
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Immunol. , 17 April 2023
Sec. Autoimmune and Autoinflammatory Disorders: Autoinflammatory Disorders
Volume 14 - 2023 | https://doi.org/10.3389/fimmu.2023.1152513
This article is part of the Research Topic Primary Immune-mediated Cicatricial and Non-cicatricial Alopecias View all 4 articles
Background: Alopecia areata (AA) is an immune disease characterized by non-scarring hair loss. With the widespread application of JAK inhibitors in immune-related diseases, attention is being given to their role in the treatment of AA. However, it is unclear which JAK inhibitors have a satisfactory or positive effect on AA. This network meta-analysis aimed to compare the efficacy and safety of different JAK inhibitors in the treatment of AA.
Methods: The network meta-analysis was performed according to the PRISMA guidelines. We included randomized controlled trials as well as a small number of cohort studies. The differences in efficacy and safety between the treatment and control groups were compared.
Results: Five randomized controlled trials, two retrospective studies, and two prospective studies involving 1689 patients were included in this network meta-analysis. In terms of efficacy, oral baricitinib and ruxolitinib significantly improved the response rate of patients compared to placebo [MD = 8.44, 95% CI (3.63, 19.63)] and [MD = 6.94, 95% CI, (1.72, 28.05)],respectively. Oral baricitinib treatment significantly improved the response rate compared to non-oral JAK inhibitor treatment [MD=7.56, 95% CI (1.32,43.36)]. Oral baricitinib, tofacitinib, and ruxolitinib treatments significantly improved the complete response rate compared to placebo [MD = 12.21, 95% CI (3.41, 43.79)], [MD = 10.16, 95% CI (1.02, 101.54)], and [MD = 9.79, 95% CI, (1.29, 74.27)], respectively. In terms of safety, oral baricitinib, tofacitinib, and ruxolitinib treatments significantly reduced treatment-emergent adverse event rates compared with conventional steroid treatment [MD = 0.08, 95% CI (0.02, 0.42)], [MD = 0.14, 95% CI (0.04, 0.55)], and [MD = 0.35, 95% CI, (0.14, 0.88)], respectively.
Conclusion: Oral baricitinib and ruxolitinib are excellent options for the treatment of AA owing to their good efficacy and safety profiles. In contrast, non-oral JAK inhibitors do not appear to have satisfactory efficacy in treating AA. However, further studies are required to verify the optimal dose of JAK inhibitors for AA therapy.
Alopecia areata (AA) is a common, non-scarring, autoimmune hair loss disorder mediated by the attack on hair follicles (1). The prevalence of AA in China is approximately 0. 27%. However, its prevalence can vary from 0.1% to 6.9% depending on the population and study area (2). Common clinical manifestations include sudden round alopecia spots, mostly without conscious symptoms, and a few patients may have mild scalp itching or tightness. Those with a small area of alopecia tend to self-manage the condition. However, some individuals experience more severe symptoms, such as alopecia totalis (AT) (full loss of hair on the scalp) and alopecia universalis (AU) (full loss of hair on the scalp and body) (3). Persistent AA and its variants can have devastating effects on the mental health and quality of life of the patient (4).
Clinically, topical, intralesional, and systemic corticosteroids, glycyrrhizin, minoxidil, diphenylcyclopropenone, and systemic agents such as methotrexate are used to alleviate AA in patients (3). Unfortunately, there is currently no cure for AA. Further, corticosteroid therapy, the most commonly used strategy in clinical practice, is associated with side effects such as acne, weight gain, and endocrine disorders. Owing to this, more effective and safe treatment options are urgently needed for the treatment of AA.
The Janus kinase/signal transducer and activator of transcription (JAK/STAT) signaling pathway is a key communication hub for cell function (5). Janus kinase inhibitors are a class of small-molecule compounds that can block one or more intracellular tyrosine kinases in the JAK-STAT signaling pathway, including JAK1, JAK2, JAK3, and tyrosine kinase 2 (TYK2). They thus block various cytokine and inflammatory pathways and induce immune suppression (6). A recent study (7) showed that JAK inhibitors can block the T lymphocyte-mediated immune response in hair follicles, promote the formation of hair follicle stem cells, and trigger angiogenesis, both of which occur during the hair growth phase. JAK inhibitors can also accelerate the transition of hair follicles from the telogen phase to the anagen phase (8).
Many clinical studies have shown that the use of JAK inhibitors to treat AA has achieved satisfactory outcomes with an acceptable or tolerable side effect profile. To date, no meta-analyses comparing the efficacy of different JAK inhibitors in the treatment of AA have been published. Therefore, this study aimed to indirectly compare the efficacy and safety of various JAK inhibitors in the treatment of AA using a network meta-analysis to lay a solid foundation for the clinical treatment of the condition.
The network meta-analysis was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) extension of the Network Meta-check Analysis List. Electronic searches were performed across four electronic databases, viz. PubMed, Embase, Web of Science, and the Cochrane Library, from inception until January 2023.
The search strategy for the aforementioned databases is summarized in Supplementary Material.
(1) Study type. Randomized controlled trials (RCT), prospective trials, and retrospective studies on JAK inhibitors for the treatment of patients with AA. The studies included both control and experimental groups.
(2) Participants Definitively diagnosed with AA/AT/AU.
(3) Intervention measures. The control group was treated with a placebo or conventional steroid therapy. The experimental groups were treated with oral or topical JAK inhibitors. At least two trials were required to confirm the efficacy of the same AA treatment regimen. The included trials provided efficacy (scalp hair regrowth rate) and safety (adverse events) outcomes.
(1) Studies including patients with only eyelash and eyebrow involvement, but no scalp involvement.
(2) Inability to extract data or missing data.
(3) Case reports or case series (fewer than six cases); abstracts; conference presentations; editorials; reviews; or expert opinions.
Two researchers independently screened for inclusion and crosschecked the results. Differences between the researchers were resolved through consultation.
Data extraction standards included the following: main author, study type, sample size, sex ratio, mean age, intervention measures in the experimental group, intervention measures in the control group, and initial SALT score.
The main treatment outcomes were good response (defined as a > 50% decrease in SALT score), complete response (defined as a > 90% decrease in SALT score), and percent change from baseline in the SALT score.
Safety outcomes included the rate of adverse events.
The quality of the included studies was evaluated according to the Cochrane 5.1 risk of bias assessment tool, which includes seven aspects: the random sequence method; whether the assignment was hidden; whether the implementation process was blinded; whether the evaluators were blinded; whether there were missing data; whether the outcome indicators were selectively reported; and whether there were other biases. We divided the above quality assessment into three grades: “unclear” (lack of relevant data), “low” (low risk), and “high” (high risk). The risk profile of each item was determined, and the results were visualized using th Review Manager 5.4.1 software. For non-randomized control trials (RCTs), the Newcastle–Ottawa Scale (NOS) was used (9, 10). The evaluation is comprised of three domains: selection, comparability, and outcome. The NOS adopts a semi-quantitative principle of the star system to evaluate the quality of literature, with a full score being nine stars.
RevMan v5.4 was used to evaluate the quality of the literature, and the risk of bias map was determined. Stata17.0 was used for the traditional meta-analysis. The odds ratio (OR) and 95% confidence interval (CI) were the effect size indicators for dichotomous variables (good response rate, complete response rate, and incidence of adverse reactions). The mean difference (MD) and 95% CI were regarded as effect size indicators for continuous variables (percentage change in the SALT score from baseline).
I2 and P values were used to evaluate the heterogeneity between the results of the studies. P≥ 0.05 or I2≤ 50% indicated that the heterogeneity between the studies was small, and the fixed effect model was used to combine the effect size. When P < 0.05 or I2 > 50%, substantial heterogeneity was considered to exist (1). Subgroup analysis was used to determine the source of heterogeneity, and sensitivity analysis was used to judge the stability and strength of the results. If the source of heterogeneity could not be found, the random-effects model was used to pool the effect size, or the meta-analysis was abandoned and only descriptive analysis was performed. Stata17.0 software, based on the frequency method, was used to conduct a network meta-analysis in which group commands were used for network analysis of the study outcome measures. Data processing, network evidence plots, funnel plots, surface under the cumulative ranking curve (SUCRA) ranking, and forest plots were generated. SUCRA was used to visually analyze the advantages and disadvantages of the interventions for each outcome indicator. SUCRA values range from 0 to 100, and the closer it is to 100, the better the intervention (11). A comparison-corrected funnel plot was drawn to evaluate whether there was a small-sample effect or publication bias.
A total of 859 relevant studies were identified after preliminary retrieval. After excluding duplicates and unrelated articles, 487 potentially relevant articles were identified. After reading titles and abstracts, 25 articles were included in the analysis. After further reading the full texts, articles without control group studies or relevant outcome indicators were excluded, and eight articles (12–19) made the final selection, comprising five RCTs (one (18) involved two RCTs), two retrospective studies, and two prospective studies (Figure 1).
The eight studies included 1689 patients. Table 1 provides basic information on the relevant literature.
Among the five RCTs included, three compared oral baricitinib with a placebo, one compared oral CTP-543 (deuterated ruxolitinib) with a placebo, and one compared topical ruxolitinib ointment with a placebo. Two retrospective studies comparing oral tofacitinib with conventional steroid therapy were included. Between the two prospective studies included, one compared sublingual tofacitinib with cyclosporine and the other compared oral tofacitinib with oral ruxolitinib.
Figure 2 depicts the risk of bias assessment of the included studies.
1) Evidence network. The sizes of the dots and lines in the network are proportional to the number of studies, with thicker lines indicating more studies comparing JAK inhibitors to other therapies. The results show that the number of studies comparing oral baricitinib with placebo was the largest, followed by those comparing oral tofacitinib with conventional hormonal therapy. Relatively few studies have, to date, compared the non-oral JAK inhibitor ruxolitinib with other treatments (Figure 3A).
2) Publication bias. The funnel plot results show that most scattering points are located on both sides of the vertical line. Some studies fall outside the funnel plot, indicating that there may have been publication bias or small sample effects in the included literature (Figure 4A).
3) Network meta-analyses. Network comparisons were performed for six treatment modalities, three of which were statistically significant. Compared with placebo, the MD and 95% CI of any doses of oral baricitinib and ruxolitinib were 8.44 and [3.63, 19.63] and 6.94 and [1.72, 28.05], respectively. Compared to non-oral JAK inhibitor treatment, the MD and 95% CI of any dose of oral baricitinib treatment was 7.56 and [1.32, 43.36], respectively (Figure 5A and Table 2A).
4) SUCRA probability ranking. The probability of a high or low response rate to JAK inhibitor treatment ranked according to the SUCRA (Figure 6A) was as follows: any dose of oral baricitinib treatment (83.6%) > any dose of oral ruxolitinib treatment (80.1%) > any dose of oral tofacitinib treatment (63.4%) > conventional steroid therapy (38.8%) > nonoral JAK inhibitor treatment (19.4%) > placebo (14.5%).
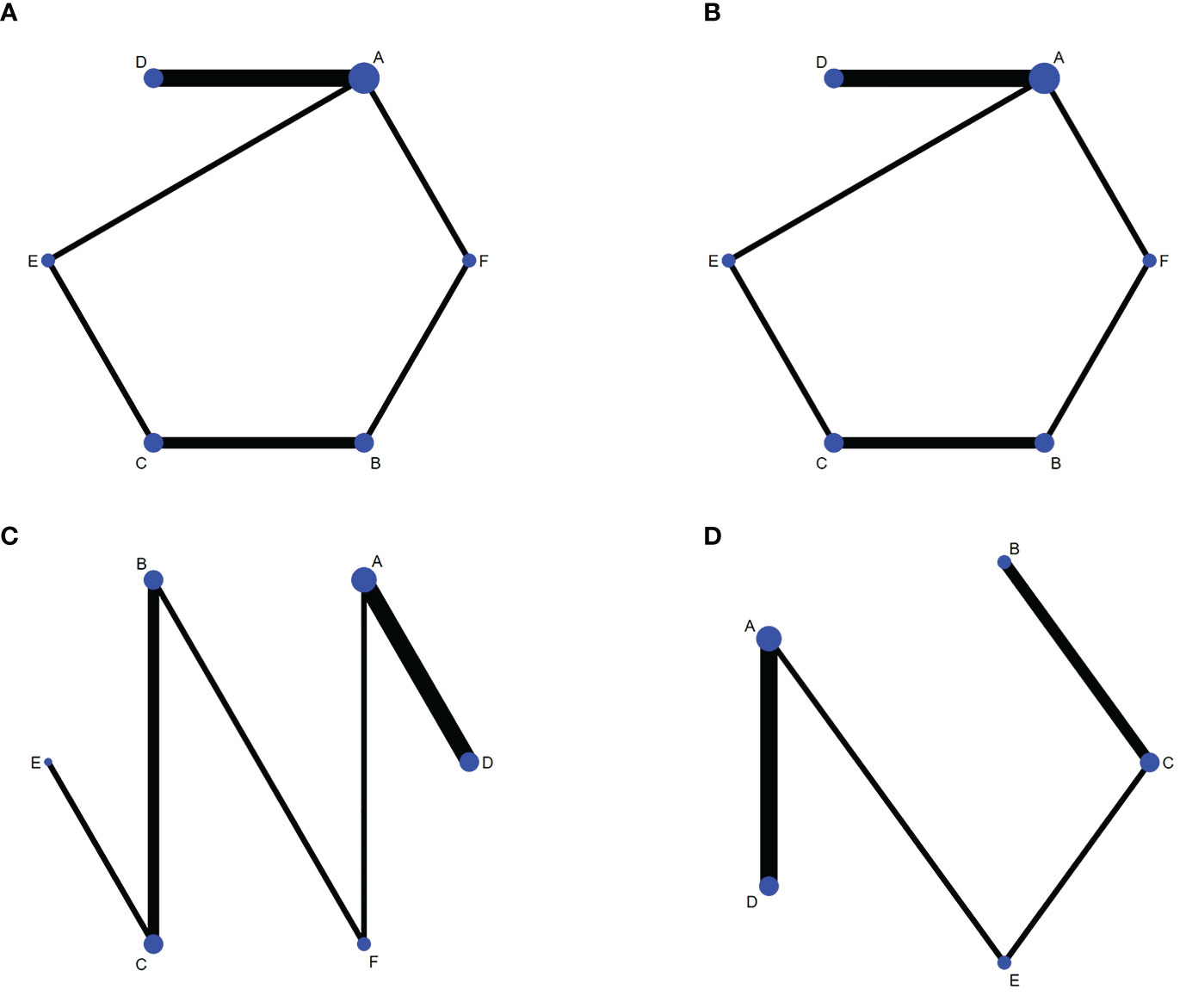
Figure 3 Network diagrams of outcome indicators. (A) good response rate; (B) complete response rate; (C) percent change form baseline in SALT scorer; (D) TEAE (treatment emergent adverse events) rate. A: placebo treatment B: conventional steroid therapy C: any dose of oral tofacitinib treatment D: any dose of oral baricitinib treatment E: any dose of oral ruxolitinib treatment F: nonoral JAK inhibitor treatment.
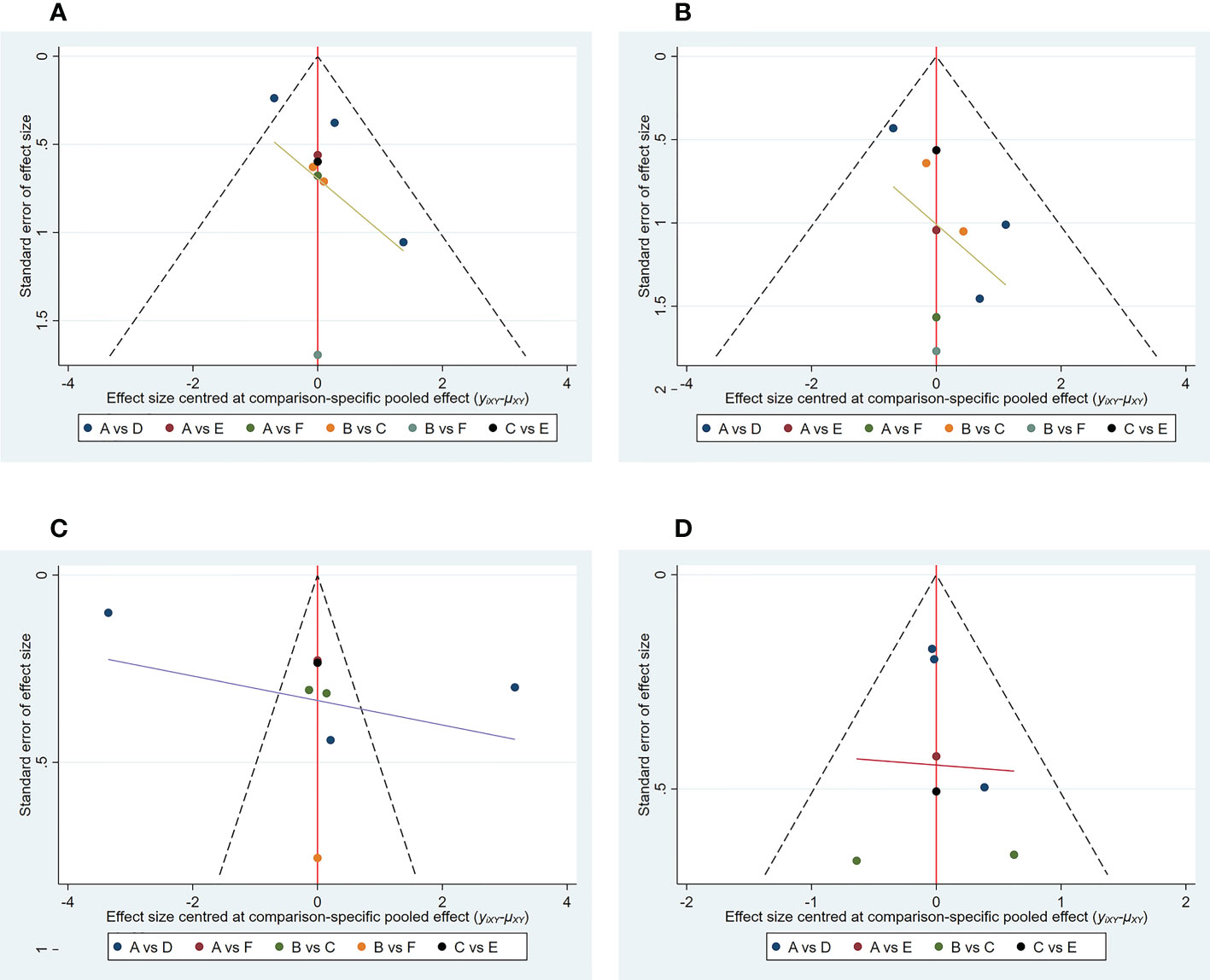
Figure 4 Funnel plot of outcome indicators. (A) good response rate; (B) complete response rate; (C) percent change from baseline in SALT score; (D) TEAE (treatment emergent adverse events) rate. A: placebo treatment B: conventional steroid therapy C: any dose of oral tofacitinib treatment D: any dose of oral baricitinib treatment E: any dose of oral ruxolitinib treatment F: nonoral JAK inihibitor treatment.
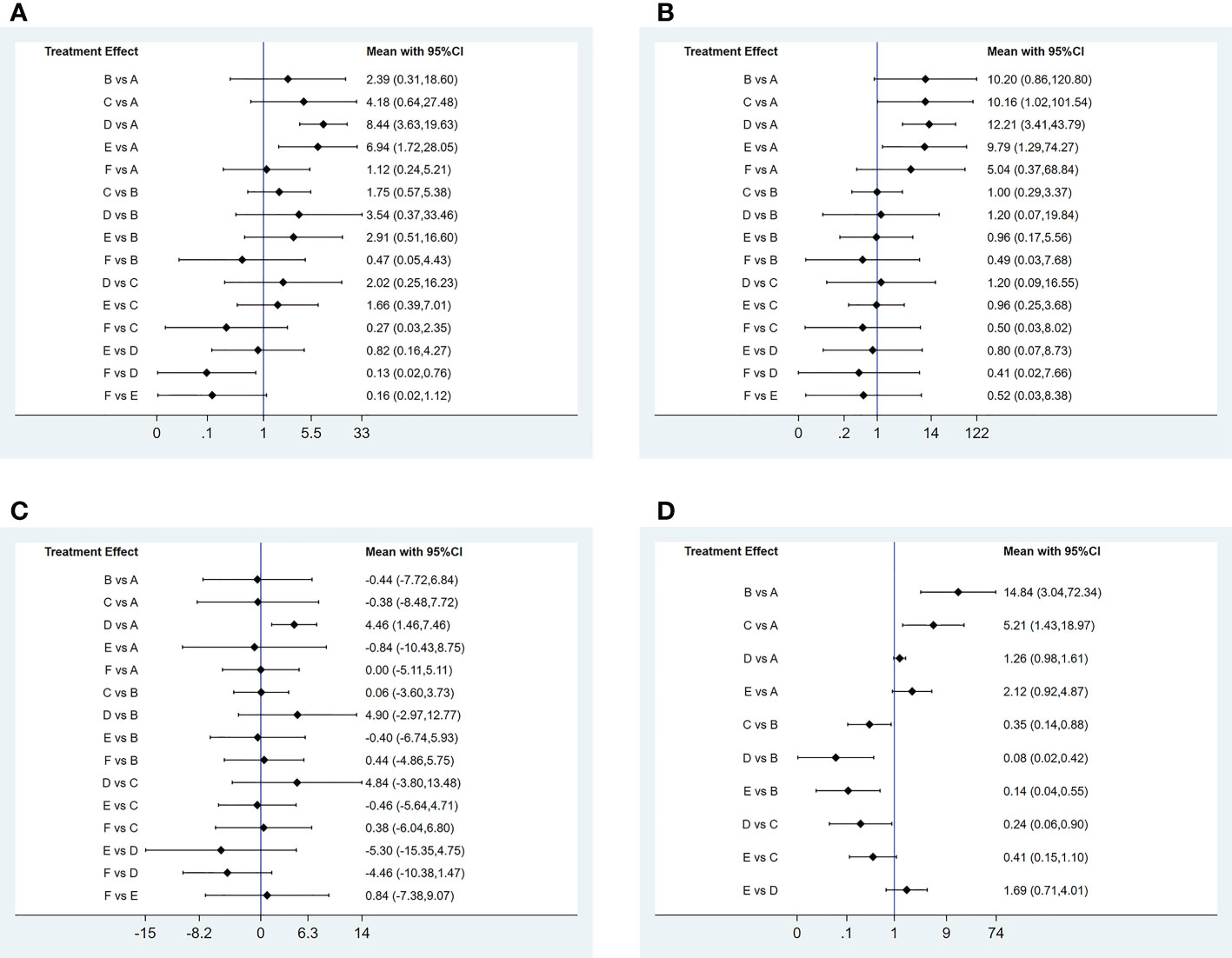
Figure 5 Pairwise comparison forest graph of outcome indicators. (A) good response rate; (B) complete response rate; (C) percent change from baseline in SALT score; (D) TEAE (treatment emergent adverse events) rate. A: placebo treatment B: conventional steroid therapy C: any dose of oral tofocitinib treatment D: any dose of oral baricitinib treatment E: any dose of oral ruxolitinib treatment : nonoral JAK inihibitor treatment.
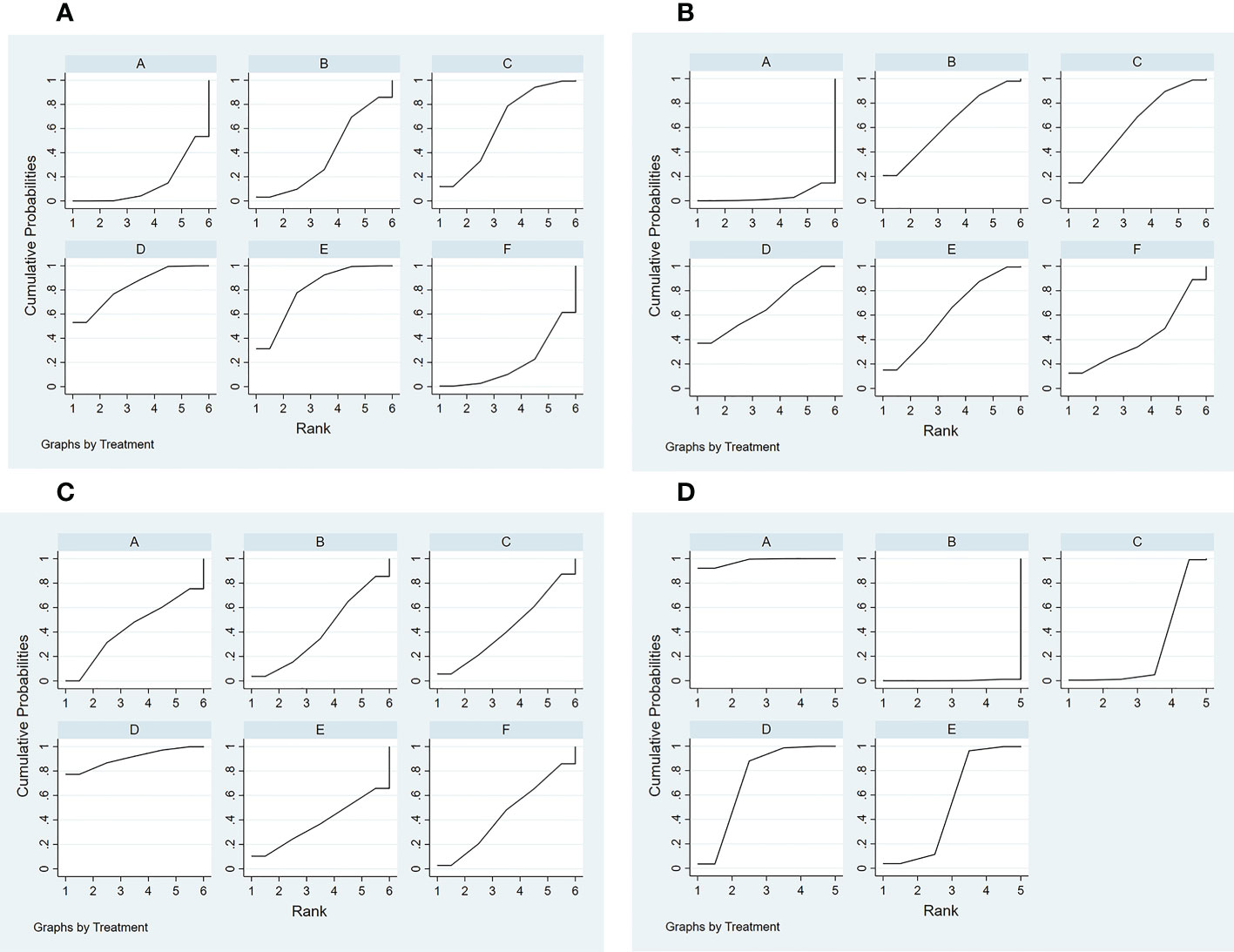
Figure 6 Curve diagram of SUCRA of outcome indicators. (A) good response rate; (B) complete response rate; (C) percent change from baseline in SALT score; (D) TEAE (treatment emergent adverse events) rate. A: placebo treatment B: conventional steroid therapy C: any dose of oral tofacitinib treatment D: any dose of oral baricitinib treatment E: any dose of oral ruxolitinib treatment F: nonoral JAK inhibitor treatment.
1) Evidence network. The sizes of the dots and lines in the network are proportional to the number of studies, with thicker lines indicating more studies comparing JAK inhibitors to other therapies. The results show that the number of studies comparing oral baricitinib with placebo was the largest, followed by those comparing oral tofacitinib with conventional hormonal therapy. Relatively few studies have, to date, compared the non-oral JAK inhibitor ruxolitinib with other treatments (Figure 3B).
2) Publication bias. The funnel plot results show that most scattering points are located on both sides of the vertical line. Some studies fall outside the funnel plot, indicating that there may have been publication bias or small sample effects in the included literature (Figure 4B).
3) Network meta-analyses. Network comparisons were performed for six treatment modalities, three of which were statistically significant. Compared with the placebo, the MD and 95% CI of any dose of oral baricitinib, tofacitinib, and ruxolitinib treatments were 12.21 and [3.41,43.79], 10.16 and [1.02,101.54], and 9.79 and [1.29,74.27], respectively (Figure 5B and Table 2B).
4) SUCRA probability ranking. The probability of a high or low complete response rate for JAK inhibitor treatment was ranked according to the SUCRA (Figure 6B) as follows: any dose of oral baricitinib (67.6%) > conventional steroid therapy (62.9%) > any dose of oral tofacitinib (62.7%) > any dose of oral ruxolitinib (61.2%) > non-oral JAK inhibitor (41.9%) > placebo (3.7%).
1) Evidence network. The sizes of the dots and lines in the network are proportional to the number of studies, with thicker lines indicating more studies comparing JAK inhibitors to other therapies. The results show that the number of studies comparing oral baricitinib with placebo was the largest, followed by those comparing oral tofacitinib with conventional hormonal therapy. Relatively few studies, to date, have compared the non-oral JAK inhibitor ruxolitinib with other treatments (Figure 3C).
2) Publication bias. The funnel plot results show that most scattering points are located on both sides of the vertical line. Some studies fall outside the funnel plot, indicating that there may have been publication bias or small sample effects in the included literature (Figure 4C).
3) Network meta-analyses. Network comparisons were performed for six treatment modalities, one of which was statistically significant. Compared with the placebo, the MD and 95% CI of any dose of oral baricitinib treatment was 86.50 and [4.31, 1737.70], respectively (Figure 5C and Table 2C).
4) SUCRA probability ranking. In accordance with the SUCRA (Figure 6C), the percent change from baseline in SALT score of JAK inhibitor treatment was ranked from high to low as follows: any dose of oral baricitinib (90.5%) > non-oral JAK inhibitor (44.8%) > placebo (43.0%) > any dose of oral tofacitinib (43.0%) > conventional steroid therapy (40.8%) > any dose of oral ruxolitinib (37.9%).
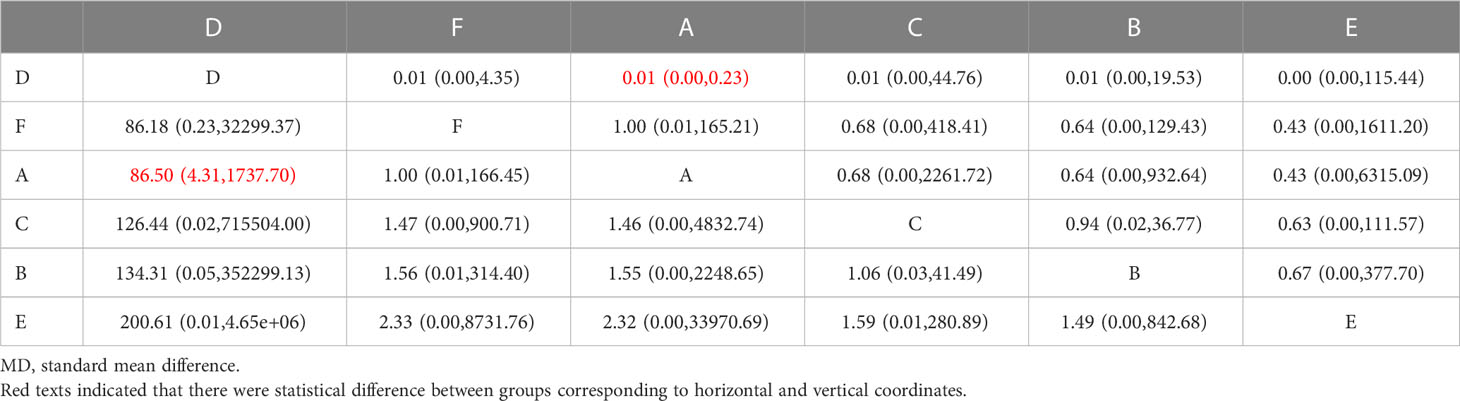
Table 2C Matrix of percent change in SALT scPercent change in SALT score after different JAKi treatment(shown as SMD and 95% Cls).
1) Evidence network. The sizes of the dots and lines in the network are proportional to the number of studies, with thicker lines indicating more studies comparing JAK inhibitors to other therapies. The results show that the number of studies comparing oral baricitinib with placebo was the largest, followed by those comparing oral tofacitinib with conventional hormonal therapy. Relatively few studies have, to date, compared ruxolitinib with other treatments (Figure 3D).
2) Publication bias. The funnel plot results show that most scattering points are located on both sides of the vertical line (Figure 4D).
3) Network meta-analyses. Network comparisons were performed for five treatment modalities, six of which were statistically significant. Compared with conventional steroid therapy, the MD and 95% CI of placebo treatment for any dose of oral baricitinib treatment, any dose of oral tofacitinib treatment, and any dose of oral ruxolitinib treatment were 0.07 and [0.01,0.33], 0.08 and [0.02,0.42], 0.14 and [0.04,0.55], and 0.35 and [0.14,0.88)], respectively. Compared with any dose of oral tofacitinib, the MD and 95% CI of placebo treatment and any dose of oral baricitinib were 0.19 and [0.05, 0.70] and 0.24 and [0.06, 0.90], respectively (Figure 5D and Table 2D).
4) SUCRA probability ranking. Based on the SUCRA (Figure 6D), the TEAE rate of JAK inhibitor treatment was ranked by likelihood from low to high as follows: placebo (97.9%) > any dose of oral baricitinib (72.5%) > any dose of oral ruxolitinib (52.8%) > any dose of oral tofacitinib (26.4%) > conventional steroid therapy (0.3%).
Details of the adverse events associated with the different treatments are shown in Table 3. Acneiform eruption, hyperlipidemia, upper respiratory infection, and headache appear to be the most common adverse reactions to oral JAK inhibitors for the treatment of AA (Table 3). In the included trials, one cancer event was reported in the oral baricitinib group. However, similar events were reported in the placebo group, with no statistically significant difference. Therefore, we believe that there is no clear link between the occurrence of neoplastic events and the treatment of AA with oral JAK inhibitors. A small proportion of patients discontinued JAK inhibitors because of severe side effects; most of these patients were in the high-dose oral JAK inhibitor group.
AA is a T cell-mediated autoimmune disease phenotypically characterized by alopecia and histologically by T cell infiltration around hair follicles (20). Steroid therapy is the most widely used treatment strategy for this condition; however, with the deepening of the mechanistic understanding of the potential key T-cell inflammatory pathways in AA, IFN-γ has been identified as an important pathogenic cytokine (21), and its related JAK-STAT pathway has become a new therapeutic target. Owing to this, JAK inhibitors have become a new option for the clinical treatment of AA. However, no clear conclusion has been reached as to which JAK inhibitor has the best therapeutic effect against AA. This study is the first to use a network meta-analysis to compare the efficacy and safety of different JAK inhibitors in the treatment of AA with the aim of providing a reference for the clinical use of JAK inhibitors and the design of subsequent related studies.
The efficacy outcomes in this study demonstrated that oral baricitinib and ruxolitinib significantly increased the frequency of good responses compared to placebo. Baricitinib has been proposed as a dual JAK1/JAK2 inhibitor given its strong binding interactions with both proteins. Ruxolitinib is generally considered a JAK1/JAK2 inhibitor that inhibits both γc family cytokine signaling (JAK1/JAK3) and IFNγ signaling (JAK1/JAK2). We hypothesized that baricitinib and ruxolitinib would display good efficacy, which may be related to their inhibition of IFNγ production. JAK inhibition regulates the activation of key hair follicle populations such as hair embryos and increases the induction rate of cultured human hair papilla cells by controlling molecular signals enriched in intact, fully induced hair papillae (22).
In our analysis, we found no significant difference in the frequency of good response between conventional steroid therapy and placebo, probably because most patients enrolled in the trial chose other treatments after the failure of conventional treatment. Therefore, we hypothesize that oral JAK inhibitors may be one of the most effective treatments currently available for AA, especially in patients for whom conventional steroid therapy has failed.
We found that topical JAK inhibitors had no significant therapeutic effects. Bokhari et al. (23), in a prospective, placebo-controlled, double-blind phase I study, proposed that topical JAK inhibitors may become a new strategy for the treatment of AA. We postulate that this contradiction with our current findings may be because the sample size of the previous study was too small, allowing the spontaneous remission of AA and placebo effects to be incorrectly attributed to the use of topical JAK inhibitors, as reflected in a study by Olsen et al. (16).
Despite the advantages of oral JAK inhibitors over conventional steroid therapy in terms of good response rates, we found no significant difference in the complete response rate between the commonly used JAK inhibitor, tofacitinib, and conventional steroid therapy. On the one hand, the pathogenesis of AA is not completely clear, and steroid-insensitive immune cell lines such as innate lymphocytes (ILCs) may be involved in the occurrence and development of inflammation in AA (24, 25), making conventional steroid therapy an unsuitable strategy to completely cure AA. Nonetheless, AA is often combined with several other chronic inflammatory skin diseases such as atopic dermatitis and vitiligo, which are both chronic inflammatory diseases dominated by inflammatory factors. When AA patients suffer from other immune-related diseases, there is often excessive activation of Th2, which causes the increase of IgE. Therefore, a combination of targeted drugs (such as dupilumab) is required (26, 27). In addition, the neuroendocrine mechanism of mental stress (28), genetic background (29), and other factors have an impact on the progression of AA. The complex pathogenesis of AA remains to be explored to elucidate direct and more effective therapeutic targets.
In addition, a variety of JAK inhibitors are being tested in clinical trials for the treatment of alopecia areata. King et al. has performed a phase 2a randomized, placebo-controlled study to evaluate the efficacy and safety of the oral JAK inhibitors ritlecitinib(JAK3 selective inhibitor) and brepocitinib(JAK1/TYK2 inhibitor) in AA (30). In this clinical trial, we observed significant improvements in both good response rate and complete response rate in patients treated with ritlecitinib or brepocitinib. Guttman-Yassky et al. tested biomarkers of scalp alopecia areata in patients who participated in the clinical trial (31), presenting that although brepocitinib can directly inhibit IFN-γ signaling through JAK1/2 activation, ritlecitinib can indirectly affect IFN-γ production through inhibition of TEC family kinases. This is also consistent with our hypothesis. However, we did not analyse the difference in efficacy and safety between ritlecitinib and other JAK inhibitors in the treatment of AA this time because there were not enough high-quality literatures to include in the discussion.
Taken together, our study findings suggest that the addition of oral baricitinib or ruxolitinib to the drug regimen may be helpful for the treatment of AA. In terms of the therapeutic dose, an increasing trend with treatment duration was observed in the included studies. However, increasing the dose of JAK inhibitors may increase the risk of adverse effects (32). An increase in adverse reactions often causes patients to stop using medication. In addition, a previous meta-analysis (33, 34) showed that there is a close relationship between drug withdrawal and the recurrence of AA. Therefore, more dose-related studies are needed to clarify the maximum tolerated and therapeutic doses for treating patients with AA.
Safety outcomes demonstrated that the incidence of adverse effects was significantly lower with oral JAK inhibitors than with conventional steroid therapy. The reported adverse effects were mostly limited to mild symptoms and relatively manageable. The most common adverse events were upper respiratory tract infections, headache, acne, and biochemical abnormalities. Among them, the incidence of mild infection was the highest. Despite the selectivity of different JAK inhibitors in their pathways of action, there is considerable overlap in their safety profiles (35). Although the frequency of serious adverse events associated with oral JAK inhibitors was low in previous studies, we recommend that patients with AA treated with JAK inhibitors regularly check their liver function, complete blood count, and other laboratory indicators during treatment.
This study compared the efficacy and safety of various JAK inhibitors in the treatment of AA through a network meta-analysis, providing a reference for clinical practice. However, it has the following limitations: (1) The sample size of most of the included studies (both prospective and retrospective studies) was small; therefore, the test power may have been insufficient. (2) There was heterogeneity in the included studies, including drug dosage, intervention course, and follow-up period, which may have led to bias in the results. (3) Most trials did not involve blinding of the subjects and interveners. In addition, there may have been selectivity bias during object selection. (4) Some of the included studies were still in the clinical trial stage at the time of compiling our findings, with further relevant clinical verification experiments needing completion. (5)
In conclusion, the clinical application of JAK inhibitors may be a new treatment strategy for AA. Oral JAK inhibitors are more effective than topical and sublingual JAK inhibitors in the treatment of AA. Oral baricitinib appears to be the best treatment option for patients with AA who desire a good response. In terms of side effects, oral JAK inhibitors are more tolerable in comparison to traditional steroid therapy. The adverse reactions to oral JAK inhibitors are mild and treatable; however, more high-quality, large-sample, multicenter, randomized controlled double-blind trials are needed to confirm these results.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
DW and XZ conceived this meta-analysis. DW and YC selected the studies included in this meta-analysis. DW and YS performed statistical analyses. DW drafted this meta-analysis. BX edited language and grammar. All authors contributed to the article and approved the submitted version.
This study was granted by the National Natural Science Foundation of China (81872517) and Hangzhou Medical Key Discipline Construction Project (No [2021]21-3).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1152513/full#supplementary-material
1. Fukuyama M, Ito T, Ohyama M. Alopecia areata: current understanding of the pathophysiology and update on therapeutic approaches, featuring the Japanese dermatological association guidelines. J Dermatol (2022) 49(1):19–36. doi: 10.1111/1346-8138.16207
2. Wolff H, Fischer TW, Blume-Peytavi U. The diagnosis and treatment of hair and scalp diseases. Dtsch Arztebl Int (2016) 113(21):377–86. doi: 10.3238/arztebl.2016.0377
3. Strazzulla LC, Wang EHC, Avila L, Lo Sicco K, Brinster N, Christiano AM, et al. Alopecia areata: disease characteristics, clinical evaluation, and new perspectives on pathogenesis. J Am Acad Dermatol (2018) 78(1):1–12. doi: 10.1016/j.jaad.2017.04.1141
4. Korta DZ, Christiano AM, Bergfeld W, Duvic M, Ellison A, Fu J, et al. Alopecia areata is a medical disease. J Am Acad Dermatol (2018) 78(4):832–4. doi: 10.1016/j.jaad.2017.09.011
5. Hu X, Li J, Fu M, Zhao X, Wang W. The JAK/STAT signaling pathway: from bench to clinic. Signal Transduct Target Ther (2021) 6(1):402. doi: 10.1038/s41392-021-00791-1
6. Fragoulis GE, Brock J, Basu N, McInnes IB, Siebert S. The role for JAK inhibitors in the treatment of immune-mediated rheumatic and related conditions. J Allergy Clin Immunol (2021) 148(4):941–52. doi: 10.1016/j.jaci.2021.08.010
7. Meephansan J, Thummakriengkrai J, Ponnikorn S, Yingmema W, Deenonpoe R, Suchonwanit P. Efficacy of topical tofacitinib in promoting hair growth in non-scarring alopecia: possible mechanism via VEGF induction. Arch Dermatol Res (2017) 309(9):729–38. doi: 10.1007/s00403-017-1777-5
8. Park KS. Pharmacological effects of centella asiatica on skin diseases: evidence and possible mechanisms. Evid Based Complement Alternat Med (2021) 2021:5462633. doi: 10.1155/2021/5462633
9. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25(9):603–5. doi: 10.1007/s10654-010-9491-z
10. Margulis AV, Pladevall M, Riera-Guardia N, Varas-Lorenzo C, Hazell L, Berkman ND, et al. Quality assessment of observational studies in a drug-safety systematic review, comparison of two tools: the Newcastle-Ottawa scale and the RTI item bank. Clin Epidemiol. (2014) 6:359–68. doi: 10.2147/CLEP.S66677
11. Rucker G, Schwarzer G. Ranking treatments in frequentist network meta-analysis works without resampling methods. BMC Med Res Methodol (2015) 15:58. doi: 10.1186/s12874-015-0060-8
12. Zhang W, Li X, Chen B, Zhang J, Torres-Culala KMT, Zhou C. Oral tofacitinib and systemic corticosteroids, alone or in combination, in patients with moderate-to-Severe alopecia areata: a retrospective study. Front Med (Lausanne). (2022) 9:891434. doi: 10.3389/fmed.2022.891434
13. Shin JW, Huh CH, Kim MW, Lee JS, Kwon O, Cho S, et al. Comparison of the treatment outcome of oral tofacitinib with other conventional therapies in refractory alopecia totalis and universalis: a retrospective study. Acta Derm Venereol (2019) 99(1):41–6. doi: 10.2340/00015555-3057
14. Lai VWY, Bokhari L, Sinclair R. Sublingual tofacitinib for alopecia areata: a roll-over pilot clinical trial and analysis of pharmacokinetics. Int J Dermatol (2021) 60(9):1135–9. doi: 10.1111/ijd.15657
15. Almutairi N, Nour TM, Hussain NH. Janus kinase inhibitors for the treatment of severe alopecia areata: an open-label comparative study. Dermatology. (2019) 235(2):130–6. doi: 10.1159/000494613
16. Olsen EA, Kornacki D, Sun K, Hordinsky MK. Ruxolitinib cream for the treatment of patients with alopecia areata: a 2-part, double-blind, randomized, vehicle-controlled phase 2 study. J Am Acad Dermatol (2020) 82(2):412–9. doi: 10.1016/j.jaad.2019.10.016
17. King B, Ko J, Forman S, Ohyama M, Mesinkovska N, Yu G, et al. Efficacy and safety of the oral janus kinase inhibitor baricitinib in the treatment of adults with alopecia areata: phase 2 results from a randomized controlled study. J Am Acad Dermatol (2021) 85(4):847–53. doi: 10.1016/j.jaad.2021.05.050
18. King B, Ohyama M, Kwon O, Zlotogorski A, Ko J, Mesinkovska NA, et al. Two phase 3 trials of baricitinib for alopecia areata. N Engl J Med (2022) 386(18):1687–99. doi: 10.1056/NEJMoa2110343
19. King B, Mesinkovska N, Mirmirani P, Bruce S, Kempers S, Guttman-Yassky E, et al. Phase 2 randomized, dose-ranging trial of CTP-543, a selective janus kinase inhibitor, in moderate-to-severe alopecia areata. J Am Acad Dermatol (2022) 87(2):306–13. doi: 10.1016/j.jaad.2022.03.045
20. Gilhar A, Etzioni A, Paus R. Alopecia areata. N Engl J Med (2012) 366(16):1515–25. doi: 10.1056/NEJMra1103442
21. Xing L, Dai Z, Jabbari A, Cerise JE, Higgins CA, Gong W, et al. Alopecia areata is driven by cytotoxic T lymphocytes and is reversed by JAK inhibition. Nat Med (2014) 20(9):1043–9. doi: 10.1038/nm.3645
22. Harel S, Higgins CA, Cerise JE, Dai Z, Chen JC, Clynes R, et al. Pharmacologic inhibition of JAK-STAT signaling promotes hair growth. Sci Adv (2015) 1(9):e1500973. doi: 10.1126/sciadv.1500973
23. Bokhari L, Sinclair R. Treatment of alopecia universalis with topical janus kinase inhibitors - a double blind, placebo, and active controlled pilot study. Int J Dermatol (2018) 57(12):1464–70. doi: 10.1111/ijd.14192
24. Petukhova L, Duvic M, Hordinsky M, Norris D, Price V, Shimomura Y, et al. Genome-wide association study in alopecia areata implicates both innate and adaptive immunity. Nature. (2010) 466(7302):113–7. doi: 10.1038/nature09114
25. Guo H, Cheng Y, Shapiro J, McElwee K. The role of lymphocytes in the development and treatment of alopecia areata. Expert Rev Clin Immunol (2015) 11(12):1335–51. doi: 10.1586/1744666X.2015.1085306
26. Kageyama R, Ito T, Hanai S, Morishita N, Nakazawa S, Fujiyama T, et al. Immunological properties of atopic dermatitis-associated alopecia areata. Int J Mol Sci (2021) 22(5):2618. doi: 10.3390/ijms22052618
27. Guttman-Yassky E, Renert-Yuval Y, Bares J, Chima M, Hawkes JE, Gilleaudeau P, et al. Phase 2a randomized clinical trial of dupilumab (anti-IL-4Ralpha) for alopecia areata patients. Allergy. (2022) 77(3):897–906. doi: 10.1111/all.15071
28. Mostaghimi A, Napatalung L, Sikirica V, Winnette R, Xenakis J, Zwillich SH, et al. Patient perspectives of the social, emotional and functional impact of alopecia areata: a systematic literature review. Dermatol Ther (Heidelb). (2021) 11(3):867–83. doi: 10.1007/s13555-021-00512-0
29. Foo J, Redler S, Forstner A, Basmanav F, Pethukova L, Guo J, et al. Exploring the overlap between alopecia areata and major depressive disorder: epidemiological and genetic perspectives. J Eur Acad Dermatol Venereol (2023). doi: 10.1111/jdv.18921
30. King B, Guttman-Yassky E, Peeva E, Banerjee A, Sinclair R, Pavel AB, et al. A phase 2a randomized, placebo-controlled study to evaluate the efficacy and safety of the oral janus kinase inhibitors ritlecitinib and brepocitinib in alopecia areata: 24-week results. J Am Acad Dermatol (2021) 85(2):379–87.
31. Guttman-Yassky E, Pavel AB, Diaz A, Zhang N, Del Duca E, Estrada Y, et al. Ritlecitinib and brepocitinib demonstrate significant improvement in scalp alopecia areata biomarkers. J Allergy Clin Immunol (2022) 149(4):1318–28. doi: 10.1016/j.jaci.2021.10.036
32. Taylor PC, Weinblatt ME, Burmester GR, Rooney TP, Witt S, Walls CD, et al. Cardiovascular safety during treatment with baricitinib in rheumatoid arthritis. Arthritis Rheumatol (2019) 71(7):1042–55. doi: 10.1002/art.40841
33. Yu DA, Kim YE, Kwon O, Park H. Treatment outcome of oral tofacitinib and ruxolitinib in patients with alopecia areata: a systematic review and meta-analysis. Indian J Dermatol Venereol Leprol. (2021) 87(5):621–7. doi: 10.25259/IJDVL_975_19
34. Yan D, Fan H, Chen M, Xia L, Wang S, Dong W, et al. The efficacy and safety of JAK inhibitors for alopecia areata: a systematic review and meta-analysis of prospective studies. Front Pharmacol (2022) 13:950450. doi: 10.3389/fphar.2022.950450
Keywords: alopecia areata, JAK inhibitors, baricitinib, ruxolitinib, tofacitinib, network meta-analysis
Citation: Wei D, Chen Y, Shen Y, Xie B and Song X (2023) Efficacy and safety of different JAK inhibitors in the treatment of alopecia areata: a network meta-analysis. Front. Immunol. 14:1152513. doi: 10.3389/fimmu.2023.1152513
Received: 27 January 2023; Accepted: 29 March 2023;
Published: 17 April 2023.
Edited by:
Neusa Sakai Valente, University of São Paulo, BrazilReviewed by:
Sho Hiroyasu, Osaka City University, JapanCopyright © 2023 Wei, Chen, Shen, Xie and Song. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiuzu Song, c29uZ3hpdXp1QHNpbmEuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.