
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Immunol. , 30 May 2023
Sec. Cancer Immunity and Immunotherapy
Volume 14 - 2023 | https://doi.org/10.3389/fimmu.2023.1177085
This article is part of the Research Topic Mechanism and Application of Synergistic Effect of Radiotherapy and Immunotherapy View all 7 articles
Esophageal squamous cell carcinoma (ESCC) is a common malignancy worldwide and often diagnosed at advanced stages with poor prognosis. Combination of radiotherapy and immunotherapy seems to be a promising approach for treating ESCC. This comprehensive review article summarizes the current state of combination of radiotherapy and immunotherapy in locally advanced/metastatic ESCC, delineates the clinical trials that merit attention, and outlines unresolved issues and future research directions in this field. The clinical trial findings suggest that radio-immunotherapy combination may improve tumor response and overall survival with manageable side effects, highlighting the importance of patient selection and the necessity for further research to optimize treatment strategies. Issues such as irradiation dosage, fractionation regimen, irradiation site and technique of radiotherapy, as well as the timing, sequence and duration of combination therapy will all affect treatment outcomes, justifying further in-depth investigation.
Esophageal cancer, a malignancy that poses a significant threat to human health, is ranked seventh in incidence and sixth in mortality worldwide (1). Based on their histological features, they can be divided into esophageal squamous cell carcinoma (ESCC) and esophageal adenocarcinoma (EAC), which differ substantially in pathogenesis, biological behavior, treatment and prognosis. Locally advanced esophageal cancer refers to those with tumor invasion of local structures or regional lymph node metastasis without distant metastasis (ie, American Joint Committee on Cancer stage ≥T2 or N+, M0) (2), accounting for the majority of clinical cases, and can be divided into resectable and unresectable groups based on the feasibility of radical resection.
Although significant progress has been achieved in the treatment of esophageal cancer, its efficacy remains unsatisfactory. Immunotherapy, exemplified by immune checkpoint inhibitors (ICIs), has emerged as a promising new therapeutic strategy that continues to revolutionize the treatment of esophageal cancer. However, the employment of a single-agent ICI approach is constrained by a response rate of only approximately 20% (3–6). To overcome this limitation, researchers have explored the combination of immunotherapy with other treatment modalities. Radiotherapy is widely applied in each stage of esophageal cancer and has garnered considerable attention in combination with immunotherapy, following the KEYNOTE-001 study, which revealed a superior efficacy of ICI in patients who had previously received radiotherapy (7).
For resectable or potentially resectable locally advanced ESCC, the current treatment paradigm established by the CROSS study and the NEOCRTEC5010 study is neoadjuvant chemoradiotherapy followed by surgery, which has achieved a pathologic complete response (pCR) rate of 43-49%, a 5-year overall survival (OS) rate of 60%, and a 10-year OS rate of 46% (8–11). Despite significant advances, neoadjuvant chemoradiotherapy remains a topic of debate, with ongoing discussions on the optimal modality of neoadjuvant therapy. Could the addition of immunotherapy to neoadjuvant chemoradiotherapy further improve the outcomes? Alternatively, could neoadjuvant chemoradiotherapy be replaced by neoadjuvant chemotherapy or immunotherapy? Within the framework of trimodality therapy (radiotherapy, chemotherapy and surgery), distant metastasis remains the most predominant mode of subsequent failure (12), and minimal residual disease (MRD) may be the source of relapse and metastasis; could it be eradicated by intensified postoperative adjuvant immunotherapy? For unresectable locally advanced esophageal cancer, definitive chemoradiotherapy is the current standard treatment. Despite multiple studies have explored the optimal chemotherapy regimens and alternative radiotherapy dose fractionation, the complete response rate remains low and the local recurrence rate remains high, with a 3-year OS rate of 40-55% and a 5-year OS rate of only 20-25% (13–17). Might the integration of immunotherapy, administered concurrently with or subsequent to definitive chemoradiotherapy, emulate the favorable outcomes of the PACIFIC protocol in lung cancer? Metastatic esophageal cancer is currently treated with ICI combined with chemotherapy as the standard first-line treatment (18, 19), but the efficiency has attained a plateau. Empirical evidence indicates that supplementing well-managed systemic therapy with aggressive local radiotherapy can not only enhance the nutritional status and improve the quality of life but also stimulate the systemic anti-tumor immune response, leading to substantial survival advantages (20–23). The relatively high proportion of oligometastasis in ESCC patients highlights the importance of exploring the combination of radiotherapy and systemic therapy as an urgent research topic. Resolutions to these inquiries can only be attained through ongoing preclinical and clinical investigations.
In China, squamous cell carcinoma is the predominant histological subtype of esophageal cancer, accounting for more than half of the global morbidity and mortality (1). Despite the abundance of publications reporting the synergistic effects and underlying mechanisms of radiotherapy and immunotherapy (24, 25), research on radio-immunotherapy combinations remains in its infancy and is confronted with various challenges, including several controversial issues that need to be resolved before its potential widespread clinical application. In this article, we present a comprehensive review of the application of radiotherapy in combination with ICIs in patients with locally advanced/metastatic ESCC, delineate the clinical trials that merit particular attention, and synthesize some of the unresolved issues and future research directions in this field.
Radiotherapy is not only efficacious in directly eradicating tumor cells, but also intricately associated with the local immune microenvironment and systemic immune status. In recent years, there have been more and more basic research and clinical trials focusing on the combination of radiotherapy with ICIs. A multitude of studies has demonstrated that the combination can enhance the abscopal effect and antitumor immune memory, leading to a favorable therapeutic outcome (26, 27). The synergistic mechanism of radiotherapy combined with immunotherapy can be summarized as follows (28, 29): Radiation causes cancer cells to undergo immunogenic cell death, releases tumor-associated antigens, which are recognized by antigen-presenting cells and presented to T cells, generating an effect similar to “in situ vaccines” and activating systemic adaptive immune responses to eliminate tumors. Furthermore, radiotherapy reprograms the tumor microenvironment by upregulating the expression of PD-L1 in the tumor microenvironment, regulating various immune cells through cytokines and chemokines, resulting in the conversion of immunologically “cold” tumors into “hot” tumors, making them more amenable to immunotherapy (30, 31). Reciprocally, ICIs not only activate cytotoxic T cells to attack tumor cells, but also normalize tumor vasculature, enhance tissue perfusion to mitigate tumor hypoxia and increase sensitivity to radiotherapy (32, 33).
Systematic searches were performed in PubMed, Google Scholar, and ClinicalTrial.gov databases up to February 2023 to gather information on clinical trials that explore the efficacy of the combination of radiotherapy and ICIs for the treatment of ESCC. Our findings have been collated in Table 1.
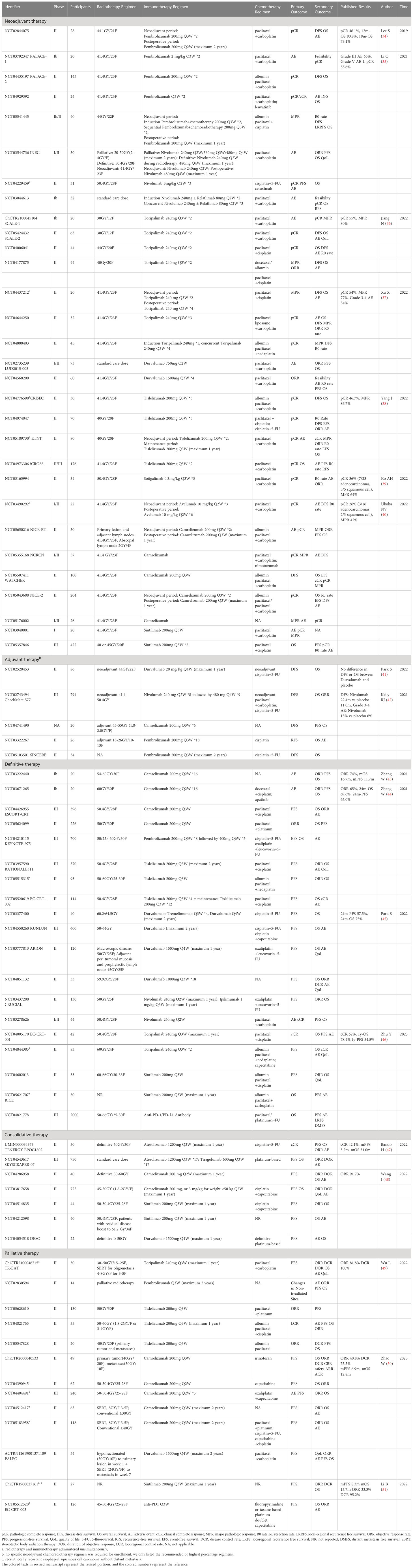
Table 1 Clinical trials investigating the combination of radiotherapy and immune checkpoint inhibitors in esophageal squamous cell carcinoma.
The Korean phase II trial of neoadjuvant chemoradiotherapy combined with Pembrolizumab for ESCC showed a pCR rate of 46.1% (34), which is comparable to the squamous cell subgroup in the CROSS study, as well as in the NEOCRTEC5010 study (8, 10). Among 28 enrolled patients, two died before surgery due to hematemesis and another two died after surgery due to acute lung injury (34). Although the statistics indicate no statistical difference in surgical risk or postoperative complications when compared to patients who underwent conventional neoadjuvant chemoradiotherapy during the same hospitalization period, the adverse effects of the combination should not be overlooked (52). In 2021, Chinese scholars reported results from the similarly designed PALACE-1 trial, which enrolled 20 patients and achieved a promising pCR rate of 55.6% and a notable major pathologic response (MPR) rate of 89%. However, 65% of patients experienced grade 3 or higher adverse events (AEs), and one patient died due to toxicity (35). The subsequent multicenter PALACE-2 study is currently recruiting to further investigate the efficacy and safety of neoadjuvant chemoradiotherapy combined with Pembrolizumab in resectable ESCC (53). In the phase Ib SCALE-1 trial, short-course neoadjuvant chemoradiotherapy (30GY/12F) plus anti-PD-1 Toripalimab followed by esophagectomy achieved an impressive pCR rate of 55% and MPR rate of 80%. Severe treatment-related consisted primarily of myelosuppression (leukopenia and neutropenia) and gastrointestinal toxicity (anorexia and nausea). Grade 3 perioperative complications occurred in 3 of the 20 patients who underwent surgery (36). A novel CD40 agonistic monoclonal antibody, Sotigalimab, in combination with chemoradiotherapy, has shown a pCR rate of up to 60% in squamous cell carcinoma. However, the sample size is too limited, and further studies involving larger sample sizes are required to validate the findings (39).
In consideration of safety concerns, several studies have investigated the sequential administration of ICIs and radiotherapy rather than simultaneous treatment. The interim results of phase II clinical trial of neoadjuvant chemoradiotherapy followed by sequential Tislelizumab showed that among 15 patients undergoing radical surgery, the pCR rate was 46.7% and the MPR rate was 86.7%. During neoadjuvant therapy, no grade 3 or higher AEs were reported, and the grade 3 postoperative complication rate was 20.0% (38). In the neoadjuvant chemoradiotherapy plus sequential perioperative Toripalimab study, the pCR rate was 54%, MPR rate was 77%, and grade 3-4 treatment-related AEs occurred in 54% patients (37). Although long-term survival outcomes are not yet available, it has been suggested that paradigm of neoadjuvant chemoradiotherapy plus sequential ICIs may reduce the incidence and severity of AEs.
To further enhance the efficacy of treatment for high-risk patients and avoid overtreatment of low-risk patients, precise individualized multi-modality treatment is under exploration. Participants with a decrease in positron emission tomography (PET) Standardized Uptake Value (SUV)max < 35% will receive Tislelizumab plus chemoradiotherapy as neoadjuvant treatment, while those with a decrease ≥35% will receive Tislelizumab plus chemotherapy without radiotherapy (54). Alternatively, non-clinical complete responders after neoadjuvant chemoradiotherapy will receive additional neoadjuvant immunochemotherapy (55).
In summary, the addition of ICI to neoadjuvant chemoradiotherapy did increase the pCR rate, but also led to increased toxicity, albeit under manageable. Whether the slightly improved pCR and objective remission rates (ORR) could ultimately translate into overall survival benefit still requires long-term follow-up. Two phase III multicenter randomized trials providing insights into the relative benefits of neoadjuvant chemoradiotherapy combined with or without ICI on response rate, overall survival, and safety, is expected to provide more information on this question (56, 57).
In the context of adjuvant therapy for resectable ESCC, the randomized, double-blind, placebo-controlled phase III CheckMate 577 study demonstrated that 1-year adjuvant Nivolumab significantly prolonged disease-free survival (DFS) (22.4m vs 11.0 m) and OS (29.7m vs 11.0m) in postoperative non-pCR ESCC patients who underwent radical surgery after standard neoadjuvant chemoradiotherapy (42). However, some scholars have questioned the apparently shorter DFS time observed in the placebo group of CheckMate 577. A retrospective study in the Netherlands collected esophageal patients meeting the inclusion criteria of CheckMate 577 but not receiving adjuvant Nivolumab, revealing a median DFS of 19.7 months, which was much longer than that in placebo population of CheckMate 577 (58). It is also noteworthy that ESCC accounted for only 29% of participants in CheckMate 577, and no DFS benefit was observed in the Asian population or Asia region subgroup (42). In addition, a single-center, randomized, double-blind adjuvant Durvalumab study conducted in Korea yielded negative results, possibly due to the fact that the trial did not exclude pCR patients who were not susceptible to benefit from adjuvant immunotherapy (41).
The CheckMate 577 study represents a significant milestone in the exploration of postoperative adjuvant immunotherapy for esophageal cancer, however, further evidence and investigation are necessary to determine appropriate treatment indications, regimens, and courses. The OS data for CheckMate 577 is not yet available, and the results of additional studies specifically evaluating ICI adjuvant therapy for ESCC are eagerly awaited. The ongoing clinical trial NCT04741490 is evaluating the efficacy of Camrelizumab in combination with radiotherapy in adjuvant phase for ESCC (59). NCT03322267 is enrolling patients who remained at high risk of recurrence (resection margin closure or involvement or ypN+) after neoadjuvant chemoradiotherapy, receiving adjuvant chemoradiotherapy followed by 1-year Pembrolizumab treatment (60). In SINCERE Study, ESCC patients who do not respond to initial neoadjuvant therapy (TRG3 and TRG4) will receive adjuvant chemotherapy combined with 2-years adjuvant pembrolizumab (61).
In a clinical study of radiotherapy combined with anti-PD-1 Camrelizumab for locally advanced ESCC, 1 (7.1%) and 13 (92.9%) patients achieved CR and PR, respectively, with no grade 3/4 AEs (62). In a similar phase Ib study, 20 locally advanced ESCC patients treated with radiotherapy plus Camrelizumab achieved an ORR of 74%, with a median OS and progression-free survival (PFS) of 16.7 and 11.7 months, respectively, and 24-month OS and PFS rates of 31.6% and 35.5%, respectively (43). Furthermore, a phase II clinical trial enrolled patients over 70 years old to receive radiotherapy plus Durvarlumab to explore the safety and efficacy of radical radiotherapy combined with immunotherapy in elderly patients (63).
The combination of radiotherapy and ICI has demonstrated success, resulting in a growing interest in integrating ICI into definitive chemoradiotherapy to further improve prognosis. Concurrent administration of Camrelizumab with chemoradiotherapy has shown promising results, with 85.0% and 69.6% 12-month and 24-month OS rates respectively, and 80.0% and 65.0% 12-month and 24-month PFS rates respectively, albeit with 40% of patients experiencing serious treatment-related AEs (44). In the EC-CRT-001 study, 62% of patients receiving Toripalimab combined with chemoradiotherapy achieved complete remission, and the 1-year PFS and OS rates were 54.5% and 78.4%, respectively (46). The randomized, double-blinded, placebo-controlled, multicenter phase III ESCORT-CRT study has been launched to compare the efficacy and safety of Camrelizumab or placebo combined with definitive chemoradiotherapy (64). Other large-scale clinical trials are also underway to assess ICIs administered concurrently with definitive chemoradiotherapy, including Keynote-975 (NCT0421011, Pembrolizumab) (65), RATIONALE 311 (NCT03957590, Tislelizumab) (66), KUNLUN (NCT04550260, Durvalumab) (67, 68), ARION (NCT03777813, Durvalumab) (69). In addition to concurrent administration of ICIs and definitive chemoradiotherapy, clinical studies have explored sequential chemoradiotherapy after immunotherapy to achieve survival benefits on a safe basis (70, 71). The EC-CRT-002 trial compared outcomes of Tislelizumab plus definitive chemoradiotherapy followed by Tislelizumab maintenance or not (72).
Dual immunotherapy (Durvalumab and Tremelimumab) with definitive chemoradiotherapy demonstrated a 2-year PFS of 57.5% and OS of 75%, with the lowest in-field failure rate (17.5%) among all articles published to date (45). However, another trial involving Nivolumab ± Ipilimumab plus chemoradiotherapy had to be terminated due to poor accrual (73). Despite this setback, the favorable prognosis of dual immunotherapy significantly surpasses historical data and does not give rise to a significant increase in toxicity, thus justifying further in-depth investigation.
The TENERGY study evaluated the efficacy of 1-year consolidative Atezolizumab following definitive chemoradiotherapy for unresectable locally advanced ESCC. The interim analysis revealed a clinical complete response rate of 42.1%, with a median PFS of 3.2 months and a median OS of 31.0 months. Furthermore, the 12-month PFS and OS rates were 29.6% and 65.8%, respectively (47, 74). Another study evaluating Camrelizumab as consolidation immunotherapy also demonstrated promising efficacy and manageable toxicity. The interim analysis included 12 patients with a median follow-up of 15 months, 11 of whom had stable disease and one patient had progressive disease, with no grade 3 or 4 AEs (48, 75). In the phase III SKYSCRAPER-07 study (76), participants with locally advanced ESCC who had completed radical chemoradiotherapy without progression were randomized to three groups: a single-immunotherapy group (Atezolizumab), a dual-immunotherapy group (Atezolizumab and the TIGIT inhibitor Tiragolumab), and a placebo-controlled group. The primary endpoints were PFS and OS, and data is not yet available. There are two other studies evaluating the efficacy and safety of consolidation Sintilimab after radical chemoradiotherapy that are worthy of attention. Patients will be evaluated for treatment response 6 weeks after the end of radiotherapy. For patients with residual disease, in addition to 1 year of Sintilimab consolidation (77, 78).
For metastatic and recurrent esophageal cancer, immunotherapy combined with chemotherapy is the current first-line standard of care. Additionally, several clinical trials have investigated the potential benefits of adding irradiation to the primary lesion (50, 79–85). These trials typically enrolled patients with oligometastases (no more than 3-5 metastases in less than 2 organs/lymphatic drainage regions) in good physical status to receive radiation regimens using conventional fractionation with 40-60GY (50, 79–85). Although preclinical studies suggest that hypo fractionated radiotherapy with relatively high daily doses may induce stronger immunostimulating signals, it may not be practical for serial organ like the esophagus. Hence, the optimal dosage of palliative radiotherapy for primary esophageal lesions requires further investigation.
In the TR-EAT study, patients with stage IVB ESCC underwent induction Toripalimab plus chemotherapy, followed by sequential chemoradiotherapy (30–50GY/15–25F, target only the primary esophageal foci and metastatic lymph nodes), and 1-year Toripalimab maintenance (86). The preliminary results revealed an ORR of 81.8%, a disease control rate (DCR) of 100%, with no grade 3 or higher AEs (49). The PALEO study developed a protocol integrating Durvarlumab with chemotherapy and two courses of radiotherapy, giving hypo fractionated radiotherapy (30GY/10F) for primary esophageal lesion in week 1-2, and stereotactic body radiation therapy (SBRT, 24GY/3F) for a single metastasis in week 7 (87). Stage IVB ESCC patients who had failed first-line immunochemotherapy received primary tumor radiotherapy (40GY/20F)/metastatic lesions radiotherapy (30GY/10F) combined with Camrelizumab and irinotecan achieved an ORR of 40.8%, a DCR of 75.5%, with median PFS and OS of 6.9 and 12.8 months, respectively (50). For locoregional recurrent ESCC without distant metastasis, several studies have evaluated the efficacy and safety of Camrelizumab combined with concurrent chemoradiotherapy (80, 88). When Sintilimab was used as consolidation therapy after second-line chemoradiotherapy in locoregional recurrent ESCC, the median PFS and OS were 8.3 and 15.7 months, respectively (51).
Clinical studies evaluating the combination of systemic therapy with metastatic lesion SBRT are scarce, with the ESO-shanghai10 study being the only one with published results, reporting a median PFS of 13.3 months, median OS of 24.6 months, and a 2-year local control rate of 92.1% (89). However, the study design lacked the participation of immunotherapy. The multicenter ESO-shanghai13 trial, which included a larger number of participants, allowed for the incorporation of immunotherapy and served as a basis for studying the synergistic effect of immunotherapy and radiotherapy (90). Studies on the combination of ICI and SBRT typically involve pan-tumor studies. One such study, the multisite SBRT (30-50GY/3-5F, 2-4 metastases) combined with Pembrolizumab in the treatment of metastasis solid tumors showed an ORR of 13.2%, median PFS of 3.1 months, and median OS of 9.6 months (91). The protocol of the phase II PraG study includes hypo fractionated radiotherapy (5-8GY*3F) for metastatic lesions, followed by PD-1 inhibitors administered within one week after the completion of radiotherapy and a subsequent two-week regimen of Granulocyte macrophage-colony stimulating factor (GM-CSF) (92). The majority of the 54 participants were in poor general condition with high tumor burden after multiple lines of treatment, and had an ORR of 16.7%, DCR of 46.3%, and median PFS and median OS of 4 months and 10.5 months, respectively. This is a noteworthy benefit in the refractory population after multiple therapy, and further clinical research of radiotherapy combined with PD-1 inhibitors combined with GM-CSF and IL-2 is being launched (92, 93).
Unlike high-dose radiotherapy, which directly kills tumor cells and may cause immunogenic cell death, low-dose radiotherapy is intended to reprogram tumor immune microenvironment and reverse tumor immune desertification and immunotherapy resistance (94–96). A post-hoc analysis revealed that 58% of the lesions treated with low-dose radiotherapy (total dose of 1-20GY) combined ICI achieved a favorable response (97). In the phase I RACIN study, all visible tumor lesions were administered low-dose radiotherapy (0.5 or 1GY per fraction, every two weeks, total dose 6GY or 13GY), in combination with Nivolumab, low-dose cyclophosphamide or Ipilimumab, aspirin or celecoxib (for activation of antigen-presenting cells). In immunotherapy-naïve patients, 37.5% of irradiated lesions shrank, with overall DCR of 87.5% (94). Low-dose radiotherapy, as a less toxic treatment option, may become a new immunomodulatory modality, especially in patients with large-volume tumors or high metastatic burden.
Given the heterogeneity of locally advanced ESCC, a single treatment model for “one-size-fits-all” management cannot be relied upon, and individualized treatment is critical. The screening of dominant populations and predictive biomarkers has emerged as a hot topic in immunotherapy. While PD-L1, as well as tumor mutational load (TMB) and microsatellite instability (MSI are presently the most recognized molecular markers, they are not yet considered ideal predictive markers. The expression levels of the markers fluctuate dynamically, and predictive efficacy can be affected by factors such as which sample, assay, and cut-off value are utilized. In the Korean study of adjuvant Durvalumab, it was found that 73% of patients exhibited alterations in PD-L1 tumor proportion score before and after neoadjuvant therapy, and the post-neoadjuvant PD-L1 expression proved to be more effective in predicting the efficacy of adjuvant treatment compared to the pre-neoadjuvant (41). In CheckMate 577, there was no significant difference in the risk of disease recurrence or death between groups with PD-L1 expression ≥1% and <1%. However, a post-hoc analysis stratified by a PD-L1 expression (combined positive score >=5) showed that high PD-L1 expression was associated with longer DFS in the Nivolumab group (42). Furthermore, high PD-L1 expression in peripheral circulating tumor-associated cells, tumor microenvironment, and extracellular vesicles has been demonstrated to be more effective in identifying dominant populations than PD-L1 on the surface of tumor cells (98–101). Dynamic monitoring of changes in PD-L1 expression may also be more valuable than static baseline expression. The use of circulating tumor DNA (ctDNA) to assess MRD shows promise in screening suitable populations for postoperative adjuvant therapy and consolidation therapy after definitive chemoradiotherapy (102–105). Various molecular biomarkers have been identified to predict the response to neoadjuvant chemoradiotherapy for esophageal cancer, as well as various lymphocyte subsets and lymphocyte ratios in the peripheral blood/tumor area, dendritic cells, cytokines, and gut microbiota, have also been reported to have predictive value (35, 44, 106–110). However, these biomarkers are not yet widely utilized in clinical practice, and further research is required to determine whether they can predict the effectiveness of immunotherapy in combination with radiotherapy.
Functional imaging modalities, including pre-, mid-, and post-treatment PET-CT and MRI, offer great potential for providing clues to predict treatment response or prognosis. However, optimization of specific technology and parameters is essential to improve the accuracy of predictions (111–115). Several clinical trials have been conducted to guide treatment decisions based on imaging response (54, 78, 116–118), and there is even novel PET imaging technique that targets tumor PD-L1, which can achieve in vivo, noninvasive, and real-time monitoring (119–122).
Advanced ESCC exhibits significant heterogeneity with varying degrees of organ metastases, regional or non-regional lymph node metastases, and therefore the value of radiotherapy application varies. Several large retrospective studies have confirmed that the addition of aggressive primary tumor radiotherapy to the treatment of advanced esophageal cancer has positive implications for patients, but further research is needed on patient selection (22, 123). In the real-world study, the addition of radiotherapy to immunochemotherapy in locally recurrent ESCC significantly prolonged OS (median OS 19.5 vs 7.7 months), while no difference in PFS or OS was observed with or without radiotherapy in the entire cohort (124). This underscores the importance of patient selection. Oligometastatic, as well as limited regional lymph node recurrence in advanced esophageal cancer, are known to have a better prognosis, and aggressive local therapy combined with systemic therapy may offer patients the opportunity for radical treatment and may be a consideration in patient selection. The ESO-shanghai10 trial enrolled highly selected oligometastatic patients with controlled primary sites (≤3 metastases, limited to ≤2 organs), and most patients had only one oligorecurrent and oligometastatic lesion, which may be associated with the impressive outcomes (89). The EC-CRT-003 study included non-regional lymph node metastasis only as a stratification factor that may suggest prognosis and guide treatment (85). Decision tree models have also been used to divide oligometastatic ESCC into different risk groups and screen high-risk patients for early intensive treatment, such as immunochemoradiotherapy (125).
Radiotherapy-induced immune response varies widely with the irradiation dose, fractionation regimen, and irradiation site. Therefore, it is essential to evaluate the safety, efficacy, and optimal timing of combined therapy in the context of utilizing radiotherapy in conjunction with ICI for the treatment of ESCC.
In general, there are three modes of integrating radiotherapy and ICI: concurrent radiotherapy with ICI, consolidation of ICI following radiotherapy, and radiotherapy subsequent to induction ICI. While there is a theoretical potential for reducing toxicity by separating radiotherapy and ICI non-simultaneously, there is no definite conclusion regarding the optimal interval time between them. Data on combination therapy for ESCC are limited, and we can draw upon our experience treating lung cancer to inform our decisions. Notably, the PACIFIC study revealed marginally improved outcomes for patients who initiated Durvalumab within two weeks after radiotherapy (126). In the real-world PACIFIC- R study, a more favorable prognosis was observed for patients who commenced Durvalumab treatment within 42 days compared to those who delayed Durvalumab initiation (127). Nevertheless, a meta-analysis also demonstrated that the interval between radiotherapy and Durvalumab frequently exceeded 42 days in practical use and did not affect 12-month PFS/OS (128). Therefore, in ongoing clinical trials investigating consolidation therapy for ESCC, the interval between the completion of radiotherapy and the initiation of ICI generally extends to 6 weeks (74, 78). The interval between esophagectomy and adjuvant immunotherapy may be longer. Perioperative immunophenotype analysis revealed that cytotoxic did not begin to grow until 6 weeks after surgery (129). The subgroup analysis of CheckMate 577 study demonstrated a superior survival benefit for commencing Nivolumab beyond 10 weeks after surgery, as opposed to initiating treatment within 10 weeks, which could be attributed to the extended period of time needed for the immune system to recuperate after neoadjuvant chemoradiotherapy and radical surgery (42). Real-world study of metastatic/recurrent ESCC found that patients treated with radiation within 90 days before and after immunotherapy exhibited extended median PFS and OS than those treated with radiation beyond 90 days (124). Clinical trials for metastatic ESCC necessitate an interval of no more than 8 weeks between conventional radiotherapy and ICI (79, 81), and one week between SBRT and ICI (91–93). A multicenter retrospective study in Italy discovered that an interval of less than 7 days between SBRT and ICIs resulted in a longer OS with slightly higher but manageable toxicity (130, 131). When utilizing stereotactic radiosurgery (SRS) to address brain metastases, concurrent ICI therapy within four weeks was found to provide the most optimal benefit (132). The most effective schedule for incorporating ICI with radiotherapy remains unknown and may vary across different multimodal treatments.
The sequencing of radio-immunotherapy combinations is also a critical determinant to their efficacy. In mouse models, investigations have demonstrated that concurrent application of PD-1 inhibitors with or soon after local radiotherapy has the potential to augment the expansion of multifunctional intratumoral CD8+ T cells and reduce peripheral CD8+ T cell death, leading to a more favorable systemic antitumor response and abscopal effect (133, 134). Patients who received ICI after or concurrently with SBRT/SRS also showed better outcomes than those who received it previously (135–137), possibly related to radiotherapy-induced neoantigen release and increased PD-L1 expression. However, certain clinical studies have found no statistical difference between simultaneous and sequential administration of SBRT and Ipilimumab (138). Ongoing clinical trials are specifically comparing the effects of different sequences (139, 140).
It remains unclear whether the exact sequence of combinations or the interval between them has a greater impact on efficacy. While most studies tend to administer ICI simultaneously with or sequentially after radiotherapy, there have been reports that anti-PD-1 administered after esophageal radiotherapy is more susceptible to perforation, necessitating careful monitoring (141). Particularly for patients with ulcerated, giant, thin-walled or large-vessel invaded esophageal cancer, induction immunotherapy can be considered first to alleviate symptoms and improve nutritional status, thus reducing the irradiation volume and associated side effects, and better protecting surrounding normal tissues.
The optimal irradiation dose for esophageal cancer has always been controversial, with higher doses being administered in regions where ESCC is more prevalent than EAC. However, as systemic therapy has intensified in recent years, several large-scale phase III studies have compared the efficacy and safety of a 50GY radiation dose with higher doses in ESCC (13, 14, 142, 143). The current preference is for 50GY as the recommended dose of radical radiotherapy for ESCC (19). We have pondered whether the radiation dose range of 50-60GY may be too high in combination with intensified systemic therapy, and whether a lower dose would be more appropriate. To explore this question, the KEYNOTE-975 study was designed to compare the effectiveness of two radiation dose groups, 50GY and 60GY, in combination with Pembrolizumab (65). In the field of neoadjuvant therapy, the guidelines recommend a radiotherapy dose of 41.4-50.4GY (19). Higher doses do not improve the efficacy and survival (144, 145), and most of the current clinical trials employ the CROSS regimen of 41.4GY/23F. The neoadjuvant radiotherapy in the SCALE study used a short course of 30GY/12F, achieving a promising pCR rate of 55% (36). In NICE-RT study, the neoadjuvant radiotherapy regimen consisted of conventional fractionated irradiation (41.4GY/23F) for the primary lesion and adjacent lymph nodes, plus low-dose irradiation (0.5GY*4) for abscopal lymph nodes (146). Regarding metastatic ESCC, radiotherapy for the primary site mostly involves definitive or neoadjuvant radiotherapy regimens. Regimens for targeting metastatic sites are more varied, including attempts at low-dose radiotherapy (94), combination radiotherapy at different doses (147), as well as SBRT/SRS. Integration of high- and low-dose radiation (20-70GY total 3-12.5GY/F; 1-10GY total 0.5-2GY/F) has demonstrated the potential to reverse immune resistance in metastatic patients who have progressed after immunotherapy treatment (147). The ongoing PALEO study used Durvarlumab plus hypo fractionated radiotherapy (30GY/10F) in week 1-2 for primary esophageal lesions and SBRT (24GY/3F) in week 7 for a single metastasis lesion (87).
There has long been a contentious debate surrounding the selection of involved-field irradiation versus elective nodal irradiation for delineating radiotherapy targets in esophageal cancer. Given the dependence of immunotherapy on lymphocytes, especially T-lymphocytes, and the negative impact of lymphopenia on prognosis (148–150), the prevailing tendency is to opt for involved field irradiation to preserve lymphocyte function and minimize lymph node irradiation, while minimizing side effects. Fewer studies have explored prophylactic lymph node irradiation (69).
Chang YJ has advocated for a multi-site irradiation strategy over single-site irradiation to overcome the tumor-associated antigen heterogeneity, thus improving the efficacy of radiotherapy combined with ICI (151). Several ongoing clinical studies of combined radiotherapy with ICI are utilizing multi-technique and multi-target irradiation schemes (81, 87, 93, 94, 146). The radiotherapy-induced immune response varies depending on irradiation site, highlighting the significance of selecting appropriate metastatic sites for irradiation in radio-immunotherapy. It has been observed that SBRT to parenchymal (brain, liver, lung) rather than nonparenchymal (bone) metastases is more likely to trigger systemic immune activation, and is associated with improved prognosis, possibly due to different inherent microenvironment of different metastatic organs (152–154). The optimal irradiation site remains uncertain. A phase I trial investigating the combination of SBRT and Ipilimumab demonstrated that liver metastasis irradiation was associated with a more favorable T-cell activation compared to lung metastasis irradiation (155). Moreover, other studies have shown that irradiation to brain metastases may result in the best synergism effect (153).
With the evolution of anti-tumor drugs, there has been a growing interest in investigating the potential of drug therapy to replace radiotherapy or surgery. However, previous clinical trials have shown that the pCR rate of neoadjuvant immunochemotherapy typically ranges between 25-45% (156–164), which was lower compared to conventional chemoradiotherapy (165). The pCR rate of neoadjuvant chemoradiotherapy combined with ICI is mostly above 45%, with the highest rate of 55.6% observed in the PALACE-1 study (35). A meta-analysis indicated that neoadjuvant immunochemoradiotherapy led to a higher pCR rate compared to immunochemotherapy for ESCC, and increasing the cycles of ICI did not appear to improve the pCR rate (166). Given that the absence of preoperative radiotherapy may result in a lower pCR and subsequently impact the prognosis, the role of radiotherapy in neoadjuvant therapy is still considered essential.
Several ongoing clinical trials are comparing neoadjuvant immunochemotherapy with neoadjuvant chemoradiotherapy, such as KEYSTONE-002 study (NCT04807673) (167), NICE2 study (NCT05043688) (168) and REVO study (NCT05007145) (169). The NICE 2 study was divided into three groups to compare the efficacy and safety of three neoadjuvant treatment regimens: immunotherapy combined with chemotherapy, immunotherapy combined with chemoradiotherapy and conventional chemoradiotherapy (168). Another study (NCT05624099) compared Camrelizumab plus chemoradiotherapy versus Camrelizumab plus chemotherapy (170). The VESTIGE trial is evaluating the efficacy of adjuvant chemotherapy with adjuvant Nivolumab plus Ipilimumab in esophageal cancer with high risk of recurrence after neoadjuvant chemotherapy and surgery. Compared with CheckMate 577 trial, this trial excluded patients who had received radiotherapy, and may provide insights into the role of radiotherapy and its synergistic effect with immunotherapy (171). Additionally, the clinical trials NCT05637268 and NCT04741490 are recruiting patients with R0-resected ESCC and comparing the outcomes of adjuvant therapy using either immunotherapy combined with chemotherapy or immunotherapy combined with radiotherapy (59, 172). The results of these studies will provide further evidence to determine the optimal combination strategy for neoadjuvant or adjuvant treatment of ESCC and clarify the role of radiotherapy in this setting.
For resectable or potentially resectable locally advanced ESCC, the current standard of care is neoadjuvant chemoradiotherapy followed by surgery. However, due to concerns about surgical complications and reduced postoperative quality of life, not all patients ultimately undergo surgery, especially those who achieve clinical complete remission (cCR) after neoadjuvant chemoradiotherapy. Based on data from several large clinical studies, approximately half of ESCC patients can achieve pCR after neoadjuvant chemoradiotherapy (8, 10), and this high pCR rate provides a theoretical basis for exploring regular surveillance without surgery. Can esophageal cancer learn from the “watch and wait” strategy of rectal cancer in complete remission after neoadjuvant chemoradiotherapy, and use esophagectomy as a salvage method for local recurrence? Multiple retrospective studies have demonstrated that among good responders after neoadjuvant therapy, active surveillance of patients who refused esophagectomy or were ultimately deemed unsuitable for surgery had comparable survival rates to those who underwent surgery (173–178). In the ongoing prospective SANO trial and ESOSTRATE trial, patients with a clinical response assessment of cCR after standard neoadjuvant chemoradiotherapy will be randomly assigned to either the active surveillance group or the immediate surgery group, comparing survival and quality of life for both groups (179–181). Neoadjuvant chemoradiotherapy combined with immunotherapy has achieved a pCR rate exceeding 50% potentially enabling more responders to bypass surgery, thereby retaining their esophageal function and improving survival with a higher quality of life. The ongoing Phase II WATCHER trial enrolled patients who achieved cCR after neoadjuvant chemoradiotherapy plus Camrelizumab, comparing survival differences between the surgery group and watch-and-wait group, and assessing the effect of immunotherapy maintenance (182).
Until the results of clinical trials are published, the standard treatment for locally advanced ESCC with a clinically complete response remains primarily surgical. The decision to withdraw surgery must be made with caution, with adequate communication with the patient, enhanced surveillance, and prompt remediation if necessary. Additionally, it is essential to acknowledge that accurate and safe response assessment after neoadjuvant therapy is crucial for clinical decision-making, and a single test is insufficient. A combination of CT/MRI findings, endoscopic ultrasonography, bite-on-bite biopsies, fine-needle aspiration of suspicious lymph nodes, and serial PET-CT for dynamic monitoring of distant metastases is necessary (183–185). Even with the combined application of CT, PET-CT and endoscopic biopsy, half of the patients eligible for cCR for surgery still have pathological residual tumors, especially in the lymph nodes, which are more likely to be underestimated (186). Under current conditions, surgery can provide accurate pathologic staging information and facilitate clinicians to assess risk stratification and target adjuvant therapy.
The incidence of high-grade AEs in combination therapy ranges from 20% to 50%, with lymphocytopenia being the most common, followed by esophagitis, anastomotic leak, and esophageal fistula (131, 166, 187–191). According to data from the Food and Drug Administration of the United States, grade 3-4 pneumonitis occurred in 1.1%, 1.9%, and 1.2% of patients who did not receive radiotherapy, received ICI within 90 days of radiotherapy, and received ICI more than 90 days after radiotherapy, respectively. Although radiotherapy combined with ICI may increase the incidence of severe radiation pneumonitis, the absolute percentage increase is so small that the administration of ICI within 90 days after radiotherapy appears to be safe (192). No statistic difference was observed in the risk of major complications, such as pulmonary complications, anastomotic leakage, and other complications, as well as death or readmission, among patients who received neoadjuvant chemoradiotherapy combined with or without immunotherapy (193). However, it was reported that 61% of patients with immune-related AEs history developed grade 2 or higher radiation pneumonitis after radiotherapy, and 83% of patients with prior ICI pneumonitis developed grade 2 or higher radiation pneumonitis (194). A retrospective study also found that radiotherapy combined with anti-PD-1 increased the incidence of esophageal perforation (18% vs. 3.1% p=0.002) (141). Although most clinical trials have deemed the safety of combination therapy to be acceptable, the populations enrolled in these trials typically have lower tumor burdens and better performance status, which may not reflect the daily clinical practice where AEs may be more common. Despite most clinical trials deeming the safety of combination therapy acceptable, the enrolled populations typically have lower tumor burdens and better performance status, which may not accurately reflect the occurrence of AEs in daily clinical practice.
The combination of immunotherapy and radiotherapy has emerged as a promising treatment approach in esophageal cancer that warrants further investigation and optimization. For locally advanced resectable ESCC, adding immunotherapy to standard care of the neoadjuvant chemoradiotherapy followed by surgery has not significantly increased the pCR rate but has increased toxicity to some extent. Evidence for neoadjuvant immunotherapy is limited to small-scale single-arm phase I/II trials and is not yet suitable for widespread application. Further research is needed to explore the optimal combination strategy for neoadjuvant treatment. In terms of postoperative adjuvant immunotherapy, the ChckMate577 trial has provided conclusive evidence, yet further exploration is necessary in terms of target populations, dosing regimens, and predictive biomarkers. For locally advanced unresectable ESCC, the addition of immunotherapy to definitive chemoradiotherapy during the concurrent or consolidation phase holds promise for improved long-term survival. For metastatic ESCC, aggressive multidisciplinary treatment combing radiotherapy to immunochemotherapy is important for symptom improvement and survival prolongation, especially for ESCC with oligometastasis.
Preliminary results of radio-immunotherapy for ESCC are promising, with a succession of large-scale studies currently underway. Issues such as irradiation dosage, fractionation regimen, irradiation site and technique of radiotherapy, the timing, sequence and duration of combination therapy, and the selection of immunotherapeutic agents will all affect treatment outcomes. We need to keep on exploring the anti-tumor mechanism and predictive markers of radio-immunotherapy. With the development of multi-omics and pharmaceutical technology, precision screening, accurate assessment and multidisciplinary treatment will certainly improve significantly, and the day of preserving organ function with high quality of life and long-term survival for esophageal cancer patients will not be far away.
MJ and HL contributed to the conception and design of the review. MJ, YH, GL and CC contributed to the data acquisition, data analysis and interpretation, original draft preparation. MJ, CC and HL contributed to review and revise the manuscript. All authors contributed to the article and approved the submitted version.
Medical Health Science and Technology Project of Zhejiang Provincial Health Commission, NO. 2023577572.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. Shah MA, Kennedy EB, Catenacci DV, Deighton DC, Goodman KA, Malhotra NK, et al. Treatment of locally advanced esophageal carcinoma: ASCO guideline. J Clin Oncol (2020) 38(23):2677–94. doi: 10.1200/JCO.20.00866
3. Kato K, Cho BC, Takahashi M, Okada M, Lin CY, Chin K, et al. Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol (2019) 20(11):1506–17. doi: 10.1016/S1470-2045(19)30626-6
4. Kojima T, Shah MA, Muro K, Francois E, Adenis A, Hsu CH, et al. Randomized phase III KEYNOTE-181 study of pembrolizumab versus chemotherapy in advanced esophageal cancer. J Clin Oncol (2020) 38(35):4138–48. doi: 10.1200/JCO.20.01888
5. Huang J, Xu J, Chen Y, Zhuang W, Zhang Y, Chen Z, et al. Camrelizumab versus investigator’s choice of chemotherapy as second-line therapy for advanced or metastatic oesophageal squamous cell carcinoma (ESCORT): a multicentre, randomised, open-label, phase 3 study. Lancet Oncol (2020) 21(6):832–42. doi: 10.1016/S1470-2045(20)30110-8
6. Shen L, Kato K, Kim SB, Ajani JA, Zhao K, He Z, et al. Tislelizumab versus chemotherapy as second-line treatment for advanced or metastatic esophageal squamous cell carcinoma (RATIONALE-302): a randomized phase III study. J Clin Oncol (2022) 40(26):3065–76. doi: 10.1200/JCO.21.01926
7. Shaverdian N, Lisberg AE, Bornazyan K, Veruttipong D, Goldman JW, Formenti SC, et al. Previous radiotherapy and the clinical activity and toxicity of pembrolizumab in the treatment of non-small-cell lung cancer: a secondary analysis of the KEYNOTE-001 phase 1 trial. Lancet Oncol (2017) 18(7):895–903. doi: 10.1016/S1470-2045(17)30380-7
8. Yang H, Liu H, Chen Y, Zhu C, Fang W, Yu Z, et al. Neoadjuvant chemoradiotherapy followed by surgery versus surgery alone for locally advanced squamous cell carcinoma of the esophagus (NEOCRTEC5010): a phase III multicenter, randomized, open-label clinical trial. J Clin Oncol (2018) 36(27):2796–803. doi: 10.1200/JCO.2018.79.1483
9. Yang H, Liu H, Chen Y, Zhu C, Fang W, Yu Z, et al. Long-term efficacy of neoadjuvant chemoradiotherapy plus surgery for the treatment of locally advanced esophageal squamous cell carcinoma: the NEOCRTEC5010 randomized clinical trial. JAMA Surg (2021) 156(8):721–9. doi: 10.1001/jamasurg.2021.2373
10. van Hagen P, Hulshof MC, van Lanschot JJ, Steyerberg EW, van Berge Henegouwen MI, Wijnhoven BP, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med (2012) 366(22):2074–84. doi: 10.1056/NEJMoa1112088
11. Eyck BM, van Lanschot JJB, Hulshof M, van der Wilk BJ, Shapiro J, van Hagen P, et al. Ten-year outcome of neoadjuvant chemoradiotherapy plus surgery for esophageal cancer: the randomized controlled CROSS trial. J Clin Oncol (2021) 39(18):1995–2004. doi: 10.1200/JCO.20.03614
12. Chen D, Kong M, Sun J, Yang H, Chen Y, Fang W, et al. Prognostic value of recurrence pattern in locally advanced esophageal squamous cell carcinoma: results from the phase III trial NEOCRTEC5010. J Thorac Cardiovasc Surg (2022) S0022–5223(22)00896-0. doi: 10.1016/j.jtcvs.2022.08.009
13. Hulshof M, Geijsen ED, Rozema T, Oppedijk V, Buijsen J, Neelis KJ, et al. Randomized study on dose escalation in definitive chemoradiation for patients with locally advanced esophageal cancer (ARTDECO study). J Clin Oncol (2021) 39(25):2816–24. doi: 10.1200/JCO.20.03697
14. Xu Y, Dong B, Zhu W, Li J, Huang R, Sun Z, et al. A phase III multicenter randomized clinical trial of 60 gy versus 50 gy radiation dose in concurrent chemoradiotherapy for inoperable esophageal squamous cell carcinoma. Clin Cancer Res (2022) 28(9):1792–9. doi: 10.1158/1078-0432.CCR-21-3843
15. Zhu H, Rivin Del Campo E, Ye J, Simone CB 2nd, Zhu Z, Zhao W, et al. Involved-field irradiation in definitive chemoradiotherapy for locoregional esophageal squamous cell carcinoma: results from the ESO-shanghai 1 trial. Int J Radiat Oncol Biol Phys (2021) 110(5):1396–406. doi: 10.1016/j.ijrobp.2021.02.053
16. Chen Y, Ye J, Zhu Z, Zhao W, Zhou J, Wu C, et al. Comparing paclitaxel plus fluorouracil versus cisplatin plus fluorouracil in chemoradiotherapy for locally advanced esophageal squamous cell cancer: a randomized, multicenter, phase III clinical trial. J Clin Oncol (2019) 37(20):1695–703. doi: 10.1200/JCO.18.02122
17. van Rossum PSN, Mohammad NH, Vleggaar FP, van Hillegersberg R. Treatment for unresectable or metastatic oesophageal cancer: current evidence and trends. Nat Rev Gastroenterol Hepatol (2018) 15(4):235–49. doi: 10.1038/nrgastro.2017.162
18. Patel MA, Kratz JD, Lubner SJ, Loconte NK, Uboha NV. Esophagogastric cancers: integrating immunotherapy therapy into current practice. J Clin Oncol (2022) 40(24):2751–62. doi: 10.1200/JCO.21.02500
19. National Comprehensive Cancer Network. Esophageal and esophagogastric junction cancers (Version 5.2022-December 5 (2022). Available at: https://www.nccn.org/professionals/physician_gls/pdf/esophageal.pdf.
20. Kroese TE, van Laarhoven HWM, Nilsson M, Lordick F, Guckenberger M, Ruurda JP, et al. Definition of oligometastatic esophagogastric cancer and impact of local oligometastasis-directed treatment: a systematic review and meta-analysis. Eur J Canc (2022) 166:254–69. doi: 10.1016/j.ejca.2022.02.018
21. Kroese TE, Buijs GS, Burger MDL, Ruurda JP, Mook S, Brosens LAA, et al. Metastasectomy or stereotactic body radiation therapy with or without systemic therapy for oligometastatic esophagogastric cancer. Ann Surg Oncol (2022) 29(8):4848–57. doi: 10.1245/s10434-022-11541-0
22. Seyedin SN, Parekh KR, Ginader T, Caster JM. The role of definitive radiation and surgery in metastatic esophageal cancer: an NCDB investigation. Ann Thorac Surg (2021) 112(2):459–66. doi: 10.1016/j.athoracsur.2020.08.034
23. Li J, Wen Y, Xiang Z, Du H, Geng L, Yang X, et al. Radical radiotherapy for metachronous oligometastasis after initial treatment of esophageal cancer. Radiother Oncol (2021) 154:201–6. doi: 10.1016/j.radonc.2020.09.042
24. Herrera FG, Bourhis J, Coukos G. Radiotherapy combination opportunities leveraging immunity for the next oncology practice. CA: Cancer J For Clin (2017) 67(1):65–85. doi: 10.3322/caac.21358
25. Jagodinsky JC, Harari PM, Morris ZS. The promise of combining radiation therapy with immunotherapy. Int J Radiat Oncol Biol Phys (2020) 108(1):6–16. doi: 10.1016/j.ijrobp.2020.04.023
26. Park SS, Dong H, Liu X, Harrington SM, Krco CJ, Grams MP, et al. PD-1 restrains radiotherapy-induced abscopal effect. Cancer Immunol Res (2015) 3(6):610–9. doi: 10.1158/2326-6066.CIR-14-0138
27. Deng L, Liang H, Burnette B, Beckett M, Darga T, Weichselbaum RR, et al. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest (2014) 124(2):687–95. doi: 10.1172/JCI67313
28. Zhang Z, Liu X, Chen D, Yu J. Radiotherapy combined with immunotherapy: the dawn of cancer treatment. Signal Transduct Target Ther (2022) 7(1):258. doi: 10.1038/s41392-022-01102-y
29. Wang Y, Liu Z-G, Yuan H, Deng W, Li J, Huang Y, et al. The reciprocity between radiotherapy and cancer immunotherapy. Clin Cancer Res: an Off J Am Assoc For Cancer Res (2019) 25(6):1709–17. doi: 10.1158/1078-0432.CCR-18-2581
30. Lim SH, Hong M, Ahn S, Choi YL, Kim KM, Oh D, et al. Changes in tumour expression of programmed death-ligand 1 after neoadjuvant concurrent chemoradiotherapy in patients with squamous oesophageal cancer. Eur J Canc (2016) 52:1–9. doi: 10.1016/j.ejca.2015.09.019
31. Sato H, Niimi A, Yasuhara T, Permata TBM, Hagiwara Y, Isono M, et al. DNA Double-strand break repair pathway regulates PD-L1 expression in cancer cells. Nat Commun (2017) 8(1):1751. doi: 10.1038/s41467-017-01883-9
32. Tian L, Goldstein A, Wang H, Ching Lo H, Sun Kim I, Welte T, et al. Mutual regulation of tumour vessel normalization and immunostimulatory reprogramming. Nature (2017) 544(7649):250–4. doi: 10.1038/nature21724
33. Zheng X, Fang Z, Liu X, Deng S, Zhou P, Wang X, et al. Increased vessel perfusion predicts the efficacy of immune checkpoint blockade. J Clin Invest (2018) 128(5):2104–15. doi: 10.1172/JCI96582
34. Lee S, Ahn BC, Park SY, Kim DJ, Lee CG, Cho J, et al. A phase II trial of preoperative chemoradiotherapy and pembrolizumab for locally advanced esophageal squamous cell carcinoma (ESCC). Ann Oncol (2019) 30:v754. doi: 10.1093/annonc/mdz266.018
35. Li C, Zhao S, Zheng Y, Han Y, Chen X, Cheng Z, et al. Preoperative pembrolizumab combined with chemoradiotherapy for oesophageal squamous cell carcinoma (PALACE-1). Eur J Canc (2021) 144:232–41. doi: 10.1016/j.ejca.2020.11.039
36. Jiang N, Jiang M, Zhu X, Ren B, Zhang J, Guo Z, et al. SCALE-1: safety and efficacy of short course neoadjuvant chemo-radiotherapy plus toripalimab for locally advanced resectable squamous cell carcinoma of esophagus. J Clin Oncol (2022) 40(16_suppl):4063–. doi: 10.1200/JCO.2022.40.16_suppl.4063
37. Xu X, Sun Z, Zhang Y, Shen L, Liu Q, Zhang C, et al. Neoadjuvant chemoradiotherapy combined with perioperative toripalimab in locally advanced esophageal cancer. J Clin Oncol (2022) 40(16_suppl):e16065-e. doi: 10.1200/JCO.2022.40.16_suppl.e16065
38. Yang J, Zhang Z, Wu B, Qin Y, Wei J, Qu Y, et al. 163P neoadjuvant chemoradiotherapy plus tislelizumab followed by surgery for esophageal carcinoma: an interim analysis of the prospective, single-arm, phase II trial. Immuno-Oncol Technol (2022) 16:24. doi: 10.1016/j.iotech.2022.100275
39. Ko AH, Noel M, Chao J, Sohal D, Crow M, Oberstein PE, et al. 1229P a multicenter phase II study of sotigalimab (CD40 agonist) in combination with neoadjuvant chemoradiation for resectable esophageal and gastroesophageal junction (GEJ) cancers. Ann Oncol (2022) 33(SUPPLEMENT 7):S1111. doi: 10.1016/j.annonc.2022.07.1347
40. Uboha NV, Eickhoff JC, Maloney JD, McCarthy D, DeCamp M, Deming DA, et al. Phase I/II trial of perioperative avelumab in combination with chemoradiation (CRT) in the treatment of stage II/III resectable esophageal and gastroesophageal junction (E/GEJ) cancer. J Clin Oncol (2022) 40(16_suppl):4034–. doi: 10.1200/JCO.2022.40.16_suppl.4034
41. Park S, Sun JM, Choi YL, Oh D, Kim HK, Lee T, et al. Adjuvant durvalumab for esophageal squamous cell carcinoma after neoadjuvant chemoradiotherapy: a placebo-controlled, randomized, double-blind, phase II study. ESMO Open (2022) 7(1):100385. doi: 10.1016/j.esmoop.2022.100385
42. Kelly RJ, Ajani JA, Kuzdzal J, Zander T, Van Cutsem E, Piessen G, et al. Adjuvant nivolumab in resected esophageal or gastroesophageal junction cancer. N Engl J Med (2021) 384(13):1191–203. doi: 10.1056/NEJMoa2032125
43. Zhang W, Yan C, Gao X, Li X, Cao F, Zhao G, et al. Safety and feasibility of radiotherapy plus camrelizumab for locally advanced esophageal squamous cell carcinoma. Oncologist (2021) 26(7):e1110–e24. doi: 10.1002/onco.13797
44. Zhang W, Yan C, Zhang T, Chen X, Dong J, Zhao J, et al. Addition of camrelizumab to docetaxel, cisplatin, and radiation therapy in patients with locally advanced esophageal squamous cell carcinoma: a phase 1b study. Oncoimmunology (2021) 10(1):1971418. doi: 10.1080/2162402X.2021.1971418
45. Park S, Oh D, Choi YL, Chi SA, Kim K, Ahn MJ, et al. Durvalumab and tremelimumab with definitive chemoradiotherapy for locally advanced esophageal squamous cell carcinoma. Cancer (2022) 128(11):2148–58. doi: 10.1002/cncr.34176
46. Zhu Y, Wen J, Li Q, Chen B, Zhao L, Liu S, et al. Toripalimab combined with definitive chemoradiotherapy in locally advanced oesophageal squamous cell carcinoma (EC-CRT-001): a single-arm, phase 2 trial. Lancet Oncol (2023) 24(4):371–82. doi: 10.1016/S1470-2045(23)00060-8
47. Bando H, Kumagai S, Kotani D, Saori M, Habu T, Tsushima T, et al. 1211P a multicenter phase II study of atezolizumab monotherapy following definitive chemoradiotherapy for unresectable locally advanced esophageal squamous cell carcinoma (EPOC1802). Ann Oncol (2022) 33:S1102–S3. doi: 10.1016/j.annonc.2022.07.1329
48. Wang J, Cheng Y, Wu Y, Cao F, Liu Q, Gao G. 1262TiP efficacy and safety of consolidative camrelizumab following definitive concurrent chemoradiotherapy in patients with locally advanced esophageal squamous cell cancer. Ann Oncol (2022) 33(SUPPLEMENT 7):S1124. doi: 10.1016/j.annonc.2022.07.1880
49. Wu L, Li B, Wan G, Liang L, Li T, Lang J, et al. Phase II study of toripalimab in combination with induction chemotherapy and subsequent chemo- radiation in patients with Advanced/Metastatic esophageal carcinoma (TR-EAT trial). Int J Radiat Oncol Biol Phys (2022) 114(3):e167–e8. doi: 10.1016/j.ijrobp.2022.07.1047
50. Zhao W, Ke S, Cai X, Zuo Z, Shi W, Qiu H, et al. Radiotherapy plus camrelizumaband irinotecan for oligometastatic esophageal squamous cell carcinoma patients after first-line immunotherapy plus chemotherapy failure: an open-label, single-arm, phase II trial. Radiother Oncol (2023) 184:109679. doi: 10.1016/j.radonc.2023.109679
51. Li B. Sintilimab after concurrent chemoradiotherapy in locoregional recurrent ESCC after definitive treatment: a single-arm, phase II study. Int J Radiat Oncol Biol Phys (2022) 114(3):e162. doi: 10.1016/j.ijrobp.2022.07.1033
52. Park SY, Hong MH, Kim HR, Lee CG, Cho JH, Cho BC, et al. The feasibility and safety of radical esophagectomy in patients receiving neoadjuvant chemoradiotherapy with pembrolizumab for esophageal squamous cell carcinoma. J Thorac Dis (2020) 12(11):6426–34. doi: 10.21037/jtd-20-1088
53. Zheng Y, Li C, Yu B, Zhao S, Li J, Chen X, et al. Preoperative pembrolizumab combined with chemoradiotherapy for esophageal squamous cell carcinoma: trial design. JTCVS Open (2022) 9:293–9. doi: 10.1016/j.xjon.2021.11.003
54. BeiGene. Study of tislelizumab in participants with resectable esophageal squamous cell carcinoma (2021). Available at: https://ClinicalTrials.gov/show/NCT04974047.
55. He W, Wang C, Wu L, Wan G, Li B, Han Y, et al. Tislelizumab plus chemotherapy sequential neoadjuvant therapy for non-cCR patients after neoadjuvant chemoradiotherapy in locally advanced esophageal squamous cell carcinoma (ETNT): an exploratory study. Front Immunol (2022) 13:853922. doi: 10.3389/fimmu.2022.853922
56. Hospital SZ, Hospital PUC, Institute, Hospital SC, University FAHoWM, Institute TMUC, et al. Neoadjuvant immunotherapy plus CRT versus neoadjuvant CRT for locally advanced resectable ESCC (2022). Available at: https://ClinicalTrials.gov/show/NCT04973306.
57. University SY-s, College ACHoSUM. PD-1 inhibitor combined with neoadjuvant chemoradiotherapy plus surgery for locally advanced ESCC (2022). Available at: https://ClinicalTrials.gov/show/NCT05357846.
58. Pape M, Vissers PAJ, Beerepoot LV, van Berge Henegouwen MI, Lagarde SM, Mook S, et al. A population-based study in resected esophageal or gastroesophageal junction cancer aligned with CheckMate 577. Ther Adv Med Oncol (2022) 14:17588359221075495. doi: 10.1177/17588359221075495
59. Hospital FG. Camrelizumab combined with radiotherapy for adjuvant treatment of esophageal squamous cell carcinoma after surgery (2021). Available at: https://ClinicalTrials.gov/show/NCT04741490.
60. Hospital NTU, Sharp M, LLC D. Adjuvant pembrolizumab for patients with locally advanced esophageal squamous cell carcinoma at high risk of recurrence (2018). Available at: https://ClinicalTrials.gov/show/NCT03322267.
61. Hospital SC. Salvage immunotherapy and chemotherapy in esophageal squamous cell carcinoma patients nonresponding to initial neoadjuvant chemoradiotherapy (2021). Available at: https://ClinicalTrials.gov/show/NCT05103501.
62. Jing Z, Du D, Zhang N, Dai H, Wang X, Hua Y, et al. Combination of radiation therapy and anti-PD-1 antibody SHR-1210 in treating patients with esophageal squamous cell cancer. Int J Radiat OncologyBiologyPhys (2018) 102(3):SUPPLEMENT, E31. doi: 10.1016/j.ijrobp.2018.07.520
63. Institute C, Hospital CAoMS. Radiotherapy plus durvalumab in elderly esophageal squamous cell carcinoma (2021). Available at: https://ClinicalTrials.gov/show/NCT04851132.
64. Jiangsu HengRui medicine co. l. study of camrelizumab (SHR-1210) in combination with concurrent chemoradiotherapy in locally advanced esophageal cancer (2020). Available at: https://ClinicalTrials.gov/show/NCT04426955.
65. Shah MA, Bennouna J, Doi T, Shen L, Kato K, Adenis A, et al. KEYNOTE-975 study design: a phase III study of definitive chemoradiotherapy plus pembrolizumab in patients with esophageal carcinoma. Future Oncol (2021) 17(10):1143–53. doi: 10.2217/fon-2020-0969
66. Yu R, Wang W, Li T, Li J, Zhao K, Wang W, et al. RATIONALE 311: tislelizumab plus concurrent chemoradiotherapy for localized esophageal squamous cell carcinoma. Future Oncol (2021) 17(31):4081–9. doi: 10.2217/fon-2021-0632
67. AstraZeneca. Study of durvalumab versus placebo in combination with definitive chemoradiation therapy in patient with ESCC (2020). Available at: https://ClinicalTrials.gov/show/NCT04550260.
68. Wang L, Chen M, Kato K, Wyrwicz L, Smyth EC, Jiang A, et al. A phase 3 randomized, double-blind, placebo-controlled, multicenter, global study of durvalumab with and after chemoradiotherapy in patients with locally advanced, unresectable esophageal squamous cell carcinoma: KUNLUN. J Clin Oncol (2022) 40(4_suppl):TPS373–TPS. doi: 10.1200/JCO.2022.40.4_suppl.TPS373
69. UNICANCER. Association of radiochemotherapy and immunotherapy for the treatment of unresectable oesophageal caNcer (2018). Available at: https://ClinicalTrials.gov/show/NCT03777813.
70. Hospital FG. Induction tislelizumab combined with chemotherapy followed by definitive chemoradiotherapy in the treatment of locally unresectable esophageal squamous cell carcinoma (2022). Available at: https://ClinicalTrials.gov/show/NCT05515315.
71. University SY-s. Toripalimab plus neoadjuvant chemotherapy combined with chemoradiotherapy for locally advanced unresectable esophageal squamous cell carcinoma (2021). Available at: https://ClinicalTrials.gov/show/NCT04844385.
72. University SY-s. First affiliated hospital SY-SU, zhongshan people’s hospital G, China, hospital JPs. combination of tislelizumab and chemoradiotherapy in esophageal cancer (EC-CRT-002) (2022). Available at: https://ClinicalTrials.gov/show/NCT05520619.
73. Research EOf, EORTC ToC-. Combination of chemoradiation with immunotherapy in inoperable œsophageal cancer (2019). Available at: https://ClinicalTrials.gov/show/NCT03437200.
74. Bando H, Kotani D, Tsushima T, Hara H, Kadowaki S, Kato K, et al. TENERGY: multicenter phase II study of atezolizumab monotherapy following definitive chemoradiotherapy with 5-FU plus cisplatin in patients with unresectable locally advanced esophageal squamous cell carcinoma. BMC Canc (2020) 20(1):336. doi: 10.1186/s12885-020-06716-5
75. Hospital HMUF. a study of camrelizumab as consolidation therapy after radical concurrent chemoradiotherapy in locally advanced ESCC (2020). Available at: https://ClinicalTrials.gov/show/NCT04286958.
76. Roche H-L. A study of atezolizumab with or without tiragolumab in participants with unresectable esophageal squamous cell carcinoma whose cancers have not progressed following definitive concurrent chemoradiotherapy (2020). Available at: https://ClinicalTrials.gov/show/NCT04543617.
77. University TFAHoZ. A phase II trials of sintilimab as consolidation therapy after radical concurrent chemoradiotherapy in locally advanced unresectable ESCC (2021). Available at: https://ClinicalTrials.gov/show/NCT04514835.
78. University W. The value of sintilimab consolidation therapy after definitive concurrent chemoradiotherapy for locally advanced thoracic esophageal cancer (2020). Available at: https://ClinicalTrials.gov/show/NCT04212598.
79. Hospital ZC, Hospital ZPPs, Univercity TFAHoWM, Hospital JMC, Hospital LMC, Univercity TAPshoN, et al. A multicenter, randomized controlled, phase II clinical study of first-line chemotherapy and camrelizumab with or without radiotherapy in the treatment of oligometastatic esophageal cancer (2021). Available at: https://ClinicalTrials.gov/show/NCT05183958.
80. Science TFAHoHUo. Technology. clinical control study of immunotherapy and concurrent chemoradiotherapy in patients with esophageal cancer recurrence (2020). Available at: https://ClinicalTrials.gov/show/NCT04404491.
81. Hospital ZC. A clinical study of camrelizumab with or without radiotherapy in the treatment of esophageal cancer (2020). Available at: https://ClinicalTrials.gov/show/NCT04512417.
82. College TFAHoXM, BeiGene. Efficacy and safety of tislelizumab and nab-paclitaxel combined with low-dose radiotherapy in patients with stage IVb esophageal squamous cell carcinoma (2022). Available at: https://ClinicalTrials.gov/show/NCT05547828.
83. Hospital FC. Tislelizumab combined with chemotherapy or radiotherapy in the treatment of advanced or recurrent metastatic elderly esophageal cancer (2022). Available at: https://ClinicalTrials.gov/show/NCT05628610.
84. Institute C, Hospital CAoMS. Study of PD-1 antibody combined with chemoradiotherapy in oligometastatic esophageal cancer (2020). Available at: https://ClinicalTrials.gov/show/NCT04821765.
85. University SY-s. Concurrent chemoradiotherapy for stage IVB esophageal squamous cell Carcinoma(EC-CRT-003) (2022). Available at: https://ClinicalTrials.gov/show/NCT05512520.
86. Wu L, Wang Y, Li B, Wan G, Liang L, Li T, et al. Toripalimab in combination with induction chemotherapy and subsequent chemoradiation as first-line treatment in patients with Advanced/Metastatic esophageal carcinoma: protocol for a single-arm, prospective, open-label, phase II clinical trial (TR-EAT). Front Oncol (2022) 12:878851. doi: 10.3389/fonc.2022.878851
87. Day F, Sridharan S, Lynam J, Gedye C, Johnson C, Fraser A, et al. Chemoradiotherapy with concurrent durvalumab for the palliative treatment of oligometastatic oesophageal and gastrooesophageal carcinoma with dysphagia: a single arm phase II clinical trial (PALEO, sponsored by the Australasian gastro-intestinal trials group). BMC Canc (2022) 22(1):1324. doi: 10.1186/s12885-022-10407-8
88. Science TFAHoHUo, Technology. Camrelizumab combined with CRT for treatment of patients with local recurrence of esophageal cancer (2021). Available at: https://ClinicalTrials.gov/show/NCT04390945.
89. Liu Q, Zhu Z, Chen Y, Deng J, Ai D, Liu Q, et al. Phase 2 study of stereotactic body radiation therapy for patients with oligometastatic esophageal squamous cell carcinoma. Int J Radiat Oncol Biol Phys (2020) 108(3):707–15. doi: 10.1016/j.ijrobp.2020.05.003
90. Liu Q, Chen J, Li B, Ye J, Wei S, Wang Y, et al. Local therapy for oligometastatic esophageal squamous cell carcinoma: a prospective, randomized, phase II clinical trial. Future Oncol (2021) 17(11):1285–93. doi: 10.2217/fon-2020-0873
91. Luke JJ, Lemons JM, Karrison TG, Pitroda SP, Melotek JM, Zha Y, et al. Safety and clinical activity of pembrolizumab and multisite stereotactic body radiotherapy in patients with advanced solid tumors. J Clin Oncol (2018) 36(16):1611–8. doi: 10.1200/JCO.2017.76.2229
92. Kong Y, Zhao X, Xu M, Pan J, Ma Y, Zou L, et al. PD-1 inhibitor combined with radiotherapy and GM-CSF (PRaG) in patients with metastatic solid tumors: an open-label phase II study. Front Immunol (2022) 13:952066. doi: 10.3389/fimmu.2022.952066
93. University SAHoS. PRaG regimens rechallenge for patients with resistance to PD1/PD-L1 inhibitors in refractory advanced solid tumors (2022). Available at: https://ClinicalTrials.gov/show/NCT05530200.
94. Herrera FG, Ronet C, Ochoa de Olza M, Barras D, Crespo I, Andreatta M, et al. Low-dose radiotherapy reverses tumor immune desertification and resistance to immunotherapy. Cancer Discov (2022) 12(1):108–33. doi: 10.1158/2159-8290.CD-21-0003
95. Ochoa-de-Olza M, Bourhis J, Coukos G, Herrera FG. Low-dose irradiation for reversing immunotherapy resistance: how to translate? J Immunother Canc (2022) 10(7):e004939. doi: 10.1136/jitc-2022-004939
96. Ochoa de Olza M, Bourhis J, Irving M, Coukos G, Herrera FG. High versus low dose irradiation for tumor immune reprogramming. Curr Opin Biotechnol (2020) 65:268–83. doi: 10.1016/j.copbio.2020.08.001
97. Menon H, Chen D, Ramapriyan R, Verma V, Barsoumian HB, Cushman TR, et al. Influence of low-dose radiation on abscopal responses in patients receiving high-dose radiation and immunotherapy. J Immunother Canc (2019) 7(1):237. doi: 10.1186/s40425-019-0718-6
98. Yu ZL, Liu JY, Chen G. Small extracellular vesicle PD-L1 in cancer: the knowns and unknowns. NPJ Precis Oncol (2022) 6(1):42. doi: 10.1038/s41698-022-00287-3
99. Zhu M, Chen C, Foster NR, Hartley C, Mounajjed T, Salomao MA, et al. Pembrolizumab in combination with neoadjuvant chemoradiotherapy for patients with resectable adenocarcinoma of the gastroesophageal junction. Clin Cancer Res (2022) 28(14):3021–31. doi: 10.1158/1078-0432.CCR-22-0413
100. Moran JA, Adams DL, Edelman MJ, Lopez P, He J, Qiao Y, et al. Monitoring PD-L1 expression on circulating tumor-associated cells in recurrent metastatic non-Small-Cell lung carcinoma predicts response to immunotherapy with radiation therapy. JCO Precis Oncol (2022) 6:e2200457. doi: 10.1200/PO.22.00457
101. Huang TC, Liang CW, Li YI, Guo JC, Lin CC, Chen YJ, et al. Prognostic value of PD-L1 expression on immune cells or tumor cells for locally advanced esophageal squamous cell carcinoma in patients treated with neoadjuvant chemoradiotherapy. J Cancer Res Clin Oncol (2022) 148(7):1803–11. doi: 10.1007/s00432-021-03772-7
102. Azad TD, Chaudhuri AA, Fang P, Qiao Y, Esfahani MS, Chabon JJ, et al. Circulating tumor DNA analysis for detection of minimal residual disease after chemoradiotherapy for localized esophageal cancer. Gastroenterology (2020) 158(3):494–505 e6. doi: 10.1053/j.gastro.2019.10.039
103. Wang X, Yu N, Cheng G, Zhang T, Wang J, Deng L, et al. Prognostic value of circulating tumour DNA during post-radiotherapy surveillance in locally advanced esophageal squamous cell carcinoma. Clin Transl Med (2022) 12(11):e1116. doi: 10.1002/ctm2.1116
104. Eyck BM, Jansen MP, Noordman BJ, Atmodimedjo PN, van der Wilk BJ, Martens JW, et al. Detection of circulating tumour DNA after neoadjuvant chemoradiotherapy in patients with locally advanced oesophageal cancer. J Pathol (2023) 259(1):35–45. doi: 10.1002/path.6016
105. Ococks E, Frankell AM, Masque Soler N, Grehan N, Northrop A, Coles H, et al. Longitudinal tracking of 97 esophageal adenocarcinomas using liquid biopsy sampling. Ann Oncol (2021) 32(4):522–32. doi: 10.1016/j.annonc.2020.12.010
106. Li Y, Liu J, Cai XW, Li HX, Cheng Y, Dong XH, et al. Biomarkers for the prediction of esophageal cancer neoadjuvant chemoradiotherapy response: a systemic review. Crit Rev Oncol Hematol (2021) 167:103466. doi: 10.1016/j.critrevonc.2021.103466
107. van den Ende T, de Clercq NC, van Berge Henegouwen MI, Gisbertz SS, Geijsen ED, Verhoeven RHA, et al. Neoadjuvant chemoradiotherapy combined with atezolizumab for resectable esophageal adenocarcinoma: a single-arm phase II feasibility trial (PERFECT). Clin Cancer Res (2021) 27(12):3351–9. doi: 10.1158/1078-0432.CCR-20-4443
108. Wang S, Zhou Z, Tian D, Huang S, Wang C, Gao Z, et al. A validated nomogram integrating hematological indicators to predict response to neoadjuvant therapy in esophageal squamous cell carcinoma patients. Ann Transl Med (2021) 9(8):703. doi: 10.21037/atm-21-1628
109. Soeratram TT, Creemers A, Meijer SL, de Boer OJ, Vos W, Hooijer GK, et al. Tumor-immune landscape patterns before and after chemoradiation in resectable esophageal adenocarcinomas. J Pathol (2022) 256(3):282–96. doi: 10.1002/path.5832
110. Xu L, Qi Y, Jiang Y, Ji Y, Zhao Q, Wu J, et al. Crosstalk between the gut microbiome and clinical response in locally advanced thoracic esophageal squamous cell carcinoma during neoadjuvant camrelizumab and chemotherapy. Ann Transl Med (2022) 10(6):325. doi: 10.21037/atm-22-1165
111. Sakin A, Ozcelik M, Sahin S, Aydemir O, Aldemir MN, Iliklerden UH, et al. The prognostic effect of pretreatment 18F-FDG PET/CT metabolic parameters in locally advanced esophageal squamous cell carcinoma treated with definitive chemoradiotherapy. Surg Oncol (2022) 43:101809. doi: 10.1016/j.suronc.2022.101809
112. Marr L, Haller B, Pyka T, Peeken JC, Jesinghaus M, Scheidhauer K, et al. Predictive value of clinical and 18F-FDG-PET/CT derived imaging parameters in patients undergoing neoadjuvant chemoradiation for esophageal squamous cell carcinoma. Sci Rep (2022) 12(1):7148. doi: 10.1038/s41598-022-11076-0
113. Lee BM, Lee CG. Significance of mid-radiotherapy 18F-fluorodeoxyglucose positron emission tomography/computed tomography in esophageal cancer. Radiother Oncol (2022) 171:114–20. doi: 10.1016/j.radonc.2022.04.009
114. Lee S, Choi Y, Park G, Jo S, Lee SS, Park J, et al. 18F-FDG PET/CT parameters for predicting prognosis in esophageal cancer patients treated with concurrent chemoradiotherapy. Technol Cancer Res Treat (2021) 20:15330338211024655. doi: 10.1177/15330338211024655
115. Borggreve AS, Mook S, Verheij M, Mul VEM, Bergman JJ, Bartels-Rutten A, et al. Preoperative image-guided identification of response to neoadjuvant chemoradiotherapy in esophageal cancer (PRIDE): a multicenter observational study. BMC Canc (2018) 18(1):1006. doi: 10.1186/s12885-018-4892-6
116. Cowzer D, Wu AJ-C, Sihag S, Walch HS, Park BJ, Jones DR, et al. Durvalumab (D) and PET-directed chemoradiation (CRT) after induction FOLFOX for esophageal adenocarcinoma: final results. J Clin Oncol (2022) 40(16_suppl):4029–. doi: 10.1200/JCO.2022.40.16_suppl.4029
117. Goodman KA, Ou FS, Hall NC, Bekaii-Saab T, Fruth B, Twohy E, et al. Randomized phase II study of PET response-adapted combined modality therapy for esophageal cancer: mature results of the CALGB 80803 (Alliance) trial. J Clin Oncol (2021) 39(25):2803–15. doi: 10.1200/JCO.20.03611
118. Hospital SC, Institute R. Selected chemotherapy combined immunotherapy treated high risk patient after NCRT in resected locally advanced ESCC (2021). Available at: https://ClinicalTrials.gov/show/NCT05189730.
119. Laengle J, Tamandl D, Schmid R, Widder J, Bergmann M, Haug A. P04.04 programmed death-ligand 1 positron emission tomography imaging during neoadjuvant (chemo)radiotherapy in esophageal and rectal cancer (PETNEC): a prospective non-randomized open-label single-center pilot study. J ImmunoTher Cancer (2021) 9(Suppl 1):A17–A8. doi: 10.1136/jitc-2021-ITOC8.33
120. Zhou M, Wang X, Chen B, Xiang S, Rao W, Zhang Z, et al. Preclinical and first-in-human evaluation of 18F-labeled d-peptide antagonist for PD-L1 status imaging with PET. Eur J Nucl Med Mol Imag (2022) 49(13):4312–24. doi: 10.1007/s00259-022-05876-9
121. Hospital WNPs. 89Zr-labeled KN035 PET imaging in patients with PD-L1 positive solid tumors (2021). Available at: https://ClinicalTrials.gov/show/NCT04977128.
122. Servier IdRI. ADIR aSGc, servier. immuno-positron emission tomography study of 89Zr-S095012 in patients with advanced solid tumours (2022). Available at: https://ClinicalTrials.gov/show/NCT05638334.
123. Guttmann DM, Mitra N, Bekelman J, Metz JM, Plastaras J, Feng W, et al. Improved overall survival with aggressive primary tumor radiotherapy for patients with metastatic esophageal cancer. J Thorac Oncol: Off Publ Int Assoc For Stud Lung Canc (2017) 12(7):1131–42. doi: 10.1016/j.jtho.2017.03.026
124. Wu X, Li Y, Zhang K, Guo Z, Li Y, Zhao F, et al. Immunotherapy with or without radiotherapy for metastatic or recurrent esophageal squamous cell carcinoma: a real-world study. Clin Transl Radiat Oncol (2023) 38:130–7. doi: 10.1016/j.ctro.2022.10.011
125. Shi Z, Zhu X, Ruan C, Wei G, Li J, Qiu H, et al. Evaluation of concurrent chemoradiotherapy for survival outcomes in patients with synchronous oligometastatic esophageal squamous cell carcinoma. JAMA Netw Open (2022) 5(12):e2244619. doi: 10.1001/jamanetworkopen.2022.44619
126. Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med (2018) 379(24):2342–50. doi: 10.1056/NEJMoa1809697
127. Girard N, Bar J, Garrido P, Garassino MC, McDonald F, Mornex F, et al. Treatment characteristics and real-world progression-free survival in patients with unresectable stage III NSCLC who received durvalumab after chemoradiotherapy: findings from the PACIFIC-r study. J Thorac Oncol (2022) 18(2):181–93. doi: 10.1016/j.jtho.2022.10.003
128. Wang Y, Zhang T, Huang Y, Li W, Zhao J, Yang Y, et al. Real-world safety and efficacy of consolidation durvalumab after chemoradiation therapy for stage III non-small cell lung cancer: a systematic review and meta-analysis. Int J Radiat Oncol Biol Phys (2022) 112(5):1154–64. doi: 10.1016/j.ijrobp.2021.12.150
129. Donlon NE, Davern M, Sheppard AD, O’Connell F, Dunne MR, Hayes C, et al. The impact of esophageal oncological surgery on perioperative immune function; implications for adjuvant immune checkpoint inhibition. Front Immunol (2022) 13:823225. doi: 10.3389/fimmu.2022.823225
130. Scoccianti S, Olmetto E, Pinzi V, Osti MF, Di Franco R, Caini S, et al. Immunotherapy in association with stereotactic radiotherapy for non-small cell lung cancer brain metastases: results from a multicentric retrospective study on behalf of AIRO. Neuro Oncol (2021) 23(10):1750–64. doi: 10.1093/neuonc/noab129
131. Duan Y, Qin W, Yang L, Zou B, Qie W, Song R, et al. Safety and efficacy of concurrent or sequential radiotherapy plus (PD-1) inhibitors in oligometastatic esophageal cancer. Cancer Manag Res (2023) 15:55–65. doi: 10.2147/CMAR.S391529
132. Lehrer EJ, Peterson J, Brown PD, Sheehan JP, Quinones-Hinojosa A, Zaorsky NG, et al. Treatment of brain metastases with stereotactic radiosurgery and immune checkpoint inhibitors: an international meta-analysis of individual patient data. Radiother Oncol (2019) 130:104–12. doi: 10.1016/j.radonc.2018.08.025
133. Wei J, Montalvo-Ortiz W, Yu L, Krasco A, Ebstein S, Cortez C, et al. Sequence of alphaPD-1 relative to local tumor irradiation determines the induction of abscopal antitumor immune responses. Sci Immunol (2021) 6(58):eabg0117. doi: 10.1126/sciimmunol.abg0117
134. Dovedi SJ, Adlard AL, Lipowska-Bhalla G, McKenna C, Jones S, Cheadle EJ, et al. Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD-L1 blockade. Cancer Res (2014) 74(19):5458–68. doi: 10.1158/0008-5472.CAN-14-1258
135. Woody S, Hegde A, Arastu H, Peach MS, Sharma N, Walker P, et al. Survival is worse in patients completing immunotherapy prior to SBRT/SRS compared to those receiving it concurrently or after. Front Oncol (2022) 12:785350. doi: 10.3389/fonc.2022.785350
136. Kiess AP, Wolchok JD, Barker CA, Postow MA, Tabar V, Huse JT, et al. Stereotactic radiosurgery for melanoma brain metastases in patients receiving ipilimumab: safety profile and efficacy of combined treatment. Int J Radiat Oncol Biol Phys (2015) 92(2):368–75. doi: 10.1016/j.ijrobp.2015.01.004
137. Schoenfeld JD, Mahadevan A, Floyd SR, Dyer MA, Catalano PJ, Alexander BM, et al. Ipilmumab and cranial radiation in metastatic melanoma patients: a case series and review. J For Immunother Canc (2015) 3:50. doi: 10.1186/s40425-015-0095-8
138. Welsh JW, Tang C, de Groot P, Naing A, Hess KR, Heymach JV, et al. Phase II trial of ipilimumab with stereotactic radiation therapy for metastatic disease: outcomes, toxicities, and low-dose radiation-related abscopal responses. Cancer Immunol Res (2019) 7(12):1903–9. doi: 10.1158/2326-6066.CIR-18-0793
139. Peter MacCallum Cancer Centre A. Sequencing of stereotactic ablative body radiotherapy in combination with PD-1 blockade using pembrolizumab in metastatic non-small cell lung carcinoma (2017). Available at: https://ClinicalTrials.gov/show/NCT03307759.
140. University SKCCaTJ, Sharp M, LLC D, University TJ. Radiation therapy and MK-3475 for patients with Recurrent/Metastatic head and neck cancer, renal cell cancer, melanoma, and lung cancer(2015). Available at: https://ClinicalTrials.gov/show/NCT02318771.
141. Peng F, Bao Y, Lian HM, Niu SQ. Increased esophageal perforation after combination of anti-PD-1 immunotherapy with radiotherapy for esophageal carcinoma. Int J Radiat Oncol Biol Phys (2021) 111(3):S102. doi: 10.1016/j.ijrobp.2021.07.237
142. Minsky BD, Pajak TF, Ginsberg RJ, Pisansky TM, Martenson J, Komaki R, et al. INT 0123 (Radiation therapy oncology group 94-05) phase III trial of combined-modality therapy for esophageal cancer: high-dose versus standard-dose radiation therapy. J Clin Oncol (2002) 20(5):1167–74. doi: 10.1200/JCO.2002.20.5.1167
143. Crehange G, M’Vondo C, Bertaut A, Pereira R, Rio E, Peiffert D, et al. Exclusive chemoradiotherapy with or without radiation dose escalation in esophageal cancer: multicenter phase 2/3 randomized trial CONCORDE (PRODIGE-26). Int J Radiat Oncol Biol Phys (2021) 111(3):S5. doi: 10.1016/j.ijrobp.2021.07.045
144. Engel S, Awerbuch A, Kwon D, Picado O, Yechieli R, Yakoub D, et al. Optimal radiation dosing in concurrent neoadjuvant chemoradiation for resectable esophageal cancer: a meta-analysis. J Gastrointest Oncol (2019) 10(3):391–9. doi: 10.21037/jgo.2019.01.02
145. Worrell SG, Towe CW A, Dorth J, Machtay M, Perry Y, Linden PA. Higher doses of neoadjuvant radiation for esophageal cancer do not affect the pathologic complete response rate or survival: a propensity-matched analysis. Ann Surg Oncol (2020) 27(2):500–8. doi: 10.1245/s10434-019-07849-z
146. Hospital SC. Camrelizumab combined with neoadjuvant concurrent chemoradiotherapy for resectable locally advanced ESCC(NICE-RT) (2022). Available at: https://ClinicalTrials.gov/show/NCT05650216.
147. Patel RR, He K, Barsoumian HB, Chang JY, Tang C, Verma V, et al. High-dose irradiation in combination with non-ablative low-dose radiation to treat metastatic disease after progression on immunotherapy: results of a phase II trial. Radiother Oncol (2021) 162:60–7. doi: 10.1016/j.radonc.2021.06.037
148. Cho Y, Park S, Byun HK, Lee CG, Cho J, Hong MH, et al. Impact of treatment-related lymphopenia on immunotherapy for advanced non-small cell lung cancer. Int J Radiat Oncol Biol Phys (2019) 105(5):1065–73. doi: 10.1016/j.ijrobp.2019.08.047
149. Chen D, Verma V, Patel RR, Barsoumian HB, Cortez MA, Welsh JW. Absolute lymphocyte count predicts abscopal responses and outcomes in patients receiving combined immunotherapy and radiation therapy: analysis of 3 phase 1/2 trials. Int J Radiat Oncol Biol Phys (2020) 108(1):196–203. doi: 10.1016/j.ijrobp.2020.01.032
150. Pike LRG, Bang A, Mahal BA, Taylor A, Krishnan M, Spektor A, et al. The impact of radiation therapy on lymphocyte count and survival in metastatic cancer patients receiving PD-1 immune checkpoint inhibitors. Int J Radiat Oncol Biol Phys (2019) 103(1):142–51. doi: 10.1016/j.ijrobp.2018.09.010
151. Brooks ED, Chang JY. Time to abandon single-site irradiation for inducing abscopal effects. Nat Rev Clin Oncol (2019) 16(2):123–35. doi: 10.1038/s41571-018-0119-7
152. McGee HM, Daly ME, Azghadi S, Stewart SL, Oesterich L, Schlom J, et al. Stereotactic ablative radiation therapy induces systemic differences in peripheral blood immunophenotype dependent on irradiated site. Int J Radiat Oncol Biol Phys (2018) 101(5):1259–70. doi: 10.1016/j.ijrobp.2018.04.038
153. Wu M, Liu J, Wu S, Liu J, Wu H, Yu J, et al. Systemic immune activation and responses of irradiation to different metastatic sites combined with immunotherapy in advanced non-small cell lung cancer. Front Immunol (2021) 12:803247. doi: 10.3389/fimmu.2021.803247
154. D’Rummo KA, Al-Jumayli M, Huang CH, Wang F. The effect of radiotherapy on clinical outcomes for patients receiving immunotherapy for metastatic non-small cell lung carcinoma. Int J Radiat Oncol Biol Phys (2019) 105(1):E501–E2. doi: 10.1016/j.ijrobp.2019.06.1318
155. Tang C, Welsh JW, de Groot P, Massarelli E, Chang JY, Hess KR, et al. Ipilimumab with stereotactic ablative radiation therapy: phase I results and immunologic correlates from peripheral T cells. Clin Cancer Res (2017) 23(6):1388–96. doi: 10.1158/1078-0432.CCR-16-1432
156. Yan X, Duan H, Ni Y, Zhou Y, Wang X, Qi H, et al. Tislelizumab combined with chemotherapy as neoadjuvant therapy for surgically resectable esophageal cancer: a prospective, single-arm, phase II study (TD-NICE). Int J Surg (2022) 103:106680. doi: 10.1016/j.ijsu.2022.106680
157. Liu J, Yang Y, Liu Z, Fu X, Cai X, Li H, et al. Multicenter, single-arm, phase II trial of camrelizumab and chemotherapy as neoadjuvant treatment for locally advanced esophageal squamous cell carcinoma. J For Immunother Cancer (2022) 10(3):e004291. doi: 10.1136/jitc-2021-004291
158. Shang X, Zhang C, Zhao G, Zhang W, Liu L, Duan X, et al. LBA3 safety and efficacy of pembrolizumab combined with paclitaxel and cisplatin as a neoadjuvant treatment for locally advanced resectable (stage III) esophageal squamous cell carcinoma (Keystone-001): interim analysis of a prospective, single-arm, single-center, phase II trial. Ann Oncol (2021) 32:S1428–S9. doi: 10.1016/j.annonc.2021.10.218
159. Wu Z, Zheng Q, Chen H, Xiang J, Hu H, Li H, et al. Efficacy and safety of neoadjuvant chemotherapy and immunotherapy in locally resectable advanced esophageal squamous cell carcinoma. J Thorac Dis (2021) 13(6):3518–28. doi: 10.21037/jtd-21-340
160. Yamamoto S, Kato K, Daiko H, Kojima T, Hara H, Abe T, et al. FRONTiER: a feasibility trial of nivolumab with neoadjuvant CF or DCF therapy for locally advanced esophageal carcinoma (JCOG1804E)–the short-term results of cohort a and b. J Clin Oncol (2021) 39(3_suppl):202–. doi: 10.1200/JCO.2021.39.3_suppl.202
161. Matsuda S, Yamamoto S, Kato K, Daiko H, Kojima T, Hara H, et al. FRONTiER: a feasibility trial of nivolumab with neoadjuvant CF or DCF, FLOT therapy for locally advanced esophageal carcinoma (JCOG1804E)–short-term results for cohorts c and d. J Clin Oncol (2022) 40(4_suppl):286–. doi: 10.1200/JCO.2022.40.4_suppl.286
162. Zhao L, Xing W, Yang Y, Zhang Y, Ma B, Fu X, et al. The sequence of chemotherapy and anti-PD-1 antibody influence the efficacy of neoadjuvant immunochemotherapy in locally advanced esophageal squamous cell cancer: a phase II study. J Clin Oncol (2021) 39(15_suppl):4051–. doi: 10.1200/JCO.2021.39.15_suppl.4051
163. Zhang Z, Ye J, Li H, Gu D, Du M, Ai D, et al. Neoadjuvant sintilimab and chemotherapy in patients with resectable esophageal squamous cell carcinoma: a prospective, single-arm, phase 2 trial. Front Immunol (2022) 13:1031171. doi: 10.3389/fimmu.2022.1031171
164. Duan H, Shao C, Pan M, Liu H, Dong X, Zhang Y, et al. Neoadjuvant pembrolizumab and chemotherapy in resectable esophageal cancer: an open-label, single-arm study (PEN-ICE). Front Immunol (2022) 13:849984. doi: 10.3389/fimmu.2022.849984
165. Xiao X, Yang YS, Zeng XX, Shang QX, Luan SY, Zhou JF, et al. The comparisons of neoadjuvant chemoimmunotherapy versus chemoradiotherapy for oesophageal squamous cancer. Eur J Cardiothorac Surg (2022) 62(1):ezac341. doi: 10.1093/ejcts/ezac341
166. Wang H, Li S, Liu T, Chen J, Dang J. Neoadjuvant immune checkpoint inhibitor in combination with chemotherapy or chemoradiotherapy in resectable esophageal cancer: a systematic review and meta-analysis. Front Immunol (2022) 13:998620. doi: 10.3389/fimmu.2022.998620
167. Shang X, Zhang W, Zhao G, Liang F, Zhang C, Yue J, et al. Pembrolizumab combined with neoadjuvant chemotherapy versus neoadjuvant chemoradiotherapy followed by surgery for locally advanced oesophageal squamous cell carcinoma: protocol for a multicentre, prospective, randomized-controlled, phase III clinical study (Keystone-002). Front Oncol (2022) 12:831345. doi: 10.3389/fonc.2022.831345
168. Yang Y, Zhu L, Cheng Y, Liu Z, Cai X, Shao J, et al. Three-arm phase II trial comparing camrelizumab plus chemotherapy versus camrelizumab plus chemoradiation versus chemoradiation as preoperative treatment for locally advanced esophageal squamous cell carcinoma (NICE-2 study). BMC Canc (2022) 22(1):506. doi: 10.1186/s12885-022-09573-6
169. Hospital FC, Suzhou Suncadia Biopharmaceuticals Co. L. PD-1 inhibitor combined with neoadjuvant chemotherapy in subjects with resectable locally advanced thoracic esophageal squamous cell carcinoma (2021). Available at: https://ClinicalTrials.gov/show/NCT05007145.
170. Hospital FC. Camrelizumab combined with chemoradiotherapy in advanced esophageal cancer (2022). Available at: https://ClinicalTrials.gov/show/NCT05624099.
171. Smyth E, Knodler M, Giraut A, Mauer M, Nilsson M, Van Grieken N, et al. VESTIGE: adjuvant immunotherapy in patients with resected esophageal, gastroesophageal junction and gastric cancer following preoperative chemotherapy with high risk for recurrence (N+ and/or R1): an open label randomized controlled phase-2-Study. Front Oncol (2019) 9:1320. doi: 10.3389/fonc.2019.01320
172. Institute TMUC, Hospital. Carrelizumab combined with chemotherapy for adjuvant therapy of esophageal squamous cell carcinoma (2020). Available at: https://ClinicalTrials.gov/show/NCT05637268.
173. Castoro C, Scarpa M, Cagol M, Alfieri R, Ruol A, Cavallin F, et al. Complete clinical response after neoadjuvant chemoradiotherapy for squamous cell cancer of the thoracic oesophagus: is surgery always necessary? J Gastrointest Surg (2013) 17(8):1375–81. doi: 10.1007/s11605-013-2269-3
174. Taketa T, Xiao L, Sudo K, Suzuki A, Wadhwa R, Blum MA, et al. Propensity-based matching between esophagogastric cancer patients who had surgery and who declined surgery after preoperative chemoradiation. Oncology (2013) 85(2):95–9. doi: 10.1159/000351999
175. Piessen G, Messager M, Mirabel X, Briez N, Robb WB, Adenis A, et al. Is there a role for surgery for patients with a complete clinical response after chemoradiation for esophageal cancer? an intention-to-treat case-control study. Ann Surg (2013) 258(5):793-9; discussion 799-800. doi: 10.1097/SLA.0000000000000228
176. Wang J, Qin J, Jing S, Liu Q, Cheng Y, Wang Y, et al. Clinical complete response after chemoradiotherapy for carcinoma of thoracic esophagus: is esophagectomy always necessary? a systematic review and meta-analysis. Thorac Canc (2018) 9(12):1638–47. doi: 10.1111/1759-7714.12874
177. van der Wille BJ, Noordman BJ, Neijenhuis LKA, Nieboer D, Nieuwenhuijzen GAP, Sosef MN, et al. Active surveillance versus immediate surgery in clinically complete responders after neoadjuvant chemoradiotherapy for esophageal cancer: a multicenter propensity matched study. Ann Surg (2021) 274(6):1009–16. doi: 10.1097/SLA.0000000000003636
178. van der Wilk BJ, Eyck BM, Hofstetter WL, Ajani JA, Piessen G, Castoro C, et al. Chemoradiotherapy followed by active surveillance versus standard esophagectomy for esophageal cancer: a systematic review and individual patient data meta-analysis. Ann Surg (2022) 275(3):467–76. doi: 10.1097/SLA.0000000000004930
179. Noordman BJ, Wijnhoven BPL, Lagarde SM, Boonstra JJ, Coene P, Dekker JWT, et al. Neoadjuvant chemoradiotherapy plus surgery versus active surveillance for oesophageal cancer: a stepped-wedge cluster randomised trial. BMC Canc (2018) 18(1):142. doi: 10.1186/s12885-018-4034-1
180. Dijon CHU. Comparison of systematic surgery versus surveillance and rescue surgery in operable oesophageal cancer with a complete clinical response to radiochemotherapy . Available at: https://ClinicalTrials.gov/show/NCT02551458.
181. van der Zijden CJ, Lagarde SM, Hermus M, Kranenburg LW, van Lanschot JJB, Mostert B, et al. A prospective cohort study on active surveillance after neoadjuvant chemoradiotherapy for esophageal cancer: protocol of surgery as needed for oesophageal cancer-2. BMC Canc (2023) 23(1):327. doi: 10.1186/s12885-023-10747-z
182. Hospital ZC, Jiangsu HengRui Medicine Co. L. Watch and wait for NeoAdjuvant concurrent radiochemotherapy combined with camrelizumab in patients with resectable ESCC (2022). Available at: https://ClinicalTrials.gov/show/NCT05507411.
183. Noordman BJ, Spaander MCW, Valkema R, Wijnhoven BPL, van Berge Henegouwen MI, Shapiro J, et al. Detection of residual disease after neoadjuvant chemoradiotherapy for oesophageal cancer (preSANO): a prospective multicentre, diagnostic cohort study. Lancet Oncol (2018) 19(7):965–74. doi: 10.1016/S1470-2045(18)30201-8
184. Eyck BM, Onstenk BD, Noordman BJ, Nieboer D, Spaander MCW, Valkema R, et al. Accuracy of detecting residual disease after neoadjuvant chemoradiotherapy for esophageal cancer: a systematic review and meta-analysis. Ann Surg (2020) 271(2):245–56. doi: 10.1097/SLA.0000000000003397
185. Valkema MJ, van der Wilk BJ, Eyck BM, Wijnhoven BPL, Spaander MCW, Doukas M, et al. Surveillance of clinically complete responders using serial 18F-FDG PET/CT scans in patients with esophageal cancer after neoadjuvant chemoradiotherapy. J Nucl Med: Off Publicat Soc Nucl Med (2021) 62(4):486–92. doi: 10.2967/jnumed.120.247981
186. Ting YC, Hsu PK, Chen HS, Lin CH, Chuang CY, Hsu HS, et al. Surgery or surveillance for esophageal squamous cell carcinoma with clinical complete response after neoadjuvant chemoradiotherapy. Semin Thorac Cardiovasc Surg (2022) S1043–0679(22)00074–0. doi: 10.1053/j.semtcvs.2022.04.003
187. Wu J, Deng R, Ni T, Zhong Q, Tang F, Li Y, et al. Efficacy and safety of radiotherapy/chemoradiotherapy combined with immune checkpoint inhibitors for locally advanced stages of esophageal cancer: a systematic review and meta-analysis. Front Oncol (2022) 12:887525. doi: 10.3389/fonc.2022.887525
188. Xu T, Liu Y, Lu X, Liang J. Toxicity profile of combined immune checkpoint inhibitors and thoracic radiotherapy in esophageal cancer: a meta-analysis and systematic review. Front Immunol (2022) 13:1039020. doi: 10.3389/fimmu.2022.1039020
189. Sha CM, Lehrer EJ, Hwang C, Trifiletti DM, Mackley HB, Drabick JJ, et al. Toxicity in combination immune checkpoint inhibitor and radiation therapy: a systematic review and meta-analysis. Radiother Oncol (2020) 151:141–8. doi: 10.1016/j.radonc.2020.07.035
190. Zhou X, Yao Z, Bai H, Duan J, Wang Z, Wang X, et al. Treatment-related adverse events of PD-1 and PD-L1 inhibitor-based combination therapies in clinical trials: a systematic review and meta-analysis. Lancet Oncol (2021) 22(9):1265–74. doi: 10.1016/S1470-2045(21)00333-8
191. Voong KR, Hazell SZ, Fu W, Hu C, Lin CT, Ding K, et al. Relationship between prior radiotherapy and checkpoint-inhibitor pneumonitis in patients with advanced non-Small-Cell lung cancer. Clin Lung Canc (2019) 20(4):e470–e9. doi: 10.1016/j.cllc.2019.02.018
192. Anscher MS, Arora S, Weinstock C, Amatya A, Bandaru P, Tang C, et al. Association of radiation therapy with risk of adverse events in patients receiving immunotherapy: a pooled analysis of trials in the US food and drug administration database. JAMA Oncol (2022) 8(2):232–40. doi: 10.1001/jamaoncol.2021.6439
193. Sihag S, Ku GY, Tan KS, Nussenzweig S, Wu A, Janjigian YY, et al. Safety and feasibility of esophagectomy following combined immunotherapy and chemoradiotherapy for esophageal cancer. J Thorac Cardiovasc Surg (2021) 161(3):836–43 e1. doi: 10.1016/j.jtcvs.2020.11.106
Keywords: esophageal squamous cell carcinoma (ESCC), radiotherapy, immune checkpoint inhibitor (ICI), combination efficacy, adverse event (AE)
Citation: Jiang M, Hu Y, Lin G, Chen C and Li H (2023) Radiotherapy combined with immune checkpoint inhibitors in locally advanced/metastatic esophageal squamous cell carcinoma: clinical trials, efficacy and future directions. Front. Immunol. 14:1177085. doi: 10.3389/fimmu.2023.1177085
Received: 01 March 2023; Accepted: 22 May 2023;
Published: 30 May 2023.
Edited by:
Liyuan Zhang, Second Affiliated Hospital of Soochow University, ChinaReviewed by:
Nan Bi, Chinese Academy of Medical Sciences and Peking Union Medical College, ChinaCopyright © 2023 Jiang, Hu, Lin, Chen and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mengjie Jiang, am1qNTQ5OEB6Y211LmVkdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.