
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol., 14 August 2023
Sec. Inflammation
Volume 14 - 2023 | https://doi.org/10.3389/fimmu.2023.1190380
Background: Inflammation plays an integral role in the development of cardiovascular disease, and few studies have identified different biomarkers to predict the prognosis of cardiac surgery. But there is a lack of reliable and valid evidence to determine the optimal systemic inflammatory biomarkers to predict prognosis.
Methods: From December 2015 and March 2021, we collected 10 systemic inflammation biomarkers among 820 patients who underwent cardiac surgery. Time-dependent receiver operating characteristic curves (ROC) curve at different time points and C-index was compared at different time points. Kaplan–Meier method was performed to analyze overall survival (OS). Cox proportional hazard regression analyses were used to assess independent risk factors for OS. A random internal validation was conducted to confirm the effectiveness of the biomarkers.
Results: The area under the ROC of lymphocyte-to-C-reactive protein ratio (LCR) was 0.655, 0.620 and 0.613 at 1-, 2- and 3-year respectively, and C-index of LCR for OS after cardiac surgery was 0.611, suggesting that LCR may serve as a favorable indicator for predicting the prognosis of cardiac surgery. Patients with low LCR had a higher risk of postoperative complications. Besides, Cox proportional hazard regression analyses indicated that LCR was considered as an independent risk factor of OS after cardiac surgery.
Conclusion: LCR shows promise as a noteworthy representative among the systemic inflammation biomarkers in predicting the prognosis of cardiac surgery. Screening for low LCR levels may help surgeons identify high-risk patients and guide perioperative management strategies.
Cardiac surgery is the therapy of choice to treat complex or three-vessel coronary artery disease, congenital heart defects and valvular heart disease (1). Despite the decade-long effort of surgeons to improve cardiac surgery safety, it remains a high-risk procedure (2). Existing literature offers little guidance on best practices for identifying errors and improving procedure safety. Currently available risk models, such as EuroSCORE II and STS Risk Score, might not account for all prognostically significant risk factors (3). Therefore, it is essential to make every effort to identify additional risk factors and perform a comprehensive preoperative assessment to guide perioperative management strategies.
Systemic inflammation biomarkers are more commonly used in cancer patients, and they have been demonstrated to be predictive of tumor progression and overall survival (OS) (4–6). Systemic inflammation biomarkers take advantage of being non-invasive, inexpensive and easily accessible, and they have the potential to be cost-effective prognostic markers. However, there are few studies on inflammation biomarkers and the prognosis of cardiac surgery. Considering that inflammation plays an integral role in the pathogenesis and the development of cardiovascular disease (CVD) (7–9), it is necessary to assess the inflammatory status of patients underwent cardiac surgery. Moreover, inflammation is not only a causal agent of CVD but also a surrogate prognostic biomarker, implying the feasibility of anti-inflammatory therapy to improve CVD outcomes (10). Therefore, early detection and assessment of patients’ inflammatory status is of great significance in identifying patients with high inflammatory status, thus improving clinical outcomes and guiding anti-inflammatory intervention. Given the lack of reliable and valid evidence to determine the best combination of systemic inflammation biomarkers to predict prognosis, this study aims to compare various combination of systemic inflammation biomarkers systematically and comprehensively to identify the optimal systemic inflammation markers to assess prognosis in patients underwent cardiac surgery. To our knowledge, this is the first study to report the prognostic value of lymphocyte-to-C-reactive protein ratio (LCR) in patients underwent cardiac surgery. We also conducted a randomized internal validation to confirm the reliability of the conclusions.
This study encompassed patients who underwent coronary artery bypass grafting (CABG) and/or valve surgery at Shanghai Tenth People’s Hospital between December 2015 and March 2021. Adult patients (≥18 years) who possessed complete measurements of serum systemic inflammatory markers and received first major cardiac surgery through midline sternotomy met the inclusion criteria of the study. Aspirin antiplatelet therapy was routinely discontinued 5 days prior to surgery. Antibiotics were also not administered preoperatively. The exclusion criteria were as follows: (1) patients in emergency surgery or admitted to intensive care unit at the start of recruitment; (2) patients with significant clinical evidence of infection or inflammation; (3) cancer patients; (4) patients with incomplete data on peripheral systemic inflammatory features; (5) patients who are unable to communicate or provide informed consent. This project was registered at http://www.chictr.org.cn (registration number: ChiCTR2200056468) and ethical approval was obtained from the Ethics Review Committee of Shanghai Tenth People’s Hospital. All participants signed a written informed consent to participate in this study.
For each patient, demographic information was acquired by researchers trained in the project on admission, including age, sex, height, weight, comorbidities and lifestyle (smoking and drinking). During hospitalization, disease- and treatment-related information was recorded for all patients, including surgical type, operative time, postoperative complications (classified on the basis of the Clavien-Dindo classification), length of hospitalization, hospitalization expenses, and readmissions. Only complications categorized as grade II or higher were analyzed.
Blood samples were collected from each patient within 48 hours prior to receiving surgery and serological information was tested, including white blood cells, red blood cells, platelets, neutrophils, lymphocytes, monocytes, C-reactive protein (CRP), total protein, albumin, blood urea nitrogen (BUN), and creatinine levels. The inflammatory immune indicators in our study were calculated as follows: CRP-to-albumin ratio (CAR) = CRP/albumin; platelet-to-albumin ratio (PAR) = platelet/albumin; neutrophil-to-albumin ratio (NAR) = neutrophil/albumin; LCR = lymphocyte/CRP; platelet-to-lymphocyte ratio (PLR) = platelet/lymphocyte; neutrophil-to-lymphocyte ratio (NLR) = neutrophil/lymphocyte; systemic-immune-inflammation index (SII) = platelet×neutrophil/lymphocyte; Glasgow prognostic score (GPS), modified GPS (mGPS), and lymphocyte-CRP score (LCS) were determined based on previous reports (11, 12).
After discharge, follow-up was performed by telephone interviews or outpatient visits, which took place 1 month after surgery, then every 3 months for the first 2 years and every 6 months thereafter. OS was defined as the interval from the surgery date to death from any cause or to the last follow-up visit (January 31, 2022).
Continuous variables were presented as mean ± SD or median (25th and 75th percentiles) depending on their distribution, categorical variables were presented as numbers and percentages. Student’s t-test, Mann–Whitney U-test and Chi-squared or Fisher’s exact test were used to analyze the differences between groups. The time-dependent receiver operating characteristic (ROC) curve was calculated to compare the predicting ability of systemic inflammation biomarkers at different time points. The optimal cut-off of the inflammation biomarker was determined by the surv_cutpoint function of the R package survminer. Kaplan–Meier method was performed to analyze OS. Several incremental models were established to control for confounding bias, and the Cox proportional hazard regression analyses were used to assess independent risk factors for OS. Restricted cubic spline regression with four knots was used to evaluate the relationship between continuous covariates and OS of patients underwent cardiac surgery in the adjustment models mentioned above. The association of biomarkers and OS in subgroups were analyzed by stratification analysis. Finally, an internal validation was conducted to confirm the effectiveness of the biomarkers by random resampling with a ratio of 70%. A 2-tailed p-value < 0.05 was considered statistically significant. All statistical analyses were performed with SPSS software version 26 (Armonk, NY, USA) and R version 4.1.3 (Vienna, Austria).
According to the inclusion and exclusion criteria, 47 patients were excluded and a total of 820 patients underwent cardiac surgery were enrolled in this study (Table 1). The patient group included 542 men and 278 women with a median age of 64 (p25–p75: 57–69) years. Of these patients, 56.7% underwent CABG, 37.7% underwent valve surgery, and 5.6% underwent both CABG and valve surgery.
Time-dependent ROC curve at different time points was conducted to compare the effectiveness of the ten systemic inflammation biomarkers in predicting the OS of patients underwent cardiac surgery (Figure 1). The area under the ROC of LCR was 0.655, 0.620 and 0.613 at 1-, 2- and 3-year, respectively. The effectiveness of LCR was also compared with CRP alone, as well as with different populations of white blood cells (Supplementary Figure 1). Among these systemic inflammation biomarkers, LCS (0.619 [0.494, 0.743]) had the highest C-index for OS after cardiac surgery, followed by LCR (0.611 [0.548, 0.674]) (Table 2). Based on these findings, it appears that LCR may serve as a favorable indicator for predicting the prognosis of cardiac surgery. The optimal cut-off of LCR was 0.35, determined by the standardized log-rank statistic based on the survival status (Supplementary Figure 2). Thus, 241 and 579 patients were identified as low and high LCR respectively. The two groups were comparable in terms of sex, body mass index (BMI), comorbidities and lifestyle. Although the age and left ventricular ejection (LVEF) of the two groups were quite close, patients with low LCR were elder (65 [59–70] vs 63 [56–68] years, p = 0.002), and had lower LVEF (60 [53–64] vs 60 [57–64] %, p=0.002). Besides, patients with low LCR had higher white blood cells (p<0.001), neutrophil percentage (p<0.001), monocytes (p<0.001) and lower red blood cells (p<0.001), hemoglobin (p<0.001), hematocrit (HCT, p<0.001), albumin (p<0.001).
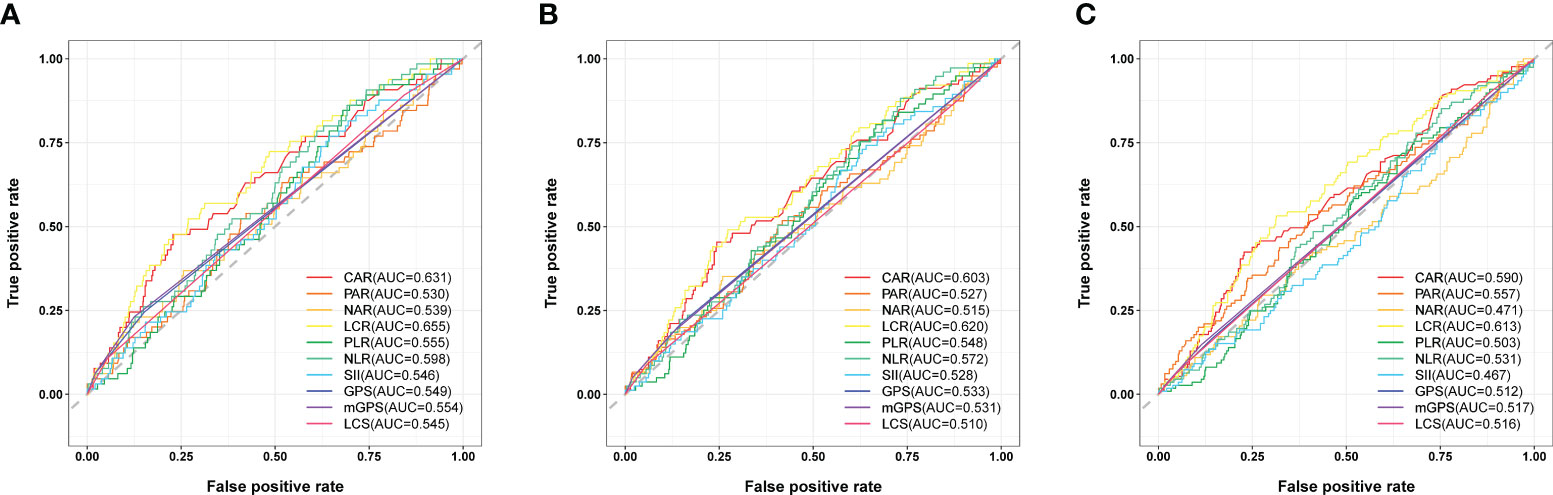
Figure 1 Comparison the effectiveness of systemic inflammation biomarkers in predicting the prognosis after cardiac surgery. (A) ROC curve analysis for survival prediction at 1-year. (B) ROC curve analysis for survival prediction at 2-year. (C) ROC curve analysis for survival prediction at 3-year.
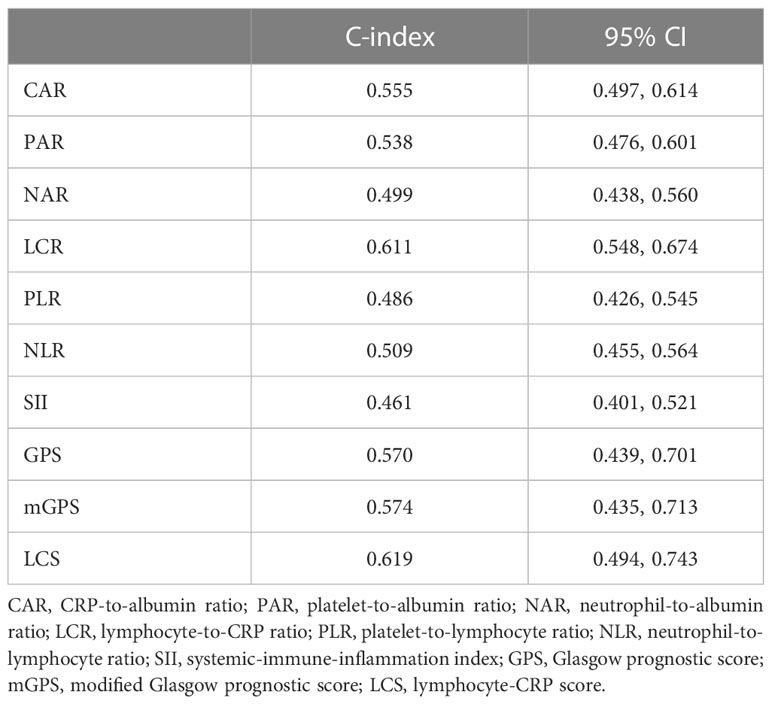
Table 2 The C-index of systemic inflammation biomarkers for OS in patients underwent cardiac surgery.
Table 3 presented the short-term outcomes of patients underwent cardiac surgery, with 367 (44.8%) experiencing postoperative complications. Low LCR was associated with a higher incidence of total complications (50.6% vs 42.3%, p = 0.029) and in-hospital mortality (9.1% vs 3.3%, p<0.001), with corresponding increases in hospitalization expenses (135145 [116721–161630] vs 128244 [111187–152505] Yuan, p = 0.009).
During the follow-up period (median 3.36 [2.18–4.51] years), 113 (13.8%) patients died. Patients with low LCR had a significantly higher mortality rate than those with high LCR (21.6% vs 10.5%, log-rank p <0.001, Figure 2). Restricted cubic spline regression revealed a significant nonlinearity between LCR and the OS of patients underwent cardiac surgery (Figure 3). Patients with lower LCR may experience a worse prognosis, and this trend persisted across all three models. Low LCR was defined as an independent risk factor for poor OS through multivariate Cox regression (Table 4). When LCR was treated as a continuous variable, the decrease based on standard deviation may not reflect the increased risk of mortality (p = 0.556). When LCR was divided into a categorical variable, low LCR demonstrated a close relationship with a poor prognosis in model c (HR = 0.468, 95% CI = 0.319–0.687, p<0.001). When LCR was divided into quartiles, the risk of poor prognosis gradually decreased compared to the lowest quintile Q1 (<0.29) and the HRs for OS in model c were 0.597 (95% CI =0.369–0.967), 0.507 (95% CI = 0.300–0.857) and 0.389 (95% CI = 0.220–0.686), respectively. When patients were stratified by age, sex, BMI, comorbidities, drinking status, smoking status, LVEF and surgical type, stratification analysis showed that the relationship between low LCR and poor OS remained stable in most of the subgroups (Supplementary Figure 3). None of the covariates had an interaction with low LCR (all p for interaction >0.050).
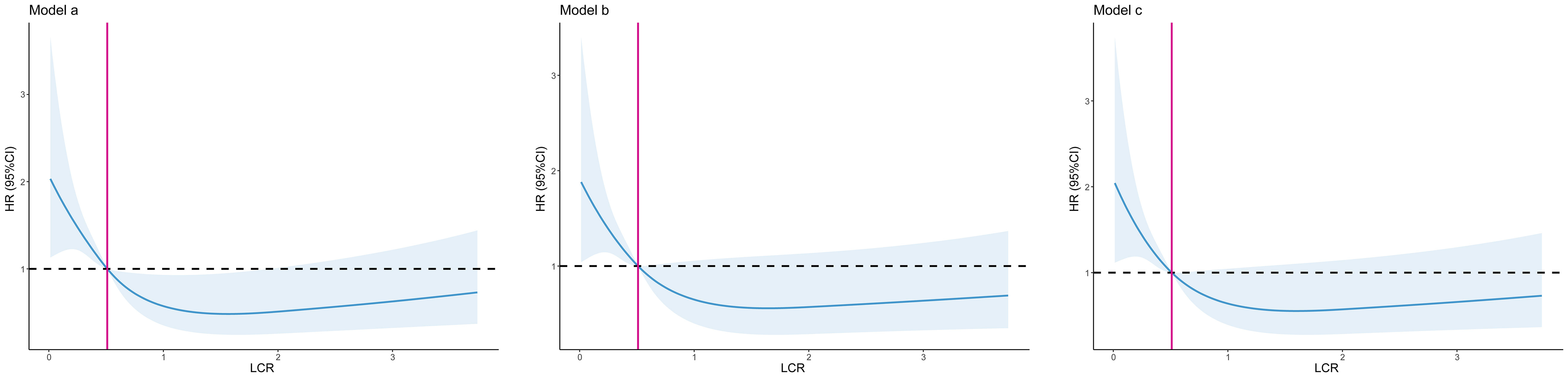
Figure 3 The association between LCR and all-cause mortality after cardiac surgery. Model a: No adjusted. Model b: Adjusted for age, sex, BMI, surgical type. Model c: Adjusted for age, sex, BMI, surgical type, hypertension, diabetes, chronic heart failure, cerebrovascular disease, operation time.
Random resampling with a ratio of 70% was performed to simulate outsource validation cohort and 574 patients were enrolled (Supplementary Table 1). Consistent with previous findings, Kaplan–Meier curve showed that LCR can effectively stratify the prognosis of patients underwent cardiac surgery in validation cohort (Supplementary Figure 4). Additionally, restricted cubic spline regression demonstrated a reverse L-shaped relationship between LCR and HRs of OS across all three models (Supplementary Figure 5). Supplementary Table 2 presented the prognostic value of LCR when analyzed as a continuous variable, categorical variable or quartiles. Patients with low LCR have poorer OS than those with high LCR in model c (HR = 0.445, 95% CI = 0.279–0.709, p<0.001).
In this study, we conducted a comprehensive comparative analysis to investigate the prognostic value of multiple systemic inflammation biomarkers in patients underwent cardiac surgery. Time-dependent ROC curve at different time points and C-index were conducted to compare the effectiveness of these ten systemic inflammation biomarkers in predicting the OS of patients underwent cardiac surgery. The effectiveness of LCR was also compared with CRP alone, as well as with different populations of white blood cells. To our knowledge, this is the first study to identify LCR as a promising systemic inflammation biomarker for predicting prognosis after cardiac surgery. Patients with low LCR had a higher risk of postoperative complications and incurred higher hospitalization expenses. Moreover, LCR was deemed as an independent risk factor of OS after cardiac surgery, which has been further confirmed in the randomized internal validation.
Patients underwent cardiac surgery are likely to be in a state of preoperative chronic inflammation triggered by cardiac disease itself. Atherosclerosis is an inflammatory disease, with chronic inflammation of the arterial wall causing slow formation of lesions and progressive narrowing of arterial lumen (7, 8). Degenerative valve disease involves inflammatory factors that affect the interaction between valvular endothelial cells, valvular interstitial cells and extracellular matrix, leading to destruction of normal structure-function correlations (9). Valve ageing and disease brings abnormal blood flow patterns, which damages the endothelium and produces chronic inflammation. Laura et al. reported that patients with aortic stenosis have reduced levels of complement C4, verifying that a pro-inflammatory state exists in calcific aortic valve disease (13). In addition, traditional risk factors of CVD, such as hypertension, diabetes and hyperlipidemia, trigger vascular inflammatory response and lead to plaque disruption and the occurrence of major adverse cardiac and cerebrovascular event (MACCE) (14–16). Elevated proinflammatory cytokine levels have been detected in early deterioration of cardiac function, which is more sensitive than neurohormonal biomarkers (17). Increasing circulating inflammatory cytokines, such as tumor necrosis factor (TNF)-α, interleukin (IL)-1 and IL-6, has been observed in patients with heart failure, which may predict clinical outcomes, but the application of these cytokines is limited by the low circulating volumes and expensive assays (18–21). All this evidence points to a strong relationship between CVD and inflammation.
Due to the chronic inflammation present in patients underwent cardiac surgery, inflammation-induced catabolic stress exacerbates the patients’ depletion and worsens their nutritional status, ultimately leading to poor clinical outcomes. Therefore, there is a pressing need for simple and readily available markers to predict the clinical outcomes of patients underwent cardiac surgery. Some single inflammatory factors appear to be available to predict clinical outcomes (22, 23), which is consistent with our study. However, the predictive value of individual inflammatory factors, such as CRP and white blood cells, was mostly confined to 1-year OS, which declined rapidly over time. Moreover, the predictive value of a single inflammatory factor is not as effective as a combination of multiple factors (24–26). Serum systemic inflammation biomarkers are derived from a simple calculation of the existing preoperative examination and offer the advantage of convenience and low cost. Among the 10 biomarkers analyzed in this study, LCR was the most effective tool for the prediction of clinical outcomes after cardiac surgery.
LCR is derived from the ratio of lymphocyte count to CRP. Lymphocytes play a key role in the regulation of the immune response, including both innate and adaptive immunity (27). The lymphocyte count reflects the immune-nutrition status of the body. Since activated effector lymphocytes primarily rely on glycolysis for their high energy requirements, they are particularly sensitive to poor nutritional status (28, 29). The number and function of lymphocytes are altered in malnutrition. Lymphopenia demonstrates low immune function and increases susceptibility to infection, while infections worsen nutritional status by increasing the demand for nutrients and decreasing appetite. Malnutrition further increase susceptibility to infection, creating a vicious cycle and resulting in adverse effects on the clinical outcomes of patients. CRP is widely used to determine the presence of acute inflammation in clinical practice (30). Muscle and visceral protein breakdown increase and they are used to provide energy during the acute phase, thought to exacerbate malnutrition (31). Studied have reported that CRP is associated with the prognosis of CVD, such as atherosclerotic disease and aortic valve disease, suggesting an active role in the pathophysiology of CVD (32–34). Meanwhile, CRP activates the classical complement pathway and mediates recognition and phagocytosis (35). Therefore, LCR, which combines CRP with lymphocyte count, comprehensively reflects the immunology, nutrition and inflammatory state of the host.
Current researches in LCR primarily focus on solid tumor and sarcoma (4–6), COVID-19 (36) and hemiarthroplasty (37). Only a few studies have reported its predictive value in CVD, such as STEMI (38) or patients underwent heart transplantation (39). Our study is the first to report the prognostic value of LCR in predicting the clinical outcomes after cardiac surgery, suggesting the potential benefit of anti-inflammatory intervention in improving the prognosis of cardiac surgery. A meta-analysis demonstrated that the impact of prophylactic steroids on clinical outcomes after cardiac surgery is still controversial (40). Statins and other lipid-lowering agents have anti-inflammatory effects, and current evidence has provided enough facts for their beneficial effects after cardiac surgery (41, 42). Novel anti-inflammatory medications in CVD also shows the potential benefit for cardiovascular risk reduction (43).
This study has several limitations. As a retrospective, single-institutional study, no external data was used to validate the prediction model. So we require further validation of multicenter, multiethnic studies in the future. However, our study has the strength of having complete biomarker data since every patient has undergone preoperative blood tests. Besides, all systemic inflammation markers were detected only once before surgery. We will focus on the impact of trajectory changes of these biomarkers in our further study.
The present study has confirmed that LCR holds considerable potential as a representative systemic inflammation biomarker for prognosticating outcomes after cardiac surgery. Patients with low LCR had a higher risk of postoperative complications and worse overall survival. Detecting low LCR levels could prove beneficial for surgeons in identifying patients at higher risk and tailoring perioperative management strategies accordingly.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by the Ethics Committee of Shanghai Tenth People’s Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
ZL, WZ and ZS designed the study. PZ, GZ and YZ collected the data. ZL and WZ did the analysis and interpretation of data. ZS wrote and revised the article. All authors contributed to the article and approved the submitted version.
This study was supported by the Shanghai Municipal Commission of Health and Family Planning (No. 201640203) and Shanghai Science and Technology Committee (No. 16140901600).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1190380/full#supplementary-material
1. Dreyfus G, Windecker S. How to shape the future of cardiology and cardiac surgery? Eur Heart J (2020) 41(38):3693–701. doi: 10.1093/eurheartj/ehaa707
2. Martinez EA, Thompson DA, Errett NA, Kim GR, Bauer L, Lubomski LH, et al. Review article: high stakes and high risk: a focused qualitative review of hazards during cardiac surgery. Anesth Analg (2011) 112(5):1061–74. doi: 10.1213/ANE.0b013e31820bfe8e
3. Thalji NM, Suri RM, Greason KL, Schaff HV. Risk assessment methods for cardiac surgery and intervention. Nat Rev Cardiol (2014) 11(12):704–14. doi: 10.1038/nrcardio.2014.136
4. Okugawa Y, Toiyama Y, Yamamoto A, Shigemori T, Ichikawa T, Yin C, et al. Lymphocyte-to-C-reactive protein ratio and score are clinically feasible nutrition-inflammation markers of outcome in patients with gastric cancer. Clin Nutr (2020) 39(4):1209–17. doi: 10.1016/j.clnu.2019.05.009
5. Zhang KP, Zhang X, Zhang Q, Ruan GT, Song MM, Xie HL, et al. Association between the lymphocyte-to-C-reactive protein ratio and survival outcomes in cancer patients with GLIM-defined malnutrition: A multicenter study. J Nutr Health Aging (2022) 26(9):847–55. doi: 10.1007/s12603-022-1835-3
6. Nakamura T, Hagi T, Asanuma K, Sudo A. Is lymphocyte C-reactive protein ratio useful for predicting survival in patients with non-metastatic soft tissue sarcoma? Cancers (Basel) (2022) 14(21):5214. doi: 10.3390/cancers14215214
7. Weber C, Noels H. Atherosclerosis: current pathogenesis and therapeutic options. Nat Med (2011) 17(11):1410–22. doi: 10.1038/nm.2538
8. Kong P, Cui ZY, Huang XF, Zhang DD, Guo RJ, Han M. Inflammation and atherosclerosis: signaling pathways and therapeutic intervention. Signal Transduct Target Ther (2022) 7(1):131. doi: 10.1038/s41392-022-00955-7
9. Schoen FJ. Mechanisms of function and disease of natural and replacement heart valves. Annu Rev Pathol (2012) 7:161–83. doi: 10.1146/annurev-pathol-011110-130257
10. Golia E, Limongelli G, Natale F, Fimiani F, Maddaloni V, Pariggiano I, et al. Inflammation and cardiovascular disease: from pathogenesis to therapeutic target. Curr Atheroscler Rep (2014) 16(9):435. doi: 10.1007/s11883-014-0435-z
11. Abacioglu OO, Koyunsever NY, Kilic S, Yildirim A, Kurt IH. Glasgow prognostic score as a marker of mortality after TAVI. Braz J Cardiovasc Surg (2021) 36(6):796–801. doi: 10.21470/1678-9741-2020-0269
12. Ruan GT, Xie HL, Zhang HY, Zhang Q, Deng L, Wang ZW, et al. Association of systemic inflammation and low performance status with reduced survival outcome in older adults with cancer. Clin Nutr (2022) 41(10):2284–94. doi: 10.1016/j.clnu.2022.08.025
13. Mourino-Alvarez L, Baldan-Martin M, Gonzalez-Calero L, Martinez-Laborde C, Sastre-Oliva T, Moreno-Luna R, et al. Patients with calcific aortic stenosis exhibit systemic molecular evidence of ischemia, enhanced coagulation, oxidative stress and impaired cholesterol transport. Int J Cardiol (2016) 225:99–106. doi: 10.1016/j.ijcard.2016.09.089
14. Libby P, Crea F. Clinical implications of inflammation for cardiovascular primary prevention. Eur Heart J (2010) 31(7):777–83. doi: 10.1093/eurheartj/ehq022
15. Guzik TJ, Touyz RM. Oxidative stress, inflammation, and vascular aging in hypertension. Hypertension (2017) 70(4):660–7. doi: 10.1161/HYPERTENSIONAHA.117.07802
16. Biondi-Zoccai GG, Abbate A, Liuzzo G, Biasucci LM. Atherothrombosis, inflammation, and diabetes. J Am Coll Cardiol (2003) 41(7):1071–7. doi: 10.1016/s0735-1097(03)00088-3
17. Rubaj A, Rucinski P, Oleszczak K, Trojnar MK, Wojcik M, Wysokinski A, et al. Inflammatory activation following interruption of long-term cardiac resynchronization therapy. Heart Vessels (2013) 28(5):583–8. doi: 10.1007/s00380-012-0285-y
18. Dick SA, Epelman S. Chronic heart failure and inflammation: What do we really know? Circ Res (2016) 119(1):159–76. doi: 10.1161/CIRCRESAHA.116.308030
19. Deswal A, Petersen NJ, Feldman AM, Young JB, White BG, Mann DL. Cytokines and cytokine receptors in advanced heart failure: an analysis of the cytokine database from the Vesnarinone trial (VEST). Circulation (2001) 103(16):2055–9. doi: 10.1161/01.cir.103.16.2055
20. Orus J, Roig E, Perez-Villa F, Pare C, Azqueta M, Filella X, et al. Prognostic value of serum cytokines in patients with congestive heart failure. J Heart Lung Transplant (2000) 19(5):419–25. doi: 10.1016/s1053-2498(00)00083-8
21. Gullestad L, Ueland T, Vinge LE, Finsen A, Yndestad A, Aukrust P. Inflammatory cytokines in heart failure: mediators and markers. Cardiology (2012) 122(1):23–35. doi: 10.1159/000338166
22. Li L, Wang J, Meng S, Li Z, Huang Z, Sun J, et al. Peripheral blood leukocytes and platelets serve as prognostic factors in breast cancer. Cancer Biother Radiopharm (2021) 36(2):167–73. doi: 10.1089/cbr.2019.3032
23. Ocana A, Nieto-Jimenez C, Pandiella A, Templeton AJ. Neutrophils in cancer: prognostic role and therapeutic strategies. Mol Cancer (2017) 16(1):137. doi: 10.1186/s12943-017-0707-7
24. Messerich NM, Uda NR, Volken T, Cogliatti S, Lehmann T, Holbro A, et al. CRP/albumin ratio and glasgow prognostic score provide prognostic information in myelofibrosis independently of MIPSS70-A retrospective study. Cancers (Basel) (2023) 15(5):1479. doi: 10.3390/cancers15051479
25. Socha MW, Malinowski B, Puk O, Wartega M, Bernard P, Nowaczyk M, et al. C-reactive protein as a diagnostic and prognostic factor of endometrial cancer. Crit Rev Oncol Hematol (2021) 164:103419. doi: 10.1016/j.critrevonc.2021.103419
26. Hu H, Yao X, Xie X, Wu X, Zheng C, Xia W, et al. Prognostic value of preoperative NLR, dNLR, PLR and CRP in surgical renal cell carcinoma patients. World J Urol (2017) 35(2):261–70. doi: 10.1007/s00345-016-1864-9
27. Larosa DF, Orange JS. Lymphocytes. J Allergy Clin Immunol (2008) 121(2 Suppl):S364–369; quiz S412. doi: 10.1016/j.jaci.2007.06.016
28. Cohen S, Danzaki K, MacIver NJ. Nutritional effects on T-cell immunometabolism. Eur J Immunol (2017) 47(2):225–35. doi: 10.1002/eji.201646423
29. Gerriets VA, MacIver NJ. Role of T cells in malnutrition and obesity. Front Immunol (2014) 5:379. doi: 10.3389/fimmu.2014.00379
30. Sproston NR, Ashworth JJ. Role of C-reactive protein at sites of inflammation and infection. Front Immunol (2018) 9:754. doi: 10.3389/fimmu.2018.00754
31. Kaysen GA. Inflammation: cause of vascular disease and malnutrition in dialysis patients. Semin Nephrol (2004) 24(5):431–6. doi: 10.1016/j.semnephrol.2004.06.009
32. Osman R, L'Allier PL, Elgharib N, Tardif JC. Critical appraisal of C-reactive protein throughout the spectrum of cardiovascular disease. Vasc Health Risk Manag (2006) 2(3):221–37. doi: 10.2147/vhrm.2006.2.3.221
33. Ridker PM. Role of inflammatory biomarkers in prediction of coronary heart disease. Lancet (2001) 358(9286):946–8. doi: 10.1016/S0140-6736(01)06112-8
34. Galante A, Pietroiusti A, Vellini M, Piccolo P, Possati G, De Bonis M, et al. C-reactive protein is increased in patients with degenerative aortic valvular stenosis. J Am Coll Cardiol (2001) 38(4):1078–82. doi: 10.1016/s0735-1097(01)01484-x
35. Mortensen RF. C-reactive protein, inflammation, and innate immunity. Immunol Res (2001) 24(2):163–76. doi: 10.1385/IR:24:2:163
36. Erdogan A, Can FE, Gonullu H. Evaluation of the prognostic role of NLR, LMR, PLR, and LCR ratio in COVID-19 patients. J Med Virol (2021) 93(9):5555–9. doi: 10.1002/jmv.27097
37. Zhu J, Cheng X, Li Y, Bai L, Su Z. Low lymphocyte-to-C-reactive protein ratio relates to high 1-year mortality in elderly patients undergoing hemiarthroplasty for displaced femoral neck facture. J Orthop Surg Res (2022) 17(1):512. doi: 10.1186/s13018-022-03406-9
38. Liu Y, Ye T, Chen L, Xu B, Wu G, Zong G. Preoperative lymphocyte to C-reactive protein ratio: A new prognostic indicator of post-primary percutaneous coronary intervention in patients with ST-segment elevation myocardial infarction. Int Immunopharmacol (2023) 114:109594. doi: 10.1016/j.intimp.2022.109594
39. Seropian IM, Romeo FJ, Pizarro R, Vulcano NO, Posatini RA, Marenchino RG, et al. Neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio as predictors of survival after heart transplantation. ESC Heart Fail (2018) 5(1):149–56. doi: 10.1002/ehf2.12199
40. Dvirnik N, Belley-Cote EP, Hanif H, Devereaux PJ, Lamy A, Dieleman JM, et al. Steroids in cardiac surgery: a systematic review and meta-analysis. Br J Anaesth (2018) 120(4):657–67. doi: 10.1016/j.bja.2017.10.025
41. Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med (2017) 376(18):1713–22. doi: 10.1056/NEJMoa1615664
42. Sousa-Uva M, Head SJ, Milojevic M, Collet JP, Landoni G, Castella M, et al. 2017 EACTS Guidelines on perioperative medication in adult cardiac surgery. Eur J Cardiothorac Surg (2018) 53(1):5–33. doi: 10.1093/ejcts/ezx314
Keywords: systemic inflammation, biomarkers, cardiac surgery, prognosis, lymphocyte-to-C-reactive protein ratio
Citation: Liu Z, Zhu G, Zhang Y, Zhang P, Zang W and Shen Z (2023) Comprehensive comparative analysis of the prognostic impact of systemic inflammation biomarkers for patients underwent cardiac surgery. Front. Immunol. 14:1190380. doi: 10.3389/fimmu.2023.1190380
Received: 20 March 2023; Accepted: 31 July 2023;
Published: 14 August 2023.
Edited by:
Mark Slevin, Manchester Metropolitan University, United KingdomReviewed by:
Vladimir M. Pisarev, Federal Research and Clinical Center of Intensive Care Medicine and Rehabilitation, RussiaCopyright © 2023 Liu, Zhu, Zhang, Zhang, Zang and Shen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wangfu Zang, emFuZ3dmQHRvbmdqaS5lZHUuY24=; Zile Shen, MTkxMDg1N0B0b25namkuZWR1LmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.