
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Immunol., 09 May 2023
Sec. Cancer Immunity and Immunotherapy
Volume 14 - 2023 | https://doi.org/10.3389/fimmu.2023.1171981
This article is part of the Research TopicImmune-checkpoint Inhibitors in Anti-cancer Armamentarium: a Double-edged Sword in Risk of Developing Autoimmunity and Immune-related Adverse EffectsView all 10 articles
Background: Immune checkpoint inhibitor (ICI) treatment has become important for treating various cancer types, including Hodgkin’s lymphoma. However, ICI can overstimulate the immune system, leading to a broad range of immunological side effects, known as immune-related adverse events (irAEs). Here, we report a case of optic neuropathy caused by pembrolizumab.
Case presentation: A patient with Hodgkin’s lymphoma received pembrolizumab every three weeks. Twelve days after the sixth cycle of pembrolizumab, the patient was admitted to the emergency department with blurred vision, visual field impairment and altered color perception affecting the right eye. The diagnosis of immune-related optic neuropathy was established. Pembrolizumab was stopped permanently and high-dose steroid treatment was immediately started. This emergency treatment led to a satisfactory binocular vision and an improvement of visual acuity testing results. After another 7 months, the left eye was affected with the same symptoms. At this time, only an extended immunosuppressive therapy consisting of high-dose steroid treatment, plasmapheresis, immunoglobulin treatment, retrobulbar injection of steroids and mycophenolate mofetil, successfully reduced the symptoms.
Conclusions: This case highlights the need for prompt recognition and treatment of rare irAEs, such as optic neuropathy. Urgent treatment with initial high-dose steroid treatment is required to avoid persistent loss of visual acuity. Options for further treatment are mainly based on small case series and case reports. In our case, a retrobulbar injection of steroids in combination with mycophenolate mofetil showed significant success in treating steroid-refractory optic neuropathy.
Immune checkpoint inhibitor (ICI) treatment has established its relevance in refractory and relapsed Hodgkin’s lymphoma (1, 2). While being effective, ICI treatment is associated with a broad spectrum of immune-related adverse events (irAEs) (3–5). Thereby, ocular irAEs are rare side effects, but can have a major impact on the quality of life in the case of impaired vision or complete loss of vision, respectively (3–7). In this report, we present a challenging case of pembrolizumab-induced bilateral optic neuropathy. It first occurred in the right eye, which was successfully treated with high-dose steroids. Seven months later, the left eye was affected too and despite high-dose steroids, plasmapheresis and immunoglobuline treatment, no persistent clinical benefit was achieved. Only the administration of a retrobulbar injection of steroids in combination with mycophenolate mofetil led to a sustained success.
A 67-year-old woman was diagnosed with Hodgkin’s lymphoma (initial disease stage according to Ann Arbor classification: IIA, without risk factors) which continued to progress after initial standard treatment with ABVD (liposomal doxorubicin, bleomycin, vinblastine, dacarbazine). Due to disease progression, two cycles of ICE (ifosfamide, carboplatin, etoposide) were applied, followed by brentuximab vedotin which had to be stopped after four months due to polyneuropathy. Stem cell transplantation was not possible due to a relevant pre-existing medical condition (congestive heart failure). Pembrolizumab was then started in this refractory situation. It was administered every three weeks with a dosage of 200mg intravenously. Two months after starting pembrolizumab a complete remission was achieved.
Twelve days after the sixth cycle of pembrolizumab, the patient presented herself to the emergency department with blurred vision, visual field impairment and altered color perception affecting the right eye. Fundus examination showed papilledema in the right temporal optic disc (Figure 1A). Further testing revealed a visual acuity of 0.05 on the right side and a horizontal visual field loss (Figure 1B). The brain magnetic resonance imaging (MRI) showed no abnormality. The cerebrospinal fluid (CSF) analysis including extensive infectious parameters (including Borrelia species, Flavirus, Treponema pallidum, different herpes virus, measles and mumps virus), neuronal and paraneoplastic antibodies (including anti-amphiphysin antibodies, anti-Hu/anti-Yo/anti-Ri/anti-Ma/anti-Tr antibodies, anti-SOX1 antibodies, anti-ZiC4 antibodies, anti-collapsin-responsive mediator protein 5 antibodies [CRMP 5], anti-glutamic acid decarboxylase antibodies [anti-GAD], anti-angiotensin converting enzyme [ACE] antibodies), oligoclonal bands, malignant cells, flow cytometry remained without any relevant findings, despite a slightly elevated total protein level. Moreover, aquaporin-4 immunoglobulin G antibodies (ACQ4) as well as myelin oligodendrocyte glycoprotein antibodies (MOG) were not pathologically elevated. Considering these diagnostic results, the patient was diagnosed with an immune-related optic neuropathy due to PD-1 blockade with pembrolizumab. High-dose steroid treatment was immediately initiated (125mg intravenous methylprednisolone for five days), followed by tapering of prednisone over a period of four months. The symptoms showed partial regression (visual acuity of 0.3). Additional treatment was not necessarily due to a satisfactory clinical outcome regarding binocular vision. The treatment with pembrolizumab was stopped permanently. There was no sign of tumor activity.
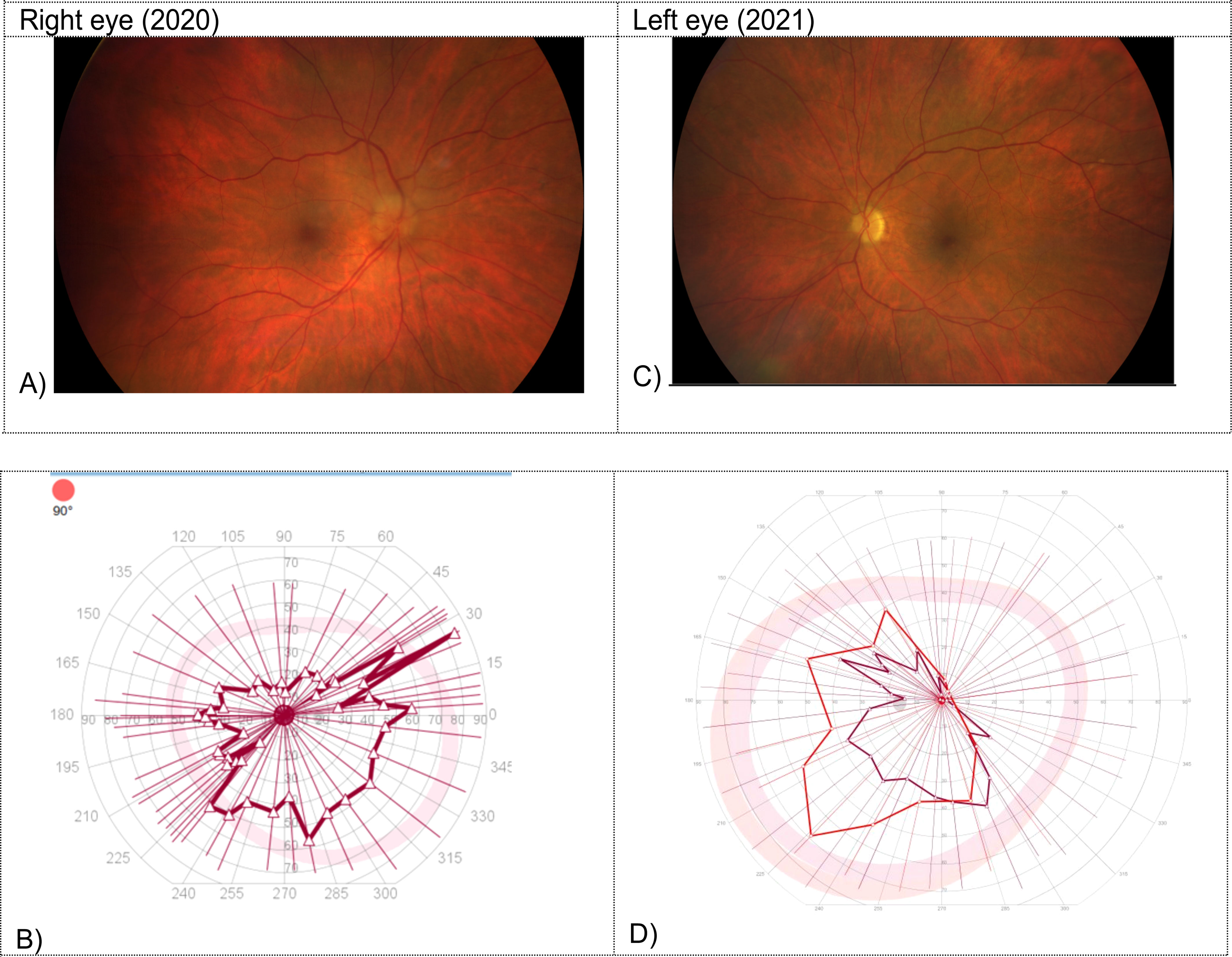
Figure 1 Fundus examination and visual field testing on the right in 2020 (A, B) and on the left side in 2021 (C, D). (A) Papilledema in the right temporal optic disc. (B) Horizontal visual field loss in the visual field testing on the right side. (C) Sectorial papilledema in the left optic disc. (D) Horizontal visual field loss in the visual field testing on the left side.
Seven months after the initial presentation with optic neuropathy on the right side, the patient was admitted to the hospital again with complaints of blurred vision, visual field impairment and altered color perception, affecting now the left eye. Fundus examination revealed sectorial papilledema in the left optic disc (Figure 1C). A visual acuity of 0.4 in the left side (preliminary recording: 0.8) with horizontal visual field loss was documented (Figure 1D). The brain MRI revealed an increasing cerebrospinal fluid (CSF) signal along the left optic nerve with continued minimal contrast uptake (Figure 2). Again, an extensive examination of the CSF (same parameters as in the initial examination) showed no abnormal finding, despite a slightly elevated total protein level. In the imaging of the whole body, there was no signs of lymphoma activity. The patient was diagnosed with immune-related optic neuropathy on the left side. Treatment with high-dose steroids was immediately started (500mg intravenous methylprednisolone). In addition, plasmapheresis was performed five times after a deterioration in the symptoms as a result of a reduced high-dose steroid treatment (250mg intravenous methylprednisolone). The symptoms improved and the steroid dosage was gradually reduced. At a dosage of 80mg prednisone (1mg per kg bodyweight) the symptoms worsened again (visual acuity 0.2) and the irAE was interpreted as steroid-refractory. Immunoglobulins were intravenously administered for five days. Due to the absence of relevant clinical improvement, a retrobulbar injection of steroids was administered and treatment with mycophenolate mofetil (1000mg peroral twice daily) was started. This treatment improved the symptoms so that the steroid treatment could finally be stopped after six months of tapering. The dosage of mycophenolate mofetil could gradually be reduced (after 12 months: current dosage of 250mg peroral twice daily) without recurrence of symptoms. A visual acuity of 0.8 could be measured on the left side, corresponding to the baseline assessment. Six months after affecting the left eye the patient is independent again in everyday life. So far, there is no sign of tumor activity.
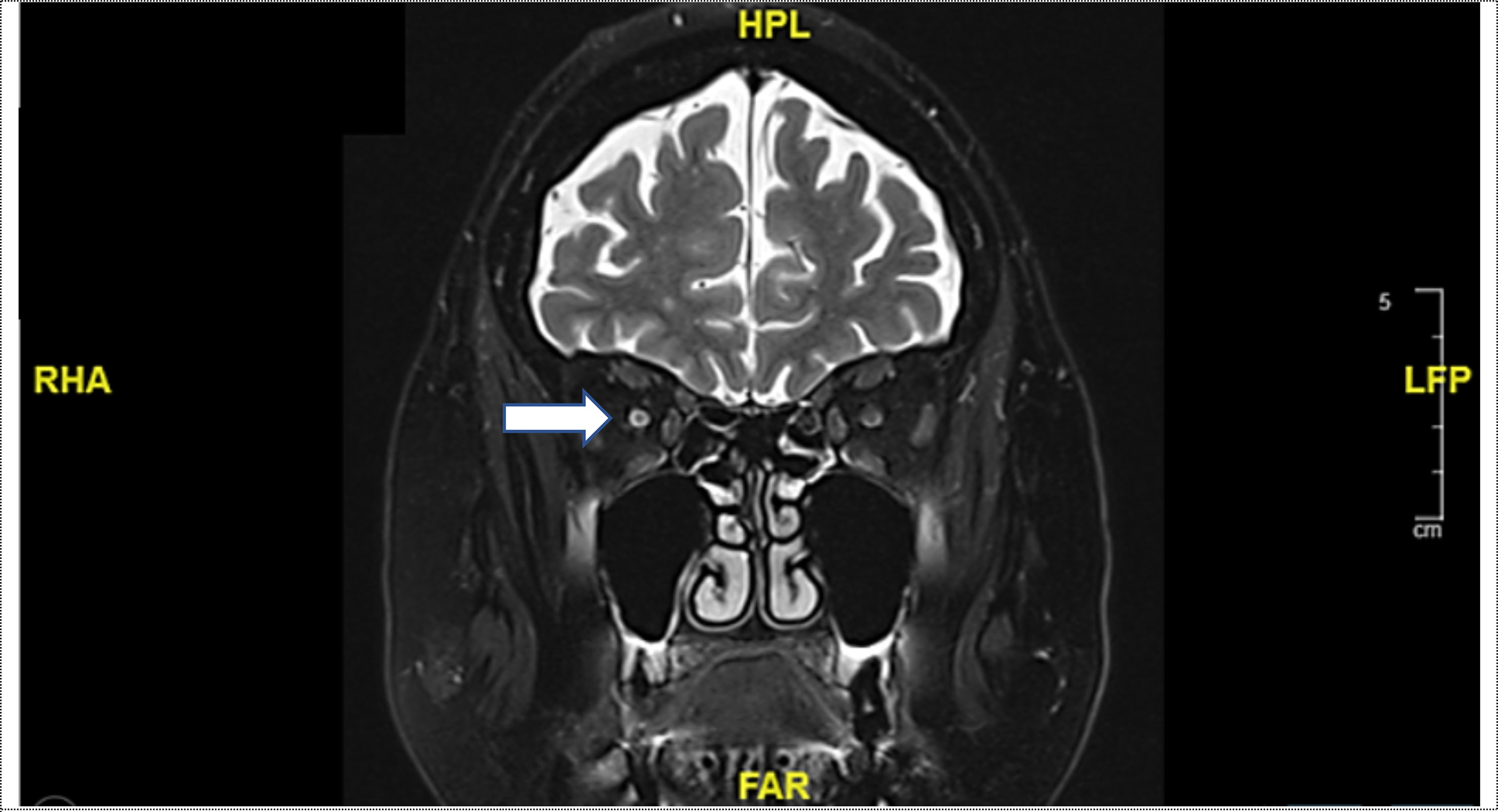
Figure 2 Coronal T2-weighted magnetic resonance imaging (MRI): Increasing cerebrospinal fluid (CSF) signal along the left optic nerve with continued minimal contrast uptake (white arrow).
Ocular side effects are rare after ICI treatment (3–7). They occur in approximately 3% of patients treated with ICI, according to the FDA Adverse Event Reporting System (FAERS) pharmacovigilance database (7). It is believed that this rare occurrence is primarily due to the immune-privileged location (8). However, this can be nullified under certain conditions leading to ocular irAEs (4). Hereby, the combination of anti-CTLA-4 and PD-(L)1 is associated with a higher risk of the occurrence of these side effects compared to a monotherapy of ICI (7). The spectrum of ocular irAEs ranges from the most common ocular side effects, including ophthalmoplegia, uveitis and keratoconjunctivitis sicca (dry eye syndrome), to optic neuropathy which is much rarer (3–7, 9). In our case report, we highlight the rare complication of ICI-treatment leading to an optic neuropathy.
The clinical presentation of ICI-induced optic neuropathy is not entirely consistent with the classic triad (unilateral decreased vision, dyschromatopsia, pain), known in optic neuropathy associated with multiple sclerosis. The leading symptoms of our patient were complaints of blurred vision, visual field impairment and altered color perception which occurred with a time delay on the right, then seven months later on the left side. Pain was not reported. This is also supported by a study with 11 patients, whereas the vast majority showed painless decreased vision, floaters or both (10). Sixty-four percent of these patients showed bilateral optic neuropathy (10). Optic neuropathy occurs on average 10-20 weeks after the initiation of ICI treatment, but can also have the potential of later occurrence (6, 9, 10). The diagnosis requires observed abnormalities in optic nerve enhancement on the MRI and the clinical presentation which is consistent with the diagnosis of an optic neuropathy (3–5, 11). Therefore, prompt involvement of an ophthalmologist is mandatory (3–5). To exclude other aetiologies of optic neuropathy, such as neuromyelitis optica, autoimmune diseases, infectious or parainfectious causes, an extensive diagnostic pathway is necessary, including laboratory examinations of the blood as well as cerebral fluid analysis (3–5, 12).
After the diagnostics, the initial treatment has to be started immediately. Steroid treatment remains the backbone of this treatment, mentioned in the publications and guidelines cited below (3–6, 9, 10, 13–20) (Table 1). Options for further treatment are mainly based on case series and case reports. In the literature, the following additional interventions are described: plasmapheresis (n=4), intravenous immunoglobulin (n=2), infliximab (n=1), rituximab (n=1), mycophenolate mofetil (MPA) (n=1) (10, 13, 16, 20) (Table 1).
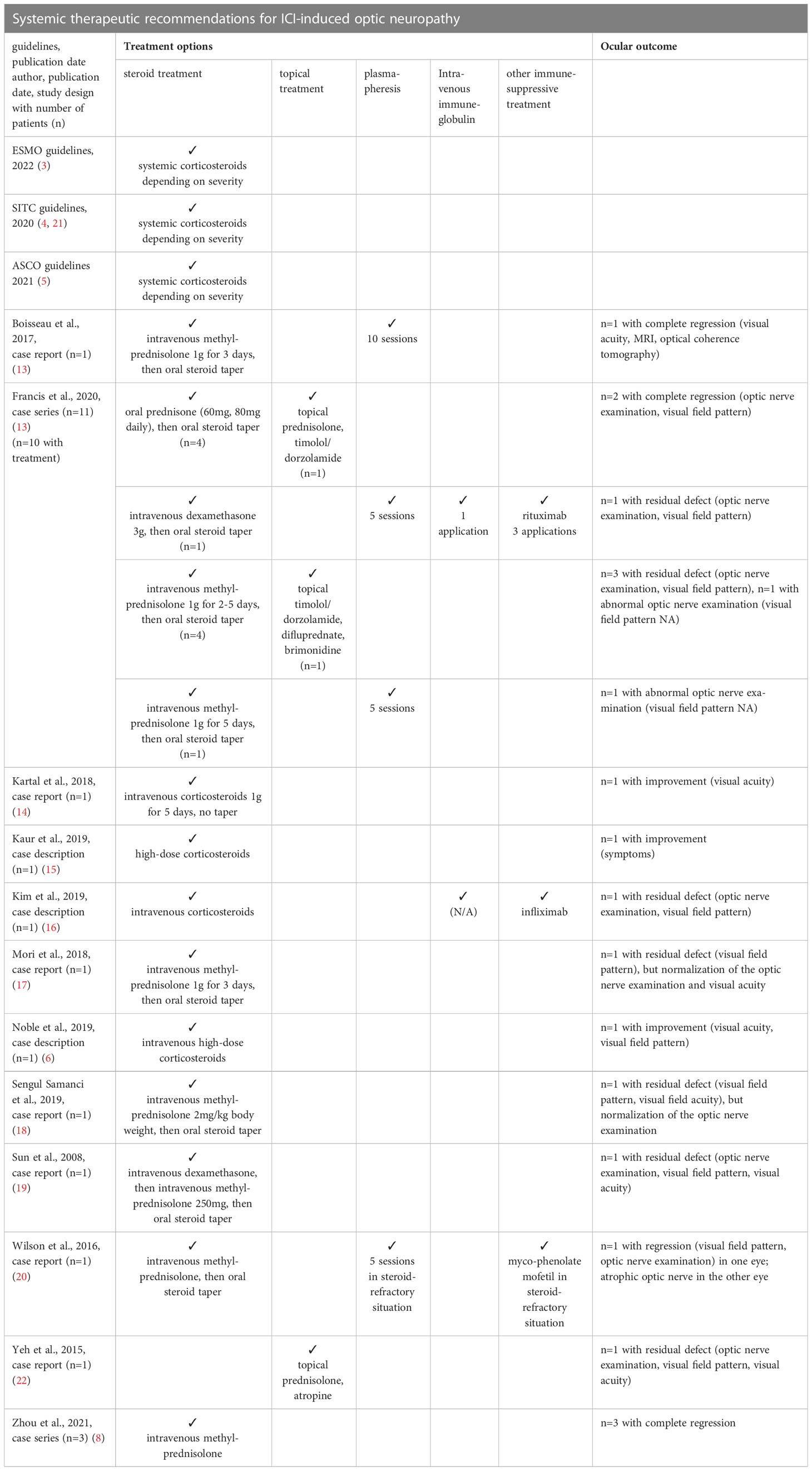
Table 1 Systemic therapeutic recommendations for ICI-induced optic neuropathy in the literature, according to the guidelines and publications (N/A = not applicable).
MPA was used in our patient due to its convincing drug characteristics, including the good tolerability and safety profile as well as its simple oral intake (23, 24). MPA reversibly inhibits the inosine monophosphate dehydrogenase which is involved in guanosine nucleotide synthesis, on which the T and B lymphocytes are exclusively dependent for proliferation (25). In addition, MPA also affects intracellular signalling pathways for lymphocyte metabolic programming (25). The efficacy of MPA is also described in optic neuropathy associated with autoimmune inflammatory disorder (23, 24). Moreover, MPA is the only drug described in steroid-refractory optic neuropathy (20). However, further investigations into the agents themselves and into the optimal sequence of these immunosuppressive interventions are required.
In conclusion, we report a case with severe optic neuropathy due to PD-1 blockade showing a bilateral involvement, first of the right and then some months later of the left eye. On the right side, prompt high-dose steroid treatment showed partial success. On the left side, despite the initiation of high-dose steroid treatment at the beginning, followed by plasmapheresis and immunoglobulin treatment, the situation could only be stabilized with a retrobulbar injection of steroids and the start of treatment with mycophenolate mofetil (MPA). We emphasize the need of prompt recognition, involvement of ophthalmologists and necessity of urgent treatment to avoid substantial morbidity.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethical review and approval were not required for the study on human participants in accordance with the local legislation and institutional requirements. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
ED, AZ, PM, HL participated in the care of the patient. ED drafted the manuscript. AZ, PM and HL have revised the manuscript. All authors contributed to the article and approved the submitted version.
AZ: received consulting/advisor fees from Bristol-Myers Squibb, Merck Sharp & Dohme, Hoffmann–La Roche, NBE Therapeutics, Secarna, ACM Pharma, and Hookipa, and maintains further non-commercial research agreements with Secarna, Roche, Vectorbiopharma, T3 Pharma and Bright Peak Therapeutics. HL: received travel grants and consultant fees from Bristol-Myers Squibb, Alector, and MSD. HL received research support from Bristol-Myers Squibb, Novartis, GlycoEra and Palleon Pharmaceuticals.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Chen R, Zinzani PL, Lee HJ, Armand P, Johnson NA, Brice P, et al. Pembrolizumab in relapsed or refractory Hodgkin lymphoma: 2-year follow-up of KEYNOTE-087. Blood. (2019) 134(14):1144–53. doi: 10.1182/blood.2019000324
2. Kuruvilla J, Ramchandren R, Santoro A, Paszkiewicz-Kozik E, Gasiorowski R, Johnson NA, et al. Pembrolizumab versus brentuximab vedotin in relapsed or refractory classical Hodgkin lymphoma (KEYNOTE-204): an interim analysis of a multicentre, randomised, open-label, phase 3 study. Lancet Oncol (2021) 22(4):512–24. doi: 10.1016/S1470-2045(21)00005-X
3. Haanen J, Obeid M, Spain L, Carbonnel F, Wang Y, Robert C, et al. Management of toxicities from immunotherapy: ESMO clinical practice guideline for diagnosis, treatment and follow-up☆. Ann Oncol (2022) 33(12):1217–38. doi: 10.1016/j.annonc.2022.10.001
4. SITC immunotherapy.pdf . Available at: http://dx.doi.org/jitc-2021-002435.
5. Schneider BJ, Naidoo J, Santomasso BD, Lacchetti C, Adkins S, Anadkat M, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: ASCO guideline update. J Clin Orthod (2021) 39(36):4073–126. doi: 10.1200/JCO.21.01440
6. Noble CW, Gangaputra SS, Thompson IA, Yuan A, Apolo AB, Lee J-M, et al. Ocular adverse events following use of immune checkpoint inhibitors for metastatic malignancies. Ocul Immunol Inflamm (2020) 28(6):854–9. doi: 10.1080/09273948.2019.1583347
7. Bomze D, Meirson T, Hasan Ali O, Goldman A, Flatz L, Habot-Wilner Z. Ocular adverse events induced by immune checkpoint inhibitors: a comprehensive pharmacovigilance analysis. Ocul Immunol Inflamm (2022) 30(1):191–7. doi: 10.1080/09273948.2020.1773867
8. Zhou R, Caspi RR. Ocular immune privilege. F1000 Biol Rep (2010) 2. http://dx.doi.org/10.3410/B2-3. doi: 10.3410/B2-3
9. Zhou L, Wei X. Ocular immune-related adverse events associated with immune checkpoint inhibitors in lung cancer. Front Immunol (2021) 12:701951. doi: 10.3389/fimmu.2021.701951
10. Francis JH, Jaben K, Santomasso BD, Canestraro J, Abramson DH, Chapman PB, et al. Immune checkpoint inhibitor-associated optic neuritis. Ophthalmology. (2020) 127(11):1585–9. doi: 10.1016/j.ophtha.2020.05.003
11. Yu CW, Yau M, Mezey N, Joarder I, Micieli JA. Neuro-ophthalmic complications of immune checkpoint inhibitors: a systematic review. Eye Brain. (2020) 12:139–67. doi: 10.2147/EB.S277760
12. Hoorbakht H, Bagherkashi F. Optic neuritis, its differential diagnosis and management. Open Ophthalmol J (2012) 6:65–72. doi: 10.2174/1874364101206010065
13. Boisseau W, Touat M, Berzero G, Savatovsky J, Marabelle A, Touitou V, et al. Safety of treatment with nivolumab after ipilimumab-related meningoradiculitis and bilateral optic neuropathy. Eur J Cancer. (2017) 83:28–31. doi: 10.1016/j.ejca.2017.05.036
14. Kartal Ö, Ataş E. Bilateral optic neuritis secondary to nivolumab therapy: a case report. Medicina (2018) 54(5). doi: 10.3390/medicina54050082
15. Kaur A, Doberstein T, Amberker RR, Garje R, Field EH, Singh N. Immune-related adverse events in cancer patients treated with immune checkpoint inhibitors: a single-center experience. Med . (2019) 98(41):e17348. doi: 10.1097/MD.0000000000017348
16. Kim JM, Materin MA, Sznol M, Kluger HM, Weiss S, Chow J, et al. Ophthalmic immune-related adverse events of immunotherapy: a single-site case series. Ophthalmology. (2019) 126(7):1058–62. doi: 10.1016/j.ophtha.2019.01.031
17. Mori S, Kurimoto T, Ueda K, Enomoto H, Sakamoto M, Keshi Y, et al. Optic neuritis possibly induced by anti-PD-L1 antibody treatment in a patient with non-small cell lung carcinoma. Case Rep Ophthalmol (2018) 9(2):348–56. doi: 10.1159/000491075
18. Sengul Samanci N, Ozan T, Çelik E, Demirelli FH. Optic neuritis related to atezolizumab treatment in a patient with metastatic non-Small-Cell lung cancer. JCO Oncol Pract (2020) 16(2):96–8. doi: 10.1200/JOP.19.00438
19. Sun J, Schiffman J, Raghunath A, Ng Tang D, Chen H, Sharma P. Concurrent decrease in IL-10 with development of immune-related adverse events in a patient treated with anti-CTLA-4 therapy. Cancer Immun (2008) 8:9.
20. Wilson MA, Guld K, Galetta S, Walsh RD, Kharlip J, Tamhankar M, et al. Acute visual loss after ipilimumab treatment for metastatic melanoma. J Immunother Cancer. (2016) 4:66. doi: 10.1186/s40425-016-0170-9
21. Maus MV, Alexander S, Bishop MR, Brudno JN, Callahan C, Davila ML, et al. Society for immunotherapy of cancer (SITC) clinical practice guideline on immune effector cell-related adverse events. J Immunother Cancer (2020) 8(2). doi: 10.1136/jitc-2020-001511
22. Yeh OL, Francis CE. Ipilimumab-associated bilateral optic neuropathy. J Neuroophthalmol. (2015) 35(2):144–7. doi: 10.1097/WNO.0000000000000217
23. Huh S-Y, Kim S-H, Hyun J-W, Joung A-R, Park MS, Kim B-J, et al. Mycophenolate mofetil in the treatment of neuromyelitis optica spectrum disorder. JAMA Neurol (2014) 71(11):1372–8. doi: 10.1001/jamaneurol.2014.2057
24. Wang Y, Ma J, Chang H, Zhang X, Yin L. Efficacy of mycophenolate mofetil in the treatment of neuromyelitis optica spectrum disorders: an update systematic review and meta -analysis. Mult Scler Relat Disord (2021) 55:103181. doi: 10.1016/j.msard.2021.103181
Keywords: immune-related adverse event(s), optic neuropathy, immune checkpoint inhibitor (ICI), neuropathic, PD-1/L1, case report
Citation: Daetwyler E, Zippelius A, Meyer P and Läubli H (2023) Pembrolizumab-induced optic neuropathy – a case report. Front. Immunol. 14:1171981. doi: 10.3389/fimmu.2023.1171981
Received: 22 February 2023; Accepted: 27 April 2023;
Published: 09 May 2023.
Edited by:
Giacomo Cafaro, University of Perugia, ItalyReviewed by:
Dimitra Grapsa, National and Kapodistrian University of Athens, GreeceCopyright © 2023 Daetwyler, Zippelius, Meyer and Läubli. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Heinz Läubli, aGVpbnoubGFldWJsaUB1c2IuY2g=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.