- 1Department of Adolescent Rheumatology, University College London Hospital (UCLH), London, United Kingdom
- 2Medical School, University College London (UCL), London, United Kingdom
- 3Centre for Adolescent Rheumatology Versus Arthritis at UCL, UCLH and Great Ormond Street (GOS) Hospital (GOSH), London, United Kingdom
- 4UCL GOS Institute of Child Health, UCL, London, United Kingdom
- 5Department of Paediatric Rheumatology GOSH, London, United Kingdom
- 6National Institute of Health Research - Biomedical Research Centre, UCLH, London, United Kingdom
Background: Despite children and young people (CYP) having a low risk for severe coronavirus disease 2019 (COVID-19) outcomes, there is still a degree of uncertainty related to their risk in the context of immunodeficiency or immunosuppression, primarily due to significant reporting bias in most studies, as CYP characteristically experience milder or asymptomatic COVID-19 infection and the severe outcomes tend to be overestimated.
Methods: A comprehensive systematic review to identify globally relevant studies in immunosuppressed CYP and CYP in general population (defined as younger than 25 years of age) up to 31 October 2021 (to exclude vaccinated populations) was performed. Studies were included if they reported the two primary outcomes of our study, admission to intensive therapy unit (ITU) and mortality, while data on other outcomes, such as hospitalization and need for mechanical ventilation were also collected. A meta-analysis estimated the pooled proportion for each severe COVID-19 outcome, using the inverse variance method. Random effects models were used to account for interstudy heterogeneity.
Findings: The systematic review identified 30 eligible studies for each of the two populations investigated: immunosuppressed CYP (n = 793) and CYP in general population (n = 102,022). Our meta-analysis found higher estimated prevalence for hospitalization (46% vs. 16%), ITU admission (12% vs. 2%), mechanical ventilation (8% vs. 1%), and increased mortality due to severe COVID-19 infection (6.5% vs. 0.2%) in immunocompromised CYP compared with CYP in general population. This shows an overall trend for more severe outcomes of COVID-19 infection in immunocompromised CYP, similar to adult studies.
Interpretation: This is the only up-to-date meta-analysis in immunocompromised CYP with high global relevance, which excluded reports from hospitalized cohorts alone and included 35% studies from low- and middle-income countries. Future research is required to characterize individual subgroups of immunocompromised patients, as well as impact of vaccination on severe COVID-19 outcomes.
Systematic Review Registration: PROSPERO identifier, CRD42021278598.
Introduction
Coronavirus disease 2019 (COVID-19) caused by the respiratory virus SARS-CoV-2 has, to date, resulted in over 600 million confirmed cases and 6.5 million deaths (1). However, children and young people (CYP), defined by the World Health Organization as aged 0–24 years (2), remain at low risk for severe outcomes of COVID-19 infection such as hospitalization, admission to intensive therapy units (ITUs), and death, reported in a large international meta-analysis as just 3.3%, 0.3%, and 0.02%, respectively (3).
The role of the immune system in SARS-CoV-2 transmission, clearance, and disease severity and the impact of immunocompromised states on COVID-19 infection severity in CYP are not entirely clear as more research has been directed toward investigating adult immunosuppressed populations (4, 5) especially as COVID-19 infection is associated with significantly poorer outcomes in older adults. However, it has already been established in large population studies that older adolescents and young adults, as well as the ones from ethnic minorities and with underlying medical conditions, are at risk for more severe outcomes following infection with SARS-CoV-2 (6).
A better understanding of the impact of immunocompromise on the severity of COVID-19 is important for risk stratification to guide strategies for administration of COVID-19 vaccines and therapeutics, immunosuppressive treatment management during infection and immunisation, as well as wider public health policies.
Immunocompromise has been investigated as an independent risk factor for COVID-19 in CYP, and studies have shown that immunocompromised patients are over-represented in cohorts of patients admitted to ITU or receiving invasive ventilation, 23% and 17%, respectively (7–9). Identifying the impact of immunocompromise on COVID-19 disease severity in CYP is challenging. Studies tend to have low sample sizes due to low prevalence of COVID-19 infection in CYP and were heterogeneous in relation to the type and severity of immunocompromise. In addition, the low rates of COVID-19 infection in immunocompromised CYP, likely due to shielding, and the low rates of SARS-CoV-2–related complications in CYP overall pose challenges for reaching the statistical certainty needed to draw definite conclusions. In CYP, non-specific symptoms, asymptomatic carriage of the virus, and variation in testing, in addition to bias in retrospective data collection and exclusive inclusion of hospitalized patients, may result in over-estimation of severity of COVID-19 infection.
A single meta-analysis investigating comorbidities associated with severe COVID-19 infection in children (defined as requiring ITU admission or invasive ventilation or resulting in death) demonstrated significantly higher rates of severe infection in immunocompromised children compared with general population controls (17.5% vs. 11.0%; RR, 1.44; 95% CI, 1.01–2.04), although there was no significant difference in disease severity in subgroups of hemato-oncology patients, patients on immunosuppressant drugs, or mixed immunosuppression when compared with general population controls (10). This meta-analysis included 154 immunocompromised children from 10 studies performed in Europe/USA, of which five studies included only hospitalized children with COVID-19.
Objective
This paper addresses the need for a more comprehensive meta-analysis with global relevance based on an updated systematic review of the literature, aiming to minimize the risk for selection bias by including reports from low- and middle-income countries (LMICs), as well as studies not exclusively focused on hospitalized patients, in order to ensure the relevance of findings for children all over the world.
Methods
Search strategy and selection criteria
This is a systematic review and meta-analysis that was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (11). The review protocol was registered with the International Prospective Register of Systematic Reviews (PROSPERO - CRD42021278598). To identify studies on immunocompromised CYP, we performed a systematic search of the literature from 31 December 2019 to 31 October 2021 (prior to the widespread of rollout of COVID-19 vaccination in CYP) in the electronic databases PubMed and Scopus using MeSH terms COVID-19, child, infant, adolescent, pediatric, young adult, immunosuppressant, immunosuppression, immunocompromised, and immunologic deficiency syndrome (Supplementary Table S1). To identify studies capturing CYP in general population, we first performed a search of the literature for pre-existing systematic reviews on COVID-19 in CYP and identified a study published by Ifran et al, which included studies from 1 December 2019 to 8 January 2021 (12). We then performed a systematic search of the literature from 8 January 2021 to 31 October 2021 in PubMed and Scopus to update it. We identified other relevant studies by searching Google Scholar and reviewing the references of included studies (snowballing) (Supplementary Table S2). There were no restrictions on language, and reports that were not in English were translated using Google translate.
Eligible studies for inclusion were cohort or cross-sectional studies that included CYP under 25 years of age with COVID-19 infection, which reported the two primary outcomes of our study: admission to ITU and mortality in general population and in immunocompromised CYP. The definition of COVID-19 infection was based on either positive polymerase chain reaction (PCR) testing, antigen testing, serological testing, or if highly clinically suspected (based on compatible symptoms, radiology, and contact with confirmed case) to avoid reporting bias as access to PCR testing varied between countries and was restricted in the early pandemic. Where studies reported both adult and pediatric patients, the study was included only if it was possible to manually identify and remove patients aged 25 years or older. The immunocompromised CYP were defined as having chemotherapy or immunosuppressant therapy currently or within past 6 months, being post-haematopoietic stem-cell transplantation (HSCT) and on immunosuppression without reaching immune reconstitution, having primary immunodeficiency, bone marrow failure, sickle-cell disease, or being classified as immunocompromised.
Studies were excluded if they only reported data on patients admitted to hospital or ITU to avoid selection bias, as these cohorts would likely have poorer outcomes. Reports on oncology patients who were not immunocompromised (e.g., not on chemotherapy/immunosuppressants or patients who had immune reconstitution post-HRCT) or case studies of fewer than five patients were also excluded as not representative. Studies which only investigated age subgroups (e.g., neonates) were excluded to avoid skewing the results. Where reports overlapped, only the most recent study was included. Studies that included CYP who had received a vaccine to SARS-CoV-2 were excluded, and the search period was limited to the end of October 2021 to minimize inclusion of vaccinated CYP.
The identified studies were screened independently by two authors (J.G.B. and S.A.) based on titles and abstracts before full texts were screened. Studies were then selected for inclusion using the inclusion and exclusion criteria above and agreed on between both authors. Any disagreements were discussed with a third author (C.C.), and a consensus was agreed.
Data analysis
Two authors (J.G.B. and S.A.) extracted data on epidemiological features, type of immunocompromise and outcomes (hospitalization, ITU admission, requirement for mechanical ventilation, and death) from eligible studies and entered it into a structured data extraction table. Where studies included patients aged 25 years or older, or not immunocompromised as per the aforementioned criteria, they were manually removed if per patient data was available. For general population CYP studies, if any immunocompromised patients were included, they were also manually removed where possible. Patients admitted to hospital or ITU for reasons unrelated to COVID-19 infection (e.g., routine chemotherapy) were excluded from the analysis. Where information of interest was not stated in the main paper or included in Supplementary Data, the corresponding authors were contacted. To evaluate study quality, we used the validated Newcastle-Ottawa scale (NOS) for cohort studies, which assesses the study selection, comparability, and outcomes (13)generating scores between 0 and 9, with 9 representing the highest study quality. Only one study included both immunocompromised and non-immunocompromised CYP (14).
The number of CYP with severe outcomes of COVID-19 infection in each study in immunocompromised versus general populations was used to evaluate the COVID-19–related morbidity (hospitalization, ITU admission, or mechanical ventilation requirement) and mortality and to compare outcomes. It was not possible to compare the overall estimated prevalence of outcomes of interest between the immunosuppressed CYP versus CYP in the general population or calculate the relative risk for each outcome as only one study reported data on both (14). The proportion of each outcome of interest was calculated for each study, in which, a continuity correction by adding 0.5 was applied when a study contained zero events.
A pooled proportion was then estimated for each outcome using meta-analysis, using the inverse variance method. Random effects models were used to account for interstudy heterogeneity, and studies were weighted according to their size and variance. Study-specific heterogeneity was assessed using the l (2) statistic (0%–100%), in which a lower value implies less heterogeneity. The between-study variance tau (2) was computed using the maximum likelihood method and tested for the assumption of homogeneity using the Wald test. The possibility of reporting bias influencing results was addressed through a combination of visual inspection of funnel plots for asymmetry, formal tests of bias using the weighted linear regression method (Egger’s test), and sensitivity analyses using the “trim-and-fill” method. All statistical analyses were carried out using the meta library version 4.14-0 in R version 4.0.2.
Results
Only one eligible study concomitantly reported COVID-19 outcomes in immunosuppressed CYP versus CYP in general population (14).
Systematic review of severe COVID-19 infection outcomes in immunosuppressed CYP
The electronic search identified 1,544 studies of immunocompromised CYP, out of which 1,532 were excluded after screening, and another three after assessment of the eligibility criteria. Nine studies identified electronically, and 21 further studies identified manually from other sources were finally included in the systematic review (Figure 1A) (14–43).
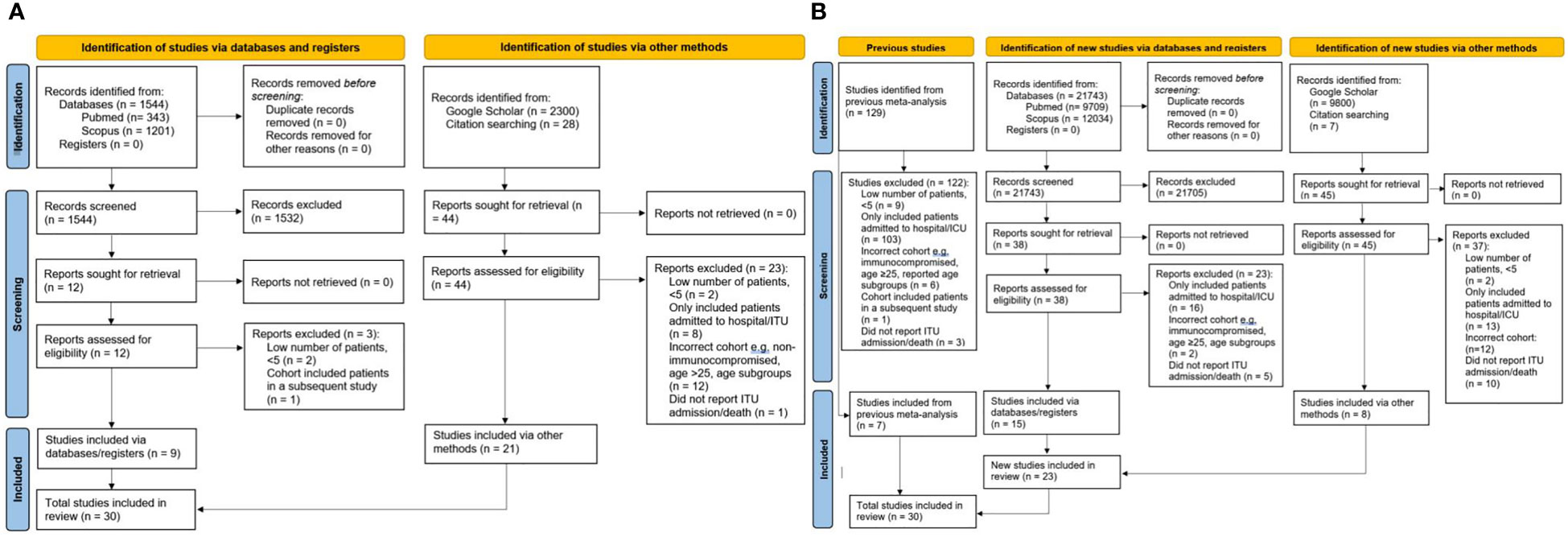
Figure 1 PRISMA flow charts for all studies in the systematic analysis. (A) studies of immunocompromised CYP (B) general population studies of CYP. CYP, children and young people; ITU, Intensive Therapy Unit.
The studies varied in size and included from five to 113 patients. In total, 793 immunocompromised CYP were included in our systematic review and meta-analysis (Table 1).
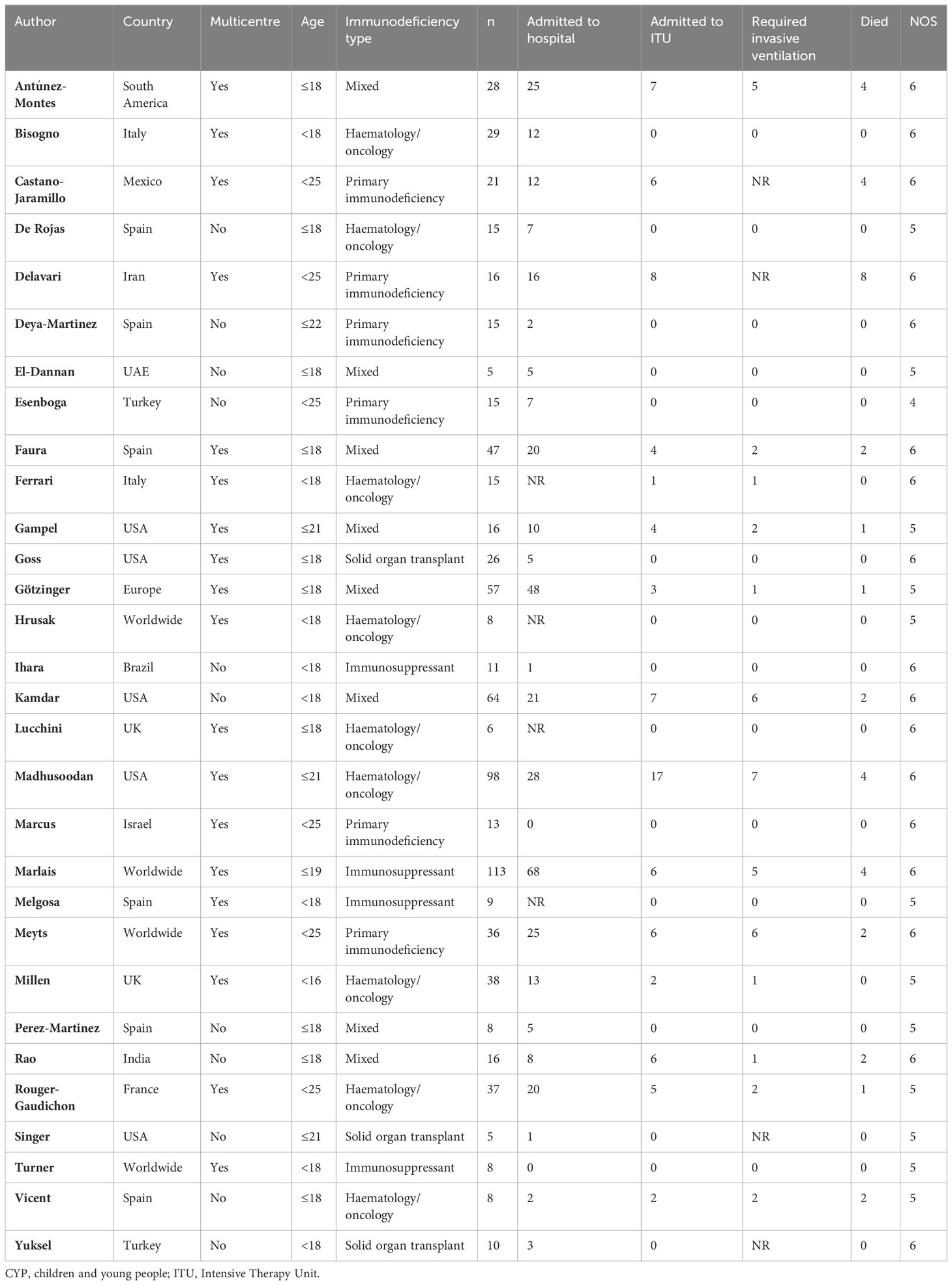
Table 1 Studies included in the systematic review of severe COVID-19 infection outcomes in immunosuppressed CYP.
Of the 30 eligible studies on immunocompromised patients, 19 (63.3%) were multicenter, six (20.0%) were multinational, and 12 (40.0%) were from LMIC. Eight (26.7%) studies included patients with hematological or oncological malignancies on chemotherapy or immunotherapy, six (20.0%) included patients with primary immunodeficiency, three (10.0%) included patients with solid organ transplant (SOT) on immunosuppression, four (13.3%) included other patients on immunosuppression, and eight (26.7%) included a mix of immunocompromised patients. The age cutoffs for patient inclusion are detailed in Table 1.
In addition to the mandatory reported outcomes (ITU admission and death), 26 studies (86.7%) reported hospitalization and 26 studies (86.7%) reported invasive ventilation. The quality of the studies was poor moderate (NOS scores 4–6/9) (Table 1).
Systematic review of severe COVID-19 infection outcomes in CYP in general population
The previous systematic review in CYP in general population by Ifran et al. identified 129 studies of which seven fulfilled inclusion criteria (12). The additional literature search we performed identified 21,743 studies in CYP in general population, of which 21,705 were excluded after screening, leading to 38 studies which were assessed for eligibility, out of which 15 studies were eligible for inclusion. Eight additional eligible studies were retrieved manually from other sources (Figure 1B). Our final analysis included 23 eligible studies identified by our searched and seven studies captured by the previous systematic analysis (14, 44–72).
The studies varied in size from 14 to 43,465 patients. In total, 102,022 non–immunocompromised patients were included in our systematic review and meta–analysis (Table 2).
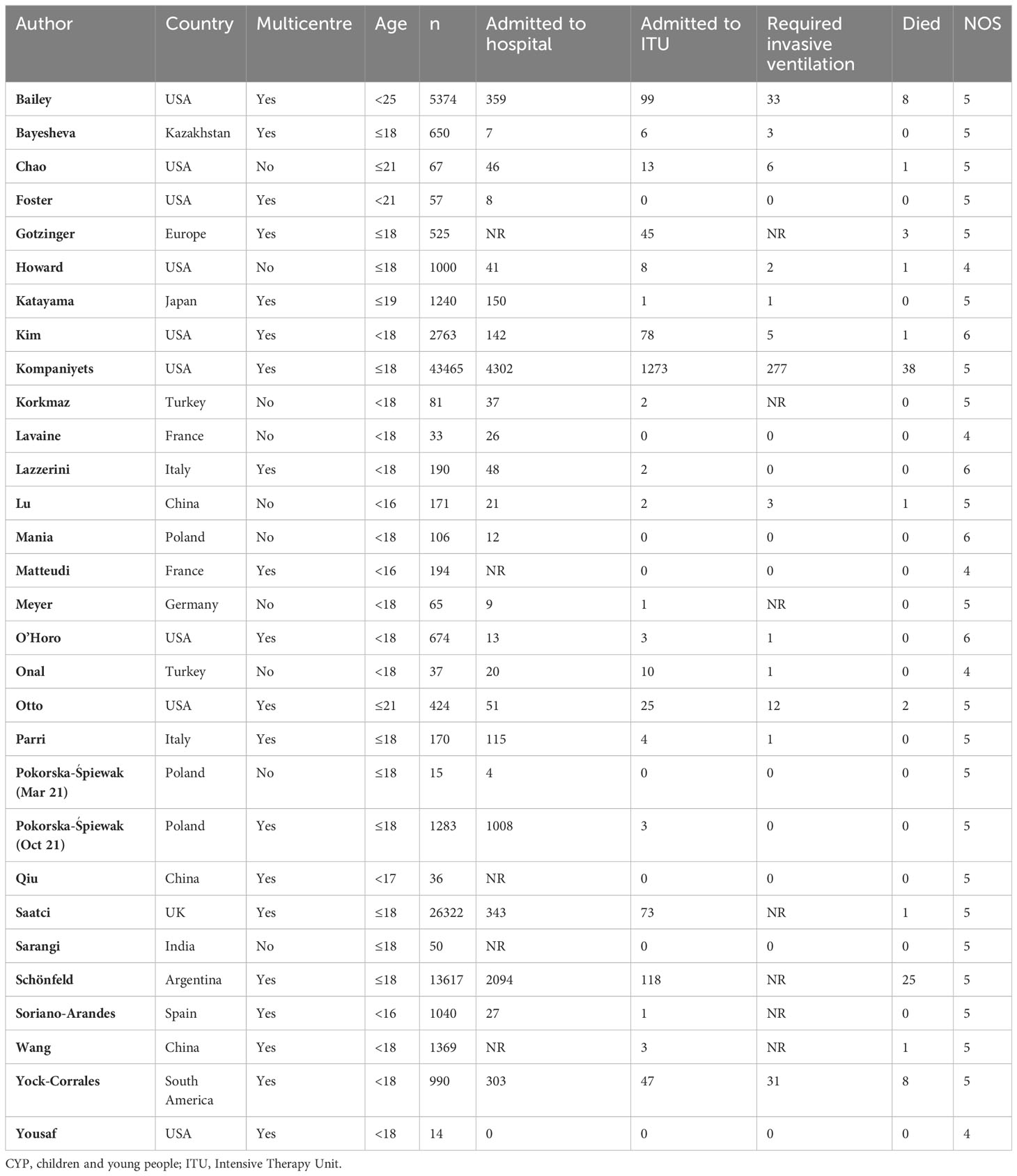
Table 2 Studies included in the systematic review of severe COVID-19 infection in CYP in general population.
Of the 30 included studies in CYP in general population, 19 were multicenter (63.3%), two (6.7%) were multinational, and nine (30%) were from LMIC. The age cutoffs are detailed in Table 2.
In addition to the mandatory reported outcomes (ITU admission and death), 25 studies (83.3%) reported hospitalization and 23 studies (76.7%) reported invasive ventilation. four studies (13.3%) were scored six stars in the NOS, 21 (70%) were scored five stars and five (16.7%) was scored four stars, out of a total of nine.
Meta–analysis of severe COVID–19 infection outcomes in immunosuppressed CYP
There were 30 studies included in the meta–analysis (Supplementary Tables S1, S3).
The pooled proportion estimate for hospital admission due to COVID–19 infection was 46% (95% CI 37%–56%, Figure 2A) and for ITU admission due to severe COVID–19 infection was 12.0% (95% CI 9%–17%, Figure 2B). The estimated proportion of patients that required invasive ventilation was 8% (95% CI 6%–10%, Figure 2C), and the mortality rate was estimated to be 6.5% (4.2%–9.9%, Figure 2D).
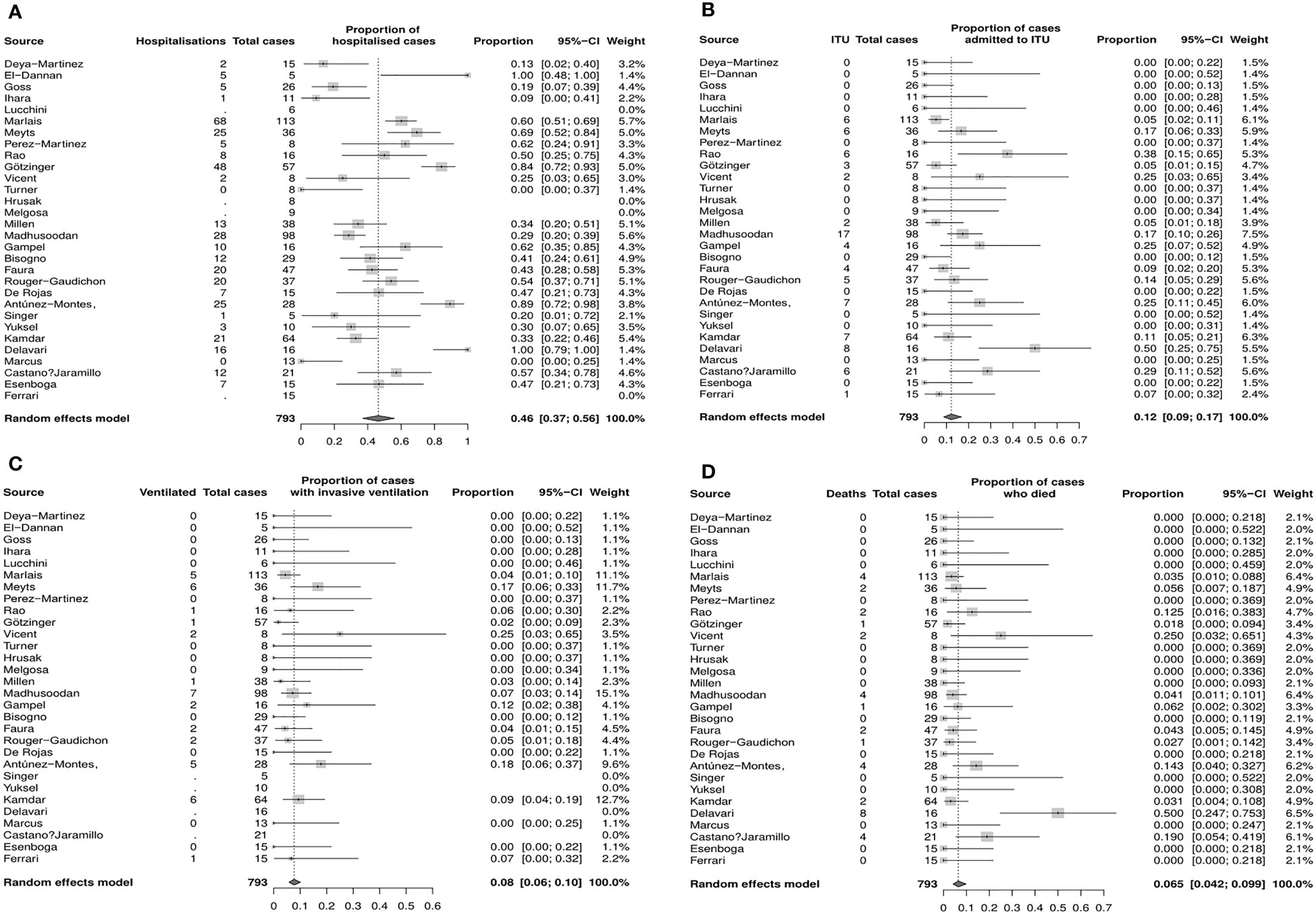
Figure 2 Estimated proportions of COVID–19 outcomes of interest in immunocompromised CYP (random effects model, 95% CI), (A) Hospitalization (B) Admission to ITU (C) Mechanical ventilation (D) Death. CI, confidence interval; CYP, children young people; ITU, intensice therapy unit.
Funnel plots and sensitivity analyses indicated that the proportion admitted to hospital was unlikely to be affected by reporting bias, however, the estimated proportion of patients admitted to ITU or requiring invasive ventilation and mortality rate may be significantly affected by bias (Supplementary Figure S1).
Meta–analysis of severe COVID–19 infection outcomes in CYP in general population
There were 30 studies included in the meta–analysis (Supplementary Tables S2, S3).
The pooled proportion estimate for hospital admission due to COVID–19 infection was 16% (95% CI 11%–23%, Figure 3A), while the estimate for ITU admission due to severe COVID–19 infection was 2.0% (95% CI 1%–2%, Figure 3B). The proportion of CYP who required mechanical ventilation requirements was 1% (95% CI 0%–1%, Figure 3C), and the mortality rate was estimated to be 0.2% (95% CI 0.2%–0.4%, Figure 3D).
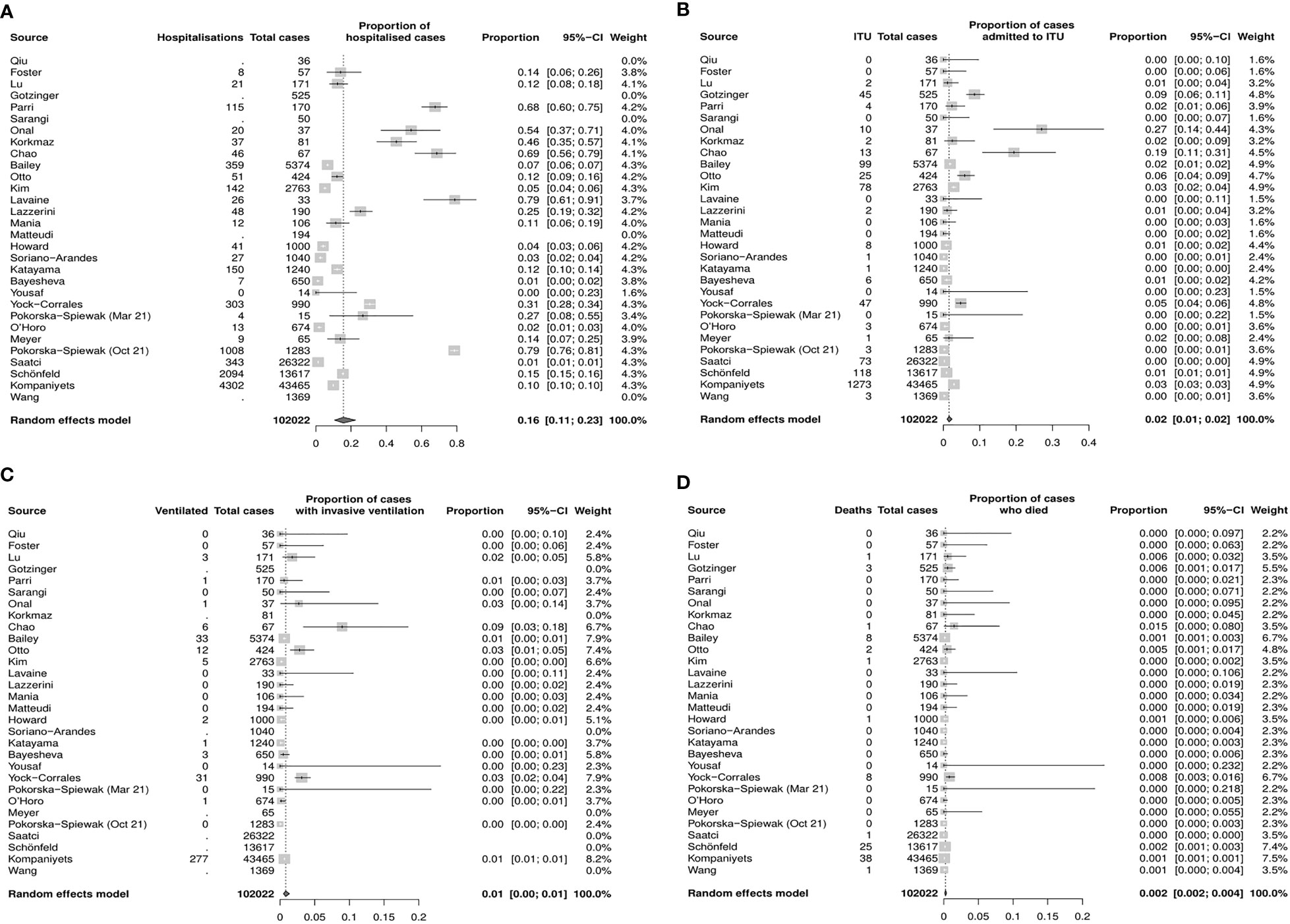
Figure 3 Estimated proportions of Covid–19 outcomes of interest inCYP in the general population (random effects model, 95% CI) (A) Hospitalization (B) Admission to ITU (C) Mechanical ventilation (D) Death. CI, confidence interval; CYP, children and young people; ITU, intensive therapy unit.
Funnel plots and sensitivity analyses indicated that the proportion admitted to hospital, requiring ITU or invasive ventilation, was unlikely to be affected by reporting bias, however, the mortality rate may be significantly affected by bias (Supplementary Figure S2).
Discussion
This is the most up–to–date systematic review and meta–analysis of outcomes of severe COVID–19 infection in CYP and the only one assessing immunosuppressed non–hospitalized cohorts in comparison with general population.
Our meta–analysis found higher estimated prevalence for hospitalization (46% vs. 16%), ITU admission (12% vs. 2%), mechanical ventilation (8% vs. 1%), and increased mortality due to severe COVID–19 infection (6.5% vs. 0.2%) in immunocompromised CYP compared with CYP in general population. This shows an overall trend for more severe outcomes of COVID–19 infection in immunocompromised CYP, such as found in adults. Comparisons with published literature are influenced by the type of populations analyzed (adults vs. children) as well as setting (hospitalized cohorts vs. general population cohorts). Adult rheumatology registry studies have shown that glucocorticoids or cyclophosphamide treatment increased the risk of severe COVID–19 outcomes (73, 74). Cohort studies of 13,206 Spanish and 6,435 Korean patients demonstrated statistically significant higher inpatient mortality in immunocompromised compared with non–immunocompromised patients, 31.3% versus 19.3% and 6.4% versus 2.0%, respectively (75, 76). A meta–analysis of 2,777 pediatric and adult SOT patients showed very high rates of hospitalization (81%), ITU admission among hospitalized patients (29%) and mortality (18.6%) (77). A meta–analysis comparing both pediatric and adult SOT patients hospitalized with COVID–19 to the general population demonstrated significantly higher rates of ITU admission (35.8% vs. 23.1%) and mortality (23.2% vs. 12.5%) (4). Another meta–analysis also found higher mortality from COVID–19 in oncology patients on chemotherapy (OR: 1.85, 95% CI: 1.26–2.7) but no difference in oncology patients on immunotherapy (78).
There are several studies that found lower rates of severe COVID–19 outcomes. A prospective cohort of immunocompromised children in the UK found that 4 of 38 children (10.5%) with COVID–19 were hospitalized, with no cases requiring ITU admission or resulting in death, with the caveat that the outcomes were self–reported and the sample size was small (79). A meta–analysis comparing risks of severe COVID–19 infection in immunodeficient and immunosuppressed pediatric and adult patients to general population did not demonstrate statistically significant differences, although included only 28 immunodeficient and 11 immunosuppressed patients (80). A cohort study of Italian children and adults with primary immunodeficiencies did not demonstrate statistically different rates of COVID–19 mortality compared with the general population (3.81% vs. 3.28%), although the mortality rates in this study were significantly higher than in other population based cohorts (81).
Immunocompromised patients are heterogeneous and include a wide variety of suppressed or defective immune system responses. The innate immune system may be affected in primary immunodeficiencies (type I interferon response abnormalities) or in the context of immunosuppressive therapies (e.g., glucocorticosteroids, which inhibit the macrophage function, biologic therapies, which block pro–inflammatory cytokines, or small molecule targeting transcription factors implicated in the innate immune cell responses). The detection of viral RNA by dendritic cells using toll–like receptors, leading to subsequent interferon signaling is postulated to be vital in the early defence to SARS–CoV–2, and this response can be significantly altered in the context of immunodeficiency or immunosuppression. The adaptive immune response can be affected by other forms of immunocompromise (e.g., various B– and T–cell primary immunodeficiencies or iatrogenic immunosuppression affecting B– and T–cell function) and is important in clearing SARS–CoV–2 infection as well as regulating the overall immune response to infection. A good cytotoxic CD8+ T–cell response is thought to be important for early viral clearance, while memory T cells and B cells are vital for developing protective immunity after infection or vaccination (82).
This meta–analysis has several strengths. It is the only up–to–date meta–analysis in CYP that addressed the risk of selection bias by excluding papers reporting only on hospitalized cohorts. It is a large meta–analysis, including 793 immunocompromised and 102,022 non–immunocompromised CYP from a total of 60 studies, many of which were multicenter and multinational. Additionally, this meta–analysis included many studies from outside Europe/USA and 35% of studies were from LMIC, therefore, the results are likely relevant to immunocompromised CYP globally. A particular strength of this study was the stringent inclusion criteria and selection of truly immunocompromised CYP and exclusion vaccination as major confounder. The sensitivity analysis showed low risk of bias in reporting hospitalization in both groups, as well as ITU admission and mechanical ventilation in CYP in the general population.
This meta–analysis also has limitations, which suggest a need for cautious interpretation of our findings. The estimated prevalence of severe outcomes of COVID–19 infection in CYP in general population from our analyses is higher than outcomes from surveillance studies, for example, a UK national database study found 2.7% of children with confirmed COVID–19 were hospitalized (83), compared with 16% in our study. This difference is likely due to differences in the study settings, with lower rates of severe disease in “community–based studies” that use public health reporting systems compared with “healthcare–based studies” that recruit COVID–19 patients who present to healthcare services. This disparity was demonstrated in another meta–analysis that found that rates of hospitalization, ITU admission and death from COVID–19 in children in community–based general population studies were 3.3%, 0.3%, and 0.1%, respectively, which was strikingly different from findings in healthcare–based studies (23.9%, 2.9%, and 1.3%, respectively) (3), which matches the estimates from our meta–analysis. The explanation for this may be that asymptomatic or mildly symptomatic patients or who have less severe COVID–19 infection are underrepresented in healthcare–based studies. In our meta–analysis, most studies were healthcare based as many community–based studies did not report both ITU admission and death and the studies were quite heterogeneous, reporting variable estimates of severe COVID–19 outcomes, which may explain our higher–than–expected rates of severe outcomes of COVID–19 infection in non–immunocompromised CYP. Although the quality of outcome reporting in some studies led to a degree of selection bias, importantly, the same process of study selection was used for both the immunocompromised and non–immunocompromised meta–analyses in our study.
Retrospective study designs and clinician reporting of cases (especially in multicenter studies) will also result in reporting bias due to over–representation of severe cases. Additionally, although the non–immunocompromised CYP in the general population studies included in this analysis were carefully selected to avoid inclusion of immunocompromised CYP, they may have had other comorbidities that we could not account for.
There were large variations in COVID–19 clinical practice between healthcare centers, between countries, and over time, including variations in indications for testing, with some studies capturing populations tested only if symptomatic and unable to account for mild or asymptomatic cases, which are very common in CYP. There were also variations in practice in relation to hospital/ITU admission, as well as potentially more cautious approaches taken for immunocompromised patients (e.g., admitting them to hospital for monitoring), while more objective outcomes, such as rates of ventilation and death were less likely to be affected.
Additionally, the impact of COVID–19 therapeutics on outcomes of disease is unknown as this was variably reported. None of the patients included in this meta–analysis was reported to be vaccinated, as assessing the impact of immunocompromise on vaccinated CYP was beyond the scope of this paper. This analysis covers different waves of the pandemic in different countries, with variable access to treatments for which we could not account for, although there is some evidence for stable outcomes in COVID–19 outcomes in hospitalized patients over time (84).
Although this study demonstrated an overall trend for more severe outcomes of COVID–19 infection in immunocompromised CYP, it is not possible to attribute causality solely to the immunocompromise itself as most patients with have co–existent comorbidities, such as additional genetic abnormalities or comorbidities. Additionally, there is likely significant inter– and intra–group heterogeneity within immunocompromised cohorts (e.g., differences in types and doses of immunosuppressive medications), which may influence COVID–19 outcomes and was not possible to investigate in this analysis.
Future research must investigate the risk of severe COVID–19 in individual subgroups of immunocompromised patients and establishing the impact of immunosuppression type or dose as well as that of various comorbidities and other factors, such as age, sex, ethnicity, and socioeconomic status on COVID–19 risk. Further research is needed to investigate the efficacy of vaccination in preventing COVID–19 infection and the role of various therapeutics in treating COVID–19 infection in subgroups of immunocompromised CYP to support evidence–based recommendations for risk stratification and tailored management. Finally, it is vital that we continue to investigate how COVID–19 interacts with the immune system and the biological mechanism by which immunosuppression affects viral replication.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
Design of the study, JG–B, CC. Electronic searches and paper screening, JG–B, SA. Study selection and inclusion, JG–B, SA, CC. Data extraction and analysis for the systematic review, JG–B, SA, CC. Meta–analysis, CD. Writing the manuscript, JG–B, CC, CD: Review of the manuscript, CC, JG–B, CD. All authors approved the final version of the manuscript.
Funding
CC is supported by a National Institute of Health Research (NIHR) Biomedical Research Centre (BRC) at University College London Hospital (UCLH). The study was performed within the Centre for Adolescent Rheumatology Versus Arthritis at University College London (UCL), UCL Hospital and Great Ormond Street Hospital (GOSH) supported by grants from Versus Arthritis (21593 and 20164), Great Ormond Street Children’s Charity, and the NIHR–BRC at both GOSH and UCLH.
Acknowledgments
Special thanks to Prof. Lucy Wedderburn, Professor of Pediatric Rheumatology at University College London Institute of Child Health for reviewing the manuscript and providing useful comments. We are grateful to the following authors of various studies included in this review who provided additional data or clarifications on request, Danilo Buonsenso (Center for Global Health Research and Studies, Università Cattolica del Sacro Cuore, Italy), Anna Faura (Pediatric Oncology and Hematology Department, Hospital Sant Joan de Déu, Spain), Marc Tebruegge and the ptbnet COVID–19 study group (Department of Infection, Immunity and Inflammation, UCL Great Ormond Street Institute of Child Health, UK), Gerard Millen (Institute of Cancer and Genomic Sciences, University of Birmingham, UK), Sunil Kumar Rao (Department of Pediatrics, Banaras Hindu University, India), Marta González Vicent (Stem Cell Transplant Unit, Hospital Universitario Niño Jesús, Spain), Cigdem Arikan (Pediatric GI and Hepatology, Koç University School of Medicine, Turkey), Ildar Fakhradiyev (Asfendiyarov Kazakh National Medical University, Kazakhstan), John Charles O’Horo (Division of Infectious Diseases, Mayo Clinic, USA), Maria Pokorska–Śpiewak (Department of Children’s Infectious Diseases, Regional Hospital of Infectious Diseases in Warsaw, Medical University of Warsaw, Poland), Antoni Soriano–Arandes (Pediatric Infectious Diseases and Immunodeficiencies Unit, Hospital Universitari Valld’Hebron, Spain), Adriana Yock–Corrales (Pediatric Emergency Department, Hospital Nacional de Niños “Dr. Carlos Sáenz Herrera”, Costa Rica).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1159269/full#supplementary-material
References
1. University CfSSaEaJH. COVID–19 dashboard baltimore, maryland. United States: Johns Hopkins University. (2022). Available at: https://coronavirus.jhu.edu/map.html.
2. Organisation WH. Adolescent health in the South–East Asia Region (2022). Available at: https://www.who.int/southeastasia/health–topics/adolescent–health (Accessed 23rd October 2022).
3. Sumner MW, Kanngiesser A, Lotfali–Khani K, Lodha N, Lorenzetti D, Funk AL, et al. Severe outcomes associated with SARS–coV–2 infection in children: A systematic review and meta–analysis. Front Pediatr (2022) 10:916655. doi: 10.3389/fped.2022.916655
4. Belsky JA, Tullius BP, Lamb MG, Sayegh R, and Stanek JR, Auletta JJ. COVID–19 in immunocompromised patients: A systematic review of cancer, hematopoietic cell and solid organ transplant patients. J Infect (2021) 82(3):329–38. doi: 10.1016/j.jinf.2021.01.022
5. Tassone TA, Connell D, Lee W, Ungaro T, An R, Ding P, et al. Immunosuppression as a risk factor for COVID–19: a meta–analysis. Intern Med J (2021) 51(2):199–205. doi: 10.1111/imj151422021
6. Bixler D MA, Mattison CP, Taylor B, Komatsu K, Peterson Pompa X, Moon S, et al. Pediatric mortality investigation team. SARS–coV–associated deaths among persons aged <21 years – United States, february 12–july 31, 2020. SARS–coV–associated deaths among persons aged <21 years – United States, february 12–july 31, 2020. MMWR Morb Mortal Wkly Rep (2020) 69(37):1324–9. doi: 10.15585/mmwrmm6937e42020
7. Shekerdemian LS, Mahmood NR, Wolfe KK, Riggs BJ, Ross CE, McKiernan CA, et al. Characteristics and outcomes of children with coronavirus disease 2019 (COVID–19) infection admitted to US and canadian pediatric intensive care units. JAMA Pediatr (2020) 174(9):868–73. doi: 10.1001/jamapediatrics.2020.1948
8. Williams N, Radia T, Agrawal P, Cook J, Gupta A. COVID–19 Severe acute respiratory syndrome coronavirus 2 (SARS–CoV–2) infection in children and adolescents: a systematic review of critically unwell children and the association with underlying comorbidities. Eur J Pediatr (2021) 180(3):689–97. doi: 10.1007/s00431–020–03801–6
9. Hernández–Garduño E. Comorbidities that predict acute respiratory syndrome coronavirus 2 test positivity in Mexican Children: A case–control study. Pediatr Obes (2021) 16(5):e12740. doi: 10.1111/ijpo.12740
10. Choi JH, Choi SH, Yun KW. Risk factors for severe COVID–19 in children: A systematic review and meta–analysis. J Korean Med Sci (2022) 37(5):e35. doi: 10.3346/jkms.2022.37.e35
11. Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ (2021) 372:n160. doi: 10.1136/bmj.n160
12. Irfan O, Muttalib F, Tang K, Jiang L, Lassi ZS, Bhutta Z. Clinical characteristics, treatment and outcomes of paediatric COVID–19: a systematic review and meta–analysis. Arch Dis Child (2021) 106(5):440–8. doi: 10.1136/archdischild–2020–321385
13. Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle–Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta–analyses. Oxford (2000). Available at: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
14. Gotzinger F, Santiago–Garcia B, Noguera–Julian A, Lanaspa M, Lancella L, Carducci Calo FI, et al. COVID–19 in children and adolescents in Europe: a multinational, multicentre cohort study. Lancet Child Adolesc Health (2020) 4(9):653–61. doi: 10.1016/S2352–4642(20)30177–2
15. Antunez–Montes OY, Escamilla MI, Figueroa–Uribe AF, Arteaga-Menchaca E, Lavariega-Sarachaga M, Salcedo-Lozada P, et al. COVID–19 and multisystem inflammatory syndrome in latin american children: A multinational study. Pediatr Infect Dis J (2021) 40(1):e1–6. doi: 10.1097/INF.0000000000002949
16. Bisogno G, Provenzi M, Zama D, Tondo A, Meazza C, Colombini A, et al. Clinical Characteristics and Outcome of Severe Acute Respiratory Syndrome Coronavirus 2 Infection in Italian Pediatric Oncology Patients: A Study From the Infectious Diseases Working Group of the Associazione Italiana di Oncologia e Ematologia Pediatrica. J Pediatr Infect Dis Soc (2020) 9(5):530–34. doi: 10.1093/jpids/piaa088
17. Castano–Jaramillo LM, Yamazaki–Nakashimada MA, O’Farrill–ROmanillos PM, Zermeno Muzquiz D, Mendoza Scheffler SC, Montoya Venegas E, et al. COVID–19 in the context of inborn errors of immunity: a case series of 31 patients from Mexico. J Clin Immunol (2021) 41(7):1463–78. doi: 10.1007/s10875–021–01077–5
18. de Rojas T, Perez–Martinez A, Cela E, Baragano M, Galan V, Mata C, et al. COVID–19 infection in children and adolescents with cancer in Madrid. Pediatr Blood Cancer (2020) 67(7):e28397. doi: 10.1002/pbc.28397
19. Delavari S, Abolhassani H, Abolnezhadian F, Babaha F, Iranparast S, Ahanchian H, et al. Impact of SARS–coV–2 pandemic on patients with primary immunodeficiency. J Clin Immunol (2021) 41(2):345–55. doi: 10.1007/s10875–020–00928–x
20. Deya–Martinez A, Garcia–Garcia A, Gonzalez–Navarro EA, Yiyi L, Vlagea A, Jordan I, et al. COVID–19 in children and young adults with moderate/severe inborn errors of immunity in a high burden area in pre–vaccine era. Clin Immunol (2021) 230:108821. doi: 10.1016/j.clim.2021.108821
21. El Dannan H, Al Hassani M, Ramsi M. Clinical course of COVID–19 among immunocompromised children: a clinical case series. BMJ Case Rep (2020) 13(10). doi: 10.1136/bcr–2020–237804
22. Esenboga S, Ocak M, Akarsu A, Bildik HN, Cagdas D, Iskit AT, et al. COVID–19 in patients with primary immunodeficiency. J Clin Immunol (2021) 41(7):1515–22. doi: 10.1007/s10875–021–01065–9
23. Faura A, Rives S, Lassaletta A, Sebastian E, Madero L, Huerta J, et al. Initial report on Spanish pediatric oncologic, hematologic, and post stem cell transplantation patients during SARS–CoV–2 pandemic. Pediatr Blood Cancer (2020) 67(9):e28557. doi: 10.1002/pbc.28557
24. Ferrari A, Zecca M, Rizzari C, Porta F, Provenzi M, Marinoni M, et al. Children with cancer in the time of COVID–19: An 8–week report from the six pediatric onco–hematology centers in Lombardia, Italy. Pediatr Blood Cancer (2020) 67(8):e28410. doi: 10.1002/pbc.28410
25. Gampel B, Troullioud Lucas AG, Broglie L, Gartrell-Corrado RD, Lee MT, Levine J, et al. COVID–19 disease in New York City pediatric hematology and oncology patients. Pediatr Blood Cancer (2020) 67(9):e28420. doi: 10.1002/pbc.28420
26. Goss MB, Galvan NTN, Ruan W, Munoz FM, Brewer ED, O'Mahony CA, et al. The pediatric solid organ transplant experience with COVID–19: An initial multi–center, multi–organ case series. Pediatr Transplant (2021) 25(3):e13868. doi: 10.1111/petr.13868
27. Hrusak O, Kalina T, Wolf J, Balduzzi A, Provenzi M, Rizzari C, et al. Flash survey on severe acute respiratory syndrome coronavirus–2 infections in paediatric patients on anticancer treatment. Eur J Cancer (2020) 132:11–6. doi: 10.1016/j.ejca.2020.03.021
28. Ihara BP, Strabelli CA, Simon JR, Viana VS, Sallum AM, Kozu KT, et al. Laboratory–confirmed pediatric COVID–19 in patients with rheumatic diseases: A case series in a tertiary hospital. Lupus (2021) 30(5):856–60. doi: 10.1177/0961203321998427
29. Kamdar KY, Kim TO, Doherty EE, Pfeiffer TM, Qasim SL, Suell MN, et al. COVID–19 outcomes in a large pediatric hematology–oncology center in Houston, Texas. Pediatr Hematol Oncol (2021) 38(8):695–706. doi: 10.1080/08880018.2021.1924327
30. Lucchini G, Furness C, Lawson S, Gibson B, Wynn R, Slatter M, et al. COVID–19 infection in paediatric recipients of allogeneic stem cell transplantation: the UK experience. Br J Haematol (2021) 194(4):e74–7. doi: 10.1111/bjh.17547
31. Madhusoodhan PP, Pierro J, Musante J, Kothari P, Gampel B, Appel B, et al. Characterization of COVID–19 disease in pediatric oncology patients: The New York–New Jersey regional experience. Pediatr Blood Cancer (2021) 68(3):e28843. doi: 10.1002/pbc.28843
32. Marcus N, Frizinsky S, Hagin D, Ovadia A, Hanna S, Farkash M, et al. Minor clinical impact of COVID–19 pandemic on patients with primary immunodeficiency in Israel. Front Immunol (2020) 11:614086. doi: 10.3389/fimmu.2020.614086
33. Marlais M, Wlodkowski T, Al–Akash S, Ananin P, Bandi VK, Baudouin V, et al. COVID–19 in children treated with immunosuppressive medication for kidney diseases. Arch Dis Child (2020) 106(8):798–801. doi: 10.1136/archdischild–2020–320616
34. Melgosa M, Madrid A, Alvarez O, Lumbreras J, Nieto F, Parada E, et al. SARS–CoV–2 infection in Spanish children with chronic kidney pathologies. Pediatr Nephrol (2020) 35(8):1521–24. doi: 10.1007/s00467–020–04597–1
35. Meyts I, Bucciol G, Quinti I, Neven B, Fischer A, Seoane E, et al. Coronavirus disease 2019 in patients with inborn errors of immunity: An international study. J Allergy Clin Immunol (2021) 147(2):520–31. doi: 10.1016/j.jaci.2020.09.010
36. Millen GC, Arnold R, Cazier JB, Curley H, Feltbower RG, Gamble A, et al. Severity of COVID–19 in children with cancer: Report from the United Kingdom Paediatric Coronavirus Cancer Monitoring Project. Br J Cancer (2021) 124(4):754–59. doi: 10.1038/s41416–020–01181–0
37. Perez–Martinez A, Guerra–Garcia P, Melgosa M, Frauca E, Fernandez-Camblor C, Remesal A, et al. Clinical outcome of SARS–CoV–2 infection in immunosuppressed children in Spain. Eur J Pediatr (2021) 180(3):967–71. doi: 10.1007/s00431–020–03793–3
38. Rao SK, Kumar A, Prasad R, Gupta V, Mishra OP. Clinical profile and outcome of COVID–19 among immunocompromised children. Indian Pediatr (2021) 58(7):686–87. doi: 10.1007/s13312–021–2267–6
39. Rouger–Gaudichon J, Thebault E, Felix A, Phulpin A, Paillard C, Alimi A, et al. Impact of the first wave of COVID–19 on pediatric oncology and hematology: A report from the french society of pediatric oncology. Cancers (Basel) (2020) 12(11). doi: 10.3390/cancers12113398
40. Singer PS, Sethna C, Molmenti E, Fahmy A, Grodstein E, Castellanos-Reyes L, et al. COVID–19 infection in a pediatric kidney transplant population: A single–center experience. Pediatr Transplant (2021) 25(4):e14018. doi: 10.1111/petr.14018
41. Turner D, Huang Y, Martin–de–Carpi J, Aloi M, Focht G, Kang B, et al. Corona virus disease 2019 and paediatric inflammatory bowel diseases: global experience and provisional guidance (March 2020) from the paediatric IBD porto group of european society of paediatric gastroenterology, hepatology, and nutrition. J Pediatr Gastroenterol Nutr (2020) 70(6):727–33. doi: 10.1097/MPG.0000000000002729
42. Vicent MG, Martinez AP, Trabazo Del Castillo M, Molina B, Sisini L, Moron-Cazalilla G, et al. COVID–19 in pediatric hematopoietic stem cell transplantation: The experience of Spanish Group of Transplant (GETMON/GETH). Pediatr Blood Cancer (2020) 67(9):e28514. doi: 10.1002/pbc.28514
43. Yuksel M, Akturk H, Mizikoglu O, Toroslu E, Arikan C. A single–center report of COVID–19 disease course and management in liver transplanted pediatric patients. Pediatr Transplant (2021) 25(7):e14061. doi: 10.1111/petr.14061
44. Bailey LC, Razzaghi H, Burrows EK, Bunnell HT, Camacho PEF, Christakis DA, et al. Assessment of 135 794 pediatric patients tested for severe acute respiratory syndrome coronavirus 2 across the United States. JAMA Pediatr (2021) 175(2):176–84. doi: 10.1001/jamapediatrics.2020.5052
45. Bayesheva D, Boranbayeva R, Turdalina B, Fakhradiyev I, Saliev T, Tanabayeva S, et al. COVID–19 in the paediatric population of Kazakhstan. Paediatr Int Child Health (2021) 41(1):76–82. doi: 10.1080/20469047.2020.1857101
46. Chao JY, Derespina KR, Herold BC, Goldman DL, Aldrich M, Weingarten J, et al. Clinical characteristics and outcomes of hospitalized and critically ill children and adolescents with coronavirus disease 2019 at a tertiary care medical center in new york city. J Pediatr (2020) 223:14–19.e2. doi: 10.1016/j.jpeds.2020.05.006
47. Foster CE, Moulton EA, Munoz FM, Hulten KG, Versalovic J, Dunn J, et al. Coronavirus disease 2019 in children cared for at texas children’s hospital: initial clinical characteristics and outcomes. J Pediatr Infect Dis Soc (2020) 9(3):373–77. doi: 10.1093/jpids/piaa072
48. Howard LM, Garguilo K, Gillon J, LeBlanc K, Seegmiller AC, Schmitz JE, et al. The first 1000 symptomatic pediatric SARS–CoV–2 infections in an integrated health care system: a prospective cohort study. BMC Pediatr (2021) 21(1):403. doi: 10.1186/s12887–021–02863–1
49. Katayama Y, Zha L, Kitamura T, Hirayama A, Takeuchi T, Tanaka K, et al. Characteristics and outcomes of pediatric COVID–19 patients in osaka, Japan. Int J Environ Res Public Health (2021) 18(11). doi: 10.3390/ijerph18115911
50. Kim TY, Kim EC, Agudelo AZ, Friedman L. COVID–19 hospitalization rate in children across a private hospital network in the United States: COVID–19 hospitalization rate in children. Arch Pediatr (2021) 28(7):530–32. doi: 10.1016/j.arcped.2021.07.004
51. Kompaniyets L, Agathis NT, Nelson JM, Preston LE, Ko JY, Belay B, et al. Underlying medical conditions associated with severe COVID–19 illness among children. JAMA Netw Open (2021) 4(6):e2111182. doi: 10.1001/jamanetworkopen.2021.11182
52. Korkmaz MF, Ture E, Dorum BA, Kilic ZB. The epidemiological and clinical characteristics of 81 children with COVID–19 in a pandemic hospital in Turkey: an observational cohort study. J Korean Med Sci (2020) 35(25):e236. doi: 10.3346/jkms.2020.35.e236
53. Lavaine O, Spizzo J, Arbitre C, Muller J, Kuhn P, Laugel V, et al. COVID–19 in children at Strasbourg University Hospital: A retrospective study of the first 2months of the epidemic. Arch Pediatr (2021) 28(5):405–10. doi: 10.1016/j.arcped.2021.03.013
54. Lazzerini M, Sforzi I, Trapani S, Biban P, Silvagni D, Villa G, et al. Characteristics and risk factors for SARS–CoV–2 in children tested in the early phase of the pandemic: a cross–sectional study, Italy, 23 February to 24 May 2020. Euro Surveill (2021) 26(14). doi: 10.2807/1560–7917.ES.2021.26.14.2001248
55. Lu X, Zhang L, Du H, Zhang J, Li YY, Qu J, et al. SARS–coV–2 infection in children. N Engl J Med (2020) 382(17):1663–65. doi: 10.1056/NEJMc2005073
56. Mania A, Mazur–Melewska K, Lubarski K, Kuczma-Napierala J, Mazurek J, Jonczyk-Potoczna K, et al. Wide spectrum of clinical picture of COVID–19 in children – From mild to severe disease. J Infect Public Health (2021) 14(3):374–79. doi: 10.1016/j.jiph.2020.12.029
57. Matteudi T, Luciani L, Fabre A, Minodier P, Boucekine M, Bosdure E, et al. Clinical characteristics of paediatric COVID–19 patients followed for up to 13 months. Acta Paediatr (2021) 110(12):3331–33. doi: 10.1111/apa.16071
58. Meyer M, Holfter A, Ruebsteck E, Gruell H, Dewald F, Koerner RW, et al. The alpha variant (B.1.1.7) of SARS–coV–2 in children: first experience from 3544 nucleic acid amplification tests in a cohort of children in Germany. Viruses (2021) 13(8). doi: 10.3390/v13081600
59. O’Horo JC, Cerhan JR, Cahn EJ, Bauer PR, Temesgen Z, Ebbert J, et al. Outcomes of COVID–19 with the mayo clinic model of care and research. Mayo Clin Proc (2021) 96(3):601–18. doi: 10.1016/j.mayocp.2020.12.006
60. Onal P, Kilinc AA, Aygun F, Durak C, Cokugras H. COVID–19 in Turkey: A tertiary center experience. Pediatr Int (2021) 63(7):797–805. doi: 10.1111/ped.14549
61. Otto WR, Geoghegan S, Posch LC, Bell LM, Coffin SE, Sammons JS, et al. The epidemiology of severe acute respiratory syndrome coronavirus 2 in a pediatric healthcare network in the United States. J Pediatr Infect Dis Soc (2020) 9(5):523–29. doi: 10.1093/jpids/piaa074
62. Parri N, Lenge M, Cantoni B, Arrighini A, Romanengo M, Urbino A, et al. COVID–19 in 17 italian pediatric emergency departments. Pediatrics (2020) 146(6). doi: 10.1542/peds.2020–1235
63. Pokorska–Spiewak M, Talarek E, Popielska J, Nowicka K, Oldakowska A, Zawadka K, et al. Comparison of clinical severity and epidemiological spectrum between coronavirus disease 2019 and influenza in children. Sci Rep (2021) 11(1):5760. doi: 10.1038/s41598–021–85340–0
64. Pokorska–Spiewak M, Talarek E, Mania A, Pawlowska M, Popielska J, Zawadka K, et al. Clinical and epidemiological characteristics of 1283 pediatric patients with coronavirus disease 2019 during the first and second waves of the pandemic–results of the pediatric part of a multicenter polish register SARSTer. J Clin Med (2021) 10(21). doi: 10.3390/jcm10215098[published Online First: 20211030
65. Qiu H, Wu J, Hong L, Luo Y, Song Q, Chen D. Clinical and epidemiological features of 36 children with coronavirus disease 2019 (COVID–19) in Zhejiang, China: an observational cohort study. Lancet Infect Dis (2020) 20(6):689–96. doi: 10.1016/S1473–3099(20)30198–5
66. Saatci D, Ranger TA, Garriga C, Clift AK, Zaccardi F, Tan PS, et al. Association between race and COVID–19 outcomes among 2.6 million children in england. JAMA Pediatr (2021) 175(9):928–38. doi: 10.1001/jamapediatrics.2021.1685
67. Sarangi B, Reddy VS, Oswal JS, Malshe N, Patil A, Chakraborty M, et al. Epidemiological and clinical characteristics of COVID–19 in Indian children in the initial phase of the pandemic. Indian Pediatr (2020) 57(10):914–17. doi: 10.1007/s13312–020–1994–4
68. Schonfeld D, Arias S, Bossio JC, Fernandez H, Gozal D, Perez-Chada D. Clinical presentation and outcomes of the first patients with COVID–19 in Argentina: Results of 207079 cases from a national database. PloS One (2021) 16(2):e0246793. doi: 10.1371/journal.pone.0246793
69. Soriano–Arandes A, Gatell A, Serrano P, Biosca M, Campillo F, Capdevila R, et al. Household severe acute respiratory syndrome coronavirus 2 transmission and children: A network prospective study. Clin Infect Dis (2021) 73(6):e1261–e69. doi: 10.1093/cid/ciab228
70. Wang M, Nie X, Huang S, Pi W, Wang D, Zhou M, et al. Epidemiological characteristics and transmission dynamics of paediatric cases with coronavirus disease 2019 in Hubei province, China. J Paediatr Child Health (2021) 57(5):637–45. doi: 10.1111/jpc.15287
71. Yock–Corrales A, Lenzi J, Ulloa–Gutierrez R, Gomez-Vargas J, Antunez-Montes OY, Aida Rios JA, et al. High rates of antibiotic prescriptions in children with COVID–19 or multisystem inflammatory syndrome: A multinational experience in 990 cases from Latin America. Acta Paediatr (2021) 110(6):1902–10. doi: 10.1111/apa.15847
72. Yousaf AR, Duca LM, Chu V, Reses HE, Fajans M, Rabold EM, et al. A prospective cohort study in nonhospitalized household contacts with severe acute respiratory syndrome coronavirus 2 infection: symptom profiles and symptom change over time. Clin Infect Dis (2021) 73(7):e1841–e49. doi: 10.1093/cid/ciaa1072
73. Sattui SE, Conway R, Putman MS, Seet AM, Gianfrancesco MA, Beins K, et al. Outcomes of COVID–19 in patients with primary systemic vasculitis or polymyalgia rheumatica from the COVID–19 Global Rheumatology Alliance physician registry: a retrospective cohort study. Lancet Rheumatol (2021) 3(12):e855–e64. doi: 10.1016/s2665–9913(21)00316–7
74. Marques CDL, Kakehasi AM, Pinheiro MM, Mota LMH, Albuquerque C.P, Silva CR, et al. High levels of immunosuppression are related to unfavourable outcomes in hospitalised patients with rheumatic diseases and COVID–19: first results of ReumaCoV Brasil registry. RMD Open (2021) 7(1). doi: 10.1136/rmdopen-2020-001461
75. Baek MS, Lee MT, Kim WY, Choi JC, Jung SY. COVID–related outcomes in immunocompromised patients: A nationwide study in Korea. PloS One (2021) 16(10):e0257641. doi: 10.1371/journal.pone
76. Suárez–García I, Perales–Fraile I, González–García A, Muñoz-Blanco A, Manzano L, Fabregate M, et al. In–hospital mortality among immunosuppressed patients with COVID–19: Analysis from a national cohort in Spain. PloS One (2021) 16(8):e0255524. doi: 10.1371/journal.pone.0255524
77. Raja MA, Mendoza MA, Villavicencio A, Anjan S, Reynolds JM, Kittipibul V, et al. COVID–19 in solid organ transplant recipients: A systematic review and meta–analysis of current literature. Transplant Rev (Orlando) (2021) 35(1):100588. doi: 10.1016/j.trre.2020.100588
78. Yekedüz E, Utkan G, Ürün Y. A systematic review and meta–analysis: the effect of active cancer treatment on severity of COVID–19. Eur J Cancer (2020) 141:92–104. doi: 10.1016/j.ejca.2020.09.028
79. Chappell H, Patel R, Driessens C, Tarr AW, Irving WL, Tighe PJ, et al. Immunocompromised children and young people are at no increased risk of severe COVID–19. J Infect (2022) 84(1):31–9. doi: 10.1016/j.jinf.2021.11.005
80. Gao Y, Chen Y, Liu M, Shi S, Tian J. Impacts of immunosuppression and immunodeficiency on COVID–19: A systematic review and meta–analysis. J Infect (2020) 81(2):e93–5. doi: 10.1016/j.jinf.2020.05.017
81. Milito C, Lougaris V, Giardino G, Punziano A, Vultaggio A, Carrabba M, et al. Clinical outcome, incidence, and SARS–CoV–2 infection–fatality rates in Italian patients with inborn errors of immunity. J Allergy Clin Immunol Pract (2021) 9(7):2904–06.e2. doi: 10.1016/j.jaip.2021.04.017
82. Moss P. The T cell immune response against SARS–CoV–2. Nat Immunol (2022) 23(2):186–93. doi: 10.1038/s41590–021–01122–w
83. Thelwall S, Aiano F, Harman K, Dabrera G, Ladhani SN. Risk of hospitalisation and death in children with SARS–CoV–2 delta (B.1.612.2) infection. Lancet Child Adolesc Health (2022) 6(5):e16–7. doi: 10.1016/s2352–4642(22)00096–7
84. Carbonell R, Urgelés S, Rodríguez A, Bodí M, Martín-Loeches I, Solé-Violán J, et al. Mortality comparison between the first and second/third waves among 3,795 critical COVID–19 patients with pneumonia admitted to the ICU: A multicentre retrospective cohort study. Lancet Reg Health Eur (2021) 11:100243. doi: 10.1016/j.lanepe.2021.100243
Keywords: COVID - 19, immunosuppression, ITU - (intensive therapy unit), hospitalization, death, children, young people
Citation: Greenan-Barrett J, Aston S, Deakin CT and Ciurtin C (2023) The impact of immunocompromise on outcomes of COVID-19 in children and young people—a systematic review and meta-analysis. Front. Immunol. 14:1159269. doi: 10.3389/fimmu.2023.1159269
Received: 08 February 2023; Accepted: 04 August 2023;
Published: 25 August 2023.
Edited by:
Claudio Pignata, University of Naples Federico II, ItalyReviewed by:
Nianqiao Gong, Huazhong University of Science and Technology, ChinaHiroyuki Suzuki, The University of Iowa, United States
Copyright © 2023 Greenan-Barrett, Aston, Deakin and Ciurtin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Coziana Ciurtin, Yy5jaXVydGluQHVjbC5hYy51aw==
†These authors share senior authorship
‡ORCID: Coziana Ciurtin, orcid.org/0000-0002-8911-4113
 James Greenan-Barrett1
James Greenan-Barrett1 Claire T. Deakin
Claire T. Deakin Coziana Ciurtin
Coziana Ciurtin