- 1Department of Neurosciences, Mental Health and Sensory Organs, Centre for Experimental Neurological Therapies (CENTERS), Sapienza University of Rome, Rome, Italy
- 2Merck Serono S.p.A., An Affiliate of Merck KGaA, Rome, Italy
- 3Department of Health Sciences, University of Genoa, Genoa, Italy
- 4Istituti di Ricovero e Cura a Carattere Scientifico (IRCCS) Istituto Neurologico Mediterraneo Neuromed, Pozzilli, Italy
- 5Neuroimmunology Unit, Istituti di Ricovero e Cura a Carattere Scientifico (IRCCS) Fondazione Santa Lucia, Rome, Italy
Interferon-beta (IFN-β) for Multiple Sclerosis (MS) is turning 30. The COVID-19 pandemic rejuvenated the interest in interferon biology in health and disease, opening translational opportunities beyond neuroinflammation. The antiviral properties of this molecule are in accord with the hypothesis of a viral etiology of MS, for which a credible culprit has been identified in the Epstein-Barr Virus. Likely, IFNs are crucial in the acute phase of SARS-CoV-2 infection, as demonstrated by inherited and acquired impairments of the interferon response that predispose to a severe COVID-19 course. Accordingly, IFN-β exerted protection against SARS-CoV-2 in people with MS (pwMS). In this viewpoint, we summarize the evidence on IFN-β mechanisms of action in MS with a focus on its antiviral properties, especially against EBV. We synopsize the role of IFNs in COVID-19 and the opportunities and challenges of IFN-β usage for this condition. Finally, we leverage the lessons learned in the pandemic to suggest a role of IFN-β in long-COVID-19 and in special MS subpopulations.
Introduction
Multiple Sclerosis (MS) is a chronic demyelinating disease in which inflammation and neurodegeneration cause accumulating central nervous system (CNS) damage and clinical disability. It is a multifactorial disorder with a complex etiology and pathophysiology: multiple genetic and environmental factors interact to establish and maintain a dysimmune attack, which halts neural homeostasis (1). Among environmental factors, the causal role of the Epstein-Barr Virus (EBV) is getting increasing support: from both large seroepidemiological studies (2) indicating that EBV infection is necessary for and precedes disease onset, and experimental evidence of biological mechanisms involving EBV in disease processes (3, 4).
The COVID-19 pandemic challenged researchers in a rush for actionable evidence on viral infections biology, to rapidly reach the goal of efficient therapeutic and preventive strategies against SARS-CoV-2. Lots of work focused on managing people at risk for severe forms of COVID-19, including “fragile” people suffering from neurological disorders and immune-suppressed people. Since the beginning of the pandemic, there has been a special interest in the implications of COVID-19 in people with MS, which are being treated with disease-modifying therapies (DMTs) that impact the immune system.
The largest studies showed a similar incidence of COVID-19 in people with MS (pwMS) compared with the general population, but a higher risk for severe course (ICU admission and death), that correlated with age, higher EDSS, comorbidities and treatment with anti-CD20 monoclonal antibodies (5–9).
Meanwhile, most studies highlighted a protective effect of IFN formulations (8, 10, 11). This fact was traced back to the biology of these endogenous antivirals – which are the forerunners of MS DMTs, having been approved almost thirty years ago (12) – linked to the hypothesis of a viral etiology of MS.
In this review, we discuss the value of IFN-β in MS through the lens of its antiviral properties. We then point out analogies and differences between possible activities against EBV and SARS-CoV-2, considering the data on IFN-β repositioning in COVID-19. Finally, we outline the potential uses of this molecule for selected MS patients and beyond.
Methods
A comprehensive literature search was performed between April 1, 2022 and April, 15 2023 in the PubMed database with the following terms: “Interferon”, “Interferon β” or “β interferon”, “multiple sclerosis”, “Covid-19” or “SARS-CoV-2”, “Epstein Barr Virus” or “EBV”. We retrieved articles published in English; only papers with full text available were included. The reference lists of the selected articles and the relevant links were also manually reviewed for additional eligible papers.
The inclusion criteria for article selection were to include papers regarding: the role of viral infections in MS etiology, biological activities of IFNs in viral infections, the MoA of IFN-β in MS, in COVID-19 and in the context of EBV biology.
Biology and mechanism of action of IFN-β in multiple sclerosis
IFNs are classified into three groups (type I, II, III) based on their structure, origin, cellular receptors and final effects. Interferon-β belongs to the type I IFN family, like IFN-α, and 15 other molecules which constitute a major line of host defense against viruses and other microorganisms. IFN-γ is the only member of the type II family; IFN-λ1 to λ4 constitute the recently discovered type III group.
Almost all cells in the body can produce type I IFNs; however, the greatest amount derives from plasmacytoid dendritic cells (pDCs), lymphocytes, and a few non-immune cells (such as fibroblasts and endothelial cells). Two patterns of IFN-I production can be distinguished: (a) a physiological, tonic, low-dose constitutive production, aiding in immune homeostasis; (b) a high-dose, phasic secretion, mainly by the innate cells, that follows the interaction of pathogen-associated molecular patterns (PAMPS) with pattern recognition receptors (PRRs) (13). Indeed, “IFNs” were named by Isaacs and Lindemann (14) due to their capacity to interfere with viral replication.
The binding of secreted type I IFNs with membrane IFNAR1/2 receptors induces the JAK-STAT pathway activation, leading to the nuclear translocation of IRF9, which guides the regulation of the so-called interferon-stimulated genes (ISGs) (15) whose products orchestrate an antiviral state in bystander cells and tackle the replication cycle in infected cells.
Furthermore, IFN-β exerts broad immune-modulating activities. It has been shown to reduce the expression of Th1 cytokines (TNFα, LTα, IFN) and MCH-II molecules on antigen-presenting cells (APCs), thus diminishing lymphocyte activation, while favoring an anti-inflammatory setup through the secretion of IL4, IL10 and TGF-β. These cytokines decrease the expression of matrix metalloproteinases (MMPs), adhesion molecules (VLA-4) and endothelial vascular cell adhesion protein (VCAM), lessening the ability of lymphocytes and monocytes to infiltrate tissues, including the CNS through the blood-brain barrier (BBB) (12). The effect on B lymphocytes is mediated by the augmented expression of apoptotic markers like Annexin-V and active caspase-3, promoting the death of memory B cells (16). This pathway may be altered in MS, whereby a pro-survival status of B cells was reported to be due to down-regulation of the interferon response factor (IRF) 1 and C-X-C motif chemokine 10 axis (17).
These wide-acting, modulating effects are thought to be the main mechanism of action (MoA) of IFN-β in MS treatment. Since a low serum level of IFN-β was found in the majority of MS patients (18), its therapeutic supplementation could serve as a rescue of this defect. A profound dysregulation of ISG transcription has been confirmed in both MS and its experimental models, more markedly in B cells (19, 20). This signature may reflect an MS-specific genetic predisposition: indeed, an enrichment of type I IFN signaling and antiviral pathways were found in the SNPs associated with disease development by several GWAS (21, 22).
Accordingly, the antiviral action of IFN-β could contribute to its efficacy in MS. The hypothesis of a viral etiology of MS stands on solid epidemiological, clinical and experimental evidence linking primarily EBV (but also HHV6 among Herpesviridae, and human endogenous retroviruses, HERVs) to disease development and progression (2–4, 23). This evidence and the above-mentioned immunomodulatory actions of IFNs may contribute to explain its beneficial effects as a therapeutic approach in MS.
Antiviral effects against Epstein-Barr virus
EBV is a herpesvirus infecting 95% of individuals worldwide and >99% of people with MS. Following the infection, which is usually asymptomatic or can lead to infectious mononucleosis, the virus establishes a lifelong latency in human B cells, with sporadic reactivations and lytic replication (24).
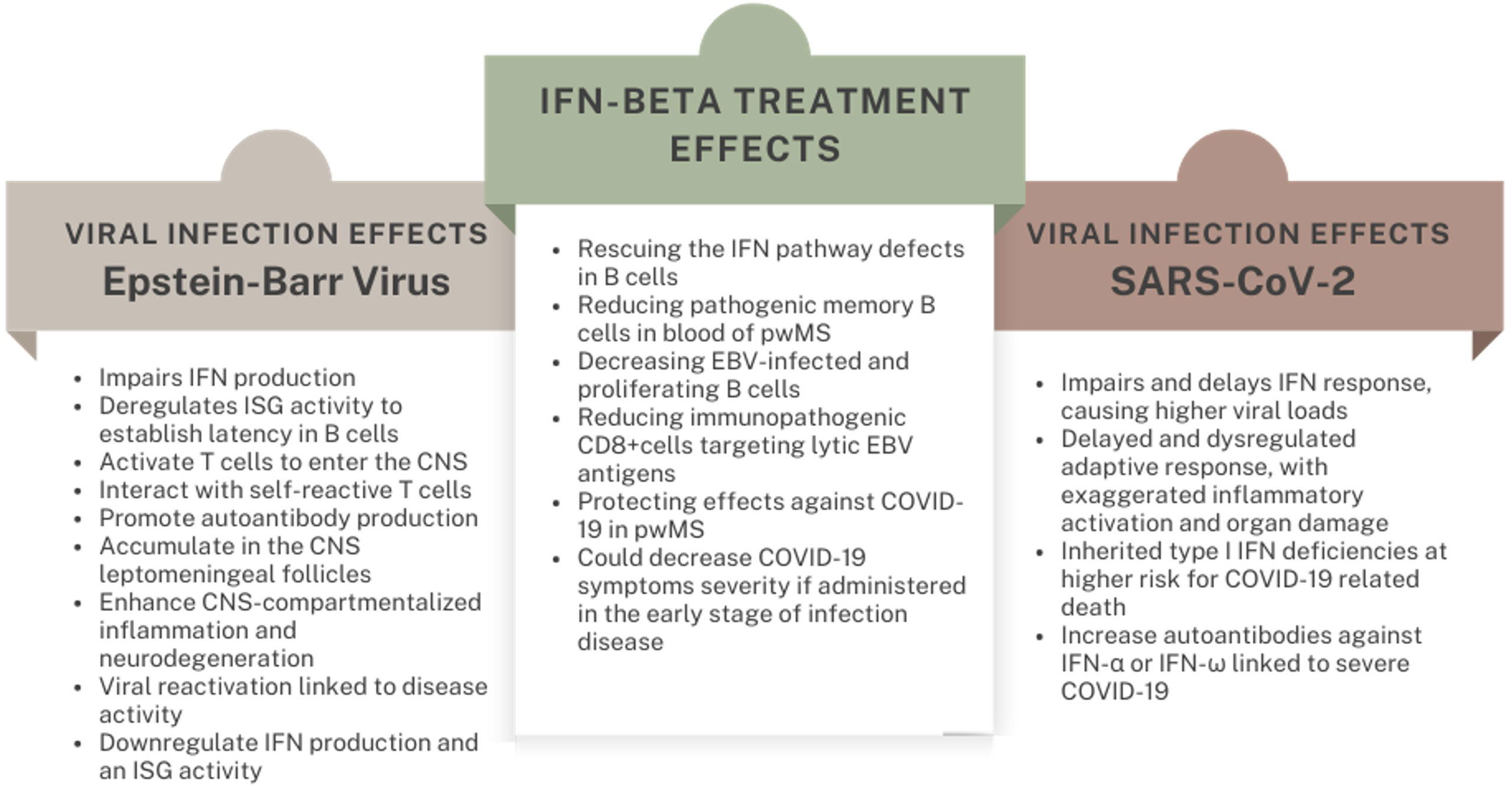
Several mechanisms have been proposed through which EBV initiates and sustains MS pathogenic processes. EBV infection may unleash autoimmune clones through molecular mimicry between viral proteins and CNS antigens (3, 25, 26). EBV-infected memory B cells in the periphery may activate T cells to enter the CNS (27), also by producing an EBV-encoded interleukin-10-like protein that counteracts the human immunomodulatory IL-10 (28). Inadequate CD8+ killing of EBV-infected cells may result in the accumulation of EBV-infected autoreactive B cells in the CNS leptomeningeal follicles (29). There, EBV-infected B cells may fuel CNS-compartmentalized inflammation and neurodegeneration through cytotoxic bystander injury, interaction with self-reactive T cells and autoantibody production (30). Furthermore, broad effects on the immune response may arise from host-viral interactions involved in genetic regulation (31), influenced by both the host’s and viral genetic asset (32, 33). EBV may institute pathological synergies with MS-associated Human-Herpesvirus 6 (HHV6) and human-endogenous retroviruses, contributing to a neurotoxic antiviral response (34, 35). Additionally, EBV reactivation has been linked to relapse triggering: antibodies targeting EBV early antigen (EA), which is expressed during the lytic phase, and EBV-DNA have been detected during clinical and radiological MS activity (36–38).
At first, when EBV attacks the host’s cells, viral lipids and proteins are sensed by transmembrane TRL2; viral DNA and transcribed RNA are recognized in endosomes by TRL7 and TRL9; viral RNA also binds RIG-I-like receptors triggering a robust IFN production. However, as for other pathogens, EBV has evolved strategies to evade and counteract the antiviral IFN response, extensively reviewed in Lange et al. (39). Sensors downregulation and inhibition is mostly operated by BGLF5, BPLF1, LMP-1 and the miR-BART6-3p. Kinase cascade signaling is counteracted by EBNA1, LMP-1 and LMP2; then, IRFs, which induce genetic transcription of IFN genes, are tackled at various levels by BGLF4, BFRF1, BRLF1 and BZLF1 (40–43). The result is both a reduced IFN production and an impaired ISG activity that favor the establishment and persistence of viral latency, as well as viral replication during reactivation.
IFN-β administration could therefore counterweigh such signature at multiple points. A deficit in the TRL7-induced pathway has been found in B cells derived from pwMS, which is reverted in vivo by IFN-β administration (44). Severa and colleagues showed that the in vitro exposure to IFN-β rescues the IFN pathway defects in B cells derived from MS patients, sustaining antiviral processes, leading to a reduction of EBV-infected and proliferating B cells (19). This data mirrors the evidence of a reduction in pathogenic, memory B cells in the blood of pwMS receiving IFN-β (16) suggesting that both the interference with viral machinery and the depletion of viral reservoir mediate the anti-EBV activity of IFN-β.
Virus-specific CD8+ T cell responses play a pivotal role in the control of EBV infection (45): if quantitatively or functionally deficient, EBV reactivation would be unrestrained; an excessive cellular activation during the lytic phase would cause immunopathology. In MS, such dysregulation is hypothesized to happen within the CNS, whereby latently infected B cells would establish a “sanctuary” evading the virus-specific response; viral reactivation would trigger an enhanced immune activation, with bystander damage of the CNS (46). Indeed, a high frequency of CD8+ T cells specific for lytic EBV antigens has been identified in the peripheral blood of pwMS with active disease (47) which is reduced by IFN-β. A similar effect was found for CD4+ T cell responses against EBNA-1 (48).
Overall, these data suggest a scenario in which the efficacy of IFN-β in the treatment of MS rests on wide immunomodulatory effects resulting, at least in part, from its antiviral properties (Figure 1).
Antiviral effects against SARS-CoV-2
As of September 2022, SARS-CoV-2, the causative agent of COVID-19, has led to more than 6.5 million deaths worldwide. The novel virus emerged in late 2019 evolving from the clades of Sarbecoviruses, which also includes SARS-CoV (49). COVID-19 is a multifaceted disease, with primary respiratory involvement and systemic consequences, whose severity depends on a dysregulated host’s immune response to the viral infection: a delayed or impaired IFN response, favoring viral replication and diffusion, and an exaggerated inflammatory activation, leading to immunopathology (50).
The induction of IFN response by SARS-CoV-2 is started through TRL3 and TLR7-mediated sensing of viral RNAs in endosomes and by MDA5 in the cytosol (51, 52) initiating MAVs-IRFs cascade and type I and III IFN production.
Similar to other Coronaviruses, SARS-CoV-2 leverages several mechanisms to hijack IFN response (53, 54). IFN-I production is antagonized by NSP6 and NSP13, which limit TBK1 action on IRF3, and ORF6, which impedes IRF3 translocation into the nucleus.
Additionally, IFN-I signaling is tackled by NSP1, NSP6, NSP13, ORF6, ORF7a, and ORF7b by interfering with STAT1/STAT2 phosphorylation and consequent nuclear translocation with IRF9 (53, 55); meanwhile, the structural Membrane (M) protein suppresses the expression of IFN-β and ISGs interfering with RIG-I/MDA5/IKKϵ/TBK1 signaling (56). The ancestral strains of SARS-CoV-2 were far more sensitive to IFNs than SARS-CoV (57). Nonetheless, nowadays IFN evasion is among the factors guiding the emergence and selection of SARS-CoV-2 variants of concerns. A systematic in vitro screening of antiviral activities of 17 IFN formulations against 5 SARS-CoV-2 strains revealed a trend of increasing (17 to 122-fold) IFN resistance of evolving variants, with the B.1.1.7 strain exhibiting the highest IFN-I antagonism. Furthermore, this study highlighted IFN-alpha-8 and IFN-β as the most potent SARS-CoV-2 replication inhibitors among this class of molecules, also acting on Delta and Omicron strains – even if at a lesser extent compared to ancestral isolates (58).
Data from COVID-19 patients underline the relevance of an early, efficient IFN response to block disease progression. Patients with severe COVID-19 display low induction of systemic and local (i.e., in the airway epithelium) IFN secretion (59) in the early phases, which traces back to a hypo responsiveness of IFN-producing plasmacytoid dendritic cells to viral infection (60). Conversely, children and people with mild disease show strong IFN-mediated innate activation in the upper airways, driven by MDA5 and RIG-I impeding viral dissemination to the lungs (61–64).
Age is a risk factor for COVID-19-related death. Perhaps, the immune-senescence processes also involve poorer and delayed IFN-mediated innate responses, plausibly due to an impairment in RIG-I signaling (65, 66). Comparing adult and aged COVID-19 mouse models, Beer and colleagues demonstrated that an age-dependent increase in disease severity depends on an impaired IFN I-II response which caused a higher viral load and a delayed, uncoordinated adaptive immunity (in terms of antigen presentation, NK lymphocytes activation, and immunoglobulin production) (67).
Broadening this concept, “IFN deficiency” is associated with a worse prognosis from COVID-19 at all ages. Indeed, several inborn defects of IFN immunity have been found in critical (especially younger) COVID-19 patients: autosomal recessive deficiencies of IFNAR1 and IRF7 (1.8% of cases), X-linked deficiency of TLR7 (1.3%), newly described or known autosomal dominant deficiencies of IFNAR1, IFNAR2, TLR3, IRF3, TRF, TBK1, UNC93B1 (2.9%), enhancing the risk of a severe disease course 20 to 34-fold (68–70). Recessive deficiencies of type I IFN immunity may underlie ∼10% of pediatric COVID-19 hospitalizations (70). Smaller effects (odds ratios ~1.5 to 2.1) are attributed to common variants identified by COVID-19 GWAS studies, linked to genes involved in IFN signaling: risk SNPs in TYK2 and IFNAR2 genes, protective polymorphisms in the OAS1, OAS2, OAS3 cluster of genes, which are ISGs (71–74). Other variants impacting IFN induction, such as those of the RNA sensor MDA5, exert their effects on disease severity in an age-dependent fashion, plausibly due to the failure of compensatory immunological synergies in the elderly (75, 76)
Acquired autoimmune phenocopies of IFN deficiencies may account for ~20% of COVID-19-related deaths. At first, Bastard and colleagues identified autoantibodies against IFN-α or IFN-ω in ~13.6% of patients with critical COVID-19, with age-related prevalence (21% in patients >80 years), while anti-IFN-β antibodies were found in ~1.3% severe COVID-19 patients, independently from age. Considering a significantly lower prevalence in the general population (0.18-3.4%), the presence of anti-IFN antibodies appeared to increase the susceptibility towards a severe SARS-CoV-2 infection (77). The result of anti-IFN α/ω was replicated in independent cohorts, allowing to mechanistically link the presence of auto-antibodies to a dampened IFN signature at the transcriptomic level as well as to lymphopenia, higher systemic inflammation indexes and poorer outcomes (78–80). The presence and role of anti-IFN-β antibodies did not receive such a strong confirmation (81), supporting the possibility of a fruitful usage of IFN-β as a therapeutic solution in the course of COVID-19 (82) (Figure 1).
Since the first months of the pandemic, several trials have tested IFN-β formulations (either IFN-β1a or IFN-β1b) in COVID-19, whose results have been reviewed and meta-analyzed recently (83–87). Overall, the data indicate that IFN-β administration could decrease symptoms severity, ICU admission, and - in some studies - overall hospital stay; however, effects on mortality reduction did not reach statistical significance in recent pooled analysis (83, 84).
To interpret such data some caveats should be considered. There was substantial heterogeneity in enrollment criteria and in co-administered drugs: for example, the contemporary usage of corticosteroids could hamper IFN-β immune-modulating efficacy (88). Most importantly, the timing of IFN-β treatment initiation could be crucial: while a benefit is expected in the early phase of the infection to halt virus spread and enhance immunity, delayed dosing could perhaps foster immunopathology and cytokine overproduction (89, 90). Indeed, in most of the trials, the benefit on symptoms and mortality emerged more clearly in patients receiving IFN-β in the first days of symptomatic disease (86); contrariwise, in a recently published RCT showing no superiority of Interferon β-1a plus remdesivir to remdesivir alone in hospitalized patients with COVID-19 pneumonia, the mean duration of symptoms before enrolment was 8.7 days in the IFN-β/remdesivir arm (91). In conclusion, the use of IFN-β would appear more appropriate in recently infected subjects, ideally screened for IFN signature, with direct (such as plasma IFN-B level) or surrogate biomarkers (such as neutrophil/lymphocyte ratio) (92–94).
Conclusions and future directions
While IFN-β in MS is turning 30, the last three years taught about its timeliness, for both pwMS and the general population. The renewed interest in IFN biology related to viral infections (and vaccinations) has led to a deeper knowledge on the effects of these molecules on the immune system, which could be rapidly translated into practice thanks to solid experience in the MS field.
In the last decades, there has been a striking expansion of MS therapeutic armamentarium, improving the prognosis and quality of life of pwMS. Currently approved DMTs can be classified based on their efficacy (in reducing relapses and disability accumulation) in first-line/moderate efficacy drugs, such as IFNs, glatiramer acetate, teriflunomide and dymethil fumarate) and second-line/high efficacy therapies (HET) (S1PR modulators, cladribine, natalizumab, anti-CD20 monoclonal antibodies and alemtuzumab). While traditionally first-line agents were chosen at disease onset and patients were shift to higher efficacy drugs in case of treatment failure or suboptimal response (“escalation” strategy), there is increasing evidence supporting the early use of HET (“induction” strategy) to guarantee a better control of disability accumulation in the long term (95–97). However, the increasing efficacy in preventing disease activity comes along with increased risks of adverse events, such as infections and, rarely, neoplasms, related to broad immunosuppressive effects of HET. Beyond safety and tolerability and the risk-benefit profile, the choice of DMT should be tailored on each patient accounting for disease phenotype, comorbidities, patient’s age, personal preferences, reproductive planning, costs, and access (98). In such a scenario, IFN-β shows an excellent safety profile paired with moderate efficacy; also, it is approved for pregnancy and during breastfeeding, allowing its use in women with MS who are planning a pregnancy (99). The low infectious risks - and the suggested effect against viral infections (100–103), as for COVID-19 - also make it suitable for elderly pwMS, such as in late-onset MS. Indeed, as the average age of onset of MS increases (104), a greater number of elderly pwMS is facing the need for DMTs that account for higher risks of neoplasms and infections in this subpopulation (105). As discussed for SARS-CoV-2, impaired type I IFN production and response is part of immunosenescence and contributes to the increased susceptibility to infections, not only due to the lack of a prompt innate antimicrobial activation but also because of weakened T and B adaptive responses to vaccinations (106). IFN dysregulation in the elderly is not simply quantitative, as it involves a wider unbalance between “tonic” and “phasic” IFN secretion that can lead to a paradoxical chronic, low-grade inflammatory state fueling tissue aging (107). Intriguingly, a lower type I IFN activity was also linked to an accelerated cognitive decline toward overt dementia syndrome (108). Therefore, IFN-β could represents a smart therapeutic option for elderly pwMS, combining an optimal safety profile with broad immunomodulating effects that go beyond neuroinflammation. New and more convenient therapeutic schedules may also be explored (109).
Efficient vaccines and direct antivirals have been developed to prevent and treat SARS-CoV-2 infection, quenching the urge for IFN-β repurposing in COVID-19. However, a future challenge will be the management of the multisystemic post-acute COVID-19 symptoms (PASC, or long COVID-19) (110), whose prevalence is estimated to exceed 40% of infected people (111). Its pathophysiology is not fully understood, but persistent dysimmunity links this condition to other post-viral diseases, including the chronic fatigue syndrome that follows infectious mononucleosis (112). It is of interest that EBV reactivation in the acute phase emerged as a prominent risk factor for long-COVID-19 (113, 114). Future studies evaluating whether IFN-β protects pwMS also from long-COVID-19 could shed light on a possible role of this molecule in this setting. Also, it will be crucial to evaluate the interplay between SARS-CoV-2 and EBV in-depth and aside from COVID-19, as potential viral synergies could have implications for MS’s natural history (115).
Author contributions
GB: conceptualization, data curation, formal analysis, investigation, methodology, writing – original draft, writing – review and editing. AA: Conceptualization, formal analysis, investigation, methodology, writing – original draft, writing – review and editing. CR: Conceptualization, formal analysis, investigation, methodology, writing – original draft, writing – review and editing. VR: Conceptualization, writing – review and editing. MS: Conceptualization, methodology, supervision, validation, writing – review and editing. GR: Conceptualization, methodology, supervision, validation, writing – review and editing. All authors contributed to the article and approved the submitted version.
Acknowledgments
Giulio D’Alfonso, an independent medical writer on behalf of Fullcro S.r.l., provided medical writing assistance, funded by Merck Serono S.p.A., Rome, Italy, an affiliate of Merck (CrossRef Funder ID: 10.13039/100009945).
Conflict of interest
MS receives research support and has received fees as speaker from Sanofi, Biogen, Roche, Novartis, Bayer Schering, and Merck Serono. VR, GB and GR declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. AA and CR are employees of Merck Serono S.p.A., Rome, Italy, an affiliate of Merck KGaA, Darmstadt, Germany.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Charabati M, Wheeler MA, Weiner HL, Quintana FJ. Multiple sclerosis: neuroimmune crosstalk and therapeutic targeting. Cell (2023) 186:1309–27. doi: 10.1016/j.cell.2023.03.008
2. Bjornevik K, Cortese M, Healy BC, Kuhle J, Mina MJ, Leng Y, et al. Longitudinal analysis reveals high prevalence of Epstein-Barr virus associated with multiple sclerosis. Science (2022) 375:296–301. doi: 10.1126/SCIENCE.ABJ8222/SUPPL_FILE/SCIENCE.ABJ8222_MDAR_REPRODUCIBILITY_CHECKLIST.PDF
3. Lanz TV, Brewer RC, Ho PP, Moon JS, Jude KM, Fernandez D, et al. Clonally expanded b cells in multiple sclerosis bind EBV EBNA1 and GlialCAM. Nature (2022) 603:321–7. doi: 10.1038/s41586-022-04432-7
4. Aloisi F, Giovannoni G, Salvetti M. Epstein-Barr Virus as a cause of multiple sclerosis: opportunities for prevention and therapy. Lancet Neurol (2023) 22:338–49. doi: 10.1016/S1474-4422(22)00471-9
5. Pugliatti M, Berger T, Hartung HP, Oreja-Guevara C, Bar-Or A. Multiple sclerosis in the era of COVID-19: disease course, DMTs and SARS-CoV2 vaccinations. Curr Opin Neurol (2022) 35:319–27. doi: 10.1097/WCO.0000000000001066
6. Reder AT, Centonze D, Naylor ML, Nagpal A, Rajbhandari R, Altincatal A, et al. COVID-19 in patients with multiple sclerosis: associations with disease-modifying therapies. CNS Drugs (2021) 35:317–30. doi: 10.1007/S40263-021-00804-1
7. Simpson-Yap S, De Brouwer E, Kalincik T, Rijke N, Hillert JA, Walton C, et al. Associations of disease-modifying therapies with COVID-19 severity in multiple sclerosis. Neurology (2021) 97:E1870–85. doi: 10.1212/WNL.0000000000012753
8. Sormani MP, De Rossi N, Schiavetti I, Carmisciano L, Cordioli C, Moiola L, et al. Disease-modifying therapies and coronavirus disease 2019 severity in multiple sclerosis. Ann Neurol (2021) 89:780–9. doi: 10.1002/ana.26028
9. Sormani MP, Schiavetti I, Carmisciano L, Cordioli C, Filippi M, Radaelli M, et al. COVID-19 severity in multiple sclerosis: putting data into context. Neurology(R) Neuroimmunol Neuroinflamm (2022) 9(1):e1105. doi: 10.1212/NXI.0000000000001105
10. Iaffaldano P, Lucisano G, Manni A, Paolicelli D, Patti F, Capobianco M, et al. Risk of getting COVID-19 in people with multiple sclerosis. Neurol - Neuroimmunol Neuroinflamm (2022) 9:1141. doi: 10.1212/NXI.0000000000001141
11. Sormani MP, Salvetti M, Labauge P, Schiavetti I, Zephir H, Carmisciano L, et al. DMTs and covid-19 severity in MS: a pooled analysis from Italy and France. Ann Clin Trans Neurol (2021) 8(8):1738–44. doi: 10.1002/ACN3.51408
12. Jakimovski D, Kolb C, Ramanathan M, Zivadinov R, Weinstock-Guttman B. Interferon β for multiple sclerosis. Cold Spring Harbor Perspect Med (2018) 9(1):e1105. doi: 10.1101/CSHPERSPECT.A032003
13. McNab F, Mayer-Barber K, Sher A, Wack A, O'Garra A. Type I interferons in infectious disease. Nat Rev Immunol (2015) 15:87–103. doi: 10.1038/nri3787
14. Isaacs A, Lindemann J. Virus interference. i. the interferon. Proc R Soc Lond Ser B Biol Sci (1957) 147:258–67.
15. Schneider WM, Chevillotte MD, Rice CM. Interferon-stimulated genes: a complex web of host defenses. Annu Rev Immunol (2014) 32:513–45. doi: 10.1146/annurev-immunol-032713-120231
16. Rizzo F, Giacomini E, Mechelli R. Interferon-β therapy specifically reduces pathogenic memory b cells in multiple sclerosis patients by inducing a FAS-mediated apoptosis. Immunol Cell Biol (2016) 94:886–94. doi: 10.1038/icb.2016.55
17. Annibali V, Umeton R, Palermo A, Severa M, Paola M, Giglio S, et al. Analysis of coding and non-coding transcriptome of peripheral b cells reveals an altered interferon response factor (IRF) -1 pathway in multiple sclerosis patients. J Neuroimmunol (2018) 324:165–71. doi: 10.1016/j.jneuroim.2018.09.005
18. Feng X, Petraglia AL, Chen M, Byskosh PV, Boos MD, Reder AT. Low expression of interferon-stimulated genes in active multiple sclerosis is linked to subnormal phosphorylation of STAT1. J Neuroimmunol (2002) 129:205–15. doi: 10.1016/S0165-5728(02)00182-0
19. Severa M, Rizzo F, Srinivasan S, Di Dario M, Giacomini E, Buscarinu MC, et al. A cell type-specific transcriptomic approach to map b cell and monocyte type I interferon-linked pathogenic signatures in multiple sclerosis. J Autoimmun (2019) 101:1–16. doi: 10.1016/j.jaut.2019.04.006
20. Srinivasan S, Severa M, Rizzo F, Menon R, Brini E, Mechelli R, et al. Transcriptional dysregulation of interferome in experimental and human multiple sclerosis. Sci Rep (2017) 7:8981. doi: 10.1038/s41598-017-09286-y
21. Beecham AH, Patsopoulos NA, Xifara DK, Davis MF, Kemppinen A, Cotsapas C, et al. Analysis of immune-related loci identifies 48 new susceptibility variants for multiple sclerosis. Nat Genet (2013) 45:1353–60. doi: 10.1038/ng.2770
22. Patsopoulos NA, Baranzini SE, Santaniello A, Shoostari P, Cotsapas C, Wong G, et al. Multiple sclerosis genomic map implicates peripheral immune cells and microglia in susceptibility. Sci (New York NY) (2019) 365. doi: 10.1126/SCIENCE.AAV7188
23. Mechelli R, Romano C, Reniè R, Manfrè G, Buscarinu MC, Romano S, et al. Viruses and neuroinflammation in multiple sclerosis. Neuroimmunol Neuroinflamm (2021) 8:269. doi: 10.20517/2347-8659.2021.01
24. Damania B, Kenney SC, Raab-Traub N. Epstein-Barr Virus: biology and clinical disease. Cell (2022) 185(20):3652–70. doi: 10.1016/j.cell.2022.08.026
25. Jog NR, McClain MT, Heinlen LD, Gross T, Towner R, Guthridge JM, et al. Epstein Barr Virus nuclear antigen 1 (EBNA-1) peptides recognized by adult multiple sclerosis patient sera induce neurologic symptoms in a murine model. J Autoimmun (2019) 1:102332. doi: 10.1016/j.jaut.2019.102332
26. Tengvall K, Huang J, Hellström C, Kammer P, Biström M, Ayoglu B, et al. Molecular mimicry between anoctamin 2 and Epstein-Barr virus nuclear antigen 1 associates with multiple sclerosis risk. Proc Natl Acad Sci United States America (2019) 116:16955–60. doi: 10.1073/PNAS.1902623116
27. D’Addario M, Libermann TA, Xu J, Ahmad A, Menezes J. Epstein-Barr Virus and its glycoprotein-350 upregulate IL-6 in human b-lymphocytes via CD21, involving activation of NF-κB and different signaling pathways. J Mol Biol (2001) 308(3):501–14. doi: 10.1006/jmbi.2001.4589
28. Kang MS, Kieff E. Epstein-Barr Virus latent genes. Exp Mol Med (2015) 47:e131. doi: 10.1038/emm.2014.84
29. Serafini B, Rosicarelli B, Franciotta D, Magliozzi R, Reynolds R, Cinque P, et al. Dysregulated Epstein-Barr virus infection in the multiple sclerosis brain. J Exp Med (2007) 204(12):2899–912. doi: 10.1084/jem.20071030
30. Bar-Or A, Pender MP, Khanna R, Steinman L, Hartung HP, Maniar T, et al. Epstein–Barr Virus in multiple sclerosis: theory and emerging immunotherapies. Trends Mol Med (2019) 8(23):296–310. doi: 10.1016/j.molmed.2019.11.003
31. Mechelli R, Umeton R, Policano C, Annibali V, Coarelli G, Ricigliano VAG, et al. A “Candidate-interactome” aggregate analysis of genome-wide association data in multiple sclerosis. PloS One (2013) 8(5):e63300. doi: 10.1371/journal.pone.0063300
32. Mechelli R, Manzari C, Policano C, Annese A, Picardi E, Umeton R, et al. Epstein-Barr Virus genetic variants are associated with multiple sclerosis. Neurology (2015) 84(13):1362–8. doi: 10.1212/WNL.0000000000001420
33. Harley JB, Chen X, Pujato M, Miller D, Maddox A, Forney C, et al. Transcription factors operate across disease loci, with EBNA2 implicated in autoimmunity. Nat Genet (2018) 50:699–707. doi: 10.1038/s41588-018-0102-3
34. Fierz W. Multiple sclerosis: an example of pathogenic viral interaction? Virol J (2017) 14:42. doi: 10.1186/s12985-017-0719-3
35. Küry P, Nath A, Créange A, Dolei A, Marche P, Gold J, et al. Human endogenous retroviruses in neurological diseases. Trends Mol Med (2018) 24(4):379–94. doi: 10.1016/j.molmed.2018.02.007
36. Höllsberg P, Kusk M, Bech E, Hansen HJ, Jakobsen J, Haahr S. Presence of Epstein-Barr virus and human herpesvirus 6B DNA in multiple sclerosis patients: associations with disease activity. Acta Neurol Scand (2005) 112:395–402. doi: 10.1111/j.1600-0404.2005.00516.x
37. Torkildsen, Nyland H, Myrmel H, Myhr KM. Epstein-Barr Virus reactivation and multiple sclerosis. Eur J Neurol (2008) 15:106–8. doi: 10.1111/j.1468-1331.2007.02009.x
38. Wandinger KP, Jabs W, Siekhaus A, Bubel S, Trillenberg P, Wagner HJ, et al. Association between clinical disease activity and Epstein-Barr virus reactivation in MS. Neurology (2000) 55:178–84. doi: 10.1212/WNL.55.2.178
39. Lange PT, White MC, Damania B. Activation and evasion of innate immunity by gammaherpesviruses. J Mol Biol (2022) 434:167214. doi: 10.1016/J.JMB.2021.167214
40. Bentz GL, Liu R, Hahn AM, Shackelford J, Pagano JS. Epstein-Barr Virus BRLF1 inhibits transcription of IRF3 and IRF7 and suppresses induction of interferon-β. Virology (2010) 402:121–8. doi: 10.1016/J.VIROL.2010.03.014
41. Liu X, Sadaoka T, Krogmann T, Cohen JI. Epstein-Barr Virus (EBV) tegument protein BGLF2 suppresses type I interferon signaling to promote EBV reactivation. J Virol (2020) 94. doi: 10.1128/JVI.00258-20
42. Wang J-T, Doong S-L, Teng S-C, Lee C-P, Tsai C-H, Chen M-R. Epstein-Barr Virus BGLF4 kinase suppresses the interferon regulatory factor 3 signaling pathway. J Virol (2009) 83:1856–69. doi: 10.1128/JVI.01099-08
43. Wu L, Fossum E, Joo CH, Inn K-S, Shin YC, Johannsen E, et al. Epstein-Barr Virus LF2: an antagonist to type I interferon. J Virol (2009) 83:1140–6. doi: 10.1128/JVI.00602-08
44. Giacomini E, Severa M, Rizzo F, Mechelli R, Annibali V, Ristori G, et al. IFN-β therapy modulates b-cell and monocyte crosstalk via TLR7 in multiple sclerosis patients. Eur J Immunol (2013) 43:1963–72. doi: 10.1002/eji.201243212
45. Hislop AD, Taylor GS, Sauce D, Rickinson AB. Cellular responses to viral infection in humans: lessons from Epstein-Barr virus. Annu Rev Immunol (2007) 25:587–617. doi: 10.1146/annurev.immunol.25.022106.141553
46. Recher M, Lang KS, Navarini A, Hunziker L, Lang PA, Fink K, et al. Extralymphatic virus sanctuaries as a consequence of potent T-cell activation. Nat Med (2007) 13:1316–23. doi: 10.1038/nm1670
47. Angelini DF, Serafini B, Piras E, Severa M, Coccia EM, Rosicarelli B, et al. Increased CD8+ T cell response to Epstein-Barr virus lytic antigens in the active phase of multiple sclerosis. PloS Pathog (2013) 9(4):e1003220. doi: 10.1371/journal.ppat.1003220
48. Comabella M, Kakalacheva K, Río J, Münz C, Montalban X, Lünemann JD. EBV-specific immune responses in patients with multiple sclerosis responding to IFNβ therapy. Multiple Sclerosis J (2012) 18:605–9. doi: 10.1177/1352458511426816
49. Hu B, Guo H, Zhou P. Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol (2021) 19:141–54. doi: 10.1038/s41579-020-00459-7
50. Lucas C, Wong P, Klein J, Castro TBR, Silva J, Sundaram M, et al. Longitudinal analyses reveal immunological misfiring in severe COVID-19. Nat 2020 584:7821 (2020) 584:463–9. doi: 10.1038/s41586-020-2588-y
51. Lamers MM, Haagmans BL. SARS-CoV-2 pathogenesis. Nat Rev Microbiol 2022 20:5 (2022) 20:270–84. doi: 10.1038/s41579-022-00713-0
52. Jouvenet N, Goujon C, Banerjee A. Clash of the titans: interferons and SARS-CoV-2. Trends Immunol (2021) 42:1069–72. doi: 10.1016/j.it.2021.10.009
53. Lei X, Dong X, Ma R, Wang W, Xiao X, Tian Z, et al. Activation and evasion of type I interferon responses by SARS-CoV-2. Nat Commun (2020) 11:3810. doi: 10.1038/S41467-020-17665-9
54. Minkoff JM, tenOever B. Innate immune evasion strategies of SARS-CoV-2. Nat Rev Microbiol (2023) 21:178–94. doi: 10.1038/s41579-022-00839-1
55. Xia H, Cao Z, Xie X, Zhang X, Chen JYC, Wang H, et al. Evasion of type I interferon by SARS-CoV-2. Cell Rep (2020) 33:108234. doi: 10.1016/j.celrep.2020.108234
56. Sui L, Zhao Y, Wang W, Wu P, Wang Z, Yu Y, et al. SARS-CoV-2 membrane protein inhibits type I interferon production through ubiquitin-mediated degradation of TBK1. Front Immunol (2021) 12:662989. doi: 10.3389/FIMMU.2021.662989
57. Lokugamage KG, Hage A, de Vries M, Valero-Jimenez AM, Schindewolf C, Dittmann M, et al. Type I interferon susceptibility distinguishes SARS-CoV-2 from SARS-CoV. J Virol (2020) 94(23):e01410–20. doi: 10.1128/JVI.01410-20
58. Guo K, Barrett BS, Morrison JH, Mickens KL, Vladar EK, Hasenkrug KJ, et al. Interferon resistance of emerging SARS-CoV-2 variants. Proc Natl Acad Sci United States America (2022) 119:e2203760119. doi: 10.1073/PNAS.2203760119/SUPPL_FILE/PNAS.2203760119.SAPP.PDF
59. Park A, Iwasaki A. Type I and type III interferons – induction, signaling, evasion, and application to combat COVID-19. Cell Host Microbe (2020) 27:870–8. doi: 10.1016/J.CHOM.2020.05.008
60. Venet M, Ribeiro MS, Décembre E, Bellomo A, Joshi G, Nuovo C, et al. Severe COVID-19 patients have impaired plasmacytoid dendritic cell-mediated control of SARS-CoV-2. Nat Commun (2023) 14:694. doi: 10.1038/s41467-023-36140-9
61. Hadjadj J, Yatim N, Barnabei L, Corneau A, Boussier J, Smith N, et al. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science (2020) 369:718–24. doi: 10.1126/science.abc6027
62. Loske J, Röhmel J, Lukassen S, Stricker S, Magalhães VG, Liebig J, et al. Pre-activated antiviral innate immunity in the upper airways controls early SARS-CoV-2 infection in children. Nat Biotechnol (2022) 40:319–24. doi: 10.1038/S41587-021-01037-9
63. Sungnak W, Huang N, Bécavin C, Berg M, Queen R, Litvinukova M, et al. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat Med (2020) 26:681–7. doi: 10.1038/s41591-020-0868-6
64. Yoshida M, Worlock KB, Huang N. Local and systemic responses to SARS-CoV-2 infection in children and adults. Nature (2022) 602:321–7. doi: 10.1038/s41586-021-04345-x
65. Bartleson JM, Radenkovic D, Covarrubias AJ, Furman D, Winer DA, Verdin E. SARS-CoV-2, COVID-19 and the aging immune system. Nat Aging 2021 1:9 (2021) 1:769–82. doi: 10.1038/s43587-021-00114-7
66. Deng J, Zheng S-N, Xiao Y, Nan M-L, Zhang J, Han L, et al. SARS-CoV-2 NSP8 suppresses type I and III IFN responses by modulating the RIG-I/MDA5, TRIF, and STING signaling pathways. J Med Virol (2023) 95(4). doi: 10.1002/jmv.28680
67. Beer J, Crotta S, Breithaupt A, Ohnemus A, Becker J, Sachs B, et al. Impaired immune response drives age-dependent severity of COVID-19. J Exp Med (2022) 219(12):e20220621. doi: 10.1084/JEM.20220621
68. Zhang Q, Bastard P, Karbuz A, Gervais A, Tayoun AA, Aiuti A, et al. Human genetic and immunological determinants of critical COVID-19 pneumonia. Nat 2022 603:7902 (2022) 603:587–98. doi: 10.1038/s41586-022-04447-0
69. Zhang Q, Bastard P, Liu Z. Inborn errors of type I IFN immunity in patients with life-threatening COVID-19. Science (2020) 370:eabd4570. doi: 10.1126/science.abd4570. 2020 09 26.
70. Zhang Q, Matuozzo D, Le Pen J, Lee D, Moens L, Asano T, et al. Recessive inborn errors of type I IFN immunity in children with COVID-19 pneumonia. J Exp Med (2022) 219. doi: 10.1084/JEM.20220131/213287
71. Asano T, Boisson B, Onodi F, Matuozzo D, Moncada-Velez M, Renkilaraj MRLM, et al. X-Linked recessive TLR7 deficiency in ~1% of men under 60 years old with life-threatening COVID-19. Sci Immunol (2021) 6:eabl4348. doi: 10.1126/sciimmunol.abl4348
72. Niemi MEK, Karjalainen J, Liao RG, Neale BM, Daly M, Ganna A, et al. Mapping the human genetic architecture of COVID-19. Nature (2021) 600:472–7. doi: 10.1038/s41586-021-03767-x
73. Pairo-Castineira E, Clohisey S, Klaric L, Bretherick AD, Rawlik K, Pasko D, et al. Genetic mechanisms of critical illness in COVID-19. Nature (2021) 591:92–8. doi: 10.1038/s41586-020-03065-y
74. Matuozzo D, Talouarn E, Marchal A, Zhang P, Manry J, Seeleuthner Y, et al. Rare predicted loss-of-function variants of type I IFN immunity genes are associated with life-threatening COVID-19. Genome Med (2023) 15:22. doi: 10.1186/s13073-023-01173-8
75. Muñiz-Banciella MG, Albaiceta GM, Amado-Rodríguez L, Del Riego ES, Alonso IL, López-Martínez C, et al. Age-dependent effect of the IFIH1/MDA5 gene variants on the risk of critical COVID-19. Immunogenetics (2023) 75:91–8. doi: 10.1007/s00251-022-01281-6
76. Amado-Rodríguez L, Salgado Del Riego E, Gomez de Ona J, López Alonso I, Gil-Pena H, López-Martínez C, et al. Effects of IFIH1 rs1990760 variants on systemic inflammation and outcome in critically ill COVID-19 patients in an observational translational study. Elife (2022) 11:e73012. doi: 10.7554/eLife.73012
77. Bastard P, Gervais A, Voyer TL, Rosain J, Philippot Q, Manry J, et al. Autoantibodies neutralizing type I IFNs are present in ~4% of uninfected individuals over 70 years old and account for ~20% of COVID-19 deaths. Sci Immunol (2021) 6:eabl4340. doi: 10.1126/sciimmunol.abl4340
78. Frasca F, Scordio M, Santinelli L, Gabriele L, Gandini O, Criniti A, et al. Anti-IFN-α/-ω neutralizing antibodies from COVID-19 patients correlate with downregulation of IFN response and laboratory biomarkers of disease severity. Eur J Immunol (2022) 52:1120–8. doi: 10.1002/EJI.202249824
79. Goncalves D, Mezidi M, Bastard P, Perret M, Saker K, Fabien N, et al. Antibodies against type I interferon: detection and association with severe clinical outcome in COVID-19 patients. Clin Trans Immunol (2021) 10(8):e1327. doi: 10.1002/CTI2.1327
80. van der Wijst MGP, Vazquez SE, Hartoularos GC, Bastard P, Grant T, Bueno R, et al. Type I interferon autoantibodies are associated with systemic immune alterations in patients with COVID-19. Sci Transl Med (2021) 13:eabh2624. doi: 10.1126/SCITRANSLMED.ABH2624
81. Troya J, Bastard P, Planas-Serra L, Ryan P, Ruiz M, de Carranza M, et al. Neutralizing autoantibodies to type I IFNs in >10% of patients with severe COVID-19 pneumonia hospitalized in Madrid, Spain. J Clin Immunol (2021) 41:914–22. doi: 10.1007/S10875-021-01036-0
82. Hale BG. Autoantibodies targeting type I interferons: prevalence, mechanisms of induction, and association with viral disease susceptibility. Eur J Immunol (2023), e2250164. doi: 10.1002/eji.202250164
83. Crichton ML, Goeminne PC, Tuand K, Vandendriessche T, Tonia T, Roche N, et al. The impact of therapeutics on mortality in hospitalised patients with COVID-19: systematic review and meta-analyses informing the European respiratory society living guideline. Eur Respir Rev (2021) 30. doi: 10.1183/16000617.0171-2021
84. Kumar S, Saurabh MK, Narasimha VL, Maharshi V. Efficacy of interferon-β in moderate-to-Severe hospitalised cases of COVID-19: a systematic review and meta-analysis. Clin Drug Invest (2021) 41:1037–46. doi: 10.1007/S40261-021-01092-9/TABLES/2
85. Nakhlband A, Fakhari A, Azizi H. Interferon-beta offers promising avenues to COVID-19 treatment: a systematic review and meta-analysis of clinical trial studies. Naunyn-Schmiedeberg’s Arch Pharmacol (2021) 394:829–38. doi: 10.1007/S00210-021-02061-X
86. Sosa JP, Caceres MMF, Comptis JR, Quiros J, Príncipe-Meneses FS, Riva-Moscoso A, et al. Effects of interferon beta in covid-19 adult patients: systematic review. Infection Chemother (2021) 53:247–60. doi: 10.3947/IC.2021.0028
87. Ryoo S, Koh D-H, Yu S-Y, Choi M, Huh K, Yeom J-S, et al. Clinical efficacy and safety of interferon (Type I and type III) therapy in patients with COVID-19: a systematic review and meta-analysis of randomized controlled trials. PloS One (2023) 18:e0272826. doi: 10.1371/journal.pone.0272826
88. Jalkanen J, Pettilä V, Huttunen T, Hollmén M, Jalkanen S. Glucocorticoids inhibit type I IFN beta signaling and the upregulation of CD73 in human lung. Intensive Care Med (2020) 46:1937–40. doi: 10.1007/S00134-020-06086-3
89. Akamatsu MA, de Castro JT, Takano CY, Ho PL. Off balance: interferons in COVID-19 lung infections. EBioMedicine (2021) 73. doi: 10.1016/J.EBIOM.2021.103642
90. Galani IE, Rovina N, Lampropoulou V, Triantafyllia V, Manioudaki M, Pavlos E, et al. Untuned antiviral immunity in COVID-19 revealed by temporal type I/III interferon patterns and flu comparison. Nat Immunol (2021) 22:32–40. doi: 10.1038/S41590-020-00840-X
91. Kalil AC, Mehta AK, Patterson TF, Erdmann N, Gomez CA, Jain MK, et al. Efficacy of interferon beta-1a plus remdesivir compared with remdesivir alone in hospitalised adults with COVID-19: a double-bind, randomised, placebo-controlled, phase 3 trial. Lancet Respir Med (2021) 9:1365–76. doi: 10.1016/S2213-2600(21)00384-2
92. Galbraith MD, Kinning KT, Sullivan KD, Araya P, Smith KP, Granrath RE, et al. Specialized interferon action in COVID-19. Proc Natl Acad Sci United States America (2022) 119(11):e2116730119. doi: 10.1073/PNAS.2116730119
93. Darif D, Ejghal R, Desterke C, Outlioua A, Hammi I, Lemrani M, et al. Type I and III interferons are good markers to monitor COVID-19 pathophysiology. Cytokine (2023) 165:156172. doi: 10.1016/j.cyto.2023.156172
94. Berri F, N’Guyen Y, Callon D, Lebreil A-L, Glenet M, Heng L, et al. Early plasma interferon-β levels as a predictive marker of COVID-19 severe clinical events in adult patients. J Med Virol (2023) 95:e28361. doi: 10.1002/jmv.28361
95. Harding K, Williams O, Willis M, Hrastelj J, Rimmer A, Joseph F, et al. Clinical outcomes of escalation vs early intensive disease-modifying therapy in patients with multiple sclerosis. JAMA Neurol (2019) 76(5):536–41. doi: 10.1001/jamaneurol.2018.4905
96. Prosperini L, Mancinelli CR, Solaro CM, Nociti V, Haggiag S, Cordioli C, et al. Induction versus escalation in multiple sclerosis: a 10-year real world study. Neurotherapeutics (2020) 17:994–1004. doi: 10.1007/s13311-020-00847-0
97. Casanova B, Quintanilla-Bordás C, Gascón F. Escalation vs. early intense therapy in multiple sclerosis. J Pers Med (2022) 12:119. doi: 10.3390/jpm12010119
98. Berger T, Adamczyk-Sowa M, Csépány T, Fazekas F, Fabjan TH, Horáková D, et al. Factors influencing daily treatment choices in multiple sclerosis: practice guidelines, biomarkers and burden of disease. Ther Adv Neurol Disord (2020) 13:1756286420975223. doi: 10.1177/1756286420975223
99. Varytė G, Arlauskienė A, Ramašauskaitė D. Pregnancy and multiple sclerosis: an update. Curr Opin Obstet Gynecol (2021) 33:378–83. doi: 10.1097/GCO.0000000000000731
100. Evans C, Zhu F, Kingwell E, Shirani A, van der Kop ML, Petkau J, et al. Association between beta-interferon exposure and hospital events in multiple sclerosis. Pharmacoepidemiol Drug Saf (2014) 23:1213–22. doi: 10.1002/pds.3667
101. Dhib-Jalbut S, Marks S. Interferon-β mechanisms of action in multiple sclerosis. Neurology (2010) 74. doi: 10.1212/WNL.0B013E3181C97D99
102. de Jong HJI, Kingwell E, Shirani A, Cohen Tervaert JW, Hupperts R, Zhao Y, et al. Evaluating the safety of β-interferons in MS: a series of nested case-control studies. Neurology (2017) 88:2310–20. doi: 10.1212/WNL.0000000000004037
103. Oikonen MK, Erälinna JP. Beta-interferon protects multiple sclerosis patients against enhanced susceptibility to infections caused by poor air quality. Neuroepidemiology (2008) 30:13–9. doi: 10.1159/000113301
104. Prosperini L, Lucchini M, Ruggieri S, Tortorella C, Haggiag S, Mirabella M, et al. Shift of multiple sclerosis onset towards older age. J Neurol Neurosurg Psychiatry (2022) 93:1137–9. doi: 10.1136/JNNP-2022-329049
105. Buscarinu MC, Reniè R, Morena E, Romano C, Bellucci G, Marrone A, et al. Late-onset MS: disease course and safety-efficacy of DMTS. Front Neurol (2022) 13:829331/BIBTEX. doi: 10.3389/FNEUR.2022.829331/BIBTEX
106. Feng E, Balint E, Poznanski SM, Ashkar AA, Loeb M. Aging and interferons: impacts on inflammation and viral disease outcomes. Cells (2021) 10(3):708. doi: 10.3390/CELLS10030708
107. Cao W. IFN-aging: coupling aging with interferon response. Front Aging (2022) 0:870489. doi: 10.3389/FRAGI.2022.870489
108. Song L, Chen J, Lo CYZ, Guo Q, Feng J, Zhao XM. Impaired type I interferon signaling activity implicated in the peripheral blood transcriptome of preclinical alzheimer’s disease. eBioMedicine (2022) 82. doi: 10.1016/j.ebiom.2022.104175
109. Romano S, Ferraldeschi M, Bagnato F, Mechelli R, Morena E, Caldano M, et al. Drug holiday of interferon beta 1b in multiple sclerosis: a pilot, randomized, single blind study of non-inferiority. Front Neurol (2019) 10:695. doi: 10.3389/fneur.2019.00695
110. Nalbandian A, Sehgal K, Gupta A, Madhavan MV, McGroder C, Stevens JS, et al. Post-acute COVID-19 syndrome. Nat Med 2021 27:4 (2021) 27:601–15. doi: 10.1038/s41591-021-01283-z
111. Chen C, Haupert SR, Zimmermann L, Shi X, Fritsche LG, Mukherjee B. Global prevalence of post COVID-19 condition or long COVID: a meta-analysis and systematic review. J Infect Dis (2022) 226(9):1593–607. doi: 10.1093/INFDIS/JIAC136
112. Choutka J, Jansari V, Hornig M, Iwasaki A. Unexplained post-acute infection syndromes. Nat Med (2022) 28:911–23. doi: 10.1038/s41591-022-01810-6
113. Gold JE, Okyay RA, Licht WE, Hurley DJ. Investigation of long COVID prevalence and its relationship to Epstein-Barr virus reactivation. Pathogens (Basel Switzerland) (2021) 10(6):763. doi: 10.3390/PATHOGENS10060763
114. Su Y, Yuan D, Chen DG, Ng RH, Wang K, Choi J, et al. Multiple early factors anticipate post-acute COVID-19 sequelae. Cell (2022) 185:881–895.e20. doi: 10.1016/J.CELL.2022.01.014/ATTACHMENT/B1FB9AE1-6442-495C-A1DF-6A5E16CA88FA/MMC7.XLSX
Keywords: multiple Sclerosis, interferon β, SARS-CoV-2, Epstein-Barr virus, immune cells
Citation: Bellucci G, Albanese A, Rizzi C, Rinaldi V, Salvetti M and Ristori G (2023) The value of Interferon β in multiple sclerosis and novel opportunities for its anti-viral activity: a narrative literature review. Front. Immunol. 14:1161849. doi: 10.3389/fimmu.2023.1161849
Received: 10 February 2023; Accepted: 24 May 2023;
Published: 02 June 2023.
Edited by:
Makoto Inoue, University of Illinois at Urbana-Champaign, United StatesReviewed by:
Lil Meyer-Arndt, Charité University Medicine Berlin, GermanyOmid Mirmosayyeb, University at Buffalo, United States
Copyright © 2023 Bellucci, Albanese, Rizzi, Rinaldi, Salvetti and Ristori. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gianmarco Bellucci, Z2lhbm1hcmNvLmJlbGx1Y2NpQHVuaXJvbWExLml0
 Gianmarco Bellucci
Gianmarco Bellucci Angela Albanese
Angela Albanese Caterina Rizzi2
Caterina Rizzi2 Virginia Rinaldi
Virginia Rinaldi Marco Salvetti
Marco Salvetti Giovanni Ristori
Giovanni Ristori