
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Immunol., 08 March 2023
Sec. Antigen Presenting Cell Biology
Volume 14 - 2023 | https://doi.org/10.3389/fimmu.2023.1140749
This article is part of the Research TopicInsights in Antigen Presenting Cell Biology: 2022View all 7 articles
 Lili Wu1,2
Lili Wu1,2 Ziqi Yan1,2
Ziqi Yan1,2 Yiyang Jiang1,2
Yiyang Jiang1,2 Yingyi Chen1,2
Yingyi Chen1,2 Juan Du1,2
Juan Du1,2 Lijia Guo3
Lijia Guo3 Junji Xu1,2
Junji Xu1,2 Zhenhua Luo1,2*
Zhenhua Luo1,2* Yi Liu1,2*
Yi Liu1,2*Dendritic cells (DCs) are antigen-presenting cells that bridge innate and adaptive immune responses. Multiple cell types, including DCs, rely on cellular metabolism to determine their fate. DCs substantially alter cellular metabolic pathways during activation, such as oxidative phosphorylation, glycolysis, fatty acid and amino acid metabolism, which have crucial implications for their functionality. In this review, we summarize and discuss recent progress in DC metabolic studies, focusing on how metabolic reprogramming influences DC activation and functionality and the potential metabolic differences among DC subsets. Improving the understanding of the relationship between DC biology and metabolic regulation may provide promising therapeutic targets for immune-mediated inflammatory diseases.
Dendritic cells (DCs) are antigen-presenting cells that coordinate innate and adaptive immune responses (1). DCs not only recognize pathogens and danger signals through pattern recognition receptors (PRRs), activate intracellular cascade signals, and release antimicrobial mediators and inflammatory cytokines to initiate the innate immune response but also take up, process, and present antigens to T lymphocytes, promoting adaptive immune response activation (1, 2).
Cells acquire and use nutrients through metabolism to fulfill their energy and biosynthetic demands for physiological processes (3). Studies have indicated that in human and mouse DCs, DC activation is followed by different metabolic alterations that regulate their survival and immune functions (4–6). Active oxidative phosphorylation (OXPHOS) in mitochondria is related to immature DCs (5), whereas enhanced glycolysis after pathogen sensing can boost immunogenic DC activity (5–7). However, increasing evidence suggests that DC activation involves multiple metabolic pathways, including glycolysis, amino acid, and fatty acid metabolism. In various pathological situations, the regulation of DC metabolism after immunogenic activation is complex, i.e. changes in metabolic pathways, molecular signaling pathways regulating cellular metabolism, as well as metabolites and nutrients may affect the function of DCs (4, 8). In addition, different subsets of DCs exhibit distinct metabolic response specializations (4).
Thus, improving the understanding of the impact of metabolic regulation on quiescent DCs and immunogenic DC activation is important. This review highlights the relationship between metabolic adaptations and functional DCs, particularly in inflammation, to identify new therapeutic prospects for inflammatory and immune diseases.
DCs are a heterogeneous group of immune cells found in lymphoid tissues (e.g., the lymph nodes [LNs], spleen, and bone marrow [BM]), as well as in the majority of nonlymphoid tissues (9, 10). Classical DCs (cDCs) can be divided into two major subsets: IRF8-dependent cDC1s and IRF4-dependent cDC2s (9). cDC1s are efficient at cross-presenting antigens to CD8+ T cells, and cDC2s specialize in CD4+ T-cell activation and cytokine generation (9). By contrast, several non-classical DC subsets play a crucial role in peripheral immune surveillance and the infection response. Plasmacytoid DCs (pDCs) are powerful Type-I interferon makers that play a crucial role in viral defense. The subpopulations also contain monocyte-derived “inflammatory DCs” (infDCs), which have a consequence of inflammation or infection (11). The principal characteristics of DC subsets have been described (9, 12–14), and in this section, we briefly review the current models of DCs development (11, 15).
As DCs are relatively few in vivo, several ex vivo experimental models have been established to investigate DC biology (Table 1). For studying human DC physiology, monocyte-derived DCs (moDCs) (16), generated from circulating monocytes stimulated with granulocyte-macrophage colony-stimulating factor (GM-CSF) and interleukin-4 (IL-4), have become a commonly used model (17–19). In addition, mouse DCs in vitro are typically produced from BM induced by GM-CSF and IL-4 (BMDCs). MoDCs and BMDCs are the most functionally similar to immature DCs (20, 21), and they are critical for studying DC metabolism and biology.
During homeostasis, DCs are mostly quiescent in peripheral tissues (Figure 1). In resting DCs, glucose is converted to pyruvate through glycolysis (22–24). Some pyruvate is metabolized to lactate, but the majority is sent to the tricarboxylic acid (TCA) cycle via acetyl-CoA (24, 25) (Figure 2). The mitochondrial electron transport chain receives electron donations from NADH, which is produced by the TCA cycle and predominantly regulated by the Liver Kinase B1 (LKB1)-AMP-activated protein kinase (AMPK) axis (3, 7, 25–27). LKB1 plays a key role in cellular metabolism by controlling AMPK activation (28). LKB1 activates AMPK and AMPK-related kinases, which leads to the upregulation of catabolic pathways and mitochondrial biogenesis while inhibiting anabolic processes (29). Activated AMPK can inhibit mTOR complex 1 (mTORC1) either directly or indirectly via its downstream target Tuberous Sclerosis Complex 1 and 2 (TSC1/TSC2) (29). In addition, LKB1 maintains mouse CD11c+ DC quiescence in an mTOR-dependent manner, and LKB1 deficiency promotes CD11c+ DC activation and metabolic profiles, indicating that LKB1 coordinates immunological and metabolic quiescence in DCs (30). However, in LKB1-deficient CD11c+DCs, mTOR inhibition only partially compensated for the loss of LKB1, implying that LKB1 has other targeting pathways to maintain the quiescent state (28, 30, 31).
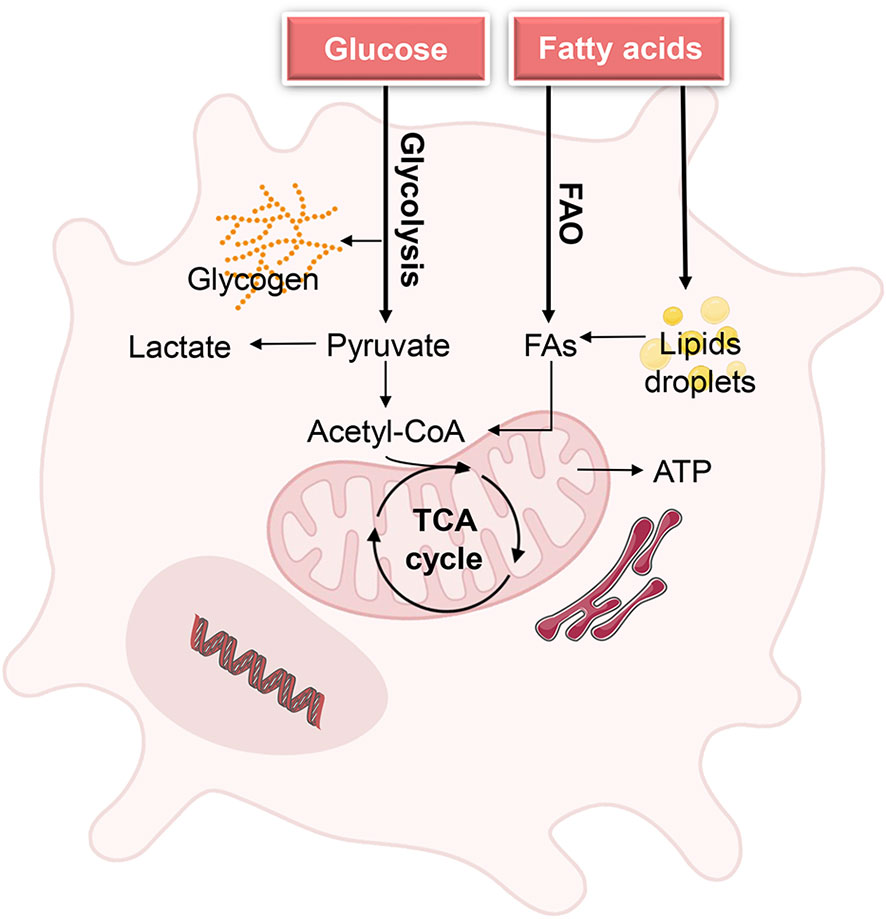
Figure 1 Metabolic demands of DCs during homeostasis. Quiescent DCs in peripheral tissues require glucose and fatty acids as fuels to generate energy for cell maintenance and to build up intracellular glycogen and lipid storage. The metabolic state of quiescence is characterized by active oxidative phosphorylation (OXPHOS), which is driven by the tricarboxylic acid (TCA) cycle Biorender.com.
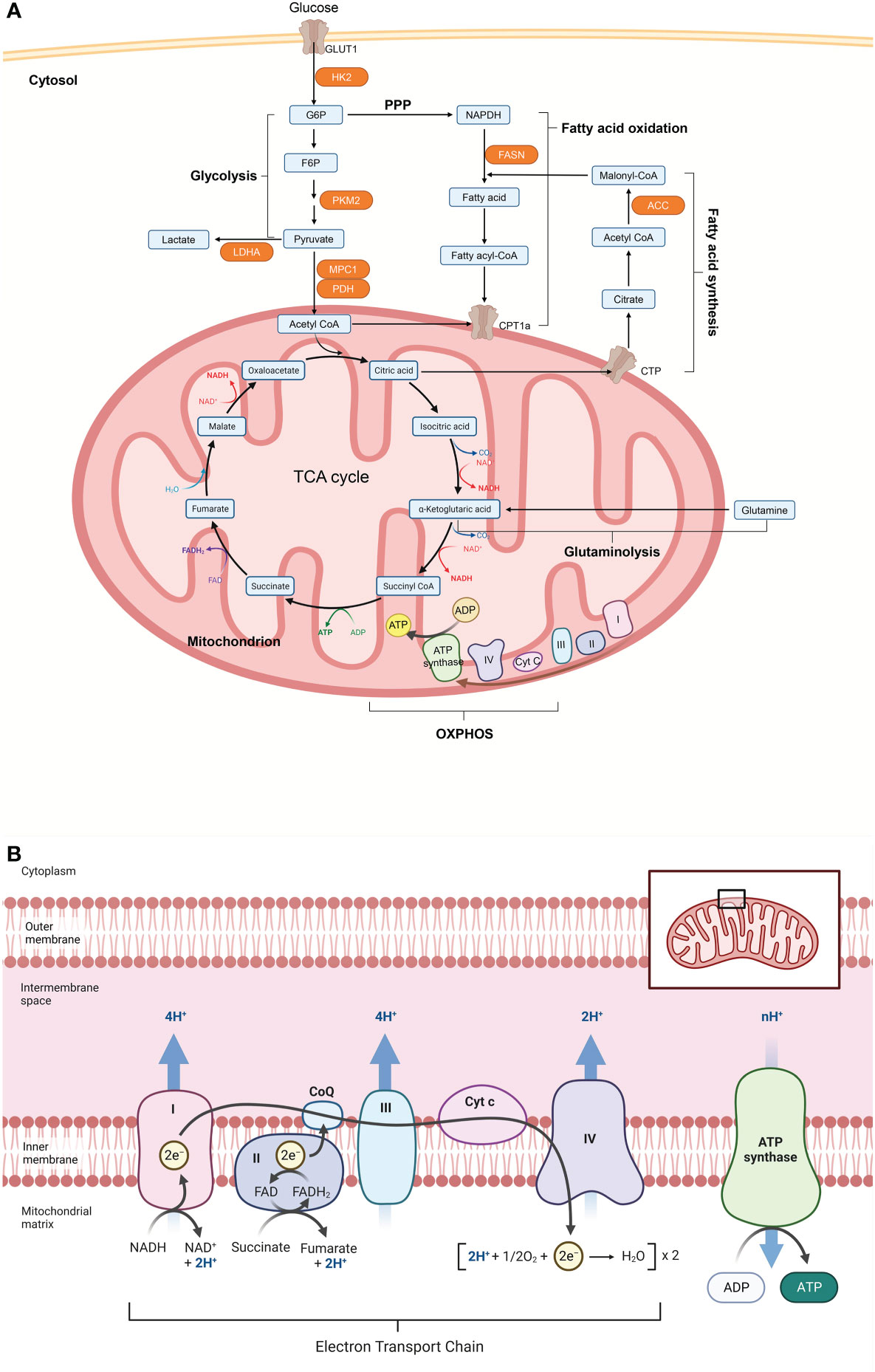
Figure 2 Cellular metabolism networks. (A) Glucose is imported from the extracellular environment and can either be converted to glycogen or oxidized during glycolysis to produce adenosine triphosphate (ATP). Pyruvate produced by glycolysis can be partially oxidized to lactate to quickly regenerate the consumed nicotinamide adenine dinucleotide (NADH), or it can be translocated into the mitochondria and completely oxidized via the tricarboxylic acid (TCA) cycle. The TCA cycle can also be powered by fatty acids through fatty acid oxidation or glutamine through glutaminolysis. Electrons released by glycolysis and the TCA cycle enter the electron transport chain, which is made up of complex I-V (CI-CV), where ATP is produced through oxidative phosphorylation (OXPHOS). (B) Acetyl-CoA from pyruvate enters the TCA cycle in mitochondria. Reactions in the cycle produce NADH and FADH, which act as substrates for the electron transport chain (ETC) and thus support OXPHOS and ATP production. Figure 2 was created with Biorender.com. ACC, acetyl-CoA carboxylase; CoA, coenzyme A; CPT1a, carnitine palmitoyltransferase 1; CTP, citrate transport protein; F6P, fructose 6 phosphate; FASN, fatty acid synthase; G6P, glucose 6 phosphate; GLUT1, glucose transporter 1; HK2, hexokinase 2; LDHA, lactate dehydrogenase A; MPC1, mitochondrial pyruvate carrier 1; NADPH, nicotinamide adenine dinucleotide phosphate; PDH, pyruvate dehydrogenase; PKM2, pyruvate kinase isozyme M2;PPP, pentose phosphate pathway.
Furthermore, resting DCs rely on catabolic metabolism to degrade nutrients and generate energy for cell maintenance. The degradation of proteins and triacylglycerols, for instance, yields amino acids (AAs) and fatty acids (FAs), respectively, as fuels for the TCA cycle within mitochondria (7). Additionally, fatty acid oxidation (FAO) was reported to be a key energy metabolic pathway in immature BMDCs, sustaining their survival (7). In steady-state DCs, lipids from the local microenvironment can serve as essential fuel for FAO. Furthermore, resting DCs accumulate glycogen intracellularly, utilize it to fulfill basal glycolytic requirements, and provide metabolic substrates for OXPHOS (32).
When DCs detect changes in the homeostatic state caused by pathogens or tissue-derived inflammatory signals, they shift from the resting state to the active state. Research has shown that metabolic pathways regulate immunogenic DC activation and the subsequent immune responses (6, 19, 33). Here, we discuss the results of recent studies on the metabolic regulation of DC activation, particularly glycolysis. Notably, most of the data on DC metabolism are acquired from DC culture models, namely, BMDCs (mouse systems) (34) and moDCs (human systems) (11).
Upon immunogenic activation, DCs frequently convert catabolic metabolism, marked by FAO and mitochondrial respiration, to anabolic metabolism, with increased glycolytic activity and decreased OXPHOS (33). Glycolysis is a key component of glucose metabolism that transforms glucose into pyruvate in the cytoplasm (22–24). The majority of the generated pyruvate transforms into lactate—instead of entering the TCA cycle in the mitochondria—even if oxygen is available. These are classic characteristics of aerobic glycolysis, often known as Warburg metabolism.
Growing evidence suggests that increased glycolysis promotes DC activation and pro-inflammatory function (Figure 3), even in different DC cultures and subsets in/ex vivo (7, 27, 35, 36). When the methodologies for extracellular acidification and oxygen consumption rates were employed to evaluate BMDC metabolism, stimulation with TLR agonists such as LPS (TLR4) increased glycolytic flux in BMDCs within minutes (7, 37–39). A rapid increase in glycolysis has also been observed in response to LPS activation in moDCs (39). In addition, pharmacological inhibition of glycolysis with 2-deoxyglucose (2-DG) or deficiencies in glycolytic enzymes such as alpha-enolase (ENO1) can significantly impair BMDC maturation and subsequent T-cell activation (7, 36, 40, 41). Similarly, glycolysis is important for DC function in vivo. LPS-driven activation of splenic cDC subsets in vivo was effectively diminished when mice were given a 2-DG injection to block glycolysis at the same time. Their ability to release IL12 and prime ovalbumin (OVA)-specific CD4 and CD8 T cells in response to LPS was diminished in the presence of 2-DG (41). However, another study found that glucose has a contrasting function in DCs, as it represses the proinflammatory output of LPS-stimulated BMDCs, and negatively affects DC-induced T-cell responses (42). Directly limiting the rate of glycolysis or switching BMDCs from glucose to galactose prevented LPS-stimulated BMDCs from glycolytic reprogramming (42). Furthermore, glucose-deprived BMDCs expressed more costimulatory molecules (CD80, CD86) and IL12 (42), which are known to be important for the induction of T-cell proliferation and the regulation of T-cell effector functions (43).
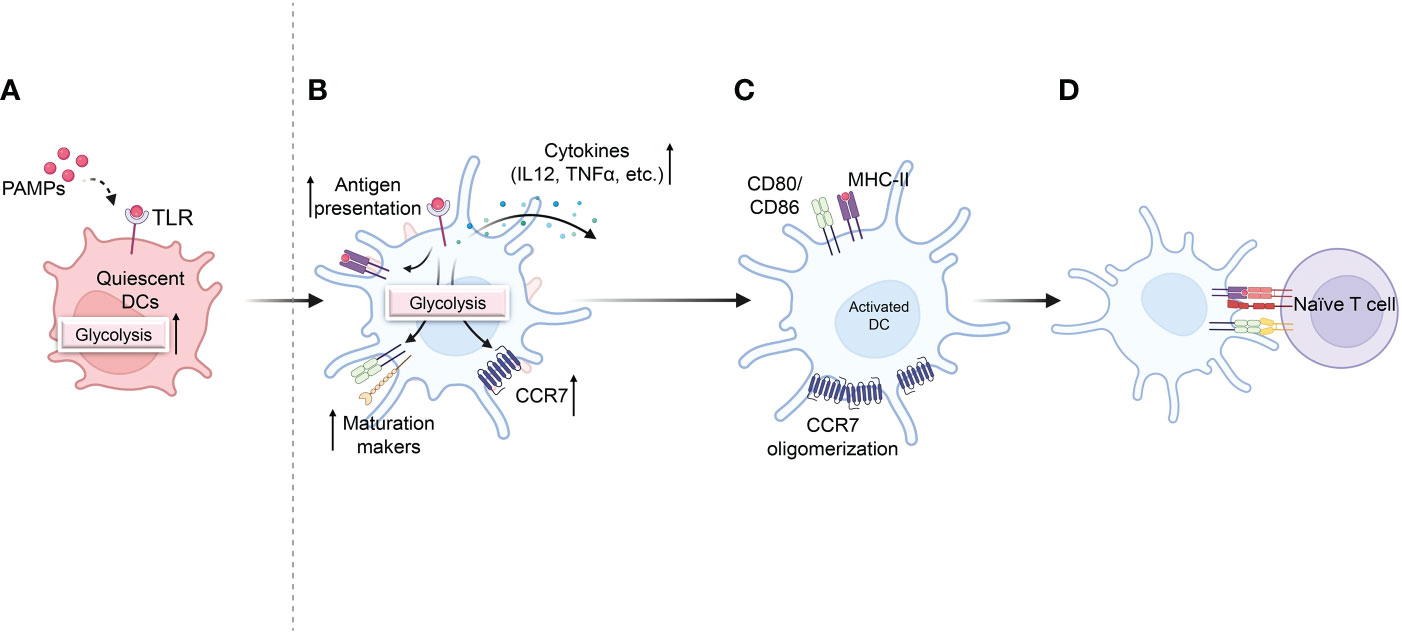
Figure 3 Effect of glycolysis on DC activation and pro-inflammatory activity. (A) Quiescent DCs recognize pathogen-associated molecular patterns (PAMPs) that are required for DC maturation, along with a rapid increase in glycolysis. (B) Increased glycolysis increases DC activation and antigen presentation after TLR activation. (C) Glycolysis also promotes the migration of activated DCs by stimulating CCR7 oligomerization. (D) Activated DCs in lymph nodes express co-stimulatory markers, initiating T-cell priming Biorender.com.
Furthermore, glycolysis is required for cytoskeletal modifications that allow activated BMDCs to shape and migrate (44). DCs upregulate CCR7 and migrate into lymphatic capillaries, producing CCR7 ligand CCL21 upon TLR activation (Figure 3) (45). Suppression of glycolysis impairs dendritic cell shape maintenance, CCR7 oligomerization, and BMDC migration to draining LNs (41, 44). To determine the effect of glycolysis on DC migration in vivo, the ability of differentially activated DCs injected in the footpad to migrate to draining LNs was investigated using CFSE-labelled BMDCs (7). BMDCs stimulated with OVA and LPS were more abundant in the LNs than BMDCs stimulated with OVA alone. 2-DG treatment reduced the number of LPS-activated OVA-pulsed DCs in the draining LNs but did not eliminate the effects of LPS (7). Finally, an animal model of allergic asthma induced by HDM was established to monitor DC migration from the lung to the mediastinal LN to investigate the migration of endogenous DCs in vivo (46). Administration of 2-DG during allergic inflammation did not significantly impair the accumulation and migration of total immune cells but reduced the migration of endogenous CD11c+MHCIIhi DCs to the lungs in response to HDM (44). These findings suggest that initiating glycolytic metabolism is critical for full DC maturation and subsequent migration.
Moreover, the effects of glycolysis on DC phagocytosis have not been consistently described. After the BMDCs had been exposed to LPS, hypoxia, or hypoxia and LPS for 24 hours, fluorescently labeled OVA was added to assess the uptake capacity of the differentially treated DC (36). LPS and/or hypoxia decrease the ability of BMDCs to engulf antigens but increase glycolytic activity (36), which has also been reported in moDCs after pathogen-associated molecular patterns (PAMPs) stimulation (47). In the presence of PAMPs, 2-DG activated the inositol-requiring protein 1 (IRE1)/X-box-binding protein 1 (XBP1) arm of the unfolded protein response (UPR) in moDCs, whereas moDCs showed robust phagocytosis as well as a robust ability to release arachidonic acid (47). Nevertheless, a separate study examined the effect of aging on Ag acquisition, processing, and presentation by DCs using a well-established model of cross-presentation (the expression of MHC-peptide on the DC surface). And the study revealed that reduced phagocytic activity in aged mouse splenic cDC1s is associated with mitochondrial dysfunction but not glycolysis (48).
In conclusion, these studies indicate that glycolysis is important for DC activation and pro-inflammatory activity.
DCs undergo two rounds of metabolic reprogramming after activation. These events are triggered by different signaling pathways. TBK1/IKKε/Akt signaling axis mediates early glycolytic reprogramming in BMDCs (Figure 4) (41). Within minutes of TLR stimulation, the glycolytic rate of BMDCs doubled and remained elevated for several hours, independent of iNOS signaling (41). It stimulates a non-classical AKT signaling pathway, specifically the TBK1/IKKε pathway, which phosphorylates the glycolysis rate-limiting enzyme, hexokinase 2 (HK2). HK2 then binds to voltage-dependent anion channels on the outer mitochondrial membrane, promoting hexokinase activity (41). These processes improve the glycolytic rate and support early glycolysis induction in LPS-stimulated BMDCs and mouse splenic cDCs (41). Targeted inhibition of TBK1, IKK, or AKT or blocking the binding of HK2 to mitochondria significantly suppressed TLR agonist-induced activation of BMDCs (41). Metabolite tracking experiments have demonstrated that early glycolysis promotes the activation of the pentose phosphate pathway and the preferential production of citrate (41, 49). The citrate generated stimulated fatty acid synthesis (FAS) for endoplasmic reticulum (ER) and Golgi body enlargement to support the increased demand for protein synthesis and transport necessary for BMDC maturation (6, 41, 49, 50). In addition, citrate metabolism promotes the formation of acetyl-CoA, which is required for the epigenetic regulation of glycolytic enzymes such as HK2 (25, 51, 52).
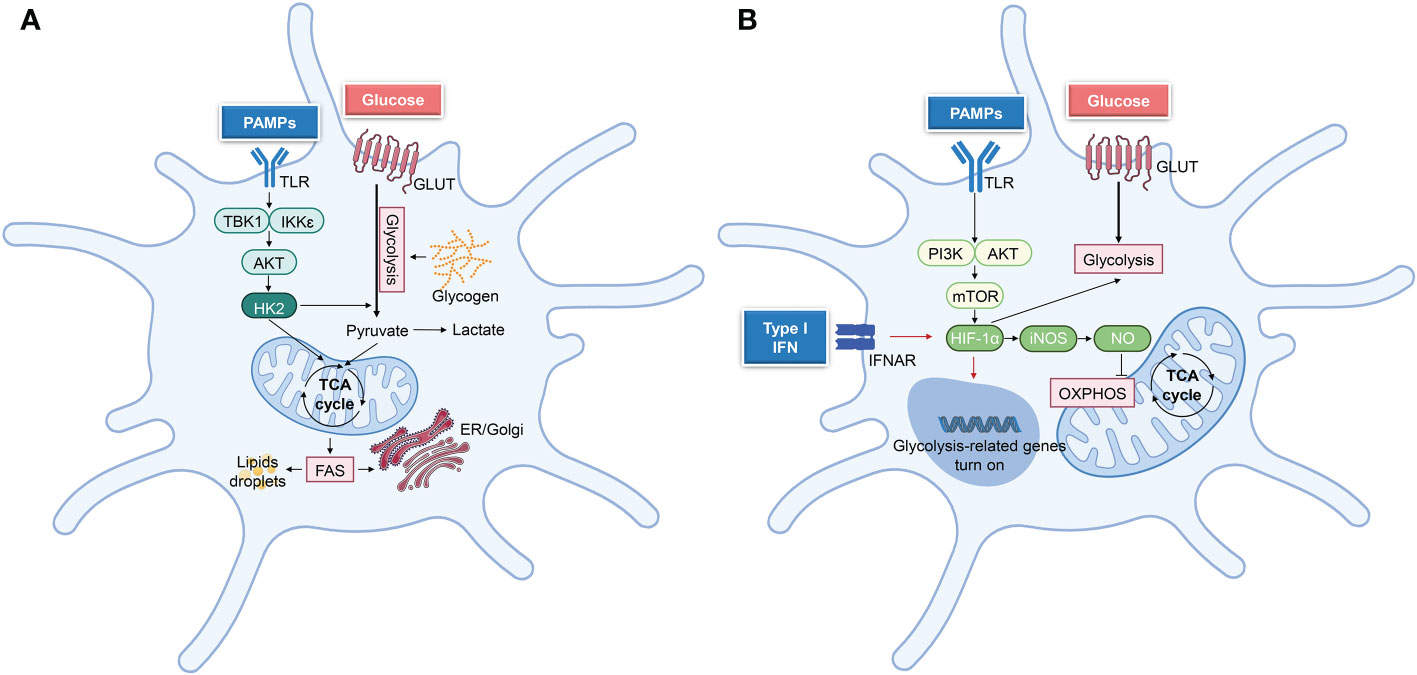
Figure 4 Principal metabolic reprogramming pathways after DCs activation. (A) TBK1-IKKε-Akt-HK2 signaling regulates the short-term (minutes to hours following TLR ligation) metabolic regulation in DCs, which undergo rapid metabolic reprogramming. This signaling pathway stimulates glycolysis, which in turn supports other metabolic processes, such as fatty acid synthesis (FAS) and lipid synthesis, hence facilitating DC activation. (B) In the later stage of DCs activation, iNOS-dependent glycolysis was performed. TLR ligation triggers PI3K/AKT, and mTOR-HIF1α pathways to promote glycolysis, whereas iNOS-NO suppresses OXPHOS. While activated cDCs do not undergo iNOS-dependent glycolysis in vivo, autocrine type-I IFN signaling triggers HIF-1α-mediated glycolytic reprogramming Biorender.com.
Subsequently, a NO-dependent second wave of glycolytic metabolism is driven by mTOR/HIF1α/iNOS in iNOS-expressing DCs (BMDCs) (Figure 3) (38, 53, 54). After LPS stimulation of BMDCs (14 h or more), the PI3K/AKT/mTOR pathway is activated, which upregulates glycolytic enzymes, including phosphofructokinase (PFK), pyruvate kinase 2 (PKM2), and glucose transporters such as GLUT1 (7, 44), which may ultimately increase the extracellular acidification rate (ECAR) (42, 55). In addition, mTOR induces glycolysis through the activation of HIF1α, which upregulates iNOS expression (42, 56) and suppresses NO-mediated mitochondrial activity and OXPHOS in TLR-activated BMDCs (38, 54). Mitochondrial impairment induces a sustained glycolytic metabolism in activated BMDCs to maintain cellular ATP generation and cell growth in the absence of a functional respiratory chain (32, 41, 55). Moreover, iNOS inhibitors can rescue mitochondrial respiration in LPS-activated BMDCs (38).
Unlike BMDCs, the majority of DC subsets, such as natural mouse cDCs and moDCs, do not exhibit detectable levels of iNOS (53). To maintain glycolysis, iNOS-deficient DCs depend on additional mechanisms, such as type-I interferon (IFN) signaling and HIF1α (Figure 4). HIF1α contributes to the increased glycolytic activity of mouse cDCs in vivo, BMDCs, and moDCs in vitro (35, 36, 57). Additionally, intracellular pyruvate or lactate generated by glycolysis can activate HIF1α (58, 59). Activated CD11c+MHCII+ cDCs promote autocrine IFN-I signaling via HIF1α, which decreases mitochondrial OXPHOS levels, increases glycolytic flow, and supplies sufficient ATP for cell activation and survival (57). HIF1α regulates multiple glycolytic genes, including lactate dehydrogenase A (LDHA) and GLUT1, which may be downregulated in DCs once HIF1α loses (36, 42, 47, 60). However, weak activation fails to stabilize HIF1α and induce its target genes, but strong BMDC stimulation results in long-term activation of glycolysis (44). Weak stimulation nevertheless elicits early glycolysis (44), indicating that HIF1α is involved in the maintenance rather than the initial induction of glycolysis (47). However, LPS-activated BMDCs have been shown to exhibit increased IL-12 levels and CD8+ T-cell activation in the absence of HIF1α (42). Hence, additional research is required to determine the precise contribution of HIF1α to DC metabolism.
Extracellular glucose and intracellular glycogen serve as the energy source for glycolysis, which is crucial for the survival and function of activated DCs (7, 44, 61). At later stages after LPS stimulation, overexpression of glucose transporters such as GLUT1 increases extracellular glucose absorption (7, 32, 44), whereas inhibition of GLUT1 suppresses the expression of co-stimulatory molecules in BMDCs (31). In addition to the direct utilization of extracellular glucose, intracellular glycogen can be utilized to fulfill the metabolic demands of BMDCs and moDCs through the glycolysis pathway. During the first 6 h after TLRs activation, glycogenolysis of intracellular glycogen stores may sustain enhanced glycolysis more than extracellular glucose (31). Additionally, glycogen phosphorylase inhibitor CP91149 inhibits BMDC maturation and function, particularly during the early stage of activation (31).
FA metabolism is important for the development and function of DCs (62). FAS causes BMDCs to increase lipid storage in lipid bodies (LBs) (41). Increased lipid concentrations in LPS-stimulated BMDCs are closely associated with improved antigen presentation and T-cell activation, suggesting that the de novo synthesis of FAs may regulate the immunogenicity of BMDCs (62). Correspondingly, downregulating lipid levels on the membranes of BMDCs through high-density lipoprotein (HDL) and ApoA-I can result in tolerant DCs and reduce T-cell responses (63). Moreover, FAS blockade in LPS-stimulated BMDCs by fatty acid synthase (FASN) or acetyl-CoA carboxylase inhibitors C75 and TOFA, or by the suppression of the mitochondria–cytosol citrate shuttle citrate transport protein, inhibits BMDC activation and pro-inflammatory functions (41).
In LPS-stimulated BMDCs, de novo synthesis of FAs is the basis for the Golgi apparatus and ER enlargement (33, 41). CD11c+MHCII+ DCs isolated from mouse and human livers with high concentrations of lipid increased ER stress and limited their capacity to trigger an immunological response (62). Additionally, PAMP-stimulated moDCs release IL-23 by activating the ER stress response (47). Another study showed that ER stress, characterized by the accumulation of unfolded proteins in the ER lumen, may cause cell death in activated BMDCs (64). However, the UPR can prevent this (64). The UPR coordinates ER expansion and promotes cellular viability by targeting mRNAs encoding XBP1 and IRE1 to increase the synthesis of FAs for ER membranes and proteins that comprise the folding machinery (33, 64).
Lipid accumulation in DCs could be due to increased FAS or increased lipid uptake. Electrospray ionization mass spectroscopy (ESI-MS) analysis of lipid content revealed that BMDCs cultured with tumor explant supernatants (TES) had higher levels of triacylglycerol (TAG), while no changes in the levels of phospholipids and cholesteryl-esters were observed in these DCs (65). The accumulation of oxidized lipids, particularly TAG, can lead to BMDC dysfunction and shorten its lifespan (66). Scavenger receptors (SRs) are an effective route for DCs to acquire fatty acids (67). Experiments with the soluble SR ligand fucoidan and specific antibody to block macrophage scavenger receptor (Msr 1), as well as experiments with Msr1−/− mice, demonstrated that up-regulation of Sra was primarily responsible for increased uptake of exogenous lipids by BMDCs and cDCs (65, 68). When compared to wild-type cells, Msr1-deficient BMDCs displayed a more mature phenotype after LPS stimulation (69, 70), were more responsive to inflammatory stimuli, and had a more effective antigen-presenting capability (69). Fatty acids are most likely transferred to DCs in the form of modified lipoproteins. The molecular mechanisms remain unclear and require further research.
Thus, the regulation of FAS and ER stress, and lipid uptake can affect the function of activated DCs in cytokine release and T-cell activation (27, 33, 65), and further research is required to improve the understanding of the regulation of their metabolic pathways.
AAs are involved in various metabolic activities and are crucial for controlling DCs’ function. DCs are vulnerable to environmental changes caused by AA concentrations. In immature moDCs, an imbalance in intracellular AAs impairs mitochondrial activity, decreasing ATP production and increasing glucose uptake, which cannot be further increased by LPS stimulation (71). The plasma of liver cirrhosis patients typically reveals an imbalance between lower levels of branched-chain amino acids (BCAAs) and higher levels of aromatic AAs. moDCs cultured in a medium containing similar AA concentrations showed impaired maturation, IL-12 secretion, and migratory potential after LPS stimulation (71, 72). LPS treatment increased BCAAs uptakes, such as isoleucine, leucine, and valine, in moDCs. BCAAs deficiency, particularly valine deficiency, can inhibit the maturation of moDCs, evidenced by decreased co-stimulated molecular expression (CD40, CD80, CD86, HLA-DR) (71). Another study revealed that BCAAs might regulate human moDC metabolism through the mTOR pathway to affect moDC maturation (73).
It was also demonstrated that LPS-stimulated moDCs increase the absorption of glutamate, cysteine, and aspartate (71). However, blocking the activity of the cystine or glutamate antiporter decreased glutathione synthesis but had no impact on the maturation and antigen uptake of moDCs (74). Additionally, inhibiting glutaminolysis did not affect the metabolic activities that occurred 6 h after LPS treatment, and reducing glutamine in BMDCs culture media (from 2 to 0 mM) did not affect co-stimulated molecular expression (32, 41). However, treatment with l-homocysteine acid (LHC), a glutathione production inhibitor, impairs the ability of murine spleen CD11c +DCs to present antigens to CD4+ and CD8 + T lymphocytes (74).
The role of AAs in DC activation has not been elucidated, and further research is required to determine the significance of various AAs in DC metabolism.
DCs are extremely rare in tissues (<2%), and the isolated procedures are complex, necessitating lengthy enzymatic digestion steps that may affect their phenotype and activity. Furthermore, once cultured ex vivo, they are vulnerable to spontaneous activation and cell death (75, 76). Hence, the majority of the data on DC metabolism are based on the use of in vitro-generated DCs. There are several differences in cellular metabolic programs between in vitro-generated DC models and ex vivo primary DC subsets (Tables 1, 2).
The most widely used in vitro DC models are BMDCs. Notably, BMDCs in culture are heterogeneous and contain a population of CD11c+MHC-II+ Macrophages (GM-Macs) (34). In contrast with BMDCs, GM Macs are mainly immobile and release high quantities of inflammatory cytokines and chemokines in response to microbial stimulation (34). BMDCs and GM Macs have distinct functional properties, which means that their metabolic requirements may be different. Notably, differences in culture conditions can change the ratio of cultured BMDCs to GM-Macs, which may result in different experimental results in the field of cellular metabolism. Furthermore, activated GM-Macs express iNOS, which suppresses mitochondrial activity and OXPHOS by producing NO (77, 78), changing the cellular metabolic machinery. After LPS activation, an increase in glycolysis is caused by NO-induced inhibition of OXPHOS in LPS-stimulated BMDCs cultures (7, 38). Therefore, using a BMDCs model to study DC metabolism is not ideal.
Later, a protocol that relies on the addition of Flt3L to bone marrow progenitors was developed (79). Flt3L is the main growth factor driving DC differentiation, and its addition results in DC subsets similar to those found in the spleen under resting conditions. However, the culture of hematopoietic progenitors with Flt3L generates a mixture of cDC1, cDC2, and pDCs (79, 80). If the study of a single population is desired, additional purification steps may be required. A variation of the original Flt3L-DC culture that co-culture with OP9 stromal cells expressing the Notch ligand Delta-like 1 (OP9-DL1), was recently proposed (80). The authors supporting this modified protocol claim that the addition of the Notch ligand DL1 can induce IRF8-dependent cDC1s with a phenotype and expression profile similar to primary splenic cDC1s (80). Besides, another protocol showed that bone marrow cells cultured in the presence of Flt3L and GM-CSF produced a high number of CD103+ cDC1s (81). However, few metabolic studies have been performed based on these DC cultures.
moDCs, generated from GM-CSF+IL-4 cultured blood mononuclear cells, is the most commonly used model for human DC studies in vitro (16). Despite several functional and phenotypic similarities with the human DC population found in vivo, moDCs are derived from a different precursor cell monocyte (cDC-CDP) and have a low migratory capacity in vivo. In addition, transcriptome research has revealed that human moDCs are more strongly linked to monocytes and macrophages than blood DCs are (82).
DCs generated in vitro can be produced quickly in large quantities and are particularly useful in the field of cell metabolism, in which high cell counts are necessary for experiments (27); however, they have limitations. In further research, new models closer to the in vivo situation are required to study the metabolic requirements of DCs.
As described, cellular metabolic programs can influence DC function. However, early metabolic investigations using in vitro BMDCs and moDCs cultures failed to correspond with natural DCs subsets. Next, we discuss the research in the field of metabolism of different mouse and human DCs subsets, focusing on the differences between cultured models (Table 2). The combination of these two aspects may contribute to the progress in understanding DC metabolism and deepen the physiological understanding, which is critical for developing effective DC-based therapeutics.
Little is known about the metabolic pathways that support multiple stages of differentiation of BM progenitors to fully differentiated DCs. DC subpopulations were generated with different energy requirements for different functions (Table 1).
The LKB1-AMPK-mTOR axis plays a crucial role in maintaining DC quiescence and can be activated during DC differentiation. Studies have shown that AMPK and LKB1 play important roles in DC differentiation. A reduced proportion of cDC1 was observed in the LN of AMPKa gene-deficient mice, suggesting a dominant role for AMPK in terminal cDC1 differentiation (83). A similar effect has been observed in conditional gene-deficient mice with LKB1-deficient CD11c+ cDCs, with a higher proportion of cDC2 in the mouse thymus (28, 30). The LKB1-AMPK axis helps determine cell differentiation during DC development, and deletion of either molecule is detrimental to IRF8+ cDC1 differentiation (83). Notably, cDC1 and cDC2 have been shown to have extremely pronounced metabolic differences at baseline, where splenic cDC1 appears to be more reliant on OXPHOS and functional mitochondrial metabolism than cDC2 or pDCs (48, 83).
Indeed, in vitro and in vivo, cDC1 had a larger mitochondrial mass and mitochondrial membrane potential (ΔΨm) than cDC2s (48, 83, 84), consistent with higher AMPK activity and increased oxidative metabolism (27). Inhibiting catabolic processes such as AMPK signaling, FAO, or mitochondrial clearance did not affect total cDC/pDC development but significantly increased the frequency of IRF4+ cDC2 cells while decreasing the frequency of IRF8+ cDC1 cells. Scavenging anabolism-associated reactive oxygen species (ROS), by contrast, tilted differentiation toward cDC1 cells (83). In addition, a data-driven systems biology algorithm (NetBID) study revealed significant enrichment of Mst1 and Mst2 (Mst1/2) activity, the non-canonical Hippo pathway kinases, in cDC1 cells relative to cDC2 cells. Mechanistically, cDC1 has a substantially greater oxidative metabolism than cDC2 and relies heavily on Mst1/2 signaling to maintain metabolic activity and mitochondrial integrity for immunogenic function (84). However, in the steady state, CD11c-Cre Mst1/2flox/flox mice have increased splenic cDC1 frequencies, unaffected pDCs, and decreased cDC2s (84), implying that understanding the precise role of Hippo/Mst signaling in DC formation requires further exploration.
Information on the metabolic pathways involved in cDCs activation in vivo is limited. Next, we briefly outline the literature on cDC subsets in the metabolic field. Splenic mouse cDC1 and cDC2 enhance their ECAR quickly after in vivo LPS stimulation (41); nevertheless, after ex vivo LPS stimulation for 24 h, they do not differ in their ECAR/OCR ratio (38). In vivo stimulation with poly(I: C) decreased the OCR and ΔΨm of total spleen cDCs, which was blocked by IFNAR elimination (57). TLR activation decreases the mitochondrial content, increases OXPHOS activity, and stimulates glycolysis in human blood cDC2. TLR-stimulated glycolysis and cDC2 activation are impaired when mitochondrial fragmentation is inhibited or when mitochondrial fusion is promoted. TLR stimulation induces BNIP3-dependent mitophagy, which is essential for glycolysis induction and cDC2 activation (85).
The understanding of metabolic reprogramming in pDCs is relatively restricted, but recently, studies have demonstrated that pDCs exhibit differential rewiring of their mitochondrial energy metabolism in different environments. After ex vivo infection with influenza or rhinovirus, OCR decreases in human pDCs (86), whereas TLR7/8 stimulation boosts their glutaminolysis and OXPHOS (85). TLR7/8-stimulated pDC activation requires autophagy-supplemented glutaminolysis to fuel OXPHOS, which is necessary for CD80 and IFNα expression (85). In addition, mouse pDCs isolated from FLT3L-DC culture exhibited enhanced glycolytic flux and OXPHOS approximately 24 h after TLR9 stimulation. Increased FAO of de novo-produced fatty acids drives an increase in mitochondrial metabolism. This impact is the result of autocrine or paracrine type I IFNs, with IFNα regulating FAO in pDCs (87). TLR-induced pDC activation is suppressed by pharmaceuticals that inhibit FAS or block the action of Cpt1a (87, 88). TLR3 stimulation increases mitochondrial-derived ROS in pDC, allowing them to stimulate CD8 + T-cell responses via cross-presentation (89).
Additionally, activated pDCs consumed more glucose than unstimulated pDCs with increased ECAR. pDC activation is linked to alterations in glycolysis and mTORC1 activity (90). The presence of appropriate amounts of AAs in the environment is required for mTORC1 activation, with leucine and methionine being particularly important (91, 92). Induction of the system L amino acid transporters SLC7A5 and SLC3A2 in pDCs, as well as leucine uptake mediated by these transporters, is required for priming future mTORC1 activation and cytokine production by activated pDCs (90). These results demonstrate that the coordinated actions of mitochondria, glycolysis and fatty acid metabolism are essential for pDC function.
Although new knowledge on the metabolic control of DCs has recently been revealed, many intriguing questions remain unresolved because the exploration of DC metabolism is nascent.
Current investigations on the impact of the metabolic microenvironment on DCs are primarily conducted with BMDCs (murine) or moDCs (human) in vitro and generally involve alterations to only a single gene or metabolite. Although these studies have provided a foundation for understanding the metabolic regulation of DC function, culture conditions in vitro frequently do not accurately reflect the complexity of different metabolites in situ in vivo. DCs are commonly cultured in DMEM and RPMI 1640 with higher levels of glucose and lower levels of electrolytes, such as magnesium and calcium (93). Furthermore, whether various DC subsets have different metabolic demands or whether their functions depend on similar metabolic programs remains unclear. The effects of other metabolic processes, including the pentose phosphate pathway and nitrogen metabolism pathways, on DC differentiation remain unknown. Moreover, little is known about the cross-talk between metabolic pathways and other epigenetic or molecular regulatory pathways, such as microRNAs, cytokines, and transcription factors, for determining the function or differentiation of DCs.
The metabolic microenvironment has a substantial impact on DC function and dysregulated DC metabolism can contribute to various diseases, including cancers (94, 95), autoimmune diseases (96), and inflammatory disorders (97, 98).
Consequently, reprogramming the metabolic status of DCs could be an efficient means of regulating inflammation. For example, limiting glucose and fat uptake could decrease their pro-inflammatory effects on tissue-associated DCs and subsequently reduce diabetes-associated inflammation (99–101). Additionally, approaches that directly target critical regulators of certain metabolic pathways, such as AMPK, mTOR, or the addition of specific nutrients, restrict the susceptibility of DCs to extracellular environment alterations and hence control inflammation (55, 102).
In conclusion, understanding how DC immunological activity is regulated by metabolism is essential. Using metabolic modulation will advance the understanding of DC biology and immune regulation in inflammatory diseases and facilitate the exploration of effective DC-based immunotherapies.
LW contributed to the design and conception, the search of the literature, the creation of figures, drafting and critically revising the manuscript; ZY and YJ contributed to the search of the literature and critically revising the manuscript; YC contributed to the creation of figures and revising the manuscript; JD, LG, and JX contributed to the revision of the manuscript; ZL and YL contributed to the conception, design, and critically revising the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by grants from the National Nature Science Foundation of China (81991504 and 81974149 to YL, 82122015 to JX), the Beijing Stomatological Hospital, Capital Medical University Young Scientist Program (YSP20200904 to LJ), the Beijing Municipal Administration of Hospitals Clinical Medicine Development of Special Funding Support (ZYLX202121 to YL), Beijing Municipal Administration of Hospitals’ Ascent Plan (DFL20181501 to YL), the Innovation research team project of Beijing Stomatological Hospital, Capital Medical University (CXTD202202 to YL).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Yin X, Chen S, Eisenbarth SC. Dendritic cell regulation of T helper cells. Annu Rev Immunol (2021) 39:759–90. doi: 10.1146/annurev-immunol-101819-025146
2. Shortman K, Liu YJ. Mouse and human dendritic cell subtypes. Nat Rev Immunol (2002) 2(3):151–61. doi: 10.1038/nri746
3. O'Neill LA, Kishton RJ, Rathmell J. A guide to immunometabolism for immunologists. Nat Rev Immunol (2016) 16(9):553–65. doi: 10.1038/nri.2016.70
4. Du X, Chapman NM, Chi H. Emerging roles of cellular metabolism in regulating dendritic cell subsets and function. Front Cell Dev Biol (2018) 6:152. doi: 10.3389/fcell.2018.00152
5. Wculek SK, Khouili SC, Priego E, Heras-Murillo I, Sancho D. Metabolic control of dendritic cell functions: Digesting information. Front Immunol (2019) 10:775. doi: 10.3389/fimmu.2019.00775
6. O'Neill LA, Pearce EJ. Immunometabolism governs dendritic cell and macrophage function. J Exp Med (2016) 213(1):15–23. doi: 10.1084/jem.20151570
7. Krawczyk CM, Holowka T, Sun J, Blagih J, Amiel E, DeBerardinis RJ, et al. Toll-like receptor-induced changes in glycolytic metabolism regulate dendritic cell activation. Blood (2010) 115(23):4742–9. doi: 10.1182/blood-2009-10-249540
8. He Z, Zhu X, Shi Z, Wu T, Wu L. Metabolic regulation of dendritic cell differentiation. Front Immunol (2019) 10:410. doi: 10.3389/fimmu.2019.00410
9. Murphy TL, Grajales-Reyes GE, Wu X, Tussiwand R, Briseno CG, Iwata A, et al. Transcriptional control of dendritic cell development. Annu Rev Immunol (2016) 34:93–119. doi: 10.1146/annurev-immunol-032713-120204
10. Guilliams M, Ginhoux F, Jakubzick C, Naik SH, Onai N, Schraml BU, et al. Dendritic cells, monocytes and macrophages: A unified nomenclature based on ontogeny. Nat Rev Immunol (2014) 14(8):571–8. doi: 10.1038/nri3712
11. Anderson DA 3rd, Dutertre CA, Ginhoux F, Murphy KM. Genetic models of human and mouse dendritic cell development and function. Nat Rev Immunol (2021) 21(2):101–15. doi: 10.1038/s41577-020-00413-x
12. Clark GJ, Silveira PA, Hogarth PM, Hart DNJ. The cell surface phenotype of human dendritic cells. Semin Cell Dev Biol (2019) 86:3–14. doi: 10.1016/j.semcdb.2018.02.013
13. Macri C, Pang ES, Patton T, O'Keeffe M. Dendritic cell subsets. Semin Cell Dev Biol (2018) 84:11–21. doi: 10.1016/j.semcdb.2017.12.009
14. Cabeza-Cabrerizo M, Cardoso A, Minutti CM, Pereira da Costa M, Reis e Sousa C. Dendritic cells revisited. Annu Rev Immunol (2021) 39:131–66. doi: 10.1146/annurev-immunol-061020-053707
15. Coillard A, Segura E. In vivo differentiation of human monocytes. Front Immunol (2019) 10:1907. doi: 10.3389/fimmu.2019.01907
16. Villar J, Segura E. Decoding the heterogeneity of human dendritic cell subsets. Trends Immunol (2020) 41(12):1062–71. doi: 10.1016/j.it.2020.10.002
17. Woltman AM, van der Kooij SW, Coffer PJ, Offringa R, Daha MR, van Kooten C. Rapamycin specifically interferes with gm-csf signaling in human dendritic cells, leading to apoptosis Via increased P27kip1 expression. Blood (2003) 101(4):1439–45. doi: 10.1182/blood-2002-06-1688
18. Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by Granulocyte/Macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med (1994) 179(4):1109–18. doi: 10.1084/jem.179.4.1109
19. Sander J, Schmidt SV, Cirovic B, McGovern N, Papantonopoulou O, Hardt AL, et al. Cellular differentiation of human monocytes is regulated by time-dependent interleukin-4 signaling and the transcriptional regulator Ncor2. Immunity (2017) 47(6):1051–66.e12. doi: 10.1016/j.immuni.2017.11.024
20. Segura E. Review of mouse and human dendritic cell subsets. Methods Mol Biol (2016) 1423:3–15. doi: 10.1007/978-1-4939-3606-9_1
21. Chandel NS. Glycolysis. Cold Spring Harb Perspect Biol (2021) 13(5):a040535. doi: 10.1101/cshperspect.a040535
22. Mulukutla BC, Yongky A, Le T, Mashek DG, Hu WS. Regulation of glucose metabolism - A perspective from cell bioprocessing. Trends Biotechnol (2016) 34(8):638–51. doi: 10.1016/j.tibtech.2016.04.012
24. Kelly B, O'Neill LA. Metabolic reprogramming in macrophages and dendritic cells in innate immunity. Cell Res (2015) 25(7):771–84. doi: 10.1038/cr.2015.68
25. Ryan DG, O'Neill LAJ. Krebs Cycle rewired for macrophage and dendritic cell effector functions. FEBS Lett (2017) 591(19):2992–3006. doi: 10.1002/1873-3468.12744
26. Minarrieta L, Velasquez LN, Sparwasser T, Berod L. Dendritic cell metabolism: Moving beyond in vitro-Culture-Generated paradigms. Curr Opin Biotechnol (2021) 68:202–12. doi: 10.1016/j.copbio.2020.12.010
27. Pelgrom LR, Patente TA, Sergushichev A, Esaulova E, Otto F, Ozir-Fazalalikhan A, et al. Lkb1 expressed in dendritic cells governs the development and expansion of thymus-derived regulatory T cells. Cell Res (2019) 29(5):406–19. doi: 10.1038/s41422-019-0161-8
28. Herzig S, Shaw RJ. Ampk: Guardian of metabolism and mitochondrial homeostasis. Nat Rev Mol Cell Biol (2018) 19(2):121–35. doi: 10.1038/nrm.2017.95
29. Wang Y, Du X, Wei J, Long L, Tan H, Guy C, et al. Lkb1 orchestrates dendritic cell metabolic quiescence and anti-tumor immunity. Cell Res (2019) 29(5):391–405. doi: 10.1038/s41422-019-0157-4
30. Chen S, Fang L, Guo W, Zhou Y, Yu G, Li W, et al. Control of treg cell homeostasis and immune equilibrium by Lkb1 in dendritic cells. Nat Commun (2018) 9(1):5298. doi: 10.1038/s41467-018-07545-8
31. Thwe PM, Pelgrom LR, Cooper R, Beauchamp S, Reisz JA, D'Alessandro A, et al. Cell-intrinsic glycogen metabolism supports early glycolytic reprogramming required for dendritic cell immune responses. Cell Metab (2019) 30(1):225. doi: 10.1016/j.cmet.2019.05.017
32. Pearce EJ, Everts B. Dendritic cell metabolism. Nat Rev Immunol (2015) 15(1):18–29. doi: 10.1038/nri3771
33. Helft J, Bottcher J, Chakravarty P, Zelenay S, Huotari J, Schraml BU, et al. Gm-csf mouse bone marrow cultures comprise a heterogeneous population of Cd11c(+)Mhcii(+) macrophages and dendritic cells. Immunity (2015) 42(6):1197–211. doi: 10.1016/j.immuni.2015.05.018
34. Collin M, Bigley V. Human dendritic cell subsets: An update. Immunology (2018) 154(1):3–20. doi: 10.1111/imm.12888
35. Perrin-Cocon L, Aublin-Gex A, Diaz O, Ramiere C, Peri F, Andre P, et al. Toll-like receptor 4-induced glycolytic burst in human monocyte-derived dendritic cells results from P38-dependent stabilization of hif-1alpha and increased hexokinase ii expression. J Immunol (2018) 201(5):1510–21. doi: 10.4049/jimmunol.1701522
36. Jantsch J, Chakravortty D, Turza N, Prechtel AT, Buchholz B, Gerlach RG, et al. Hypoxia and hypoxia-inducible factor-1 alpha modulate lipopolysaccharide-induced dendritic cell activation and function. J Immunol (2008) 180(7):4697–705. doi: 10.4049/jimmunol.180.7.4697
37. Basit F, de Vries IJM. Dendritic cells require Pink1-mediated phosphorylation of Bckde1alpha to promote fatty acid oxidation for immune function. Front Immunol (2019) 10:2386. doi: 10.3389/fimmu.2019.02386
38. Everts B, Amiel E, van der Windt GJ, Freitas TC, Chott R, Yarasheski KE, et al. Commitment to glycolysis sustains survival of no-producing inflammatory dendritic cells. Blood (2012) 120(7):1422–31. doi: 10.1182/blood-2012-03-419747
39. Malinarich F, Duan K, Hamid RA, Bijin A, Lin WX, Poidinger M, et al. High mitochondrial respiration and glycolytic capacity represent a metabolic phenotype of human tolerogenic dendritic cells. J Immunol (2015) 194(11):5174–86. doi: 10.4049/jimmunol.1303316
40. Ryans K, Omosun Y, McKeithen DN, Simoneaux T, Mills CC, Bowen N, et al. The immunoregulatory role of alpha enolase in dendritic cell function during chlamydia infection. BMC Immunol (2017) 18(1):27. doi: 10.1186/s12865-017-0212-1
41. Everts B, Amiel E, Huang SC, Smith AM, Chang CH, Lam WY, et al. Tlr-driven early glycolytic reprogramming Via the kinases Tbk1-ikkvarepsilon supports the anabolic demands of dendritic cell activation. Nat Immunol (2014) 15(4):323–32. doi: 10.1038/ni.2833
42. Lawless SJ, Kedia-Mehta N, Walls JF, McGarrigle R, Convery O, Sinclair LV, et al. Glucose represses dendritic cell-induced T cell responses. Nat Commun (2017) 8:15620. doi: 10.1038/ncomms15620
43. Bakdash G, Sittig SP, van Dijk T, Figdor CG, de Vries IJ. The nature of activatory and tolerogenic dendritic cell-derived signal ii. Front Immunol (2013) 4:53. doi: 10.3389/fimmu.2013.00053
44. Guak H, Al Habyan S, Ma EH, Aldossary H, Al-Masri M, Won SY, et al. Glycolytic metabolism is essential for Ccr7 oligomerization and dendritic cell migration. Nat Commun (2018) 9(1):2463. doi: 10.1038/s41467-018-04804-6
45. Randolph GJ, Ochando J, Partida-Sanchez S. Migration of dendritic cell subsets and their precursors. Annu Rev Immunol (2008) 26:293–316. doi: 10.1146/annurev.immunol.26.021607.090254
46. Plantinga M, Guilliams M, Vanheerswynghels M, Deswarte K, Branco-Madeira F, Toussaint W, et al. Conventional and monocyte-derived Cd11b(+) dendritic cells initiate and maintain T helper 2 cell-mediated immunity to house dust mite allergen. Immunity (2013) 38(2):322–35. doi: 10.1016/j.immuni.2012.10.016
47. Marquez S, Fernandez JJ, Teran-Cabanillas E, Herrero C, Alonso S, Azogil A, et al. Endoplasmic reticulum stress sensor Ire1alpha enhances il-23 expression by human dendritic cells. Front Immunol (2017) 8:639. doi: 10.3389/fimmu.2017.00639
48. Chougnet CA, Thacker RI, Shehata HM, Hennies CM, Lehn MA, Lages CS, et al. Loss of phagocytic and antigen cross-presenting capacity in aging dendritic cells is associated with mitochondrial dysfunction. J Immunol (2015) 195(6):2624–32. doi: 10.4049/jimmunol.1501006
49. Everts B, Pearce EJ. Metabolic control of dendritic cell activation and function: Recent advances and clinical implications. Front Immunol (2014) 5:203. doi: 10.3389/fimmu.2014.00203
50. Rehman A, Hemmert KC, Ochi A, Jamal M, Henning JR, Barilla R, et al. Role of fatty-acid synthesis in dendritic cell generation and function. J Immunol (2013) 190(9):4640–9. doi: 10.4049/jimmunol.1202312
51. Cheng SC, Quintin J, Cramer RA, Shepardson KM, Saeed S, Kumar V, et al. Mtor- and hif-1alpha-Mediated aerobic glycolysis as metabolic basis for trained immunity. Science (2014) 345(6204):1250684. doi: 10.1126/science.1250684
52. Wellen KE, Hatzivassiliou G, Sachdeva UM, Bui TV, Cross JR, Thompson CB. Atp-citrate lyase links cellular metabolism to histone acetylation. Science (2009) 324(5930):1076–80. doi: 10.1126/science.1164097
53. Thwe PM, Amiel E. The role of nitric oxide in metabolic regulation of dendritic cell immune function. Cancer Lett (2018) 412:236–42. doi: 10.1016/j.canlet.2017.10.032
54. Amiel E, Everts B, Fritz D, Beauchamp S, Ge B, Pearce EL, et al. Mechanistic target of rapamycin inhibition extends cellular lifespan in dendritic cells by preserving mitochondrial function. J Immunol (2014) 193(6):2821–30. doi: 10.4049/jimmunol.1302498
55. Amiel E, Everts B, Freitas TC, King IL, Curtis JD, Pearce EL, et al. Inhibition of mechanistic target of rapamycin promotes dendritic cell activation and enhances therapeutic autologous vaccination in mice. J Immunol (2012) 189(5):2151–8. doi: 10.4049/jimmunol.1103741
56. Harris AJ, Thompson AR, Whyte MK, Walmsley SR. Hif-mediated innate immune responses: Cell signaling and therapeutic implications. Hypoxia (Auckl) (2014) 2:47–58. doi: 10.2147/HP.S50269
57. Pantel A, Teixeira A, Haddad E, Wood EG, Steinman RM, Longhi MP. Direct type I ifn but not Mda5/Tlr3 activation of dendritic cells is required for maturation and metabolic shift to glycolysis after poly ic stimulation. PloS Biol (2014) 12(1):e1001759. doi: 10.1371/journal.pbio.1001759
58. Colegio OR, Chu NQ, Szabo AL, Chu T, Rhebergen AM, Jairam V, et al. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature (2014) 513(7519):559–63. doi: 10.1038/nature13490
59. Lu H, Forbes RA, Verma A. Hypoxia-inducible factor 1 activation by aerobic glycolysis implicates the warburg effect in carcinogenesis. J Biol Chem (2002) 277(26):23111–5. doi: 10.1074/jbc.M202487200
60. Fliesser M, Morton CO, Bonin M, Ebel F, Hunniger K, Kurzai O, et al. Hypoxia-inducible factor 1alpha modulates metabolic activity and cytokine release in anti-aspergillus fumigatus immune responses initiated by human dendritic cells. Int J Med Microbiol (2015) 305(8):865–73. doi: 10.1016/j.ijmm.2015.08.036
61. Snyder JP, Amiel E. Regulation of dendritic cell immune function and metabolism by cellular nutrient sensor mammalian target of rapamycin (Mtor). Front Immunol (2018) 9:3145. doi: 10.3389/fimmu.2018.03145
62. Ibrahim J, Nguyen AH, Rehman A, Ochi A, Jamal M, Graffeo CS, et al. Dendritic cell populations with different concentrations of lipid regulate tolerance and immunity in mouse and human liver. Gastroenterology (2012) 143(4):1061–72. doi: 10.1053/j.gastro.2012.06.003
63. Wang SH, Yuan SG, Peng DQ, Zhao SP. Hdl and apoa-I inhibit antigen presentation-mediated T cell activation by disrupting lipid rafts in antigen presenting cells. Atherosclerosis (2012) 225(1):105–14. doi: 10.1016/j.atherosclerosis.2012.07.029
64. Walter P, Ron D. The unfolded protein response: From stress pathway to homeostatic regulation. Science (2011) 334(6059):1081–6. doi: 10.1126/science.1209038
65. Herber DL, Cao W, Nefedova Y, Novitskiy SV, Nagaraj S, Tyurin VA, et al. Lipid accumulation and dendritic cell dysfunction in cancer. Nat Med (2010) 16(8):880–6. doi: 10.1038/nm.2172
66. Gardner JK, Mamotte CD, Patel P, Yeoh TL, Jackaman C, Nelson DJ. Mesothelioma tumor cells modulate dendritic cell lipid content, phenotype and function. PloS One (2015) 10(4):e0123563. doi: 10.1371/journal.pone.0123563
67. Veglia F, Tyurin VA, Mohammadyani D, Blasi M, Duperret EK, Donthireddy L, et al. Lipid bodies containing oxidatively truncated lipids block antigen cross-presentation by dendritic cells in cancer. Nat Commun (2017) 8(1):2122. doi: 10.1038/s41467-017-02186-9
68. Jin JO, Park HY, Xu Q, Park JI, Zvyagintseva T, Stonik VA, et al. Ligand of scavenger receptor class a indirectly induces maturation of human blood dendritic cells Via production of tumor necrosis factor-alpha. Blood (2009) 113(23):5839–47. doi: 10.1182/blood-2008-10-184796
69. Wang XY, Facciponte J, Chen X, Subjeck JR, Repasky EA. Scavenger receptor-a negatively regulates antitumor immunity. Cancer Res (2007) 67(10):4996–5002. doi: 10.1158/0008-5472.CAN-06-3138
70. Becker M, Cotena A, Gordon S, Platt N. Expression of the class a macrophage scavenger receptor on specific subpopulations of murine dendritic cells limits their endotoxin response. Eur J Immunol (2006) 36(4):950–60. doi: 10.1002/eji.200535660
71. Kakazu E, Kondo Y, Kogure T, Ninomiya M, Kimura O, Ueno Y, et al. Plasma amino acids imbalance in cirrhotic patients disturbs the tricarboxylic acid cycle of dendritic cell. Sci Rep (2013) 3:3459. doi: 10.1038/srep03459
72. Kakazu E, Ueno Y, Kondo Y, Fukushima K, Shiina M, Inoue J, et al. Branched chain amino acids enhance the maturation and function of myeloid dendritic cells ex vivo in patients with advanced cirrhosis. Hepatology (2009) 50(6):1936–45. doi: 10.1002/hep.23248
73. Kakazu E, Kanno N, Ueno Y, Shimosegawa T. Extracellular branched-chain amino acids, especially valine, regulate maturation and function of monocyte-derived dendritic cells. J Immunol (2007) 179(10):7137–46. doi: 10.4049/jimmunol.179.10.7137
74. D'Angelo JA, Dehlink E, Platzer B, Dwyer P, Circu ML, Garay J, et al. The Cystine/Glutamate antiporter regulates dendritic cell differentiation and antigen presentation. J Immunol (2010) 185(6):3217–26. doi: 10.4049/jimmunol.1001199
75. Vremec D, Hansen J, Strasser A, Acha-Orbea H, Zhan Y, O'Keeffe M, et al. Maintaining dendritic cell viability in culture. Mol Immunol (2015) 63(2):264–7. doi: 10.1016/j.molimm.2014.07.011
76. Vremec D, O'Keeffe M, Wilson A, Ferrero I, Koch U, Radtke F, et al. Factors determining the spontaneous activation of splenic dendritic cells in culture. Innate Immun (2011) 17(3):338–52. doi: 10.1177/1753425910371396
77. Clementi E, Brown GC, Feelisch M, Moncada S. Persistent inhibition of cell respiration by nitric oxide: Crucial role of s-nitrosylation of mitochondrial complex I and protective action of glutathione. Proc Natl Acad Sci U.S.A. (1998) 95(13):7631–6. doi: 10.1073/pnas.95.13.7631
78. Giuffre A, Sarti P, D'Itri E, Buse G, Soulimane T, Brunori M. On the mechanism of inhibition of cytochrome c oxidase by nitric oxide. J Biol Chem (1996) 271(52):33404–8. doi: 10.1074/jbc.271.52.33404
79. Naik SH, Proietto AI, Wilson NS, Dakic A, Schnorrer P, Fuchsberger M, et al. Cutting edge: Generation of splenic Cd8+ and Cd8- dendritic cell equivalents in fms-like tyrosine kinase 3 ligand bone marrow cultures. J Immunol (2005) 174(11):6592–7. doi: 10.4049/jimmunol.174.11.6592
80. Kirkling ME, Cytlak U, Lau CM, Lewis KL, Resteu A, Khodadadi-Jamayran A, et al. Notch signaling facilitates in vitro generation of cross-presenting classical dendritic cells. Cell Rep (2018) 23(12):3658–72.e6. doi: 10.1016/j.celrep.2018.05.068
81. Mayer CT, Ghorbani P, Nandan A, Dudek M, Arnold-Schrauf C, Hesse C, et al. Selective and efficient generation of functional Batf3-dependent Cd103+ dendritic cells from mouse bone marrow. Blood (2014) 124(20):3081–91. doi: 10.1182/blood-2013-12-545772
82. Robbins SH, Walzer T, Dembele D, Thibault C, Defays A, Bessou G, et al. Novel insights into the relationships between dendritic cell subsets in human and mouse revealed by genome-wide expression profiling. Genome Biol (2008) 9(1):R17. doi: 10.1186/gb-2008-9-1-r17
83. Kratchmarov R, Viragova S, Kim MJ, Rothman NJ, Liu K, Reizis B, et al. Metabolic control of cell fate bifurcations in a hematopoietic progenitor population. Immunol Cell Biol (2018) 96(8):863–71. doi: 10.1111/imcb.12040
84. Du X, Wen J, Wang Y, Karmaus PWF, Khatamian A, Tan H, et al. Hippo/Mst signalling couples metabolic state and immune function of Cd8alpha(+) dendritic cells. Nature (2018) 558(7708):141–5. doi: 10.1038/s41586-018-0177-0
85. Basit F, Mathan T, Sancho D, de Vries IJM. Human dendritic cell subsets undergo distinct metabolic reprogramming for immune response. Front Immunol (2018) 9:2489. doi: 10.3389/fimmu.2018.02489
86. Bajwa G, DeBerardinis RJ, Shao B, Hall B, Farrar JD, Gill MA. Cutting edge: Critical role of glycolysis in human plasmacytoid dendritic cell antiviral responses. J Immunol (2016) 196(5):2004–9. doi: 10.4049/jimmunol.1501557
87. Wu D, Sanin DE, Everts B, Chen Q, Qiu J, Buck MD, et al. Type 1 interferons induce changes in core metabolism that are critical for immune function. Immunity (2016) 44(6):1325–36. doi: 10.1016/j.immuni.2016.06.006
88. Qiu CC, Lee MH, Gallucci S. Modulation of the energy metabolism inhibits plasmacytoid dendritic cell activation and delays onset of autoantibody production in murine models of systemic lupus erythematosus. J Immunol (2017) 198(1):80.12. doi: 10.4049/jimmunol.198.Supp.80.12
89. Oberkampf M, Guillerey C, Mouries J, Rosenbaum P, Fayolle C, Bobard A, et al. Mitochondrial reactive oxygen species regulate the induction of Cd8(+) T cells by plasmacytoid dendritic cells. Nat Commun (2018) 9(1):2241. doi: 10.1038/s41467-018-04686-8
90. Grzes KM, Sanin DE, Kabat AM, Stanczak MA, Edwards-Hicks J, Matsushita M, et al. Plasmacytoid dendritic cell activation is dependent on coordinated expression of distinct amino acid transporters. Immunity (2021) 54(11):2514–30.e7. doi: 10.1016/j.immuni.2021.10.009
91. Valvezan AJ, Manning BD. Molecular logic of Mtorc1 signalling as a metabolic rheostat. Nat Metab (2019) 1(3):321–33. doi: 10.1038/s42255-019-0038-7
92. Condon KJ, Sabatini DM. Nutrient regulation of Mtorc1 at a glance. J Cell Sci (2019) 132(21). doi: 10.1242/jcs.222570
93. McKee TJ, Komarova SV. Is it time to reinvent basic cell culture medium? Am J Physiol Cell Physiol (2014) 312(5):C624–C6. doi: 10.1152/ajpcell.00336.2016
94. Wculek SK, Cueto FJ, Mujal AM, Melero I, Krummel MF, Sancho D. Dendritic cells in cancer immunology and immunotherapy. Nat Rev Immunol (2020) 20(1):7–24. doi: 10.1038/s41577-019-0210-z
95. Le Gall CM, Weiden J, Eggermont LJ, Figdor CG. Dendritic cells in cancer immunotherapy. Nat Mater (2018) 17(6):474–5. doi: 10.1038/s41563-018-0093-6
96. Morante-Palacios O, Fondelli F, Ballestar E, Martinez-Caceres EM. Tolerogenic dendritic cells in autoimmunity and inflammatory diseases. Trends Immunol (2021) 42(1):59–75. doi: 10.1016/j.it.2020.11.001
97. Agrawal A, Agrawal S, Gupta S. Role of dendritic cells in inflammation and loss of tolerance in the elderly. Front Immunol (2017) 8:896. doi: 10.3389/fimmu.2017.00896
98. Qian C, Cao X. Dendritic cells in the regulation of immunity and inflammation. Semin Immunol (2018) 35:3–11. doi: 10.1016/j.smim.2017.12.002
99. Vitale M, Masulli M, Rivellese AA, Babini AC, Boemi M, Bonora E, et al. Influence of dietary fat and carbohydrates proportions on plasma lipids, glucose control and low-grade inflammation in patients with type 2 diabetes-the Tosca.It study. Eur J Nutr (2016) 55(4):1645–51. doi: 10.1007/s00394-015-0983-1
100. Sainsbury E, Kizirian NV, Partridge SR, Gill T, Colagiuri S, Gibson AA. Effect of dietary carbohydrate restriction on glycemic control in adults with diabetes: A systematic review and meta-analysis. Diabetes Res Clin Pract (2018) 139:239–52. doi: 10.1016/j.diabres.2018.02.026
101. Jonasson L, Guldbrand H, Lundberg AK, Nystrom FH. Advice to follow a low-carbohydrate diet has a favourable impact on low-grade inflammation in type 2 diabetes compared with advice to follow a low-fat diet. Ann Med (2014) 46(3):182–7. doi: 10.3109/07853890.2014.894286
Keywords: metabolic reprogramming, glycolysis, inflammation, dendritic cells (DC), cellular metabolism
Citation: Wu L, Yan Z, Jiang Y, Chen Y, Du J, Guo L, Xu J, Luo Z and Liu Y (2023) Metabolic regulation of dendritic cell activation and immune function during inflammation. Front. Immunol. 14:1140749. doi: 10.3389/fimmu.2023.1140749
Received: 09 January 2023; Accepted: 03 February 2023;
Published: 08 March 2023.
Edited by:
Elodie Segura, Institut Curie, FranceReviewed by:
Connie M. Krawczyk, Van Andel Research Institute (VARI), United StatesCopyright © 2023 Wu, Yan, Jiang, Chen, Du, Guo, Xu, Luo and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhenhua Luo, bGFyeWh1YUAxNjMuY29t; Yi Liu, bGlsaWxpdXlpQDE2My5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.