- Centre for Advanced Neurological Research, K.S Hegde Medical Academy, NIitte University, Karnataka, India
Background: Helicobacter pylori (Hp) persists after colonizing the gut in childhood, and potentially regulates host immune system through this process. Earlier studies have shown that Hp infection in childhood, may protect against MS in later life. Such an association was not seen with AQP4-IgG positive NMOSD, while the association with MOGAD is unclear.
Objective: To evaluate frequency of Hp IgG among patients with MOGAD, MS, NMOSD and matched controls and its effect on disease course. To ascertain whether childhood socio economic factors were linked to prevalence of Hp infection.
Methods: In all, 99 patients diagnosed to have MOGAD, 99 AQP4 IgG+ NMOSD, 254MS and 243 matched controls were included. Patient demographics, diagnosis, age at disease onset, duration and the last recorded expanded disability status scale (EDSS) were obtained from our records. Socioeconomic and educational status was queried using a previously validated questionnaire. Serum HpIgG was detected using ELISA kits (Vircell, Spain).
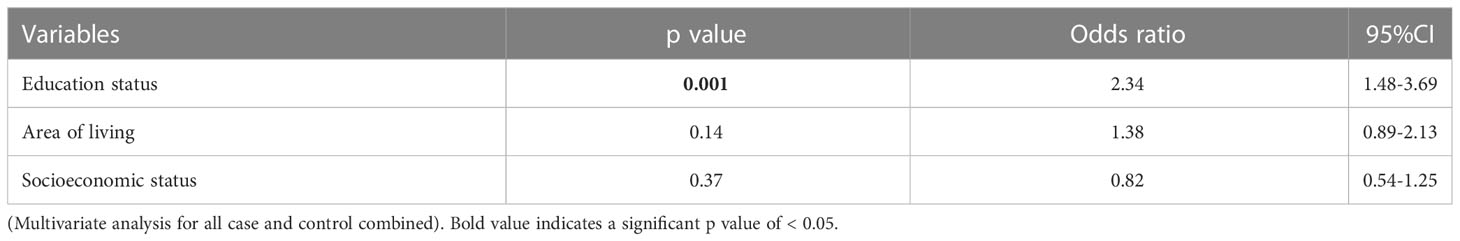
Result: Frequency of Hp IgG was significantly lower among MOGAD (28.3% vs 44%, p-0.007) and MS (21.2% vs 44%, p-0.0001) but not AQP4-IgG+ NMOSD patients (42.4% vs 44%, p-0.78) when compared to controls. Frequency of Hp IgG in MOGAD & MS patients combined (MOGAD-MS) was significantly lower than those with NMOSD (23.2% vs 42.4%, p- 0.0001). Seropositive patients with MOGAD- MS were older (p-0.001. OR -1.04, 95% CI- 1.01- 1.06) and had longer disease duration (p- 0.04, OR- 1.04, 95% CI- 1.002- 1.08) at time of testing. Educational status was lower among parents/caregivers of this study cohort (p- 0.001, OR -2.34, 95% CI- 1.48-3.69) who were Hp IgG+.
Conclusions: In developing countries Hp infection may be a significant environmental factor related to autoimmune demyelinating CNS disease. Our preliminary data suggests that Hp may exert a differential influence - a largely protective role for MS-MOGAD but not NMOSD and may influence disease onset and course. This differential response maybe related to immuno-pathological similarities between MOGAD and MS in contrast to NMOSD. Our study further underscores the role of Hp as a surrogate marker for poor gut hygiene in childhood and its association with later onset of autoimmune diseases.
Introduction
Traditionally recognized as a human pathogen, Helicobacter pylori (Hp)is a gram-negative microaerophilic bacterium that colonizes the human gut in early childhood and persists most often for life (1). Infection may be acquired through oral-oral or faecal- oral transmission. Improving hygienic conditions and socio economic status has reduced Hp status in developed/industrialised nations. This declining trend was noted to be associated with an increase in autoimmune disorders in the developed world (2, 3) and supports the “hygiene hypothesis”. The latter refers to the inverse relationship between infection and atopy that was first proposed by Strachan (4) in 1989. He observed an increased frequency of allergic rhinitis and atopic dermatitis among first born children who were less likely to have been exposed to infection compared to younger siblings. The term “hygiene hypothesis” coined in 2000 (5), laid emphasis on the broader environmental infection burden that showed a negative correlation between overall infection frequency and a substantial increase in frequency of allergic and autoimmune diseases, observed in industrialized countries. A variety of pathogens, parasites and commensal microorganism have been observed to protect against different autoimmune conditions and includes Hp (6).
Helicobacter pylori infection in childhood possibly contributes to the development of the immune system and may be protective against later onset of some autoimmune diseases such as atopy, allergy and MS. Despite high prevalence in the developing world, < 10% develop a chronic inflammatory state which leads to symptomatic gastroduodenal disease later in the life of the human host (7). This phase of hp infection is also associated with extra-gastric diseases including certain autoimmune disorders such as rheumatoid arthritis, inflammatory bowel disease (8) and NMOSD (9, 10). Several studies have reported the protective role of Hp in MS, including from Japan (9, 10,) Iran (11), India (12) and Australia (13). There were two studies that failed to show this protective effect, one of which had included patients with opticospinal MS along with conventional MS (14). The other study had a poorly designed control arm (15, 16). Recently 2 meta analysis which included several new studies also concurred with the protective role of hp in MS (17, 18). As early as 2007, there were reports of a higher frequency of Hp among opticospinal form of MS (which in all probability was NMOSD) when compared to conventional MS patients (9, 14) This was confirmed by one of the authors (14) in a later study that incorporated AQP4-IgG assay that showed an association with NMOSD but not MS.
Myelin oligodendrocyte glycoprotein antibody associated disorders (MOGAD) have been recently discovered. Antibodies targeting MOG which is a surface expressed protein in myelin results in demyelination similar to MS (19) with which it also shares pathological similarity (20). The association of Hp with MOGAD has not been reported before. The present study explores the frequency of Hp in all three primary autoimmune demyelinating CNS disorders in a developing country where there is high Hp seroprevalence, to determine its possible association.
Materials and methods
Four hundred and fifty two patients, which included all 99 myelin oligodendrocyte glycoprotein antibody associated disorder (MOGAD) (21), all 99 aquaporin-4 antibody positive (AQP4 IgG+) NMOSD (22) and 254 consecutive patients with MS (23) from the Mangalore demyelinating disease registry [MANDDIR]were selected. Two hundred and forty three healthy volunteers matched by age and gender were included as controls for this study. Patient demographics, diagnosis, age at disease onset, duration and the last recorded expanded disability status scale (EDSS) was obtained from our data base. Socioeconomic and educational status (24) was queried using a previously validated questionnaire (12). Testing for serum Hp IgG was done using Vircell (Granada, Spain) ELISA kits as per manufacturer’s instructions. Antibody index was determined (by dividing optical density values of samples by optical density for cut-off control samples, multiplied by 10). Antibody index was positive if >11, equivocal if between 9 -11 and negative if < 9. All equivocal results were retested and if found to remain equivocal reported as negative for Hp IgG. All patients were tested for both AQP4 IgG (25) and MOG IgG using “in house” cell based assays. This study was approved by the institutional ethics committee and patients and healthy volunteers signed an informed consent form.
Statistics
Categorical variables were expressed in percentages and continuous variables as mean and standard deviations. Patients and controls were stratified by Hpserology. Frequency of Hp in different subtypes of demyelinating disorders were compared with matched controls and then amongst each sub group using Chisquare test. Univariate analysis was performed on following variables namely age at onset,disease duration, gender, EDSS, socio economic and educational state and area of living.Independent variables that showed a p value of ≤ 0.20 were included in the multivariate analysis. A p value < 0.05 was taken to be significant. Strength of association was expressed as odds ratios (OR) and 95%confidence intervals (CI). Analysis was performed on SPSS statistical software program (IBM, USA).
Results
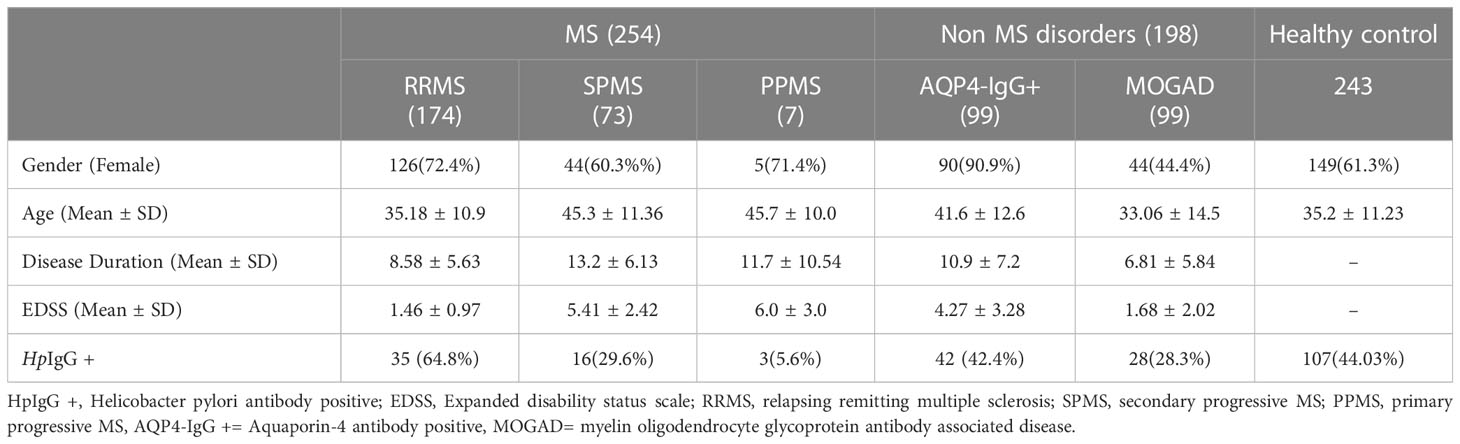
Clinical and Demographic features are listed in Table 1. Patients in the MS group were predominantly of the relapsing remitting (RR). The non MS group comprised of equal number of AQP4IgG + NMOSD and MOGAD patients. Patients were predominantly female among MS and NMOSD patients. All patients had comparable age and disease duration at time of Hp serology testing.
Frequency of Hp IgG serology
Helicobacter pylori antibody frequency was significantly low in MOGAD (28.3% vs 44% p- 0.007) and MS patients (21.2% vs 44%, p- 0.0001) when compared to matched controls. Comparison of NMOSD patients did not show a difference from controls (42.4% vs 44% p=0.78).Seroprevalence of Hp was similar between MS and MOGAD (p- 0.16) while it was significantly different for both subtypes when compared to NMOSD (Supplementary Table 1).
Hp serological prevalence and disease association
For determining association with potential disease modifying variables, MOGAD and MS patients were combined together (MOGAD-MS) and analysed after stratification based on Hp serology. In univariate analysis (Supplementary Table 2), age at disease onset was significantly higher in seropositive patients (p- 0.001, OR- 1.03, 95% CI -1.01-1.05) who were also less educated (p-0.009, OR-1.99, 95% CI -1.18- 3.36). In multivariate analysis- age at disease onset (p-0.001, OR-1.04, 95% CI- 1.01-1.06), duration of disease (p-0.04, OR-1.04, 95%CI- 1.002- 1.08) and educational status (p-0.02, OR- 2.12, 95% CI- 1.11-4.06) were significant association after controlling for gender, disability measured by EDSS, socioeconomic status and area of living (Supplementary Table 3). Among NMOSD patients, seropositive patients showed a trend for greater disability (p- 0.08, OR-1.14, 95%CI – 0.97-1.33) compared to seronegative patients. Lower socioeconomic status was significantly associated with Hp seropositivity (p=0.01, OR-3.83, 95% CI – 1.31 -11.23). Age of disease onset did not differ between the groups. Though statistically insignificant, our data suggested that seropositive patients may have had an early disease onset. Multivariate analysis did not show any variable to be significant (data not shown).
Hp serology and association with socioeconomic and educational status
We combined all patients and controls, to determine whether there were commonalities among those harbouring Hp in our study cohort. In univariate analysis a significant number of seropositive patients had low educational levels(p- 0.001, OR-2.3, 95% CI 1.57- 3.38) and lived in rural areas(p- 0.002, OR 1.79, 95% CI – 1.23- 2.61). After adjusting for other variables, (Tables 2, 3) multivariate analysis showed that educational background of care givers and patients (p- 0.001, OR -2.34, 95% CI- 1.48-3.69)was a significant determinant that differentiated seropositive patients and controls from their seronegative counterparts.
Discussion
Helicobacter pylori prevalence remains high in developing nations and offers an opportunity to study its association with autoimmune demyelinating CNS disorders in these regions. This is particularly relevant for Indian MS patients in whom there were no definitive environmental associations detected that were traditionally associated among white populations. These include Epstein Barr virus infection (26), smoking (12), obesity (12) and Vitamin D deficiency (27). In an earlier study we reported that Hp infection was protective against MS patients (12) and we have currently replicated the results in a larger cohort. Seropositive patients with MS- MOGAD had a later age of disease onset in our study. A similar delay in disease onset was reported in Hp seropositive patients with bronchial asthma (28). Despite a longer duration of disease, seropositive patients had disability that was comparable with seronegative patients (Supplementary Table 2). Previously two case control studies have reported similar results in Hp seropositive MS patients (9, 11). Our study also suggests that Hp infection may confer a protective effect against MOGAD similar to MS. In contrast, Hp infection was detected to be frequent in NMOSD patients. An effect on disease onset and course could not be clearly determined in the latter.
The differential association of CNS autoimmune disorders with Hp infection may in part be due to the differential immune response mounted by Hp in the human host (29). It is evident from human gut mucosal studies that Hp behaves as a commensal in early infection with a potential for pathogenicity in later life. Compared to adults, gastric mucosa in paediatric patients showed minimal inflammation, increased T regulatory (reg) cells and elevated levels of Treg related cytokines namely transforming growth factor- beta (TGFβ) and interleukin 10 (IL10) (30). Limited studies in experimental allergic encephalitis (EAE) models demonstrated that prior infection with Hp induced Foxp3+Treg cell upregulation (31) and inhibition of MOG specific Th1 and Th17 responses (32). In adult patients, peptic ulceration was noted to be associated with reduced Tregs (33).
We have hypothesised possible mechanisms by which Hp infection could modulate the host immune response. Recent insights (34) suggest that Hp may possess inflammasone ligands that trigger caspase- 1 activation in dendritic cells lining the gastric lumen (Figure 1). Activation of the Toll like receptor2/Nucleotide-Binding Domain, Leucine-Rich–Containing Family, Pyrin Domain–Containing-3/Caspace1/Interleukin-18 axis (TLR2/NLRP3/CASP1/IL-18) axis may promote IL18 mediated Treg upregulation in the early phase of infection. The resulting immune tolerance may be the basis for protection against allergy and possibly autoimmune disorders such as MS and MOGAD. On the other hand, chronic gastritis is characterized by upregulated T helper 17(Th17) response, neutrophil recruitment and expression of B cell activating factor (BAFF) in macrophages (35, 36). Chronic Hp infection has also been noted to be associated with gut dysbiosis and associated pro inflammatory state (37). We have postulated that pro- inflammatory state of chronic gastritis accompanied by gut dysbiosis may upregulate Th1/Th17 driven immune response initially in the gut and later systemically, facilitating the development of NMOSD in later life. The role of gastric derived antibodies that cross react with neural elements cannot be excluded. A previous publication from our group has shown this to be a potential pathway for disease pathogenesis in NMOSD (38). Helicobacter pylori related pro-inflammatory proteins particularly neutrophil activating protein (NAP) may contribute to AQP4 –IgG associated neural damage and severity of disease.In a recent study a significant correlation was noted between seropositive AQP4 IgG + NMOSD, Hp- NAP and disability (10). Bacterial virulence genes of Hp have not been studied in detail in the context of CNS autoimmune disorders. An association between Hp- cytotoxin associated gene A (cag-A) and autoimmune thyroiditis (39) and asthma was previously reported (28). Additionally the underlying genetic susceptibility for autoimmune disorders is also important.
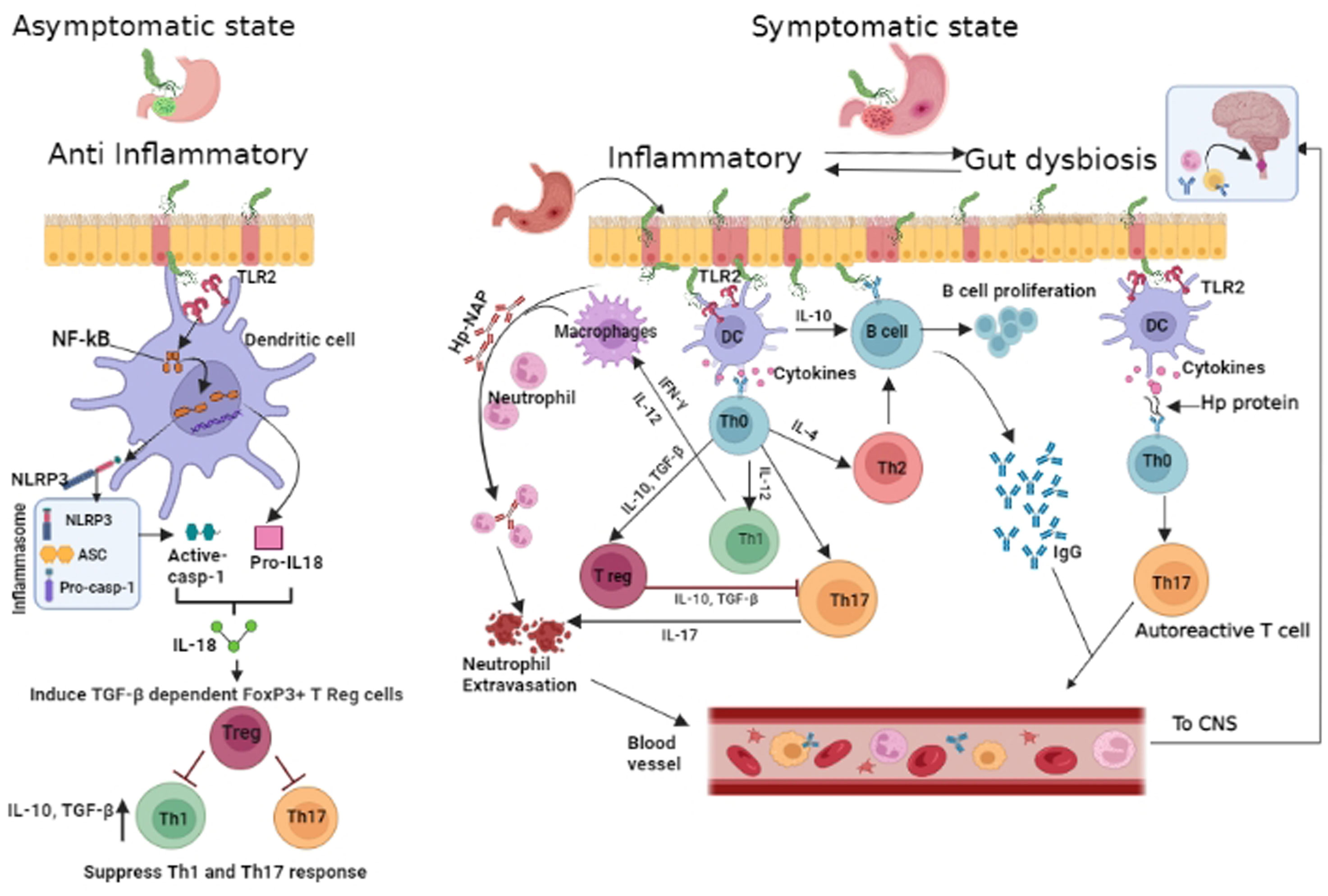
Figure 1 Proposed schematic of Helicobacter pylori mediated modulation of host immunity: In early phase of Hp infection(depicted on left of cartoon), Hptriggers Nucleotide-Binding Domain, Leucine-Rich–Containing Family, Pyrin Domain–Containing-3(NLRP3)inflammasome and caspace 1 activity in antigen presenting cell([APC]dendritic cells and macrophages) via Toll like receptor2 (TLR2) and Nuclear factor kappa B(NF-kB). Processing of pro interleukin -18 (pro-IL-18) and release of mature IL-18 results in T regulatory (Treg) cell differentiation and production of anti inflammatory cytokines – Interleukin-10 (IL10) and transforming growth factor Beta (TGF-β) with further expansion of Treg cells, resulting in immunosuppression. In the inflammatory state (depicted on right),APCafter processing Hp,signals naïve Tcells to differentiate (through release of cytokines - IL12 &IL17) into T helper (Th1 &Th17) cells andB cells are driven by interleukin 10 (IL10)to generate Hp-specific antibodies. Th17 induced neutrophil recruitment is further enhanced by Hp– neutrophil activating protein (NAP). Gut derived antibodies with potential for cross reaction with neural elements, Hp specific autoreactive T cells, neutrophils and Hp specific antibodies may target the central nervous system and induce/aggravate CNS inflammation. Created in BioRender.com.
We reviewed the relevance of hygiene hypothesis in a low middle income (LMIC) setting in relation to Hp infection and CNS autoimmune disorders. For this purpose,we analysed educational status and income levels of patients and primary caregivers in childhood in order to correlate poor sanitary conditions in childhood with early Hp colonization in the gut. Our study showed a significant association between lower educational status among all cases and controls stratified by Hp serology. Our hospital caters to patients from lower economic status which may explain the lack of difference in economic status between the two groups.
In conclusion, in developing countries Hpylori may be a significant environmental factor associated with autoimmune demyelinating disorders. Our study was limited by the small number of study participants and the cross sectional nature of the analysis. It is not clear at this time whether analysis of risk/benefit has to be carefully undertaken before Hp eradication is contemplated, in populations harbouring high Hp seroprevalence. Therefor the role of H pylori in modulating human immunity and its protective/deleterious effects need to be understood through larger studies that will additionally evaluate the role of Hp virulence genes.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by Central Ethics Committe, Nitte University. The patients/participants provided their written informed consent to participate in this study.
Author contributions
LP developed the concept, study design, analysis, interpretation, drafting and revising the work. CM contributed to study design, data acquisition, analysis, interpretation of results, manuscript drafting and revision. AD’C contributed to data acquisition, analysis and manuscript drafting. AS contributed to data collection, data analysis and interpretation. All authors contributed to the article and approved the submitted version.
Acknowledgments
We would like to acknowledge the help of Dr Neevan D’Souza PhD, Associate Professor of Biostatistics, KS Hegde Medical Academy, Nitte University for supervising our statistical analysis for this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1162248/full#supplementary-material
References
1. Wroblewski LE, Peek RM Jr., Wilson KT. Helicobacter pylori and gastric cancer: factors that modulate disease risk. Clin Microbiol Rev (2010) 23:713–39. doi: 10.1128/CMR.00011-10
2. Arnold IC, Hitzler I, Muller A. The immunomodulatory properties of helicobacter pylori confer protection against allergic and chronic inflammatory disorders. Front Cell Infect Microbiol (2012) 2:10. doi: 10.3389/fcimb.2012.00010
3. Robinson K. Helicobacter pylori-mediated protection against extra-gastric immune and inflammatory disorders: the evidence and controversies. Diseases (2015) 3:34–55. doi: 10.3390/diseases3020034
4. Strachan DP. Hay fever, hygiene, and household size. BMJ (1989) 299:1259– 60. doi: 10.1136/bmj.299.6710.1259
5. Strachan DP. Family size, infection and atopy: the first decade of the “hygiene hypothesis”. Thorax (2000) 55:S2–S10. doi: 10.1136/thorax.55.suppl_1.S2
6. Bach JF. The hygiene hypothesis in autoimmunity: the role of pathogens and commensals. Nat Rev Immunol (2018) 105:105–20. doi: 10.1038/nri.2017.111
7. Atherton JC. The pathogenesis of Helicobacter pylori-induced gastro-duodenal diseases. Annu Rev Pathol (2006) 1:63–96. doi: 10.1146/annurev.pathol.1.110304.100125
8. Youssefi M, Tafaghodi M, Farsiani H, Ghazvini K, Keikha M. Helicobacter pylori infection and autoimmune diseases; is there an association with systemic lupus erythematosus, rheumatoid arthritis, autoimmune atrophy gastritis and autoimmune pancreatitis? a systematic review and meta-analysis study. J Microbiol Immunol Infect (2021) 54:359–69. doi: 10.1016/j.jmii.2020.08.011
9. Li W, Minohara M, Su JJ, Matsuoka T, Osoegawa M, Ishizu T, et al. Helicobacter pylori infection is a potential protective factor against conventional multiple sclerosis in the Japanese population. J Neuroimmunol (2007) 184:227–31. doi: 10.1016/j.jneuroim.2006.12.010
10. Li W, Minohara M, Piao H, Matsushita T, Masaki K, Matsuoka T, et al. Association of anti-helicobacter pylori neutrophil-activating protein antibody response with anti-aquaporin-4 autoimmunity in Japanese patients with multiple sclerosis and neuromyelitisoptica. Mult Scler (2009) 12:1411–21. doi: 10.1177/1352458509348961
11. Mohebi N, Mamarabadi M, Mohgaddasi M. Relation of helicobacter pylori and multiple sclerosis in Iranian patients. Neurol Int (2013) 5:31–3. doi: 10.4081/ni.2013.e10
12. Malli C, Pandit L, D’Cunha A, Mustafa S. Environmental factors related to multiple sclerosis in Indian population. PloS One (2015) 10:1–7. doi: 10.1371/journal.pone.0124064
13. Pedrini MJ, Seewann A, Bennett KA, Wood AJ, James I, Burton J, et al. Helicobacter pylori infection as a protective factor against multiple sclerosis risk in females. J NeurolNeurosurg Psychiatry (2015) 86(6):603–7. doi: 10.1136/jnnp-2014-309495
14. Long Y, Gao C, Qiu W, Hu X, Shu Y, Peng F, et al. Helicobacter pylori infection in neuromyelitisoptica and multiple sclerosis. Neuroimmunomodul (2013) 20:107–12. doi: 10.1159/000345838
15. Gavalas E, Kountouras J, Deretzi G, Boziki M, Grigoriadis N, Zavos C, et al. Helicobacter pylori and multiple sclerosis. J Neuroimmunol (2007) 188:187–9. doi: 10.1016/j.jneuroim.2007.06.007
16. Gavalas E, Kountouras J, Boziki M, Zavos C, Polyzos SA, Vlachaki E, et al. Relationship between helicobacter pylori infection and multiplesclerosis. Annof Gastroenterol (2015) 28:353–56.
17. Jaruvongvanich V, Sanguankeo A, Jaruvongvanich S, Upala S. Assocation between helicobacter pylori infection and multiple sclerosis: a systematic review and meta-analysis. Mult Scler Relat Disord (2016) 7:92–7. doi: 10.1016/j.msard.2016.03.013
18. Yao G, Wang P, Luo XD, Yu TM, Harris RA, Zhang XM. Meta-analysis of association between Helicobacter pylori infection and multiple sclerosis. Neurosci Lett (2016) 620:1–7. doi: 10.1016/j.neulet.2016.03.037
19. Kaneko K. Myelin injury without astrocytopathy in neuroinflammatory disorders with MOG antibodies. J Neurol Neurosurg Psychiatry (2016) 87:1257–59. doi: 10.1136/jnnp-2015-312676
20. Peschl P, Bradl M, Hoftberger R, Berger T, Reindl M. Myelin oligodendrocyte glycoprotein: deciphering a target in inflammatory demyelinating diseases. Front Immunol (2017) 8:529. doi: 10.3389/fimmu.2017.00529
21. López-Chiriboga AS, Majed M, Fryer J, Dubey D, McKeon A, Flanagan EP, et al. Association of MOG-IgG serostatus with relapse after acute disseminated encephalomyelitis and proposed diagnostic criteria for MOG-IgG-associated disorders. JAMA Neurol (2018) 75:1355. doi: 10.1001/jamaneurol.2018.1814
22. Wingerchuk DM, Banwell B, Bennett JL, Cabre P, Carroll W, Chitnis T, et al. International panel for NMO diagnosis. international consensus diagnostic criteria for neuromyelitisoptica spectrum disorders. Neurology (2015) 85:177–89. doi: 10.1212/WNL.0000000000001729
23. Thompson AJ, Banwell BL, Barkhof F, Carroll WM, Coetzee T, Comi G, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol (2018) 17:162–73. doi: 10.1016/S1474-4422(17)30470-2
24. Dudala SR, Reddy KAK, Prabhu GR. Prasad's socio-economic status classification- an update for 2014. Int J Res Health Sci (2014) 2:875–8.
25. Pandit L, Malli C, D’Cunha A, Sudhir A. Overcoming the challenges in diagnosis of AQP4-IgG positive neuromyelitisoptica spectrum disorders in resource poor settings using an indigenized and cost effective cell based assay. J Neuroimmunol (2021) 360:577706. doi: 10.1016/j.jneuroim.2021.577706
26. Pandit L, Malli C, D'Cunha A, Shetty R, Singhal B. Association of Epstein-Barr virus infection with multiple sclerosis in India. J Neurol Sci (2013) 325:86–9. doi: 10.1016/j.jns.2012.12.004
27. Pandit L, Ramagopalan SV, Malli C, D’Cunha A, Kunder R, Shetty R. Association of vitamin d and multiple sclerosis in India. Mult Scler (2013) 19:1592–6. doi: 10.1177/1352458513482375
28. Reibman J, Marmon M, Filner J, Fernandez-Beros ME, Rogers L, Perez-Perez GI, et al. Asthma is inversely associated with Helicobacter pylori.Status in urban population. PloS One (2008) 3:e4060. doi: 10.1371/journal.pone.0004060
29. White JR, Winter JA, Robinson K. Differential inflammatory response to helicobacter pylori infection: aetiology and clinical outcomes. J Inflammation (2015) 8:137–47. doi: 10.2147/JIR.S64888
30. Harris PR, Wright SW, Serrano C, Riera F, Duarte I, Torres J, et al. Helicobacter pylori gastritis in children is associated with a regulatory T-cell response. Gastroenterol (2008) 134:491–99. doi: 10.1053/j.gastro.2007.11.006
31. Cook KW, Letley DP, Ingram RJ, Staples E, Skjoldmose H, Atherton JC, et al. CCL20/CCR6-mediated migration of regulatory T cells to the Helicobacter pylori-infected human gastric mucosa. Gut (2014) 63:1550–59. doi: 10.1136/gutjnl-2013-306253
32. Cook KW, Crooks J, O'Brien K, O'Brien K, Braitch M, Kareem H, et al. Helicobacter pylori infection reduces disease severity in an experimental model of multiple sclerosis. Front Microbiol (2015) 6:00052. doi: 10.3389/fmicb.2015.00052
33. Robinson K, Kenefeck R, Pidgeon EL, Shakib S, Patel S, Polson RJ, et al. Helicobacter pylori-induced peptic ulcer disease is associated with inadequate regulatory T cell responses. Gut (2008) 57:1375–85. doi: 10.1136/gut.2007.137539
34. Koch KN, Muller A. Helicobacter pylori activates the TLR2/NLRP3/caspase-1/IL-18 axisto induce regulatory T-cells, establish persistent infection and promote tolerance to allergens. Gut Microbes (2015) 6:382–7. doi: 10.1080/19490976.2015.1105427
35. Shi Y, Liu XF, Zhuang Y, Zhang JY, Liu T, Yin Z, et al. Helicobacter pylori-induced Th17 responses modulate Th1 cell responses, benefit bacterial growth, and contribute to pathology in mice. J Immunol (2010) 184:5121–29. doi: 10.4049/jimmunol.0901115
36. Munari F, Fassan M, Capitani N, Codolo G, Vila-Caballer M, Pizzi M, et al. Cytokine BAFF released by Helicobacter pylori-infected macrophages triggers the Th17 response in human chronic gastritis. J Immunol (2014) 193:5584–94. doi: 10.4049/jimmunol.1302865
37. Klymiuk I, Bilgilier C, Stadlmann A, Thannesberger J, Kastner MT, Högenauer C, et al. The human gastric microbiome is predicated upon infection with Helicobacterpylori”. Front Microbiol (2017) 8:2508. doi: 10.3389/fmicb.2017.02508
38. Wang Y, Zhu S, Xu Y, Wang X, Zhu Y. Interaction between gene a-positive helicobacter pylori and human leukocyte antigen II alleles increase the risk of graves disease in Chinese han population: an association study. Gene (2013) 531:84–9. doi: 10.1016/j.gene.2013.07.069
Keywords: Helicobacter pylori, environmental factor, MS, MOGAD, NMOSD
Citation: Malli C, Pandit L, D’Cunha A and Sudhir A (2023) Helicobacter pylori infection may influence prevalence and disease course in myelin oligodendrocyte glycoprotein antibody associated disorder (MOGAD) similar to MS but not AQP4-IgG associated NMOSD. Front. Immunol. 14:1162248. doi: 10.3389/fimmu.2023.1162248
Received: 09 February 2023; Accepted: 30 March 2023;
Published: 26 May 2023.
Edited by:
Jorge Correale, Fundación Para la Lucha Contra las Enfermedades Neurológicas de la Infancia (FLENI), ArgentinaReviewed by:
Mariano Marrodan, Fundación Para la Lucha Contra las Enfermedades Neurológicas de la Infancia (FLENI), ArgentinaMaria Celica Ysrraelit, Fundación Para la Lucha Contra las Enfermedades Neurológicas de la Infancia (FLENI), Argentina
Copyright © 2023 Malli, Pandit, D’Cunha and Sudhir. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lekha Pandit, cGFuZGl0bW5nQGdtYWlsLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Chaithra Malli†
Chaithra Malli† Lekha Pandit
Lekha Pandit