- 1Department of Oncology, Jinan Central Hospital, Shandong University, Jinan, Shandong, China
- 2Pulmonary and Critical Care Medicine, Central Hospital Affiliated to Shandong First Medical University, Jinan, Shandong, China
- 3Department of Oncology, Central Hospital Affiliated to Shandong First Medical University, Jinan, Shandong, China
Objective: This review aims to determine the incidence and risk of pancreatic adverse events (AEs) associated with immune checkpoint inhibitors (ICIs) therapy for solid tumors.
Methods: We conducted a comprehensive systematic literature search in PubMed, Embase, and Cochrane Library up to March 15, 2023, to identify all randomized controlled trials comparing ICIs with standard treatment in solid tumors. We included studies that reported immune-related pancreatitis or elevation of serum amylase or lipase levels. Following protocol registration in PROSPERO, we conducted a systematic review and meta-analysis.
Results: 59 unique randomized controlled trials with at least one ICI-containing arm (41 757 patients) were retrieved. The incidences for all-grade pancreatitis, amylase elevation and lipase elevation were 0.93% (95% CI 0.77-1.13), 2.57% (95% CI 1.83-3.60) and 2.78% (95% CI 1.83-4.19), respectively. The incidences for grade ≥3 pancreatitis, amylase elevation and lipase elevation were 0.68% (95% CI 0.54-0.85), 1.17% (95% CI 0.83-1.64) and 1.71% (95% CI 1.18-2.49), respectively. The use of ICIs was associated with an increased risk of all-grade pancreatic immune-related AEs (irAEs) including pancreatitis (OR=2.04, 95% CI 1.42-2.94, P =0.0001), amylase elevation (OR=1.91, 95% CI 1.47-2.49, P < 0.0001) and lipase elevation (OR=1.77, 95% CI 1.37-2.29, P < 0.0001). In addition to these, the post-hoc analysis found that PD-1 inhibitors had a significant higher risk of pancreatic AEs compared with PD-L1 inhibitors and the patients undergoing dual ICI therapy were at a significantly higher risk of pancreatic AEs than the patients receiving single ICI therapy.
Conclusion: Our study provides an overview of the incidence and risk of ICI-associated pancreatitis and pancreatic enzyme elevations in the treatment of solid tumors. Our findings may help raise awareness among clinicians of the potential for ICI-associated pancreatic AEs in clinical practice.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO, identifier 345350.
Introduction
Immune checkpoint inhibitors (ICIs) including programmed cell death 1 (PD-1) inhibitors, programmed cell death 1 ligand 1(PD-L1)inhibitors and cytotoxic T- lymphocyte-associated antigen 4 (CTLA-4) inhibitors have revolutionized cancer therapy and become the standard treatment for a number of malignancies in the past few years (1, 2). While ICIs activate the immune system against tumor cells, they can also lead to adverse events due to the imbalance of immunologic homeostasis in normal tissues (3). IrAEs can range from mild self-limiting symptoms to severe life-threatening events that can affect nearly all organ systems. These adverse events include but are not limited to, colitis, hepatitis, dermatitis, pneumonia, endocrine disorders, nephritis, myocarditis, and neuropathy (4). As the use of immunotherapy in cancer patients continues to rise, uncommon irAEs present a significant clinical challenge (5). Pancreatic AEs are rare but often overlooked, requiring clinician attention due to their adverse impact on the quality of life of cancer patients.
Despite early clinical studies confirming the immune-related toxicity of ICIs in the pancreas (6), several questions remain unanswered. Firstly, how to effectively recognize pancreatic irAEs, as they may present as asymptomatic elevations in amylase and/or lipase levels, as per the guidelines of the National Comprehensive Cancer Network (NCCN) (7). Furthermore, it is unclear whether the incidence of pancreatic AEs increases with the widespread use of ICIs and whether different types of combination therapy affect the risk of incidence. Therefore, our study aims to address these knowledge gaps and provide insights into predicting and managing pancreatic irAEs through a systematic review and meta-analysis.
Methods
Search strategy
This systematic review and meta-analysis was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines (8). The statement was registered at the International Prospective Register of Systematic Reviews (number 345350). We conducted a comprehensive systematic literature search in PubMed, Embase, and Cochrane Library up to March 15, 2023, for all randomized controlled trials(RCTs)that compared ICIs with standard treatment in solid tumors. Based on PICOS (participants, interventions, comparisons, outcomes, and study design) guidelines (9), the keywords and Medical Subject Headings (MeSH) terms were used as follows: “neoplasms”; “immune checkpoint inhibitor”, “PD-1 inhibitors”, “PD-L1 inhibitors”,” CTLA4 inhibitors” “pembrolizumab”, “nivolumab”, “tislelizumab”, “sintilimab”, “camrelizumab”, “toripalimab”, “atezolizumab”, “avelumab”, “durvalumab”, “cemiplimab”, “tremelimumab”, “Ipilimumab” “drug-related side effects and adverse reactions”, “adverse reactions”, and “randomized controlled trials”.
Selection criteria
Studies eligible for inclusion met all the following criteria: (1) phase III RCTs including at least one ICI-containing arm (ICIs as monotherapy or in combination with another ICIs or standard treatment) in adult patients (age >_18 years) with solid cancer; (2) clinical trials reporting immune-related pancreatitis or elevation of serum amylase or lipase levels; and (3) studies published in English. The exclusion criteria were as follows: (1) studies published as abstracts, letters, or conference reports; (2) studies published repeatedly; (3)both treatment arms were immunotherapy.
Data extraction
Two investigators (ZZ and WZ) independently evaluated the titles, abstracts, and full texts to select the potentially eligible publications. The following data were obtained from the included study: basic information (first author, publication year, trial name, and Clinical Trial number), participants(disease diagnosis, treatment arms, and the number of included patients), and the number of patients with pancreatitis, amylase elevation, and lipase elevation for all-grade (G1–5) and for grade 3 or higher (G3–5). The severity of the AE was graded on a scale from 0 to 5, with grade 0 being no toxicity and grade 5 being death according to the Common Terminology Criteria for Adverse Events(CTCAE) (10). Additional data included ICI regimen, control arm regimen, previous lines of chemotherapy, blindness, and median/mean follow-up (months). The primary outcome of our meta-analysis was the summary risk of pancreatic AEs associated with ICI exposure (ICIs as monotherapy or in combination with other ICIs or standard treatment) vs. controls in RCTs. If disagreement occurred, it was resolved by discussion with the corresponding author. All included studies represented unique trials.
Statistical analysis
To conduct a meta-analysis of the incidence and profile of pancreatic AEs, a random effect model with logit transformation was applied. All models are fitted by restricted maximum likelihood estimation with a classic continuity correction of 0.5 for zero cells and the corresponding sample sizes. Multiple groups of a trial were combined separately. The outcome measure is the incidence with its 95% confidence interval (CI). Based on previous studies (11), we hypothesized that pancreatic AEs are not a frequent event (incidence < 10%), and we interpreted the odds ratio(OR) as a measure of risk (12, 13). Pooled ORs and 95% CIs were estimated with a random effects model using the Mantel–Haenszel method (14). If a study included more than one intervention arm, we separately compared each intervention arm with the control arm. In addition to that, we conducted subgroup analyses to examine studies by cancer type and combination type.
Post-hoc analyses were used to assess the pancreatic AEs differences between anti-PD-1 drugs and anti-PD-L1 drugs, as well as, between dual- and single -ICI therapies. We matched the included RCTs with their tumor type and intervention type, or tumor type and design of control groups to form several mirror groups for the adjusted indirect comparison (15). An OR (95% CI) was derived from each mirror group and then pooled across all ICI groups using a random-effects model.
We used the inconsistency index ι2 statistic and χ2 test with its P-value to evaluate the heterogeneity between studies. According to the Cochrane Handbook for Systematic Reviews of Interventions, substantial heterogeneity between studies was defined by ι2 value > 50%, and significant heterogeneity was defined by χ2 test P-value <0.10 (16). Publication bias was assessed using Peter tests with funnel plots, which is a recommended method for dichotomous data with low heterogeneity (17, 18). The risk of bias of included studies were evaluated with the Cochrane risk of bias tool (19). All analyses were done using Review Manager 5.3 software (Cochrane Collaboration 2014, Nordic Cochrane Center, Copenhagen, Denmark) and R statistical software (version 4.1.3; with the metafor_v3.0–2 packages) (20). A two-sided P-value of <0.05 in Z-tests (for overall effect) or χ2 test (for overall subgroup comparison) in all analyses was considered statistically significant.
Results
Eligible studies and characteristics
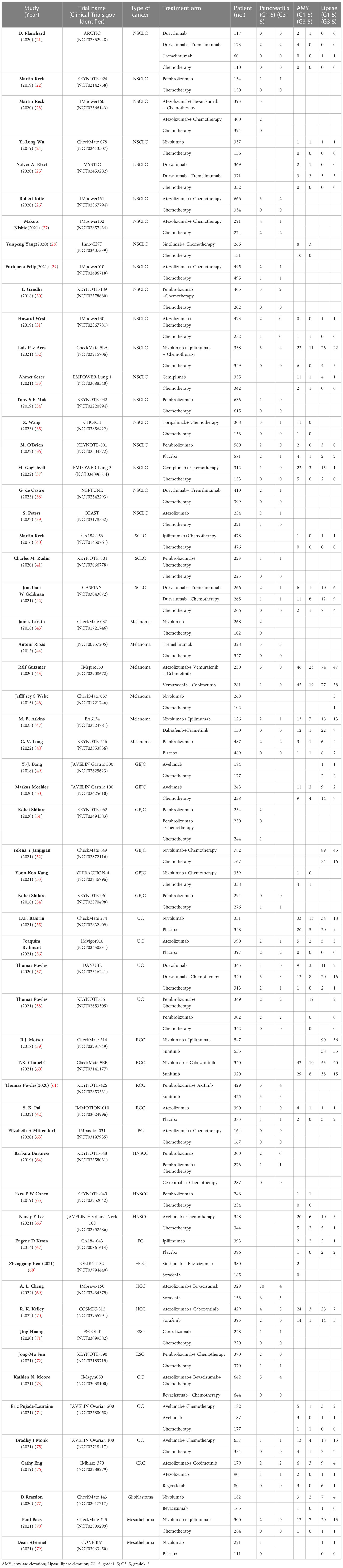
We identified 25 874 records from PubMed, Embase, and Cochrane Library. Figure 1 and Supplementary Table 1 illustrate the details of the study screening and selection procedures. Finally, 59 eligible studies involving 41 757 patients for quantitative analysis were included. Details of the study characteristics are presented in Table 1. Among these 59 RCTs, one was a four-arm study and 9 RCTs were three-arm. The mean follow-up time for the entire population ranged from 7.3 to 41.2 months. According to the type of combination therapy, there were 30 arms of ICI monotherapy 32 arms of ICI plus chemotherapy or targeted therapy, and 8 arms of dual-ICI therapy. In our study, we incorporated multiple tumor types including non-small cell lung cancer (NSCLC, n =19) (21–39), small cell lung cancer(SCLC, n = 3) (40–42), melanoma (n = 6) (43–48), gastroesophageal junction cancer (GEJC, n = 6) (49, 51, 52, 54, 80, 81), urothelial carcinoma (UC, n = 4) (55–58), renal cell carcinoma (RCC, n=4) (59–62), breast cancer(BC,n=1) (63), head and neck squamous cell carcinoma (HNSCC,n=3) (64–66), prostate cancer (PC,n=1) (67), hepatocellular carcinoma (HCC,n=3) (68–70), esophageal carcinoma (ESO,n=2) (71, 72), ovarian cancer (OC,n=3) (73–75), colorectal cancer (CRC,n=1) (76), glioblastoma (n=1) (77) and mesothelioma (n=2) (78, 79). Among the 41 757 patients in the 59 trials that reported information on treatment-related deaths, no pancreatic-related deaths occurred. All included RCTs had a low risk of bias. A detailed evaluation of the risk of bias for each randomized controlled trial is presented in Supplementary Table 2.
Figure 1 Study flow diagrams. PRISMA flow diagram of systematic review and meta-analysis in PubMed, Emabse, and Cochrane Library up to March 15, 2023. AEs, adverse events; RCTs, randomized controlled trials; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Incidence of pancreatic AEs
A total of 41 757 patients were enrolled in the 59 included RCTs (70 ICI-containing arms), including 23 334 (55.9%) patients in the ICI-containing arms and 18 423 patients in the control arms (44.1%). ICI-containing arms included ICI monotherapy in 30/70 arms, ICI plus chemotherapy or targeted therapy in 32/70 arms, and ICI dual therapy in 8/70 arms. In the included 70 arms, NSCLC was the most common tumor type, accounting for 32.9% (23/70), and GEJC accounted for 10.0% (7/70) as the second most common type.
The incidence was 0.93% (95% CI 0.77-1.13, I²=3.4%) for all-grade pancreatitis and 0.68% (95% CI 0.54-0.85; I²=0) for grade ≥3 pancreatitis. (Figure 2) Compared with ICI monotherapy, dual-ICI therapy had significantly higher incidences of all-grade pancreatitis (1.10% vs 0.70%) and grade ≥3 pancreatitis (0.94% vs 0.58%) (P< 0.05). (Supplementary Table 3) However, it was not observed in the patients undergoing ICI plus chemotherapy or targeted therapy. An overview of the pancreatitis incidence in different tumor types was shown in Supplementary Table 3. Pancreatitis has a roughly similar incidence in different tumor types (G1-5: 0.30-1.79%, G3-5: 0.17-1.12%).
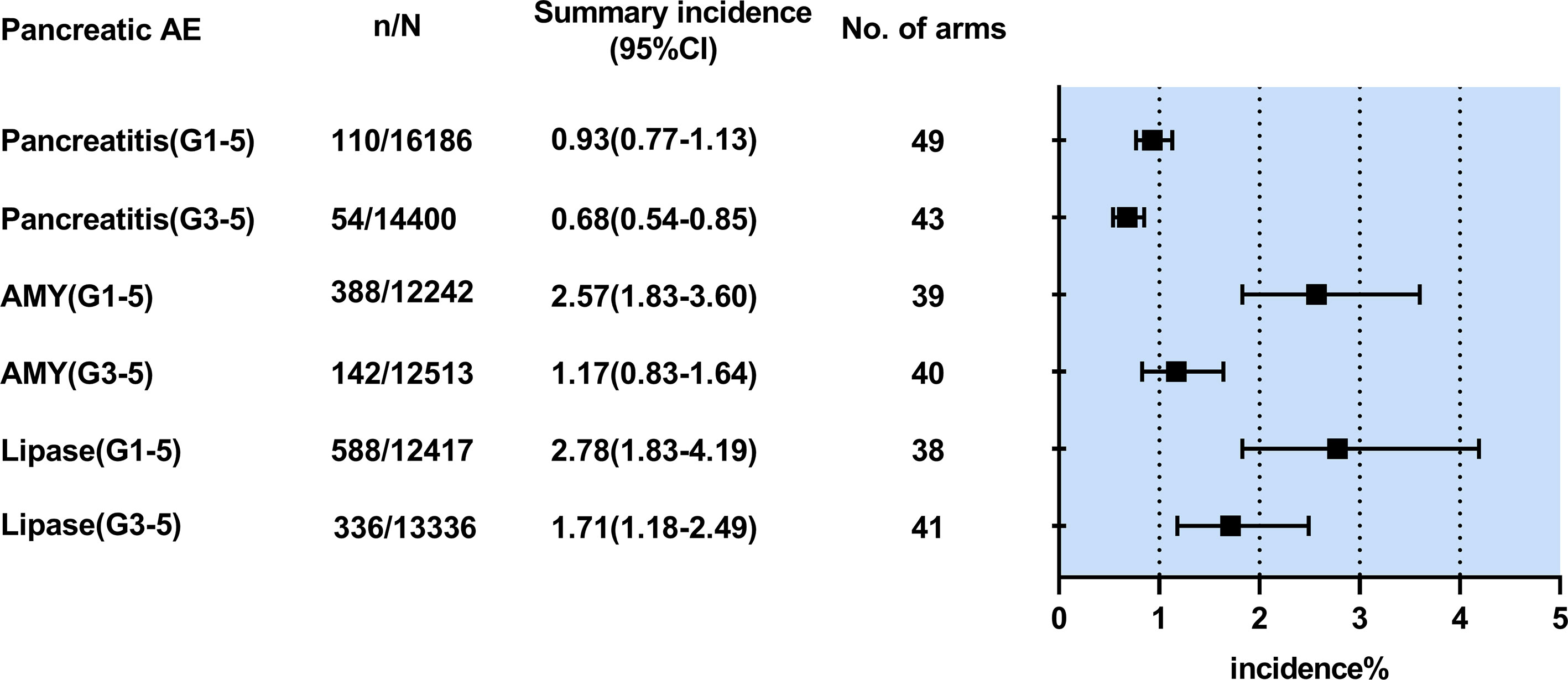
Figure 2 Summary pooled incidence analysis of pancreatic adverse events associated with immune checkpoint inhibitor therapy. n/N refers to the number of events (n) observed for the outcome regarding the overall number of patients (N) in patients treated with immune checkpoint inhibitor therapy. AE,adverse event; CI, confidence interval; AMY,amylase elevation;Lipase, lipase elevation;G1-5,grade1-5;G3-5.grade3-5.
The incidence was 2.57% (1.83-3.60; I²=89.2%) for all-grade amylase elevation and 1.17% (0.83-1.64; I2 =76%) for grade ≥3 amylase elevation. (Figure 2) Compared with ICI monotherapy, dual-ICI therapy had significantly higher incidences of all-grade amylase elevation (3.01% vs 1.66%) and grade ≥3 amylase elevation (1.79% vs 0.78%) (P< 0.05). (Supplementary Table 4)Similar results were found in the patients treated with ICI plus chemotherapy or targeted therapy (G1-5: 3.78% vs 1.66%, G3-5: 1.57% vs 0.78%, P< 0.05). An overview of the amylase elevation incidence in different treatment regimens and tumor types is shown in Supplementary Table 4. The results showed an increased incidence of all-grade and grade ≥3 amylase elevation in patients with melanoma (5.62%,2.75% respectively) and Mesothelioma (5.67%, 2.33% respectively).
The incidence was 2.78% (1.83-4.19, I2 =93%) for all-grade lipase elevation and 1.71% (1.18-2.49, I2 =89%) for grade ≥3 lipase elevation. (Figure 2) Compared with ICI monotherapy, dual-ICI therapy had significantly higher incidences of all-grade lipase elevation (4.08% vs 1.45%) and grade ≥3 lipase elevation (3.28% vs 1.01%) (P< 0.05). (Supplementary Table 5)We found similar outcomes in the patients receiving ICI plus chemotherapy or targeted therapy compared with ICI monotherapy (G1-5: 5.34% vs 1.45%, G3-5: 2.23% vs 1.01%, P< 0.05). An overview of the amylase elevation incidences in different treatment regimens and tumor types is shown in Supplementary Table 5. The patients with melanoma (9.28%,6.14%) are most likely to develop all-grade and grade ≥3 lipase elevation.
Risk of pancreatitis associated with ICI exposure
Pancreatitis as a treatment-related adverse effect was reported in 40 studies (49 ICI-containing arms) and graded using CTCAE. A total of 28 097 patients were evaluated with 16 186 in the ICI-containing arms and 11 911 in the control arms. As shown in Table 2, ICIs significantly increased the risk of all-grade pancreatitis (OR=2.04, 95% CI 1.42-2.94, P = 0.0001; I²=0) and grade ≥3 pancreatitis (OR=1.90, 95% CI 1.15-3.13, P=0.01; I²=0). Subgroup analysis suggested that dual-ICI therapy was associated with a higher incidence risk of all-grade pancreatitis (OR=3.47, 95%CI 1.22-9.91, P=0.02). (Supplementary Table 6) A similar statistically significant difference was found in grade ≥3 pancreatitis(OR=3.56, 95%CI 1.09-11.56, P=0.04). Tumor type-stratified analyses showed an increased risk of all-grade pancreatitis(OR=2.55, 95%CI 1.32-4.92, P=0.005) in patients with NSCLC.
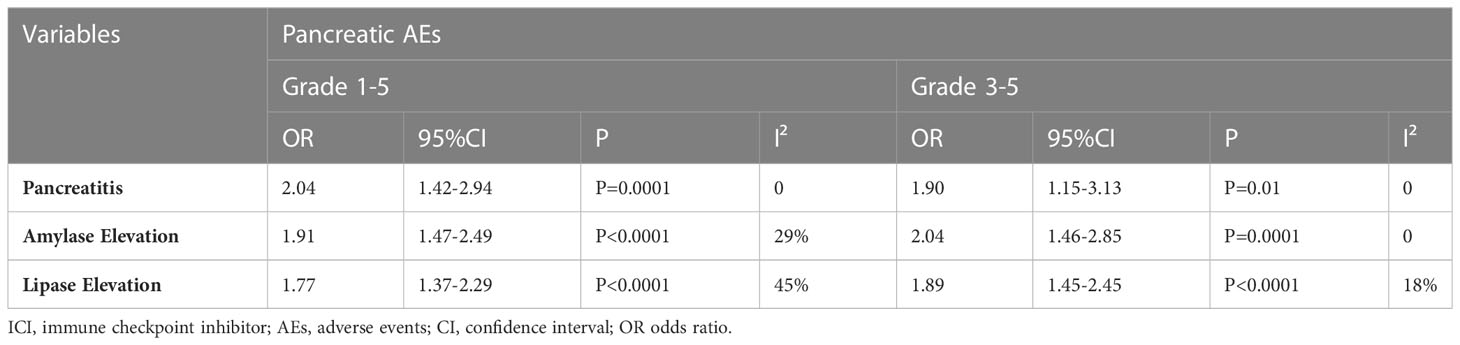
Table 2 Summary pooled analysis on the risk of ICI therapy-associated pancreatic adverse events vs. controls in randomized controlled trials.
Risk of amylase elevation associated with ICI exposure
Amylase elevation as a treatment-related adverse effect was reported in 33 studies (41 ICI-containing arms) and graded using CTCAE. A total of 22 390 patients were evaluated with 12 893 in the ICI-containing arms and 9 497 in the control arms. As shown in Table 2, ICIs significantly increased the risk of all-grade amylase elevation (OR=1.91, 95% CI 1.47-2.49, P < 0.0001; I²= 29%) and grade ≥3 amylase elevation (OR=2.04, 95% CI 1.46-2.85, P=0.0001; I² = 0). Subgroup analysis suggested that all three therapies that include ICI could significantly increase the incidence risk of all-grade amylase elevation (OR=1.86, 95% CI 1.28-2.69, P=0.001; OR=1.60, 95% CI 1.09-2.35, P=0.02 and OR=3.79, 95% CI 1.68-8.57, P=0.001, respectively). (Supplementary Table 7) Tumor type-stratified analyses showed an increased risk of all-grade amylase elevation in patients with SCLC (OR=4.10,95% CI 1.44-11.63, P=0.008), UC (OR=4.64,95% CI 1.30-16.49, P=0.02), RCC (OR=1.71,95% CI 1.06-2.74, P=0.03), HNSCC (OR=4.00,95% CI 1.55-10.33, P=0.004) and mesothelioma (OR=17.00,95% CI 2.25-128.60, P=0.006).
Risk of lipase elevation associated with ICI exposure
Lipase elevation as a treatment-related adverse effect was reported in 32 studies (40 ICI-containing arms) and graded using CTCAE. A total of 23 461 patients were evaluated with 13 336 in the ICI-containing arms and 10 125 in control arms. As shown in Table 2, ICIs significantly increased the risk of all-grade lipase elevation (OR=1.77, 95% CI 1.37-2.29, P < 0.0001; I²= 45%) and grade ≥3 lipase elevation (OR=1.89, 95% CI 1.45-2.45, P< 0.0001; I² = 18%). Subgroup analysis suggested that both ICI plus chemotherapy or targeted therapy and dual-ICI therapy could significantly increase the incidence risk of all-grade lipase elevation (OR=1.72, 95% CI 1.34-2.20, P<0.0001, and OR=2.92, 95% CI 1.37-6.20, P=0.005 respectively). (Supplementary Table 8) As for grade ≥3 lipase elevation, the trends are similar to those of the all-grade lipase elevation groups. At the same time, we observed a significant increase in the risk of all-grade lipase elevation in the patient with NSCLC (OR=4.23,95% CI 2.14-8.34, P<0.0001), UC (OR=4.20,95% CI 1.46-12.09, P=0.008), RCC (OR=1.53,95% CI 1.16-2.01, P=0.003), and OC (OR=3.42,95% CI 1.17-9.97, P=0.02).
Post-hoc analyses
In this study, we conducted post-hoc analyses of PD-1/PD-L1 inhibitors related to pancreatic AEs. As shown in Table 3, the patients with UC undergoing PD-1 inhibitors were at a significantly higher risk of all-grade amylase elevation (OR=5.24,95% CI 2.59-10.57, P<0.0001), all-grade lipase elevation (OR=4.90,95% CI 1.97-12.18, P=0.0006) and grade ≥3 lipase elevation (OR=3.88,95% CI 1.50-10.04, P=0.005), than the patients with UC receiving PD-L1 inhibitors. We conducted post-hoc analyses of dual ICI therapy/single ICI therapy-related pancreatic AEs. As shown in Table 4, the patients with NSCLC undergoing dual ICI therapy were at a significantly higher risk of all-grade pancreatitis (OR=4.72,95% CI 1.11-20.17, P=0.04), grade ≥3 pancreatitis (OR= 14.98,95% CI 1.82-123.34, P= 0.01), grade ≥3 amylase elevation (OR=5.95,95% CI 1.30-27.24, P=0.02) and all-grade lipase elevation (OR=4.99,95% CI 1.99-12.55, P=0.0006), than the patients with NSCLC receiving single ICI therapy.

Table 3 Odds ratios comparing pancreatic irAEs in patients who received anti-PD-1- vs anti-PD-L1-based therapies.
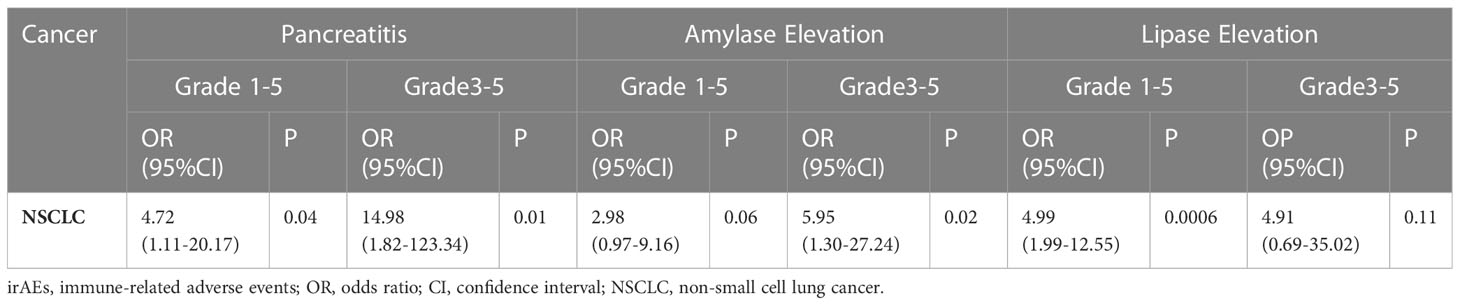
Table 4 Odds ratios comparing pancreatic irAEs in patients who received dual ICI therapy - vs single ICI therapy -based therapies.
Quality of included studies
Given the significant heterogeneity in the meta-analysis of all the included studies, we performed subgroup analyses to better understand the heterogeneity. (Supplementary Table 9) Some study heterogeneity was suggested by the assessment of all-grade amylase elevation (I² = 36%), which appeared to be concentrated in the studies of NSCLC (I² = 59%), GJEC (I² = 42%) and UC (I² = 54%). A similar situation could also be observed with the group of all-grade lipase elevation (I²=46%) and grade 3 or higher lipase elevation (I²=26%).
No obvious asymmetry was seen in classic funnel plots, indicating that no evidence of significant publication bias existed. Beyond this, the above view was confirmed by Peter’s test. (Supplementary Table 10).
Discussion
In our meta-analysis, we investigated the incidence and risk of pancreatic irAEs associated with ICIs, including pancreatitis, amylase elevation, and lipase elevation. Our findings demonstrated that the incidence of all-grade and grade≥3 pancreatitis with ICIs were 0.93% and 0.68%, respectively. These rates were consistent with previous studies reporting rates of pancreatitis (CTLA-4: 0.9–3%, PD-1: 0.5–1.6%, CTLA4 + PD-1: 1.2–2.1%) (11). Our results also showed that patients treated with dual ICIs therapy had a higher incidence of pancreatitis compared to those treated with monotherapy, and the combination of ICI monotherapy with chemotherapy, targeted therapy, or immunotherapy increased the incidence of pancreatic enzyme elevation. Moreover, our study revealed that melanoma patients had the highest incidence of amylase elevation (G1-5: 5.62%, G3-5: 2.75%) and all-grade and grade 3 or higher lipase elevation (G1-5: 9.28%, G3-5: 6.14%) after receiving immunotherapy.
Our study findings revealed a significant increase in the incidence of pancreatitis, regardless of all grades or grades 3-5, in the ICI group compared to standard chemotherapy or targeted therapy. Further, our subgroup analysis identified a tumor-specific preference for pancreatitis, which was more likely to occur in HCC. Our data suggested that ICI monotherapy did not increase the risk of immune-related pancreatitis, whereas ICI combination therapy did. This may be attributed to the potential of chemotherapeutic agents and targeted drugs to exacerbate pancreatic damage from ICIs. Notably, our data indicated a higher likelihood of pancreatitis in the ICI dual therapy group (G1-5: OR=3.47, 95% CI 1.22-9.91, P=0.02; G3-5: OR=3.56, 95% CI 1.09-11.65, P=0.04). Therefore, additional multi-center RCTs are warranted to confirm its statistical significance. Our results align with previous studies (11, 82, 83).
According to many experts, pancreatitis is more likely to occur in the early stages with low grades, but can be controlled with aggressive intravenous fluid replacement (84, 85). Routine monitoring of amylase and lipase is not recommended for asymptomatic patients unless pancreatitis is clinically suspected (85). However, one study suggests that the use of ICI may increase the risk of developing grade 3 or higher pancreatitis, with clinical symptoms including loss of appetite, vomiting, and abdominal pain (86). Additionally, a case report described a 65-year-old man with stage IV melanoma who developed grade 3 pancreatitis while receiving ipilimumab and pembrolizumab (87). Despite the resolution of clinical signs and symptoms, the patient was diagnosed with pancreatic insufficiency. Interestingly, it seemed that diabetes was also associated with pancreatitis. One study showed that both immune-related pancreatitis and immune-related diabetes occurred earlier than monotherapy when two ICIs were combined, and immune-related diabetes had a later onset than immune-related pancreatitis (88), suggesting that the onset of diabetes might also be a complication of immune-related pancreatitis (89). In order to improve the quality of life and to avoid the long-term sequelae of pancreatitis in patients who have used ICI, vigilant monitoring should be warranted (90).
So far, the exact mechanism of immune-related pancreatitis remains under investigation, and the potential mechanisms may include the increased activity of T cells against antigens present on tumors and normal tissues,the increase in the concentration of pre-existing autoimmune antibodies and the increased levels of inflammatory cytokines (91). Immunohistochemical staining demonstrated a large infiltration of CD3+ T lymphocytes in the non-tumor regions of the pancreas from patients with immune-related pancreatitis (92, 93), which suggested that the potential asscociation of immune-related pancreatitis with autoimmune pancreatitis (AIP) (94). The clinical presentation of AIP differred from that of acute pancreatitis in that abdominal pain and nausea was milder, and positive imaging might be delayed (95).
It is worth noting that despite their widespread use, steroids were not found to be effective in treating immune-related pancreatitis in terms of preventing short- or long-term adverse outcomes, or improving overall survival (84). In fact, exposure to a baseline dose of prednisone equivalent to at least 10 mg/d was found to reduce the efficacy benefit of ICI and significantly shorten progression-free survival (PFS) and overall survival (OS) in NSCLC patients (96). Patients with immune-related pancreatitis were reported to be at risk of relapse upon the resumption of ICI therapy (97). Nonetheless, in general, immunotherapy may be resumed when toxicity returns to grade 1 or lower (85). Our study found that amylase and lipase elevations were more frequent in the ICI group, suggesting a potential immune-related mechanism. Subgroup analyses revealed a significantly higher incidence of all-grade amylase and lipase elevations in melanoma patients. The tumor-specific preference for immune-related elevation of pancreatic enzymes and pancreatitis was similar, with both showing a predilection for NSCLC and UC, as demonstrated by grouping methods based on tumor type or ICI regimen. However, non-specific elevations of pancreatic enzymes due to factors such as alcohol consumption, bowel obstruction, or kidney failure may also occur, leading to a potential overestimation of the incidence of immune-related elevations (98, 99). Nevertheless, unlike pancreatitis, our study provided compelling evidence of a plausible causal association between ICI therapy and elevations of amylase and lipase. We hypothesized that ICI therapy may result in weak pancreatic injury, such as enzyme elevations, rather than robust injury like immune-related pancreatitis. Nonetheless, the decision to continue ICI therapy in patients with grade 3 or higher amylase or lipase elevations without clinical or imaging evidence of pancreatitis after immunotherapy requires further investigation.
It is assumed that the elevation of pancreatic enzymes is associated with pancreatitis and could implicate its development. Research has shown that elevated amylase levels increase the risk of pancreatitis (100). Additionally, 39% of patients with grade 3 or higher lipase elevations had significant clinical symptoms of pancreatitis (84), which was consistent with a retrospective study of 21 cases of immune-related lipase elevations (101). Patients with clinically symptomatic immune-related pancreatitis had higher mean peak serum lipase levels than those without clinical symptoms, but this was not the case in patients with other causes of acute pancreatitis (100). These studies demonstrated that elevated pancreatic enzyme values do not determine the severity of pancreatitis but indicate an increased risk. However, another study found that the true incidence of pancreatitis in patients with immune-related lipase elevations was only 14%, suggesting that in patients with elevated immune-related lipase without clinical symptoms, pancreatic X-ray abnormalities, and diabetes mellitus by fasting blood glucose, the lipase increase may be regarded as a non-clinically significant event (101). Further clinical trials are needed to confirm these findings.
In the post-hoc analysis, the findings indicated that PD-1 inhibitors had a significantly higher risk of pancreatic AEs compared to PD-L1 inhibitors, consistent with other immune-related adverse events, such as pneumonitis (15). Furthermore, the study revealed a statistically significant increase in the incidence of pancreatic AEs with dual-ICI therapy relative to single-ICI therapy, possibly due to the similarity in toxicity profiles of CTLA-4 inhibitors and PD-1 inhibitors. In Phase II and III trials of patients with nonresectable melanoma who were randomized to combination versus monotherapy, grade 3 or 4 adverse events occurred in 55–59% of the patients receiving combination therapy, as compared with 16–21% with nivolumab alone and 27–28% with ipilimumab alone (102, 103). Therefore, it is important to be vigilant about the occurrence of irAEs when using dual-ICI therapy, including monitoring pancreatic enzymes.
The study had several limitations. Firstly, our meta-analysis was based on phase III RCTs with strict inclusion criteria, which may limit the generalizability of the findings to real-world settings. Secondly, we may have missed some pancreatic AE cases, as we only analyzed cases recorded in the main text and appendix, which could result in reporting bias (104). Furthermore, some studies included in the analysis were open-label. Thirdly, individual patient data was not available, which prevented us from analyzing the relationship between pancreatic enzyme elevations and pancreatitis or linking immune-related pancreatitis with other irAEs. Lastly, although we acknowledged that drug dose might affect the incidence of irAEs, we were unable to conduct subgroup analyses due to the wide variation in drugs and doses across studies.
Conclusion
Our study offers a comprehensive overview of the incidence and risk of ICI-associated pancreatitis and pancreatic enzyme elevations in various solid tumor types and treatment combinations. Moreover, the post-hoc analysis revealed that PD-1 inhibitors have a significantly higher risk of pancreatic AEs than PD-L1 inhibitors, and patients receiving dual ICI therapy have a significantly higher risk of pancreatic AEs than those receiving single ICI therapy. These findings should enhance clinicians’ awareness of ICI-associated pancreatic AEs in their clinical practice.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
JL, ZZ, and LP designed the search strategy and confirmed the inclusion criteria. ZZ, WZ, LZ, and SL searched the database, selected the articles, and collected the data. ZZ, LP, WZ, LZ, and SL completed the quality assessment that JL checked. ZZ, LP, WZ, LZ, and SL finished data synthesis and statistics. ZZ write-original draft preparation. JL revised the manuscript carefully. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Natural Science Foundation of Shandong Province (ZR2020MH210).
Acknowledgments
Thanks to the support of the Natural Science Foundation of Shandong Province.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1166299/full#supplementary-material
References
1. Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science (2018) 359(6382):1350–5. doi: 10.1126/science.aar4060
2. Tang J, Shalabi A, Hubbard-Lucey V. Comprehensive analysis of the clinical immuno-oncology landscape. Ann Oncol (2018) 29(1):84–91. doi: 10.1093/annonc/mdx755
3. Tirumani SH, Ramaiya NH, Keraliya A, Bailey ND, Ott PA, Hodi FS, et al. Radiographic profiling of immune-related adverse events in advanced melanoma patients treated with ipilimumab. Cancer Immunol Res (2015) 3(10):1185–92. doi: 10.1158/2326-6066.CIR-15-0102
4. Darnell EP, Mooradian MJ, Baruch EN, Yilmaz M, Reynolds KL. Immune-related adverse events (irAEs): diagnosis, management, and clinical pearls. Curr Oncol Rep (2020) 22(4):39. doi: 10.1007/s11912-020-0897-9
5. Postow MA, Callahan MK, Wolchok JD. Immune checkpoint blockade in cancer therapy. J Clin Oncol (2015) 33(17):1974–82. doi: 10.1200/JCO.2014.59.4358
6. Wang PF, Chen Y, Song SY, Wang TJ, Ji WJ, Li SW, et al. Immune-related adverse events associated with anti-PD-1/PD-L1 treatment for malignancies: a meta-analysis. Front Pharmacol (2017) 8:730. doi: 10.3389/fphar.2017.00730
7. Thompson JA. New NCCN guidelines: recognition and management of immunotherapy-related toxicity. J Natl Compr Canc Netw (2018) 16(5s):594–6. doi: 10.6004/jnccn.2018.0047
8. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Internal Med (2009) 151(4):264–9, w64. doi: 10.1371/journal.pmed.1000097
9. Amir-Behghadami M, Janati A. Population, intervention, comparison, outcomes and study (PICOS) design as a framework to formulate eligibility criteria in systematic reviews. Emerg Med J (2020) 37(6):387. doi: 10.1136/emermed-2020-209567
10. Cancer therapy evaluation program (CTEP) . Available at: https://ctep.cancer.gov/.
11. Su Q, Zhang XC, Zhang CG, Hou YL, Yao YX, Cao BW. Risk of immune-related pancreatitis in patients with solid tumors treated with immune checkpoint inhibitors: systematic assessment with meta-analysis. J Immunol Res (2018) 2018:1027323. doi: 10.1155/2018/1027323
12. Sedgwick P. Relative risks versus odds ratios. BMJ (Online) (2014) 348(feb07 2):g1407. doi: 10.1136/bmj.g1407
13. Ranganathan P, Aggarwal R, Pramesh CS. Common pitfalls in statistical analysis: odds versus risk. Perspect Clin Res (2015) 6(4):222–4. doi: 10.4103/2229-3485.167092
14. DerSimonian R, Laird N. Meta-analysis in clinical trials revisited. Contemp Clin Trials (2015) 45(Pt A):139–45. doi: 10.1016/j.cct.2015.09.002
15. Duan J, Cui L, Zhao X, Bai H, Cai S, Wang G, et al. Use of immunotherapy with programmed cell death 1 vs programmed cell death ligand 1 inhibitors in patients with cancer: a systematic review and meta-analysis. JAMA Oncol (2020) 6(3):375–84. doi: 10.1001/jamaoncol.2019.5367
16. Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane handbook for systematic reviews of interventions version 6.3 (updated February 2022). Cochrane (2022). Available at: www.training.cochrane.org/handbook.
17. Harbord RM, Egger M, Sterne JA. A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Stat Med (2006) 25(20):3443–57. doi: 10.1002/sim.2380
18. Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ (Clinical Res ed) (2011) 343:d4002. doi: 10.1136/bmj.d4002
19. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The cochrane collaboration’s tool for assessing risk of bias in randomised trials. Bmj (2011) 343:d5928. doi: 10.1136/bmj.d5928
20. Viechtbauer W. Conducting meta-analyses in r with the metafor package. J Stat Software (2010) 36(3):1–48. doi: 10.18637/jss.v036.i03
21. Planchard D, Reinmuth N, Orlov S, Fischer JR, Sugawara S, Mandziuk S, et al. ARCTIC: durvalumab with or without tremelimumab as third-line or later treatment of metastatic non-small-cell lung cancer. Ann Oncol (2020) 31(5):609–18. doi: 10.1016/j.annonc.2020.02.006
22. Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, et al. Updated analysis of KEYNOTE-024: pembrolizumab versus platinum-based chemotherapy for advanced non-Small-Cell lung cancer with PD-L1 tumor proportion score of 50% or greater. J Clin Oncol (2019) 37(7):537–46. doi: 10.1200/JCO.18.00149
23. Reck M, Wehler T, Orlandi F, Nogami N, Barone C, Moro-Sibilot D, et al. Safety and patient-reported outcomes of atezolizumab plus chemotherapy with or without bevacizumab versus bevacizumab plus chemotherapy in non-small-cell lung cancer. J Clin Oncol (2020) 38(22):2530–42. doi: 10.1200/JCO.19.03158
24. Wu YL, Lu S, Cheng Y, Zhou C, Wang J, Mok T, et al. Nivolumab versus docetaxel in a predominantly Chinese patient population with previously treated advanced NSCLC: CheckMate 078 randomized phase III clinical trial. J Thorac Oncol (2019) 14(5):867–75. doi: 10.1016/j.jtho.2019.01.006
25. Rizvi NA, Cho BC, Reinmuth N, Lee KH, Luft A, Ahn MJ, et al. Durvalumab with or without tremelimumab vs standard chemotherapy in first-line treatment of metastatic non-small cell lung cancer: the MYSTIC phase 3 randomized clinical trial. JAMA Oncol (2020) 6(5):661–74. doi: 10.1001/jamaoncol.2020.0237
26. Jotte R, Cappuzzo F, Vynnychenko I, Stroyakovskiy D, Rodríguez-Abreu D, Hussein M, et al. Atezolizumab in combination with carboplatin and nab-paclitaxel in advanced squamous NSCLC (IMpower131): results from a randomized phase III trial. J Thorac Oncol (2020) 15(8):1351–60. doi: 10.1016/j.jtho.2020.03.028
27. Nishio M, Barlesi F, West H, Ball S, Bordoni R, Cobo M, et al. Atezolizumab plus chemotherapy for first-line treatment of nonsquamous NSCLC: results from the randomized phase 3 IMpower132 trial. J Thorac Oncol (2021) 16(4):653–64. doi: 10.1016/j.jtho.2020.11.025
28. Yang Y, Wang Z, Fang J, Yu Q, Han B, Cang S, et al. Efficacy and safety of sintilimab plus pemetrexed and platinum as first-line treatment for locally advanced or metastatic nonsquamous NSCLC: a randomized, double-blind, phase 3 study (Oncology pRogram by InnovENT anti-PD-1-11). J Thorac Oncol (2020) 15(10):1636–46. doi: 10.1016/j.jtho.2020.07.014
29. Felip E, Altorki N, Zhou C, Csőszi T, Vynnychenko I, Goloborodko O, et al. Adjuvant atezolizumab after adjuvant chemotherapy in resected stage IB-IIIA non-small-cell lung cancer (IMpower010): a randomised, multicentre, open-label, phase 3 trial. Lancet (2021) 398(10308):1344–57. doi: 10.1016/S0140-6736(21)02098-5
30. Gandhi L, Rodríguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, et al. Pembrolizumab plus chemotherapy in metastatic non-Small-Cell lung cancer. N Engl J Med (2018) 378(22):2078–92. doi: 10.1056/NEJMoa1801005
31. West H, McCleod M, Hussein M, Morabito A, Rittmeyer A, Conter HJ, et al. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol (2019) 20(7):924–37. doi: 10.1016/S1470-2045(19)30167-6
32. Paz-Ares L, Ciuleanu TE, Cobo M, Schenker M, Zurawski B, Menezes J, et al. First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in patients with non-small-cell lung cancer (CheckMate 9LA): an international, randomised, open-label, phase 3 trial. Lancet Oncol (2021) 22(2):198–211. doi: 10.1016/S1470-2045(20)30641-0
33. Sezer A, Kilickap S, Gümüş M, Bondarenko I, Özgüroğlu M, Gogishvili M, et al. Cemiplimab monotherapy for first-line treatment of advanced non-small-cell lung cancer with PD-L1 of at least 50%: a multicentre, open-label, global, phase 3, randomised, controlled trial. Lancet (2021) 397(10274):592–604. doi: 10.1016/S0140-6736(21)00228-20
34. Mok TSK, Wu YL, Kudaba I, Kowalski DM, Cho BC, Turna HZ, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet (2019) 393(10183):1819–30. doi: 10.1016/S0140-6736(18)32409-7
35. Wang Z, Wu L, Li B, Cheng Y, Li X, Wang X, et al. Toripalimab plus chemotherapy for patients with treatment-naive advanced non-Small-Cell lung cancer: a multicenter randomized phase III trial (CHOICE-01). J Clin Oncol (2023) 41(3):651–63. doi: 10.1200/JCO.22.00727
36. O’Brien M, Paz-Ares L, Marreaud S, Dafni U, Oselin K, Havel L, et al. Pembrolizumab versus placebo as adjuvant therapy for completely resected stage IB-IIIA non-small-cell lung cancer (PEARLS/KEYNOTE-091): an interim analysis of a randomised, triple-blind, phase 3 trial. Lancet Oncol (2022) 23(10):1274–86. doi: 10.1016/S1470-2045(22)00518-6
37. Gogishvili M, Melkadze T, Makharadze T, Giorgadze D, Dvorkin M, Penkov K, et al. Cemiplimab plus chemotherapy versus chemotherapy alone in non-small cell lung cancer: a randomized, controlled, double-blind phase 3 trial. Nat Med (2022) 28(11):2374–80. doi: 10.1038/s41591-022-01977-y
38. de Castro G Jr., Rizvi NA, Schmid P, Syrigos K, Martin C, Yamamoto N, et al. NEPTUNE: phase 3 study of first-line durvalumab plus tremelimumab in patients with metastatic NSCLC. J Thorac Oncol (2023) 18(1):106–19. doi: 10.1016/j.jtho.2022.09.223
39. Peters S, Dziadziuszko R, Morabito A, Felip E, Gadgeel SM, Cheema P, et al. Atezolizumab versus chemotherapy in advanced or metastatic NSCLC with high blood-based tumor mutational burden: primary analysis of BFAST cohort c randomized phase 3 trial. Nat Med (2022) 28(9):1831–9. doi: 10.1038/s41591-022-01933-w
40. Reck M, Luft A, Szczesna A, Havel L, Kim SW, Akerley W, et al. Phase III randomized trial of ipilimumab plus etoposide and platinum versus placebo plus etoposide and platinum in extensive-stage small-cell lung cancer. J Clin Oncol (2016) 34(31):3740–8. doi: 10.1200/JCO.2016.67.6601
41. Rudin CM, Awad MM, Navarro A, Gottfried M, Peters S, Csőszi T, et al. Pembrolizumab or placebo plus etoposide and platinum as first-line therapy for extensive-stage small-cell lung cancer: randomized, double-blind, phase III KEYNOTE-604 study. J Clin Oncol (2020) 38(21):2369–79. doi: 10.1200/JCO.20.00793
42. Goldman JW, Dvorkin M, Chen Y, Reinmuth N, Hotta K, Trukhin D, et al. Durvalumab, with or without tremelimumab, plus platinum-etoposide versus platinum-etoposide alone in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): updated results from a randomised, controlled, open-label, phase 3 trial. Lancet Oncol (2021) 22(1):51–65. doi: 10.1016/S1470-2045(20)30539-8
43. Larkin J, Minor D, D’Angelo S, Neyns B, Smylie M, Miller WH Jr., et al. Overall survival in patients with advanced melanoma who received nivolumab versus investigator’s choice chemotherapy in CheckMate 037: a randomized, controlled, open-label phase III trial. J Clin Oncol (2018) 36(4):383–90. doi: 10.1200/JCO.2016.71.8023
44. Ribas A, Kefford R, Marshall MA, Punt CJ, Haanen JB, Marmol M, et al. Phase III randomized clinical trial comparing tremelimumab with standard-of-care chemotherapy in patients with advanced melanoma. J Clin Oncol (2013) 31(5):616–22. doi: 10.1200/JCO.2012.44.6112
45. Gutzmer R, Stroyakovskiy D, Gogas H, Robert C, Lewis K, Protsenko S, et al. Atezolizumab, vemurafenib, and cobimetinib as first-line treatment for unresectable advanced BRAFV600 mutation-positive melanoma (IMspire150): primary analysis of the randomised, double-blind, placebo-controlled, phase 3 trial. Lancet (2020) 395(10240):1835–44. doi: 10.1016/S0140-6736(20)30934-X
46. Weber JS, D’Angelo SP, Minor D, Hodi FS, Gutzmer R, Neyns B, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol (2015) 16(4):375–84. doi: 10.1016/S1470-2045(15)70076-8
47. Atkins MB, Lee SJ, Chmielowski B, Tarhini AA, Cohen GI, Truong TG, et al. Combination dabrafenib and trametinib versus combination nivolumab and ipilimumab for patients with advanced BRAF-mutant melanoma: the DREAMseq trial-ECOG-ACRIN EA6134. J Clin Oncol (2023) 41(2):186–97. doi: 10.1200/JCO.22.01763
48. Long GV, Luke JJ, Khattak MA, de la Cruz Merino L, Del Vecchio M, Rutkowski P, et al. Pembrolizumab versus placebo as adjuvant therapy in resected stage IIB or IIC melanoma (KEYNOTE-716): distant metastasis-free survival results of a multicentre, double-blind, randomised, phase 3 trial. Lancet Oncol (2022) 23(11):1378–88. doi: 10.1016/S0140-6736(22)00562-1
49. Bang YJ, Ruiz EY, Van Cutsem E, Lee KW, Wyrwicz L, Schenker M, et al. Randomised trial of avelumab versus physician’s choice of chemotherapy as third-line treatment of patients with advanced gastric or gastro-oesophageal junction cancer: primary analysis of JAVELIN gastric 300. Ann Oncol (2018) 29(10):2052–60. doi: 10.1093/annonc/mdy264
50. Moehler Markus. Phase III trial of avelumab maintenance after first-line induction chemotherapy versus continuation of chemotherapy in patients with gastric cancers: results from JAVELIN gastric 100. J Clin Oncol (2020) 39(9):966–77. doi: 10.1200/JCO.20.00892
51. Shitara K, Van Cutsem E, Bang YJ, Fuchs C, Wyrwicz L, Lee KW, et al. Efficacy and safety of pembrolizumab or pembrolizumab plus chemotherapy vs chemotherapy alone for patients with first-line, advanced gastric cancer: the KEYNOTE-062 phase 3 randomized clinical trial. JAMA Oncol (2020) 6(10):1571–80. doi: 10.1001/jamaoncol.2020.3370
52. Janjigian YY, Shitara K, Moehler M, Garrido M, Salman P, Shen L, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet (2021) 398(10294):27–40. doi: 10.1016/S0140-6736(21)00797-2
53. Kang Yoon-Koo. Nivolumab plus chemotherapy versus placebo plus chemotherapy in patients with HER2-negative, untreated, unresectable advanced or recurrent gastric or gastro-oesophageal junction cancer (ATTRACTION-4): a randomised, multicentre, double-blind, placebo-controlled, phase 3 trial. (2021). doi: 10.1016/S1470-2045(21)00692-6
54. Shitara K, Özgüroğlu M, Bang YJ, Di Bartolomeo M, Mandalà M, Ryu MH, et al. Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): a randomised, open-label, controlled, phase 3 trial. Lancet (2018) 392(10142):123–33. doi: 10.1016/S0140-6736(18)31257-1
55. Bajorin DF, Witjes JA, Gschwend JE, Schenker M, Valderrama BP, Tomita Y, et al. Adjuvant nivolumab versus placebo in muscle-invasive urothelial carcinoma. N Engl J Med (2021) 384(22):2102–14. doi: 10.1056/NEJMoa2034442
56. Bellmunt J, Hussain M, Gschwend JE, Albers P, Oudard S, Castellano D, et al. Adjuvant atezolizumab versus observation in muscle-invasive urothelial carcinoma (IMvigor010): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol (2021) 22(4):525–37. doi: 10.1016/S1470-2045(21)00004-8
57. Powles T, van der Heijden MS, Castellano D, Galsky MD, Loriot Y, Petrylak DP, et al. Durvalumab alone and durvalumab plus tremelimumab versus chemotherapy in previously untreated patients with unresectable, locally advanced or metastatic urothelial carcinoma (DANUBE): a randomised, open-label, multicentre, phase 3 trial. Lancet Oncol (2020) 21(12):1574–88. doi: 10.1016/S1470-2045(20)30541-6
58. Powles T, Csőszi T, Özgüroğlu M, Matsubara N, Géczi L, Cheng SY, et al. Pembrolizumab alone or combined with chemotherapy versus chemotherapy as first-line therapy for advanced urothelial carcinoma (KEYNOTE-361): a randomised, open-label, phase 3 trial. Lancet Oncol (2021) 22(7):931–45. doi: 10.1016/S1470-2045(21)00152-2
59. Motzer RJ, Tannir NM, McDermott DF, Arén Frontera O, Melichar B, Choueiri TK, et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med (2018) 378(14):1277–90. doi: 10.1056/NEJMoa1712126
60. Choueiri TK, Powles T, Burotto M, Escudier B, Bourlon MT, Zurawski B, et al. Nivolumab plus cabozantinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med (2021) 384(9):829–41. doi: 10.1056/NEJMoa2026982
61. Powles T, Plimack ER, Soulières D, Waddell T, Stus V, Gafanov R, et al. Pembrolizumab plus axitinib versus sunitinib monotherapy as first-line treatment of advanced renal cell carcinoma (KEYNOTE-426): extended follow-up from a randomised, open-label, phase 3 trial. Lancet Oncol (2020) 21(12):1563–73. doi: 10.1016/S1470-2045(20)30436-8
62. Pal SK, Uzzo R, Karam JA, Master VA, Donskov F, Suarez C, et al. Adjuvant atezolizumab versus placebo for patients with renal cell carcinoma at increased risk of recurrence following resection (IMmotion010): a multicentre, randomised, double-blind, phase 3 trial. Lancet (2022) 400(10358):1103–16. doi: 10.1016/S0140-6736(22)01658-0
63. Mittendorf EA, Zhang H, Barrios CH, Saji S, Jung KH, Hegg R, et al. Neoadjuvant atezolizumab in combination with sequential nab-paclitaxel and anthracycline-based chemotherapy versus placebo and chemotherapy in patients with early-stage triple-negative breast cancer (IMpassion031): a randomised, double-blind, phase 3 trial. Lancet (2020) 396(10257):1090–100. doi: 10.1016/S0140-6736(20)31953-X
64. Burtness B, Harrington KJ, Greil R, Soulières D, Tahara M, de Castro G Jr., et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet (2019) 394(10212):1915–28. doi: 10.1016/S0140-6736(19)32591-7
65. Cohen EEW, Soulières D, Le Tourneau C, Dinis J, Licitra L, Ahn MJ, et al. Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): a randomised, open-label, phase 3 study. Lancet (2019) 393(10167):156–67. doi: 10.1016/S0140-6736(18)31999-8
66. Lee NY, Ferris RL, Psyrri A, Haddad RI, Tahara M, Bourhis J, et al. Avelumab plus standard-of-care chemoradiotherapy versus chemoradiotherapy alone in patients with locally advanced squamous cell carcinoma of the head and neck: a randomised, double-blind, placebo-controlled, multicentre, phase 3 trial. Lancet Oncol (2021) 22(4):450–62. doi: 10.1016/S1470-2045(20)30737-3
67. Kwon ED, Drake CG, Scher HI, Fizazi K, Bossi A, van den Eertwegh AJ, et al. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol (2014) 15(7):700–12. doi: 10.1016/S1470-2045(14)70189-5
68. Ren Z, Xu J, Bai Y, Xu A, Cang S, Du C, et al. Sintilimab plus a bevacizumab biosimilar (IBI305) versus sorafenib in unresectable hepatocellular carcinoma (ORIENT-32): a randomised, open-label, phase 2–3 study. Lancet Oncol (2021) 22(7):977–90. doi: 10.1016/S1470-2045(21)00252-7
69. Cheng AL, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Updated efficacy and safety data from IMbrave150: atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J Hepatol (2022) 76(4):862–73. doi: 10.1016/j.jhep.2021.11.030
70. Kelley RK, Rimassa L, Cheng AL, Kaseb A, Qin S, Zhu AX, et al. Cabozantinib plus atezolizumab versus sorafenib for advanced hepatocellular carcinoma (COSMIC-312): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol (2022) 23(8):995–1008. doi: 10.1016/S1470-2045(22)00326-6
71. Huang J, Xu J, Chen Y, Zhuang W, Zhang Y, Chen Z, et al. Camrelizumab versus investigator’s choice of chemotherapy as second-line therapy for advanced or metastatic oesophageal squamous cell carcinoma (ESCORT): a multicentre, randomised, open-label, phase 3 study. Lancet Oncol (2020) 21(6):832–42. doi: 10.1016/S1470-2045(20)30110-8
72. Sun JM, Shen L, Shah MA, Enzinger P, Adenis A, Doi T, et al. Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): a randomised, placebo-controlled, phase 3 study. Lancet (2021) 398(10302):759–71. doi: 10.1016/S0140-6736(21)01234-4
73. Moore KN, Bookman M, Sehouli J, Miller A, Anderson C, Scambia G, et al. Atezolizumab, bevacizumab, and chemotherapy for newly diagnosed stage III or IV ovarian cancer: placebo-controlled randomized phase III trial (IMagyn050/GOG 3015/ENGOT-OV39). J Clin Oncol (2021) 39(17):1842–55. doi: 10.1200/JCO.21.00306
74. Pujade-Lauraine E, Fujiwara K, Ledermann JA, Oza AM, Kristeleit R, Ray-Coquard IL, et al. Avelumab alone or in combination with chemotherapy versus chemotherapy alone in platinum-resistant or platinum-refractory ovarian cancer (JAVELIN ovarian 200): an open-label, three-arm, randomised, phase 3 study. Lancet Oncol (2021) 22(7):1034–46. doi: 10.1016/S1470-2045(21)00216-3
75. Monk BJ, Colombo N, Oza AM, Fujiwara K, Birrer MJ, Randall L, et al. Chemotherapy with or without avelumab followed by avelumab maintenance versus chemotherapy alone in patients with previously untreated epithelial ovarian cancer (JAVELIN ovarian 100): an open-label, randomised, phase 3 trial. Lancet Oncol (2021) 22(9):1275–89. doi: 10.1016/S1470-2045(21)00342-9
76. Eng C, Kim TW, Bendell J, Argilés G, Tebbutt NC, Di Bartolomeo M, et al. Atezolizumab with or without cobimetinib versus regorafenib in previously treated metastatic colorectal cancer (IMblaze370): a multicentre, open-label, phase 3, randomised, controlled trial. Lancet Oncol (2019) 20(6):849–61. doi: 10.1016/S1470-2045(19)30027-0
77. Reardon DA, Brandes AA, Omuro A, Mulholland P, Lim M, Wick A, et al. Effect of nivolumab vs bevacizumab in patients with recurrent glioblastoma: the CheckMate 143 phase 3 randomized clinical trial. JAMA Oncol (2020) 6(7):1003–10. doi: 10.1001/jamaoncol.2020.1024
78. Baas P, Scherpereel A, Nowak AK, Fujimoto N, Peters S, Tsao AS, et al. First-line nivolumab plus ipilimumab in unresectable malignant pleural mesothelioma (CheckMate 743): a multicentre, randomised, open-label, phase 3 trial. Lancet (2021) 397(10272):375–86. doi: 10.1016/S0140-6736(20)32714-8
79. Fennell DA, Ewings S, Ottensmeier C, Califano R, Hanna GG, Hill K, et al. Nivolumab versus placebo in patients with relapsed malignant mesothelioma (CONFIRM): a multicentre, double-blind, randomised, phase 3 trial. Lancet Oncol (2021) 22(11):1530–40. doi: 10.1016/S1470-2045(21)00471-X
80. Moehler M, Dvorkin M, Boku N, Özgüroğlu M, Ryu MH, Muntean AS, et al. Phase III trial of avelumab maintenance after first-line induction chemotherapy versus continuation of chemotherapy in patients with gastric cancers: results from JAVELIN gastric 100. J Clin Oncol (2021) 39(9):966–77. doi: 10.1200/JCO.20.00892
81. Kang YK, Chen LT, Ryu MH, Oh DY, Oh SC, Chung HC, et al. Nivolumab plus chemotherapy versus placebo plus chemotherapy in patients with HER2-negative, untreated, unresectable advanced or recurrent gastric or gastro-oesophageal junction cancer (ATTRACTION-4): a randomised, multicentre, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol (2022) 23(2):234–47. doi: 10.1016/S1470-2045(21)00692-6
82. George J, Bajaj D, Sankaramangalam K, Yoo JW, Joshi NS, Gettinger S, et al. Incidence of pancreatitis with the use of immune checkpoint inhibitors (ICI) in advanced cancers: a systematic review and meta-analysis. Pancreatology (2019) 19(4):587–94. doi: 10.1016/j.pan.2019.04.015
83. Friedman CF, Clark V, Raikhel AV, Barz T, Shoushtari AN, Momtaz P, et al. Thinking critically about classifying adverse events: incidence of pancreatitis in patients treated with nivolumab + ipilimumab. J Natl Cancer Inst (2017) 109(4):djw260. doi: 10.1093/jnci/djw260
84. Abu-Sbeih H, Tang T, Lu Y, Thirumurthi S, Altan M, Jazaeri AA, et al. Clinical characteristics and outcomes of immune checkpoint inhibitor-induced pancreatic injury. J immunother cancer (2019) 7(1):31. doi: 10.1186/s40425-019-0502-7
85. Brahmer JR, Lacchetti C, Schneider BJ, Atkins MB, Brassil KJ, Caterino JM, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American society of clinical oncology clinical practice guideline. J Clin Oncol (2018) 36(17):1714–68. doi: 10.1200/JCO.2017.77.6385
86. Ikeuchi K, Okuma Y, Tabata T. Immune-related pancreatitis secondary to nivolumab in a patient with recurrent lung adenocarcinoma: a case report. Lung Cancer (Amsterdam Netherlands) (2016) 99:148–50. doi: 10.1016/j.lungcan.2016.07.001
87. Hofmann L, Forschner A, Loquai C, Goldinger SM, Zimmer L, Ugurel S, et al. Cutaneous, gastrointestinal, hepatic, endocrine, and renal side-effects of anti-PD-1 therapy. Eur J Cancer (Oxford England: 1990) (2016) 60:190–209. doi: 10.1016/j.ejca.2016.02.025
88. Zhang Y, Fang Y, Wu J, Huang G, Bin J, Liao Y, et al. Pancreatic adverse events associated with immune checkpoint inhibitors: a Large-scale pharmacovigilance analysis. Front Pharmacol (2022) 13:817662. doi: 10.3389/fphar.2022.817662
89. Marchand L, Disse E, Dalle S, Reffet S, Vouillarmet J, Fabien N, et al. The multifaceted nature of diabetes mellitus induced by checkpoint inhibitors. Acta diabetologica (2019) 56(12):1239–45. doi: 10.1007/s00592-019-01402-w
90. Hsu C, Marshall JL, He AR. Workup and management of immune-mediated hepatobiliary pancreatic toxicities that develop during immune checkpoint inhibitor treatment. oncologist (2020) 25(2):105–11. doi: 10.1634/theoncologist.2018-0162
91. Passat T, Touchefeu Y, Gervois N, Jarry A, Bossard C, Bennouna J. Physiopathological mechanisms of immune-related adverse events induced by anti-CTLA-4, anti-PD-1 and anti-PD-L1 antibodies in cancer treatment. Bull du cancer (2018) 105(11):1033–41. doi: 10.1016/j.bulcan.2018.07.005
92. Yoneda S, Imagawa A, Hosokawa Y, Baden MY, Kimura T, Uno S, et al. T-Lymphocyte infiltration to islets in the pancreas of a patient who developed type 1 diabetes after administration of immune checkpoint inhibitors. Diabetes Care (2019) 42(7):e116–e8. doi: 10.2337/dc18-2518
93. Weber J, Kähler K, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol (2012) 30(21):2691–7. doi: 10.1200/JCO.2012.41.6750
94. Darvin P, Toor SM, Sasidharan Nair V, Elkord E. Immune checkpoint inhibitors: recent progress and potential biomarkers. Exp Mol Med (2018) 50(12):1–11. doi: 10.1038/s12276-018-0191-1
95. Nagpal S, Sharma A, Chari S. Autoimmune pancreatitis. Am J gastroenterol (2018) 113(9):1301. doi: 10.1038/s41395-018-0146-0
96. Arbour KC, Mezquita L, Long N, Rizvi H, Auclin E, Ni A, et al. Impact of baseline steroids on efficacy of programmed cell death-1 and programmed death-ligand 1 blockade in patients with non-Small-Cell lung cancer. J Clin Oncol (2018) 36(28):2872–8. doi: 10.1200/JCO.2018.79.0006
97. Pollack MH, Betof A, Dearden H, Rapazzo K, Valentine I, Brohl AS, et al. Safety of resuming anti-PD-1 in patients with immune-related adverse events (irAEs) during combined anti-CTLA-4 and anti-PD1 in metastatic melanoma. Ann Oncol (2018) 29(1):250–5. doi: 10.1093/annonc/mdx642
98. Spector D, Perry Z, Shah S, Kim JJ, Tarnoff ME, Shikora SA. Roux-en-Y gastric bypass: hyperamylasemia is associated with small bowel obstruction. Surg Obes related Dis (2015) 11(1):38–43. doi: 10.1016/j.soard.2014.04.030
99. Coté GA, Gottstein JH, Daud A, Blei AT. The role of etiology in the hyperamylasemia of acute liver failure. Am J Gastroenterol (2009) 104(3):592–7. doi: 10.1038/ajg.2008.84
100. Ismail OZ, Bhayana V. Lipase or amylase for the diagnosis of acute pancreatitis? Clin Biochem (2017) 50(18):1275–80. doi: 10.1016/j.clinbiochem.2017.07.003
101. Michot JM, Ragou P, Carbonnel F, Champiat S, Voisin AL, Mateus C, et al. Significance of immune-related lipase increase induced by antiprogrammed death-1 or death ligand-1 antibodies: a brief communication. J immunother (Hagerstown Md: 1997) (2018) 41(2):84–5. doi: 10.1097/CJI.0000000000000202
102. Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med (2015) 373(1):23–34. doi: 10.1056/NEJMoa1504030
103. Wolchok JD, Chiarion-Sileni V, Gonzalez R, Rutkowski P, Grob JJ, Cowey CL, et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med (2017) 377(14):1345–56. doi: 10.1056/NEJMoa1709684
Keywords: pancreatic adverse events, drug-related adverse events, immune checkpoint inhibitors, immunotherapy, meta - analysis
Citation: Zhao Z, Zhang W, Pang L, Zeng L, Liu S and Liu J (2023) Pancreatic adverse events of immune checkpoint inhibitors therapy for solid cancer patients: a systematic review and meta-analysis. Front. Immunol. 14:1166299. doi: 10.3389/fimmu.2023.1166299
Received: 15 February 2023; Accepted: 30 May 2023;
Published: 09 June 2023.
Edited by:
Megan Barnet, St Vincent’s Hospital Sydney, AustraliaReviewed by:
Fan Xu, Chengdu Medical College, ChinaRishat Ruzi, Peking Union Medical College Hospital (CAMS), China
Ketao Wang, Capital Medical University, China
Antonio Giovanni Solimando, University of Bari Aldo Moro, Italy
Copyright © 2023 Zhao, Zhang, Pang, Zeng, Liu and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jie Liu, c2RqbmxqamllQDEyNi5jb20=
 Zhe Zhao
Zhe Zhao Weike Zhang1
Weike Zhang1 Surui Liu
Surui Liu Jie Liu
Jie Liu