- 1Student Research Committee, School of Medicine, Iran University of Medical Sciences, Tehran, Iran
- 2Hematology, Oncology and Stem Cell Transplantation Research Center, Research Institute for Oncology, Hematology and Cell Therapy, Tehran University of Medical Sciences, Tehran, Iran
- 3Colorectal Research Center, Iran University of Medical Sciences, Tehran, Iran
- 4Cancer Immunology Project (CIP), Universal Scientific Education and Research Network (USERN), Tehran, Iran
- 5Network of Immunity in Infection, Malignancy and Autoimmunity (NIIMA), Universal Scientific Education and Research Network (USERN), Tehran, Iran
- 6Digestive Diseases Research Center, Digestive Diseases Research Institute, Tehran University of Medical Sciences, Tehran, Iran
- 7Department of Hematology and Blood Banking, School of Allied Medical Sciences, Shahid Beheshti University of Medical Sciences, Tehran, Iran
- 8Department of Urology, Shariati Hospital, Tehran University of Medical Sciences, Tehran, Iran
- 9Department of Urology, Medical University of Vienna, Vienna, Austria
- 10Clinical Research Development Unit, Shariati Hospital, Tehran University of Medical Sciences, Tehran, Iran
Immunotherapy has revolutionized the treatment paradigm of many cancers, however, its effectiveness in prostate cancer patients is still under question. In the present systematic review and meta-analysis, we sought for assessing the efficacy and safety of Immune checkpoint inhibitors (ICIs) in patients with prostate cancer. PubMed, Scopus, Web of Science, and EMBASE databases were searched on Aguste 19, 2022. Thirty five studies met the eligibility criteria. The median overall survival (mOS) of all treatments was 14.1 months, with the longest and shortest mOS was seen among patients who received anti-CTLA-4 monotherapy and anti-PD-1/PD-L1+anti-CTLA-4 regimen at 24.9 and 9.2 months, respectively. Noteworthy, all types of adverse events had the lowest incidence in the anti-PD-1/PD-L1 monotherapy group. Considering the ICI monotherapy regimens, we found that fatigue, diarrhea, and infusion reaction had the highest incidence rates. Future studies evaluating the efficacy and safety of novel combination therapies with ICIs are warranted.
1 Introduction
With about 375 thousand deaths in 2020, prostate cancer is the fifth major cause of cancer death worldwide. Furthermore, approximately 1.5 million new cases were reported in 2020, which makes it the second most commonly diagnosed cancer (1). The majority of patients presented with localized disease which is mainly curable through radiation therapy, radical prostatectomy, or active surveillance (2). Patients who are diagnosed with metastatic hormone sensitive prostate cancer can be efficiently treated which led to a significant increase in their overall survival (3). Nevertheless, a number of patient specially those affected by castration-resistant prostate cancer progress to advanced metastatic disease which have poor outcomes. In such situation, the overall survival falls within nearly two years, highlighting the importance of finding more efficient treatments approaches (4, 5). Apart from chemotherapy and hormone therapies, radiopharmaceutical agents such as Radium-223 dichloride and 177-Lu-PSMA-617 were approved for the treatment of metastatic castration-resistant prostate cancer (6). However, there is still room for hope with more efficient medications.
Many trials using immunotherapy have been conducted for different types of tumors for over a decade, and they have expanded our knowledge of interactions between the immune system and diseases like cancer and its progression. Immunotherapy with immune checkpoint inhibitors (ICIs) has revolutionized the treatment paradigm of several tumors (7–10). In this case, cytotoxic T lymphocyte antigen 4 (CTLA-4), programmed-death 1 (PD-1), and programmed death-ligand 1 (PD-L1) inhibitors indicated promising results in melanoma, non-small cell lung cancer, and gastrointestinal cancers (11–13). In contrast, so-called “cold tumors”, such as prostate cancer, exhibit an immunosuppressive tumor microenvironment (TME) which results in a very restricted response to ICIs (14). Randomized controlled trials (RCTs) on patients with metastatic prostate cancer reported that using ICIs resulted in modest antitumor activity and suggested that combination therapy may enhance survival of these patients (10, 15, 16). Hence, in the present study, we systematically reviewed the clinical trials reporting the efficacy and safety of ICIs for patients with advanced prostate cancer and compared the finding of different regimens.
2 Methods
Present systematic review and meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (17).
2.1 Search strategy
Eligible trials that evaluated the efficacy and safety of ICIs for patient suffering from prostate cancer were identified through a comprehensive literature search in PubMed, Scopus, Web of Science, and EMBASE databases. We searched the trials that were published in English as of Aguste 19, 2022, using the key terms including (“prostate neoplasm” OR “prostate cancer” OR “adenocarcinoma of prostate” OR “squamous cell carcinoma of prostate” OR “transitional cell carcinoma of prostate” OR “castration-resistant prostate neoplasm”) AND (“PD-L1 inhibitor” OR “PD-1 inhibitor” OR “CTLA-4 inhibitor” OR “Pembrolizumab” OR “Nivolumab” OR “Durvalumab” OR “Camrelizumab” OR “Atezolizumab” OR “Ipilimumab”) AND (“trial” OR “clinical trial”). The detailed information on search strategy was outlined in Supplementary Table 1. In addition, we reviewed the published abstracts from annual conferences of the American Society of Clinical Oncology (ASCO), the European Society of Medical Oncology (ESMO), and the American Association for Cancer Research (AACR). In the case where duplicate studies were identified, the most recent and complete version of the data was included.
2.2 Study selection
Obtained records were exported to EndNote software (Clarivate Analytics, Philadelphia, PA, USA). After removing the duplicate publications, two review authors independently reviewed the title/abstract of the articles according to the inclusion and exclusion criteria. Afterward, the same two authors screened the full-texts of the selected records, independently. Discrepancies were resolved by consulting a third author.
2.3 Eligibility criteria
Trials were included if the following criteria were met (1): patients with locally advanced or metastatic prostate adenocarcinoma aged 18 years or older were enrolled (2); a PD-1/PD-L1/CTLA-4 inhibitor with or without standard of care combination treatments was given to one of the study arms; and (3) outcomes of interest in terms of efficacy (i.e. overall survival [OS], progression-free survival [PFS], prostate specific antigen response [PSAR], objective response rate [ORR], disease control rate [DCR], complete response [CR], partial response [PR], stable disease [SD], or progressive disease [PD]) and safety (i.e. treatment-related adverse events (TRAEs), ≥ grade 3 TRAEs, immune-related adverse events (irAEs), serious adverse events (AEs), AEs led to treatment discontinuation, and AEs led to death) were reported.
The exclusion criteria were as follows (1): trials including a mixed cohort of patients with different cancer types (2); trials that administrated immunotherapeutic agents other than ICIs; and (3) other types of studies such as case reports, case series, case-controls, cohorts, cross-sectionals, editorials, letters to the editor, commentaries, re-analysis of previously published articles, and any types of review articles.
2.4 Data extraction
The following data were extracted by two authors independently from included trials (1): study characteristics including the name of the first author, year of publication or conference presentation, study title, clinical trial identification number, the acronym of the trial, country of origin, and phase of the trial (2); characteristics of participants including the total number of patients, inclusion and exclusion criteria, age, median follow up duration, PSA level (ng/ml), median duration of treatment, and Eastern Cooperative Oncology Group (ECOG) performance status scale (3); characteristics of intervention medications including type, dose, and schedule of ICI medication(s) along with concomitant treatment(s); and (4) efficacy and safety measures. Disagreements were addressed by consulting a third reviewer.
2.5 Quality assessment
All included studies were treated as non-randomized trials. Therefore, the Risk Of Bias In Non-randomized Studies of Interventions (ROBINS-I) tool was used for assessing the quality of trials (18). Two independent reviewers assessed the quality of the included papers. This tool examines the risk of bias according to the following domains: bias due to confounding, bias in selection of participants into the study, bias in classification of interventions, bias due to deviations from intended interventions, bias due to missing data, bias in measurement of outcomes, and bias in selection of the reported results.
2.6 Data synthesis
The primary efficacy endpoint was to estimate the median OS and PFS after receiving ICI treatment regimens and the secondary efficacy endpoint was to estimate the pooled rate of ORR, DCR, CR, PR, SD, and PD. The safety outcome was the pooled rate of TRAEs, ≥ grade 3 TRAEs, irAEs, serious AEs, AEs led to treatment discontinuation, and AEs led to death. We used Cochrane’s Q statistic to assess between-study heterogeneity and calculated the I-square statistic. A random-effect model was applied if obvious heterogeneity was present (I2 >50%), otherwise, a fixed-effect model was chosen (19).
The subgroup analysis was conducted according to the target of ICI medication and the type of concomitant treatments. Differences between groups were tested by the chi-square test. The survival data were retrieved from Kaplan-Meyer curves via online plot digitizer tool (20). The pooled Kaplan-Meyer curves were plotted and analyzed using the package MetaSurvival (21) of software R version 3.6.3. Moreover, we used STATA version 17.0 (22) to calculate the pooled rates with metaprop command, which requires a nominator and a denominator (which is the total sample size) and some other options like random or fixed effects model. This command was built on the existing Stata command metan, which is routinely used to pool ratios and differences of means (23). A p-value less than 0.05 were treated as statistically significant.
3 Results
3.1 Study selection and characteristics of the included studies
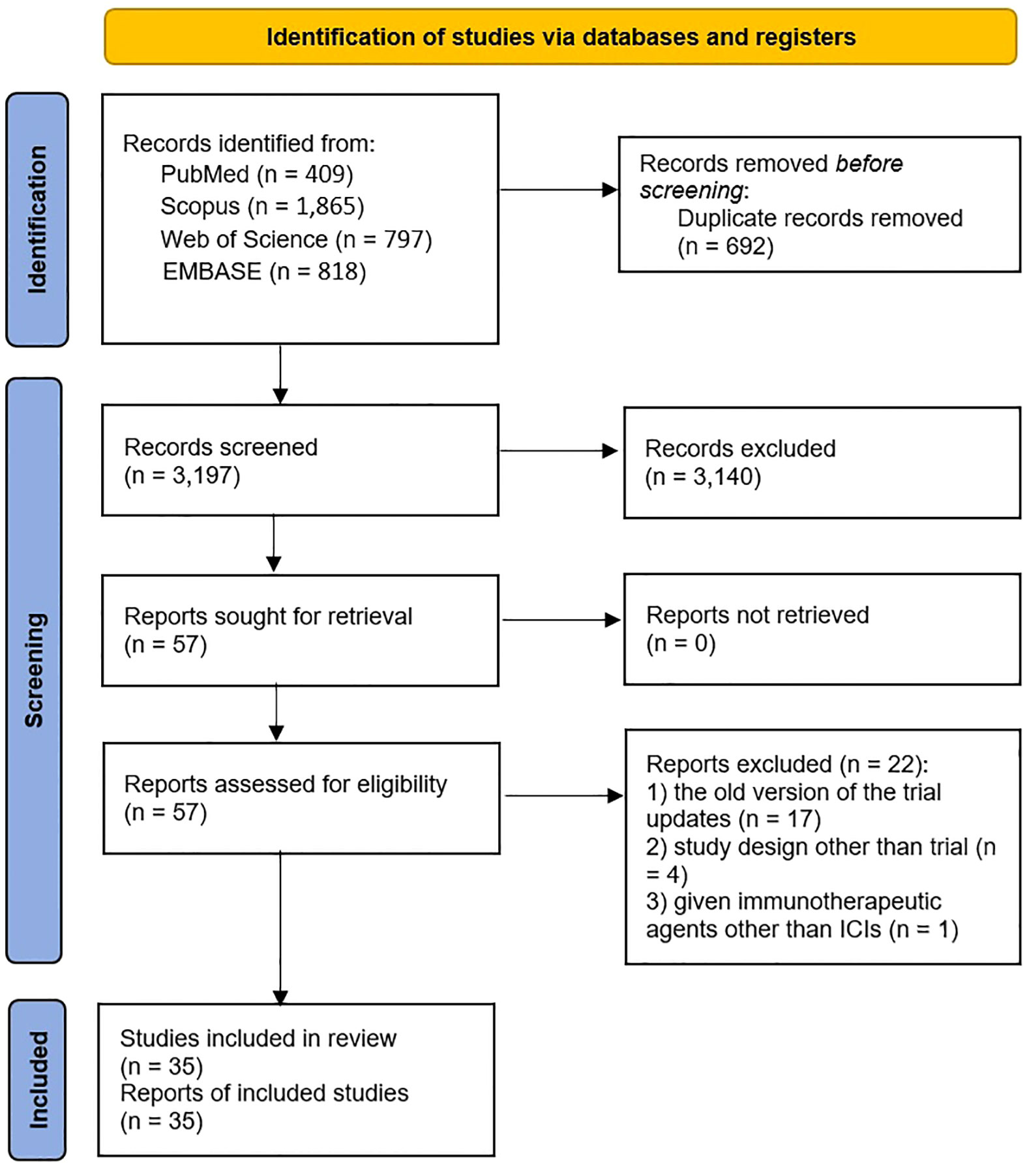
As illustrated in the PRISMA flow diagram (Figure 1), 3,889 studies were identified through initial database searching. Following the removal of 692 duplicated records, the remaining 3,197 articles underwent title and abstract screening. After a detailed full-text evaluation of 57 potentially relevant studies, 22 studies were excluded, among which 17 studies were the old version of updated trials (24–40), four were not trials (41–44), and one article reported administration of an immunotherapeutic agent other than ICIs (45). Ultimately, 35 studies met the eligibility criteria and were included in the meta-analysis (10, 15, 16, 46–77).
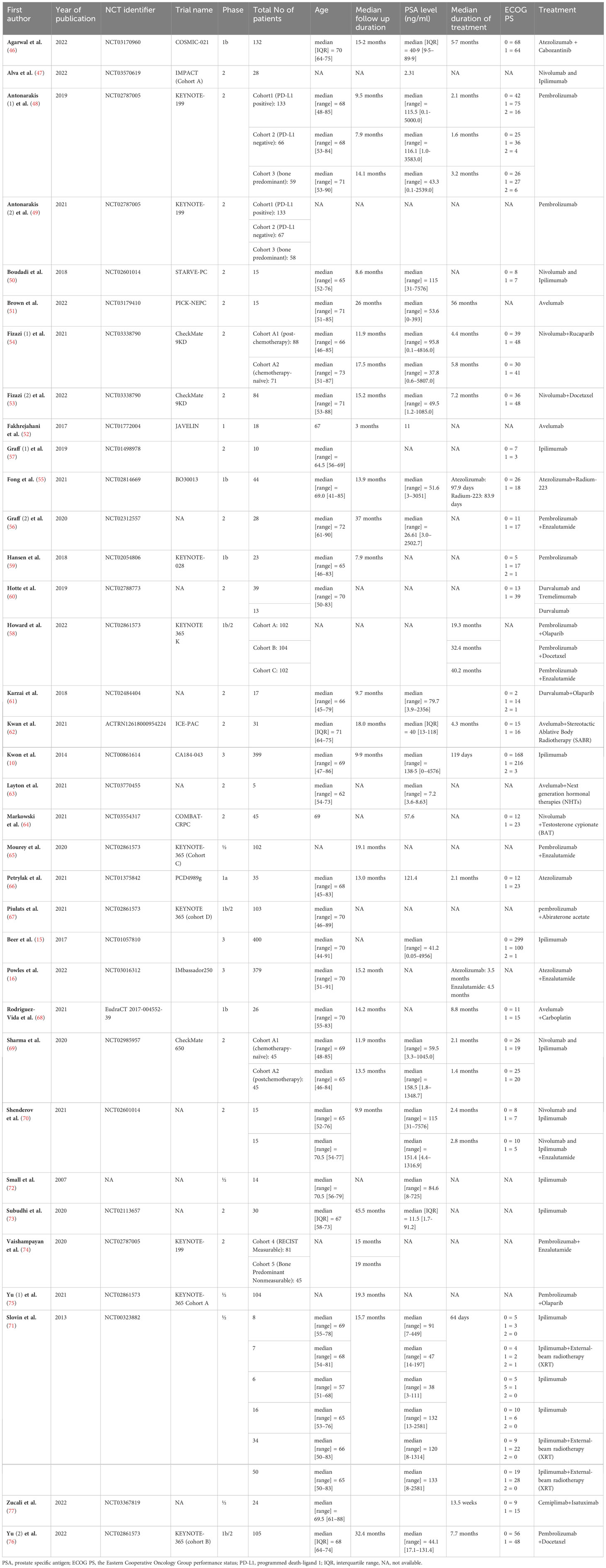
The eligible articles were published between 2007 and 2022. All included trials were non-randomized studies except for three randomized trials (10, 15, 78) that we considered their experimental groups as single-arm trial. One study was in phase I, one in phase Ia, four in phase Ib, five in phase I/II, three in phase Ib/II, eighteen in phase II, and three in phase III. A total of 3,618 prostate cancer patients who received treatments comprised of ICIs were enrolled in the present meta-analysis. The treatment approaches in the eligible studies included anti PD-1/PD-L1+TKI (46), anti PD-1/PD-L1+radiotherapy (55, 62), anti PD-1/PD-L1+PARP inhibitor (54, 58, 61, 75), anti PD-1/PD-L1+hormone therapy (16, 56, 58, 63–65, 67, 74), anti PD-1/PD-L1+chemotherapy (53, 58, 68, 76), anti PD-1/PD-L1+anti CTLA-4 (47, 50, 60, 69, 70), anti PD-1/PD-L1+anti CTLA-4+hormone therapy (70), anti CTLA-4+radiotherapy (71), anti PD-1/PD-L1+anti CD-38 (77), anti PD-1/PD-L1 monotherapy (48, 49, 51, 52, 59, 60, 66), and anti CTLA-4 monotherapy (10, 15, 57, 71–73). The characteristics of the included studies and treatment doses and schedules are summarized in Table 1 and Supplementary Table 2. In addition, the criteria of enrolling patients reported in the included studies are outlined in Supplementary Table 3.
3.2 Quality assessment
According to the ROBINS-I tool, 23 trials were rated as having a moderate methodological quality. In addition, no precise information was reported in 12 trials for assessing the risk of bias. Bias due to confounding was the domain that had the highest rate of moderate risk of bias items, whereas bias due to deviations from intended Intervention was the domain that had the highest rate of low risk of bias items Supplementary Figure 1.
3.3 Efficacy
3.3.1 Overall survival
The pooled overall OS among all patients receiving ICI monotherapy or in combination with other therapies was calculated, and the Kaplan Meier curve was built (Table 2 and Figure 2A). The mOS was 14.1 months (95% CI: 11.7-17.0), and the 6-, 12-, 18-, and 24-months OS rates were 78.4%, 56.0%, 41.0%, and 29.2%, respectively.
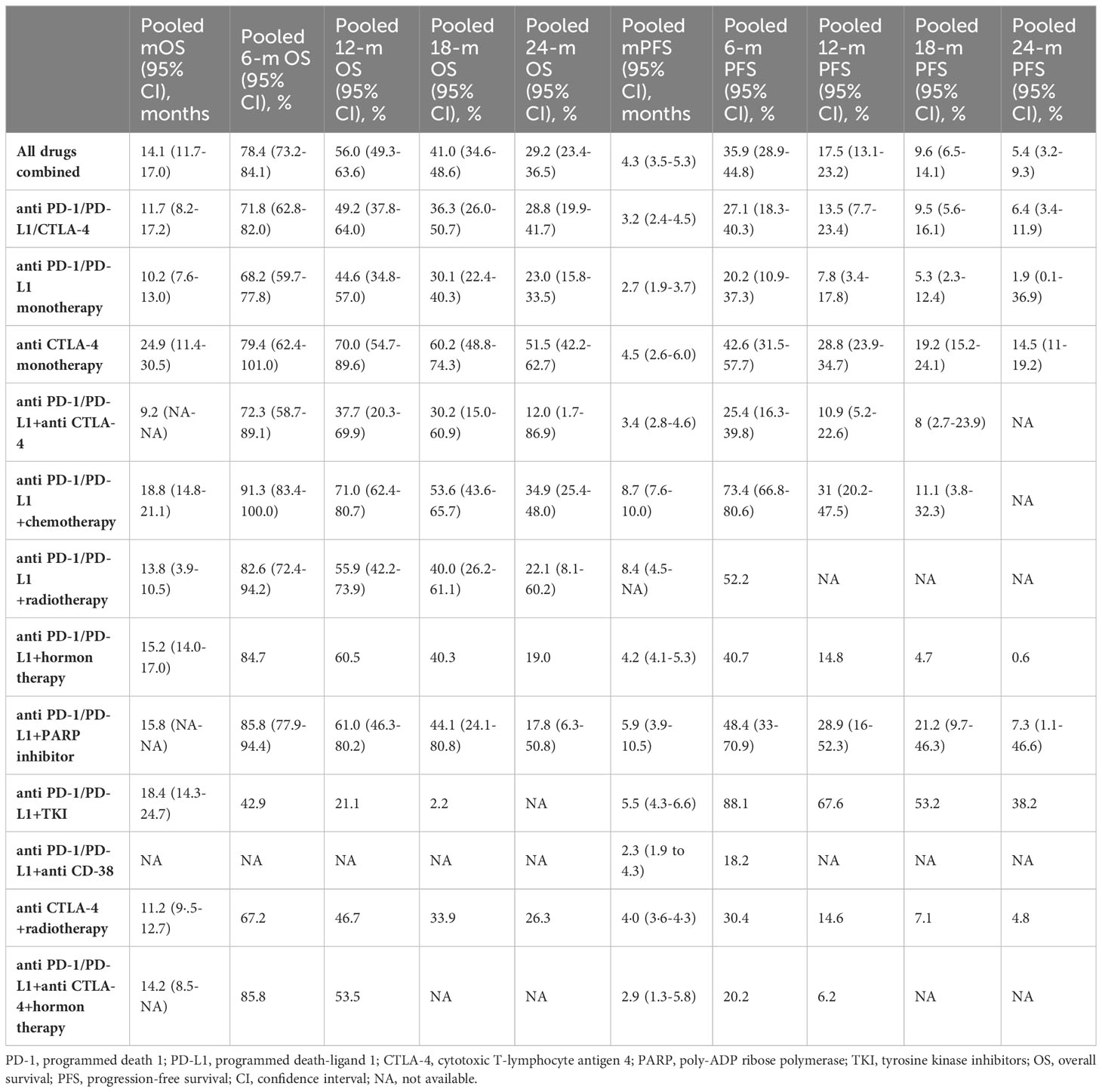
Table 2 Pooled results of overall survival and progression-free survival in total and by immune checkpoint inhibitor treatment regimen subgroups.
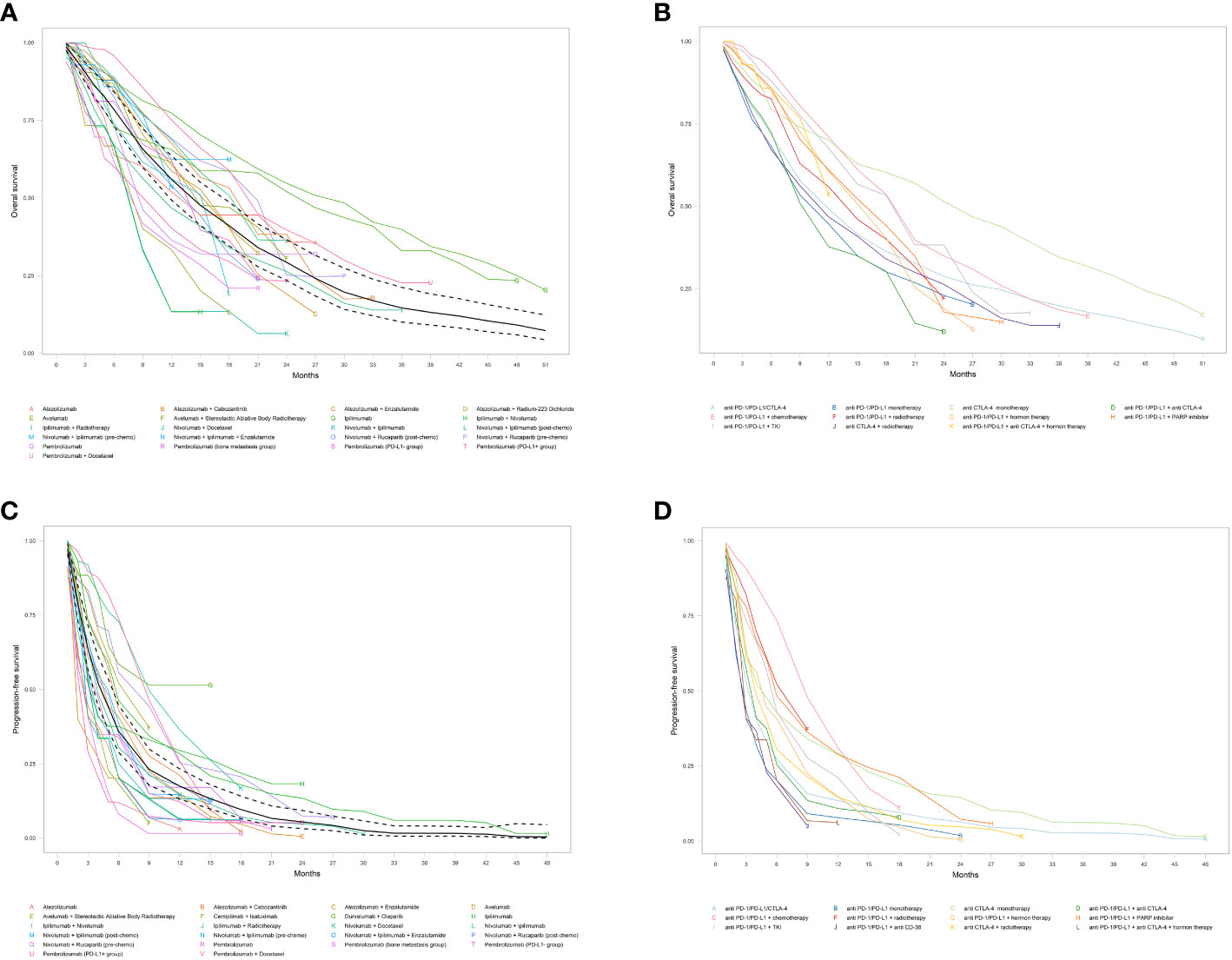
Figure 2 Pooled Kaplan-Meier estimates of overall survival (OS) and progression-free survival (PFS). (A) Overall OS; (B) OS subgroups by different immune checkpoint inhibitor treatment regimen; (C) Overall PFS; and (D) PFS by different immune checkpoint inhibitor treatment regimen. Solid and dashed black lines indicate pooled overall estimate and its 95% confidence intervals, respectively.
Subgroup analysis among different ICI regimens demonstrated that anti-CTLA-4 monotherapy had by far the longest mOS at 24.9 months (95% CI: 11.4-30.5), followed by anti-PD-1/PD-L1+chemotherapy regimen at 18.8 months (95% CI: 14.8-21.1), and anti-PD-1/PD-L1+TKI group at 18.4 months (95% CI: 14.3-24.7). Patients in the anti-PD-1/PD-L1+PARP, anti-PD-1/PD-L1+hormone therapy, anti-PD-1/PD-L1+anti-CTLA-4+hormone therapy, and anti-PD-1/PD-L1+radiotherapy groups ended up with similar mOS of 15.8 (95% CI: NA) and 15.2 (95% CI: 14.0-17.0), 14.2 (95% CI: 8.5-NA), and 13.8 (95% CI: NA) months, respectively. On the contrary, the anti-PD-1/PD-L1+anti-CTLA-4 regimen had the poorest mOS, with just 9.2 months (95% CI: NA), inferior to anti-PD-1/PD-L1/CTLA-4 (11.7 months, 95% CI: 8.2-17.2), anti-CTLA-4+radiotherapy (11.2 months, 95% CI: 9.5-12.7), and anti-PD-1/PD-L1 monotherapy (10.2 months, 95% CI: 7.6-13.0). The mOS and OS rates for different ICI monotherapy and in combination with other therapies and their Kaplan-Meyer curve are represented in Table 2 and Figure 2B.
3.3.2 Progression-free survival
The pooled mPFS was 4.3 months (95% CI: 3.5-5.3) with 6-, 12-, 18-, and 24-months PFS rates of 35.9%, 17.5%, 9.6%, and 5.4%, respectively (Table 2 and Figure 2C). The evaluation of PFS in different ICI regimens showed that the longest mPFS was observed in the anti-PD-1/PD-L1+chemotherapy regimen at 8.7 months (95% CI: 7.6-10.0). The pooled mPFS among patients receiving anti-PD-1/PD-L1+radiotherapy was 8.4 months (95% CI: 4.5-NA). For patients taking anti-PD-1/PD-L1+PARP inhibitor, the mPFS was 5.9 months (95% CI: 3.9-10.5) and for those taking anti PD-1/PD-L1+TKI, the mPFS was 5.5 months (95% CI: 4.3-6.6). The anti-CTLA-4 monotherapy, anti-PD-1/PD-L1+hormone therapy, and anti-CTLA-4+radiotherapy groups ended up with comparable mPFS of 4.5 (95% CI: 2.6-6.0), 4.2 (95% CI: 4.1-5.3), and 4.0 months (95% CI: 3.6-4.3), respectively. The shortest mPFS belonged to the anti-PD-1/PD-L1+anti CD-38 group at 2.3 months (95% CI: 1.9-4.3), marginally lower than anti-PD-1/PD-L1+anti CTLA-4, anti-PD-1/PD-L1/CTLA-4, anti-PD-1/PD-L1+anti CTLA-4+hormone therapy, and anti-PD-1/PD-L1 monotherapy, at 3.4 (95% CI: 2.8-4.6), 3.2 (95% CI: 2.4-4.5), 2.9 (95% CI: 1.3-5.8), and 2.7 months (95% CI: 1.9-3.7), respectively. The detailed results of PFS among different ICI regimen groups and their Kaplan-Meyer curve are presented in Table 2 and Figure 2D.
3.3.3 Response rates
The ORR, DCR, and PSAR rates were analyzed according to the ICI therapy regimen type. The bar chart illustrated in Figure 3 describes the aforementioned response rates between different treatment regimens containing ICIs. Supplementary Table 4 and Supplementary Figure 2 show that the pooled PSAR rates in anti-PD-1/PD-L1+chemotherapy and anti-PD-1/PD-L1+hormone therapy groups were significantly higher than in other ICI regimens groups, at 32.68% (95% CI: 14.52-53.90) and 26.76% (95% CI: 12.43-43.76), respectively. In contrast, the lowest PSAR rates were at 0.0%, 4.17%, and 4.46% among patients receiving anti-PD-1/PD-L1+anti-CTLA-4+hormone therapy, anti-PD-1/PD-L1+anti-CD38, and anti-PD-1/PD-L1 monotherapy, respectively. The PSAR in anti-PD-1/PD-L1 monotherapy was considerably inferior to almost all other ICI regimen groups. Moreover, the pooled PSARs among patients receiving anti PD-1/PD-L1/CTLA-4, anti CTLA-4 monotherapy, anti PD-1/PD-L1+anti CTLA-4, anti PD-1/PD-L1+radiotherapy, and anti PD-1/PD-L1+PARP inhibitor were 10.98%, 15.32%, 13.60%, 10.61%, and 23.24%, respectively. Significant heterogeneity was observed in pooled PSAR rate analyses of anti-PD-1/PD-L1/CTLA-4 and anti-PD-1/PD-L1+hormone therapy (I2 = 44.12%, p=0.04 and I2 = 89.03%, p<0.01, respectively).
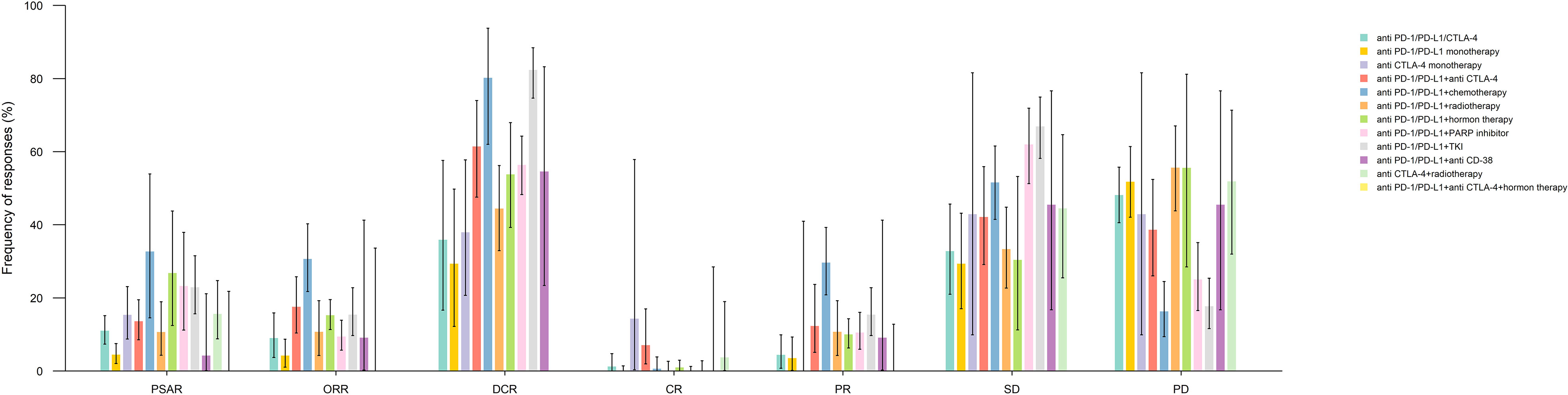
Figure 3 Pooled response rates for prostate specific antigen response (PSAR), objective response rate (ORR), disease control rate (DCR), complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD) by immune checkpoint inhibitor medication subgroups.
The highest pooled ORR was observed in the anti-PD-1/PD-L1+chemotherapy group at 30.61% (95% CI: 21.71-40.28), which was significantly higher than in other ICI therapy groups, except in anti-PD-1/PD-L1+anti CD-38 group (9.09%, 95% CI: 0.23-41.28) (Supplementary Table 5). Comparatively, patients taking anti-PD-1/PD-L1 monotherapy and anti-PD-1/PD-L1+anti-CTLA-4+hormone therapy had ORRs of 4.20% (95% CI: 1.06-8.67) and 0% (95% CI: 0-33.63), significantly lower than in anti-PD-1/PD-L1+anti CTLA-4 (17.51%), anti-PD-1/PD-L1+chemotherapy (30.61%), anti-PD-1/PD-L1+hormone therapy (15.23%), and anti-PD-1/PD-L1+TKI (15.38%) groups. Moreover, patients receiving anti-PD-1/PD-L1/CTLA-4 (9.01%), anti-PD-1/PD-L1+PARP inhibitor (9.41%), and anti-PD-1/PD-L1+ radiotherapy (10.67%) had comparable ORRs. The ORRs reported in studies evaluating the anti-PD-1/PD-L1/CTLA-4 receiving patients had significant heterogeneity (I2 = 58.86, p=0.01) (Supplementary Figure 3).
Although similar to ORR, the DCR observed in the anti-PD-1/PD-L1+chemotherapy group was considerably high (80.21%, 95% CI: 61.98-93.77), the highest DCR was detected among the anti-PD-1/PD-L1+TKI group (82.31%, 95% CI: 74.65-88.44). On the contrary, the anti-PD-1/PD-L1 monotherapy group had the lowest DCR, at 29.31% (95% CI: 12.18-49.78), which was significantly lower than that in all the other ICI regimen groups. Likewise, patients taking the anti-PD-1/PD-L1/CTLA-4 regimen also had a DCR of 35.86% (95% CI: 16.61-57.60), significantly lower than in other regimen groups, except in the anti-CTLA-4 monotherapy group (37.93%, 95% CI: 20.69-57.74). Moreover, the DCR in the anti-PD-1/PD-L1+radiotherapy (44.41%), anti-PD-1/PD-L1+hormone therapy (53.77%), anti-PD-1/PD-L1+CD-38 (54.55%), anti-PD-1/PD-L1+PARP inhibitor (56.34), and anti-PD-1/PD-L1+anti-CTLA-4 (61.4%) groups were alike. Considerable heterogeneity was detected in the pooled analysis of DCR in the anti-PD-1/PD-L1/CTLA-4 (I2 = 92.09%, p<0.01), anti-PD-1/PD-L1 monotherapy (I2 = 83.93%, p<0.01), and anti-PD-1/PD-L1+hormone therapy (I2 = 72.71%, p=0.01) (Supplementary Figure 4 and Supplementary Table 6).
The pooled results of CR, PR, SD, and PD by ICI treatment regimen groups, as well as the tables of associated p-values, are presented in Supplementary Figures 5-8 and Supplementary Tables 7-10.
3.4 Safety
Any grade TRAEs, ≥ grade3 AEs, irAEs, serious AEs, AEs led to discontinuation, and AEs led to death were analyzed according to the ICI therapy regimen subgroup. The bar chart presented in Figure 4 shows the earlier mentioned AEs between different treatment regimens containing ICIs. Noteworthy, all the AEs mentioned before had the lowest incidence in the anti-PD-1/PD-L1 monotherapy group. The anti-PD-1/PD-L1+TKI and anti-PD-1/PD-L1+chemotherapy had the highest incidence of ≥ grade3 and any grade TRAEs, respectively. The anti-PD-1/PD-L1+radiotherapy group had the highest any grade irAEs, and AEs led to death, whereas anti-CTLA-4 monotherapy was the leading subgroup in serious AEs and AEs led to discontinuation. The detailed pooled results of different AE types by ICI medication subgroup and their associated p-values are manifested in Supplementary Figures 9-14 and Supplementary Tables 11-16.
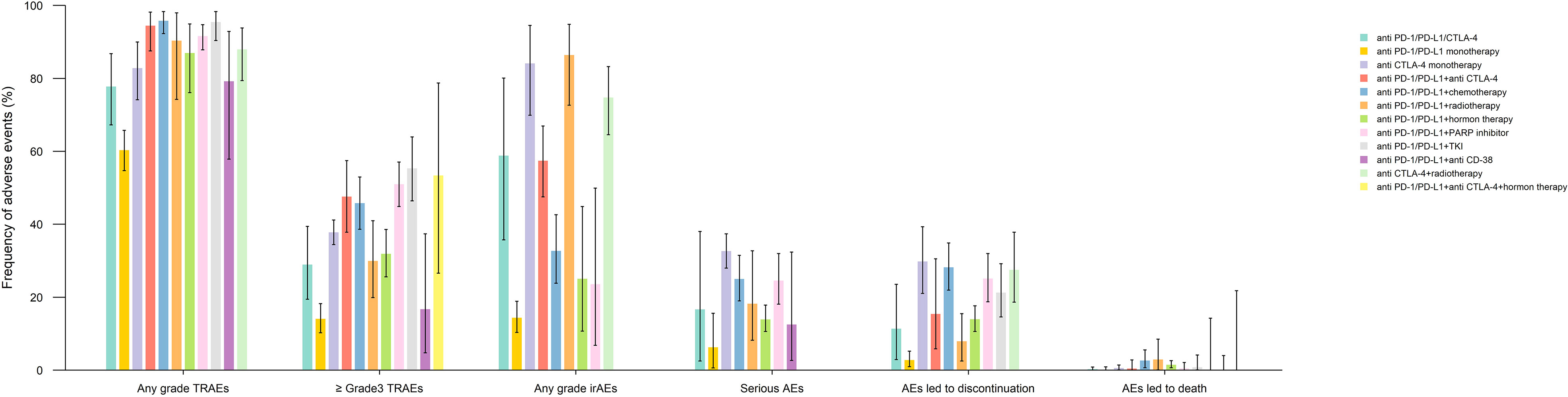
Figure 4 Pooled frequency of treatment-related adverse events (TRAEs), ≥ grade 3 TRAEs, immune-related adverse events (irAEs), serious adverse events (AEs), AEs led to treatment discontinuation, and AEs led to death.
To eliminate the AEs related to components of treatment other than ICIs, an additional analysis was carried out to determine the frequency of TRAEs in treatment regimens consisting of ICIs alone (anti-PD-1/PD-L1+/anti CTLA-4). The most commonly reported any grade TRAEs included fatigue (24.88%), diarrhea (20.61%), infusion reaction (17.67%), and decreased appetite (15.06%). Moreover, the most frequent ≥ grade 3 TRAE was diarrhea (2.8%), followed by AST increase (11.07%), fatigue (0.97%), and dizziness (0.79%) (Figure 5 and Supplementary Figures 15, 16).
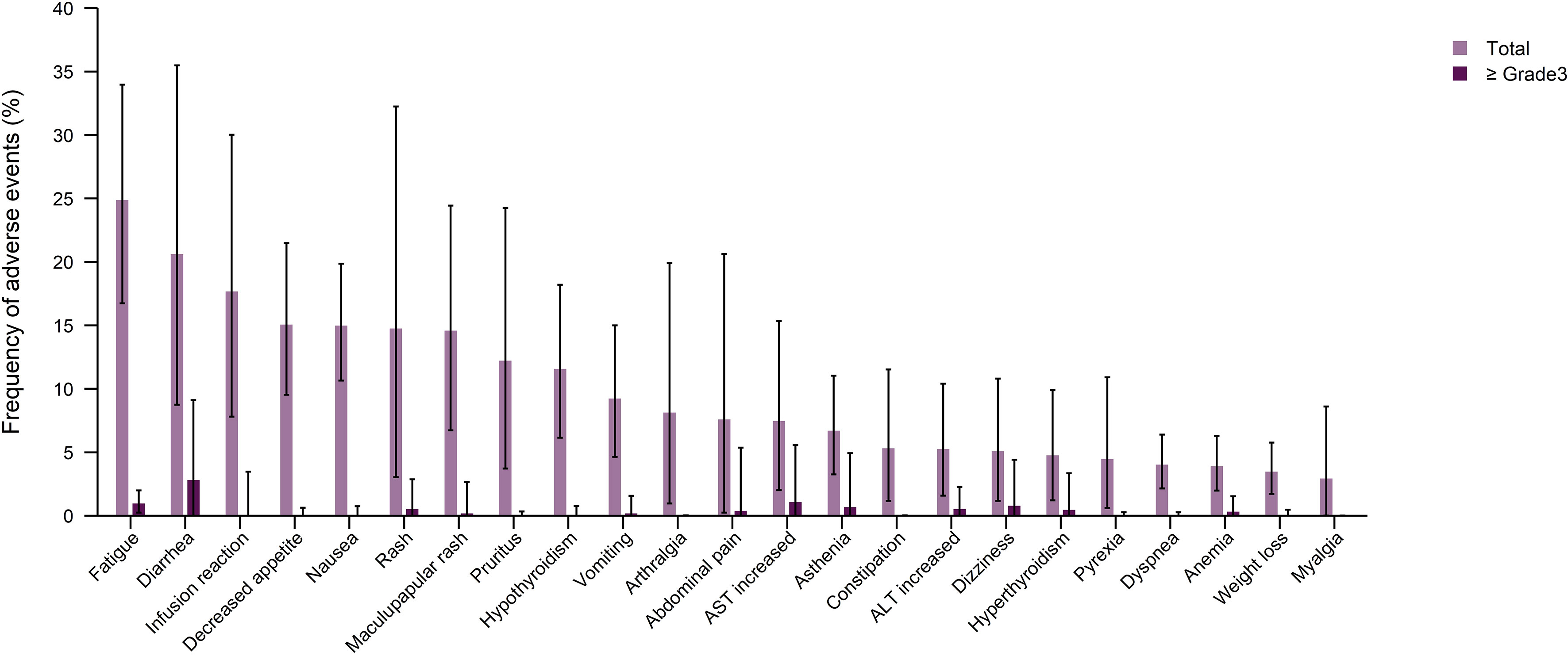
Figure 5 Pooled frequency of any grade treatment-related adverse events (TRAEs) and ≥ grade 3 TRAEs caused by immune checkpoint inhibitor monotherapy treatment regimen groups.
4 Discussion
ICI medications have been long investigated for urological cancers, leading to FDA approval of Nivolumab, Pembrolizumab, and Avelumab for urothelial carcinoma (79). Likewise, Ipilimumab, Nivolumab, Pembrolizumab, and Avelumab gained FDA approval for advanced renal cell carcinoma, mainly in combination with other medications in the class TKIs such as Axitinib, Cabozantinib, or Lenvatinib (79). Nevertheless, the benefit of ICIs in prostate cancer remain unclear (80). There has been several single-arm trials on efficacy and safety of ICIs as monotherapy or in combinations with other therapeutic modalities including androgen inhibitors, PARP inhibitors, TKIs, chemotherapy, radiotherapy, or other ICIs (81, 82). However, only the results of 3 phase III trials using the Ipilimumab or Atezolizumab have been published for mCRPC patients (10, 15, 16); and due to non-promising findings, none of ICIs have been approved by FDA and fit the treatment paradigm for prostate cancer patients.
The present systematic review is the first study aiming at pooling the results of clinical trials administrating ICIs for prostate cancer patients. We compared the effectiveness and AEs of several ICI monotherapy and combination therapy regimens in order to be used in clinical practice. Notably, anti-PD-1/PD-L1 monotherapy or combination of anti-PD-1/PD-L1 with CTLA-4 inhibitors showed 4.2% and 17.5% ORR in our study, respectively. In agreement, the results of a recent meta-analysis showed that Pembrolizumab monotherapy or Ipilimumab-plus-Nivolumab regimen resulted in an ORR of 5% and 17%, among prostate cancer patients, respectively (83). Regarding PSAR, similar results were obtained; while the PSAR for Pembrolizumab monotherapy, Ipilimumab monotherapy, and Ipilimumab-plus-Nivolumab were 11%, 19%, and 14%, respectively in the mentioned study (83), it was 4.5%, 15.3%, and 13.6% for anti-PD-1/PD-L1 monotherapy, anti-CTLA-4 monotherapy, and anti-PD-1/PD-L1+anti-CTLA-4 regimen in accordance to our findings, respectively.
Although initial studies have proposed that immunotherapy with an autologous active cellular vaccine (Sipuleucel-T) provide promising outcomes for men with prostate cancer (84), the body of evidence came into the conclusion that prostate tumor is less immunogenic than thought before (85–87). Immunologically, prostate cancer is a cold tumor which represents with low tumor mutational burden (TMB) (73, 88). It has been reported that efficacy of ICIs for prostate cancer patients outstripped the chemotherapy only when the TMB exceeded 10 mutations per megabase, detected only in a low proportion of patients (42). Low level of TMB and subsequently lower level of neoantigen expression reflects decreasing rate of immune cell infiltration particularly T cells into the prostate tumor tissue. Additionally, the hypoxic zone of the prostate tumor microenvironment prohibits T-cell attraction by causing an acidic pH, depleting important nutrients, promoting transforming growth factor-Beta (TGF-B) signaling, and activating myeloid-derived suppressor cells (89, 90). Thus, by inhibition of CD8+ T cell infiltration, a compromised response to ICIs would be observed in prostate cancer patients.
In order to combat the immunosuppressive microenvironment and override tumor escape mechanisms, multiple approaches have been examined to improve the efficacy of ICIs for prostate cancer patients. According to our study, a number of trials attempted to enhance the chance of better outcomes following ICI therapy by combining it with other medications. In this case, we found that administration of ICIs with chemotherapy regimens had desirable outcomes in a way presenting with the longest PFS as well as highest ORR and PSAR among prostate cancer patients. Indeed, killing tumor cells by chemotherapeutic agents can result in releasing tumor neoantigens, overcoming compensatory immunosuppressive mechanisms, and subsequently improving the function of effector immune cells (91–93). Therefore, combining immunotherapy with chemotherapy can induce addictive or synergic clinical activity. Apart from chemotherapy, studies have shown that a combination of CTLA-4 and PD-1/PD-L1 blockade has better antitumor outcome than monotherapy regimens alone (94); however, our results showed that anti-PD-1/PD-L1+anti-CTLA-4 regimen had the lowest mOS among all other regimens. This might be due to the intrinsic characteristics of the enrolled patients in trials that were associated with worse outcomes and need to be investigated further in future trials.
It has been reported that ICIs, in spite of their efficacies, may induce a wide range of AEs which should be monitored closely. In the present review, we assessed the safety of ICIs as well as the severity of side effects in prostate cancer patients. The anti-PD-1/PD-L1 monotherapy had the lowest incidence of all types of AEs, while anti-PD-1/PD-L1+TKI and anti-PD-1/PD-L1+chemotherapy had the highest incidence of ≥grade 3 and any grade TRAEs, respectively. Therefore, although combinational therapy with standard medications might improve the outcomes, it accompanies by more AEs, which should be considered carefully in prostate cancer patients who are typically of older ages. A recent systematic review sought for evaluating the AEs following ICI therapy in patients with urologic cancers (95), demonstrating that prostate cancer patients had the highest rate of irAEs and ≥grade3 irAEs among all other urologic malignancies with a rate of 48.3% and 17.6%, respectively (95). The immunological reason why irAEs are more common in prostate cancer must be investigated in ongoing experimental studies. According to ICI monotherapy trials, we found that the most commonly reported any grade TRAEs included fatigue, diarrhea, and Infusion reactions. In this regard, Wang and colleagues performed a systematic review to assess the rate of different AEs related to ICI therapy for all human cancers (96); similarly, they found that the incidence of fatigue was the highest (18.3%) among all other AEs, which was lower relative to our estimates (24.9%). In addition, diarrhea and infusion reactions ranked third and fourteenth in this study, respectively with lower incidence rates relative to our findings (9.5% vs. 20.6% and 3.6% vs. 17.7%, respectively) (96).
5 Strength and limitations
Our systematic review was the first study intended to comprehensively investigate the efficacy and safety of ICIs for patients with prostate cancer. We included 35 trials, pooling the results of 3,618 participants into the data analysis. Applying novel approaches, we calculated the mOS and mPFS for the overall treatments as well as for categorized treatment regimens, separately. However, we faced a few limitations which should be considered when interpreting our findings. Firstly, we pooled the proportions obtained from single arms which might be potentially susceptible to bias as a result of variations in previous treatment lines, baseline differences in patient characteristics, target of medications, and dose and schedule of treatments. Secondly, inclusion of conference abstracts which were not peer-reviewed might be the another source of bias. Thirdly, we only included studies that examined the ICI combination therapies with conventional medications, while many trials investigated addition of ICIs to the state of the art immunotherapeutic agents. Therefore, future systematic reviews can also include novel combination approaches.
6 Conclusion and future perspective
Immunotherapy using monoclonal antibodies against immune checkpoint proteins assumed to be a promising opportunity for patients with prostate cancer; however, real-world data is not convincing even with ICI combination with conventional medications. We found that patients with advanced prostate cancer responded differently to ICI regimens and a variety of AEs were observed according to the type of administrated medications. While our findings may provide important guidance to clinicians in management of patients with prostate cancers, introduction of ICIs as the standard treatment of advanced prostate cancer requires further studies. Currently, trials are directed toward advanced combination approaches with novel immunotherapeutic medications. For instance, a bispecific T cell engagers (BITE) have been recently engineered to simultaneously bind to prostate-specific membrane antigen (PSMA) on tumor cells and CD3 on T cells, resulting in direct T cell activation and tumor cell lysis; the safety and efficacy of this BITE is being evaluated in combination with Pembrolizumab in the trial NCT03792841. Besides, conjugation of lutetium-177 to the PSMA ligand has been provided by Lu-PSMA-617 which is a radiopharmaceutical agent constructed to directly deliver radiation to prostate tumor cells; currently, addition of Lu-PSMA-617 to Pembrolizumab is being studied in the NCT03658447 trial. Furthermore, the other treatment modalities such as chimeric antigen receptor T cell (CAR-T) therapy and cancer vaccines have been developed for prostate cancer patients which are currently at early phases of clinical trials and can be given as combination therapy with ICIs in near future (97–99). All in all, while ICIs :either as single agents or in a combined-modal strategy with conventional medications:may partially improve survival of patients with advanced prostate cancer, advanced combination approaches with novel immunotherapeutic medications may pave the way for successful therapeutic strategies using immunotherapy in this malignancy.
Author contributions
MN designed the study. MN, SA, and FA performed the study selection and data extraction. MN conducted the analysis. MN, SA, FA, FF, AM, and DB wrote the first draft of the manuscript. DB, MK, and AK critically revised the manuscript. All authors reviewed the drafted manuscript for critical content. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1181051/full#supplementary-material
References
1. Wang L, Lu B, He M, Wang Y, Wang Z, Du L. Prostate cancer incidence and mortality: global status and temporal trends in 89 countries from 2000 to 2019. Front Public Health (2022) 10:811044. doi: 10.3389/fpubh.2022.811044
2. Keyes M, Crook J, Morton G, Vigneault E, Usmani N, Morris WJ. Treatment options for localized prostate cancer. Can Fam Physician (2013) 59(12):1269–74.
3. Merseburger AS, Krabbe LM, Krause BJ, Böhmer D, Perner S, Amsberg GV. The treatment of metastatic, hormone-sensitive prostatic carcinoma. Dtsch Arztebl Int (2022) 119(37):622–32. doi: 10.3238/arztebl.m2022.0294
4. Zheng H, Chen J, Qiu W, Lin S, Chen Y, Liang G, et al. Safety and efficacy of first-line treatments for chemotherapy-naive metastatic castration-resistant prostate cancer: A systematic review and indirect comparison. BioMed Res Int (2017) 2017:3941217. doi: 10.1155/2017/3941217
5. Lowrance WT, Roth BJ, Kirkby E, Murad MH, Cookson MS. Castration-resistant prostate cancer: AUA guideline amendment 2015. J Urol (2016) 195(5):1444–52. doi: 10.1016/j.juro.2015.10.086
6. Achard V, Putora PM, Omlin A, Zilli T, Fischer S. Metastatic prostate cancer: treatment options. Oncology (2022) 100(1):48–59. doi: 10.1159/000519861
7. Motzer RJ, Tannir NM, McDermott DF, Arén Frontera O, Melichar B, Choueiri TK, et al. Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. N Engl J Med (2018) 378(14):1277–90. doi: 10.1056/NEJMoa1712126
8. Wolchok JD, Chiarion-Sileni V, Gonzalez R, Rutkowski P, Grob JJ, Cowey CL, et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med (2017) 377(14):1345–56. doi: 10.1056/NEJMoa1709684
9. Beer TM, Kwon ED, Drake CG, Fizazi K, Logothetis C, Gravis G, et al. Randomized, double-blind, phase III trial of ipilimumab versus placebo in asymptomatic or minimally symptomatic patients with metastatic chemotherapy-naive castration-resistant prostate cancer. J Clin Oncol (2016) 35(1):40–7. doi: 10.1200/JCO.2016.69.1584
10. Kwon ED, Drake CG, Scher HI, Fizazi K, Bossi A, van den Eertwegh AJ, et al. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol (2014) 15(7):700–12. doi: 10.1016/S1470-2045(14)70189-5
11. Robert C. A decade of immune-checkpoint inhibitors in cancer therapy. Nat Commun (2020) 11(1):1–3. doi: 10.1038/s41467-020-17670-y
12. Pennock GK, Chow LQ. The evolving role of immune checkpoint inhibitors in cancer treatment. oncolo (2015) 20(7):812–22. doi: 10.1634/theoncologist.2014-0422
13. Thallinger C, Füreder T, Preusser M, Heller G, Müllauer L, Höller C, et al. Review of cancer treatment with immune checkpoint inhibitors. Wiener Klinische Wochenschrift (2018) 130(3):85–91. doi: 10.1007/s00508-017-1285-9
14. von Amsberg G, Alsdorf W, Karagiannis P, Coym A, Kaune M, Werner S, et al. Immunotherapy in advanced prostate cancer—Light at the end of the tunnel? Int J Mol Sci (2022) 23(5):2569. doi: 10.3390/ijms23052569
15. Beer TM, Kwon ED, Drake CG, Fizazi K, Logothetis C, Gravis G, et al. Randomized, double-blind, phase III trial of ipilimumab versus placebo in asymptomatic or minimally symptomatic patients with metastatic chemotherapy-naive castration-resistant prostate cancer. J Clin Oncol (2017) 35(1):40–7. doi: 10.1200/JCO.2016.69.1584
16. Powles T, Yuen KC, Gillessen S, Kadel EE, Rathkopf D, Matsubara N, et al. Atezolizumab with enzalutamide versus enzalutamide alone in metastatic castration-resistant prostate cancer: a randomized phase 3 trial. Nat Med (2022) 28(1):144–+. doi: 10.1038/s41591-021-01600-6
17. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ (2021) 372:n71. doi: 10.1016/j.ijsu.2021.105906
18. Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. bmj (2016) 355. doi: 10.1136/bmj.i4919
19. Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane Handbook for Systematic Reviews of Interventions. John Wiley & Sons (2019). doi: 10.1002/9781119536604
20. Web plot digitizer. Available at: https://apps.automeris.io/wpd/.
21. Shubhram P. metaSurvival: Meta-analysis of asingle survival curve using the multivariate methodology of DerSimonian and Laird. R package version 0.1.0 (2022). Available At: https://github.com/shubhrampandey/metaSurvival.
23. Nyaga VN, Arbyn M, Aerts M. Metaprop: a Stata command to perform meta-analysis of binomial data. Arch Public Health (2014) 72(1):39. doi: 10.1186/2049-3258-72-39
24. Sharma P, Pachynski RK, Narayan V, Flechon A, Gravis G, Galsky MD, et al. Initial results from a phase II study of nivolumab (NIVO) plus ipilimumab (IPI) for the treatment of metastatic castration-resistant prostate cancer (mCRPC; CheckMate 650). Journal of Clinical Oncology (2019) 37(7_suppl):142–142. doi: 10.1200/JCO.2019.37.7_suppl.142
25. Arranz Arija JA, Yu EY, Piulats JM, Gravis G, Laguerre B, Oudard S, et al. Pembrolizumab (pembro) plus olaparib in patients (pts) with docetaxel-pretreated metastatic castration-resistant prostate cancer (mCRPC): KEYNOTE-365 Cohort A update. Ann Oncol (2020) 31:S513–S4. doi: 10.1016/j.annonc.2020.08.880
26. Brown LC, Halabi S, Humeniuk MS, Wu Y, Oyekunle T, Huang J, et al. Efficacy of the PD-L1 inhibitor avelumab in neuroendocrine or aggressive variant prostate cancer: Results from a phase II, single-arm study. J Clin Oncol (2021) 39(6 SUPPL). doi: 10.1200/JCO.2021.39.6_suppl.89
27. De Bono JS, Goh JCH, Ojamaa K, Piulats Rodriguez JM, Drake CG, Hoimes CJ, et al. KEYNOTE-199: Pembrolizumab (pembro) for docetaxel-refractory metastatic castrationresistant prostate cancer (mCRPC). J Clin Oncol (2018) 36(15). doi: 10.1200/JCO.2018.36.15_suppl.5007
28. Fizazi K, Gonzalez Mella P, Castellano D, Minatta JN, Rezazadeh Kalebasty A, Shaffer D, et al. Efficacy and safety of nivolumab in combination with docetaxel in men with metastatic castration-resistant prostate cancer in CheckMate 9KD. Ann Oncol (2019) 30:v885–v6. doi: 10.1093/annonc/mdz394.045
29. Fizazi K, Mella PG, Castellano D, Minatta JN, Rezazadeh A, Shaffer DR, et al. CheckMate 9KD Arm B final analysis: Efficacy and safety of nivolumab plus docetaxel for chemotherapy-naïve metastatic castration-resistant prostate cancer. J Clin Oncol (2021) 39(6 SUPPL). doi: 10.1200/JCO.2021.39.6_suppl.12
30. Goh JCH, Piulats JM, Gross-Goupil M, Vaishampayan UN, de Wit R, Alanko TV, et al. Phase II study of pembrolizumab in docetaxel-pretreated patients with metastatic castration-resistant prostate cancer (mCRPC): Updated follow-up of cohorts (C) 1-3 from KEYNOTE-199. Ann Oncol (2020) 31:S1331–S2. doi: 10.1016/j.annonc.2020.10.451
31. Graff JN, Antonarakis ES, Hoimes CJ, Tagawa ST, Hwang C, Kilari D, et al. Pembrolizumab (pembro) plus enzalutamide (enza) for enza-resistant metastatic castration-resistant prostate cancer (mCRPC): KEYNOTE-199 cohorts 4-5. J Clin Oncol (2020) 38(6). doi: 10.1200/JCO.2020.38.6_suppl.15
32. Hansen A, Massard C, Ott PA, Haas N, Lopez J, Ejadi S, et al. Pembrolizumab for patients with advanced prostate adenocarcinoma: Preliminary results from the KEYNOTE-028 study. Ann Oncol (2016) 27. doi: 10.1093/annonc/mdw372.09
33. Higa J, Wilenius K, Weidhaas JB, Larsen C, Lam RY, Turner J, et al. Pembrolizumab for recurrent or advanced prostate cancer. J Clin Oncol (2018) 36(6). doi: 10.1200/JCO.2018.36.6_suppl.250
34. Hoimes CJ, Graff JN, Tagawa ST, Hwang C, Kilari D, Tije AJT, et al. KEYNOTE-199 cohorts (C) 4 and 5: Phase II study of pembrolizumab (pembro) plus enzalutamide (enza) for enzaresistant metastatic castrationresistant prostate cancer (mCRPC). J Clin Oncol (2020) 38(15). doi: 10.1200/JCO.2020.38.15_suppl.5543
35. McDermott R, Graff JN, Antonarakis ES, Hoimes CJ, Tagawa ST, Hwang C, et al. KEYNOTE-199 cohorts 4 and 5: Pembrolizumab (pembro) plus enzalutamide (enza) for enza-resistant metastatic castration-resistant prostate cancer (mCRPC). Eur Urol Open Sci (2020) 19:e885–e6. doi: 10.1016/S2666-1683(20)33171-2
36. Morris MJ, Fong L, Petrylak DP, Sartor AO, Higano CS, Pagliaro LC, et al. Safety and clinical activity of atezolizumab (atezo) + radium-223 dichloride (r-223) in 2L metastatic castration-resistant prostate cancer (mCRPC): Results from a phase Ib clinical trial. J Clin Oncol (2020) 38(15). doi: 10.1200/JCO.2020.38.15_suppl.5565
37. Omlin AG, Graff JN, Hoimes CJ, Tagawa ST, Hwang C, Kilari D, et al. KEYNOTE-199 phase II study of pembrolizumab plus enzalutamide for enzalutamide-resistant metastatic castration-resistant prostate cancer (mCRPC): Cohorts (C) 4 and 5 update. Ann Oncol (2020) 31:S514–S5. doi: 10.1016/j.annonc.2020.08.882
38. Pachynski RK, Retz M, Goh JC, Burotto M, Gravis G, Castellano D, et al. CheckMate 9KD cohort A1 final analysis: Nivolumab (NIVO) + rucaparib for post-chemotherapy (CT) metastatic castrationresistant prostate cancer (mCRPC). J Clin Oncol (2021) 39(15 SUPPL). doi: 10.1200/JCO.2021.39.15_suppl.5044
39. Romano E, Sridhar SS, Kolinsky MP, Gravis G, Mourey L, Piulats JM, et al. Pembrolizumab (pembro) plus docetaxel and prednisone in patients with abiraterone acetate (abi)- or enzalutamide (enza)–pretreated metastatic castration-resistant prostate cancer (mCRPC): KEYNOTE-365 cohort B update. Ann Oncol (2020) 31:S512–S3. doi: 10.1016/j.annonc.2020.08.879
40. Slovin SF, Hamid O, Tejwani S, Higano CS, Harzstark A, Alumkal JJ, et al. Ipilimumab (IPI) in metastatic castrate-resistant prostate cancer (mCRPC): Results from an open-label, multicenter phase I/II study. J Clin Oncol (2012) 30(5). doi: 10.1200/jco.2012.30.5_suppl.25
41. Barata P, Agarwal N, Nussenzveig R, Gerendash B, Jaeger E, Hatton W, et al. Clinical activity of pembrolizumab in metastatic prostate cancer with microsatellite instability high (MSI-H) detected by circulating tumor DNA. J ImmunoTher Cancer (2020) 8(2). doi: 10.1136/jitc-2020-001065
42. Graf RP, Fisher V, Weberpals J, Gjoerup O, Tierno MB, Huang RS, et al. Comparative effectiveness of immune checkpoint inhibitors vs chemotherapy by tumor mutational burden in metastatic castration-resistant prostate cancer. JAMA network Open (2022) 5(3):e225394–e. doi: 10.1001/jamanetworkopen.2022.5394
43. Subudhi SK, Aparicio A, Troncoso P, Zhang J, Gumbs C, Wu CJ, et al. Linking tumor mutational load to clinical responses to ipilimumab (IPI) in men with advanced prostate cancer (PCa). J Clin Oncol (2017) 35(15). doi: 10.1200/JCO.2017.35.15_suppl.5065
44. Yuan ZG, Fernandez D, Dhillon J, Abraham-Miranda J, Awasthi S, Kim Y, et al. Proof-of-principle Phase I results of combining nivolumab with brachytherapy and external beam radiation therapy for Grade Group 5 prostate cancer: safety, feasibility, and exploratory analysis. Prostate Cancer Prostatic Dis (2021) 24(1):140–9. doi: 10.1038/s41391-020-0254-y
45. Dorff T, Hirasawa Y, Acoba J, Pagano I, Tamura D, Pal S, et al. Phase Ib study of patients with metastatic castrate-resistant prostate cancer treated with different sequencing regimens of atezolizumab and sipuleucel-T. J Immunother Cancer (2021) 9(8). doi: 10.1136/jitc-2021-002931
46. Agarwal N, McGregor B, Maughan BL, Dorff TB, Kelly W, Fang B, et al. Cabozantinib in combination with atezolizumab in patients with metastatic castration-resistant prostate cancer: results from an expansion cohort of a multicentre, open-label, phase 1b trial (COSMIC-021). Lancet Oncol (2022) 23(7):899–909. doi: 10.1016/S1470-2045(22)00278-9
47. Alva AS, Li J, Chou J, Reimers MA, McKay RR, Zhang J, et al. Phase 2 trial of immunotherapy in tumors with CDK12 inactivation (IMPACT): Results from cohort A of patients (pts) with metastatic castration resistant prostate cancer (mCRPC) receiving dual immune checkpoint inhibition (ICI). J Clin Oncol (2022) 40(6 SUPPL). doi: 10.1200/JCO.2022.40.6_suppl.103
48. Antonarakis ES, Piulats JM, Gross-Goupil M, Goh J, Ojamaa K, Hoimes CJ, et al. Pembrolizumab for treatment-refractory metastatic castration-resistant prostate cancer: multicohort, open-label phase II KEYNOTE-199 study. J Clin Oncol (2020) 38(5). doi: 10.1200/JCO.19.01638
49. Antonarakis ES, Piulats JM, Gross-Goupil M, Goh JC, Vaishampayan UN, De Wit R, et al. Pembrolizumab (pembro) monotherapy for docetaxel-pretreated metastatic castration-resistant prostate cancer (mCRPC): Updated analyses with 4 years of follow-up from cohorts 1-3 of the KEYNOTE-199 study. Ann Oncol (2021) 32:S651–S2. doi: 10.1016/j.annonc.2021.08.1124
50. Boudadi K, Suzman DL, Anagnostou V, Fu W, Luber B, Wang H, et al. Ipilimumab plus nivolumab and DNA-repair defects in AR-V7-expressing metastatic prostate cancer. Oncotarget (2018) 9(47):28561–71. doi: 10.18632/oncotarget.25564
51. Brown LC, Halabi S, Somarelli JA, Humeniuk M, Wu Y, Oyekunle T, et al. A phase 2 trial of avelumab in men with aggressive-variant or neuroendocrine prostate cancer. Prostate Cancer Prostatic Dis (2022) 25, 762–9. doi: 10.1038/s41391-022-00524-7
52. Fakhrejahani F, Madan RA, Dahut WL, Karzai F, Cordes LM, Schlom J, et al. Avelumab in metastatic castration-resistant prostate cancer (mCRPC). J Clin Oncol (2017) 35(6). doi: 10.1200/JCO.2017.35.6_suppl.159
53. Fizazi K, González Mella P, Castellano D, Minatta JN, Rezazadeh Kalebasty A, Shaffer D, et al. Nivolumab plus docetaxel in patients with chemotherapy-naïve metastatic castration-resistant prostate cancer: results from the phase II CheckMate 9KD trial. Eur J Cancer (2022) 160:61–71. doi: 10.1016/j.ejca.2021.09.043
54. Fizazi K, Retz M, Petrylak DP, Goh JC, Perez-Gracia J, Lacombe L, et al. Nivolumab plus rucaparib for metastatic castration-resistant prostate cancer: results from the phase 2 CheckMate 9KD trial. J Immunother Cancer (2022) 10(8). doi: 10.1136/jitc-2022-004761
55. Fong L, Morris MJ, Sartor O, Higano CS, Pagliaro L, Alva A, et al. A phase Ib study of atezolizumab with radium-223 dichloride in men with metastatic castration-resistant prostate cancer. Clin Cancer Res (2021) 27(17):4746–56. doi: 10.1158/1078-0432.CCR-21-0063
56. Graff JN, Beer TM, Alumkal JJ, Slottke RE, Redmond WL, Thomas GV, et al. A phase II single-arm study of pembrolizumab with enzalutamide in men with metastatic castration-resistant prostate cancer progressing on enzalutamide alone. J Immunother Cancer (2020) 8(2). doi: 10.1136/jitc-2020-000642
57. Graff JN, Stein MN, Surana R, Al Rabadi L, Liu ER, Fong L, et al. Phase II study of ipilimumab in men with metastatic prostate cancer with an incomplete response to androgen deprivation therapy. Front Oncol (2020) 10. doi: 10.3389/fonc.2020.01381
58. Gurney H. Multicohort phase 1b/2 study of pembrolizumab combination therapies in patients with metastatic castration-resistant prostate cancer: updated results from KEYNOTE 365 cohorts A-C. Asia-Pacific J Clin Oncol (2021) 17(SUPPL 7):59–60.
59. Hansen AR, Massard C, Ott PA, Haas NB, Lopez JS, Ejadi S, et al. Pembrolizumab for advanced prostate adenocarcinoma: findings of the KEYNOTE-028 study. Ann Oncol (2018) 29(8):1807–13. doi: 10.1093/annonc/mdy232
60. Hotte SJ, Winquist E, Chi KN, Ellard SL, Sridhar S, Emmenegger U, et al. CCTG IND 232: A phase II study of durvalumab with or without tremelimumab in patients with metastatic castration resistant prostate cancer (mCRPC). Ann Oncol (2019) 30:v885. doi: 10.1093/annonc/mdz394.044
61. Karzai F, Vanderweele D, Madan RA, Owens H, Cordes LM, Hankin A, et al. Activity of durvalumab plus olaparib in metastatic castration-resistant prostate cancer in men with and without DNA damage repair mutations 11 Medical and Health Sciences 1112 Oncology and Carcinogenesis. J ImmunoTher Cancer (2018) 6(1). doi: 10.1186/s40425-018-0463-2
62. Kwan EM, Spain L, Anton A, Gan CL, Garrett L, Chang D, et al. Avelumab combined with stereotactic ablative body radiotherapy in metastatic castration-resistant prostate cancer: the phase 2 ICE-PAC clinical trial. Eur Urol (2022) 81(3):253–62. doi: 10.1016/j.eururo.2021.08.011
63. Layton JL, Manogue C, Light M, Jaeger E, Cotogno P, Ledet EM, et al. PD-L1 inhibition with avelumab plus abiraterone acetate or enzalutamide in African Americans with metastatic castrate-resistant prostate cancer (mCRPC). J Clin Oncol (2021) 39(6 SUPPL). doi: 10.1200/JCO.2021.39.6_suppl.87
64. Markowski MC, Taplin ME, Aggarwal RR, Wang H, Lalji A, Paller CJ, et al. COMBAT-CRPC: Concurrent administration of bipolar androgen therapy (BAT) and nivolumab in men with metastatic castration-resistant prostate cancer (mCRPC). J Clin Oncol (2021) 39(15 SUPPL). doi: 10.1200/JCO.2021.39.15_suppl.5014
65. Mourey L, Conter HJ, Shore N, Berry WR, Fong PC, Piulats JM, et al. Pembrolizumab (pembro) plus enzalutamide (enza) in patients with abiraterone acetate (abi)-pretreated metastatic castration-resistant prostate cancer (mCRPC): KEYNOTE-365 Cohort C update. Ann Oncol (2020) 31:S516–S7. doi: 10.1016/j.annonc.2020.08.884
66. Petrylak DP, Loriot Y, Shaffer DR, Braiteh F, Powderly J, Harshman LC, et al. Safety and clinical activity of atezolizumab in patients with metastatic castration-resistant prostate cancer: A phase I study. Clin Cancer Res (2021) 27(12):3360–9. doi: 10.1158/1078-0432.CCR-20-1981
67. Piulats J, Ferrario C, Linch M, Stoeckle M, Laguerre B, Arranz J, et al. 351 KEYNOTE-365 cohort D: pembrolizumab plus abiraterone acetate and prednisone in patients with chemotherapy-naive metastatic castration-resistant prostate cancer (mCRPC). Journal forImmunoTherapy of Cancer (2021) 9. doi: 10.1136/jitc-2021-SITC2021.351
68. Rodriguez-Vida A, Maroto Rey JP, Font Pous A, Martin C, Mellado B, Corbera Lloret A, et al. Safety and efficacy of avelumab plus carboplatin in patients with metastatic castration resistant prostate cancer in an open-label phase Ib study. Ann Oncol (2021) 32:S665. doi: 10.1016/j.annonc.2021.08.1144
69. Sharma P, Pachynski RK, Narayan V, Flechon A, Gravis G, Galsky MD, et al. Nivolumab plus ipilimumab for metastatic castration-resistant prostate cancer: preliminary analysis of patients in the checkMate 650 trial. Cancer Cell (2020) 38(4):489–+. doi: 10.1016/j.ccell.2020.08.007
70. Shenderov E, Boudadi K, Fu W, Wang H, Sullivan R, Jordan A, et al. Nivolumab plus ipilimumab, with or without enzalutamide, in AR-V7-expressing metastatic castration-resistant prostate cancer: A phase-2 nonrandomized clinical trial. Prostate (2021) 81(6):326–38. doi: 10.1002/pros.24110
71. Slovin SF, Higano CS, Hamid O, Tejwani S, Harzstark A, Alumkal JJ, et al. Ipilimumab alone or in combination with radiotherapy in metastatic castration-resistant prostate cancer: results from an open-label, multicenter phase I/II study. Ann Oncol (2013) 24(7):1813–21. doi: 10.1093/annonc/mdt107
72. Small EJ, Tchekmedyian NS, Rini BI, Fong L, Lowy I, Allison JP. A pilot trial of CTLA-4 blockade with human anti-CTLA-4 in patients with hormone-refractory prostate cancer. Clin Cancer Res (2007) 13(6):1810–5. doi: 10.1158/1078-0432.CCR-06-2318
73. Subudhi SK, Vence L, Zhao H, Blando J, Yadav SS, Xiong Q, et al. Neoantigen responses, immune correlates, and favorable outcomes after ipilimumab treatment of patients with prostate cancer. Sci Trans Med (2020) 12(537):eaaz3577. doi: 10.1126/scitranslmed.aaz3577
74. Vaishampayan UN, Elliott T, Omlin AG, Graff JN, Hoimes CJ, Tagawa ST, et al. Phase II study of pembrolizumab (pembro) plus enzalutamide for enzalutamide (enza)-resistant metastatic castration-resistant prostate cancer (mCRPC): Cohorts (C) 4 and 5 update from KEYNOTE-199. Ann Oncol (2020) 31:S1330. doi: 10.1016/j.annonc.2020.10.447
75. Yu E, Piulats JM, Gravis G, Fong PCC, Todenhöfer T, Laguerre B, et al. Pembrolizumab (pembro) plus olaparib in patients with docetaxel-pretreated metastatic castration-resistant prostate cancer (mCRPC): Update of KEYNOTE-365 cohort A with a minimum of 11 months of follow-up for all patients. Ann Oncol (2021) 32:S652–S3. doi: 10.1016/j.annonc.2021.08.1125
76. Yu EY, Kolinsky MP, Berry WR, Retz M, Mourey L, Piulats JM, et al. Pembrolizumab plus docetaxel and prednisone in patients with metastatic castration-resistant prostate cancer: long-term results from the phase 1b/2 KEYNOTE-365 cohort B study. Eur Urol (2022) 82(1):22–30. doi: 10.1016/j.eururo.2022.02.023
77. Zucali PA, Lin CC, Carthon BC, Bauer TM, Tucci M, Italiano A, et al. Targeting CD38 and PD-1 with isatuximab plus cemiplimab in patients with advanced solid Malignancies: results from a phase I/II open-label, multicenter study. J Immunother Cancer (2022) 10(1). doi: 10.1136/jitc-2021-003697
78. Thomas F, Howard G, Piotr T, Se Hoon P, Balaji V, Stefan S, et al. Adjuvant pembrolizumab for patients with renal cell carcinoma at increased risk of recurrence postnephrectomy: updated results from KEYNOTE-564. Asia-Pacific J Clin Oncol (2022) 18:56–7.
79. Drugs@FDA: FDA-Approved Drugs (2022). Available at: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm.
80. Venkatachalam S, McFarland TR, Agarwal N, Swami U. Immune checkpoint inhibitors in prostate cancer. Cancers (2021) 13(9):2187. doi: 10.3390/cancers13092187
81. Heidegger I, Necchi A, Pircher A, Tsaur I, Marra G, Kasivisvanathan V, et al. A systematic review of the emerging role of immune checkpoint inhibitors in metastatic castration-resistant prostate cancer: will combination strategies improve efficacy? Eur Urol Oncol (2021) 4(5):745–54. doi: 10.1016/j.euo.2020.10.010
82. Fahmy O, Alhakamy NA, Khairul-Asri MG, Ahmed OAA, Fahmy UA, Fresta CG, et al. Oncological response and predictive biomarkers for the checkpoint inhibitors in castration-resistant metastatic prostate cancer: A systematic review and meta-analysis. J Pers Med (2021) 12(1). doi: 10.3390/jpm12010008
83. Wang X-H, Wang Z-Q, Mu Z-Y, Zhu L-P, Zhong C-F, Guo S. The efficacy and safety of immune checkpoint inhibitors in metastatic castration-resistant prostate cancer: A systematic review and meta-analysis. Medicine (2022) 101(31). doi: 10.1097/MD.0000000000029715
84. Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. New Engl J Med (2010) 363(5):411–22. doi: 10.1056/NEJMoa1001294
85. De Velasco MA, Uemura H. Prostate cancer immunotherapy: where are we and where are we going? Curr Opin Urol (2018) 28(1):15–24. doi: 10.1097/MOU.0000000000000462
86. Vitkin N, Nersesian S, Siemens DR, Koti M. The tumor immune contexture of prostate cancer. Front Immunol (2019) 10:603. doi: 10.3389/fimmu.2019.00603
87. solipuram V, Pokharel K, Venkatesulu BP, Samanapally H. Effect of immunotherapy on survival outcomes in prostate cancer: Systematic review and meta-analysis. J Clin Oncol (2021) 39(15_suppl):e17030–e. doi: 10.1200/JCO.2021.39.15_suppl.e17030
88. Berger MF, Lawrence MS, Demichelis F, Drier Y, Cibulskis K, Sivachenko AY, et al. The genomic complexity of primary human prostate cancer. Nature (2011) 470(7333):214–20. doi: 10.1038/nature09744
89. Jayaprakash P, Ai M, Liu A, Budhani P, Bartkowiak T, Sheng J, et al. Targeted hypoxia reduction restores T cell infiltration and sensitizes prostate cancer to immunotherapy. J Clin Invest (2018) 128(11):5137–49. doi: 10.1172/JCI96268
90. Chouaib S, Noman M, Kosmatopoulos K, Curran M. Hypoxic stress: obstacles and opportunities for innovative immunotherapy of cancer. Oncogene (2017) 36(4):439–45. doi: 10.1038/onc.2016.225
91. Galluzzi L, Buque A, Kepp O, Zitvogel L, Kroemer G. Immunological effects of conventional chemotherapy and targeted anticancer agents. Cancer Cell (2015) 28(6):690–714. doi: 10.1016/j.ccell.2015.10.012
92. Apetoh L, Ladoire S, Coukos G, Ghiringhelli F. Combining immunotherapy and anticancer agents: the right path to achieve cancer cure? Ann Oncol (2015) 26(9):1813–23. doi: 10.1093/annonc/mdv209
93. Zitvogel L, Galluzzi L, Smyth MJ, Kroemer G. Mechanism of action of conventional and targeted anticancer therapies: reinstating immunosurveillance. Immunity (2013) 39(1):74–88. doi: 10.1016/j.immuni.2013.06.014
94. Rotte A. Combination of CTLA-4 and PD-1 blockers for treatment of cancer. J Exp Clin Cancer Res (2019) 38(1):255. doi: 10.1186/s13046-019-1259-z
95. Wu Z, Chen Q, Qu L, Li M, Wang L, Mir MC, et al. Adverse events of immune checkpoint inhibitors therapy for urologic cancer patients in clinical trials: a collaborative systematic review and meta-analysis. Eur Urol (2022) 81(4):414–25. doi: 10.1016/j.eururo.2022.01.028
96. Wang Y, Zhou S, Yang F, Qi X, Wang X, Guan X, et al. Treatment-related adverse events of PD-1 and PD-L1 inhibitors in clinical trials: a systematic review and meta-analysis. JAMA Oncol (2019) 5(7):1008–19. doi: 10.1001/jamaoncol.2019.0393
97. Runcie KD, Dallos MC. Prostate cancer immunotherapy—Finally in from the cold? Curr Oncol Rep (2021) 23(8):88. doi: 10.1007/s11912-021-01084-0
98. Cha HR, Lee JH, Ponnazhagan S. Revisiting immunotherapy: A focus on prostate cancer. Cancer Res (2020) 80(8):1615–23. doi: 10.1158/0008-5472.CAN-19-2948
Keywords: immune checkpoint inhibitors, prostate cancer, immunotherapy, PD-1, PDL, CTLA-4
Citation: Noori M, Azizi S, Mahjoubfar A, Abbasi Varaki F, Fayyaz F, Mousavian A-H, Bashash D, Kardoust Parizi M and Kasaeian A (2023) Efficacy and safety of immune checkpoint inhibitors for patients with prostate cancer: a systematic review and meta-analysis. Front. Immunol. 14:1181051. doi: 10.3389/fimmu.2023.1181051
Received: 06 March 2023; Accepted: 04 September 2023;
Published: 31 October 2023.
Edited by:
Laura Ridolfi, Scientific Institute for Research, Hospitalization and Healthcare (IRCCS), ItalyReviewed by:
Giuseppe Schepisi, Scientific Institute of Romagna for the Study and Treatment of Tumors (IRCCS), ItalyH. Atakan Ekiz, Izmir Institute of Technology, Türkiye
Copyright © 2023 Noori, Azizi, Mahjoubfar, Abbasi Varaki, Fayyaz, Mousavian, Bashash, Kardoust Parizi and Kasaeian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Amir Kasaeian, YW1pcl9rYXNhZWlhbkB5YWhvby5jb20=; YWthc2FlaWFuQHNpbmEudHVtcy5hYy5pcg==
†These authors have contributed equally to this work
 Maryam Noori
Maryam Noori Shadi Azizi1†
Shadi Azizi1† Aref Mahjoubfar
Aref Mahjoubfar Amir-Hossein Mousavian
Amir-Hossein Mousavian Davood Bashash
Davood Bashash Mehdi Kardoust Parizi
Mehdi Kardoust Parizi Amir Kasaeian
Amir Kasaeian