
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol. , 09 August 2023
Sec. Cancer Immunity and Immunotherapy
Volume 14 - 2023 | https://doi.org/10.3389/fimmu.2023.1195659
This article is part of the Research Topic Research, Clinical Trials, Drug Resistance and Safety for Cancer Immunotherapy in Head and Neck Cancers and Brain Tumors View all 10 articles
 Huageng Huang1†
Huageng Huang1† Yuyi Yao1†
Yuyi Yao1† Xinyi Deng2†
Xinyi Deng2† Huawei Weng1
Huawei Weng1 Zegeng Chen1
Zegeng Chen1 Le Yu3
Le Yu3 Zhao Wang1
Zhao Wang1 Xiaojie Fang1
Xiaojie Fang1 Huangming Hong3
Huangming Hong3 He Huang1*‡
He Huang1*‡ Tongyu Lin1,3*‡
Tongyu Lin1,3*‡Background: Immunotherapy has been a hotspot in nasopharyngeal carcinoma (NPC) in recent years. This study aimed to provide a comprehensive landscape of the characteristics of immunotherapy clinical trials in NPC and to determine whether contemporary studies are of sufficient quality to demonstrate therapeutic value.
Methods: This is a cross-sectional analysis of NPC trials registered on ClinicalTrials.gov in the last 15 years (Jan 1, 2008-Nov 20, 2022). Only interventional trials with a primary purpose of treatment were included in the final analysis. Characteristics of immunotherapy trials were compared with those of other NPC trials. Chronological shifts in NPC immunotherapy trials were also analyzed.
Results: Of the 440 NPC studies selected, 161 (36.6%) were immunotherapy trials and 279 (63.4%) were other NPC trials. NPC immunotherapy trials were more likely than other NPC trials to be phase 1-2 (82.6% vs. 66.7%, P < 0.001), single-arm (51.3% vs. 39.6%, P = 0.020), non-randomized (64.8% vs. 44.4%, P < 0.001), and enroll fewer than 50 participants (46.3% vs. 34.4%, P = 0.015). Blinding was used in 8.8% of NPC immunotherapy trials. Also, 90.7% of NPC immunotherapy trials were recruited nationally and 82.6% were Asia-centric. Although academic institutions and governments (72.7%) were the major sponsors of NPC trials, immunotherapy trials were more likely to be industry-funded than other NPC trials (34.2% vs. 11.5%, P < 0.001). The number of NPC immunotherapy trials increased exponentially after 2017, attributed to the exploration of immune checkpoint inhibitors. Immunotherapy combined with chemotherapy was the most commonly investigated regimen.
Conclusion: NPC immunotherapy trials over a 15-year period were predominantly exploratory. To generate high-quality evidence and advance the clinical application of immunotherapy in NPC, more attention and concerted efforts are needed.
Nasopharyngeal carcinoma (NPC) is an Epstein-Barr virus (EBV)-related cancer that is particularly prevalent in South East Asia and Southern China (1). Unlike other head and neck cancers, NPC is susceptible to radiotherapy (RT), which has become the mainstay of treatment for this disease. Despite advances in RT techniques and optimization of chemotherapy regimens, about 20% of patients with locally advanced NPC (LANPC) will recur (2). Moreover, recurrent and/or metastatic (R/M) NPC remains the most serious challenge because the median overall survival (OS) of these patients is only 15.7 months (3). Current conventional treatments, including RT, chemotherapy and surgery, are often accompanied by serious adverse effects and limited efficacy (4). Therefore, there is an urgent need for novel treatment strategies to improve the prognosis of patients with NPC.
In recent years, immunotherapy has sparked a revolution in the clinical management of cancer (5, 6). NPC is regarded as a typical “immune-hot” tumor due to the expression of EBV antigen and CD4+/CD8+ T-cell target proteins (7, 8), massive lymphocytic infiltration (9), the expression of programmed death ligand-1 (PD-L1) up to 89-95% (10), and the presence of several key immune molecules (CD40, CD70, CD80, and CD86) that regulate T-cell activation (11). Several clinical studies on NPC immunotherapy have shown early successes (12–14). Nevertheless, aside from individual reports, the overall characteristics of NPC immunotherapy clinical trials and whether contemporary studies are of sufficient quality to demonstrate the therapeutic value of immunotherapy in personalized NPC practice are unclear.
ClinicalTrials.gov, a publicly available registry and results database for human clinical studies, provides the most comprehensive clinical study information worldwide. In 2004, the International Committee of Medical Journal Editors (ICMJE) announced a policy as a prerequisite for publication that requires the registration of clinical trials before enrolling participants (15, 16). As of Nov 20, 2022, ClinicalTrials.gov contains detailed information on more than 430 000 clinical trials conducted in over 200 countries. ClinicalTrials.gov is recognized as a promising information source for facilitating the systematic evaluation of clinical trials (16).
In this study, we examined all of the interventional NPC studies registered on ClinicalTrials.gov in 15 years (Jan 1, 2008-Nov 20, 2022). We compared the fundamental characteristics of NPC trials focusing on immunotherapy with the characteristics of other non-immunotherapy NPC trials, and we evaluated the changes over time.
This is a cross-sectional analysis of immunotherapy trials for NPC. We searched ClinicalTrials.gov on Nov 20, 2022 using the keyword “nasopharyngeal carcinoma”. In total, 803 registered clinical studies were identified and downloaded. We restricted our selection to interventional trials with a primary purpose of treatment that were registered between Jan 1, 2008 and Nov 20, 2022 (n = 440) (Figure 1). This study was considered exempt by the institutional review board of Sun Yat-sen University Cancer Center because it did not involve human participants.

Figure 1 Flowchart identifying trials registered on ClinicalTrials.gov from Jan 1, 2008 to Nov 20, 2022. NPC, nasopharyngeal carcinoma.
Study data accuracy was ensured by independent verification of all data by three investigators. Two oncologists (H.-G.H. and Y.-Y.Y.) manually and independently reviewed all of the selected trials, and a third author (T.-Y.L.) adjudicated any disagreements. Trials were then categorized according to treatment type, identified by the term “Intervention/treatment”, “Brief Summary”, or “Official Title”. If the treatment type was not clear, other registration information (e.g., “detailed description” and “eligibility”) was reviewed.
We defined immunotherapy trials as studies that (1) added immunotherapy to the standard of care (2); compared any treatment regimens with or without immunotherapy; (3) investigated novel immunotherapy regimens, such as new agents, usages, or dosages; (4) compared different immunotherapy regimens; and (5) evaluated interventions for immunotherapy-related complications. Although EBV-specific monoclonal antibody is a type of immunotherapy tool, some of them work in a more targeted way and partly overlap with ICIs and targeted therapy. Therefore, we excluded EBV-specific monoclonal antibody trials from the immunotherapy study.
Immunotherapy is categorized into four types in this study: (1) immune checkpoint inhibitors (ICIs), including but not limited to programmed cell death protein-1 (PD-1)/PD-L1/cytotoxic T-lymphocyte associated antigen-4 (CTLA-4)/lymphocyte activation gene-3 (LAG-3) inhibitors; (2) adoptive cell therapy (ACT), including adoptive cell transfer of autologous cytotoxic T lymphocytes (CTLs), tumor-infiltrating lymphocytes (TILs), cytokine-induced killer (CIK) cells, and genetically modified cellular immunotherapy such as chimeric antigen receptor-modified T (CAR-T) cell therapy and T cell receptor-engineered T (TCR-T) cell therapy; (3) vaccines; and (4) immunomodulators, including cytokines and oncolytic viruses. The remaining eligible studies constituted the other NPC trials, investigating RT, chemotherapy, surgery, targeted therapy, etc.
We extracted the following information for each trial: (1) whether the trial was registered before participant enrollment; (2) study phase; (3) sample size; (4) number of arms; (5) masking; (6) allocation methods; (7) number of centers; (8) national or international recruitment; (9) age selection; (10) funding source; (11) site location; and (12) recruitment status. As previously described (17–20), if a trial reported only one treatment arm, the allocation methods (if missing) were classified as non-randomized, and the blinding category (if missing) was classified as open-label.
Funding sources were assigned as an industry, National Institutes of Health (NIH), and other academic institutions or governments based on the recorded lead sponsor and/or collaborator for each clinical trial. A trial was classified as industry-funded if its lead sponsor or one of its collaborators was from the industry with no NIH involvement or NIH-funded if its lead sponsor or one of its collaborators was from the NIH with no industry involvement (21). All other trials were classified as other-funded studies.
Descriptive statistics were primarily used to summarize the clinical trial characteristics. Categorical variables were reported as frequencies and percentages. Missing values were excluded from the analyses unless they could be inferred from other relevant data. Trial characteristics were compared using the Pearson χ2 test, as well as Fisher’s exact test, if indicated. The statistical significance level was set at P < 0.05 (two-sided). Analyses were undertaken using SPSS, version 25.0 (IBM Corp).
Of the 440 NPC trials eligible for analysis, 161 (36.6%) were immunotherapy trials and 279 (63.4%) were other NPC trials (Figure 1).
Table 1 shows the trial characteristics of immunotherapy and other NPC trials included in this study. Immunotherapy trials were more likely than other NPC trials to be registered before participant enrollment (116 of 161 [72.0%] vs. 128 of 279 [45.9%], P < 0.001). In addition, immunotherapy trials tended to have more phase 1-2 studies (133 of 161 [82.6%] vs. 164 of 246 [66.7%], P < 0.001) and less likely to be phase 3 studies (23 of 161 [14.3%] vs. 68 of 246 [27.6%], P = 0.002) than the other NPC trials. Furthermore, immunotherapy trials were more likely to be single-arm (80 of 156 [51.3%] vs. 109 of 275 [39.6%], P = 0.020), non-randomized (103 of 159 [64.8%] vs. 123 of 277 [44.4%], P < 0.001), and enroll fewer than 50 participants (74 of 160 [46.3%] vs. 96 of 279 [34.4%], P = 0.015) compared with other NPC trials. Blinding was used in 8.8% (14 of 159) of NPC immunotherapy trials and 90.7% (146 of 161) of NPC immunotherapy trials recruited nationally.
Although other funding sources accounted for the highest proportion of immunotherapy and other NPC trials (97 of 161 [60.2%] vs. 223 of 279 [79.9%], P < 0.001), immunotherapy trials were more likely to be industry-funded than the other NPC trials (55 of 161 [34.2%] vs. 32 of 279 [11.5%], P < 0.001). Asia was the most common study location for the NPC immunotherapy trials (133 of 161 [82.6%]), followed by the United States (US)/Canada (32 of 161 [19.9%]). The most commonly recruited population for NPC immunotherapy trials was distributed in China (120 of 161 [74.5%]), followed by the US (31 of 161 [19.3%]) and Singapore (16 of 161 [9.9%]) (Figure 2)
With regard to recruitment status, immunotherapy trials were more likely to be ongoing (118 of 161 [73.3%] vs. 89 of 279 [31.9%], P < 0.001) and less likely to be completed (19 of 161 [11.8%] vs. 77 of 279 [27.6%], P < 0.001) than other NPC trials. Despite the marginal difference, immunotherapy trials had a lower proportion of trials that stopped early than the other NPC trials (6 of 161 [3.7%] vs. 24 of 279 [8.6%], P = 0.075).
Figure 3A shows chronological shifts in the number of NPC immunotherapy trials. Between 2008 and 2017, the number of NPC immunotherapy trials remained relatively stable, ranging between 2 and 7 annually. The number of NPC immunotherapy trials increased from 15 in 2018 to 32 in 2021 (P = 0.001). As of Nov 20, 2022, the number of NPC immunotherapy trials in 2022 had reached 32, the same as in 2021.
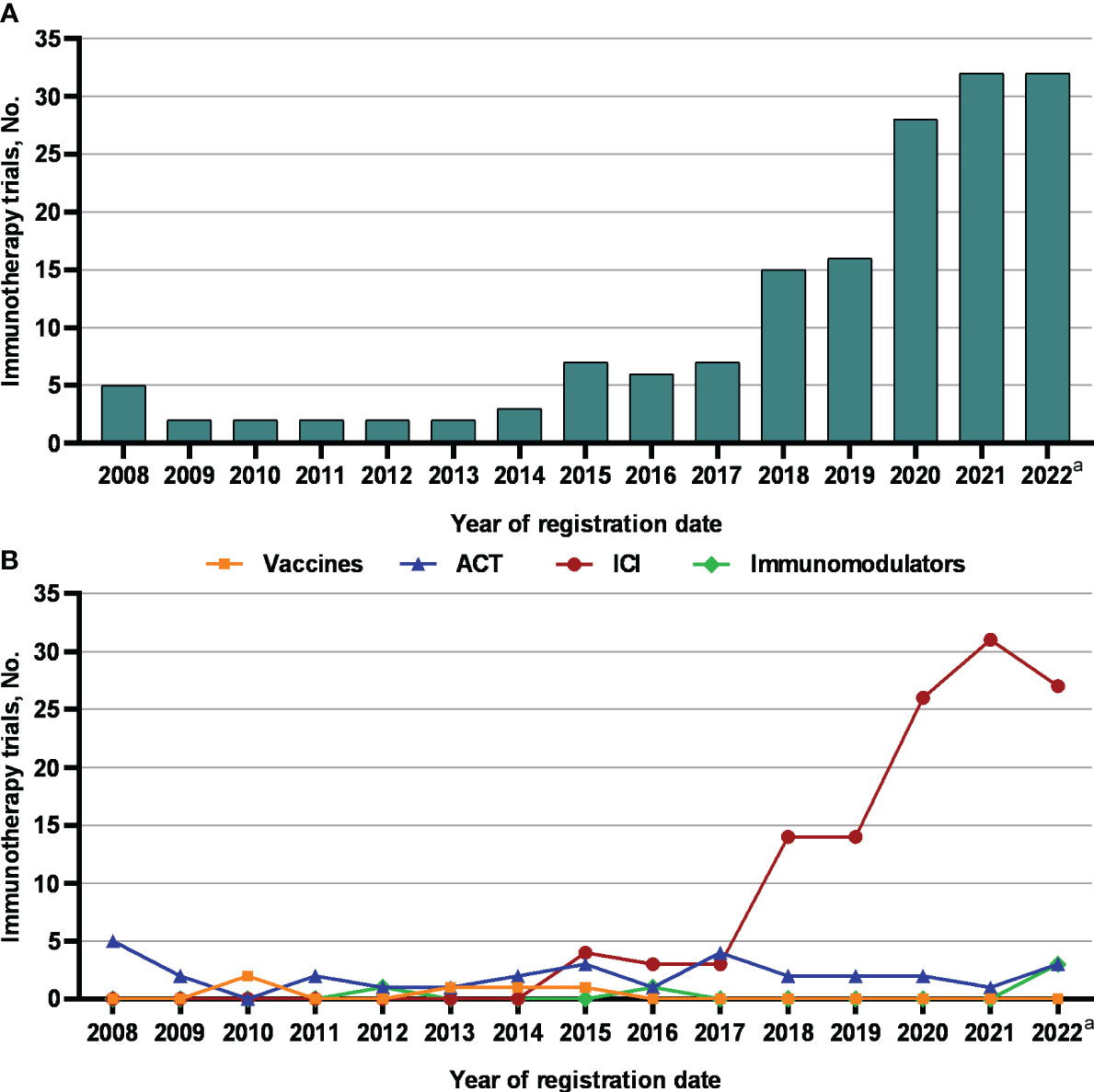
Figure 3 The number of (A) immunotherapy trials and (B) different types of immunotherapy trials for nasopharyngeal carcinoma registered on ClinicalTrials.gov between 2008 and 2022. ACT, adoptive cell therapy; ICIs, immune checkpoint inhibitors. a Observed period was Jan 1, 2022 to Nov 20, 2022.
Furthermore, we looked at the chronological shifts in the number of different types of immunotherapy trials for NPC (Figure 3B). From 2008 to 2017, the numbers of ICIs, ACT, vaccine and immunomodulator trials were relatively stable (fewer than 5 annually). Notably, the number of ICI trials rapidly increased to 14 in 2018 and 2019 and doubled to approximately 30 from 2020 to 2022. Toripalimab was the most commonly investigated ICIs in NPC (30 of 121 [24.8%]), followed by camrelizumab (20 of 121 [16.5%]) (Figure 4). But the numbers of ACT, vaccine and immunomodulator trials stayed stagnant.
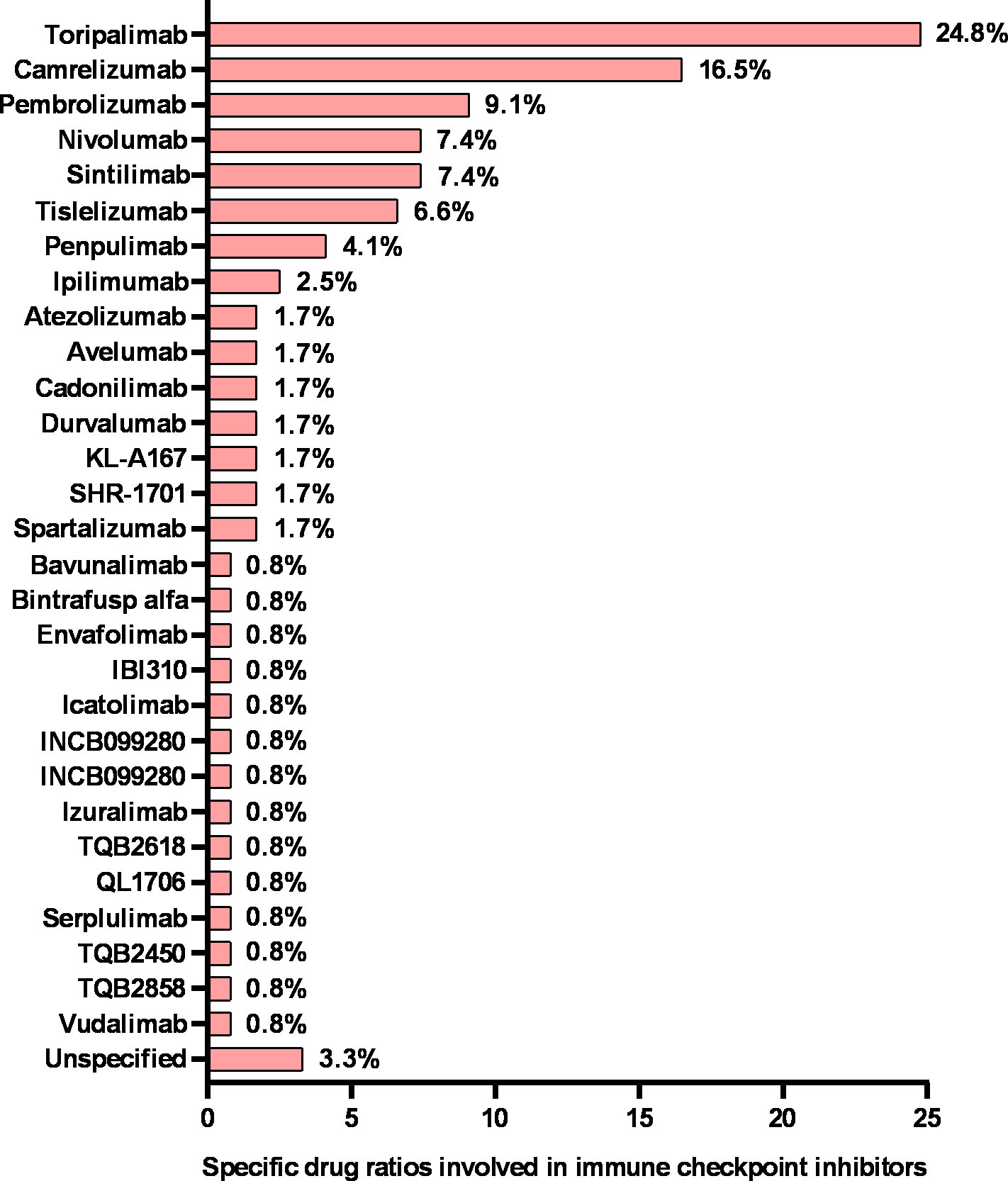
Figure 4 Specific drug ratios in nasopharyngeal cancer clinical trials involving immune checkpoint inhibitors. The sum of the percentages may exceed 100% because categories are not mutually exclusive.
Because NPC immunotherapy trials increased exponentially in number after 2017, we analyzed chronological shifts in the characteristics of NPC immunotherapy trials in the two periods Jan 1, 2008 to Dec 31, 2017 (n = 38, 23.6%) and Jan 1, 2018 to Nov 20, 2022 (n = 123, 76.4%) (Table 2). Compared to 2008-2017, a higher proportion of immunotherapy trials were registered before first participant enrollment in 2018-2022 (94 of 123 [76.4%] vs. 22 of 38 [57.8%], P = 0.038). Immunotherapy trials were more likely to be phase 2-3 in 2018-2022 than in 2008-2017 (96 of 123 [78.0%] vs. 17 of 38 [44.7%], P < 0.001). Despite the marginal difference, more immunotherapy trials had a sample size of more than 100 patients in 2018-2022 than in 2008-2017 (52 of 123 [42.3%] vs. 9 of 37 [24.3%], P = 0.055). The two periods’ basic trial characteristics remained unchanged (all P > 0.05).

Table 2 Trend changes in characteristics of immunotherapy trials for nasopharyngeal carcinoma registered on ClinicalTrials.gov between Jan 1, 2008 to Dec 31, 2017, and Jan 1, 2018 to Nov 20, 2022.
The number of industry-funded NPC immunotherapy trials increased marginally from 8 of 38 studies (21.1%) in 2008-2017 to 47 of 123 studies (38.2%) in 2018-2022 (P = 0.077), but the number of NIH-funded immunotherapy trials decreased significantly from 7 of 38 studies (18.4%) in 2008-2017 to 2 of 123 studies (1.6%) in 2018-2022 (P = 0.001). The proportion of other-funded immunotherapy trials remained stable at approximately 60%. In terms of study locations, there was a significant decrease in US/Canada centric from 16 of 38 studies (42.1%) in 2008-2017 to 16 of 123 studies (13.0%) in 2018-2022 (P < 0.001) but a significant increase in Asia centric from 22 of 38 studies (57.9%) in 2008-2017 to 111 of 123 studies (90.2%) in 2018-2022 (P < 0.001).
Among the 161 NPC immunotherapy trials, 46 (28.6%) evaluated single agents and 115 (71.4%) were designed to investigate immunotherapy combination strategies (Table 3). Monotherapy (34 of 46 [73.9%]) was the most commonly explored immunotherapy regimen in single usage, followed by immunotherapy maintenance after standard treatment (11 of 46 [23.9%]). The highest proportion of immunotherapy combination strategies investigated was combination chemotherapy (39 of 115 [33.9%]), followed by radiochemotherapy (27 of 115 [23.5%]) and targeted therapy (19 of 115 [16.5%]).
Well-designed clinical trials are desperately needed to validate the clinical applications of immunotherapy in NPC, given its promising efficacy. However, with an overall low incidence rate worldwide for its unique epidemiology, NPC does not attract much attention from most research. To the best of our knowledge, this is the first study assessing the critical characteristics of NPC immunotherapy trials over a 15-year period. By evaluating a comprehensive landscape, we found that NPC immunotherapy trials were predominantly phase 1-2 trials of limited sample size and tended to be single-arm, non-randomized and industry-funded. Blinding was rarely used. Asia was the major study location and clinical trials with international collaboration were lacking as well. The number of NPC immunotherapy trials increased exponentially after 2017, attributed to the exploration of ICIs. But the progress in trial design over time was slow and the basic trial characteristics largely remained unchanged. These findings raise concerns that trials evaluating the therapeutic role of immunotherapy in NPC may not be received the attention or efforts necessary to generate high-quality data. As a result, this orientation toward a less robust design may compromise evidence-based care for NPC.
As an EBV-associated malignancy, NPC is frequently infiltrated with varied stromal cells, making its microenvironment a highly heterogeneous and suppressive harbor that protects NPC cells from drug penetration and immune attack and promotes tumor progression (22, 23). This general immune landscape of NPC renders patients suitable for immunotherapy. In the past 15 years, immunotherapy trials accounted for 36.6% of all NPC trials. Unfortunately, 82.6% of NPC immunotherapy trials were phase 1-2 studies and tended to be single-arm, non-randomized, and enrolled less than 50 participants. Actually, the high proportion of single-arm, non-randomized, early-phase studies may either be because these studies are exploratory, hypotheses generating to fuel future randomized trials involving more patients, or because they were studying more highly innovative expensive cellular-based studies where funding was often inadequate for larger studies with more patients. In addition, the well-defined geographic distribution of NPC might further limit clinicians from conducting large-scale immunotherapy trials. Similarly, Xu et al. (24) tracked the evolving landscape of global immuno-oncology trials in 2007-2019 and found that most immunotherapy trials worldwide were phase 2 studies. Fortunately, NPC immunotherapy trials in 2018-2022 were more likely to be phase 2-3 (78.0% vs. 44.7%, P < 0.001) and had a sample size of more than 100 patients (42.3% vs. 24.3%, P = 0.055) than in 2008-2017. However, the other basic trial characteristics did not improve in an obvious manner over time.
Establishing international collaborative groups to foster research networks is an effective way to enroll more participants and improve the power of a study. However, 90.7% of NPC immunotherapy trials were conducted in only one region without sufficient international collaboration. Furthermore, in contrast to the findings that the US leads global immunotherapy research with stable growth (24), Asia (82.6%) is the major study location for NPC immunotherapy trials. And the proportion of US/Canada-centric decreased from 42.1% to 13.0% (P < 0.001) while the proportion of Asia-centric increased from 57.9% to 90.2% (P < 0.001) over the two periods. It’s not surprising because the Asian centricity is concordant with the unique epidemiology of NPC as a predominantly Asian disease. The patterns of NPC (incidence, histology) are different in South East Asia and the rest of the world. It would be helpful if clinical trials could address this discrepancy in future study designs.
Generally, the lengthy duration and high cost of immunotherapy trials may suppress industry enthusiasm. However, our findings showed that NPC immunotherapy trials were more likely to be industry-funded compared with other NPC trials (34.2% vs. 11.5%, P < 0.001) and the proportion has increased over time (21.1% vs. 38.2%, P = 0.077). It indicates a large market potential in the field of NPC immunotherapy, thus raising financial interests and industrial enthusiasm for sponsorship of such trials. Moreover, 60.2% of NPC immunotherapy trials were other-funded, and this proportion has remained stable over time. It implies that academic institutions and governments continue to play an important role in supporting immunotherapy clinical research for NPC and shoulder vital public health responsibility. Still, allocating more resources to NPC immunotherapy from all relevant parties is essential to improve the effective leveraging of the constrained resources.
It is noteworthy that there was an increasing number of NPC immunotherapy trials after 2017. Actually, this reflects more recent successes in other major tumor types, and therefore there is an increasing interest in studying this intervention in an EBV-related tumor type like NPC that does not have many mutational targets. However, only the number of ICI trials increased significantly, while the numbers of ACT, vaccine and immunomodulator trials remained stagnant. A potential explanation is that ICIs as pan-cancerous antitumor agents were found to have equally promising efficacy in NPC, thus spurring enthusiasm for research. Furthermore, the recognition of ICI-based immunotherapy by the 2018 Nobel Prize in Physiology or Medicine might have further increased researchers’ interest in the exploration of ICIs in NPC. In contrast, the exploration and further application of EBV-specific ACTs and vaccines and immunomodulators were hampered by the lack of specific and effective targets, generally low and transient immune responses, technical limitations and financial shortages. According to the results of published NPC studies (Supplementary Table 1), ICIs monotherapy achieves 17.1-34.0% of the objective response rate in the second or later-line treatment of R/M NPC (14, 25–30). In the first-line treatment, the addition of ICIs to chemotherapy also significantly improved progression-free survival and OS in R/M NPC (31–33). Further studies are needed to assess the therapeutic value of ICIs in LANPC and early-stage disease. Notably, CAR-T/TCR-T cell therapy and antibody-drug conjugates may be another promising immunotherapy modality for NPC, as they have shown promising efficacy in a variety of other cancers (34, 35). Therefore, concerted efforts by oncologists, sponsors and other concerned parties are still needed to advance the development of immunotherapy for NPC.
Integration with conventional treatment modalities is one of the trends in immunotherapy. In this study, we found that the most commonly investigated immunotherapy regimen in NPC was combination chemotherapy, followed by combination radiochemotherapy. A recently published study reported on the promising antitumor activity and a manageable toxicity profile of immunotherapy combined with antiangiogenic therapy in R/M NPC (36). In addition, future NPC studies could consider more novel combination strategies to enhance the clinical responses, for example, ICIs combined with ACT (37) or CAR-T cell therapy combined with the oncolytic virus (38).
Limitations of this study should also be acknowledged. First, not all investigators choose ClinicalTrials.gov to register their projects. There are many alternative registries available around the world (39). Nevertheless, ClinicalTrials.gov is the most robust database to date, accounting for 70–80% of the unique clinical trials recorded by the World Health Organization (39). Second, partial NPC trials have not yet been registered on ClinicalTrials.gov, which hindered us from more fully reflecting current global trends in NPC immunotherapy trials. Third, the National Library of Medicine, which operates ClinicalTrials.gov, is unable to validate all registered data. The accuracy of the data relies on the study sponsor. Fourth, we did not include noninterventional trials in our analysis.
In conclusion, NPC Immunotherapy trials over a 15-year period have been largely exploratory. Advancing the clinical application of immunotherapy in NPC requires more attention and concerted efforts to improve the quality of trials.
Publicly available datasets were analyzed in this study. This data can be found here: https://clinicaltrials.gov/.
TL had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. HGH, YY, and XD contributed equally to this work. Concept and design: HGH, YY, XD, HH, and TL. Acquisition, analysis, or interpretation of data: HGH, YY, XD, and TL. Drafting of the manuscript: HGH, YY, and XD. Critical revision of the manuscript for important intellectual content: All authors. Statistical analysis: HGH and XD. Obtained funding: HH and TL. Administrative, technical, or material support: HH and TL. Supervision: HH and TL. All authors contributed to the article and approved the submitted version.
This study is supported by the National Natural Science Foundation of China-Science and Technology Development Fund, Macau SAR (No. 81661168011), and the Regional Innovation and Cooperation Project of Sichuan Province (No. 2021YFQ0037).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer YG declared a shared parent affiliation with the authors HGH, YY, HW, ZC, ZW, XF, HH, and TL to the handling editor at the time of review.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1195659/full#supplementary-material
1. Wong KCW, Hui EP, Lo KW, Lam WKJ, Johnson D, Li L, et al. Nasopharyngeal carcinoma: an evolving paradigm. Nat Rev Clin Oncol (2021) 18(11):679–95. doi: 10.1038/s41571-021-00524-x
2. Huang CL, Guo R, Li JY, Xu C, Mao YP, Tian L, et al. Nasopharyngeal carcinoma treated with intensity-modulated radiotherapy: clinical outcomes and patterns of failure among subsets of 8th AJCC stage IVa. Eur Radiol (2020) 30(2):816–22. doi: 10.1007/s00330-019-06500-5
3. Prawira A, Oosting SF, Chen TW, Delos Santos KA, Saluja R, Wang L, et al. Systemic therapies for recurrent or metastatic nasopharyngeal carcinoma: a systematic review. Br J cancer. (2017) 117(12):1743–52. doi: 10.1038/bjc.2017.357
4. Poh SS, Soong YL, Sommat K, Lim CM, Fong KW, Tan TW, et al. Retreatment in locally recurrent nasopharyngeal carcinoma: Current status and perspectives. Cancer Commun (London England). (2021) 41(5):361–70. doi: 10.1002/cac2.12159
6. Hargadon KM, Johnson CE, Williams CJ. Immune checkpoint blockade therapy for cancer: An overview of FDA-approved immune checkpoint inhibitors. Int immunopharmacol (2018) 62:29–39. doi: 10.1016/j.intimp.2018.06.001
7. Smith C, Wakisaka N, Crough T, Peet J, Yoshizaki T, Beagley L, et al. Discerning regulation of cis- and trans-presentation of CD8+ T-cell epitopes by EBV-encoded oncogene LMP-1 through self-aggregation. Blood (2009) 113(24):6148–52. doi: 10.1182/blood-2009-02-203687
8. Münz C, Bickham KL, Subklewe M, Tsang ML, Chahroudi A, Kurilla MG, et al. Human CD4(+) T lymphocytes consistently respond to the latent Epstein-Barr virus nuclear antigen EBNA1. J Exp Med (2000) 191(10):1649–60. doi: 10.1084/jem.191.10.1649
9. Lee AZE, Tan LSY, Lim CM. Cellular-based immunotherapy in Epstein-Barr virus induced nasopharyngeal cancer. Oral Oncol (2018) 84:61–70. doi: 10.1016/j.oraloncology.2018.07.011
10. Outh-Gauer S, Alt M, Le Tourneau C, Augustin J, Broudin C, Gasne C, et al. Immunotherapy in head and neck cancers: A new challenge for immunologists, pathologists and clinicians. Cancer Treat Rev (2018) 65:54–64. doi: 10.1016/j.ctrv.2018.02.008
11. Agathanggelou A, Niedobitek G, Chen R, Nicholls J, Yin W, Young LS. Expression of immune regulatory molecules in Epstein-Barr virus-associated nasopharyngeal carcinomas with prominent lymphoid stroma. Evidence for a functional interaction between epithelial tumor cells and infiltrating lymphoid cells. Am J pathology. (1995) 147(4):1152–60.
12. Li F, Song D, Lu Y, Zhu H, Chen Z, He X. Delayed-type hypersensitivity (DTH) immune response related with EBV-DNA in nasopharyngeal carcinoma treated with autologous dendritic cell vaccination after radiotherapy. J immunother (Hagerstown Md: 1997). (2013) 36(3):208–14. doi: 10.1097/CJI.0b013e31828bd87b
13. Smith C, Lee V, Schuessler A, Beagley L, Rehan S, Tsang J, et al. Pre-emptive and therapeutic adoptive immunotherapy for nasopharyngeal carcinoma: Phenotype and effector function of T cells impact on clinical response. Oncoimmunology (2017) 6(2):e1273311. doi: 10.1080/2162402x.2016.1273311
14. Ma BBY, Lim WT, Goh BC, Hui EP, Lo KW, Pettinger A, et al. Antitumor activity of nivolumab in recurrent and metastatic nasopharyngeal carcinoma: an international, multicenter study of the mayo clinic phase 2 consortium (NCI-9742). J Clin Oncol (2018) 36(14):1412–8. doi: 10.1200/jco.2017.77.0388
15. De Angelis C, Drazen JM, Frizelle FA, Haug C, Hoey J, Horton R, et al. Clinical trial registration: a statement from the International Committee of Medical Journal Editors. New Engl J Med (2004) 351(12):1250–1. doi: 10.1056/NEJMe048225
16. De Angelis C, Drazen JM, Frizelle FA, Haug C, Hoey J, Horton R, et al. Clinical trial registration: a statement from the International Committee of Medical Journal Editors. Lancet (London England). (2004) 364(9438):911–2. doi: 10.1016/s0140-6736(04)17034-7
17. Chen YP, Lv JW, Liu X, Zhang Y, Guo Y, Lin AH, et al. The landscape of clinical trials evaluating the theranostic role of PET imaging in oncology: insights from an analysis of clinicalTrials. gov Database. Theranostics (2017) 7(2):390–9. doi: 10.7150/thno.17087
18. Califf RM, Zarin DA, Kramer JM, Sherman RE, Aberle LH, Tasneem A. Characteristics of clinical trials registered in ClinicalTrials. gov 2007-2010. JAMA (2012) 307(17):1838–47. doi: 10.1001/jama.2012.3424
19. Hirsch BR, Califf RM, Cheng SK, Tasneem A, Horton J, Chiswell K, et al. Characteristics of oncology clinical trials: insights from a systematic analysis of ClinicalTrials. gov. JAMA Internal Med (2013) 173(11):972–9. doi: 10.1001/jamainternmed.2013.627
20. Liu X, Zhang Y, Tang LL, Le QT, Chua MLK, Wee JTS, et al. Characteristics of radiotherapy trials compared with other oncological clinical trials in the past 10 years. JAMA Oncol (2018) 4(8):1073–9. doi: 10.1001/jamaoncol.2018.0887
21. Liu X, Zhang Y, Li WF, Vokes E, Sun Y, Le QT, et al. Evaluation of oncology trial results reporting over a 10-year period. JAMA network Open (2021) 4(5):e2110438. doi: 10.1001/jamanetworkopen.2021.10438
22. Tsang CM, Lui VWY, Bruce JP, Pugh TJ, Lo KW. Translational genomics of nasopharyngeal cancer. Semin Cancer Biol (2020) 61:84–100. doi: 10.1016/j.semcancer.2019.09.006
23. Chen YP, Yin JH, Li WF, Li HJ, Chen DP, Zhang CJ, et al. Single-cell transcriptomics reveals regulators underlying immune cell diversity and immune subtypes associated with prognosis in nasopharyngeal carcinoma. Cell Res (2020) 30(11):1024–42. doi: 10.1038/s41422-020-0374-x
24. Xu C, Zhang S, Zhang Y, Tang SQ, Fang XL, Zhu GL, et al. Evolving landscape and academic attitudes toward the controversies of global immuno-oncology trials. Int J cancer. (2021) 149(1):108–18. doi: 10.1002/ijc.33503
25. Hsu C, Lee SH, Ejadi S, Even C, Cohen RB, Le Tourneau C, et al. Safety and antitumor activity of pembrolizumab in patients with programmed death-ligand 1-positive nasopharyngeal carcinoma: results of the KEYNOTE-028 study. J Clin Oncol (2017) 35(36):4050–6. doi: 10.1200/jco.2017.73.3675
26. Fang W, Yang Y, Ma Y, Hong S, Lin L, He X, et al. Camrelizumab (SHR-1210) alone or in combination with gemcitabine plus cisplatin for nasopharyngeal carcinoma: results from two single-arm, phase 1 trials. Lancet Oncol (2018) 19(10):1338–50. doi: 10.1016/s1470-2045(18)30495-9
27. Yang Y, Zhou T, Chen X, Li J, Pan J, He X, et al. Efficacy, safety, and biomarker analysis of Camrelizumab in Previously Treated Recurrent or Metastatic Nasopharyngeal Carcinoma (CAPTAIN study). J Immunother Cancer (2021) 9(12):e003790. doi: 10.1136/jitc-2021-003790
28. Wang FH, Wei XL, Feng J, Li Q, Xu N, Hu XC, et al. Efficacy, safety, and correlative biomarkers of toripalimab in previously treated recurrent or metastatic nasopharyngeal carcinoma: A phase II clinical trial (POLARIS-02). J Clin Oncol (2021) 39(7):704–12. doi: 10.1200/jco.20.02712
29. Even C, Wang HM, Li SH, Ngan RK, Dechaphunkul A, Zhang L, et al. Randomized study of spartalizumab (PDR001), an anti-PD-1 antibody, versus chemotherapy in patients with recurrent/metastatic nasopharyngeal cancer. Clin Cancer Res (2021) 27(23):6413–23. doi: 10.1158/1078-0432.Ccr-21-0822
30. Shi Y, Qin X, Peng X, Zeng A, Li J, Chen C, et al. Efficacy and safety of KL-A167 in previously treated recurrent or metastatic nasopharyngeal carcinoma: a multicenter, single-arm, phase 2 study. Lancet regional Health Western Pacific (2023) 31:100617. doi: 10.1016/j.lanwpc.2022.100617
31. Yang Y, Qu S, Li J, Hu C, Xu M, Li W, et al. Camrelizumab versus placebo in combination with gemcitabine and cisplatin as first-line treatment for recurrent or metastatic nasopharyngeal carcinoma (CAPTAIN-1st): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol (2021) 22(8):1162–74. doi: 10.1016/s1470-2045(21)00302-8
32. Mai HQ, Chen QY, Chen D, Hu C, Yang K, Wen J, et al. Toripalimab or placebo plus chemotherapy as first-line treatment in advanced nasopharyngeal carcinoma: a multicenter randomized phase 3 trial. Nat Med (2021) 27(9):1536–43. doi: 10.1038/s41591-021-01444-0
33. Yang Y, Pan J, Wang H, Zhao Y, Qu S, Chen N, et al. Tislelizumab plus chemotherapy as first-line treatment for recurrent or metastatic nasopharyngeal cancer: A multicenter phase 3 trial (RATIONALE-309). Cancer Cell (2023) 41(6):1061–72.e4. doi: 10.1016/j.ccell.2023.04.014
34. Norberg SM, Hinrichs CS. Engineered T cell therapy for viral and non-viral epithelial cancers. Cancer Cell (2023) 41(1):58–69. doi: 10.1016/j.ccell.2022.10.016
35. Thomas A, Teicher BA, Hassan R. Antibody-drug conjugates for cancer therapy. Lancet Oncol (2016) 17(6):e254–e62. doi: 10.1016/s1470-2045(16)30030-4
36. Ding X, Zhang WJ, You R, Zou X, Wang ZQ, Ouyang YF, et al. Camrelizumab plus apatinib in patients with recurrent or metastatic nasopharyngeal carcinoma: an open-label, single-arm, phase II study. J Clin Oncol (2023), Jco2201450. doi: 10.1200/jco.22.01450
37. Smith C, McGrath M, Neller MA, Matthews KK, Crooks P, Le Texier L, et al. Complete response to PD-1 blockade following EBV-specific T-cell therapy in metastatic nasopharyngeal carcinoma. NPJ Precis Oncol (2021) 5(1):24. doi: 10.1038/s41698-021-00162-7
38. Rezaei R, Esmaeili Gouvarchin Ghaleh H, Farzanehpour M, Dorostkar R, Ranjbar R, Bolandian M, et al. Combination therapy with CAR T cells and oncolytic viruses: a new era in cancer immunotherapy. Cancer Gene Ther (2022) 29(6):647–60. doi: 10.1038/s41417-021-00359-9
Keywords: nasopharyngeal carcinoma, immunotherapy, Clinicaltrials.gov, clinical trial, immune checkpoint inhibitor
Citation: Huang H, Yao Y, Deng X, Weng H, Chen Z, Yu L, Wang Z, Fang X, Hong H, Huang H and Lin T (2023) Characteristics of immunotherapy trials for nasopharyngeal carcinoma over a 15-year period. Front. Immunol. 14:1195659. doi: 10.3389/fimmu.2023.1195659
Received: 28 March 2023; Accepted: 24 July 2023;
Published: 09 August 2023.
Edited by:
Takumi Kumai, Asahikawa Medical University, JapanReviewed by:
Ying Guo, Third Affiliated Hospital of Sun Yat-sen University, ChinaCopyright © 2023 Huang, Yao, Deng, Weng, Chen, Yu, Wang, Fang, Hong, Huang and Lin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tongyu Lin, bGludHlAc3lzdWNjLm9yZy5jbg==; He Huang, aHVhbmdoZUBzeXN1Y2Mub3JnLmNu
†These authors have contributed equally to this work and share first authorship
‡These authors have contributed equally to this work and share last authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.