- 1Division of Infectious Diseases, Department of Internal Medicine, State Key Laboratory of Complex Severe and Rare Disease, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
- 2Clinical Epidemiology Unit, Peking Union Medical College, International Clinical Epidemiology Network, Beijing, China
- 3Center for Tuberculosis Research, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
- 4School of Population Medicine and Public Health, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
- 54 + 4 Medical Doctor Program, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, China
- 6Peking Union Medical College Hospital, Peking Union Medical College, Beijing, China
- 7Department of Internal Medicine, State Key Laboratory of Complex Severe and Rare Disease, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
- 8Key Laboratory of Rheumatology & Clinical Immunology, Department of Rheumatology and Clinical Immunology, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, Ministry of Education, Beijing, China
Objectives: Both burdens of tuberculosis (TB) and systemic lupus erythematosus (SLE) in China are ranked as top three in the world. SLE patients are at high risk for TB, but so far, there are no guidelines for TB prevention and management targeting this population in China. This study aims to investigate the incidence of active tuberculosis (ATB) and to explore the risk factors for developing ATB in SLE patients, and to provide evidence for TB prevention and management for SLE patients in China.
Methods: A multi-center prospective cohort study was conducted. SLE patients were enrolled from clinics and wards of 13 tertiary hospitals in Eastern, Middle, and Western China from September 2014 to March 2016. Baseline demographic features, TB infection status, clinical information, and laboratory data were collected. ATB development was examined during follow-up visits. Kaplan-Meier method was applied to plot survival curves, and Log-rank test was used to evaluate differences. Cox proportional-hazards model was used to explore the risk factors for ATB development.
Results: With a median follow-up time of 58 months [interquartile range (IQR): 55-62], 16 out of 1361 SLE patients developed ATB. The 1-year incidence of ATB was 368 [95% confidence interval (CI): 46-691] per 100,000. Over a 5-year period, the cumulative incidence of ATB was 1141 [95% CI: 564-1718] per 100,000, and the incidence density was 245 per 100,000 person-years. Cox regression models were constructed with maximum daily dose of glucocorticoids (GCs) as a continuous variable and a categorical variable, respectively. In model 1, maximum daily dose of GCs (pills per day) [adjusted hazard ratio (aHR)=1.16, 95%CI: 1.04-1.30, p=0.010] and TB infection (aHR=8.52, 95%CI: 3.17-22.92, p<0.001) were independent risk factors for ATB development. In model 2, maximum daily dose of GCs≥30 mg/d (aHR =4.81, 95%CI: 1.09-22.21, P=0.038) and TB infection (aHR=8.55, 95%CI: 3.18-23.00, p<0.001] were independent risk factors for ATB development.
Conclusions: SLE patients had a higher incidence of ATB compared to the general population. The risk of developing ATB was even higher with increased daily dose of GCs or in a status of TB infection, in which case TB preventive treatment should be considered.
1 Introduction
Tuberculosis (TB) is a chronic respiratory infectious disease caused by Mycobacterium tuberculosis (MTB). WHO estimated that China had 780,000 new TB patients in 2021, with an incidence of 55 per 100,000 and disease burden ranked third in the world (1). Systemic lupus erythematosus (SLE) is a representative of common chronic systemic rheumatic immune diseases that occurs frequently in young women of childbearing age. The prevalence of SLE in China is 30 to 70 per 100,000, ranking second in the world. It is estimated that there are 1 million SLE patients in China. Unlike European and American countries, infection is the leading cause of death for SLE patients in China (2).
The difficult control of TB is related to special properties of MTB. Instead of developing ATB directly, 90% of people infected with MTB are in the status of latent tuberculosis infection (LTBI) without clinical symptoms. The prevalence of LTBI in China is as high as 20%, with an estimation of 300 million LTBI patients (3). An LTBI patient has a 10% chance of progressing to ATB and becoming a new source of infection (4).
The pathogenesis of SLE has not yet been elucidated. The main risk of developing ATB is the use of glucocorticoids (GCs) or immunosuppressants in patients with SLE, which could already manifest abnormalities in the number and function of T and B lymphocytes. Disease characteristics and therapeutic strategies place SLE patients at high risk for LTBI and ATB (5). Studies showed that the ATB prevalence among SLE patients was as high as 2.3%-19.6% (6–8) and the mortality rate of SLE patients with ATB was 15.6%-54.5% (6–11), which was 4-15 times that of ordinary ATB patients. Moreover, since SLE is chronic and hard to cure, patients need long-term follow-up visits in general hospitals, which further increase the risk of TB infection and transmission.
TB preventive treatment (TPT) can reduce the risk of ATB by about 70% (12), but its common adverse reactions such as liver toxicity should not be ignored (13). The proportion of adverse reactions of anti-TB drugs in patients with rheumatic immune diseases was as high as 36%, and 19.4% of the patients had to discontinue or change medications due to severe adverse reactions (14). Concomitant medication is an independent risk factor for adverse reactions of TPT (15). Due to a large population base, China has high burdens of both TB (both ATB and LTBI) and SLE. For SLE patients, unselective prevention is a waste of medical resources and may lead to serious adverse reactions. Therefore, accurate identification and management of SLE patients at high risk of ATB, is not only crucial for SLE patients but also one of the breakthrough points in achieving the two major goals of reducing the incidence and mortality rates of TB prevention and control in China.
There are limited existing studies on the incidence of ATB in SLE patients, which are either retrospective, single-center, or small in sample size (16–22). No relevant reports in the mainland of China have yet been published, much less epidemiological data on the occurrence of ATB in SLE patients nationwide. With multi-stage cluster sampling, this study is a multi-center prospective cohort study based on tertiary general hospitals in China. The objectives are investigating incidence and exploring risk factors of ATB in SLE patients in China, which are valuable for TB prevention and management targeting this population.
2 Methods
2.1 Study population
This study was based on the Epidemiological Study and Therapeutic Evaluation of Rheumatic Patients with Tuberculosis (ETHERTB) (23). Research subjects were selected from outpatients and inpatients with SLE in 13 tertiary general hospitals in eastern, central, and western China from September 2014 to March 2016. Inclusion criteria: 1) age>15 years; 2) meeting the 1997 American College of Rheumatology (ACR) SLE classification criteria (24). Exclusion criteria: 1) patients with suspected or confirmed ATB; 2) pregnant women; 3) refusal to follow-up visits. Whether a given patient met enrollment conditions or not was independently reviewed and verified by two rheumatologists; in case of disagreement, a third rheumatologist must be consulted for a decision.
2.2 Sampling
According to geographical location and economic status, China was divided into three major regions: eastern, central, and western. Then multi-stage cluster sampling was adopted nationwide to select 13 tertiary general hospitals, from which SLE outpatients and inpatients were continuously included in a cross-sectional survey. Patients who agreed to follow-up visits were enrolled in the cohort.
2.3 Data collection
Patient data collection was performed by trained investigators using a unified questionnaire. All enrolled SLE patients were tested for T-SPOT.TB or TST to identify LTBI at the time of enrollment. Demographic information, course of disease, SLE disease activity index (SLEDAI)-2000 (25), the use of medications such as GCs or immunosuppressants (including cyclophosphamide (CTX), mycophenolate mofetil (MMF), methotrexate (MTX), azathioprine (AZA), leflunomide (LEF), cyclosporine A (CsA), tacrolimus (FK506)), TB infection status, and laboratory results such as routine blood test were collected at baseline. Follow-up visits were conducted in the first and fifth years, requesting patients to attend a face-to-face interview at the clinic or a telephone follow-up if they were unable to come to the appointment. The follow-up evaluation included assessments of medication regarding GCs/immunosuppressants usage, history of TB exposure, the presence of suspicious TB symptoms such as coughing, fever, chest pain or night sweats, and whether ATB was diagnosed. If a patient was suspected or confirmed to have ATB, the relevant medical records must be reviewed and discussed by an expert panel before confirmation. Course of disease was defined as the time from the first diagnosis of SLE to enrollment. TB infection status was defined as positive T-SPOT.TB, positive TST, or evidence of previous TB (including past TB history and imaging showing old TB lesions such as fiber strips). Medication including GCs and immunosuppressants usage referred to the period from enrollment to the end of follow-up. The occurrence of ATB referred to etiological or clinical diagnosis of ATB during the follow-up period, with the same standard as that of the ETHERTB study (23). In the study, determination of a patient’s enrollment was taken as the initial event, while ATB occurrence was the end event. Professionally trained researchers used a double-entry method to enter data. When the entered data was discrepant or obviously illogical, it would be re-checked; if necessary, the corresponding patient would be contacted again to ensure accuracy of information.
2.4 Statistical analysis
Kolmogorov-Smirnov test was used to test the normality of continuous variables. Median and interquartile range were used to describe continuous variables following a non-normal distribution. Categorical variables were described by frequency and percentage. Life table method was used to calculate cumulative incidences and corresponding confidence intervals. Kaplan-Meier method was used to draw survival curves, and Log-rank test was used to evaluate risk differences of ATB. Cox proportional hazards regression model was used to analyze influencing factors of ATB incidence in this population. Variables considered to be clinically significant or significantly related to ATB infection in the univariate Cox regression analysis (P<0.1) were included in the multivariate Cox regression model (26). In view of the number of events available, we carefully screened variables to ensure compactness of the final model (Backward LR, entry 0.05, removal 0.10). All statistical results above were obtained using SPSS 26 (IBM Crop, SPSS Inc., Chicago, IL, USA) software.
2.5 Ethical approval
This study was approved by the Ethics Committees of Peking Union Medical College Hospital (No. S-715) and 12 participating hospitals. Written informed consents were obtained from all patients and their legal guardians if necessary.
3 Results
3.1 General characteristics
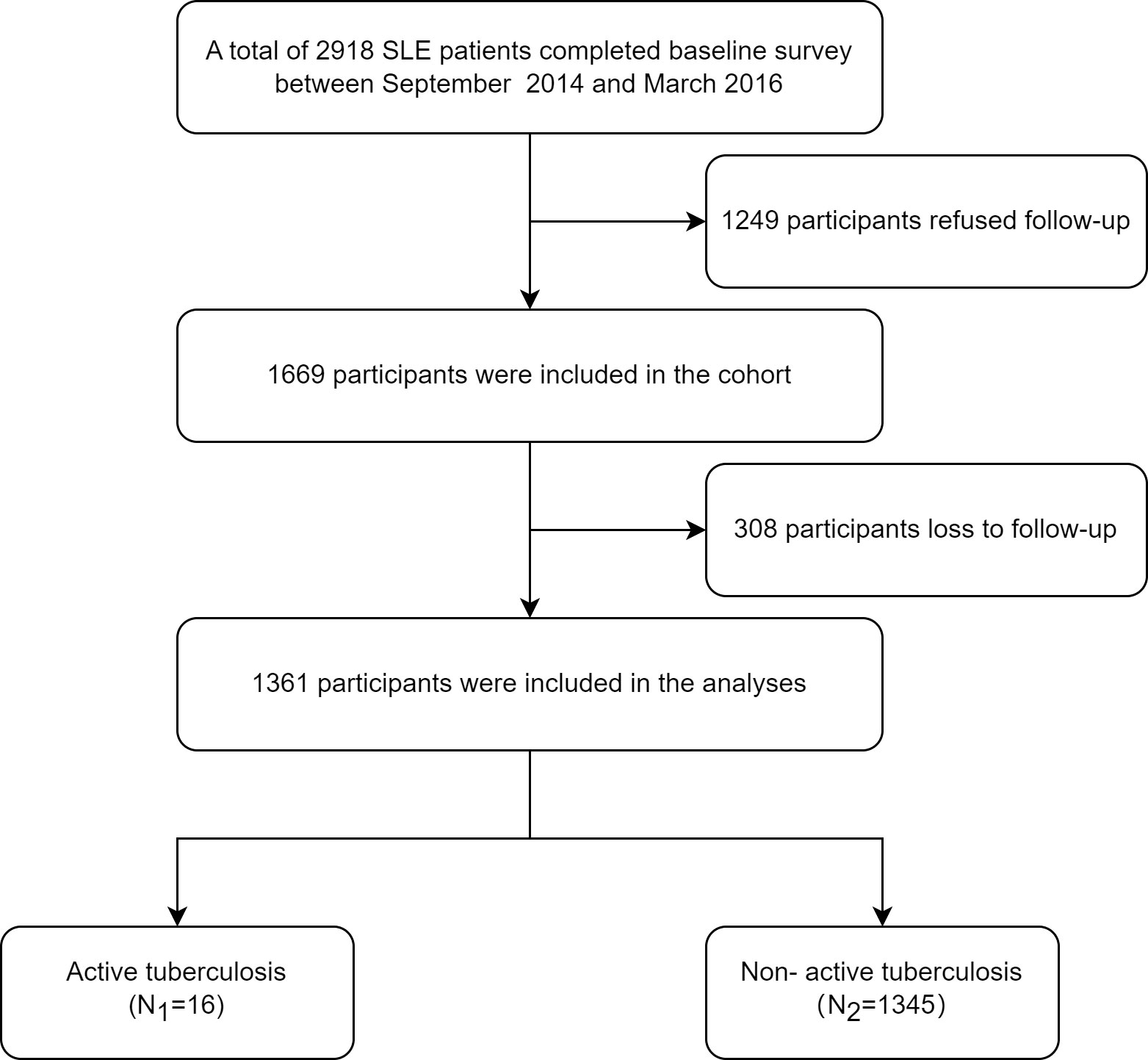
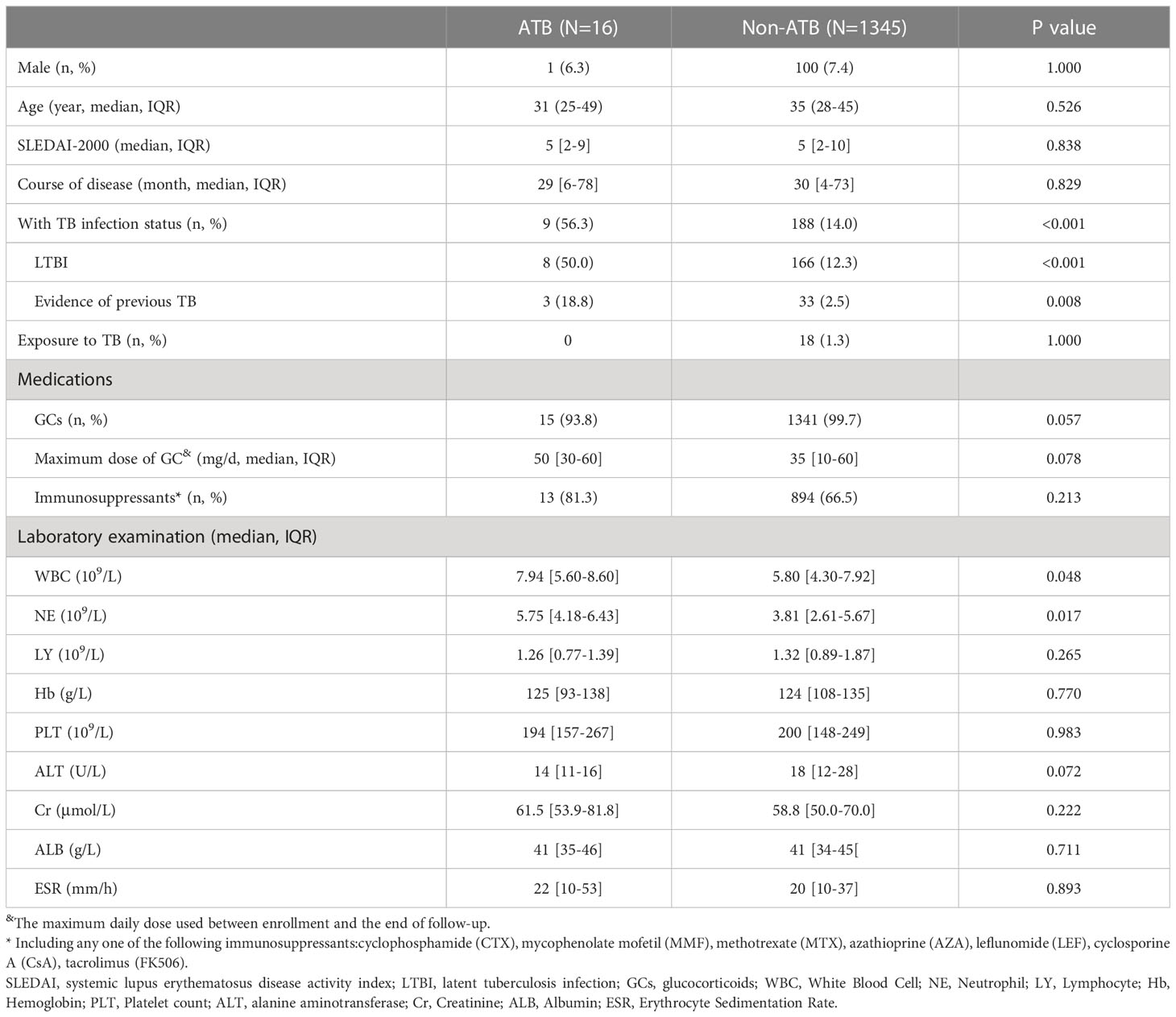
A total of 2,918 eligible patients were recruited in the ETHERTB study, of which 1,249 patients were not included because of refusal to follow-up visits. 1,669 patients were finally enrolled. During the study, 308 patients lost to follow-up, with a drop-out rate of 18.5%. Baseline data of patients who refused follow-up, who dropped out, and who responded are shown in Supplementary Table 1. Among the 1361 SLE patients who completed follow-up, the positive rates of T-SPOT.TB and TST (induration diameter≥5 mm) were 15.6% (172/1103) and 9.4% (32/341), respectively. Until the end of observation, 16 out of 1361 SLE patients developed ATB (Figure 1). Baseline information is shown in Table 1.
3.2 ATB incidence in SLE patients
Among the 1361 SLE patients, the median follow-up time was 58 months (IQR: 55-62), during which 16 patients developed ATB. Among the 16 ATB patients, 10 cases (62.5%) were microbiologically or pathologically confirmed, while 6 cases (37.5%) were clinically diagnosed. According to the classification based on disease site, 11 cases (69%) had lung involved, including 10 cases of pulmonary TB and 1 case of pulmonary TB combined with lymphatic TB. The others were 2 cases of pleural TB, 2 cases of TB meningitis, 1 case of lymphatic TB.
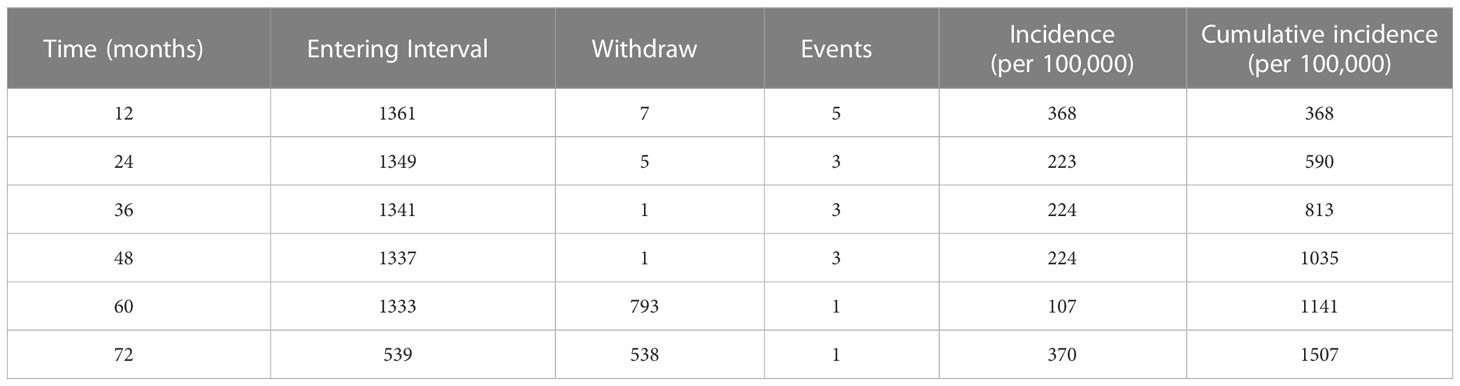
The 1-year incidence of ATB in this cohort was 368 (95%CI 46-691) per 100,000, and the five-year cumulative incidence was 1141 (95%CI 564-1718) per 100,000 (Table 2). The incidence density of ATB in SLE patients was 245 per 100,000 person-years during the follow-up period. Compared to SLE patients without MTB infection, SLE patients with TB infection were at higher risk of developing ATB (Figure 2). The ATB incidence density of SLE patients without MTB infection was 125 per 100,000 person-years, while the ATB incidence density of those with TB infection was 971 per 100,000 person-years.
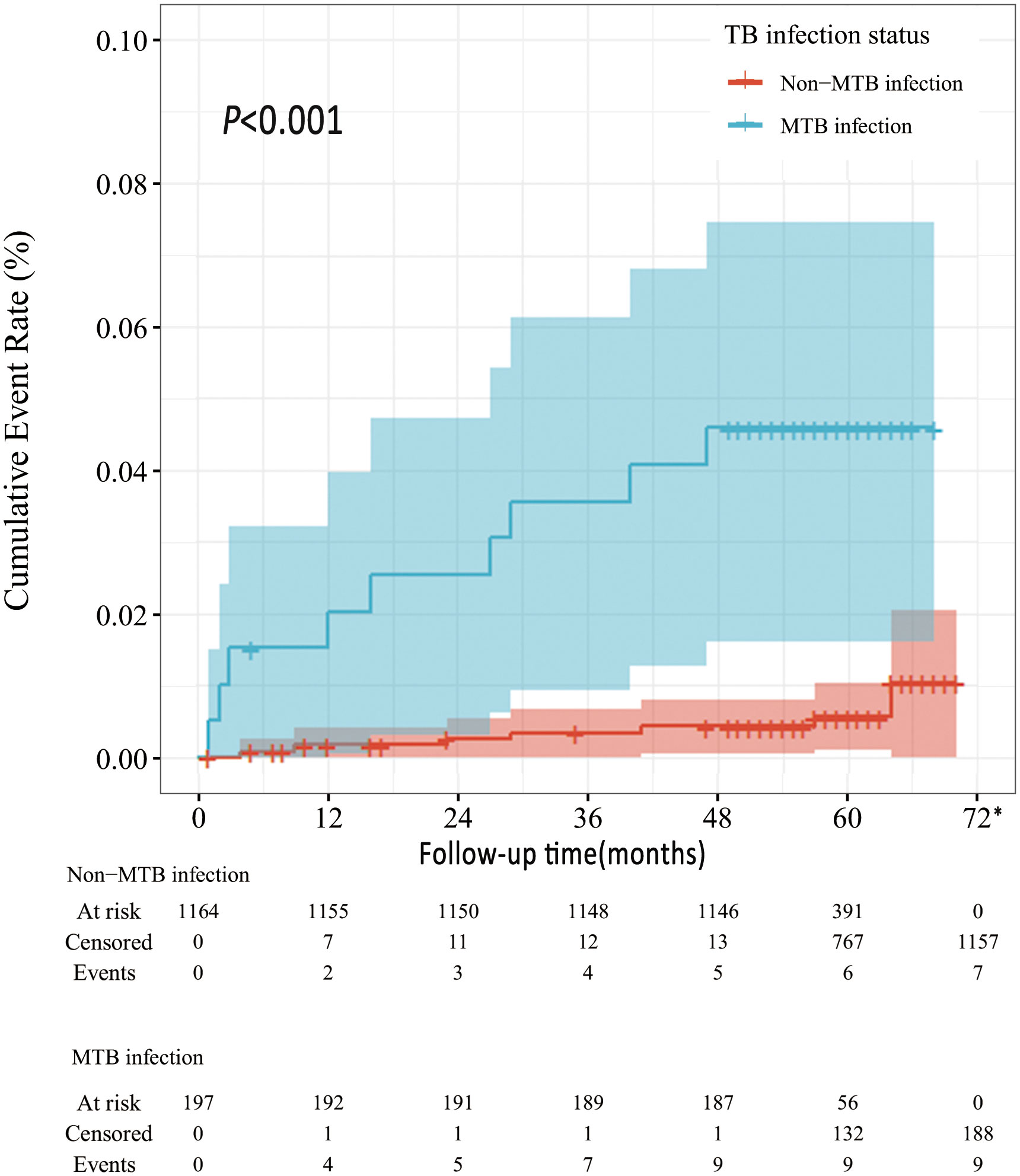
Figure 2 Survival curves of ATB development in MTB vs. non-MTB infection status *All the patients censored at this time point due to the end of follow-up.
3.3 Risk factors for ATB in SLE patients
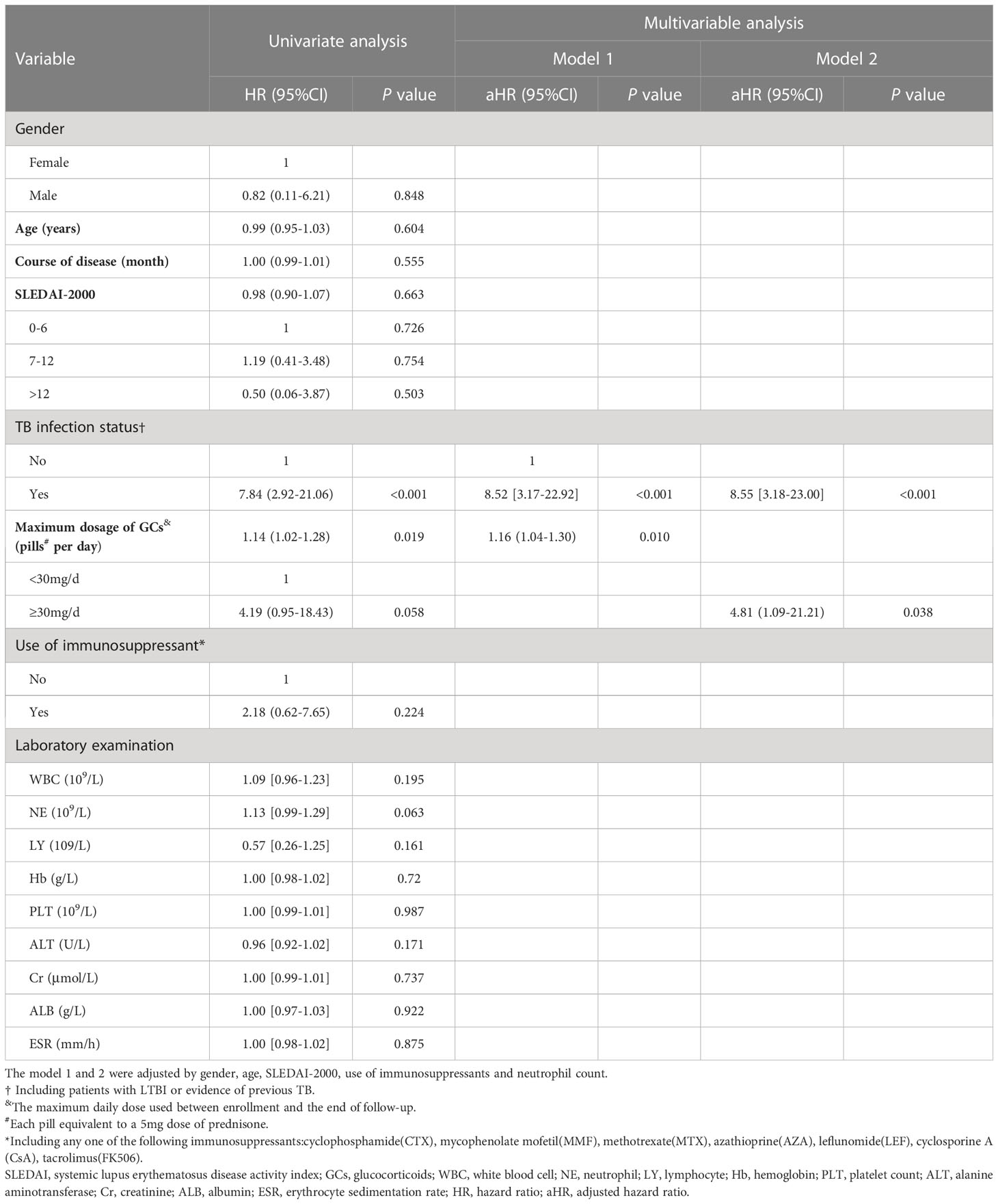
Risk factors for ATB were analyzed in the 1361 SLE patients who completed follow-up. Survival curves corresponding to subgroups of each covariate were parallel, satisfying the hazard ratio condition of Cox regression model. Univariate Cox regression analysis was performed on gender, age, course of disease, SLEDAI-2000, TB infection status, maximum daily dosage of GCs, the use of immunosuppressants, and laboratory examinations such as routine blood test. The results are shown in Table 3. Gender, age, SLEDAI-2000, TB infection status, maximum daily dose of GCs, the use of immunosuppressants and neutrophil count were included in a multivariable Cox regression model 1. The results showed that TB infection status (adjusted hazard ratio (aHR)=8.52, 95%CI: 3.17-22.92, P<0.001) and maximum daily dose of GCs (pills per day, each tablet is equivalent to 5mg of prednisone) (aHR=1.16, 95%CI: 1.04-1.30, p=0.010) were independent risk factors for ATB. Specifically, each additional pill in maximum daily dose of glucocorticoids would increase the risk of ATB by 16%. Maximum daily dose of GCs was stratified according to whether it exceeded 30 mg (equivalent to the dose of prednisone) and included in a multivariable Cox regression model 2. The results showed that TB infection status (aHR=8.55, 95%CI: 3.18 -23.00, P<0.001) and maximum daily dose of GCs≥30mg (equivalent to the dose of prednisone) (aHR=4.81, 95%CI: 1.09-21.21, p=0.038) were independent risk factors for ATB.
4 Discussion
This is the first multi-center prospective cohort study in the world investigating incidence and exploring risk factors of ATB in the population of SLE patients. With a large sample size and long follow-up period, this study supplements the epidemiological data of TB incidence as well as risk factors for ATB development in SLE patients, providing evidence for precise TB prevention in this population.
This study showed that the incidence of ATB in SLE patients within one year was 368 per 100,000, about 7 times that of China in 2021 (55 per 100,000 population). The incidence density of ATB in SLE patients with TB infection was as high as 971 per 100,000 person-years, about 18 times that of the general population (27). Data from prospective cohort studies are very limited. A single-center study from Spain (2006), a low-endemic area of TB, showed that the annual incidence of ATB in SLE patients was 187 cases per 100,000 persons (95% CI 39-547), about 6 times that of the general population in the same period (30 per 100 000 person-years) (21). There are also some single-center retrospective studies done in areas with high TB prevalence. A study in Indonesia (2022) showed that the incidence of ATB in SLE patients was 2873 cases per 100,000 person years (95% confidence interval [CI], 2400-3345), about 8 times that of the general population (354 per 100,000 population) (20). A study from India (2021) showed that the incidence of ATB in adult SLE patients was 6.1 per 100 patients, nearly 30 times that of the general population (211 per 100,000 population) (28); while another study from India in the same year showed that the incidence of ATB in SLE patients was 733 per 100,000 patient years, about 3 times that of the general population (18). Thus, no matter in high or low TB prevalence areas, the incidence of ATB in SLE patients is higher than that in the general population. In addition, single-center studies may have selection bias leading to greater heterogeneity in research results, while multi-center prospective cohort studies may provide relatively stable and accurate estimates.
This study found that TB infection status, including LTBI or evidence of previous TB, was an independent risk factor for ATB in SLE population (aHR=8.5). Many previous studies have confirmed that the incidence of ATB in the LTBI population is higher than that of the general population (29, 30). A chronic autoimmune disease, SLE causes abnormalities of innate and adaptive immunity, both of which play important roles in the process of TB infection and pathogenesis (31, 32). LTBI is the result of check and balance between immune system and MTB. When the balance is broken, MTB, which cannot be effectively limited or eliminated, will increase the risk of TB activity (33, 34). Calcified nodules and fibrotic lesions increase the risk of TB recurrence, the incidence of ATB in patients with past TB history and fibrotic lesions ranges from 2.0 to 13.6 per 1000 person-years (35). A multi-center prospective cohort study in China also confirmed that people with evidence of previous TB had a higher risk of developing ATB than those without previous TB infection (HR=5.4) (36). Our cross-sectional study also found that evidence of previous TB (OR = 6.2) was an independent risk factor for ATB in patients with rheumatic immune diseases (23). Therefore, SLE patients with either LTBI or evidence of previous TB have a greatly increased risk of developing ATB and deserve sufficient clinical attention.
The use of SLE treatment drugs is also an important reason for the high incidence of ATB in this population. GCs are commonly used drugs for the treatment of SLE. Their anti-inflammatory and immunosuppressive effects interfere with the function of phagocytes and suppress cellular immunity, increasing the risk of TB infection and morbidity (37, 38). Numerous studies have shown that the use of GCs pulse therapy (7, 20), dose and duration of GCs (8), and cumulative dose of GCs (7, 39, 40) are all related to the development of ATB in SLE patients. In this study, maximum daily dose of GCs was included as a continuous variable and a categorical variable respectively in Cox regression models, and it turned out to be an independent risk factor for ATB development in both cases. An increase in maximum daily dose of GCs was associated with an increase in the risk of ATB. Under maximum daily dose≥30 mg/d, the risk of ATB was increased by nearly 5 times, which might provide a reference for initiating preventive treatment. When SLE treatment with GCs alone shows poor efficacy, immunosuppressants are often used in combination, which may also affect the HR for developing ATB. There are many types of immunosuppressants with different mechanisms of immunosuppression and inconsistent research conclusions. LEF (OR 4.0-11.7), MTX (OR 1.6-4.6), CsA (OR=3.8, 95% CI 0.9- 16.6), AZA (OR=2.27, 95% CI 1.32-3.89), and other immunosuppressants have been reported to be associated with the onset of ATB (41). However, this study did not find that an association between the use of immunosuppressants and the incidence of ATB in SLE patients. Due to the small number of outcome events in our study, this conclusion needs to be confirmed by further studies.
The severity of SLE disease was a factor of great concern to us, However, the data from this study showed that the SLEDAI-2000 score at baseline is not a risk factor for the developing of ATB. We think this is explicable. The SLEDAI-2000 obtained at baseline only evaluates the disease activity of patients at the time of enrollment, while the status of SLE disease is dynamic and the factors affecting the incidence of ATB are diverse. In clinical practice, a considerable number of SLE patients develop ATB during the stable phase of the disease. Several case-control studies have also shown no significant difference in SLEDAI-2000 scores between the SLE/ATB+ and SLE/ATB- groups (7, 8, 39).
This study inevitably has some limitations. First, about 40% of SLE patients in this study refused to be followed up, and the follow-up cohort had a drop-out rate of 18.5%. Both groups of patients might lead to selection bias. We compared baseline data of patients who refused follow-up, who dropped out, and who responded and found that baseline characteristics of the three were similar, with a presumably negligible impact on the estimation of TB incidence. Secondly, 37.5% (6/16) of ATB patients were clinically diagnose. However, we followed a strict clinical diagnostic process and followed up the efficacy of anti-TB treatment to ensure diagnostic accuracy. Thirdly, due to some of the follow-up visits were conducted by telephone, we were unable to obtain accurate information on some important details, such as the duration of GCs use. In addition, due to the small number of outcome events, estimated incidences had wide confidence intervals that would need further studies to verify.
5 Conclusion
The incidence of ATB in SLE patients was higher than that in the general population. TB infection status and maximum daily dose of GCs were related to the risk of developing ATB. When SLE patients are in TB infection status or treated with a maximum daily dose of GCs≥30mg/d, it is recommended to initiate TB preventive treatment after considering benefits and risks of the patients.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
This study involving human participants reviewed and approved by the Ethics Committees of Peking Union Medical College Hospital (No.S-715) and 12 participating hospitals. Written informed consents were obtained from all patients and their legal guardians if necessary.
Author contributions
LZ, XShi and XL contributed to the study conception and design. FZ, YZ, XZeng, SL, XZuo, HW, LW, HL, ZZ, SC, PZ, MZ, WQ, YL, HL, XShi and XL recruited the study subjects and performed the clinical assessment. LX, JL, ZY, QC, CL, XSun and XZou conducted the patient follow-up. LZ and XZou performed the data analysis. XZ and NJ wrote the first draft of the manuscript. LZ, XShi and XL revised the manuscript. All authors read and approved the final manuscript.
Funding
This study was supported by the National Science and Technology Major Project of the People’s Republic of China (2014ZX10003003 and 2017ZX10201302-003) and National High Level Hospital Clinical Research Funding (2022-PUMCH-C-013 and 2022-PUMCH-A-119).
Acknowledgments
We acknowledge the contribution from other members of the ETHERTB study team in recruiting the study subjects and performing the clinical assessment. Those members include Shengyun Liu (Department of Rheumatology and Immunology, First Affiliated Hospital of Zhengzhou University, Zhengzhou, People’s Republic of China), Xiaoxia Zuo (Department of Rheumatology and Immunology, Xiangya Hospital, Central South University, Changsha, People’s Republic of China), Huaxiang Wu(Department of Rheumatology, Second Affiliated Hospital of Zhejiang University School of Medicine, Hangzhou, People’s Republic of China), Lijun Wu (Department of Rheumatology, People’s Hospital of Xinjiang Uygur Autonomous Region, Urumchi, People’s Republic of China), Hongbin Li (Department of Rheumatology and Immunology, Affiliated Hospital of Inner Mongolia Medical University, Hohhot, People’s Republic of China), Zhiyi Zhang (Department of Rheumatology and Immunology, First Affiliated Hospital of Harbin Medical University, Harbin, People’s Republic of China), Sheng Chen (Department of Rheumatology, Renji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, People’s Republic of China), Ping Zhu (Department of Clinical Immunology, Xijing Hospital, Fourth Military Medical University, Xi’an, People’s Republic of China), Miaojia Zhang(Department of Rheumatology, First Affiliated Hospital of Nanjing Medical University, Nanjing, People’s Republic of China), Wencheng Qi (Department of Rheumatology, Tianjin First Central Hospital, Tianjin, People’s Republic of China), Yi Liu (Department of Rheumatology and Immunology, West China Hospital, Sichuan University, Chengdu, People’s Republic of China), and Huaxiang Liu (Department of Rheumatology, Qilu Hospital of Shandong University, Ji’nan, People’s Republic of China).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1157157/full#supplementary-material
References
1. WHO: global tuberculosis report. Geneva: World Health Organization (2021). Available at: https://www.who.int/teams/global-tuberculosis-programme/data.
2. Xiaofeng Z, Mengtao L, Xinping T. National clinical research center for dermatologic and immunologic diseases: systemic lupus erythematosus in China: a national report of 2020. Shenyang: Liaoning Science and Technology Press (2021).
3. Institute of Pathogen Biology, Chinese Academy of Medical Sciences and Peking Union Medical College, Chinese Center for Disease Control and Prevention, Union Medical Institute of Geographic Sciences and Natural Resources Research, Chinese Academy of Sciences. Expert consensus on the estimation of the national burden on latent tuberculosis infection. Chin J Antituberculosis (2022) 44(1):4–8. doi: 10.19982/j.issn.1000-6621.20210662
4. Hartman-Adams H, Clark K, Juckett G. Update on latent tuberculosis infection. Am Fam Physician (2014) 89(11):889–96.
5. Zhao Di, Deng D. Correlation between systemic lupus erythematosus and tuberculosis. China J Leprosy Skin Dis (2021) 37(8):549–52. doi: 10.12144/zgmfskin202108549
6. Wang D, Ma Li. Literature review analysis of systemic lupus erythematosus complicated with tuberculosis infection in China. Chin J Rheumatol (2009) 13(9):599–602. doi: 10.3760/cma.j.issn.1007-7480.2009.09.005
7. Xiao X, Da G, Xie X, Liu X, Zhang L, Zhou B, et al. Tuberculosis in patients with systemic lupus erythematosus-a 37-year longitudinal survey-based study. J Intern Med (2021) 290(1):101–15. doi: 10.1111/joim.13218
8. Lao M, Chen D, Wu X, Chen H, Qiu Q, Yang X, et al. Active tuberculosis in patients with systemic lupus erythematosus from southern China: a retrospective study. Clin Rheumatol (2019) 38(2):535–43. doi: 10.1007/s10067-018-4303-z
9. Zhang R, Liu G, Sun Y. Clinical analysis of 93 cases of systemic lupus erythematosus complicated with tuberculosis. J South Med Univ (2007) 27(5):615–7. doi: 10.3321/j.issn:1673-4254.2007.05.035
10. Cheng CF, Huang YM, Lu CH, Hsieh SC, Li KJ. Prednisolone dose during treatment of tuberculosis might be a risk factor for mortality in patients with systemic lupus erythematosus: a hospital-based cohort study. Lupus (2019) 28(14):1699–704. doi: 10.1177/0961203319882759
11. Yang H. The clinical characteristics of 11 systemic lupus erythematosus patients with central nervous system tuberculosis infection. Chin J Clin (Electronic Edition) (2011) 05(22):6603–7. doi: 10.3877/cma.j.issn.1674-0785.2011.22.017
12. Zenner D, Beer N, Harris RJ, Lipman MC, Stagg HR, van der Werf MJ. Treatment of latent tuberculosis infection: an updated network meta-analysis. Ann Intern Med (2017) 167(4):248–55. doi: 10.7326/M17-0609
13. WHO. WHO operational handbook on tuberculosis. In: Module 1: prevention - tuberculosis preventive treatment. Geneva: World Health Organization. Available at: https://www.who.int/publications/i/item/9789240002906.
14. Park DW, Chung SJ, Yeo Y, Park TS, Lee H, Moon JY, et al. Therapeutic issues with, and long-term outcomes of, pulmonary mycobacterial tuberculosis treatment in patients with autoimmune rheumatic diseases. J Thorac Dis (2019) 11(11):4573–82. doi: 10.21037/jtd.2019.10.74
15. Gao L, Zhang H, Xin H, Liu J, Pan S, Li X, et al. Short-course regimens of rifapentine plus isoniazid to treat latent tuberculosis infection in older Chinese patients: a randomised controlled study. Eur Respir J (2018) 52(6):1801470. doi: 10.1183/13993003.01470-2018
16. Yun JE, Lee SW, Kim TH, Jun JB, Jung S, Bae SC, et al. The incidence and clinical characteristics of mycobacterium tuberculosis infection among systemic lupus erythematosus and rheumatoid arthritis patients in Korea. Clin Exp Rheumatol (2002) 20(2):127–32.
17. Hodkinson B, Musenge E, Tikly M. Osteoarticular tuberculosis in patients with systemic lupus erythematosus. QJM (2009) 102(5):321–8. doi: 10.1093/qjmed/hcp015
18. Muhammed H, Jain A, Pattanaik SS, Chatterjee R, Naveen R, Kabeer H, et al. Clinical spectrum of active tuberculosis in patients with systemic lupus erythematosus. Rheumatol Int (2021) 41(12):2185–93. doi: 10.1007/s00296-021-04933-0
19. Victorio-Navarra ST, Dy EE, Arroyo CG, Torralba TP. Tuberculosis among Filipino patients with systemic lupus erythematosus. Semin Arthritis Rheum (1996) 26(3):628–34. doi: 10.1016/s0049-0172(96)80013-8
20. Hamijoyo L, Sahiratmadja E, Ghassani NG, Darmawan G, Susandi E, van Crevel R, et al. Tuberculosis among patients with systemic lupus erythematosus in Indonesia: a cohort study. Open Forum Infect Dis (2022) 9(7):ofac201. doi: 10.1093/ofid/ofac201
21. Erdozain JG, Ruiz-Irastorza G, Egurbide MV, Martinez-Berriotxoa A, Aguirre C. High risk of tuberculosis in systemic lupus erythematosus? Lupus (2006) 15(4):232–5. doi: 10.1191/0961203306lu2289xx
22. Tam LS, Li EK, Wong SM, Szeto CC. Risk factors and clinical features for tuberculosis among patients with systemic lupus erythematosus in Hong Kong. Scand J Rheumatol (2002) 31(5):296–300. doi: 10.1080/030097402760375205
23. Liu X, Zhang L, Zhang F, Zeng X, Zhao Y, Wang Q, et al. Prevalence and risk factors of active tuberculosis in patients with rheumatic diseases: a multi-center, cross-sectional study in China. Emerg Microbes Infect (2021) 10(1):2303–12. doi: 10.1080/22221751.2021.2004864
24. Hochberg MC. Updating the American college of rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum (1997) 40(9):1725. doi: 10.1002/art.1780400928
25. Uribe AG, Vila LM, McGwin G Jr., Sanchez ML, Reveille JD, Alarcon GS. The systemic lupus activity measure-revised, the Mexican systemic lupus erythematosus disease activity index (SLEDAI), and a modified SLEDAI-2K are adequate instruments to measure disease activity in systemic lupus erythematosus. J Rheumatol (2004) 31(10):1934–40.
26. Zhou ZR, Wang WW, Li Y, Jin KR, Wang XY, Wang ZW, et al. In-depth mining of clinical data: the construction of clinical prediction model with r. Ann Transl Med (2019) 7(23):796. doi: 10.21037/atm.2019.08.63
27. WHO. Tuberculosis profile: China. World Health Organization. Available at: https://worldhealthorg.shinyapps.io/tb_profiles/?_inputs_&entity_type=%22country%22&lan=%22EN%22&iso2=%22CN%22.
28. Gupta L, Aggarwal R, Naveen R, Lawrence A, Zanwar A, Misra DP, et al. High prevalence of active tuberculosis in adults and children with idiopathic inflammatory myositis as compared with systemic lupus erythematosus in a tuberculosis endemic country: retrospective data review from a tertiary care centre in India. Mediterr J Rheumatol (2021) 32(2):134–42. doi: 10.31138/mjr.32.2.134
29. Shah M, Dorman SE. Latent tuberculosis infection. N Engl J Med (2021) 385(24):2271–80. doi: 10.1056/NEJMcp2108501
30. WHO. WHO consolidated guidelines on tuberculosis: module 1. In: Prevention: tuberculosis preventive treatment. Geneva: World Health Organization (2020). Available at: https://www.who.int/publications/i/item/9789240001503.
31. Roy Chowdhury R, Vallania F, Yang Q, Lopez Angel CJ, Darboe F, Penn-Nicholson A, et al. A multi-cohort study of the immune factors associated with m. tuberculosis infection outcomes. Nat (2018) 560(7720):644–8. doi: 10.1038/s41586-018-0439-x
32. Pai M, Behr MA, Dowdy D, Dheda K, Divangahi M, Boehme CC, et al. Tuberculosis. Nat Rev Dis Primers (2016) 2:16076. doi: 10.1038/nrdp.2016.76
33. Prezzemolo T, Guggino G, La Manna MP, Di Liberto D, Dieli F, Caccamo N. Functional signatures of human CD4 and CD8 T cell responses to mycobacterium tuberculosis. Front Immunol (2014) 5:180. doi: 10.3389/fimmu.2014.00180
34. Gideon HP, Phuah J, Myers AJ, Bryson BD, Rodgers MA, Coleman MT, et al. Variability in tuberculosis granuloma T cell responses exists, but a balance of pro- and anti-inflammatory cytokines is associated with sterilization. PloS Pathog (2015) 11(1):e1004603. doi: 10.1371/journal.ppat.1004603
35. Targeted tuberculin testing and treatment of latent tuberculosis infection. this official statement of the American thoracic society was adopted by the ATS board of directors, July 1999. this is a joint statement of the American thoracic society (ATS) and the centers for disease control and prevention (CDC). this statement was endorsed by the council of the infectious diseases society of america. (IDSA), September 1999, and the sections of this statement. Am J Respir Crit Care Med (2000) 161(4 Pt 2):S221–247. doi: 10.1164/ajrccm.161.supplement_3.ats600
36. Gao L, Li X, Liu J, Wang X, Lu W, Bai L, et al. Incidence of active tuberculosis in individuals with latent tuberculosis infection in rural China: follow-up results of a population-based, multicentre, prospective cohort study. Lancet Infect Dis (2017) 17(10):1053–61. doi: 10.1016/S1473-3099(17)30402-4
37. Xie Y, Xie J, Meijer AH, Schaaf MJM. Glucocorticoid-induced exacerbation of mycobacterial infection is associated with a reduced phagocytic capacity of macrophages. Front Immunol (2021) 12:618569. doi: 10.3389/fimmu.2021.618569
38. Cain DW, Cidlowski JA. Immune regulation by glucocorticoids. Nat Rev Immunol (2017) 17(4):233–47. doi: 10.1038/nri.2017.1
39. Torres-Gonzalez P, Romero-Diaz J, Cervera-Hernandez ME, Ocampo-Torres M, Chaires-Garza LG, Lastiri-Gonzalez EA, et al. Tuberculosis and systemic lupus erythematosus: a case-control study in Mexico city. Clin Rheumatol (2018) 37(8):2095–102. doi: 10.1007/s10067-018-4109-z
40. Gonzalez-Naranjo LA, Coral-Enriquez JA, Restrepo-Escobar M, Munoz-Vahos CH, Jaramillo-Arroyave D, Vanegas-Garcia AL, et al. Factors associated with active tuberculosis in Colombian patients with systemic lupus erythematosus: a case-control study. Clin Rheumatol (2021) 40(1):181–91. doi: 10.1007/s10067-020-05225-x
41. Cantini F, Niccoli L, Capone A, Petrone L, Goletti D. Risk of tuberculosis reactivation associated with traditional disease modifying anti-rheumatic drugs and non-anti-tumor necrosis factor biologics in patients with rheumatic disorders and suggestion for clinical practice. Expert Opin Drug Saf (2019) 18(5):415–25. doi: 10.1080/14740338.2019.1612872
Keywords: tuberculosis, systemic lupus erythematosus, risk facors, cohort study, epidemiology
Citation: Zhang L, Zou X, Jiang N, Xie L, Liu J, Yang Z, Cao Q, Li C, Sun X, Zhang F, Zhao Y, Zeng X, Shi X and Liu X (2023) Incidence and risk factors of tuberculosis in systemic lupus erythematosus patients: a multi-center prospective cohort study. Front. Immunol. 14:1157157. doi: 10.3389/fimmu.2023.1157157
Received: 02 February 2023; Accepted: 12 May 2023;
Published: 14 June 2023.
Edited by:
Rossella De Angelis, Università Politecnica delle Marche, ItalyReviewed by:
Vikas Agarwal, Sanjay Gandhi Post Graduate Institute of Medical Sciences (SGPGI), IndiaAnno Saris, Leiden University Medical Center (LUMC), Netherlands
María B. Arriaga, Vanderbilt University Medical Center, United States
Copyright © 2023 Zhang, Zou, Jiang, Xie, Liu, Yang, Cao, Li, Sun, Zhang, Zhao, Zeng, Shi and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaochun Shi, c2hpeGNoNzcyMkAxNjMuY29t; Xiaoqing Liu, bGl1eHFAcHVtY2guY24=
†These authors have contributed equally to this work
 Lifan Zhang1,2,3†
Lifan Zhang1,2,3† Nan Jiang
Nan Jiang Fengchun Zhang
Fengchun Zhang Yan Zhao
Yan Zhao Xiaofeng Zeng
Xiaofeng Zeng Xiaochun Shi
Xiaochun Shi Xiaoqing Liu
Xiaoqing Liu