Corrigendum: The role of 18F−FDG PET in predicting the pathological response and prognosis to unresectable HCC patients treated with lenvatinib and PD-1 inhibitors as a conversion therapy
- 1Department of Nuclear Medicine, The First Medical Centre, Chinese People's Liberation Army (PLA) General Hospital, Beijing, China
- 2Nuclear Medicine Department, Beijing Friendship Hospital, Capital Medical University, Beijing, China
- 3Faculty of Hepato-Pancreato-Biliary Surgery, Chinese People's Liberation Army (PLA) General Hospital/Institute of Hepatobiliary Surgery of Chinese People's Liberation Army/Key Laboratory of Digital Hepetobiliary Surgery, People's Liberation Army, Beijing, China
- 4Graduate School, Medical School of Chinese People's Liberation Army (PLA), Beijing, China
- 5Department of Pathology, The First Medical Centre, Chinese People's Liberation Army (PLA) General Hospital, Beijing, China
Purpose: To investigate the diagnostic value of 18F-fluorodeoxyglucose positron emission tomography (18F-FDG PET), as an imaging biomarker, for predicting pathological response and prognosis of unresectable hepatocellular carcinoma (HCC) patients treated with Lenvatinib and programmed cell death protein 1 (PD-1) inhibitors as a conversion therapy.
Methods: A total of 28 unresectable HCC patients with BCLC stage B or C were treated with Lenvatinib and PD-1 inhibitors before surgery. The 18F-FDG PET/CT scans were acquired before pre- (scan-1) and post-conversion therapy (scan-2). The maximum standardized uptake value (SUVmax), TLR (tumor-to-normal liver standardized uptake value ratio), and the percentages of post-treatment changes in metabolic parameters (ΔSUVmax [%] and ΔTLR [%]) were calculated. Major pathological response (MPR) was identified based on the residual viable tumor in the resected primary tumor specimen (≤10%). Differences in the progression-free survival (PFS) and overall survival (OS) stratified by ΔTLR were examined by the Kaplan-Meier method.
Results: 11 (11/28, 39.3%) patients were considered as MPR responders and 17 (17/28, 60.7%) patients as non-MPR responders after conversion therapy. ΔSUVmax (-70.0 [-78.8, -48.8] vs. -21.7 [-38.8, 5.7], respectively; P<0.001) and ΔTLR (-67.6 [-78.1, -56.8] vs. -18.6 [-27.9, 4.0], respectively; P<0.001) were reduced in the responder group than those in the non-responder group. According to the results of the receiver operating characteristic curve analysis, ΔTLR showed an excellent predictive value for the MPR of primary HCC lesions (area under curve=0.989, with the optimal diagnostic threshold of -46.15). When using ΔTLR of -21.36% as a threshold, patients with ΔTLR-based metabolic response had superior PFS (log-rank test, P=0.001) and OS (log-rank test, P=0.016) compared with those without ΔTLR-based metabolic response.
Conclusion: 18F-FDG PET is a valuable tool for predicting pathological response and prognosis of unresectable HCC patients treated by Lenvatinib combined with PD-1 as a conversion therapy.
Introduction
Primary liver cancer is the sixth most common cancer and the third leading cause of cancer death worldwide (1). Hepatocellular carcinoma (HCC), as the most common type of primary liver malignancy in the world (75-85% of cases), has shown an increasing prevalence rate globally (2). Although surgical resection is a potentially curative treatment for patients with HCC, the majority of these patients are already in the advanced stage of HCC, and only 40-50% of patients in developed countries with regular physical examination are diagnosed at an early stage (3). Because of liver dysfunction, advanced stage or poor performance, more than half of HCC patients are not candidates of radical resection, resulting in poor prognosis (4, 5).
Non-surgical local or systemic treatment is the predominant choice for most advanced HCC patients (6). In recent years, non-surgical treatment of liver cancer, particularly systemic therapy, has progressed. Especially, for some advanced HCC patients, the original unresectable lesions can be changed to resectable lesions through systemic therapy, which is also called conversion therapy (6). Anti-angiogenic drugs, such as tyrosine kinase inhibitors (TKIs), combined with immunotherapies (e.g., programmed cell death protein 1 [PD-1]) have become an important choice for unresectable or intermediate and advanced HCC, and for conversion therapy of potentially resectable HCC (7). Lenvatinib, a multi-target TKI, was approved for the treatment of unresectable HCC in European countries, USA, Japan, and China (8). Lenvatinib inhibited vascular endothelial growth factor (VEGF) and fibroblast growth factor (FGF) pathways, and suppressed the proliferation signals from VEGF receptor (VEGFR) and FGF receptor (FGFR), which were overexpressed in cancer cells (9, 10). As a type of immunotherapy, the PD-1 blocking monoclonal antibodies act directly on immune cells and block the inhibitory T-cell receptor PD-1, and have also been proven to be effective for the treatment of liver cancer (4). Anti-angiogenic drugs combined with immunotherapy can achieve an objective response rate (ORR) of about 30%, and the median survival for patients receiving this type of therapy can be up to 20 months (11–14). As one of the TKIs combined with immunotherapy, Lenvatinib combined with PD-1 inhibitors have also been confirmed to show a certain therapeutic effect (11, 15–20).
When an unresectable HCC patient successfully receives TKIs combined with immunotherapy and surgery, pathological response is a very important indicator for the postoperative recurrence and long-term survival of the patient (6). Studies have shown that the tumor-free survival of HCC patients after resection is related to pathological response, and the tumor-free survival of patients with pathological response is longer (20, 21). However, how to predict pathological response remains to be investigated. In terms of imaging evaluation, the modified Response Evaluation Criteria in Solid Tumors (mRECIST) criteria were the most common standard to evaluate the therapeutic response of liver lesions (22, 23). However, it is still unclear whether mRECIST can predict pathological response and prognosis of HCC patients after conversion therapy. Although 18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) has exhibited a poor sensitivity for the detection of HCC compared with other solid tumors (24), 18F-FDG PET/CT has still been used for accurate staging, predicting therapeutic response, and detecting recurrence of HCC (25). In recent years, the metabolic parameters of 18F-FDG PET have shown a great value in predicting pathological response and prognosis of various malignant tumors after neoadjuvant therapy (26–28). However, there is no study on the metabolic parameters of 18F-FDG PET in predicting pathological response and prognosis of unresectable HCC patients undergoing conversion therapy. The present study aimed to explore the value of 18F-FDG PET in predicting pathological response and prognosis of unresectable HCC patients treated with Lenvatinib combined with PD-1 inhibitors as conversion therapy.
Materials and methods
Patients
This single-center retrospective study was based on a prospective, single-center, single-arm, investigator-initiated, clinical trial study, which was registered at http://www.chictr.org.cn/(ChiCTR1900023914), and it was approved by the Ethics Committee of the General Hospital of the People’s Liberation Army (Beijing, China). All patients were informed and signed the informed consent form before 18F-FDG PET/CT. The study was performed in accordance with the Declaration of Helsinki.
Between July 2019 and March 2023, unresectable HCC patients who underwent pre-treatment and post-treatment 18F-FDG PET/CT in the General Hospital of the People’s Liberation Army were retrospectively recruited. The inclusion criteria were as follows:(a) Patients older than 18 years and without a history of other malignance; (b) The diagnosis of HCC was pathologically confirmed by fine-needle biopsy or in accordance with the clinical diagnosis criteria of the American Association for the Study of Liver Diseases (AASLD) (29); (c) Patients who were diagnosed with unresectable HCC, and conversion therapy (combination of Lenvatinib and PD-1 inhibitors) could be performed after clinical evaluation; (d) 18F-FDG PET/CT was performed within 2 weeks prior to conversion therapy and within 3 weeks prior to surgery; I No other anti-tumor therapy was given during the treatment using Lenvatinib combined with PD-1 inhibitors, and the drugs were not terminated or changed during the therapy; (f) All patients underwent surgery and had definite postoperative pathological diagnosis; (g) High-quality 18F-FDG PET/CT images that could be used for diagnosis.
PET/CT scanning
All patients underwent 18F-FDG PET/CT (Biograph 64; GE Healthcare, New York, NY, USA). Patients were fasted for 6 h with plasma glucose levels under 11.1 mmol/L, and rested for at least 20 min in a quiet waiting room before intravenous administration of 18F-FDG (18F-FDG was produced by our department, with a radiochemical purity of >95%). Patients were injected with 18F-FDG at a dose of 3.70-4.44 MBq/kg (0.10-0.12 mCi/kg). PET/CT scan was performed after 60 min, beginning from the skull base to the upper femur in free-breathing mode. The low-dose CT (LDCT) parameters were as follows: voltage=120 kV, current=100 mAs, rotation=0.8, layer thickness=5 mm, and pitch=1. The parameters of PET included 3-dimensional mode, 2 min/bed (30% overlap), 4-5 beds/person, three iterations, 21 subsets, and Gaussian filter half-height width of 4.0 mm. Images were reconstructed with CT attenuation correction (CTAC) using the ordered subset expectation maximization (OSEM) algorithm.
Image analysis
Multiparametric analysis prototype (GE Healthcare), a dedicated prototypic post-processing tool, was used for image analysis. Quantitative analyses were performed by two experienced nuclear medicine physicians (WGY and MGY) who were blinded to patients’ clinical data. If there were discrepancies between the two physicians, the process would be repeated two weeks later to reach a consensus. Areas with abnormal uptake of 18F-FDG on PET and/or abnormal density on CT were defined as lesions. A two-dimensional region of interest (ROI) was delineated manually according to the boundary of the HCC lesion and portal vein tumor thrombus (PVTT) on each layer of transaxial CT images to form a three-dimensional volume of interest (VOI). Contrast-enhanced magnetic resonance imaging (MRI)/CT was used to accurately determine the VOI. The VOI was applied to the corresponding PET images, which were registered to CT images. To measure a normal liver activity, 3 non-overlapping spherical 1-cm (3)-sized VOIs were drawn in the normal liver on the axial PET images, avoiding the HCC areas on dynamic CT. The SUVmax (maximum standard uptake value) in HCC and PVTT for each patient was calculated by placing a spherical VOI over the sites of the HCC lesions and PVTT. Using the SUVmax of HCC and PVTT and mean SUV of the normal liver, TLR (tumor-to-normal liver standardized uptake value ratio, SUVmax of the tumor/SUVmean of the normal liver parenchyma) and PLR (PVTT-to-normal liver standardized uptake ratio, SUVmax of the PVTT/SUVmean of the normal liver parenchyma) were calculated for each patient. There were no significant differences in terms of SUVmean of the liver parenchyma between the MPR responder group and non-MPR responder group (pre-treatment: 2.41 ± 0.25 vs. 2.60 ± 0.33, P = 0.122; post-treatment: 2.38 ± 0.4 vs. 2.31 ± 0.41, P = 0.637).
The percentages of post-treatment changes in metabolic parameters were calculated as follows:
Furthermore, ΔSUVmax and ΔTLR of primary HCC lesions, and ΔPLR of PVTT were recorded, respectively.
Systemic therapy
Conversion therapy mainly included Lenvatinib and PD-1 inhibitors. Patients were treated with intravenous infusion of anti-PD-1 antibodies (dose, 200-240 mg for different drugs), and the vast majority of the data were collected under four treatment regimens (Pembrolizumab 200 mg/q3w, Sintilimab 200 mg/q3w, Toripalimab 240 mg/q3w, and Tislelizumab 200mg/q3w). Lenvatinib was given orally (8 or 12 mg/day, depending on the patient’s weight < 60 or ≥ 60 kg.
Follow-up during systemic therapy and radiological assessment
All patients were treated regularly and were monitored to assess their response to systemic therapy. Patients’ complete blood count, thyroid, cardiac, liver, renal, adrenal functions, and tumor markers prior to each cycle of PD-1 treatment were assessed. After 3 cycles of treatment with PD-1 inhibitors, tumor response of the patients was evaluated (according to RECIST ver. 1.1 (30) and mRECIST (22) criteria: complete response [CR], partial response [PR], stable disease [SD], and progressive disease [PD], and the resectability of liver cancer was investigated by contrast-enhanced MRI/CT and chest CT. The patients were categorized into responders (CR or PR) and non-responders (SD or PD) according to mRECIST. Immune-related adverse events (irAEs) were assessed using the National Cancer Institute’s Common Terminology Criteria for Adverse Events (ver. 4.0) (31, 32) (Table S1).
Criteria for successful conversion therapy
The criteria for successful conversion therapy were summarized as follows (33): (a) Child-Pugh grade A; (b) Eastern Cooperative Oncology Group Performance Status (ECOG PS) score ≤ 1; (c) Shrinkage or disappearance of metastatic lymph nodes, and the remaining lymph nodes can be removed; (d) No new extrahepatic metastases; I Intact vascular structure (including the inflow and outflow) of the reserved liver; (f) The expected ratio of future liver remnant volume to standard liver volume (FLR/SLV) after resection of tumor-bearing liver is ≥40% in compromised livers and 35% in normal livers. All patients who met the criteria for successful conversion therapy would be informed of the benefits and risks of surgery.
Histopathological assessment of tumor regression
Surgical specimens were analyzed by two experienced pathologists who were blinded to the patients’ treatment and outcomes. The pathological treatment response (PTR) was classified based on the tumor cellularity. The primary tumors and PPVT were recorded. Major pathological response (MPR, ≤10% residual viable tumor) or complete pathological response (CPR, no residual viable tumor) following immunotherapy was used as endpoints in the great majority of clinical trials (34, 35). Whether patients reached CPR or MPR through HCC lesions and PVTT (if present) was comprehensively considered.
We categorized all patients according to their pathological response into MPR responder and non-MPR responder groups.
Postoperative therapy and follow-up
Patients continued to receive therapy according to the pathological results and their personal conditions at 4-6 weeks after surgery and clinical evaluation. Serum tumor biomarkers were examined every cycle, and imaging examinations (contrast-enhanced MRI/CT or abdominal ultrasound) were performed every 3 months to monitor HCC recurrence. HCC recurrence was defined as the presence of radiological evidence of new intra- and/or extra-hepatic tumors (36). According to the guidelines, post-recurrence treatments were administered (6). The time of recurrence and death was recorded, respectively.
Statistical analysis
Quantitative data were expressed as median (interquartile range [IQR]) or mean ± standard deviation (SD). Qualitative data were expressed as number of cases and percentage (n [%]). Homogeneity of variance of the data was verified using Levene’s test, and normal distribution of the data by Shapiro–Wilk test. The student’s t-test or Mann-Whitney U test was used to compare 18F-FDG PET/CT metabolic parameters among different groups. The categorical variables were analyzed by the Fisher’s exact test or the Chi-square test. The optimal cut-off values for continuous variables were estimated using receiver operating characteristic (ROC) curve analysis with the area under the curve (AUC), and sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were calculated, respectively. PTR was compared with 18F-FDG PET/CT metabolic parameters using Spearman correlation analysis.
The metabolic parameters were dichotomized according to specific cutoff values, which were determined by using ROC curve analysis. Progression-free survival (PFS) was determined as the interval from the start of conversion therapy to the date of disease relapse/progression. Overall survival (OS) was defined as the interval between the conversion therapy and death from any cause. All patients were followed up for at least 6 months (i.e., 2 of 28 patients who was followed up for shorter than 8 months was excluded). Kaplan Meier was used to plot the survival curve and log-rank test of PFS and OS difference was used to evaluate the significance.
The statistical analysis was performed using SPSS 24.0 (IBM, Armonk, NY, USA) and R 4.0.2 (Bell Laboratories, Holmdel, NJ, USA) software. All statistical tests were two-sided and the significance level was set at P=0.05.
Results
Patients’ characteristics
Eventually, 28 patients underwent surgical excision after successful conversion therapy in our study (24 men; median age: 58.0 years, IQR: 51.8–61.8 years; Figure 1). Among them, 11 (11/28, 39.3%) and 17 (17/28, 60.7%) patients were assigned to MPR responder group and non-MPR responder group, respectively. In addition, 5 of 11 patients in the MPR responder group achieved CPR. There was no significant difference in baseline characteristics between MPR responder group and non-MPR responder group in terms of general status (age, gender, body mass index [BMI], alcohol abuse, history of liver diseases, and ECOG PS score), clinical data (Barcelona clinic liver cancer [BCLC] stage, Child-Pugh score, and baseline alpha fetoprotein [AFP] level), imaging findings (tumor diameter, cirrhosis, macroscopic portal vein invasion, extrahepatic metastases), surgical findings (strategy of hepatectomy and R0 resection) and the type of PD-1 inhibitors. The post-treatment AFP level (normal or abnormal), number of tumor and the distribution of mRECIST were significantly different between the two groups (P=0.025, P=0.025 and P=0.001, respectively; Table 1). Due to the impact of conversion therapy, only two patients in MPR responder group determined the degree of pathological differentiation, both of whom were poorly differentiated; Among patients in non-MPR responder group, 10 were moderately differentiated, 5 were moderately poorly differentiated, and 1 was poorly differentiated. The median time between the start of conversion therapy and surgery was 107.0 days (IQR: 92.3-133.8 days), the median cycle of conversion therapy was 5.0 (IQR: 4.0-5.8), the median time between the pre-treatment 18F-FDG PET/CT and the start of conversion therapy was 4.0 days (IQR: 2.0-7.0 days), the median time between post-treatment 18F-FDG PET/CT and surgery was 6 days (IQR: 3.3-8.8 days), and the median time between two 18F-FDG PET/CT was 104.5 days (IQR: 90.0-132.3 days). Supplementary Table 2 shows the details of patients’ conversion therapy and surgery.
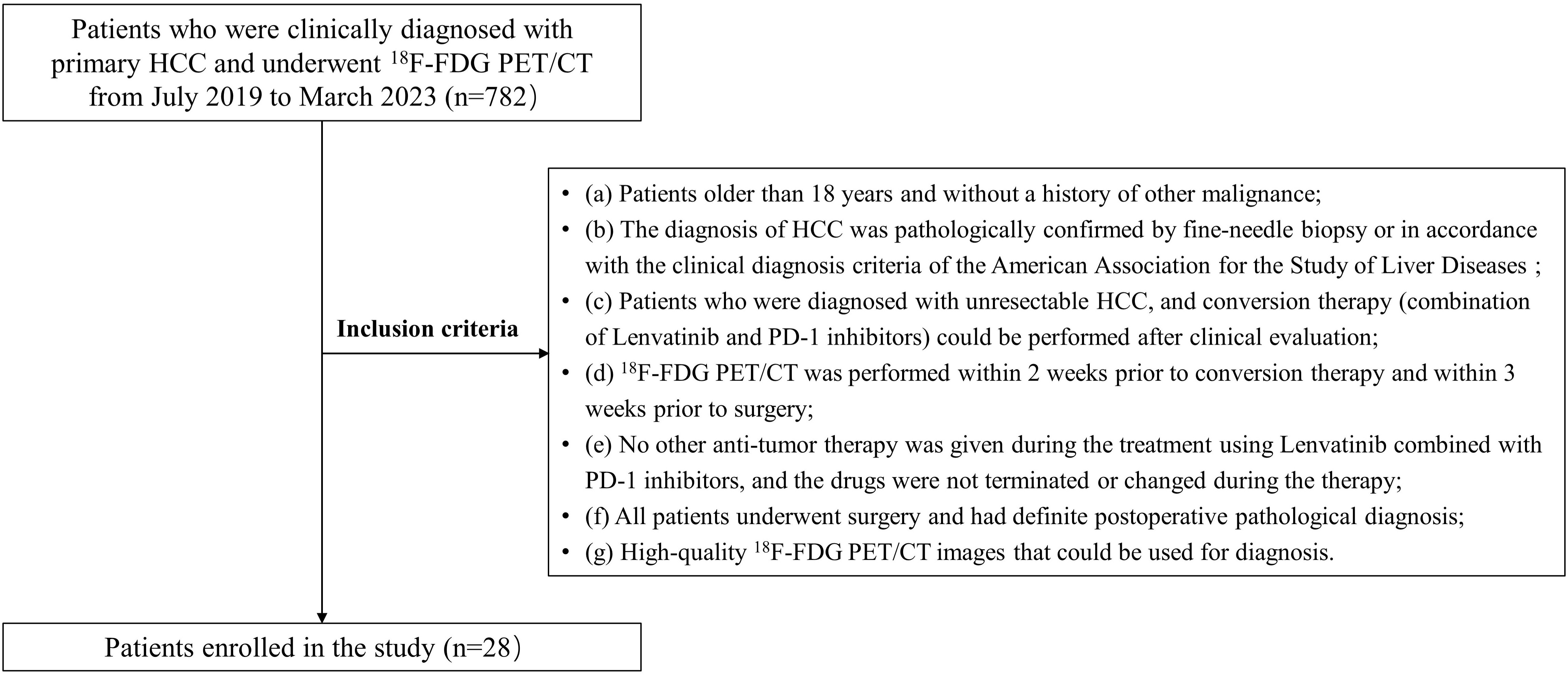
Figure 1 The flow diagram of study. HCC, Hepatocellular carcinoma; 18F-FDG PET/CT, 18F-fluorodeoxyglucose positron emission tomography/computed tomography.
Tumor metabolic parameters of 18F−FDG PET indicated a significant difference between MPR responder and non−MPR responder groups and predicted pathological response of MPR patients
Pre-treatment 18F-FDG PET metabolic parameters were compared between responder group and non-responder group, and there was a significant difference in SUVmax (11.6 [8.7, 16.7] vs. 6.7 [4.5, 10.8], respectively; P=0.028) and TLR (5.1 [3.9, 6.5] vs. 2.3 [1.8, 4.0], respectively; P=0.022) on pre-treatment scan. The metabolic parameters of post-treatment scan showed no significant difference between MPR responder group and non−MPR responder group (P=0.053 and 0.059 for SUVmax and TLR, respectively). ΔSUVmax (%) (-70.0 [-78.8, -48.8] vs. -21.7 [-38.8, 5.7], respectively; P<0.001) and ΔTLR (%) (-67.6 [-78.1, -56.8] vs. -18.9 [-27.9, 2.6], respectively; P<0.001) were significantly lower in the MPR responder group than those in the non−MPR responder group after conversion therapy (Table 2).
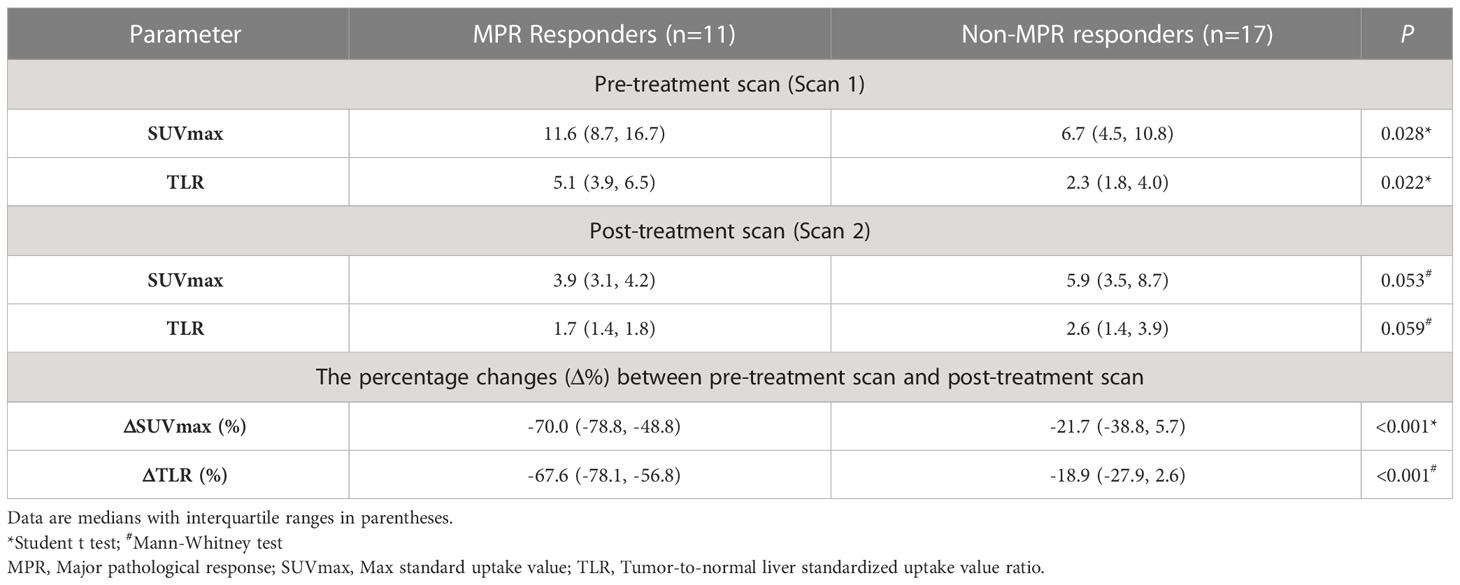
Table 2 The difference of 18F-FDG PET parameters between MPR responders and non-MPR responders in primary lesion.
Compared mRECIST and other 18F-FDG PET metabolic parameters, ΔTLR (%) showed the largest AUC (AUC=0.989, 95% confidence interval [CI]: 0.962-1.000), with the optimal diagnostic threshold of -46.15. The sensitivity, specificity, PPV, and NPV were 0.909 (0.571-0.995), 1.000 (0.771-1.000), 1.000 (0.655-1.000), and 0.944 (0.706-0.997), respectively (Table 3 and Figure 2). The relationship between the ΔTLR (%) and the mRECIST criteria and pathological response is detailed in Figure 3.
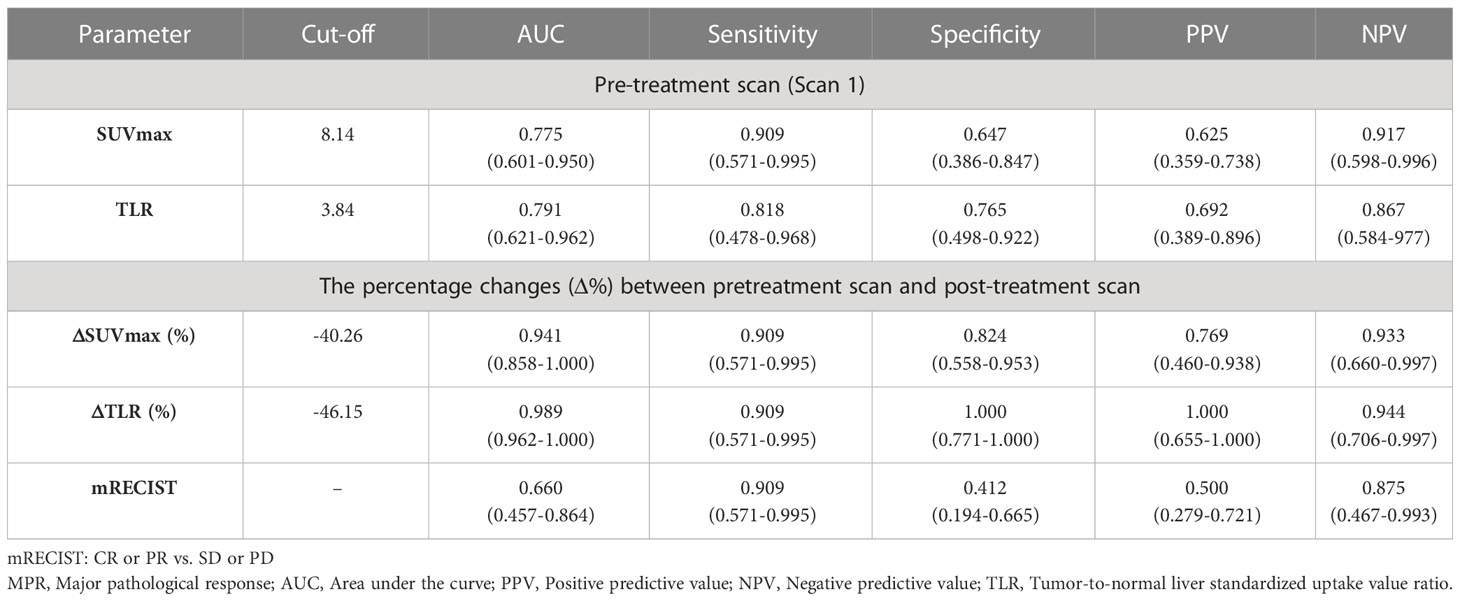
Table 3 Differential diagnostic efficiency of 18F-FDG PET metabolic parameters and mRECIST criteria between MPR-responders and MPR-non-responders.
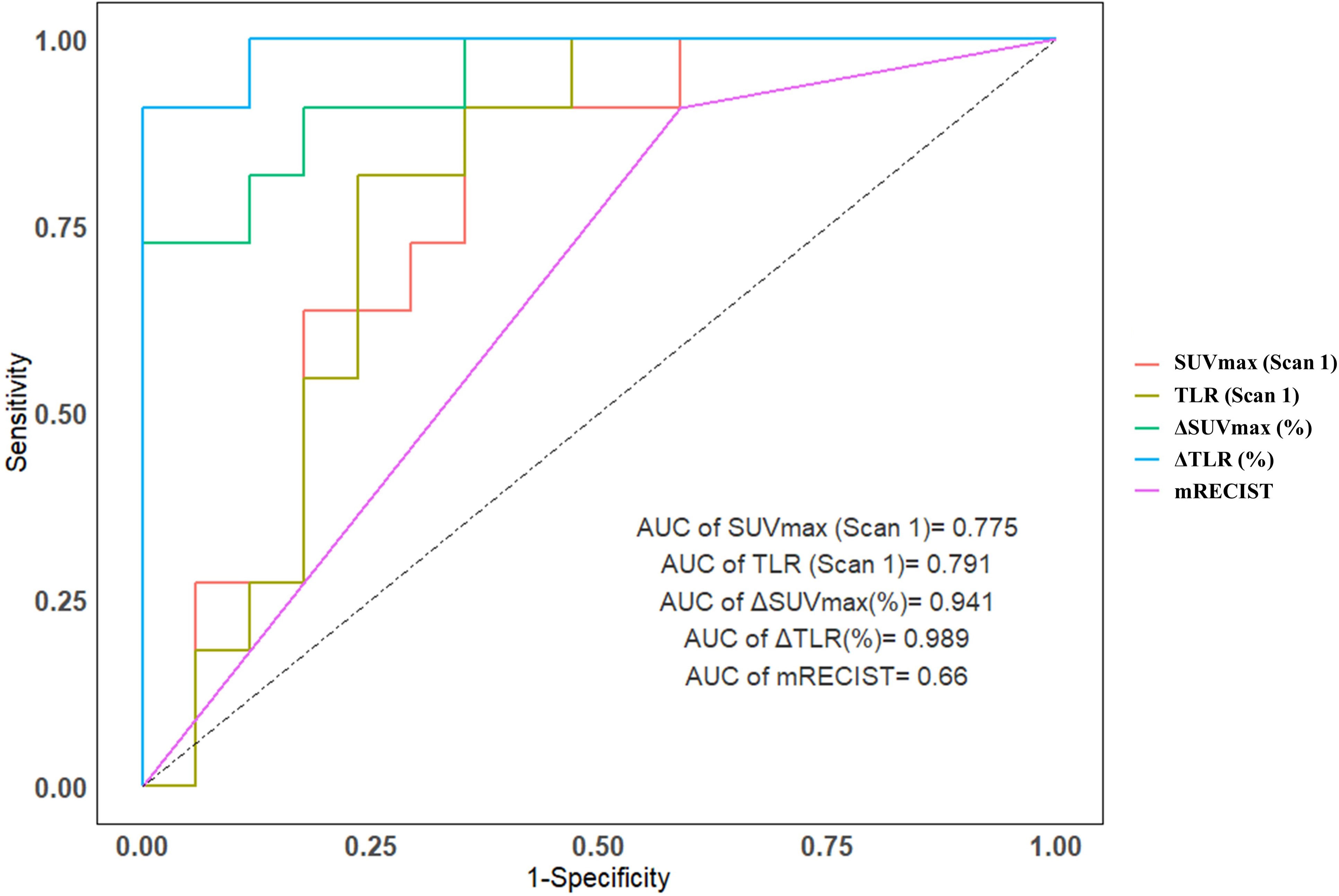
Figure 2 The area under the ROC curve for predicting major pathological response for SUVmax (Scan 1), TLR (Scan 1), ΔTLRand ΔSUVmax was 0.775, 0.791, 0.941 and 0.989, respectively, both are above the mRECIST criteria (0.660).
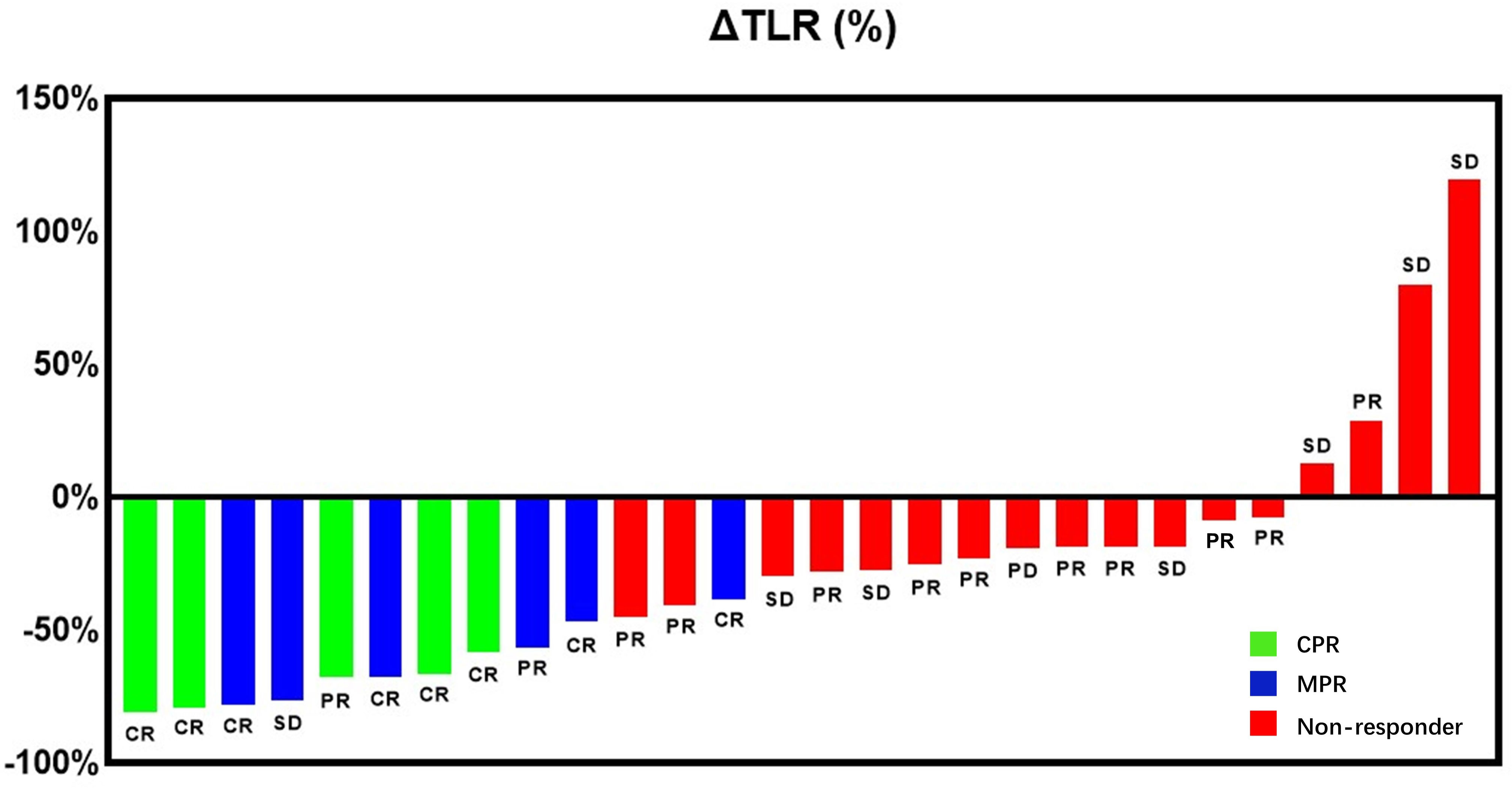
Figure 3 Waterfall plot presenting the percentage of change in primary tumor TLR from baseline to on-treatment per individual patient and the preoperative primary HCC lesions of patients determined by mRECIST criteria. Bar color indicates CPR response (green), MPR response (blue) or non-response (red).
Correlation between 18F-FDG PET metabolic parameters and pathological response
The Spearman correlation analysis was carried out to explore the relationship between 18F-FDG PET metabolic parameters and pathological response. The results showed that ΔTLR (%), ΔSUVmax (%), TLR (Scan 1), SUVmax (Scan 1), and SUVmax (Scan 2) were correlated with pathological response, with correlation coefficients (rs) of -0.83, -0.75, 0.49, 0.47, and -0.38, respectively (P<0.05). The TLR(Scan 2) showed a lower correlation (r=-0.37), whereas no significant difference was found (P>0.05). The results are displayed in Figure 4.
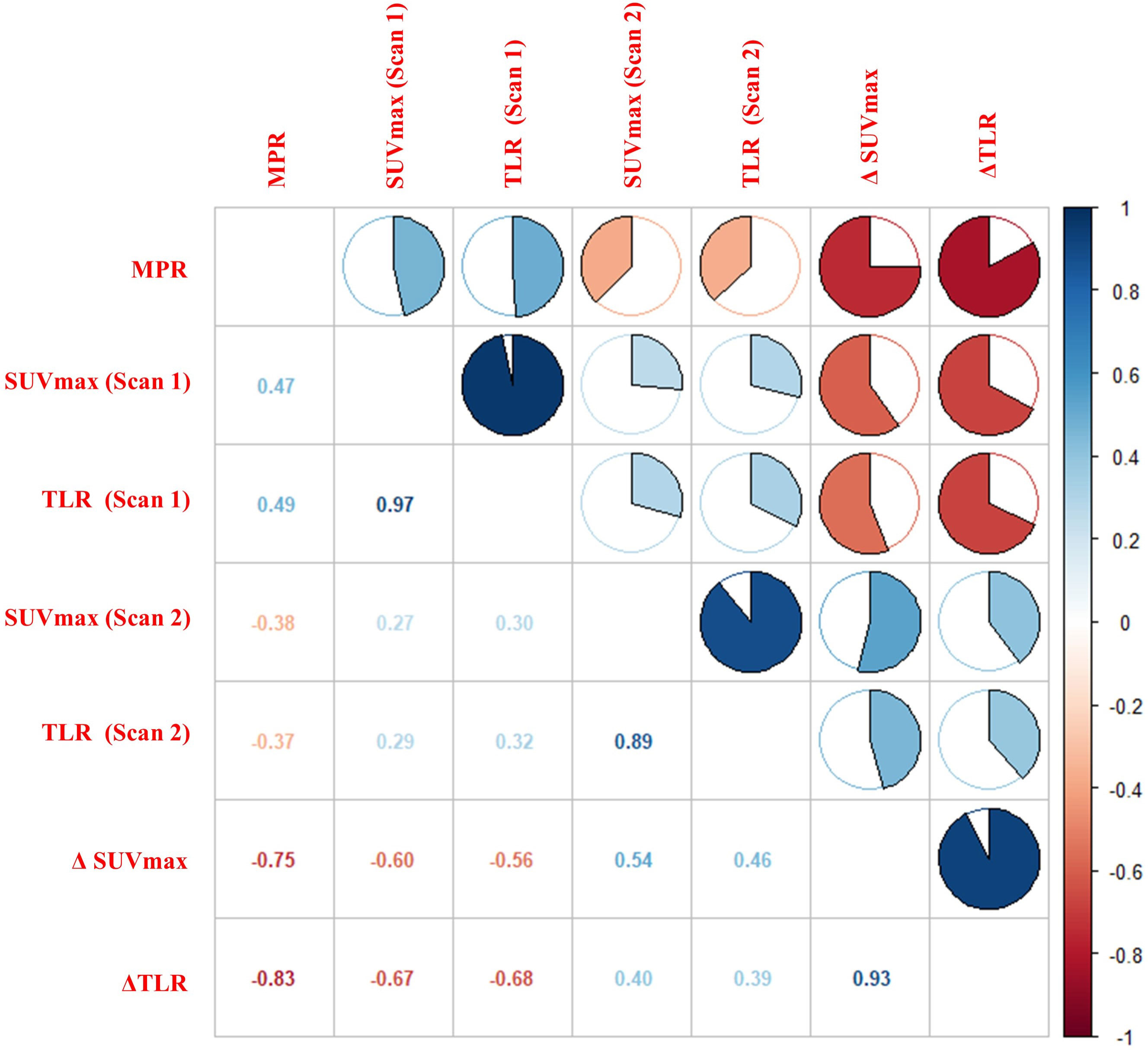
Figure 4 Correlation between 18F-FDG PET metabolic parameters and pathological response. The figure showed a strong correlation between ΔTLR and MPR (r = -0.83, P< 0.01).
Prognostic value of 18F-FDG PET on PFS and OS
The follow-up ended in February 15, 2023. Twopatients waere not included in the analysis due to short follow-up time. During the follow-up period, 19/26 (73.1%) patients showed a disease progression, and median follow-up was 14.7 (IQR:6.6-23.6) months; 7/26 (26.9%) patients died, and median follow-up was 27.6 (IQR: 12.7-31.1) months. When ΔTLR of -46.15% was used as a threshold, patients with ΔTLR-based metabolic response had no superior PFS (log-rank test, P=0.112) and OS (log-rank test, P=0.218) compared with those without a ΔTLR-based metabolic response. According to ROC analysis, when ΔTLR of -21.36% was used as a threshold, patients with ΔTLR-based metabolic response had superior PFS (log-rank test, P=0.001) and OS (log-rank test, P=0.016) compared with those without ΔTLR-based metabolic response (Figure 5). Patients’ follow-up data are summarized in Supplementary Table S3.
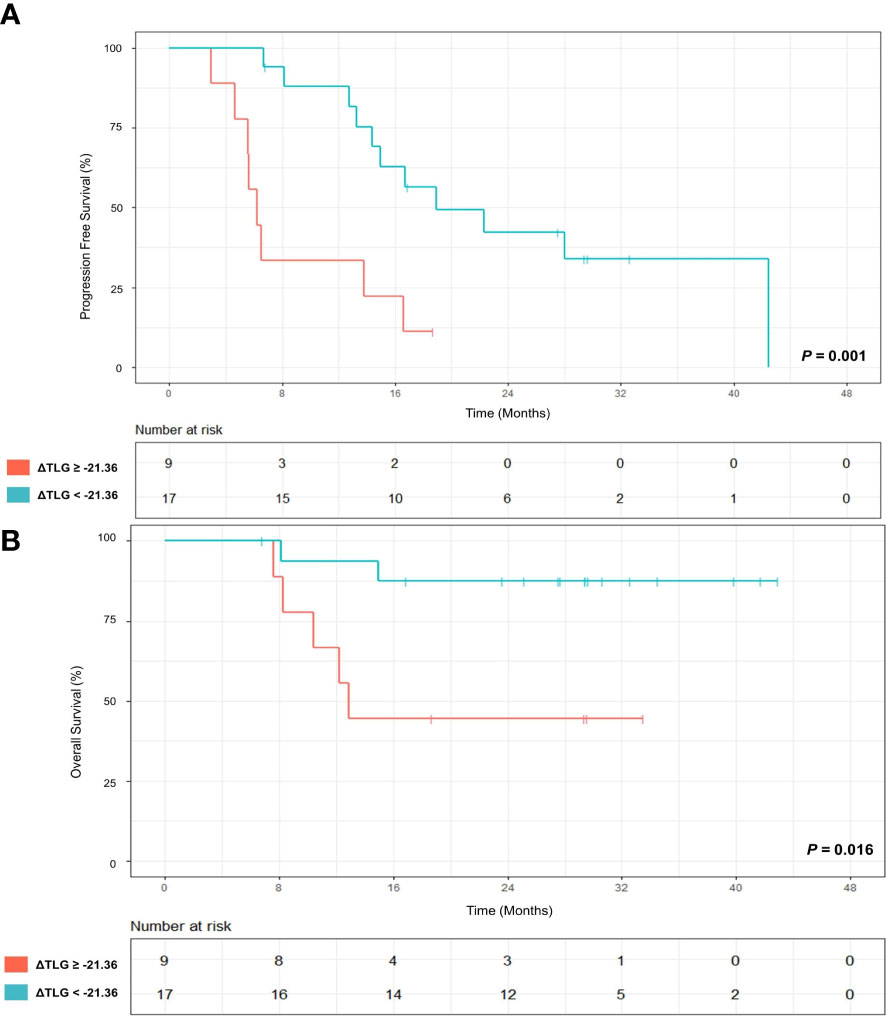
Figure 5 (A) Progression-free survival (PFS) of patients with ≥ 21.36% decrease in TLR at primary tumor site (red) from baseline to on-treatment and patients without ≥ 21.36% decrease (green). (B) Overall survival (OS) of patients with ≥21.36% decrease in TLR at primary tumor site (red) from baseline to on-treatment and patients without ≥21.36% decrease (green). P values were calculated using log-rank test.
18F-FDG PET identified PVTT involvement
In this study, 17 of 28 (60.7%) patients had macroscopic portal vein invasion. The number of residual tumor cells in PVTT was analyzed in 14 patients. Among them, 9 (9/14, 64.3%) patients were considered as PVTT CPR-responders and 5 (5/14, 35.7%) patients were PVTT CPR-non-responders. The metabolic parameters of pre-treatment scan, post-treatment scan, and the percentage of change in pre-treatment scan and post-treatment scan showed no significant difference between the responder group and the non-responder group (Table 4).
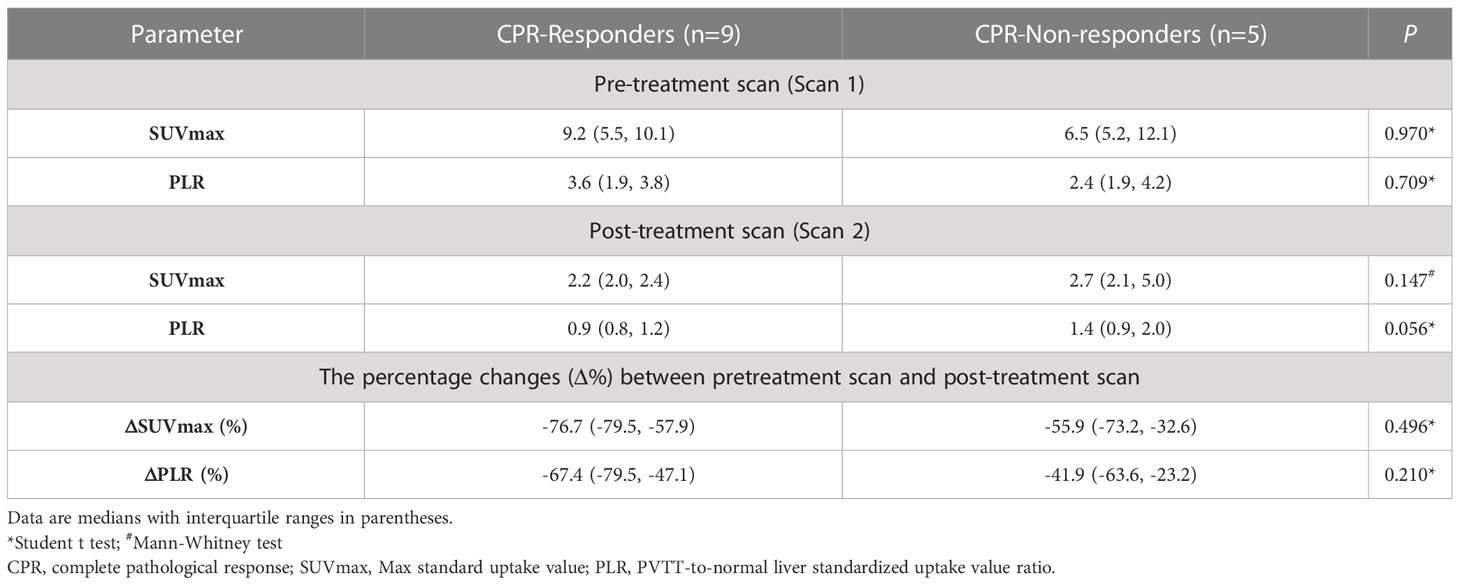
Table 4 The difference of 18F-FDG PET metabolic parameters between CPR-responders and CPR-non-responders in PVTT.
Discussion
To our knowledge, this is the first study to report the role of 18F-FDG PET in predicting pathological response and prognosis of unresectable HCC patients after treated by Lenvatinib in combination with PD-1 inhibitors as conversion therapy. The results suggested that the differences between the TLR (ΔTLR, %) of pre-treatment 18F-FDG PET and post-treatment 18F-FDG PET were promising imaging biomarkers for pathological response and prognosis of primary unresectable HCC after treated with the PD-1 blockade in combination with Lenvatinib as conversion therapy. However, 18F-FDG PET was not a predictive factor of PVTT pathological response.
CPR has been proven to be an important prognostic factor for patients with multiple malignancies after treatment and surgery, including HCC (37, 38). However, for patients with unresectable HCC, there are few options to achieve CPR, thus, MPR is a good alternative. MPR, defined as equal to 10% residual tumor following neoadjuvant therapy, has also been used as a prognostic factor of malignant tumors, such as non-small cell lung cancer (39), pancreatic cancer (40), and melanoma (41). The possible reason is that patients may not need a complete pathological resolution of the tumor burden to experience clinical benefits, because the main mechanism of the clinical benefits of immunotherapy-based conversion therapy is to initiate an anti-tumor immune response that may systematically seek and destroy microscopic tumor deposits that may lead to tumor recurrence (34). Compared with the traditional RECIST (ver. 1.1) criteria, mRECIST criteria based on CT or MRI were developed to better evaluate the response of liver lesions (6), and they possess some advantages in terms of assessing the degree of pathological response (42). After the treatment takes effect, tumor necrosis appears first, while absorption is relatively slow. Due to the histological and biological changes caused by tumor necrosis, mRECIST criteria are more appropriate for imaging evaluation of conversion therapy (7). However, some studies have shown that mRECIST criteria are only appropriate for assessing pathological response of HCC patients receiving neo-adjuvant therapy before liver transplantation (43). For treatment response, the metabolic parameters of 18F-FDG PET also play an important role in predicting HCC (44–46). However, no study has analyzed the pathological response of 18F-FDG PET in patients with unresectable HCC after receiving Lenvatinib in combination with PD-1 inhibitors as conversion therapy.
Our previous study indicated that pre-treatment TLR was a potent marker to predict pathological response of HCC patients (BCLC stage C) treated with Lenvatinib and PD-1 inhibitors as conversion therapy (33). In the present study, it was found that pre-treatment TLR could predict MPR (AUC=0.791, sensitivity=81.8%, specificity=76.5%), which is similar to our previous study. One explanation is that the FDG uptake is positively correlated with the content of tumor-infiltrating lymphocytes (TILs), especially T cells (47–49). Besides, the high FDG uptake in HCC may be a valuable predictor of an extremely rapid response to Lenvatinib (50). This may explain the relationship between the high FDG uptake and pathological response, and it is also because more TILs are accumulated in responders’ HCC lesions, and they may more strongly promote the local and systematic enhancement of T cell-mediated anti-tumor immunity by TKIs combined with immunotherapy than non-responders. Therefore, the therapeutic effect of responders was better. This suggested to some extent why there was a greater difference in FDG uptake between pre-treatment and post-treatment 18F-FDG PET, and the patient was more likely to achieve MPR. Our results showed that ΔTLR (cut-off value: -46.15%) was the best parameter to predict pathological response of primary HCC lesions, and it was more accurate than mRECIST criteria (Figure 6). However, in our patients, four patients showed an increase in ΔTLR. But all four patients were in the non−MPR responder group, and their treatment cycles were relatively short, ranging from 3-5 cycles. All four patients had relapsed, and two died. The reason may be that although the volume of the tumor has decreased, the surviving tumor cells have stronger activity and stronger metabolism compared to before, leading to an increase in FDG uptake, which may lead to postoperative recurrence in these patients. Therefore, using 18F-FDG PET to evaluate the conversion therapy effectiveness of unresectable HCC patients at different time points may also help to find a more accurate surgical time. 18F-FDG PET may provide more reliable imaging predictors for the timing of operation for unresectable HCC patients treated with Lenvatinib and PD-1 inhibitors as conversion therapy.
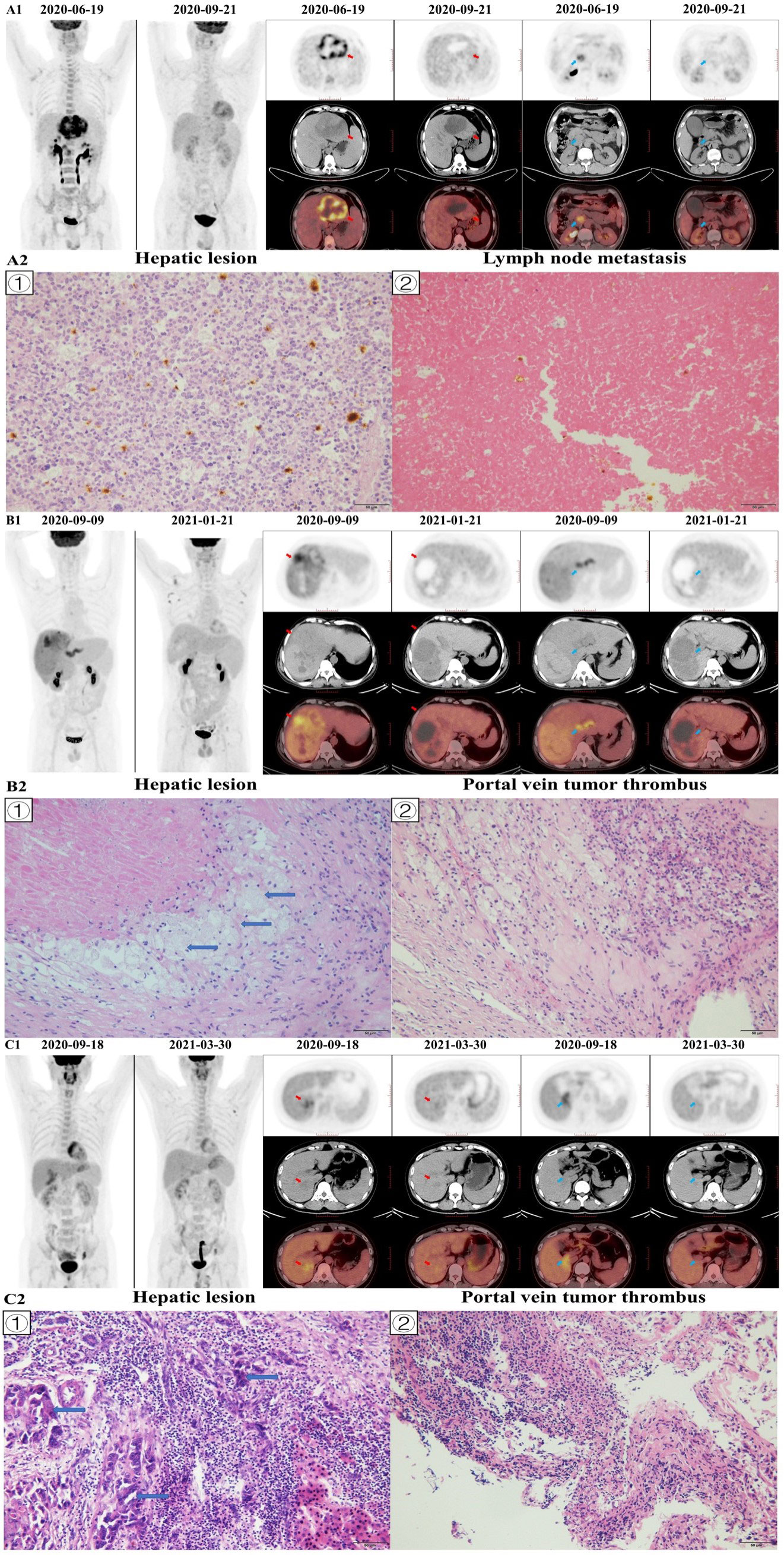
Figure 6 Image A1 shows a 51-year-old man with BCLC-C stage hepatocellular carcinoma in the left hepatic lobe (red arrow), and the patient was accompanied by lymph node metastasis (blue arrow). The hepatic lesion of pre-treatment 18F-FDG PET/CT (2020–06–19) showed that tumor-to-normal liver standardized uptake value ratio (TLR) was 8.21, and the hepatic lesion of post-treatment 18F-FDG PET/CT (2020–09–21) showed that TLR was 1.80. The percentage of change in TLR was -78.08. The baseline AFP level was 960.4 ng/mL, the baseline tumor diameter was 106 mm, and the Child-Pugh score was 5. The patient had no history of hepatitis and drinking, while had a history of liver cirrhosis. After conversion therapy (4 cycles of Lenvatinib and Sintilimab), the AFP level decreased to 2.95 ng/mL and the tumor diameter decreased to 85 mm. The patient underwent left hemihepatectomy and lymph node dissection, and histopathological evaluation of response revealed major histopathological response to therapy (residual viable tumor cells rate =8%; Image A2 ①), and no residual tumor tissue was found in metastatic lymph nodes; Image A2 ②). The patient died of myocardial infarction 14.9 months later, and there was no recurrence during the follow-up period. Image A2 shows: ① the hepatic tumor, with a small number of tumor cells, some visible mitotic figures, surrounded by a large number of lymphocyte infiltration (×200); ② showed a large number of necrotic tissues in metastatic lymph nodes and cell aggregation (×200). Image B1 shows a 51-year-old man with BCLC-C stage hepatocellular carcinoma in the right hepatic lobe (red arrow), and the patient was accompanied by portal vein tumor thrombus (PVTT; blue arrow). The hepatic lesion of pre-treatment 18F-FDG PET/CT (2020–09–09) showed that TLR was 4.22, and the hepatic lesion of post-treatment 18F–FDG PET/CT (2021–01–21) showed that TLR was 1.37. The hepatic lesion of pre-treatment 18F-FDG PET/CT (2020–09–09) showed that PVTT-to-normal liver standardized uptake value ratio (PLR) was 3.85, and the hepatic lesion of post-treatment 18F-FDG PET/CT (2021–01–21) showed that PLR was 0.87. The percentage of change in TLR and PLR was -67.61 and -77.44, respectively. The baseline AFP level was 86.78 ng/mL, the baseline tumor diameter 190 mm, and the Child-Pugh score 6. The patient had no history of hepatitis, while had a history of liver cirrhosis. After conversion therapy (4 cycles of Lenvatinib and Sintilimab), the AFP level decreased to 1.88 ng/mL and the tumor diameter decreased to 127 mm. The patient underwent right hemihepatectomy and PVTT resection. The histopathologic evaluation of primary liver lesion response indicated major histopathological response to therapy (residual viable tumor cells rate<5%; Image B2 ①) and the histopathological evaluation of PVTT response revealed complete histopathological response to therapy (residual viable tumor cells rate=0%; Image B2 ②). No recurrence or death occurred during the follow-up period. Image B2 shows: ① showed the hepatic tumor with no viable tumor cells but foam cells aggregation (blue arrows), and scattered lymphocyte infiltration (×200); ② showed a large area of necrosis in the PVTT, with a large number of inflammatory cell infiltration and foam cell reaction around it, and no obvious viable tumor cells (×200). Image C1 shows a 38-year-old man with BCLC-C stage hepatocellular carcinoma in the right hepatic lobe (red arrow), and the patient was accompanied by portal vein tumor thrombus (blue arrow). The hepatic lesion of pre-treatment 18F-FDG PET/CT (2020–09–18) showed that TLR was 1.94, and the hepatic lesion of post-treatment 18F-FDG PET/CT (2021–03–30) showed that TLR was 1.39. The hepatic lesion of pre-treatment 18F-FDG PET/CT (2020–09–18) showed that PLR was 2.20, and the hepatic lesion of post-treatment 18F-FDG PET/CT (2021–03–30) showed that PLR was 1.35. The percentage of change in TLR and PLR was -28.08 and -38.59, respectively. The baseline AFP level was 289.4 ng/mL, the baseline tumor diameter 48 mm, and the Child-Pugh score 5. The patient had no history of hepatitis, while had a history of liver cirrhosis. After conversion therapy (9 cycles of Lenvatinib and Sintilimab), the AFP level decreased to 35.04 ng/mL and the tumor diameter decreased to 23 mm. The patient underwent S7 segmentectomy and PVTT resection. The histopathological evaluation of response revealed no major histopathological response to therapy (residual viable tumor cells rate =85%; Image C2 ①) and the histopathological evaluation of portal vein tumor thrombus response indicated no complete histopathological response to therapy (residual viable tumor cells rate=50%; Image C2 ②). No recurrence or death occurred during the follow-up period. Image C2 shows: ① showed the tumor cell with degeneration, deep staining of the nucleus, obvious atypia (blue arrows), and a large number of lymphocyte infiltration around (×200); ② showed PVTT, visible tumor cells with some cancer tissue degeneration, visible hemorrhage necrosis and foam cell aggregation (×200).
As pathological response is associated with prognosis of HCC patients, we hypothesized that FDG metabolic parameters can also predict the prognosis of unresectable HCC patients after receiving Lenvatinib in combination with PD-1 inhibitors as conversion therapy. There are limited data of biomarkers to help decision-making and guide the treatment of advanced HCC (51), and there is no imaging biomarker for prognosis of patients with unresectable HCC after conversion therapy. The present study revealed that ΔTLR (cut-off value: -21.36%) was also an indicator to predict PFS and OS of patients receiving Lenvatinib in combination with PD-1 inhibitors as conversion therapy. Previous studies have shown that the more obvious the reduction of FDG uptake, the better the prognosis (PSF or/and OS) of patients with other malignant tumors treated with TKIs or immunotherapy (52–55). Our present study indicated a potential imaging biomarker of the therapeutic efficacy and prognosis of patients with advanced HCC after treated by conversion therapy.
However, our study found that metabolic parameters of PET could not predict pathological response of PVTT. PVTT plays a major role in the prognosis and clinical staging of HCC (56, 57), some studies have shown that HCC patients with PVTT after neo-adjuvant therapy still have better survival outcomes than those without neo-adjuvant therapy (58, 59). Huang et al. demonstrated that the ORR of Lenvatinib combined with PD-1 inhibitors was 54.5% for macrovascular tumor thrombi (MVTT) and 32.8% for hepatic tumors, and among 17 MVTT patients who achieved ORR, 6 (18.1%) patients underwent surgery (60). Postoperative pathology indicated that 66.7% of patients with PVTT achieved pathological complete necrosis. This confirmed that the conversion therapy of Lenvatinib combined with PD-1 inhibitors had a promising therapeutic effect on PVTT. Therefore, biomarkers are also needed to evaluate pathological response of patients with PVTT. It has been reported that FDG uptake has diagnostic and prognostic value for HCC PVTT (61, 62). However, the components in the tumor thrombus are more complex than the original tumor. After treatment for the tumor thrombus, there may still be many tumor-infiltrating inflammatory cells, which may lead to the increased FDG uptake, disabling metabolic parameters to predict pathological response of patients with PVTT. It is noteworthy that fewer patients were included in this study, and bias was inevitable. More studies are still required to verify our findings.
The present study has some limitations. First, it was a retrospective single-center study and the number of enrolled patients was small. This may bias the study results. However, due to the low proportion of unresectable HCC patients treated with Lenvatinib and PD-1 Inhibitors as a conversion therapy and successfully undergo conversion surgery (63–65), few patients could be included in our study. Second, the follow-up was short, and a longer follow-up period is needed to examine whether 18F-FDG PET metabolic parameters on primary tumors can predict survival outcomes of HCC patients after treated with Lenvatinib in combination with PD-1 inhibitors as conversion therapy, followed by surgery. Third, only pathological treatment response of the primary tumor and PVTT was assessed, and there is still a lack of evidence on extrahepatic metastases. Especially, in our study, except for 18F-FDG PET, we found that there was a significant difference between MPR responder group and non-MPR responder group in whether the post-treatment AFP levels were normal. This may also provide biomarkers for predicting pathological response, but more research is still needed. Fourth, due to the small number of patients, we were unable to analyze more related factors and predicting biomarker in the survival analysis, such as tumor responses, PVTT, male, baseline AFP level and liver disease history (51, 60, 65, 66). It is therefore essential to comprehensively analyze the related factors in the future large-scale research. Fifthly, since the pathological results of most patients in MPR responder group did not indicate the tumor differentiation, the impact of HCC differentiation on 18F-FDG uptake could not be considered. In the future, we will design prospective studies with a longer follow-up and a larger sample size to verify the role of 18F-FDG PET in predicting pathological response and prognosis of unresectable HCC patients after treated by Lenvatinib in combination with PD-1 inhibitors as conversion therapy.
Conclusions
In conclusion, the results of this study suggest that 18F-FDG PET is a precious tool for predicting pathological response and prognosis of patients with primary unresectable HCC after treated by Lenvatinib combined with PD-1 inhibitors as conversion therapy. Our study provided valuable markers for clinical decision-making, preoperative evaluation and prognostic prediction of patients with unresectable HCC.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The study involving human participants was reviewed and approved by The Ethics Committee of the General Hospital of the People’s Liberation Army (Beijing, China). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
Conception and design: GYW, WWZ, BXX, SCL, RMW and GYM. Data collation: GYW, WWZ, XHL, ZBW, JJL and JMZ. Statistical Analysis: GYW, RMW and GYM. Article writing: GYW, WWZ, XHL and XDX. Article revision: JJL, JMZ, BXZ, SCL, RMW and GYM. All authors contributed to the article and approved the submitted version.
Acknowledgments
The authors would like to thank the staffs at Department of Nuclear Medicine and Faculty of Hepato-Pancreato-Biliary Surgery (The First Medical Centre, Chinese PLA General Hospital, Beijing, China) for their assistance in carrying out this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1151967/full#supplementary-material
Abbreviations
18F-FDG, 18F-fluorodeoxyglucose; AASLD, American Association for the Study of Liver Diseases; AC, attenuation correction; AFP, alpha fetoprotein; AUC, area under the curve;BCLC, Barcelona clinic liver cancer; BMI, body mass index; CPR, complete pathological response; ECOG PS, Eastern Cooperative Oncology Group Performance Status; FGF, fibroblast growth factor; FLR/SLV, future liver remnant volume to standard liver volume; HCC, hepatocellular carcinoma; irAEs, Immune-related adverse events; mRECIST, modified Response Evaluation Criteria in Solid Tumors; MPR, major pathological response; MVTT, macrovascular tumor thrombi; NPV, negative predictive value; ORR, objective response rate; OS, overall survival; OSEM, ordered subset expectation maximization algorithm; PD-1, programmed cell death protein 1; PET, positron emission tomography; PFS, progression free survival; PLR, portal vein tumor thrombus-to-normal liver standardized uptake value ratio; PVTT, portal vein tumor thrombus; PPV, positive predictive value; PTR, pathological treatment response; ROC, receiver operating characteristic; ROI, region of interest; SUVmax, maximum standardized uptake value; TILs, tumor-infiltrating lymphocytes; TKIs, tyrosine kinase inhibitors; TLR, tumor-to-normal liver standardized uptake value ratio; VEGF, vascular endothelial growth factor; VOI, volume of interest.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. Clark T, Maximin S, Meier J, Pokharel S, Bhargava P. Hepatocellular carcinoma: review of epidemiology, screening, imaging diagnosis, response assessment, and treatment. Curr Probl Diagn Radiol (2015) 44(6):479–86. doi: 10.1067/j.cpradiol.2015.04.004
3. Pinter M, Peck-Radosavljevic M. Review article: systemic treatment of hepatocellular carcinoma. Aliment Pharmacol Ther (2018) 48(6):598–609. doi: 10.1111/apt.14913
4. Zhang L, Ding J, Li HY, Wang ZH, Wu J. Immunotherapy for advanced hepatocellular carcinoma, where are we? Biochim Biophys Acta Rev Cancer (2020) 1874(2):188441. doi: 10.1016/j.bbcan.2020.188441
5. Bruix J, Reig M, Sherman M. Evidence-based diagnosis, staging, and treatment of patients with hepatocellular carcinoma. Gastroenterology (2016) 150(4):835–53. doi: 10.1053/j.gastro.2015.12.041
6. Sun HC, Zhou J, Wang Z, Liu X, Xie Q, Jia W, et al. Chinese expert consensus on conversion therapy for hepatocellular carcinoma (2021 edition). Hepatobiliary Surg Nutr (2022) 11(2):227–52. doi: 10.21037/hbsn-21-328
7. Tang H, Cao Y, Jian Y, Li X, Li J, Zhang W, et al. Conversion therapy with an immune checkpoint inhibitor and an antiangiogenic drug for advanced hepatocellular carcinoma: a review. Biosci Trends (2022) 16(2):130–41. doi: 10.5582/bst.2022.01019
8. Al-Salama ZT, Syed YY, Scott LJ. Lenvatinib: a review in hepatocellular carcinoma. Drugs (2019) 79(6):665–74. doi: 10.1007/s40265-019-01116-x
9. Matsuki M, Hoshi T, Yamamoto Y, Ikemori-Kawada M, Minoshima Y, Funahashi Y, et al. Lenvatinib inhibits angiogenesis and tumor fibroblast growth factor signaling pathways in human hepatocellular carcinoma models. Cancer Med (2018) 7(6):2641–53. doi: 10.1002/cam4.1517
10. Tohyama O, Matsui J, Kodama K, Hata-Sugi N, Kimura T, Okamoto K, et al. Antitumor activity of lenvatinib (e7080): an angiogenesis inhibitor that targets multiple receptor tyrosine kinases in preclinical human thyroid cancer models. J Thyroid Res (2014) 2014:638747. doi: 10.1155/2014/638747
11. Finn RS, Ikeda M, Zhu AX, Sung MW, Baron AD, Kudo M, et al. Phase ib study of lenvatinib plus pembrolizumab in patients with unresectable hepatocellular carcinoma. J Clin Oncol (2020) 38(26):2960–70. doi: 10.1200/JCO.20.00808
12. Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med (2020) 382(20):1894–905. doi: 10.1056/NEJMoa1915745
13. Xu J, Shen J, Gu S, Zhang Y, Wu L, Wu J, et al. Camrelizumab in combination with apatinib in patients with advanced hepatocellular carcinoma (RESCUE): a nonrandomized, open-label, phase II trial. Clin Cancer Res (2021) 27(4):1003–11. doi: 10.1158/1078-0432.CCR-20-2571
14. Ren Z, Xu J, Bai Y, Xu A, Cang S, Du C, et al. Sintilimab plus a bevacizumab biosimilar (IBI305) versus sorafenib in unresectable hepatocellular carcinoma (ORIENT-32): a randomised, open-label, phase 2-3 study. Lancet Oncol (2021) 22(7):977–90. doi: 10.1016/S1470-2045(21)00252-7
15. Kudo M, Ikeda M, Motomura K, Okusaka T, Kato N, Dutcus CE, et al. A phase ib study of lenvatinib (LEN) plus nivolumab (NIV) in patients (Pts) with unresectable hepatocellular carcinoma (uHCC): study 117. J Clin Oncol (2020) 38(4_suppl):513–3. doi: 10.1200/JCO.2020.38.4_suppl.513
16. Zhu AX, Finn RS, Ikeda M, Sung MW, Baron AD, Kudo M, et al. A phase ib study of lenvatinib (LEN) plus pembrolizumab (PEMBRO) in unresectable hepatocellular carcinoma (uHCC). J Clin Oncol (2020) 38(2020):4519. doi: 10.1200/JCO.2020.38.15_suppl.4519
17. Zhang W, Lu S, Hu B, Wan T, Wang H, Han J, et al. PD-1 inhibitor combined with lenvatinib for unresectable liver cancer as the conversion therapy: an open-label, nonrandomized, phase IV study. J Clin Oncol (2021) 39(suppl 15):e16173. doi: 10.1200/JCO.2021.39.15_suppl.e16173
18. Zhang W, Hu B, Han J, Wang Z, Ma G, Ye H, et al. Surgery after conversion therapy with PD-1 inhibitors plus tyrosine kinase inhibitors are effective and safe for advanced hepatocellular carcinoma: a pilot study of ten patients. Front Oncol (2021) 11:747950. doi: 10.3389/fonc.2021.747950
19. Zhu XD, Huang C, Shen YH, Ji Y, Ge NL, Qu XD, et al. Downstaging and resection of initially unresectable hepatocellular carcinoma with tyrosine kinase inhibitor and anti-PD-1 antibody combinations. Liver Cancer (2021) 10(4):320–9. doi: 10.1159/000514313
20. Zhang W, Hu B, Han J, Wang H, Wang Z, Ye H, et al. A real-world study of PD-1 inhibitors combined with TKIs for HCC with major vascular invasion as the conversion therapy: a prospective, non-randomized, open-label cohort study. Ann Oncol (2020) 31:S1307. doi: 10.1016/j.annonc.2020.10.195
21. Yarchoan M, Zhu Q, Durham JN, Gross N, Charmsaz S, Leatherman JM, et al. Feasibility and efficacy of neoadjuvant cabozantinib and nivolumab in patients with borderline resectable or locally advanced hepatocellular carcinoma (HCC). J Clin Oncol (2021) 39(3_suppl):335. doi: 10.1200/JCO.2021.39.3_suppl.335
22. Lencioni R, Llovet JM. (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis (2010) 30(1):52–60. doi: 10.1055/s-0030-1247132
23. Llovet JM, Lencioni R. mRECIST for HCC: performance and novel refinements. J Hepatol (2020) 72(2):288–306. doi: 10.1016/j.jhep.2019.09.026
24. Hong CM, Ahn BC, Jang YJ, Jeong SY, Lee SW, Lee J. Prognostic value of metabolic parameters of 18F-FDG PET/CT and apparent diffusion coefficient of MRI in hepatocellular carcinoma. Clin Nucl Med (2017) 42(2):95–9. doi: 10.1097/RLU.0000000000001478
25. Kim YI, Paeng JC, Cheon GJ, Suh KS, Lee DS, Chung JK, et al. Prediction of posttransplantation recurrence of hepatocellular carcinoma using metabolic and volumetric indices of 18F-FDG PET/CT. J Nucl Med (2016) 57(7):1045–51. doi: 10.2967/jnumed.115.170076
26. Tian F, Shen G, Deng Y, Diao W, Jia Z. The accuracy of (18)F-FDG PET/CT in predicting the pathological response to neoadjuvant chemotherapy in patients with breast cancer: a meta-analysis and systematic review. Eur Radiol (2017) 27(11):4786– 96. doi: 10.1007/s00330-017-4831-y
27. Vos JL, Zuur CL, Smit LA, de Boer JP, Al-Mamgani A, van den Brekel MWM, et al. [(18)F]FDG-PET accurately identifies pathological response early upon neoadjuvant immune checkpoint blockade in head and neck squamous cell carcinoma. Eur J Nucl Med Mol Imaging (2022) 49(6):2010–22. doi: 10.1007/s00259-021-05610-x
28. Rufini V, Collarino A, Calcagni ML, Meduri GM, Fuoco V, Pasciuto T, et al. The role of (18)F-FDG-PET/CT in predicting the histopathological response in locally advanced cervical carcinoma treated by chemo-radiotherapy followed by radical surgery: a prospective study. Eur J Nucl Med Mol Imaging (2020) 47(5):1228–38. doi: 10.1007/s00259-019-04436-y
29. Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the american association for the study of liver diseases. Hepatology (2018) 68(2):723–50. doi: 10.1002/hep.29913
30. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer (2009) 45(2):228–47. doi: 10.1016/j.ejca.2008.10.026
31. Institute NC. Common terminology criteria for adverse events. Available at: http://ctepcancergov/protocolDevelopment/electronic_applications/ctchtm. (Accessed 14 Jun 2011).
32. Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 noninferiority trial. Lancet (2018) 391(10126):1163–73. doi: 10.1016/S0140-6736(18)30207-1
33. Wang G, Zhang W, Chen J, Luan X, Wang Z, Wang Y, et al. Pretreatment metabolic parameters measured by (18)F-FDG PET to predict the pathological treatment response of HCC patients treated with PD-1 inhibitors and lenvatinib as a conversion therapy in BCLC stage c. Front Oncol (2022) 12:884372. doi: 10.3389/fonc.2022.884372
34. Stein JE, Lipson EJ, Cottrell TR, Forde PM, Anders RA, Cimino-Mathews A, et al. Pan-tumor pathologic scoring of response to PD-(L)1 blockade. Clin Cancer Res (2020) 26(3):545–51. doi: 10.1158/1078-0432.CCR-19-2379
35. Xia Y, Tang W, Qian X, Li X, Cheng F, Wang K, et al. Efficacy and safety of camrelizumab plus apatinib during the perioperative period in resectable hepatocellular carcinoma: a single-arm, open label, phase II clinical trial. J Immunother Cancer (2022) 10:e004656. doi: 10.1136/jitc-2022-004656
36. Lim C, Salloum C, Chalaye J, Lahat E, Costentin CE, Osseis M, et al. 18F-FDG PET/CT predicts microvascular invasion and early recurrence after liver resection for hepatocellular carcinoma: a prospective observational study. HPB (Oxford) (2019) 21(6):739–47. doi: 10.1016/j.hpb.2018.10.007
37. Allard MA, Sebagh M, Ruiz A, Guettier C, Paule B, Vibert E, et al. Does pathological response after transarterial chemoembolization for hepatocellular carcinoma in cirrhotic patients with cirrhosis predict outcome after liver resection or transplantation? J Hepatol (2015) 63(1):83–92. doi: 10.1016/j.jhep.2015.01.023
38. Yang Y, Dang Z, Lu P, Qian Y, Lin K, Pan Z, et al. Impact of pathological response after preoperative transcatheter arterial chemoembolization (TACE) on incidences of microvascular invasion and early tumor recurrence in hepatocellular carcinoma: a multicenter propensity score matching analysis. Hepatobiliary Surg Nutr (2022) 11(3):386–99. doi: 10.21037/hbsn-20-700
39. Hellmann MD, Chaft JE, William WN Jr, Rusch V, Pisters KM, Kalhor N, et al. Pathological response after neoadjuvant chemotherapy in resectable non-small-cell lung cancers: proposal for the use of major pathological response as a surrogate endpoint. Lancet Oncol (2014) 15(1):e42–50. doi: 10.1016/S1470-2045(13)70334-6
40. Truty MJ, Kendrick ML, Nagorney DM, Smoot RL, Cleary SP, Graham RP, et al. Factors predicting response, perioperative outcomes, and survival following total neoadjuvant therapy for Borderline/Locally advanced pancreatic cancer. Ann Surg (2021) 273(2):341–9. doi: 10.1097/SLA.0000000000003284
41. Stein JE, Soni A, Danilova L, Cottrell TR, Gajewski TF, Hodi FS, et al. Major pathologic response on biopsy (MPRbx) in patients with advanced melanoma treated with anti-PD-1: evidence for an early, on-therapy biomarker of response. Ann Oncol (2019) 30(4):589–96. doi: 10.1093/annonc/mdz019
42. Edeline J, Boucher E, Rolland Y, Vauléon E, Pracht M, Perrin C, et al. Comparison of tumor response by response evaluation criteria in solid tumors (RECIST) and modified RECIST in patients treated with sorafenib for hepatocellular carcinoma. Cancer (2012) 118(1):147–56. doi: 10.1002/cncr.26255
43. Vicentin I, Mosconi C, Garanzini E, Sposito C, Serenari M, Buscemi V, et al. Inter-center agreement of mRECIST in transplanted patients for hepatocellular carcinoma. Eur Radiol (2021) 31(12):8903–12. doi: 10.1007/s00330-021-08088-1
44. Song MJ, Bae SH, Lee SW, Song DS, Kim HY, Yoo IeR, et al. 18F-fluorodeoxyglucose PET/CT predicts tumour progression after transarterial chemoembolization in hepatocellular carcinoma. Eur J Nucl Med Mol Imaging (2013) 40(6):865–73. doi: 10.1007/s00259-013-2366-2
45. Kucuk ON, Soydal C, Araz M, Bilgic S, Ibis E. Prognostic importance of 18F-FDG uptake pattern of hepatocellular cancer patients who received SIRT. Clin Nucl Med (2013) 38(7):e283–289. doi: 10.1097/RLU.0b013e3182867f17
46. Song MJ, Bae SH, Yoo leR, Park CH, Jang JW, Chun HJ, et al. Predictive value of (1)(8)F-fluorodeoxyglucose PET/CT for transarterial chemolipiodolization of hepatocellular carcinoma. World J Gastroenterol (2012) 18(25):3215–22. doi: 10.3748/wjg.v18.i25.3215
47. Lopci E, Toschi L, Grizzi F, Rahal D, Olivari L, Castino GF, et al. Correlation of metabolic information on FDG-PET with tissue expression of immune markers in patients with non-small cell lung cancer (NSCLC) who are candidates for upfront surgery. Eur J Nucl Med Mol Imaging (2016) 43(11):1954–61. doi: 10.1007/s00259-016-3425-2
48. Wofford JA, Wieman HL, Jacobs SR, Zhao Y, Rathmell JC. IL-7 promotes Glut1 trafficking and glucose uptake via STAT5-mediated activation of akt to support t-cell survival. Blood (2008) 111(4):2101–11. doi: 10.1182/blood-2007-06-096297
49. Itoh S, Yoshizumi T, Kitamura Y, Yugawa K, Iseda N, Shimagaki T, et al. Impact of metabolic activity in hepatocellular carcinoma: association with immune status and vascular formation. Hepatol Commun (2021) 5(7):1278–89. doi: 10.1002/hep4.1715
50. Kawamura Y, Kobayashi M, Shindoh J, Kobayashi Y, Kasuya K, Sano T, et al. (18)F-fluorodeoxyglucose uptake in hepatocellular carcinoma as a useful predictor of an extremely rapid response to lenvatinib. Liver Cancer (2020) 9(1):84–92. doi: 10.1159/000503577
51. Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S, et al. Hepatocellular carcinoma. Nat Rev Dis Primers (2021) 7(1):6. doi: 10.1038/s41572-020-00240-3
52. Valerio L, Guidoccio F, Giani C, Tardelli E, Puccini G, Puleo L, et al. [18F]-FDG-PET/CT correlates with the response of radiorefractory thyroid cancer to lenvatinib and patient survival. J Clin Endocrinol Metab (2021) 106(8):2355–66. doi: 10.1210/clinem/dgab278
53. Shao D, Cheng Y, Yuan ZS, Jiang BY, Wang SX. Value of interim (18)F-FDG PET/CT for predicting progression-free survival in stage B/IV EGFR-mutant non-small-cell lung cancer patients with EGFR-TKI therapy. Lung Cancer (2020) 149:137–43. doi: 10.1016/j.lungcan.2020.09.020
54. Sachpekidis C, Kopp-Schneider A, Pan L, Papamichail D, Haberkorn U, Hassel JC. Interim [(18)F]FDG PET/CT can predict response to anti-PD-1 treatment in metastatic melanoma. Eur J Nucl Med Mol Imaging (2021) 48(6):1932–43. doi: 10.1007/s00259-020-05137-7
55. Ito K, Teng R, Schöder H, Humm JL, Ni A, Michaud L, et al. (18)F-FDG PET/CT for monitoring of ipilimumab therapy in patients with metastatic melanoma. J Nucl Med (2019) 60(3):335–41. doi: 10.2967/jnumed.118.213652
56. Li SH, Wei W, Guo RP, Shi M, Guo ZX, Chen ZY, et al. Long-term outcomes after curative resection for patients with macroscopically solitary hepatocellular carcinoma without macrovascular invasion and an analysis of prognostic factors. Med Oncol (2013) 30(4):696. doi: 10.1007/s12032-013-0696-3
57. Sun J, Guo R, Bi X, Wu M, Tang Z, Lau WY, et al. Guidelines for diagnosis and treatment of hepatocellular carcinoma with portal vein tumor thrombus in china (2021 edition). Liver Cancer (2022) 11(4):315–28. doi: 10.1159/000523997
58. Li N, Feng S, Xue J, Wei XB, Shi J, Guo WX, et al. Hepatocellular carcinoma with main portal vein tumor thrombus: a comparative study comparing hepatectomy with or without neoadjuvant radiotherapy. HPB (Oxford) (2016) 18(6):549–56. doi: 10.1016/j.hpb.2016.04.003
59. Wei X, Jiang Y, Zhang X, Feng S, Zhou B, Ye X, et al. Neoadjuvant three-dimensional conformal radiotherapy for resectable hepatocellular carcinoma with portal vein tumor thrombus: a randomized, open-label, multicenter controlled study. J Clin Oncol (2019) 37(24):2141–51. doi: 10.1200/JCO.18.02184
60. Huang C, Zhu XD, Shen YH, Wu D, Ji Y, Ge NL, et al. Organ specific responses to first-line lenvatinib plus anti-PD-1 antibodies in patients with unresectable hepatocellular carcinoma: a retrospective analysis. biomark Res (2021) 9(1):19. doi: 10.1186/s40364-021-00274-z
61. Ahn SJ, Park MS, Kim KA, Park JY, Kim I, Kang WJ, et al. (1)(8)F-FDG PET metabolic parameters and MRI perfusion and diffusion parameters in hepatocellular carcinoma: a preliminary study. PloS One (2013) 8(8):e71571. doi: 10.1371/journal.pone.0071571
62. Lee JW, Hwang SH, Kim DY, Han KH, Yun M. Prognostic value of FDG uptake of portal vein tumor thrombosis in patients with locally advanced hepatocellular carcinoma. Clin Nucl Med (2017) 42(1):e35–40. doi: 10.1097/RLU.0000000000001422
63. Zhu XD, Huang C, Shen YH, Xu B, Ge NL, Ji Y, et al. Hepatectomy after conversion therapy using tyrosine kinase inhibitors plus anti-PD-1 antibody therapy for patients with unresectable hepatocellular carcinoma. Ann Surg Oncol (2023) 30(5):2782–90. doi: 10.1245/s10434-022-12530-z
64. Xu B, Zhu XD, Shen YH, Zhu JJ, Liu J, Li ML, et al. Criteria for identifying potentially resectable patients with initially oncologically unresectable hepatocellular carcinoma before treatment with lenvatinib plus an anti-PD-1 antibody. Front Immunol (2022) 13:1016736. doi: 10.3389/fimmu.2022.1016736
65. Yi Y, Sun BY, Weng JL, Zhou C, Zhou CH, Cai MH, et al. Lenvatinib plus anti-PD-1 therapy represents a feasible conversion resection strategy for patients with initially unresectable hepatocellular carcinoma: a retrospective study. Front Oncol (2022) 12:1046584. doi: 10.3389/fonc.2022.1046584
Keywords: unresectable hepatocellular carcinoma, conversion therapy, major pathological response, prognosis, 18F-FDG PET
Citation: Wang G, Zhang W, Luan X, Wang Z, Liu J, Xu X, Zhang J, Xu B, Lu S, Wang R and Ma G (2023) The role of 18F−FDG PET in predicting the pathological response and prognosis to unresectable HCC patients treated with lenvatinib and PD-1 inhibitors as a conversion therapy. Front. Immunol. 14:1151967. doi: 10.3389/fimmu.2023.1151967
Received: 27 January 2023; Accepted: 25 April 2023;
Published: 05 May 2023.
Edited by:
Takaji Matsutani, Repertoire Genesis, Inc., JapanReviewed by:
Xiaoli Lan, Huazhong University of Science and Technology, ChinaHongcheng Shi, Fudan University, China
Copyright © 2023 Wang, Zhang, Luan, Wang, Liu, Xu, Zhang, Xu, Lu, Wang and Ma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guangyu Ma, bWd5MzAxQDE2My5jb20=; Ruimin Wang, d3JtQHllYWgubmV0; Shichun Lu, bHVzY18zMDFAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Guanyun Wang
Guanyun Wang Wenwen Zhang
Wenwen Zhang Xiaohui Luan
Xiaohui Luan Zhanbo Wang
Zhanbo Wang Jiajin Liu
Jiajin Liu Xiaodan Xu
Xiaodan Xu Jinming Zhang1
Jinming Zhang1 Baixuan Xu
Baixuan Xu Shichun Lu
Shichun Lu Ruimin Wang
Ruimin Wang Guangyu Ma
Guangyu Ma