
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Immunol., 11 July 2023
Sec. Immunological Memory
Volume 14 - 2023 | https://doi.org/10.3389/fimmu.2023.1204231
This article is part of the Research TopicExploring Immune Memory Dynamics in Chronic Antigen Exposure and Disease Progression: Implications for ImmunotherapyView all 8 articles
Memory T cells are conventionally subdivided into T central memory (TCM) and T effector memory (TEM) cells. However, a new subset of memory T cells named T memory stem cell (TSCM) cells has been recognized that possesses capabilities of both TCM and TEM cells including lymphoid homing and performing effector roles through secretion of cytokines such as interleukin-2 (IL-2) and interferon-gamma (IFN-γ). The TSCM subset has some biological properties including stemness, antigen independency, high proliferative potential, signaling pathway and lipid metabolism. On the other hand, memory T cells are considered one of the principal culprits in the pathogenesis of autoimmune diseases. TSCM cells are responsible for developing long-term defensive immunity against different foreign antigens, alongside tumor-associated antigens, which mainly derive from self-antigens. Hence, antigen-specific TSCM cells can produce antitumor responses that are potentially able to trigger autoimmune activities. Therefore, we reviewed recent evidence on TSCM cell functions in autoimmune disorders including type 1 diabetes, systemic lupus erythematosus, rheumatoid arthritis, acquired aplastic anemia, immune thrombocytopenia, and autoimmune uveitis. We also introduced TSCM cell lineage as an innovative prognostic biomarker and a promising therapeutic target in autoimmune settings.
T cells are identified as key members of the adaptive immune system defending against a wide range of pathogens while making a sharp distinction between self- and non-self-antigens (Ags) (1, 2). The feature “memory” potentiates T cells to produce stronger and more rapid responses during reexposure to the corresponding Ag (1). Memory T cells are also capable of preserving their protectiveness against recognized Ags for several decades without restimulation by those Ags (2, 3). Conventionally, memory T cells are divided into T central memory (TCM) and T effector memory (TEM) cells according to the expression of their phenotypic markers such as CD45RO/RA, CCR7, and CD62L (3). However, a new subset of memory T cells has been recently recognized during the investigation of the graft-versus-host disease translational model, which possesses features of both TCM and TEM cells including lymphoid homing and effector role performance through secreting effector cytokines like interleukin-2 (IL-2) and interferon-gamma (IFN-γ) named T memory stem cell (TSCM) cells. Unlike other memory T-cell subsets, TSCM cells have two main characteristics including stemness and Ag independency along with some biological properties such as high proliferative potential and lipid metabolism (1, 2, 4). Recent investigations extensively reviewed the roles and applications of TSCM cells in malignancies, including melanoma, gastric cancer, B-cell lymphoma, and adult T-cell leukemia as well as infectious disorders such as human immunodeficiency virus type 1 and simian immunodeficiency virus (1–3), but few literatures have focused on properties of TSCM cells in the setting of autoimmune diseases.
An autoimmune disorder is a well-known condition that typically stems from hyperactivation of cells producing inflammatory cytokines accompanied by the disruption of immunoregulatory pathways leading to a persistent response to self-Ags (1). Despite immunosuppressor and anti-inflammatory drugs being routinely used to control autoimmune diseases, no complete success has been achieved with this approach, which can be pertinent to the existence of immune memory cells, specifically TSCM cells (2). Gaining insight into the novel concept of TSCM cells, developing our knowledge regarding TSCM cell’s differentiation, molecular mechanism, signal transduction, and regulation pathways can aid clinicians in designing efficient immunotherapeutic strategies against autoimmune diseases. Therefore, in this study, we review the characteristic features of these innovative memory T cells with their recognized roles in some autoimmune diseases.
TSCM cells constitute approximately 2%–3% of circulating T cells (5), with a distinctive gene expression profile that is closely related to that of conventional memory T cells (1, 2, 4). Further investigations showed that TSCM functional roles are apparently different from those of classical memory T subsets (3). TSCM cells emerge mostly in peripheral blood and secondary lymphoid organs (SLOs) and quietly fade at mucosal surfaces (3, 6). Currently, TSCM cells were identified in mice, humans, and nonhuman primates (NHPs) (1–3). According to their life span, TSCM cells are categorized into two subgroups; shorter-lived TSCM cells survive less than 1 year and can be reconstituted rapidly, but another subgroup that is estimated to have at least 9 years of longevity can perfectly preserve their self-renewal and memory abilities even above 25 years (1, 2, 4). The difference between the life span of these two subgroups can be attributed to methylation/demethylation of the promoters of their transcriptional factors, which can switch on/off their self-renewal molecular machinery (1, 4, 7). More importantly, the telomeres of the long-lived TSCM cells are protected by the high levels of telomerase against erosion (1, 2).
Different models are proposed on the conversion of TSCM into effector cells. Even though some literature acknowledges the linear model of TSCM cell differentiation (6, 8, 9), circular (on-off-on) and asymmetric models are also suggested (6). Herein, we briefly explain each model and discuss which one is more plausible at least in the context of autoimmune diseases like type 1 diabetes (T1D) (10).
In the linear model, the cell differentiation in each phase depends on the T-cell receptor (TCR) potential signal and extent and persistence of antigenic stimulation on the cells. This model explains that while T cells are directed straightly toward the memory and effector phases, they gradually lose their memory strength and develop remarkably effector capabilities, which is also termed as the “decreasing potential” model (Figure 1A). However, it seems that this model is in conflict with the primary definition of TSCM cells in regard to the preservation of memory capacities over long periods of time (6).
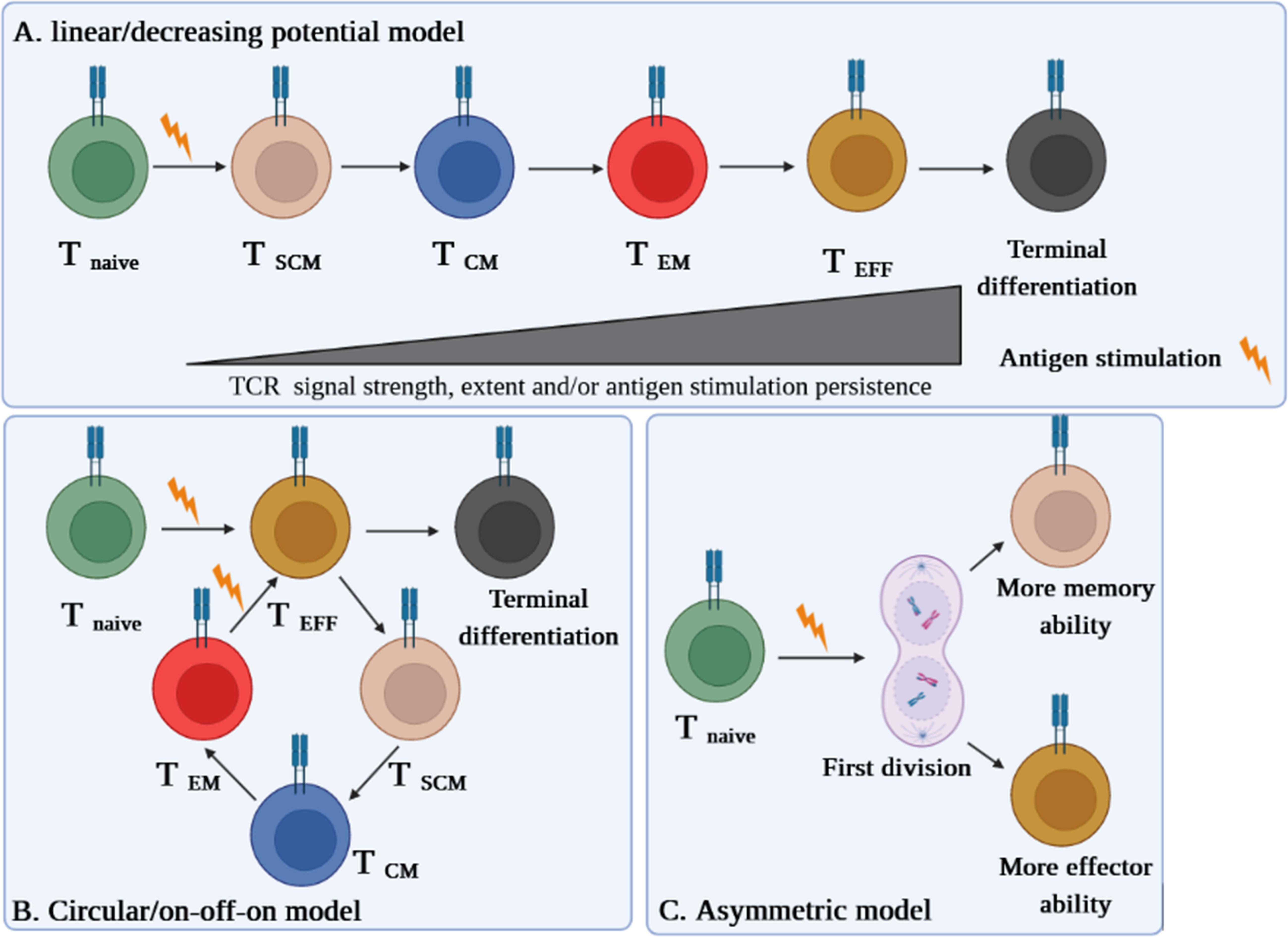
Figure 1 Three suggested models of TSCM cell differentiation. (A) The “linear model” stipulates that a T cell loses its memory strength and develops remarkable effector capabilities during differentiation based on the T-cell receptor (TCR) potential signal and extent and persistence of antigenic stimulation on the cells. (B) The “circular model” claims that effector T cells should emerge before memory T-cell dedifferentiation. (C) The “asymmetric division model” proposes that the formation of memory and effector T cells is predetermined from the first division via asymmetric distribution of key modulators between two daughter cells.
The “circular” or “on-off-on” model is opposite the linear one, as it explains that once T cells are exposed to an Ag, they differentiate into effector T cells, and upon the response contraction, the participated effector T cells dedifferentiate into different memory subsets until reencountering with the cognate Ag, by which the cells are able to remember and redifferentiate into the effector T cells (Figure 1B). This model could be relatively accepted in the context of some infectious diseases (6).
The third model proposes that the formation of memory and effector T cells is predetermined from the first division via asymmetric distribution of critical transcriptional and epigenetic modulators between two daughter cells whereby one acquires memory ability and another one develops its effector potency. This pattern, called the “asymmetric division model,” can be more conceivable in autoimmune diseases such as T1D in which the full activation signal is received by one daughter cell, while the weak stimulation signal is picked up by another one (Figure 1C) (6, 10).
Likewise, in other immune cells, immunophenotyping is a helpful technique to identify the TSCM cell subset (3). The subset is characterized in humans and NHPs by expressing the combination of effector and memory T-cell markers, including CD45RA+, CD45RO–, CD27+, CD28+, CCR7+, CD62L+, CD95+, CD122+, and CD127+ (1, 2, 4). Nevertheless, CD45RA+, CD45RO–, CD27+, CD28+, CCR7+, CD62L+, and particularly CD95+ are considered distinctive markers between naive and TSCM cells (Figure 2) (1, 2, 4). The equivalent markers of TSCM cells in mice are known as CD62L+, stem cell marker (Sca-1)+, CD122+, antiapoptotic marker molecule (Bcl-2)+, CCR5+, and CXCR3+ (Figure 2). Given that memory T cells are typically identified by CD45RO+ and CD27+, contrary to effector T cells expressing CD45RA (1–4), the markers CD27+ and CD45RA+ by TSCM cells indicate both memory and effector abilities of this subset (4). CCR7 (through binding to CCL19 and CCL21) along with CD62L drive TSCM cells to SLO homing. Not only does CD57 expression reflect telomere shortage (cell senescence) due to repetitive proliferation, but it also enhances either degranulation ability or inflammatory cytokine secretion of TSCM cells (1, 2, 4). Cell imaging techniques showed that normal T cells that express CXCR3 have higher proliferation, multipotency, and polyfunctionality alongside highly released cytokines tumor necrosis factor-alpha (TNF-α), IFN-γ, and IL-2, which are also seen in TSCM cells (2, 4). Obviously, the chemokine receptor helps TSCM cells in lymph node homing (2, 4). Moreover, the expression of CD122 (IL-2R) and CD127 (IL-7R) on TSCM cells indicates that IL-2, IL-15, and IL-7 play critical roles in boosting proliferation and survival of the cells (1–4). The function of TSCM cell human markers has been expressed in Table 1.
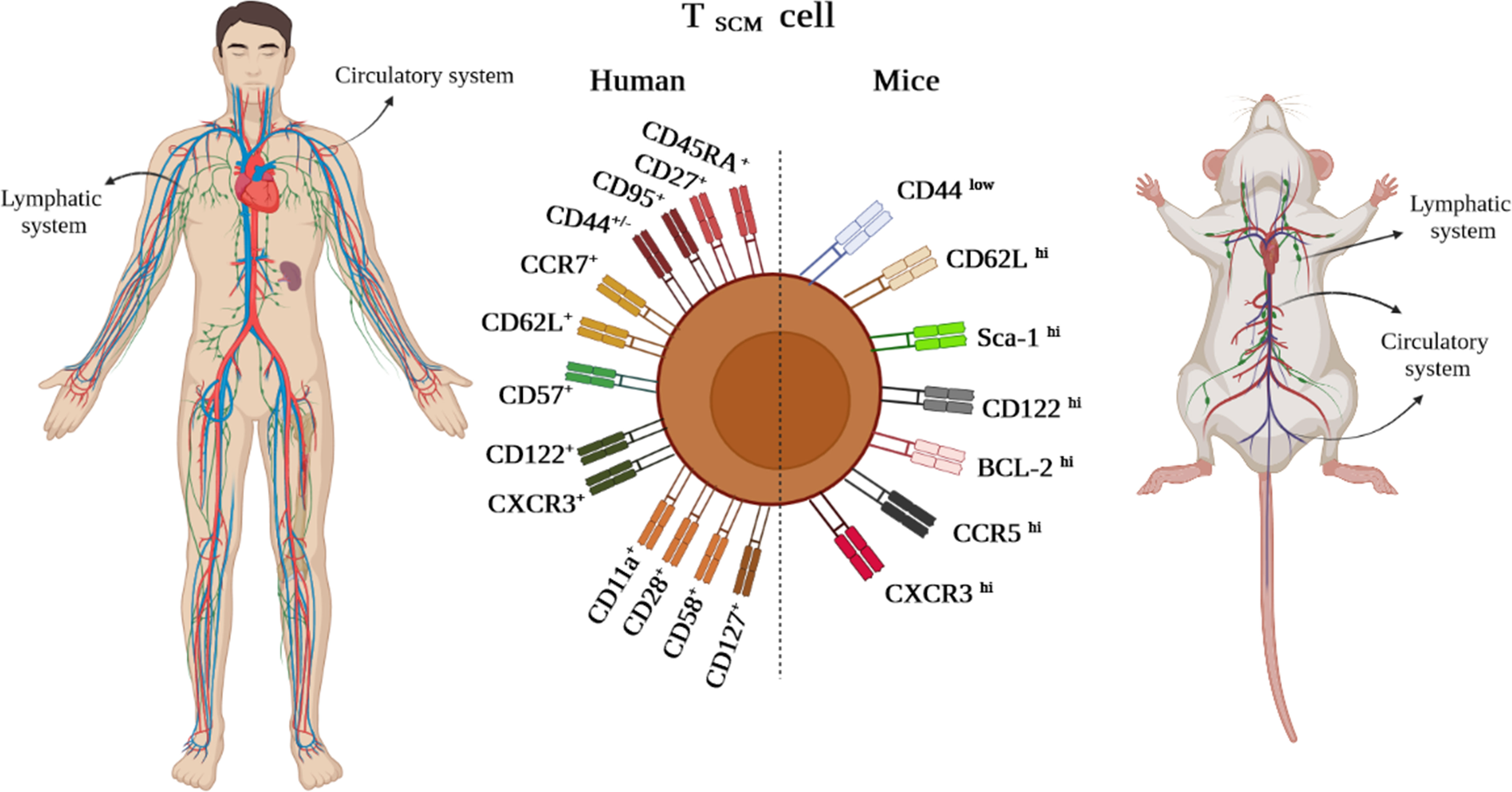
Figure 2 TSCM cell phenotypic expression markers in humans and mice existing in lymphatic and circulatory systems.
Although it has been demonstrated that human TSCM cells are capable of immediately releasing TNF-α, IFN-γ, perforin, and high amounts of IL-2, Zhang et al. demonstrated that murine TSCM cells are not able to produce cytotoxic molecules and IFN-γ (1, 5). Gene expression profiling studies revealed alterations in the genes expressed in human and mouse TSCM cells. Among them, higher expression levels of the transcription factors including TCF-1/LEF, Forkhead box protein O1 (FOXO-1), the inhibitor of DNA binding-3 (Id-3), and B-cell lymphoma-6 (BCL-6) were reported in TSCM cells. In contrast, the levels of T-bet, B lymphocyte-induced maturation protein 1 (BLIMP-1), signal transducer and activator of transcription (STAT)-4, inhibitor of DNA binding-2 (Id-2), Eomes, and zinc finger E-box binding homeobox 2 (ZEB-2) genes were revealed to be partially low (Figure 3A). Notably, TCF-1/LEF, Eomes, and Id-3 are regarded as master regulators of the wnt-β-catenin signaling pathway (1, 2, 4, 13).
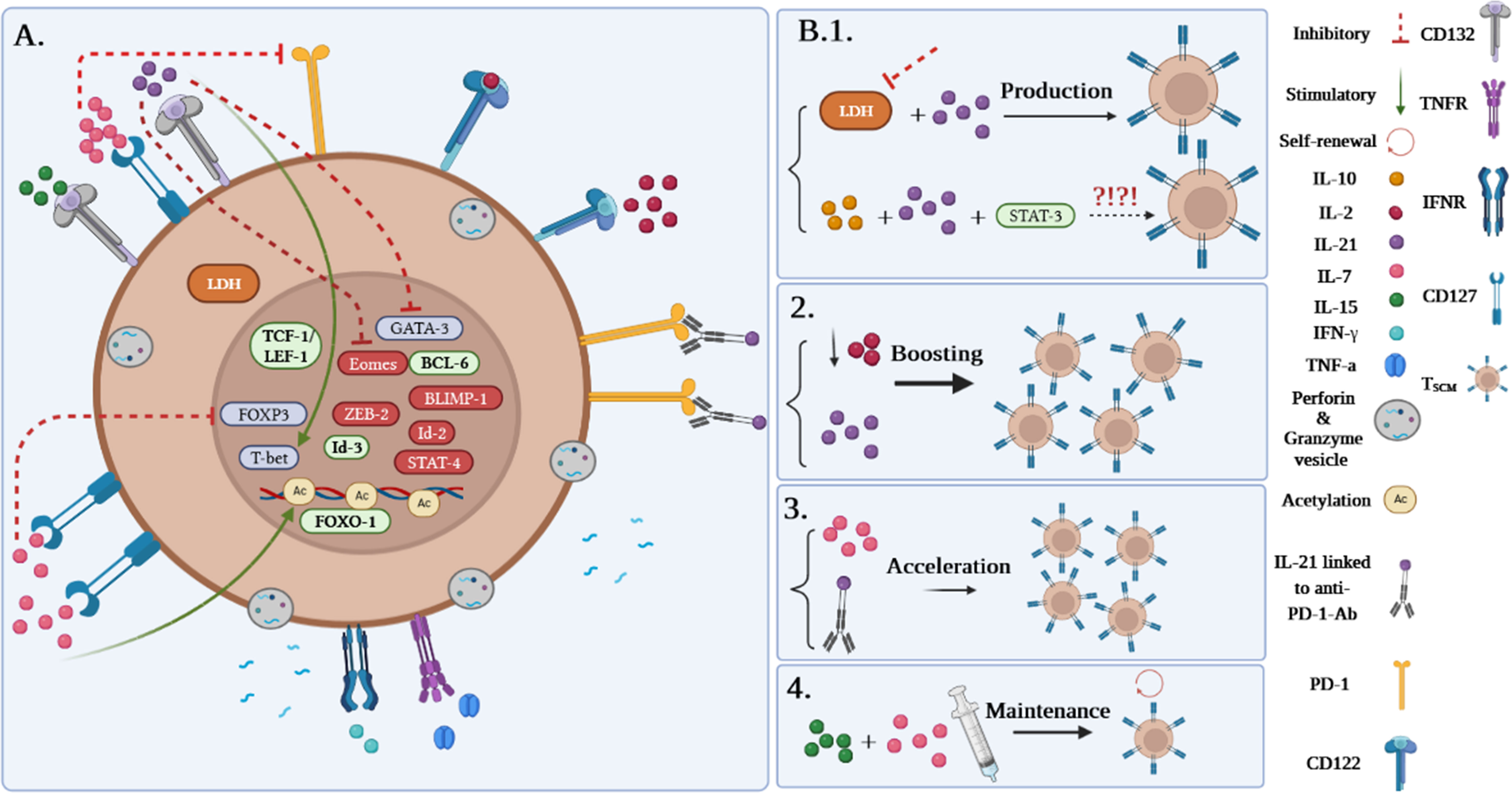
Figure 3 (A) Gene profile and cytokine receptor expression on TSCM cells. (B1–B4) Different approaches to produce TSCM cells.
Among the molecules involved in the development of TSCM, it has been suggested that inhibition of lactate dehydrogenase (LDH) along with IL-21 could be effective in this process. Notably, pieces of literature reported that TSCM cells can be generated via the IL-10-IL-21-STAT-3 signaling pathway (Figure 3B.1) (4, 14); however, it is unclear how the IL-10-IL-21-STAT-3 signaling pathway plays a proliferative role in this cell subset production. Although IL-2 is defined as the most prevalent growth factor for T cells, it has been demonstrated that high concentrations of IL-2 lead to the expansion of effector T-cell subsets while decreasing early memory T-cell generation through abating TCM cell populations (1, 4). Some studies reported that lower levels of IL-2 in the presence of IL-21 could improve the early memory T-cell proliferation and also could be effective in boosting TSCM cell populations (Figure 3B.2) (1, 4). Additionally, IL-7 can accelerate the proliferation of TSCM cells through different mechanisms (Figures 3A, B.3). These mechanisms consist of 1) inhibition of programmed cell death-1 (PD-1) and forkhead box p3 (FoxP3) expressions, 2) epigenetic modification through histone acetylation of gene promoters of effector T cells in order to convert to “naive-revertant cells” that can be phenotypically considered TSCM cells, and, more importantly, 3) maintenance of TSCM cell’s phenotype by IL-7 and IL-15 supplementation in the cell culture (Figure 3B.4). Similar to IL-7, IL-21 can directly and indirectly enhance TSCM cell populations (4). According to Chen et al., IL-21 coincidently upregulates T-bet and suppressor of cytokine signaling gene expression and downregulates Eomes and GATA binding protein 3 to promote TSCM cell proliferation (Figure 3A) (4, 14). Another study has also demonstrated that IL-21 linked to anti-PD-1 antibody (Ab) can prompt TSCM cell development (Figure 3B.3) (4, 15).
Human CD8+ TSCM cells express not only the surface antigens related to naive T lymphocytes (CD45RO−CD62L+CCR7+) but also markers exclusive for the memory subclass, including CD95, CXCR3, lymphocyte function-associated antigen 1 (LFA-1), and the β chain of IL-2 and IL-15 receptors (16). These cells enjoy similar superior potential for self-renewal and multipotentiality realized in CD4+ TSCM (17). CD8+ TSCM cells also show a robust response to IL-7 and possess a memory function that causes instant cytokine release after TCR stimulation (18). Meanwhile, from a comparative point of view, in vivo studies show that CD4+ TSCM cells favorably respond to IL-7, while CD8+ TSCM cells are amplified by both IL-7 and IL-15 (19). Gattinoni et al. (20) found that the glycogen synthase kinase-3β (GSK-3β) inhibitor TWS119 or Wnt3a prompts the formation of CD8+ TSCM cells through the Wnt/β-catenin/TCF-1 signaling pathway. However, CD8+ and CD4+ TSCM cells proliferated more efficiently once cocultured with anti-CD3/anti-CD28 conjugated beads alongside low concentrations of IL-7 and IL-15 in comparison with TWS119 exposure (20). Likewise, the generation of CD8+ TSCM cells can be improved by IL-21 through the Janus kinase 2 (JAK-2)/STAT-3 pathway (14). Nevertheless, suppressors of cytokine signaling (SOCS) can restrict the impacts of these cytokines. For instance, upon activation of SOCS1, the formation of TSCM cells from naive T cells via IL-21 induction is intensely inhibited (21).
Despite sporadic literature on the characterization of the CD4+ subset, a study revealed that CD4+ TSCM cells are sensitive to the external environment. That is, during aging and chronic infections, the numbers and the functions of CD4+ TSCM cells defect in the circulation. Inflammation can also affect CD4+ TSCM cells via the induction of Wnt/β-catenin signaling, which culminates in an improved CD4+ TSCM cell proliferative rate. Thus, the TSCM differentiation by promotion of the Wnt/β-catenin pathway with a high concentration of agonist drives the acquisition of a CD4+ TSCM phenotype (22).
Naive T cells are inactive in the peripheral blood and have low metabolic supplies. Thus, they principally use oxidative phosphorylation (OXPHOS) to produce ATP. However, differentiated T cells employ glycolysis to multiply, whereas memory T cells tend to benefit from fatty acid oxidation-dependent OXPHOS to provide ATP, which aids in performing prolonged immune response and heightened longevity (23, 24). Another study on T1D reported that in vitro formation of TSCM cells from naive T cells occurred under IL-7 stimulation through overexpression of the glucose transporter GLUT1 to sustain glycolysis and subsequent oxidation of pyruvate in the mitochondria. Thus, targeting glucose metabolism by means of the selective inhibitor for GLUT1 (WZB117) can efficiently diminish the TSCM differentiation in T1D subjects (25). Pilipow et al. (26) displayed that circulating T SCM cells possess an important reservoir of reduced glutathione (GSH) ex vivo and limiting ROS with antioxidants in activated CD8+ T cells stops terminal differentiation while permitting the generation of long-lived TSCM cells. In this case, N-acetylcysteine was capable of inducing CD8+ TSCM cells from naive T precursors in vitro (26). In the research conducted by Kondo et al., (27) it has been observed that coculturing T cells with stromal OP9 cells expressing the NOTCH ligand professionally differentiated conventional human T cells into TSCM cells through mitochondrial metabolic reprogramming. NOTCH signaling along with its downstream target, forkhead box M1 (FOXM1), stimulated mitochondrial biogenesis and fatty acid synthesis during TSCM formation, notifying that NOTCH/FOXM1 pathway might be a beneficial target for TSCM cell formation via metabolic alternations (27).
It is stated that imperative transcription factors and cytokines, accompanied by some inhibitors during the process of T-cell differentiation, stimulate TSCM cell production through regulating T cell-related metabolic enzymes (28, 29). Good et al. (30) have declared that blocking the mTOR pathway such as inhibitors of Bruton’s tyrosine kinase (BTK) and IL-2-inducible T-cell kinase (ITK) can direct T cells to TSCM cell differentiation (31). Scholz et al. (31) achieved that inhibition of mTOR complex 1 (mTORC1) by rapamycin or TWS119 in activated human naive T cells eventuates in the induction of TSCM cells via T-cell metabolism alternation toward fatty acid oxidation. As rapamycin is routinely used in the treatment of autoimmune diseases (32), it seems that the usage of rapamycin may adversely exaggerate autoimmune diseases in the long run. Additionally, recent evidence indicates that TSCM cells can be induced by Mek1/2 inhibitor (Meki) through regulating the metabolism regardless of affecting TCR-mediated activation (33). These studies point out that the regulation of metabolism and glycolysis is the fundamental factor in prompting the TSCM formation. Hence, targeted metabolic checkpoints can bring about T cells differentiating into memory and afford more fresh T cells for immunotherapy.
Undoubtedly, memory T cells are one of the principal culprits in the pathogenesis of autoimmune diseases. TSCM cells are responsible for developing long-term defensive immunity against different foreign Ags involving viral, bacterial, parasitic, and, in particular, tumor-associated Ags (1, 4). Since tumor-associated Ags mainly derive from self-Ags, once TSCM cells trigger antitumor responses, it can ultimately cause autoimmune diseases (1, 2, 4). Notably, Hosokawa et al. (34) and our team separately showed that TSCM cells are the least exhausted population than other memory T-cell subsets, which may be due to self-renewal potency and possessing high-length telomeres (34, 35). This evidence may justify the chronic and progressive hallmarks of autoimmune disorders. Meanwhile, Hosokawa et al. (34) exposed that upregulation of PD‐1 on CD8+ TSCM cells in aplastic anemia patients parallel to elevated IFN-γ secretion could be an indicator of autoreactive CD8+ TSCM cell’s clonal expansion. PD‐1, as one of the core costimulatory molecules (36), is expressed on various immune cells, in particular, exhausted and activated T cells to derive inhibitory signals, modulate T-cell response, and maintain peripheral tolerance (36, 37). Recent studies demonstrated that CD4+ and/or CD8+ TSCM cells play a vital role in the pathogenesis of autoimmune diseases such as systemic lupus erythematosus (SLE) (34, 38), aplastic anemia (AA) (34), autoimmune uveitis (34), T1D (25, 35), rheumatoid arthritis (RA) (39, 40), and immune thrombocytopenia (ITP) (41). However, the question whether the increased frequency of TSCM cells results from immune activation or vice versa remains unanswered. Most investigators in the context of autoimmune diseases consistently speculated that due to the ability of TSCM cells to recreate all memory and effector T-cell subsets, the increased frequency of TSCM cells could lead to autoimmune disease progression. Therefore, TSCM cell subsets can be a potential biomarker for autoimmune diseases and their response prediction (25, 34, 35, 38–41). Here, we explain the role of TSCM cells in some autoimmune diseases (Table 2).
Acquired AA is a rare condition of bone marrow failure syndrome in which hematopoietic stem/progenitor cells (HSPCs) are destroyed by mechanisms unrelated to the inherited syndrome (42). Despite the exact pathophysiology mechanism of AA still being blurred and the specificity of some recognized auto-Ags such as diazepam-binding related protein-1 not being proven in vivo, the immune attack to allogeneic hematopoietic cells by autoreactive T cells is considered as an underlying mechanism of autoimmunity in AA (43). Some features of autoreactive T cells in AA encouraged Hosokawa et al. (34) to explore the role of TSCM cells in the immunopathogenesis of AA. It has been shown that the recognition of HSPC-restricted Ags through major histocompatibility complex (MHC) class I or II by oligoclonal CD8+ autoreactive T cells leads to pro-inflammatory cytokine secretion like IFN-γ against HSPC cells. Following immunosuppressive therapy (IST) with anti-thymocyte globulin (ATG) and cyclosporine A (CsA), the regeneration of oligoclonal T cells and even the new ones can occur (34). Accordantly, Hosokawa et al. (34) displayed that, in comparison to healthy individuals, the rate of CD8+ TSCM cells in AA patients was higher at the onset of diagnosis and after IST in responder (complete and partial response) and non-responder patients, respectively. This indicates a favorable response to IST when CD8+ TSCM cell frequency is high at the time of diagnosis in responders and inversely bringing about disease aggravation in non-responders to IST. Moreover, their intracellular staining revealed that both CD4+ and CD8+ TSCM cells have more elevated levels of IL-2 and IFN-γ than those in healthy controls (34).
AU is an organ-specific disorder in which immune cells and, in particular, CD4+ and CD8+ Ag-specific memory T cells reside within the ocular tissue (44). The study of Hosokawa et al. (34) on AU patients showed that CD8+ TSCM cell frequencies in these patients are significantly higher than those in healthy individuals. They also displayed that IST (combination of prednisolone and anti-TNF-α antibody) can be effective in reducing the CD8+ TSCM cell population in patients with AU (34), suggesting CD8+ TSCM cells as a potential marker associating with a better response to IST following lower frequency of the cells.
Systemic lupus erythematosus (SLE) is a complex autoimmune disease affecting multiple organs. Despite that the pathogenesis of SLE is still not fully understood, it is believed that factors, including environmental, hormonal, genetic, and immunological, serve a role in SLE development. Among others, the role of immunological dysregulation seems more prominent. In fact, immunological dysregulation can disrupt the balance of T helper (TH)1/TH2 and TH17/T regulatory (Treg) cell and ultimately shift them toward autoreactive T cells (45). T follicular helper (TFH) cells also interact with B cells, to boost their autoantibody (auto-Ab) production (38, 45). Auto-Abs and autoreactive T-cell activity are considered the major hindrance to achieving complete remission in SLE patients, despite long-lasting IST that indicates the emergence of the TSCM cell population in SLE patients (38, 45). Recently, Hosokawa et al. (34) demonstrated that the CD4+ TSCM cell population in SLE patients is lower than that in healthy controls due to receiving IST during sampling (34). Furthermore, Lee et al. (38) found that CD4+ and CD8+ TSCM cell frequencies in SLE patients were remarkably elevated than those in the controls. They also observed that the CD4+ TSCM cells of SLE patients can differentiate into TFH cells through BCL6, CXCR5, PD1, ICOS, LEF1, TCF-1, and IL-21 (as TFH cell inducer) gene overexpression, and BLIMP-1 gene encoding downregulation, leading to pathogenic auto-Ab formation (38). Strikingly, they declared that the CD4+ TSCM cell population in SLE patients participates in the inflammatory process by producing higher levels of TNF-α, IFN-γ, IL-2, and IFN-α than those in normal individuals (38).
ITP is an acquired autoimmune disease that is characterized by platelet devastation due to T-cell dysfunction (46). Indeed, autoreactive T cells, including TH1 and TH17, give rise to auto-Ab production against platelets, and cytotoxic CD8+ T cells invade the surface glycoprotein GPIIb/IIIa of platelets. Additionally, the number and function of CD4+CD25+Treg cells decrease, resulting in the development and progression of ITP (46). In the investigation conducted by Cao et al., (41) it was disclosed that the population of CD8+ TSCM cells in ITP patients outnumbered that of controls. Moreover, they found that prednisolone prescription, an IST, reduces the CD8+ TSCM cell frequency and alleviates platelet destruction in responder groups (complete and partial) (41). They suggested that the higher frequency of this T-cell memory subset can lead to ITP exacerbation (41).
T1D is another chronic autoimmune disease in which autoreactive T cells attack β-cell auto-Ags such as glutamic acid decarboxylase 65 (GAD65), (pro)insulin, and islet-specific glucose-6-phosphatase catalytic subunit-related protein (IGRP) (25). Some autoreactive T cells in T1D patients exhibit distinguishable characteristics, including memory marker expression like CD95 and the presence of IL-7, which prolongs the survival and maintenance of autoreactive T cells, particularly TSCM cells. More importantly, similar to other autoimmune diseases, a high dosage of IST during and after pancreas transplantation fails to eliminate autoreactive memory T cells in these patients (25). The mechanism of TSCM cells in T1D is explainable by the “asymmetric division model” by which activation of T cells through β-cell auto-Ag presentation in an immune synapse between DC and naive T-cell undertakes mitosis simultaneously in the immune synapse. After polarization, the daughter cell receiving a continuous strong signal from the immune synapse becomes fully activated and consequently differentiates into CD25hi CD127low effector T cell (6). Another daughter cell with a faint signal expressing CD25low CD127hi (TSCM cell) migrates via chemokine receptor CXCR-4 upregulation linking to CXCL-12 (stromal derived factor-1) into bone marrow (BM) where the stromal cells immensely produce IL-7 (6). A hemostatic cytokine IL-7 not only upregulates CXCR-4 on T cells but also assists TSCM cells in self-renewal. It is postulated that the T1D autoreactive T cells arrested in BM and decreased their turnover by IL-7 can reconstitute specific effector and memory autoreactive T cells (Figure 4) (6). Vignali et al. (25) measured the frequency of autoreactive CD8+ TSCM cells against GAD65, insulin, and IGRP in new-onset (<6 months T1D) and long-term (>20 years T1D) patients. They observed that the frequency of circulating specific CD8+ TSCM cells against GAD65 and insulin in the new-onset patients was significantly higher than that in healthy controls (25). They also found that IL-7 can considerably amplify their frequency by GLUT-1 upregulation in T1D patients compared to the controls (25). Our study revealed that in new-onset (<1 year T1D), the frequency of CD4+ TSCM cells is noticeably higher than that in long-term (>5 years T1D) and normal individuals (35). Therefore, both studies stated that TSCM cell subsets can lead to disease progression (25, 35).
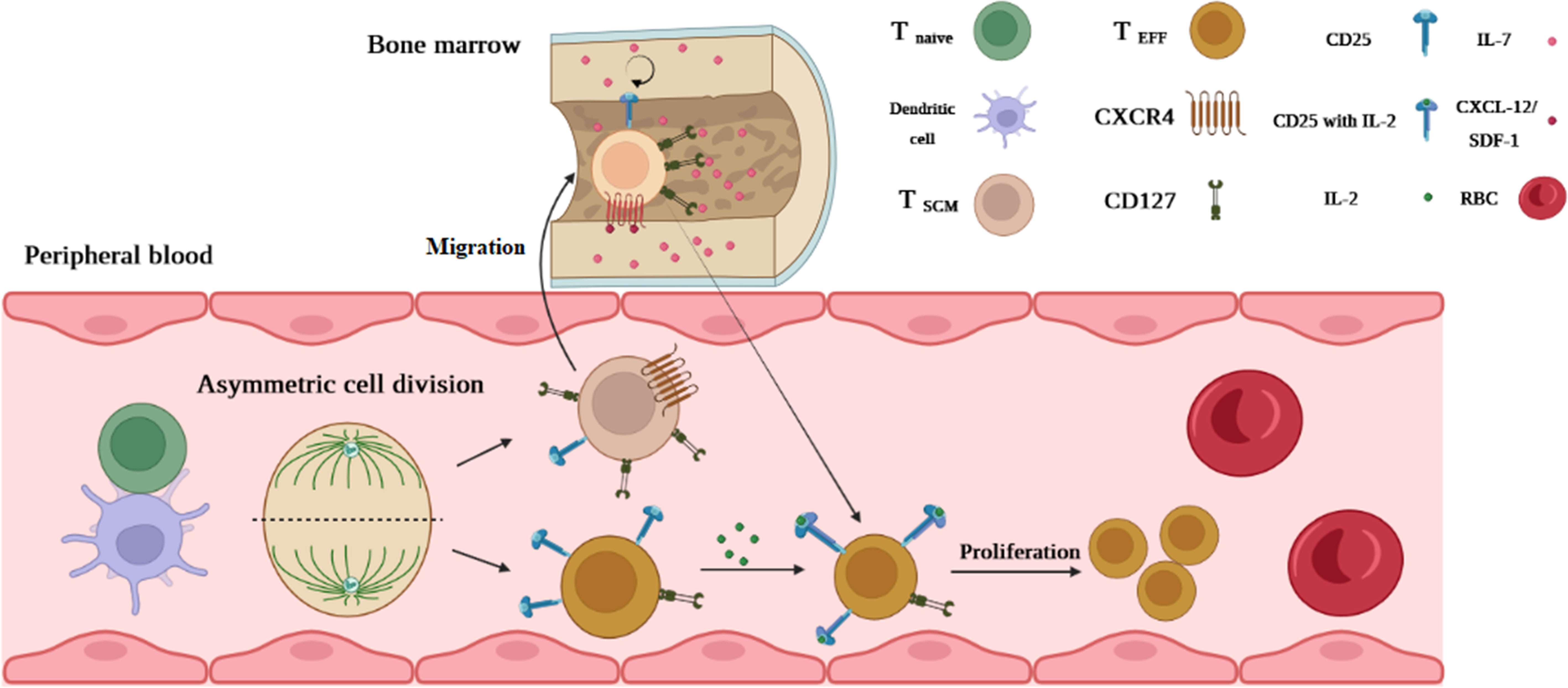
Figure 4 The possible mechanism of TSCM cell generation in T1D. An activated T cell undergoes mitosis coincidentally in the immune synapse forming between DC and naive T cells. After polarization, one of which becomes a fully activated daughter cell (because of permanent interaction with DC and receiving stimulatory signals) differentiating into CD25hi CD127low effector T cells. Another daughter cell (resting cell) without receiving a full-activation signal expressing CD25low CD127hi (TSCM cell) migrates via chemokine receptor CXCR-4 upregulation linking to CXCL-12 into the bone marrow (BM). IL-7 in BM assists TSCM cells in self-renewal by reducing their turnover. Once a second Ag stimulation occurs, T1D TSCM cells that arrested in BM can reconstitute specific effector and memory autoreactive T cells.
RA is a chronic inflammatory autoimmune disease in which synovial fluid (SF) is predominantly targeted by autoreactive T cells and leads to arthropathy (47). Besides autoreactive CD4+ T cells serving a key role in the immunopathogenesis of RA through their TH1, TH17, and TFH cell subsets, autoreactive CD8+ T cells are observed in the SF of new-onset patients (40). The study performed by Takeshita et al. (40) showed that the frequency of CD4+ and CD8+ TSCM cells elevated in RA patients’ peripheral blood and SF. Moreover, they found that among immunosuppressant drugs, not only does methotrexate (MTX) decline TSCM cell frequency, but it also reduces other T-cell subsets (except for TFH cells), resulting in MTX’s suppression effect on T-cell proliferation pathways including E2F, IL-2-STAT5, and mTORC1 (40). Consistent with this study, Cianciotti et al. (39) displayed that the citrullinated vimentin (Cit-vimentin)-specific CD4+ TSCM cell population is higher in the circulation of RA patients than that in controls. They also found that TNF-α blockade can attenuate this subset frequency and prohibit differentiation of circulating TH17 from CD4+ TSCM cells through blocking TNFII receptor (TNFRII) signaling, suggesting TNF-α as a prosurvival factor for TSCM cells. Thereby, both mentioned studies implicated that the frequency of TSCM cells can be a beneficial marker for disease development and response prediction (39).
Chronic hepatitis C virus (HCV) is one of the most important viruses related to autoimmune diseases that can inflict destructive effects on the liver, thyroid tissue, and platelets (48). Although the disease etiology is still not clarified, TSCM cells, as a less-differentiated memory T-cell subset, play a fundamental role in the long-term immune defense against HCV (49, 50). Lu et al. (49) observed that the CD8+ TSCM cell population was raised in both monoinfected HCV and coinfected HCV/HIV patients. Their investigation also showed that the CD8+ TSCM cell population respectively has direct and indirect correlations with TCM cells and TEM cells, which can help maintain T-cell hemostasis (49). Moreover, they reported that a high incidence of CD8+ TSCM cells can effectively control HCV replication in monoinfected HCV patients, indicating that the CD8+ TSCM cell population has a protective impact in HCV infection and paving the way for TSCM cell-based vaccine design to attain HCV clearance (49).
Sickle cell disease (SCD) is a nonspecific chronic inflammation that may occur due to environmental factors like transfusions leading to red blood cell (RBC) deformation, hemolysis, and vaso-occlusion development (34, 51, 52). Indeed, the physicochemical alterations of RBCs culminate in hemolysis and erythrocyte rupture, which in turn trigger the inflammatory responses and lymphocyte activation through necrotic particle production (52). Although SCD is not recognized as an autoimmune disease, Hosokawa et al. (34) surprisingly observed that the CD8+ TSCM cell frequency in SCD was significantly higher in comparison with controls despite their limited sample size (five patients). This can be justified by previous studies that demonstrated that permanent inflammation in SCD likely induces memory T-cell formation alongside various pro-inflammatory and inflammatory cytokines such as IL-2, IL-7, and IL-15 (51), requiring cytokines for CD8+ TSCM cell generation (1, 4). Meanwhile, future studies are essential to definitively prove our explanation.
The emerging role of TSCM cells in the pathogenesis of autoimmune diseases presents new opportunities for prevention or even treatment of these diseases. Eliminating TSCM cells, which are detected in high levels in various autoimmune disorders, can improve the efficacy of immunosuppressive therapeutics and alleviate autoreactive symptoms. Molecular regulation of the proliferation, metabolic behavior, and self-renewal of TSCM cells can provide promising targets for treating autoimmune illnesses. In this regard, pharmaceutical inhibition of Wnt-β-catenin signaling, which is a crucial driver for the induction of TSCM cells (20), might limit the expansion of these cells. Notwithstanding, few attempts have been made to target these key molecules in research. Implicating current treatment approaches, including adoptive cell transfer and gene therapy, will be noteworthy in the setting of targeted therapy of molecules restricting TSCM cell generation. Ongoing studies might open new doors in this era for the treatment of autoimmune diseases.
On the other side, it is unclear what the limitations of manipulating TSCM cells are in the setting of autoimmune diseases. Some questions that will arise include whether downregulation of these cells to treat autoimmunity induces detrimental effects such as the development of tumors and infections or how much these cells should be reduced and after how long will the amount of memory cells be restored in the context of various autoimmune diseases. Whether the manipulation of TSCM cells will affect the function of other T cells in the circulation. It is speculated that targeting of TSCM cells in autoimmune diseases is a form of personalized medicine, which can be prescribed based on the patient’s age, weight, and disease condition. Further experiments are necessary to answer various questions surrounding the targeting of TSCM cells in autoimmune diseases.
TSCM cells possess a unique capability for enhanced self-renewal and multidifferentiation along with performing effector functions. Given that TSCM cells are newly discovered T cells that play roles in autoimmune diseases, gaining a deep understanding of their importance in the development and progression of autoimmune disorders may be at the forefront of research interests. Future attempts are also needed to analyze transcriptome profiles and effector molecules of TSCM cells, with a precise exploration of pathways determining T-cell differentiation and function, and suggest strategies targeting specific molecules in the control or treatment of autoimmune disorders.
All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Gao S, Liang X, Wang H, Bao B, Zhang K, Zhu Y, et al. Stem cell-like memory T cells: a perspective from the dark side. Cell Immunol (2021) 361:104273. doi: 10.1016/j.cellimm.2020.104273
2. Wang Y, Qiu F, Xu Y, Hou X, Zhang Z, Huang L, et al. Stem cell-like memory T cells: the generation and application. J Leukoc Biol (2021) 110(6):1209–23. doi: 10.1002/JLB.5MR0321-145R
3. Gattinoni L, Speiser DE, Lichterfeld M, Bonini C. T Memory stem cells in health and disease. Nat Med (2017) 23(1):18–27. doi: 10.1038/nm.4241
4. Li Y, Wu Y, Yang X, Zhou S. Immunotherapeutic potential of T memory stem cells. Front Oncol (2021) 11:723888. doi: 10.3389/fonc.2021.723888
5. Zhang Y, Joe G, Hexner E, Zhu J, Emerson SG. Host-reactive CD8+ memory stem cells in graft-versus-host disease. Nat Med (2005) 11(12):1299–305. doi: 10.1038/nm1326
6. Kedzierska K, Koutsakos M. The ABC of major histocompatibility complexes and T cell receptors in health and disease. Viral Immunol (2020) 33(3):160–78. doi: 10.1089/vim.2019.0184
7. Abdelsamed HA, Moustaki A, Fan Y, Dogra P, Ghoneim HE, Zebley CC, et al. Human memory CD8 T cell effector potential is epigenetically preserved during in vivo homeostasis. J Exp Med (2017) 214(6):1593–606. doi: 10.1084/jem.20161760
8. Buchholz VR, Schumacher TN, Busch DH. T Cell fate at the single-cell level. Annu Rev Immunol (2016) 34:65–92. doi: 10.1146/annurev-immunol-032414-112014
9. Henning AN, Roychoudhuri R, Restifo NP. Epigenetic control of CD8+ T cell differentiation. Nat Rev Immunol (2018) 18(5):340–56. doi: 10.1038/nri.2017.146
10. Monti P, Heninger A-K, Bonifacio E. Differentiation, expansion, and homeostasis of autoreactive T cells in type 1 diabetes mellitus. Curr Diab Rep (2009) 9(2):113–8. doi: 10.1007/s11892-009-0020-y
11. Schumann J, Stanko K, Schliesser U, Appelt C, Sawitzki B. Differences in CD44 surface expression levels and function discriminates IL-17 and IFN-γ producing helper T cells. PLoS One. (2015) 10(7). doi: 10.1371/journal.pone.0132479
12. Lee J-B, Chang J. CD43 expression regulated by IL-12 signaling is associated with survival of CD8 T cells. Immune Netw (2010) 10(5):153–63. doi: 10.4110/in.2010.10.5.153
13. Zehn D, Thimme R, Lugli E, de Almeida GP, Oxenius A. ‘Stem-like’precursors are the fount to sustain persistent CD8+ T cell responses. Nat Immunol (2022) 23(6):836–47. doi: 10.1038/s41590-022-01219-w
14. Chen Y, Yu F, Jiang Y, Chen J, Wu K, Chen X, et al. Adoptive transfer of interleukin-21-stimulated human CD8+ T memory stem cells efficiently inhibits tumor growth. J Immunother (Hagerstown Md: 1997) (2018) 41(6):274. doi: 10.1097/CJI.0000000000000229
15. Li Y, Cong Y, Jia M, He Q, Zhong H, Zhao Y, et al. Targeting IL-21 to tumor-reactive T cells enhances memory T cell responses and anti-PD-1 antibody therapy. Nat Commun (2021) 12(1):951. doi: 10.1038/s41467-021-21241-0
16. Gattinoni L, Lugli E, Ji Y, Pos Z, Paulos CM, Quigley MF, et al. A human memory T cell subset with stem cell–like properties. Nat Med (2011) 17(10):1290–7. doi: 10.1038/nm.2446
17. Takeshita M, Suzuki K, Kassai Y, Takiguchi M, Nakayama Y, Otomo Y, et al. Polarization diversity of human CD4+ stem cell memory T cells. Clin Immunol (2015) 159(1):107–17. doi: 10.1016/j.clim.2015.04.010
18. Bishop EL, Gudgeon N, Dimeloe S. Control of T cell metabolism by cytokines and hormones. Front Immunol (2021) 12:653605. doi: 10.3389/fimmu.2021.653605
19. Lugli E, Gattinoni L, Roberto A, Mavilio D, Price DA, Restifo NP, et al. Identification, isolation and in vitro expansion of human and nonhuman primate T stem cell memory cells. Nat Protoc (2013) 8(1):33–42. doi: 10.1038/nprot.2012.143
20. Gattinoni L, Zhong X-S, Palmer DC, Ji Y, Hinrichs CS, Yu Z, et al. Wnt signaling arrests effector T cell differentiation and generates CD8+ memory stem cells. Nat Med (2009) 15(7):808–13. doi: 10.1038/nm.1982
21. Palmer DC, Restifo NP. Suppressors of cytokine signaling (SOCS) in T cell differentiation, maturation, and function. Trends Immunol (2009) 30(12):592–602. doi: 10.1016/j.it.2009.09.009
22. Kared H, Tan SW, Lau MC, Chevrier M, Tan C, How W, et al. Immunological history governs human stem cell memory CD4 heterogeneity via the wnt signaling pathway. Nat Commun (2020) 11(1):821. doi: 10.1038/s41467-020-14442-6
23. Pearce EL, Walsh MC, Cejas PJ, Harms GM, Shen H, Wang LS, et al. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature (2009) 460(7251):103–7. doi: 10.1038/nature08097
24. Lécuyer E, Rakotobe S, Lengliné-Garnier H, Lebreton C, Picard M, Juste C, et al. Segmented filamentous bacterium uses secondary and tertiary lymphoid tissues to induce gut IgA and specific T helper 17 cell responses. Immunity (2014) 40(4):608–20. doi: 10.1016/j.immuni.2014.03.009
25. Vignali D, Cantarelli E, Bordignon C, Canu A, Citro A, Annoni A, et al. Detection and characterization of CD8+ autoreactive memory stem T cells in patients with type 1 diabetes. Diabetes (2018) 67(5):936–45. doi: 10.2337/db17-1390
26. Pilipow K, Scamardella E, Puccio S, Gautam S, Paoli FD, Mazza EM, et al. Antioxidant metabolism regulates CD8+ T memory stem cell formation and antitumor immunity. JCI Insight (2018) 3(18):e122299. doi: 10.1172/jci.insight.122299
27. Kondo T, Ando M, Nagai N, Tomisato W, Srirat T, Liu B, et al. The NOTCH–FOXM1 axis plays a key role in mitochondrial biogenesis in the induction of human stem cell memory–like CAR-T CellsNotch–FOXM1 axis induces stem cell memory–like CAR-T cells. Cancer Res (2020) 80(3):471–83. doi: 10.1158/0008-5472.CAN-19-1196
28. Hu Z, Zou Q, Su B. Regulation of T cell immunity by cellular metabolism. Front Med (2018) 12:463–72. doi: 10.1007/s11684-018-0668-2
29. Mousset CM, Hobo W, Ji Y, Fredrix H, De Giorgi V, Allison RD, et al. Ex Vivo AKT-Inhibition Facilitates Generation of Polyfunctional Stem Cell Memory-Like CD8(+) T Cells for Adoptive Immunotherapy. Oncoimmunology (2018) 7(10):e1488565. doi: 10.1080/2162402X.2018.1488565
30. Good Z, Borges L, Vivanco Gonzalez N, Sahaf B, Samusik N, Tibshirani R, et al. Proliferation tracing with single-cell mass cytometry optimizes generation of stem cell memory-like T cells. Nat Biotechnol (2019) 37, 259–266. doi: 10.1038/s41587-019-0033-2
31. Scholz G, Jandus C, Zhang L, Grandclément C, Lopez-Mejia IC, Soneson C, et al. Modulation of mTOR signalling triggers the formation of stem cell-like memory T cells. EBioMedicine (2016) 4:50–61. doi: 10.1016/j.ebiom.2016.01.019
32. Xiang M, Kim H, Ho VT, Walker SR, Bar-Natan M, Anahtar M, et al. Gene expression–based discovery of atovaquone as a STAT3 inhibitor and anticancer agent. Blood J Am Soc Hematol (2016) 128(14):1845–53. doi: 10.1182/blood-2015-07-660506
33. Verma V, Jafarzadeh N, Boi S, Kundu S, Jiang Z, Fan Y, et al. MEK inhibition reprograms CD8+ T lymphocytes into memory stem cells with potent antitumor effects. Nat Immunol (2021) 22(1):53–66. doi: 10.1038/s41590-020-00818-9
34. Hosokawa K, Muranski P, Feng X, Townsley DM, Liu B, Knickelbein J, et al. Memory stem T cells in autoimmune disease: high frequency of circulating CD8+ memory stem cells in acquired aplastic anemia. J Immunol (2016) 196(4):1568–78. doi: 10.4049/jimmunol.1501739
35. Fazeli P, Talepoor AG, Faghih Z, Nasser Gholijani N, Ataollahi MR, Hassanzadeh MA, et al. The frequency of CD4+ and CD8+ circulating T stem cell memory in type 1 diabetes. Immun Inflamm Dis (2022) 10(10). doi: 10.1002/iid3.715
36. Fujisawa R, Haseda F, Tsutsumi C, Hiromine Y, Noso S, Kawabata Y, et al. Low programmed cell death-1 (PD-1) expression in peripheral CD4+ T cells in Japanese patients with autoimmune type 1 diabetes. Clin Exp Immunol (2015) 180(3):452–7. doi: 10.1111/cei.12603
37. Zhong T, Li X, Zhou Z. 1175-p: circulating CD4+/CD8+ PD-1+ T cells are increased during the partial remission phase in patients with type 1 diabetes. Diabetes Care (2019) 68(Supplement_1):. doi: 10.2337/db19-1175-P
38. Lee YJ, Park JA, Kwon H, Choi YS, Jung KC, Park SH, et al. Role of stem cell–like memory T cells in systemic lupus erythematosus. Arthritis Rheumatol (2018) 70(9):1459–69. doi: 10.1002/art.40524
39. Cianciotti BC, Ruggiero E, Campochiaro C, Oliveira G, Magnani ZI, Baldini M, et al. CD4+ memory stem T cells recognizing citrullinated epitopes are expanded in patients with rheumatoid arthritis and sensitive to tumor necrosis factor blockade. Arthritis Rheumatol (2020) 72(4):565–75. doi: 10.1002/art.41157
40. Takeshita M, Suzuki K, Kondo Y, Morita R, Okuzono Y, Koga K, et al. Multi-dimensional analysis identified rheumatoid arthritis-driving pathway in human T cell. Ann Rheum Dis (2019) 78(10):1346–56. doi: 10.1136/annrheumdis-2018-214885
41. Cao J, Zhang C, Han X, Cheng H, Chen W, Qi K, et al. Emerging role of stem cell memory-like T cell in immune thrombocytopenia. Scand J Immunol (2019) 89(3):. doi: 10.1111/sji.12739
42. Schoettler ML, Nathan DG. The pathophysiology of acquired aplastic anemia: current concepts revisited. Hematol Oncol Clin North Am (2018) 32(4):581–94. doi: 10.1016/j.hoc.2018.03.001
43. Zeng Y, Katsanis EJC, Immunology E. The complex pathophysiology of acquired aplastic anaemia. Clin Exp Immunol (2015) 180(3):361–70. doi: 10.1111/cei.12605
44. Lee RW, Nicholson LB, Sen HN, Chan CC, Wei L, Nussenblatt RB, et al. Autoimmune and autoinflammatory mechanisms in uveitis. In: Seminars in immunopathology. (Germany: Springer) (2014) 36(5):581–94. doi: 10.1007/s00281-014-0433-9
45. Pan L, Lu M-P, Wang J-H, Yang SR. Immunological pathogenesis and treatment of systemic lupus erythematosus. World J Pediatr (2020) 16:19–30. doi: 10.1007/s12519-019-00229-3
46. Swinkels M, Rijkers M, Voorberg J, Vidarsson G, Leebeek FWG, Jansen AJG. Emerging concepts in immune thrombocytopenia. Front Immunol (2018) 9:880. doi: 10.3389/fimmu.2018.00880
47. Firestein GS, McInnes IB. Immunopathogenesis of rheumatoid arthritis. Immunity (2017) 46(2):183–96. doi: 10.1016/j.immuni.2017.02.006
48. Pastore F, Martocchia A, Stefanelli M, et al. Hepatitis c virus infection and thyroid autoimmune disorders: a model of interactions between the host and the environment. World J Hepatol (2016) 8(2):83. doi: 10.4254/wjh.v8.i2.83
49. Lu X, Song B, Weng W, et al. CD8+ stem cell-like memory T cell subset is associated with disease progression in chronic hepatitis c virus infection. Viral Immunol (2022) 36(1):25–32. doi: 10.21203/rs.3.rs-1533048/v1
50. Asadipour M, Fazeli P, Zohouri M, Bemani P, Mohebbiniya M, Khansalar S, et al. IL-18 in blood serum of hepatitis c patients might be of predictive value for individual outcomes. Infect Disord Drug Targets (2021) 21(3):389–93. doi: 10.2174/1871526520666200707113401
51. Silva-Junior AL, Garcia NP, Cardoso EC, Dias S, Tarragô AM, Fraiji NA, et al. Immunological hallmarks of inflammatory status in vaso-occlusive crisis of sickle cell anemia patients. Front Immunol (2021) 12:559925. doi: 10.3389/fimmu.2021.559925
Keywords: T memory stem cell, autoimmune diseases, type 1 diabetes, rheumatoid arthritis, sickle cell disease, hepatitis C virus
Citation: Fazeli P, Kalani M and Hosseini M (2023) T memory stem cell characteristics in autoimmune diseases and their promising therapeutic values. Front. Immunol. 14:1204231. doi: 10.3389/fimmu.2023.1204231
Received: 11 April 2023; Accepted: 16 June 2023;
Published: 11 July 2023.
Edited by:
Emiliano Marasco, Bambino Gesù Children’s Hospital (IRCCS), ItalyReviewed by:
Qixiang Shao, Jiangsu University, ChinaCopyright © 2023 Fazeli, Kalani and Hosseini. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maryam Hosseini, SG9zZWluaXNtQHN1bXMuYWMuaXI=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.