- 1Department of Critical Care Medicine, Shengjing Hospital of China Medical University, Shenyang, Liaoning, China
- 2Cancer Institute, First Affiliated Hospital, China Medical University, Shenyang, Liaoning, China
Sepsis is a multiple organ dysfunction syndrome caused by the host’s immune response to infection, with extremely high incidence and mortality. Immunosuppression is an essential pathophysiological alteration that influences the clinical treatment and prognosis of sepsis. Recent studies have suggested that the programmed cell death 1 signaling pathway is involved in the formation of immunosuppression in sepsis. In this review, we systematically present the mechanisms of immune dysregulation in sepsis and elucidate the expression and regulatory effects of the programmed cell death 1 signaling pathway on immune cells associated with sepsis. We then specify current research developments and prospects for the application of the programmed cell death 1 signaling pathway in immunomodulatory therapy for sepsis. Several open questions and future research are discussed at the end.
1 Introduction
Sepsis is the most serious threat to human health worldwide, affecting more than 40 million people worldwide each year and killing roughly one in five of them (1). Its core mechanism is the host immune response disorder caused by infection, which leads to multiple organ dysfunction (2). Current studies believe that immunosuppression originates from the very early stage of sepsis, which is caused by the excessive release of anti-inflammatory cytokines and the decline in the number and function of immune cells, and continuous immunosuppression will lead to increased mortality (3). Programmed cell death 1 (PD-1) signaling pathway plays an crucial role in immune regulation of tumor and autoimmune diseases (4). It has recently been reported that the PD-1 pathway is also involved in the formation of immunosuppression in sepsis, and blocking this pathway has been shown to have promising therapeutic effects in animal experiments. Therefore, the purpose of this article is to introduce the mechanism of immune dysregulation in sepsis, demonstrate the regulatory effects of PD-1 signaling pathway on various kinds of sepsis-related immune cells, and explore the application prospect of blocking PD-1 signaling pathway in immunomodulatory therapy of sepsis and problems to be solved in future research. All references cited in this paper are from the PubMed database.
2 Immune dysregulation in sepsis
The pathogenesis of sepsis is the immune disorder caused by infection, which includes the excessive release of pro-inflammatory cytokines and the immunosuppression, both of which occur simultaneously at the early stage of infection (5). With the help of antibiotics and infection control measures, the body will return to homeostasis if the host’s immune system is able to rid itself of the pathogen quickly. However, some hosts showed continuous immunosuppression, whose immune system could not clear the pathogens in time, and even developed to secondary infections, eventually into multiple organ dysfunction syndrome (MODS) and death (6).
In the early stage of sepsis, the body’s innate immune system first recognizes the pathogen-associated molecular patterns (PAMPs) after the pathogen has invaded, causing the release of a series of pro-inflammatory cytokines such as tumor necrosis factor-alpha (TNF-α), interleukin-1 (IL-1) and interleukin-6 (IL-6). These pro-inflammatory factors promote downstream cytokine release, causing tissue damage, disseminated intravascular coagulation (DIC), and organ dysfunction (7), also called a cytokines storm. At the same time, immune cells secrete anti-inflammatory cytokines like interleukin-4 (IL-4) and interleukin-10 (IL-10). Secreted mainly by activated T lymphocytes, IL-4 can induce CD4+ T cells converting into T helper 2 (Th2) cells, and indirectly inhibit the release of interleukin-2 (IL-2), interferon-gamma (IFN-γ) and other pro-inflammatory factors (8). IL-10 is mainly secreted by monocytes and macrophages, and can promote the proliferation of immunosuppressive cells like regulatory T cells (Tregs) and myeloid-derived suppressor cells (MDSCs) (9). Thus, the negative regulatory effect of anti-inflammatory cytokines is a major cause of immunosuppression in sepsis.
In addition, multiple factors contribute to the reduction in the number and function of immune cells associated with sepsis. In patients with sepsis, over-expressed caspase-1 or caspase-4/5 combined with lipopolysaccharide (LPS) recognizes and activates gasdermin-D (GSDMD), an actor of pyroptosis, which can cause immune cell death (10). Patients with sepsis showed significantly reduced expression of human leukocyte antigen-DR (HLA-DR), which resulted in decreased antigen presenting cell function, and further inhibited subsequent T cell activation and adaptive immune response (11). Negative costimulatory molecules, such as PD-1, T-cell immunoglobulin and mucin domain-containing protein-3 (TIM-3) and B and T lymphocyte attenuator (BTLA) is also highly expressed in sepsis, leading to immunosuppression (12). The precise role of PD-1 will be discussed later.
3 Expression of PD-1 pathway related molecules in sepsis
PD-1, a member of the B7-CD28 immunoglobulin superfamily, is a transmembrane receptor protein that acts as an essential negative costimulatory molecule, also known as an immune checkpoint, and is widely expressed on the surfaces of lymphocytes, dendritic cells, monocytes and other immune cells. After binding with Programmed death-ligand 1(PD-L1), PD-1 inhibits proliferation and cytokins secretion of T cells by dephosphorylation of the T cell receptors (TCR) related molecules, and promotes the conversion of T cells to Tregs, thus transmits negative immune regulatory signals (13). Since PD-L1 is widely expressed on tumor cells, immunosuppression mediated by PD-1 pathway is an critical mechanism for immune escape of tumor cells (14).
Similar to tumor cells, PD-1 and its ligands are also highly expressed in various immune cells associated with sepsis. The expression of PD-1 in spleen T cells and PD-L1 in macrophages increases in candidiemic mice (15).CD4+ T cells in septic mice showed a special depletion phenotype, which was characterized by increased expression of PD-1, TIM-3 and the proportion of Tregs (16). PD-L1 expression also increases in Myeloid derived suppressor cells (MDSCs) in septic mice (17). Human immune cells also highly express PD-1 related molecules during sepsis. PD-1 expression increases in CD4+T cells in patients with sepsis and is associated with a poor prognosis. The cutoff value for predicting death in 28 days with the expression frequency of PD-1 was 22.46% (18). Expression of immune checkpoints including PD-1 by peripheral blood mononuclear cells (PBMCs) increased in patients with aggressive candida infection, especially in patients who died (19). The expression of PD-L1 in natural killer (NK) cells in septic patients is significantly increased, and its frequency is an independent risk factor for death at 28 days (20). Soluble PD-L1(sPD-L1) in circulation is still increased even after recovery from sepsis, with significantly higher readmissions and all-cause mortality within six months (21). PD-1/PD-L1 expression was even increased in various subsets of memory B cells and T cells in patients with sepsis (22). These findings confirm that PD-1 pathway related molecules are widely expressed in immune cells associated with sepsis.
4 Regulatory effects of the PD-1 pathway on immune cells associated with sepsis
It has been established that the PD-1 signaling pathway, based on extensive expression, plays a regulatory role in various types of innate and adaptive immune cells associated with sepsis.
4.1 Regulatory effects on neutrophils
PD-L1 expression was negatively correlated with the apoptosis rate of neutrophils in septic patients by prolongating neutrophil survival through activation of PI3K/Akt signaling pathway (23). PD-L1 expression also reduced the migration ability of neutrophils and induced apoptosis of co-cultured lymphocytes in septic mice (24).
4.2 Regulatory effects on monocytes/macrophages
In septic mice with PD-1 gene deletion, Kupffer cells expressed more major histocompatibility complex II (MHC-II), engulf function was significantly enhanced, and after LPS stimulation, the secretion of IL-6 and monocyte chemoattractant protein-1 also increased significantly. In addition, PD-1 gene deletion was found to decrease cleaved caspase-3 levels and reduce LPS induced Kupffer cell apoptosis (25). In septic mice, blocking PD-1 with antibodies resulted in aggregation of macrophage cytoskeleton proteins α-actitinin and F-actin, enhancing their phagocytosis and mobility. It is speculated that the PD-1 signaling pathway achieves this effect by inducing cytoskeletal rearrangement (26). Avendano-Ortiz et al. found that the expression of PD-1 in monocytes of septic patients is related to endotoxin tolerance, and blocking this pathway can enhance the immune response of monocytes in sepsis patients (27).
4.3 Regulatory effects on dendritic cells
In septic mice, TNF-α induced protein 8 like 1 (TIPE1) can inhibit dendritic cell maturation by activating PD-1 signaling pathway, thus affecting its antigen presentation ability and subsequent T cell activation (28).
4.4 Regulatory effects on adaptive immune cells
Besides the typical effects of inducing T-cell exhaustion and promoting the transformation from efficient T cells to Tregs that mentioned previously (13), PD-1 pathway can also inhibit the cytokines secretion of T cells. The ability of CD4+T cells to secrete TNF-α was decreased in septic mice with elevated PD-1 expression (29). Similar phenomena have also been observed from the in vitro experiments of septic patients, and PD-1 blockers can enhance the ability of T cells to secrete IFN-γ (30, 31). In addition, the PD-1 pathway can also inhibit activity and secretion ability of CD19(+) B cells (32), but its specificity in sepsis is still lacking.
5 Blocking the PD-1 pathway in immunomodulatory therapy for sepsis
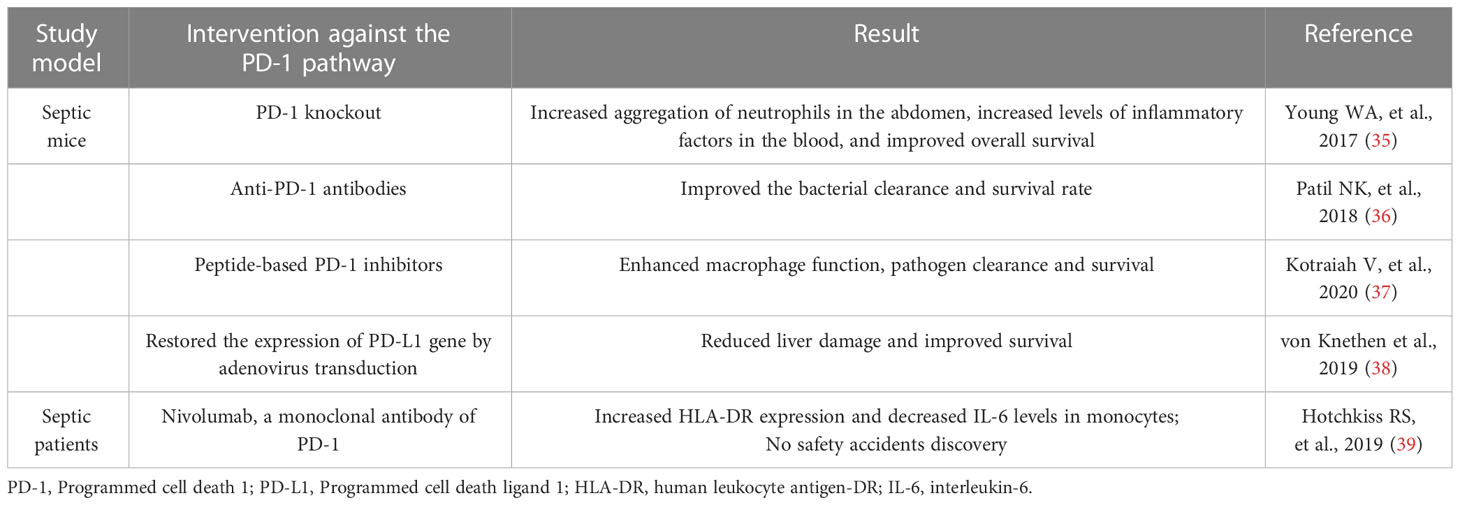
It was previously believed that the main harm of sepsis was systemic inflammatory response syndrome (SIRS) caused by infection, which was induced by excessive release of inflammatory cytokines in the early stage of the disease. Therefore, the immunotherapeutic strategies of sepsis in the past were mainly to inhibit the inflammatory response and block the pro-inflammatory cytokines such as PAMPs, Toll-like cytokines (TLRs), TNF-α, IL-6, etc., but the results were not as expected. Although the fatality rate at the early stage of sepsis decreased, the overall prognosis did not continue to improve, and more patients showed immunosuppression, uncontrolled infection and organ failure in the late stage of the disease (3, 33, 34). This led to a deeper understanding of the core mechanism of sepsis, the now widely accepted theory of immune dysregulation. It also provides a theoretical basis for animal and clinical trials of immunomodulatory therapies for sepsis, which attenuate immunosuppression by blocking the PD-1 pathway, as shown in Table 1.
5.1 Animal trials
Multiple animal trials have demonstrated that blocking the PD-1 pathway or knockout of related genes can improve the prognosis of sepsis. Compared with wild-type, PD-1 knockout mice showed increased aggregation of neutrophils in the abdomen after sepsis, increased levels of inflammatory factors in the blood, and significantly improved overall survival (35). After receiving PD-L1 antibody treatment, septic mice caused by burns showed an improvement in clearance of pathogenic bacteria and survival rate (36). Treatment of septic mice with peptide-based PD-1 inhibitors enhanced their macrophage function, pathogen clearance, and survival (37). However, not all animal trials have reached similar conclusions. One study found that PD-L1 expression was down-regulated in liver cells of septic mice. But after the expression of PD-L1 gene was restored by adenovirus transduction, the damage of liver cells in mice was reduced and the survival rate was significantly improved (38).Possible reasons for this discrepancy will be discussed later.
5.2 Clinical trials
Clinical trials of therapies that block the PD-1 pathway are in their infancy compared to animal trials. Hotchkiss et al. (39) selected 31 septic patients from the Intensive care unit (ICU) of 10 hospitals in the United States and conducted a randomized controlled trial on nivolumab, a monoclonal antibody of PD-1, at different doses. Basic pharmacokinetic parameters were obtained and increased HLA-DR expression and decreased IL-6 levels were observed in monocytes in all patients. But the study was a Phase 1 trial that demonstrated the safety of nivolumab only in patients with sepsis, not its efficacy. Another randomized controlled clinical trial of anti-PD-L1 drugs and placebo conducted by the same team also reached a similar conclusion (40). No follow-up clinical trials were reported at the time of submission.
6 Problems and prospects
As previously mentioned, PD-1/PD-L1 plays an essential regulatory role in the immunosuppression induced by sepsis by affecting the function of various immune cells and is closely related to the immune status and prognosis of sepsis. Most animal studies have shown that blocking the PD-1 pathway improves survival, and preliminary clinical studies have demonstrated the safety of related drugs in humans. However, a variety of fundamental and clinical issues remain to be addressed in this field.
6.1 Role of PD-L2
PD-L2 is another ligand of PD-1, which is mainly expressed on the surface of macrophages and dendritic cells and has a stronger affinity for PD-1, with particular expression in some tumor cells (41). In septic mice with PD-L2 gene deficiency, hepatic vascular permeability increased and liver damage was more severe, but inflammatory factors such as IL-6 decreased instead, and there was no difference in survival rate overall (42). Currently, research on PD-L2 is extremely limited and its regulatory role in sepsis is unclear.
6.2 Influencing factors of PD-1 signaling pathway
The PD-1 signaling pathway, which plays an immunomodulatory role in sepsis, is regulated by a variety of factors. The pathogenic components such as lipopolysaccharide (LPS) (43) and the secreted toxins such as Streptococcal Pyrogenic Exotoxin A (SPEA) (44) can enhance the expression of PD-L1 in immune cells. Tissue hypoxia and its consequent hyperlactemia are also significant factors. The expression of PD-L1 in monocytes of septic patients increases under the regulation of transcription factor hypoxia-inducible factor-1α (HIF1α) (27). In septic mice, blood lactic acid can increase the expression of PD-L1 in the kidney and increase kidney injury (45). Some hormones can also affect the expression of molecules in this pathway, for example, human ghrelin and growth hormone can reduce the expression of PD-1 and the number of Tregs in the spleen of sepsis rats (46), and Vitamin D related signaling pathways can affect the expression of PD-1 and other molecules (47). The molecular mechanisms of these known influences and their effects on the expression of PD-1 signaling pathways in different cells need further investigation. In addition, there may be additional potential influences that have not yet been identified.
6.3 PD-1 signaling pathway and exosomes
Exosomes are the extracellular vesicles (EVs) with a diameter of 40 to 160nm, which are formed by the lipid bilayer coated with DNA, RNA and proteins, and play an essential role in mediating intercellular signal transmission and influencing gene expression in recipient cells (48). Exosome PD-L1 from tumor cells can bind to PD-1 on the surface of T cells, inhibiting the activity of the latter and creating an immunosuppressive microenvironment for tumors (49). Removal of exosome PD-L1 can inhibit the growth of tumor cells and has a synergistic anti-tumor effect with PD-L1 antibody (50). EVs are involved in the formation and organ function impairment of sepsis (51), and β2 integrin and PD-L2 expression are significantly increased in exosomes of sepsis patients (52). However, whether expression of PD-1 pathway molecules in the exosome has a direct regulatory effect on immune cells associated with sepsis and the specific mechanism remains to be investigated.
6.4 Soluble PD-1 pathway molecules
Besides the usual membrane-binding form, PD-1 and PD-L1 are dissolved in the circulating serum called soluble PD-1/sPD-L1 (sPD-1/SPD-L1), including the exosome PD-L1 mentioned above. Human sPD-1 level is positively correlated with the expression of PD-1 in CD4+ or CD8+T cells and the severity of sepsis (53). Serum sPD-L1 has also been shown to be associated with sepsis severity and prognosis (54). As soluble PD-1 pathway molecules are easy to extract and detect, they have the potential to be used as risk and prognostic indicators of sepsis. However, large-scale prospective clinical trials are currently lacking.
6.5 Metabolomics studies of PD-1 pathway in sepsis
Metabolomics can investigate the pathogenesis of sepsis and evaluate biomarkers and prognosis by detecting and analyzing metabolites (55). Bu et al. used a non-targeted metabolomics approach to investigate differences in metabolites in sepsis patients with different prognoses and PD-1 expression groups. They identified three metabolites: PC (P-18:0/14:0), 2-ethyl-2-hydroxybutyric acid and aldehyde increased significantly in 7-day death group and PD-1 high expression group. And 2-ethyl-2-hydroxybutyric acid is positively correlated with IL-2 and lactic acid concentration (56). These three metabolites may be involved in the mechanism of regulation of sepsis by the PD-1 pathway, and are closely related to environmental factors such as IL-2 and lactate. However, due to the limited number of samples, this is only a preliminary conclusion and further research is needed.
6.6 Immunomodulatory therapy
The therapeutic idea of improving immune function, destroying immune tolerance and immune escape by blocking the PD-1 signaling pathway was originally derived in tumor biotherapy. Up to now, more than 1700 clinical trials have been successfully conducted on tumor immunomodulatory therapy, and such drugs have been widely used in clinics, confirming the effectiveness and safety of immunomodulatory therapy (57). However, as mentioned above, the application of immunomodulatory therapies to sepsis has not been extensively studied, and current animal experiments targeting the PD-1 signaling pathway have not achieved fully consistent results.
The timing of treatment may be a key factor. Unlike the chronic course and low heterogeneity of tumors, sepsis occurs and develops quite rapidly. The pathogenic mechanism of different pathogens, the immune response intensity of the host individual and the basic organ function vary considerably, and the overall immune status is also continually changing (58). As a result, different timing of interventions may lead to differences in overall outcomes. Professor von Knethen et al. (38) enhanced PD-L1 expression in septic mice receiving cecal-ligation and puncture (CLP) under 24 hours, which inhibited the function of CD8+T cells to secrete inflammatory cytokines. In the early stages of sepsis, the main lethal factor is direct organ damage caused by excessive inflammatory cytokines. This intervention suppresses the early inflammatory response and protects organ function, making it reasonable to expect an improved prognosis. On the other hand, immunoregulatory therapies targeting the PD-1 pathway focus on persistent immunosuppression and double infection in late-stage sepsis, which are not contradictory. Of course, more research is needed to see if the results of animal trials can be applied in clinical applications.
In addition, it is difficult to accurately distinguish “early” and “late” sepsis in clinical cases, and the timing of immunoregulatory treatment still needs to be guided by indicators that accurately reflect the immune status of the host. Besides sPD-1/sPD-L1 mentioned above, Th17/Treg ratio and neutrophil/lymphocyte ratio (NLR) can be used as indicators of immune status (3). And the emerging artificial intelligence and machine learning technologies in recent years also have great application potential to guide the immunoregulatory therapy of sepsis (59).
7 Conclusion
In summary, the PD-1 signaling pathway plays an essential regulatory role in immunosuppression in sepsis by affecting immune cell function. Immunomodulatory therapies targeting the PD-1 pathway in sepsis have great potential for applications, but there are still many fundamental and clinical issues to be further investigated.
Author contributions
YY designed the review and SZ wrote the manuscript. All authors contributed to the article and approved the submitted version.
Acknowledgments
We thank Prof. Bin Zang for his kind support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Rudd KE, Johnson SC, Agesa KM, Shackelford KA, Tsoi D, Kievlan DR, et al. Global, regional, and national sepsis incidence and mortality, 1990-2017: analysis for the global burden of disease study. Lancet (2020) 395(10219):200–11. doi: 10.1016/S0140-6736(19)32989-7
2. Evans L, Rhodes A, Alhazzani W, Antonelli M, Coopersmith CM, French C, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med (2021) 47(11):1181–247. doi: 10.1007/s00134-021-06506-y
3. Liu D, Huang SY, Sun JH, Zhang HC, Cai QL, Gao C, et al. Sepsis-induced immunosuppression: mechanisms, diagnosis and current treatment options. Mil Med Res (2022) 9(1):56. doi: 10.1186/s40779-022-00422-y
4. Kuchroo JR, Hafler DA, Sharpe AH, Lucca LE. The double-edged sword: harnessing PD-1 blockade in tumor and autoimmunity. Sci Immunol (2021) 6(65):eabf4034. doi: 10.1126/sciimmunol.abf4034
5. Kerrigan SW, Devine T, Fitzpatrick G, Thachil J, Cox D. Early host interactions that drive the dysregulated response in sepsis. Front Immunol (2019) 10:1748. doi: 10.3389/fimmu.2019.01748
6. Yao RQ, Ren C, Zheng LY, Xia ZF, Yao YM. Advances in immune monitoring approaches for sepsis-induced immunosuppression. Front Immunol (2022) 13:891024. doi: 10.3389/fimmu.2022.891024
7. Tang D, Wang H, Billiar TR, Kroemer G, Kang R. Emerging mechanisms of immunocoagulation in sepsis and septic shock. Trends Immunol (2021) 42(6):508–22. doi: 10.1016/j.it.2021.04.001
8. Gao K, Jin J, Huang C, Li J, Luo H, Li L, et al. Exosomes derived from septic mouse serum modulate immune responses via exosome-associated cytokines. Front Immunol (2019) 10:1560. doi: 10.3389/fimmu.2019.01560
9. Neumann C, Scheffold A, Rutz S. Functions and regulation of T cell-derived interleukin-10. Semin Immunol (2019) 44:101344. doi: 10.1016/j.smim.2019.101344
10. Wang L, Zhang J, Zhang L, Hu L, Tian J. Significant difference of differential expression pyroptosis-related genes and their correlations with infiltrated immune cells in sepsis. Front Cell Infect Microbiol (2022) 12:1005392. doi: 10.3389/fcimb.2022.1005392
11. Leijte GP, Rimmelé T, Kox M, Bruse N, Monard C, Gossez M, et al. Monocytic HLA-DR expression kinetics in septic shock patients with different pathogens, sites of infection and adverse outcomes. Crit Care (2020) 24(1):110. doi: 10.1186/s13054-020-2830-x
12. Luperto M, Zafrani L. T Cell dysregulation in inflammatory diseases in ICU. Intensive Care Med Exp (2022) 10(1):43. doi: 10.1186/s40635-022-00471-6
13. Gao Z, Ling X, Shi C, Wang Y, Lin A. Tumor immune checkpoints and their associated inhibitors. J Zhejiang Univ Sci B (2022) 23(10):823–43. doi: 10.1631/jzus.B2200195
14. van der Heide V, Humblin E, Vaidya A, Kamphorst AO. Advancing beyond the twists and turns of T cell exhaustion in cancer. Sci Transl Med (2022) 14(670):eabo4997. doi: 10.1126/scitranslmed.abo4997
15. Wurster S, Albert ND, Kontoyiannis DP. Candida auris bloodstream infection induces upregulation of the PD-1/PD-L1 immune checkpoint pathway in an immunocompetent mouse model. mSphere (2022) 7(2):e0081721. doi: 10.1128/msphere.00817-21
16. He W, Xiao K, Xu J, Guan W, Xie S, Wang K, et al. Recurrent sepsis exacerbates CD4+ T cell exhaustion and decreases antiviral immune responses. Front Immunol (2021) 12:627435. doi: 10.3389/fimmu.2021.627435
17. Ruan WS, Feng MX, Xu J, Xu YG, Song CY, Lin LY, et al. Early activation of myeloid-derived suppressor cells participate in sepsis-induced immune suppression via PD-L1/PD-1 axis. Front Immunol (2020) 11:1299. doi: 10.3389/fimmu.2020.01299
18. Chen J, Wang H, Guo R, Li H, Cui N. Early expression of functional markers on CD4+ T cells predicts outcomes in ICU patients with sepsis. Front Immunol (2022) 13:938538. doi: 10.3389/fimmu.2022.938538
19. Mellinghoff SC, Thelen M, Bruns C, Garcia-Marquez M, Hartmann P, Lammertz T, et al. T-Cells of invasive candidiasis patients show patterns of T-cell-exhaustion suggesting checkpoint blockade as treatment option. J Infect (2022) 84(2):237–47. doi: 10.1016/j.jinf.2021.12.009
20. Jiang W, Li X, Wen M, Liu X, Wang K, Wang Q, et al. Increased percentage of PD-L1+ natural killer cells predicts poor prognosis in sepsis patients: a prospective observational cohort study. Crit Care (2020) 24(1):617. doi: 10.1186/s13054-020-03329-z
21. Yende S, Kellum JA, Talisa VB, Peck Palmer OM, Chang CH, Filbin MR, et al. Long-term host immune response trajectories among hospitalized patients with sepsis. JAMA Netw Open (2019) 2(8):e198686. doi: 10.1001/jamanetworkopen.2019.8686
22. Wilson JK, Zhao Y, Singer M, Spencer J, Shankar-Hari M. Lymphocyte subset expression and serum concentrations of PD-1/PD-L1 in sepsis - pilot study. Crit Care (2018) 22(1):95. doi: 10.1186/s13054-018-2020-2
23. Wang JF, Wang YP, Xie J, Zhao ZZ, Gupta S, Guo Y, et al. Upregulated PD-L1 delays human neutrophil apoptosis and promotes lung injury in an experimental mouse model of sepsis. Blood (2021) 138(9):806–10. doi: 10.1182/blood.2020009417
24. Wang JF, Li JB, Zhao YJ, Yi WJ, Bian JJ, Wan XJ, et al. Up-regulation of programmed cell death 1 ligand 1 on neutrophils may be involved in sepsis-induced immunosuppression: an animal study and a prospective case-control study. Anesthesiology (2015) 122(4):852–63. doi: 10.1097/ALN.0000000000000525
25. Wang F, Huang X, Chung CS, Chen Y, Hutchins NA, Ayala A. Contribution of programmed cell death receptor (PD)-1 to kupffer cell dysfunction in murine polymicrobial sepsis. Am J Physiol Gastrointest Liver Physiol (2016) 311(2):G237–45. doi: 10.1152/ajpgi.00371.2015
26. Ayala A, Elphick GF, Kim YS, Huang X, Carreira-Rosario A, Santos SC, et al. Sepsis-induced potentiation of peritoneal macrophage migration is mitigated by programmed cell death receptor-1 gene deficiency. J Innate Immun (2014) 6(3):325–38. doi: 10.1159/000355888
27. Avendaño-Ortiz J, Maroun-Eid C, Martín-Quirós A, Toledano V, Cubillos-Zapata C, Gómez-Campelo P, et al. PD-L1 overexpression during endotoxin tolerance impairs the adaptive immune response in septic patients via HIF1α. J Infect Dis (2018) 217(3):393–404. doi: 10.1093/infdis/jix279
28. Luan YY, Zhang L, Zhu FJ, Dong N, Lu JY, Yao YM. Effect of TIPE1 on immune function of dendritic cells and its signaling pathway in septic mice. J Infect Dis (2019) 220(4):699–709. doi: 10.1093/infdis/jiz158
29. Xie J, Robertson JM, Chen CW, Zhang W, Coopersmith CM, Ford ML. Pre-existing malignancy results in increased prevalence of distinct populations of CD4+ T cells during sepsis. PloS One (2018) 13(1):e0191065. doi: 10.1371/journal.pone.0191065
30. Patera AC, Drewry AM, Chang K, Beiter ER, Osborne D, Hotchkiss RS. Frontline science: defects in immune function in patients with sepsis are associated with PD-1 or PD-L1 expression and can be restored by antibodies targeting PD-1 or PD-L1. J Leukoc Biol (2016) 100(6):1239–54. doi: 10.1189/jlb.4HI0616-255R
31. Thampy LK, Remy KE, Walton AH, Hong Z, Liu K, Liu R, et al. Restoration of T cell function in multi-drug resistant bacterial sepsis after interleukin-7, anti-PD-L1, and OX-40 administration. PloS One (2018) 13(6):e0199497. doi: 10.1371/journal.pone.0199497
32. Buermann A, Römermann D, Baars W, Hundrieser J, Klempnauer J, Schwinzer R. Inhibition of b-cell activation and antibody production by triggering inhibitory signals via the PD-1/PD-ligand pathway. Xenotransplantation (2016) 23(5):347–56. doi: 10.1111/xen.12261
33. Roger C, Morel J, Leone M. Low level of evidence in surviving sepsis campaign guidelines: should we throw the baby out with the bathwater? Anaesth Crit Care Pain Med (2020) 39(4):491–2. doi: 10.1016/j.accpm.2020.07.005
34. Munroe ES, Prescott HC. Web exclusive. annals for hospitalists inpatient notes - understanding the 2021 surviving sepsis campaign guidelines recommendations on fluid resuscitation in sepsis. Ann Intern Med (2022) 175(7):HO2–3. doi: 10.7326/M22-1679
35. Young WA, Fallon EA, Heffernan DS, Efron PA, Cioffi WG, Ayala A. Improved survival after induction of sepsis by cecal slurry in PD-1 knockout murine neonates. Surgery (2017) 161(5):1387–93. doi: 10.1016/j.surg.2016.11.008
36. Patil NK, Luan L, Bohannon JK, Hernandez A, Guo Y, Sherwood ER. Frontline science: anti-PD-L1 protects against infection with common bacterial pathogens after burn injury. J Leukoc Biol (2018) 103(1):23–33. doi: 10.1002/JLB.5HI0917-360R
37. Kotraiah V, Phares TW, Browne CD, Pannucci J, Mansour M, Noe AR, et al. Novel peptide-based PD1 immunomodulators demonstrate efficacy in infectious disease vaccines and therapeutics. Front Immunol (2020) 11:264. doi: 10.3389/fimmu.2020.00264
38. von Knethen A, Schäfer A, Kuchler L, Knape T, Christen U, Hintermann E, et al. Tolerizing CTL by sustained hepatic PD-L1 expression provides a new therapy approach in mouse sepsis. Theranostics (2019) 9(7):2003–16. doi: 10.7150/thno.28057
39. Hotchkiss RS, Colston E, Yende S, Crouser ED, Martin GS, Albertson T, et al. Immune checkpoint inhibition in sepsis: a phase 1b randomized study to evaluate the safety, tolerability, pharmacokinetics, and pharmacodynamics of nivolumab. Intensive Care Med (2019) 45(10):1360–71. doi: 10.1007/s00134-019-05704-z
40. Hotchkiss RS, Colston E, Yende S, Angus DC, Moldawer LL, Crouser ED, et al. Immune checkpoint inhibition in sepsis: a phase 1b randomized, placebo-controlled, single ascending dose study of antiprogrammed cell death-ligand 1 antibody (BMS-936559). Crit Care Med (2019) 47(5):632–42. doi: 10.1097/CCM.0000000000003685
41. Xiao L, Guan X, Xiang M, Wang Q, Long Q, Yue C, et al. B7 family protein glycosylation: promising novel targets in tumor treatment. Front Immunol (2022) 13:1088560. doi: 10.3389/fimmu.2022.1088560
42. Rossi AL, Le M, Chung CS, Chen Y, Fallon EA, Matoso A, et al. A novel role for programmed cell death receptor ligand 2 in sepsis-induced hepatic dysfunction. Am J Physiol Gastrointest Liver Physiol (2019) 316(1):G106–14. doi: 10.1152/ajpgi.00204.2018
43. Peng L, Pan B, Zhang X, Wang Z, Qiu J, Wang X, et al. Lipopolysaccharide facilitates immune escape of hepatocellular carcinoma cells via m6A modification of lncRNA MIR155HG to upregulate PD-L1 expression. Cell Biol Toxicol (2022) 38(6):1159–73. doi: 10.1007/s10565-022-09718-0
44. Giesbrecht K, Förmer S, Sähr A, Heeg K, Hildebrand D. Streptococcal pyrogenic exotoxin a-stimulated monocytes mediate regulatory T-cell accumulation through PD-L1 and kynurenine. Int J Mol Sci (2019) 20(16):3933. doi: 10.3390/ijms20163933
45. Xu J, Ma X, Yu K, Wang R, Wang S, Liu R, et al. Lactate up-regulates the expression of PD-L1 in kidney and causes immunosuppression in septic acute renal injury. J Microbiol Immunol Infect (2021) 54(3):404–10. doi: 10.1016/j.jmii.2019.10.006
46. Zhou M, Yang WL, Aziz M, Ma G, Wang P. Therapeutic effect of human ghrelin and growth hormone: attenuation of immunosuppression in septic aged rats. Biochim Biophys Acta Mol Basis Dis (2017) 1863(10 Pt B):2584–93. doi: 10.1016/j.bbadis.2017.01.014
47. Luo J, Chen H, Ma F, Xiao C, Sun B, Liu Y, et al. Vitamin d metabolism pathway polymorphisms are associated with efficacy and safety in patients under anti-PD-1 inhibitor therapy. Front Immunol (2022) 13:937476. doi: 10.3389/fimmu.2022.937476
48. Fang Y, Ni J, Wang YS, Zhao Y, Jiang LQ, Chen C, et al. Exosomes as biomarkers and therapeutic delivery for autoimmune diseases: opportunities and challenges. Autoimmun Rev (2022) 21:103260. doi: 10.1016/j.autrev.2022.103260
49. Xie F, Xu M, Lu J, Mao L, Wang S. The role of exosomal PD-L1 in tumor progression and immunotherapy. Mol Cancer (2019) 18(1):146. doi: 10.1186/s12943-019-1074-3
50. Poggio M, Hu T, Pai CC, Chu B, Belair CD, Chang A, et al. Suppression of exosomal PD-L1 induces systemic anti-tumor immunity and memory. Cell (2019) 177(2):414–427.e13. doi: 10.1016/j.cell.2019.02.016
51. Tian C, Wang K, Zhao M, Cong S, Di X, Li R. Extracellular vesicles participate in the pathogenesis of sepsis. Front Cell Infect Microbiol (2022) 12:1018692. doi: 10.3389/fcimb.2022.1018692
52. Kawamoto E, Masui-Ito A, Eguchi A, Soe ZY, Prajuabjinda O, Darkwah S, et al. Integrin and PD-1 ligand expression on circulating extracellular vesicles in systemic inflammatory response syndrome and sepsis. Shock (2019) 52(1):13–22. doi: 10.1097/SHK.0000000000001228
53. Zhao Y, Jia Y, Li C, Shao R, Fang Y. Predictive value of soluble programmed death-1 for severe sepsis and septic shock during the first week in an intensive care unit. Shock (2019) 51(3):289–97. doi: 10.1097/SHK.0000000000001171
54. Yende S, Kellum JA, Talisa VB, Peck Palmer OM, Chang CH, Filbin MR, et al. Long-term host immune response trajectories among hospitalized patients with sepsis. JAMA Netw Open (2019) 2(8):e198686. doi: 10.1001/jamanetworkopen.2019.8686
55. Lee J, Banerjee D. Metabolomics and the microbiome as biomarkers in sepsis. Crit Care Clin (2020) 36(1):105–13. doi: 10.1016/j.ccc.2019.08.008
56. Bu Y, Wang H, Ma X, Han C, Jia X, Zhang J, et al. Untargeted metabolomic profiling of the correlation between prognosis differences and PD-1 expression in sepsis: a preliminary study. Front Immunol (2021) 12:594270. doi: 10.3389/fimmu.2021.594270
57. Hotchkiss RS, Opal SM. Activating immunity to fight a foe - a new path. N Engl J Med (2020) 382(13):1270–2. doi: 10.1056/NEJMcibr1917242
58. Wiersinga WJ, van der Poll T. Immunopathophysiology of human sepsis. EBioMedicine (2022) 86:104363. doi: 10.1016/j.ebiom.2022.104363
Keywords: sepsis, programmed cell death 1, immunosuppression, immunomodulation, immunotherapy
Citation: Zhong S and Yin Y (2023) Regulatory role of the programmed cell death 1 signaling pathway in sepsis induced immunosuppression. Front. Immunol. 14:1183542. doi: 10.3389/fimmu.2023.1183542
Received: 10 March 2023; Accepted: 15 May 2023;
Published: 24 May 2023.
Edited by:
Ricardo Pujol Borrell, Autonomous University of Barcelona, SpainReviewed by:
Mónica Martínez-Gallo, Vall d’Hebron University Hospital, SpainCopyright © 2023 Zhong and Yin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuanqin Yin, Y211Y2NtQHFxLmNvbQ==
 Shubai Zhong
Shubai Zhong Yuanqin Yin2*
Yuanqin Yin2*