2016) 16(6):1080–4. doi: 10.1016/j.pan.2016.09.007
65. Polk N, Budai B, Hitre E, Patócs A, Mersich T. Corrigendum: high neutrophil-to-lymphocyte ratio (NLR) and systemic immune-inflammation index (SII) are markers of longer survival after metastasectomy of patients with liver-only metastasis of rectal cancer. Pathol Oncol Res (2022) 28:1610658. doi: 10.3389/pore.2022.1610658
66. Kumagai Y, Ohzawa H, Miyato H, Horie H, Hosoya Y, Lefor AK, et al. Kitayama J. Surgical stress increases circulating low-density neutrophils which may promote tumor recurrence. J Surg Res (2020) 246:52–61. doi: 10.1016/j.jss.2019.08.022
67. Wen S, Chen Y, Hu C, Du X, Xia J, Wang X, et al. Combination of tertiary lymphoid structure and neutrophil-to-lymphocyte ratio predicts survival in patients with hepatocellular carcinoma. Front Immunol (2022) 12:788640. doi: 10.3389/fimmu.2021.788640
68. Xu ZG, Ye CJ, Liu LX, Wu G, Zhao ZX, Wang YZ, et al. The pretransplant neutrophil-lymphocyte ratio as a new prognostic predictor after liver transplantation for hepatocellular cancer: a systematic review and meta-analysis. biomark Med (2018) 12(2):189–99. doi: 10.2217/bmm-2017-0307
69. Zhang X, Svn Z, Liv M, Liu M, Zhang Y, Sun Q. Assessment of prognostic value of aspartate aminotransferase-to-platelet ratio index in patients with hepatocellular carcinoma: meta-analysis of 28 cohort studies. Front Med (Lausanne) (2021) 8:756210. doi: 10.3389/fmed.2021.756210
Keywords: neutrophil-lymphocyte ratio, hepatocellular carcinoma, inflammation, prognostic value, poor prognosis
Citation: Xu C, Wu F, Du L, Dong Y and Lin S (2023) Significant association between high neutrophil-lymphocyte ratio and poor prognosis in patients with hepatocellular carcinoma: a systematic review and meta-analysis. Front. Immunol. 14:1211399. doi: 10.3389/fimmu.2023.1211399
Received: 24 April 2023; Accepted: 05 September 2023;
Published: 21 September 2023.
Edited by:
Youqiang Ke, Royal Liverpool University Hospital, United KingdomReviewed by:
Rohit Seth, Guru Ghasidas Vishwavidyalaya, IndiaCopyright © 2023 Xu, Wu, Du, Dong and Lin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shan Lin, YmlvdGVjaGxpbkBmb3htYWlsLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.