
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol., 11 April 2023
Sec. Viral Immunology
Volume 14 - 2023 | https://doi.org/10.3389/fimmu.2023.1162208
This article is part of the Research TopicCommunity Series in Antiviral Innate Immune Sensing, Regulation, and Viral Immune Evasion, volume IIView all 16 articles
 Chunchen Wu1
Chunchen Wu1 Luzhi Zeng1,2
Luzhi Zeng1,2 Wenfu Yi3
Wenfu Yi3 Yuanjiu Miao3
Yuanjiu Miao3 Yihan Liu1,2
Yihan Liu1,2 Qiming Wang2
Qiming Wang2 Shi Liu4
Shi Liu4 Guoping Peng2
Guoping Peng2 Zhenhua Zheng3
Zhenhua Zheng3 Jianbo Xia1*
Jianbo Xia1*Among enteroviruses, echovirus can cause severe illnesses in neonates or infants, with high morbidity and mortality. Autophagy, a central component of host defense mechanisms, can function against diverse infections. In the present study, we investigated the interplay between echovirus and autophagy. We demonstrated that echovirus infection increases LC3-II expression dose-dependently, accompanied by an increased intracellular LC3 puncta level. In addition, echovirus infection induces the formation of autophagosome. These results suggest that echovirus infection induces autophagy machinery. Furthermore, phosphorylated mTOR and ULK1 were both decreased upon echovirus infection. In contrast, both levels of the vacuolar protein sorting 34 (VPS34) and Beclin-1, the downstream molecules which play essential roles in promoting the formation of autophagic vesicles, increased upon virus infection. These results imply that the signaling pathways involved in autophagosome formation were activated by echovirus infection. Moreover, induction of autophagy promotes echovirus replication and viral protein VP1 expression, while inhibition of autophagy impairs VP1 expression. Our findings suggest that autophagy can be induced by echovirus infection via regulating mTOR/ULK1 signaling pathway and exhibits a proviral function, revealing the potential role of autophagy in echovirus infection.
Human enteroviruses (HEVs) are small, nonenveloped, positive single-strand RNA viruses belonging to the genus Enterovirus within the family Picornaviridae. More than 100 enterovirus types have been identified and classified into four species, A to D, according to molecular and antigenic properties (1). Echovirus was discovered when the first tissue-culture techniques were introduced into laboratories (2). Currently, echovirus has been classified within the B species, the largest group of the Enterovirus genus, together with coxsackievirus group B, coxsackie A9, and several novel enteroviruses. Like other enteroviruses, echovirus infections are associated with a broad spectrum of illnesses, ranging from minor symptoms (e.g., febrile rash, mild hand, foot, and mouth diseases (HFMD)) to severe, potentially fatal conditions (e.g., aseptic meningitis, encephalitis and acute flaccid paralysis (AFP)) (3). Among 30 serotypes, echovirus 11 was one of the most commonly identified serotypes that cause severe illnesses in neonates or infants, with high morbidity and mortality (4–8). In addition, echovirus 11 has frequently been found to be associated with outbreaks in neonatal intensive units (NICUs) or postpartum care centers, causing public health threats globally (9–12). However, the pathogenic mechanisms of echovirus are poorly understood, limiting the development of antiviral strategies against echovirus.
Autophagy is an evolutionarily conserved intracellular degradation process by which misfolded proteins, damaged organelles, and various invading pathogens are sequestered in the cytoplasm and removed to maintain cellular homeostasis (13). A key initial event in autophagy is the formation of the autophagosome, a unique double-membrane organelle that engulfs the cytosolic cargo destined for degradation. A series of autophagy-related genes (ATG) has been identified to participate in these processes (14). As a part of autonomous innate immunity, autophagy functions to defend individual cells against invading pathogens such as bacteria, fungi, parasites, and viruses (15, 16). For example, autophagy can exert antiviral functions during Sindbis virus or tobacco mosaic virus infection by selectively targeting viral particles or components to the lysosomes for degradatio (17). However, many viruses, for example, poliovirus, coxsackievirus, and hepatitis C virus, have evolved various strategies to hijack and subvert host autophagy for their life cycles and pathogenesis (18–20). Although previous studies suggested a potential role for autophagy in echovirus 7 entry (21), the interplay between echovirus and autophagy remains unclear.
In the present study, we explored the induction of autophagy machinery during EchoV infection by monitoring the activation of LC3 and the presence of autophagosome-like structures. Also, we checked the effects of inducing or perturbing the autophagy pathway, using pharmacological inducers or inhibitors, respectively, on viral replication. Our data revealed that autophagy is induced during echovirus infection and is involved in echovirus replication.
Human rhabdomyosarcoma (RD; CCL-136; ATCC) cells were maintained in Dulbecco’s modified Eagle Medium (DMEM) containing 10% fetal bovine serum (FBS; Life Technologies) at 37°C in a 5% CO2 incubator. Echovirus 11 strain (NCBI Accession No. OP764694) was isolated from a feces sample of a 24-day-old female neonate with enterovirus infection after passaging in the RD cells. RD cells were infected with echovirus at various multiplicity of infection (MOI) for 1.5 h in serum-free DMEM. The cells were washed with phosphate-buffered saline (PBS) and cultured in a completely fresh medium for various times as indicated until they were harvested. For autophagy induction experiments, cells were infected or mock-infected with echovirus for 1.5 h, then cultured in a complete medium containing rapamycin (Selleck, AY-22989) at indicated concentrations for the indicated times. For autophagy inhibition experiments, cells were cultured in DMEM containing indicated concentrations of 3-methyladenine (3-MA) (Selleck, S2767) for 2 h, followed by echovirus infection for 1.5 h, and then incubated with fresh DMEM for 16 h. Cell counting kit-8 (CCK8) (Vazyme, A311-01) assay was performed to examine the cytotoxicity of rapamycin or 3-MA to RD cells.
Western blotting was performed as described previously (22). The used antibodies were as follows: anti-LC3 (Cell Signaling Technology, 3868), anti-p62 (Proteintech, 18420-1-AP), anti-mTOR (Cell Signaling Technology, 2972), anti-phospho-mTOR (Cell Signaling Technology, 2971), anti-ULK1 (Cell Signaling Technology, 8054), anti-phospho-ULK1 (Ser757) (Cell Signaling Technology, 6888), anti-VPS34 (Proteintech, 12452-1-AP), anti-Beclin-1 (Proteintech, 11306-1-AP) and anti-β-actin (Santa Cruz Biotechnology, sc-47778). Anti-VP1, a rabbit polyclonal antibody, was produced by Wuhan Abclonal Biotechnology. The relative band intensities of the proteins were quantified using the NIH ImageJ software.
RD cells were transiently transfected with a GFP-tagged LC3 expression vector (GFP-LC3) as described previously (23) using Lipofectamine (Invitrogen). At 24 h post-transfection, cells were infected with echovirus for 1.5 h, then cultured in a complete medium in the presence or absence of rapamycin for 16 h. The fluorescence of GFP-LC3 was observed under a Nikon A1 confocal fluorescence microscope. The nuclei were stained with Hoechst 33258.
Echovirus-infected cells were fixed with 0.5% glutaraldehyde at 4°C for 10 min and then fixed with 2.5% glutaraldehyde at 4°C for 1 h. After that, cells were further fixed with 0.1% osmium tetroxide. The cells were then dehydrated in a gradient series of ethanol and embedded with LR White (Agar Shin sections were cut on a Leica EM FC7 UC7 ultramicrotome and viewed under an FEI Tecani G20 TWIN transmission electron microscope.
Total RNA was extracted from cells using TRIzol (Invitrogen) and then reverse transcribed into cDNA using FastKing gDNA Dispelling RT SuperMix (TIANGEN, KR118-02). Quantitative reverse transcription-polymerase chain reaction (qRT-PCR) was performed to detect viral RNA using Taq Pro Universal SYBR qPCR Master Mix (Vazyme, Q712-02). The following primers were used: EchoV-F: 5’-AAAGTGG CCAAAGGCAAGTC-3’; EchoV-R: 5’-GGTCAGGATCACACCCAACC-3’; GAPDH-F: 5’-TGGTATCGTGGAAGGACTCA-3’; GAPDH-R: 5’-CCAGTAGAGG CAGGGATGAT-3’. The relative levels of EV-D68 RNA in different samples were determined using a comparative 2-ΔΔCT method and normalized to the GAPDH gene.
Statistical analysis was conducted using GraphPad Prism 5.0 software (GraphPad Software). A one-way ANOVA was used to determine statistically significant differences in multiple comparisons. P<0.05 was considered statistically significant. The results are presented as the mean ± standard deviations (n≥3).
A hallmark of autophagy induction is that the LC3-I protein undergoes a lipidation modification, and phosphatidylethanolamine (PE) is conjugated to LC3-I to generate LC3-II, which becomes membrane-associated and participates in autophagosome formation (24). To determine whether echovirus infection regulates autophagy, we examined the conversion of endogenous LC3-I to LC3-II. Echovirus-infected cells exhibited an increase in LC3-II and viral VP1 protein when compared with uninfected Mock cells (Figure 1A). The densitometry ratio of LC3-II to β-actin showed an increase (Figure 1B). In contrast, the autophagic receptor p62 level correspondingly decreased (Figures 1A, C). These results suggested that echovirus infection may induce autophagy.
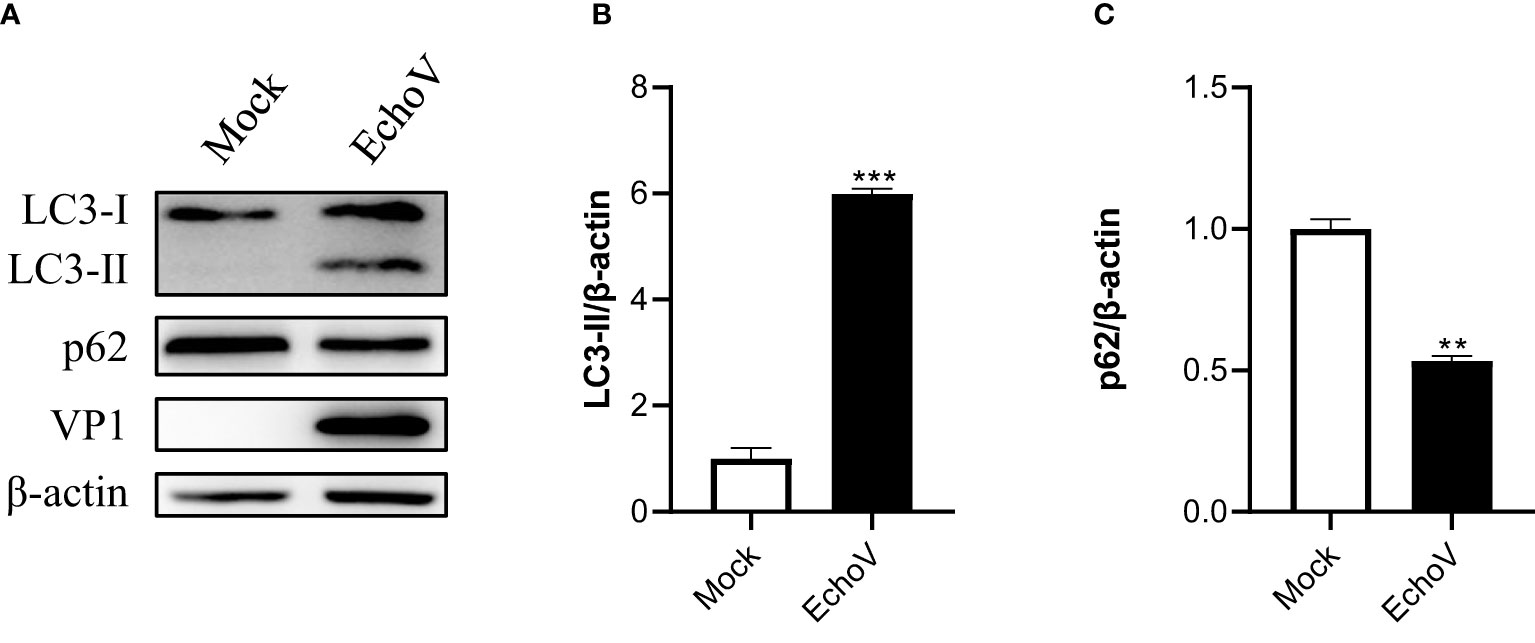
Figure 1 Echovirus infection induces autophagy. RD cells were infected or not (Mock) with echovirus 11 at 1 MOI for 1.5 h. At 16 h post-infection, cells were then harvested. (A) Intracellular LC3 and p62 proteins were detected via western blotting. β-actin served as a loading control. This result is representative of three independent experiments. The relative band intensities of detected proteins were calculated using ImageJ from NIH, and the result was represented as the ratio of LC3-II to β-actin (B) or p62 to β-actin (C). A one-way ANOVA was used to determine statistically significant differences in multiple comparisons. **p<0.01, ***P<0.001.
To explore the relationship between viral concentration and the degree of autophagy, RD cells were infected with echovirus at indicated MOIs, and the expression of LC3-II was determined by Western blotting. As the viral MOIs increased, the level of viral capsid protein (VP1) gradually increased. Meanwhile, the level of LC3-II expression gradually increased in echovirus-infected cells. In contrast, the level of p62 expression decreased (Figure 2A). The densitometry ratio of LC3-II to LC3-I showed an increase. In contrast, the densitometry ratio of p62 showed a decrease, especially under virus infection at 1 MOI (Figures 2B, C). These results indicated that echovirus infection induced-autophagy was positively correlated with viral load.
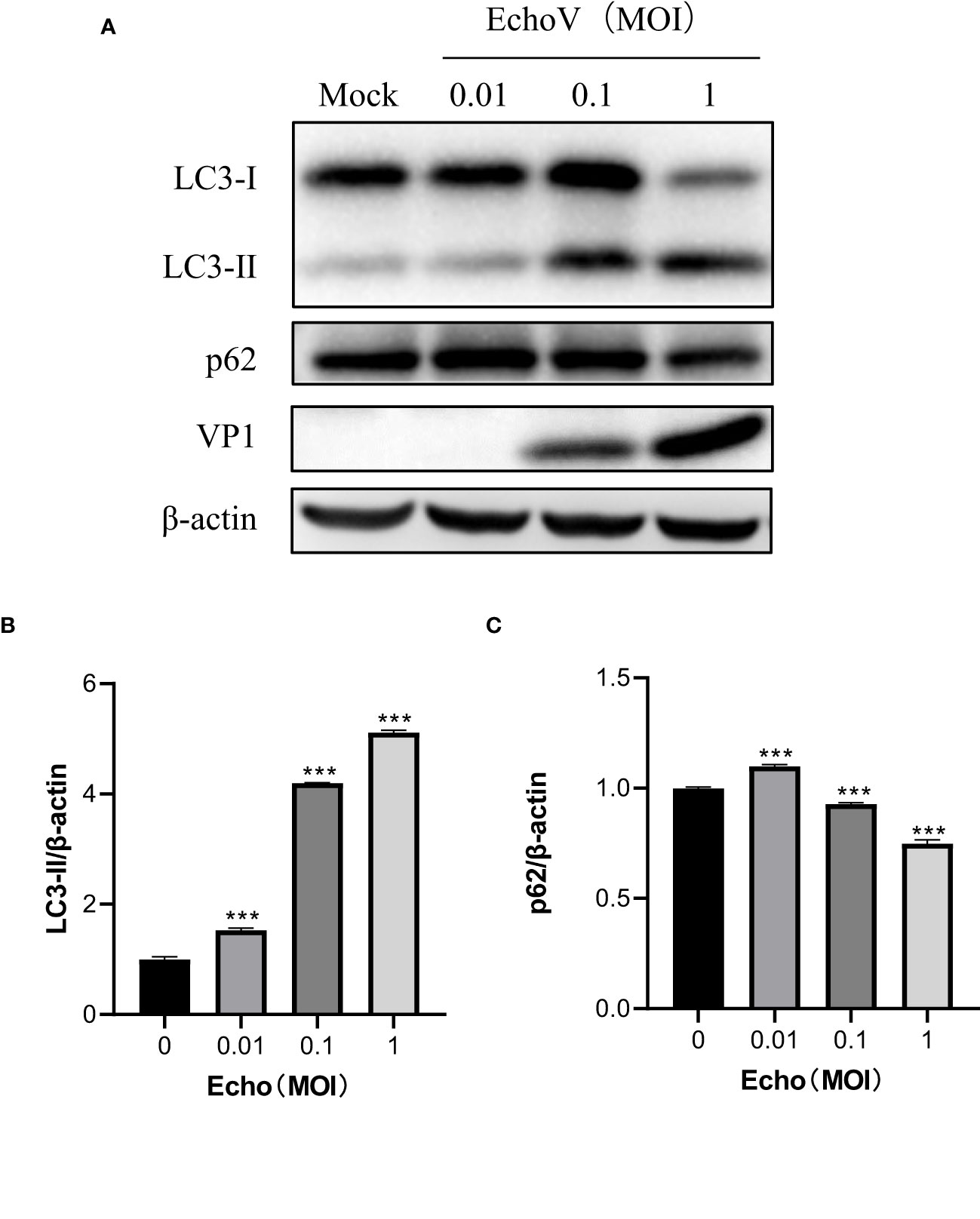
Figure 2 Echovirus infection induced-autophagy is positively correlated with viral load. RD cells were infected or not (Mock) with echovirus 11 at different MOI for 1.5 h. At 16 h post-infection, cells were then harvested. (A) Intracellular LC3 and p62 proteins were detected via western blotting. β-actin served as a loading control. This result is representative of three independent experiments. The relative band intensities of detected proteins were calculated using ImageJ from NIH, and the result was represented as the ratio of LC3-II to β-actin (B) or p62 to β-actin (C). A one-way ANOVA was used to determine statistically significant differences in multiple comparisons. ***P<0.001.
The redistribution of LC3 from a diffuse cytoplasmic localization to a characteristic punctate cytoplasmic pattern, which reflects the recruitment of LC3 to autophagic vesicles, is another hallmark of autophagy (25). Therefore, a GFP-tagged LC3 expression vector (GFP-LC3), as described previously (23), was used to assess other autophagy induced by echovirus infection. In cells transfected with GFP-LC3, the level of LC3 puncta formation was increased by echovirus infection (Figures 3A, B). These findings further confirmed that autophagy was induced by echovirus infection.
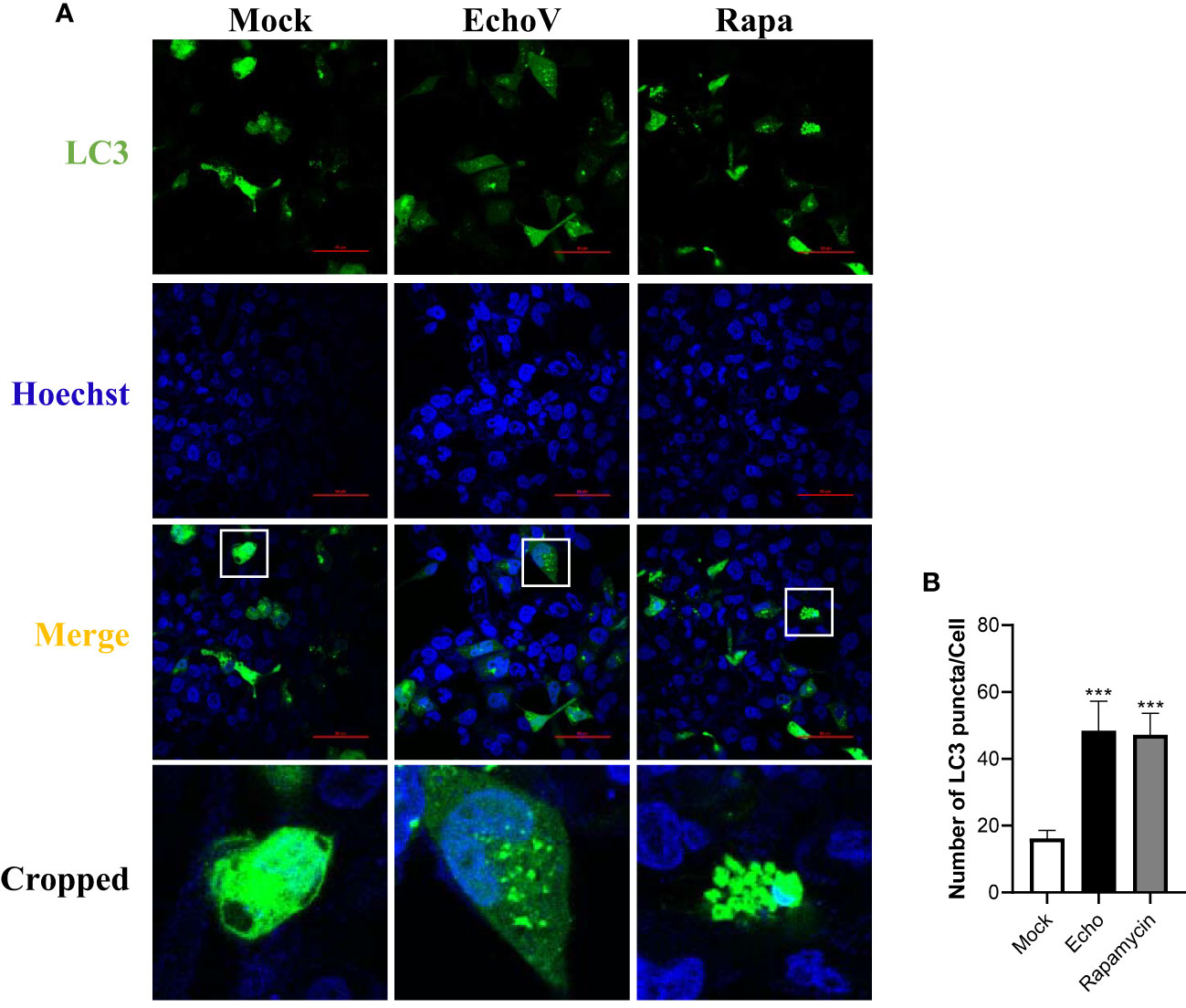
Figure 3 Echovirus infection increases the level of LC3 puncta formation. RD cells were transfected with GFP-LC3 plasmid for 24 h, followed by infected or not (Mock) with echovirus 11 at 1 MOI for 1.5 h. (A) At 16 h post-infection, cells were fixed and analyzed by confocal fluorescence microscopy. RD cells were treated with rapamycin (500 nM) (Rapa) for 16 h as a positive control. (B) Quantitation of the numbers of LC3 puncta in RD cells. Data shown represent the number of LC3 puncta per cell under each condition. A one-way ANOVA was used to determine statistically significant differences in multiple comparisons. ***P<0.001.
A key initial event in autophagy is the formation of the autophagosome, a unique double-membrane organelle that engulfs the cytosolic cargo destined for degradation. Therefore, ultrastructural analysis was performed with RD cells with or without echovirus infection. Double-membrane vesicles engulfing cytosolic materials were observed in the cytoplasm of infected cells but not in uninfected cells under transmission electron microscopy (Figure 4). The data revealed that echovirus infection induced autophagosome formation.
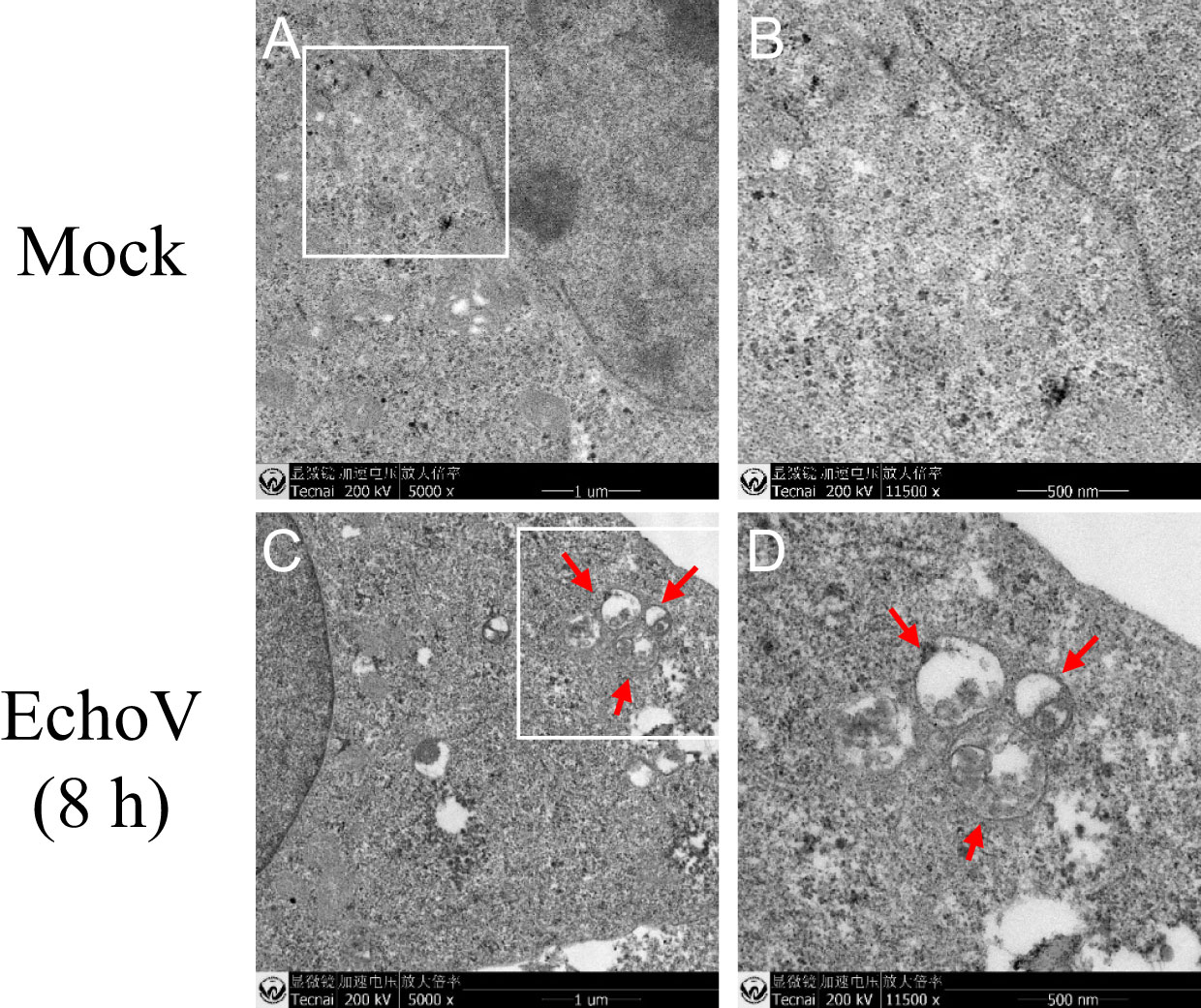
Figure 4 Echovirus infection induces autophagosome formation. RD cells were infected or not (Mock) with echovirus 11 at 1 MOI for 1.5 h. At 8 h post-infection, cells were then fixed and subjected to transmission electron microscopy analysis. Lower magnification of Mock (A) and EchoV-infected (C) cells. (B) Higher magnification of the area in white square of (A); (D) Higher magnification of the area in white square of (C). Scale bar in A and C, 1 µm; Scale bar in B and D, 500 nm. The autophagosomes are denoted by red arrows.
The mTOR/ULK1 signaling pathway plays a key role in mediating the initiation and formation of an autophagosome (26). The phosphorylation levels of mTOR were decreased in echovirus-infected cells (Figure 5A). Both levels of ULK1 and phosphorylated ULK1 protein on S757 were also decreased in virus-infected cells (Figure 5A). Upon autophagy induction, the ULK1 complex translocates to autophagy initiation sites and regulates the recruitment of a second kinase complex, the vacuolar protein sorting 34 (VPS34) complex consisting of VPS34, as well as Beclin-1, VPS15, and ATG14L, which promotes the formation of autophagic vesicles (26, 27). Therefore, we further checked the levels of VPS34 and Beclin-1. As shown in Figure 5B, both VPS34 and Beclin-1 protein levels increased in echovirus-infected cells in a dose-dependent manner. The results indicated that echovirus infection induced the activation of signaling pathways involved in autophagosome formation.
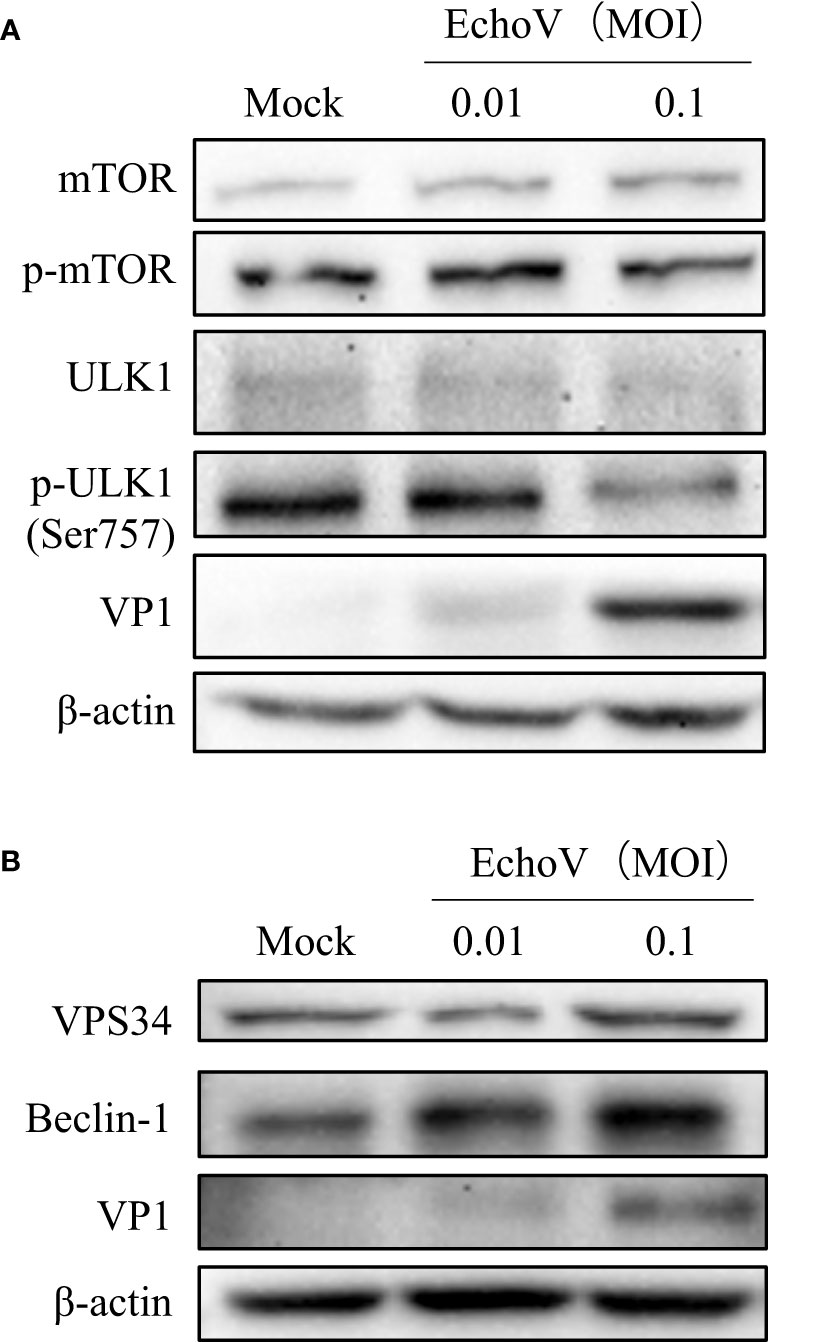
Figure 5 Echovirus infection induces the activation of signaling pathways involved in autophagosome formation. RD cells were infected or not (Mock) with echovirus 11 at different MOI for 1.5 h. At 16 h post-infection, cells were then harvested. (A) Intracellular mTOR, phosphorylated mTOR (p-mTOR), ULK1, phosphorylated ULK1 (p-ULK1), and viral VP1 proteins were detected via western blotting. (B) Intracellular VPS34, Beclin-1, and viral VP1 proteins were detected via western blotting. β-actin served as a loading control. This result is representative of three independent experiments.
Previous studies have demonstrated that autophagy may serve an antiviral or proviral function during diverse viral infections (14). Therefore, to investigate the impact of autophagy induction on echovirus infection, we monitored virus expression and replication under autophagy inducer rapamycin treatment. Rapamycin at indicated concentrations showed no toxicity to RD cells (Figure 6A). Induction of autophagy through rapamycin treatment significantly increased viral protein VP1 expression in a dose-dependent manner (Figures 6B–C). Moreover, the levels of viral RNA were also increased under rapamycin treatment in a dose-dependent manner (Figure 6D). These results suggested that induction of autophagy promoted echovirus replication.
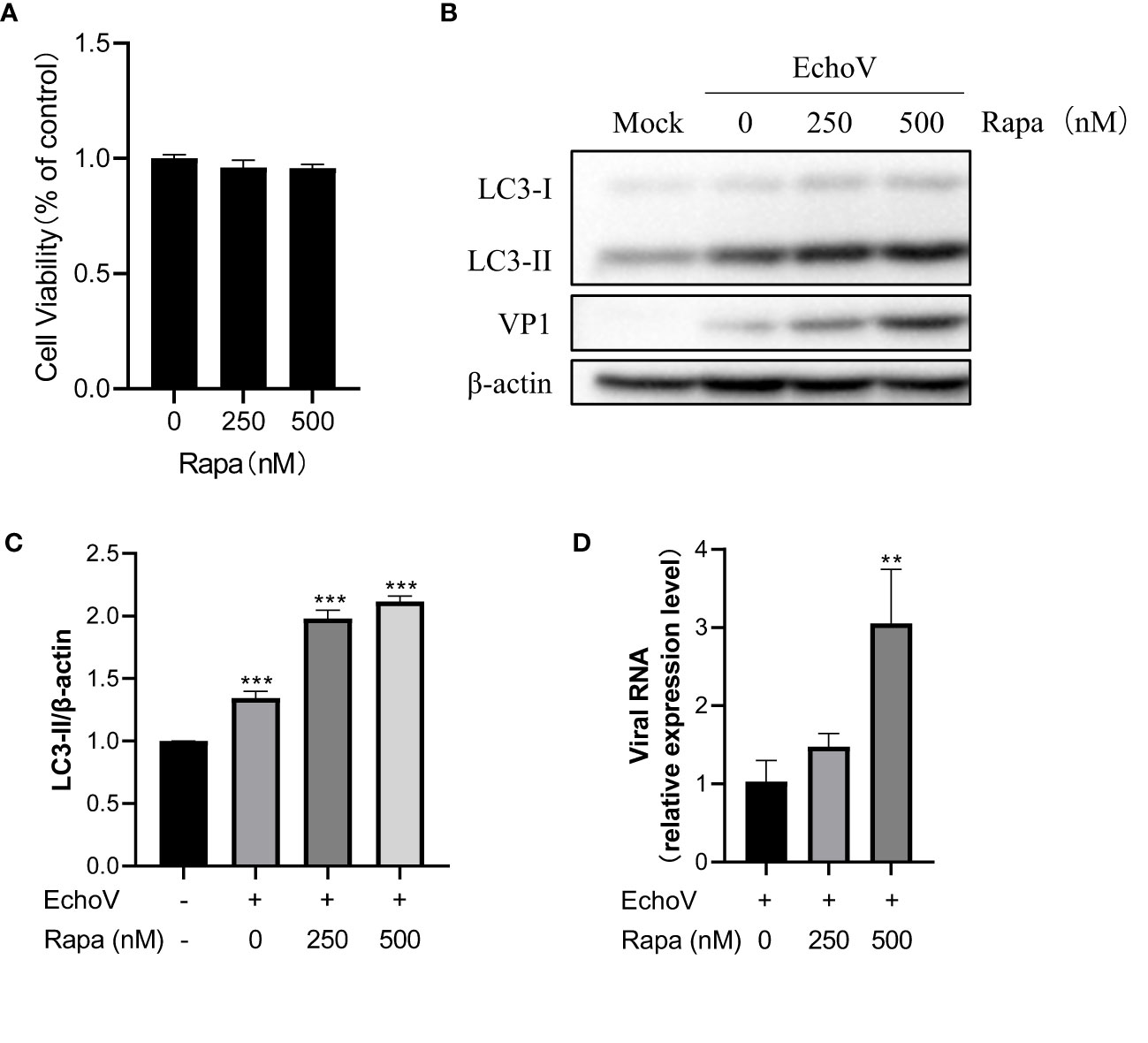
Figure 6 Induction of autophagy promotes Echovirus replication. RD cells were infected or not (Mock) with echovirus 11 at 0.1 MOI for 1.5 h and then untreated (Echo) or treated with rapamycin (Rapa) at indicated concentrations for 16 h. Cells were then harvested. (A) Cell counting kit-8 (CCK8) assay was performed to examine the cytotoxicity of rapamycin to RD cells. (B) Intracellular LC3 and viral VP1 proteins were detected via western blotting. β-actin served as a loading control. This result is representative of three independent experiments. The relative band intensities of detected proteins were calculated using ImageJ from NIH, and the result was represented as the ratio of LC3-II to β-actin (C). (D) Total RNA was extracted from cells and subjected to a quantitative reverse transcription-polymerase chain reaction (qRT-PCR) to detect viral RNA. A one-way ANOVA was used to determine statistically significant differences in multiple comparisons. **P<0.01; ***P<0.001.
To further validate the effect of autophagy on echovirus replication, 3-MA, a widely used selective autophagy inhibitor, was used. As shown in Figure 7, 3-MA at indicated concentrations showed no toxicity to RD cells (Figure 7A). Treatment with 3-MA impaired the level of LC3-II expression, suggesting the inhibition of autophagy (Figure 7B). Correspondingly, viral VP1 protein expression reduction was observed (Figures 7C). This finding confirmed that autophagy might serve a proviral function during echovirus infection.
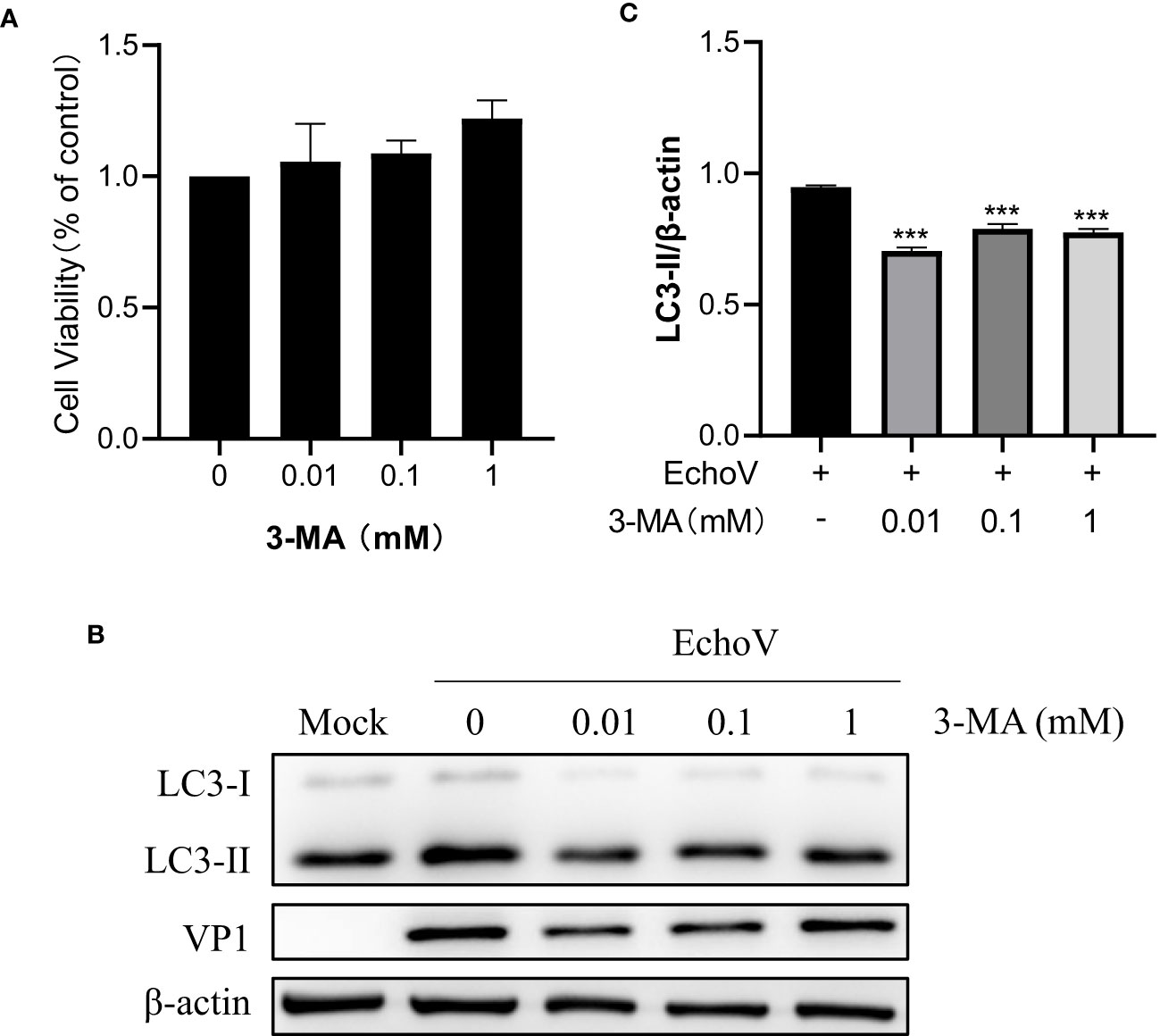
Figure 7 Inhibition of Autophagy inhibits EchoV replication. RD cells were pre-untreated or pre-treated with 3-MA at indicated concentrations for 2 h, followed by infected (EchoV) or not (Mock) with echovirus 11 at 0.1 MOI for 1.5 h. At 16 h post-infection, cells were then harvested. (A) Cell counting kit-8 (CCK8) assay was performed to examine the cytotoxicity of 3-MA to RD cells. (B) Intracellular LC3 and viral VP1 proteins were detected via western blotting. β-actin served as a loading control. This result is representative of three independent experiments. The relative band intensities of detected proteins were calculated using ImageJ from NIH, and the result was represented as the ratio of LC3-II to β-actin (C). A one-way ANOVA was used to determine statistically significant differences in multiple comparisons. ***P<0.001.
Autophagy is an evolutionarily conserved cellular process through which the lysosome could degrade long-lived proteins, damaged organelles, or invading pathogens to maintain cellular homeostasis and host health (13, 17). However, viruses have evolved strategies during a long evolutionary process by which they can hijack and subvert host autophagy to favor their benefits (14). Echovirus is one of the most common worldwide causes of severe illnesses in neonates or infants. The interplay between echovirus and autophagy needs to be better understood. Here, we demonstrated that echovirus 11 induced autophagy to promote its replication via regulating mTOR/ULK1 signaling pathway (Figure 8).
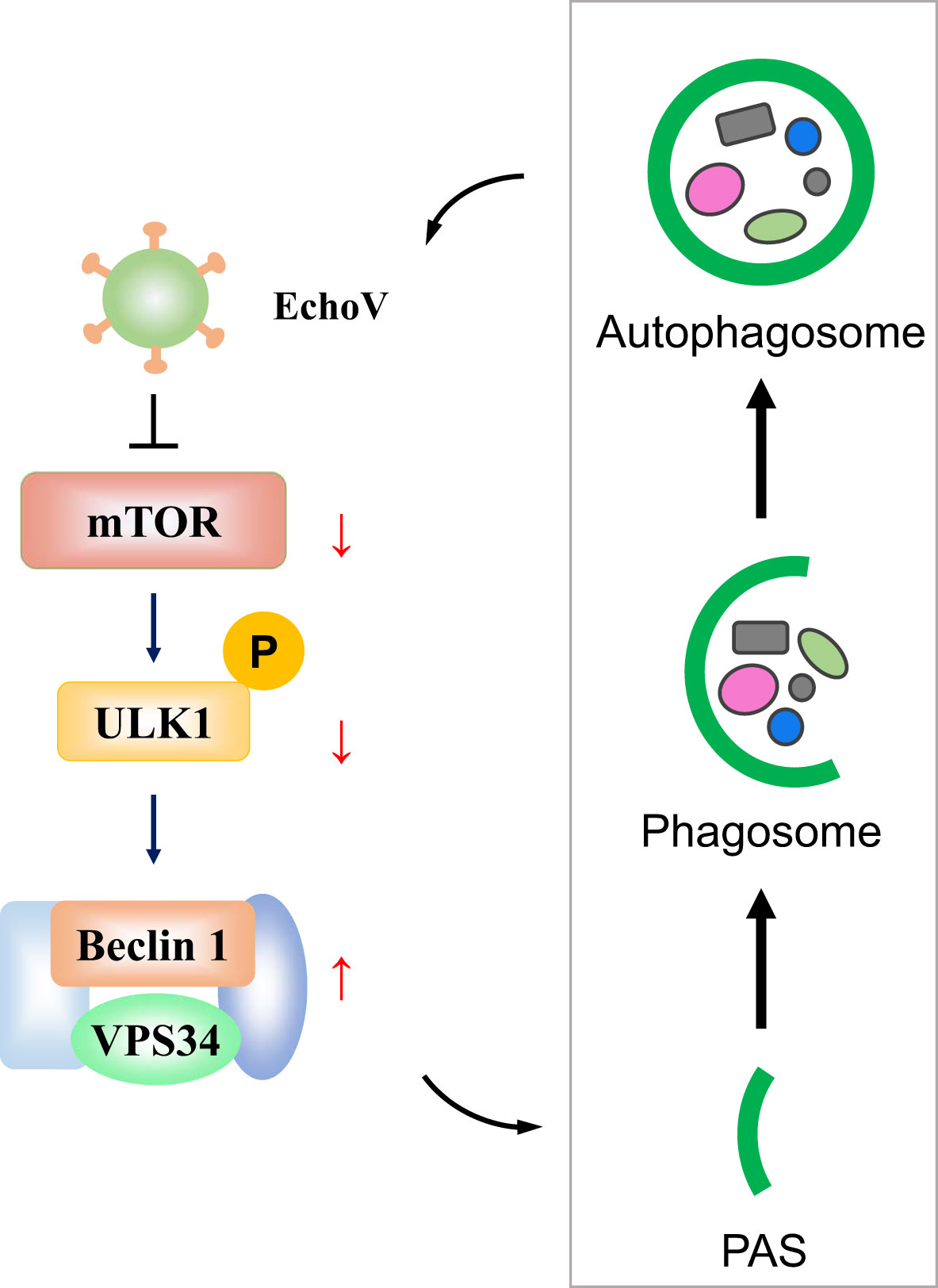
Figure 8 A proposed model depicting the interplay between echovirus infection and autophagy via regulating mTOR/ULK1 signaling pathway. Upon echovirus infection, the levels of phosphorylated mTOR and phosphorylated ULK1 protein on S757 decreased. Furthermore, both VPS34 and Beclin-1 protein levels increased, which promotes the formation of autophagic vesicles. Echovirus-induced autophagy promoted viral replication.
Previous studies have indicated that several core components of the autophagy machinery, including Beclin-1, Atg12, Atg14, Atg16L1, and LC3, are important for echovirus 7 entry into polarized Caco-2 cells (21). However, the impact of autophagy on echovirus replication after virus entry was not discussed in that paper. Here, our data provided experimental evidence that autophagy also participates in echovirus replication. Replication of positive-stranded RNA viruses requires intracellular membrane surfaces on which they assemble their replication complexes (28). Double-membrane compartments formed during autophagy can provide a physical platform for the viral replication machinery. For example, the influenza A virus triggers the accumulation of autophagosomes for viral replication (29). Zika virus infection results in membrane rearrangements and induction of autophagy, which are considered to be the sites of viral RNA replication and virion assembly (30). CVB3, another important enterovirus classified within the B species, also uses autophagy for replication (31). Similar to these results, echovirus infection can also induce autophagy. In virus-infected RD cells, autophagosome can be observed. Presumably, echovirus exploited the autophagic membrane to support its replication.
Mechanically, membrane compartments formed during autophagy can locally concentrate essential intermediates and protect viral RNAs from detection by innate immune sensors and degradation (14). For example, HCV induces autophagosome formation but blocks lysosomal fusion, resulting in the accumulation of autophagosomes in support of HCV replication (32). CVB3-induced accumulation of autophagosomes via blockage of autophagosome-lysosome fusion (33). However, in this study, p62, a marker of autophagy-mediated protein degradation or autophagic flux, decreased during echovirus infection, indicating that echovirus infection may not interfere with the fusion of autophagosomes with lysosomes. Therefore, further studies may be needed to elucidate the mechanism by which echovirus-induced autophagy promotes viral replication. Notably, the expression level of viral VP1 protein increased upon autophagy induction and decreased upon autophagy inhibition. This result suggested that autophagy may play a role in viral protein synthesis.
The process of autophagosome formation is tightly controlled. The serine/threonine protein kinase mTOR is one of the key regulators and negatively controls autophagosome formation (34). We consistently showed that mTOR phosphorylation levels decreased after echovirus infection, suggesting that echovirus-induced autophagy is triggered by mTOR dephosphorylation. Furthermore, we observed that the level of phosphorylated ULK1 on S757 decreased. As phosphorylation of the major autophagy activator ULK1 on S757 by mTORC1 inhibits ULK1 activity and represses autophagy (35), our findings thus suggested that mTOR/ULK1 axis participates in echovirus-induced autophagy. Subsequently, Beclin-1 (a homolog of yeast ATG6) is the first identified mammalian autophagy protein critical for the signaling pathways involved in autophagosome formation (27). Some viral proteins, like hepatitis B virus X protein, can sensitize cells to starvation-induced autophagy via up-regulation of Beclin-1 expression. In the present study, we also observed the up-regulation of Beclin-1 during echovirus infection. In addition, Beclin-1 forms the class III PI3K complex with VPS34, which promotes autophagosome formation (36). Our data also showed that the levels of VPS34 increased upon echovirus infection. These results further demonstrated that echovirus could induce autophagy via activating autophagy signaling. Our preliminary data showed that VP1, a capsid protein of echovirus, did not participate in echovirus-induced autophagy. Therefore, further studies may be needed to elucidate the mechanism by which echovirus infection regulates mTOR/ULK1 signaling pathway.
In conclusion, our study demonstrates that autophagy can be induced by echovirus infection and exhibits a proviral function, revealing the potential role of autophagy in echovirus infection. These findings not only shed light on the molecular mechanisms underlying how echovirus hijacks cellular components and pathways for its benefits but also provide therapeutic options against echovirus infection.
The original contributions presented in the study are included in the article/supplementary materials. Further inquiries can be directed to the corresponding author.
CW and JX designed the research, wrote and revised the paper. LZ, WY, YM and YL performed the experiments. QW, SL, GP and ZZ analyzed the data. All authors contributed to the article and approved the submitted version.
This study was supported by grants from the Natural Science Foundation of Hubei Province of China (2022CFB564), the Health Commission Foundation of Hubei Province of China (WJ2023M108), the Open project of National Virus Resource Center (NVRC-PY-03), Sino-German mobility programme (M-0060), Traditional Chinese Medicine Administration Foundation of Hubei Province of China (ZY2023F027) and the Young and Middle-aged Medical Professionals in Wuhan (grant number 1030000301). This work was also supported by the International Cooperation Base of Hubei Province for Infection and Immunity.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer YX declared a shared parent affiliation with the author SL at the time of review.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Pons-Salort M, Parker EP, Grassly NC. The epidemiology of non-polio enteroviruses: Recent advances and outstanding questions. Curr Opin Infect Dis (2015) 28(5):479–87. doi: 10.1097/QCO.0000000000000187
2. Committee ON the Echo Viruses. ENTERIC cytopathogenic human orphan (ECHO) viruses. Science (1955) 122(3181):1187–8. doi: 10.1126/science.122.3181.1187
3. Rotbart HA. Enteroviral infections of the central nervous system. Clin Infect Dis (1995) 20(4):971–81. doi: 10.1093/clinids/20.4.971
4. Nagington J. Echovirus 11 infection and prophylactic antiserum. Lancet (1982) 1(8269):446. doi: 10.1016/S0140-6736(82)91642-7
5. Nagington J, Wreghittt TG, Gandy G, Roberton NR, Berry PJ. Fatal echovirus 11 infections in outbreak in special-care baby unit. Lancet (1978) 2(8092 Pt 1):725–8. doi: 10.1016/S0140-6736(78)92714-9
6. Nagington J, Gandy G, Walker J, Gray JJ. Use of normal immunoglobulin in an echovirus 11 outbreak in a special-care baby unit. Lancet (1983) 2(8347):443–6. doi: 10.1016/S0140-6736(83)90402-6
7. Rabkin CS, Telzak EE, Ho MS, Goldstein J, Bolton Y, Pallansch M, et al. Outbreak of echovirus 11 infection in hospitalized neonates. Pediatr Infect Dis J (1988) 7(3):186–90.
8. Chevaliez S, Szendroi A, Caro V, Balanant J, Guillot S, Berencsi G, et al. Molecular comparison of echovirus 11 strains circulating in Europe during an epidemic of multisystem hemorrhagic disease of infants indicates that evolution generally occurs by recombination. Virology (2004) 325(1):56–70. doi: 10.1016/j.virol.2004.04.026
9. Khetsuriani N, Lamonte A, Oberste MS, Pallansch M. Neonatal enterovirus infections reported to the national enterovirus surveillance system in the united states, 1983-2003. Pediatr Infect Dis J (2006) 25(10):889–93. doi: 10.1097/01.inf.0000237798.07462.32
10. Chuang YY, Huang YC. Enteroviral infection in neonates. J Microbiol Immunol Infect (2019) 52(6):851–7. doi: 10.1016/j.jmii.2019.08.018
11. Li J, Yan D, Chen L, Zhang Y, Song Y, Zhu S, et al. Multiple genotypes of echovirus 11 circulated in mainland China between 1994 and 2017. Sci Rep (2019) 9(1):10583. doi: 10.1038/s41598-019-46870-w
12. Lu J, Kang M, Zeng H, Zhong Y, Fang L, Zheng X, et al. Tracking echovirus eleven outbreaks in guangdong, China: A metatranscriptomic, phylogenetic, and epidemiological study. Virus Evol (2020) 6(1):veaa029. doi: 10.1093/ve/veaa029
13. Mizushima N, Komatsu M. Autophagy: Renovation of cells and tissues. Cell (2011) 147(4):728–41. doi: 10.1016/j.cell.2011.10.026
14. Choi Y, Bowman JW, Jung JU. Autophagy during viral infection - a double-edged sword. Nat Rev Microbiol (2018) 16(6):341–54. doi: 10.1038/s41579-018-0003-6
15. Deretic V, Levine B. Autophagy balances inflammation in innate immunity. Autophagy (2018) 14(2):243–51. doi: 10.1080/15548627.2017.1402992
16. Deretic V, Saitoh T, Akira S. Autophagy in infection, inflammation and immunity. Nat Rev Immunol (2013) 13(10):722–37. doi: 10.1038/nri3532
17. Liang S, Wu YS, Li DY, Tang JX, Liu HF. Autophagy in viral infection and pathogenesis. Front Cell Dev Biol (2021) 9:766142. doi: 10.3389/fcell.2021.766142
18. Wong J, Zhang J, Si X, Gao G, Mao I, McManus BM, et al. Autophagosome supports coxsackievirus B3 replication in host cells. J Virol (2008) 82(18):9143–53. doi: 10.1128/JVI.00641-08
19. Dreux M, Gastaminza P, Wieland SF, Chisari FV. The autophagy machinery is required to initiate hepatitis c virus replication. Proc Natl Acad Sci USA (2009) 106(33):14046–51. doi: 10.1073/pnas.0907344106
20. Taylor MP, Kirkegaard K. Potential subversion of autophagosomal pathway by picornaviruses. Autophagy (2008) 4(3):286–9. doi: 10.4161/auto.5377
21. Kim C, Bergelson JM. Echovirus 7 entry into polarized caco-2 intestinal epithelial cells involves core components of the autophagy machinery. J Virol (2014) 88(1):434–43. doi: 10.1128/JVI.02706-13
22. Yuan Y, Zhao K, Yao Y, Liu C, Chen Y, Li J, et al. HDAC11 restricts HBV replication through epigenetic repression of cccDNA transcription. Antiviral Res (2019) 172:104619. doi: 10.1016/j.antiviral.2019.104619
23. Xia J, Cao L, Zhao Z, Deng Y, Lu M, Wu C, et al. HBV sL13H mutation impairs its surface antigen expression and ability to induce autophagy. Genes Dis (2022) 9(6):1401–4. doi: 10.1016/j.gendis.2022.01.004
24. Klionsky DJ, Abeliovich H, Agostinis P, Agrawal DK, Aliev G, Askew DS, et al. Guidelines for the use and interpretation of assays for monitoring autophagy in higher eukaryotes. Autophagy (2008) 4(2):151–75. doi: 10.4161/auto.5338
25. Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, et al. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J (2000) 19(21):5720–8. doi: 10.1093/emboj/19.21.5720
26. Zachari M, Ganley IG. The mammalian ULK1 complex and autophagy initiation. Ess. Biochem (2017) 61(6):585–96. doi: 10.1042/EBC20170021
27. Cao Y, Klionsky DJ. Physiological functions of Atg6/Beclin 1: A unique autophagy-related protein. Cell Res (2007) 17(10):839–49. doi: 10.1038/cr.2007.78
28. Wileman T. Aggresomes and autophagy generate sites for virus replication. Science (2006) 312(5775):875–8. doi: 10.1126/science.1126766
29. Zhou Z, Jiang X, Liu D, Fan Z, Hu X, Yan J, et al. Autophagy is involved in influenza a virus replication. Autophagy (2009) 5(3):321–8. doi: 10.4161/auto.5.3.7406
30. Chiramel AI, Best SM. Role of autophagy in zika virus infection and pathogenesis. Virus Res (2018) 254:34–40. doi: 10.1016/j.virusres.2017.09.006
31. Robinson SM, Tsueng G, Sin J, Mangale V, Rahawi S, McIntyre LL, et al. Coxsackievirus b exits the host cell in shed microvesicles displaying autophagosomal markers. PloS Pathogens (2014) 10(4):e1004045. doi: 10.1371/journal.ppat.1004045
32. Wang L, Tian Y, Ou JH. HCV induces the expression of Rubicon and UVRAG to temporally regulate the maturation of autophagosomes and viral replication. PloS Pathogens (2015) 11(3):e1004764. doi: 10.1371/journal.ppat.1004764
33. Tian L, Yang Y, Li C, Chen J, Li Z, Li X, et al. The cytotoxicity of coxsackievirus B3 is associated with a blockage of autophagic flux mediated by reduced syntaxin 17 expression. Cell Death Dis (2018) 9(2):242. doi: 10.1038/s41419-018-0271-0
34. Kim YC, Guan KL. mTOR: a pharmacologic target for autophagy regulation. J Clin Invest (2015) 125(1):25–32. doi: 10.1172/JCI73939
35. Kim J, Kundu M, Viollet B, Guan KL. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol (2011) 13(2):132–41. doi: 10.1038/ncb2152
Keywords: enterovirus, echovirus, autophagy, viral replication, virus-host interaction
Citation: Wu C, Zeng L, Yi W, Miao Y, Liu Y, Wang Q, Liu S, Peng G, Zheng Z and Xia J (2023) Echovirus induces autophagy to promote viral replication via regulating mTOR/ULK1 signaling pathway. Front. Immunol. 14:1162208. doi: 10.3389/fimmu.2023.1162208
Received: 09 February 2023; Accepted: 21 March 2023;
Published: 11 April 2023.
Edited by:
Huifang Zhu, First Affiliated Hospital of Gannan Medical University, ChinaCopyright © 2023 Wu, Zeng, Yi, Miao, Liu, Wang, Liu, Peng, Zheng and Xia. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianbo Xia, eGpiOTE1QDEyNi5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.