- Hepatic Surgery Center, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
Background: The clinical value of postoperative adjuvant therapy (PAT) for hepatocellular carcinoma (HCC) remains unclear. This study aimed to explore the effect of PAT with tyrosine kinase inhibitors (TKIs) and anti-PD-1 antibodies on the surgical outcomes of HCC patients with high-risk recurrent factors (HRRFs).
Methods: HCC patients who underwent radical hepatectomy at Tongji Hospital between January 2019 and December 2021 were retrospectively enrolled, and those with HRRFs were divided into PAT group and non-PAT group. Recurrence-free survival (RFS) and overall survival (OS) were compared between the two groups after propensity score matching (PSM). Prognostic factors associated with RFS and OS were determined by Cox regression analysis, and subgroup analysis was also conducted.
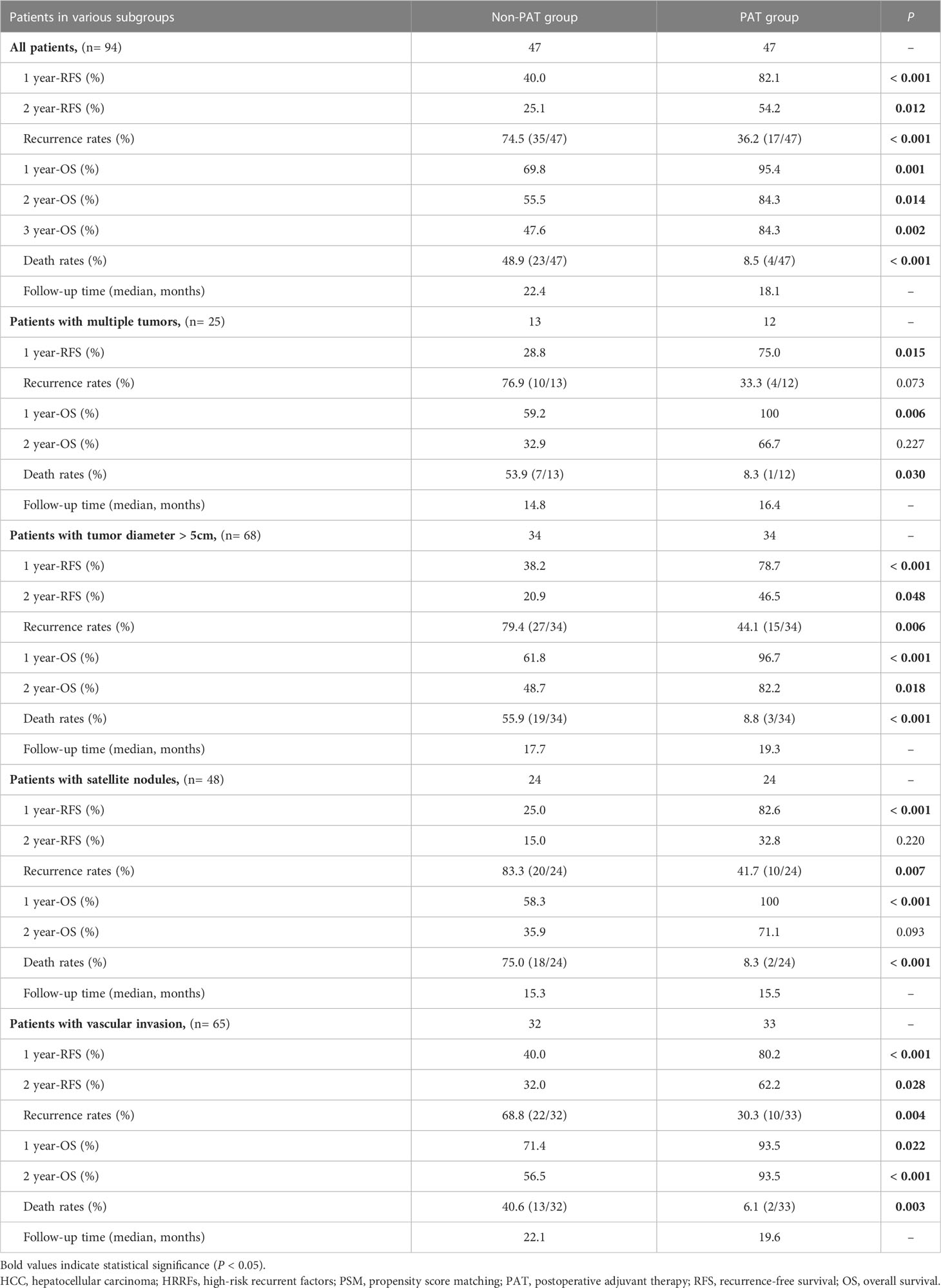
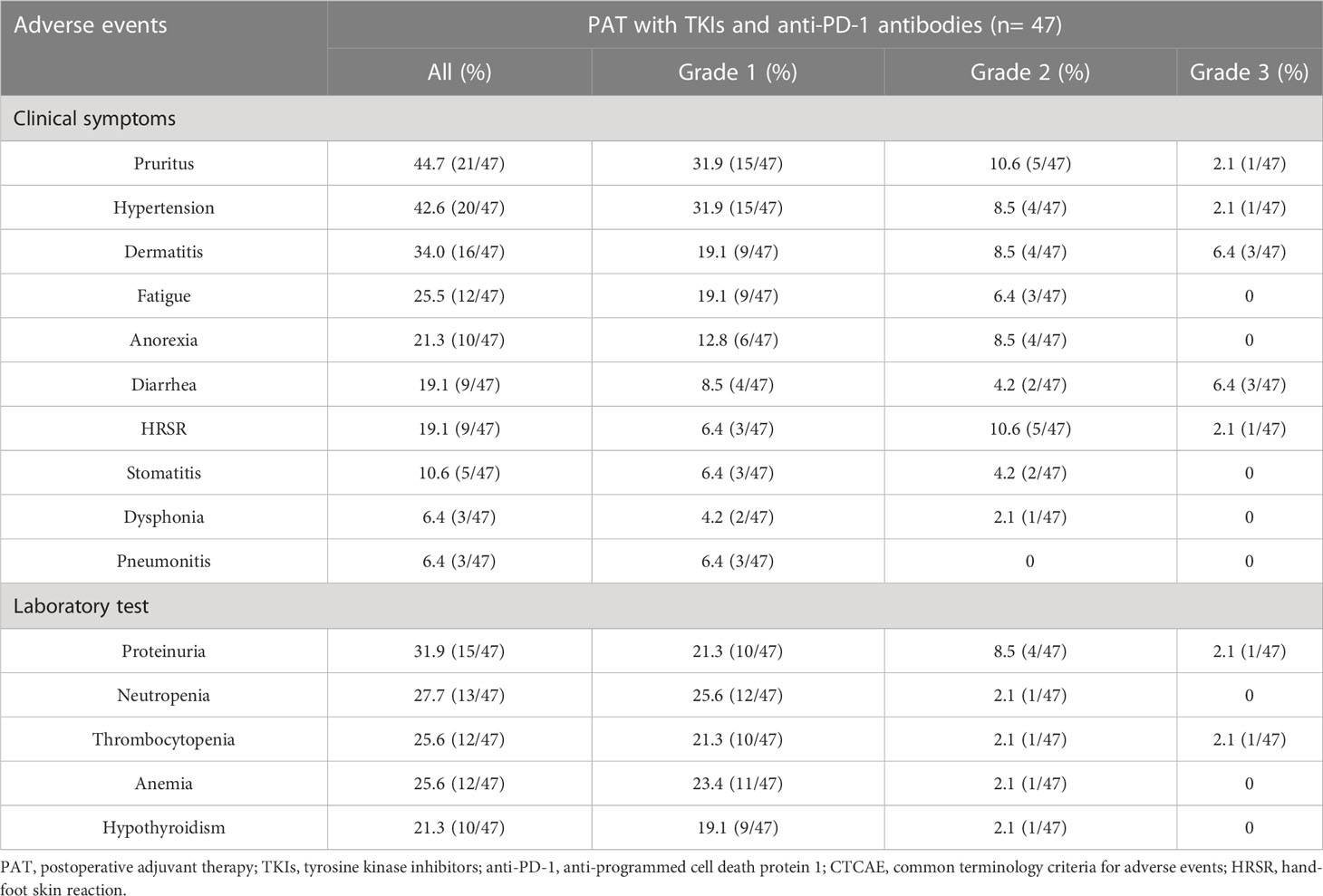
Results: A total of 250 HCC patients were enrolled, and 47 pairs of patients with HRRFs in the PAT and non-PAT groups were matched through PSM. After PSM, the 1- and 2-year RFS rates in the two groups were 82.1% vs. 40.0% (P < 0.001) and 54.2% vs. 25.1% (P = 0.012), respectively. The corresponding 1- and 2-year OS rates were 95.4% vs. 69.8% (P = 0.001) and 84.3% vs. 55.5% (P = 0.014), respectively. Multivariable analyses indicated that PAT was an independent factor related to improving RFS and OS. Subgroup analysis demonstrated that HCC patients with tumor diameter > 5 cm, satellite nodules, or vascular invasion could significantly benefit from PAT in RFS and OS. Common grade 1-3 toxicities, such as pruritus (44.7%), hypertension (42.6%), dermatitis (34.0%), and proteinuria (31.9%) were observed, and no grade 4/5 toxicities or serious adverse events occurred in patients receiving PAT.
Conclusions: PAT with TKIs and anti-PD-1 antibodies could improve surgical outcomes for HCC patients with HRRFs.
Introduction
Hepatocellular carcinoma (HCC) is one of the most common malignancies and the third leading cause of cancer-related death worldwide (1, 2). At present, hepatectomy remains the preferred treatment for HCC. However, the 5-year recurrence rate after radical hepatectomy in HCC patients is up to 70%, with 5-year survival rate less than 50% (3, 4). Especially for HCC patients with high-risk recurrent factors (HRRFs), such as tumor diameter > 5 cm, multiple tumors, satellite nodules, microvascular invasion (MVI) or portal vein tumor thrombus (PVTT) suffered remarkably higher rate of early recurrence, which directly contributed to worse long-term survival (5–12). Previous studies demonstrated that the 4-month recurrence rate of patients with PVTT was 78.3%, the 1-year recurrence rate of patients with MVI was nearly 50%, while the 6-month recurrence rate of patients with multiple tumors was 60% (5–7). The 5-year survival rates for HCC patients with PVTT, MVI, and multiple tumors were only 32.9%, 33.3%, and 31.9%, respectively (6, 11, 12). Therefore, reducing the early recurrence rate after radical resection is crucial to improve the long-term survival of HCC patients with HRRFs.
Nowadays, there is no uniform and standardized adjuvant treatments to reduce early postoperative recurrence rate in HCC patients (13). Therefore, it is imperative to explore effective strategies to prevent early recurrence of HCC. Previous studies indicated that postoperative adjuvant therapy (PAT) with transcatheter arterial chemoembolization (TACE), hepatic arterial infusion of chemotherapy (HAIC), molecular targeted therapy, adoptive immunotherapy or tumor vaccine could lower the early recurrence rate and further improve the long-term outcomes of HCC patients with HRRFs (14–16). However, the efficacy of the above strategies was still unsatisfactory.
In recent years, molecular targeted therapy combined with immune checkpoint inhibitors (ICIs) have been fruitful for treating advanced HCC (17–19). However, this emerging combination modality has not yet been reported in the settings of adjuvant therapy for HCC patients with HRRFs after radical resection. Several ongoing randomized clinical trials (RCT) exploring the efficacy of the combination therapy in preventing early recurrence of HCC patients with HRRFs undergoing radical resection are still no confirming results (16, 20).
HCC patients with HRRFs after radical resection still had residual small, disseminated foci that could not be removed surgically, which might be responsible for early recurrence and metastasis (21). Tyrosine kinase inhibitors (TKIs) target the vascular endothelial growth factor receptors, with an overall inhibitory effect on tumor angiogenesis (22), thereby potentially clearing the residual micrometastases and preventing tumor recurrence and metastasis after surgery in patients with HRRFs. Anti-programmed death receptor 1 (PD-1) antibodies regulate the immune microenvironment and induce the expansion of T lymphocytes, conducive to the elimination of small metastases in the liver (23). This effect might aid in maximizing the long-term efficacy in patients with HRRFs after radical resection. The combination therapy with TKIs and ICIs represents an attractive therapeutic option to eliminate microscopic tumor foci and disseminated tumor cells. Therefore, we supposed that the combination therapy might reduce the early recurrence rates and further improve the long-term survival in HCC patients with HRRFs.
Herein, this study aimed to investigate whether PAT with TKIs and anti-PD-1 antibodies could improve the surgical outcomes for HCC patients with HRRFs.
Materials and methods
Patient selection
This retrospective study included HCC patients who underwent radical resection at the Hepatic Surgery Center, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology between January 2019 and December 2021. This study was in line with the requirements of the Helsinki Declaration and was approved by the hospital’s Ethics Committee (TJ-IRB20230127). Due to the retrospective nature of this study, the committee abandoned the requirement of informed consent for all patients.
The inclusion criteria for this study were as follows: (1) age ≥ 18 years; (2) histopathology confirmed HCC; (3) all patients underwent radical hepatectomy according to the Chinese guidelines for diagnosis and treatment of HCC; (4) not receiving any anti-tumor treatment before radical hepatectomy; (5) Child-Pugh grade A or B7. The exclusion criteria for this study were as follows: (1) number of tumors ≥ 4 or extrahepatic metastasis; (2) spontaneous rupture of tumor; (3) PAT with other adjuvant strategies, such as TKIs or anti-PD-1 antibodies monotherapy, TACE; (4) loss to follow-up.
Data collection and definition
The demographic and clinical features were extracted from the electronic medical record system in our hospital. Vascular invasion was defined as concomitant PVTT or MVI. PVTT mainly included the following types in this study: type I (vp1): tumor thrombus invading the tertiary branch of the portal vein; type II (vp2): tumor thrombus invading the secondary branch of the portal vein; type III (vp3): tumor thrombus invading the primary branch (left or right branch) of the portal vein (24). MVI was defined as nests of cancer cells in the lumen of blood vessels lined by endothelial cells observed microscopically, usually detectable in adjacent liver tissue (25, 26). Satellite nodules were defined as small tumor foci that appear macroscopically or microscopically within the peritumoral liver tissue. The distance between the tumor foci and the primary tumor was < 2 cm. Satellite nodules are considered as intrahepatic micrometastases that occurred on the basis of MVI. They could be classified as MVI, when it was challenging to pathologically distinguish satellite nodules and MVI within the pericancerous liver tissues (26). In this study, HRRFs were defined as including at least one of the following factors: tumor diameter > 5 cm, multiple tumors, satellite nodules, or vascular invasion.
Surgical resection
All enrolled patients underwent radical hepatectomy, which was defined as: (1) complete resection of tumor nodules found by preoperative imaging or intraoperative exploration, and no cancer cells or residual cancer tissues were detected at the surgical margin of the submitted specimens; (2) no gross tumor thrombus in the main portal vein, hepatic vein, or bile duct, and without intrahepatic or extrahepatic metastasis; (3) no recurrence of HCC within two months after surgery, and for patients with baseline alpha-fetoprotein (AFP) positive, AFP-levels decreased to the normal reference range (26, 27). All operations were performed by the same experienced surgeon with the assistance of other members of the medical team.
All patients received a careful preoperative evaluation. The Eastern Cooperative Oncology Group performance status score assessed the patient’s general condition, and necessary examinations were performed to evaluate the functioning of the vital organs, such as the patients’ heart, lungs and kidneys. The tumor status (location and stage) was judged by color Doppler ultrasound, contrast-enhanced computed tomography (CT), or magnetic resonance imaging (MRI). Indocyanine green retention for 15 min and Child-Pugh grading estimated the liver function reserve. Wide or narrow surgical margin was defined as the shortest distance from the tumor margin to the hepatectomy plane ≥ 1 cm (wide margin) or < 1 cm (narrow margin) (28). Major hepatectomy was defined as the resection of 3 or more liver segments, and minor hepatectomy was defined as the resection of 1 or 2 liver segments (29).
Postoperative adjuvant therapy
HCC patients with HRRFs would be recommended adjuvant therapies after radical hepatectomy, and the decision ultimately depended on the patient’s own wishes (26). Patients who did not receive adjuvant therapy after hepatectomy were routinely managed according to the guidelines (26, 30). For patients who chose PAT with TKIs and anti-PD-1 antibodies, the TKIs included sorafenib, lenvatinib, donafenib, regorafenib, and apatinib. The anti-PD-1 antibodies included pembrolizumab, sintilimab, camrelizumab, toripalimab, and tislelizumab. TKIs and anti-PD-1 antibodies were administered according to the recommended dosages from four weeks after surgery until HCC recurrence or serious adverse events, or until the patient withdrew automatically. Generally, 3 weeks was taken as one course, and patients in PAT group received at least 3 courses of treatment. Intermittent or reduced dosage was allowed during treatment to reduce drug-related toxicities. Adverse events were classified according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 5.0.
Postoperative follow-up
All patients were followed up regularly after radical hepatectomy to monitor recurrence, survival status and drug-related toxicities. In the first year of the surgery, blood biochemical (mainly including liver function and AFP), abdominal ultrasound or contrast-enhanced CT/MRI, chest radiograph, or lung CT were performed every two months. In the second year, routine reviews were conducted every 3-4 months and then every half a year. Recurrence was diagnosed according to the typical imaging findings of HCC and/or persistently elevated serum AFP-levels (26). Early recurrence was defined as HCC recurrence within two years after radical resection (31).
Recurrence-free survival (RFS) was defined as the interval between receiving radical hepatectomy and the first diagnosis of recurrence, or the last follow-up. Overall survival (OS) was defined as the period from surgery to the date of death or the last follow-up. The last follow-up date for this study was on August 1, 2022.
Statistical analysis
Statistical analysis in this study was performed using R software version 4.2.0 (http://www.R-project.org). Continuous variables with normality were presented as mean ± standard deviation (SD), and those without normality were reported as medians and interquartile ranges (IQR). Comparisons between continuous variables were performed using the independent samples t-test or Mann-Whitney U test, as deemed appropriate. Categorical variables were described as numbers (n) or percentages (%) and compared using the appropriate Chi-square test or Fisher’s exact test. A 1:1 propensity score matching (PSM) was performed to adjust for confounding factors between the two groups. The continuous propensity scores from 0 to 1 were generated by binary logistic regression with selected variables. Nearest-neighbor matching between the PAT and non-PAT groups was done to choose patients for subsequent analyses. The pairs on the propensity-score logit were matched to within a range of 0.2 of SD. Survival curves were created and compared between groups using the Kaplan-Meier method and the log-rank test. Independent prognostic factors for RFS and OS were identified by univariable and multivariable Cox regression analyses. Variables with P < 0.05 in the univariable Cox regression were entered into multivariable Cox regression for further analysis. Subgroup survival analyses were conducted using univariable Cox regression, stratified by different clinical variables (gender, age, AFP, number of tumors, tumor diameter, satellite nodules, grade, and vascular invasion), and forest plots were drawn with hazard ratio (HR) and 95% confidence interval (CI). The differences between groups with a two-tailed P < 0.05 were considered statistically significant.
Results
Baseline characteristics for HCC patients
A total of 315 HCC patients underwent hepatectomy between January 2019 and December 2021, of whom 65 cases were excluded, including intrahepatic/extrahepatic metastasis (n= 8), spontaneous rupture of HCC (n= 12), previous received hepatectomy, local or systemic treatments (n= 23), PAT with other adjuvant treatments (n= 13), and loss to follow-up (n= 9). Finally, 250 eligible patients were included in this study (Figure 1). The clinicopathological characteristics of enrolled patients being depicted in Table S1. In these patients, age was 54.3 ± 11.6 years, 89.2% cases were male, 16.8% cases were with multiple tumors, 51.2% cases were with tumor diameter > 5 cm, 25.2% cases were with satellite nodules, and 30.8% cases were with vascular invasion.
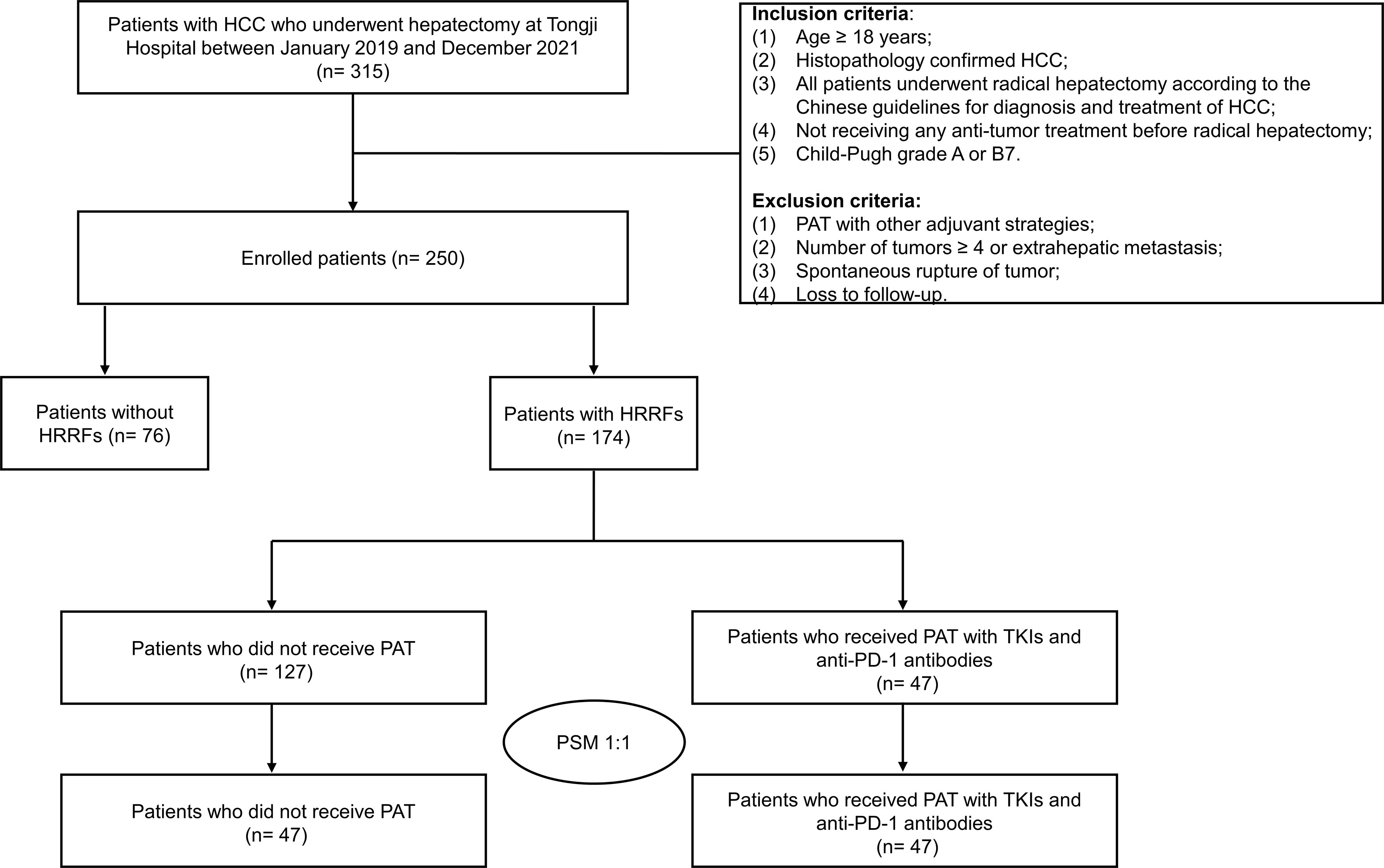
Figure 1 Flow chart of patient selection in this study. HCC, hepatocellular carcinoma; PAT, postoperative adjuvant therapy; TKIs, tyrosine kinase inhibitors; anti-PD-1, anti-programmed death receptor 1; HRRFs, high-risk recurrent factors; PSM, propensity score matching.
Before and after PSM for HCC patients with HRRFs
As shown in Table 1, among the 174 HCC patients with HRRFs before PSM, 47 (27%) were assigned to the PAT group, and 127 (73%) were assigned to the non-PAT group. Significant differences in four variables (AFP, satellite nodules, vascular invasion, and extent of resection) (all P < 0.05) were observed upon comparing the baseline characteristics of the two groups. Considering that these confounding factors may interfere with the comparative analysis of survival outcomes between the two groups, PSM was conducted in this study. There were 47 pairs of patients with HRRFs in the PAT and non-PAT groups after PSM, with no significant difference between the two groups (all P > 0.05).
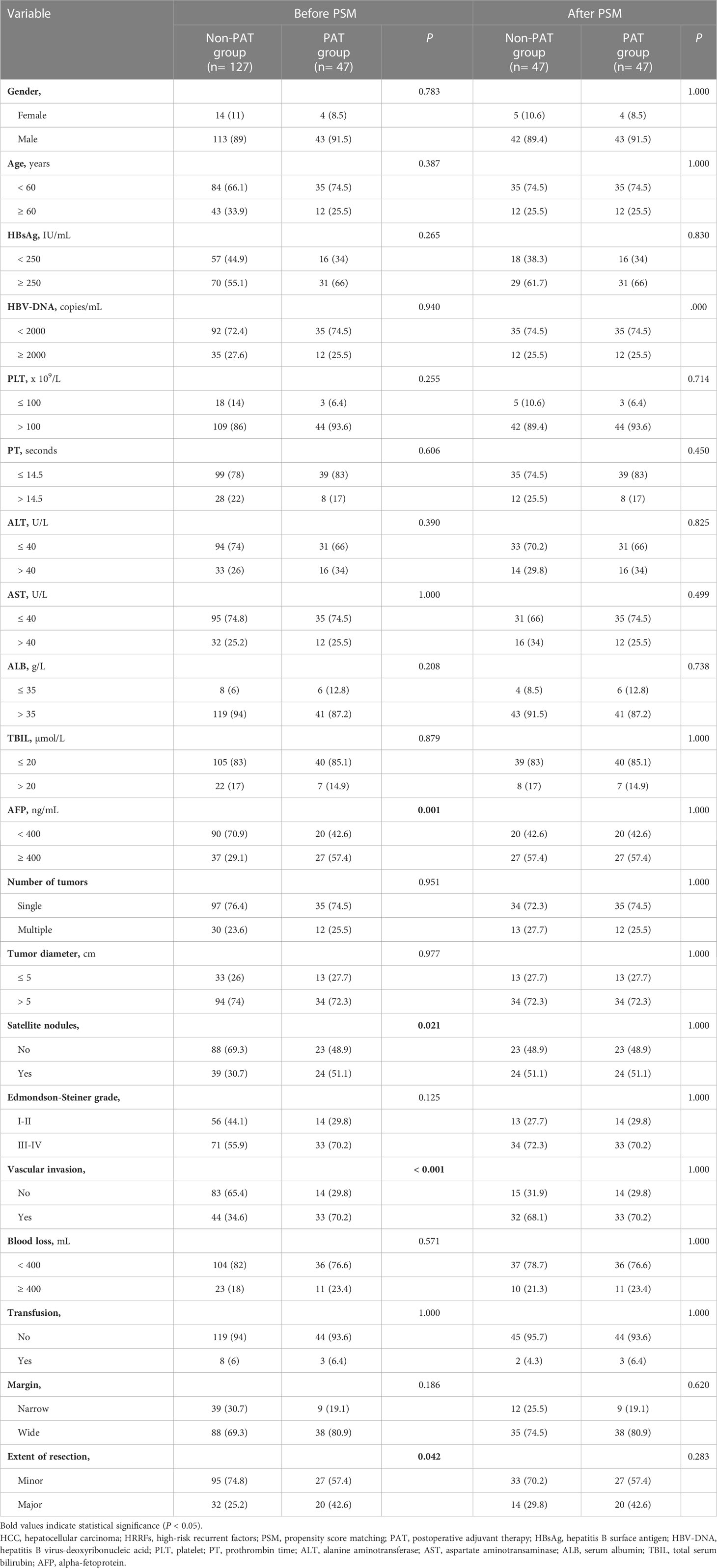
Table 1 Basal clinicopathological characteristics of 174 HCC patients with HRRFs before and after PSM.
Survival analysis
The median follow-up for the 250 HCC patients was 22.4 (IQR: 14.3-34.1) months, including 84 (33.6%) recurrences and 39 (15.6%) deaths; and the 90-day mortality was 0 (Table S1). As shown in Figures 2A, B, the RFS and OS of patients without HRRFs were significantly longer than those with HRRFs (all P < 0.001). Moreover, before PSM, there was no significant difference in RFS and OS between PAT and non-PAT groups of HCC patients with HRRFs. However, the OS of the PAT group of patients tended to be longer than those of the non-PAT group (all P > 0.05, Figure S1). This observation might be attributed to the imbalance of baseline characteristics of the two groups of patients. In contrast, the PAT group patients had higher tumor malignancy (AFP ≥ 400 ng/mL, satellite nodules, and vascular invasion) than those in the non-PAT group.
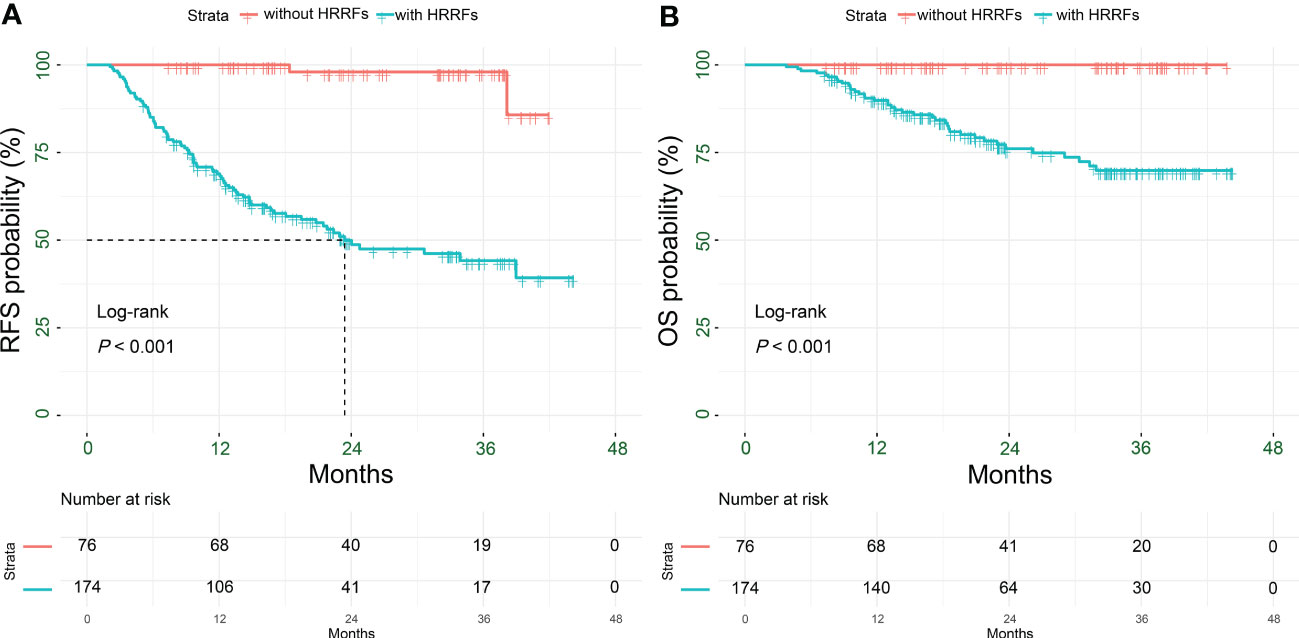
Figure 2 Kaplan-Meier analysis for survival outcomes in HCC patients with or without HRRFs after radical resection. RFS (A) and OS (B) for patients. HCC, hepatocellular carcinoma; HRRFs, high-risk recurrent factors; RFS, recurrence-free survival; OS, overall survival.
After PSM, there were 17 patients (36.2%), 35 patients (74.5%) recurrences (P < 0.001), and 4 patients (8.5%), 23 patients (48.9%) deaths (P < 0.001) in the in PAT and non-PAT groups, respectively. Patients in the PAT group had significantly longer RFS and OS than those in the non-PAT group (all P < 0.05). The 1- and 2-year RFS rates of the patients in the two groups were 82.1% vs. 40.0% (P < 0.001) and 54.2% vs. 25.1% (P = 0.012), respectively; and the corresponding 1- and 2-year OS rates were 95.4% vs. 69.8% (P = 0.001) and 84.3% vs. 55.5% (P = 0.014), respectively (Figure 3, Table 2).
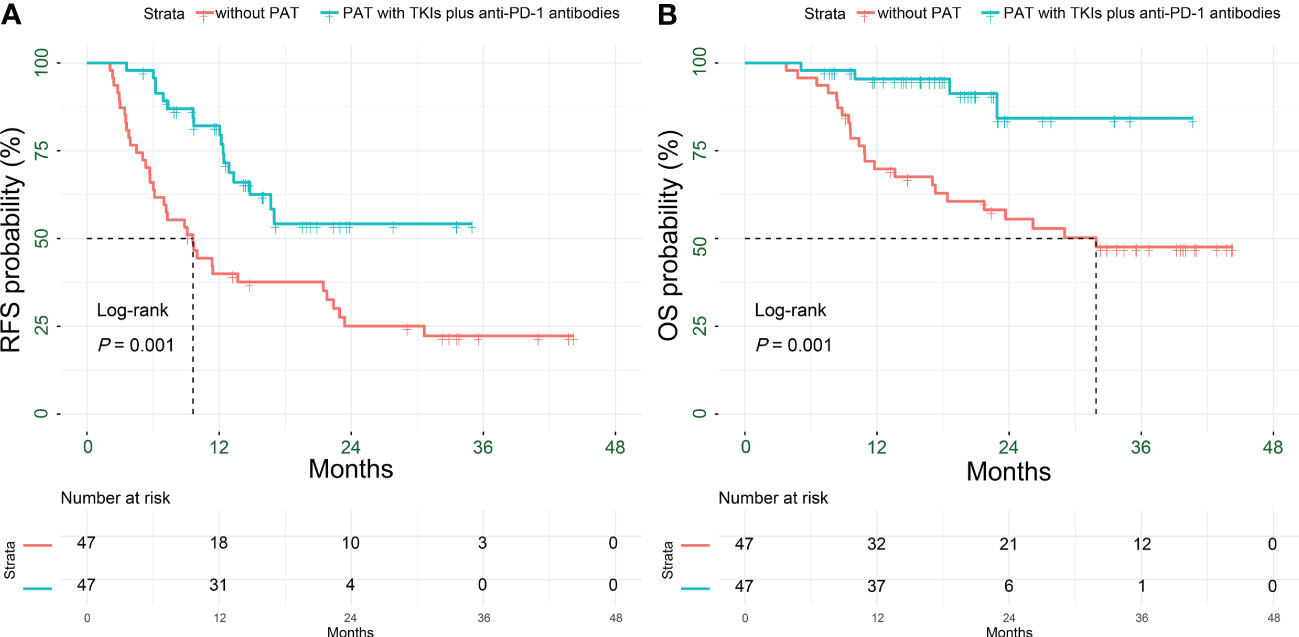
Figure 3 Kaplan-Meier analysis for survival outcomes in HCC patients with HRRFs who underwent radical resection after PSM. RFS (A) and OS (B) for patients. HCC, hepatocellular carcinoma; HRRFs, high-risk recurrent factors; RFS, recurrence-free survival; OS, overall survival; PSM, propensity score matching; PAT, postoperative adjuvant therapy; TKIs, tyrosine kinase inhibitors; anti-PD-1, anti-programmed death receptor 1.
Subgroup survival analysis
To further explore the potential value of PAT for improving RFS and OS, subgroup analyses were performed. The results indicated that PAT could significantly improve RFS in HCC patients with multiple tumors (HR: 0.30, 95% CI: 0.09-0.98, P = 0.046), tumor diameter > 5 cm (HR: 0.45, 95% CI: 0.24-0.86, P = 0.015), satellite nodules (HR: 0.32, 95% CI: 0.15-0.70, P = 0.004) or vascular invasion (HR: 0.36, 95% CI: 0.17-0.76, P = 0.007) (Figure 4A).
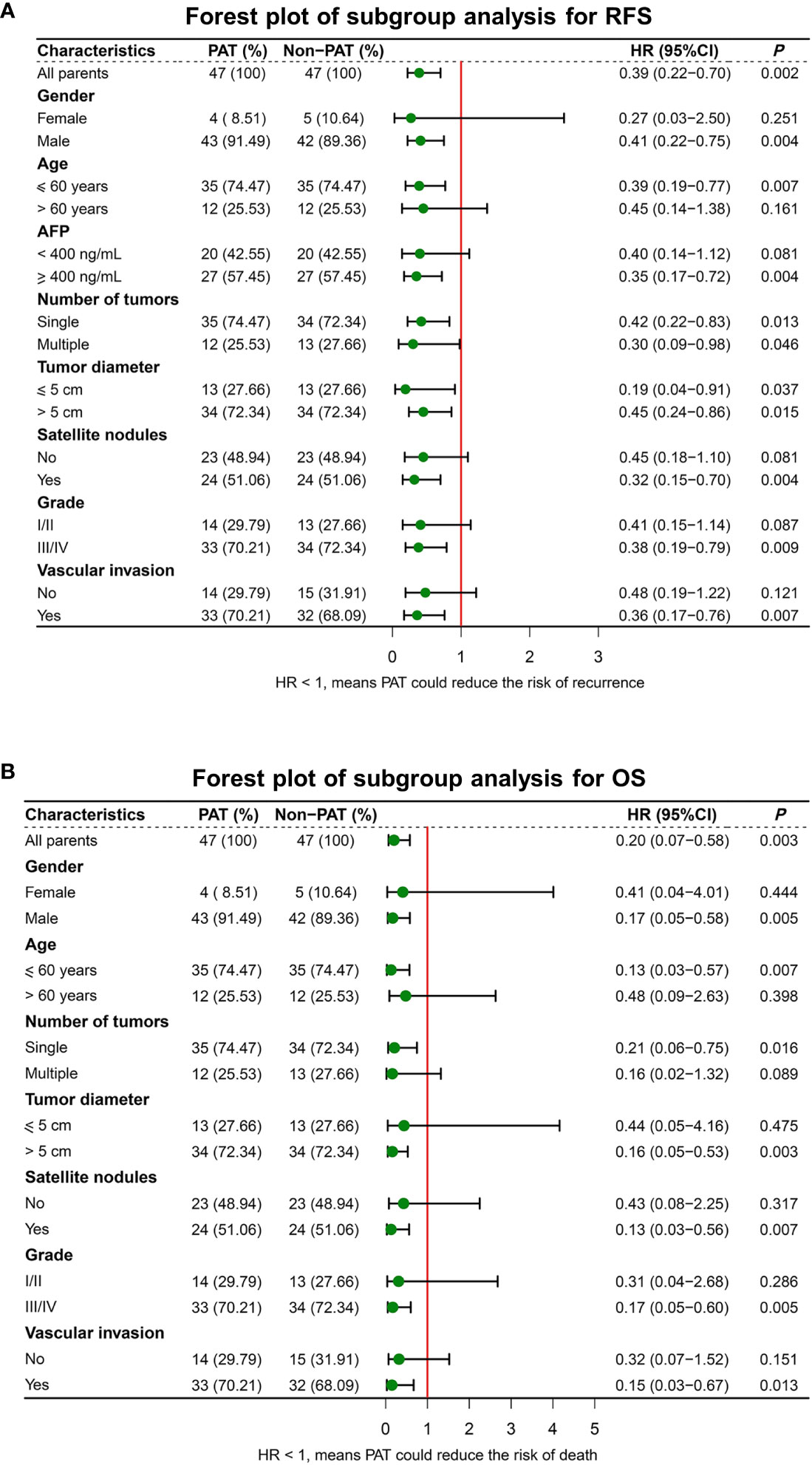
Figure 4 Forest plot for subgroup survival analysis for RFS (A) and OS (B). RFS, recurrence-free survival; OS, overall survival; PAT, postoperative adjuvant therapy; AFP, alphafetoprotein.
In addition, PAT significantly improved OS in HCC patients with tumor diameter > 5 cm (HR: 0.16, 95% CI: 0.05-0.53, P = 0.003), satellite nodules (HR: 0.13, 95% CI: 0.03-0.56, P = 0.007) or vascular invasion (HR: 0.15, 95% CI: 0.03-0.67, P = 0.013) (Figure 4B).
PAT significantly prolonged the RFS (P = 0.034), and relatively extended the OS (P = 0.053) for HCC patients with multiple tumors. The 1-year RFS rate of patients in the PAT and non-PAT groups was 75% and 28.8%, respectively (P = 0.015); and the 1- and 2-year OS rates were 100% vs. 59.2% (P = 0.006) and 66.7% vs. 32.9% (P = 0.227), respectively (Figures 5A, B, Table 2).
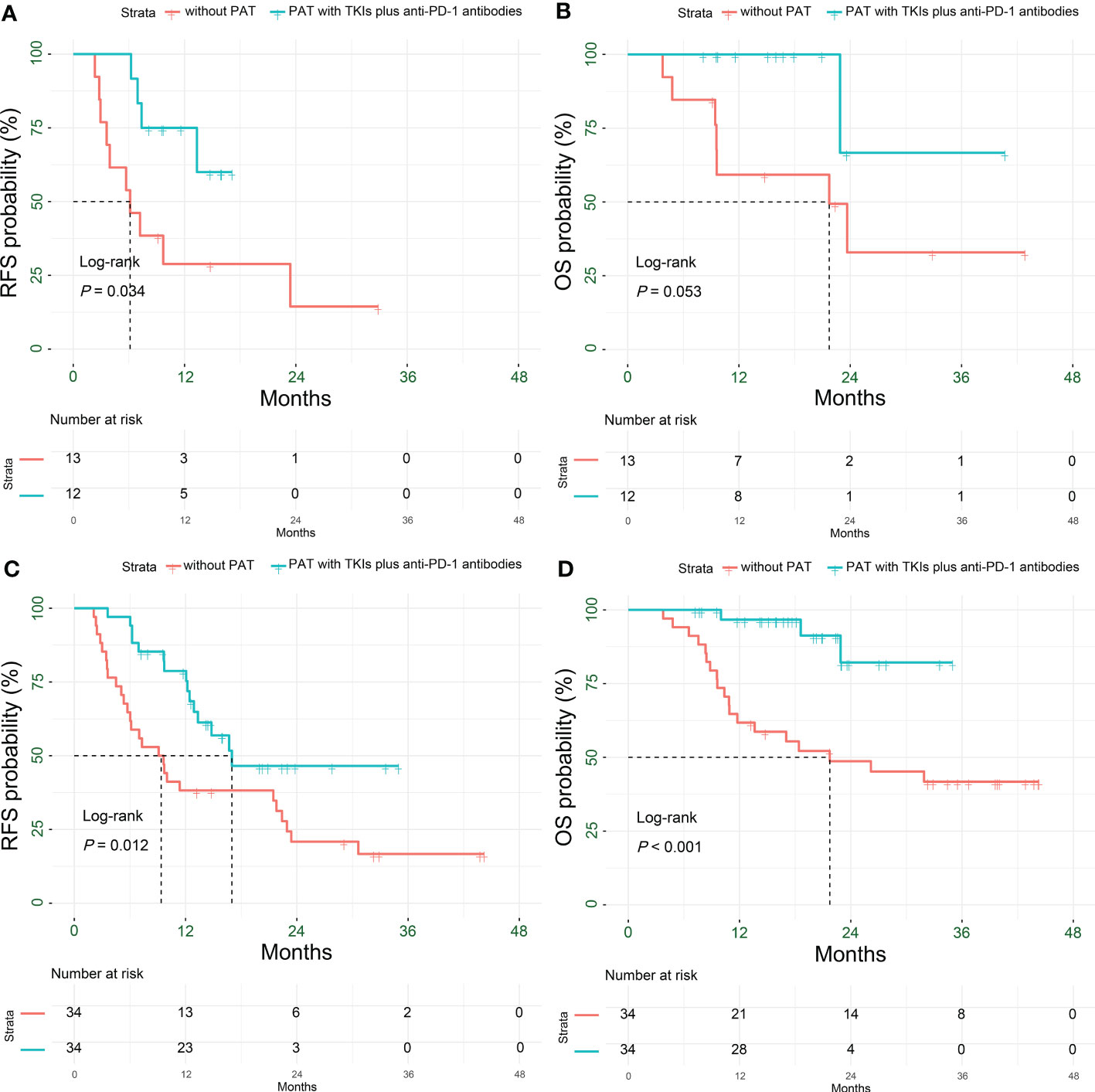
Figure 5 Kaplan-Meier subgroup analysis for survival outcomes in HCC patients with HRRFs who underwent radical resection after PSM. RFS (A) and OS (B) for patients with multiple tumors; RFS (C) and OS (D) for patients with tumor diameter > 5 cm. HCC, hepatocellular carcinoma; HRRFs, high-risk recurrent factors; RFS, recurrence-free survival; OS, overall survival; PSM, propensity score matching; PAT, postoperative adjuvant therapy; TKIs, tyrosine kinase inhibitors; anti-PD-1, anti-programmed death receptor 1.
PAT significantly improved the RFS and OS for HCC patients with tumor diameter > 5 cm (P= 0.012 and P < 0.001, respectively). The 1- and 2-year RFS rates of patients in the two groups were 78.7% vs. 38.2% (P < 0.001) and 46.5% vs. 20.9% (P = 0.048), respectively; while the 1- and 2-year OS rates were 96.7% vs. 61.8% (P < 0.001) and 82.2% vs. 48.7% (P = 0.018), respectively (Figures 5C, D, Table 2).
The RFS and OS of HCC patients with satellite nodules in the PAT group were significantly longer than those in the non-PAT group (P = 0.003 and P = 0.001, respectively). The 1- and 2-year RFS rates of patients in the two groups were 82.6% vs. 25% (P < 0.001) and 32.8% vs. 15% (P = 0.220), respectively. The 1- and 2-year OS were 100% vs. 58.3% (P < 0.001) and 71.1% vs. 35.9% (P = 0.093), respectively (Figures 6A, B, Table 2).
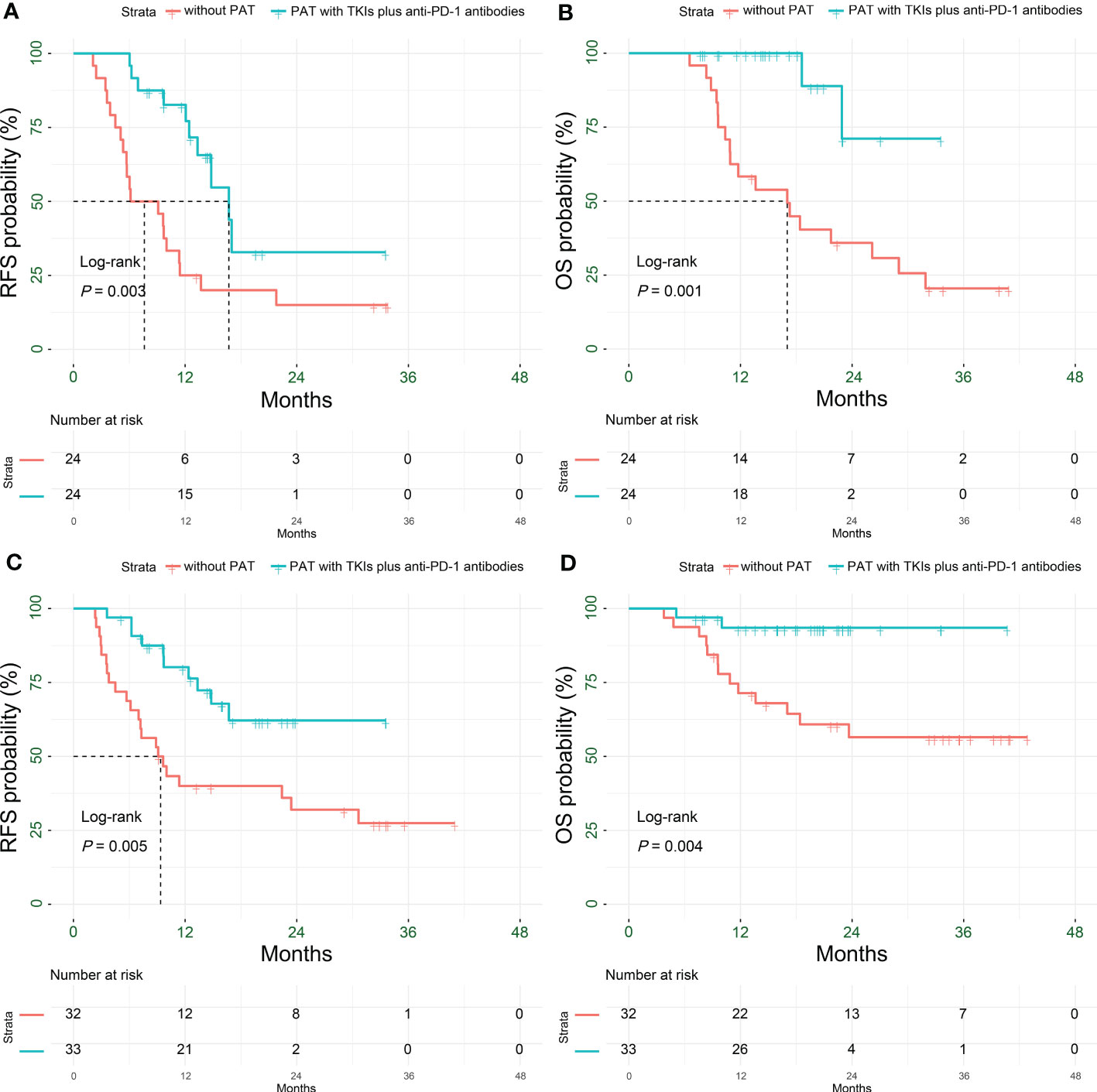
Figure 6 Kaplan-Meier subgroup analysis for survival outcomes in HCC patients with HRRFs who underwent radical resection after PSM. RFS (A) and OS (B) for patients with satellite nodules; RFS (C) and OS (D) for patients with vascular invasion. HCC, hepatocellular carcinoma; HRRFs, high-risk recurrent factors; RFS, recurrence-free survival; OS, overall survival; PSM, propensity score matching; PAT, postoperative adjuvant therapy; TKIs, tyrosine kinase inhibitors; anti-PD-1, anti-programmed death receptor 1.
The RFS and OS of patients in the PAT group were significantly longer than those of HCC patients with vascular invasion in the non-PAT group (P = 0.005 and P = 0.004, respectively). The 1- and 2-year RFS rates were 80.2% vs. 40% (P < 0.001) and 62.2% vs. 32% (P = 0.028), respectively. The 1- and 2-year OS rates were 93.5% vs. 71.4% (P = 0.022) and 93.5% vs. 56.5% (P < 0.001), respectively (Figures 6C, D, Table 2).
Independent prognostic factors related to RFS and OS
Multivariable Cox regression analysis indicated two variables to closely associated with RFS in HCC patients with HRRFs: AFP ≥ 400 ng/mL (HR: 2.28, 95% CI: 1.27-4.11, P = 0.006) and PAT (HR: 0.33, 95% CI: 0.18-0.60, P < 0.001) (Table S2). Moreover, three factors were strongly related to OS in HCC patients with HRRFs: AFP ≥ 400 ng/mL (HR: 2.91, 95% CI: 1.19-7.11, P = 0.019), satellite nodules (HR: 2.76, 95% CI: 1.05-7.22, P = 0.039), and PAT (HR: 0.18, 95% CI: 0.06-0.54, P = 0.002) (Table S3). The above results significantly established PAT as a favorable factor in improving the RFS and OS of HCC patients with HRRFs.
Safety
The occurrence of adverse events in 47 patients receiving PAT was summarized in Table 3. No grade 4/5 toxicities or serious adverse events were observed, and common grade 1-3 toxicities included pruritus (44.7%), hypertension (42.6%), dermatitis (34.0%), and proteinuria (31.9%). Grade 3 toxicities included dermatitis (6.4%), diarrhea (6.4%), pruritus (2.1%), hypertension (2.1%), hand-foot skin reaction (2.1%), proteinuria (2.1%), and thrombocytopenia (2.1%).
Discussion
Nowadays, the administration of adjuvant therapy to HCC patients after radical resection is still highly debatable. Western guidelines suggested that adjuvant therapy should not be routinely recommended for HCC patients undergoing radical resection due to a lack of solid evidence demonstrating adjuvant therapy improving survival outcomes (32, 33). A large-sample RCT (STORM, phase III trial) result indicated that PAT with sorafenib failed to effectively improve the RFS and OS in patients with HCC (34). On the contrary, several findings revealed that PAT with sorafenib extended RFS and OS for patients with high recurrence risk (35–38). The ending of the STORM trial with negative results might be attributed to the inclusion of early-stage HCC patients with low recurrence risk in the study, making it difficult to observe differences in the survival curves of patients between the adjuvant sorafenib group and the control group.
This study found that HCC patients with HRRFs were more likely to experience early recurrence and suffered worse survival prognosis than those without HRRFs. Previous studies also confirmed that higher rates of early recurrence and metastasis after curative resection in HCC patients with HRRFs, severely affecting their long-term survival (5–7, 39). Accordingly, Asian Pacific scholars advocated that for HCC patients with HRRFs, adjuvant therapeutic measures should be taken after curative resection to prevent early recurrence and metastasis (26, 40).
As far as we know, the results of this study revealed for the first time that PAT using TKIs in combination with anti-PD-1 antibodies showed a strong association with reduced early recurrence rate and prolonged OS in HCC patients with HRRFs. Compared with the non-PAT group, the early recurrence rate of patients in the PAT group could be reduced by 38.3%, and the 3-year OS rate could be increased by 36.7%. To avoid the interference of confounding factors (such as AFP, satellite nodules, vascular invasion, etc.) on RFS and OS, this study balanced the baseline characteristics of the two groups through 1:1 PSM. Therefore, the markedly lower risk of early recurrence and the significantly prolonged OS should be attributed to PAT. In addition, multivariable results showed that PAT was an independent prognostic factor for improving RFS and OS. This finding of the present study sufficiently demonstrates that PAT has great potential to be an effective modality for HCC patients with HRRFs to prevent early recurrence and prolong the long-term survival.
An RCT by Wang et al. indicated that adjuvant TACE only reduced the early recurrence rate by 15.8% and improved the 3-year OS rate by 7.8% for patients with HRRFs (15). Another multicenter RCT result showed that PAT with HAIC could only lower the recurrence rate by 15.6% and increase the 3-year OS rate by 5.5% in patients with MVI (41). Zhang et al. reported that adjuvant sorafenib could reduce the early recurrence rate by 17% and improve the 3-year OS rate by 15% for patients with MVI (38). A recent study illustrated that adjuvant lenvatinib could improve the early RFS rates of patients with MVI by 17.1%-27.3% (42). Chen et al. demonstrated that adjuvant anti-PD-1 antibodies ameliorated the early RFS rates by 22.8%-24.4% in patients with HRRFs (43). However, the results of this study indicated that PAT using TKIs combined with anti-PD-1 antibodies could improve the early RFS rates by 29.1%-42.1% in patients with HRRFs. Compared with adjuvant TACE, HAIC, or TKIs and anti-PD-1 antibodies monotherapy, adjuvant TKIs combined with anti-PD-1 antibodies have shown an overwhelming advantage in improving the survival prognosis of patients with HRRFs. This may be attributed to the synergistic anti-tumor effects of the combined regimens, which is more beneficial for clearing the residual micrometastatic lesions in the remaining liver, thus achieving satisfactory effects of preventing early recurrence and prolonging long-term survival (43–46). Notably, the present study showed that the combination therapy would not increase the probability of treatment-related adverse events compared with monotherapy (34, 42, 43).
Early recurrence (≤ 2 years) is the most common type of HCC recurrence, mainly related to the biologic features of tumor (such as tumor diameter > 5 cm, multiple tumors, satellite nodules, vascular invasion, or poor differentiation, etc.), while late recurrence (> 2 years) is generally considered to be associated with the underlying liver disease background (31). PAT with TKIs and anti-PD-1 antibodies could effectively control early recurrence caused by the dissemination of intrahepatic micrometastatic lesions, but its curative effect on late recurrence might be limited owing to the inability to alter the underlying liver diseases. The median follow-up was relatively short (22.4 months), so this study mainly observed the influencing factors of early recurrence. In the overall cohort, multivariable results showed that tumor diameter > 5 cm, multiple tumors, satellite nodules, and vascular invasion were high-risk factors associated with early recurrence, which was consistent with previous studies (5, 7, 9) (Table S4). Excitingly, subgroup analysis suggested that PAT could significantly improve RFS and OS for patients with tumor diameter > 5 cm, satellite nodules, or vascular invasion. PAT failed to result in a significant improvement in OS for patients with multiple tumors, which might be related to the insufficient sample size of subgroups.
This study had several limitations. First, although PSM and multivariable analysis were adopted, the influence of selection bias on the findings could not be completely excluded due to the inherent deficiencies of single center, retrospective design. Therefore, the conclusion of this study still needs to be further validated by several ongoing RCT results. Second, the sample size of this study was limited, and the duration of follow-up was relatively short, thus the 5-year survival results were unavailable. Therefore, future studies still need to expand the sample size and extend the follow-up time. Third, this study was limited to clinical observation, and the mechanism of postoperative adjuvant combination therapy to prevent early recurrence in HCC patients with HRRFs deserves thorough exploration.
Conclusions
In conclusion, the present study demonstrated that adjuvant TKIs combined with anti-PD-1 antibodies after radical resection closely associated with improved surgical outcomes for HCC patients with HRRFs. Moreover, the adverse events of adjuvant therapy were controllable, providing more confidence in using this line of therapy. However, multicenter studies with large-sample sizes still need to be carried out to validate this study’s results further.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
This study was approved by the Tongji Hospital Ethics Committee (TJ-IRB20230127). Due to the retrospective nature of this study, the committee waived the requirement of informed consent for all patients.
Author contributions
Conception and design, Z-YH, E-LZ, and JL. Administrative support, Z-YH, E-LZ, and B-YL. Provision of study materials or patients, Z-YH, E-LZ, and B-YL. Collection and assembly of data, JL, W-QW, XL, J-LW, and R-HZ. Data analysis and interpretation, JL, E-LZ, and Z-YH. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Hubei provincial special grants for scientific and technical innovation (No. 2021BCA115) and the National Natural Science Foundation of China (No. 81902839).
Acknowledgments
We thank Bullet Edits Limited for the linguistic editing and proofreading of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1202039/full#supplementary-material
References
1. Vogel A, Meyer T, Sapisochin G, Salem R, Saborowski A. Hepatocellular carcinoma. Lancet (2022) 400:1345–62. doi: 10.1016/S0140-6736(22)01200-4
2. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71:209–49. doi: 10.3322/caac.21660
3. Tang ZY. Hepatocellular carcinoma surgery–review of the past and prospects for the 21st century. J Surg Oncol (2005) 91:95–6. doi: 10.1002/jso.20291
4. Altekruse SF, McGlynn KA, Dickie LA, Kleiner DE. Hepatocellular carcinoma confirmation, treatment, and survival in surveillance, epidemiology, and end results registries, 1992-2008. Hepatology (2012) 55:476–82. doi: 10.1002/hep.24710
5. Choi KK, Kim SH, Choi SB, Lim JH, Choi GH, Choi JS, et al. Portal venous invasion: the single most independent risk factor for immediate postoperative recurrence of hepatocellular carcinoma. J Gastroenterol Hepatol (2011) 26:1646–51. doi: 10.1111/j.1440-1746.2011.06780.x
6. Chen ZH, Zhang XP, Feng JK, Li LQ, Zhang F, Hu YR, et al. Actual long-term survival in hepatocellular carcinoma patients with microvascular invasion: a multicenter study from China. Hepatol Int (2021) 15:642–50. doi: 10.1007/s12072-021-10174-x
7. Kim BW, Kim YB, Wang HJ, Kim MW. Risk factors for immediate post-operative fatal recurrence after curative resection of hepatocellular carcinoma. World J Gastroenterol (2006) 12:99–104. doi: 10.3748/wjg.v12.i1.99
8. Shinkawa H, Tanaka S, Takemura S, Amano R, Kimura K, Kinoshita M, et al. Nomograms predicting extra- and early intrahepatic recurrence after hepatic resection of hepatocellular carcinoma. Surgery (2021) 169:922–28. doi: 10.1016/j.surg.2020.10.012
9. Tung-Ping Poon R, Fan ST, Wong J. Risk factors, prevention, and management of postoperative recurrence after resection of hepatocellular carcinoma. Ann Surg (2000) 232:10–24. doi: 10.1097/00000658-200007000-00003
10. Chen ZH, Zhang XP, Lu YG, Li LQ, Chen MS, Wen TF, et al. Actual long-term survival in HCC patients with portal vein tumor thrombus after liver resection: a nationwide study. Hepatol Int (2020) 14:754–64. doi: 10.1007/s12072-020-10032-2
11. Orimo T, Kamiyama T, Kakisaka T, Nagatsu A, Asahi Y, Aiyama T, et al. Hepatectomy is beneficial in select patients with multiple hepatocellular carcinomas. Ann Surg Oncol (2022) 29:8436–45. doi: 10.1245/s10434-022-12495-z
12. Kokudo T, Hasegawa K, Matsuyama Y, Takayama T, Izumi N, Kadoya M, et al. Survival benefit of liver resection for hepatocellular carcinoma associated with portal vein invasion. J Hepatol (2016) 65:938–43. doi: 10.1016/j.jhep.2016.05.044
13. Foerster F, Galle PR. The current landscape of clinical trials for systemic treatment of HCC. Cancers (Basel) (2021) 13. doi: 10.3390/cancers13081962
14. Liu S, Guo L, Li H, Zhang B, Sun J, Zhou C, et al. Postoperative adjuvant trans-arterial chemoembolization for patients with hepatocellular carcinoma and portal vein tumor thrombus. Ann Surg Oncol (2018) 25:2098–104. doi: 10.1245/s10434-018-6438-1
15. Wang Z, Ren Z, Chen Y, Hu J, Yang G, Yu L, et al. Adjuvant transarterial chemoembolization for HBV-related hepatocellular carcinoma after resection: a randomized controlled study. Clin Cancer Res (2018) 24:2074–81. doi: 10.1158/1078-0432.CCR-17-2899
16. Zhang W, Zhang B, Chen XP. Adjuvant treatment strategy after curative resection for hepatocellular carcinoma. Front Med (2021) 15:155–69. doi: 10.1007/s11684-021-0848-3
17. Finn RS, Ikeda M, Zhu AX, Sung MW, Baron AD, Kudo M, et al. Phase ib study of lenvatinib plus pembrolizumab in patients with unresectable hepatocellular carcinoma. J Clin Oncol (2020) 38:2960–70. doi: 10.1200/JCO.20.00808
18. Xu J, Shen J, Gu S, Zhang Y, Wu L, Wu J, et al. Camrelizumab in combination with apatinib in patients with advanced hepatocellular carcinoma (RESCUE): a nonrandomized, open-label, phase II trial. Clin Cancer Res (2021) 27:1003–11. doi: 10.1158/1078-0432.CCR-20-2571
19. Gordan JD, Kennedy EB, Abou-Alfa GK, Beg MS, Brower ST, Gade TP, et al. Systemic therapy for advanced hepatocellular carcinoma: ASCO guideline. J Clin Oncol (2020) 38:4317–45. doi: 10.1200/JCO.20.02672
20. Hack SP, Spahn J, Chen M, Cheng AL, Kaseb A, Kudo M, et al. IMbrave 050: a phase III trial of atezolizumab plus bevacizumab in high-risk hepatocellular carcinoma after curative resection or ablation. Future Oncol (2020) 16:975–89. doi: 10.2217/fon-2020-0162
21. Calderaro J, Petitprez F, Becht E, Laurent A, Hirsch TZ, Rousseau B, et al. Intra-tumoral tertiary lymphoid structures are associated with a low risk of early recurrence of hepatocellular carcinoma. J Hepatol (2019) 70:58–65. doi: 10.1016/j.jhep.2018.09.003
22. Qin S, Li A, Yi M, Yu S, Zhang M, Wu K. Recent advances on anti-angiogenesis receptor tyrosine kinase inhibitors in cancer therapy. J Hematol Oncol (2019) 12:27. doi: 10.1186/s13045-019-0718-5
23. Versluis JM, Long GV, Blank CU. Learning from clinical trials of neoadjuvant checkpoint blockade. Nat Med (2020) 26:475–84. doi: 10.1038/s41591-020-0829-0
24. Ikai I, Yamamoto Y, Yamamoto N, Terajima H, Hatano E, Shimahara Y, et al. Results of hepatic resection for hepatocellular carcinoma invading major portal and/or hepatic veins. Surg Oncol Clin N Am (2003) 12:65–75, ix. doi: 10.1016/s1055-3207(02)00082-0
25. Rodriguez-Peralvarez M, Luong TV, Andreana L, Meyer T, Dhillon AP, Burroughs AK. A systematic review of microvascular invasion in hepatocellular carcinoma: diagnostic and prognostic variability. Ann Surg Oncol (2013) 20:325–39. doi: 10.1245/s10434-012-2513-1
26. Zhou J, Sun H, Wang Z, Cong W, Wang J, Zeng M, et al. Guidelines for the diagnosis and treatment of hepatocellular carcinoma (2019 edition). Liver Cancer (2020) 9:682–720. doi: 10.1159/000509424
27. Bruix J, Han KH, Gores G, Llovet JM, Mazzaferro V. Liver cancer: approaching a personalized care. J Hepatol (2015) 62:S144–56. doi: 10.1016/j.jhep.2015.02.007
28. Yang P, Si A, Yang J, Cheng Z, Wang K, Li J, et al. A wide-margin liver resection improves long-term outcomes for patients with HBV-related hepatocellular carcinoma with microvascular invasion. Surgery (2019) 165:721–30. doi: 10.1016/j.surg.2018.09.016
29. Jang JS, Cho JY, Ahn S, Han HS, Yoon YS, Choi Y, et al. Comparative performance of the complexity classification and the conventional Major/Minor classification for predicting the difficulty of liver resection for hepatocellular carcinoma. Ann Surg (2018) 267:18–23. doi: 10.1097/SLA.0000000000002292
30. Vogel A, Cervantes A, Chau I, Daniele B, Llovet JM, Meyer T, et al. Hepatocellular carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol (2018) 29:iv238–iv55. doi: 10.1093/annonc/mdy308
31. Wang MD, Li C, Liang L, Xing H, Sun LY, Quan B, et al. Early and late recurrence of hepatitis b virus-associated hepatocellular carcinoma. Oncologist (2020) 25:e1541–e51. doi: 10.1634/theoncologist.2019-0944
32. Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American association for the study of liver diseases. Hepatology (2018) 68:723–50. doi: 10.1002/hep.29913
33. European Association for the Study of the Liver. Electronic address eee, European Association for the Study of the L. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol (2018) 69:182–236. doi: 10.1016/j.jhep.2018.03.019
34. Bruix J, Takayama T, Mazzaferro V, Chau GY, Yang J, Kudo M, et al. Adjuvant sorafenib for hepatocellular carcinoma after resection or ablation (STORM): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol (2015) 16:1344–54. doi: 10.1016/S1470-2045(15)00198-9
35. Li J, Hou Y, Cai XB, Liu B. Sorafenib after resection improves the outcome of BCLC stage c hepatocellular carcinoma. World J Gastroenterol (2016) 22:4034–40. doi: 10.3748/wjg.v22.i15.4034
36. Xia F, Wu LL, Lau WY, Huan HB, Wen XD, Ma KS, et al. Adjuvant sorafenib after heptectomy for Barcelona clinic liver cancer-stage c hepatocellular carcinoma patients. World J Gastroenterol (2016) 22:5384–92. doi: 10.3748/wjg.v22.i23.5384
37. Wang SN, Chuang SC, Lee KT. Efficacy of sorafenib as adjuvant therapy to prevent early recurrence of hepatocellular carcinoma after curative surgery: a pilot study. Hepatol Res (2014) 44:523–31. doi: 10.1111/hepr.12159
38. Zhang XP, Chai ZT, Gao YZ, Chen ZH, Wang K, Shi J, et al. Postoperative adjuvant sorafenib improves survival outcomes in hepatocellular carcinoma patients with microvascular invasion after R0 liver resection: a propensity score matching analysis. HPB (Oxford) (2019) 21:1687–96. doi: 10.1016/j.hpb.2019.04.014
39. Zhang XP, Chen ZH, Zhou TF, Li LQ, Chen MS, Wen TF, et al. A nomogram to predict early postoperative recurrence of hepatocellular carcinoma with portal vein tumour thrombus after R0 liver resection: a large-scale, multicenter study. Eur J Surg Oncol (2019) 45:1644–51. doi: 10.1016/j.ejso.2019.03.043
40. Liu Y, Wang Y, Guo X, He Y, Zhou J, Lv Q, et al. Comparative effectiveness of adjuvant treatment for resected hepatocellular carcinoma: a systematic review and network meta-analysis. Front Oncol (2021) 11:709278. doi: 10.3389/fonc.2021.709278
41. Li SH, Mei J, Cheng Y, Li Q, Wang QX, Fang CK, et al. Postoperative adjuvant hepatic arterial infusion chemotherapy with FOLFOX in hepatocellular carcinoma with microvascular invasion: a multicenter, phase III, randomized study. J Clin Oncol (2023) 41:1898–908. doi: 10.1200/JCO.22.01142
42. Bai S, Hu L, Liu J, Sun M, Sun Y, Xue F. Prognostic nomograms combined adjuvant lenvatinib for hepatitis b virus-related hepatocellular carcinoma with microvascular invasion after radical resection. Front Oncol (2022) 12:919824. doi: 10.3389/fonc.2022.919824
43. Chen W, Hu S, Liu Z, Sun Y, Wu J, Shen S, et al. Adjuvant anti-PD-1 antibody for hepatocellular carcinoma with high recurrence risks after hepatectomy. Hepatol Int (2023) 17(2): 406–416. doi: 10.1007/s12072-022-10478-6
44. Dong Q, Diao Y, Sun X, Zhou Y, Ran J, Zhang J. Evaluation of tyrosine kinase inhibitors combined with antiprogrammed cell death protein 1 antibody in tyrosine kinase inhibitor-responsive patients with microsatellite stable/proficient mismatch repair metastatic colorectal adenocarcinoma: protocol for open-label, single-arm trial. BMJ Open (2022) 12:e049992. doi: 10.1136/bmjopen-2021-049992
45. Marmorino F, Boccaccino A, Germani MM, Falcone A, Cremolini C. Immune checkpoint inhibitors in pMMR metastatic colorectal cancer: a tough challenge. Cancers (Basel) (2020) 12. doi: 10.3390/cancers12082317
Keywords: hepatocellular carcinoma, high-risk recurrent factors, tyrosine kinase inhibitors, anti-PD-1 antibodies, postoperative adjuvant therapy, surgical outcomes
Citation: Li J, Wang W-q, Zhu R-h, Lv X, Wang J-l, Liang B-y, Zhang E-l and Huang Z-y (2023) Postoperative adjuvant tyrosine kinase inhibitors combined with anti-PD-1 antibodies improves surgical outcomes for hepatocellular carcinoma with high-risk recurrent factors. Front. Immunol. 14:1202039. doi: 10.3389/fimmu.2023.1202039
Received: 07 April 2023; Accepted: 24 May 2023;
Published: 08 June 2023.
Edited by:
Franz Rödel, University Hospital Frankfurt, GermanyReviewed by:
Lv Jun, First Affiliated Hospital of Zhengzhou University, ChinaSongqing He, Guangxi Medical University, China
Jiang Chen, Zhejiang University, China
Copyright © 2023 Li, Wang, Zhu, Lv, Wang, Liang, Zhang and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhi-yong Huang, aHVhbmd6eUB0amgudGptdS5lZHUuY24=; Er-lei Zhang, YmFpeXUxOTg2MTEwNEAxNjMuY29t
 Jian Li
Jian Li Wen-qiang Wang
Wen-qiang Wang Xing Lv
Xing Lv Er-lei Zhang
Er-lei Zhang Zhi-yong Huang
Zhi-yong Huang