
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol. , 14 April 2023
Sec. Vaccines and Molecular Therapeutics
Volume 14 - 2023 | https://doi.org/10.3389/fimmu.2023.1161507
Toxoplasma gondii, a specialized intracellular parasite, causes a widespread zoonotic disease and is a severe threat to social and economic development. There is a lack of effective drugs and vaccines against T. gondii infection. Recently, mRNA vaccines have been rapidly developed, and their packaging materials and technologies are well established. In this study, TGGT1_216200 (TG_200), a novel molecule from T. gondii, was identified using bioinformatic screening analysis. TG_200 was purified and encapsulated with a lipid nanoparticle (LNP) to produce the TG_200 mRNA-LNP vaccine. The immune protection provided by the new vaccine and its mechanisms after immunizing BABL/C mice via intramuscular injection were investigated. There was a strong immune response when mice were vaccinated with TG_200 mRNA-LNP. Elevated levels of anti-T. gondii-specific immunoglobulin G (IgG), and a higher IgG2a-to-IgG1 ratio was observed. The levels of interleukin-12 (IL-12), interferon-γ (IFN-γ), IL-4, and IL-10 were also elevated. The result showed that the vaccine induced a mixture of Th1 and Th2 cells, and Th1-dominated humoral immune response. Significantly increased antigen-specific splenocyte proliferation was induced by TG_200 mRNA-LNP immunization. The vaccine could also induce T. gondii-specific cytotoxic T lymphocytes (CTLs). The expression levels of interferon regulatory factor 8 (IRF8), T-Box 21 (T-bet), and nuclear factor kappa B (NF-κB) were significantly elevated after TG_200 mRNA-LNP immunization. The levels of CD83, CD86, MHC-I, MHC-II, CD8, and CD4 molecules were also higher. The results indicated that TG_200 mRNA-LNP produced specific cellular and humoral immune responses. Most importantly, TG_200 mRNA-LNP immunized mice survived significantly longer (19.27 ± 3.438 days) than the control mice, which died within eight days after T. gondii challenge (P< 0.001). The protective effect of adoptive transfer was also assessed, and mice receiving serum and splenocytes from mice immunized with TG_200 mRNA-LNP showed improved survival rates of 9.70 ± 1.64 days and, 13.40 ± 2.32 days, respectively (P< 0.001). The results suggested that TG_200 mRNA-LNP is a safe and promising vaccine against T. gondii infection.
Toxoplasma gondii, as an obligate intracellular apicomplexan protozoan, is capable of infecting almost all warm-blooded animals, and causes zoonotic infections in humans. It is generally assumed that T. gondii infects approximately 25% to 30% of the world’s human population. Latin America and tropical African countries have higher prevalences of T. gondii infection (1, 2). Infection in humans usually occurs via ingesting tissue cysts in infected meat or ingesting water or food contaminated with sporulated oocysts produced in the environment. Besides, some infections are iatrogenic and congenital and are transmitted vertically.
Toxoplasmosis is a common opportunistic infection, and primary infection is usually subclinical; however, cervical lymphadenopathy or eye disease might be present in some patients. It can cause ocular toxoplasmosis and even schizophrenia and bipolar disorders by accumulating in the central nervous system (3–5). Immunocompromised individuals (patients with AIDS or recipients of organ transplants) or pregnant women infected with T. gondii would develop significant clinical symptoms (6). Infection of pregnant women can be detrimental to the fetus and even lead to stillbirth. The gold standard for treating toxoplasmosis is the combination of pyrimethamine and sulfadiazine, which can inhibit the synthesis of parasite DNA by inhibiting dihydrofolate reductase (DHFR) (7). However, this treatment can cause folate deficiency states, which can be responsible for significant side effects on the blood system and embryonic diseases, in addition to the risk of causing diseases such as agranulocytosis and Stevens-Johnson syndrome (8). Vaccination for disease prevention is the best way to control diseases, for example, human papillomavirus vaccines and COVID-19 vaccines; therefore, the development of toxoplasmosis vaccine candidates is imperative (9, 10).
Among several candidate vaccines under investigation, including live attenuated, DNA, mRNA, epitope, carbohydrate-based, and subunit vaccines, only one vaccine, classified as a live attenuated vaccine, has been commercially licensed and used in the sheep industry: Toxovax® (MSD, Wellington, New Zealand). It was obtained from the non-cyst-forming S48 strain. Until now, there have been no other vaccines with significant protective efficiency for commercial licensing (11–13). Furthermore, these vaccines still have some shortcomings. For example, attenuated vaccines can be counterproductive due to their unknown genetic background, causing diseases they are supposed to prevent because of the possibility of reversion to the toxic wild-type. Moreover, the integration of potential genomic plasmids of DNA vaccines can activate relevant proteins and produce antibodies against the vaccine itself. Epitope vaccines lack the secondary and tertiary structures of the native proteins and are small molecules with poorly immunogenicity. In carbohydrate-based vaccines, the structure of the carbohydrate tends to be similar to that of the host, which might lead to autoimmunity. Carrier or adjuvant delivery is usually required for subunit vaccines, but this provides less immune protection (12, 14). mRNA vaccines theoretically avoid the risk of genetic recombination as they will be confined to the cytoplasm. Moreover, they are relatively safe, with little risk of misfolding recombinant proteins. In addition, independent cell expansion shortens production time and is more conducive to preventing epidemic diseases (15). The COVID-19 mRNA vaccine was developed and has been used in clinical settings in just two years, showing the possibility of developing a T. gondii mRNA vaccine (16).
In this study, BALB/c mice were used as the animal models for immunization to study the immunogenicity and protective efficiency of a novel vaccine, comprising TGGT1_216200 (TG_200) mRNA, a novel molecule from T. gondii, encapsulated in a lipid nanoparticle (TG_200 mRNA-LNP vaccine). We evaluated antibody levels, cytokine levels, and immune-related transcription factor levels. The lymphocyte proliferation and cytotoxic T lymphocyte (CTL) activity were also assessed. Survival time was analyzed after T. gondii RH strain challenge, including vaccine-immunized mice and mice receiving adoptive transfer. Herein, the potential mechanisms and prospects of the vaccine were also discussed.
Bioinformatic prediction was carried out using DNASTAR software (Madison, WI, USA) and the Immune Epitope Database (IEDB, http://tools.immuneepitope.org/mhcii/). Protean software in the DNASTAR package predicted the biochemical indicators (surface probability, hydrophilicity, flexibility, and antigenicity), and the half-maximum inhibitory concentration (IC50) value of a peptide that bound to the major histocompatibility complex (MHC)-II molecule TG_200 was analyzed in the IEDB. Applying the IEDB methodology, the prediction results were ranked by percentiles based on the suggested methodology.
BALB/c mice (6–8 weeks old) and New Zealand white rabbits (7–9 weeks old) were purchased from the Zhejiang Experimental Animal Center (Zhejiang, China). All animals were kept in standard pathogen-free conditions with stable temperatures and were used for the vaccination study. The procedures in this study involving the mice and rabbits were performed in accordance with Chinese legislation on the use and care of laboratory animals (GB/T35823-2018). The animal experiments were approved by Hangzhou Medical College Institutional Animal Care and Use Committee (Approval No: 2021-152). Regarding euthanasia, mice were euthanized by intraperitoneal injection of sodium pentobarbital (150 mg/kg).
We used the human foreskin fibroblast (HFF) cells to maintain and proliferate the T. gondii RH strain (type I) tachyzoites. The obtained tachyzoites were used to prepared the soluble tachyzoite antigens (STAg). Furthermore, the tachyzoites were used for total RNA extraction, and in the mice challenge experiments. HFF cells, cultured muscle cells (C2C12), 293T cells from the human embryonic kidney (293T cells), and a strain of T. gondii RH were stored at −80°C in our laboratory. 293T and C2C12 cells were used for the transfection experiments, which used the lipo2000 reagent (Invitrogen, Carlsbad, CA, USA). The HFF, 293T, and C2C12 cells purchased from American Type Culture Collection (ATCC; Manassas, VA, USA) and maintained in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal bovine serum (FBS; Gibco, New Zealand) in an incubator with 5% CO2 at 37°C.
Using the Trizol reagent (Invitrogen), we extracted the total RNA of T. gondii from the tachyzoites of the RH strain, according to the manufacturer’s protocol. The GoScript™ Reverse Transcription System (Promega, Madison, WI, USA) was used to synthesize cDNA from the total RNA. The whole TG_200 open reading frame (ORF) was amplified from the T. gondii RH tachyzoite cDNA using PCR with the following primers: forward: 5’-CGGGATCCATGGCGTTAACCATGTTGGCA-3’; reverse: 5’-CCAAGCTTTCAGGCGGCCTACTGGCCGTGCAC-3’, which were designed using the DNASTAR software and incorporated BamHI and HindIII restriction sites. The recombinant plasmid pET28a-TG_200 was constructed by ligating the PCR product into vector pET-28a via the BamHI/HindIII sites. Escherichia coli BL21(DE3) was chosen to express recombinant TG_200 (rTG_200), which was purified using Ni2+-NTA agarose columns (Qiagen, Hilden, Germany).
Two rabbits were immunized with 200 μg of purified rTG_200 protein intravenously to obtain polyclonal antiserum. Three injections in each rabbit were administered at intervals of two weeks. We collected blood from the rabbit ear vein the day before each injection.
The mRNA construct comprised five structures to make it more complete and stable, including a 5′ cap structure, a 5′ untranslated region (UTR), the open reading frame (ORF) of TG_200, a 3′ UTR, and a Poly-A tail (Figures 1A, S1). The 300 bp 5′ UTR (the sequence was chosen by searching in ToxoDB http://toxodb.org/toxo/) was placed before the TG_200 coding sequence, and a 300 bp sequence was placed after the TG_200 coding sequence as the 3′ UTR. The T7 promoter was chosen for in vitro transcription of the mRNA, and the modified mRNA was synthesized from a linearized DNA template in vitro using a T7-FlashScribe Transcription Kit (CellScript, Madison, WI, USA). The 5′ cap and poly-A tail structure were added enzymatically, contributing to the recognition and binding of the mRNA by ribosomes and the stabilization of the mRNA structure during protein expression. To perform IVT, standard unmodified nucleotides were used as described by the manufacturer. We generated mRNA for the encapsulation in LNPs after replacing uridine with pseudouridine using the Incognito mRNA synthesis kit (CellScript). The mRNA was stored frozen at −80°C until use.
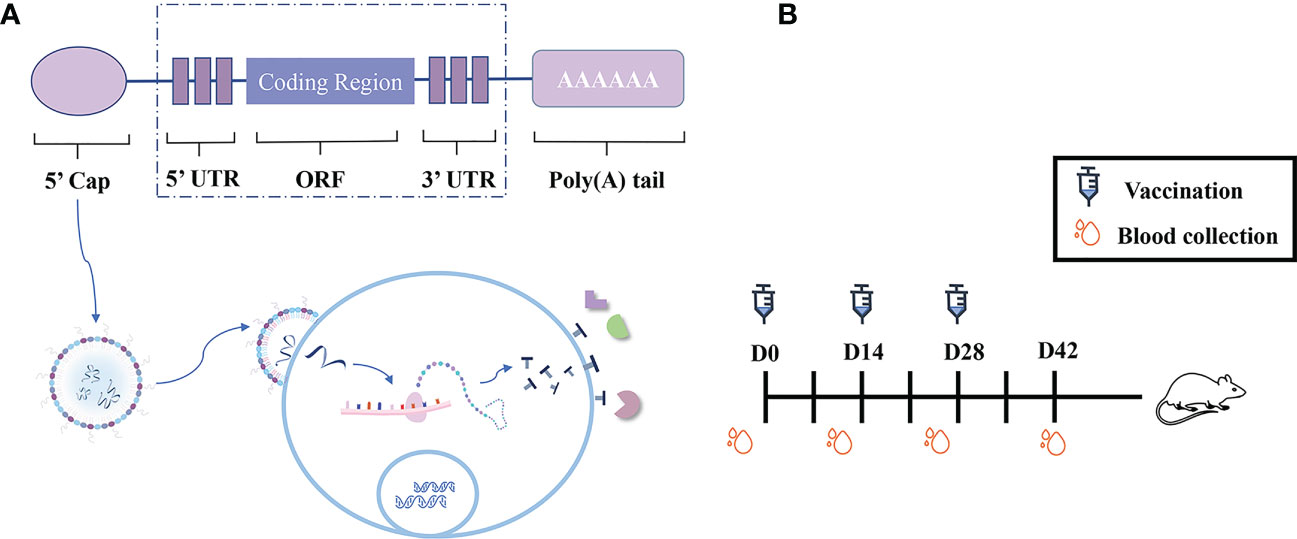
Figure 1 Experimental design and immunization schematic diagram. (A) Schematic diagram of the construction of TG_200 mRNA-LNP vaccine candidates and a schematic diagram showing the proposed mechanism for mRNA vaccine candidate translation in the cytoplasm. (B) Scheme of the TG_200 mRNA-LNP vaccination schedule.
The mRNA-LNP formulation was prepared using a GenVoy ionizable lipid mixture (GenVoy-ILM) (Precision NanoSystems (PNI), Santa Rosa, CA, USA) on the benchtop. GenVoy-ILM is the simplest way to make LNPs. GenVoy-ILM is a mixture of molar ratio 50 (PNI Ionizable Lipid):10 Distearoylphosphatidylcholine (DSPC):37.5 (cholesterol):2.5 (PNI Stabilizer). The program was set with a total flow rate of 12 mL/min and a flow ratio of 3:1 to pass the mRNA and GenVoy ILM in PNI buffer through a laminar tube that encapsulates lipids to generate mRNA-LNPs. As described by the manufacturer, the mRNA encapsulation efficiency was calculated using a quantit™ RiboGreen™ RNA Reagent and Kit (Thermo Fisher Scientific, Waltham, MA, USA). The fluorescence intensity was converted to concentration using a standard curve drawn using standard solutions. Finally, the encapsulation efficiency (EE) was calculated according to the following formula (in which “W” represents weight): EE% = W(total RNA)-W(free RNA)/W(total RNA).
According to the manufacturer’s instructions, the mRNA synthesized in vitro was transfected into 293T cells using the lipo2000 reagent, ensuring the correct translation of mRNA in the cell. The skeletal muscle myoblast C2C12 cells were transfected to ensure that the synthesized mRNA could be expressed by intramuscular injection. The collected cell lysate and supernatant were analyzed using western blotting with rabbit anti-TG_200 polyclonal antibodies. In vitro synthesized mRNA (pseudouridine in place of uridine) was purified and encapsulated in LNPs for administration to mice.
Female BALB/c mice were randomly divided into three groups of 10 mice per group. Ten mice were immunized with the TG_200 mRNA-LNP vaccine three times intramuscularly in a 100 μL volume (1 mg/mL) as an experimental group (Figure 1B). Ten mice were injected with LNP as negative control and the rest, without treatment, formed the BLANK control group. Before each immunization and three weeks after the final injection, we collected serum samples from all mice. Two weeks after the last injection, each group was challenged with 102 tachyzoites of the RH strain. The mice challenged by RH T. gondii were observed and their survival was recorded.
Before blood collection, the tail of the mice was placed in water at about 45°C to fill up the tail vessels. The tail was wiped with 75% alcohol, a transverse incision was made using a razor blade to cut through the caudal vein, and about 0.1 mL of blood was taken. Finally the wound was disinfected and pressure was applied to stop the bleeding. Total IgG and subtype antibodies (IgG1, IgG2a) in mice serum after TG_200 mRNA-LNP immunization were determined indirectly using ELISA with 10 μg/mL rTG_200 protein-coated flat-bottom 96-well plates in bicarbonate buffer (pH 9.6) at 4 °C overnight. The coated plates were blocked with 5% skim milk in phosphate-buffered saline with 0.05% Tween 20 (PBST) for 1 h at 37°C, washed with PBST three times, then incubated with 100 μL of serum samples (1:100 in PBST) for 2 h at 37°C after adding, followed by incubation with 100 μL of horseradish peroxidase (HRP)-conjugated goat-mouse IgG, IgG1 or IgG2a (Abcam, Waltham, MA, USA) for 1 h at 37°C. The plates were then washed three times and incubated with 100 μL of tetramethylbenzidine (TMB) for 10 min. Then, 50 μL of 2 M H2SO4 was added to stop the reaction. An automatic ELISA reader (BioTek, Winooski, VT, USA) was used to detect the absorbance values at 450 nm. Each sample contained three replicates.
Splenic lymphocytes were removed from five mice in each group after euthanasia at two weeks after final immunization. The spleen was pushed through a nylon sieve to harvest the spleen cells. To remove the red blood cells, red blood cell lysis buffer (Sigma, St. Louis, MO, USA) was used. Moreover, DMEM, which was supplemented with 10% fetal bovine serum, was used to resuspend the purified spleen cells. The purified spleen cells were cultured in 96-well plates (2 × 105 cells/well) in triplicate in the presence of 10 μg/mL rTG_200 protein. The negative control comprised cells incubated in complete medium, and the positive control comprised cells incubated in 5 μg/mL concanavalin A (ConA). The plates were incubated for four days at 37°C under 5% CO2. The proliferative activity of spleen lymphocytes was detected using the Cell Counting Kit-8 (CCK-8) reagent (Dojindo, Kumamoto, Japan) following the manufacturer’s protocol. The absorbance was read and recorded at 570 nm. Then, the stimulation index (SI) was calculated using the following formula:
Following a previously described protocol, five mice spleens were taken from each group to detect cytokine levels at two weeks after the last injection (17). Spleen cells in 96-well plates were cultured at 37°C, 5% CO2 and cell-free supernatants were collected. Interleukin (IL)-4 levels at 24 h, IL-10 levels at 72 h, and interferon-gamma (IFN-γ) and IL-12 levels at 96 h were detected using commercially available kits (eBioscience, San Diego, CA, USA) according to the manufacturer’s instructions. All samples were assayed three times.
Splenocytes from five mice per group were isolated two weeks after the last immunization. The isolated lymphocytes were then cultured in DMEM with10% fetal bovine serum and 1% penicillin-streptomycin overnight. The nonadherent cells were removed and the remaining cells were collected in PBS after three washes. 106 cells were conditioned in 100 μL of PBS and stained with anti-mouse CD11c-fluorescein isothiocyanate (FITC), CD83-Phycoerythrin (PE), and CD86-PE (eBioscience) at 4°C in dark conditions for 40 min. Flow cytometry (Beckman Coulter Inc., Brea, CA, USA) was used to sort and count cells after washing and collection to investigate the surface markers of dendritic cells (DCs). Consistent with the above method, the molecular changes of MHCs in DCs were analyzed using anti-mouse CD11c-FITC, MHC-I-PE, and MHC-II-PE (eBioscience). After adjusting the cell numbers, two cell tubes were collected and respectively stained with anti-mouse CD3e-FITC, CD4-PE (eBioscience) and anti-mice CD3e-PE, CD8-PE to detect the subsets of CD4+ T and CD8+ T lymphocytes. The ratio of CD4+ and CD8+ T lymphocyte subsets was also analyzed. All samples were assayed in triplicate by performing the staining strategy and flow cytometry analysis. All labeled cells were analyzed on a FACSAria III flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA), and the data were analyzed using CellQuest software (BD Biosciences).
The rverse transcription step of the qRT-PCR protocol was consistent with the preparation of polyclonal antibodies as described in section 2.4. Using CFX96 connect apparatus (Bio-Rad, Hercules, CA, USA), the qPCR step of the qRT-PCR protocol was caried out using the prepared cDNA as the template. The reactions were conducted in triplicate using intercalating dye SYBR Green-based GoTaq® qPCR Master Mix (Promega), following the manufacturer’s instructions. The experimental results were normalized by selecting H3 (encoding Histone H3) as an internal reference gene. The primers used are listed in Table 1.

Table 1 qRT-PCR primers used to amplify the p65, Tbet, Irf8, and Actb genes designed by DNASTAR software.
The expression of the TG_200 protein in 293T cells and C2C12 cells were analyzed by western blotting (WB). The level of β-actin in these cell lysates served as an internal control. Detection of TG_200 was performed using rabbit anti-TG_200 polyclonal antibodies as the primary antibodies. Moreover, WB was used to validate the levels of downstream molecules T-Box 21 (T-bet), interferon regulatory factor 8 (IRF8), and the nuclear factor kappa B (NF-κB) p65 subunit. Antibodies against T-bet, p65, and IRF8 were purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA). The nuclear and cytoplasmic isolation kit (Beyotime Institute, Haimen, China) was used to isolate transcription factors (NF-κB p65, T-bet, and IRF8) in the nucleus, which were assessed using WB. The antibody was diluted with 0.05% skim milk in a ratio of 1:1000. The detailed assay steps refer to a previous publication (17).
A CytoTox 96® Non-Radioactive Cytotoxicity Assay Kit (Promega) measured the CTL activity. The spleen cells were cultured with 100 U/mL recombinant murine IL-12 for five days as effector cells. The target cells were Sp2/0 mice cells that were transfected with TG_200 mRNA-LNP. Effector and target cells were mixed at ratios of 10:1, 20:1, 40:1, and 80:1. The percentage of specific cell lysis was calculated at 6 h after incubation as follows: Cytotoxicity % = (Experimental-Effector Spontaneous-Target Spontaneous)/(Target Maximum-Target Spontaneous) × 100.
Sera from three groups of mice (TG_200 mRNA-LNP, LNP, and BLANK) were collected and transferred to naive mice by tail vein injection (100 µL per mice) to assess the protective effect of the immune sera. Thirty mice were randomly divided equally into three groups, TG_200 mRNA-LNP (TG_200 immunized serum injection), LNP (LNP immunized serum injection), BLANK (BLANK serum injection), respectively. After five daily transfers, each group was challenged with 102 tachyzoites of the RH strain. Survival time was recorded after the mice were challenged with T. gondii and their survival was monitored daily.
Splenocytes from the three groups of mice were collected and transferred to naive mice by tail vein injection (5 × 107 cells mice) to assess the protective of the immune splenocytes. Thirty mice were randomly divided equally into three groups, TG_200 mRNA-LNP (TG_200 immunized splenocytes injection), LNP (LNP immunized splenocytes injection), BLANK (BLANK splenocytes injection), respectively. Each group was challenged with 102 tachyzoites of the RH strain at 24 h after transfer. Survival time was recorded after the mice were challenged with T. gondii and their survival was monitored daily.
An independent sample T-test was performed for the levels of antibodies, cytokines, lymphocyte proliferation, flow cytometry analysis; and a Log-rank test was for survival analysis in GraphPad 8.0 software (GraphPad Inc., San Diego, CA, USA). Differences were considered statistically significant when P<0.05.
Numerous antigens have been identified as potential vaccines against T. gondii, such as surface antigen proteins (TgSAGs), rhoptry protein (TgROPs), microneme proteins (TgMICs), and others (18). Despite choosing diverse immunization routes, animal models, doses, and vaccine production processes, none of the vaccines developed based on these candidates have demonstrated complete protection against T. gondii. Secreted proteins play a crucial role in mediating the interaction between host and pathogen (19); therefore we focused on analyzing T. gondii secreted proteins at the genome-wide level to find possible vaccine candidates. Using the ToxoDB, we first screened secreted proteins containing both transmembrane domain and signal peptide at the whole-genome level. Out of over 8,000 genes in the T. gondii genome, only 233 genes had both transmembrane domains and signal peptides. A vaccine candidate with a high potential for success is often identified by a bioinformatic analysis that predicts a high T/B cell epitope score (20, 21). Based on published data, T. gondii surface antigen 1 (SAG1) has strong immunogenicity and immunoprotective effects, and is considered a promising candidate antigen and a major target for inducing host immune responses (22). Therefore, SAG1 was chosen as the reference protein for this experiment. We used DNASTAR (PROTEAN program) and IEDB database to perform T/B cell epitope analysis on the 233 genes encoding proteins that were screened, and compared them with SAG1. We obtained several candidate proteins, among which TGGT1_216200 proteins showed the best T/B cell epitope prediction scores. TG_200 and SAG1 scores for surface probability, antigenic index, hydrophilicity, and flexible regions were compared. Flexible amino acid residues are malleable sites that readily form antigenic epitopes. Hydrophilic amino acids are enriched on the surface of hydrophilic regions, and these sites have evolved to become the primary amino acid insertion sites for proteins, which also provide the basis for protein secretion into the cytoplasm and extracellular compartments. Surface probability indicates the probability that an antigenic site is located on an exposed region of the protein surface. The antigenic index can reflect the antigenicity scale by analyzing amino acids at contiguous sites of well-studied proteins. According to Figure 2, in comparison with SAG1, each TG_200 indicator showed a high score. The regions with hydrophilicity of TG_200 are amino acids 10–60, 67–86, 92–102, 135–137, and 142–160. The surface probability regions of TG_200 are amino acids 13–27, 32–36, 39–54, 57, 68–77, 80–82, 94–97, 137, 147–156. The antigen index regions of TG_200 are amino acids 11–60, 68–78, 80–87, 93–100, 113–114, 116–118, 122, 124–126, 135–160. The flexibility regions of TG_200 are amino acids 10–27, 32–59, 73–85, 96–98, 115, 134–138, 142–155. The TG_200 protein has good surface probability, hydrophilicity, a high antigenic index, and more variable regions, indicating that vaccines based on TG_200 protein might be promising.
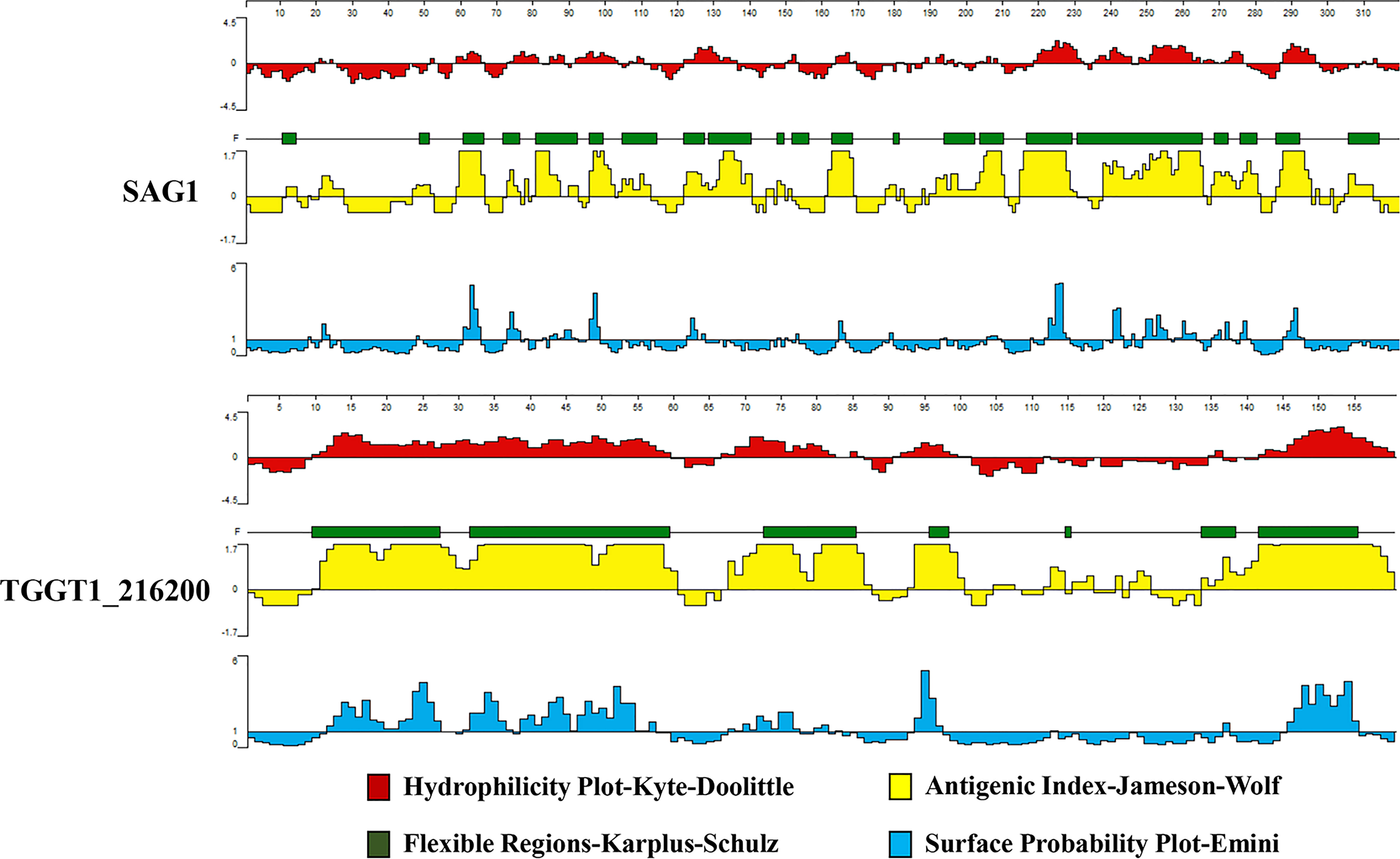
Figure 2 The plot of the DNASTAR-predicted hydrophilicity, flexible regions, antigenic index, and surface probability of the linear-B cell epitopes of TG_200 compared with those of SAG1.
In addition, both TG_200 and SAG1 T-cell epitopes were analyzed using the IEDB online service. The MHC II molecules were correlated with the half-maximum inhibitory concentration (IC50) values of the TG_200 peptides, and generally, the smaller the value, the stronger the epitope and the binding ability of MHC-II. Table 2 shows that there are lower percentile classes of TG_200 peptides than those of SAG1. The IC50 values of H2-IAd, H2-IEd, and HLA-DRB1*01:01 from TG_200 were lower than those of SAG1. This result indicated that the TG_200 protein might have strong binding to MHC-II.
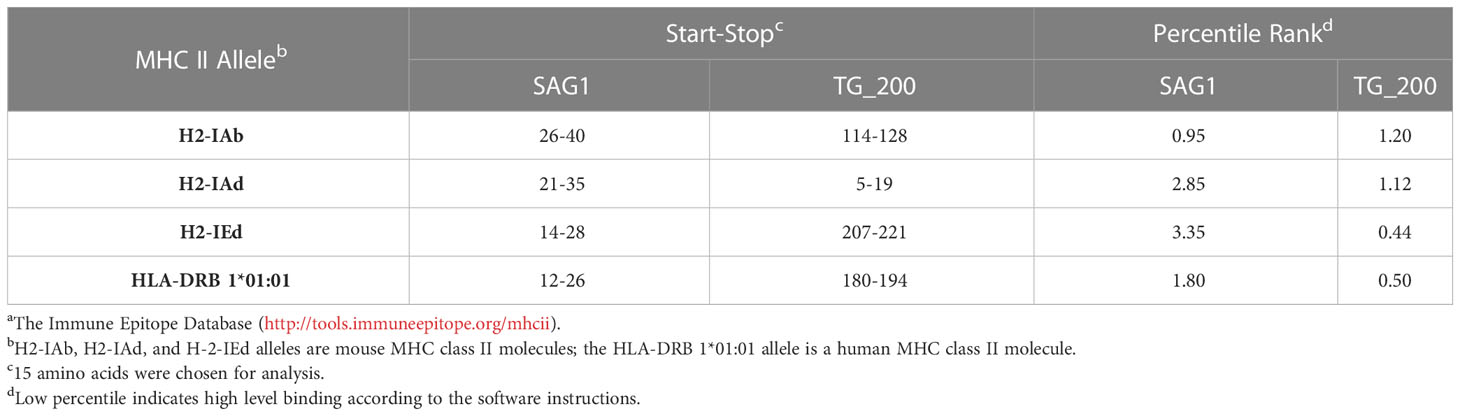
Table 2 IC50 values for TG_200 and SAG1 binding to MHC class II molecules, obtained using IEDBa.
The bioinformatic analysis showed that the TG_200 peptide has a higher linear B-cell epitope score and lower IC50 value percentile compared with those of SAG1, suggesting that TG_200 might be more promising as a candidate vaccine molecule.
The construct was designed based on the TG_200 coding sequence and sequences were added to make it more complete and stable. The UTRs contributed to mRNA stability and translation regulation, which was critical for optimal protein expression. We transcribed mRNA from the T7 RNA polymerase promoter site upstream of the 5′ UTR in vitro. The modified mRNA was synthesized from a linearized DNA template in vitro.
TG_200 mRNA construct was transfected in 293T cells, and its expression was analyzed by WB (Figure 3A). In WB, the rabbit anti-TG_200 polyclonal antibody detected single specific protein bands in 293T cells transfected with rTG_200, STAg, TG_200 mRNA, and supernatant. The first band was detected, indicating that the antibody was correct and viable. The presence of a band in the STAg sample (second band) indicated that the TG_200 protein is soluble. No bands were detected in untransfected 293T cells. Additionally, we detected bands in the supernatant of transfected cells; however, no internal control bands were detected in the supernatant sample, indicating that the sample was not contaminated by cells and that the TG_200 protein was a secreted protein.
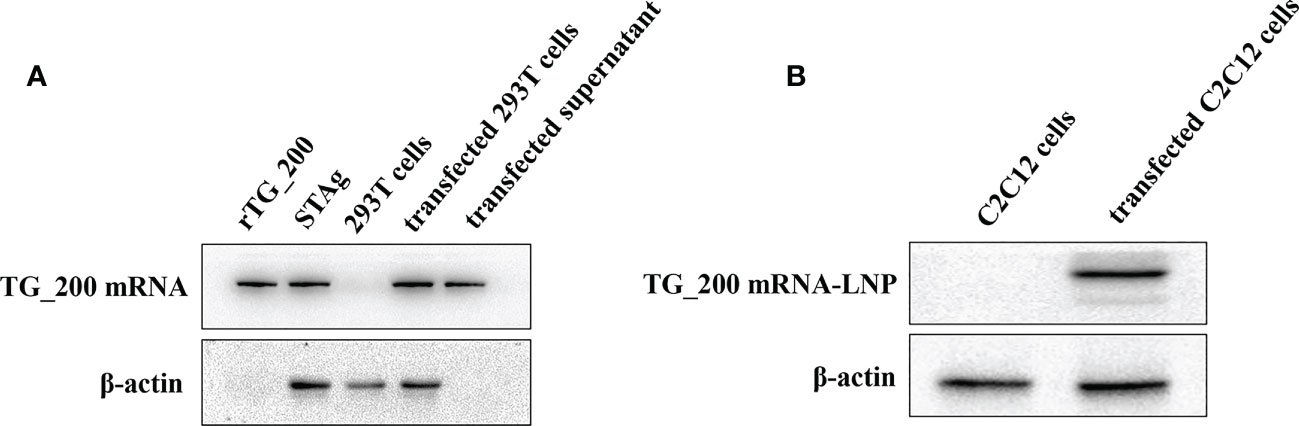
Figure 3 Expression of TG_200 mRNA in 293T cells and TG_200 mRNA-LNP in C2C12 cells. (A) Western blotting detection of the expression of TG_200 protein in rTG_200, STAg, 293T cell lysates and TG_200 mRNA transfected 293T cells supernatant. rTG_200: the recombinant pET28a-TG_200 acting as a positive control; STAg: soluble antigen of T. gondii; 293T cells; transfected 293T cells: TG_200 mRNA transfected 293T cells; transfected supernatant: TG_200 mRNA transfected 293T cell supernatant. (B) Western blotting detection of TG_200 mRNA-LNP in C2C12 cells. C2C12 cells: C2C12 cell lysates; transfected C2C12 cells: TG_200 mRNA-LNP transfected C2C12 cells lysates.
C2C12 cells were selected for transfection to ensure that TG_200 mRNA-LNP could be expressed in myocytes for subsequent intramuscular injection. In Figure 3B, TG_200 mRNA-LNP levels were measured indirectly by WB detection of TG_200. In C2C12 cells transfected with TG_200 mRNA-LNP, a single specific protein band was detected, and there was no band in the untransfected cells. The result indicated that the TG_200 mRNA-LNP was successfully transfected and expressed. Assessment of the encapsulation efficiency using Quant-iT™ RiboGreen™ RNA Reagent and Kit to measure the concentration of free and total RNA in the post-encapsulation solution showed that the encapsulation efficiency obtained was about 95.67%.
ELISA was conducted to detect serum IgG, IgG2a, and IgG1. A higher IgG titer was detected in the serum of mice immunized with TG_200 mRNA-LNP than in the BLANK and LNP groups (Figure 4A). Significant differences in IgG levels began to appear between the TG_200 mRNA-LNP immunized group and the control at two weeks after the first immunization (P< 0.001). There was no significant difference in antibody levels between the groups before immunization, and antibody titer increased with the number of immunizations (until week 6). No significant difference was observed between the BLANK and LNP groups from week 0 to week 6 (P > 0.05). Immunization with the TG_200 mRNA-LNP vaccine increased IgG antibody levels in mice, inducing corresponding humoral immunity. Figure 4B shows that compared with those in the control group, IgG1 and IgG2a in the TG_200 mRNA-LNP immunized group were significantly elevated (P< 0.001). The IgG2a/IgG1 ratio in the TG_200 mRNA-LNP immunized group was higher than that in the controls, indicating a dominant T helper type 1 (Th1-type) cellular immune response induced by the TG_200 mRNA-LNP vaccine. Increased antibody levels following immunization suggested increased protection against T. gondii infection.
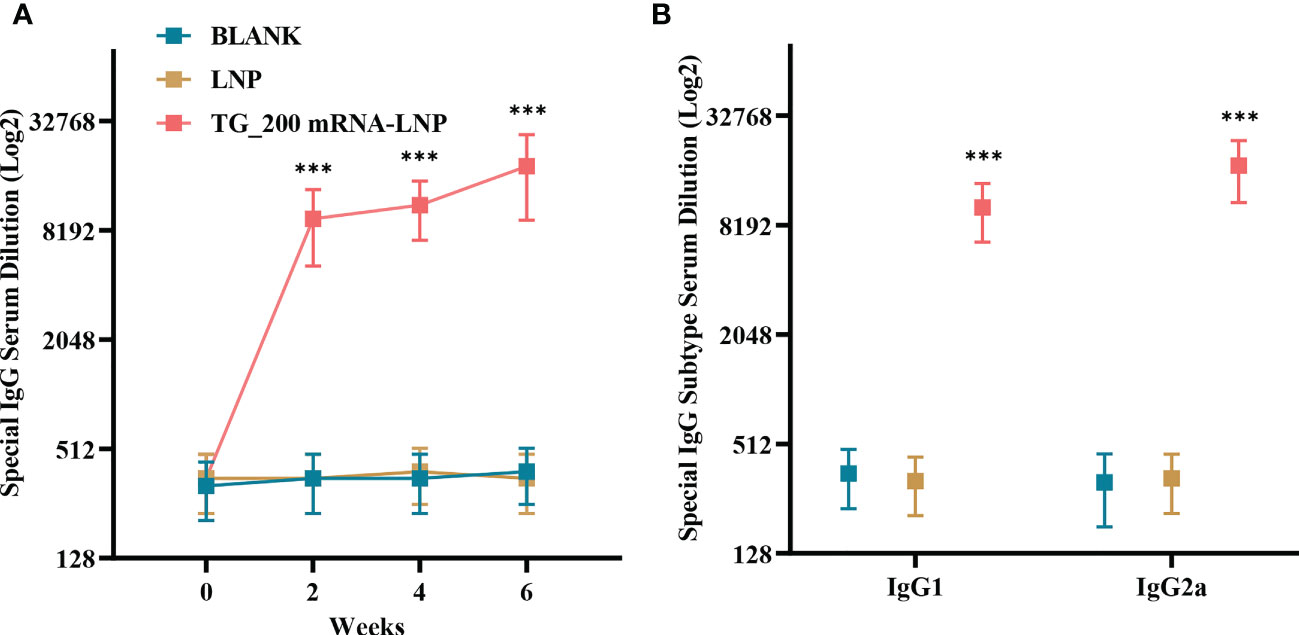
Figure 4 Detection of T. gondii specific humoral immune responses induced by TG_200 mRNA-LNP immunization. (A) Serum from vaccinated mice was isolated at 0, 2, 4, and 6 weeks after vaccination and analyzed for IgG neutralization of serially diluted serum samples, n = 8/group. ***P< 0.001. (B) Serum from vaccinated mice was isolated 2 weeks after the boost and analyzed for IgG1 and IgG2a neutralization of serially diluted serum samples, n = 8/group. ***P< 0.001. Data are presented as the means ± SD.
Immune cell responses to TG_200 mRNA-LNP were evaluated using spleen cell suspensions prepared from each mice two weeks after the last immunization. Figure 5A shows that compared with the medium and LNP groups, splenocytes from mice immunized with TG_200 mRNA-LNP showed an enhanced proliferative response (P< 0.001). There was no significant difference between the control groups (P > 0.05). Lymphocyte proliferation and differentiation are essential stages in the immune response process, and the lymphocyte proliferation test is the classical test to detect cellular immune function. A significant increase in lymphocyte proliferation indicated the production of specific cellular immunity against the TG_200 mRNA-LNP molecule.
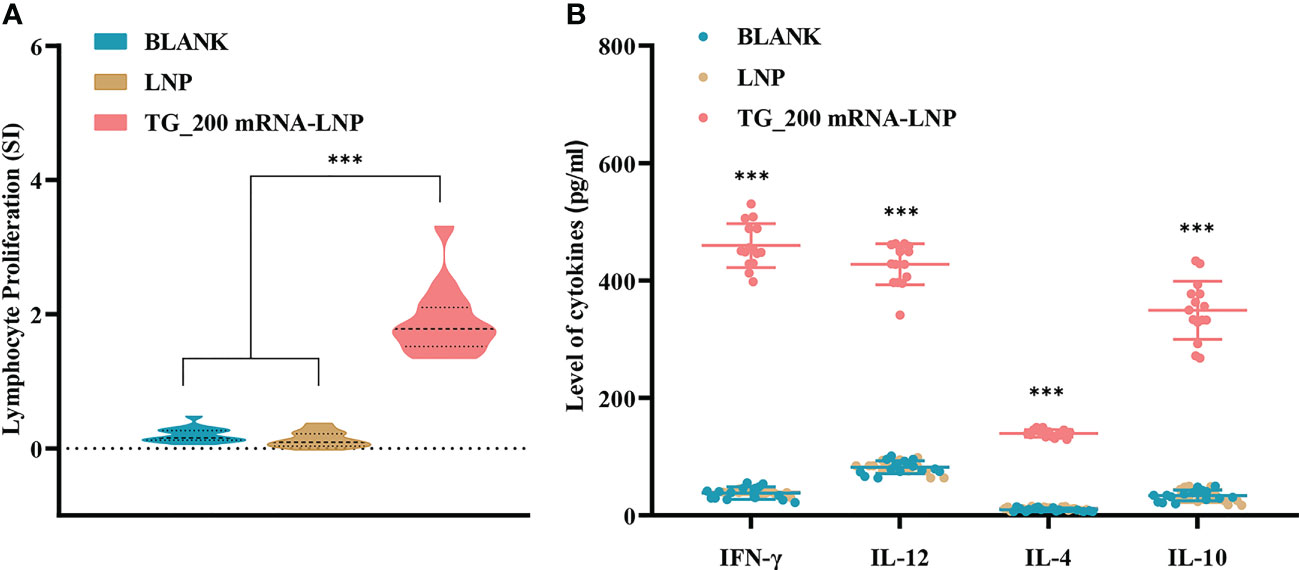
Figure 5 Splenocyte proliferation and cytokine production levels after TG_200 mRNA-LNP immunization. (A) Proliferative responses of splenocytes of BALB/c mice immunized with LNP, TG_200 mRNA-LNP, or the BLANK control, n = 5/group, ***P< 0.001. (B) Cytokine production by splenocytes of mice immunized with LNP, TG_200 mRNA-LNP, or the BLANK control. The vertical axis is the cytokine concentration (pg/mL). The horizontal axis shows IFN-γ, IL-12, IL-4, and IL-10, n = 5/group, ***P< 0.001. Data are shown as the means ± SD of three independent experiments.
IL-12 can induce IFN-γ release. In turn, IFN-γ plays an essential immunomodulatory role as a cytokine secreted by immunoreactive cells in the induction of immunity against infection, including the activation of natural killer cells (NK), CTLs, and phagocytes. IFN-γ and IL-12 are Th1 cytokines, and IL-4 and IL-10 are Th2 cytokines. Th2 cytokines regulate the pathological inflammatory response of Th1 cytokines due to strong immunity during the fight against infection to balance the stability of the normal immune system. Therefore in the current experiment, Th1 and Th2 cytokines were tested separately. Levels of IL-12, IFN-γ, IL-10, and IL-4 were measured in the spleen cell supernatant from spleens taken from mice after the last two weeks of immunization. Figure 5B shows that compared with those in the BLANK and LNP groups, higher levels of IFN-γ (459.5 ± 37.52), IL-12 (427.7 ± 34.91), IL-4 (139.7 ± 6.363), and IL-10 (349.2 ± 49.46) were detected in TG_200 immunized mice (P< 0.001). The result indicated that a mixed Th1 and Th2 immune response was produced in mice immunized with TG_200 mRNA-LNP. Thus, an effective immune response was produced against T. gondii infection.
CTLs plays a vital role in the fight against intracellular pathogen infection. The killing activity of CTLs is antigen-specific and is limited by MHC class I molecules. It requires the same MHC allele product for initial sensitization and resensitization or the same antigenic peptide-binding common motif. One CTL can kill multiple target cells in succession and is highly efficient. Therefore, we evaluated the specific cellular immunity induced by the vaccine by measuring the cytotoxic lysis activity of splenic lymphocytes. Figure 6 shows that the CTL activity of splenocytes from mice immunized with TG_200 mRNA-LNP increased progressively as the effector-to-target cell ratio increased. A significant difference was observed when the effector-target cell ratio was 40:1 and above compared with that in the controls (P< 0.001). When the ratio was 80:1, CTL activity reached higher values (T. gondii-specific lysis accounted for more than 30% of total possible lysis). Splenic lymphocyte CTL activity between the control groups was not significantly different (P > 0.05). The result showed that pathogen-specific CTL responses were induced and produced effective immune responses to intracellular pathogens.
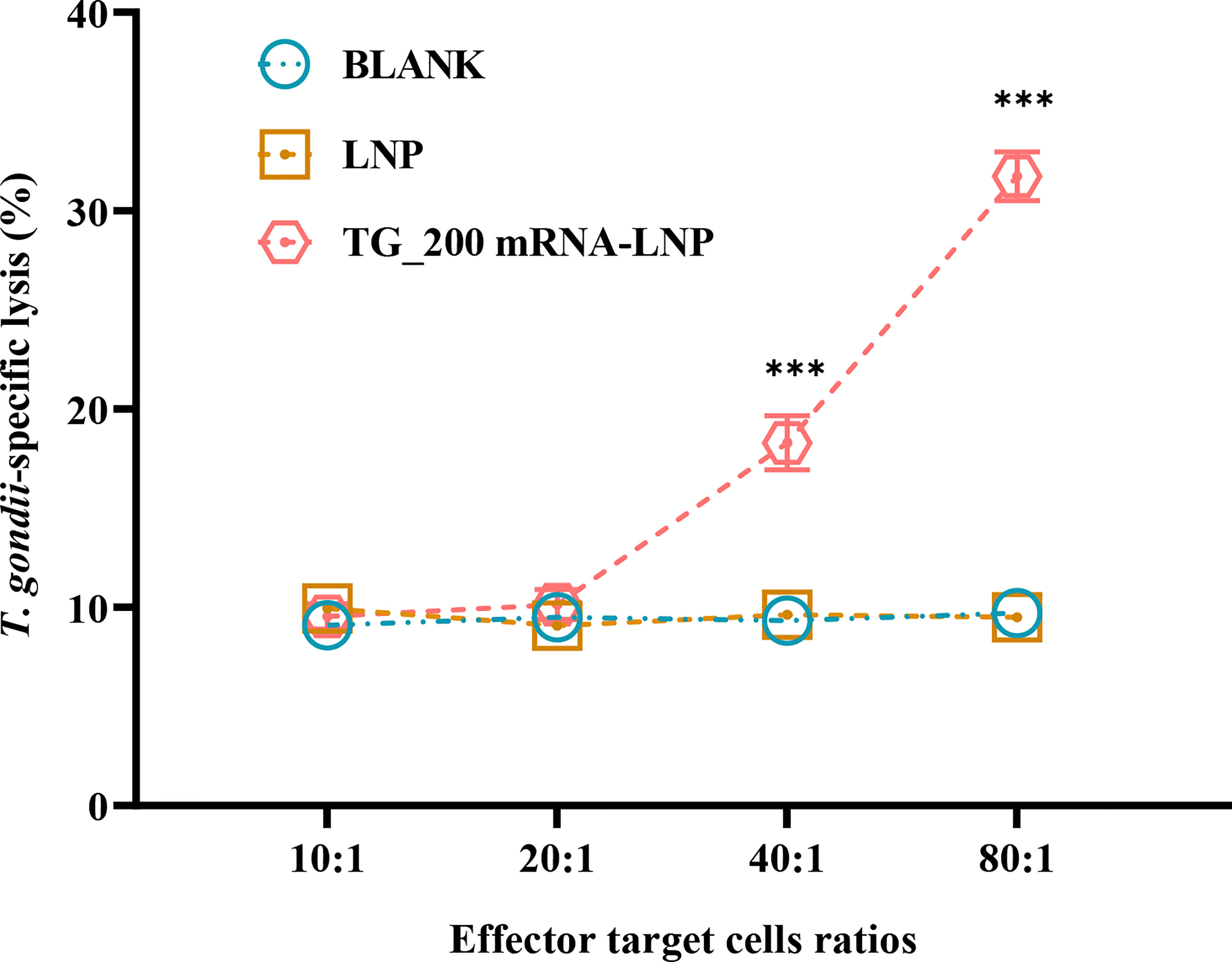
Figure 6 CTL activities of spleen lymphocytes in TG_200 mRNA-LNP immunized mice. The vertical axis shows T. gondii-specific lysis as a percentage of the total possible lysis (%). The horizontal axis is the ratio of effector cells to target cells. n = 5/group, ***P< 0.001.
The gathering of inflammatory mononuclear cells at the site of infection is also essential for controlling T. gondii infection. We determined whether DC differentiation was activated by detecting elevated expression levels of CD83, CD86, MHC-II, and MHC-I on the surface of DCs in mice after immunization with TG_200 mRNA-LNP. CD4+ T cells play an important role in the acute phase of infection. CD8+ T cells and CD4+ T cells were the main cells controlling parasitic IFN-γ production in chronically infected mice. Therefore, flow cytometry was performed to determine whether TG_200 mRNA-LNP vaccination activated DC cells and to analyze the MHC molecules in DCs and the proportion of CD4+ and CD8+ T lymphocytes subsets. Figures 7A, B show higher DC surface expression of CD83+ and CD86+ in the TG_200 mRNA-LNP group than in the control groups (P< 0.001). This indicated that DC cells are efficiently activated and differentiated. Figures 7C, D show that after TG_200 mRNA-LNP immunization, there was a higher level of MHC-I and MHC-II molecules than those in the controls (P< 0.001). In addition, Figures 7E, F show that mice immunized with TG_200 mRNA-LNP had a significant increase in the percentage of CD8+ and CD4+ T cells compared with those in the control group (P< 0.001). There were no significant differences between the control groups (P > 0.05). The results showed that the vaccine activated CD4+ T cells and CD8+ T cells, eliciting an effective cellular immune response. Combined with the CTL results, this suggested that the vaccine induced an effective and specific cellular immune response, which is critical for killing intracellular parasites.
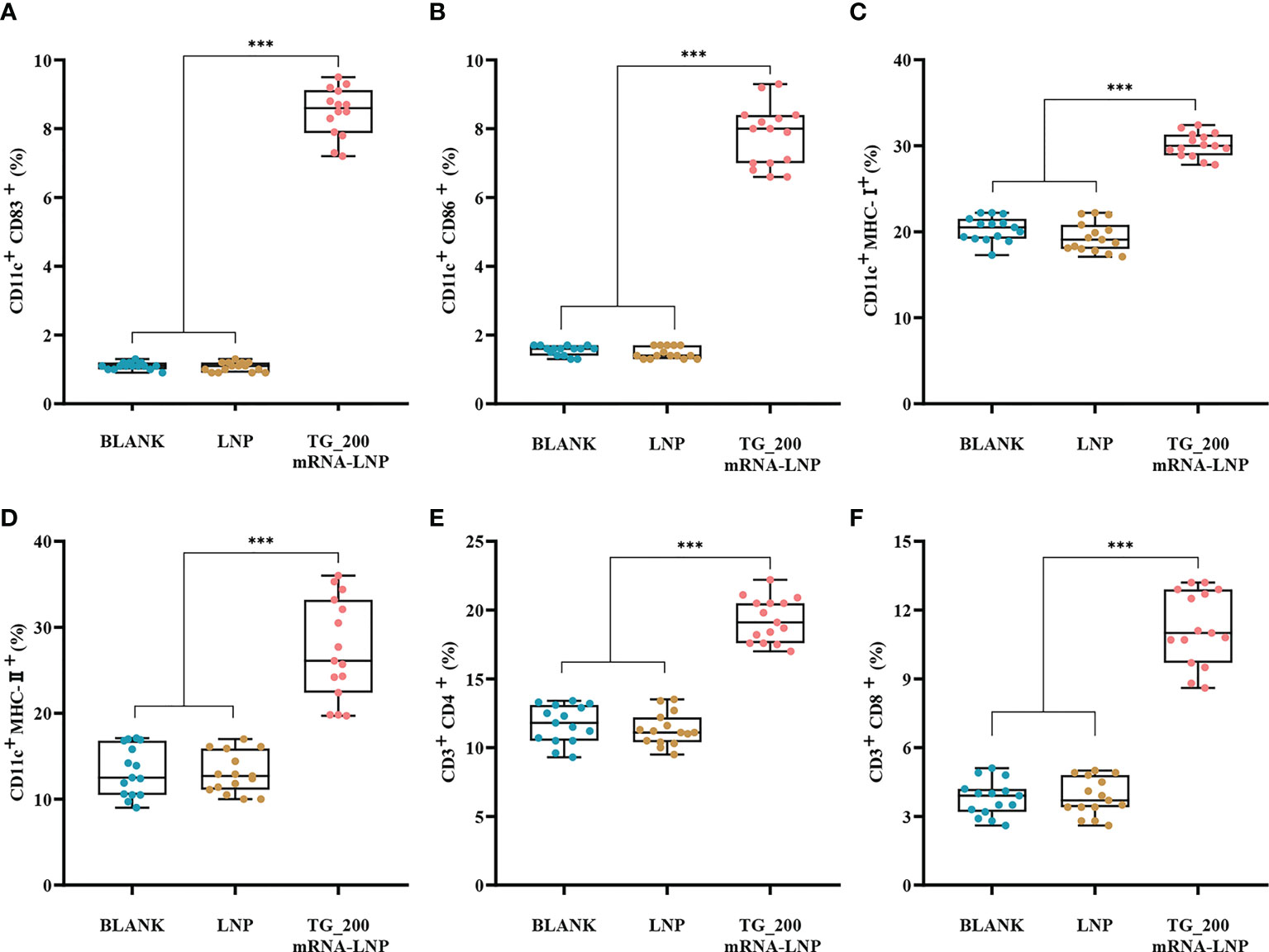
Figure 7 Flow cytometry analysis of dendritic cells (DCs) and T lymphocytes in immunized BALB/c mice. (A) The box graph shows the percentages of CD11c+ CD83+ DCs in mice splenocytes. (B) The box graph shows the percentages of CD11c+ CD86+ DCs in mice splenocytes. (C) The box graph shows the percentages of CD11c+ MHC-I+ DCs in mice splenocytes. (D) The box graph shows the percentages of CD11c+ MHC-II+ DCs in mice splenocytes. (E) The box graph shows the percentages of CD3+ CD4+ T lymphocytes. (F) The box graph shows the percentages of CD3+ CD8+ T lymphocytes. n = 5/group, ***P< 0.001. The data are shown as the means ± SD of three independent experiments.
The p65 molecule is a member of the NF-κB family, and NF-κB transcription factors are essential in the fight against T. gondii infection. Interferon regulatory factors (IRFs) can activate DCs to produce IFNs and cytokines. T-bet can specifically promote Th0 differentiation into Th1 cells, which is important in intracellular pathogen infection. qRT-PCR was used to detect the mRNA expression of transcription factors Irf8, p65, and Tbet. Compared with those in the control groups, Irf8, p65, and Tbet mRNA levels were significantly higher in the TG_200 mRNA-LNP immunization group (P< 0.001) (Figure 8A). There was no significant difference between the LNP and the BLANK groups (P > 0.05). Furthermore, WB showed that the levels of the IRF8, p65, and T-bet were higher in the TG_200 mRNA-LNP group compared with those in the two control groups (Figure 8B). At the same time, the expression of internal control gene (H3) was consistent among the three groups of samples. The WB results were consistent with the qRT-PCR results, indicating that TG_200 mRNA-LNP immunization can effectively activate the NF-κB pathway and DC cells, in addition to promoting the specific cellular immunity dominated by a Th1-type immune response.
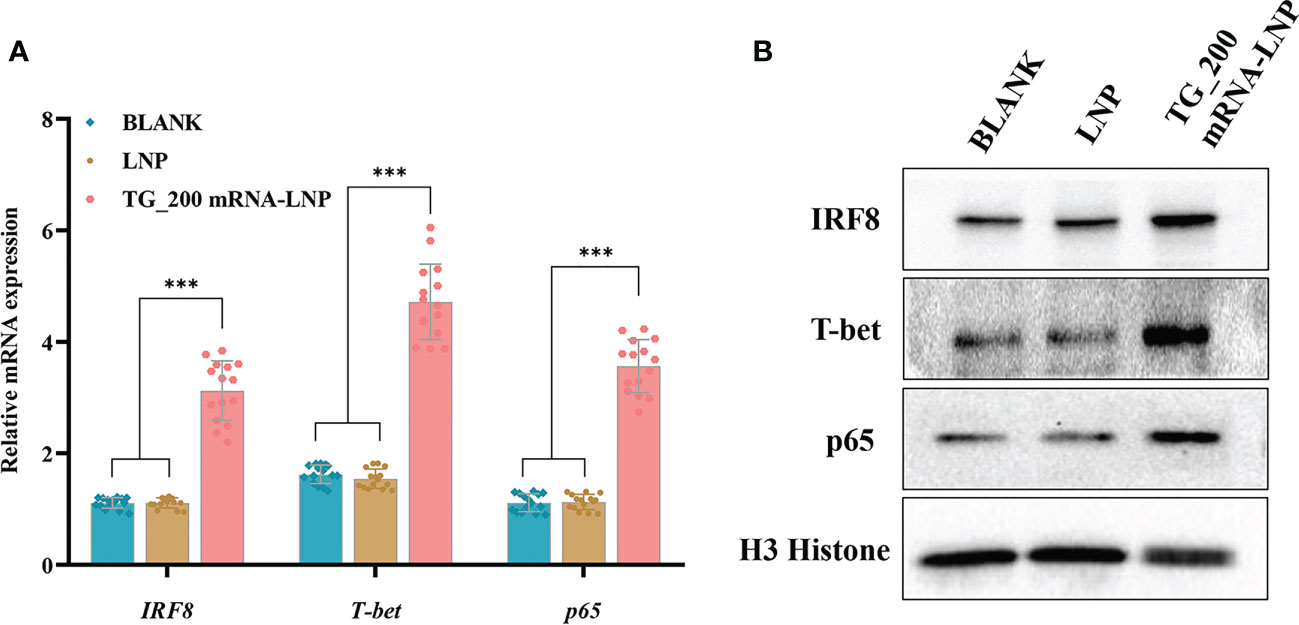
Figure 8 The mRNA and protein expression levels of IRF8, T-bet, and p65 in mice splenocytes. (A) mRNA levels of Irf8, Tbet, and p65. n = 15/group, ***P< 0.001. (B) Protein levels of IRF8, T-bet, and p65, BLANK: Splenocytes from untreated mice; LNP: Splenocytes lysates from the LNP injected mice; TG_200 mRNA-LNP: Splenocytes lysates from mice injected with TG_200 mRNA-LNP.
Mice were challenged with T. gondii RH strain to assess the protective effect of the TG_200 mRNA-LNP vaccine. The survival curves in Figure 9A show that the survival time of TG_200 mRNA-LNP immunized mice was prolonged after challenge with 1 × 102 T. gondii RH compared with that of the mice that received LNP or BLANK controls (19.27 ± 3.438 days, P< 0.001).
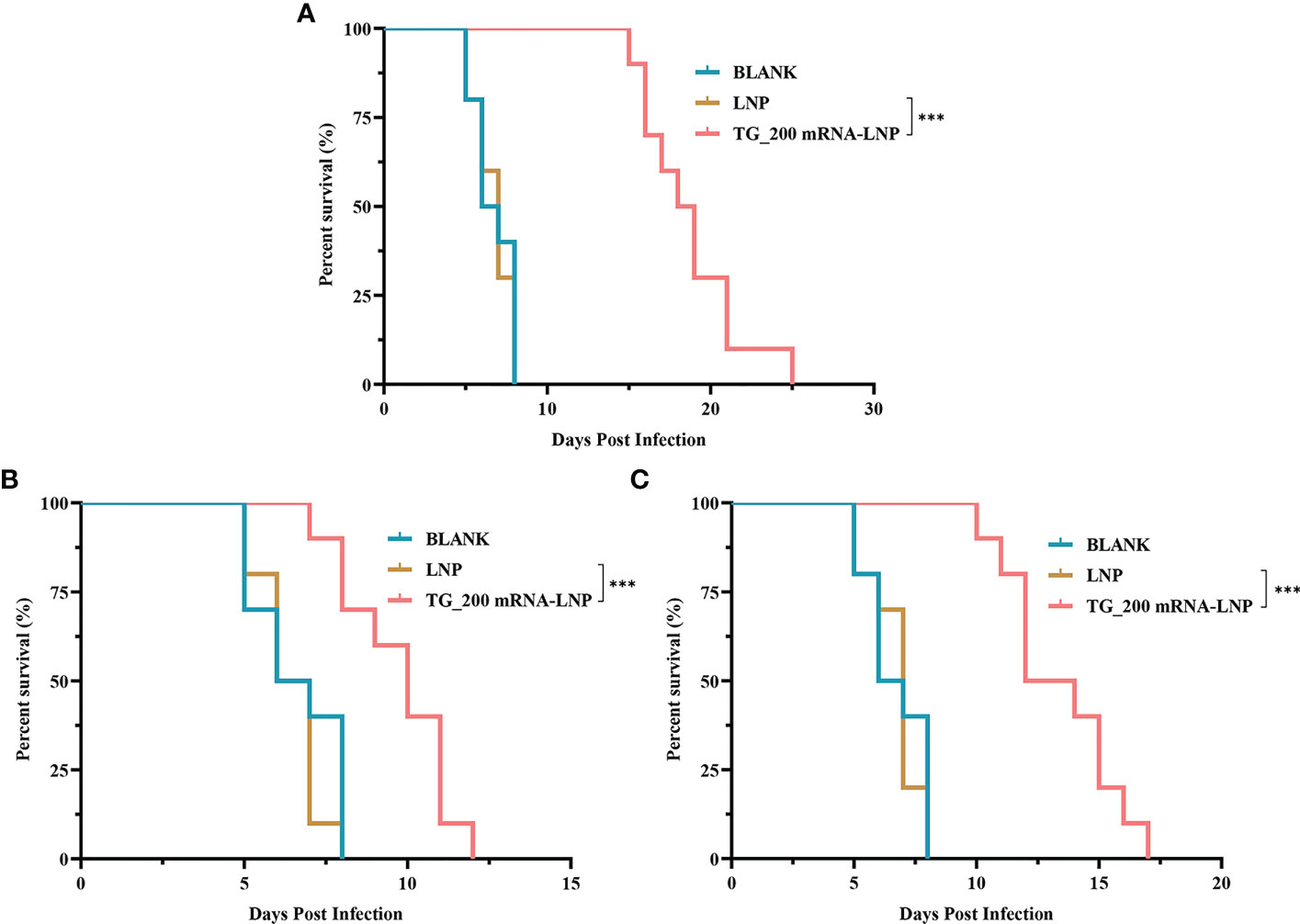
Figure 9 The survival rate of mice immunized with TG_200 mRNA-LNP and adoptively transferred serum or splenocytes from vaccinated mice. (A) The survival rate of BALB/c mice immunized with TG_200 mRNA-LNP, LNP and BLANK group and challenged with 1×102 T. gondii RH tachyzoites two weeks after the last immunization, n = 10/group. (B) The survival rate of mice receiving adoptively transferred serum (daily for five days) from mice vaccinated with TG_200 mRNA-LNP, LNP and BLANK group and challenged with 1×102 T. gondii RH tachyzoites, n = 10/group. (C) The survival rate of mice receiving adoptively transferred splenocytes (5×107) from mice vaccinated with TG_200 mRNA-LNP, LNP and BLANK group and challenged with 1×102 T. gondii RH tachyzoites, n = 10/group. ***P< 0.001.
Although sera from TG_200 mRNA-LNP vaccinated mice have antibodies that inhibit T. gondii reproduction, the protection offered by the immunized sera is limited. Figure 9B shows that the survival of mice treated with adoptively transferred serum from vaccinated mice was increased after challenge with 1 × 102 T. gondii RH compared with that of the mice treated with serum from control mice (9.70 ± 1.64 days, P< 0.001).
Splenocytes (5 × 107) from TG_200 mRNA-LNP inoculated mice were transferred successively to mice, and the survival of the successively transferred mice was monitored after challenge with 1 × 102 T. gondii RH tachyzoites. Figure 9C shows that compared with the mice receiving LNP or BLANK controls, the survival time of splenocyte-immunized mice was prolonged (13.40 ± 2.32 days, P< 0.001).
T. gondii can infect all warm-blooded animals. Human infections are acquired from contaminated food or water, and it is estimated that this parasite persists chronically in 25–30% of the global human population. Zoonotic parasitic diseases cause serious public health problems and threaten economies and productivity. To date, combination therapy with pyrimethamine and sulfadiazine has been used to treat toxoplasmosis; however, these drugs have unavoidable side effects. Therefore, researchers have sought to develop T. gondii vaccines; however, although there are many types under research, few vaccines offer complete protection and there are problems related to their safety and efficacy. Therefore, a safe and effective T. gondii vaccine is urgently required. Compared with conventional vaccines, mRNA vaccines have the potential for high potency, rapid development, safe delivery, and low-cost manufacturing (23). Furthermore, successful mRNA vaccines have been clinically developed and licensed in a short time (for example, the COVID-19 mRNA vaccine) (24, 25). There are also many mRNA vaccines developed to prevent parasitic diseases, for example, the mRNA vaccine protection against pre-erythrocytic malaria, which shows good protection rates (26). In anti-T. gondii mRNA vaccine research, only one article has been published, which described a significantly enhanced protective effect of the vaccine (27). We studied the possibility of using new T. gondii molecule, TG_200, as an mRNA vaccine molecule to prevent toxoplasmosis in mice. The results showed that mice immunized with TG_200 mRNA-LNP generated cellular and humoral immunity, which prolonged the survival of mice challenged with T. gondii.
There are many T. gondii vaccine research platforms, and in comparison, mRNA vaccines theoretically avoid the risk of genetic recombination and are relatively safe, with little risk of misfolding recombinant proteins. In addition, independent cell expansion shortens the production time and is more conducive to preventing epidemic diseases (28). In this study, the survival time of the mice was prolonged during T. gondii challenge and appeared to be more effective compared with some other platform vaccines: epitope vaccines, recombinant protein vaccines, DNA vaccines and carbohydrate-based vaccines (29–33). However, comparisons between different laboratories are difficult due to the lack of standardized protocols for T. gondii vaccine studies (28). There are variations in immunization routes, animal models, dosages, and vaccine production processes.
Secretory proteins play an important role in host-pathogen interactions (19); therefore, the search for vaccine candidate molecules on the T. gondii database (ToxoDB, http://ToxoDB.org) focused on predicting T. gondii secretory proteins. After screening for genome-wide secreted proteins containing transmembrane structural domains and signal peptides, further T/B cell epitope prediction of the screened candidate molecules revealed that the TG_200 molecule had better predicted scores of T/B cell epitopes. Epitope prediction plays an important role in the analysis of peptide immunogenicity and plasmid vaccine construction (20, 21). The epitopes of many T. gondii vaccine molecules, such as MIC4 (34), SAG1 (35), and GRA24 (17), have been predicted using bioinformatics. Besides, adaptive immune responses are usually mediated by T and B cells to provide immune protection. In the present study, bioinformatic analysis of TG_200 predicted its potential as a vaccine. The DNASTAR software suggest that TG_200 has good surface probability, hydrophilicity, a high antigenic index, and more variable regions. Moreover, the T-cell epitopes of TG_200 were predicted to be capable of binding strongly to MHC II molecules. Bioinformatic analysis also showed that the percentile IC50 value was lower than that of SAG1 and the linear B-cell epitope score of the TG_200 peptide was higher, suggesting that TG_200 has promising potential for vaccine production.
Unfortunately, mRNA vaccines are easily degraded in vivo and have a short intracellular half-life. In addition, it is almost impossible for naked mRNA to cross the cell membrane successfully because of its physicochemical properties, such as the large size of the mRNA molecule and its dense negative charge (23). Thus, effective and appropriate delivery systems are needed to induce robust humoral and cellular immunity and prevent antigen degradation in mRNA vaccines (36). Many materials have been developed to deliver mRNA, including lipids, polymers, protein derivatives, and lipid-like materials (23, 37–40). Among them, as one of the most attractive and widely used delivery tools for mRNA, LNPs have been thoroughly investigated and successfully applied in the clinic (41). BNT162b21 (42) and mRNA-1273 (43) are two currently approved anti-coronavirus disease 2019 (COVID-19) vaccines that use LNPs to deliver antigenic mRNA. LNPs have been evaluated as effective and safe, representing a mature technology. Therefore, LNPs were chosen as the TG_200 mRNA vaccine delivery system. The encapsulation efficiency of the TG_200 mRNA vaccine in LNPs was about 95.67%. 293T cells were selected to test the expression of TG_200 mRNA in vitro. In clinical practice, most vaccines are administered by intramuscular injection and myocytes dominate muscle tissue. Therefore, C2C12 cells were chosen to detect the expression of TG_200 mRNA-LNP in muscle cells. The results show that the TG_200 protein is correctly expressed in cells (293T and C2C12) and is a secreted protein. And the protein might be taken up by cells and presented by the MHC-II molecule, which is mainly involved in the presentation of exogenous antigens and is usually expressed on antigen presenting cells (APCs) such as monocytes-macrophages, B cells, and DCs.
In the anti-T. gondii infection response, humoral immunity plays a critical role. Moreover, the B-cell response is important in infection by toxoplasmosis (44). Anti-T. gondii IgG antibodies control T. gondii infection by inhibiting T. gondii replication. IgG allows macrophages to kill intracellular parasites through the antibody-dependent cell-mediated cytotoxicity (ADCC) pathway by promoting parasite attachment to immune cells (45). In addition, the Th1 immune response plays an important role in resistance to T. gondii infection. One indicator of the Th1 immune response is the IgG2a level, while IgG1 is an indicator of the Th2 immune response. Levels of IgG2a and IgG1 antibodies were significantly increased in the TG_200 mRNA-LNP group. The IgG2a/IgG1 ratio also increased, indicating that TG_200 mRNA-LNP induced a mixed humoral Th1 and Th2 (Th1 dominated) immune response. This response is associated with increased protection against infection, which is similar to DNA vaccines, such as that of TgDOC2C (46), GRA7 (47), GRA24 (17), and other antigens.
The production of Th1-type cytokines IFN-γ and IL-12 mediates Th1 cell activity against intracellular pathogens (including T. gondii), representing the main anti-infection effector (48). IL-12 and IFN-γ expression levels in the splenocyte culture supernatant of TG_200 mRNA-LNP immunized mice were significantly higher than those of the control group (P< 0.001). IL-12 can induces IFN-γ release and Th1 cell differentiation, and thus control T. gondii infection. Blocking IL-12 leads to a weak Th1 immune response and promotes T. gondii growth and transmission (49). IFN-γ is secreted by NK cells through a toll-like receptor-myeloid differentiation factor 88 (MyD88)-dependent signaling pathway that is synergistically triggered by T. gondii antigen and IL-12 (50). IFN-γ is important for limiting the growth of tachyzoites in the early stages of infection, inhibiting the reactivation of dormant T. gondii cysts (51), inhibiting the growth and reproduction of T. gondii through the degradation of amino acids (tryptophan, L-arginine) essential for growth, and by inducing immune-related GTPases (IRGs), which can destroy vacuoles and eliminate parasites by lysosome-mediated degradation (48, 52). Immunization with the vaccine also significantly induced Th2-type cytokines, such as IL-10 and IL-4 (P< 0.001). Immunopathology can also be fatal during acute T. gondii infection, and the accompanying IL-10 and IL-4 responses can prevent an intense inflammatory response by suppressing systemic Th1 cytokine production and preventing the development of immunopathology (48). Increased inflammatory cell infiltration and intense necrosis were found in KO IL-10 mice, and their mortality was associated with enhanced liver pathology (53). IFN-γ performs an essential role in the early stages of T. gondii infection, and IL-4 is generally antagonistic to IFN-γ. During acute and chronic infection with T. gondii, IL-4 is necessary to suppress severe immunopathology. IL-4 has is also important in the late stages of infection, in which it can promote IFN-γ production. KO IL-4 mice lead to increased susceptibility to severe T. gondii encephalitis, of impaired IFN-γ production (52). In our experiments, Th1 and Th2 immune cells were stimulated to produce effective protection, prevent severe immunopathology caused by killing pathogens, and control host immune responses. There are some vaccine candidates whose results are consistent with our results: GRA24-based DNA Vaccine (17), MYR1-based DNA Vaccine (54). However, some vaccine candidates showed that they only produce a Th1-type immune response to control pathogenic infections: the GRA7-based DNA vaccines (47), and TgMIC5 and TgMIC16-based DNA vaccines (29).
DCs can produce type I cytokines and IFN, which are important in innate immunity. An essential checkpoint, CD83, is often expressed on the surface of mature DCs. It plays an important role in the regulation of immunity and the induction of inflammation regression (55). CD86 binds to CD28 molecules to provide co-stimulatory signals from T cells, thereby lowering their naive activation threshold (56). In the current study, significant enhancement of CD83 and CD86 molecules was observed in animals immunized with TG_200 mRNA-LNP vaccine, suggesting the promotion of DC maturation and increased expression of co-stimulatory molecules. Previous studies indicated that T. gondii promotes DC maturation by detecting its marker molecules, such as CD86 (57). Mature DCs can produce MHC-II molecules that present antigens and activate CD4+ T cells. This study observed significantly high levels of MHC-II molecules in mice immunized with TG_200 mRNA-LNP, suggesting an important role in antigen presentation. MHC-I molecules are often expressed in nucleated cells, and are essential for endogenous antigen presentation, leading to CD8+ T cell activation (58). The present study indicated that antigen presentation is induced in a mainly MHC-II, and slightly MHC-I, dependent manner. This suggests that TG_200 mRNA-LNP promotes DC maturation. Resistance to T. gondii is dominated by innate immunity based on NK cells and adaptive immunity based on CD4+ T lymphocytes. Activated CD4+ T lymphocytes can activate macrophages, and then produce cytokines that are recruited to the infection site (59). In addition, activated CD8+ T cells differentiate into CTLs, exhibiting immunotoxicity against the parasite (60). In the present study, a significant increase in CD4+ and CD8+ T lymphocytes compared with that in the control group suggested that TG_200 mRNA-LNP plays an important role in the induction of CD4+ and CD8+ T lymphocytes against toxoplasmosis. Studies of nano vaccines ribosomal P2 (61) and GRA7 (17) of T. gondii showed a significant increase in CD4+ and CD8+ T lymphocytes, similar to this study. In addition, mice immunized with TG_200 mRNA-LNP were found to have higher CTL activity than the controls. Our results indicated that TG_200 mRNA-LNP induced a pathogen-specific CTL response and an effective immune response to intracellular pathogens. These results are similar to those reported for the GRA24-based DNA Vaccine (17) and the MYR1-based DNA Vaccine (21).
IFN-γ can stimulate innate immune cells to upregulate immunity-related GTPases, such as p65 guanylate-binding protein (Gbp) family genes. p65, also known as RelA, is one of the five components of NF-κB. NF-κB is a transcription factor and enhancer of activated B cells. Host NF-κB plays a key role in blocking T. gondii-mediated apoptosis, and this inhibition is lost in p65 KO cells (62). This indicated that p65 is an important component of NF-κB, which plays an important role in the control of T. gondii infection. IRF family proteins activate DCs to produce IFN and cytokines (IL-10 and IL-12) (63). IL-12 is also critical to the control of T. gondii infection. T. gondii profilin can induce IL-12 through MyD88 and IRF8-dependent signaling pathways, and IRF8 is a critical transcription factor that regulates IL-12 activation of downstream TLR11 and MYD88 expression. Failure to upregulate IRF8 in DCs leads to acute susceptibility to T. gondii infection (62). T-bet plays a key role in the Th1/Th2 transition. It can specifically regulate Th0 differentiation and is selectively expressed in Th1 cells. Innate IFN-γ, which is T-bet dependent, is critical for the induction of IRF8. Thus, IRF8, T-bet, and p65 molecules have a crucial role in the immunoprotective response to T. gondii; therefore, we assessed their expression levels after vaccination. The spleen lymphocytes of TG_200 mRNA-LNP immunized mice showed a significant increase in IRF8, T-bet, and p65 expression. The results suggested that TG_200 mRNA-LNP can induce an immune response against T. gondii infection. The promotion of IFN-γ production is also mediated through activation of the IRF8 pathway, T-bet, and NF-κB pathways (64, 65), which is consistent with our results.
After T. gondii challenge, the survival of vaccinated mice was assessed. Survival time is a direct and credible indicator of the safety and efficacy of candidate T. gondii vaccines. We evaluated the survival of vaccinated mice by intraperitoneal infection with the RH strain of T. gondii two weeks after the last immunization with TG_200 mRNA-LNP. Compared with that of the controls, the survival time of the mice immunized with TG_200 mRNA-LNP increased significantly (19.27 ± 3.438 days, P< 0.001), although after infection, the mice did not survive. Thus, after infection with highly virulent T. gondii, the TG_200 mRNA-LNP vaccine induces better partial protection, although it does not induce complete protection; however, its induced survival time was better than some other T. gondii vaccines. GRA14-based DNA vaccination induced high levels of IFN-γ, total IgG and IgG2a, and Th1-type dominant humoral cellular immune responses. However, although BALB/c mice challenged with a lethal dose of RH strain tachyzoites had the longest survival time, it was only up to 17 days (66). The survival time of mice immunized with recombinant calcium-dependent protein kinases (rTgCDPK1) was significantly prolonged (14.90 ± 2.89 days) with a higher level of IgG antibodies and significantly increased levels of Th1-type cytokines (67). The survival time of BALB/c mice injected with pVAX-rhoptry proteins 21 (TgROP21) was also significantly prolonged (13.50 ± 1.65 days) (68). Survival time was also analyzed using immunized mice sera and splenocytes to assess the protective effect of the vaccine. Herein, after challenge with 1 × 102 RH tachyzoites, the mice survival time was prolonged when accepting immunized mice splenocytes (13.40 ± 2.32 days). After receiving transfer of CD8+ or CD4+ splenocytes from Hsp70-immunized mice, the T. gondii load was limited in the acute and chronic phases of toxoplasmosis (69). Compared with the control group, adoptive transfer of the splenocytes of KO tyrosine kinase (KO tkl1) immunized mice enhanced the survival time (70). The results indicated that CD8+ and CD4+ CTL are functional effectors of host resistance to T. gondii. Successive splenocyte transfers might better control disease recurrence and are important to develop better immunotherapies against T. gondii. The mice challenge with 1×102 RH tachyzoites also showed a prolonged survival time when accepting immunized mice serum daily for five days (9.70 ± 1.64 days, P< 0.001). A study also indicated that the survival time of mice accepting serum from KO tkll immunized mice was increased compared with the controls (70). For better immune protection against T. gondii infection, appropriate adjuvants might be required or the vaccines might be administered in combination with other important molecules of T. gondii. It has been shown that the protection produced by multiple proteins or epitopes is better than that by single proteins or epitopes (29, 71, 72).
In this study, a mRNA vaccine based on a novel T. gondii molecule-TG_200 was successfully constructed and BABL/C mice were used as the experimental model to assess immune protection through humoral, cellular immunity, and survival rates induced by the vaccine. The TG_200 mRNA-LNP vaccine elicits high levels of IL-12, IFN-γ, IL-10, IL-4, and increased IgG2a/IgG1 ratios. The results indicate that the novel vaccine can elicit Th1 and Th2 immune responses, with Th1 immunity being predominant. Furthermore, under immunization by the TG_200 mRNA-LNP vaccine, the ability to increase lymphocyte proliferation was significantly enhanced. After T. gondii challenge, mice immunized with the TG_200 mRNA-LNP vaccine had a significantly prolonged survival time. After receiving serum and splenocytes from immunized mice, the survival time was also significantly prolonged. Thus, TG_200 mRNA-LNP might be a promising vaccine against T. gondii infection after adjustment of the adjuvant or combination with other T. gondii critical molecules.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The animal study was reviewed and approved by Chinese legislation on the use and care of laboratory animals (GB/T35823-2018). Hangzhou Medical College Institutional Animal Care and Use Committee (No: 2021-152).
BZ and SHL developed the study protocol. YZ, DL, YS, and SYL did the experiments. YZ, DL, and YS analyzed the data and wrote the paper. BZ and SHL revised the paper. All authors contributed to the article and approved the submitted version.
This work was supported by grants from the Zhejiang Medical and Health Science and Technology Plan (2020KY102), the Scientific Research Project of Zhejiang Provincial Department of Education (Y202146047), the Special Funding Program in Hangzhou Medical College (YS2021003), the Provincial Key R & D program of Zhejiang Department of Science and Technology (2019C03057), the Central Leading Local Science and Technology Development Fund Project (2023ZY1019), and Key Discipline of Zhejiang Province in Public Health and Preventive Medicine (First Class, Category A), Hangzhou Medical College.
We would like to thank the native English speaking scientists of Elixigen Company (Huntington Beach, California) for editing our manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1161507/full#supplementary-material
1. Robert-Gangneux F, Dardé ML. Epidemiology of and diagnostic strategies for toxoplasmosis. Clin Microbiol Rev (2012) 25(2):264–96. doi: 10.1128/CMR.05013-11
2. Montoya JG, Liesenfeld O. Toxoplasmosis. Lancet (2004) 363(9425):1965–76. doi: 10.1016/S0140-6736(04)16412-X
3. Xiao J, Prandovszky E, Kannan G, Pletnikov MV, Dickerson F, Severance EG, et al. Toxoplasma gondii: biological parameters of the connection to schizophrenia. Schizophr bulletin. (2018) 44(5):983–92. doi: 10.1093/schbul/sby082
4. Schlüter D, Barragan A. Advances and challenges in understanding cerebral toxoplasmosis. Front Immunol (2019) 10:242. doi: 10.3389/fimmu.2019.00242
5. Smith JR, Ashander LM, Arruda SL, Cordeiro CA, Lie S, Rochet E, et al. Pathogenesis of ocular toxoplasmosis. Prog retinal eye Res (2021) 81:100882. doi: 10.1016/j.preteyeres.2020.100882
6. Milne G, Webster JP, Walker M. Toxoplasma gondii: AnUnderestimated Threat?. Trends Parasitol (2020) 36(12):959–69. doi: 10.1016/j.pt.2020.08.005
7. Lapinskas PJ, Ben-Harari RR. Perspective on current and emerging drugs in the treatment of acute and chronic toxoplasmosis. Postgraduate Med (2019) 131(8):589–96. doi: 10.1080/00325481.2019.1655258
8. Dunay IR, Gajurel K, Dhakal R, Liesenfeld O, Montoya JG. Treatment of toxoplasmosis: historical perspective, animal models, and current clinical practice. Clin Microbiol Rev (2018) 31(4):00057–17. doi: 10.1128/CMR.00057-17
9. Kamolratanakul S, Pitisuttithum P. Human papillomavirus vaccine efficacy and effectiveness against cancer. Vaccines (Basel). (2021) 9(12):1413–34. doi: 10.3390/vaccines9121413
10. Lamb YN. BNT162b2 mRNA COVID-19 vaccine: first approval. Drugs (2021) 81(4):495–501. doi: 10.1007/s40265-021-01480-7
11. Buxton D, Innes EA. A commercial vaccine for ovine toxoplasmosis. Parasitology (1995) 110 Suppl:S11–6. doi: 10.1017/S003118200000144X
12. Wang JL, Zhang NZ, Li TT, He JJ, Elsheikha HM, Zhu XQ. Advances in the development of anti-toxoplasma gondii vaccines: challenges, opportunities, and perspectives. Trends Parasitol (2019) 35(3):239–53. doi: 10.1016/j.pt.2019.01.005
13. Chu KB, Quan FS. Advances in toxoplasma gondii vaccines: current strategies and challenges for vaccine development. Vaccines (Basel). (2021) 9(5):413–29. doi: 10.3390/vaccines9050413
14. Kutzler MA, Weiner DB. DNA Vaccines: ready for prime time? Nat Rev Genet (2008) 9(10):776–88. doi: 10.1038/nrg2432
15. Pardi N, Hogan MJ, Weissman D. Recent advances in mRNA vaccine technology. Curr Opin Immunol (2020) 65:14–20. doi: 10.1016/j.coi.2020.01.008
16. Pilkington EH, Suys EJA, Trevaskis NL, Wheatley AK, Zukancic D, Algarni A, et al. From influenza to COVID-19: lipid nanoparticle mRNA vaccines at the frontiers of infectious diseases. Acta biomaterialia. (2021) 131:16–40. doi: 10.1016/j.actbio.2021.06.023
17. Zheng B, Lou D, Ding J, Zhuo X, Ding H, Kong Q, et al. GRA24-based DNA vaccine prolongs survival in mice challenged with a virulent toxoplasma gondii strain. Front Immunol (2019) 10:418. doi: 10.3389/fimmu.2019.00418
18. Mamaghani AJ, Fathollahi A, Arab-Mazar Z, Kohansal K, Fathollahi M, Spotin A, et al. Toxoplasma gondii vaccine candidates: a concise review. Irish J Med sci (2023) 192(1):231–61. doi: 10.1007/s11845-022-02998-9
19. Xu X, Zhang Y, Lin D, Zhang J, Xu J, Liu YM, et al. Serodiagnosis of schistosoma japonicum infection: genome-wide identification of a protein marker, and assessment of its diagnostic validity in a field study in China. Lancet Infect diseases. (2014) 14(6):489–97. doi: 10.1016/S1473-3099(14)70067-2
20. Zhou J, Wang L, Lu G, Zhou A, Zhu M, Li Q, et al. Epitope analysis and protection by a ROP19 DNA vaccine against toxoplasma gondii. Parasite (2016) 23:17. doi: 10.1051/parasite/2016017
21. Zhou J, Lu G, He S. Analysis of structures and epitopes of a novel secreted protein MYR1 in toxoplasma gondii. Folia parasitol (2016) 63:028–32. doi: 10.14411/fp.2016.028
22. Pagheh AS, Sarvi S, Sharif M, Rezaei F, Ahmadpour E, Dodangeh S, et al. Toxoplasma gondii surface antigen 1 (SAG1) as a potential candidate to develop vaccine against toxoplasmosis: a systematic review. Comp Immunol Microbiol Infect Dis (2020) 69:101414. doi: 10.1016/j.cimid.2020.101414
23. Le T, Sun C, Chang J, Zhang G, Yin X. mRNA vaccine development for emerging animal and zoonotic diseases. Viruses (2022) 14(2):401–20. doi: 10.3390/v14020401
24. Teijaro JR, Farber DL. COVID-19 vaccines: modes of immune activation and future challenges. Nat Rev Immunol (2021) 21(4):195–7. doi: 10.1038/s41577-021-00526-x
25. Sharif N, Alzahrani KJ, Ahmed SN, Dey SK. Efficacy, immunogenicity and safety of COVID-19 vaccines: a systematic review and meta-analysis. Front Immunol (2021) 12:714170. doi: 10.3389/fimmu.2021.714170
26. Mallory KL, Taylor JA, Zou X, Waghela IN, Schneider CG, Sibilo MQ, et al. Messenger RNA expressing PfCSP induces functional, protective immune responses against malaria in mice. NPJ Vaccines (2021) 6(1):84. doi: 10.1038/s41541-021-00345-0
27. Luo F, Zheng L, Hu Y, Liu S, Wang Y, Xiong Z, et al. Induction of protective immunity against toxoplasma gondii in mice by nucleoside triphosphate hydrolase-II (NTPase-II) self-amplifying RNA vaccine encapsulated in lipid nanoparticle (LNP). Front Microbiol (2017) 8:605. doi: 10.3389/fmicb.2017.00605
28. Zhang Y, Li D, Lu S, Zheng B. Toxoplasmosis vaccines: what we have and where to go? NPJ Vaccines (2022) 7(1):131. doi: 10.1038/s41541-022-00563-0
29. Zhu YC, Ma LJ, Zhang JL, Liu JF, He Y, Feng JY, et al. Protective immunity induced by TgMIC5 and TgMIC16 DNA vaccines against toxoplasmosis. Front Cell Infect Microbiol (2021) 11:686004. doi: 10.3389/fcimb.2021.686004
30. Zhang NZ, Xu Y, Wang M, Petersen E, Chen J, Huang SY, et al. Protective efficacy of two novel DNA vaccines expressing toxoplasma gondii rhomboid 4 and rhomboid 5 proteins against acute and chronic toxoplasmosis in mice. Expert Rev Vaccines (2015) 14(9):1289–97. doi: 10.1586/14760584.2015.1061938
31. Foroutan M, Barati M, Ghaffarifar F. Enhancing immune responses by a novel multi-epitope ROP8 DNA vaccine plus interleukin-12 plasmid as a genetic adjuvant against acute toxoplasma gondii infection in BALB/c mice. Microb Pathog (2020) 147:104435. doi: 10.1016/j.micpath.2020.104435
32. Guo J, Sun X, Yin H, Wang T, Li Y, Zhou C, et al. Chitosan microsphere used as an effective system to deliver a linked antigenic peptides vaccine protect mice against acute and chronic toxoplasmosis. Front Cell Infect Microbiol (2018) 8:163. doi: 10.3389/fcimb.2018.00163
33. Götze S, Reinhardt A, Geissner A, Azzouz N, Tsai YH, Kurucz R, et al. Investigation of the protective properties of glycosylphosphatidylinositol-based vaccine candidates in a toxoplasma gondii mouse challenge model. Glycobiology (2015) 25(9):984–91. doi: 10.1093/glycob/cwv040
34. Ghaffari AD, Dalimi A, Ghaffarifar F, Pirestani M, Majidiani H. Immunoinformatic analysis of immunogenic b- and T-cell epitopes of MIC4 protein to designing a vaccine candidate against toxoplasma gondii through an in-silico approach. Clin Exp Vaccine Res (2021) 10(1):59–77. doi: 10.7774/cevr.2021.10.1.59
35. Wang Y, Wang G, Zhang D, Yin H, Wang M. Screening and identification of novel b cell epitopes of toxoplasma gondii SAG1. Parasit Vectors. (2013) 6:125. doi: 10.1186/1756-3305-6-125
36. Zhang NZ, Wang M, Xu Y, Petersen E, Zhu XQ. Recent advances in developing vaccines against toxoplasma gondii: an update. Expert Rev Vaccines (2015) 14(12):1609–21. doi: 10.1586/14760584.2015.1098539
37. Li B, Zhang X, Dong Y. Nanoscale platforms for messenger RNA delivery. Wiley Interdiscip Rev Nanomed nanobiotechnol (2019) 11(2):e1530. doi: 10.1002/wnan.1530
38. Guan S, Rosenecker J. Nanotechnologies in delivery of mRNA therapeutics using nonviral vector-based delivery systems. Gene Ther (2017) 24(3):133–43. doi: 10.1038/gt.2017.5
39. Kowalski PS, Rudra A, Miao L, Anderson DG. Delivering the messenger: advances in technologies for therapeutic mRNA delivery. Mol Ther (2019) 27(4):710–28. doi: 10.1016/j.ymthe.2019.02.012
40. Kim J, Eygeris Y, Gupta M, Sahay G. Self-assembled mRNA vaccines. Advanced Drug delivery Rev (2021) 170:83–112. doi: 10.1016/j.addr.2020.12.014
41. Akinc A, Maier MA, Manoharan M, Fitzgerald K, Jayaraman M, Barros S, et al. The onpattro story and the clinical translation of nanomedicines containing nucleic acid-based drugs. Nat nanotechnol (2019) 14(12):1084–7. doi: 10.1038/s41565-019-0591-y
42. Vergnes JN. Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine. N Engl J Med (2021) 384(16):1577. doi: 10.1056/NEJMc2036242
43. Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med (2021) 384(5):403–16. doi: 10.1056/NEJMoa2035389
44. Zhou J, Wang L. SAG4 DNA and peptide vaccination provides partial protection against t. gondii infection in BALB/c mice. Front Microbiol (2017) 8:1733. doi: 10.3389/fmicb.2017.01733
45. Pifer R, Yarovinsky F. Innate responses to toxoplasma gondii in mice and humans. Trends Parasitol (2011) 27(9):388–93. doi: 10.1016/j.pt.2011.03.009
46. Zhang NZ, Gao Q, Wang M, Hou JL, Zhang FK, Hu LY, et al. Protective efficacy against acute and chronic toxoplasma gondii infection induced by immunization with the DNA vaccine TgDOC2C. Front Microbiol (2018) 9:2965. doi: 10.3389/fmicb.2018.02965
47. Sun HC, Huang J, Fu Y, Hao LL, Liu X, Shi TY. Enhancing immune responses to a DNA vaccine encoding toxoplasma gondii GRA7 using calcium phosphate nanoparticles as an adjuvant. Front Cell Infect Microbiol (2021) 11:787635. doi: 10.3389/fcimb.2021.787635
48. Dupont CD, Christian DA, Hunter CA. Immune response and immunopathology during toxoplasmosis. Semin immunopathol (2012) 34(6):793–813. doi: 10.1007/s00281-012-0339-3
49. Morgado P, Sudarshana DM, Gov L, Harker KS, Lam T, Casali P, et al. Type II toxoplasma gondii induction of CD40 on infected macrophages enhances interleukin-12 responses. Infect Immun (2014) 82(10):4047–55. doi: 10.1128/IAI.01615-14
50. Ge Y, Chen J, Qiu X, Zhang J, Cui L, Qi Y, et al. Natural killer cell intrinsic toll-like receptor MyD88 signaling contributes to IL-12-dependent IFN-γ production by mice during infection with toxoplasma gondii. Int J parasitol (2014) 44(7):475–84. doi: 10.1016/j.ijpara.2014.03.004
51. MacMicking JD. Interferon-inducible effector mechanisms in cell-autonomous immunity. Nat Rev Immunol (2012) 12(5):367–82. doi: 10.1038/nri3210
52. Suzuki Y, Sa Q, Gehman M, Ochiai E. Interferon-gamma- and perforin-mediated immune responses for resistance against toxoplasma gondii in the brain. Expert Rev Mol Med (2011) 13:e31. doi: 10.1017/S1462399411002018
53. Gazzinelli RT, Wysocka M, Hieny S, Scharton-Kersten T, Cheever A, Kühn R, et al. In the absence of endogenous IL-10, mice acutely infected with toxoplasma gondii succumb to a lethal immune response dependent on CD4+ T cells and accompanied by overproduction of IL-12, IFN-gamma and TNF-alpha. J Immunol (1996) 157(2):798–805. doi: 10.4049/jimmunol.157.2.798
54. Zheng B, Ding J, Lou D, Tong Q, Zhuo X, Ding H, et al. The virulence-related MYR1 protein of toxoplasma gondii as a novel DNA vaccine against toxoplasmosis in mice. Front Microbiol (2019) 10:734. doi: 10.3389/fmicb.2019.00734
55. Grosche L, Knippertz I, König C, Royzman D, Wild AB, Zinser E, et al. The CD83 molecule - an important immune checkpoint. Front Immunol (2020) 11:721. doi: 10.3389/fimmu.2020.00721
56. Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol (2005) 23:515–48. doi: 10.1146/annurev.immunol.23.021704.115611
57. Aosai F, Rodriguez Pena MS, Mun HS, Fang H, Mitsunaga T, Norose K, et al. Toxoplasma gondii-derived heat shock protein 70 stimulates maturation of murine bone marrow-derived dendritic cells via toll-like receptor 4. Cell Stress chaperones. (2006) 11(1):13–22. doi: 10.1379/CSC-138R.1
58. Lyons RE, Anthony JP, Ferguson DJ, Byrne N, Alexander J, Roberts F, et al. Immunological studies of chronic ocular toxoplasmosis: up-regulation of major histocompatibility complex class I and transforming growth factor beta and a protective role for interleukin-6. Infect Immun (2001) 69(4):2589–95. doi: 10.1128/IAI.69.4.2589-2595.2001
59. Tubo NJ, Jenkins MK. CD4+ T cells: guardians of the phagosome. Clin Microbiol Rev (2014) 27(2):200–13. doi: 10.1128/CMR.00097-13
60. Halle S, Halle O, Förster R. Mechanisms and dynamics of T cell-mediated cytotoxicity in vivo. Trends Immunol (2017) 38(6):432–43. doi: 10.1016/j.it.2017.04.002
61. Yu Z, He K, Cao W, Aleem MT, Yan R, Xu L, et al. Nano vaccines for t. gondii ribosomal P2 protein with nanomaterials as a promising DNA vaccine against toxoplasmosis. Front Immunol (2022) 13:839489. doi: 10.3389/fimmu.2022.839489
62. Molestina RE, Payne TM, Coppens I, Sinai AP. Activation of NF-kappaB by toxoplasma gondii correlates with increased expression of antiapoptotic genes and localization of phosphorylated IkappaB to the parasitophorous vacuole membrane. J Cell Sci (2003) 116(Pt 21):4359–71. doi: 10.1242/jcs.00683
63. Tailor P, Tamura T, Ozato K. IRF family proteins and type I interferon induction in dendritic cells. Cell Res (2006) 16(2):134–40. doi: 10.1038/sj.cr.7310018
64. Zhu J, Jankovic D, Oler AJ, Wei G, Sharma S, Hu G, et al. The transcription factor T-bet is induced by multiple pathways and prevents an endogenous Th2 cell program during Th1 cell responses. Immunity (2012) 37(4):660–73. doi: 10.1016/j.immuni.2012.09.007
65. López-Yglesias AH, Burger E, Camanzo E, Martin AT, Araujo AM, Kwok SF, et al. T-Bet-dependent ILC1- and NK cell-derived IFN-γ mediates cDC1-dependent host resistance against toxoplasma gondii. PloS Pathog (2021) 17(1):e1008299. doi: 10.1371/journal.ppat.1008299
66. Pagheh AS, Sarvi S, Gholami S, Asgarian-Omran H, Valadan R, Hassannia H, et al. Protective efficacy induced by DNA prime and recombinant protein boost vaccination with toxoplasma gondii GRA14 in mice. Microb Pathog (2019) 134:103601. doi: 10.1016/j.micpath.2019.103601
67. Huang SY, Chen K, Wang JL, Yang B, Zhu XQ. Evaluation of protective immunity induced by recombinant calcium-dependent protein kinase 1 (TgCDPK1) protein against acute toxoplasmosis in mice. Microb Pathog (2019) 133:103560. doi: 10.1016/j.micpath.2019.103560
68. Zhang Z, Li Y, Wang M, Xie Q, Li P, Zuo S, et al. Immune protection of rhoptry protein 21 (ROP21) of toxoplasma gondii as a DNA vaccine against toxoplasmosis. Front Microbiol (2018) 9:909. doi: 10.3389/fmicb.2018.00909
69. Chu D, Moroda M, Piao LX, Aosai F. CTL induction by DNA vaccine with toxoplasma gondii-HSP70 gene. Parasitol Int (2014) 63(2):408–16. doi: 10.1016/j.parint.2014.01.002
70. Wang JL, Liang QL, Li TT, He JJ, Bai MJ, Cao XZ, et al. Toxoplasma gondii tkl1 deletion mutant is a promising vaccine against acute, chronic, and congenital toxoplasmosis in mice. J Immunol (2020) 204(6):1562–70. doi: 10.4049/jimmunol.1900410
71. El Bissati K, Chentoufi AA, Krishack PA, Zhou Y, Woods S, Dubey JP, et al. Adjuvanted multi-epitope vaccines protect HLA-A*11:01 transgenic mice against toxoplasma gondii. JCI Insight (2016) 1(15):e85955. doi: 10.1172/jci.insight.85955
72. Dodangeh S, Fasihi-Ramandi M, Daryani A, Valadan R, Asgarian-Omran H, Hosseininejad Z, et al. Protective efficacy by a novel multi-epitope vaccine, including MIC3, ROP8, and SAG1, against acute toxoplasma gondii infection in BALB/c mice. Microb Pathog (2021) 153:104764. doi: 10.1016/j.micpath.2021.104764
Keywords: Toxoplasma gondii, vaccine, mRNA, lipid nanoparticle, immune response
Citation: Zhang Y, Li D, Shen Y, Li S, Lu S and Zheng B (2023) Immunization with a novel mRNA vaccine, TGGT1_216200 mRNA-LNP, prolongs survival time in BALB/c mice against acute toxoplasmosis. Front. Immunol. 14:1161507. doi: 10.3389/fimmu.2023.1161507
Received: 08 February 2023; Accepted: 31 March 2023;
Published: 14 April 2023.
Edited by:
Ann E. Sluder, Massachusetts General Hospital and Harvard Medical School, United StatesReviewed by:
Ramaswamy Kalyanasundaram, University of Illinois at Chicago, United StatesCopyright © 2023 Zhang, Li, Shen, Li, Lu and Zheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shaohong Lu, bGxzc2hoMjAwM0AxNjMuY29t; Bin Zheng, YmluX3poZW5nQGZveG1haWwuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.