- 1Department of Pediatrics, Hallym University Kangnam Sacred Heart Hospital, Seoul, Republic of Korea
- 2Department of Pediatrics, Kyung Hee University Medical Center, Seoul, Republic of Korea
- 3Department of Computer Science and Engineering, Sungkyunkwan University, Suwon, Republic of Korea
- 4Department of Software, Sejong University, Seoul, Republic of Korea
- 5Department of Pediatrics, Yeouido St. Mary’s Hospital, The Catholic University of Korea, Seoul, Republic of Korea
- 6Department of Pediatrics, Kangdong Sacred Heart Hospital, Hallym University College of Medicine, Seoul, Republic of Korea
- 7Department of Pediatrics, Kyung Hee University Hospital at Gangdong, Seoul, Republic of Korea
- 8Department of Pediatrics, Bundang CHA Medical Center, CHA University School of Medicine, Seongnam, Republic of Korea
Background: Mycoplasma pneumoniae infection is common in the general population and may be followed by immune dysfunction, but links with subsequent autoimmune disease remain inconclusive.
Objective: To estimate the association of M. pneumoniae infection with the risk of subsequent autoimmune disease.
Methods: This retrospective cohort study examined the medical records of South Korean children from 01/01/2002 to 31/12/2017. The exposed cohort was identified as patients hospitalized for M. pneumoniae infection. Each exposed patient was matched with unexposed controls based on birth year and sex at a 1:10 ratio using incidence density sampling calculations. The outcome was subsequent diagnosis of autoimmune disease, and hazard ratios (HRs) were estimated with control for confounders. Further estimation was performed using hospital-based databases which were converted to a common data model (CDM) to allow comparisons of the different databases.
Results: The exposed cohort consisted of 49,937 children and the matched unexposed of 499,370 children. The median age at diagnosis of M. pneumoniae infection was 4 years (interquartile range, 2.5–6.5 years). During a mean follow-up time of 9.0 ± 3.8 years, the incidence rate of autoimmune diseases was 66.5 per 10,000 person-years (95% CI: 64.3–68.8) in the exposed cohort and 52.3 per 10,000 person-years (95% CI: 51.7–52.9) in the unexposed cohort, corresponding to an absolute rate of difference of 14.3 per 10,000 person-years (95% CI: 11.9–16.6). Children in the exposed cohort had an increased risk of autoimmune disease (HR: 1.26; 95% CI: 1.21–1.31), and this association was similar in the separate analysis of hospital databases (HR: 1.25; 95% CI 1.06–1.49).
Conclusion: M. pneumoniae infection requiring hospitalization may be associated with an increase in subsequent diagnoses of autoimmune diseases.
Introduction
Mycoplasma pneumoniae (M. pneumoniae) is one of the most frequent causes of respiratory diseases and is responsible for 10% to 40% of all cases of community-acquired pneumonia (CAP) in children. M. pneumoniae, a gram-negative bacterium lacking cell walls and characterized by its small size, depends on host cell association as an extracellular pathogen. It develops a specialized attachment organelle that potentially leads to the formation of capsular material outside the cell membrane. This bacterium is a significant respiratory pathogen in children (1). Although many infected individuals completely recover without complications, some experience sustained chronic diseases (2–4). More specifically, some children experience extrapulmonary manifestations that influence specific organs or whole-body systems after an infection. Some evidence suggests that a M. pneumoniae infection may lead to an increased development of autoimmune diseases (5).
There is increasing interest in the impact of M. pneumonia infection on autoimmune diseases (2, 6), and many case reports have examined this relationship (7, 8). However, there is no definitive evidence of a causal relationship because these studies had cross-sectional designs (9, 10), observational studies lacking appropriate controls (11), and examined small numbers of patients who were mostly from specialist clinics (12). There are also conflicting results from case–control studies regarding the relationship of M. pneumoniae infection with common childhood autoimmune diseases, such as Kawasaki disease and Guillain–Barre’s syndrome (13). We are unaware of any rigorous epidemiologic investigations that examined the effect of M. pneumoniae infection on subsequent autoimmune diseases.
The purpose of this study was to determine the association of M. pneumoniae infection with the risk of subsequent autoimmune diseases using a nationwide population-based database that has information on all medical diagnoses and use of healthcare resources, with control for confounding by comparison with hospital-based data.
Methods
Study design
This retrospective population-based cohort study enrolled all individuals born in South Korea between 2002 and 2005, as identified by the National Health Insurance Service (NHIS), with linked Statistics Korea census data. The NHIS provides healthcare services coverage for over 98% of the South Korean population and includes claims-based medical data. These data provided information on demographic characteristics, healthcare utilization (with diagnostic codes from the 10th version of the International Classification of Diseases, ICD-10), prescriptions, and relevant procedures. All guidelines for observational studies that use routinely collected health data were followed (Supplementary Table 1).
Study population
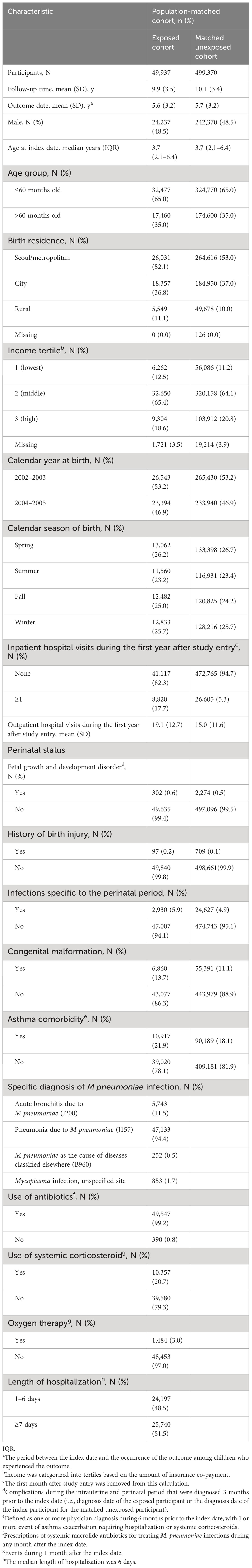
A total of 1,914,461 subjects were included and followed up from birth until 31/12/2017, using all included participants who were less than 18 years old on that date (Figure 1). The index date was defined as the date of first hospitalization due to M. pneumoniae infection. Individuals with underlying history of lower respiratory tract infection due to other pathogens (ICD-10 code J12X-J18X), respiratory and cardiovascular disorders specific to the perinatal period (ICD-10 code P20X-P29X), congenital malformations of the respiratory system (ICD-10 code Q30-Q34), chromosomal anomaly (ICD-10 code Q90-Q99), and lower respiratory tract infection due to solids and liquids (ICD-10 code J69X) were excluded. Individuals diagnosed as autoimmune diseases prior to the index date were also excluded. Lower respiratory tract infections due to other viral pathogens and infections due to ingestion of solids or liquids were also excluded, considering the potential inclusion of M. pneumoniae diagnoses or insufficient differentiation within these categories. Individuals in the exposed cohort were matched with unexposed controls at a 1:10 ratio based on birth year and sex using incidence density sampling. The unexposed cohort participants were randomly matched based on birth year and sex and had no M. pneumoniae infection or autoimmune diseases at the index patient’s diagnosis date.
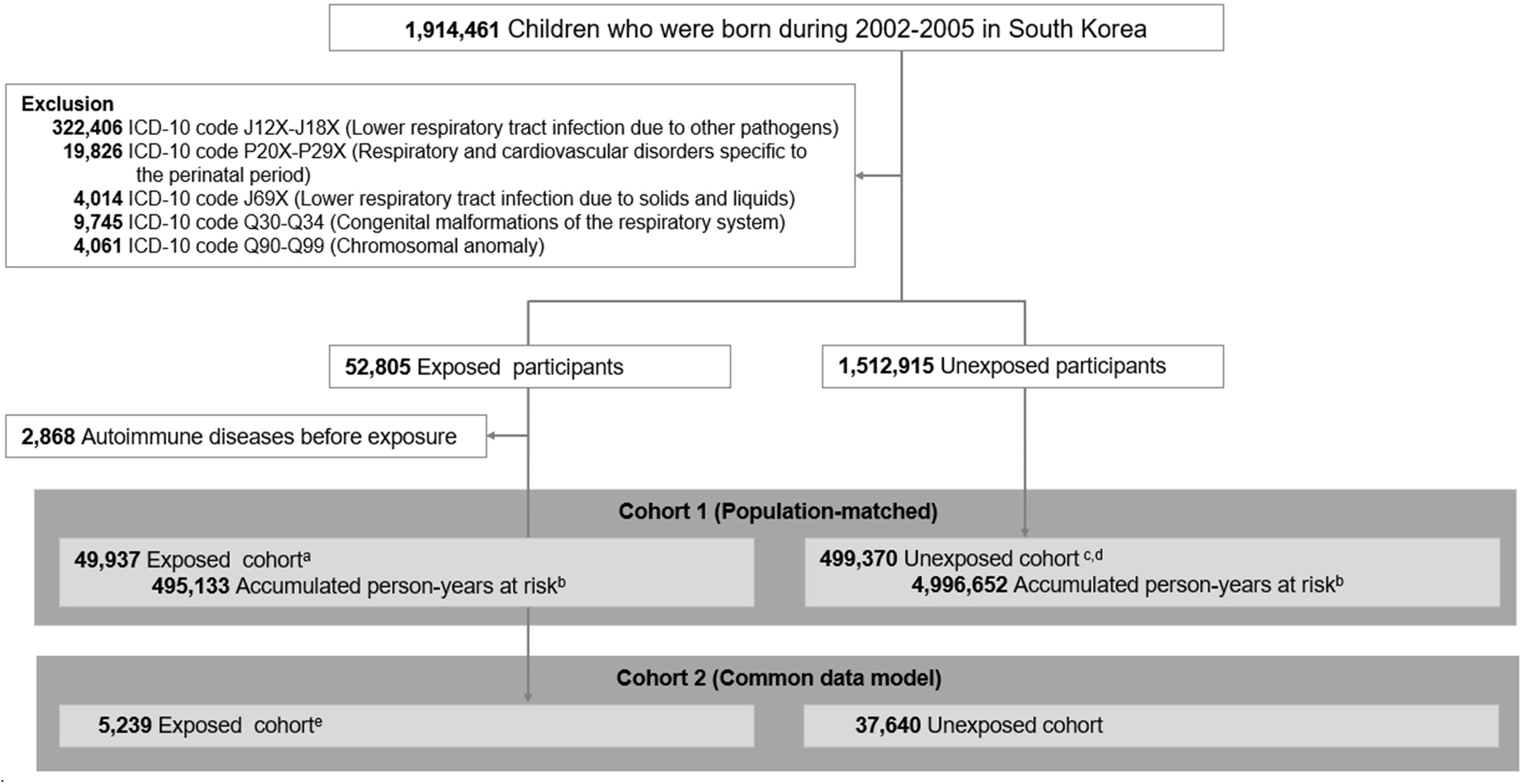
Figure 1 Study design. Individuals born in South Korea from 2002 to 2005 were identified from the database of the National Health Insurance Service (NHIS) and linked information on death from Statistics Korea. After application of exclusion criteria, there were 49,937 children in the exposed cohort and 1,277,613 matched children in the unexposed cohort. aThere were 265,430 born in 2002–2003, 233,940 born in 2004–2005. bThe first year of follow-up was excluded for calculation of accumulated person-years. cEligible unexposed individuals had no M. pneumonia infection and no autoimmune disease at the time of diagnosis of the matched index patient. Matching was performed using density sampling. There were 26,543 born in 2002–2003, and 23,394 born in 2004–2005. dAmong individuals in the unexposed cohort, 10,420 (2.1%) who received diagnoses of M. pneumonia infections during follow-up were reallocated to the exposed group after diagnosis. eFor the CDM, the exposed cohort included hospitalized patients from four hospitals without M. pneumonia infection and without autoimmune disease at the date of diagnosis of the index patient.
Exposure
The exposure in this study was defined as hospitalization due to M. pneumoniae infection, with relevant ICD-10 codes including J200 (acute bronchitis due to M. pneumoniae); J157 (pneumonia due to M. pneumoniae); B960 (M. pneumoniae as the cause of diseases classified elsewhere), or A493 (Mycoplasma infection, unspecified site) (14–16) (Supplementary Table 2). We referred to existing studies to define the exposure to enhance validity and minimize the possibility of misclassification (Supplementary Tables 3-5; literature search in Supplementary Table 6). Moreover, two pediatricians (Ha and Han) performed separate analyses to validate the predictive value of ICD-10 codes for the diagnosis of M. pneumonia infections (J200, J157, B960, or A493) by reviewing electronic medical records (EMRs) at Bundang CHA Medical Center and Kangnam Sacred Heart Hospital (17). The positive predictive values (PPVs) were 97.8% (95 CI, 95.8%–99.9%) and 97.5% (95% CI, 95.3%–99.7%), indicating that the diagnostic assessment used herein had high accuracy and was suitable for use in this analysis.
In addition, a Common Database Model (CDM) comparison, consisting of hospital-based data from four different hospitals that had data of patients with M. pneumonia infections and a matched unexposed cohort, was conducted to determine the robustness of the main results. These data were from different healthcare institutions and allowed large-scale distributed comparative effectiveness analysis (18). Evidence was generated using standard analytic tools by converting data to a CDM. For this analysis, patient-based retrospective cohort data were from four hospitals (Myongji Hospital [MJ], Kyung Hee University Hospital at Gangdong [KH], Bundang CHA Medical Center [CHA], Pusan National University Hospital [PS]). Heterogeneous data sources were standardized and analyzed using the analysis program that was distributed to these four institutions.
Follow-up
All participants were followed from 01/01/2002 until the first diagnosis of an autoimmune disease, death, or the end of the study (31/12/2017), whatever happened first. The follow-up of unexposed individuals included the determination of a M. pneumonia infection later; an individual who was censored due to a M. pneumonia infection was transferred to the exposed group. The first year of follow-up was excluded from the analyses to reduce the probabilities of reverse causality and surveillance bias. The exposed individuals were followed for an average of 9.9 years (SD 3.5) and the unexposed cohort for 10.1 years (SD 3.4).
Autoimmune diseases
Information on autoimmune diseases was from the NHIS database. The 41 autoimmune diseases (19, 20) were identified by ICD-10 codes (Supplementary Table 2).
Covariates
Data were examined for age, sex, follow-up time, birth residence, household income, calendar year/season at birth, any medical condition during the perinatal period, asthma comorbidity, and inpatient and outpatient hospital visits during the first year after study entry (21).. The most updated information before the index date was used for all analyses, and data on inpatient and outpatient visits were collected as a surrogate for use of medical resources during follow-up. Asthma was defined as one or more physician diagnoses 6 months prior to the index date, with one or more events of asthma exacerbation requiring hospitalization and/or systemic corticosteroids (22, 23). Use of systemic macrolide antibiotics infections during any month after the index date, duration of hospitalization, and use of oxygen therapy for treating M. pneumoniae during the first year after study entry were also assessed. We removed the first month after study entry from this calculation considering that exposed patients are likely to receive intensive medical care during the first month after M. pneumoniae infection.
Statistical analysis
Conditional Cox models were used to estimate hazard ratios (HRs) with 95% CIs for developing an autoimmune disease due to previous M. pneumonia infection based on time after the index date. For this analysis, specific autoimmune diseases with fewer than 100 observed cases were not analyzed separately, but it was used in calculations of hazard ratios (HRs) for the corresponding main category. We performed stratified analyses by matching identifiers (birth year and sex) and adjusted for age group at index date (≤60 months or >60 months), time since index date (1 to <5 years or ≥5 years), calendar year of birth (2002–2003 or 2004–2005), birth residence (Seoul and metropolitan, city, or rural area), household income (by tertiles, low, middle, or high), calendar season at birth date (spring, summer, fall, or winter), any medical condition during the perinatal period (yes or no), asthma comorbidity (yes or no), inpatient hospital visits during the first year after study entry (yes or no), and outpatient hospital visits during the first year after study entry (≤13 or >13). All analyses were stratified by matching identifiers (birth year and sex) and adjusted for birth residence (Seoul/metropolitan, city, or rural area), household income (low, middle, or high), and perinatal history (disorder related to the length of gestation and fetal growth, birth trauma, infections specific to the perinatal period, congenital malformation/deformation, and chromosomal abnormalities). HRs were separately calculated for sex, age at the index date (≤60 months or >60 months), time since the index date (1–5 years or ≥5 years), calendar year of birth (2002–2003 or 2004–2005), birth residence (Seoul/metropolitan, city, or rural area), household income (low, middle, or high), the season of birth (spring, summer, fall, or winter), disorders related to any perinatal status (yes or no), history of asthma (yes or no), history of hospital admission (yes or no), and the number of outpatient visits (≤13 or >14, based on the median) during the first year after study entry. In the stratified analysis, information from the first month after study entry was not considered, as medical service utilization within that month was likely attributed to the exposure itself. Differences in HRs by variables were assessed by introducing an interaction term into the Cox models. The difference in log (HR) between strata was used to calculate the z-score and P value for this comparison. The absolute rate differences with 95% CIs were also determined for all associations.
Sensitivity analyses were performed to examine the effect of using a more stringent definition of M. pneumonia infection (ICD-10 code J157 alone), two or more and three or more autoimmune syndromes, individual autoimmune diseases, any of the major groups of autoimmune diseases (Supplementary Table 2) (20), or a more stringent definition of the outcome (use of medication such as non-steroidal anti-inflammatory drugs [NSAIDs], systemic steroid, intravenous immunoglobulin (24, 25), thyroid medications).
To determine the possible effects of reverse causality and surveillance bias, the analyses were repeated by excluding participants who were enrolled during the first 2 years (2002–2003) or during the first 5 years (2002–2007). In this analysis, the cumulative incidence curves of autoimmune diseases among participants with more than 1 year of follow-up were analyzed.
Furthermore, we performed an analysis using the CDM to assess the reliability of our results. We utilized four hospital-based cohorts that had been transformed into the OMOP-CDM (Observational Medical Outcomes Partnership Common Data Model) format. This format enabled the utilization of deidentified patient data extracted from EMRs. The hospitals included Myongji Hospital (MJ), Kyung Hee University Hospital at Gangdong (KH), CHA University Bundang CHA Medical Center (CHA), and Pusan National University Hospital (PS). This allowed for the multicenter analysis of disparate databases. This consisted of the following information: person, drug exposure, drug era, condition occurrence, condition error, observation period, observation, procedure occurrence, visit occurrence, death, drug cost, procedure cost, location, provider, organization, care site, payment plan period, and cohort (26). The analysis period and total number of patients were 2003 to 2020 and 880,392 from MJ, 2006 to 2017 and 822,183 from KH, 2006 to 2019 and 2,363,386 from CHA, and 2011 to 2018 and 1,753,001 from PS. In total, 5,818,962 patients were enrolled across all hospitals, consisting of 5,239 individuals in the exposed cohort (M. pneumonia-related hospitalization) and 37,640 in the unexposed cohort (Figure 2). All analyses were conducted in SAS statistical software version 9.4 (SAS Institute), and a two-sided P value below 0.05 was considered statistically significant.
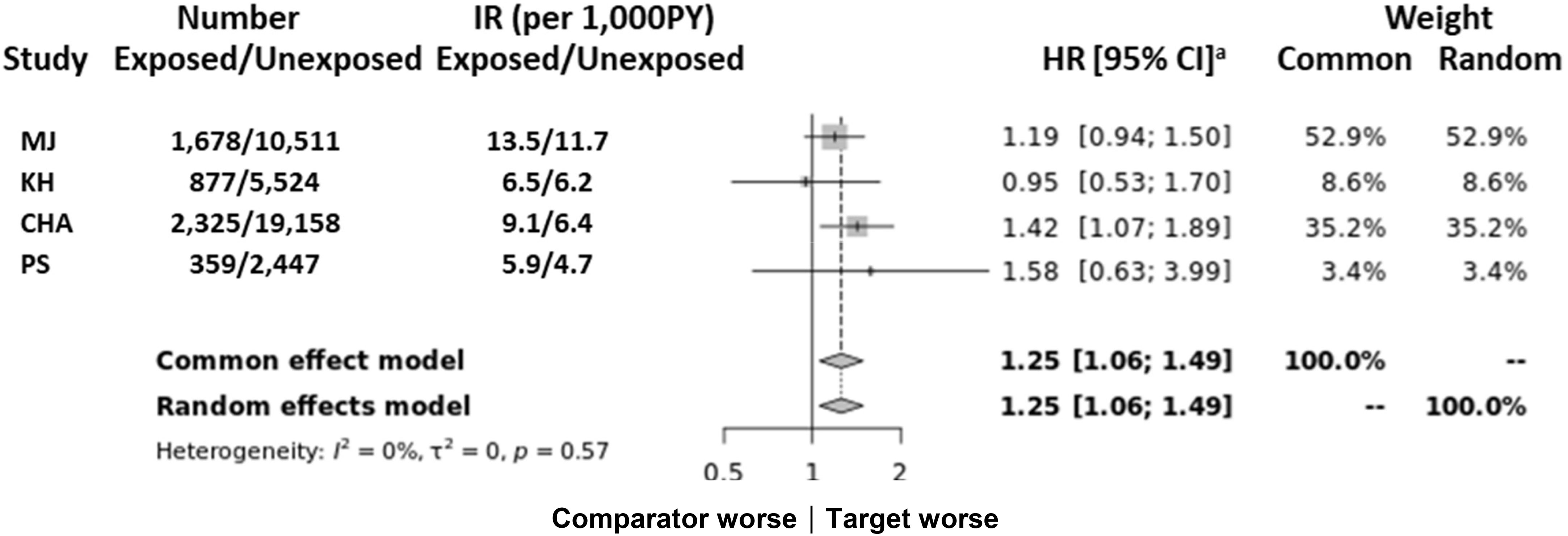
Figure 2 Multicenter analysis of results from the Common Data Model (Supplementary Table 7) of the association between M. pneumonia-related hospitalization and autoimmune disease. CHA, Bundang CHA Medical Center; CI, confidence interval; KH, Kyung Hee University Hospital at Gangdong; MJ, Myongji Hospital; N, number; PS, Pusan National University Hospital; PY, per year. aThe first year of follow-up was excluded from all analyses.
Ethics statement
The study was approved by the Institutional Review Board and Ethics Committees of Hallym University Kangnam Sacred Heart Hospital (IRB No. [2022-05-001]), Hallym University Kangdong Sacred Heart Hospital (IRB No. [2019-09-005]), and CHA University Bundang CHA Medical Center (IRB No. [2022-06-009]). The requirement for informed consent was waived because the study utilized a deidentified database open to the public in Korea.
Results
Baseline characteristics of the participants
All individuals who required hospitalization due to an initial M. pneumonia infection between 2002 and 2005 were identified (Figure 1). We excluded 348,741 individuals who met our predefined criteria, including those with a history of respiratory or cardiovascular complications during the intrauterine and perinatal period (n = 19,826), congenital malformations (n = 9,745), and chromosomal anomalies (n = 4,061). This left us with 52,805 children with M. pneumonia infections who met our criteria for inclusion in the study. We further excluded 2,868 children who had been diagnosed with autoimmune diseases, leaving us with 49,937 individuals in the exposed cohort. For the unexposed cohort, we identified individuals who had not been hospitalized for M. pneumonia infections and who met our inclusion criteria for population matching. Overall, there were 49,937 participants in the exposed cohort and 499,370 in the matched unexposed cohort.
The median age at diagnosis of M. pneumoniae infection was 3.67 years, and 48.54% of all patients were men (Table 1). Compared with the unexposed cohort, the exposed cohort had more inpatient and outpatient hospital visits and a higher rate of asthma comorbidity. The incidence of M. pneumoniae infection was greatest (0.8%) in children who were 2 and 3 years old and decreased steadily with age (Supplementary Figure 1). Trends in M. pneumoniae infection-related hospitalization from 2002 to 2016, analyzed using our database, are presented in Supplementary Figure 2. The variations in prevalence might be explained by the M. pneumonia epidemics in Korea during specific periods (27).
Relationship between hospitalization due to M. pneumoniae infection and increased risks to autoimmune diseases
Elevated HRs of autoimmune diseases in the exposed group with history of hospitalization due to M. pneumoniae infection was shown by the Cox model with adjustment of multiple confounders, compared with the unexposed group (Table 2). During a mean follow-up time of 10 years, we identified newly diagnosed autoimmune diseases in 3295 individuals in the exposed cohort (incidence rate: 66.5 per 10,000 person-years; 95% CI: 64.3–68.8) and in 26,145 individuals in the unexposed cohort (incidence rate: 52.3 per 10,000 person-years; 95% CI: 51.7–52.9). This corresponded to an absolute difference of 14.3 per 10,000 person-years (95% CI: 11.9–16.6), with an HR of 1.26 (95% CI: 1.215–1.308).
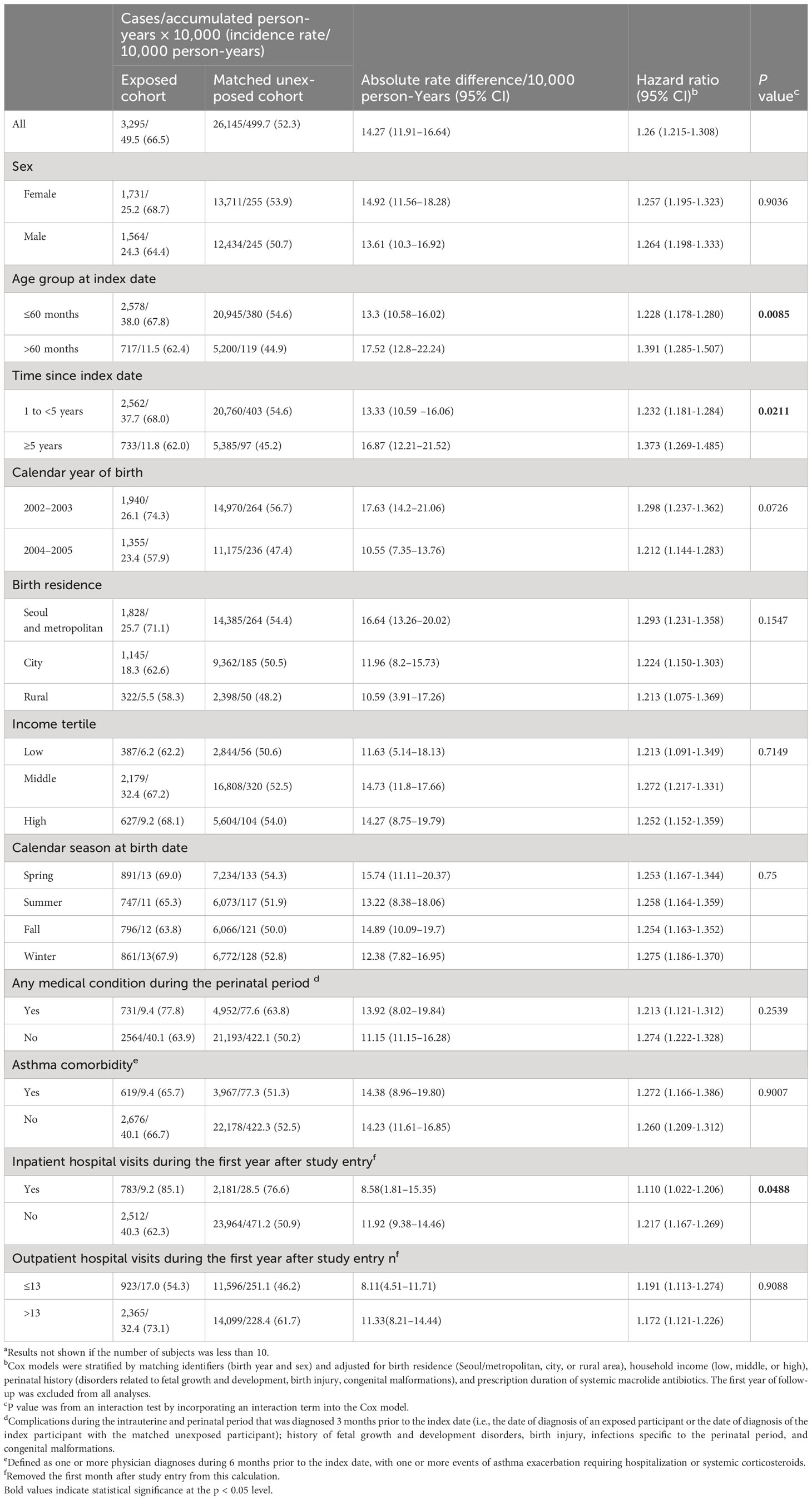
Table 2 Risk of autoimmune disease in the exposed cohort (patients hospitalized with M. pneumoniae infection) relative to the matched unexposed cohort.
Sex, calendar year of birth, asthma comorbidity, perinatal status, and the number of outpatient visits had no significant effects on the HR for autoimmune disease. However, the HR was less in patients infected at an age of 60 months or less (HR: 1.228; 95% CI: 1.178–1.280 vs. HR: 1.391; 95% CI: 1.285–1.507; P for interaction = 0.0085) and in patients with an index date of 1 to 5 years (HR: 1.232; 95% CI: 1.181–1.284 vs. HR: 1.373; 95% CI: 1.269–1.485; P for interaction = 0.021). The HR was marginally smaller for those with a history of hospital admission during the first year after study entry (HR: 1.10; 95% CI: 1.022–1.206 vs. HR: 1.217; 95% CI: 1.167–1.269; P for interaction = 0.049).
M. pneumoniae infection was also associated with elevated risks in each of the nine major groups of autoimmune diseases (Figure 3). The population-matched analysis indicated that these associations were especially notable for four groups of autoimmune diseases. Several associations between M. pneumoniae infection and several autoimmune diseases were notable, including autoimmune thyroiditis (HR 1.258, 95% CI 1.142–1.386), juvenile idiopathic arthritis (HR 1.403, 95% CI 1.276–1.543), ankylosing spondylitis (HR 1.72, 95% CI 1.253–2.361), Kawasaki disease (HR 1.449, 95% CI 1.280–1.640), Henoch-Schonlein purpura (HR 1.314, 95% CI 1.168–1.478), systemic lupus erythematosus (SLE) (HR 1.559, 95% CI 1.169–2.077), psoriasis vulgaris (HR 1.268, 95% CI 1.092–1.472), vitiligo (HR 1.185, 95% CI 1.085–1.295), idiopathic thrombocytopenic purpura (ITP) (HR 1.474, 95% CI 1.14–1.907), Crohn’s disease (HR 1.26, 95% CI 1.006–1.586), and IgA nephropathy (HR 1.167, 95% CI 1.048–1.299). Among these, the risk for SLE was the highest in the exposed cohort, and no significantly lower risks were observed in any of the other autoimmune diseases.
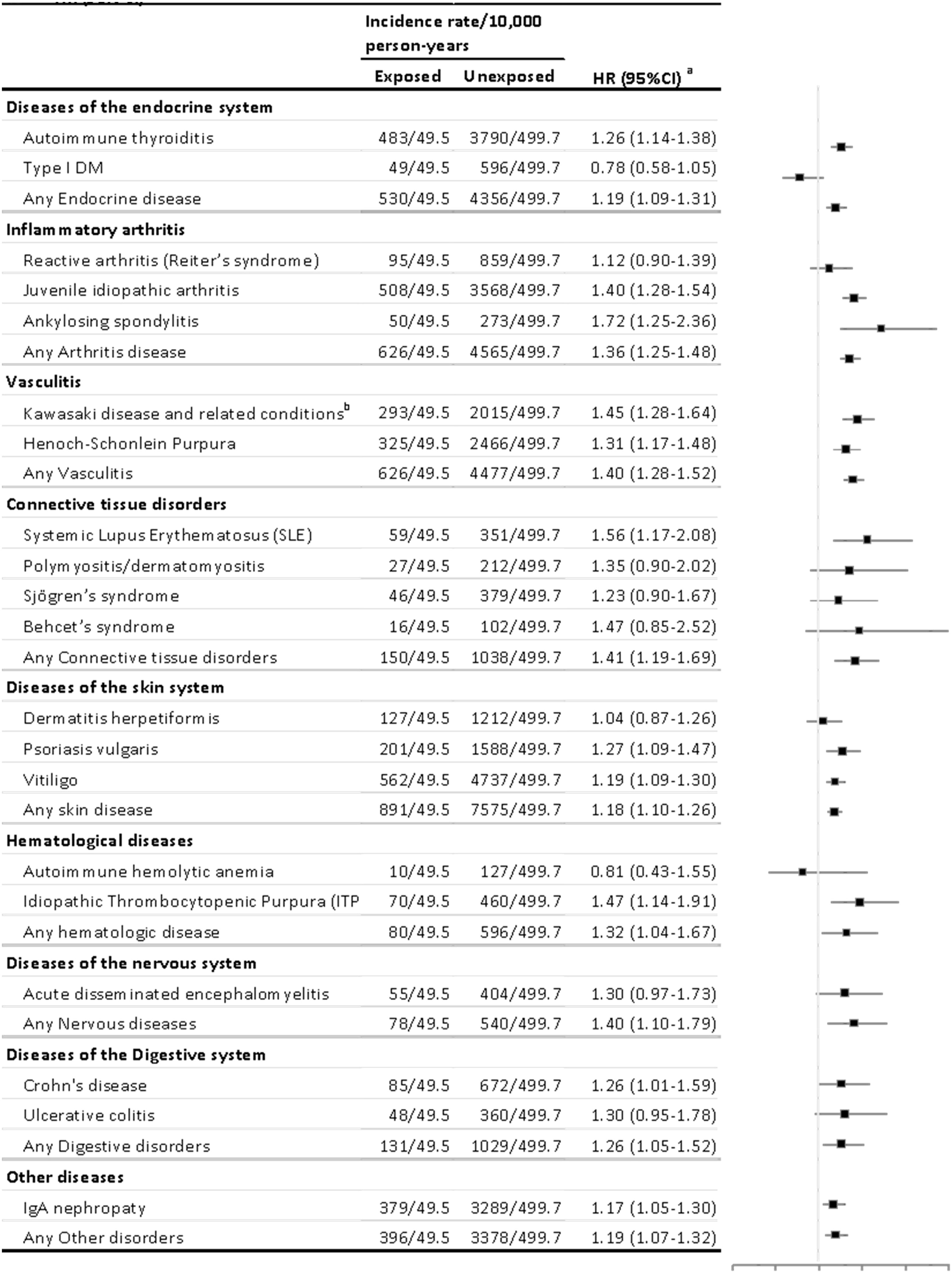
Figure 3 Risk estimates for the association of M. pneumonia-related hospitalization with different types of autoimmune diseases. HR, hazard ratio. Autoimmune diseases with fewer than 100 cases were not analyzed separately but were used for calculations of hazard ratios (HRs) for the main categories. Cox models were stratified by matching identifiers (birth year and sex) and adjusted for birth residence (Seoul/metropolitan, city, or rural area), household income (low, middle, or high), and perinatal history (disorder related to length of gestation and fetal growth, birth trauma, infections specific to the perinatal period, congenital malformation/deformation, and chromosomal abnormalities). The first year of follow-up was excluded from all analyses. aAutoimmune diseases with fewer than 100 cases were not analyzed separately but were included in calculations of hazard ratios (HRs) for the main categories. bIncludes autoimmune vascular disorders, such as polyarteritis nodosa and Churg–Strauss syndrome.
Effects of healthcare history, age at infection, and antibiotic use on autoimmune diseases hospitalization due to M. pneumoniae infection
We examined the effects of use of antibiotics, oxygen therapy, and hospital visits on the association between hospitalization due to M. pneumoniae infection and autoimmune disease. The association was influenced by the prescription of antibiotics from 1 month before to 1 month after the index date, whereas the significance interaction was marginal. Oxygen therapy and frequency of outpatient visits within a month after the index date did not show an effect (Supplementary Table 7).
Multiple sensitivity analyses
The CDM comparison corroborated the observed associations (Figure 2) with higher estimates noted for autoimmune diseases (HR: 1.25; 95% CI: 1.06–1.49) although there were differences in statistical values among the hospitals.
The results of further sensitivity analysis yielded consisted results (Table 3). The association of hospitalization for M. pneumoniae infection with autoimmune disease was stronger for those with two or more autoimmune syndromes (HR: 1.506; 95% CI: 1.314–1.727; P for difference = 0.0011) and with three or more autoimmune syndromes (HR: 1.789; 95% CI: 1.178–2.716; P for difference <0.001) (Table 3). The sensitivity analysis also indicated greater risk when excluding cases diagnosed 2 years after study entry or 5 years after study entry; when exposure was defined more stringently (only ICD-10 code J157); and when the outcome was defined more stringently (diagnosis and treatment for autoimmune disease). In addition, when participants were restricted as individuals followed up for more than 5 years after entry, the exposed group (history of hospitalization for M. pneumoniae infection) had a higher cumulative incidence of autoimmune diseases compared with their matched unexposed group across the follow-up period (Supplementary Figure 3).
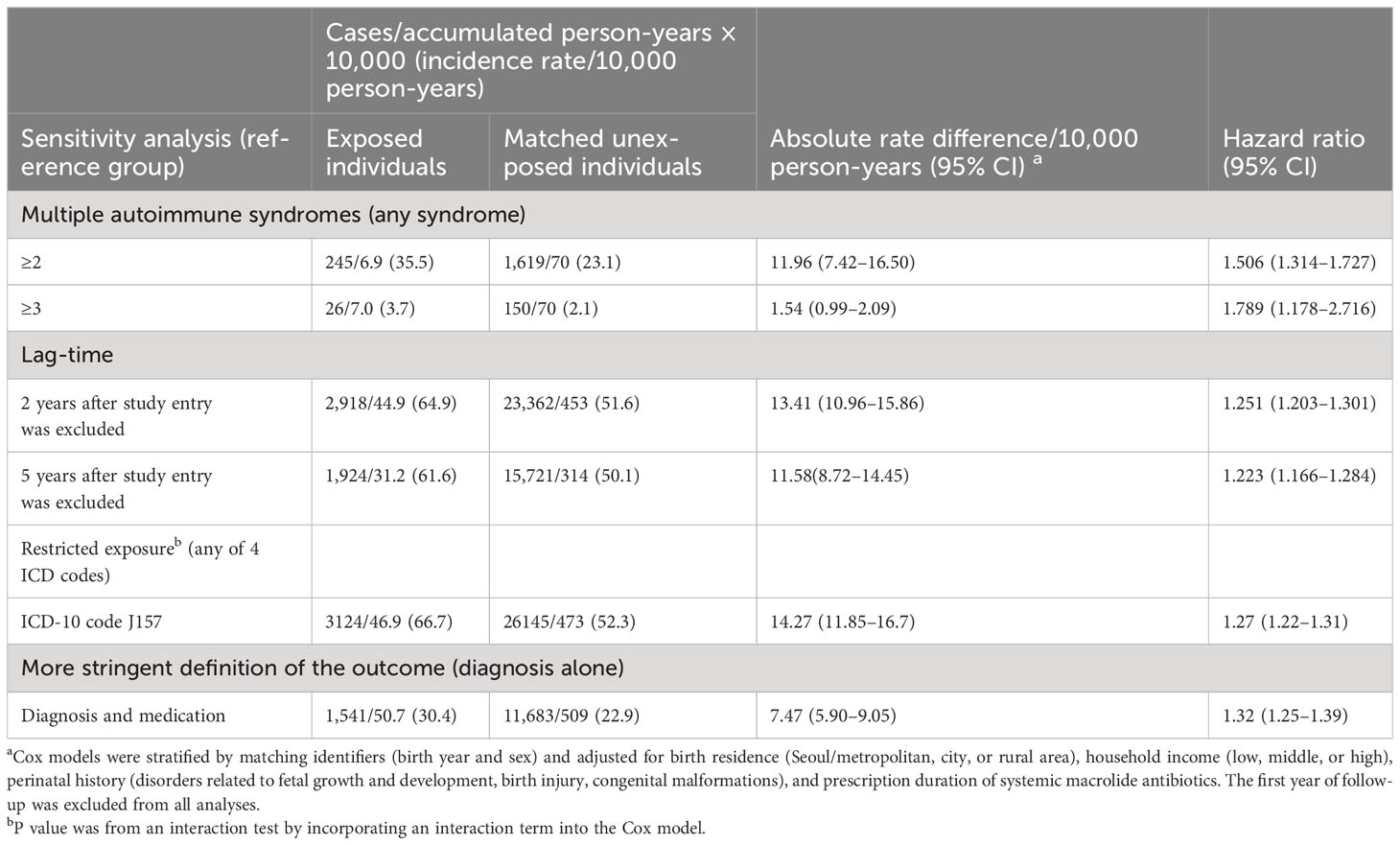
Table 3 Sensitivity analysis of the association between M. pneumonia-related hospitalization and autoimmune disease.
Discussion
In this large-scaled population-based cohort study, we observed a higher risk of autoimmune disease following hospitalization for M. pneumoniae infection. This association was particularly pronounced among patients who were older at the time of admission (as determined by diagnosis data) and whose index date was beyond 60 months. We found that the association persisted regardless of hospitalization duration, oxygen therapy, and antibiotic use. To the best of our knowledge, this is the first study to examine the relationship of hospitalization for M. pneumoniae infection with 19 distinct autoimmune in a large pediatric population, using a population-based comparison. By shedding light on this possible link, our study underscores the need for continued research regarding the potential link between severe M. pneumoniae infection and autoimmune and immune-mediated diseases (28, 29).
Our findings also suggest that degree of association differed for the various autoimmune diseases examined in our study. For instance, the hazard ratio was 1.40 for juvenile idiopathic arthritis and 1.26 for autoimmune thyroid disease. These differences may reflect variations in the prevalence and pathogenicity of different autoimmune diseases in children Moreover, our results are consistent with previous studies that have reported associations between M. pneumoniae infection and autoimmune diseases such as Guillain–Barre’s syndrome (GBS) (30, 31) and polyarthritis (32). However, these previous studies were limited by a cross-sectional design, which precluded causal inferences. While our study provides compelling evidence for a potential link between severe infection and autoimmune and immune-mediated diseases, it is important to acknowledge that further research is needed to fully understand the mechanisms underlying this association (28).
Our findings are consistent with previous evidence that reported links of severe cases of M. pneumoniae infection and related respiratory diseases with immune system dysfunction and suggest that autoimmune reactions may contribute to some of the extrapulmonary complications in patients who have these infections (33). Previous evidence suggests that this may be attributed to immune responses related to cytokine activation and immune cell reaction due to severe infection. For example, inflammatory reflexes precipitated by M. pneumoniae infection might activate the sympathetic nervous system, possibly leading to impaired immune function or a widespread inflammatory response. Moreover, the adhesin proteins of M. pneumoniae (P1, P30, and P116) enable the adhesion of this pathogen to host epithelial cells, and this leads to the production of antibodies that target cells in the brain, liver, kidney, smooth muscle, and lungs that could induce autoimmune disorders in the affected organs (34, 35). The release of cytokines and inflammatory mediators during M. pneumoniae infection can lead to worse disease outcomes and overactivation of the immune system (1, 36). This supports our finding that patients hospitalized for M. pneumoniae infection were more likely to develop autoimmune and immune-mediated diseases involving different organs and multiple autoimmune syndromes. This might also explain the stronger association of infection with an autoimmune disease in children exposed after the age of 60 months, whose clinical course is more severe. Immune reactions induced by M. pneumoniae infections might occur because of the amino-acid sequence homology of mycoplasmal adhesins with antigens (P1, P30, and perhaps P65 and P116) in diverse human tissues and the formation of immune complexes (37). M. pneumoniae infections can activate T cells and increase B-cell proliferation, and these play important roles in the pathogenesis of extrapulmonary manifestations during pneumonia and autoimmune diseases (3). However, another study found no significant difference in the age of occurrence of extrapulmonary symptoms possibly related with immune-mediated reactions between M. pneumoniae infection and respiratory infections caused by M. pneumoniae in children. Caution is warranted in interpreting this finding due to the inherent limitations of epidemiological studies (38).
The strengths of our study are that we used a population-based cohort design and performed a complete follow-up of nearly 50,000 patients who were diagnosed with M. pneumoniae infections during a 10-year period. Furthermore, the large sample size allowed us to perform several sensitivity analyses, so we analyzed the effect of age at exposure and the use of multiple outcome measures. The availability of detailed sociodemographic and medical information also allowed us to control for a large number of potential confounders.
This study has several limitations. First, there may have been some surveillance bias. To address this issue, we performed sensitivity analyses using extended lag times, measured different outcomes, and adjusted for estimated levels of medical surveillance. Second, we relied on diagnoses of infection registered in the NHIS. This could have been an underestimation if infection occurred as a comorbidity of a different severe condition, if there was delayed identification of the pathogen, or if there was a missed diagnosis. Furthermore, since exposures included inpatient cases, the associations between Mycoplasma pneumoniae infection and autoimmune diseases may be limited to severe cases and may not be applicable to non-hospitalized infections. Third, since this is an observational retrospective cohort study, we could not exclude potential confounders that may affect the causal relationship because of the nature of this data. As an example, changes in epidemics over time such as age, sex, and peak season that might influence the pathogenesis of M. pneumoniae infection were not assessable. Furthermore, unmeasured factors such as unreported health conditions preceding an autoimmune disease (39), alterations in health-related behaviors, or the use of certain medications should be addressed in future studies. Fourth, although we identified a stronger association for older children, we did not assess the association between M. pneumoniae infections with autoimmune diseases that occurred later in life.
Conclusions
This nationwide population-based cohort study may indicate that exposure to M. pneumoniae infection requiring hospitalization may be associated with an increase in subsequent diagnoses of autoimmune diseases. Although our findings provide insight into the relationship between hospitalizations due to M. pneumonia infection and the increased occurrence of autoimmune disease, further studies are needed to better understand the underlying mechanisms.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Institutional Review Board and Ethics Committees of Hallym University Kangnam Sacred Heart Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
MH and SC contributed to the conception of the work, design, analysis, interpretation of the data, and final approval of the version to be published; EH mainly drafted the manuscript and final approval of the version to be published; H-SB and YS contributed to the design of the work, interpretation of the data, drafting of the work, and final approval of the version to be published; HS-B, and YS contributed to the interpretation and analysis of the data, revising it critically for important intellectual content and final approval of the version to be published; HC and MH contributed to data collection, and analysis for the work, revising it critically for important intellectual content and final approval of the version to be published.
Funding
Supported by the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (HR22C1605030022). The funders had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1165586/full#supplementary-material
References
1. Cho HK. Consideration in treatment decisions for refractory Mycoplasma pneumoniae pneumonia. Clin Exp Pediatr (2021) 64(9):459–67. doi: 10.3345/cep.2020.01305
2. Atkinson TP, Balish MF, Waites KB. Epidemiology, clinical manifestations, pathogenesis and laboratory detection of Mycoplasma pneumoniae infections. FEMS Microbiol Rev (2008) 32(6):956–73. doi: 10.1111/j.1574-6976.2008.00129.x
3. Waites KB. New concepts of Mycoplasma pneumoniae infections in children. Pediatr Pulmonol. (2003) 36(4):267–78. doi: 10.1002/ppul.10346
4. Poddighe D. Extra-pulmonary diseases related to Mycoplasma pneumoniae in children: recent insights into the pathogenesis. Curr Opin Rheumatol (2018) 30(4):380–7. doi: 10.1097/BOR.0000000000000494
5. He J, Liu M, Ye Z, Tan T, Liu X, You X, et al. Insights into the pathogenesis of Mycoplasma pneumoniae (Review). Mol Med Rep (2016) 14(5):4030–6. doi: 10.3892/mmr.2016.5765
6. Sfriso P, Ghirardello A, Botsios C, Tonon M, Zen M, Bassi N, et al. Infections and autoimmunity: the multifaceted relationship. J Leukoc Biol (2010) 87(3):385–95. doi: 10.1189/jlb.0709517
7. Sudhakar M, Mohandoss V, Chaudhary H, Ahluwalia J, Bhattarai D, Jindal AK. Multifocal thrombosis with peripheral gangrene in a young boy: Mycoplasma infection triggered cold agglutinin disease. Immunobiology. (2021) 226(3):152075. doi: 10.1016/j.imbio.2021.152075
8. Roshan S, Tan SW. A case report of severe mycoplasma pneumonia with autoimmune haemolytic anaemia. Med J Malaysia. (2020) 75(5):600–2.
9. Liu J, He R, Wu R, Wang B, Xu H, Zhang Y, et al. Mycoplasma pneumoniae pneumonia associated thrombosis at Beijing Children's hospital. BMC Infect Dis (2020) 20(1):51. doi: 10.1186/s12879-020-4774-9
10. Poddighe D, Abdukhakimova D, Dossybayeva K, Mukusheva Z, Assylbekova M, Rakhimzhanova M, et al. Mycoplasma pneumoniae seroprevalence and total igE levels in patients with juvenile idiopathic arthritis. J Immunol Res (2021) 2021:6596596. doi: 10.1155/2021/6596596
11. Lan Y, Li S, Yang D, Zhou J, Wang Y, Wang J, et al. Clinical characteristics of Kawasaki disease complicated with Mycoplasma pneumoniae pneumonia: A retrospective study. Med (Baltimore). (2020) 99(19):e19987. doi: 10.1097/MD.0000000000019987
12. Hussein EA. Idiopathic TTP in the Middle East: Epidemiology and clinical outcomes in infection associated episodes. Transfus Apher Sci (2020) 59(6):102916. doi: 10.1016/j.transci.2020.102916
13. Doria A, Sarzi-Puttini P, Shoenfeld Y. Infections, rheumatism and autoimmunity: the conflicting relationship between humans and their environment. Autoimmun Rev (2008) 8(1):1–4. doi: 10.1016/j.autrev.2008.07.014
14. Williams DJ, Shah SS, Myers A, Hall M, Auger K, Queen MA, et al. Identifying pediatric community-acquired pneumonia hospitalizations: Accuracy of administrative billing codes. JAMA Pediatr (2013) 167(9):851–8. doi: 10.1001/jamapediatrics.2013.186
15. Skull SA, Andrews RM, Byrnes GB, Campbell DA, Nolan TM, Brown GV, et al. ICD-10 codes are a valid tool for identification of pneumonia in hospitalized patients aged > or = 65 years. Epidemiol Infect (2008) 136(2):232–40. doi: 10.1017/S0950268807008564
16. Lo Re V 3rd, Carbonari DM, Jacob J, Short WR, Leonard CE, Lyons JG, et al. Validity of ICD-10-CM diagnoses to identify hospitalizations for serious infections among patients treated with biologic therapies. Pharmacoepidemiol Drug Saf. (2021) 30(7):899–909. doi: 10.1002/pds.5253
17. Eun BW, Kim NH, Choi EH, Lee HJ. Mycoplasma pneumoniae in Korean children: the epidemiology of pneumonia over an 18-year period. J Infect (2008) 56(5):326–31. doi: 10.1016/j.jinf.2008.02.018
18. Kim JH, Lee SW, Yon DK, Ha EK, Jee HM, Sung M, et al. Association of serum lipid parameters with the SCORAD index and onset of atopic dermatitis in children. Pediatr Allergy Immunol (2021) 32(2):322–30. doi: 10.1111/pai.13391
19. Hayter SM, Cook MC. Updated assessment of the prevalence, spectrum and case definition of autoimmune disease. Autoimmun Rev (2012) 11(10):754–65. doi: 10.1016/j.autrev.2012.02.001
20. Song H, Fang F, Tomasson G, Arnberg FK, Mataix-Cols D, Fernandez de la Cruz L, et al. Association of stress-related disorders with subsequent autoimmune disease. JAMA. (2018) 319(23):2388–400. doi: 10.1001/jama.2018.7028
21. Kim JH, Lee JE, Shim SM, Ha EK, Yon DK, Kim OH, et al. Cohort profile: National Investigation of Birth Cohort in Korea study 2008 (NICKs-2008). Clin Exp Pediatr (2021) 64(9):480–8. doi: 10.3345/cep.2020.01284
22. Johnston SL, Martin RJ. Chlamydophila pneumoniae and Mycoplasma pneumoniae: a role in asthma pathogenesis? Am J Respir Crit Care Med (2005) 172(9):1078–89. doi: 10.1164/rccm.200412-1743PP
23. Biscardi S, Lorrot M, Marc E, Moulin F, Boutonnat-Faucher B, Heilbronner C, et al. Mycoplasma pneumoniae and asthma in children. Clin Infect Dis (2004) 38(10):1341–6. doi: 10.1086/392498
24. Quintero OL, Rojas-Villarraga A, Mantilla RD, Anaya JM. Autoimmune diseases in the intensive care unit. An update. Autoimmun Rev (2013) 12(3):380–95. doi: 10.1016/j.autrev.2012.06.002
25. Kolkhir P, Grakhova M, Bonnekoh H, Krause K, Maurer M. Treatment of urticarial vasculitis: A systematic review. J Allergy Clin Immunol (2019) 143(2):458–66. doi: 10.1016/j.jaci.2018.09.007
26. Lim JE, Kim HM, Kim JH, Baek HS, Han MY. Association between dyslipidemia and asthma in children: a systematic review and multicenter cohort study using a common data model. Clin Exp Pediatr (2023) 66(8):357–65. doi: 10.3345/cep.2023.00290
27. Rhim JW, Kang HM, Yang EA, Lee KY. Epidemiological relationship between Mycoplasma pneumoniae pneumonia and recurrent wheezing episode in children: an observational study at a single hospital in Korea. BMJ Open (2019) 9(4):e026461. doi: 10.1136/bmjopen-2018-026461
28. Poddighe D, Marseglia GL. Is there any relationship between extra-pulmonary manifestations of mycoplasma pneumoniae infection and atopy/respiratory allergy in children? Pediatr Rep (2016) 8(1):6395. doi: 10.4081/pr.2016.6395
29. Barge L, Pahn G, Weber N. Transient immune-mediated agranulocytosis following Mycoplasma pneumoniae infection. Case Rep (2018) 2018, bcr-2018. doi: 10.1136/bcr-2018-224537
30. Bitnun A, Ford-Jones EL, Petric M, MacGregor D, Heurter H, Nelson S, et al. Acute childhood encephalitis and Mycoplasma pneumoniae. Clin Infect Dis (2001) 32(12):1674–84. doi: 10.1086/320748
31. Christie LJ, Honarmand S, Talkington DF, Gavali SS, Preas C, Pan CY, et al. Pediatric encephalitis: what is the role of Mycoplasma pneumoniae? Pediatrics (2007) 120(2):305–13. doi: 10.1542/peds.2007-0240
32. Dionisio D, Valassina M, Uberti M, Fabbri C, Parri F, Saffi EG. Mycoplasma pneumoniae non-pulmonary infection presenting with pharyngitis, polyarthritis and localized exanthem. Scand J Infect Dis (2001) 33(10):782–3. doi: 10.1080/003655401317074662
33. Einstein A, Podolsky B, Rosen N. Can quantum-mechanical description of physical reality be considered complete? Phys Rev (2001) 47:777–80.
34. Chourasia BK, Chaudhry R, Malhotra P. Delineation of immunodominant and cytadherence segment(s) of Mycoplasma pneumoniae P1 gene. BMC Microbiol (2014) 14:108. doi: 10.1186/1471-2180-14-108
35. Waites KB, Talkington DF. Mycoplasma pneumoniae and its role as a human pathogen. Clin Microbiol Rev (2004) 17(4):697–728. doi: 10.1128/CMR.17.4.697-728.2004
36. Yang J, Hooper WC, Phillips DJ, Talkington DF. Cytokines in Mycoplasma pneumoniae infections. Cytokine Growth Factor Rev (2004) 15(2-3):157–68. doi: 10.1016/j.cytogfr.2004.01.001
37. Chaudhry R, Varshney AK, Malhotra P. Adhesion proteins of Mycoplasma pneumoniae. Front Biosci (2007) 12:690–9. doi: 10.2741/2093
38. Poddighe D, Comi EV, Brambilla I, Licari A, Bruni P, Marseglia GL. Increased total serum immunoglobulin E in children developing mycoplasma pneumoniae-related extra-pulmonary diseases. Iran J Allergy Asthma Immunol (2018) 17(5):490–6. doi: 10.18502/ijaai.v17i5.307
Keywords: autoimmune diseases, childhood, epidemiology, immune system, Mycoplasma pneumoniae, pneumonia
Citation: Ha EK, Kim JH, Cha HR, Han BE, Shin YH, Baek H-S, Choi SH and Han MY (2023) Investigating the occurrence of autoimmune diseases among children and adolescents hospitalized for Mycoplasma pneumoniae infections. Front. Immunol. 14:1165586. doi: 10.3389/fimmu.2023.1165586
Received: 14 February 2023; Accepted: 09 November 2023;
Published: 05 December 2023.
Edited by:
Magda Carneiro-Sampaio, University of São Paulo, BrazilReviewed by:
Dimitri Poddighe, Nazarbayev University, KazakhstanEun-Ae Yang, Catholic University of Korea, Republic of Korea
Copyright © 2023 Ha, Kim, Cha, Han, Shin, Baek, Choi and Han. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Man Yong Han, ZHJtZXNoQGdtYWlsLmNvbQ==; Sun Hee Choi, Y2hzaDA0MTRAa2h1LmFjLmty
†These authors have contributed equally to this work
‡These authors equally supervised this work
 Eun Kyo Ha
Eun Kyo Ha Ju Hee Kim2†
Ju Hee Kim2† Man Yong Han
Man Yong Han