- 1Department of Medical Service, The First Affiliated Hospital of Anhui Medical University, Hefei, China
- 2Department of Medical Service, The Second Affiliated Hospital of Anhui Medical University, Hefei, China
- 3Department of Epidemiology and Biostatistics, School of Public Health, Anhui Medical University, Hefei, Anhui, China
- 4Department of Human Resource, The First Affiliated Hospital of Anhui Medical University, Hefei, China
- 5School of Health Services Management, Anhui Medical University, Hefei, Anhui, China
- 6Party Committee Office, The Second Affiliated Hospital of Anhui Medical University, Hefei, China
Background: Previous observational studies have reported the striking association between ankylosing spondylitis (AS) and psoriasis, but the causal relationship between the two diseases remains unclear.
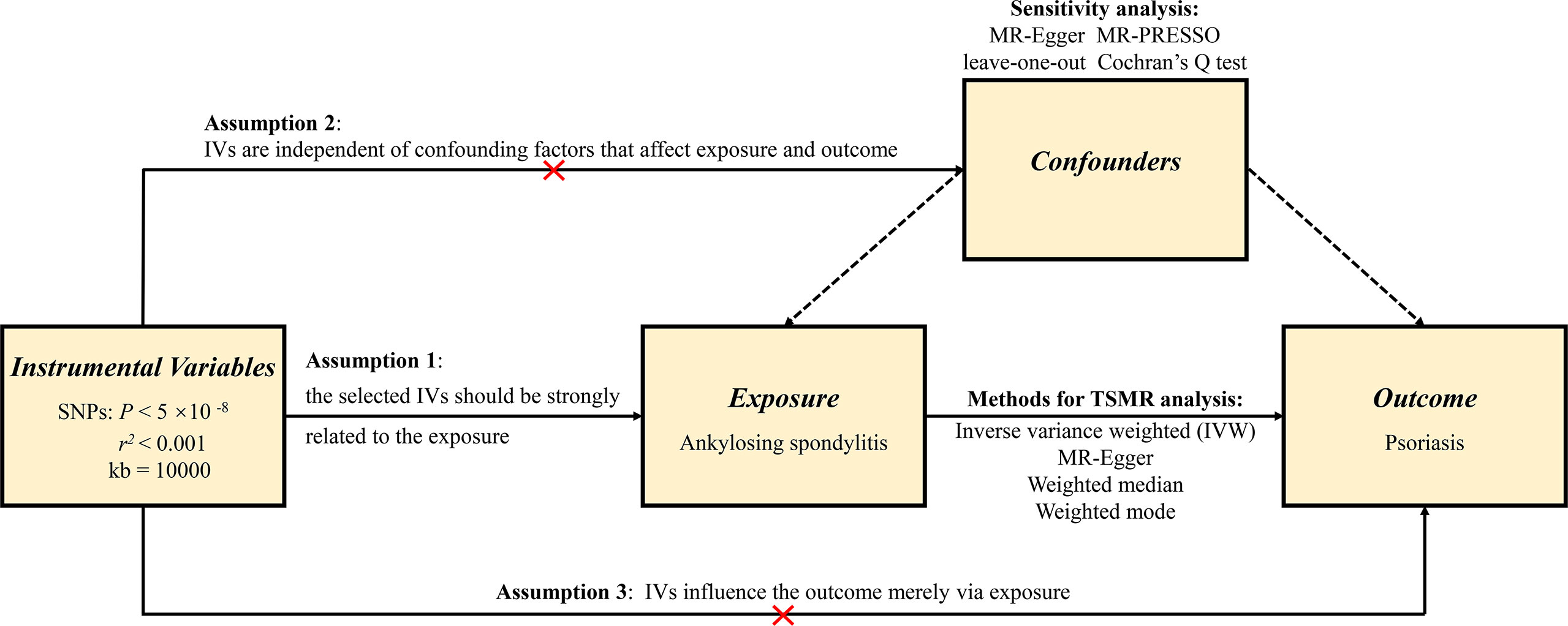
Methods: Two-sample Mendelian randomization (MR) analysis with methods of inverse-variance weighted, MR-Egger regression, weighted median, and weighted mode was conducted to evaluate the bidirectional causal associations between AS and psoriasis. Effective single-nucleotide polymorphisms (SNPs) from genome-wide association studies (GWAS) were selected as instrumental variables (IVs). Sensitivity analyses were also applied to verify whether heterogeneity and pleiotropy can bias the results.
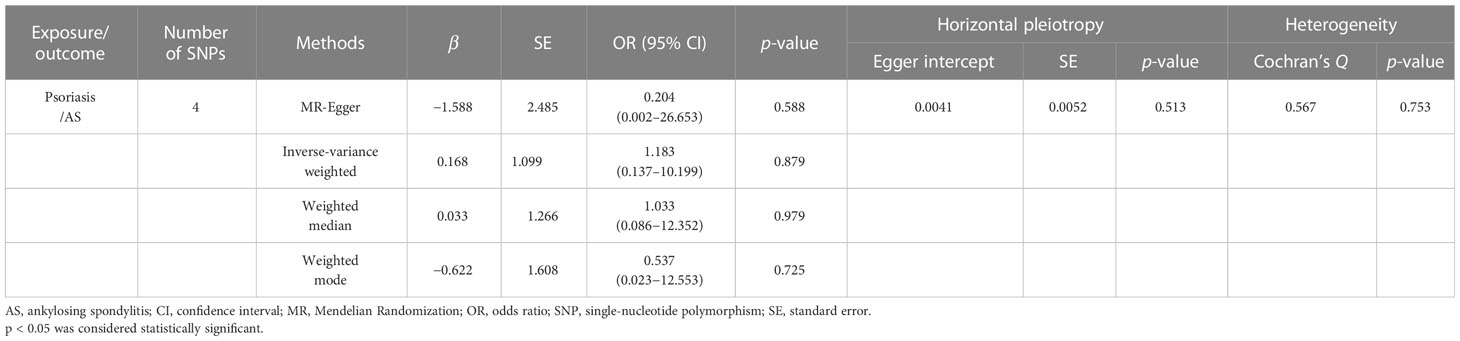
Result: We found positive causal effects of genetically increased AS risk on psoriasis (IVW: OR = 1.009, 95% CI = 1.005–1.012, p = 8.07E-07). Comparable outcomes were acquired by MR-Egger regression, weighted median, and weighted mode approaches. Nevertheless, we did not find significant causal effects of psoriasis on AS (IVW: OR = 1.183, 95% CI = 0.137–10.199, p = 0.879). The sensitivity analyses showed that the horizontal pleiotropy was unlikely to skew the causality. The leave-one-out analysis demonstrated that no single SNP can drive the MR estimates. No evidence of heterogeneity was found between the selected IVs.
Conclusion: Our findings provide evidence that AS has positive causal effects on the risk of psoriasis in the European population.
Introduction
Ankylosing spondylitis (AS) is an immune-related chronic inflammatory disorder, which mainly involves the spine and the sacroiliac joints and arouses pain and stiffness of these joints (1). AS affects approximately 0.1%–0.5% of the general population worldwide (2). Although the nosogenesis of AS remains largely unknown, numerous evidence has revealed that the interactions of genetic inheritance, environmental exposure, chronic infections, and abnormal endocrine contribute to the occurrence and development of the disease (3, 4).
Psoriasis is a common inflammatory papulosquamous skin disorder, which is caused by the interplay of genetic, immunological, and environmental factors with a wide range of clinical manifestations (5, 6). Psoriasis can occur at any age and is associated with several comorbidities, such as lymphoma and depression (7). WTO recognized psoriasis as a “chronic, non-communicable, painful, disfiguring, and disabling disorder for which there is no cure” (8). It has been estimated that over 60 million adults and children around the world are affected by psoriasis, imposing a huge burden on individuals and society (8).
The parallels between AS and psoriasis has long been recognized. For both diseases, heritability is the main risk determinant, and inflammatory cytokines, such as interleukin (IL)-17, IL-23, and tumor necrosis factor (TNF)-α are key drivers, indicating that they might share similar pathogenesis (9, 10). There is a striking association between AS and psoriasis. A variety of cross-sectional and cohort studies reported that AS patients were more likely to develop psoriasis than heathy controls (11, 12). The prevalence of psoriasis in AS was estimated at 9.3% according to a previous meta-analysis (13). Moreover, it has been suggested that psoriasis may predate the onset or diagnosis of AS (14). Studies have reported that patients with AS combined with psoriasis have higher disease activity scores (15). Notably, the hallmarks of psoriasis are the inflammation of skin and joints (16). Nevertheless, the causal relationship between the two conditions is still ambiguous, which warrants further research to increase the current understanding of the pathogeneses of these two diseases.
Mendelian randomization (MR) is a developing popular approach, which usually serves genetic variants as instrumental variables (IVs) to infer whether there is causal relationship between the exposure and the outcome (17). Since the genetic variations are stochastically arranged in meiosis and fixed after fertilization, this technology enables to conquer several shortcomings of traditional observational studies, including the interference of confounding factors, reverse causation, and selection biases.
In this current study, a bidirectional two-sample MR analysis was conducted, in order to determine whether there is a significant causal relationship between AS and psoriasis risk.
Materials and methods
Data sources
Single-nucleotide polymorphisms (SNPs), which were selected from the publicly available genome-wide association studies (GWAS) database, were severed as IVs for exposure (AS) and outcome (psoriasis). No additional ethical statement or consent was therefore required. SNPs that associated with AS were extracted from a large AS GWAS conducted by the International Genetics of Ankylosing Spondylitis Consortium (IGAS), including 9,069 cases and 13,578 controls of European ancestry (https://gwas.mrcieu.ac.uk/datasets/ebi-a-GCST005529) (18). The diagnosis of AS was based on the revised New York criteria of 1984 (19). The summary-level data on the effect of the AS-associated SNPs on psoriasis were derived from another independent GWAS by Neale laboratory, which involved 3,871 cases and 333,288 controls of European ancestry (https://gwas.mrcieu.ac.uk/datasets/ukb-a-100). Psoriasis was diagnosed by professional physicians through pattern recognition that was careful morphologic assessment of the skin lesions (20).
SNP selection
To guarantee the correctness and truth of the conclusions on the causality between AS and psoriasis risk, a series of quality control steps were carried out to select effective IVs. We selected the SNPs with a genome-wide significant association (p < 5E-08), and without linkage disequilibrium (LD) (r2 < 0.001, clumping distance = 10,000 kb) in summary statistics. When the exposure-related SNPs were not present in the outcome dataset, the proxy SNPs significantly correlated with the SNPs of interest were used (r2 > 0.8). To make sure that the impact of SNPs on exposure corresponds to the same allele as the impact on the outcome, palindromic SNPs with intermediate allele frequencies were not included in IVs. SNPs with the minor allele frequency (MAF) less than 0.01 were also excluded. Finally, eligible SNPs were selected as valid IVs for the final model.
MR assumptions
In order to minimize the bias on the outcomes, the MR analysis should comply with three pivotal assumptions (21). First, the IVs that were selected must be robustly related to the exposure. In the current study, the strength of the association between the IVs and the exposure was assessed by F value, which is expressed as R2 (n−k−1)/k (1−R2) (R2: the cumulative interpreted variance of chosen SNPs on the exposure, k: the number of chosen SNPs, n: the sample size). If F is greater than 10, the correlation is regarded as strong enough and enable to avert bias brought by weak IVs. Second, IVs need to be independent of confounding factors that can affect both exposure and outcome. Third, IVs should affect the outcome merely by exposure, which requires that there is no horizontal pleiotropy effect between the IVs and outcome. Figure 1 shows an overview of the study design.
Effect size estimate
We performed two-sample MR analysis to evaluate the causal relationship between exposure (AS) and outcome (psoriasis). Multiple complementary methods were applied, including the inverse variance weighted (IVW), the MR-Egger regression, the weighted median, and the weighted mode approaches. IVW is constitutionally a meta-analysis method, which requires all IVs to be valid and combines the Wald ratios of the causal effects of each SNP. In the absence of horizontal pleiotropy, IVW is perceived as the primary method because it provides the most accurate estimates (22). MR-Egger regression conducts a weighted linear regression and produces consistent causal estimates, even if the selected IVs are all invalid. Nevertheless, its accuracy is relatively low (23). The weighted median supplies reliable effect estimates of causal effects when less than 50% of the weight in the analysis comes from invalid IVs (24). The weighted median method possesses several important advantages over the MR-Egger regression, since it has lower type I error and higher causal estimate power. Additionally, the weighted mode approach estimates the causal effects of the subset with the maximum number of SNPs by clustering SNPs into subsets with similar causal effects (25).
Sensitivity analysis
To rule out possible breaches of the MR assumptions, multiple sensitivity analyses were conducted to verify whether pleiotropy and heterogeneity in the genetic instruments tested could bias the MR outcomes. Pleiotropy is the phenomenon that a single locus influences plural phenotypes. Horizontal pleiotropy occurs when a genetic variable is correlated with more than one phenotype by separatory pathways, which can distort the outcomes of MR analysis. Herein, we performed the MR-Egger regression and evaluated the intercept, in order to detect as well as adjust for the potential horizontal pleiotropic effects within the selected IVs (assumption 3) (26). The MR pleiotropy residual sum and outlier (MR-PRESSO) test was also performed to detect pleiotropy, exclude outliers, and reappraise the effect estimates (assumption 3) (27). Cochran’s Q statistic was used in order to quantify the heterogeneity within the selected IVs (assumption 2) (28). To make sure that the MR conclusions are not affected by certain IVs, the leave-one-out sensitivity analysis was performed by removing each SNP in turn when conducting the MR analysis (assumption 2).
A two-sided p-value lower than 0.05 was considered as statistically significant. All data analyses were completed by the “TwoSampleMR” and “MRPRESSO” packages of the R 4.2.2 software (www.r-project.org).
Results
Genetic instruments selection
Initially, a total of 1,036 genome-wide significant SNPs were identified (p < 5E-08) from the GWAS study conducted by Cortes et al. (18). After removing SNPs that had LD effects or were not available in the summary statistic of outcome, 16 SNPs explaining 1.85% of the variance for AS were selected as IVs for the further two-sample MR analysis. The detailed information of these SNPs including effect allele, other allele, beta, SE, and p-value are summarized in Supplementary Table S1. There was no LD (r2 < 0.001) between these SNPs, and the F statistics were all greater than 10 with the average F value of 134.872 for AS, indicating that the selected IVs conformed to the intense correlation assumption of MR, and the instrument bias was weak, which cannot significantly affect the estimation of MR.
Two-sample MR analysis
The IVW result demonstrated that the per-unit increase in the log-odds of developing AS was significantly associated with an increased risk of having psoriasis at p less than 0.05 (OR = 1.009, 95% CI = 1.005–1.012, p = 8.07E-07). In addition, MR-Egger (OR = 1.011, 95% CI = 1.006–1.016, p = 1.19E-03), weighted median (OR = 1.010, 95% CI = 1.006–1.013, p = 2.14E-09), and weighted mode (OR = 1.010, 95% CI = 1.007–1.013, p = 2.90E-05) also provided consistent results that AS had a positive causal effect on psoriasis risk (Table 1 and Figure 2A).
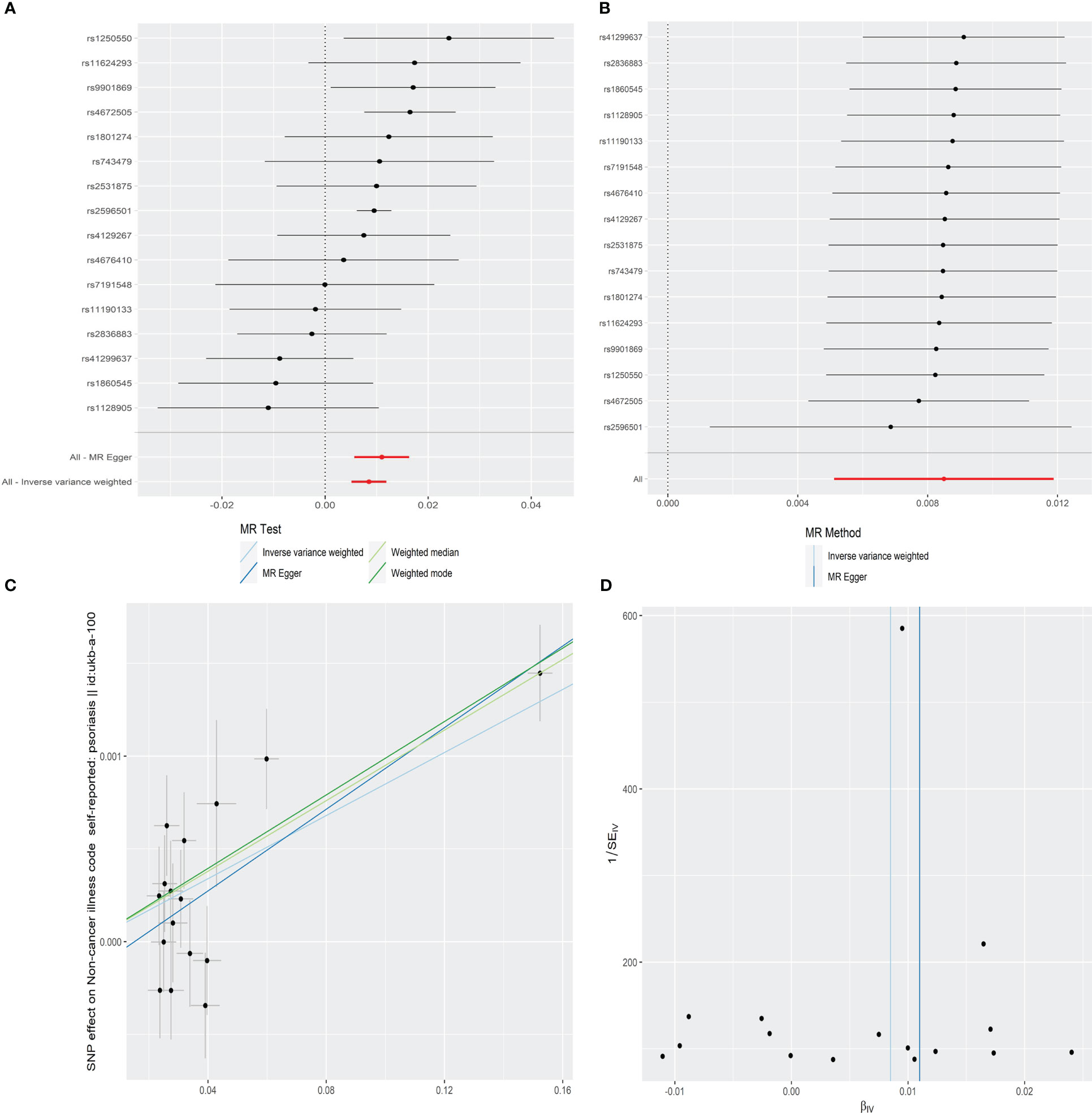
Figure 2 Forest plot (A), sensitivity analysis (B), scatter plot (C), and funnel plot (D) of the effect of AS on psoriasis.
The leave-one-out sensitivity analysis showed that there was no single SNP driving the MR estimates (Figure 2B). Furthermore, the horizontal pleiotropy between IVs and outcome was evaluated by MR-Egger regression, and the result suggested that the horizontal pleiotropy was unlikely to skew the causal relationship between AS and psoriasis (intercept = −0.0002, SE = 0.0001, p = 0.257) (Table 1 and Figure 2C). The MR-PRESSO global test also found no outlier SNPs or a horizontal pleiotropic effect of AS on the risk of psoriasis (p = 0.115). Cochran’s Q test observed no significant heterogeneity among the selected IVs (Q = 22.350, p = 0.072) (Table 1 and Figure 2D).
Further analyses
In order to explore the causal effect of psoriasis on AS risk, we further carried out the two-sample MR analysis by using psoriasis as exposure and AS as outcome. There were 21 significant and independent SNPs (p < 5E-08, r2 < 0.001) correlated with the risk of psoriasis, which were derived from the large-scale psoriasis GWAS by the Neale laboratory. However, 17 SNPs were excluded because of no corresponding outcomes in AS. Thus, four SNPs were finally selected as the IVs for the MR analysis (Supplementary Table S2). The F statistics of these SNPs were all larger than 10 with the average F value of 46.406 for psoriasis, indicating the absence of weak IV bias. According to IVW, MR-Egger, weighted median, and weighted mode approaches, there was no evidence showing causal association of an increased risk of psoriasis with the change in the risk of AS (Table 2 and Figure 3A). Sensitivity analysis using the leave-one-out method also proved no significant causal relationship of the psoriasis and AS risk (Figure 3B). No significantly horizontal pleiotropy was detected by MR-Egger regression (intercept = 0.0041, SE = 0.0052, p = 0.513) (Table 2 and Figure 3C). The MR-PRESSO global test also observed no outlier SNPs or a horizontal pleiotropic effect of psoriasis on the risk of AS (p = 0.718). Additionally, Cochran’s Q test indicated that there was no significant heterogeneity across estimates of included SNPs (MR-Egger: Q = 0.567, p = 0.753) (Table 2 and Figure 3D).
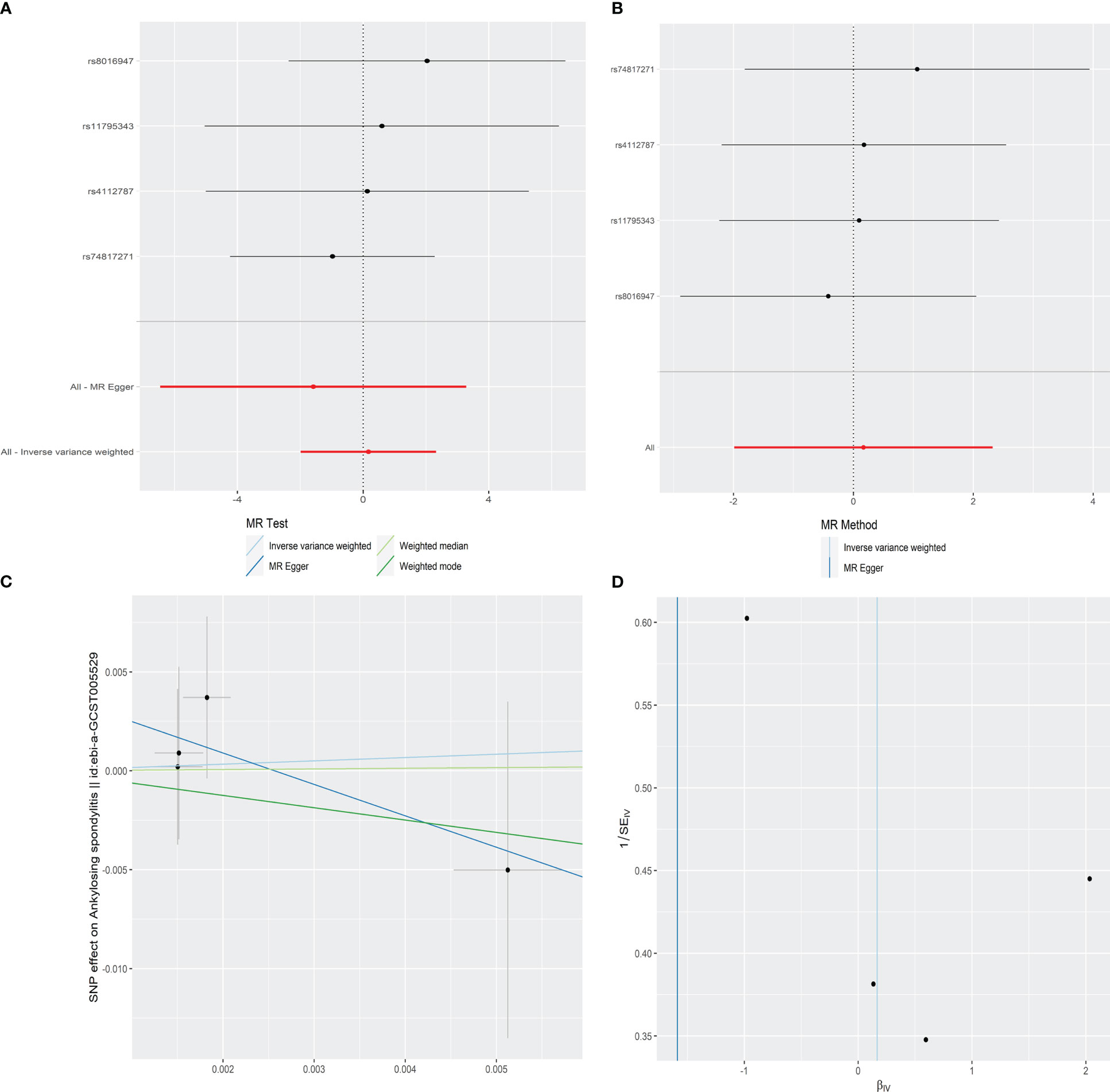
Figure 3 Forest plot (A), sensitivity analysis (B), scatter plot (C), and funnel plot (D) of the effect of psoriasis on AS.
Discussion
This is the first study using MR method and integrating large-scale GWAS data to investigate the bi-directional causal relationship between AS and psoriasis. Our findings implied that AS had positive causal effects on the risk of psoriasis, but did not show that psoriasis had positive causal effects on the risk of AS in individuals from European descent. Suffering from AS would be the causal element of the increased risk of psoriasis, indicating that the two diseases might share similar pathogenesis.
AS and psoriasis are featured by disturbances in systemic or organ-specific immune system regulatory pathways that lead to uncontrolled inflammation. These “seronegative” disorders are clinically closely related and often co-occur in both patients and families. Despite the fact that the exact mechanisms linking AS and psoriasis are not fully explained, the hyperactivity of the IL-17/IL-23 cytokine axis was proposed to account for the pathogenic association (29, 30). Numerous studies have demonstrated increased levels of IL-17 and IL-23 in the serum and synovium of AS patients, as well as the skin of psoriasis patients (31, 32). Inhibitors targeting IL-17 have shown positive effects in the treatment of AS and psoriasis (33, 34). Furthermore, the overlap between the two diseases in genetically identified pathogenic pathways has also been verified, such as the polymorphisms of ERAP1 and HLA-class I genes (35, 36).
The high risk of psoriasis in AS patients has been observed in previous epidemiological studies. In a large retrospective cohort study conducted using data from the Clinical Practice Research Datalink (CPRD) during 1987–2012, Stolwijk et al. (11) demonstrated that 4.4% of patients had psoriasis at the time of diagnosis of AS, and the incidence of psoriasis was 3.4 per 1,000 person-years. Furthermore, with the 12-year follow-up data from the Outcome in Ankylosing Spondylitis International Study (OASIS), Essers et al. (37) found that psoriasis was present in 4.1% of the 216 AS patients at baseline and psoriasis was not associated with longer disease duration. Hence, they speculated that psoriasis may already be present before the onset or diagnosis of AS (37). Given the presence of confounders in observational studies, it is unable to determine which comes first, the psoriasis or AS, and whether there is a causal relationship between the two diseases. The findings of our study provided new information on it. Briefly, AS had a causal effect on psoriasis, but psoriasis was not the cause of AS, which provided a theoretical basis for paying attention to skin involvement in AS patients.
The causal effect of AS on psoriasis is of great significance for the diagnosis, treatment and prognosis of AS. AS has the longest delay in diagnosis among rheumatic diseases, ranging from 5 to 10 years, because the diagnostic criteria of AS depend on radiographic sacroiliitis, which usually appears in the late stage of the disease (38). The presence of psoriasis increases the probability of AS in patients with chronic back pain and may contribute to the diagnosis of the disease. Furthermore, there are conflicting results regarding the impact of psoriasis on clinical manifestations and disease severity in AS patients. Several studies showed that AS patients with concomitant psoriasis had more severe disease course than patients with primary AS (15, 39). However, another research reported that there was no difference in disease phenotype between AS patients with or without psoriasis (40). Despite no consensus has been reached, there is no doubt that AS patients with concomitant psoriasis adds to the complexity of care, influences treatment decisions and patients’ quality of life, and requires cooperation with dermatologists. The results of our study also underlined the significance of preventing and managing psoriasis in patients with AS.
Several disadvantages should be noticed in the present MR study. Firstly, the data we used belonged to two large-scale GWAS analyses, so that detailed demographic information was lacking, and we cannot conduct any stratification analyses to adjusted for other covariables. Secondly, the study focused on European ancestry, so it is limited to extrapolate the results to other ethnics. Thirdly, the degree of sample overlap between the exposure and outcome is difficult to estimate. Finally, there is a lack of non-radiographic axial population, so it is unable to provide new information about whether AS and non-radiographic axial spondyloarthritis are genetically different.
Despite these disadvantages, our study also has several strengths. Firstly, this is the first MR research to investigate the causal relationship between AS and psoriasis with a large sample size. Secondly, the interference of confounding factors on results and reverse causality has been overcome by using the design of MR approach.
As a whole, this MR study suggests that AS has positive causal effects on the risk of psoriasis, but psoriasis does not have positive causal effects on the risk of AS. Further studies with updated data from large genetic research are required to confirm the findings of this study.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
DT: Conceptualization, Methodology, Software, Writing-Original draft preparation; YZ: Data curation, Visualization, Investigation; YC, YW, HW, and CJ: Supervision, Software; YY, YL, and HYW: Validation; DZ: Writing- Reviewing and Editing. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1149206/full#supplementary-material
Abbreviations
AS, ankylosing spondylitis; CPRD, Clinical Practice Research Datalink; CI, confidence interval; GWAS, genome-wide association studies; IL, interleukin; IVs, instrumental variables; IVW, inverse variance weighted; IGAS, International Genetics of Ankylosing Spondylitis Consortium; LD, linkage disequilibrium; MR, Mendelian randomization; MAF, minor allele frequency; MR-PRESSO, MR pleiotropy residual sum and outlier; OASIS, Outcome in Ankylosing Spondylitis International Study; SNPs, single-nucleotide polymorphisms; TNF, tumor necrosis factor.
References
1. Taurog JD, Chhabra A, Colbert RA. Ankylosing spondylitis and axial spondyloarthritis. New Engl J Med (2016) 374(26):2563–74. doi: 10.1056/NEJMra1406182
2. Ward MM, Deodhar A, Gensler LS, Dubreuil M, Yu D, Khan MA, et al. 2019 Update of the American college of Rheumatology/Spondylitis association of America/Spondyloarthritis research and treatment network recommendations for the treatment of ankylosing spondylitis and nonradiographic axial spondyloarthritis. Arthritis Care Res (2019) 71(10):1285–99. doi: 10.1002/acr.24025
3. Ranganathan V, Gracey E, Brown MA, Inman RD, Haroon N. Pathogenesis of ankylosing spondylitis - recent advances and future directions, nature reviews. Rheumatology (2017) 13(6):359–67.
4. Chen Y, Wu Y, Yang H, Wang J, Kong J, Yu L, et al. DNA Methylation and mRNA expression of B7-H3 gene in ankylosing spondylitis: a case-control study. Immunol investigations (2022) 51(7):2025–34. doi: 10.1080/08820139.2022.2095285
5. Hawkes JE, Chan TC, Krueger JG. Psoriasis pathogenesis and the development of novel targeted immune therapies. J Allergy Clin Immunol (2017) 140(3):645–53. doi: 10.1016/j.jaci.2017.07.004
6. Wang T, Xia Y, Zhang X, Qiao N, Ke S, Fang Q, et al. Short-term effects of air pollutants on outpatients with psoriasis in a Chinese city with a subtropical monsoon climate. Front Public Health (2022) 10:1071263. doi: 10.3389/fpubh.2022.1071263
8. Griffiths CEM, Armstrong AW, Gudjonsson JE, Barker J. Psoriasis. Lancet (London England) (2021) 397(10281):1301–15. doi: 10.1016/S0140-6736(20)32549-6
9. Wohn C, Brand A, van Ettinger K, Brouwers-Haspels I, Waisman A, Laman JD, et al. Gradual development of psoriatic skin lesions by constitutive low-level expression of IL-17A. Cell Immunol (2016) 308:57–65. doi: 10.1016/j.cellimm.2015.11.006
10. Zhou F, Zhu Z, Gao J, Yang C, Wen L, Liu L, et al. NFKB1 mediates Th1/Th17 activation in the pathogenesis of psoriasis. Cell Immunol (2018) 331:16–21. doi: 10.1016/j.cellimm.2018.04.016
11. Stolwijk C, Essers I, van Tubergen A, Boonen A, Bazelier MT, De Bruin ML, et al. The epidemiology of extra-articular manifestations in ankylosing spondylitis: a population-based matched cohort study. Ann rheumatic Dis (2015) 74(7):1373–8. doi: 10.1136/annrheumdis-2014-205253
12. Bodur H, Ataman S, Buğdaycı DS, Rezvani A, Nas K, Uzunca K, et al. Description of the registry of patients with ankylosing spondylitis in Turkey: TRASD-IP. Rheumatol Int (2012) 32(1):169–76. doi: 10.1007/s00296-010-1599-7
13. Stolwijk C, van Tubergen A, Castillo-Ortiz JD, Boonen A. Prevalence of extra-articular manifestations in patients with ankylosing spondylitis: a systematic review and meta-analysis. Ann rheumatic Dis (2015) 74(1):65–73. doi: 10.1136/annrheumdis-2013-203582
14. Essers I, Ramiro S, Stolwijk C, Blaauw M, Landewé R, van der Heijde D, et al. Do extra-articular manifestations influence outcome in ankylosing spondylitis? 12-year results from OASIS. Clin Exp Rheumatol (2016) 34(2):214–21.
15. Man S, Ji X, Hu L, Wang Y, Ma Y, Wang L, et al. Characteristics associated with the occurrence and development of acute anterior uveitis, inflammatory bowel disease, and psoriasis in patients with ankylosing spondylitis: data from the Chinese ankylosing spondylitis prospective imaging cohort. Rheumatol Ther (2021) 8(1):555–71. doi: 10.1007/s40744-021-00293-0
16. Ceccarelli M, Venanzi Rullo E, Berretta M, Cacopardo B, Pellicanò GF, Nunnari G, et al. New generation biologics for the treatment of psoriasis and psoriatic arthritis. state of the art and considerations about the risk of infection. Dermatologic Ther (2021) 34(1):e14660. doi: 10.1111/dth.14660
17. Emdin CA, Khera AV, Kathiresan S. Mendelian randomization. Jama (2017) 318(19):1925–6. doi: 10.1001/jama.2017.17219
18. Cortes A, Hadler J, Pointon JP, Robinson PC, Karaderi T, Leo P, et al. Identification of multiple risk variants for ankylosing spondylitis through high-density genotyping of immune-related loci. Nat Genet (2013) 45(7):730–8.
19. van der Linden S, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis. a proposal for modification of the new York criteria. Arthritis rheumatism (1984) 27(4):361–8. doi: 10.1002/art.1780270401
20. Raychaudhuri SK, Maverakis E, Raychaudhuri SP. Diagnosis and classification of psoriasis. Autoimmun Rev (2014) 13(4-5):490–5. doi: 10.1016/j.autrev.2014.01.008
21. Chen Y, Shen J, Wu Y, Ni M, Deng Y, Sun X, et al. Tea consumption and risk of lower respiratory tract infections: a two-sample mendelian randomization study. Eur J Nutr (2022) 62(1):385–93. doi: 10.1007/s00394-022-02994-w
22. Holmes MV, Ala-Korpela M, Smith GD. Mendelian randomization in cardiometabolic disease: challenges in evaluating causality, nature reviews. Cardiology (2017) 14(10):577–90. doi: 10.1038/nrcardio.2017.78
23. Bowden J, Del Greco MF, Minelli C, Davey Smith G, Sheehan NA, Thompson JR. Assessing the suitability of summary data for two-sample mendelian randomization analyses using MR-egger regression: the role of the I2 statistic. Int J Epidemiol (2016) 45(6):1961–74. doi: 10.1093/ije/dyw220
24. Burgess S, Bowden J, Fall T, Ingelsson E, Thompson SG. Sensitivity analyses for robust causal inference from mendelian randomization analyses with multiple genetic variants. Epidemiol (Cambridge Mass.) (2017) 28(1):30–42. doi: 10.1097/EDE.0000000000000559
25. Hartwig FP, Davey Smith G, Bowden J. Robust inference in summary data mendelian randomization via the zero modal pleiotropy assumption. Int J Epidemiol (2017) 46(6):1985–98. doi: 10.1093/ije/dyx102
26. Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through egger regression. Int J Epidemiol (2015) 44(2):512–25. doi: 10.1093/ije/dyv080
27. Verbanck M, Chen CY, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from mendelian randomization between complex traits and diseases. Nat Genet (2018) 50(5):693–8. doi: 10.1038/s41588-018-0099-7
28. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ (Clinical Res ed.) (2003) 327(7414):557–60. doi: 10.1136/bmj.327.7414.557
29. Krueger JG, Wharton KA Jr., Schlitt T, Suprun M, Torene RI, Jiang X, et al. IL-17A inhibition by secukinumab induces early clinical, histopathologic, and molecular resolution of psoriasis. J Allergy Clin Immunol (2019) 144(3):750–63. doi: 10.1016/j.jaci.2019.04.029
30. Bridgewood C, Newton D, Bragazzi N, Wittmann M, McGonagle D. Unexpected connections of the IL-23/IL-17 and IL-4/IL-13 cytokine axes in inflammatory arthritis and enthesitis. Semin Immunol (2021) 58:101520. doi: 10.1016/j.smim.2021.101520
31. Tsukazaki H, Kaito T. The role of the IL-23/IL-17 pathway in the pathogenesis of spondyloarthritis. Int J Mol Sci (2020) 21(17):6401. doi: 10.3390/ijms21176401
32. Chandrasekharan UM, Kaur R, Harvey JE, Braley C, Rai V, Lee M, et al. TNFR2 depletion reduces psoriatic inflammation in mice by downregulating specific dendritic cell populations in lymph nodes and inhibiting IL-23/IL-17 pathways. J Invest Dermatol (2022) 142(8):2159–72.e9. doi: 10.1016/j.jid.2021.12.036
33. Baeten D, Sieper J, Braun J, Baraliakos X, Dougados M, Emery P, et al. Secukinumab, an interleukin-17A inhibitor, in ankylosing spondylitis. New Engl J Med (2015) 373(26):2534–48. doi: 10.1056/NEJMoa1505066
34. Torres T, Puig L, Vender R, Lynde C, Piaserico S, Carrascosa JM, et al. Drug survival of IL-12/23, IL-17 and IL-23 inhibitors for psoriasis treatment: a retrospective multi-country, multicentric cohort study. Am J Clin Dermatol (2021) 22(4):567–79. doi: 10.1007/s40257-021-00598-4
35. Bergboer JG, Oostveen AM, de Jager ME, den Heijer M, Joosten I, . van de Kerkhof PC, et al. Paediatric-onset psoriasis is associated with ERAP1 and IL23R loci, LCE3C_LCE3B deletion and HLA-C*06. Br J Dermatol (2012) 167(4):922–5. doi: 10.1111/j.1365-2133.2012.10992.x
36. Roberts AR, Appleton LH, Cortes A, Vecellio M, Lau J, Watts L, et al. ERAP1 association with ankylosing spondylitis is attributable to common genotypes rather than rare haplotype combinations. Proc Natl Acad Sci United States America (2017) 114(3):558–61. doi: 10.1073/pnas.1618856114
37. Essers I, Ramiro S, Stolwijk C, Blaauw M, Landewé R, van der Heijde D, et al. Characteristics associated with the presence and development of extra-articular manifestations in ankylosing spondylitis: 12-year results from OASIS. Rheumatol (Oxford England) (2015) 54(4):633–40. doi: 10.1093/rheumatology/keu388
38. Ibn Yacoub Y, Amine B, Laatiris A, Bensabbah R, Hajjaj-Hassouni N. Relationship between diagnosis delay and disease features in Moroccan patients with ankylosing spondylitis. Rheumatol Int (2012) 32(2):357–60. doi: 10.1007/s00296-010-1635-7
39. Richette P, Tubach F, Breban M, Viguier M, Bachelez H, Bardin T, et al. Psoriasis and phenotype of patients with early inflammatory back pain. Ann rheumatic Dis (2013) 72(4):566–71. doi: 10.1136/annrheumdis-2012-201610
Keywords: ankylosing spondylitis, psoriasis, Mendelian randomization, genome-wide association studies, single-nucleotide polymorphisms
Citation: Tian D, Zhou Y, Chen Y, Wu Y, Wang H, Jie C, Yang Y, Liu Y, Wang H and Zhou D (2023) Genetically predicted ankylosing spondylitis is causally associated with psoriasis. Front. Immunol. 14:1149206. doi: 10.3389/fimmu.2023.1149206
Received: 23 January 2023; Accepted: 13 June 2023;
Published: 06 July 2023.
Edited by:
Santos Castañeda, Hospital de La Princesa, SpainReviewed by:
Felipe Julio Ramírez, Hospital Clinic of Barcelona, SpainXabier Michelena, Vall d’Hebron University Hospital, Spain
Clementina López-Medina, Reina Sofía Hospital, Spain
Copyright © 2023 Tian, Zhou, Chen, Wu, Wang, Jie, Yang, Liu, Wang and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dian Zhou, YWhtdV96aG91ZGlhbkAxNjMuY29t
 Di Tian
Di Tian Yuan Zhou2
Yuan Zhou2 Dian Zhou
Dian Zhou