- 1Division of Rheumatology, Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Republic of Korea
- 2Division of Rheumatology, Department of Internal Medicine, University of Ulsan, College of Medicine, Asan Medical Center, Seoul, Republic of Korea
- 3Convergence Medicine Research Center, Asan Institution for Life Science, Asan Medical Center, Seoul, Republic of Korea
Interleukin-32 (IL-32) is an important cytokine involved in the innate and adaptive immune responses. The role of IL-32 has been studied in the context of various diseases. A growing body of research has investigated the role of IL-32 in rheumatic diseases including inflammatory arthritides (rheumatoid arthritis, ankylosing spondylitis, and psoriatic arthritis) and connective tissue diseases (systemic lupus erythematosus, systemic sclerosis, granulomatosis and polyangiitis, and giant cell arteritis). IL-32 has been shown to play different roles according to the type of rheumatic diseases. Hence, the putative role of IL-32 as a biomarker is also different in each rheumatic disease: IL-32 could serve as a biomarker for disease activity in some diseases, whereas in other diseases it could be a biomarker for certain disease manifestations. In this narrative review, we summarize the associations between IL-32 and various rheumatic diseases and discuss the putative role of IL-32 as a biomarker in each disease.
Introduction
Interleukin-32 (IL-32), formerly known as the Natural Killer Cell Transcript 4 (NK4), is a cytokine that plays an important role in both innate and adaptive immune responses (1–4). In various inflammatory stimuli, IL-32 is produced by various cells, including monocytes, T cells, NK cells, fibroblasts, epithelial cells, and endothelial cells (2, 5, 6). IL-32, in turn, stimulates monocytes to differentiate into macrophage or dendritic cells and induces pro-inflammatory cytokines including tumor necrosis factor (TNF)-α, IL-1β, IL-6, and IL-8 through nuclear factor-kappa B (NF-κB) and p38 mitogen-activated protein kinase inflammatory signal pathway (2, 7). Since its identification in 1992, IL-32 has been studied in a wide range of diseases including chronic inflammatory diseases, autoimmune diseases, infection, and cancer (8–11). Rheumatic diseases are one of the disease groups that the role of IL-32 has been extensively studied on (12–18).
The IL-32 gene is found in higher mammals but not in rodents (2). The gene is located in chromosome 16p13.3 and has eight exons (2). The first exon does not translate into amino acids (3). Based on different alternative splicing sites, IL-32 has several isoforms: IL-32α, IL-32β, IL-32γ, IL-32δ, IL-32ϵ, IL-32ζ, IL-32η, IL-32θ, and IL-32small (IL-32sm) (19). The biological activity of IL-32 varies according to the isoforms with IL-32γ and IL-32θ having the most potent biological activity (7, 20, 21). Of the isoforms, IL-32γ has the longest amino acid sequence and is the isoform that is most studied (20, 22). All isoforms have a tripeptide RGD motif, which helps cell adhesion and movement (23). Through these motifs, the secreted forms of IL-32 may bind to integrin, suggesting integrin as a possible receptor for IL-32 (24). However, specific receptors for IL-32 are yet to be identified.
Biomarkers are defined as a marker that can be objectively measured and evaluated as an indicator of normal biological processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention (25). In the field of rheumatic diseases, biomarkers are increasingly used for early diagnosis of the disease, accurate assessment of disease activity, detection of certain disease manifestations, and prediction of treatment response and prognosis (26). A growing body of evidence suggests that IL-32 could be used as a possible biomarker in various rheumatic diseases. In this narrative review, we summarize the associations between IL-32 and various rheumatic diseases, and discuss the potential role of IL-32 as a biomarker in each disease.
Inflammatory arthritides
Rheumatoid arthritis
Rheumatoid arthritis (RA) is a chronic autoimmune disease characterized by inflammatory synovitis, which leads to progressive joint damage (27). The inflammatory milieu of the synovial tissue is mediated by pro-inflammatory cytokines such as TNF-α, IL-1β, and IL-6 (28). IL-32, as a pro-inflammatory cytokine, has also been implicated in the pathogenesis of synovitis in RA (29). In synovial tissue from patients with RA, IL-32 was highly expressed, whereas in that from patients with osteoarthritis (OA), expression of IL-32 was not observed (29). In addition, the level of IL-32 staining in synovial tissue from patients with RA correlated with erythrocyte sedimentation rate (ESR), indices of synovial inflammation, and synovial presence of TNF-α, IL-1β, and IL-18 (29). In a study comparing gene expression profiles of in vitro cultured fibroblast-like synoviocytes (FLS) from patients with RA and OA, IL-32 was the most prominent gene that was differentially expressed between patients with RA and OA, with higher expression in patients with RA (30). These findings together indicate that IL-32 is an important cytokine involved in the pathogenesis of synovial inflammation in RA (29, 30).
The suggested mechanism of IL-32 for inducing inflammatory synovitis in RA is induction of other pro-inflammatory cytokines that are involved in the development of RA (28). When human IL-32γ was injected into knee joints of C57/BL6 (wild-type [WT]) mice, joint swelling, infiltration of inflammatory cells, and cartilage damage were observed, whereas in TNF-α deficient mice, the injection of human IL-32γ into the knee joints did not result in joint swelling, and the inflammatory cell influx was markedly reduced (29). In another study using a collagen-induced arthritis model, transfer of IL-32β-producing CD4+ T cells aggravated arthritis, which was attenuated by TNF-α blockade (6). These findings suggest that the pathogenic role of IL-32 in RA is at least in part, dependent on TNF-α. Conversely, TNF-α also induces expression of IL-32 in synovial fibroblasts, monocyte-derived dendritic cells, and T cells (6). In response to TNF-α, FLS from patients with RA produce IL-32 in a dose-dependent manner, indicating that inflammatory cascade in the synovial tissue can be magnified via IL-32 activity through an autocrine loop (31). In addition, IL-32γ induces the maturation of dendritic cells and production of IL-12, leading to enhanced Th1 response, which may aggravate inflammation in RA (32).
In addition to its role in inducing inflammation in synovial tissue, IL-32 also plays a role in bone resorption in RA (13). IL-32α induces differentiation of osteoclast precursors into multinucleated osteoclasts, partially independent of the receptor activator of nuclear factor kappa B (RANK)/RANK ligand (RANKL) pathway (33). However, IL-32α is not capable of activating the multinucleated osteoclasts into bone-resorbing osteoclasts (33). In contrast, IL-32γ, which is the isoform with a stronger biological activity, potently stimulates both the differentiation of osteoclast precursor into multinucleated osteoclast and the activation of multinucleated osteoclast into bone-resorbing osteoclasts, with synergistic effect with RANKL (34). Furthermore, IL-32γ increases the expression of RANKL but decreases the expression of osteoprotegerin in FLS from patients with RA, which is a favorable condition for osteoclastogenesis, leading to bone resorption (34).
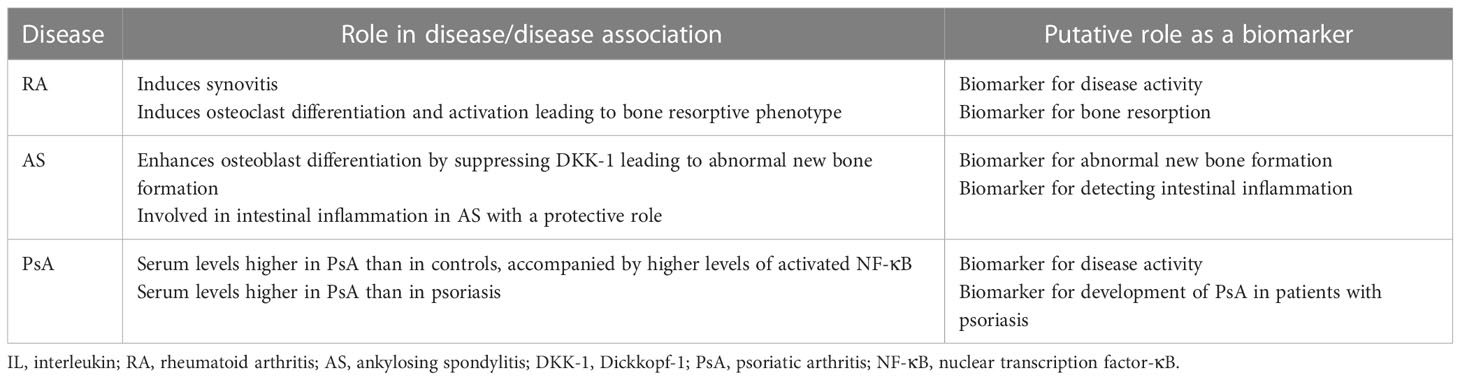
Taken together, IL-32 could be a putative biomarker for assessing inflammatory burden (i.e., disease activity) and the isoform IL-32γ could be a biomarker for bone resorption in RA (Table 1).
Ankylosing spondylitis
Ankylosing spondylitis (AS) is a chronic inflammatory arthritis that mainly affects the axial skeleton with a characteristic bone phenotype of abnormal new bone formation (35). The pathogenic role of IL-32γ in bone formation in AS has been suggested (36, 37). Compared with patients with OA and even those with RA, patients with AS have higher levels of IL-32γ in synovial fluid and higher expression of IL-32 in synovial tissue (36). When osteoblast precursors from WT (C57/BL6) mice were stimulated with IL-32γ, osteoblast differentiation was potently enhanced (36). Furthermore, IL-32γ transgenic mice had higher rates of osteoblast differentiation than the WT mice (36). Mechanistically, IL-32γ reduced the expression of Dickkopf-1 (DKK-1), an inhibitor of Wnt/β-catenin signaling pathway in osteoblast precursors (36). The reduced expression of DKK-1 was mediated by upregulation of miR-29a in osteoblast precursors (37). These data indicate that IL-32γ is involved in abnormal new bone formation in AS by suppressing DKK-1 expression, resulting in enhanced osteoblast differentiation.
IL-32 has also been implicated in intestinal inflammation in AS (38). Ileal tissue from patients with AS who had chronic intestinal inflammation revealed higher expression of IL-32 than that from patients with AS without chronic intestinal inflammation, and healthy controls (38). Functionally, IL-32 stimulated IL-10 production in human intestinal epithelial cell line, suggesting that IL-32 is involved in intestinal inflammation in AS with a protective role (38).
Collectively, IL-32γ could be a biomarker for abnormal new bone formation and IL-32 could be a biomarker for detecting intestinal inflammation in AS (Table 1).
Psoriatic arthritis
Psoriatic arthritis (PsA) is a chronic inflammatory arthritis that affects approximately 30% of patients with psoriasis (39). A study using plasma samples has shown that the levels of IL-32 were higher in the plasma from patients with PsA than that from healthy controls (14). Moreover, levels of activated NF-κB were higher in peripheral blood mononuclear cells (PBMCs) from patients with PsA than that from healthy controls: although direct evidence of association between IL-32 and activation of NF-κB has not been provided, the study suggested a possible role of IL-32 in inflammatory process in PsA (14).
Psoriasis precedes PsA in a majority of the cases of PsA (39). Interestingly, the levels of IL-32 in the plasma and IL-32 mRNA in the PBMCs from patients with psoriasis are already higher than those from healthy controls (14, 40). In comparison between patients with PsA and those with psoriasis, the levels of IL-32 in plasma from patients with PsA were higher than that from patients with psoriasis (14). These findings suggest that levels of IL-32 in the plasma from patients with psoriasis increase before PsA develops, which further increase on the development of PsA. Therefore, IL-32 could be a biomarker for assessing disease activity of PsA and for detecting development of PsA in patients with psoriasis (Table 1).
Connective tissue diseases
Systemic lupus erythematosus
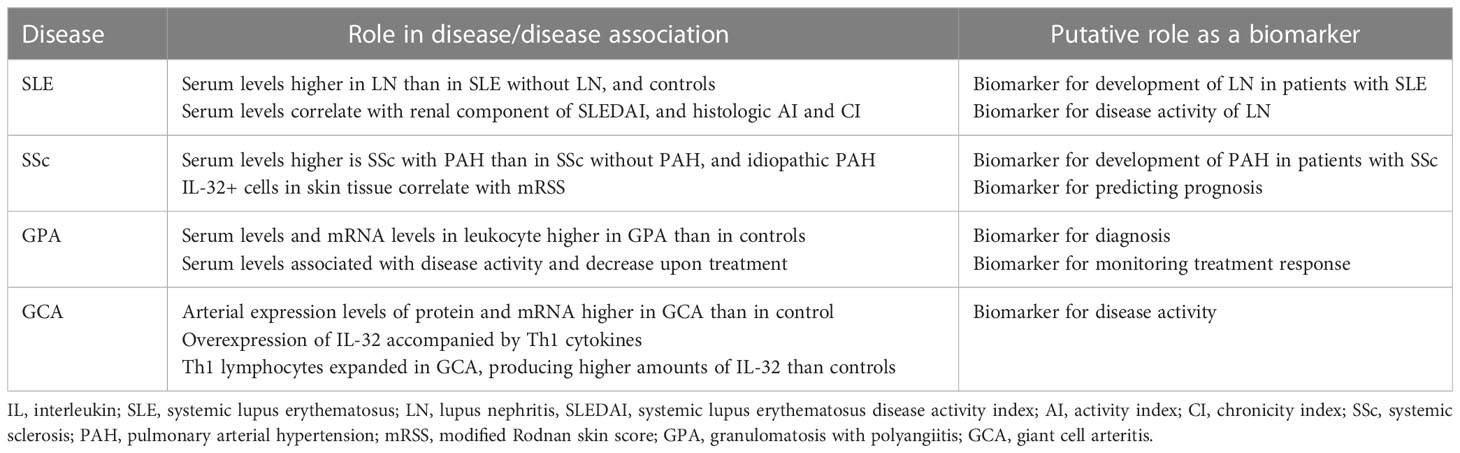
Systemic lupus erythematosus (SLE) is a systemic autoimmune disease that can cause inflammation and damage in virtually all organs throughout the body (41). In a study from China comparing serum levels of various cytokines between patients with SLE and healthy controls, there was no significant difference in the serum levels of IL-32 (42). Another study, also from China, reported lower plasma levels of IL-32 in patients with SLE than in healthy controls (43). The authors speculated that the levels of IL-32 could have been measured low in patients with SLE because of the treatment they received (42, 43). There is evidence suggesting that IL-32 is involved in the pathogenesis of SLE, in particular, lupus nephritis (LN) (15, 44). In a study that measured serum levels of IL-32γ in patients with SLE and healthy controls, serum levels of IL-32γ were detectable in 18.8% of patients with LN, whereas the levels were not detectable in patients with SLE without history of LN and in healthy controls, suggesting a possible pathogenic role of IL-32γ in LN (44). Similarly, in a study with larger sample size, serum levels of IL-32γ were higher in patients with LN than in patients with SLE without LN and healthy controls (15). The serum level of IL-32γ positively correlated with renal component of SLE disease activity index, histologic activity index, and histologic chronicity index of LN (15). Moreover, the expression of IL-32 in renal tissue was higher in patients with LN than in healthy controls (15). Taken together, IL-32γ could be a biomarker for detecting development of LN in patients with SLE and for assessing disease activity of LN (Table 2).
Systemic sclerosis
Systemic sclerosis (SSc) is an autoimmune disease characterized by microvascular damage and progressive fibrosis of the skin and internal organs (45). IL-32 has been tested as a biomarker for detection of pulmonary arterial hypertension (PAH) in patients with SSc (16). Compared with patients with SSc without PAH and those with idiopathic PAH, patients with SSc with PAH had higher levels of serum IL-32, suggesting IL-32 as a promising biomarker for detecting PAH in patients with SSc (16). Moreover, the serum levels of IL-32 correlated with mean pulmonary arterial pressure and systolic pulmonary arterial pressure indicating that IL-32 could be used as a new screening tool for PAH in patients with SSc (16). The number of IL-32+ cells was higher in skin tissue derived from patients with SSc with PAH than that from patients with SSc without PAH (16). In addition, the number of IL-32+ cells in the skin tissue correlated with the modified Rodnan skin score (mRSS) (16). Given that mRSS is considered as a surrogate outcome measure for severity and mortality in patients with SSc (46, 47), IL-32+ cells in the skin tissue could have prognostic value. Therefore, IL-32 could be a biomarker for detecting development of PAH and predicting prognosis in SSc (Table 2).
Granulomatosis with polyangiitis
Granulomatosis with polyangiitis (GPA) is a systemic vasculitis characterized by necrotizing granulomatous inflammation involving the respiratory tract and necrotizing vasculitis affecting small vessels (48). Proteinase 3 (PR3) is a major autoantigen targeted by anti-neutrophil cytoplasmic antibodies in GPA (49). PR3 is also known to specifically bind and activate IL-32 (50). On this basis, a study has evaluated the role of IL-32 in GPA (17). The serum levels of IL-32 were higher in patients with GPA than in healthy controls, attributable to the higher levels IL-32 mRNA in leukocytes from patients with GPA than that from healthy controls (50). The serum levels of IL-32 were associated with disease activity as assessed by Birmingham vasculitis activity score, which decreased in response to treatment (50). These data suggest that IL-32 could be a biomarker for diagnosing GPA and monitoring treatment response.
Giant cell arteritis
Giant cell arteritis (GCA) is a systemic vasculitis that primarily affects large- and medium-sized vessels (48). The levels of IL-32 and IL-32 mRNA were higher in arterial biopsy specimens from patients with GCA than that from healthy controls (18). Moreover, the expression level of IL-32 correlated with the number of inflammatory parameters (fever, weight loss, ESR ≥85 mm/h, and hemoglobin <11.0 g/dL) (18). In the inflamed arteries from patients with GCA, the overexpression of IL-32 was accompanied by overexpression of interferon-γ and IL-27p28, which are Th1 cytokines (18). Th1 lymphocytes were expanded among PBMCs from patients with GCA and produced higher amounts of IL-32 compared with that in healthy controls (18). These suggest that IL-32 has an important role in mediating arterial inflammation in GCA. Therefore, IL-32 could be a biomarker for disease activity of GCA (Table 2).
Conclusion
A number of studies have shown varying roles of IL-32 depending on the type of rheumatic diseases, indicating its different role as a putative biomarker in each disease. Although this progress is encouraging and could help clinicians in their clinical practice, we are still far from completely understanding the exact mechanism underlying the associations between IL-32 and each rheumatic disease. Considering the lack of recognized specific IL-32 receptor, identification of the IL-32 receptor could be the first step in elucidating the mechanism. Moreover, majority of the previous studies did not specify the isoform of IL-32. As different isoforms exert different biological activity, it is important to specify the isoform of IL-32 in future studies. Finally, although a variety of rheumatic diseases have been studied, data on connective tissue diseases are still relatively scarce. Further validation studies assessing IL-32 as a biomarker in the diseases summarized here are definitely needed.
Author contributions
Y-GK was responsible for the concept of the manuscript. OCK drafted the manuscript. M-CP and Y-GK revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
Y-GK received grants from the National Research Foundation of Korea (NRF- 2022M3A9G1014039).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Dahl CA, Schall RP, He HL, Cairns JS. Identification of a novel gene expressed in activated natural killer cells and T cells. J Immunol (1992) 148(2):597–603. doi: 10.4049/jimmunol.148.2.597
2. Kim SH, Han SY, Azam T, Yoon DY, Dinarello CA. Interleukin-32: A cytokine and inducer of TNFalpha. Immunity (2005) 22(1):131–42. doi: 10.1016/j.immuni.2004.12.003
3. Shim S, Lee S, Hisham Y, Kim S, Nguyen TT, Taitt AS, et al. A paradoxical effect of interleukin-32 isoforms on cancer. Front Immunol (2022) 13:837590. doi: 10.3389/fimmu.2022.837590
4. Ribeiro-Dias F, Oliveira IBN. A critical overview of interleukin 32 in leishmaniases. Front Immunol (2022) 13:849340. doi: 10.3389/fimmu.2022.849340
5. Calabrese F, Baraldo S, Bazzan E, Lunardi F, Rea F, Maestrelli P, et al. IL-32, a novel proinflammatory cytokine in chronic obstructive pulmonary disease. Am J Respir Crit Care Med (2008) 178(9):894–901. doi: 10.1164/rccm.200804-646OC
6. Shoda H, Fujio K, Yamaguchi Y, Okamoto A, Sawada T, Kochi Y, et al. Interactions between IL-32 and tumor necrosis factor alpha contribute to the exacerbation of immune-inflammatory diseases. Arthritis Res Ther (2006) 8(6):R166. doi: 10.1186/ar2074
7. Choi JD, Bae SY, Hong JW, Azam T, Dinarello CA, Her E, et al. Identification of the most active interleukin-32 isoform. Immunology (2009) 126(4):535–42. doi: 10.1111/j.1365-2567.2008.02917.x
8. Khawar B, Abbasi MH, Sheikh N. A panoramic spectrum of complex interplay between the immune system and IL-32 during pathogenesis of various systemic infections and inflammation. Eur J Med Res (2015) 20(1):7. doi: 10.1186/s40001-015-0083-y
9. Khawar MB, Abbasi MH, Sheikh N. IL-32: A novel pluripotent inflammatory interleukin, towards gastric inflammation, gastric cancer, and chronic rhino sinusitis. Mediators Inflammation (2016) 2016:8413768. doi: 10.1155/2016/8413768
10. Kim S. Interleukin-32 in inflammatory autoimmune diseases. Immune Netw (2014) 14(3):123–7. doi: 10.4110/in.2014.14.3.123
11. Hong JT, Son DJ, Lee CK, Yoon DY, Lee DH, Park MH. Interleukin 32, inflammation and cancer. Pharmacol Ther (2017) 174:127–37. doi: 10.1016/j.pharmthera.2017.02.025
12. Xu WD, Zhang M, Feng CC, Yang XK, Pan HF, Ye DQ. IL-32 with potential insights into rheumatoid arthritis. Clin Immunol (2013) 147(2):89–94. doi: 10.1016/j.clim.2013.02.021
13. Kwon OC, Kim S, Hong S, Lee CK, Yoo B, Chang EJ, et al. Role of IL-32 gamma on bone metabolism in autoimmune arthritis. Immune Netw (2018) 18(3):e20. doi: 10.4110/in.2018.18.e20
14. Al-Shobaili HA, Farhan J, Zafar U, Rasheed Z. Functional role of human interleukin-32 and nuclear transcription factor-kB in patients with psoriasis and psoriatic arthritis. Int J Health Sci (Qassim) (2018) 12(3):29–34.
15. Kwon OC, Ghang B, Lee EJ, Hong S, Lee CK, Yoo B, et al. Interleukin-32γ: Possible association with the activity and development of nephritis in patients with systemic lupus erythematosus. Int J Rheum Dis (2019) 22(7):1305–11. doi: 10.1111/1756-185x.13550
16. Di Benedetto P, Guggino G, Manzi G, Ruscitti P, Berardicurti O, Panzera N, et al. Interleukin-32 in systemic sclerosis, a potential new biomarker for pulmonary arterial hypertension. Arthritis Res Ther (2020) 22(1):127. doi: 10.1186/s13075-020-02218-8
17. Bae S, Kim YG, Choi J, Hong J, Lee S, Kang T, et al. Elevated interleukin-32 expression in granulomatosis with polyangiitis. Rheumatol (Oxford) (2012) 51(11):1979–88. doi: 10.1093/rheumatology/kes163
18. Ciccia F, Alessandro R, Rizzo A, Principe S, Raiata F, Cavazza A, et al. Expression of interleukin-32 in the inflamed arteries of patients with giant cell arteritis. Arthritis Rheum (2011) 63(7):2097–104. doi: 10.1002/art.30374
19. Kang JW, Park YS, Lee DH, Kim MS, Bak Y, Ham SY, et al. Interaction network mapping among IL-32 isoforms. Biochimie (2014) 101:248–51. doi: 10.1016/j.biochi.2014.01.013
20. Shim S, Lee S, Hisham Y, Kim S, Nguyen TT, Taitt AS, et al. Comparison of the seven interleukin-32 isoforms’ biological activities: IL-32θ possesses the most dominant biological activity. Front Immunol (2022) 13:837588. doi: 10.3389/fimmu.2022.837588
21. Joosten LA, Heinhuis B, Netea MG, Dinarello CA. Novel insights into the biology of interleukin-32. Cell Mol Life Sci (2013) 70(20):3883–92. doi: 10.1007/s00018-013-1301-9
22. Sohn DH, Nguyen TT, Kim S, Shim S, Lee S, Lee Y, et al. Structural characteristics of seven IL-32 variants. Immune Netw (2019) 19(2):e8. doi: 10.4110/in.2019.19.e8
23. de Albuquerque R, Komsi E, Starskaia I, Ullah U, Lahesmaa R. The role of interleukin-32 in autoimmunity. Scand J Immunol (2021) 93(2):e13012. doi: 10.1111/sji.13012
24. Heinhuis B, Netea MG, van den Berg WB, Dinarello CA, Joosten LA. Interleukin-32: a predominantly intracellular proinflammatory mediator that controls cell activation and cell death. Cytokine (2012) 60(2):321–7. doi: 10.1016/j.cyto.2012.07.010
25. Biomarkers Definitions Working Group. Biomarkers and surrogate endpoints: Preferred definitions and conceptual framework. Clin Pharmacol Ther (2001) 69(3):89–95. doi: 10.1067/mcp.2001.113989
26. Mohan C, Assassi S. Biomarkers in rheumatic diseases: how can they facilitate diagnosis and assessment of disease activity? Bmj (2015) 351:h5079. doi: 10.1136/bmj.h5079
27. Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet (2016) 388(10055):2023–38. doi: 10.1016/s0140-6736(16)30173-8
28. McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med (2011) 365(23):2205–19. doi: 10.1056/NEJMra1004965
29. Joosten LA, Netea MG, Kim SH, Yoon DY, Oppers-Walgreen B, Radstake TR, et al. IL-32, a proinflammatory cytokine in rheumatoid arthritis. Proc Natl Acad Sci U.S.A. (2006) 103(9):3298–303. doi: 10.1073/pnas.0511233103
30. Cagnard N, Letourneur F, Essabbani A, Devauchelle V, Mistou S, Rapinat A, et al. Interleukin-32, CCL2, PF4F1 and GFD10 are the only cytokine/chemokine genes differentially expressed by in vitro cultured rheumatoid and osteoarthritis fibroblast-like synoviocytes. Eur Cytokine Netw (2005) 16(4):289–92.
31. Mun SH, Kim JW, Nah SS, Ko NY, Lee JH, Kim JD, et al. Tumor necrosis factor alpha-induced interleukin-32 is positively regulated via the syk/protein kinase Cdelta/JNK pathway in rheumatoid synovial fibroblasts. Arthritis Rheum (2009) 60(3):678–85. doi: 10.1002/art.24299
32. Jung MY, Son MH, Kim SH, Cho D, Kim TS. IL-32gamma induces the maturation of dendritic cells with Th1- and Th17-polarizing ability through enhanced IL-12 and IL-6 production. J Immunol (2011) 186(12):6848–59. doi: 10.4049/jimmunol.1003996
33. Mabilleau G, Sabokbar A. Interleukin-32 promotes osteoclast differentiation but not osteoclast activation. PloS One (2009) 4(1):e4173. doi: 10.1371/journal.pone.0004173
34. Kim YG, Lee CK, Oh JS, Kim SH, Kim KA, Yoo B. Effect of interleukin-32gamma on differentiation of osteoclasts from CD14+ monocytes. Arthritis Rheum (2010) 62(2):515–23. doi: 10.1002/art.27197
35. Inman R. Axial spondyloarthritis: Current advances, future challenges. J Rheum Dis (2021) 28(2):55–9. doi: 10.4078/jrd.2021.28.2.55
36. Lee EJ, Lee EJ, Chung YH, Song DH, Hong S, Lee CK, et al. High level of interleukin-32 gamma in the joint of ankylosing spondylitis is associated with osteoblast differentiation. Arthritis Res Ther (2015) 17:350. doi: 10.1186/s13075-015-0870-4
37. Lee EJ, Kim SM, Choi B, Kim EY, Chung YH, Lee EJ, et al. Interleukin-32 gamma stimulates bone formation by increasing miR-29a in osteoblastic cells and prevents the development of osteoporosis. Sci Rep (2017) 7:40240. doi: 10.1038/srep40240
38. Ciccia F, Rizzo A, Accardo-Palumbo A, Giardina A, Bombardieri M, Guggino G, et al. Increased expression of interleukin-32 in the inflamed ileum of ankylosing spondylitis patients. Rheumatol (Oxford) (2012) 51(11):1966–72. doi: 10.1093/rheumatology/kes170
39. Ritchlin CT, Colbert RA, Gladman DD. Psoriatic arthritis. N Engl J Med (2017) 376(10):957–70. doi: 10.1056/NEJMra1505557
40. Al-Shobaili HA, Rasheed Z. Elevated gene expression of interleukin-32 isoforms alpha, beta, gamma, and delta in the peripheral blood of chronic psoriatic patients. Diseases (2018) 6(1). doi: 10.3390/diseases6010021
41. Lisnevskaia L, Murphy G, Isenberg D. Systemic lupus erythematosus. Lancet (2014) 384(9957):1878–88. doi: 10.1016/s0140-6736(14)60128-8
42. Zhang M, Xu WD, Zhu Y, Wen PF, Leng RX, Pan HF, et al. Serum levels of cytokines in systemic lupus erythematosus: Association study in a Chinese population. Z Rheumatol (2014) 73(3):277–80. doi: 10.1007/s00393-013-1274-y
43. Wang Y, Zhou B, Zhao Y, Yu X, Liu Y, Zhang L. Association of plasma IL-32 levels and gene polymorphisms with systemic lupus erythematosus in Chinese han population. Dis Markers (2016) 2016:2460206. doi: 10.1155/2016/2460206
44. Inoue M, Shoda H, Seri Y, Kubo K, Kanda H, Fujio K, et al. Three cases of lupus nephritis patients with serum interleukin-32γ detection. Lupus (2014) 23(11):1187–91. doi: 10.1177/0961203314538108
45. Denton CP, Khanna D. Systemic sclerosis. Lancet (2017) 390(10103):1685–99. doi: 10.1016/s0140-6736(17)30933-9
46. Khanna D, Furst DE, Clements PJ, Allanore Y, Baron M, Czirjak L, et al. Standardization of the modified rodnan skin score for use in clinical trials of systemic sclerosis. J Scleroderma Relat Disord (2017) 2(1):11–8. doi: 10.5301/jsrd.5000231
47. Matsuda KM, Yoshizaki A, Kuzumi A, Fukasawa T, Ebata S, Miura S, et al. Skin thickness score as a surrogate marker of organ involvements in systemic sclerosis: A retrospective observational study. Arthritis Res Ther (2019) 21(1):129. doi: 10.1186/s13075-019-1919-6
48. Jennette JC, Falk RJ, Bacon PA, Basu N, Cid MC, Ferrario F, et al. 2012 Revised international chapel hill consensus conference nomenclature of vasculitides. Arthritis Rheum (2013) 65(1):1–11. doi: 10.1002/art.37715
49. Nakazawa D, Masuda S, Tomaru U, Ishizu A. Pathogenesis and therapeutic interventions for ANCA-associated vasculitis. Nat Rev Rheumatol (2019) 15(2):91–101. doi: 10.1038/s41584-018-0145-y
Keywords: IL-32, biomarker, rheumatic disease, inflammatory arthritis, connective tissue disease
Citation: Kwon OC, Park M-C and Kim Y-G (2023) Interleukin-32 as a biomarker in rheumatic diseases: A narrative review. Front. Immunol. 14:1140373. doi: 10.3389/fimmu.2023.1140373
Received: 08 January 2023; Accepted: 06 February 2023;
Published: 15 February 2023.
Edited by:
Jéssica C. dos Santos, Radboud University Medical Centre, NetherlandsReviewed by:
Federico Diaz-Gonzalez, University of La Laguna, SpainCopyright © 2023 Kwon, Park and Kim. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yong-Gil Kim, YmVzdG1kMjAwMEBhbWMuc2VvdWwua3I=
 Oh Chan Kwon
Oh Chan Kwon Min-Chan Park
Min-Chan Park Yong-Gil Kim
Yong-Gil Kim