- 1Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS), MultiMedica, Milan, Italy
- 2Department of Biotechnology and Life Sciences, University of Insubria, Varese, Italy
- 3Translational Research Unit, National Institute for Infectious Diseases Lazzaro Spallanzani- Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS), Rome, Italy
- 4Stazione Zoologica Anton Dohrn, Istituto Nazionale di Biologia, Ecologia e Biotecnologie Marine, Napoli, Italy
- 5Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) European Institute of Oncology IEO-, Milan, Italy
Interleukin-10 (IL-10) is a pleiotropic cytokine that has a fundamental role in modulating inflammation and in maintaining cell homeostasis. It primarily acts as an anti-inflammatory cytokine, protecting the body from an uncontrolled immune response, mostly through the Jak1/Tyk2 and STAT3 signaling pathway. On the other hand, IL-10 can also have immunostimulating functions under certain conditions. Given the pivotal role of IL-10 in immune modulation, this cytokine could have relevant implications in pathologies characterized by hyperinflammatory state, such as cancer, or infectious diseases as in the case of COVID-19 and Post-COVID-19 syndrome. Recent evidence proposed IL-10 as a predictor of severity and mortality for patients with acute or post-acute SARS-CoV-2 infection. In this context, IL-10 can act as an endogenous danger signal, released by tissues undergoing damage in an attempt to protect the organism from harmful hyperinflammation. Pharmacological strategies aimed to potentiate or restore IL-10 immunomodulatory action may represent novel promising avenues to counteract cytokine storm arising from hyperinflammation and effectively mitigate severe complications. Natural bioactive compounds, derived from terrestrial or marine photosynthetic organisms and able to increase IL-10 expression, could represent a useful prevention strategy to curb inflammation through IL-10 elevation and will be discussed here. However, the multifaceted nature of IL-10 has to be taken into account in the attempts to modulate its levels.
Introduction
Cytokines are a broad category of soluble proteins or glycoproteins with low molecular weight (ranging from 6 to 70 kDa) that are produced transiently, in response to various biological stimuli, by nearly every cell type and affecting virtually all main cellular processes (1–3). These molecules are crucial to orchestrate cell-to-cell communication and biological functions (1–3). They can act locally, either via autocrine and paracrine signaling, respectively on the same cells that produce them or on cells close to the site of release. Several cytokines are also capable of acting systemically, via endocrine signaling, on cells located in other body districts reached through the blood or lymphatic stream (4). Cytokines act by binding to specific transmembrane and membrane-anchored receptors located on the target cells and activating downstream intracellular signaling cascades that usually result in gene expression modulation (5, 6). Some cytokine receptors also exist in soluble form and can function as either antagonists or agonists of cytokine signaling, forming decoy receptors or functional receptors, respectively (2, 6). Their activity is highly specific: the expression pattern of cytokine receptors is unique for every tissue and varies among different cell types, determining to which cytokine a particular cell/tissue will respond to (6). Cytokines also establish complex networks with each other, where one cytokine can potentiate/contrast the action or stimulate/inhibit the production of other cytokines (2).
Cytokines play a pivotal role in the regulation of many physiological processes, including cytoskeletal organization, stem cell differentiation, embryonic development, cell proliferation, activation, migration, wound healing, survival and apoptosis (6–9). They are also key regulators of the innate and adaptive immunity, coordinating humoral, cytotoxic and cellular immune response, mediating communication between immune and non-immune cells, controlling immune cell trafficking and tissue organization, affecting microenvironment and regulating inflammation (1, 10–12). Given the high pleiotropic activity of these molecules, cytokines are present at very low or undetectable concentrations in body fluids and tissues under homeostatic conditions, but, when required by the physiopathological context, they can rapidly increase up to 1000 fold (13).
Since cytokines have such a fundamental influence on immune system and body’s health, any dysregulation in their secretion or signaling, and they are critically implicated in the genesis and/or progression of several human pathological conditions, among which, cancer, infectious and immune diseases (1, 13, 14) they represent important biomarkers and targets.
A dysregulation of the immune system is a characteristic of the COronaVIrus Disease 2019 (COVID-19) global pandemic, caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (15–17). A growing body of clinical data suggests that the more severe and lethal forms of COVID-19 syndrome are associated with self-feeding massive release of pro-inflammatory cytokines, defined “cytokine storm” (18–20), which triggers and sustains a systemic hyper-activated inflammatory response that finally leads to acute respiratory distress syndrome (ARDS), multi-organ failure and even death (17, 21).
This COVID-19 pandemic has posed huge challenges to the health care system worldwide due to its widespread diffusion, the severity of clinical picture (17), and the numerous variants, with more than 765 million confirmed cases and over 6.9 million deaths reported globally (22). The rapid availability of vaccines has brought an enormous progress. Unfortunately, in case of infection, clinical interventions capable of effectively treating COVID-19 patients entering the cytokine storm phase have demonstrated a highly variable margin of success depending on timing and patient selection (23). In addition, a considerable percentage of patients who had mild or severe disease do not fully recover but continue to manifest a range of persistent debilitating multi-organ symptoms for weeks or even months after the acute infection. These conditions have been reported in literature as “long COVID”, “post-COVID-19 syndrome”, “post-acute sequelae of COVID-19 (PACS)” or “post-acute COVID-19 syndrome” and they still leave many open questions about the pathogenetic mechanisms and potential therapeutic approaches (24–28). Although the vaccines have brought substantial benefits to halt the COVID-19 pandemic, both reducing rate of SARS-CoV-2 new infection and decreasing mortality and risk of serious complications, there is actually no proven effective treatment against post-COVID-19 and the real impact of vaccines on patients who have this syndrome remains still unclear (28–30). Therefore, a thorough understanding of the molecular mechanisms underlying COVID-19 and post-COVID-19 as well as the design of novel targeted therapeutic interventions remains a priority for biomedical research.
In light of the importance demonstrated by the “cytokine storm” in driving the immunopathological process of COVID-19, numerous therapeutic strategies capable to prevent/reduce the over-production of pro-inflammatory cytokines have been proposed to suppress/attenuate COVID-19 hyper-inflammation and ameliorate its severe complications (31–33). These pharmacological agents comprise non-specific immune modulators, such as corticosteroids, hydroxychloroquine, interferons, cardiovascular drugs, such as statins and renin–angiotensin–aldosterone system (RAAS) inhibitors, and specific immune modulators, such as Janus kinase (Jak) inhibitors, humanized anti-interleukin-6 (IL-6), anti-IL-1 receptor and anti-tumor necrosis factor alpha (TNF-α) monoclonal antibodies (34–41). Very likely, they can be valid therapeutic options also for managing post-COVID-19 complications (42).
In this scenario, a novel candidate that appears to be particularly worth to be exploited as effective anti-inflammatory molecule is IL-10, a cytokine that has gained increasing interest from clinical medicine in different therapeutic settings due to its potent immunomodulatory properties on a broad spectrum of cells (43–45).
Recent evidence has outlined IL-10 as associated with severity and mortality for patients with acute or post-acute SARS-CoV-2 infection (46). IL-10 can act like an endogenous “danger signal” released in response to the peak of circulating pro-inflammatory cytokines and having the purpose to protect the organism from damage caused by harmful hyperinflammatory state (43, 47).
IL-10 structure and signaling pathway
Human IL-10 is encoded by the IL-10 gene, located on the long arm of chromosome 1 (48). The IL-10 gene promoter is characterized by the presence of positive and negative regulatory sequences as well as polymorphisms that can significantly affect IL-10 expression between individuals (49, 50). IL-10 is a member of the class II cytokine family and its biologically active form is a soluble 36 kDa homodimer, comprising two monomers with six α-helices structure and stabilized by two intrachain disulfide bonds (51). The cellular response to IL-10 starts with the binding of an IL-10 homodimer to a heterotetrametric IL-10 receptor (IL-10R) complex, belonging to the interferon receptor family and comprised of two ligand-binding IL-10R-alpha (IL-10RA) subunits and two accessory signal-transducing IL-10R-beta (IL-10RB) subunits (52, 53). IL-10RA is the main responsible for directing ligand and target specificity: it recognizes IL-10 with high-affinity (50) and it is mainly expressed by lymphocytes, macrophages and dendritic cells at basal level, but can be upregulated by various cells upon their activation (54). Instead, IL-10RB has lower affinity or no direct binding to IL-10, is constitutively expressed by nearly all cell types and is shared by the receptor complex of other IL-10 family cytokines, such as IL-22 and IL-26 (50, 55). The signaling cascade in IL-10 responding cells is mediated by theJak1/Tyrosine kinase 2 (Tyk2)/signal transducer and activator of transcription 3 (STAT3) pathway. The binding of IL-10 homodimer to the IL-10RA extracellular domain leads to its oligomerization with the IL-10RB and the following phosphorylation of the enzymes Jak1 and Tyk2, associated with the intracellular domain of alpha and beta subunits, respectively. Upon their phosphorylation, these kinases further phosphorylate two functional tyrosine motifs on the intracellular domain of the IL-10RA. This allows the recruitment of STAT3 and its subsequent phosphorylation by Jak1 and Tyk2 (43, 50). Once phosphorylated, STAT3 dimerizes and translocates into the nucleus, where it binds to STAT-consensus elements of target gene promoters and initiates their transcriptional program (43, 50, 56). One of the actions of STAT3-responsive genes is the suppression of cytokine signaling 3 (SOCS-3), which inhibits mitogen-activated protein kinase (MAPK) activation, NF-κB nuclear translocation, and the resulting expression of pro-inflammatory genes. It also functions as a negative feedback regulator of IL-10 signaling, by inhibiting Jak1 and consequently the Jak1/Tyk2/STAT3 pathway. Another element induced by STAT3 is the IL-1 receptor antagonist (IL-1RN), a decoy protein that, binding to IL-1 receptor, prevents the interaction of IL-1β with its receptor and the following activation of pro-inflammatory signaling. Moreover, STAT3 suppresses STAT6 activation and consequently inhibits the expression of IL-4/IL-13-responsive genes in monocytes and dendritic cells (DCs) (50, 57–59).
In addition to STAT3, IL-10RA may simultaneously phosphorylate and activate STAT1 and STAT5 in monocytes and regulatory T (Treg) cells. By this action, it leads to the formation of different STAT heterocomplexes and to the subsequent generation of multiple downstream transcriptional effects (60). Furthermore, additionally to Jak1/Tyk2/STAT3 pathway, IL-10 may also modulate transcription by the activation of PI3K/Akt/Glycogen Synthase Kinase 3 Beta (GSK3β) and PI3K/Akt/mTORC1 signaling cascades in macrophages (43).
There is evidence of IL-10 and IL-10R deficiencies which are monogenic inborn errors of immunity (IEI) causing early-onset inflammatory bowel diseases (IBD) (61, 62). Consanguinity is reported in all evaluable patients with IL-10 deficiency and in 38% of patients with IL-10R deficiency (23% of patients with IL-10RA, and 79% of patients with IL-10RB deficiency). The common associated pathologies are auto-inflammation and enteropathy. Dermatological manifestations as well as lymphoma not Epstein Barr Virus (EBV)-related, and failure to thrive are associated with IL-10R deficiency (63, 64).
IL-10 cellular sources
When originally described by Fiorentino and colleagues in 1989, IL-10 was classified as a cytokine specifically secreted by T helper 2 (Th2) cells (65), however, it was subsequently widely recognized that it can be produced by many myeloid and lymphoid cells (50). Among these, CD4+ Th1, Th2 and Th17 cells, and Treg cells, DCs, monocytes and macrophages are main producers of IL-10 (43, 50). Recently, microglia and cardiac macrophages have been also identified as producers of IL-10 (66, 67).
In CD4+ Th cells, IL-10 production occurs downstream of T cell receptor (TCR) activation and the subsequent activation of Ras, ERK1/2 and transcription factor AP1 (43). In Th2 cells, IL-10 synthesis is induced by IL-4/STAT6 signaling and requires GATA binding protein 3 (GATA3) transcription factor (68). Th1 and Th17 can secrete IL-10 under the correct set of conditions. In Th1 cells, IL-10 production requires STAT4, strong TCR activation (i. e. increased expression of Delta-like-4 ligand and inducible T cell co-stimulator ligand (ICOSL) on DCs) and IL-12 (69).
In Th17 cells, IL-10 expression is induced by the cytokines IL-6 (70), IL-24 (71) and IL-27 (72), and it is mediated by STAT3 and, in some cases, STAT-1 signaling (73). Several transcription factors are involved in regulating IL-10 production in T cells, including Blimp-1, cMaf, AhR, Bhlhe40 (43, 50).
Natural and induced FoxP3+ Treg cells can secrete IL-10 in a STAT3 dependent manner and use it to control immune responses against self-antigens at the environmental interfaces (74). FoxP3- Treg cells secrete IL-10 following differentiation from naive CD4+ T cells under various stimuli, including cytokines, such as interferon gamma (INF-γ), immunosuppressive drugs, stimulation with soluble antigens or immature DCs and co-stimulation with CD2, CD46 or ICOSL (75).
In macrophages and DCs, IL-10 expression is regulated by cytokines, such as type I IFN, and by the activation, downstream of Toll-like receptor (TLR) signaling, of ERK1/2, p38, NF-κB and phosphoinositide-3-kinase (PI3K) serine/threonine protein kinase B (Akt) pathways (43, 50, 76).
In addition to CD4+ T cells, DCs and macrophages, most adaptive immunity cell types, including CD8+ cytotoxic T cells and B cells, as well as various innate immunity cell types, including mast cells, natural killer (NK) cells, and eosinophils can also be sources of IL-10 in particular contexts (77).
CD8+ T‐cells become significant producers of IL-10 during hypoxia and viral infection (78), in response to TCR activation, IL-21 stimulation or interaction with CD40 ligand on activated pDCs (75). Mast cells directly induce IL-10 expression following Toll-like receptor 4 (TLR4) activation by lipopolysaccharide (LPS) or during allergic dermatitis or skin damage (75). In B cells, IL-10 production occurs following stimulation with autoantigens, ligands of TLR4 and TLR9, or vitamin D3 (79), while NK cells release IL-10 during systemic infection (80). However, unlike other myeloid cells and in contrast to mouse neutrophils, human neutrophils are unable to produce or secrete IL-10, also after stimulation with bacterial and inflammatory molecules such as serum amyloid or LPS (81). Cassatella’s and Bazzoni’s labs showed that IL-10 induced transcriptional repression of CXCL8 and TNF-α genes in human monocytes pretreated with LPS (82). The inhibitory effect of IL-10 on cytokine transcription consists of two distinct sequential phases: an early phase, occurring rapidly and in a protein synthesis-independent manner, followed by a second delayed phase, that occurs after 60 minutes and is dependent on protein synthesis (82).
In addition, some non-immune cell types, including intestinal epithelial cells, intestinal fibroblasts and skin keratinocytes, produce IL-10 in response to certain stimuli, comprising infection, UV radiation, tissue injury and damage (83–86), and even different tumor cells, such as melanoma, breast and colon carcinoma cells, have demonstrated IL-10 secretion ability (87–90).
IL-10 systemic effects
IL-10 was initially defined as “cytokine synthesis inhibitory factor” due to its inhibitory activity on IL-2 and interferon-γ (IFN-γ) release by Th1 cells (65), however it is now commonly considered as a key immunoregulatory cytokine with pleiotropic activities, exerting multiple and sometimes even opposite effects on immune cells.
IL-10 functions as a double-edged sword on the immune system: on one hand it has emerged as a potent anti-inflammatory and immunosuppressive cytokine, on the other hand it can also have immunostimulatory properties (50, 91, 92). The different sources of IL-10 and the type of target cells on which it acts, as well as the site and timing of its secretion are critical features to activate multiple signal transduction pathways, each one contributing to different functions towards the inhibition or the activation of immune cells (79).
IL-10 is a master regulator of immunity during infection with viruses, bacteria, fungi, protozoa and other pathogens, playing a key, and often essential, role in limiting or terminating inflammation and in the consequent host protection. IL-10 production by innate immune cells generally occurs later compared to that of pro-inflammatory cytokines released in the early phase of the inflammatory process. IL-10 secreted at the site of ongoing inflammation is responsible for maintaining the right balance between effective pathogen elimination and prevention of detrimental immune-mediated response against infections, resulting in the restoration of normal tissue homeostasis (47, 79, 93, 94). At the same time, numerous pathogens induce IL-10 up-regulation during the infection and exploit the immunosuppressive activity of this cytokine to escape host immune system and promote a microenvironment that favors their tolerance and long-term survival (79).
IL-10 exerts strong immunosuppressive effects on monocytes, macrophages, which are the cells with the higher expression of IL-10R, and dendritic cells (50). It inhibits the ability of these cells to produce pro-inflammatory cytokines (including IL-1α and β, IL-6, IL-12, IL-18, and TNF-α) and chemokines (CCL2, CCL12, CCL5, IL-8, CXCL10, and CXCL2) and prevents their differentiation, maturation and migration to lymphoid organs (50). It also suppresses the antigen-presenting capabilities to Th1 and Th2 of monocytes and APCs by down-regulating their expression of the class II major histocompatibility complex (MHC II) (95) and the co-stimulatory molecules CD54 (intercellular adhesion molecule-1, ICAM-1), CD80 and CD56 (96–99). Moreover, it can act on CD4+ T cells by inhibiting their antigen-specific activation and proliferation in lymph nodes, limiting their secretion of cytokines, such as IL-2, IFN-γ, IL-4, IL-5 and TNF-α, and their cytotoxic activity (45, 100, 101) and inducing their long-term anergy through the block of CD28 co-stimulatory signaling (102, 103). Therefore, through these coordinated actions, IL-10 leads to the shutdown of the inflammatory immune response, both directly, by the suppression of macrophages and dendritic cells activity, and indirectly, by limiting T cells activation, differentiation and effector function and promoting peripheral tolerance (43, 96).
On the other hand, IL-10 exhibits several immunostimulatory activities. This cytokine is a potent stimulator of B lymphocytes: it prevents apoptosis in germinal cells, enhances cell growth, proliferation and activation and drives differentiation into immunoglobulin-secreting plasma cells (15, 92, 104, 105). IL-10 plays also an important role in differentiation and functioning of the Tregs (106, 107) and promotes the survival of T cells otherwise destined to apoptotic cell death (108, 109). Regulatory B cells (Bregs), representing B cells immune-suppressive fractions, regulate inflammation primarily through an interleukin 10 mediated inhibitory mechanism (110, 111). In addition, IL-10 induces thymocytes proliferation, by upregulating the expression of CD3 and CD8 molecules (112). It also enhances the production of IFN-γ and granzyme, improves MHC expression and facilitates antigen recognition, promoting in this way the survival, expansion and cytotoxic activity of antigen activated CD8+ T cells. IL-10 is critically involved in the generation and/or sustaining of effector CD8+ memory T cells too (112).
IL-10 promotes NK cell proliferation and migration and enhances their cytolytic activity and effector functions (113–116). Furthermore, IL-10 directly stimulates mast cells, enhancing their expansion, survival, and activation, upregulating their expression of high-affinity IgE receptors (FcϵRI) and increasing their production of pro-inflammatory cytokines (117).
On murine T cells, IL-10 can function as growth cofactor, stimulating a strong proliferative response of thymocytes in presence of IL-2 and IL-4 (118), and as cytotoxic differentiation factor, promoting IL-2-driven proliferation and differentiation of precursor CD8+ splenocytes into effector CTL (119). IL-10 reveals powerful immunostimulatory properties in vivo as well: infusion of exogenous IL-10 in mice recipients of fully allogeneic donor grafts leads to increased graft rejection and graft-versus-host-disease (GVHD)-induced mortality (120). In transgenic murine models, IL-10 expression in the islets of Langerhans results in marked pancreatic inflammation and pronounced recruitment of macrophages, T and B lymphocytes to the pancreas (121). Furthermore, local production of IL-10 by islet cells induces an early development and increased prevalence of autoimmune diabetes in non-obese diabetic mice and accelerates immune-mediated destruction of beta cells (122, 123).
In addition to its broad range activity on the immune system, IL-10 also exerts critical actions on non-immune cells. IL-10 has a fundamental role in central and peripheral nervous system homeostasis, reducing neuronal injury during infection, inflammation, ischemia and trauma, and increasing neuron survival and axon regeneration as well as modulating adult neurogenesis (43, 66, 124). Furthermore, IL-10 is an important regulator of epithelial wound repair and plays a key function in gut homeostasis, promoting wound closure and stimulating intestinal epithelial cell proliferation (43). In dermal wounds, IL-10 promotes regenerative tissue repair via STAT3-dependent regulation of fibroblast-specific hyaluronan synthesis, recruits endothelial progenitor cells (EPCs) and leads to increased vascular structures and faster re-epithelialization (125).
Regulation of IL-10 production and its double role in immunological homeostasis
IL-10 plays a fundamental role in maintaining host homeostasis at both local and global level, ensuring the fine equilibrium between pro- and anti-inflammatory immune response required to achieve an effective clearance of infecting pathogens and preventing, at the same time, tissue damage occurrence (50, 79). Therefore, in physiological conditions IL-10 production is under a highly dynamic and finely balanced modulation to orchestrate the different immunological activities in a cell-specific manner and to control the inflammatory response force and duration. Several transcription factors, expressed and activated by both distinct and overlapping signaling pathways, are involved in the positive or negative modulation of IL-10 transcription. In addition, a number of common and cell-specific regulatory molecular mechanisms act at epigenetic, post-transcriptional, translational, and secretory level to silence or improve IL-10 expression in the immune effector cells and to ensure the appropriate secretion of the cytokine (79, 126–129). IL-10 expression by immune cells can be regulated, in response to bacterial toxin such as LPS and other environmental stimuli, by alterations in cell metabolic profile, or by accumulation of certain metabolites (43). Consequently, the cells that are main producers and targets of IL-10 as well as the pattern of spatial distribution and temporal availability of this cytokine, may specifically differ between tissues and even in the same tissue, depending on the particular host’s immune status (79). Given its fundamental immunoregulatory properties, IL-10 can equally promote the propagation or the shutdown of inflammatory responses and also direct the fate of parasite, bacterial and viral infections (43).
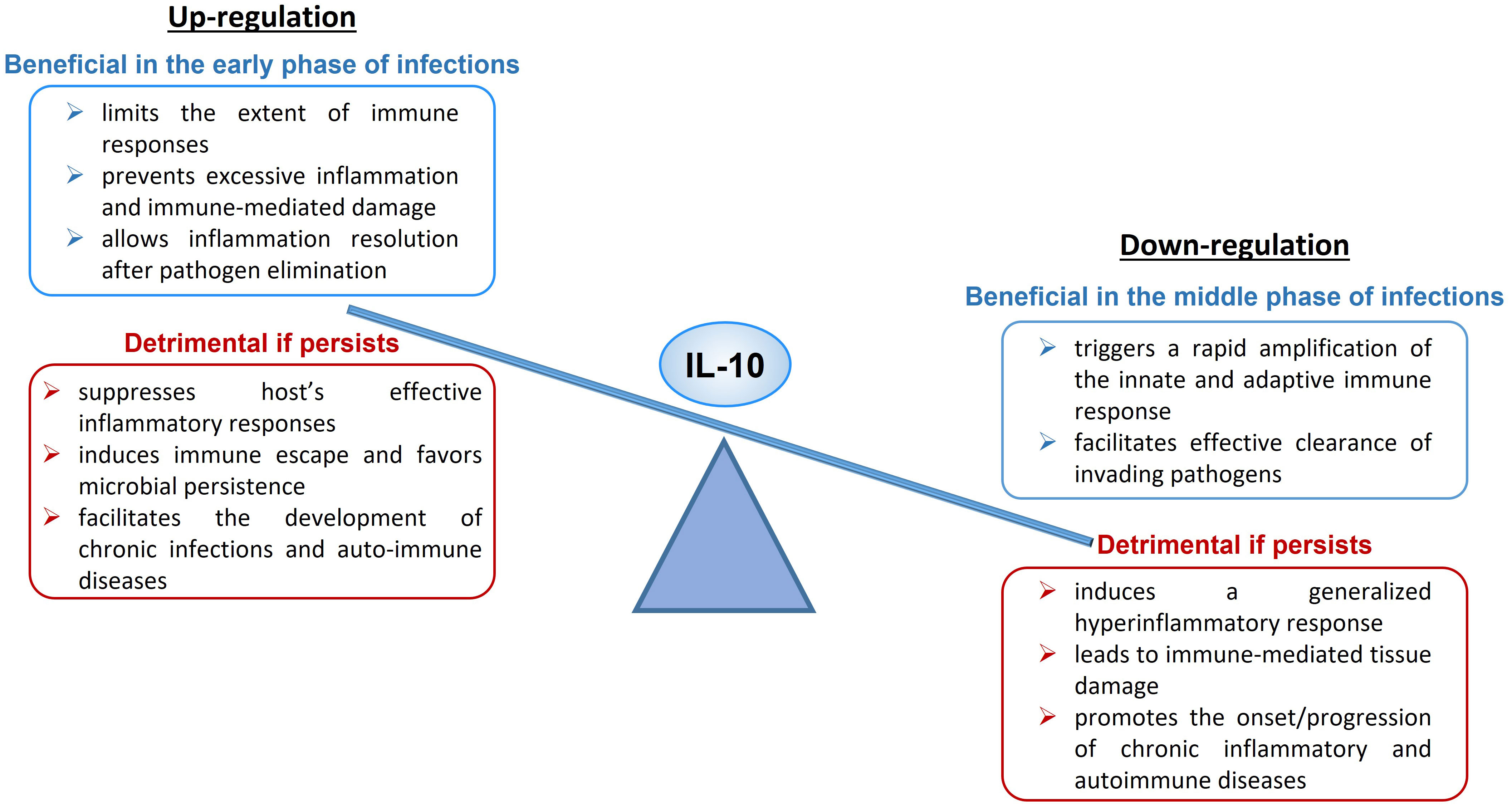
Upregulation of IL-10 expression or enhanced signaling can have both a protective and a harmful effect on the organism (Figure 1). During acute infections, IL-10 limits the magnitude of the immune responses, preventing excessive inflammation and protecting tissues from immune-mediated damage, and allows inflammation resolution when the pathogen is cleared (47). An excess of IL-10 production or signaling can suppress host’s effective inflammatory responses, induce tolerance and immune escape and favor microbial persistence, leading to the establishment of chronic or latent infections (47, 79) and facilitating the development of auto-immune diseases. As example, it has been demonstrated that IL-10 production is crucial to counter-regulate the harmful inflammatory response activated during acute infections with T. gondii (130), T. cruzi (131), H. hepaticus (132, 133) and influenza (134), while increased IL-10 expression level has been linked to reduced T cell activity and enhanced pathogen replication during chronic infections with T. gondii (130, 135), Leishmania (136, 137), EBV (138), HIV (101, 139, 140) and hepatitis B (HBV) (141, 142).
High levels of IL-10 have been documented in systemic lupus erythematosus (143, 144), multiple sclerosis (124), rheumatoid arthritis (145) and Sjogren’s syndrome (146), as well as in autoimmune lymphoproliferative syndrome (147), acute ulcerative colitis (148), and Grave’s disease (149).
On the other hand, downregulation of IL-10 expression or defective signaling can also have both a beneficial and a detrimental impact to the host (Figure 1). An IL-10 deficiency occurring in the early phase of microbe infection triggers a rapid amplification of the innate and adaptive immune response and facilitates effective clearance of invading pathogens (150). If the deficiency persists, it leads to systemic, exaggerated inflammation and immune-mediated tissue damage and participates to the onset or aggravation of chronic inflammatory diseases and several autoimmune pathologies (74, 79, 151).
As example, IL-10-deficient mice develop colitis (152, 153) and during infection with T. cruzi (154) and T. gondii (135, 155) succumb to an excessive, lethal inflammatory response. IL-10 deficiency has been also demonstrated to aggravate chronic liver and kidney disease, enhancing fibrosis and inflammation (156, 157). IL-10 expression was found lower in psoriatic (158, 159) and asthmatic patients (160) and IL-10 and IL-10R mutations, causing a loss of IL-10 function, were found to be associated with severe inflammatory bowel disease, including Crohn’s disease and ulcerative colitis (52, 161, 162).
With age the functional competence of the immune system declines, a process called immunosenescence and involves the remodeling of innate and adaptive immunity and it is associated with a higher likelihood and severity of several infections (163). Immunosuppressive cells and immunosuppressive cytokines are involved in this process including IL-10 that has been found increased in several studies (164).
The scheme is illustrating the dual immunological activities of IL-10 and the possible beneficial or detrimental impact of this cytokine at high or low levels on human health and disease (Figure 1).
IL-10 in cancer
The role of IL-10 in tumor pathogenesis is currently highly controversial, with some findings showing that IL-10 promotes tumor development and angiogenesis, while others supporting that it inhibits tumor growth and metastasis (115).
This cytokine is considered a master switch from tumor-promoting inflammation to antitumor immunity, thus dysregulation in IL-10 levels can importantly contribute to carcinogenesis and tumor progression (112, 165, 166). Elevated IL-10 level exerts tumor-promoting effects by stimulating tumor cell growth and proliferation via STAT3 activation, by inhibiting apoptosis and by allowing immune surveillance escape through inhibition of DC function, downregulation of human leukocyte antigen (HLA) class I molecules on tumor cell surface, recruitment of Treg, suppression of NK cells cytotoxic activity and impaired activation of Th1 CD4+ and cytotoxic T cells (167–172). Increased IL-10 expression in primary tumor cells and tumor-associated macrophages has been proposed as a predictor of cancer stage progression and metastatic potential development (87, 173, 174). Moreover, IL-10 circulating levels were found to be elevated in serum of various cancer patients, often accompanied by the increase of other inflammatory markers, and correlated with a poor prognosis (140, 175–183).
On the other hand, IL-10 mediates important tumor-inhibiting activities by recruiting and stimulating cytotoxic CD8+ T cells and NK cells in the tumor microenvironment, by promoting lymphocyte and antibody-dependent immune memory, by abrogating inflammatory M1 macrophage-Th17 T cells axis, by downregulating the synthesis of pro-angiogenic factors and by suppressing local release of pro-inflammatory cytokines that support tumor growth, survival, and invasion (167, 184–188).
IL-10 as a potential therapeutic opportunity
The increasing knowledge about the essential regulatory role of this cytokine has encouraged investigators to consider IL-10 as a potential therapeutic opportunity (43, 157, 189, 190). Although no therapy has been yet approved to date, systemic administration of recombinant human (rhu) IL-10 has been tested in multiple clinical trials for the treatment of autoimmune and immune-mediated inflammatory diseases (including inflammatory bowel disease, psoriasis, Crohn’s disease, rheumatoid arthritis, ulcerative colitis, pancreatitis), tissue damage, and chronic infectious diseases (such as chronic hepatitis C), due to its anti-inflammatory, wound repairing and anti-fibrotic functions, respectively (191–194).
Early phase I and II studies showed a trend toward favorable responses of systemically administered IL-10 in psoriasis and Crohn’s disease patients, but larger studies revealed only a slight clinical benefit, due to the double anti- and pro-inflammatory properties of this cytokine (43, 195). Results obtained in a mouse model of human multiple sclerosis suggested that inducing local expression of IL-10 in the site of inflammation has the potential to prevent autoimmune inflammatory process in the central nervous system (43, 195). Induction of a homogeneous population of IL-10-producing CD4 T cells by a combination of immunosuppressive drugs (vitamin D3 and dexamethasone) may represent a promising therapeutic strategy for the treatment of autoimmune and inflammatory diseases (43, 195). From the other side, the use of anti-IL-10R mAbs potentiate the Th1 response and may be useful for the development of effective vaccines and to enhance appropriate immune responses against chronic pathogens (43, 195).
Moreover, given the double tumor-promoting and tumor-repressing IL-10 action, both blocking and systemic administration of IL-10 have been explored as potential strategies for cancer immunotherapy.
Yet, IL-10’s biologically active form is an unstable homodimer with a short half-life and low in vivo stability. This represents a significant drawback of using IL-10 in therapeutic application (51). IL-10’s therapeutic potential can be increased by pegylation, a modification of IL-10 by covalent conjugation with non-toxic polymer polyethylene glycol (PEG), that increase the half-life of a protein following administration (112, 196). It was observed that systemic administration of PEGylated human IL-10 (pegilodecakin) promotes infiltration, activation and intratumor expansion of tumor-specific CD8+ T cells and restores their cytotoxic activity, resulting in enhanced granzyme B and IFN-γ production in CD8+ cells, enhanced intratumor antigen presentation and induction of anti- tumor immune response with evidence of clinical benefits in different advanced solid tumors, such as renal cell carcinoma and uveal melanoma (112, 191, 197, 198). On the other hand, cancer immunization with simultaneous IL-10 signaling blockade, using IL-10R monoclonal antibodies, soluble IL-10R, peptide-based IL-10R antagonists, or oligonucleotides, raised tumor immune response with evidence of clinical benefits in different advanced solid tumors, such as renal cell carcinoma and uveal melanoma (112, 191, 197, 198), and enhances CD8+ T cell response and potentiates vaccine-induced tumor regression (189).
Concomitant blockade of IL-10 and PD-1 immune checkpoint in a mouse model of lymphocytic choriomeningitis virus (LCMV) increases the efficacy in restoring antiviral T cell responses and controlling persistent viral infection (199). Combined treatment with IL-10 and PD-1 blockers enhances the expansion and function of tumor-infiltrating CD8+ T cells, resulting in a synergistic anti-tumor effect in metastatic melanoma and ovarian cancer (189). Recently, therapy with PEGylated-IL-10 and anti-PD-1 monoclonal antibody (pembrolizumab or nivolumab) has shown encouraging results in a phase 1b clinical trial conducted on advanced refractory renal cell carcinoma and non-small-cell carcinoma patients (200).
Potential role of IL-10 in COVID-19
Chronic viral infections are another field in which IL-10 appears as an intriguing therapeutic challenge. Studies have demonstrated that blockade of IL-10 is able to restore T cell antiviral activity, enhance vaccine efficacy and promote immune-mediated eradication of viral persistence in case of cytomegalovirus, lymphocytic choriomeningitis virus, HIV, and HCV infections (98, 201, 202).
ARDS is the most common complication of Coronavirus disease 2019, affecting approximately 75% of COVID-19 patients in intensive care units (ICU), and a leading cause of COVID-19-releated death (203). It is a progressive respiratory insufficiency, defined by a plethora of symptoms including severe hypoxemia, increased respiratory work, pulmonary embolism, microvascular thrombosis, diffuse alveolar damage with alveolar cell death, edema, fibrosis and inflammatory cells infiltrate into the lung interstitium and alveoli, which can evolve in systemic tissue damage and multiple organ failure and eventually results in a fatal outcome (204–207).
SARS-CoV-2 virus enters in the target cells through the binding of its viral spike protein (S) to the host angiotensin converting enzyme 2 (ACE2), which is present in different human organs (oronasal and nasopharyngeal mucosa, lung, stomach, colon, skin, lymph nodes, liver, kidney, brain) and mainly expressed on lung alveolar epithelial cell type II, enterocytes of the small intestine and vascular endothelium (207, 208). Even though the exact sequelae of mechanisms leading to COVID-19-mediated lung damage are still being delineated, it is widely accepted that cytokine storm plays a prominent role (15, 16, 209–211). Alveolar epithelial cells, alveolar macrophages and dendritic cells function as sensor cells of the respiratory mucosa and, upon SARS-CoV-2 infection, give rise to immune response with a first huge release of early pro-inflammatory cytokines (including IFN-α, IFN-γ, IL-1β, IL-2, IL-6, IL-12, IL-18, IL-23, TNF-α) and chemokines (such as CCL2, CCL3, CCL5, CXCL8, CXCL9, CXCL10) that activate resident lymphocytes and stimulate recruitment of effector cells (210, 212). Protracted cytokine and chemokine overproduction causes massive recruitment of neutrophils, eosinophils and NK cells in the pulmonary parenchyma. Once there, neutrophils secrete free radicals, myeloperoxidase and other proteases, eosinophils release major basic proteins and cationic proteins, while NK cells liberate granzymes and perforins. All these substances exert cytotoxic effects and lead to alveolar injury. Resident macrophages polarize to M1 phenotype and, in concert with infiltrating DCs, produce nitric oxide and additional pro-inflammatory molecules, such as TNF-α, which induce alveolar cell death and further contribute to pulmonary endothelium damage. Cytotoxic T cells, in turn, migrate to lungs upon activation by DCs, and also participate in the killing of infected cells (210, 213).
The host immune response, active in the first phase, can positively affect infection resolution, suppressing viral replication and leading to complete pathogen elimination and homeostasis restoration. However, excessive inflammation is deleterious and triggers a vicious circle that is self-sustaining of the ongoing hyper-inflammatory state. The resulting dysregulated cascade of cytokine first causes the disruption of the lung epithelial barrier and then, traveling through the bloodstream, can further amplify the cytokine storm, giving rise to systemic inflammation and potentially damaging multiple organs throughout the body (209).
Several pro-inflammatory molecules can variably participate to the cytokine storm driving ARDS in COVID-19, as demonstrated by different clinical studies reporting higher circulating levels of one or more immunoactive molecules in patients with severe form of COVID-19, including IL-1β, IL-2, IL-6, IL-7, IL-8, IL-17, TNF-α, IFN-γ, granulocyte-macrophage colony-stimulating factor (GM-CSF), CXCL10, CCL2, CCL7, CCL3, CCL4, and C-reactive protein (CRP) (209, 214–220). Overweight and obesity are considered a main risk factor for severe symptoms of COVID-19 and increased mortality. This can be explained by the finding that obese patients have altered NK cell polarization, increased levels of pro-inflammatory cytokines, such as IL-6 and TNF-α, and hyperactivation of mTOR pathway (221), besides cardiovascular co-morbidity.
The uncontrolled overproduction of pro-inflammatory chemokine/cytokines observed in SARS-CoV-2 infection is a clinical characteristic in common with that previously seen in SARS-CoV and MERS-CoV infections (211, 212). Although this pathogenic process is shared between COVID-19 and the other beta-coronavirus infections, the massive increase of IL-10 levels in patients with severe forms of the illness is a clinical feature that uniquely distinguishes SARS-CoV-2 infections (211, 222).
A large increase in the proportion of IL-10-secreting regulatory T cells has been found in peripheral blood of patients with severe COVID-19, compared to those with mild-to-moderate cases and healthy individuals (223). Several studies have also reported that circulating levels of IL-10 are significantly elevated in severe cases of COVID-19, especially in patients admitted to the ICU compared to those not requiring ICU care (224, 225) and continued to increase after admission (45, 116). In addition, elevated IL-10 levels are seen in patients developing ARDS, respiratory failure and extrapulmonary dysfunction like disseminated intravascular coagulation (116, 207) and severe acute kidney injury (46, 226), as well as a reduced patient survival (116, 227, 228).
Higher IL-10 levels have been positively correlated with increased exhaustion markers PD-1 and TIM-3 expression on T cells and lower total number of CD4+ and CD8+ T cells (225). In patients with severe forms of COVID-19 also a strong relationship between early overexpression of IL-10 and increased serum concentrations of IL-1 receptor antagonist (IL-1RA) and other proinflammatory molecules, including IL-6, IL-8 and C-reactive protein (116, 215, 228–230) was observed. Numerous clinical studies have also revealed that elevated amounts of IL-10 in the serum of COVID-19 patients, alone or with IL-6, IL-12 or IL-1RA, may accurately predict progression to more severe form of disease and increased mortality (46, 215, 229–234).
Taken together, these evidence have robustly supported the great potentiality of monitoring circulating levels of IL-10 in COVID-19 patients as reliable biomarker to rapidly predict the disease course at the first stages of infection, to early recognize patients with higher risk of developing detrimental complications (231) and to accurately determine the most suitable therapeutic options and the right time of treatment administration (228, 234).
Alternative potential scenarios were proposed to explain the clinical meaning of the increase in IL-10 levels in serum of COVID-19 patients occurring within a few days from infection. IL-10 level significantly increases one week after symptoms onset following the massive release of pro-inflammatory cytokines that occurred in the preceding days. A study showed statistically significant differences of IL-10 serum levels in the non-severe and severe groups on days 0, 3, and 6. The median concentration of IL-10 on days 3 and 6 was increased in both the non-severe and severe groups compared to day 0 (235). IL-10 is generated after acute infection and could have the ability to block the expression and production of numerous proinflammatory cytokines, preventing the development of excessive or chronic immune activation.
The first possible explanation of the COVID associated data, suggests in fact that IL-10, in the context of ongoing inflammation induced by COVID-19, behaves in a canonical way as an anti-inflammatory and immunosuppressive cytokine. High circulating levels of IL-10 could be interpreted like an endogenous danger signal, activated as a negative feedback mechanism in response to the dramatic increase of pro-inflammatory mediators and the related alveolar endothelial cell damage. Therefore, IL-10 acts as an attempt of the host organism to protect itself from the deleterious effects of an excessive inflammatory reaction, preventing further progression of tissue damage (211, 236) (Figure 2).
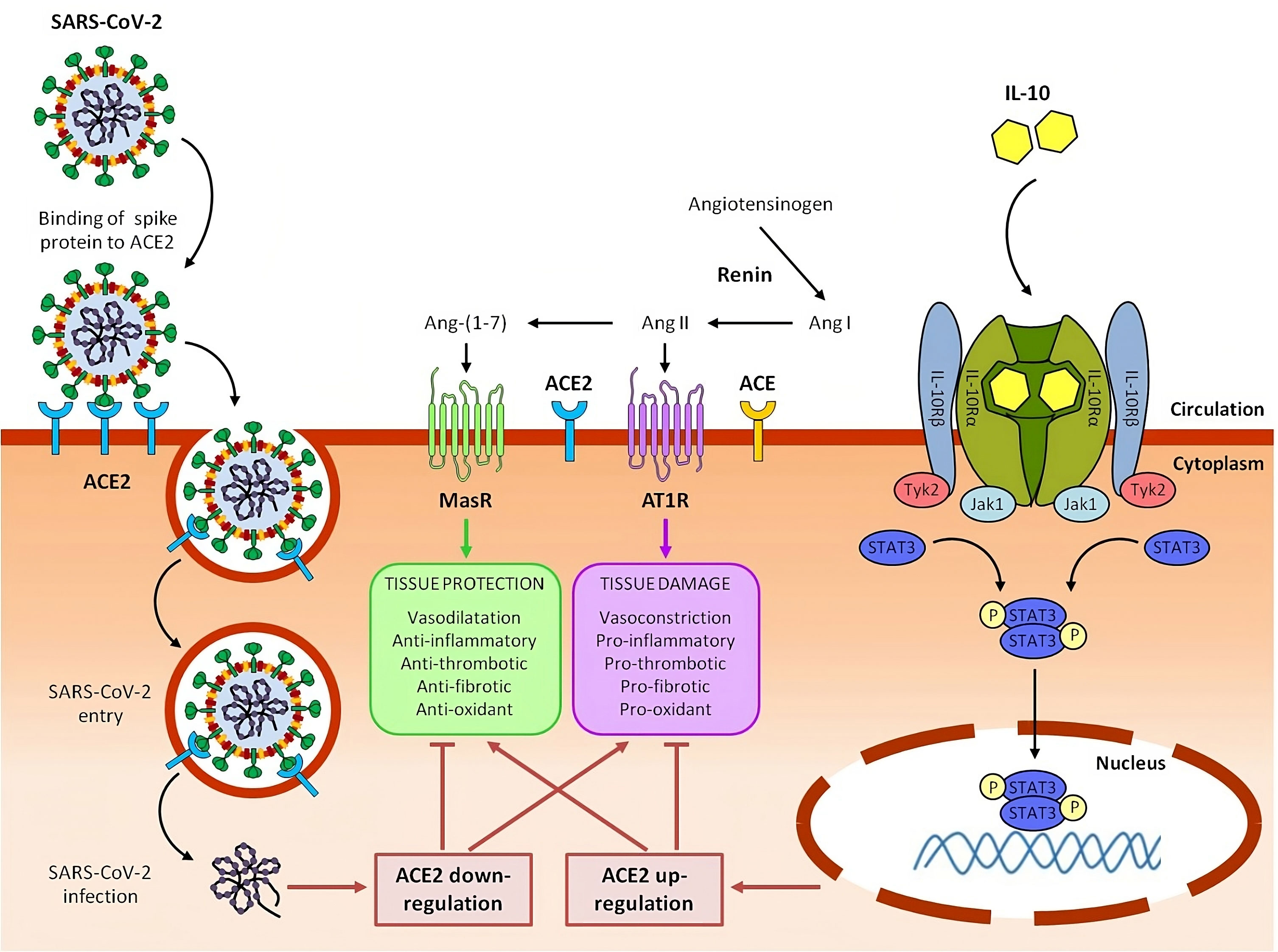
Figure 2 Potential role of IL-10 in counteracting ACE2 downregulation, rebalancing RAAS system and mitigating tissue damage caused by SARS-CoV-2 in severe COVID-19 and Long COVID syndrome.
In the renin-angiotensin system (RAAS), renin converts angiotensinogen to angiotensin I (Ang I), which is in turn converted to angiotensin II (Ang II) by angiotensin-converting enzyme (ACE). Ang II, acting through Ang II type 1 receptor (AT1R), promotes inflammation, fibrosis, vasoconstriction, thrombosis and oxidative stress, ultimately resulting in tissue injury. Detrimental effects of Ang II are counterbalanced by Angiotensin-converting enzyme 2 (ACE2), which converts Ang II to angiotensin 1-7 (Ang-(1–7)). Ang-(1-7), signaling through the Mas receptor (MasR), inhibits inflammation and mediates tissue protection.
SARS-CoV-2 infects host cells by binding its viral spike protein to the receptor ACE2. Following this binding, SARS-CoV-2 is internalized by endocytosis and ACE2 expressed on the cell plasma membrane is downregulated. Reduction of ACE2 leads to RAAS imbalance with an increase of the ACE/Ang II/AT1R axis and a parallel decrease of the ACE2/Ang-(1–7)/Mas-R axis, contributing to hyperinflammation and tissue damage of COVID-19 and Long COVID syndrome.
Circulating interleukin 10 (IL-10) binds as a homodimer to tetrameric IL-10 receptor (IL-10R) complex and induce the downstream activation of Janus kinase 1 (Jak1) and tyrosine kinase 2 (Tyk2) and the subsequent phosphorylation of signal transducer and activator of transcription 3 (STAT3). Phospho-STAT3 (p-STAT3) homodimers translocate into the nucleus, where they directly bind to specific sequences and regulate the transcription of its target genes, including anti-inflammatory genes and ACE2. Upregulating ACE2, IL-10 can help to restore RAAS balance, with a reduction of ACE/Ang II/AT1R axis and an increase of ACE2/Ang-(1–7)/MasR axis, resulting in beneficial effects on COVID-19 and post-COVID-19 symptoms. We have previously reported that, in lung-derived and endothelial cell lines, IL-10 administration increased the expression level of SARS-CoV-2 receptor, ACE2 a potent anti-inflammatory molecule (237) (Figure 2).
Blood level of IL-10 is low following SARS-CoV-2 infection during the innate immune response phase and starts to be significantly increased around 3 days/one week following the massive release of inflammatory cytokines (TNF-α, IL-1, IL-6) and symptoms onset. IL-10 raises after acute disease. During the convalescent phase, IL-10 levels slowly decrease along with the symptoms in about 2-3 weeks.
Thus, IL-10 could behave as a counter-regulator of the local endothelial inflammation as well as the systemic inflammatory process, by enhancing ACE2 expression (237). In an ex-vivo study on peripheral-blood immune cells, we have also recently demonstrated that IL-10 treatment decreased the IFN-γ specific response to spike stimulation, decreased the release of numerous pro-inflammatory cytokines, chemokines and growth factors, reduced the frequency of IFN-γ producing CD4, CD8 and NK cells and cell activation (evaluated by HLA-DR expression), in both COVID-19 patients and NO COVID-19 vaccinated subjects (45). Our study further confirmed the view of an immunomodulatory role of IL-10 in the SARS-CoV-2 specific inflammatory response and highlights the therapeutic potential of the administration of rhu IL-10 to treat ARDS in COVID-19 patients, as already investigated for solid tumors and various autoimmune and inflammatory diseases (45).
Several clinical studies have described a huge increase in IL-10 early after few days from infection, after the concomitant increase of various other pro-inflammatory cytokines (such as TNF-α, IL-6, IL-1) (Figure 3), as distinctive trait of the hyperinflammatory state developed upon SARS-CoV-2 infection (238). It has also been observed a strong association between IL-10 levels and COVID-19 severity and outcome, suggesting that IL-10 fails to adequately turn off the inflammation. A plausible explanation for this emerging evidence concerns the potential resistance or hypo-responsiveness of activated immune cells to the immunosuppressive action of IL-10, resulting in uncontrolled and self-sustained release of pro-inflammatory cytokine into circulation (239). The occurrence of this situation has already been demonstrated in vitro in high-glucose conditions and in vivo in patients diagnosed with type 2 diabetes and has been attributed to defective STAT3 activation (151). The impaired IL-10 response in presence of high glucose can justify the increased frequency of mortality and severe complication in COVID-19 patients with diabetes or hyperglycemia and the better outcomes associated with improved glycemic control (236, 240). Therefore, it is reasonable to speculate that pharmacological strategies able to overcome resistance and/or restore responsiveness to IL-10, as happens by the treatment with a small molecule agonist of SHIP1 (Src homology-2 containing inositol-5’-phosphatase 1) in macrophages under hyperglycemia (151), could give a valid therapeutic opportunity to reduce the overwhelmed inflammation in patients with severe COVID-19, especially those with diabetes.
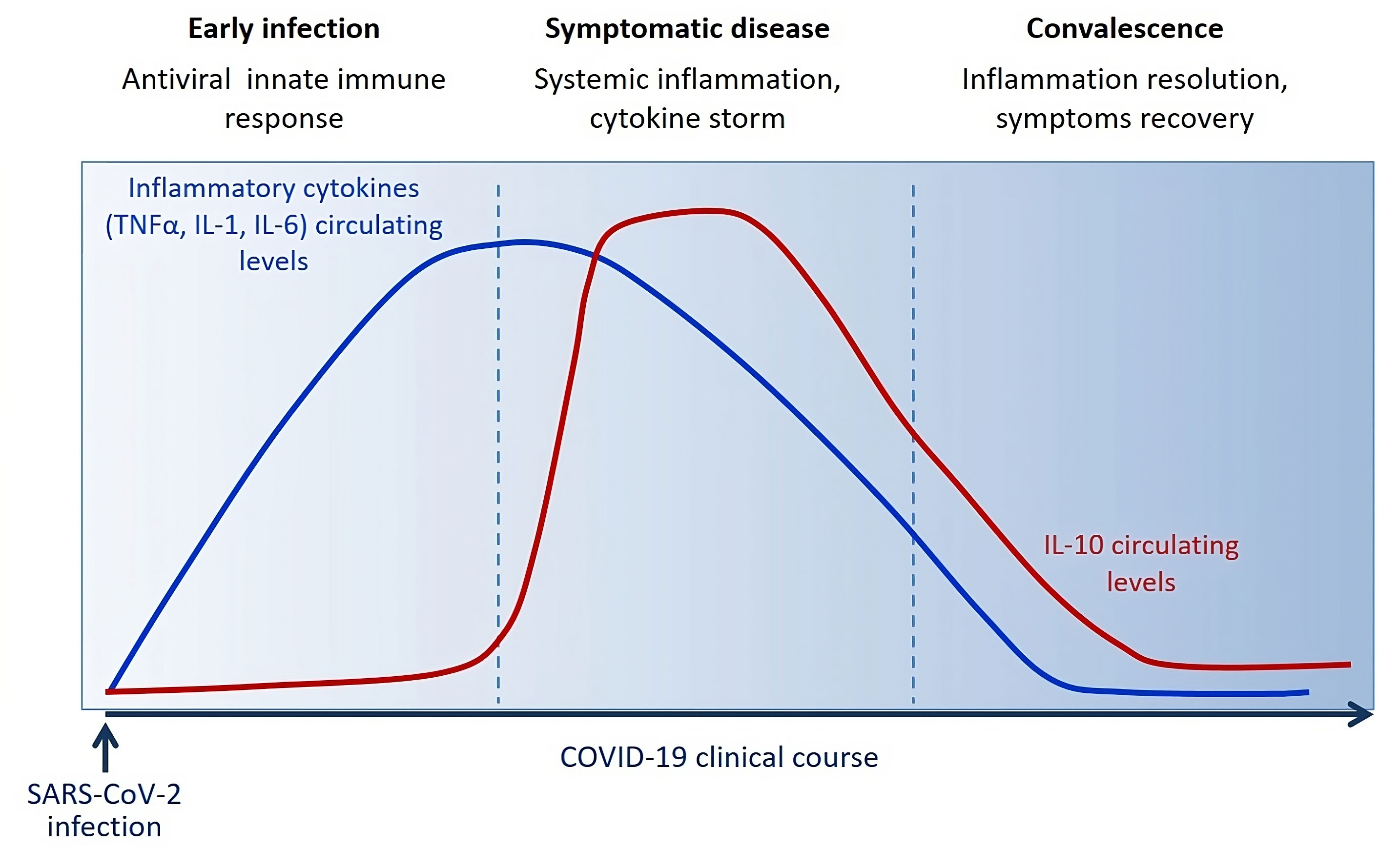
Figure 3 Circulating levels of IL-10 dynamically change during the clinical course of COVID-19 reflecting host immune/inflammatory state.
In severe COVID-19 cases a drastic early rise in IL-10 was observed, an effect that represents a paradoxical role of this cytokine in its classical anti-inflammatory role. This observation gives a convincing justification for the increased IL-10 levels in the presence of systemic inflammation such as COVID-19 condition, as well as previously observed in cancer and immunity (115, 241) (Figure 2). This can be explained with IL-10 “resistance”, as reported by Islam et al., 2021, hypothesis that requires further investigation (239).
Different studies have previously revealed hyper-activation and expansion of CD8+ T cells, enhanced production of IFN-γ and peripheral increase of various pro-inflammatory and immune-activating mediators following recombinant IL-10 administration in healthy subjects with LPS-induced endotoxemia and in patients with Crohn’s disease and some cancers. Most cytokines/chemokines are reported as upregulated in these studies (IL-2Rα, IL-4, IL-7, IL-18, IFN-γ, GM-CSF, TNF-α, CXCL10 and CXCL9) supporting the potential immunostimulatory action of this cytokine in severe COVID-19 (45). In addition, elevated levels of LPS, a potent stimulators of IL-10 secretion by macrophages, were observed in plasma of patients with severe COVID-19 (239). In this scenario, stimulation of IL-10 signaling with PEG-IL-10 or other IL-10 stimulation might result in clinical benefit for patients with severe COVID-19.
It is also possible that IL-10 plays a double role in COVID-19, depending on the timing of the secretion: after few days from infection, IL-10 produced in the lungs, after viral infection, may work as a negative feedback mechanism started by an increased proinflammatory mediators release and aimed at counter-modulating inflammation and restoring tissue homeostasis. However, in the later phases, IL-10 production becomes continuous and elevated and may act as an immune stimulating factor that promotes a further release of proinflammatory cytokines/chemokines, hyperactivates cytotoxic effector CD8+ T cells and amplifies systemic inflammation, leading to disease exacerbation (211).
Potential role of IL-10 in post-COVID-19 syndrome
One of the main problems aggravating the sanitary emergency due to SARS-CoV-2 pandemic, is the management of the estimated 10% of patients who do not undergo a complete recovery but manifest persistent post-COVID-19 symptoms for up to 12 or more weeks after initial infection (24, 242).
The plethora of post-COVID-19 symptoms is highly heterogeneous and comprises variable degrees of severity: physical symptoms as fever, fatigue, respiratory symptoms, as dyspnea, breathlessness and coughing, painful symptoms, as myalgia, arthralgia, headache and chest pain, neurological symptoms, as anosmia, dysgeusia, difficulty concentrating and sleeping, psychological symptoms, as depression, anxiety, poor memory and concentration, cardiovascular symptoms, as tachycardia and coagulation dysfunction, and gastrointestinal symptoms. These multi-organ symptoms can occur as a result of organ damage following severe COVID-19 or arise de novo after mild infection without evidence of organ injury (24, 25, 28, 242, 243).
The clinical spectrum of post-COVID-19 symptoms was classified, by Fernández-de-Las-Peñas and colleagues, into three different phases based on a temporal criterion: acute post-COVID symptoms (from 4-5 to 12 weeks after infection), long post-COVID symptoms (from 12 to 24 weeks), and persistent post-COVID symptoms (lasting more than 24 weeks) (244). The precise mechanisms responsible for post-COVID-19 pathology remains still unclear, but different causative factors have been proposed to contribute to the various clinical sequelae observed in patients (26, 245, 246). Firstly, SARS-CoV-2, by infecting and replicating into ACE2 expressing cells, can exert a direct viral toxicity and cause diffuse endothelial cell damage (247, 248). SARS-CoV-2-mediated endothelial damage, by recruiting and activating immune cells and promoting pro-inflammatory and pro-thrombotic mediators release, can trigger subsequent endothelial inflammation leading to thrombosis and vascular damage (207, 247). Viral entry into cells mediates the downregulation of ACE2 and its consequent failure to convert the angiotensin II into angiotensin 1-7, resulting in the accumulation of angiotensin II and overstimulation of RAAS that ultimately causes hypertension, electrolyte unbalancing, lung fibrosis and inflammation, vasculitis, thromboembolism and intravascular disseminated coagulation (207, 249). In addition, SARS-CoV-2 impairs the mitochondrial antioxidant function, resulting in increased reactive oxygen species (ROS) release, oxidative stress and oxidative damage, which lead to tissue damage, thrombosis, and red blood cell dysfunction (250–252). The other fundamental mechanism contributing to the pathological process is thought to be host’s immune response dysregulation. SARS-CoV-2 dissemination can trigger a massive cell activation to induce an anti-viral immune response with an exaggerated and continual production of inflammatory cytokines, that lead to alveolar edema, hypoxia, thrombosis, tissue damage, and can ultimately results in systemic inflammatory response involving the whole organism and causing a multi-organ injury (207, 247, 248).
Given the critical role played by IL-10 in the promotion of tissue repair and resolution of inflammation it is possible that this cytokine could have a useful impact in recovering physiological homeostasis and ending the post-COVID-19 symptoms. Blood level of IL-10 is significantly increased in the first week following the symptoms’ onset in patients who developed severe COVID-19. Moreover, higher serum levels of IL-10 were found in individuals who did not experience sequelae after acute infection compared to subjects with post-COVID-19. This supports the idea that elevated levels of IL-10 in the post-COVID-19 period, allow a more efficient resolution of the immunopathological process, by improving anti-inflammatory response (253).
Among the symptoms of post-COVID-19 on which IL-10 could have a beneficial effect there is the olfactory and gustatory dysfunction (OD/GD), a distinctive sign of acute COVID-19 and one of the most frequent long-lasting complications in post-COVID-19 (254). Locatello and colleagues have reported that elevated serum concentration of IL-10 on hospitalization, compared to increased levels of other cytokines or presence of clinical comorbidities, is the only significantly parameter associated with 30-day taste recovery (255). Luporini et al. has reported higher IL-6 and IL-10 levels in serum of adults over 65 with COVID-19, associated with disease severity and a higher comorbidity index (222). This evidence further supports an involvement of inflammatory process in COVID-19-associated chemosensory dysfunction and suggests a role for IL-10 as reliable predictor of OD/GD course as well as potential pharmacological strategy to reach a successful recovery in post-COVID-19 patients.
Pain is another post-COVID-associated pathological manifestation in which IL-10 may have a clinical utility. In particular, joint, muscle and chest pain represent one of the most frequently reported persistent symptoms after the resolution of acute COVID-19 infection and Bussmann et al. (256) have observed a strong inverse correlation between circulating levels of IL-10 and pain intensity in COVID-19 patients (256). This evidence suggests an analgesic function for IL-10 in the context of post-COVID-19 and proposes that this cytokine can significantly improve the patient’s quality of life, resolving the chronic pain debilitating condition (256).
Cardiovascular and respiratory symptoms are other persistent clinical signs, among those commonly affecting post-COVID-19 patients, which can be positively influenced by IL-10. Virus-mediated downregulation of ACE2, the counter-regulator of ACE, may cause dysregulation of the renin-angiotensin-aldosterone system (RAAS), resulting in a worsening of cardiovascular and respiratory condition (257). Absence of angiotensin-converting enzyme inhibitors (ACEI)/angiotensin II receptor blockers (ARBs) therapy were the main prognostic indicators of in-hospital mortality (258). As reported before, IL-10 could increase ACE2 expression in lung and endothelial cells (237). Therefore IL-10, by restoring RAAS balance, can importantly contribute to normalization of electrolyte levels and blood pressure, containment of pulmonary inflammation and fibrosis, resolution of vasculitis, thrombosis, and hyper-coagulation (207).
Although the exact function played by IL-10 in COVID-19 has not yet been fully defined, due to the multifaceted actions exerted on inflammation, IL-10 has been increasingly proposed as critical contributor during the kinetics of cytokine storm, which is considered a main responsible for the development and progression of ARDS in COVID-19 patients and a keystone factor in influencing disease morbidity and mortality (211, 212, 259).
In fact, IL-10 can also have a beneficial impact in mitigating or even suppressing the continuative systemic inflammation typically associated to post-COVID-19 syndrome. In our recent research, we have demonstrated that exogenous delivery of IL-10 to whole-blood cells downregulates SARS-CoV-2 induced exacerbated inflammatory response, by reducing several pro-inflammatory mediators correlated with COVID-19 severity and by decreasing frequency and activation of IFN-γ producing CD4, CD8 T cells and NK cells (45).
It is also possible that exogenous IL-10 plays a useful therapeutic role in counteracting neurological symptoms observed in post-COVID-19. In this regard, Trandem and colleagues (260) have shown the protective effects of elevated IL-10 levels in mice infected with a neurotropic coronavirus (260). High IL-10 production, occurring during the early phase of viral encephalitis, leads to decreased microglia activation, immune cells infiltration and proinflammatory factors release and an increased regulatory T cell rate in the site of infection. The immunomodulating actions of IL-10 were long-time lasting and manifested during the resolution phase of the infection, resulting in decreased demyelination and improved survival (260).
Natural bioactive compounds influencing IL-10 production
The therapeutic role of bioactive compounds obtained from plants in the treatment of human diseases has been extensively acknowledged (261–265).
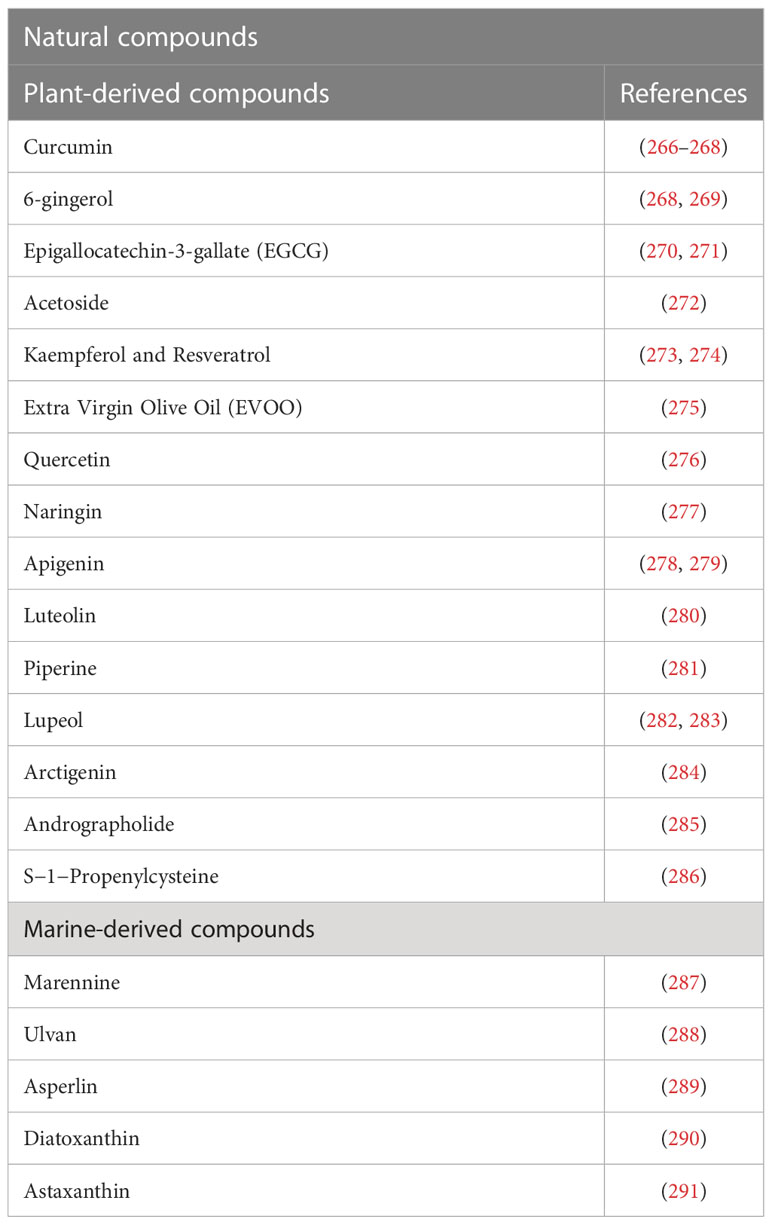
Considering the potential wide-ranging impact that Il-10 could have on complications associated to post-COVID-19 syndrome, and in other diseases, such as cancer, it is of interest to study natural bioactive compounds, able to increase IL-10 expression and enhance its action, which could represent a useful therapeutic strategy. In Table 1 we report bioactive compounds, derived from natural sources, that influence IL-10 production. Among these compounds, the polyphenol curcumin is endowed with numerous beneficial properties, including antioxidant, anti-inflammatory, anti-nociceptive, anti-fatigue and anti-fibrotic effects, by increasing the expression, production, and activity of IL-10 (266–268). Administration of nano-curcumin has been reported to provide anti-viral action and to downregulate expression and secretion of the inflammatory cytokines IL-1β and IL-6 in COVID-19 patients (292, 293). The polyphenol 6-gingerol can upregulate IL-10 production and possesses useful therapeutic effects, comprising antioxidant, anti-inflammatory, immunomodulatory, analgesic, antipyretic and anti-SARS-CoV-2 activity (268, 269). The green tea polyphenol epigallocatechin-3-gallate (EGCG) induces Treg by increasing Foxp3 and IL-10 expression in CD4 T cells (270, 271), while acteoside, a phenolic glycoside, can promote B cell-derived IL-10 production, ameliorating inflammatory process (272). The natural dietary polyphenols kaempferol and resveratrol, with known anti-inflammatory, antioxidant, antimicrobic and disease‐protective activities, stimulate IL-10 production and inhibit inflammatory cytokine secretion (273). A similar effect on oxidative stress, inflammation and IL-10 level was obtained with a diet enriched in high-polyphenols containing Extra Virgin Olive Oil (EVOO) (275). The flavonoids quercetin (276), naringin (277), apigenin (278, 279), luteolin (280), present in different vegetables and fruits, and the alkaloid piperin (281) and S-1-Propenylcysteine (286), are other examples of natural compounds able to increase IL-10 levels and exert antioxidant, anti-inflammatory, immunomodulatory, anti-cancer and antimicrobial properties (268, 274, 280, 294).
Evidence emerging from literature and clinical trials suggests that dietary-derived polyphenols could represent a helpful supplement in COVID-19 therapy, by contrasting viral load, suppressing inflammation, promoting ACE2/Ang- (1–7)/MasR axis, protecting organs from damage, preventing complications and reducing illness severity (295).
IL-10 production has been demonstrated to be significantly increased in macrophages M1 treated with lupeol (pentacyclic triterpene Lup-20(29)-en-3-ol), a secondary metabolite which is primarily present in fruit plants (282, 283). Similar results were observed by Hyam et al. in 2,4,6-trinitrobenzene sulfonic acid (TNBS) induced colitis model treated with arctigenin, present in Arctium lappa (burdock plant) seeds (284). Moreover, it has been observed that the treatment with andrographolide, a bioactive compound present in the plant known as Andrographis paniculate, increase IL-10 expression in LPS stimulated primary glial culture (285).
In addition, marine environment represents a rich reservoir of immunoactive molecules, mainly concentrated in photosynthetic organisms such as microalgae, which have been recently considered bioactive cell factories for human health benefits (296). Marine organisms have emerged as a source of bio-compounds that could be used as potential immunomodulatory drugs (297), indeed, different marine compounds show an immunomodulatory function increasing IL-10 levels.
Marennine, a blue pigment produced by Haslea ostrearia, a marine pennate diatom, acts on neuroinflammatory processes, inducing a strong up-regulation of IL-10 genes (287). Ulvan, a sulfated polysaccharide produced from a green marine algae Ulva Ohnoi, showed a mild immunomodulatory function increasing IL-10 levels (288). Zhou et al. reported that asperlin, derived from the marine fungus Aspergillus versicolor shown beneficial properties again atherosclerosis, in vitro and in vivo, due to the increase of protective cytokines (IL-10 and IL-4) (289).
In this scenario marine microalgae are emerging as rich sources of a wide range of bioactive metabolites with anti-antioxidant and anti-inflammatory properties that can serve as potential new therapeutic agents to treat or prevent the severe symptoms of COVID-19, possibly by enhancing IL-10 levels (298, 299). Marine sulfated polysaccharides, isolated from different algae, have shown anticoagulant and immunomodulatory activities as well as potent antiviral properties, both by stimulating innate immune system and mucosal barrier defense against the virus and by preventing viral entry, replication and proliferation (299–301). We have recently demonstrated that diatoxanthin, a carotenoid derived by marine diatoms, significantly upregulates IL-10 production, increases ACE2 activity, exerts an immunomodulant effect by up-regulating antiviral defense genes and by strongly inhibiting spike-induced inflammatory response in a lung cell line. Diatoxanthin, exclusively found in marine environment, decreases the release of pro-inflammatory mediators responsible for cytokines storm in SARS-COV2 disease, supporting the therapeutic potential of marine-derived bioactive compounds against COVID-19 (290).
Among bioactive molecules derived from marine microalgae, there are some polyphenols also known to exert antiviral activities, such as the two flavonoids kaempferol and apigenin that are natural down-regulators of ACE. Apigenin upregulates the expression of the ACE2 enzyme in kidneys inducing a blood pressure decrease effect, potentially effective for viral disease control (e.g. COVID-19). In addition, apigenin and kaempferol inhibit RAAS, which participates in virus entry into lung cells in the case of coronavirus infection (302).
Astaxanthin is another carotenoid of microalgal origin that provides a rationale to be investigated as a potential beneficial additive in COVID-19 therapeutics. Astaxanthin has potent antioxidant, anti-inflammatory, and immunomodulatory effects, can increase IL-10 secretion and exert a protective role against cytokine storm and hyper-inflammation, preventing severe complications (291, 303).
Conclusions
IL-10 is a critical mediator of host innate and adaptive immunity. It has a multifaceted nature in stimulating or inhibiting crucial immune pathways. IL-10 as an immune modulator can decrease detrimental inflammation, inhibit cancer progression, and curbing disease conditions. It has come to reviewed attention due to its important level changes in certain COVID-19 patients.
COVID-19 and its long-lasting complications resulting in post-COVID-19 syndrome have been and continue to be a global emergency and a severe challenge to healthcare systems around the world. It has been observed that the trigger of cytokine storm and the following hyper-inflammatory state are key causative factors for the development of severe symptoms and complications. Although different cytokines have been found as deregulated in COVID-19 patients, IL-10, due to its multifaceted role in modulating inflammation, appears as one of the most intriguing. Present findings support the potential of this cytokine as reliable predictor of the severity and the outcome in COVID-19 patients, as a possible danger factor and as novel strategy to counteract hyperinflammation, not only in the acute SARS-CoV-2 infection phase but also in the post-infection period.
Further studies are needed to elucidate whether exogenous administration of IL-10 or molecules able to act as adjuvant for the activation of anti-inflammatory IL-10 signaling may be beneficial for ameliorating COVID-19 and post-COVID-19 symptoms. More investment in investigation of IL-10 pathway therapy could be useful in cancer and other chronic diseases. Natural molecules have also been revealed to be modulators of this pivotal cytokine.
Author contributions
AA and DG conceptualization, EA, VC writing original draft, DN, AA, LC, GD, CS review and editing. All authors contributed to the article and approved the submitted version.
Funding
This research was supported by a grant from the Ministero della Salute COVID-2020-12371849 to DN and progetto RCR2021-23671212 on Long COVID. DN is also the recipient of a grant from Programmi di Ricerca Scientifica di Rilevante Interesse Nazionale (PRIN) Grant 2010 NECHBX_003. This research was partially funded by Stazione Zoologica Anton Dohrn and by “Antitumor Drugs and Vaccines from the Sea (ADViSE)” project [PG/2018/0494374]. Studies are partially funded by the Italian Ministry of Health Ricerca Corrente-IRCCS MultiMedica. The study was partially funded by the Italian Ministry of Health, Ricerca Corrente provided to National Institute for Infectious Diseases Lazzaro Spallanzani-IRCCS, Linea di Ricerca 1. AA is the recipient of a grant from Fattoria La Vialla, F.lli Lofranco (Castiglion Fibocchi AR).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Arango Duque G, Descoteaux A. Macrophage cytokines: involvement in immunity and infectious diseases. Front Immunol (2014) 5:491. doi: 10.3389/fimmu.2014.00491
2. Stenken JA, Poschenrieder AJ. Bioanalytical chemistry of cytokines–a review. Anal Chim Acta (2015) 853:95–115. doi: 10.1016/j.aca.2014.10.009
3. Oppenheim JJ. Cytokines: past, present, and future. Int J Hematol (2001) 74(1):3–8. doi: 10.1007/BF02982543
4. Altan-Bonnet G, Mukherjee R. Cytokine-mediated communication: a quantitative appraisal of immune complexity. Nat Rev Immunol (2019) 19(4):205–17. doi: 10.1038/s41577-019-0131-x
5. Morris R, Kershaw NJ, Babon JJ. The molecular details of cytokine signaling Via the Jak/Stat pathway. Protein Sci (2018) 27(12):1984–2009. doi: 10.1002/pro.3519
6. Lokau J, Garbers C. Biological functions and therapeutic opportunities of soluble cytokine receptors. Cytokine Growth Factor Rev (2020) 55:94–108. doi: 10.1016/j.cytogfr.2020.04.003
7. Dinarello CA. Historical insights into cytokines. Eur J Immunol (2007) 37 Suppl 1(Suppl 1):S34–45. doi: 10.1002/eji.200737772
8. Oppenheim JJ. The future of the cytokine discipline. Cold Spring Harb Perspect Biol (2018) 10(9):a028498. doi: 10.1101/cshperspect.a028498
9. Hughes CE, Nibbs RJB. A guide to chemokines and their receptors. FEBS J (2018) 285(16):2944–71. doi: 10.1111/febs.14466
10. Commins SP, Borish L, Steinke JW. Immunologic messenger molecules: cytokines, interferons, and chemokines. J Allergy Clin Immunol (2010) 125(2 Suppl 2):S53–72. doi: 10.1016/j.jaci.2009.07.008
11. Turner MD, Nedjai B, Hurst T, Pennington DJ. Cytokines and chemokines: At the crossroads of cell signalling and inflammatory disease. Biochim Biophys Acta (2014) 1843(11):2563–82. doi: 10.1016/j.bbamcr.2014.05.014
12. Silveira-Nunes G, Speziali E, Teixeira-Carvalho A, Vitelli-Avelar DM, Sathler-Avelar R, Figueiredo-Soares T, et al. Lifewide profile of cytokine production by innate and adaptive immune cells from Brazilian individuals. Immun Ageing (2017) 14:2. doi: 10.1186/s12979-017-0084-5
13. Kany S, Vollrath JT, Relja B. Cytokines in inflammatory disease. Int J Mol Sci (2019) 20(23):6008. doi: 10.3390/ijms20236008
14. Tayal V, Kalra BS. Cytokines and anti-cytokines as therapeutics–an update. Eur J Pharmacol (2008) 579(1-3):1–12. doi: 10.1016/j.ejphar.2007.10.049
15. Costela-Ruiz VJ, Illescas-Montes R, Puerta-Puerta JM, Ruiz C, Melguizo-Rodriguez L. Sars-Cov-2 infection: the role of cytokines in covid-19 disease. Cytokine Growth Factor Rev (2020) 54:62–75. doi: 10.1016/j.cytogfr.2020.06.001
16. Fara A, Mitrev Z, Rosalia RA, Assas BM. Cytokine storm and covid-19: a chronicle of pro-inflammatory cytokines. Open Biol (2020) 10(9):200160. doi: 10.1098/rsob.200160
17. Rabaan AA, Al-Ahmed SH, Muhammad J, Khan A, Sule AA, Tirupathi R, et al. Role of inflammatory cytokines in covid-19 patients: a review on molecular mechanisms, immune functions, immunopathology and immunomodulatory drugs to counter cytokine storm. Vaccines (Basel) (2021) 9(5):436. doi: 10.3390/vaccines9050436
18. Lange A, Lange J, Jaskula E. Cytokine overproduction and immune system dysregulation in allohsct and covid-19 patients. Front Immunol (2021) 12:658896. doi: 10.3389/fimmu.2021.658896
19. Montazersaheb S, Hosseiniyan Khatibi SM, Hejazi MS, Tarhriz V, Farjami A, Ghasemian Sorbeni F, et al. Covid-19 infection: an overview on cytokine storm and related interventions. Virol J (2022) 19(1):92. doi: 10.1186/s12985-022-01814-1
20. Cron RQ, Goyal G, Chatham WW. Cytokine storm syndrome. Annu Rev Med (2023) 74:321–37. doi: 10.1146/annurev-med-042921-112837
21. Hu B, Huang S, Yin L. The cytokine storm and covid-19. J Med Virol (2021) 93(1):250–6. doi: 10.1002/jmv.26232
22. Weekly epidemiological update on covid-19 world health organization: world health organization (2023). Available at: https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19—11-may-2023.
23. Cron RQ, Caricchio R, Chatham WW. Calming the cytokine storm in covid-19. Nat Med (2021) 27(10):1674–5. doi: 10.1038/s41591-021-01500-9
24. Aiyegbusi OL, Hughes SE, Turner G, Rivera SC, McMullan C, Chandan JS, et al. Symptoms, complications and management of long covid: a review. J R Soc Med (2021) 114(9):428–42. doi: 10.1177/01410768211032850
25. Crook H, Raza S, Nowell J, Young M, Edison P. Long covid-mechanisms, risk factors, and management. BMJ (2021) 374:n1648. doi: 10.1136/bmj.n1648
26. Proal AD, VanElzakker MB. Long covid or post-acute sequelae of covid-19 (Pasc): an overview of biological factors that may contribute to persistent symptoms. Front Microbiol (2021) 12:698169. doi: 10.3389/fmicb.2021.698169
27. Mantovani A, Morrone MC, Patrono C, Santoro MG, Schiaffino S, Remuzzi G, et al. Long covid: where we stand and challenges ahead. Cell Death Differ (2022) 29(10):1891–900. doi: 10.1038/s41418-022-01052-6
28. Nalbandian A, Sehgal K, Gupta A, Madhavan MV, McGroder C, Stevens JS, et al. Post-acute covid-19 syndrome. Nat Med (2021) 27(4):601–15. doi: 10.1038/s41591-021-01283-z
29. Ledford H. Do vaccines protect against long covid? what the data say. Nature (2021) 599(7886):546–8. doi: 10.1038/d41586-021-03495-2
30. Krause PR, Fleming TR, Peto R, Longini IM, Figueroa JP, Sterne JAC, et al. Considerations in boosting covid-19 vaccine immune responses. Lancet (2021) 398(10308):1377–80. doi: 10.1016/S0140-6736(21)02046-8
31. Cantini F, Goletti D, Benucci M, Foti R, Damiani A, Niccoli L. Tailored first-line biologic and targeted synthetic disease modifying anti-rheumatic drugs therapy in patients with rheumatoid arthritis: 2021 updated itabio statements. Expert Opin Drug Saf (2022) 21(5):613–23. doi: 10.1080/14740338.2022.2020247
32. Ferraccioli G, Gremese E, Goletti D, Petrone L, Cantini F, Ugel S, et al. Immune-guided therapy of covid-19. Cancer Immunol Res (2022) 10(4):384–402. doi: 10.1158/2326-6066.CIR-21-0675
33. Goletti D, Cantini F. Baricitinib therapy in covid-19 pneumonia - an unmet need fulfilled. N Engl J Med (2021) 384(9):867–9. doi: 10.1056/NEJMe2034982
34. Rizk JG, Kalantar-Zadeh K, Mehra MR, Lavie CJ, Rizk Y, Forthal DN. Pharmaco-immunomodulatory therapy in covid-19. Drugs (2020) 80(13):1267–92. doi: 10.1007/s40265-020-01367-z
35. Cavalli G, Larcher A, Tomelleri A, Campochiaro C, Della-Torre E, De Luca G, et al. Interleukin-1 and interleukin-6 inhibition compared with standard management in patients with covid-19 and hyperinflammation: a cohort study. Lancet Rheumatol (2021) 3(4):e253–e61. doi: 10.1016/S2665-9913(21)00012-6
36. Parisi V, Leosco D. Precision medicine in covid-19: il-1beta a potential target. JACC Basic Transl Sci (2020) 5(5):543–4. doi: 10.1016/j.jacbts.2020.04.006
37. Landi L, Ravaglia C, Russo E, Cataleta P, Fusari M, Boschi A, et al. Blockage of interleukin-1beta with canakinumab in patients with covid-19. Sci Rep (2020) 10(1):21775. doi: 10.1038/s41598-020-78492-y
38. Nasonov E, Samsonov M. The role of interleukin 6 inhibitors in therapy of severe covid-19. BioMed Pharmacother (2020) 131:110698. doi: 10.1016/j.biopha.2020.110698
39. Bell LCK, Meydan C, Kim J, Foox J, Butler D, Mason CE, et al. Transcriptional response modules characterize il-1beta and il-6 activity in covid-19. iScience (2021) 24(1):101896. doi: 10.1016/j.isci.2020.101896
40. Pinzon RT, Wijaya VO, Buana RB. Interleukin-6 (Il-6) inhibitors as therapeutic agents for coronavirus disease 2019 (Covid-19): a systematic review and meta-analysis. J Infect Public Health (2021) 14(8):1001–9. doi: 10.1016/j.jiph.2021.06.004
41. Zizzo G, Tamburello A, Castelnovo L, Laria A, Mumoli N, Faggioli PM, et al. Immunotherapy of covid-19: inside and beyond il-6 signalling. Front Immunol (2022) 13:795315. doi: 10.3389/fimmu.2022.795315
42. Pum A, Ennemoser M, Adage T, Kungl AJ. Cytokines and chemokines in sars-Cov-2 infections-therapeutic strategies targeting cytokine storm. Biomolecules (2021) 11(1):91. doi: 10.3390/biom11010091
43. Saraiva M, Vieira P, O’Garra A. Biology and therapeutic potential of interleukin-10. J Exp Med (2020) 217(1):e20190418. doi: 10.1084/jem.20190418
44. Gorby C, Sotolongo Bellon J, Wilmes S, Warda W, Pohler E, Fyfe PK, et al. Engineered il-10 variants elicit potent immunomodulatory effects at low ligand doses. Sci Signal (2020) 13(649):eabc0653. doi: 10.1126/scisignal.abc0653
45. Najafi-Fard S, Petruccioli E, Farroni C, Petrone L, Vanini V, Cuzzi G, et al. Evaluation of the immunomodulatory effects of interleukin-10 on peripheral blood immune cells of covid-19 patients: implication for covid-19 therapy. Front Immunol (2022) 13:984098. doi: 10.3389/fimmu.2022.984098
46. Henry BM, Benoit SW, Vikse J, Berger BA, Pulvino C, Hoehn J, et al. The anti-inflammatory cytokine response characterized by elevated interleukin-10 is a stronger predictor of severe disease and poor outcomes than the pro-inflammatory cytokine response in coronavirus disease 2019 (Covid-19). Clin Chem Lab Med (2021) 59(3):599–607. doi: 10.1515/cclm-2020-1284
47. Rojas JM, Avia M, Martin V, Sevilla N. Il-10: a multifunctional cytokine in viral infections. J Immunol Res (2017) 2017:6104054. doi: 10.1155/2017/6104054
48. Kim JM, Brannan CI, Copeland NG, Jenkins NA, Khan TA, Moore KW. Structure of the mouse il-10 gene and chromosomal localization of the mouse and human genes. J Immunol (1992) 148(11):3618–23. doi: 10.4049/jimmunol.148.11.3618
49. Sabat R, Grutz G, Warszawska K, Kirsch S, Witte E, Wolk K, et al. Biology of interleukin-10. Cytokine Growth Factor Rev (2010) 21(5):331–44. doi: 10.1016/j.cytogfr.2010.09.002
50. Ouyang W, O’Garra A. Il-10 family cytokines il-10 and il-22: from basic science to clinical translation. Immunity (2019) 50(4):871–91. doi: 10.1016/j.immuni.2019.03.020
51. Minshawi F, Lanvermann S, McKenzie E, Jeffery R, Couper K, Papoutsopoulou S, et al. The generation of an engineered interleukin-10 protein with improved stability and biological function. Front Immunol (2020) 11:1794. doi: 10.3389/fimmu.2020.01794
52. Zhu L, Shi T, Zhong C, Wang Y, Chang M, Liu X. Il-10 and il-10 receptor mutations in very early onset inflammatory bowel disease. Gastroenterol Res (2017) 10(2):65–9. doi: 10.14740/gr740w
53. Krawiec P, Pawlowska-Kamieniak A, Pac-Kozuchowska E. Interleukin 10 and interleukin 10 receptor in paediatric inflammatory bowel disease: from bench to bedside lesson. J Inflammation (Lond) (2021) 18(1):13. doi: 10.1186/s12950-021-00279-3
54. Llopiz D, Ruiz M, Infante S, Villanueva L, Silva L, Hervas-Stubbs S, et al. Il-10 expression defines an immunosuppressive dendritic cell population induced by antitumor therapeutic vaccination. Oncotarget (2017) 8(2):2659–71. doi: 10.18632/oncotarget.13736
55. Shouval DS, Ouahed J, Biswas A, Goettel JA, Horwitz BH, Klein C, et al. Interleukin 10 receptor signaling: master regulator of intestinal mucosal homeostasis in mice and humans. Adv Immunol (2014) 122:177–210. doi: 10.1016/B978-0-12-800267-4.00005-5
56. Laudisi F, Cherubini F, Monteleone G, Stolfi C. Stat3 interactors as potential therapeutic targets for cancer treatment. Int J Mol Sci (2018) 19(6):1787. doi: 10.3390/ijms19061787
57. Murray PJ. Understanding and exploiting the endogenous interleukin-10/Stat3-Mediated anti-inflammatory response. Curr Opin Pharmacol (2006) 6(4):379–86. doi: 10.1016/j.coph.2006.01.010
58. Verma R, Balakrishnan L, Sharma K, Khan AA, Advani J, Gowda H, et al. A network map of interleukin-10 signaling pathway. J Cell Commun Signal (2016) 10(1):61–7. doi: 10.1007/s12079-015-0302-x
59. Schulke S. Induction of interleukin-10 producing dendritic cells as a tool to suppress allergen-specific T helper 2 responses. Front Immunol (2018) 9:455. doi: 10.3389/fimmu.2018.00455
60. Mogensen TH. Irf and stat transcription factors - from basic biology to roles in infection, protective immunity, and primary immunodeficiencies. Front Immunol (2018) 9:3047. doi: 10.3389/fimmu.2018.03047
61. Sharifinejad N, Zaki-Dizaji M, Sepahvandi R, Fayyaz F, Dos Santos Vilela MM, ElGhazali G, et al. The clinical, molecular, and therapeutic features of patients with Il10/Il10r deficiency: a systematic review. Clin Exp Immunol (2022) 208(3):281–91. doi: 10.1093/cei/uxac040
62. Sasahara Y, Uchida T, Suzuki T, Abukawa D. Primary immunodeficiencies associated with early-onset inflammatory bowel disease in southeast and East Asia. Front Immunol (2021) 12:786538. doi: 10.3389/fimmu.2021.786538
63. Neven B, Mamessier E, Bruneau J, Kaltenbach S, Kotlarz D, Suarez F, et al. A mendelian predisposition to b-cell lymphoma caused by il-10r deficiency. Blood (2013) 122(23):3713–22. doi: 10.1182/blood-2013-06-508267
64. Riaz IB, Faridi W, Patnaik MM, Abraham RS. A systematic review on predisposition to lymphoid (B and T cell) neoplasias in patients with primary immunodeficiencies and immune dysregulatory disorders (Inborn errors of immunity). Front Immunol (2019) 10:777. doi: 10.3389/fimmu.2019.00777
65. Fiorentino DF, Bond MW, Mosmann TR. Two types of mouse T helper cell. iv. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J Exp Med (1989) 170(6):2081–95. doi: 10.1084/jem.170.6.2081
66. Lobo-Silva D, Carriche GM, Castro AG, Roque S, Saraiva M. Balancing the immune response in the brain: il-10 and its regulation. J Neuroinflamm (2016) 13(1):297. doi: 10.1186/s12974-016-0763-8
67. Hulsmans M, Sager HB, Roh JD, Valero-Munoz M, Houstis NE, Iwamoto Y, et al. Cardiac macrophages promote diastolic dysfunction. J Exp Med (2018) 215(2):423–40. doi: 10.1084/jem.20171274
68. Koh B, Hufford MM, Sun X, Kaplan MH. Etv5 regulates il-10 production in Th cells. J Immunol (2017) 198(5):2165–71. doi: 10.4049/jimmunol.1600801
69. Saraiva M, Christensen JR, Veldhoen M, Murphy TL, Murphy KM, O’Garra A. Interleukin-10 production by Th1 cells requires interleukin-12-Induced Stat4 transcription factor and erk map kinase activation by high antigen dose. Immunity (2009) 31(2):209–19. doi: 10.1016/j.immuni.2009.05.012
70. Jin JO, Han X, Yu Q. Interleukin-6 induces the generation of il-10-Producing Tr1 cells and suppresses autoimmune tissue inflammation. J Autoimmun (2013) 40:28–44. doi: 10.1016/j.jaut.2012.07.009
71. Sie C, Kant R, Peter C, Muschaweckh A, Pfaller M, Nirschl L, et al. Il-24 intrinsically regulates Th17 cell pathogenicity in mice. J Exp Med (2022) 219(8):e20212443. doi: 10.1084/jem.20212443
72. Chang KK, Liu LB, Jin LP, Zhang B, Mei J, Li H, et al. Il-27 triggers il-10 production in Th17 cells Via a c-Maf/Rorgammat/Blimp-1 signal to promote the progression of endometriosis. Cell Death Dis (2017) 8(3):e2666. doi: 10.1038/cddis.2017.95
73. Stumhofer JS, Silver JS, Laurence A, Porrett PM, Harris TH, Turka LA, et al. Interleukins 27 and 6 induce Stat3-mediated T cell production of interleukin 10. Nat Immunol (2007) 8(12):1363–71. doi: 10.1038/ni1537
74. Ng TH, Britton GJ, Hill EV, Verhagen J, Burton BR, Wraith DC. Regulation of adaptive immunity; the role of interleukin-10. Front Immunol (2013) 4:129. doi: 10.3389/fimmu.2013.00129
75. Saraiva M, O’Garra A. The regulation of il-10 production by immune cells. Nat Rev Immunol (2010) 10(3):170–81. doi: 10.1038/nri2711
76. Gabrysova L, Howes A, Saraiva M, O’Garra A. The regulation of il-10 expression. Curr Top Microbiol Immunol (2014) 380:157–90. doi: 10.1007/978-3-662-43492-5_8
77. Howes A, Gabryšová L, O’Garra A. Role of IL-10 and the IL-10 Receptor in Immune Responses, Reference Module in Biomedical Sciences. Elsevier (2014). doi: 10.1016/B978-0-12-801238-3.00014-3
78. Vuillefroy de Silly R, Ducimetiere L, Yacoub Maroun C, Dietrich PY, Derouazi M, Walker PR. Phenotypic switch of Cd8(+) T cells reactivated under hypoxia toward il-10 secreting, poorly proliferative effector cells. Eur J Immunol (2015) 45(8):2263–75. doi: 10.1002/eji.201445284
79. Iyer SS, Cheng G. Role of interleukin 10 transcriptional regulation in inflammation and autoimmune disease. Crit Rev Immunol (2012) 32(1):23–63. doi: 10.1615/critrevimmunol.v32.i1.30
80. Martinez-Espinosa I, Serrato JA, Ortiz-Quintero B. Role of il-10-Producing natural killer cells in the regulatory mechanisms of inflammation during systemic infection. Biomolecules (2021) 12(1):4. doi: 10.3390/biom12010004
81. Davey MS, Tamassia N, Rossato M, Bazzoni F, Calzetti F, Bruderek K, et al. Failure to detect production of il-10 by activated human neutrophils. Nat Immunol (2011) 12(11):1017–8. doi: 10.1038/ni.2111
82. Castellucci M, Rossato M, Calzetti F, Tamassia N, Zeminian S, Cassatella MA, et al. Il-10 disrupts the Brd4-docking sites to inhibit lps-induced Cxcl8 and tnf-alpha expression in monocytes: implications for chronic obstructive pulmonary disease. J Allergy Clin Immunol (2015) 136(3):781–91 e9. doi: 10.1016/j.jaci.2015.04.023
83. Nguyen HD, Aljamaei HM, Stadnyk AW. The production and function of endogenous interleukin-10 in intestinal epithelial cells and gut homeostasis. Cell Mol Gastroenterol Hepatol (2021) 12(4):1343–52. doi: 10.1016/j.jcmgh.2021.07.005
84. Mestrallet G, Rouas-Freiss N, LeMaoult J, Fortunel NO, Martin MT. Skin immunity and tolerance: focus on epidermal keratinocytes expressing hla-G. Front Immunol (2021) 12:772516. doi: 10.3389/fimmu.2021.772516
85. Piipponen M, Li D, Landen NX. The immune functions of keratinocytes in skin wound healing. Int J Mol Sci (2020) 21(22):8790. doi: 10.3390/ijms21228790
86. Ciazynska M, Olejniczak-Staruch I, Sobolewska-Sztychny D, Narbutt J, Skibinska M, Lesiak A. Ultraviolet radiation and chronic inflammation-molecules and mechanisms involved in skin carcinogenesis: a narrative review. Life (Basel) (2021) 11(4):326. doi: 10.3390/life11040326
87. Itakura E, Huang RR, Wen DR, Paul E, Wunsch PH, Cochran AJ. Il-10 expression by primary tumor cells correlates with melanoma progression from radial to vertical growth phase and development of metastatic competence. Mod Pathol (2011) 24(6):801–9. doi: 10.1038/modpathol.2011.5
88. Qian Q, Wu C, Chen J, Wang W. Relationship between il-10 and pd-L1 in esophageal carcinoma tissues and il-10 down-regulates pd-L1 expression Via met signaling pathway. J Gastrointest Oncol (2020) 11(2):337–55. doi: 10.21037/jgo.2020.01.06
89. Labani-Motlagh A, Ashja-Mahdavi M, Loskog A. The tumor microenvironment: a milieu hindering and obstructing antitumor immune responses. Front Immunol (2020) 11:940. doi: 10.3389/fimmu.2020.00940
90. Herbeuval JP, Lelievre E, Lambert C, Dy M, Genin C. Recruitment of Stat3 for production of il-10 by colon carcinoma cells induced by macrophage-derived il-6. J Immunol (2004) 172(7):4630–6. doi: 10.4049/jimmunol.172.7.4630
91. Briukhovetska D, Dorr J, Endres S, Libby P, Dinarello CA, Kobold S. Interleukins in cancer: from biology to therapy. Nat Rev Cancer (2021) 21(8):481–99. doi: 10.1038/s41568-021-00363-z
92. Wilke CM, Wei S, Wang L, Kryczek I, Kao J, Zou W. Dual biological effects of the cytokines interleukin-10 and interferon-gamma. Cancer Immunol Immunother (2011) 60(11):1529–41. doi: 10.1007/s00262-011-1104-5
93. Penaloza HF, Schultz BM, Nieto PA, Salazar GA, Suazo I, Gonzalez PA, et al. Opposing roles of il-10 in acute bacterial infection. Cytokine Growth Factor Rev (2016) 32:17–30. doi: 10.1016/j.cytogfr.2016.07.003
94. Cyktor JC, Turner J. Interleukin-10 and immunity against prokaryotic and eukaryotic intracellular pathogens. Infect Immun (2011) 79(8):2964–73. doi: 10.1128/IAI.00047-11
95. Mittal SK, Roche PA. Suppression of antigen presentation by il-10. Curr Opin Immunol (2015) 34:22–7. doi: 10.1016/j.coi.2014.12.009
96. Mittal SK, Cho KJ, Ishido S, Roche PA. Interleukin 10 (Il-10)-Mediated immunosuppression: march-I induction regulates antigen presentation by macrophages but not dendritic cells. J Biol Chem (2015) 290(45):27158–67. doi: 10.1074/jbc.M115.682708
97. Bandola-Simon J, Roche PA. Dysfunction of antigen processing and presentation by dendritic cells in cancer. Mol Immunol (2019) 113:31–7. doi: 10.1016/j.molimm.2018.03.025
98. Wilson EB, Brooks DG. The role of il-10 in regulating immunity to persistent viral infections. Curr Top Microbiol Immunol (2011) 350:39–65. doi: 10.1007/82_2010_96
99. Bedke T, Muscate F, Soukou S, Gagliani N, Huber S. Il-10-Producing T cells and their dual functions. Semin Immunol (2019) 44:101335. doi: 10.1016/j.smim.2019.101335
100. Kumar R, Ng S, Engwerda C. The role of il-10 in malaria: a double edged sword. Front Immunol (2019) 10:229. doi: 10.3389/fimmu.2019.00229
101. Goletti D, Weissman D, Jackson RW, Collins F, Kinter A, Fauci AS. The in vitro induction of human immunodeficiency virus (Hiv) replication in purified protein derivative-positive hiv-infected persons by recall antigen response to mycobacterium tuberculosis is the result of a balance of the effects of endogenous interleukin-2 and proinflammatory and antiinflammatory cytokines. J Infect Dis (1998) 177(5):1332–8. doi: 10.1086/515276
102. Taylor A, Akdis M, Joss A, Akkoc T, Wenig R, Colonna M, et al. Il-10 inhibits Cd28 and icos costimulations of T cells Via src homology 2 domain-containing protein tyrosine phosphatase 1. J Allergy Clin Immunol (2007) 120(1):76–83. doi: 10.1016/j.jaci.2007.04.004
103. Eiwegger T, Gruber S, Szepfalusi Z, Akdis CA. Novel developments in the mechanisms of immune tolerance to allergens. Hum Vaccin Immunother (2012) 8(10):1485–91. doi: 10.4161/hv.20903
104. Heine G, Drozdenko G, Grun JR, Chang HD, Radbruch A, Worm M. Autocrine il-10 promotes human b-cell differentiation into igm- or igg-secreting plasmablasts. Eur J Immunol (2014) 44(6):1615–21. doi: 10.1002/eji.201343822
105. Laidlaw BJ, Lu Y, Amezquita RA, Weinstein JS, Vander Heiden JA, Gupta NT, et al. Interleukin-10 from Cd4(+) follicular regulatory T cells promotes the germinal center response. Sci Immunol (2017) 2(16):eaan4767. doi: 10.1126/sciimmunol.aan4767
106. Rubtsov YP, Rasmussen JP, Chi EY, Fontenot J, Castelli L, Ye X, et al. Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity (2008) 28(4):546–58. doi: 10.1016/j.immuni.2008.02.017
107. Hsu P, Santner-Nanan B, Hu M, Skarratt K, Lee CH, Stormon M, et al. Il-10 potentiates differentiation of human induced regulatory T cells Via Stat3 and Foxo1. J Immunol (2015) 195(8):3665–74. doi: 10.4049/jimmunol.1402898
108. Wang S, Wang J, Ma R, Yang S, Fan T, Cao J, et al. Il-10 enhances T cell survival and is associated with faster relapse in patients with inactive ulcerative colitis. Mol Immunol (2020) 121:92–8. doi: 10.1016/j.molimm.2020.03.001
109. Yogev N, Bedke T, Kobayashi Y, Brockmann L, Lukas D, Regen T, et al. Cd4(+) T-Cell-Derived il-10 promotes cns inflammation in mice by sustaining effector T cell survival. Cell Rep (2022) 38(13):110565. doi: 10.1016/j.celrep.2022.110565
110. de Gruijter NM, Jebson B, Rosser EC. Cytokine production by human b cells: role in health and autoimmune disease. Clin Exp Immunol (2022) 210(3):253–62. doi: 10.1093/cei/uxac090
111. Matsumura Y, Watanabe R, Fujimoto M. Suppressive mechanisms of regulatory b cells in mice and humans. Int Immunol (2023) 35(2):55–65. doi: 10.1093/intimm/dxac048
112. Oft M. Immune regulation and cytotoxic T cell activation of il-10 agonists - preclinical and clinical experience. Semin Immunol (2019) 44:101325. doi: 10.1016/j.smim.2019.101325
113. Mocellin S, Panelli M, Wang E, Rossi CR, Pilati P, Nitti D, et al. Il-10 stimulatory effects on human nk cells explored by gene profile analysis. Genes Immun (2004) 5(8):621–30. doi: 10.1038/sj.gene.6364135
114. Park JY, Lee SH, Yoon SR, Park YJ, Jung H, Kim TD, et al. Il-15-Induced il-10 increases the cytolytic activity of human natural killer cells. Mol Cells (2011) 32(3):265–72. doi: 10.1007/s10059-011-1057-8
115. Mannino MH, Zhu Z, Xiao H, Bai Q, Wakefield MR, Fang Y. The paradoxical role of il-10 in immunity and cancer. Cancer Lett (2015) 367(2):103–7. doi: 10.1016/j.canlet.2015.07.009
116. Wang Z, Guan D, Huo J, Biswas SK, Huang Y, Yang Y, et al. Il-10 enhances human natural killer cell effector functions Via metabolic reprogramming regulated by Mtorc1 signaling. Front Immunol (2021) 12:619195. doi: 10.3389/fimmu.2021.619195
117. Polukort SH, Rovatti J, Carlson L, Thompson C, Ser-Dolansky J, Kinney SR, et al. Il-10 enhances ige-mediated mast cell responses and is essential for the development of experimental food allergy in il-10-Deficient mice. J Immunol (2016) 196(12):4865–76. doi: 10.4049/jimmunol.1600066
118. MacNeil IA, Suda T, Moore KW, Mosmann TR, Zlotnik A. Il-10, a novel growth cofactor for mature and immature T cells. J Immunol (1990) 145(12):4167–73. doi: 10.4049/jimmunol.145.12.4167
119. Chen WF, Zlotnik A. Il-10: a novel cytotoxic T cell differentiation factor. J Immunol (1991) 147(2):528–34. doi: 10.4049/jimmunol.147.2.528
120. Abraham S, Choi JG, Ye C, Manjunath N, Shankar P. Il-10 exacerbates xenogeneic gvhd by inducing massive human T cell expansion. Clin Immunol (2015) 156(1):58–64. doi: 10.1016/j.clim.2014.11.004
121. Vasseur P, Devaure I, Sellier J, Delwail A, Chagneau-Derrode C, Charier F, et al. High plasma levels of the pro-inflammatory cytokine il-22 and the anti-inflammatory cytokines il-10 and il-1ra in acute pancreatitis. Pancreatology (2014) 14(6):465–9. doi: 10.1016/j.pan.2014.08.005
122. Russell MA, Morgan NG. The impact of anti-inflammatory cytokines on the pancreatic beta-cell. Islets (2014) 6(3):e950547. doi: 10.4161/19382014.2014.950547
123. Li C, Zhang L, Chen Y, Lin X, Li T. Protective role of adenovirus vector-mediated interleukin-10 gene therapy on endogenous islet beta-cells in recent-onset type 1 diabetes in nod mice. Exp Ther Med (2016) 11(5):1625–32. doi: 10.3892/etm.2016.3169
124. Porro C, Cianciulli A, Panaro MA. The regulatory role of il-10 in neurodegenerative diseases. Biomolecules (2020) 10(7):1017. doi: 10.3390/biom10071017
125. Short WD, Steen E, Kaul A, Wang X, Olutoye OO 2nd, Vangapandu HV, et al. Il-10 promotes endothelial progenitor cell infiltration and wound healing Via Stat3. FASEB J (2022) 36(7):e22298. doi: 10.1096/fj.201901024RR
126. Zheng Z, Huang G, Gao T, Huang T, Zou M, Zou Y, et al. Epigenetic changes associated with interleukin-10. Front Immunol (2020) 11:1105. doi: 10.3389/fimmu.2020.01105
127. Zhang H, Kuchroo V. Epigenetic and transcriptional mechanisms for the regulation of il-10. Semin Immunol (2019) 44:101324. doi: 10.1016/j.smim.2019.101324
128. Rutz S, Ouyang W. Regulation of interleukin-10 expression. Adv Exp Med Biol (2016) 941:89–116. doi: 10.1007/978-94-024-0921-5_5
129. Stanfield BA, Purves T, Palmer S, Sullenger B, Welty-Wolf K, Haines K, et al. Il-10 and class 1 histone deacetylases act synergistically and independently on the secretion of proinflammatory mediators in alveolar macrophages. PloS One (2021) 16(1):e0245169. doi: 10.1371/journal.pone.0245169
130. Wilson EH, Wille-Reece U, Dzierszinski F, Hunter CA. A critical role for il-10 in limiting inflammation during toxoplasmic encephalitis. J Neuroimmunol (2005) 165(1-2):63–74. doi: 10.1016/j.jneuroim.2005.04.018
131. Hovsepian E, Penas F, Siffo S, Mirkin GA, Goren NB. Il-10 inhibits the nf-kappab and Erk/Mapk-mediated production of pro-inflammatory mediators by up-regulation of socs-3 in trypanosoma cruzi-infected cardiomyocytes. PloS One (2013) 8(11):e79445. doi: 10.1371/journal.pone.0079445
132. Kullberg MC, Rothfuchs AG, Jankovic D, Caspar P, Wynn TA, Gorelick PL, et al. Helicobacter hepaticus-induced colitis in interleukin-10-Deficient mice: cytokine requirements for the induction and maintenance of intestinal inflammation. Infect Immun (2001) 69(7):4232–41. doi: 10.1128/IAI.69.7.4232-4241.2001
133. Danne C, Ryzhakov G, Martinez-Lopez M, Ilott NE, Franchini F, Cuskin F, et al. A Large polysaccharide produced by helicobacter hepaticus induces an anti-inflammatory gene signature in macrophages. Cell Host Microbe (2017) 22(6):733–45 e5. doi: 10.1016/j.chom.2017.11.002
134. Sun J, Madan R, Karp CL, Braciale TJ. Effector T cells control lung inflammation during acute influenza virus infection by producing il-10. Nat Med (2009) 15(3):277–84. doi: 10.1038/nm.1929
135. Jeong YI, Hong SH, Cho SH, Park MY, Lee SE. Induction of il-10-Producing regulatory b cells following toxoplasma gondii infection is important to the cyst formation. Biochem Biophys Rep (2016) 7:91–7. doi: 10.1016/j.bbrep.2016.05.008
136. Anderson CF, Lira R, Kamhawi S, Belkaid Y, Wynn TA, Sacks D. Il-10 and tgf-beta control the establishment of persistent and transmissible infections produced by leishmania tropica in C57bl/6 mice. J Immunol (2008) 180(6):4090–7. doi: 10.4049/jimmunol.180.6.4090
137. Gautam S, Kumar R, Maurya R, Nylen S, Ansari N, Rai M, et al. Il-10 neutralization promotes parasite clearance in splenic aspirate cells from patients with visceral leishmaniasis. J Infect Dis (2011) 204(7):1134–7. doi: 10.1093/infdis/jir461
138. Samanta M, Iwakiri D, Takada K. Epstein-Barr Virus-encoded small rna induces il-10 through rig-I-Mediated irf-3 signaling. Oncogene (2008) 27(30):4150–60. doi: 10.1038/onc.2008.75
139. Brockman MA, Kwon DS, Tighe DP, Pavlik DF, Rosato PC, Sela J, et al. Il-10 is up-regulated in multiple cell types during viremic hiv infection and reversibly inhibits virus-specific T cells. Blood (2009) 114(2):346–56. doi: 10.1182/blood-2008-12-191296
140. Pereira Ribeiro S, Aid M, Dupuy FP, Chan CN, Hultquist J, Delage C, et al. Il-10 driven memory T cell survival and tfh differentiation promote hiv persistence. bioRxiv (2021). doi: 10.1101/2021.02.26.432955
141. Hyodo N, Nakamura I, Imawari M. Hepatitis b core antigen stimulates interleukin-10 secretion by both T cells and monocytes from peripheral blood of patients with chronic hepatitis b virus infection. Clin Exp Immunol (2004) 135(3):462–6. doi: 10.1111/j.1365-2249.2003.02376.x
142. Liu C, Shih YF, Liu CJ. Immunopathogenesis of acute flare of chronic hepatitis b: with emphasis on the role of cytokines and chemokines. Int J Mol Sci (2022) 23(3):1407. doi: 10.3390/ijms23031407
143. Abd Elazeem MI, Mohammed RA, Abdallah NH. Correlation of serum interleukin-10 level with disease activity and severity in systemic lupus erythematosus. Egyptian Rheumatol Rehabil (2018) 45(1):25–33. doi: 10.4103/err.err_15_17
144. Roman-Fernandez IV, Machado-Contreras JR, Munoz-Valle JF, Cruz A, Salazar-Camarena DC, Palafox-Sanchez CA. Altered Ptpn22 and Il10 mrna expression is associated with disease activity and renal involvement in systemic lupus erythematosus. Diagnostics (Basel) (2022) 12(11):2859. doi: 10.3390/diagnostics12112859
145. Shrivastava AK, Singh HV, Raizada A, Singh SK, Pandey A, Singh N, et al. Inflammatory markers in patients with rheumatoid arthritis. Allergol Immunopathol (Madr) (2015) 43(1):81–7. doi: 10.1016/j.aller.2013.11.003
146. Howard Tripp N, Tarn J, Natasari A, Gillespie C, Mitchell S, Hackett KL, et al. Fatigue in primary sjogren’s syndrome is associated with lower levels of proinflammatory cytokines. RMD Open (2016) 2(2):e000282. doi: 10.1136/rmdopen-2016-000282
147. Lopez-Nevado M, Gonzalez-Granado LI, Ruiz-Garcia R, Pleguezuelo D, Cabrera-Marante O, Salmon N, et al. Primary immune regulatory disorders with an autoimmune lymphoproliferative syndrome-like phenotype: immunologic evaluation, early diagnosis and management. Front Immunol (2021) 12:671755. doi: 10.3389/fimmu.2021.671755
148. Melgar S, Yeung MM, Bas A, Forsberg G, Suhr O, Oberg A, et al. Over-expression of interleukin 10 in mucosal T cells of patients with active ulcerative colitis. Clin Exp Immunol (2003) 134(1):127–37. doi: 10.1046/j.1365-2249.2003.02268.x
149. Takeoka K, Watanabe M, Matsuzuka F, Miyauchi A, Iwatani Y. Increase of serum interleukin-10 in intractable graves’ disease. Thyroid (2004) 14(3):201–5. doi: 10.1089/105072504773297876
150. Redpath SA, Fonseca NM, Perona-Wright G. Protection and pathology during parasite infection: il-10 strikes the balance. Parasite Immunol (2014) 36(6):233–52. doi: 10.1111/pim.12113
151. Barry JC, Shakibakho S, Durrer C, Simtchouk S, Jawanda KK, Cheung ST, et al. Hyporesponsiveness to the anti-inflammatory action of interleukin-10 in type 2 diabetes. Sci Rep (2016) 6:21244. doi: 10.1038/srep21244
152. Keubler LM, Buettner M, Hager C, Bleich A. A multihit model: colitis lessons from the interleukin-10-Deficient mouse. Inflammation Bowel Dis (2015) 21(8):1967–75. doi: 10.1097/MIB.0000000000000468
153. Gunasekera DC, Ma J, Vacharathit V, Shah P, Ramakrishnan A, Uprety P, et al. The development of colitis in Il10(-/-) mice is dependent on il-22. Mucosal Immunol (2020) 13(3):493–506. doi: 10.1038/s41385-019-0252-3
154. Pino-Martinez AM, Miranda CG, Batalla EI, Gonzalez-Cappa SM, Alba Soto CD. Il-10 participates in the expansion and functional activation of Cd8(+) T cells during acute infection with trypanosoma cruzi. J Leukoc Biol (2019) 105(1):163–75. doi: 10.1002/JLB.3A0318-111RR
155. O’Brien CA, Batista SJ, Still KM, Harris TH. Il-10 and icos differentially regulate T cell responses in the brain during chronic toxoplasma gondii infection. J Immunol (2019) 202(6):1755–66. doi: 10.4049/jimmunol.1801229
156. Zhang LJ, Wang XZ. Interleukin-10 and chronic liver disease. World J Gastroenterol (2006) 12(11):1681–5. doi: 10.3748/wjg.v12.i11.1681
157. Wei W, Zhao Y, Zhang Y, Jin H, Shou S. The role of il-10 in kidney disease. Int Immunopharmacol (2022) 108:108917. doi: 10.1016/j.intimp.2022.108917
158. Kutwin M, Migdalska-Sek M, Brzezianska-Lasota E, Zelga P, Wozniacka A. An analysis of il-10, il-17a, il-17ra, il-23a and il-23r expression and their correlation with clinical course in patients with psoriasis. J Clin Med (2021) 10(24):5834. doi: 10.3390/jcm10245834
159. Traupe H. Psoriasis and the interleukin-10 family: evidence for a protective genetic effect, but not an easy target as a drug. Br J Dermatol (2017) 176(6):1438–9. doi: 10.1111/bjd.15158
160. Raeiszadeh Jahromi S, Mahesh PA, Jayaraj BS, Madhunapantula SR, Holla AD, Vishweswaraiah S, et al. Serum levels of il-10, il-17f and il-33 in patients with asthma: a case-control study. J Asthma (2014) 51(10):1004–13. doi: 10.3109/02770903.2014.938353
161. Glocker EO, Kotlarz D, Klein C, Shah N, Grimbacher B. Il-10 and il-10 receptor defects in humans. Ann N Y Acad Sci (2011) 1246:102–7. doi: 10.1111/j.1749-6632.2011.06339.x
162. Shah N, Kammermeier J, Elawad M, Glocker EO. Interleukin-10 and interleukin-10-Receptor defects in inflammatory bowel disease. Curr Allergy Asthma Rep (2012) 12(5):373–9. doi: 10.1007/s11882-012-0286-z
163. Grifoni A, Alonzi T, Alter G, Noonan DM, Landay AL, Albini A, et al. Impact of aging on immunity in the context of covid-19, hiv, and tuberculosis. Front Immunol (2023) 14:1146704. doi: 10.3389/fimmu.2023.1146704
164. Salminen A. Immunosuppressive network promotes immunosenescence associated with aging and chronic inflammatory conditions. J Mol Med (2021) 99(11):1553–69. doi: 10.1007/s00109-021-02123-w
165. Oft M. Il-10: master switch from tumor-promoting inflammation to antitumor immunity. Cancer Immunol Res (2014) 2(3):194–9. doi: 10.1158/2326-6066.CIR-13-0214
166. Rallis KS, Corrigan AE, Dadah H, Stanislovas J, Zamani P, Makker S, et al. Il-10 in cancer: an essential thermostatic regulator between homeostatic immunity and inflammation - a comprehensive review. Future Oncol (2022) 18(29):3349–65. doi: 10.2217/fon-2022-0063
167. Mocellin S, Marincola FM, Young HA. Interleukin-10 and the immune response against cancer: a counterpoint. J Leukoc Biol (2005) 78(5):1043–51. doi: 10.1189/jlb.0705358
168. Chaudhry A, Samstein RM, Treuting P, Liang Y, Pils MC, Heinrich JM, et al. Interleukin-10 signaling in regulatory T cells is required for suppression of Th17 cell-mediated inflammation. Immunity (2011) 34(4):566–78. doi: 10.1016/j.immuni.2011.03.018
169. Qiao J, Liu Z, Dong C, Luan Y, Zhang A, Moore C, et al. Targeting tumors with il-10 prevents dendritic cell-mediated Cd8(+) T cell apoptosis. Cancer Cell (2019) 35(6):901–15 e4. doi: 10.1016/j.ccell.2019.05.005
170. Feng L, Qi Q, Wang P, Chen H, Chen Z, Meng Z, et al. Serum levels of il-6, il-8, and il-10 are indicators of prognosis in pancreatic cancer. J Int Med Res (2018) 46(12):5228–36. doi: 10.1177/0300060518800588
171. Chen L, Shi Y, Zhu X, Guo W, Zhang M, Che Y, et al. Il−10 secreted by Cancer−Associated macrophages regulates proliferation and invasion in gastric cancer cells Via C−Met/Stat3 signaling. Oncol Rep (2019) 42(2):595–604. doi: 10.3892/or.2019.7206
172. Hazini A, Fisher K, Seymour L. Deregulation of hla-I in cancer and its central importance for immunotherapy. J Immunother Cancer (2021) 9(8):e02899. doi: 10.1136/jitc-2021-002899
173. Wang R, Lu M, Zhang J, Chen S, Luo X, Qin Y, et al. Increased il-10 mrna expression in tumor-associated macrophage correlated with late stage of lung cancer. J Exp Clin Cancer Res (2011) 30(1):62. doi: 10.1186/1756-9966-30-62
174. Czajka-Francuz P, Francuz T, Cison-Jurek S, Czajka A, Fajkis M, Szymczak B, et al. Serum cytokine profile as a potential prognostic tool in colorectal cancer patients - one center study. Rep Pract Oncol Radiother (2020) 25(6):867–75. doi: 10.1016/j.rpor.2020.08.004
175. Ikeguchi M, Hatada T, Yamamoto M, Miyake T, Matsunaga T, Fukumoto Y, et al. Serum interleukin-6 and -10 levels in patients with gastric cancer. Gastric Cancer (2009) 12(2):95–100. doi: 10.1007/s10120-009-0509-8
176. Lippitz BE. Cytokine patterns in patients with cancer: a systematic review. Lancet Oncol (2013) 14(6):e218–28. doi: 10.1016/S1470-2045(12)70582-X
177. Zhao S, Wu D, Wu P, Wang Z, Huang J. Serum il-10 predicts worse outcome in cancer patients: a meta-analysis. PloS One (2015) 10(10):e0139598. doi: 10.1371/journal.pone.0139598
178. Hsu TI, Wang YC, Hung CY, Yu CH, Su WC, Chang WC, et al. Positive feedback regulation between Il10 and egfr promotes lung cancer formation. Oncotarget (2016) 7(15):20840–54. doi: 10.18632/oncotarget.7894
179. Lecot P, Alimirah F, Desprez PY, Campisi J, Wiley C. Context-dependent effects of cellular senescence in cancer development. Br J Cancer (2016) 114(11):1180–4. doi: 10.1038/bjc.2016.115
180. Xu G, Wang F. Associations of polymorphisms in interleukins with susceptibility to breast cancer: evidence from a meta-analysis. Cytokine (2020) 130:154988. doi: 10.1016/j.cyto.2020.154988
181. Lee HM, Lee HJ, Chang JE. Inflammatory cytokine: an attractive target for cancer treatment. Biomedicines (2022) 10(9):2116. doi: 10.3390/biomedicines10092116
182. Shokrzadeh M, Mohammadpour A, Hoseini V, Abediankenari S, Ghassemi-Barghi N, Tabari YS. Serum cytokine of il-2, il-10 and il-12 levels in patients with stomach adenocarcinoma. Arq Gastroenterol (2018) 55(4):385–9. doi: 10.1590/S0004-2803.201800000-83
183. Kim Y, Yang H, Lee WS, Cheon J, Sang YB, Kang B, et al. High levels of baseline serum il-10 are associated with reduced clinical benefit from first-line immune checkpoint inhibitor therapy in advanced renal cell carcinoma. J Cancer (2023) 14(6):935–42. doi: 10.7150/jca.81384
184. Kohno T, Mizukami H, Suzuki M, Saga Y, Takei Y, Shimpo M, et al. Interleukin-10-Mediated inhibition of angiogenesis and tumor growth in mice bearing vegf-producing ovarian cancer. Cancer Res (2003) 63(16):5091–4.
185. Stewart CA, Metheny H, Iida N, Smith L, Hanson M, Steinhagen F, et al. Interferon-dependent il-10 production by tregs limits tumor Th17 inflammation. J Clin Invest (2013) 123(11):4859–74. doi: 10.1172/JCI65180
186. Guo B. Il-10 modulates Th17 pathogenicity during autoimmune diseases. J Clin Cell Immunol (2016) 7(2):400. doi: 10.4172/2155-9899.1000400
187. Li Q, Anderson CD, Egilmez NK. Inhaled il-10 suppresses lung tumorigenesis Via abrogation of inflammatory macrophage-Th17 cell axis. J Immunol (2018) 201(9):2842–50. doi: 10.4049/jimmunol.1800141
188. Morris RM, Mortimer TO, O’Neill KL. Cytokines: can cancer get the message? Cancers (Basel) (2022) 14(9):2178. doi: 10.3390/cancers14092178
189. Ni G, Zhang L, Yang X, Li H, Ma B, Walton S, et al. Targeting interleukin-10 signalling for cancer immunotherapy, a promising and complicated task. Hum Vaccin Immunother (2020) 16(10):2328–32. doi: 10.1080/21645515.2020.1717185
190. Kwilasz AJ, Grace PM, Serbedzija P, Maier SF, Watkins LR. The therapeutic potential of interleukin-10 in neuroimmune diseases. Neuropharmacology (2015) 96(Pt A):55–69. doi: 10.1016/j.neuropharm.2014.10.020
191. Wang J, Yang X, Li Y, Huang JA, Jiang J, Su N. Specific cytokines in the inflammatory cytokine storm of patients with covid-19-Associated acute respiratory distress syndrome and extrapulmonary multiple-organ dysfunction. Virol J (2021) 18(1):117. doi: 10.1186/s12985-021-01588-y
192. Wei HX, Wang B, Li B. Il-10 and il-22 in mucosal immunity: driving protection and pathology. Front Immunol (2020) 11:1315. doi: 10.3389/fimmu.2020.01315
193. Steen EH, Wang X, Balaji S, Butte MJ, Bollyky PL, Keswani SG. The role of the anti-inflammatory cytokine interleukin-10 in tissue fibrosis. Adv Wound Care (New Rochelle) (2020) 9(4):184–98. doi: 10.1089/wound.2019.1032
194. Yuba E, Budina E, Katsumata K, Ishihara A, Mansurov A, Alpar AT, et al. Suppression of rheumatoid arthritis by enhanced lymph node trafficking of engineered interleukin-10 in murine models. Arthritis Rheumatol (2021) 73(5):769–78. doi: 10.1002/art.41585
195. O’Garra A, Barrat FJ, Castro AG, Vicari A, Hawrylowicz C. Strategies for use of il-10 or its antagonists in human disease. Immunol Rev (2008) 223:114–31. doi: 10.1111/j.1600-065X.2008.00635.x
196. Soderquist RG, Milligan ED, Harrison JA, Chavez RA, Johnson KW, Watkins LR, et al. Pegylation of interleukin-10 for the mitigation of enhanced pain states. J BioMed Mater Res A (2010) 93(3):1169–79. doi: 10.1002/jbm.a.32611
197. Mumm JB, Emmerich J, Zhang X, Chan I, Wu L, Mauze S, et al. Il-10 elicits ifngamma-dependent tumor immune surveillance. Cancer Cell (2011) 20(6):781–96. doi: 10.1016/j.ccr.2011.11.003
198. Naing A, Infante JR, Papadopoulos KP, Chan IH, Shen C, Ratti NP, et al. Pegylated il-10 (Pegilodecakin) induces systemic immune activation, Cd8(+) T cell invigoration and polyclonal T cell expansion in cancer patients. Cancer Cell (2018) 34(5):775–91 e3. doi: 10.1016/j.ccell.2018.10.007
199. Brooks DG, Ha SJ, Elsaesser H, Sharpe AH, Freeman GJ, Oldstone MB. Il-10 and pd-L1 operate through distinct pathways to suppress T-cell activity during persistent viral infection. Proc Natl Acad Sci U.S.A. (2008) 105(51):20428–33. doi: 10.1073/pnas.0811139106
200. Naing A, Wong DJ, Infante JR, Korn WM, Aljumaily R, Papadopoulos KP, et al. Pegilodecakin combined with pembrolizumab or nivolumab for patients with advanced solid tumours (Ivy): a multicentre, multicohort, open-label, phase 1b trial. Lancet Oncol (2019) 20(11):1544–55. doi: 10.1016/S1470-2045(19)30514-5
201. Ejrnaes M, Filippi CM, Martinic MM, Ling EM, Togher LM, Crotty S, et al. Resolution of a chronic viral infection after interleukin-10 receptor blockade. J Exp Med (2006) 203(11):2461–72. doi: 10.1084/jem.20061462
202. Brooks DG, Trifilo MJ, Edelmann KH, Teyton L, McGavern DB, Oldstone MB. Interleukin-10 determines viral clearance or persistence in vivo. Nat Med (2006) 12(11):1301–9. doi: 10.1038/nm1492
203. Tzotzos SJ, Fischer B, Fischer H, Zeitlinger M. Incidence of Ards and outcomes in hospitalized patients with covid-19: a global literature survey. Crit Care (2020) 24(1):516. doi: 10.1186/s13054-020-03240-7
204. Frohlich S, Murphy N, Boylan J. Definition of acute respiratory distress syndrome. JAMA (2012) 308(13):1321–2. doi: 10.1001/2012.jama.11895
205. Matthay MA, Zemans RL, Zimmerman GA, Arabi YM, Beitler JR, Mercat A, et al. Acute respiratory distress syndrome. Nat Rev Dis Primers (2019) 5(1):18. doi: 10.1038/s41572-019-0069-0
206. Gibson PG, Qin L, Puah SH. Covid-19 acute respiratory distress syndrome (Ards): clinical features and differences from typical pre-Covid-19 Ards. Med J Aust (2020) 213(2):54–6 e1. doi: 10.5694/mja2.50674
207. Albini A, Di Guardo G, Noonan DM, Lombardo M. The sars-Cov-2 receptor, ace-2, is expressed on many different cell types: implications for ace-inhibitor- and angiotensin ii receptor blocker-based cardiovascular therapies. Intern Emerg Med (2020) 15(5):759–66. doi: 10.1007/s11739-020-02364-6
208. Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. Tissue distribution of Ace2 protein, the functional receptor for sars coronavirus. a first step in understanding sars pathogenesis. J Pathol (2004) 203(2):631–7. doi: 10.1002/path.1570
209. Darif D, Hammi I, Kihel A, El Idrissi Saik I, Guessous F, Akarid K. The pro-inflammatory cytokines in covid-19 pathogenesis: what goes wrong? Microb Pathog (2021) 153:104799. doi: 10.1016/j.micpath.2021.104799
210. Aslan A, Aslan C, Zolbanin NM, Jafari R. Acute respiratory distress syndrome in covid-19: possible mechanisms and therapeutic management. Pneumonia (Nathan) (2021) 13(1):14. doi: 10.1186/s41479-021-00092-9
211. Lu L, Zhang H, Dauphars DJ, He YW. A potential role of interleukin 10 in covid-19 pathogenesis. Trends Immunol (2021) 42(1):3–5. doi: 10.1016/j.it.2020.10.012
212. Coperchini F, Chiovato L, Croce L, Magri F, Rotondi M. The cytokine storm in covid-19: an overview of the involvement of the Chemokine/Chemokine-receptor system. Cytokine Growth Factor Rev (2020) 53:25–32. doi: 10.1016/j.cytogfr.2020.05.003
213. Carcaterra M, Caruso C. Alveolar epithelial cell type ii as main target of sars-Cov-2 virus and covid-19 development Via nf-kb pathway deregulation: a physio-pathological theory. Med Hypotheses (2021) 146:110412. doi: 10.1016/j.mehy.2020.110412
214. Bhaskar S, Sinha A, Banach M, Mittoo S, Weissert R, Kass JS, et al. Cytokine storm in covid-19-Immunopathological mechanisms, clinical considerations, and therapeutic approaches: the reprogram consortium position paper. Front Immunol (2020) 11:1648. doi: 10.3389/fimmu.2020.01648
215. Han H, Ma Q, Li C, Liu R, Zhao L, Wang W, et al. Profiling serum cytokines in covid-19 patients reveals il-6 and il-10 are disease severity predictors. Emerg Microbes Infect (2020) 9(1):1123–30. doi: 10.1080/22221751.2020.1770129
216. Liu J, Li S, Liu J, Liang B, Wang X, Wang H, et al. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of sars-Cov-2 infected patients. EBioMedicine (2020) 55:102763. doi: 10.1016/j.ebiom.2020.102763
217. Wan S, Yi Q, Fan S, Lv J, Zhang X, Guo L, et al. Relationships among lymphocyte subsets, cytokines, and the pulmonary inflammation index in coronavirus (Covid-19) infected patients. Br J Haematol (2020) 189(3):428–37. doi: 10.1111/bjh.16659
218. Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to covid-19 based on an analysis of data of 150 patients from wuhan, China. Intensive Care Med (2020) 46(5):846–8. doi: 10.1007/s00134-020-05991-x
219. Cao X. Covid-19: immunopathology and its implications for therapy. Nat Rev Immunol (2020) 20(5):269–70. doi: 10.1038/s41577-020-0308-3
220. Luo XH, Zhu Y, Mao J, Du RC. T Cell immunobiology and cytokine storm of covid-19. Scand J Immunol (2021) 93(3):e12989. doi: 10.1111/sji.12989
221. Caci G, Albini A, Malerba M, Noonan DM, Pochetti P, Polosa R. Covid-19 and obesity: dangerous liaisons. J Clin Med (2020) 9(8):2511. doi: 10.3390/jcm9082511
222. Luporini RL, Rodolpho JMA, Kubota LT, Martin A, Cominetti MR, Anibal FF, et al. Il-6 and il-10 are associated with disease severity and higher comorbidity in adults with covid-19. Cytokine (2021) 143:155507. doi: 10.1016/j.cyto.2021.155507
223. Neumann J, Prezzemolo T, Vanderbeke L, Roca CP, Gerbaux M, Janssens S, et al. Increased il-10-Producing regulatory T cells are characteristic of severe cases of covid-19. Clin Transl Immunol (2020) 9(11):e1204. doi: 10.1002/cti2.1204
224. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in wuhan, China. Lancet (2020) 395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5
225. Diao B, Wang C, Tan Y, Chen X, Liu Y, Ning L, et al. Reduction and functional exhaustion of T cells in patients with coronavirus disease 2019 (Covid-19). Front Immunol (2020) 11:827. doi: 10.3389/fimmu.2020.00827
226. Medeiros T, Guimaraes GMC, Carvalho FR, Alves LS, Faustino R, Campi-Azevedo AC, et al. Acute kidney injury associated to covid-19 leads to a strong unbalance of circulant immune mediators. Cytokine (2022) 157:155974. doi: 10.1016/j.cyto.2022.155974
227. Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ, et al. Covid-19: consider cytokine storm syndromes and immunosuppression. Lancet (2020) 395(10229):1033–4. doi: 10.1016/S0140-6736(20)30628-0
228. Li J, Rong L, Cui R, Feng J, Jin Y, Chen X, et al. Dynamic changes in serum il-6, il-8, and il-10 predict the outcome of icu patients with severe covid-19. Ann Palliat Med (2021) 10(4):3706–14. doi: 10.21037/apm-20-2134
229. Zhao Y, Qin L, Zhang P, Li K, Liang L, Sun J, et al. Longitudinal covid-19 profiling associates il-1ra and il-10 with disease severity and rantes with mild disease. JCI Insight (2020) 5(13):e139834. doi: 10.1172/jci.insight.139834
230. Jafrin S, Aziz MA, Islam MS. Elevated levels of pleiotropic interleukin-6 (Il-6) and interleukin-10 (Il-10) are critically involved with the severity and mortality of covid-19: an updated longitudinal meta-analysis and systematic review on 147 studies. biomark Insights (2022) 17:11772719221106600. doi: 10.1177/11772719221106600
231. Udomsinprasert W, Jittikoon J, Sangroongruangsri S, Chaikledkaew U. Circulating levels of interleukin-6 and interleukin-10, but not tumor necrosis factor-alpha, as potential biomarkers of severity and mortality for covid-19: systematic review with meta-analysis. J Clin Immunol (2021) 41(1):11–22. doi: 10.1007/s10875-020-00899-z
232. Dhar SK KV, Damodar S, Gujar S, Das M. Il-6 and il-10 as predictors of disease severity in covid-19 patients: results from meta-analysis and regression. Heliyon (2021) 7(2):e06155. doi: 10.1016/j.heliyon.2021.e06155
233. Moll-Bernardes R, de Sousa AS, Macedo AVS, Lopes RD, Vera N, Maia LCR, et al. Il-10 and il-12 (P70) levels predict the risk of covid-19 progression in hypertensive patients: insights from the brace-corona trial. Front Cardiovasc Med (2021) 8:702507. doi: 10.3389/fcvm.2021.702507
234. Azaiz MB, Jemaa AB, Sellami W, Romdhani C, Ouslati R, Gharsallah H, et al. Deciphering the balance of il-6/Il-10 cytokines in severe to critical covid-19 patients. Immunobiology (2022) 227(4):152236. doi: 10.1016/j.imbio.2022.152236
235. Yudhawati R, Sakina S, Fitriah M. Interleukin-1beta and interleukin-10 profiles and ratio in serum of covid-19 patients and correlation with covid-19 severity: a time series study. Int J Gen Med (2022) 15:8043–54. doi: 10.2147/IJGM.S381404
236. Islam N, Jdanov DA, Shkolnikov VM, Khunti K, Kawachi I, White M, et al. Effects of covid-19 pandemic on life expectancy and premature mortality in 2020: time series analysis in 37 countries. BMJ (2021) 375:e066768. doi: 10.1136/bmj-2021-066768
237. Albini A, Calabrone L, Carlini V, Benedetto N, Lombardo M, Bruno A, et al. Preliminary evidence for il-10-Induced Ace2 mrna expression in lung-derived and endothelial cells: implications for sars-Cov-2 Ards pathogenesis. Front Immunol (2021) 12:718136. doi: 10.3389/fimmu.2021.718136
238. Mulchandani R, Lyngdoh T, Kakkar AK. Deciphering the covid-19 cytokine storm: systematic review and meta-analysis. Eur J Clin Invest (2021) 51(1):e13429. doi: 10.1111/eci.13429
239. Islam H, Chamberlain TC, Mui AL, Little JP. Elevated interleukin-10 levels in covid-19: potentiation of pro-inflammatory responses or impaired anti-inflammatory action? Front Immunol (2021) 12:677008. doi: 10.3389/fimmu.2021.677008
240. Gazzaz ZJ. Diabetes and covid-19. Open Life Sci (2021) 16(1):297–302. doi: 10.1515/biol-2021-0034
241. Saxena A, Khosraviani S, Noel S, Mohan D, Donner T, Hamad AR. Interleukin-10 paradox: a potent immunoregulatory cytokine that has been difficult to harness for immunotherapy. Cytokine (2015) 74(1):27–34. doi: 10.1016/j.cyto.2014.10.031
242. Vehar S, Boushra M, Ntiamoah P, Biehl M. Post-acute sequelae of sars-Cov-2 infection: caring for the ‘Long-haulers’. Cleve Clin J Med (2021) 88(5):267–72. doi: 10.3949/ccjm.88a.21010
243. Castanares-Zapatero D, Chalon P, Kohn L, Dauvrin M, Detollenaere J, Maertens de Noordhout C, et al. Pathophysiology and mechanism of long covid: a comprehensive review. Ann Med (2022) 54(1):1473–87. doi: 10.1080/07853890.2022.2076901
244. Fernandez-de-Las-Penas C, Palacios-Cena D, Gomez-Mayordomo V, Cuadrado ML, Florencio LL. Defining post-covid symptoms (Post-acute covid, long covid, persistent post-covid): an integrative classification. Int J Environ Res Public Health (2021) 18(5):2621. doi: 10.3390/ijerph18052621
245. Davis HE, Assaf GS, McCorkell L, Wei H, Low RJ, Re’em Y, et al. Characterizing long covid in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine (2021) 38:101019. doi: 10.1016/j.eclinm.2021.101019
246. Nasserie T, Hittle M, Goodman SN. Assessment of the frequency and variety of persistent symptoms among patients with covid-19: a systematic review. JAMA Netw Open (2021) 4(5):e2111417. doi: 10.1001/jamanetworkopen.2021.11417
247. Gupta A, Madhavan MV, Sehgal K, Nair N, Mahajan S, Sehrawat TS, et al. Extrapulmonary manifestations of covid-19. Nat Med (2020) 26(7):1017–32. doi: 10.1038/s41591-020-0968-3
248. Higgins V, Sohaei D, Diamandis EP, Prassas I. Covid-19: from an acute to chronic disease? potential long-term health consequences. Crit Rev Clin Lab Sci (2021) 58(5):297–310. doi: 10.1080/10408363.2020.1860895
249. Verdecchia P, Cavallini C, Spanevello A, Angeli F. The pivotal link between Ace2 deficiency and sars-Cov-2 infection. Eur J Intern Med (2020) 76:14–20. doi: 10.1016/j.ejim.2020.04.037
250. Nasi A, McArdle S, Gaudernack G, Westman G, Melief C, Rockberg J, et al. Reactive oxygen species as an initiator of toxic innate immune responses in retort to sars-Cov-2 in an ageing population, consider n-acetylcysteine as early therapeutic intervention. Toxicol Rep (2020) 7:768–71. doi: 10.1016/j.toxrep.2020.06.003
251. Grossini E, Concina D, Rinaldi C, Russotto S, Garhwal D, Zeppegno P, et al. Association between plasma redox State/Mitochondria function and a flu-like Syndrome/Covid-19 in the elderly admitted to a long-term care unit. Front Physiol (2021) 12:707587. doi: 10.3389/fphys.2021.707587
252. Kielbowski K, Herian M, Pawlik A. How to restore oxidative balance that was disrupted by sars-Cov-2 infection. Int J Mol Sci (2022) 23(12):6377. doi: 10.3390/ijms23126377
253. Queiroz MAF, Neves P, Lima SS, Lopes JDC, Torres M, Vallinoto I, et al. Cytokine profiles associated with acute covid-19 and long covid-19 syndrome. Front Cell Infect Microbiol (2022) 12:922422. doi: 10.3389/fcimb.2022.922422
254. Tan BKJ, Han R, Zhao JJ, Tan NKW, Quah ESH, Tan CJ, et al. Prognosis and persistence of smell and taste dysfunction in patients with covid-19: meta-analysis with parametric cure modelling of recovery curves. BMJ (2022) 378:e069503. doi: 10.1136/bmj-2021-069503
255. Locatello LG, Bruno C, Maggiore G, Cilona M, Orlando P, Fancello G, et al. The prognostic role of il-10 in non-severe covid-19 with chemosensory dysfunction. Cytokine (2021) 141:155456. doi: 10.1016/j.cyto.2021.155456
256. Bussmann AJC, Ferraz CR, Lima AVA, Castro JGS, Ritter PD, Zaninelli TH, et al. Association between il-10 systemic low level and highest pain score in patients during symptomatic sars-Cov-2 infection. Pain Pract (2022) 22(4):453–62. doi: 10.1111/papr.13101
257. Rysz S, Al-Saadi J, Sjostrom A, Farm M, Campoccia Jalde F, Platten M, et al. Covid-19 pathophysiology may be driven by an imbalance in the renin-Angiotensin-Aldosterone system. Nat Commun (2021) 12(1):2417. doi: 10.1038/s41467-021-22713-z
258. Sonaglioni A, Lombardo M, Albini A, Noonan DM, Re M, Cassandro R, et al. Charlson comorbidity index, neutrophil-to-Lymphocyte ratio and undertreatment with renin-Angiotensin-Aldosterone system inhibitors predict in-hospital mortality of hospitalized covid-19 patients during the omicron dominant period. Front Immunol (2022) 13:958418. doi: 10.3389/fimmu.2022.958418
259. Ragab D, Salah Eldin H, Taeimah M, Khattab R, Salem R. The covid-19 cytokine storm; what we know so far. Front Immunol (2020) 11:1446. doi: 10.3389/fimmu.2020.01446
260. Trandem K, Jin Q, Weiss KA, James BR, Zhao J, Perlman S. Virally expressed interleukin-10 ameliorates acute encephalomyelitis and chronic demyelination in coronavirus-infected mice. J Virol (2011) 85(14):6822–31. doi: 10.1128/JVI.00510-11
261. Morales-Ubaldo AL, Rivero-Perez N, Valladares-Carranza B, Madariaga-Navarrete A, Higuera-Piedrahita RI, Delgadillo-Ruiz L, et al. Phytochemical compounds and pharmacological properties of larrea tridentata. Molecules (2022) 27(17):5393. doi: 10.3390/molecules27175393
262. Aye MM, Aung HT, Sein MM, Armijos C. A review on the phytochemistry, medicinal properties and pharmacological activities of 15 selected Myanmar medicinal plants. Molecules (2019) 24(2):293. doi: 10.3390/molecules24020293
263. Diwaker D, Mishra KP, Ganju L, Singh SB. Rhodiola inhibits dengue virus multiplication by inducing innate immune response genes rig-I, Mda5 and isg in human monocytes. Arch Virol (2014) 159(8):1975–86. doi: 10.1007/s00705-014-2028-0
264. Khanna K, Mishra KP, Ganju L, Singh SB. Golden root: a wholesome treat of immunity. BioMed Pharmacother (2017) 87:496–502. doi: 10.1016/j.biopha.2016.12.132
265. Sharma SK, Bansal S, Mangal M, Dixit AK, Gupta RK, Mangal AK. Utilization of food processing by-products as dietary, functional, and novel fiber: a review. Crit Rev Food Sci Nutr (2016) 56(10):1647–61. doi: 10.1080/10408398.2013.794327
266. Mollazadeh H, Cicero AFG, Blesso CN, Pirro M, Majeed M, Sahebkar A. Immune modulation by curcumin: the role of interleukin-10. Crit Rev Food Sci Nutr (2019) 59(1):89–101. doi: 10.1080/10408398.2017.1358139
267. Babaei F, Nassiri-Asl M, Hosseinzadeh H. Curcumin (a constituent of turmeric): new treatment option against covid-19. Food Sci Nutr (2020) 8(10):5215–27. doi: 10.1002/fsn3.1858
268. Peter AE, Sandeep BV, Rao BG, Kalpana VL. Calming the storm: natural immunosuppressants as adjuvants to target the cytokine storm in covid-19. Front Pharmacol (2020) 11:583777. doi: 10.3389/fphar.2020.583777
269. Promdam N, Panichayupakaranant P. [6]-gingerol: a narrative review of its beneficial effect on human health. Food Chem Adv (2022) 1:100043. doi: 10.1016/j.focha.2022.100043
270. Wong CP, Nguyen LP, Noh SK, Bray TM, Bruno RS, Ho E. Induction of regulatory T cells by green tea polyphenol egcg. Immunol Lett (2011) 139(1-2):7–13. doi: 10.1016/j.imlet.2011.04.009
271. Brockmann L, Soukou S, Steglich B, Czarnewski P, Zhao L, Wende S, et al. Molecular and functional heterogeneity of il-10-Producing Cd4(+) T cells. Nat Commun (2018) 9(1):5457. doi: 10.1038/s41467-018-07581-4
272. Wu M, Yu S, Chen Y, Meng W, Chen H, He J, et al. Acteoside promotes b cell-derived il-10 production and ameliorates autoimmunity. J Leukoc Biol (2022) 112(4):875–85. doi: 10.1002/JLB.3MA0422-510R
273. Palacz-Wrobel M, Borkowska P, Paul-Samojedny M, Kowalczyk M, Fila-Danilow A, Suchanek-Raif R, et al. Effect of apigenin, kaempferol and resveratrol on the gene expression and protein secretion of tumor necrosis factor alpha (Tnf-alpha) and interleukin-10 (Il-10) in raw-264.7 macrophages. BioMed Pharmacother (2017) 93:1205–12. doi: 10.1016/j.biopha.2017.07.054
274. Cianciulli A, Dragone T, Calvello R, Porro C, Trotta T, Lofrumento DD, et al. Il-10 plays a pivotal role in anti-inflammatory effects of resveratrol in activated microglia cells. Int Immunopharmacol (2015) 24(2):369–76. doi: 10.1016/j.intimp.2014.12.035
275. Luisi MLE, Lucarini L, Biffi B, Rafanelli E, Pietramellara G, Durante M, et al. Effect of Mediterranean diet enriched in high quality extra virgin olive oil on oxidative stress, inflammation and gut microbiota in obese and normal weight adult subjects. Front Pharmacol (2019) 10:1366. doi: 10.3389/fphar.2019.01366
276. Liu S, Tian L, Chai G, Wen B, Wang B. Targeting heme oxygenase-1 by quercetin ameliorates alcohol-induced acute liver injury Via inhibiting Nlrp3 inflammasome activation. Food Funct (2018) 9(8):4184–93. doi: 10.1039/c8fo00650d
277. Alam MA, Subhan N, Rahman MM, Uddin SJ, Reza HM, Sarker SD. Effect of citrus flavonoids, naringin and naringenin, on metabolic syndrome and their mechanisms of action. Adv Nutr (2014) 5(4):404–17. doi: 10.3945/an.113.005603
278. Liu T, Gao H, Zhang Y, Wang S, Lu M, Dai X, et al. Apigenin ameliorates hyperuricemia and renal injury through regulation of uric acid metabolism and Jak2/Stat3 signaling pathway. Pharm (Basel) (2022) 15(11):1442. doi: 10.3390/ph15111442
279. Fu R, Wang L, Meng Y, Xue W, Liang J, Peng Z, et al. Apigenin remodels the gut microbiota to ameliorate ulcerative colitis. Front Nutr (2022) 9:1062961. doi: 10.3389/fnut.2022.1062961
280. Zhang ZT, Zhang DY, Xie K, Wang CJ, Xu F. Luteolin activates tregs to promote il-10 expression and alleviating caspase-11-Dependent pyroptosis in sepsis-induced lung injury. Int Immunopharmacol (2021) 99:107914. doi: 10.1016/j.intimp.2021.107914
281. Duan Z, Xie H, Yu S, Wang S, Yang H. Piperine derived from piper nigrum l. inhibits lps-induced inflammatory through the mapk and nf-kappab signalling pathways in Raw264.7 cells. Foods (2022) 11(19):2990. doi: 10.3390/foods11192990
282. Zhu Y, Li X, Chen J, Chen T, Shi Z, Lei M, et al. The pentacyclic triterpene lupeol switches M1 macrophages to M2 and ameliorates experimental inflammatory bowel disease. Int Immunopharmacol (2016) 30:74–84. doi: 10.1016/j.intimp.2015.11.031
283. Saqib U, Sarkar S, Suk K, Mohammad O, Baig MS, Savai R. Phytochemicals as modulators of M1-M2 macrophages in inflammation. Oncotarget (2018) 9(25):17937–50. doi: 10.18632/oncotarget.24788
284. Hyam SR, Lee IA, Gu W, Kim KA, Jeong JJ, Jang SE, et al. Arctigenin ameliorates inflammation in vitro and in vivo by inhibiting the Pi3k/Akt pathway and polarizing M1 macrophages to M2-like macrophages. Eur J Pharmacol (2013) 708(1-3):21–9. doi: 10.1016/j.ejphar.2013.01.014
285. Gupta S, Mishra KP, Singh SB, Ganju L. Inhibitory effects of andrographolide on activated macrophages and adjuvant-induced arthritis. Inflammopharmacology (2018) 26(2):447–56. doi: 10.1007/s10787-017-0375-7
286. Miki S, Suzuki JI, Takashima M, Ishida M, Kokubo H, Yoshizumi M. S-1-Propenylcysteine promotes il-10-Induced M2c macrophage polarization through prolonged activation of il-10r/Stat3 signaling. Sci Rep (2021) 11(1):22469. doi: 10.1038/s41598-021-01866-3
287. Meresse S, Gateau H, Tirnan T, Larrigaldie V, Casse N, Pasetto P, et al. Haslea ostrearia pigment marennine affects key actors of neuroinflammation and decreases cell migration in murine neuroglial cell model. Int J Mol Sci (2023) 24(6):5388. doi: 10.3390/ijms24065388
288. Kidgell JT, Glasson CRK, Magnusson M, Vamvounis G, Sims IM, Carnachan SM, et al. The molecular weight of ulvan affects the in vitro inflammatory response of a murine macrophage. Int J Biol Macromol (2020) 150:839–48. doi: 10.1016/j.ijbiomac.2020.02.071
289. Zhou Y, Chen R, Liu D, Wu C, Guo P, Lin W. Asperlin inhibits lps-evoked foam cell formation and prevents atherosclerosis in apoe(-/-) mice. Mar Drugs (2017) 15(11):358. doi: 10.3390/md15110358
290. Sansone C, Pistelli L, Del Mondo A, Calabrone L, Fontana A, Noonan DM, et al. The microalgal diatoxanthin inflects the cytokine storm in sars-Cov-2 stimulated Ace2 overexpressing lung cells. Antioxidants (Basel) (2022) 11(8):1515. doi: 10.3390/antiox11081515
291. Davinelli S, Melvang HM, Andersen LP, Scapagnini G, Nielsen ME. Astaxanthin from shrimp cephalothorax stimulates the immune response by enhancing ifn-gamma, il-10, and il-2 secretion in splenocytes of helicobacter pylori-infected mice. Mar Drugs (2019) 17(7):382. doi: 10.3390/md17070382
292. Valizadeh H, Abdolmohammadi-Vahid S, Danshina S, Ziya Gencer M, Ammari A, Sadeghi A, et al. Nano-curcumin therapy, a promising method in modulating inflammatory cytokines in covid-19 patients. Int Immunopharmacol (2020) 89(Pt B):107088. doi: 10.1016/j.intimp.2020.107088
293. Zahedipour F, Hosseini SA, Sathyapalan T, Majeed M, Jamialahmadi T, Al-Rasadi K, et al. Potential effects of curcumin in the treatment of covid-19 infection. Phytother Res (2020) 34(11):2911–20. doi: 10.1002/ptr.6738
294. Zhai WJ, Zhang ZB, Xu NN, Guo YF, Qiu C, Li CY, et al. Piperine plays an anti-inflammatory role in staphylococcus aureus endometritis by inhibiting activation of nf-kappab and mapk pathways in mice. Evid Based Complement Alternat Med (2016) 2016:8597208. doi: 10.1155/2016/8597208
295. Santos JC, Ribeiro ML, Gambero A. The impact of polyphenols-based diet on the inflammatory profile in covid-19 elderly and obese patients. Front Physiol (2020) 11:612268. doi: 10.3389/fphys.2020.612268
296. Sansone C, Bruno A, Piscitelli C, Baci D, Fontana A, Brunet C, et al. Natural compounds of marine origin as inducers of immunogenic cell death (Icd): potential role for cancer interception and therapy. Cells (2021) 10(2):231. doi: 10.3390/cells10020231
297. Montuori E, de Pascale D, Lauritano C. Recent discoveries on marine organism immunomodulatory activities. Mar Drugs (2022) 20(7):422. doi: 10.3390/md20070422
298. Asif M, Saleem M, Yaseen HS, Yehya AH, Saadullah M, Zubair HM, et al. Potential role of marine species-derived bioactive agents in the management of sars-Cov-2 infection. Future Microbiol (2021) 16(16):1289–301. doi: 10.2217/fmb-2021-0024
299. Geahchan S, Ehrlich H, Rahman MA. The anti-viral applications of marine resources for covid-19 treatment: an overview. Mar Drugs (2021) 19(8):409. doi: 10.3390/md19080409
300. Sansone C, Brunet C, Noonan DM, Albini A. Marine algal antioxidants as potential vectors for controlling viral diseases. Antioxidants (Basel) (2020) 9(5):392. doi: 10.3390/antiox9050392
301. Alam MA, Parra-Saldivar R, Bilal M, Afroze CA, Ahmed MN, Iqbal HMN, et al. Algae-derived bioactive molecules for the potential treatment of sars-Cov-2. Molecules (2021) 26(8):2134. doi: 10.3390/molecules26082134
302. Del Mondo A, Smerilli A, Ambrosino L, Albini A, Noonan DM, Sansone C, et al. Insights into phenolic compounds from microalgae: structural variety and complex beneficial activities from health to nutraceutics. Crit Rev Biotechnol (2021) 41(2):155–71. doi: 10.1080/07388551.2021.1874284
Keywords: IL-10, STAT3, cytokine storm, alarmin, COVID - 19, Post-COVID
Citation: Carlini V, Noonan DM, Abdalalem E, Goletti D, Sansone C, Calabrone L and Albini A (2023) The multifaceted nature of IL-10: regulation, role in immunological homeostasis and its relevance to cancer, COVID-19 and post-COVID conditions. Front. Immunol. 14:1161067. doi: 10.3389/fimmu.2023.1161067
Received: 07 February 2023; Accepted: 24 May 2023;
Published: 08 June 2023.
Edited by:
Andrzej Lange, Polish Academy of Sciences, PolandReviewed by:
Lorenzo Bonaguro, University of Bonn, GermanyRana A. Youness, University of Hertfordshire, United Kingdom
Marco A. Cassatella, University of Verona, Italy
Copyright © 2023 Carlini, Noonan, Abdalalem, Goletti, Sansone, Calabrone and Albini. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Adriana Albini, YWRyaWFuYS5hbGJpbmlAaWVvLml0; Luana Calabrone, bHVhbmEuY2FsYWJyb25lQG11bHRpbWVkaWNhLml0
 Valentina Carlini1
Valentina Carlini1 Douglas M. Noonan
Douglas M. Noonan Eslam Abdalalem
Eslam Abdalalem Delia Goletti
Delia Goletti Clementina Sansone
Clementina Sansone Luana Calabrone
Luana Calabrone Adriana Albini
Adriana Albini