
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol. , 22 June 2023
Sec. Autoimmune and Autoinflammatory Disorders: Autoinflammatory Disorders
Volume 14 - 2023 | https://doi.org/10.3389/fimmu.2023.1174998
Background: Psoriasis is a chronic inflammatory skin disease. Dyslipidemia may be a risk factor of psoriasis. But the causal relationship between psoriasis and blood lipid still remains uncertain.
Methods: The two data of blood lipid were obtained from UK Biobank (UKBB) and Global Lipid Genetics Consortium Results (GLGC). The primary and secondary database were from large publicly available genome-wide association study (GWAS) with more than 400,000 and 170,000 subjects of European ancestry, respectively. The psoriasis from Finnish biobanks of FinnGen research project for psoriasis, consisting of 6,995 cases and 299,128 controls. The single-variable Mendelian randomization (SVMR) and multivariable Mendelian randomization (MVMR) were used to assess the total and direct effects of blood lipid on psoriasis risk.
Results: SVMR estimates in primary data of blood lipid showed low-density lipoprotein cholesterol (LDL-C) (odds ratio (OR): 1.11, 95%, confidence interval (CI): 0.99−1.25, p = 0.082 in stage 1; OR: 1.15, 95% CI: 1.05−1.26, p = 0.002 in stage 2; OR: 1.15, 95% CI: 1.04−1.26, p = 0.006 in stage 3) and triglycerides (TG) (OR: 1.22, 95% CI: 1.10−1.35, p = 1.17E-04 in stage 1; OR: 1.15, 95% CI: 1.06−1.24, p = 0.001 in stage 2; OR: 1.14, 95% CI: 1.05−1.24, p = 0.002 in stage 3) had a highly robust causal relationship on the risk of psoriasis. However, there were no robust causal associations between HDL-C and psoriasis. The SVMR results in secondary data of blood lipid were consistent with the primary data. Reverse MR analysis showed a causal association between psoriasis and LDL-C (beta: -0.009, 95% CI: -0.016− -0.002, p = 0.009) and HDL-C (beta: -0.011, 95% CI: -0.021− -0.002, p = 0.016). The reverse causation analyses results between psoriasis and TG did not reach significance. In MVMR of primary data of blood lipid, the LDL-C (OR: 1.05, 95% CI: 0.99–1.25, p = 0.396 in stage 1; OR: 1.07, 95% CI: 1.01–1.14, p = 0.017 in stage 2; OR: 1.08, 95% CI: 1.02–1.15, p = 0.012 in stage 3) and TG (OR: 1.11, 95% CI: 1.01–1.22, p = 0.036 in stage 1; OR: 1.09, 95% CI: 1.03–1.15, p = 0.002 in stage 2; OR: 1.07, 95% CI: 1.01–1.13 p = 0.015 in stage 3) positively correlated with psoriasis, and there had no correlation between HDL-C and psoriasis. The results of the secondary analysis were consistent with the results of primary analysis.
Conclusions: Mendelian randomization (MR) findings provide genetic evidence for causal link between psoriasis and blood lipid. It may be meaningful to monitor and control blood lipid level for a management of psoriasis patients in clinic.
Psoriasis is a chronic inflammatory skin disease. It is typically reported to affect approximately 1%–3% of the people every year (1). According to most researches, the white individuals have higher morbidity rates than other ethnic groups (2, 3). However, psoriasis is not just a skin disease, it is actually related to the occurrence of other disorders, including metabolic syndrome (MetS), rheumatological, cardiovascular and inflammatory bowel disease (4). This disease causes great physical and psychological burden for patients (2). Risk factors for psoriasis are diverse, such as cardiovascular disease, MetS, diabetes mellitus, obesity, dyslipidemia and hypertension and so on (5, 6). These risk factors are a challenge to the treatment and management of psoriasis (7). Thus, it is meaningful to find modifiable risk factors to prevent psoriasis.
MetS is one of the most common comorbidities in psoriasis, and characterized by obesity, hypertension, diabetes mellitus and hyperlipidemia (8, 9). Among these, dyslipidemia is one of typical manifestations. An increasing number of studies suggested that dyslipidemia can be observed in most patients with psoriasis (10). The most common manifestations are elevated the serum cholesterol, low-density lipoprotein cholesterol (LDL-C) and triglycerides (TG) levels, and lowered value of high-density lipoprotein cholesterol (HDL- C) (11–13). Many comprehensive studies revealed an increasing graded relationship between MetS and severity of psoriasis (4, 14, 15). Dyslipidemia can also increase the risk of developing psoriasis comorbidities, such as MetS, cardiovascular disease and non-alcoholic fatty liver disease (NAFLD). In turn, the comorbidities (such as MetS) may aggravate dyslipidemia and form a vicious circle (16–19). Lotus Mallbris et al. found dyslipidemia in psoriasis may be due to genetic influence rather than acquired (12). However, another study demonstrated psoriasis is an independent risk factor for dyslipidemia (20). There still remain controversial whether blood lipid associated with increased risk of psoriasis or the psoriasis affects blood lipid level.
For the limitations of observational studies, many studies cannot determine the temporal relationship between psoriasis onset and dyslipidemia. While psoriasis may in turn affect changes in blood lipid, which makes the causal relationship between psoriasis and blood lipid more unclear. Considering the controversies, Mendelian randomization (MR) can be applied for causal inference between psoriasis and dyslipidemia. The MR is widely used to explore the causality between risk factors and diseases (21, 22), employs single-nucleotide polymorphisms (SNPs) as genetic tools and reliably estimates their effects on the outcomes of interest. It is a genetic method that assesses causality under certain assumptions, independent of confounders in the environment. Thus, we used MR to evaluate the causal relationships between psoriasis and blood lipid, hoping to provide genetic evidence for resolving the existing controversy.
The MR design consisted of the analysis of two blood lipid genome-wide association study (GWAS) databases, namely the primary database analysis and the secondary database analysis. The selection of genetic instrumental variables for blood lipid is mainly divided into three stages, each stage will select suitable SNPs for corresponding MR analysis. To improve the credibility of the results, we performed MR analysis for SNPs of blood lipid in each stage, including single-variable Mendelian randomization (SVMR) and multivariable Mendelian randomization (MVMR). Finally, we performed a reversal MR analysis to explore the effect of psoriasis on blood lipid. This reversal causation analysis will help determine whether psoriatic disease status could affect the levels of blood lipid.
We obtained the GWAS for blood lipid from two independent database, namely UK Biobank (UKBB) and Global Lipid Genetics Consortium Results (GLGC). The primary database was form UKBB. There were 441,016 for TG, 403,943 for HDL-C, and 440,546 for LDL-C included in GWAS. Detailed GWAS methods were provided in original article (23). The secondary database was from GLGC. The TG (N = 177,861), HDL-C (N = 187,167) and LDL-C (N = 173,082) were also from 188,577 European-ancestry individuals (24). Outcome data for psoriasis were obtained from Finnish biobanks of FinnGen research project, consisting of 6,995 cases and 299,128 controls. The details for used data sources were presented in Supplementary Table 1. For the reversal causation analysis, the genetic instruments consisting of 36 SNPs for psoriasis was derived from a GWAS meta-analysis, involving 10,588 cases and 22,806 controls in total (25).
Psoriasis definition in FinnGen R7 were demonstrated online (https://r7.risteys.finngen.fi/phenocode/L12_PSORIASIS). Briefly, it was diagnosed using the Hospital Discharge: ICD-10 L40 and Cause of death: ICD-10 L40. After a series of filtering, 6995 of cases (3677 Female and 3318 Male) were retained in GWAS.
For primary database analysis, we firstly selected independent SNPs from a recent reported GWAS which including 220 (LDL-C), 534 (HDL-C), and 440 (TG) independent SNPs (23). After extracting corresponding information from outcome dataset and harmonizing the exposure and outcome data, 193 SNPs for LDL-C, 193 SNPs for HDL-C and 193 SNPs for TG were retained. We would perform MR after this step, called stage 1 analysis. Secondly, to reduce the reverse association bias, we remove variants that have a larger R Squared in outcomes and perform the stage 2 analysis. Thirdly, we examined the instrumental variables’ (IVs) association with potential confounders, and removed variants that were associated at a genome-wide significance level of P < 5E-08 to any of the following confounders: type 2 diabetes (T2D), BMI (Supplementary Table 2), and performed MR-PREESO to remove outliers (if have). After this step, we performed MR again using the retained SNPs in stage 3 analysis. To make our results more robust, we performed secondary database analysis as a replication using another GWAS enrolling different populations for lipid traits (24). Initial independent SNPs were selected from online (p < 5E-08, linkage disequilibrium < 0.01) (Freq.A1.1000G.EUR). The fowling steps were the same as the primary database analysis. The detailed SNP filtering and corresponding number of SNPs was presented in Figure 1. The details of SNPs were presented in Supplementary Data 1. To avoid the effect of weak instrumental variable bias, we used the F statistic to assess the strengths of IVs. After calculating, the F statistics of all SNPs in every stage of both databases were greater than 10, indicating a smaller possibility of weak instrumental variable bias (Supplementary Table 2).
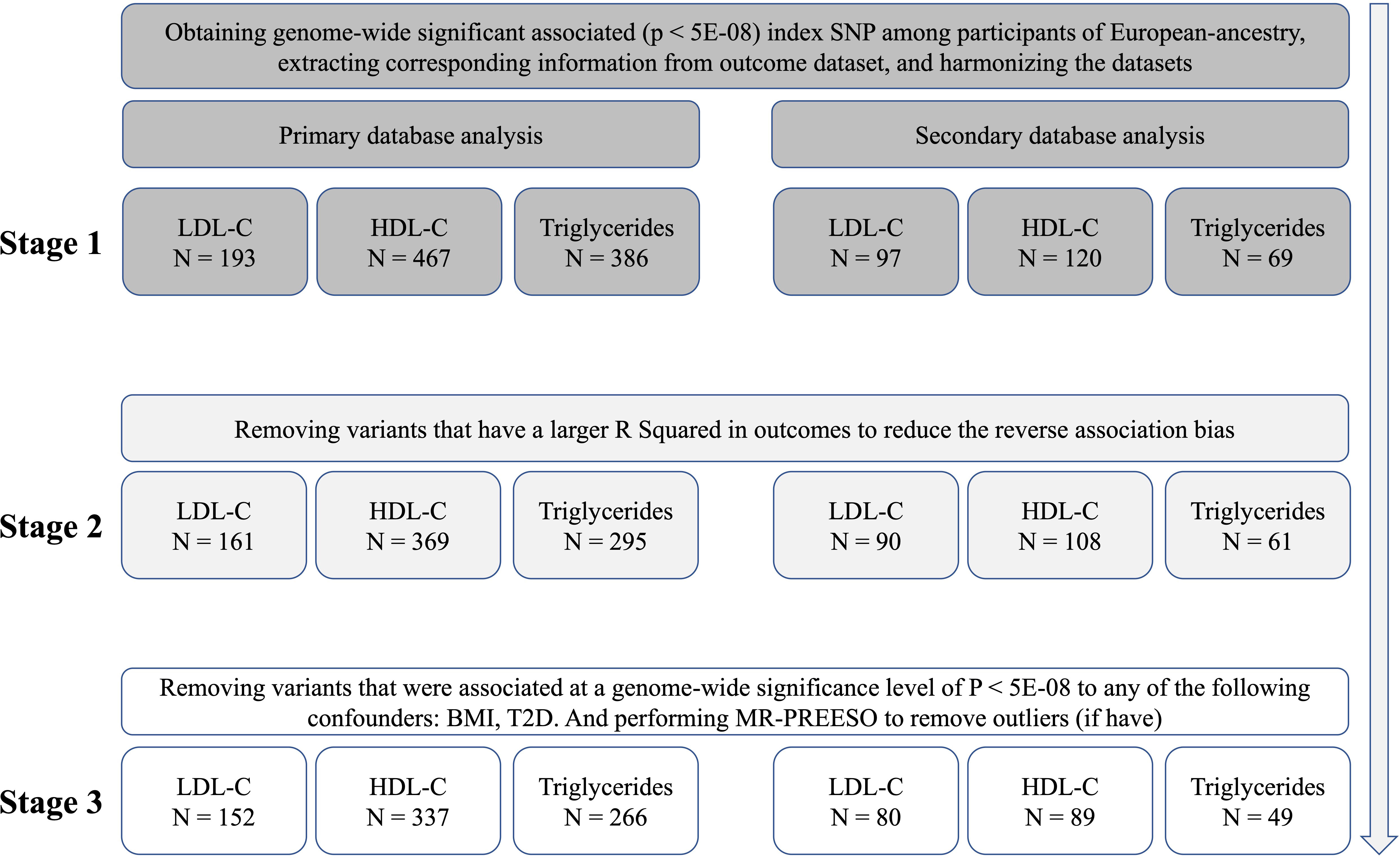
Figure 1 The identification of genetic instruments, and data and MR methods used for analyses. N, numbers of SNPs; LDL-C, Low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; SNP, single-nucleotide polymorphisms; MR, Mendelian randomization; BMI, Body Mass Index; T2D, Type 2 Diabetes.
The two-sample MR design is based on three main hypotheses of classical assumptions (26, 27):(1) the IVs with blood lipid is closely associated with the exposure of interest; (2) There are no confounders between IVs and outcomes; (3) IVs directly affect outcomes through the exposure of interest and not through other pathways. We tried to exclude some confounding factors related to blood lipid. Moreover, by rigorous screening for IVs, we reduced the weak association between potential confounders and genetic variations. Effected by horizontal pleiotropy, genetic variation may affect outcomes in ways other than exposure of interest. In this MR study, we used three MR methods to estimate the robust effects, including inverse variance weighted (IVW), Mendelian randomization-Egger (MR–Egger), weighted median, and weighted methods. We performed MR-egger intercept analysis on SNPs at each stage in primary and secondary database analysis, the results indicated there was no pleiotropy (Egger intercept p value > 0.05). The details are presented in Supplementary Tables 3, 4.
In MR analysis, the IVW and MR–Egger methods were used as the main analysis method. MR–Egger, IVW, weighted median, and weighted methods are used to estimate the robust effects if genetic variants have pleiotropic effects. The weighted median method provides reliable evidence when at least half of the valid instrumental variables have no pleiotropic effects. The MR–Egger regression provides consistent estimates when 100% of genetic variants are invalid IVs.
The packages Two-Sample MR and MVMR in R v.4.0.3 (www.r-project.org) are used to analysis. The online tool is used to perform power calculations (http://cnsgenomics.com/shiny/mRnd/).
We analyzed SNPs for each stage of LDL-C from primary and secondary databases. Overall, we found a highly robust causal associations between LDL-C and psoriasis outcomes. In primary database analysis, we observed evidence for genetically predicted LDL-C through IVW method (OR: 1.11, 95% CI: 0.99–1.25, p = 0.082 in stage 1; OR: 1.15, 95% CI: 1.05–1.26, p = 0.002 in stage 2; OR: 1.15, 95% CI: 1.04–1.26, p = 0.006 in stage 3). In secondary database analysis, LDL-C still had a strongly causal association with psoriasis (OR: 1.09, 95% CI: 0.99–1.94, p = 0.081 in stage 1; OR: 1.10, 95% CI: 1.02–1.18, p = 0.009 in stage 2; OR: 1.12, 95% CI: 1.03–1.21, p = 0.008 in stage 3) through IVW method (Table 1). Furthermore, we used three other MR methods to analyze the SNPs, and the results were basically consistent with the IVW analysis (Supplementary Tables 3, 4). Sensitivity analysis showed a consistent trend across the four MR methods (Figure 3). MR analysis of both databases revealed a positive association between LDL-C and psoriasis. This indicated that LDL-C was a risk factor for psoriasis.
Overall, we did not observe causal association between HDL-C and psoriasis in primary database and secondary database analysis. No significant result was found through IVW and MR egger method analysis in each stage from primary and secondary database (Figure 2). To exclude the effect of horizontal pleiotropy, using Weighted median and Weighted mode methods to analyze all SNPs from the two databases, there was no horizontal pleiotropy and significant result (Supplementary Tables 3, 4). Finally, the sensitivity analysis of the four MR analysis methods showed a robust consistent trend (Figure 3). Therefore, we considered there was no highly robust causal associations between HDL-C and psoriasis.
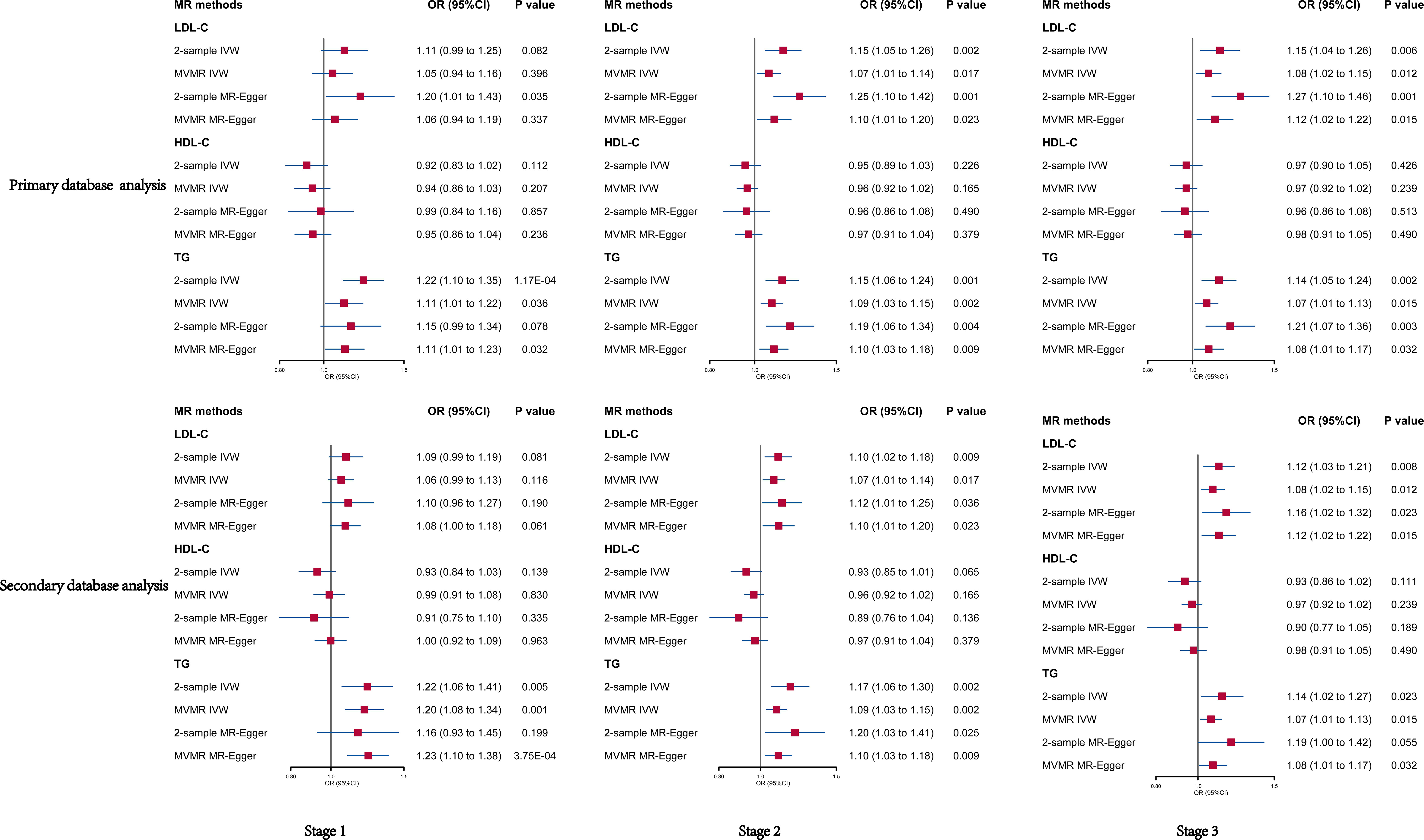
Figure 2 Univariable and multivariable MR of the effect of LDL-C, HDL-C and TG on psoriasis in primary and secondary data, respectively. OR, odds ratio; CI, confidence interval; LDL-C, Low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; TG, triglycerides.

Figure 3 The scatter plots of all MR test results in primary database and secondary database analysis, respectively represents the IVW, MR-Egger, Weighted median, and Weighted mode effect, respectively. The slope of the line represents the MR effect size. The red, purple, yellow, and black line. LDL-C, Low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; TG, triglycerides; SNP, single-nucleotide polymorphisms.
We observed a strong causal association between TG and psoriasis in primary database and secondary database analysis (Figure 2). In primary database analysis, IVW method showed TG (OR: 1.22, 95% CI: 1.10–1.35, p = 1.17E-04 in stage 1; OR: 1.15, 95% CI: 1.06–1.24, p = 0.001 in stage 2; OR: 1.14, 95% CI: 1.05–1.24, p = 0.002 in stage 3) had a significant association with psoriasis in each stage. In secondary database analysis, TG had a robust association with psoriasis (OR: 1.22, 95% CI: 1.06–1.41, p = 0.005 in stage 1; OR: 1.17, 95% CI: 1.06–1.30, p = 0.002 in stage 2; OR: 1.14, 95% CI: 1.02–1.27, p = 0.02 in stage 3) through IVW method (Table 1). The results of MR egger method, Weighted median and Weighted were still strongly association between TG and psoriasis (Supplementary Tables 3, 4). The sensitivity analysis showed a consistent trend (Figure 3). These results revealed the TG was a strong risk factor for psoriasis. Elevated TG levels may increase risk of psoriasis in European population.
In general, the results of MVMR analysis in primary and secondary database indicated that LDL-C and TG were risk factors for psoriasis, but HDL-C had no association with psoriasis. In primary database analysis, the results of IVW method showed that LDL-C (OR: 1.05, 95% CI: 0.99–1.25, p = 0.396 in stage 1; OR: 1.07, 95% CI: 1.01–1.14, p = 0.017 in stage 2; OR: 1.08, 95% CI: 1.02–1.15, p = 0.012 in stage 3) and TG (OR: 1.11, 95% CI: 1.01–1.22, p = 0.036 in stage 1; OR: 1.09, 95% CI: 1.03–1.15, p = 0.002 in stage 2; OR: 1.07, 95% CI: 1.01–1.13 p = 0.015 in stage 3) positively correlated with psoriasis, and there had no correlation between HDL-C and psoriasis (OR: 0.94, 95% CI: 0.86–1.03, p = 0.207 in stage 1; OR: 0.96 95% CI: 0.92–1.02, p = 0.165 in stage 2; OR: 0.97 95% CI: 0.92–1.02, p = 0.239 in stage 3) (Figure 2). The results of the secondary database analysis were consistent with the results of primary database analysis. The details were presented in Figure 1. In MR egger method of primary database and secondary database analysis, the association between LDL-C and TG with risk of psoriasis were still significant in each stage. It also showed that HDL-C was not significantly associated with psoriasis (Figure 2).
We performed a reversal MR analysis to explore the effect of psoriasis on blood lipid. We found a causal association between psoriasis and LDL-C (beta: -0.009, 95% CI: -0.016– -0.002, p = 0.009) and HDL-C (beta: -0.011, 95% CI: -0.021– -0.002, p = 0.016). The reverse causation analyses results between psoriasis and TG did not reach significance. The details are presented in Supplementary Table 5.
Using 2-sample and MVMR analysis, we found the strong genetic evidence that increasing the serum level of LDL-C and TG was associated with higher risk of psoriasis. The reverse MR revealed the status of psoriasis could affect the level of LDL-C and HDL-C. Overall, we found the genetic association between dyslipidemia and the risk of incident psoriasis were significant through MR method analysis.
In most clinical observational studies, dyslipidemia can be found in patients with psoriasis (28–30). As for previous clinical observational studies on LDL-C and psoriasis, elevated LDL-C levels were common. A big meta-analysis concluded that VLDL and LDL have been proved to be significantly higher in psoriatic patients (31). Other observational studies also reached the same conclusion (16, 32). In addition, in some observational studies of psoriatic arthritis (PSA), the serum TG, HDL-C, TC, and LDL-C have no significant association with psoriatic arthritis (33–35). The relationship between psoriasis and blood lipid is still unclear due to the evidence of publication bias and confounding factors in the environment. Our MR analysis results showed a strong genetic evidence show that the increase of LDL-C was associated with increased risk of psoriasis. Mehta et al. (36) have reported both the structure and function of lipoprotein were altered in psoriasis, with increased LDL-C particle concentration and deceased size. Orem A et al. (37) find a positive relationship between the serums level of autoantibodies against ox-LDL and severity of psoriasis. Of note, oxidized low-density lipoprotein (ox-LDL) is considered a marker to assess the severity of psoriasis. A study found an elevation in the ratio of anti-ox-LDL to ox-LDL could serve as a composite parameter reflecting the total oxidative lipoprotein burden in patients with psoriasis (38). Another prospective longitudinal cohort study has investigated a dependent association between elevated lectinlike ox-LDL receptor-1 and psoriasis severity (39). So far, the lipoprotein metabolism and dyslipidemia may be associated with development of psoriasis independent of hyperlipidemia status, but the actual underlying mechanisms are still unclear. We observed strong evidence for a causal link between LDL and psoriasis, and the reverse causality showed negative correlation. This may be associated with higher levels of LDL-C producing more ox-LDL. However, the LDL-C effects of mechanism by psoriasis required in-depth study.
For HDL-C, the relationship between it and psoriasis became more complex. An observational study shows the psoriasis patients have significantly higher very-low-density lipoprotein (VLDL) and HDL-C (12). However, in a population-based, cross-sectional study revealed the cholesterol, LDL-C, TG, and alanine aminotransferase are significantly higher in psoriasis. But the serum HDL-C has no association with psoriasis (13). In other large clinical observational studies, no clear relationship has been shown between the type of dyslipidemia and psoriasis. In a large cross-sectional study, there is no substantial association for total cholesterol (TC), TG and HDL-C (40). Another cross-sectional study showed the same results (16). We observed there was no causal relationship between HDL-C and psoriasis risks. Some studies have demonstrated that lipid function depend more on their structural and functional alterations than level of lipid in psoriasis (36, 41). Holzer M et al. (42) also reveal the lipid composition of HDL-C is altered from 15 patients with psoriasis. Even the efflux capacity of HDL-C and cholesterol were decreased. Furthermore, the similar changes of lipoprotein also occur in children with psoriasis (41). These findings indicate the transport defects of reverse cholesterol in HDL-C start early in life. Our MR results showed no significant difference between HDL-C levels and psoriasis. However, a positive reverse causality association was found between the psoriasis and HDL-C. Hereditarily, this suggested the level of HDL-C maybe have no correlation to the onset of psoriasis. But in turn, psoriasis may affect the structural and functional alterations of HDL-C.
Elevated TG is one of the diagnostic criteria of MetS, and it is also a risk factor for psoriasis comorbidities, such as cardiovascular diseases (43). A across-sectional controlled study demonstrates the serum cholesterol, TG and LDL-C were significantly higher between psoriasis patients with control group (20). Some observational studies reveal a similar result that TG is elevated in patients with psoriasis (13, 44). However, other studies find that TG have no significant difference between PsA group and controls group (45, 46). In addition to the LDL oxidation, S. Kaur et al. (47) suggested the TG level may be the mechanisms behind the psoriasis. But the relationship of pathogenesis between TG and psoriasis was still unclear due to lack of relevant research. In this MR study showed a strong positive correlation between TG and psoriasis, and provided genetic evidence for a link between TG and psoriasis. This risk would exist lifelong and does not change with the environment. Yet, in our MR study, there is no reverse causality association to be found between psoriasis and TG.
This study had several strengths. Firstly, two cohorts were served as primary data and secondary data in this study, including UKBB and GLGC. Furthermore, SVMR and MVMR methods were performed to analyze all SNPs of blood lipid in three stages to improve the credibility of the results. The MR analysis showed high consistent results, which added to high reliability relationship between blood lipid and psoriasis. Secondly, multiple MR analytical methods were severed in this study, and the consistent trends of sensitivity analysis indicated high reliability. Thirdly, the effects of diabetes and BMI confounders were excluded, and the results obtained were more reliable. Finally, MR-egger intercept analysis and F statistics were performed for all SNPs in blood lipid to exclude the effect of horizontal pleiotropy and weak instrumental variable bias. Overall, the analysis of multiple data and multiple MR methods improved the confidence of results.
However, there are some limitations in this study. Firstly, the definition of “psoriasis” was broad, included psoriasis subtypes such as psoriasis vulgaris, pustular psoriasis and psoriatic arthritis, among others. Secondly, the association of horizontal pleiotropy could not completely be ruled out, which could be mediated via other causal pathways. Thirdly, the sourcing of two cohorts from different databases may have resulted in data overlap, but larger cohorts may reduce this effect. Finally, the cohorts in this study were European population, caution was warranted before applying the findings to non-European populations.
MR findings suggest that an increase in the serum level of LDL-C and TG are potential causal risk factors for psoriasis. Mendelian randomization (MR) findings provide genetic evidence for causal link between psoriasis and blood lipid. It may be meaningful to monitor and control blood lipid level for a management of psoriasis patients in clinic.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
WL contributed to the conception and design of the study. YT contributed to the analysis and interpretation of data for the work, and revised the manuscript for the second time. Z-YJ contributed to the data analysis. Z-Y-OZ contributed to write the first draft of the manuscript. All authors contributed to the interpretation of the results and critical revision of the manuscript. All authors contributed to the article and approved the submitted version.
This work including the Rapid Service Fee was supported in part by the 1.3.5 project for disciplines of excellence, West China Hospital, Sichuan University (Grant. ZYJC21050) and the Key R&D project of Science and Technology Department of Sichuan Province (Grant. 2021YFG0306).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1174998/full#supplementary-material
UKBB, UK Biobank; GLGC, Global Lipid Genetics Consortium Results; GWAS, Genome-wide association study; SVMR, Single-variable Mendelian randomization; MVMR, Multivariable Mendelian randomization; LDL-C, Low-density lipoprotein cholesterol; OR, odds ratio; CI, Confidence interval; TG, triglycerides; HDL-C, high-density lipoprotein cholesterol; MR, Mendelian randomization; SNPs, Single-nucleotide polymorphisms; IVs, instrumental variables; T2D, Type 2 diabetes; IVW, Inverse variance weighted; MR–Egger, Mendelian randomization-Egger; VLDL, Very-low-density lipoprotein; TC, cholesterol; PsA, Psoriatic arthritis; ox-LDL, Oxidized low-density lipoprotein.
1. Spah F. Inflammation in atherosclerosis and psoriasis: common pathogenic mechanisms and the potential for an integrated treatment approach. Br J Dermatol (2008) 159 Suppl 2:10–7. doi: 10.1111/j.1365-2133.2008.08780.x
2. Parisi R, Symmons DP, Griffiths CE, Ashcroft DM. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol (2013) 133(2):377–85. doi: 10.1038/jid.2012.339
3. Chandran V, Raychaudhuri SP. Geoepidemiology and environmental factors of psoriasis and psoriatic arthritis. J Autoimmun (2010) 34(3):J314–21. doi: 10.1016/j.jaut.2009.12.001
4. Langan SM, Seminara NM, Shin DB, Troxel AB, Kimmel SE, Mehta NN, et al. Prevalence of metabolic syndrome in patients with psoriasis: a population-based study in the united kingdom. J Invest Dermatol (2012) 132(3 Pt 1):556–62. doi: 10.1038/jid.2011.365
5. Kamiya K, Kishimoto M, Sugai J, and Komine M, Ohtsuki M. Risk factors for the development of psoriasis. Int J Mol Sci (2019) 20(18):4347. doi: 10.3390/ijms20184347
6. Lee EB, Wu KK, Lee MP, Bhutani T, Wu JJ. Psoriasis risk factors and triggers. Cutis (2018) 102(5S):18–20.
7. Elmets CA, Leonardi CL, Davis DMR, Gelfand JM, Lichten J, Mehta NN, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with awareness and attention to comorbidities. J Am Acad Dermatol (2019) 80(4):1073–113. doi: 10.1016/j.jaad.2018.11.058
8. Takeshita J, Grewal S, Langan SM, Mehta NN, Ogdie A, Voorhees Van AS, et al. Psoriasis and comorbid diseases: epidemiology. J Am Acad Dermatol (2017) 76(3):377–90. doi: 10.1016/j.jaad.2016.07.064
9. Peralta C, Hamid P, Batool H, Achkar Al Z, Maximus P. Psoriasis and metabolic syndrome: comorbidities and environmental and therapeutic implications. Cureus (2019) 11(12):e6369. doi: 10.7759/cureus.6369
10. Murray ML, Bergstresser PR, Adams-Huet B, Cohen JB. Relationship of psoriasis severity to obesity using same-gender siblings as controls for obesity. Clin Exp Dermatol (2009) 34(2):140–4. doi: 10.1111/j.1365-2230.2008.02791.x
11. Corbetta S, Angioni R, Cattaneo A, Beck-Peccoz P, Spada A. Effects of retinoid therapy on insulin sensitivity, lipid profile and circulating adipocytokines. Eur J Endocrinol (2006) 154(1):83–6. doi: 10.1530/eje.1.02057
12. Mallbris L, Granath F, Hamsten A, Stahle M. Psoriasis is associated with lipid abnormalities at the onset of skin disease. J Am Acad Dermatol (2006) 54(4):614–21. doi: 10.1016/j.jaad.2005.11.1079
13. Koebnick C, Black MH, Smith N, Der-Sarkissian JK, Porter AH, Jacobsen SJ, et al. The association of psoriasis and elevated blood lipids in overweight and obese children. J Pediatr (2011) 159(4):577–83. doi: 10.1016/j.jpeds.2011.03.006
14. Itani S, Arabi A, Harb D, Hamzeh D, Kibbi AG. High prevalence of metabolic syndrome in patients with psoriasis in Lebanon: a prospective study. Int J Dermatol (2016) 55(4):390–5. doi: 10.1111/ijd.12811
15. Parodi A, Aste N, Calvieri C, Cantoresi F, Carlesimo M, Fabbri P, et al. Metabolic syndrome prevalence in psoriasis: a cross-sectional study in the Italian population. Am J Clin Dermatol (2014) 15(4):371–7. doi: 10.1007/s40257-014-0074-8
16. Lin IC, Heck JE, Chen L, Feldman SR. Psoriasis severity and cardiometabolic risk factors in a representative US national study. Am J Clin Dermatol (2021) 22(5):719–30. doi: 10.1007/s40257-021-00600-z
17. Miller IM, Skaaby T, Ellervik C, Jemec GB. Quantifying cardiovascular disease risk factors in patients with psoriasis: a meta-analysis. Br J Dermatol (2013) 169(6):1180–7. doi: 10.1111/bjd.12490
18. Fernandez-Armenteros JM, Gomez-Arbones X, Buti-Soler M, Sanmartin-Novell Betriu-Bars A, Ortega-Bravo M. Psoriasis, metabolic syndrome and cardiovascular risk factors. a population-based study. J Eur Acad Dermatol Venereol (2019) 33(1):128–35. doi: 10.1111/jdv.15159
19. Hao Y, Zhu YJ, Zou S, Zhou P, Hu YW, Zhao QX, et al. Metabolic syndrome and psoriasis: mechanisms and future directions. Front Immunol (2021) 12:711060. doi: 10.3389/fimmu.2021.711060
20. Bajaj DR, Mahesar SM, Devrajani BR, Iqbal MP. Lipid profile in patients with psoriasis presenting at liaquat university hospital Hyderabad. J Pak Med Assoc (2009) 59(8):512–5.
21. Davies NM, Holmes MV, Davey Smith G. Reading mendelian randomisation studies: a guide, glossary, and checklist for clinicians. BMJ (2018) 362:k601. doi: 10.1136/bmj.k601
22. Skrivankova VW, Richmond RC, Woolf BAR, Yarmolinsky J, Davies NM, Swanson SA, et al. Strengthening the reporting of observational studies in epidemiology using mendelian randomization: the STROBE-MR statement. JAMA (2021) 326(16):1614–21. doi: 10.1001/jama.2021.18236
23. Richardson TG, Sanderson E, Palmer TM, Ala-Korpela M, Ference BA, Smith Davey G, et al. Evaluating the relationship between circulating lipoprotein lipids and apolipoproteins with risk of coronary heart disease: a multivariable mendelian randomisation analysis. PloS Med (2020) 17(3):e1003062. doi: 10.1371/journal.pmed.1003062
24. Willer CJ, Schmidt EM, Sengupta S, Peloso GM, Gustafsson S, Kanoni S, et al. Discovery and refinement of loci associated with lipid levels. Nat Genet (2013) 45(11):1274–83. doi: 101038/ng2797
25. Tsoi LC, Spain SL, Knight J, Ellinghaus E, Stuart PE, Capon F, et al. Identification of 15 new psoriasis susceptibility loci highlights the role of innate immunity. Nat Genet (2012) 44(12):1341–8. doi: 10.1038/ng.2467
26. Jian Z, Yuan C, Ma Y. Blood pressure mediated the effects of urinary uromodulin levels on myocardial infarction: a mendelian randomization study. Hypertension (2022) 79(11):2430–8. doi: 10.1161/HYPERTENSIONAHA.122.19670
27. Jian Z, Huang Y, He Y, Jin X, Li H, Li S, et al. Genetically predicted lifelong circulating 25(OH)D levels are associated with serum calcium levels and kidney stone risk. J Clin Endocrinol Metab (2022) 107(3):e1159–66. doi: 10.1210/clinem/dgab758
28. Santos M, Fonseca HM, Jalkh AP, Gomes GP, Cavalcante Ade S. Obesity and dyslipidemia in patients with psoriasis treated at a dermatologic clinic in manaus. Bras Dermatol (2013) 88(6):913–6. doi: 10.1590/abd1806-4841.20132090
29. Gyldenlove M, Jensen P, Linneberg A, Thyssen JP, Zachariae C, Hansen PR, et al. Psoriasis and the framingham risk score in a Danish hospital cohort. Int J Dermatol (2014) 53(9):1086–90. doi: 10.1111/ijd.12196
30. Kimball AB, Szapary P, Mrowietz U, Reich K, Langley RG, You Y, et al. Underdiagnosis and undertreatment of cardiovascular risk factors in patients with moderate to severe psoriasis. J Am Acad Dermatol (2012) 67(1):76–85. doi: 10.1016/j.jaad.2011.06.035
31. Nowowiejska J, Baran A, Flisiak I. Aberrations in lipid expression and metabolism in psoriasis. Int J Mol Sci (2021) 22(12):6561. doi: 10.3390/ijms22126561
32. Al Harthi F, Huraib GB, Zouman A, Arfin M, Tariq M, Al-Asmari A, et al. Apolipoprotein e gene polymorphism and serum lipid profile in Saudi patients with psoriasis. Dis Markers (2014) 2014:239645. doi: 10.1155/2014/239645
33. Caso F, Puente Del A, Oliviero F, Peluso R, Girolimetto N, Bottiglieri P, et al. Metabolic syndrome in psoriatic arthritis: the interplay with cutaneous involvement. evidences from literature and a recent cross-sectional study. Clin Rheumatol (2018) 37(3):579–86. doi: 101007/s10067-017-3975-0
34. Gulati AM, Semb AG, Rollefstad S, Romundstad PR, Kavanaugh A, Gulati S, et al. On the HUNT for cardiovascular risk factors and disease in patients with psoriatic arthritis: population-based data from the nord-trondelag health study. Ann Rheum Dis (2016) 75(5):819–24. doi: 10.1136/annrheumdis-2014-206824
35. Wang Y, Ding L, Chen J, Zhang L, Yang M, Liu Z, et al. Risk factors for progression from subclinical to clinical phase of psoriatic arthritis: a case-control study. Rheumatol Ther (2021) 8(1):585–97. doi: 10.1007/s40744-021-00295-y
36. Mehta NN, Li R, Krishnamoorthy P, Yu Y, Farver W, Rodrigues A, et al. Abnormal lipoprotein particles and cholesterol efflux capacity in patients with psoriasis. Atherosclerosis (2012) 224(1):218–21. doi: 10.1016/j.atherosclerosis.2012.06.068
37. Orem A, Cimsit G, Deger O, Orem C, Vanizor B. The significance of autoantibodies against oxidatively modified low-density lipoprotein (LDL) in patients with psoriasis. Clin Chim Acta (1999) 284(1):81–8. doi: 10.1016/S0009-8981(99)00062-5
38. Sunitha S, Rajappa M, Thappa Mohan D, Chandrashekar L, Munisamy M, Revathy G, et al. Is the ratio of antibodies against oxidized LDL to oxidized LDL an indicator of cardiovascular risk in psoriasis? Oman Med J (2016) 31(5):390–3. doi: 10.5001/omj.2016.78
39. Dey AK, Gaddipati R, Elnabawi YA, Ongstad E, Goyal A, Chung JH, et al. Association between soluble lectinlike oxidized low-density lipoprotein receptor-1 and coronary artery disease in psoriasis. JAMA Dermatol (2020) 156(2):151–7. doi: 10.1001/jamadermatol.2019.3595
40. Snekvik I, Nilsen TIL, Romundstad PR, Saunes M. Psoriasis and cardiovascular disease risk factors: the HUNT study, Norway. J Eur Acad Dermatol Venereol (2018) 32(5):776–82. doi: 10.1111/jdv.14835
41. Tom WL, Playford MP, Admani S, Natarajan B, Joshi AA, Eichenfield LF, et al. Characterization of lipoprotein composition and function in pediatric psoriasis reveals a more atherogenic profile. J Invest Dermatol (2016) 136(1):67–73. doi: 10.1038/JID.2015.385
42. Holzer M, Wolf P, Curcic S, Birner-Gruenberger R, Weger W, Inzinger M, et al. Psoriasis alters HDL composition and cholesterol efflux capacity. J Lipid Res (2012) 53(8):1618–24. doi: 10.1194/jlr.M027367
43. Nordestgaard BG, Varbo A. Triglycerides and cardiovascular disease. Lancet (2014) 384(9943):626–35. doi: 10.1016/S0140-6736(14)61177-6
44. Miao C, Li J, Li Y, Zhang X. Obesity and dyslipidemia in patients with psoriasis: a case-control study. Med (Baltimore) (2019) 98(31):e16323. doi: 10.1097/MD.0000000000016323
45. Pietrzak A, Chabros P, Grywalska E, Kicinski P, Pietrzak-Franciszkiewicz K, Krasowska D, et al. Serum lipid metabolism in psoriasis and psoriatic arthritis - an update. Arch Med Sci (2019) 15(2):369–75. doi: 10.5114/aoms.2018.74021
46. Ma C, Schupp CW, Armstrong EJ, Armstrong AW. Psoriasis and dyslipidemia: a population-based study analyzing the national health and nutrition examination survey (NHANES). J Eur Acad Dermatol Venereol (2014) 28(8):1109–12. doi: 10.1111/jdv.12232
Keywords: blood lipid, psoriasis, Mendelian randomization, risk factor, GWAS - genome-wide association study
Citation: Zhang Z-Y-O, Jian Z-Y, Tang Y and Li W (2023) The relationship between blood lipid and risk of psoriasis: univariable and multivariable Mendelian randomization analysis. Front. Immunol. 14:1174998. doi: 10.3389/fimmu.2023.1174998
Received: 27 February 2023; Accepted: 07 June 2023;
Published: 22 June 2023.
Edited by:
Fusheng Zhou, Anhui Medical University, ChinaReviewed by:
Alan Remaley, National Institutes of Health (NIH), United StatesCopyright © 2023 Zhang, Jian, Tang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Li, bGl3ZWloeF9oeHl5QHNjdS5lZHUuY24=; Yin Tang, dGFuZ3lpbkBzY3UuZWR1LmNu
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.