- 1Queen Mary School, Nanchang University, Nanchang, Jiangxi, China
- 2Department of Gastroenterology, the First Affiliated Hospital of Nanchang University, Nanchang, Jiangxi, China
- 3Department of Cardiology, Zhongnan Hospital, Wuhan University, Wuhan, Hubei, China
Background: This review summarizes the factors influencing the efficacy and safety of the COVID-19 vaccine in LTR through meta-analysis, hoping to provide strategies for vaccine use.
Methods: Electronic databases were screened for studies on mRNA vaccines in LTR. The primary outcome was the pooled seroconversion rate, and the secondary outcome was the incidence of adverse events+breakthrough infections. Subgroup analyses were made based on BMI, associated comorbidities, presence of baseline leukopenia, time since transplant, and drugs used.
Result: In total, 31 articles got included. The pooled seroconversion rate after at least two doses of SARS-CoV-2 vaccination was 72% (95% CI [0.52-0.91). With significant heterogeneity among studies I2 = 99.9%, the seroconversion rate was about 72% (95%CI [0.66-0.75]), from the studies reporting two doses of vaccine slightly higher around 75%(95%CI [0.29-1.22]) from studies reporting three doses. The pooled seroconversion rate within the lower to normal BMI group was 74% (95% CI [0.22-1.27], Pi=0.005) against 67% (95% CI [0.52-0.81], Pi=0.000) in the high BMI group. The pooled seroconversion rate in the ‘‘positive leukopenia’’ group was the lowest, 59%. Leukopenia could influence the vaccine seroconversion rate in LTR. From the time since transplant analysis after setting seven years as cut off point, the pooled seroconversion rate after at least two doses of COVID-19 vaccination was 53% (95% CI [0.18-0.83], P=0.003, I2 = 99.6%) in <7years group and 83% (95% CI [0.76-0.90], P=0.000 I2 = 95.7%) in > 7years group. The only time since transplantation had reached statistical significance to be considered a risk factor predictor of poor serological response (OR=1.27 95%CI [1.03-1.55], P=0.024). The breakthrough infection rate after vaccination was very low2% (95% CI 0.01-0.03, I2 = 63.0%), and the overall incidence of adverse events, which included mainly pain at the injection site and fatigue, was 18% (95%CI [0.11-0.25], I2 = 98.6%, Pi=0.000).
Conclusion: The seroconversion rate in LTR vaccinated with at least two doses of mRNA COVID-19 vaccine could be significantly affected by the vaccine type, immunosuppressant used, BMI, leukopenia, associated comorbidities, and time since transplantation. Nevertheless, booster doses are still recommended for LTR.
1 Introduction
Coronavirus disease 19 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has infected nearly 600 million people worldwide and caused more than 6 million cumulative deaths, causing a significant global economic and medical burden. The development and application of SARS-CoV-2 vaccines are one of the most important measures to reduce the infection rate of SARS-CoV-2.
Several clinical trials have confirmed the efficacy of the SARS-CO V-2 vaccine, which is being used worldwide (1). However, these studies often excluded patients treated with immunosuppressive drugs, including LT patients (2–4).
In organ suppression or tissue transplantation, the transplant recipient’s immune system will produce an immune response to the transplanted organ of the transplant donor, so drugs are needed to suppress the excessive rejection of the immune system (5). This may have potential implications for the efficacy and safety of vaccination in these patients and may increase the risk of infection (6). Multiple questions have arisen regarding the effectiveness of vaccination against SARS-CoV-2 in LTR, including factors such as the different complications that occur in LT recipients, differences in the duration of LTation, and the use of different immunosuppressive agents (7).
Several studies have reported that the COVID-19 vaccine could work well in people who have had liver transplants, however most of the studies had either a sample sizes that were insufficient to predict outcomes accurately or didn’t include the analysis of other factors like BMI, type of comorbidities, etc. BMI is emerging as an important factor influencing the effectiveness of vaccines, evidences have suggested that high BMI levels were associated with impaired immune response leading to low vaccine response in setting of influenza, hepatitis and other vaccines. However, in the context of Covid 19 it should be noted that individual with obesity have been indexed as high risk group for severe outcomes, and preclinical data suggested that those peoples would generate a lower seroconversion rate compared to others. Nevertheless, the clinical significance of BMI in context of covid 19 vaccine among LTR still remain unelucidated. On the other side liver transplant recipients often experience leukopenia due to their immunosuppressive medication which will obviously affect their immune responses to infections. This raises concern about their ability to mount an effective immune response to COVID 19 vaccination. And therefore warrant the investigation of the impact of both leukopenia and drugs on the immunogenicity of Covid19 vaccine among LTR (8–10). Finally, studies have shown that comorbidities such as diabetes, hypertension, or heart disease may have a reduced immune response to the vaccine compared to those without underlying health conditions, but this still needs to be demonstrated in the setting of Covid-19 vaccinated LTR (11).
Up to days, the safety of the COVID-19 vaccine has remained a substantial matter of concern among LT recipients, their families, and their caretakers. Therefore, this review aims to summarize the factors that could potentially affect vaccine efficacy and safety in LT recipients and provide references for future studies.
2 Methods
This meta-analysis was conducted according to the panel recommendation of the Meta-Analysis of Observational Studies in Epidemiology (MOOSE) and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (12).
2.1 Database search
A literature search was done using PubMed and Cochrane Library databases (the last search was on March 25th, 2023) using the keywords Liver Transplantation combined using the operator ‘AND’ COVID-19, SARS-COV-2 ‘AND’ Vaccine ‘OR’ Vaccination.
((Liver transplantation) AND ((COVID-19) OR (SARS-COV-2))) AND ((vaccine) OR (vaccination))
All titles and abstracts were screened to collect studies that may be relevant to LT patients and SARS-COV-2. References for the included studies were manually searched to identify other relevant studies. Titles and abstracts were reviewed, followed by full-text screening, and quality assessment.
2.2 Inclusion and exclusion criteria
Specific inclusion criteria for the systematic review and meta-analysis were as follows: (1) describe seroconversion of LT recipients with the second, or booster doses of the messenger RNA-based Sars-Cov-2 vaccine; (2) Study reporting on novel coronavirus infection or death in LT recipients after vaccination; (3) the occurrence of local or systemic adverse reactions after vaccination in LT recipients; (4) Studies including at least 10 LT recipients only were considered to avoid significant bias caused by small sample size. The analysis excluded case reports, case series (< 10 cases), guidelines, surveys, and editorial reviews.
2.3 Data extraction
From each eligible study the following data were extracted by one author and reviewed by a second author these include: information on the authors of published literature, study population, patient characteristics (drugs, complications, BMI, comorbidities, etiologies of transplantation, comorbidities and presence of leucopenia or not), the time since liver transplantation, type and number of doses of mRNA vaccine BNT162b2 or mRNA-1273 received, post-vaccination infection rates, and presence of not of adverse events.
2.4 Outcome indicators
A single-arm meta-analysis (pooled data analysis) was performed in absence of control arms. All studies on the efficacy and safety of the Novel Coronavirus vaccine in LTR were evaluated. Since seroconversion and breakthrough infection are considered markers of the effectiveness of the SARS-CoV-2 vaccine, the primary outcomes was the rate of seroconversion and the occurrence of novel coronavirus infection following second, and booster doses of the SARS-CoV-2 vaccine administration in LTR. the secondary outcome was the occurrence of adverse events after vaccination in LTR.
Subgroup analyses were also performed to assess the effect of others factors(predominant comorbidities, BMI, presence of leukopenia, time from transplant to vaccination, effects of different drugs on the seroconversion rates in LT recipients). The comorbidities were categorized as cardiovascular (hypertension, coronary disease etc.), endocrine (diabetes mellitus), respiratory (COPD, respiratory failure etc.), renal (CKD etc.). to assess the role of BMI, Studies reporting many cases of obesity or of BMI> 25 were labelled as High BMI and those with BMI lower than 25 or few cases of obesity were labeled as low to normal BMI. As for leukopenia all the studies reporting on either the mean of white blood cell count or the presence of leukopenia in included cohorts were grouped in two categories that were no leukopenia and positive leukopenia.
2.5 Study characteristics and quality assessment
Because most of the included studies were observational prospective or retrospective investigations, the quality of each study was independently assessed by two investigators (N.F, X.L) using the Newcastle-Ottawa Scale (NOS) and inconsistencies were resolved by consensus.
2.6 Data analysis
The analyses were conducted using the Stata software 13 MP (StataCorp, College Station, TX). A random-effects model was chosen because of substantial heterogeneity accross the included studies. The pooled rates of adverse events were computed under the random-effects model and the inverse variance method. The heterogeneity was determined by the I2 and P value of heterogeneity>0.1.
2.7 Publication of bias assessment
The funnel plot asymmetry was used to confirm the existence of publication bias across the studies. Which was confirmed by Egger’s test.
2.8 Sensitivity analyses
Sensitivity analyses were generated from the Stata software via the ‘‘studies omission approach’; when the omission of selected studies didn’t affect much the pooled results; then, the results was to be considered valid.
3 Results
A total of 252 records were identified through an initial keyword search. After screening by title and abstract, 208 articles were removed for multiples reasons, including articles that did not report outcomes of interest, review articles, and articles not in English. This left 44 articles for further full-text screening, among which only 30 studies were included in our analysis. The PRISMA flow diagram gives a detailed description of the selection process (Figure 1). The baseline characteristics of included studies are shown in Tables 1, 2 (13–18, 20–26, 28–42, 44–50).
Figure 1 PRISMA 2020 flow diagram for new systematic reviews which included searches of databases and registers only.

Table 1 characteristic of included studies describing the effectiveness and safety of SARS-CoV-2 vaccines in LTR patients.
3.1 Overall seroconversion rates after SARS-CoV-2 vaccination in LT recipients
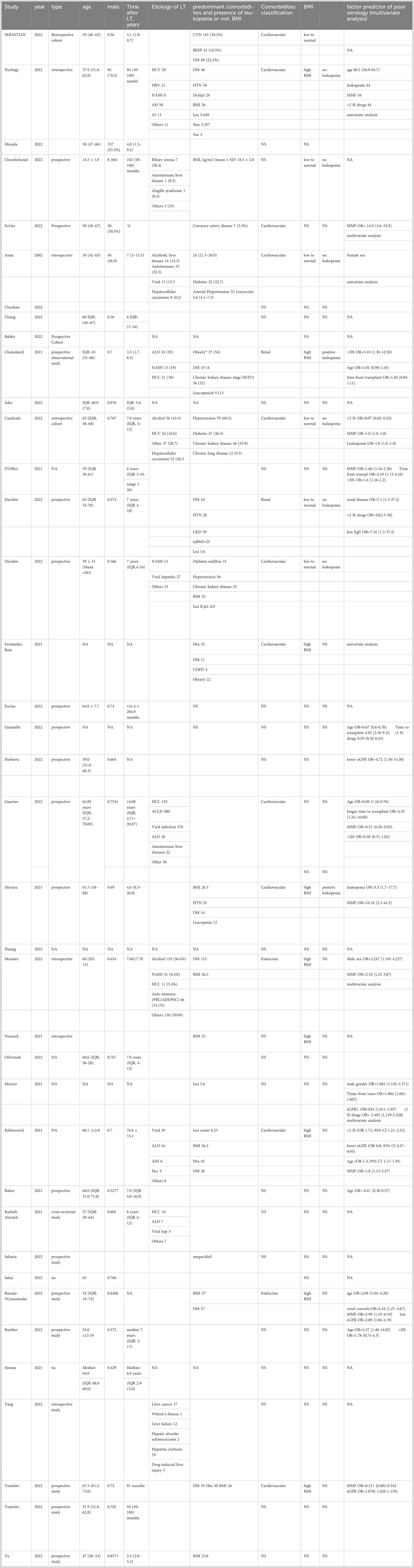
A summary of seroconversion rates after vaccination against SARS-CoV-2 is provided in Figure 2. All the included studies reported seroconversion following at least two doses of covid vaccine (13–20, 22–41, 45, 49, 50). The pooled seroconversion rate after at least two doses of SARS-CoV-2 vaccination was 72% (95% CI [0.52-0.91], P<0.05). With significant heterogeneity among studies I2 = 99.9%, and a non-significant heterogeneity between the two groups Pi=0.88. It could be observed that for studies reporting only two doses the seroconversion rate was about 72% (95%CI [0.66-0.75],P<0.05), whilst for studies reporting three doses of vaccine that rate was slightly higher around 75% (95%CI[0.29-1.22], P<0.05).
3.2 Subgroup analyses
We conducted subgroup analyses based on, major immunosuppressant drug used, BMI, major comorbidity, timing since transplantation and presence of leukopenia.
3.2.1 BMI on seroconversion rate
Studies that included BMI reports in their participant baseline characteristics were grouped as lower to normal BMI and higher BMI categories; the rest of the studies that didn’t include BMI reports on their participants’ baselines were labeled as NS (not specified). As shown in Figure S1, the pooled seroconversion rate within the lower to normal BMI group was 74% (95% CI [0.22-1.27], P<0.05) against 67% (95% CI [0.52-0.81], P<0.05) in the high BMI group. Which could mean that LTR with normal BMI may present with a better seroconversion rate after at least two doses of covid vaccine than those with high BMI. Though the heterogeneity across studies was high i2 = 99 but the heterogeneity between the subgroup was not significant Pi=0.632
3.2.2 Major comorbidities on seroconversion rate
To explore whether the presence of some sort of comorbidities at the time of vaccination could affect the seroconversion rate, we spotted the most common comorbidities across included studies and we categorized studies based on the major comorbidity (ie the comorbidity presents in a great majority of the participants included in that study). In 11 studies the majority of participants had cardiovascular comorbidity (see Table 1), the predominance of renal comorbidity was found only in two studies and two studies included endocrine disease as major comorbidity; the rest of the studies didn’t have a report on comorbidities and were categorized as NS (not specified) (Figure S2). The pooled seroconversion rate was 70% for cardiovascular, 73% for renal, and 60% for endocrine. This suggests that the presence of endocrine comorbidities could affect the seroconversion rate in LTR after at least two doses of vaccine. Statics results were significant P<0.005 for endocrine and renal with high heterogeneity across studies and a no significant heterogeneity between the group groups Pi=0.52.
3.2.3 Presence of leukopenia on seroconversion rate
Studies were further categorized as positive leukopenia, no leukopenia and NS (not specified) groups. As expected the pooled seroconversion rate in the “positive leukopenia’’ group was the slowest 59% 95%CI [0.37, 0.82] and the pooled seroconversion rate in the “no leukopenia’’ group was the highest and could reach 80% 95%CI [0.70, 0.90]; indicating that baseline leukopenia could influence the seroconversion rate in LTR after at least two doses of covid vaccine. All these results were significant P<0.005 with high heterogeneity across studies and a not significant heterogeneity between groups Pi=0.22 (see Figure S3).
3.2.4 Use of immunosuppressive agents
We conducted a subgroup analysis based on the use of immunosuppressive agents (Figure 3). After at least 2 doses of vaccines, the overall seroconversion rate in LT recipients treated with MMF was 65% (95% CI [0.55-0.74], I2 = 76%, P<0.05). The pooled seroconversion rate in patients on tacrolimus was 67% (95% CI 0.48-0.86], I2 = 96% P<0.05). The pooled seroconversion rate in patients on corticosteroids was 55% (95% CI [0.33-0.78], I2 = 77.0%). With everolimus the pooled seroconversion rate was 70% (95% CI [0.63-0.78], I2 = 59%, P<0.05). The seroconversion rates were 87% (95% CI [0.70-1.03], I2 = 98% P<0.05) and 70% (95% CI [0.52-0.88], I2 = 91%, P<0.05) for LT recipients treated with CNI and antimetabolite, respectively. There was a high heterogeneity across studies, however the heterogeneity among the subgroups was not significant Pi=0.195.
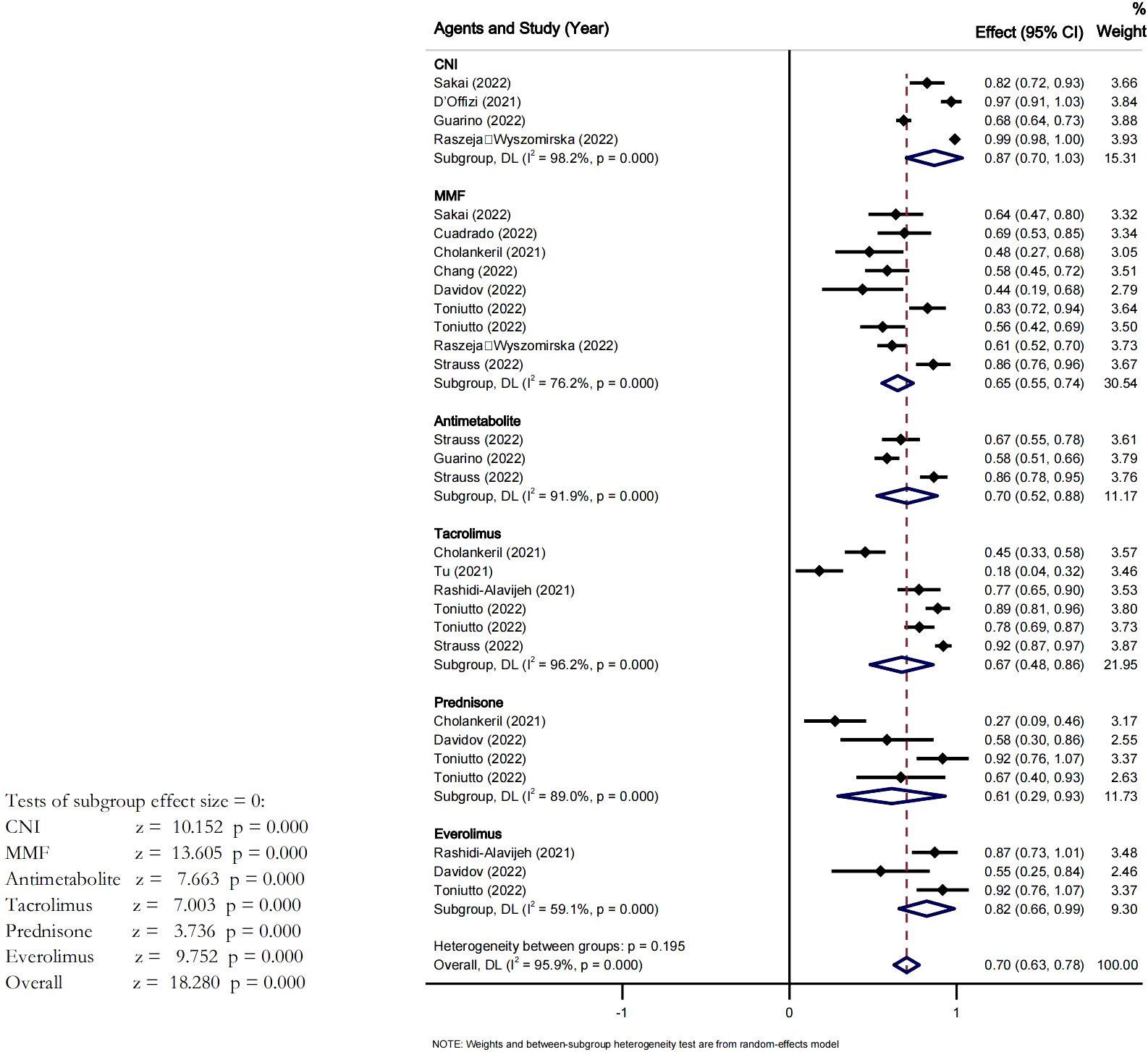
Figure 3 Effect of different drugs on the seroconversion rate of LTR after at least two dose of vaccine.
3.2.5 Time since transplantation
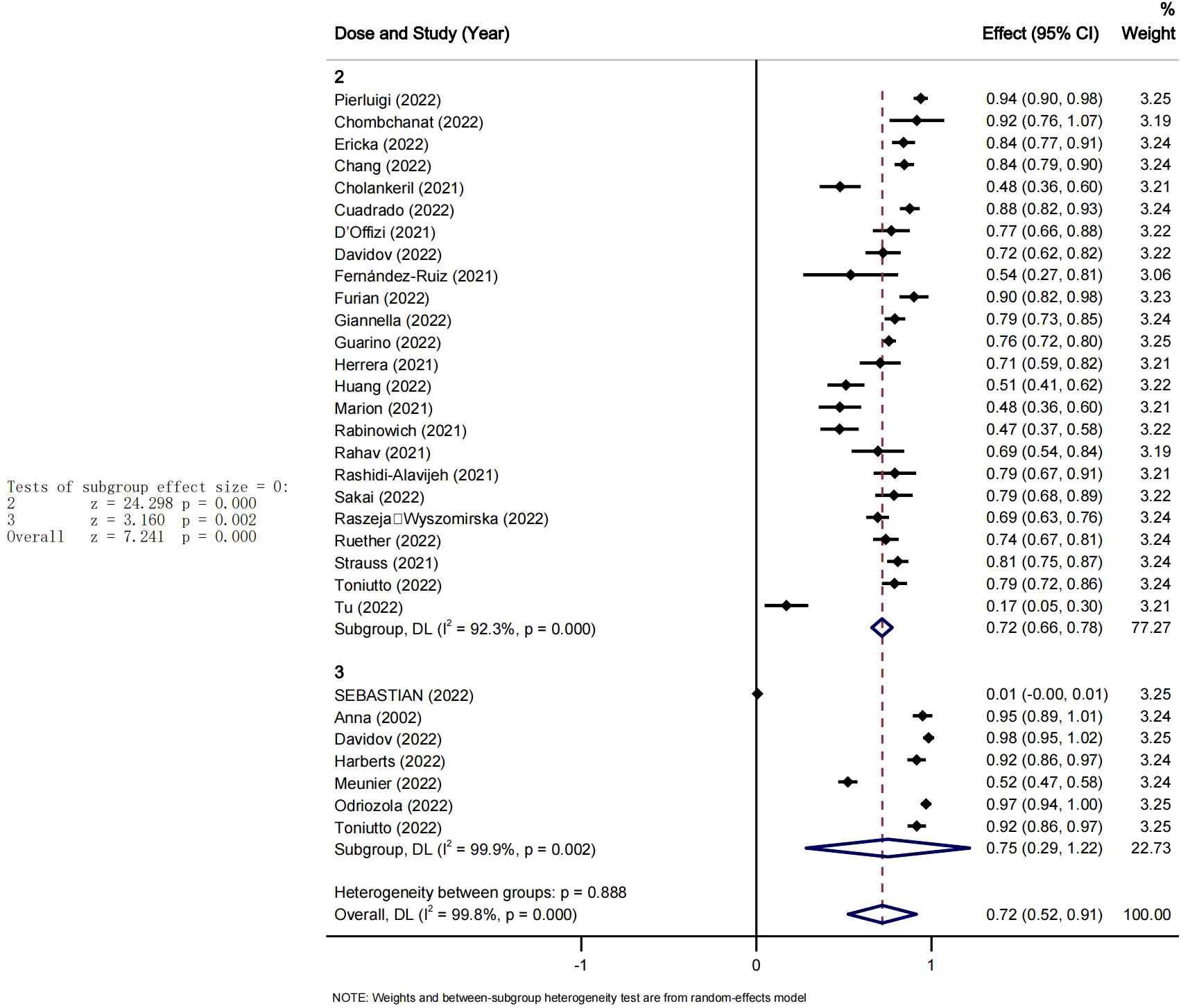
We also conducted a subgroup analysis based on the mean time from transplant to vaccination. We computed the mean duration of time since transplantation across the included studies to use it as the cut-off point. Therefore, 7 years was set as the cut-off point and the studies were divided into two groups. The pooled seroconversion rate after at least 2 doses of COVID-19 vaccination was 53% (95% CI [0.18-0.83], P<0.05, I2 = 99.6%) in <7years group and 83% (95% CI [0.76-0.90], P<0.05 I2 = 95.7%) in > 7years group, the heterogeneity between the groups was significant Pi=0.005 (see Figure 4).
3.3 Predictors of poor serological response
Analysis was performed with some studies that had used multivariate analysis to identify predictors of poor serological response to COVID-19 vaccines. A total of 8 studies 14,18,23,29,32,and 33 included age as the potential risk factor in their multivariate analysis. Meta-analysis of these studies did confirm that advanced age was a risk factor for a poor serological response, but the association did not reach statistical significance (OR=1.01 95%CI [0.78-1.29] P=0.9). There pooled OR from the 8 studies that reported MMF as significant was also not statistically significant (P=0.07); 9 studies reported on the use of >2 immunosuppressive drugs, reported on decreased GFR, 5 on time since transplantation and 2 on leukopenia as the risk factors for poor immune response in multivariate analysis. However, our analysis results showed that only time since transplantation had reached statistical significance to be considered as risk factor for poor serological response (OR=1.27 95%CI [1.03-1.55], P=0.024) (Figure 5).
3.4 Breakthrough infections and adverse events
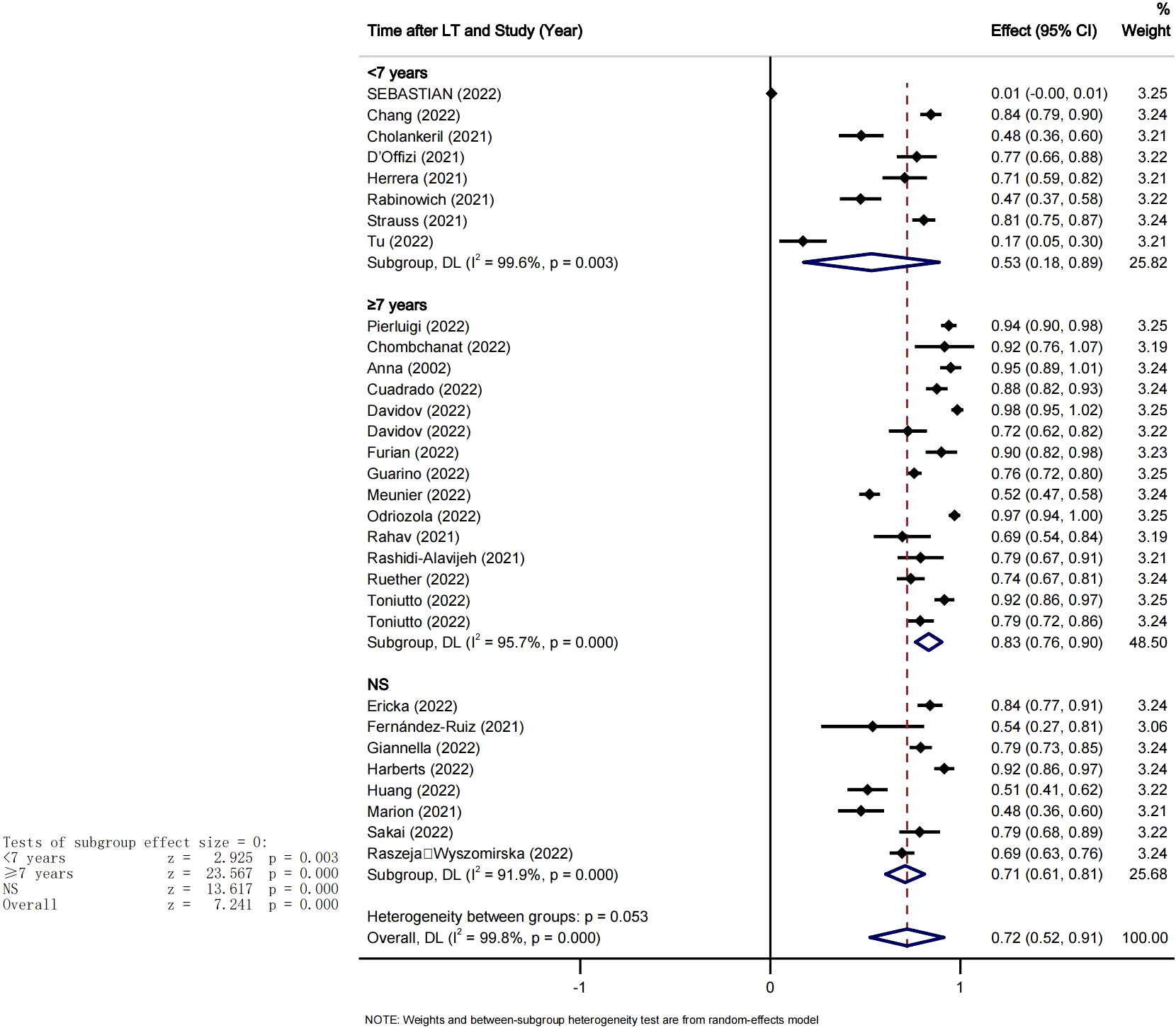
Seven studies reported on breakthrough infections after COVID-19 vaccination in LT recipients. In total, 44 infections and 2 deaths occurred in 2083 LT recipients during the follow-up period. The overall rate of breakthrough infections after complete vaccination was 2% (95% CI 0.01-0.03, P<0.05, I2 = 63.0%) (Figure 6). 3 studies reported adverse events after COVID-19 vaccination in LT recipients. The incidence of combined adverse events and breakthrough infections after COVID-19 vaccination was 18% (95%CI [0.11-0.25], I2 = 98.6%, P<0.05). The major adverse events recorded were local pain at the injection site with an incidence around 51% (95%CI [0.28-0.74], I2 = 97.9%, P<0.05) and fatigue which incidence was of 30%(95%CI: -[0.14%-0.47], I2 = 95%, P<0.05), Pi=0.00 (Figure 6).
3.5 Sensitivity analyses results
The pooled seroconversion rate and its 95% CI didn’t varie much when selected studies were omitted by the stata analysis software (72%,95%CI [0.52-0.90]. Which means that our results are reliable and were not just subject to chance. The details of all the sensitivity results are available attached to its figure generated in this analysis.
3.6 Publication bias
There was marked funnel plot asymetry confirmed by a positive Egger’s test P=0.000. This indicated that our results might be significantly impacted by threxisting publication bias among the included studies. See Supplementary Files.
4 Discussion
COVID-19 is now considered a global pandemic, currently causing millions of deaths in just a few years. At present, the pathogenesis and treatment of this disease are still under study. The widespread use of vaccines is currently considered the most important measure to control the pandemic (51).
Sars-CoV-2 infection results in an altered host immune response. T and N K cell depletion may occur during SARS-COV-2 infection (52). For transplant patients, long-term use of immunosuppressive agents may lead to an increased risk of SARS-CoV-2 infection. Since immunocompromised people are at high risk of severe disease and death after infection with a Novel coronavirus, it is recommended that solid organ transplant patients (hereinafter referred to as SOT recipients) receive the SARS-CoV-2 vaccine. In addition, many clinical studies on vaccines often exclude organ transplant patients, including a large proportion of liver transplant patients. Hence, the efficacy and safety of vaccination in liver transplant patients deserve further evaluation.
Among liver-transplant recipients, the pooled seroconversion rates was 72% (95% CI 0.66-0.78) after two doses of the SARS-CoV-2 vaccine, and there was a slight positive increase in the pooled seroconversion rate after three doses of vaccine (75% 95% CI: [0.73-1.00]), which was statistically significant. These results not only suggest that a third dose could be recommended for patients undergoing liver transplantation, even if the second dose improve the rate of seroconversion but also indicate that the immune response to the SARS-CoV-2 vaccine is not attenuated after the administration of a multiple-dose regimen in liver transplant recipients, despite prolonged use of immunosuppressive agents.
Though Previous meta-analysis did evaluate the seroconversion rate after two doses of vaccine in LTR, and evaluated on factors such as types of vaccine, the number of doses, time between booster doses, time since transplantation and presence of adverse events and breakthrough infections (53, 54). However the role of BMI and the presence or types of comorbidities on the seroconversion rate in LTR have not well been clarified in their pooled analysis and for the other factors their conclusion brought non-negligible feedback from the scientific community; therefore this matter may not yet as per say be considered as concluded and more investigation were necessary to validate previous theories made. In Our analysis the pooled seroconversion rate from studies characterized by a lower to normal BMI value was 74% (95% CI [0.22-1.27], P=0.005) against 67% (95% CI [0.52-0.81], P=0.000) in those characterized by high BMI value. This could imply that obese and overweight LTR may have a lower seroconversion rate after at least two doses of mRna vaccine. This may be explained by the fact that COVID-19 and obesity are intertwined pandemics. Those with obesity have a higher risk of severe outcomes from SARS-CoV-2 and excess weight increases the risk of adverse outcomes, regardless of comorbidities. Obesity affects metabolism causing insulin resistance, changes in adipokines (leptin increase, adiponectin decrease), and chronic inflammation. This leads to endothelial dysfunction and worsens a prothrombotic state. Obese mice shed viruses for longer, had more bacterial infections, and more respiratory damage. People with obesity may have reduced vaccine effectiveness due to the altered cytokine production and immune responses. This has been observed with influenza vaccine and preliminary reports also suggest that lower antibody concentrations can be found in obese patients after COVID-19 vaccine.
The comorbidities analysis revealed that the seroconversion rate after at least two doses of vaccine was lower in the group having endocrine pathologies as major comorbidity than the rest; however, this observation might actually not reflect the reality because of the few number of studies, howbeit previous records acknowledged that there was a risk of cardiac tissue damage following the SARS COV2 infection. Based on these facts it would be logical to assume that the presence of comorbities in LTR may affect the response to covid 19 vaccine.
In our subgroup analyses we also evaluated the influence of the presence of baseline leukopenia on the seroconversion rate and we found that as expected the pooled seroconversion rate in the “positive leukopenia’’ group was the lowest 59% and the pooled seroconversion rate in the “no leukopenia’’ group was the highest and could reach 80%. The goal of vaccination is generally to induce the formation of memory cells to prepare the body for future eventual invasion from the pathogen. Therefore, a good immunological status which could be marked by sufficient level of immunoglobulins and immune cells is essential for optimized response after vaccination. So in LTR with altered baseline immunity the positive response from the vaccine would appear to be lower than that of those with normal baseline immunity profile.
This analysis also showed that after at least two doses of vaccine in LTR the seroconversion rate was slightly higher in patients treated with CNI, and was low in patients treated with MMF, Tacrolimus and Prednisone. Therefore, CNI seemed to be the more appropriate immunosuppressant drugs for LTR after at least two vaccine doses. Studies have shown that for LTR, immunosuppressive treatment with different types of immunosuppressants (which possess different properties and mechanisms) is not a contraindication for vaccination. Therefore, vaccination remains feasible despite immunosuppressant therapy. Our findings also supported this theory. T cells are frequently inhibited by immunosuppressive drugs, and vaccine-induced immune responses also include T-cells. So the normal expectation would be a reduced seroconversion rate after vaccine.This shows that our analysis results might not be conclusive and more precise and well elaborated studies could re-evaluate, the characteristic of the immune response in patients taking immunosuppressant therapy.
. The study showed a remarkable difference in the concomitant seroconversion rate after two doses of the COVID-19 vaccine between patients with less than 7 years after liver transplantation and those with more than 7 years after liver transplantation. Studies have shown that the number of CD4~+CD25~+Foxp3~+ regulatory T cells in peripheral blood of patients who survived 6 months to 3 years, 3 years to 10 years, and more than 10 years after liver transplantation with immunosuppressive agents gradually decreased and maintained at a relatively low level with the extension of survival time (55). It is suggested that the immune response is different in patients with different survival times after transplantation. The time of transplantation also may have an impact on the vaccine conversion rate. The longer the time from transplantation, the better the vaccine seroconversion rate. It could also be said that the immune response in transplant patients may not be reduced with the extended survival time.
Another important measure of the effectiveness of SARS-CoV-2 vaccines is the incidence of breakthrough infections after vaccination against COVID-19. However, only 7 studies have evaluated infection following breakthrough infection S A R S-COV-2 infection; therefore, further research on the risk of breakthrough infection in liver transplant patients is warranted. Further studies are needed to assess the breakthrough infection rate after vaccination with SARS-COV-2 in LTR patients compared with the general population.
In addition, this meta-analysis also illustrated that the majority of adverse events after vaccine were limited to injection site side effects. Three studies reported that the major adverse events in LT recipients after COVID-19 vaccination were local pain at the injection site 51% (95%CI [0.28-0.74], I2 = 95%) and Systemic adverse events reported only in a small subset such as fatigue 30% (95%CI: [0.14-0.47], I2 = 88%). overall the incidence of adverse events following vaccination was very low and the majority did not require hospitalization. These results are suggesting that COVID-19 vaccination is safe for LTR patients.
The strength of our study is the meta-analysis of a large number of prospective studies that included data on COVID-19 vaccination in a large number of liver transplant recipients. In addition, subgroup analyses add to the robust statistical design. But there are limitations to this meta-analysis. Heterogeneity in immunosuppressive therapies, vaccine technics, regional differences in vaccination sites according to countries, may have also accounted for the heterogeneity across studies making it difficult to ascertain the assessment of some outcomes. The assessment of adverse events also presented some limitations, in part because of inherent limitations from the included studies used to assess adverse events and the small sample size, from the lack of randomized controls to accurately evaluate the incidence of adverse events after vaccination. The studies used different assays to assess SARS-CoV-2 antibodies, this may also have influenced the results. Therefore, further studies are needed to compare seroconversion rates between different assays for SARS-CoV-2 antibodies. Moreover, it is worth noting that infections could still occur despite the seroconversion post-vaccination 24, 39, 44 due to the different epidemic situations in different countries. Nevertheless, vaccination can reduce the severity of the disease. The T-cell response is also an important component of the SARS-COV-2 vaccine response (56). The first edition of the Technical Guidelines for SARS-CoV-2 Vaccination recommended inactivated and recombinant subunit vaccines for solid organ transplant patients (57). However, with the development of vaccines, mRNA vaccines have been widely used worldwide, and the difference in effectiveness among mRNA vaccines has attracted attention (8, 19, 27). For the immune response after 3 doses of the COVID-19 vaccine, the seroconversion rate of the mixed vaccine did not change much, while the seroconversion rate of the BNT162b2 and mrNA-1273 vaccines increased significantly. It is recommended to avoid mixed vaccine regimens whenever possible 53. In addition, others limitations may reside in the inaccessibility of records published in language other than English and unpublished studies. Nonetheless this study is to our knowledge the first meta-analysis on immunogenicity of mRNA covid-vaccine in LTR that included BMI, Leukopenia and associated comorbidities in its analysis. Thus, making it worth of consideration.
5 Conclusion
In conclusion, this meta-analysis showed that the overall seroconversion rate of liver transplant recipients vaccinated with COVID-19 increased after booster vaccination, irrespective of vaccine type, immunosuppressant used. This meta-analysis also demonstrated that normal BMI, absence of pre vaccination leukopenia and increased duration of transplantation was significantly positively also associated with improved seroconversion rate. Further studies are needed to investigate on the efficacy of different vaccines against SARS-CoV-2 variants infection in LTR and the T-cell response after covid19 vaccination
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
YX designed and supervised the study, ZY and XL collected data, XL and FL performed the analysis and drafted the manuscript, and FL proofread the paper. All authors contributed to the article and approved the submitted version.
Acknowledgments
We would like to send appreciation to all the authors who contributed to this paper.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1145081/full#supplementary-material
Supplementary Figure 1 | Effect of BMI on the seroconversion rate of LTR second dose vaccine.
Supplementary Figure 2 | Effect of comorbidities on the seroconversion rate of LTR after at least two doses of vaccine.
Supplementary Figure 3 | Presence of leukopenia on seroconversion rate.
Abbreviations
LTR, liver transplant recipients; LT, liver transplant; LTation, liver transplantation; MMF, mycophenolate mofetil; CNI, caleineuin inhibitor; eGFR, epidermal growth factor receptor; Pi, P value for heterogeneity.
References
1. Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, et al. Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine. N Engl J Med (2020) 383(27):2603–15. doi: 10.1056/NEJMoa2034577
2. Ali NM, Alnazari N, Mehta SA, Boyarsky B, Avery RK, Segev DL, et al. Development of COVID-19 infection in transplant recipients after SARS-CoV-2 vaccination. Transplantation (2021) 105(9):e104–6. doi: 10.1097/TP.0000000000003836
3. Havlin J, Svorcova M, Dvorackova E, Lastovicka J, Lischke R, Kalina T, et al. Immunogenicity of BNT162b2 mRNA COVID-19 vaccine and SARS-CoV-2 infection in lung transplant recipients. J Heart Lung Transplant (2021) 40(8):754–8. doi: 10.1016/j.healun.2021.05.004
4. Wadei HM, Gonwa TA, Leoni JC, Shah SZ, Aslam N, Speicher LL. COVID-19 infection in solid organ transplant recipients after SARS-CoV-2 vaccination. Am J Transplant (2021) 21(10):3496–9. doi: 10.1111/ajt.16618
5. Smith KGC, Isbel NM, Catton MG, Leydon JA, Becker GJ, Walker RG. Suppression of the humoral immune response by mycophenolate mofetil. Nephrol Dialysis Transplant (1998) 13(1):160–4. doi: 10.1093/ndt/13.1.160
6. Boyarsky BJ, Werbel WA, Avery RK, Tobian AAR, Massie AB, Segev DL, et al. Antibody response to 2-dose SARS-CoV-2 mRNA vaccine series in solid organ transplant recipients. JAMA (2021) 325(21):2204–6. doi: 10.1001/jama.2021.7489
7. Pallarés-Carratalá V, Polo García J, Martín Rioboo E, Ruíz García A, Serrano-Cumplido A, Barrios V. [COVID-19 vaccine and anticoagulation patients at high cardiovascular risk. SEMERGEN recommendations]. Semergen (2021) 47(1):1–3. doi: 10.1016/j.semerg.2020.12.005
8. Demeulemeester F, de Punder K, van Heijningen M, van Doesburg F. Obesity as a risk factor for severe COVID-19 and complications: A review. Cells (2021) 10(4):933. doi: 10.3390/cells10040933
9. Barry M, Chandra S, Hymes KB. Cytopenias in transplant patients. Principles Pract Transplant Infect Diseases (2018) 8:199–207. doi: 10.1007/978-1-4939-9034-4_10
10. Molina Perez E, Fernández Castroagudín J, Seijo Ríos S, Mera Calviño J, Tomé Martínez de Rituerto S, Otero Antón E, et al. Valganciclovir-induced leukopenia in liver transplant recipients: influence of concomitant use of mycophenolate mofetil. Transplant Proc (2009) 41(3):1047–9. doi: 10.1016/j.transproceed.2009.02.033
11. Choi WS, Cheong HJ. COVID-19 vaccination for people with comorbidities. Infect Chemother (2021) 53(1):155–8. doi: 10.3947/ic.2021.0302
12. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med (2009) 6(7):e1000097. doi: 10.1371/journal.pmed.1000097
13. Hamm SR, Rezahosseini O, Møller DL, Loft JA, Poulsen JR, Knudsen JD, et al. Incidence and severity of SARS-CoV-2 infections in liver and kidney transplant recipients in the post-vaccination era: Real-life data from Denmark. Am J Transpl (2022) 22:2637–50. doi: 10.1111/ajt.17141
14. Toniutto P, Falleti E, Cmet S, Cussigh A, Veneto L, Bitetto D, et al. Past COVID-19 and immunosuppressive regimens affect the long-term response to anti-SARS-CoV-2 vaccination in liver transplant recipients. J Hepatol (2022) 77(1):152–62. doi: 10.1016/j.jhep.2022.02.015
15. Tubjaroen C, Prachuapthunyachart S, Potjalongsilp N, Sodsai P, HIrankarn N, Jaru-Ampornpan P, et al. Immunogenicity of an mRNA-Based COVID-19 Vaccine among Adolescents with Obesity or Liver Transplants. Vaccines (2022) 10:1867. doi: 10.3390/vaccines10111867
16. Zecca E, Rizzi M, Tonello S, Matino E, Costanzo M, Rizzi E, et al. Ongoing mycophenolate treatment impairs anti-SARS-coV-2 vaccination response in patients affected by chronic inflammatory autoimmune diseases or liver transplantation recipients: results of the RIVALSA prospective cohort. Viruses (2022) 14:1766. doi: 10.3390/v14081766
17. Herting A, Jahnke-Triankowski J, Harberts A, Schaub GM, Lütgehetmann M, Ruether DF, et al. Clinical outcomes of SARS-CoV-2 breakthrough infections in liver transplant recipients during the omicron wave. Viruses (2023) 15:297. doi: 10.3390/v1502029
18. Chang A, Strauss AT, Alejo JL, Chiang TP, Hernandez NF, Zeiser LB, et al. Letter to the editor: Six-month antibody kinetics and durability in liver transplant recipients after two doses of SARS-CoV-2 mRNA vaccination. Hepatol Commun (2022) 6(10):2990–2. doi: 10.1002/hep4.2027
19. Balsby D, Nilsson AC, Petersen I, Lindvig SO, Davidsen JR, Abazi R, et al. Humoral immune response following a third SARS-CoV-2 mRNA vaccine dose in solid organ transplant recipients compared with matched controls. Front Immunol (2022) 13:1039245. doi: 10.3389/fimmu.2022.1039245
20. Cholankeril G, Al-Hillan A, Tarlow B, Abrams D, Jacobs JS, Flores NP, et al. Clinical factors associated with lack of serological response to SARS-CoV-2 messenger RNA vaccine in liver transplantation recipients. Liver Transpl (2022) 28(1):123–6. doi: 10.1002/lt.26351
21. John BV, Deng Y, Khakoo NS, Taddei TH, Kaplan DE, Dahman B. Coronavirus disease 2019 vaccination is associated with reduced severe acute respiratory syndrome coronavirus 2 infection and death in liver transplant recipients. Gastroenterology (2022) 162(2):645–7.e642. doi: 10.1053/j.gastro.2021.11.001
22. Cuadrado A, Del Barrio M, Fortea JI, Amigo L, San Segundo D, Rodriguez-Cundin MP, et al. Antibody response to the messenger RNA-1273 vaccine (Moderna) in liver transplant recipients. Hepatol Commun (2022) 6(7):1673–9. doi: 10.1002/hep4.1937
23. D'Offizi G, Agrati C, Visco-COmandini U, Castilletti C, Puro V, Piccolo P, et al. Coordinated cellular and humoral immune responses after two-dose SARS-CoV2 mRNA vaccination in liver transplant recipients. Liver Int (2022) 42(1):180–6. doi: 10.1111/liv.15089
24. Davidov Y, Indenbaum V, Tsaraf K, Cohen-Ezra O, Likhter M, Ben Yakov G, et al. A third dose of the BNT162b2 mRNA vaccine significantly improves immune responses among liver transplant recipients. J Hepatol (2022) 77(3):702–9. doi: 10.1016/j.jhep.2022.03.042
25. Davidov Y, Tsaraf K, Cohen-Ezra O, Likhter M, Ben Yakov G, Levy I, et al. Immunogenicity and adverse effects of the 2-dose BNT162b2 messenger RNA vaccine among liver transplantation recipients. Liver Transpl (2022) 28(2):215–23. doi: 10.1002/lt.26366
26. Fernandez-Ruiz M, Almendro-Vazquez P, Carretero O, Ruiz-Merlo T, Laguna-Goya R, San Juan R, et al. Discordance between SARS-CoV-2-specific cell-mediated and antibody responses elicited by mRNA-1273 vaccine in kidney and liver transplant recipients. Transplant Direct (2021) 7(12):e794. doi: 10.1097/TXD.0000000000001246
27. Furian L, Russo FP, Zaza G, Burra P, Hartzell S, Bizzaro D, et al. Differences in humoral and cellular vaccine responses to SARS-CoV-2 in kidney and liver transplant recipients. Front Immunol (2022) 13:853682. doi: 10.3389/fimmu.2022.853682
28. Giannella M, Righi E, Pascale R, Rinaldi M, Caroccia N, Gamberini C, et al. Evaluation of the kinetics of antibody response to COVID-19 vaccine in solid organ transplant recipients: the prospective multicenter ORCHESTRA cohort. Microorganisms (2022) 10(5):1021. doi: 10.3390/microorganisms10051021
29. Harberts A, Schaub GM, Ruether DF, Duengelhoef PM, Brehm TT, Karsten H, et al. Humoral and cellular immune response after third and fourth SARS-CoV-2 mRNA vaccination in liver transplant recipients. Clin Gastroenterol Hepatol (2022) 20(11):2558–66.e5. doi: 10.1016/j.cgh.2022.06.028
30. Guarino M, Esposito I, Portella G, Cossiga V, Loperto I, Tortora R, et al. Humoral response to 2-dose BNT162b2 mRNA COVID-19 vaccination in liver transplant recipients. Clin Gastroenterol Hepatol (2022) 20(7):1534–41.e1534. doi: 10.1016/j.cgh.2022.01.012
31. Herrera S, Colmenero J, Pascal M, Escobedo M, Castel MA, Sole-González E, et al. Cellular and humoral immune response after mRNA-1273 SARS-CoV-2 vaccine in liver and heart transplant recipients. Am J Transplant (2021) 21(12):3971–9. doi: 10.1111/ajt.16768
32. Huang HJ, Yi SG, Mobley CM, Saharia A, Bhimaraj A, Moore LW, et al. Early humoral immune response to two doses of severe acute respiratory syndrome coronavirus 2 vaccine in a diverse group of solid organ transplant candidates and recipients. Clin Transplant (2022) 36(5):e14600. doi: 10.1111/ctr.14600
33. Meunier L, Sanavio M, Dumortier J, Meszaros M, Faure S, Ursic Bedoya J, et al. Mycophenolate mofetil decreases humoral responses to three doses of SARS-CoV-2 vaccine in liver transplant recipients. Liver Int (2022) 42(8):1872–8. doi: 10.1111/liv.15258
34. Meunier L, Sanavio M, Dumortier J, Meszaros M, Faure S, Ursic Bedoya J, et al. Immune response after a third dose of the mRNA-1273 SARS-CoV-2 vaccine in liver transplant recipients. Transplantation (2022) 106(7):e341–2. doi: 10.1097/TP.0000000000004147
35. Marion O, Del Bello A, Abravanel F, Faguer S, Esposito L, Laure Hebral A, et al. Predictive factors for humoral response after 2-dose SARS-CoV-2 vaccine in solid organ transplant patients. Transplant Direct (2022) 8(1):e1248. doi: 10.1097/TXD.0000000000001248
36. Rabinowich L, Grupper A, Baruch R, Ben-Yehoyada M, Halperin T, Turner D, et al. Low immunogenicity to SARS-CoV-2 vaccination among liver transplant recipients. J Hepatol (2021) 75(2):435–8. doi: 10.1016/j.jhep.2021.04.020
37. Rahav G, Lustig Y, Lavee J, Ohad B, Magen H, Hod T, et al. BNT162b2 mRNA COVID-19 vaccination in immunocompromised patients: A prospective cohort study. EClinicalMedicine (2021) 41:101158. doi: 10.1016/j.eclinm.2021.101158
38. Rashidi-Alavijeh J, Frey A, Passenberg M, Korth J, Zmudzinski J, Anastasiou OE, et al. Humoral response to SARS-Cov-2 vaccination in liver transplant recipients-A single-center experience. Vaccines (Basel) (2021) 9(7):738. doi: 10.3390/vaccines9070738
39. Sakai A, Morishita T, Matsunami H. Antibody response after a second dose of the BNT162b2 mRNA COVID-19 vaccine in liver transplant recipients. Transpl Int (2022) 35:10321. doi: 10.3389/ti.2022.10321
40. Raszeja-Wyszomirska J, Janik MK, Wojcicki M, Milkiewicz P. SARSCoV2 vaccination in liver transplant recipients: factors affecting immune response and refusal to vaccine. Pol Arch Intern Med (2022) 132(7-8):16274. doi: 10.20452/pamw.16274
41. Ruether DF, Schaub GM, Duengelhoef PM, Haag F, Brehm TT, Fathi A, et al. SARS-CoV2-specific humoral and T-cell immune response after second vaccination in liver cirrhosis and transplant patients. Clin Gastroenterol Hepatol (2022) 20(1):162–72.e169. doi: 10.1016/j.cgh.2021.09.003
42. Sharma A, Tiwari S, Deb MK, Marty JL. Severe acute respiratory syndrome coronavirus 2 antibody response to a third dose of homologous messenger RNA vaccination in liver transplantation recipients. Liver Transpl (2022) 28(8):1393–6. doi: 10.1002/lt.26472
43. Tang R, Li C, Wu G, Tong X, Yu L, Hao H, et al. Safety analysis of COVID-19 vaccines in liver transplant recipients: a two-center study. Hepatobiliary Surg Nutr (2022) 11(1):166–8. doi: 10.21037/hbsn-21-392
44. Toniutto P, Cussigh A, Cmet S, Bitetto D, Fornasiere E, Fumolo E, et al. Immunogenicity and safety of a third dose of anti-SARS-CoV-2 BNT16b2 vaccine in liver transplant recipients. Liver Int (2022) 43(2):452–61. doi: 10.1111/liv.15331
45. Tu ZH, Jin PB, Chen DY, Chen ZY, Li ZW, Wu J, et al. Evaluating the response and safety of inactivated COVID-19 vaccines in liver transplant recipients. Infect Drug Resist (2022) 15:2469–74. doi: 10.2147/IDR.S359919
46. Erol Ç, Yanık Yalçın T, Sarı N, Bayraktar N, Ayvazoğlu Soy E, Yavuz Çolak M, et al. Differences in antibody responses between an inactivated SARS-CoV-2 vaccine and the BNT162b2 mRNA vaccine in solid-organ transplant recipients. Exp Clin Transplant (2021) 19(12):1334–40. doi: 10.6002/ect.2021.0402
47. Mazzola A, Todesco E, Drouin S, Hazan F, Marot S, Thabut D, et al. Poor antibody response after two doses of severe acute respiratory syndrome coronavirus 2 (SARS-coV-2) vaccine in transplant recipients. Clin Infect Dis (2022) 74(6):1093–6. doi: 10.1093/cid/ciab580
48. Saharia KK, Husson JS, Niederhaus SV, Iraguha T, Avila SV, Yoo YJ, et al. Humoral immunity against SARS-CoV-2 variants including omicron in solid organ transplant recipients after three doses of a COVID-19 mRNA vaccine. Clin Transl Immunol (2022) 11(5):e1391. doi: 10.1002/cti2.1391
49. Strauss AT, Hallett AM, Boyarsky BJ, Ou MT, Werbel WA, Avery RK, et al. Antibody response to severe acute respiratory syndrome-coronavirus-2 messenger RNA vaccines in liver transplant recipients. Liver Transpl (2021) 27(12):1852–6. doi: 10.1002/lt.26273
50. Timmermann L, Globke B, Lurje G, Schmelzle M, Schöning W, Öllinger R, et al. Humoral immune response following SARS-CoV-2 vaccination in liver transplant recipients. Vaccines (Basel) (2021) 9(12):1422. doi: 10.3390/vaccines9121422
51. Vetter P, Eckerle I, Kaiser L. Covid-19: a puzzle with many missing pieces. BMJ (2020) 368:m627. doi: 10.1136/bmj.m627
52. Hossein-Khannazer N, Shokoohian B, Shpichka A, Aghdaei HA, Timashev P, Vosough M. Novel therapeutic approaches for treatment of COVID-19. J Mol Med (Berl) (2020) 98(6):789–803. doi: 10.1007/s00109-020-01927-6
53. Luo D, Chen X, Du J, Mei B, Wang A, Kuang F, et al. Immunogenicity of COVID-19 vaccines in chronic liver disease patients and liver transplant recipients: A systematic review and meta-analysis. Liver Int (2023) 43(1):34–48. doi: 10.1111/liv.15403
54. Cheung KS, Mok CH, Mao X, Zhang R, Hung IF, Seto WK, et al. COVID-19 vaccine immunogenicity among chronic liver disease patients and liver transplant recipients: A meta-analysis. Clin Mol Hepatol (2022) 28(4):890–911. doi: 10.3350/cmh.2022.0087
55. Shi G. Variation of CD4+CD25+Foxp3+ regulatory T cells and Th17 cells in the peripheral blood of human liver allograft patients with long-term survival. Transplant Proc (2017) 49(8):1834–40. doi: 10.1016/j.transproceed.2017.06.026
56. Jarjour NN, Masopust D, Jameson SC. T cell memory: understanding COVID-19. Immunity (2021) 54(1):14–8. doi: 10.1016/j.immuni.2020.12.009
57. Novel technical guide for coronavirus vaccination (2021). Available at: http://www.nhc.gov.cn/jkj/s3582/202103/c2febfd04fc5498f916b1be080905771.shtml.
Keywords: liver transplant, SARS-CoV-2, adverse effect, Vaccine, Meta - analysis
Citation: Luo X, Lessomo FYN, Yu Z and Xie Y (2023) Factors influencing immunogenicity and safety of SARS-CoV-2 vaccine in liver transplantation recipients: a systematic review and meta-analysis. Front. Immunol. 14:1145081. doi: 10.3389/fimmu.2023.1145081
Received: 15 January 2023; Accepted: 22 August 2023;
Published: 05 September 2023.
Edited by:
Rongqian Wu, Xi’an Jiaotong University, ChinaReviewed by:
Javier Carbone, Gregorio Marañón Hospital, SpainFan Du, Huazhong University of Science and Technology, China
Copyright © 2023 Luo, Lessomo, Yu and Xie. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yong Xie, eGlleW9uZzcyMTAzMUAxNjMuY29t
†These authors have contributed equally to this work and share first authorship
 Xinyi Luo1,2†
Xinyi Luo1,2† Fabrice Yves Ndjana Lessomo
Fabrice Yves Ndjana Lessomo Zhimin Yu
Zhimin Yu Yong Xie
Yong Xie