
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol., 11 April 2023
Sec. Cancer Immunity and Immunotherapy
Volume 14 - 2023 | https://doi.org/10.3389/fimmu.2023.1168879
This article is part of the Research TopicAdvances of Novel Approaches to Enhance Therapeutic Efficacy and Safety in Human Solid Cold TumorView all 12 articles
Background: Programmed cell death-ligand 1 (PD-L1) inhibitors plus chemotherapy have made substantial progress in extensive-stage small-cell lung cancer (ES-SCLC), but the survival benefit is still limited. This study aimed to evaluate the preliminary efficacy and safety of camrelizumab plus platinum-irinotecan (IP/IC) followed by maintenance camrelizumab plus apatinib in patients with untreated ES-SCLC.
Methods: In this non-randomized clinical trial (NCT04453930), eligible patients with untreated ES-SCLC received 4-6 cycles of camrelizumab plus IP/IC, followed by maintenance with camrelizumab plus apatinib until disease progression or unmanageable toxicity. The primary endpoint was progression-free survival (PFS). Patients who received PD-L1 inhibitors (atezolizumab or durvalumab) plus platinum-etoposide (EP/EC) were selected as the historical control.
Results: Nineteen patients received IP/IC plus camrelizumab and 34 patients received EP/EC plus PD-L1 inhibitor. At a median follow-up time of 12.1 months, the median PFS was 10.25 months (95% CI: 9.40-NA) in the IP/IC plus camrelizumab group and 7.10 months (95% CI 5.79-8.40) in the EP/EC plus PD-L1 inhibitor group, respectively (HR=0.58, 95% CI 0.42-0.81). The objective response rate of IP/IC plus camrelizumab and EP/EC plus PD-L1 inhibitor was 89.6% and 82.4%, respectively. The most common treatment-related adverse events in the IP/IC plus camrelizumab group was neutropenia, followed by reactive cutaneous capillary endothelial proliferation (RCCEP) and diarrhea. The occurrence of immune-related adverse event was found to be associated with a prolonged PFS (HR=4.64, 95% CI 1.92-11.18).
Conclusions: IP/IC plus camrelizumab followed by maintenance camrelizumab plus apatinib showed preliminary efficacy and acceptable safety profile in patients with untreated ES-SCLC.
Approximately 15% of lung cancer cases are small-cell lung cancer (SCLC), which has a poor prognosis (1). Platinum-based chemotherapy has been the standard first-line treatment for both limited-stage (LS) and extensive-stage (ES) disease since the 1980s, with an objective response rate (ORR) of about 65% and a one-year survival rate of 30%-40% for ES-SCLC (2, 3).
Since the advent of immune checkpoint inhibitors (ICIs), treatment for many solid tumors has been subverted. Furthermore, SCLC has a high mutation rate, making ICIs an attractive therapeutic option (4). Two phase 3 trials evaluated the addition of programmed cell death-ligand 1 (PD-L1) inhibitor atezolizumab (IMpower133) (5, 6) or durvalumab (CASPIAN) (7, 8) to first-line chemotherapy (etoposide and cisplatin [EP]) followed by maintenance PD-L1 inhibitor in patients with ES-SCLC, and showed a significant improvement in overall survival (OS). Based on the above two studies, PD-L1 inhibitor combined with chemotherapy followed by maintenance PD-L1 inhibitor has been the standard care for untreated ES-SCLC now. However, compared with the unprecedented success of PD-L1 combined with EP in the first-line treatment of ES-SCLC patients, programmed cell death 1 (PD-1) inhibitors showed unsatisfactory efficacy outcomes. KEYNOTE-604 study reported an improvement in progression-free survival (PFS) but not in OS with first-line pembrolizumab combined with EP in patients with ES-SCLC (9). Other therapeutic regimens with PD-1 inhibitors therefore remain to be explored.
Camrelizumab is a humanized high-affinity IgG4-kappa anti-PD-1 monoclonal antibody, which has been approved in combination with chemotherapy (carboplatin plus paclitaxel or pemetrexed) for the first-line treatment of advanced non-small-cell lung cancer (NSCLC) (10, 11). However, the efficacy and safety of camrelizumab plus chemotherapy for ES-SCLC patients remains unknown. In patients with ES-SCLC, two of the most commonly used first-line chemotherapy regimens are platinum (cisplatin or carboplatin) combined with etoposide (EP/EC) or irinotecan (IP/IC). A phase III trial conducted in Japan demonstrated a survival benefit of IP regimen over EP regimen for previously untreated ES-SCLC (12). Moreover, the PASSION study found that the ORR of apatinib (an antiangiogenic agent) combined with camrelizumab was 34.0%, with a median PFS of 3.6 months and a median OS of 8.4 months in patients with ES-SCLC who failed platinum-based chemotherapy (13). Thus, based on the potential therapeutic effect of IP in Asian patients and the promising efficacy of camrelizumab plus apatinib as second-line regimen for ES-SCLC, this non-randomized trial was conducted to explore the preliminary efficacy and safety of camrelizumab plus platinum-irinotecan followed by maintenance camrelizumab plus apatinib in patients with untreated ES-SCLC, with comparison of our historical cohort who received PD-L1 inhibitors plus platinum-etoposide.
This non-randomized clinical trial was approved by the ethics committee of Peking Union Medical College Hospital, and was registered with ClinicalTrials.gov (NCT04453930). All patients signed the informed consent before any procedure. The key inclusion criteria were patients aged 18 to 75 years old; pathologically or cytologically confirmed ES-SCLC; previously untreated (including radiotherapy, chemotherapy, vascular endothelial growth factor receptor [VEGFR] inhibitors and ICIs); with an Eastern Cooperative Oncology Group performance status (ECOG PS) score of 0-1. Patients with active brain metastasis or meningeal metastasis were excluded. Detailed inclusion and exclusion criteria were presented in the Supplementary Materials.
Eligible patients were administrated camrelizumab (200 mg, day 1), irinotecan (65 mg/m2, days 1 and 8) plus platinum (cisplatin: 30 mg/m2, days 1 and 8; or carboplatin: area under curve [AUC]=4~5, day 1) every 3 weeks for 4-6 cycles as induction therapy. After induction therapy, patients were assessed for efficacy per Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST 1.1). If progressive disease (PD) was not occurred, patients then received maintenance therapy with camrelizumab (200 mg, day 1, every 3 weeks) and oral apatinib (250 mg, once daily) until disease progression or unacceptable toxicity. Prophylactic brain irradiation was allowed after the induction therapy.
Efficacy was assessed per RECIST 1.1 criteria after the first cycle, and every two cycles thereafter in both induction treatment phase and maintenance treatment phase. For patients without PD but who discontinued the treatment, efficacy was assessed every eight weeks until PD or death. Follow-up for survival was conducted every eight weeks until death or loss of follow-up. Adverse events (AEs) were recorded and graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE, version 5.0).
The primary endpoint was PFS, which defined as the time from enrollment to PD per RECIST 1.1 criteria or death from any cause, whichever occurred first. The secondary endpoints included OS (defined as the time from enrollment to death from any cause), disease control rate (DCR, defined as the percentage of patients with a complete response [CR], partial response [PR] or stable disease [SD]), ORR (defined as the percentage of patients with a CR or PR), and duration of response (DoR, defined as the time from the first document CR or PR to PD or death from any cause). The safety profiles included treatment-related AEs (TRAEs) and immune-related AEs (irAEs).
Using the electronic medical record system of Peking Union Medical College Hospital, ES-SCLC patients who received ICIs combined with chemotherapy as the first-line treatment were retrospectively reviewed. Among them, patients who received EP/EC combined with PD-L1 blockades (atezolizumab or durvalumab) were selected as the historical control. Information of baseline characteristics, treatment pattern, efficacy and safety profiles of patients were reviewed.
All statistical analyses were conducted using SPSS version 23.0 and RStudio 1.2.5001. Continuous variables were expressed as median and range, and classification variables were expressed as frequency and percentage. Kaplan-Meier method was used to evaluate PFS and DoR, and Cox proportional hazards model was used to estimate hazard ratio (HR) and 95% confidence interval (CI). PFS was analyzed in different subgroups according to age (≤65 years, >65 years), sex (male, female), metastatic sites (with or without brain metastasis, with or without liver metastasis), modified neutrophil to lymphocyte ratio (dNLR, ≤3, >3), serum sodium (<135, ≥135), serum lactate dehydrogenase (LDH, ≤260, >260), the absolute value of CD4+ cells (≤600/μl, >600/μl), the absolute value of CD8+ cells (≤450/μl, >450/μl), CD4+/CD8+ (1-2, <1 or >2) and the occurrence of irAEs (with or without irAEs).The HR and 95%CI of subgroups were calculated using the non-stratified Cox risk ratio model, and the association between different subgroups and median PFS was calculated. P < 0.05 was considered statistically significant.
From March 2020 to December 2021, 19 ES-SCLC patients were enrolled in this study and received study medications. Of them, the median age was 63 (range 51-70) years old, and 17 (89.5%) patients were male. Most patients (89.5%) were stage IV. Two patients had liver metastasis and one patient experienced brain metastasis (Table 1).
In addition, a total of 54 ES-SCLC patients received ICIs combined with chemotherapy as the first-line treatment were retrospective reviewed form January 2013 to December 2021, of which 34 patients treated with EP/EC combined with a PD-L1 inhibitor (atezolizumab or durvalumab) were selected as the historical control group. The median age of these patients was 64 (range 42-77) years old and 27 (79.4%) patients were male. Two patients had an ECOG PS score of 2 or above. The majority (97.1%) were stage IV. Six (17.6%) and three (8.8%) patients had liver metastasis and brain metastasis, respectively. There were no significant differences in the baseline characteristics of patients between two groups (Table 1; Figure S1).
At the time of data cut-off (March 16, 2022), the median follow-up time was 12.1 months. Nine (47.4%) patients experienced PD and four (21.1%) died in the IP/IC plus camrelizumab group, with a median PFS of 10.25 (95% CI 9.40-NA) months. In the EP/EC plus PD-L1 group, 18 (52.9%) patients developed PD and six (17.6%) patients died, with a median PFS of 7.10 (95% CI 5.79-8.40) months. IP/IC plus camrelizumab showed better PFS than EP/EC plus PD-L1 as first-line treatment in patients with ES-SCLC (HR=0.58, 95% CI 0.42-0.81; P=0.0013) (Figure 1). The median OS was not achieved in both groups.
In the IP/IC plus camrelizumab group, 17 (89.5%) patients had PR, and two (10.5%) had SD, with a median DoR of 8.74 months. In the EP/EC plus PD-L1 inhibitor group, 28 (82.4%) patients had PR, four (11.8%) had SD, and two (5.9%) had PD. The median DoR was 4.4 months. The DCR and ORR were 100% and 89.6% in the IP/IC plus camrelizumab group, as well as 94.1% and 82.4% in the EP/EC plus PD-L1 inhibitor group, respectively (Table 2).
As of the last follow-up, 18 (53.9%) patients with EP/EC plus PD-L1 inhibitor and nine (47.4%) patients with IP/IC plus camrelizumab developed PD. Post-progression treatment was at the discretion of the investigator depending on the patient’s condition. Of these patients with PD in the EP/EC plus PD-L1 inhibitor group, eight (23.5%) patients subsequently started receiving PD-L1 inhibitor plus other chemotherapy regimens, one (2.9%) patient received topotecan, one (2.9%) patient received albumin paclitaxel monotherapy, and eight (53.9%) patients started receiving IP/IC plus PD-1 inhibitor. In the IP/IC plus camrelizumab group, four (10.5%) patients started treatment with EP/EC plus PD-L1 inhibitor (atezolizumab/durvalumab) following progression, four (10.5%) patients began EP/EC treatment, and one patient (5.3%) received anlotinib monotherapy.
In the IP/IC plus camrelizumab group, the median cycle number was six for chemotherapy and seven for camrelizumab, respectively; while in the EP/EC plus PD-L1 inhibitor group, the median cycle number was five for chemotherapy and six for immunotherapy. The most common TRAE in the IP/IC plus camrelizumab group was neutropenia, followed by reactive cutaneous capillary endothelial proliferation (RCCEP) and diarrhea (Table 3). The irAEs in the IP/IC plus camrelizumab group included RCCEP (52.6%), abnormal liver function (21.1%), rash (10.5%), hypothyroidism (5.3%), oculomotor paralysis (5.3%) and kidney injury (5.3%) (Table 4).
The IP/IC plus camrelizumab showed better PFS than EP/EC plus PD-L1 inhibitor among all subgroups, but the difference was not statistically significant (Figure S2). For all enrolled patients, 19 patients experienced irAEs. The median PFS in patients with irAEs was 14.5 months (95% CI 7.94-NA), which was significantly longer than that in patients without irAEs (14.5 months vs. 6.3 months, HR=4.64, 95% CI 1.92-11.18) (Figure 2).
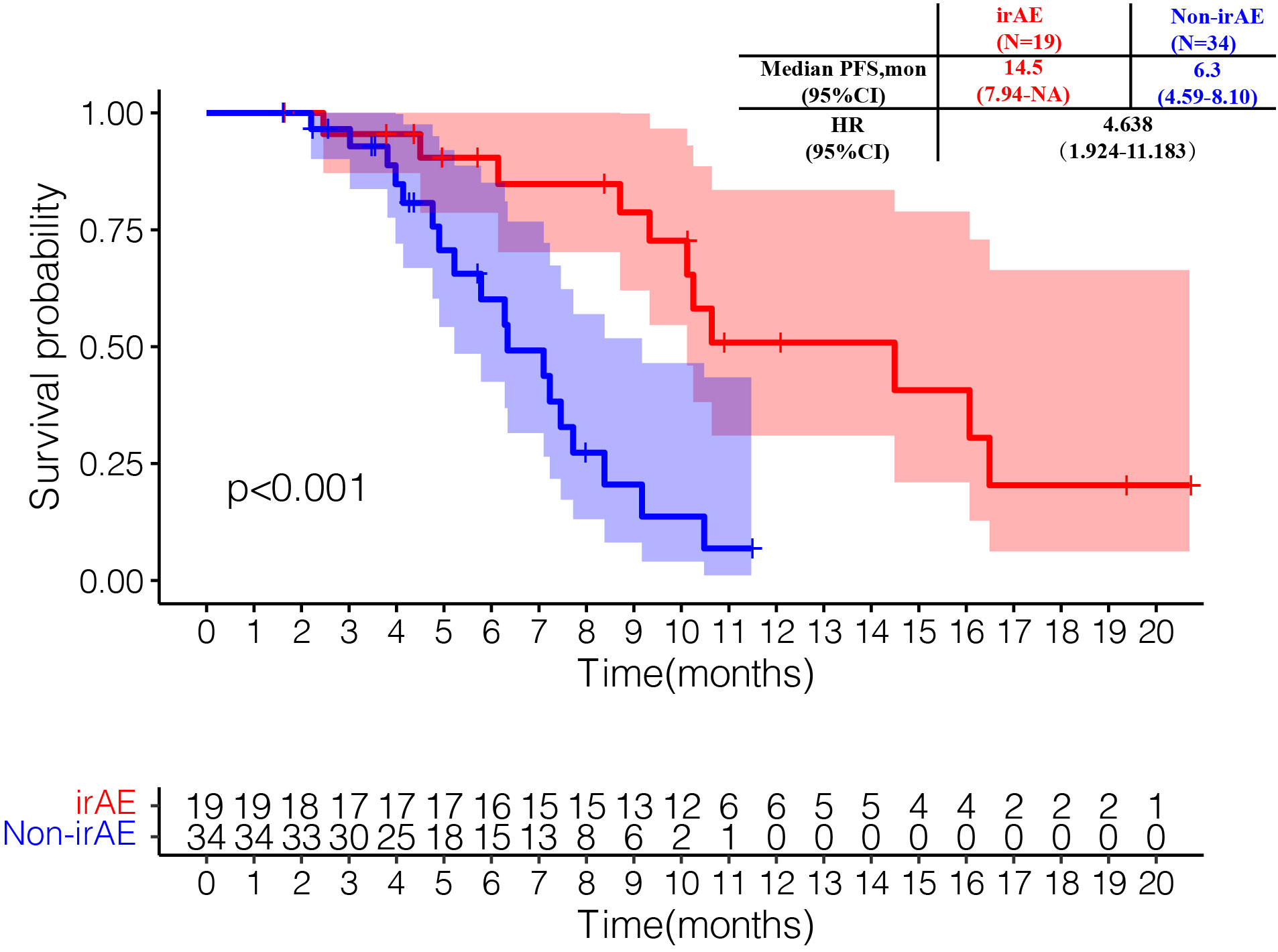
Figure 2 Kaplan-Meier curves of progression-free survival by immune-related adverse events (irAEs) in the irAE-evaluable population.
In the past 30 years, platinum-based chemotherapy has been used as a first-line treatment for ES-SCLC patients (14, 15). Because SCLC has high tumor mutation burden, ICIs offers a new way to treat this disease and prolong patient survival. There is no doubt that PD-L1 inhibitor in combination with chemotherapy has become the standard first-line treatment for ES-SCLC patients. However, the survival benefit of ICI treatment on SCLC patients is less than that on NSCLC patients, and the OS of SCLC is still limited (4, 16). Therefore, exploring new combined treatment options is urgently needed. To our knowledge, this is the first study to report the efficacy and safety of IP/IC plus camrelizumab followed by maintenance camrelizumab plus apatinib as the first-line treatment in ES-SCLC. Compare with PD-L1 inhibitor plus EP/EC (the historical control), IP/IC plus camrelizumab had the comparable ORR (89.6% vs 82.4%), but achieved a better median PFS (10.25 months vs. 7.10 months, HR=0.58, 95% CI 0.42-0.81). The OS data was immature in both groups.
PD-1 inhibitors are able to block both PD-1/PD-L1 pathways as well as PD-1/PD-L2 pathways, whereas PD-L1 inhibitors only inhibit PD-1/PD-L1 (17). Therefore, when PD-L1 inhibitors are used, the tumor can escape through the PD-1/PD-L2 axis. Based on a meta-analysis, NSCLC patients treated with PD-1 inhibitor plus chemotherapy had a lower death risk compared to those treated with PD-L1 inhibitor plus chemotherapy (RR=0.66, 95% CI 0.48-0.90) (18). However, a different scenario was shown in untreated ES-SCLC. In the first-line treatment of ES-SCLC, IMpower133 and CASPAIN studies have demonstrated the survival benefit of PD-L1 inhibitor atezolizumab or durvalumab plus chemotherapy, while KEYNOTE-604 study of PD-1 inhibitor pembrolizumab plus chemotherapy only showed a PFS benefit but failed to show an OS benefit (5–8, 19). Nevertheless, was too early to draw a conclusion that that ES-SCLC would not benefit from the first-line therapy with PD-1 inhibitor (20). In our study, a superior PFS was also observed in the IP/IC plus camrelizumab group compared with in the EP/EC plus PD-L1 inhibitor group, whereas OS data were not yet mature at the time of data cutoff. Considering that the chemotherapy regime and maintenance therapy were distinct from the above-mentioned studies, mature OS data from our study are worthy of anticipation.
Chemotherapy and radiotherapy have the ability to increase the immunogenicity of tumor cells, increase major histocompatibility class I (MHC-I) molecule expression, activate immune effector factors such as nature killer cells, and promote immune response by targeting tumor immunosuppressive cells (21–23). In addition, radiotherapy and chemotherapy can reduce the immunosuppressive properties of tumor cells, thereby stimulating T cell activation and resulting in tumor shrinkage (24). SCLC is a disease that is highly sensitive to chemotherapy and radiotherapy. The preferred chemotherapy regimen for initial treatment of patients with ES-SCLC is EP/EC, followed by IP/IC; whereas two meta-analyses of randomized controlled trials reported a favorable OS and less hematological toxicities in irinotecan/platinum regimens compared with etoposide/platinum regimens in this population (25, 26). Therefore, in contrast to the EP/EC regimen used as a combination with immunotherapy in the IMpower133 and CASPIAN studies (5, 8), we chose IP/IC regimen when combined with camrelizumab in this study.
Maintenance PD-L1 inhibitor was administrated in IMpower133 and CASPIAN studies and pembrolizumab plus chemotherapy was continued until disease progression in KEYNOTE-604 study, whereas in our study camrelizumab combined with apatinib was used for maintenance therapy. Preclinical data have shown that apatinib could relieve hypoxia, enhance CD8+ T cell infiltration, decrease the recruitment of tumor-associated macrophages in tumors and the amount of TGF-β in both tumors and serum, and generate synergistic antitumor effects with PD-L1 blockade in lung cancer (27). Moreover, the PASSION study evaluated the efficacy of camrelizumab combined with apatinib in the treatment of recurrent ES-SCLC, and showed promising efficacy and manageable safety profile (13). These suggested this combination may have promising therapeutic effects on untreated ESCLC; however, further studies are needed to elucidate its exact molecular mechanism.
The median number of chemotherapy cycles in our study was six for IP/IC and five for EP/EC, compared to the standard four cycles. Although the latest NCCN guideline for SCLC also recommends 4 cycles of treatment for untreated ES-disease, patients may receive up to 6 cycles based on response and tolerability after 4 cycles (28). Additionally, similar overall safety profiles and grade 3-4 TRAEs were observed in two groups. The most common grade 3 or higher TRAE in both groups was neutropenia, which was reversible by symptomatic treatment (such as with granulocyte-colony stimulating factor), and its incidence rate was higher with IP/IC plus camrelizumab than with EP/EC plus PD-L1 inhibitor. Neutropenia is often induced by chemotherapy. Previous retrospective study reported that the development of severe neutropenia with IP regimen might be associated with improved prognosis as opposed to EP therapy in patients with ES-SCLC (29). In addition, RCCEP and diarrhea were the two most common TRAEs of any grade in the IP/IC plus camrelizumab group, whereas none occurred in the EP/EC plus PD-L1 inhibitor group. Diarrhea was generally reversible with dose modification or antidiarrhea therapy. Given that the vast majority of diarrhea were grade 1-2, whether prophylactic loperamide is necessary deserves further exploration. RCCEP was observed in more than half of the IP/IC combined with camrelizumab group, which may be related to the activation of vascular endothelial cell proliferation by camrelizumab through regulating vascular receptor VEGFR2, leading to vascular proliferation (30, 31). No new safety signals were observed in this study.
Notably, the subgroup analysis showed that the occurrence of irAEs was associated with a longer median PFS, which was consistent with the results of SCLC with larger samples (32), NSCLC (33), malignant melanoma (34), and metastatic renal clear cell carcinoma (35). In 2020, a multicenter retrospective study showed that the occurrence of irAEs was an independent protective factor for ES-SCLC patients treated with ICIs (PFS: HR=0.44, 95% CI 0.29-0.66; OS: HR=0.47, 95%CI 0.32-0.71) (32). Besides, it was demonstrated that the development of irAEs was associated with survival outcome in patients with advanced or recurrent NSCLC treated with nivolumab (median PFS: 9.2 months vs. 4.4 months, P=0.04; median OS: NA vs. 11.1 months, P=0.01) (33). A retrospective study of 195 NSCLC patients treated with nivolumab found that the incidence of irAEs was 43.6%, and that the ORR (43.5% vs 10%, P<0.001) and median PFS (5.7 months vs 2.0 months, P<0.001) of patients with irAEs were significantly improved (36).
This study has some limitations. First, the control group was retrospectively reviewed with selective bias, so the results of this study cannot fully reflect the efficacy and safety of the EP/EC plus PD-L1 inhibitor in patients with ES-SCLC. Second, since this study was a single-center analysis, the results might be influenced by the level of diagnosis and treatment at our center. Third, the sample size was small, and finally only 53 patients with ES-SCLC were analyzed, resulting in insufficient statistical power of our study. Last, the data of OS was immature, and the patients’ survival situation should be continuously tracked. Further large-scale randomized controlled trials are warranted to confirm our results.
In conclusion, our study demonstrated the preliminary efficacy and manageable safety of IP/IC plus camrelizumab followed by maintenance camrelizumab plus apatinib in untreated ES-SCLC, compared with EP/EC plus PD-L1 inhibitor. The occurrence of irAEs was associated with a longer PFS, which may be a potential prognostic factor for patients treated with ICIs.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by Peking Union Medical College Hospital. The patients/participants provided their written informed consent to participate in this study.
LZ and XZ contributed to the conception of the study. JN, XS and HW performed the experiment. JN contributed to analysis and manuscript preparation. JN performed the data analyses and wrote the manuscript. All authors contributed to the article and approved the submitted version.
This study was sponsored by Hengrui Medicine Co, Ltd, Jiangsu, China. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article, or the decision to submit it for publication.
The authors would like to thank the patient and his family for providing information on this case.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1168879/full#supplementary-material
Supplementary Figure 1 | Baseline characteristics of patients by treatment pattern.
Supplementary Figure 2 | Forest plot of subgroup analysis of progression-free survival.
1. Rudin CM, Brambilla E, Faivre-Finn C, Sage J. Small-cell lung cancer. Nat Rev Dis Primers (2021) 7(1):3. doi: 10.1038/s41572-020-00235-0
2. Amarasena IU, Chatterjee S, Walters JA, Wood-Baker R, Fong KM. Platinum versus non-platinum chemotherapy regimens for small cell lung cancer. Cochrane Database Syst Rev (2015) 8):CD006849. doi: 10.1002/14651858.CD006849.pub3
3. Barrows ED, Blackburn MJ, Liu SV. Evolving role of immunotherapy in small cell lung cancer. Semin Cancer Biol (2022) 86(Pt 3):868–74. doi: 10.1016/j.semcancer.2022.02.021
4. Remon J, Aldea M, Besse B, Planchard D, Reck M, Giaccone G, et al. Small cell lung cancer: A slightly less orphan disease after immunotherapy. Ann Oncol (2021) 32(6):698–709. doi: 10.1016/j.annonc.2021.02.025
5. Horn L, Mansfield AS, Szczesna A, Havel L, Krzakowski M, Hochmair MJ, et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med (2018) 379(23):2220–9. doi: 10.1056/NEJMoa1809064
6. Liu SV, Reck M, Mansfield AS, Mok T, Scherpereel A, Reinmuth N, et al. Updated overall survival and pd-L1 subgroup analysis of patients with extensive-stage small-cell lung cancer treated with atezolizumab, carboplatin, and etoposide (Impower133). J Clin Oncol (2021) 39(6):619–30. doi: 10.1200/JCO.20.01055
7. Goldman JW, Dvorkin M, Chen Y, Reinmuth N, Hotta K, Trukhin D, et al. Durvalumab, with or without tremelimumab, plus platinum–etoposide versus platinum–etoposide alone in first-line treatment of extensive-stage small-cell lung cancer (Caspian): Updated results from a randomised, controlled, open-label, phase 3 trial. Lancet Oncol (2021) 22(1):51–65. doi: 10.1016/s1470-2045(20)30539-8
8. Paz-Ares L, Dvorkin M, Chen Y, Reinmuth N, Hotta K, Trukhin D, et al. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (Caspian): A randomised, controlled, open-label, phase 3 trial. Lancet (2019) 394(10212):1929–39. doi: 10.1016/S0140-6736(19)32222-6
9. Rudin CM, Awad MM, Navarro A, Gottfried M, Peters S, Csoszi T, et al. Pembrolizumab or placebo plus etoposide and platinum as first-line therapy for extensive-stage small-cell lung cancer: Randomized, double-blind, phase iii keynote-604 study. J Clin Oncol (2020) 38(21):2369–79. doi: 10.1200/JCO.20.00793
10. Zhou C, Chen G, Huang Y, Zhou J, Lin L, Feng J, et al. Camrelizumab plus carboplatin and pemetrexed versus chemotherapy alone in chemotherapy-naive patients with advanced non-squamous non-Small-Cell lung cancer (Camel): A randomised, open-label, multicentre, phase 3 trial. Lancet Respir Med (2021) 9(3):305–14. doi: 10.1016/S2213-2600(20)30365-9
11. Ren S, Chen J, Xu X, Jiang T, Cheng Y, Chen G, et al. Camrelizumab plus carboplatin and paclitaxel as first-line treatment for advanced squamous nsclc (Camel-sq): A phase 3 trial. J Thorac Oncol (2022) 17(4):544–57. doi: 10.1016/j.jtho.2021.11.018
12. Noda K, Nishiwaki Y, Kawahara M, Negoro S, Sugiura T, Yokoyama A, et al. Irinotecan plus cisplatin compared with etoposide plus cisplatin for extensive small-cell lung cancer. N Engl J Med (2002) 346(2):85–91. doi: 10.1056/NEJMoa003034
13. Fan Y, Zhao J, Wang Q, Huang D, Li X, Chen J, et al. Camrelizumab plus apatinib in extensive-stage sclc (Passion): A multicenter, two-stage, phase 2 trial. J Thorac Oncol (2021) 16(2):299–309. doi: 10.1016/j.jtho.2020.10.002
14. Fruh M, De Ruysscher D, Popat S, Crino L, Peters S, Felip E, et al. Small-cell lung cancer (Sclc): Esmo clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol (2013) 24(Suppl 6):vi99–105. doi: 10.1093/annonc/mdt178
15. Rudin CM, Ismaila N, Hann CL, Malhotra N, Movsas B, Norris K, et al. Treatment of small-cell lung cancer: American society of clinical oncology endorsement of the American college of chest physicians guideline. J Clin Oncol (2015) 33(34):4106–11. doi: 10.1200/JCO.2015.63.7918
16. Ganti AKP, Loo BW, Bassetti M, Blakely C, Chiang A, D'Amico TA, et al. Small cell lung cancer, version 2.2022, nccn clinical practice guidelines in oncology. J Natl Compr Canc Netw (2021) 19(12):1441–64. doi: 10.6004/jnccn.2021.0058
17. Chen L, Han X. Anti-Pd-1/Pd-L1 therapy of human cancer: Past, present, and future. J Clin Invest (2015) 125(9):3384–91. doi: 10.1172/JCI80011
18. Duan J, Cui L, Zhao X, Bai H, Cai S, Wang G, et al. Use of immunotherapy with programmed cell death 1 vs programmed cell death ligand 1 inhibitors in patients with cancer: A systematic review and meta-analysis. JAMA Oncol (2020) 6(3):375–84. doi: 10.1001/jamaoncol.2019.5367
19. Wang WT, Han C, Sun YM, Chen TQ, Chen YQ. Noncoding rnas in cancer therapy resistance and targeted drug development. J Hematol Oncol (2019) 12(1):55. doi: 10.1186/s13045-019-0748-z
20. Ortega-Franco A, Ackermann C, Paz-Ares L, Califano R. First-line immune checkpoint inhibitors for extensive stage small-cell lung cancer: Clinical developments and future directions. ESMO Open (2021) 6(1):100003. doi: 10.1016/j.esmoop.2020.100003
21. Aymeric L, Apetoh L, Ghiringhelli F, Tesniere A, Martins I, Kroemer G, et al. Tumor cell death and atp release prime dendritic cells and efficient anticancer immunity. Cancer Res (2010) 70(3):855–8. doi: 10.1158/0008-5472.CAN-09-3566
22. Ghiringhelli F, Apetoh L. The interplay between the immune system and chemotherapy: Emerging methods for optimizing therapy. Expert Rev Clin Immunol (2014) 10(1):19–30. doi: 10.1586/1744666X.2014.865520
23. Reits EA, Hodge JW, Herberts CA, Groothuis TA, Chakraborty M, Wansley EK, et al. Radiation modulates the peptide repertoire, enhances mhc class I expression, and induces successful antitumor immunotherapy. J Exp Med (2006) 203(5):1259–71. doi: 10.1084/jem.20052494
24. Bang A, Schoenfeld JD. Immunotherapy and radiotherapy for metastatic cancers. Ann Palliat Med (2019) 8(3):312–25. doi: 10.21037/apm.2018.07.10
25. Shao N, Jin S, Zhu W. An updated meta-analysis of randomized controlled trials comparing Irinotecan/Platinum with Etoposide/Platinum in patients with previously untreated extensive-stage small cell lung cancer. J Thorac Oncol (2012) 7(2):470–2. doi: 10.1097/JTO.0b013e31823c5a23
26. Jiang J, Liang X, Zhou X, Huang L, Huang R, Chu Z, et al. A meta-analysis of randomized controlled trials comparing Irinotecan/Platinum with Etoposide/Platinum in patients with previously untreated extensive-stage small cell lung cancer. J Thorac Oncol (2010) 5(6):867–73. doi: 10.1097/jto.0b013e3181d95c87
27. Zhao S, Ren S, Jiang T, Zhu B, Li X, Zhao C, et al. Low-dose apatinib optimizes tumor microenvironment and potentiates antitumor effect of pd-1/Pd-L1 blockade in lung cancer. Cancer Immunol Res (2019) 7(4):630–43. doi: 10.1158/2326-6066.CIR-17-0640
28. Nccn Clinical Practice Guidelines in Oncology (Nccn Guidelines®). Small cell lung cancer. version 2.2023 (2022). Available at: https://www.nccn.org/professionals/physician_gls/pdf/sclc.pdf.
29. Miyagi T, Tsuji D, Kawasakai Y, Ishikawa H, Tanaka R, Nakao M, et al. Chemotherapy-induced neutropenia as a prognostic factor in patients with extensive-stage small cell lung cancer. Eur J Clin Pharmacol (2023) 79(3):407–14. doi: 10.1007/s00228-023-03451-1
30. Finlay WJJ, Coleman JE, Edwards JS, Johnson KS. Anti-Pd1 'Shr-1210' aberrantly targets pro-angiogenic receptors and this polyspecificity can be ablated by paratope refinement. MAbs (2019) 11(1):26–44. doi: 10.1080/19420862.2018.1550321
31. Teng Y, Guo R, Sun J, Jiang Y, Liu Y. Reactive capillary hemangiomas induced by camrelizumab (Shr-1210), an anti-Pd-1 agent. Acta Oncol (2019) 58(3):388–9. doi: 10.1080/0284186X.2019.1567935
32. Ricciuti B, Naqash AR, Naidoo J, Sehgal K, Miller A, Kehl K, et al. Association between immune-related adverse events and clinical outcomes to programmed cell death protein 1/Programmed death-ligand 1 blockade in sclc. JTO Clin Res Rep (2020) 1(4):100074. doi: 10.1016/j.jtocrr.2020.100074
33. Haratani K, Hayashi H, Chiba Y, Kudo K, Yonesaka K, Kato R, et al. Association of immune-related adverse events with nivolumab efficacy in non-Small-Cell lung cancer. JAMA Oncol (2018) 4(3):374–8. doi: 10.1001/jamaoncol.2017.2925
34. Borcoman E, Nandikolla A, Long G, Goel S, Le Tourneau C. Patterns of response and progression to immunotherapy. Am Soc Clin Oncol Educ Book (2018) 38:169–78. doi: 10.1200/EDBK_200643
35. Verzoni E, Carteni G, Cortesi E, Giannarelli D, De Giglio A, Sabbatini R, et al. Real-world efficacy and safety of nivolumab in previously-treated metastatic renal cell carcinoma, and association between immune-related adverse events and survival: The Italian expanded access program. J Immunother Cancer (2019) 7(1):99. doi: 10.1186/s40425-019-0579-z
36. Ricciuti B, Genova C, De Giglio A, Bassanelli M, Dal Bello MG, Metro G, et al. Impact of immune-related adverse events on survival in patients with advanced non-small cell lung cancer treated with nivolumab: Long-term outcomes from a multi-institutional analysis. J Cancer Res Clin Oncol (2019) 145(2):479–85. doi: 10.1007/s00432-018-2805-3
Keywords: small cell lung carcinoma (SCLC), platinum, irinotecan, camrelizumab, immune checkpoint inhibitors
Citation: Ni J, Si X, Wang H, Zhang X and Zhang L (2023) Camrelizumab plus platinum-irinotecan followed by maintenance camrelizumab plus apatinib in untreated extensive-stage small-cell lung cancer: a nonrandomized clinical trial. Front. Immunol. 14:1168879. doi: 10.3389/fimmu.2023.1168879
Received: 18 February 2023; Accepted: 27 March 2023;
Published: 11 April 2023.
Edited by:
Lian Xiang Luo, Guangdong Medical University, ChinaReviewed by:
Jialin Shang, Cornell University, United StatesCopyright © 2023 Ni, Si, Wang, Zhang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Li Zhang, emhhbmdsaXB1bWNoMTAyNkBzaW5hLmNvbQ==; Xiaotong Zhang, emhhbmd4dHB1bWNoQDEyNi5jb20=
†These authors share last authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.