
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol., 18 April 2023
Sec. Inflammation
Volume 14 - 2023 | https://doi.org/10.3389/fimmu.2023.1139912
 Maritha Kasambala1,2*
Maritha Kasambala1,2* Samson Mukaratirwa1,3
Samson Mukaratirwa1,3 Arthur Vengesai4
Arthur Vengesai4 Tariro Mduluza-Jokonya5
Tariro Mduluza-Jokonya5 Luxwell Jokonya5
Luxwell Jokonya5 Herald Midzi6,7
Herald Midzi6,7 Rutendo Birri Makota2
Rutendo Birri Makota2 Arnold Mutemeri8
Arnold Mutemeri8 Emmanuel Maziti8
Emmanuel Maziti8 Bazondlile Dube-Marimbe8
Bazondlile Dube-Marimbe8 Dixon Chibanda8
Dixon Chibanda8 Francisca Mutapi9
Francisca Mutapi9 Takafira Mduluza6
Takafira Mduluza6Background: Cognitive function is negatively impacted by schistosomiasis and might be caused by systemic inflammation which has been hypothesized to be one of the mechanisms driving cognitive decline, This study explored the association of systemic inflammatory biomarkers; interleukin (IL)-10, IL-6, IL-17, transforming growth factor (TGF-β), tumor necrosis factor (TNF-α), C-reactive protein (CRP) and hematological parameters with cognitive performance of preschool-aged children (PSAC) from an Schistosoma haematobium endemic area
Methods: The Griffith III tool was used to measure the cognitive performance of 136 PSAC. Whole blood and sera were collected and used to quantify levels of IL-10, TNF-α, IL-6, TGF-β, IL-17 A and CRP using the enzyme-linked immunosorbent assay and hematological parameters using the hematology analyzer. Spearman correlation analysis was used to determine the relationship between each inflammatory biomarker and cognitive performance. Multivariate logistic regression analysis was used to determine whether systemic inflammation due to S. haematobium infection affected cognitive performance in PSAC.
Results: Higher levels of TNF-α and IL-6, were correlated with lower performance in the Foundations of Learning domain (r = -0.30; p < 0.001 and r = -0.26; p < 0.001), respectively. Low cognitive performance in the Eye-Hand-Coordination Domain was observed in PSAC with high levels of the following inflammatory biomarkers that showed negative correlations to performance; TNF-α (r = -0.26; p < 0.001), IL-6 (r = -0.29; p < 0.001), IL-10 (r = -0.18; p < 0.04), WBC (r = -0.29; p < 0.001), neutrophils (r = -0.21; p = 0.01) and lymphocytes (r = -0.25; p = 0.003) The General Development Domain correlated with TNF-α (r = -0.28; p < 0.001) and IL-6 (r = -0.30; p < 0.001). TGF-β, L-17A and MXD had no significant correlations to performance in any of the cognitive domains. The overall general development of PSAC was negatively impacted by S. haematobium infections (OR = 7.6; p = 0.008) and (OR = 5.6; p = 0.03) where the PSAC had higher levels of TNF-α and IL-6 respectively.
Conclusion: Systemic inflammation and S. haematobium infections are negatively associated with cognitive function. We recommend the inclusion of PSAC into mass drug treatment programs.
Neurodevelopment is critical during early childhood development which is a time period involving rapid brain development of neurological pathways that influence cognitive functioning that includes memory, attention, language learning, perception, decision making and problem-solving skills (1, 2). Factors such as malnutrition, poverty, inflammation and infectious diseases predispose children, particularly those that reside in resource-poor communities of developing countries to neurodevelopmental delays (3, 4). Schistosomiasis (bilharzia) is an acute and chronic neglected tropical disease caused by Schistosoma spp. and has been reported in over 70 countries where 90% of populations requiring treatment reside in the rural areas of sub-Saharan Africa (5, 6). Amongst the negative impacts of schistosomiasis to infected adults and especially children are inflammatory reactions and hematologic changes accompanied with poor cognitive performance (7–11).
To date, there are no studies that have attempted to investigate the mechanism of how schistosomiasis causes cognitive impairment especially in infected children. However, it is widely believed that chronic host systemic immune responses and inflammation to infection from diseases such as schistosomiasis are the major causes of cognitive decline and could be one of the key mechanisms through which schistosomiasis affects cognitive functioning (12). The immunopathology of acute and chronic schistosomiasis is characterized by T helper (Th1) and T helper 2 (Th2) immune responses against the different types of antigens that are released during the growth and reproductive stage of the schistosomes (13). At the onset of infection for 6 weeks, a Th1 immune response involving the production of pro-inflammatory cytokines; TNF-α, IFN-γ, IL-6 and IL-1 induce cell-mediated immunity and phagocytosis (14). IL-4, IL-5, TGF-β, IL-17A and IL-10 (anti-inflammatory cytokine) are Th2 cytokines that downregulate the Th1 response, promote granuloma formation and induce the humoral mediated immunity mostly against the egg antigens (15–17). Some of the cytokines produced during schistosome infections (interferon-gamma (IFN)-λ, TNF-α, IL-10, IL-6, TGF-β, and IL-17A) have been studied in different populations and age groups with different infectious conditions and have been associated with the alteration of cognitive functions and behavioral manifestations (18, 19). The immune cells, which are part of the hematological parameters, are the sources of inflammatory cytokines which play a key role in communicating with the central nervous system (20). Inflammatory mediators such as cytokines induce the permeability of the blood-brain barrier (BBB) which preserves the central nervous system (CNS) (21–23).
Previous studies have focused on the possible impacts of schistosomiasis on cognitive functions in children but only few studies have explored the effects of schistosomiasis on early childhood development. There are no studies to date that have examined the relationship between inflammatory biomarkers (cytokines and hematological parameters) and cognitive performance in populations infected with schistosomes in endemic areas, however, various studies on other diseases are indicative of the complex diverse relationship between different cytokines and cognitive domains. Studies have reported on contradictory results which entail a mixture of associations and no associations between cytokines levels and cognitive function in different domains (24–29).
This study thus aims to provide information that contributes towards understanding how schistosomiasis might be impacting the cognitive functioning in PSAC by exploring the role of systemic inflammation which encompasses the measurement of various immune cells, cytokines and inflammatory proteins in PSAC residing in a urinary schistosomiasis endemic area in Zimbabwe.
This case control cross sectional study was part of another study that primarily aimed at determining the impacts of schistosomiasis on the early childhood development that was conducted in Murewa, Zimbabwe (30). The main objective of the study was to determine the role of systemic inflammation on cognitive performance by investigating the following two objectives; i) Determining if there were any correlations between inflammatory biomarker levels and cognitive functioning and, ii) Determining which possible explanatory factors could be driving cognitive function in PSAC with significant correlations between inflammatory biomarker levels and cognitive function. PSAC from Zimbabwe (Magaya village in Murewa district) were screened for schistosomiasis. This area has a high prevalence of S. haematobium (> 50%). Thirty PSAC were diagnosed with S. haematobium infection through urine filtration and 106 non-infected PSAC from non-permanent residents of Magaya village were selected as controls.
Inclusion and exclusion criteria. PSAC who were eligible for recruitment into the study met the following inclusion criteria; (i) were lifelong occupants of the study area (Murewa, Zimbabwe) (ii) had never received anthelminthic medication (iii) were aged less than 72 months (iv) provided 3 urine samples for the diagnosis of S. haematobium and stool samples for soil transmitted helminths and S. mansoni diagnosis (v) written consent obtained from parent to participate in the study.
Children were excluded in the study if; (i) They had mental or physical disabilities such as attention-deficit disorder which predisposes them to attaining low cognitive scores, (ii) had a positive diagnosis of soil transmitted helminths and/or S. mansoni.
Sample size calculation was done using the assumption of 50% exposure in cases and 22% exposure in controls and an Odds Ratio of 3.5 to report a power of 80% and 95% confidence interval.
Parasitological screening for S. haematobium, soil transmitted helminths and S. mansoni eggs was conducted as described previously (30). Each PSAC provided at least 3 urine and stool samples over 3 cumulative days. Urine samples were used to microscopically screen for S. haematobium eggs using the urine filtration method (31) whilst S. mansoni and soil transmitted helminths were screened from the stool samples using the Kato-Katz method (32).
Nutritional status was scored using the WHO child growth standards from the WHO Anthro software, version 3.0.1(http://www.who.int/childgrowth/en/) as described in a previous study (33). Briefly, measurements of height, weight and the mid upper arm circumference (MUAC) were taken from PSAC using a stadiometer (Gima®), electronic scale (Gima®) and MUAC tape (AnthroFlex®) respectively. These measurements were used to obtain Z-scores and PSAC with BMI Z-scores < -2 were classified as having a low nutritional status.
Cognitive performance measures of early childhood development was determined using the Griffiths III psychometric tool which was administered by a psychologist on the same day that blood samples were collected. This tool measures 5 cognitive subscales whose average developmental quotient scores give the general development profile of a child (general quotient score). The 5 cognitive subscales that were assessed include; The Foundations of Learning, Language and Communication, Eye and Hand Coordination, Personal-Social-Emotional and Gross Motor function. The Foundations of learning subscale assesses learning during the early childhood period. The Language and Communication subscale is used to assess expressive and receptive language including the child’s ability to communicate. The Eye and Hand Coordination subscale measures the child’s small muscle coordination of eyes with hands. The Personal-Social-Emotional subscale is used to assess the child’s ability to manage emotions, peer relationships and how they articulate to tasks and the Gross Motor function which assesses a child’s development in the areas of physical skills development. Developmental Quotient (DQ) scores >88 was used to classify cognitive performance in PSAC as normal from each Griffith Mental Developmental subscale (34).
Blood samples (5ml) were collected from all the PSAC on the same day and prior to the administration of the cognitive assessment by a clinician. The serum samples were used for the determination of cytokines (IL-6, TNF-α, TGF-β, IL-10 and IL-17A) and CRP. CRP levels were measured using a commercial Duoset Enzyme-Linked Immunosorbent Assay (ELISA) kit (R & D Systems, Minneapolis, MN, USA) according to the protocol provided by the manufacturer. The cytokine levels of IL-6, TNF-α, TGF-β, IL-10 and IL-17A were measured using the ELISA according to the manufacturer’s (Mabtech Company, Stockholm, Sweden) protocol. Briefly, serum samples for each inflammatory biomarker were run in duplicates on the same microtiter plate. Standards of known concentration were used to determine the concentrations of the cytokines. The Dynatech Immulon® microtiter plates were coated overnight with the coating solution (capture monoclonal antibody mixed with phosphate-buffered saline (PBS)). The microtiter plates were washed and blocked with the Tris- buffered saline (TBS) and were incubated for 30 minutes. After another wash step, the serum samples that were diluted in the reagent diluent were micro pipetted into the microtiter plates and were incubated at 37°C for 2 hours. After washing the plates, the conjugated antibody was added. The microtiter plates were incubated for an hour after which the substrate o-phenylenediamine dihydrochloride (OPD) was added. The microtiter plates were incubated for 20 minutes in dark conditions. The optical densities were immediately measured at 450nm using a microplate reader (Micro read 1000).
The determination of total white blood cells (WBC), hemoglobin (HGB), lymphocyte (LYM), Mixed Cell Count (MXD) and neutrophil count was done using a hematology analyzer (MaxM; Coulter, Fullerton, CA) from whole blood samples that were in tubes containing ethylene diamine tetra acetic acid (EDTA).
The data obtained was checked for normality and analyzed using Stata Version 17.0 (StataCorp LLC, Texas, USA). Wilcoxon rank sum was used to determine the differences in cytokine concentration levels by cognitive status, by infection status and the differences in cognitive performance by infection status. The Spearman correlation was used to assess the relationship between the cytokine levels and cognitive performance in the cognitive subscales. Inflammatory biomarkers that were found to have significant correlations with cognitive performance were considered as predictors to cognitive function. PSAC who had higher levels of the significant inflammatory biomarkers than other PSAC were used for multivariate regression analysis. The multivariate regression analysis was done to determine the adjusted odds of S. haematobium infection having an association with cognitive performance after adjusting for possible explanatory variables (age, sex, hemoglobin concentrations and nutritional status) which were used as continuous variables. P-values < 0.05 were considered as significant.
Table 1 summarizes the characteristics of the PSAC who took part in this study, the prevalence S. haematobium infection among the study participants was 22.06% and the mean egg count/10ml of urine was 13.5. There was a higher prevalence of S. haematobium infection in female PSAC (73%) than in male PSAC (27%). No S. mansoni or soil-transmitted helminth eggs were detected in the study population. Out of the entire population, 53 PSAC (39%) did not have toilets in their households, while 40 PSAC (30%) had toilets and 21% did not respond on the presence or absence of a toilet in their households. Normal performance across the cognitive domains was observed from the majority of the PSAC.
Figures 1–6 summarizes the comparison between inflammatory biomarker levels and cognitive performance. PSAC who were categorized as low performers in the Foundations of Learning subscale (Figures 1A-E) had significantly higher levels of IL-6 (p < 0.001) and TNF-α (p = 0.007). There was no significant difference observed in the cytokine levels of IL-17A (p = 0.80), TGF-β (p = 0.21) and IL-10 (p = 0.89) between PSAC who were low and normal performers in the Foundations of Learning Domain. In the Language and Communication Domain (Figures 2A-E), there were significant differences in the inflammatory markers levels of TGF-β, IL-6, TNF-α and IL-10 between low and normal performers while there was no significant difference in performance amongst the inflammatory marker levels of IL-17A. Significant differences in IL-6, TNF-α and IL-10 levels were observed between low and normal PSAC performers in the Eye-Hand-Coordination (Figures 3C-E), in the Personal-Social-Emotional Domain (Figures 4C-E) and in the General Development Domain (Figures 6C-E).
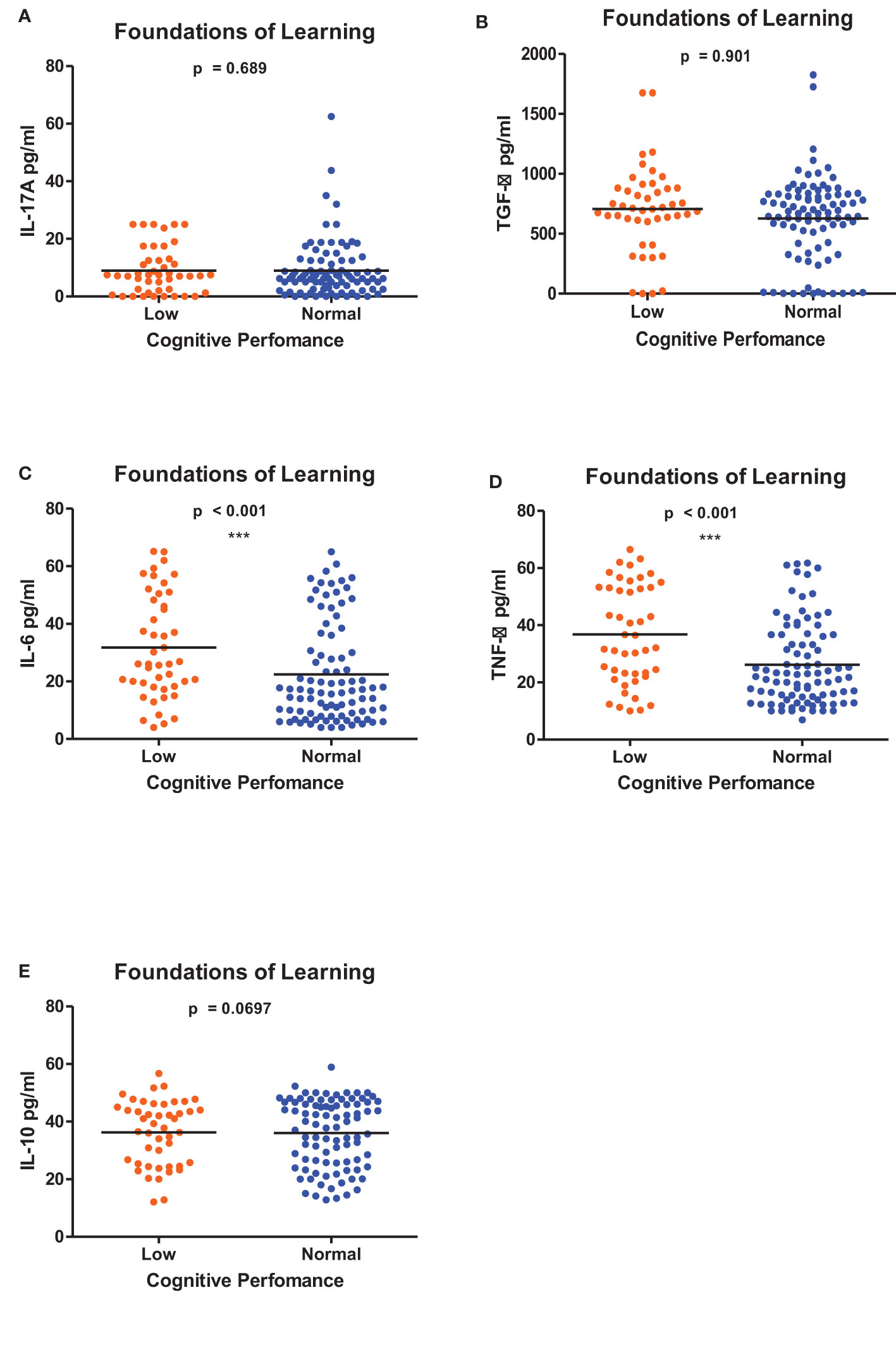
Figure 1 (A-E) Cytokine concentration level comparisons between low and normal PSAC performers in the Foundations for Learning Domain using the Wilcoxon matched-pairs signed rank test. ** = p ≤ 0.01.
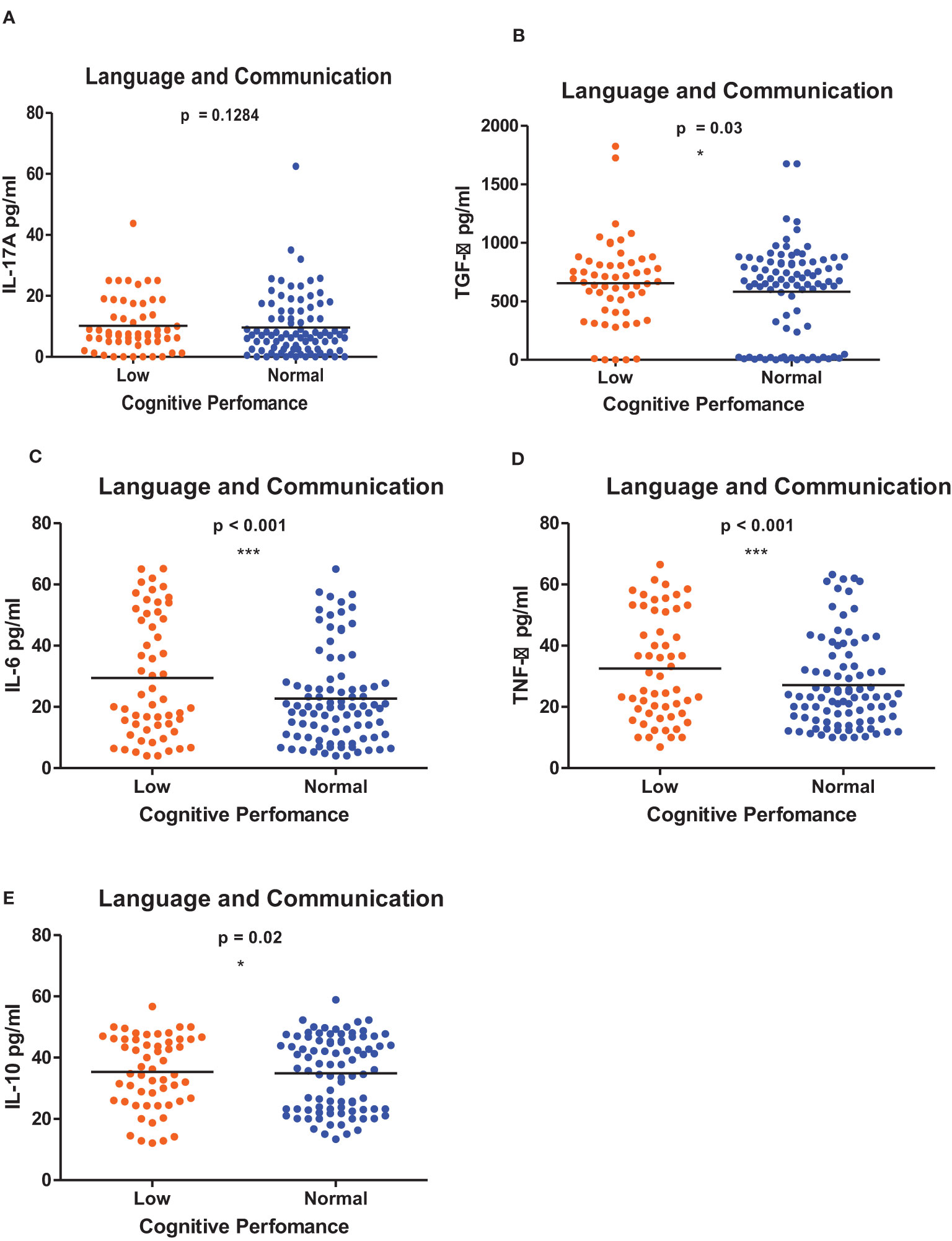
Figure 2 (A-E) Cytokine concentration level comparisons between low and normal PSAC performers in the Language and Communication Domain using the Wilcoxon matched-pairs signed rank test. * = p ≤ 0.05, *** = p ≤ 0.001.
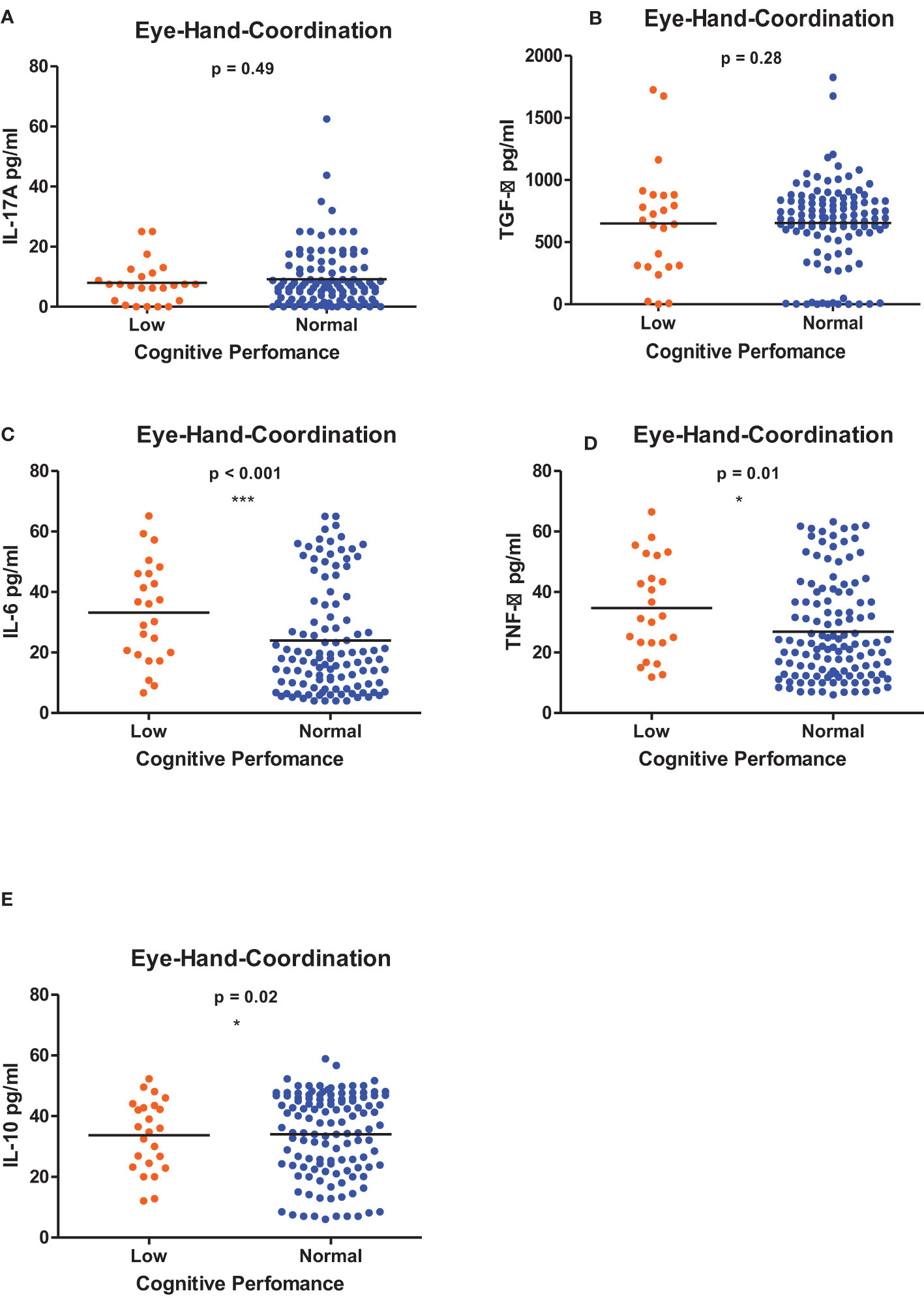
Figure 3 (A-E) Cytokine concentration level comparisons between low and normal PSAC performers in the Eye-Hand-Coordination Domain using the Wilcoxon matched-pairs signed rank test. * = p ≤ 0.05, *** = p ≤ 0.001.
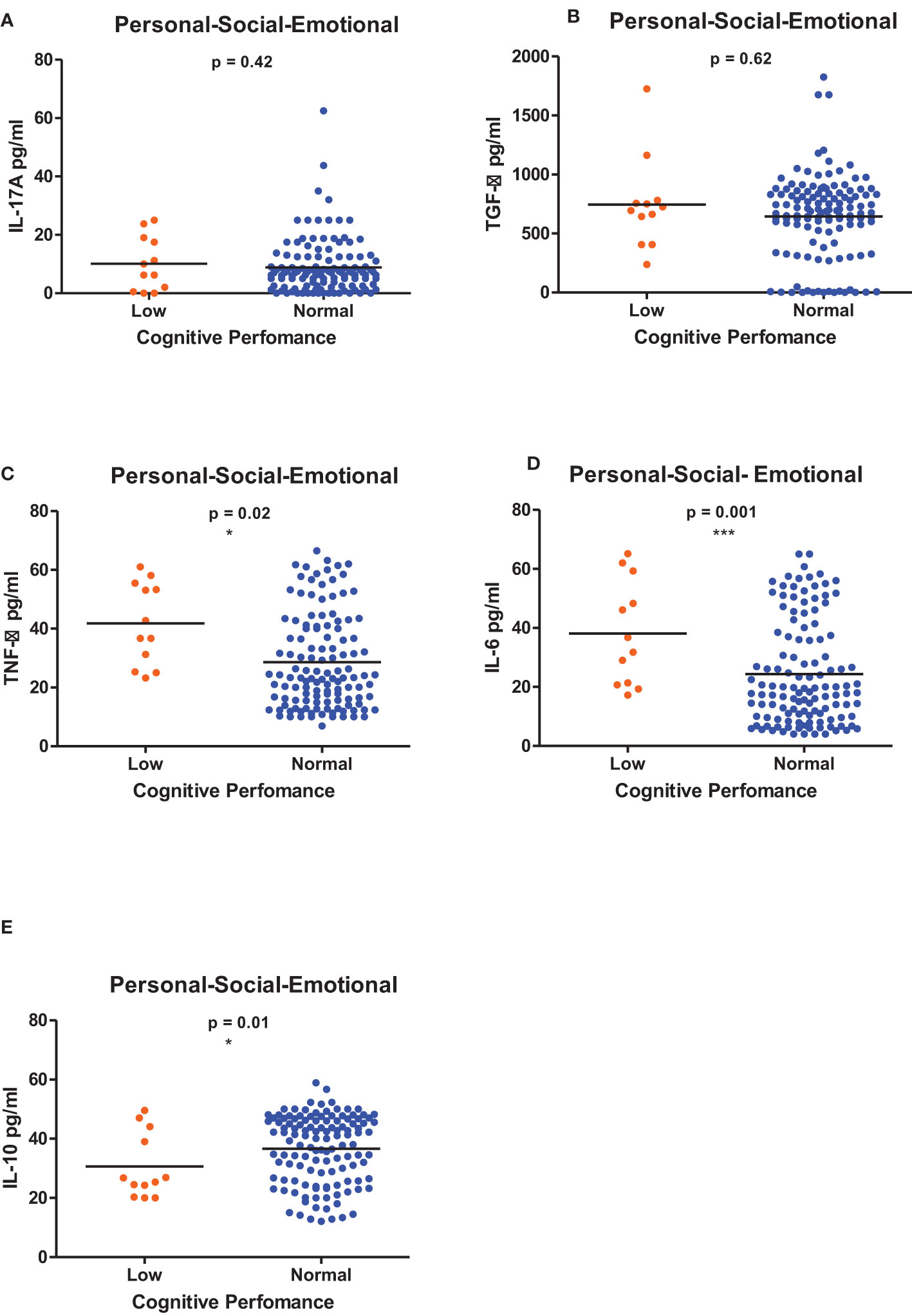
Figure 4 (A-E) Cytokine concentration level comparisons between low and normal PSAC performers in the Personal-Social-Emotional Domain using the Wilcoxon matched-pairs signed rank test. * = p ≤ 0.05, *** = p ≤ 0.001.
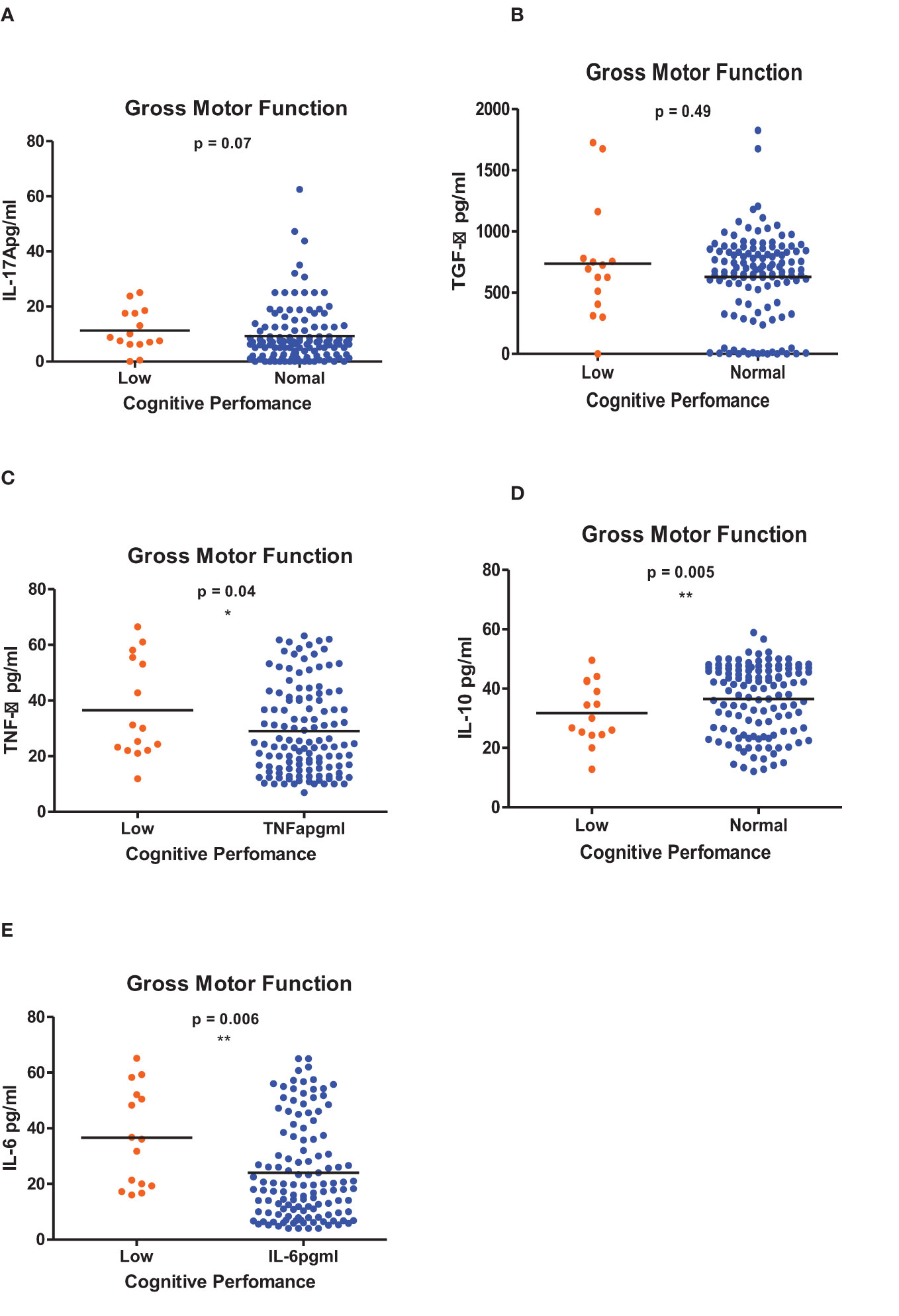
Figure 5 (A-E) Cytokine concentration level comparisons between low and normal PSAC performers in the Gross Motor Function Domain using the Wilcoxon matched-pairs signed rank test. * = p ≤ 0.05, ** = p ≤ 0.01.
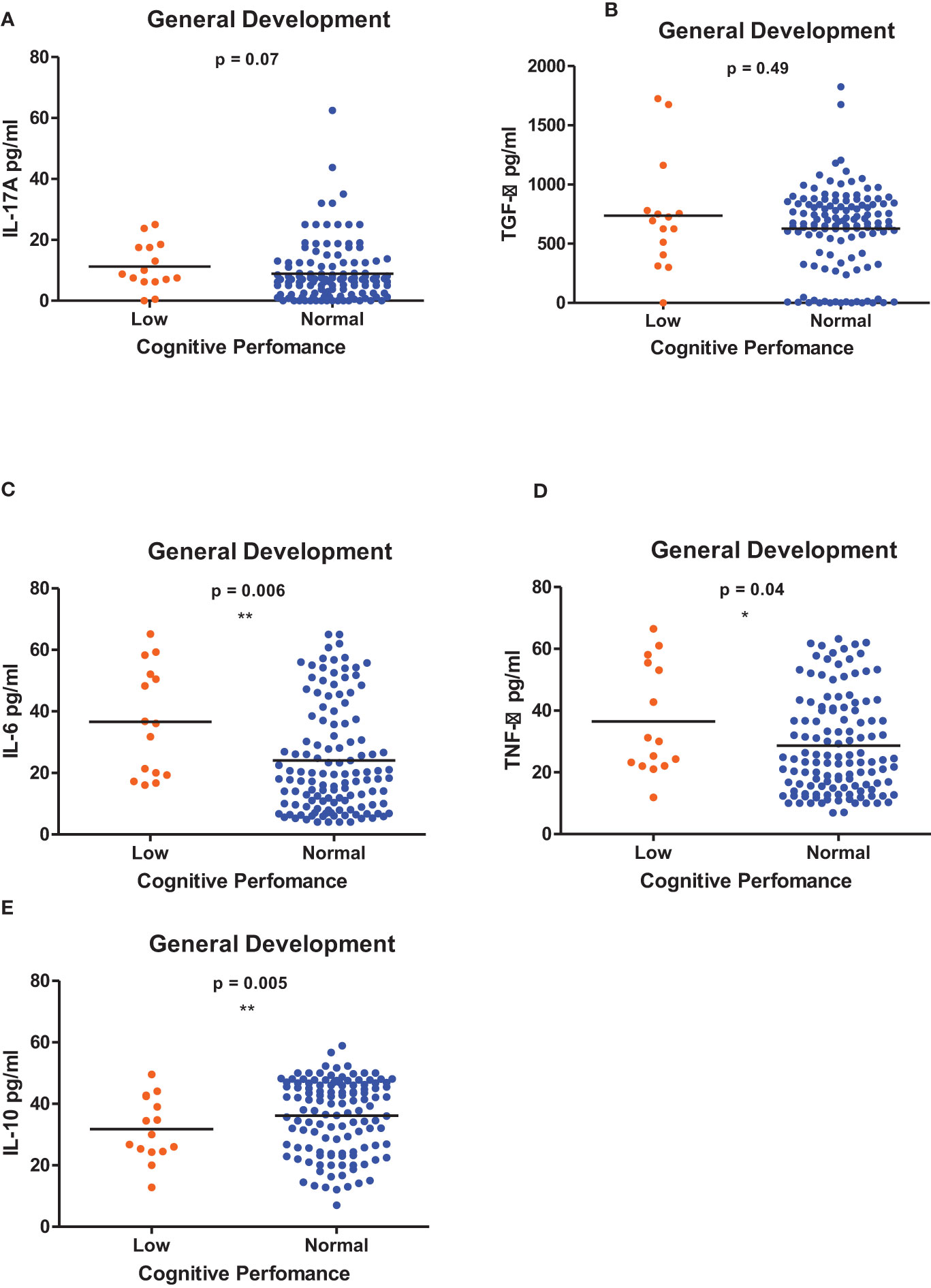
Figure 6 (A-E) Cytokine concentration level comparisons between low and normal PSAC performers in the General Development Domain using the Wilcoxon matched-pairs signed rank test. * = p ≤ 0.05, ** = p ≤ 0.01.
Significant correlations were evident between some of the inflammatory biomarkers (TNF-α, IL-6, IL-10, CRP, HGB, WBC, neutrophils and lymphocytes) and cognitive performance in various cognitive domains in the study participants (Table 2). High TNF-α concentration levels were correlated to low performance in the Foundations of Learning subscale (r = -0.30; p < 0.001), Eye-Hand-Coordination (r = -0.26; p < 0.001), General Development Domain (r = -0.28; p < 0.001). IL-6 concentration levels were also negatively correlated to cognitive performance in the Foundations of Learning (r = -0.33; p < 0.001), Eye-Hand-Coordination (r = -0.29; p < 0.001) and General Development Domain (r = -0.30; p < 0.001) suggesting that an increase in IL-6 concentration levels resulted in lower cognitive performance. Higher levels of IL-10 concentration were correlated to better performance in the Eye-Hand-Coordination (r = -0.18; p < 0.04). CRP levels were negatively correlated to performance in the Foundation of Learning Domain (r = - 0.26, p = 0.008). This indicated that increased CRP levels were associated with lower performance in the Foundation of Learning Domain.
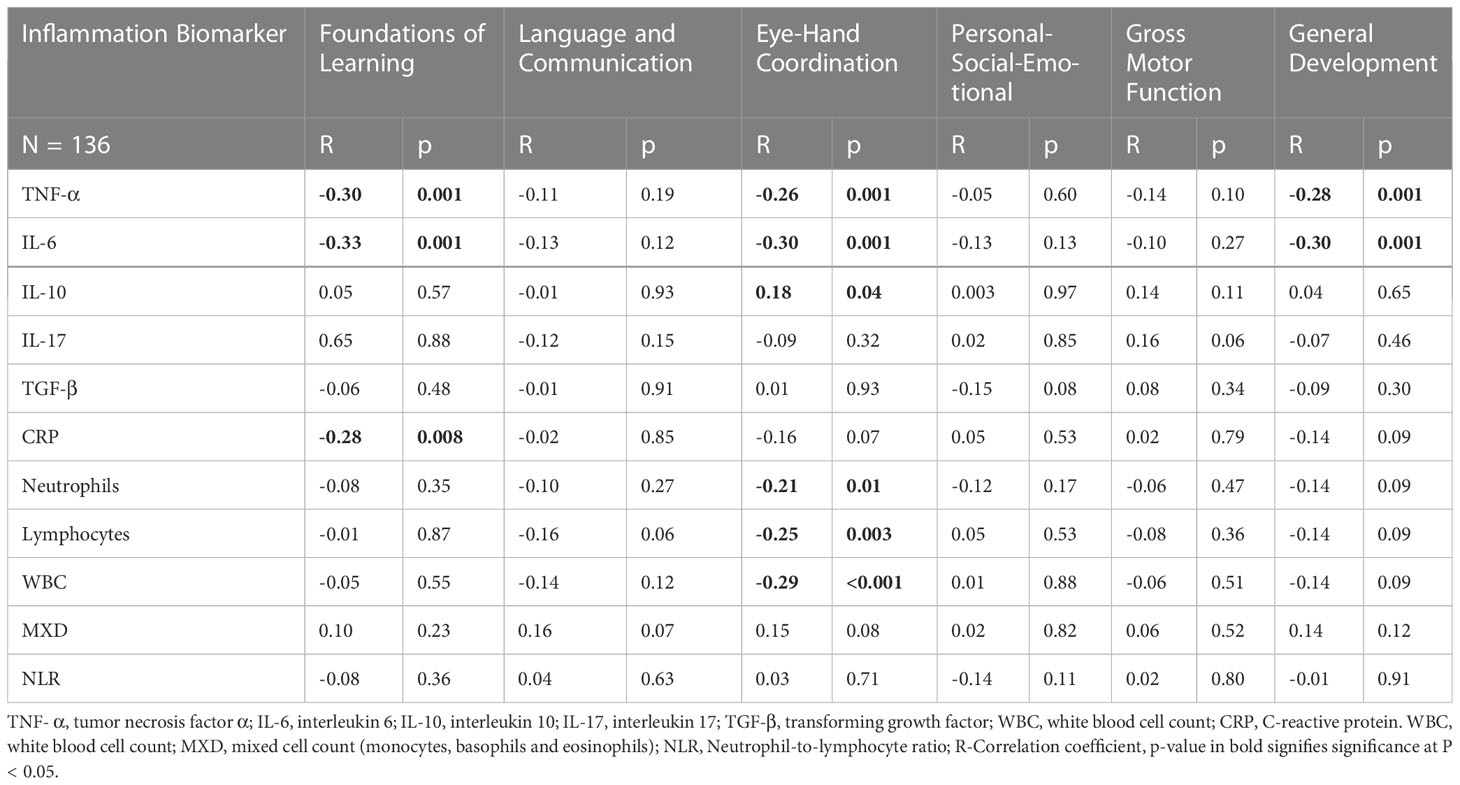
Table 2 Spearman Correlation analysis of inflammatory biomarkers and cognitive performance of preschool aged children.
WBC, neutrophils and lymphocytes were negatively correlated to cognitive performance in the Eye-Hand-Coordination Domain (r = -0.29; p < 0.001), (r = -0.21; p = 0.01) and (r = -0.25; p = 0.003 respectively). This indicates that higher levels of WBC, neutrophils and lymphocytes were associated with lower performance in the Eye-Hand-Coordination Domain. The other inflammatory biomarkers (TGF-β, L-17A and MXD had no significant correlations to performance in any of the cognitive domains.
Multivariate logistic regression was done for the inflammatory biomarker variables (TNF-α, IL-6, IL-10, CRP, HGB, WBC, MCHC, neutrophils and lymphocytes). For each inflammatory biomarker variable group, PSAC were categorized as having either high or low inflammatory biomarkers levels. PSAC with inflammatory biomarker levels that had non-significant correlations with cognitive performance were excluded from their respective multivariate logistic regression analysis.
Table 3 summarizes the multivariate analysis of cognitive function in the Foundations of Learning Domain in PSAC and the inflammatory biomarker variables (IL-6, TNF-α and CRP). There was no significant associations observed between performance in this domain and the possible explanatory variables (sex, hemoglobin, age, nutrition status and S. haematobium infection status) in PSAC who were categorized as having higher IL-6. Schistosoma haematobium infection status had significant greater odds of low performance in the Foundations of Learning Domain in PSAC who were categorized as having high TNF-α. This implies that PSAC who had S. haematobium infections were 2.8 times higher chances of performing lower in comparison to PSAC who were uninfected. There were no significant associations observed in the other explanatory variables (sex, nutritional status, hemoglobin levels and age).
PSAC with high CRP levels and with S. haematobium infections were at greater odds 2.8 of lower performance in the Foundations of Learning Domain There were no significant associations between cognitive performance and the explanatory variables (sex, nutritional status, age, and hemoglobin levels) within PSAC who had high CRP levels.
Multivariate logistic regression was done for the inflammatory biomarkers (IL-6, TNF-α, IL-10, Neutrophils, Lymphocytes and WBC). Sex, age and S. haematobium infection status had significant odds of performance in the Eye-Hand-Coordination Domain in PSAC with high IL-6 levels (Table 3). Female PSAC were 4.8 times more likely to have better scores than male PSAC. Younger PSAC were more likely to have lower scores than older PSAC. PSAC with S. haematobium infections had 4.8 odds of low performance in the Eye-Hand-Coordination Domain compared to uninfected PSAC. Schistosoma haematobium infection status, gender and age had significant odds of performance in the Eye-Hand-Coordination in PSAC who had high TNF –α levels. Older PSAC performed better than the younger PSAC. Female PSAC were 3.6 times more likely to have better scores than male PSAC. Infected PSAC had 5.9 odds of lower performance than uninfected PSAC.
In PSAC who had high IL-10 levels, age, gender and S. haematobium infection status were found to be associated with performance in the Eye-Hand-Coordination Domain. Younger PSAC performed lower than the older PSAC. Female PSAC had greater odds of better scores than male PSAC and infected PSAC were 6.8 times likely to have lower scores. In PSAC with high lymphocyte levels, sex, age and S. haematobium infection status had significant odds of cognitive performance. Male PSAC were 6 times likely to have lower performance than female PSAC. Infected PSAC were 9 times likely to have lower scores than uninfected PSAC while the younger PSAC had greater odds of low performance than the older PSAC. Age, sex and S. haematobium infection status were significant factors affecting performance in PSAC who had higher levels of neutrophils and lymphocyte counts in the Eye-Hand-Coordination Domain.
Multivariate logistic regression analysis in Table 3 indicates that both age and S. haematobium infection status had significant odds of performance in the General Development Domain in PSAC who had higher TNF-α and IL-6 levels. Infected PSAC had greater odds of having lower performance scores in comparison to uninfected PSAC. Younger PSAC had lower scores than the older PSAC.
Figures 7A-J shows the comparisons of the inflammatory biomarker profiles between S. haematobium infected and uninfected PSAC. The levels of IL-17A (Figure 7A) and TGF-β Figure 7B) were comparable in both infected and uninfected PSAC. Infected PSAC had significantly higher concentration of IL-6 (Figure 7C) and TNF-α (Figure 7D) in comparison to uninfected PSAC while the levels of IL-10 were higher in the uninfected PSAC group (Figure 7E).
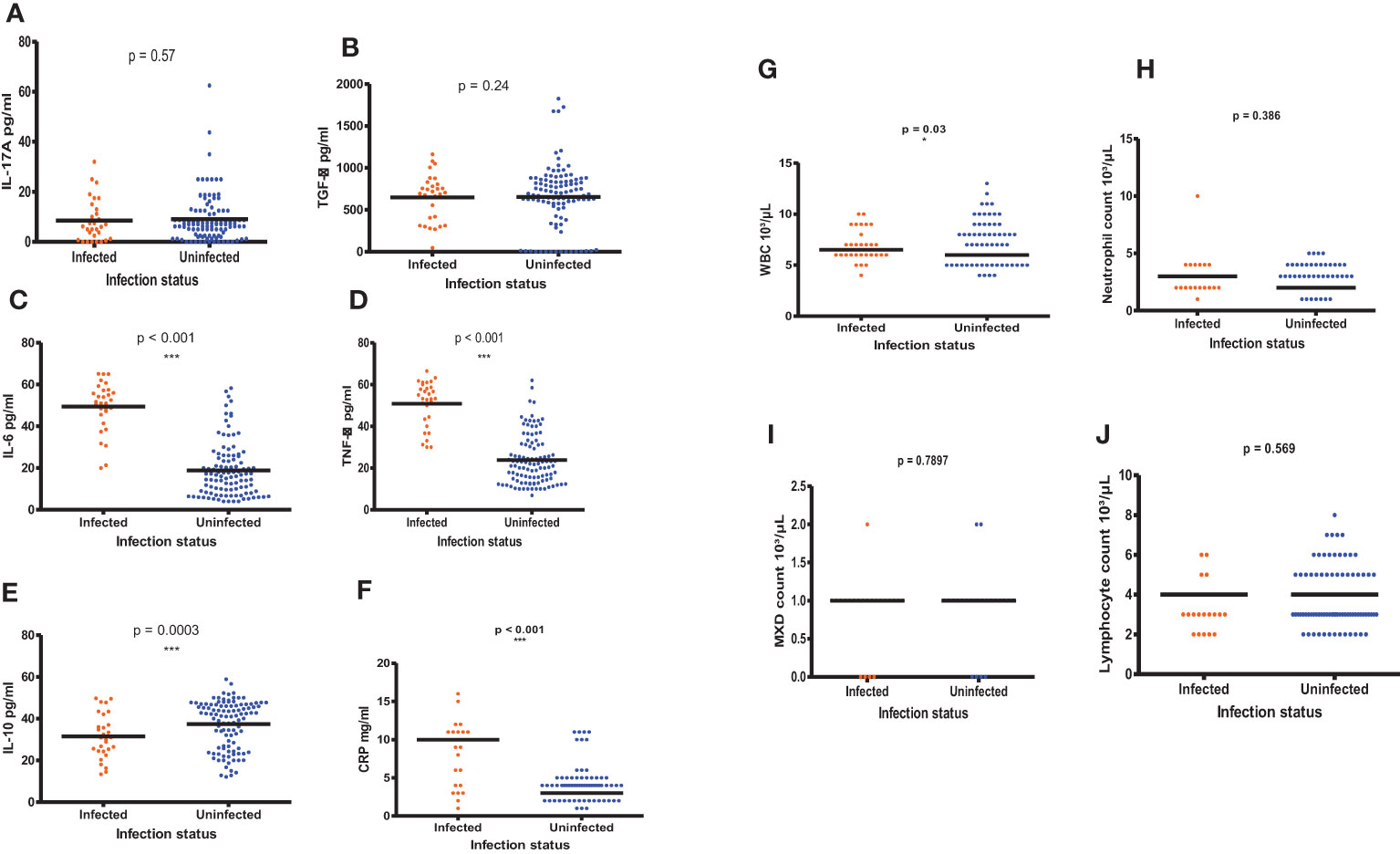
Figure 7 (A-J) Comparisons of the inflammatory biomarker levels in infected and uninfected PSAC using the Wilcoxon matched-pairs signed rank test. * = p ≤ 0.05, *** = p ≤ 0.001.
There were significant higher WBC counts (p = 0.03, Wilcoxon- test) and MXD (p < 0.001, Wilcoxon- test) in PSAC with S. haematobium infections compared to the uninfected PSAC. The CRP levels (p < 0.001) and NLR (p = 0.03, Wilcoxon- test) were also high in infected PSAC while the MXD were surprisingly high in uninfected PSAC (p = <0.001, Wilcoxon- test). The other hematological parameters (neutrophils and lymphocytes) did not significantly differ between infected and uninfected PSAC.
There is a paucity of studies that have explored the relationship between systemic inflammation and cognition in children and the few existing studies have contradictory and inconclusive results (38–43). Our study is the first to date that has attempted to explore the relationship between ten systemic inflammatory biomarkers and cognitive performance in PSAC during schistosomiasis infections. We report two key findings; the complex relationship between inflammatory biomarkers and reduced cognitive performance as well as the varied negative effects of S. haematobium infections on performance in the cognitive domains.
Significant correlations of high levels of systemic inflammatory biomarkers (TNF-α, IL-6, IL-10, CRP, HGB, WBC, neutrophils and lymphocytes) with lower performance in various cognitive domains (Foundations of Learning, Language and Communication, Eye-Hand-Coordination and General Development Domain) were observed in our study and this in agreement with previous studies (44–48). The cognitive performance in the PSAC from our study infected with S. haematobium could have been affected by the interaction of systemic cytokines with the central nervous system through the hypothesized routes which include the interaction of systemic cytokines with the receptors on the blood-brain barrier, passage via the neural afferent pathway, and/or the circumventricular organs through perivascular spaces (49). Consequently, their passage triggers the brain immune cells (microglia and astrocytes) to produce cytokines that modulate neuronal responses and learning (50–52).
Contrary to our results, IL-6 concentrations were found not to affect cognitive performance in other studies (53, 54). We also found no significant associations between IL-17A concentration and performance in any of the cognitive domains contrary to a study in patients with breast cancer who had lower IL-17A levels which were associated with better performance in the Psychomotor/Gross Motor Function and memory domain (55). We observed the absence of correlations between either of the inflammatory markers TGF-β and IL-17A and cognitive performance and this was likely due to the PSAC having undetectable low levels of TGF-β and IL-17A.
Discrepancies in the results from studies that have investigated the relationship between systemic inflammatory markers and cognitive performance could be because of the differences in study design, study participants, age, the use of different cognitive tools, different timeframes of blood collection, cognitive assessment and quantification of inflammatory markers. In our study, we reduced the risk of bias by ensuring that blood samples were collected on the same day that the cognitive assessments were done, PSAC with schistosomiasis (cases) were matched and compared to PSAC without schistosomiasis (controls), we controlled for the most common confounding factors of cognitive performance and the production of inflammatory biomarkers.
After analysis of the potential explanatory variables that could be influencing cognitive performance and the inflammatory biomarker concentrations, our results indicate that although hemoglobin levels and nutritional status did not have any relationship with cognitive performance, age and gender were found to influence cognitive function which was in agreement to results from previous studies (56, 57). This further emphasizes the importance of controlling for the common potential confounding factors during the analysis of results from cognition based studies as cognitive performance is affected by various factors (8). We also report comparable results of significant greater odds of low performance in PSAC with S. haematobium infection in comparison to uninfected PSAC in the Foundations of Learning, Eye-Hand Coordination and General Development Domain (11, 58).
Furthermore, there were significant differences between infected and uninfected PSAC’s TNF-α, IL-6, WBC, CRP and NLR in our study, which is indicative of the immune response towards the different types of antigens (cercariae, growing schistosomula, adult worms and eggs) exposed to the host body. The immune response to schistosomiasis is characterized by the formation of granulomas that consists of immune cells such as eosinophils and lymphocytes around egg antigens. Comparable results have been reported were there was increased WBC (59–61) IL-6 and TNF-α (62–64) in population groups with schistosomiasis in comparison to those without. Contrary to other studies we did not observe significant differences in neutrophils and lymphocytes counts between infected and uninfected populations (60, 65).
Uninfected PSAC were observed to have significantly higher concentration levels of IL-10 than infected PSAC and these results are comparable to studies that have reported on the presence of lowIL-10 levels in children with soil-transmitted helminths which could be due to the acute nature of S. haematobium infections in PSAC (66). Chronic infection would be required to induce high IL-10 immune responses.
We acknowledge the limitations of our study which include the inability of our results to infer casual relationships between the inflammatory biomarkers, schistosomiasis and cognitive performance. Our study findings might also differ with different ethnicity groups as inflammatory biomarkers such as cytokines have been reported to vary by race and there could have been the presence of other infectious diseases such as HIV which were not screened for in our study population (67). Nonetheless, our findings are indicative of the negative association of systemic inflammation on the cognitive performance of PSAC with schistosomiasis.
In this study, high levels of inflammatory biomarkers (TNF-α, IL-6, IL-10, CRP, HGB, WBC, neutrophils and lymphocytes) were significantly correlated to lower cognitive performance in some of the Griffiths III cognitive domains (Foundations of Learning, Language and Communication, Eye-Hand-Coordination and General Development Domain). Schistosoma haematobium infections had a negative impact on the cognitive performance of PSAC in the Foundations of Learning, Eye-Hand-Coordination and General Development Domain. As this is the first study assessing the association of systemic inflammation with reduced cognitive performance in PSAC with schistosomiasis, we recommend further research to determine the relationships between inflammation and cognitive performance in diverse study populations groups across different ethnicities that have schistosome infections.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Medical Research Council of Zimbabwe. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Conceptualization of the study, MK, SM, FM, DC and TM. Parasitological analysis, MK, TM-J, AV, LJ and HM. Psychological clinical examination, AM, EM and BD-M. Serology lab work, MK. Data analysis, MK and RM. Manuscript writing, MK. Supervision, SM and TM. All authors contributed to the article and approved the submitted version.
The study was commissioned by the National Institute of Health Research (NIHR), Global Health Research Programme (16/136/33) using UK aid from UK government. The field and laboratory activities were funded by the British Academy (BA).
The authors give special acknowledgment to the parents/guardians and PSAC who took part in the study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
CI, confidence interval; CRP, C reactive protein; CNS, Central nervous system; DQ, Developmental Quotient; EHCDQ, Eye and Hand Coordination Development Quotient; ELISA, Enzyme-linked immunosorbent assay; FLDQ, Foundations of Leaning Development Quotient; GDDQ, General Development Quotient; GMDQ, Gross Motor Function Development Quotient; HGB, Haemoglobin; IFN –γ, Interferon gamma; IL-6, Interleukin-6; IQR, Interquartile range; MDA, Mass Drug Administration; ml, Millilitres; MRCZ, Medical Research Council of Zimbabwe; nm, nanometres; OR, odds ratios; OPD, o-phenylene diamine dihydrochloride; PSAC, Preschool-aged children; PSEDQ, Personal-Social-Emotional Development Quotient; PZQ, Praziquantel; S. haematobium, Schistosoma haematobium; TGF, Transforming growth factor; Th1, T helper 1; Th2, T helper 2; TNF, Tumor Necrosis Factor; WBC, Total white blood cells; WHO, World Health Organization.
1. Fisher GG, Chacon M, Chaffee DS. Theories of cognitive aging and work. In: Work across the lifespan. Elsevier (2019). p. 17–45.
2. Bach AM, Xie W, Piazzoli L, Jensen SK, Afreen S, Haque R, et al. Systemic inflammation during the first year of life is associated with brain functional connectivity and future cognitive outcomes. Dev Cogn Neurosci (2022) 53:101041. doi: 10.1016/j.dcn.2021.101041
3. Krebs NF, Lozoff B, Georgieff MK. Neurodevelopment: the impact of nutrition and inflammation during infancy in low-resource settings. Pediatrics (2017) 139(Supplement_1):S50–S8. doi: 10.1542/peds.2016-2828G
4. Johnson SB, Riis JL, Noble KG. State of the art review: poverty and the developing brain. Pediatrics (2016) 137(4). doi: 10.1542/peds.2015-3075
5. Aluise CD, Robinson RAS, Beckett TL, Murphy MP, Cai J, Pierce WM, et al. Preclinical Alzheimer disease: brain oxidative stress, aβ peptide and proteomics. Neurobiol Dis (2010) 39(2):221–8. doi: 10.1016/j.nbd.2010.04.011
6. Angeli V, Faveeuw C, Roye O, Fontaine J, Teissier E, Capron A, et al. Role of the parasite-derived prostaglandin D2 in the inhibition of epidermal langerhans cell migration during schistosomiasis infection. J Exp Med (2001) 193(10):1135–48. doi: 10.1084/jem.193.10.1135
7. Nelwan ML. Schistosomiasis: life cycle, diagnosis, and control. Curr Ther Res (2019) 91:5–9. doi: 10.1016/j.curtheres.2019.06.001
8. Ezeamama AE, Bustinduy AL, Nkwata AK, Martinez L, Pabalan N, Boivin MJ, et al. Cognitive deficits and educational loss in children with schistosome infection–a systematic review and meta-analysis. PloS Negl Trop Dis (2018) 12(1):e0005524. doi: 10.1371/journal.pntd.0005524
9. Mazibuko XI, Chimbari MJ. Parasitic infections and environmental influences on verbal memory and learning potential of isiZulu speaking pre-school children. J Psychol Africa (2020) 30(5):471–4. doi: 10.1080/14330237.2020.1821318
10. King CH, Galvani AP. Underestimation of the global burden of schistosomiasis. Lancet (2018) 391(10118):307–8. doi: 10.1016/S0140-6736(18)30098-9
11. Ezeamama AE, Friedman JF, Acosta LP, Bellinger DC, Langdon GC, Manalo DL, et al. Helminth infection and cognitive impairment among Filipino children. Am J Trop Med Hygiene (2005) 72(5):540. doi: 10.4269/ajtmh.2005.72.540
12. Xie W, Kumar S, Kakon SH, Haque R, Petri WA, Nelson CA. Chronic inflammation is associated with neural responses to faces in Bangladeshi children. Neuroimage (2019) 202:116110. doi: 10.1016/j.neuroimage.2019.116110
13. Colley D, Secor W. Immunology of human schistosomiasis. Parasite Immunol (2014) 36(8):347–57. doi: 10.1111/pim.12087
14. Romagnani S. T-Cell subsets (Th1 versus Th2). Ann Allergy Asthma Immunol (2000) 85(1):9–21. doi: 10.1016/S1081-1206(10)62426-X
15. Masamba P, Kappo AP. Immunological and biochemical interplay between cytokines, oxidative stress and schistosomiasis. Int J Mol Sci (2021) 22(13):7216. doi: 10.3390/ijms22137216
16. Dunne DW, Cooke A. A worm’s eye view of the immune system: consequences for evolution of human autoimmune disease. Nat Rev Immunol (2005) 5(5):420–6. doi: 10.1038/nri1601
17. Pearce EJ, MacDonald AS. The immunobiology of schistosomiasis. Nat Rev Immunol (2002) 2(7):499–511. doi: 10.1038/nri843
18. Huang C, Irwin MG, Wong GTC, Chang RCC. Evidence of the impact of systemic inflammation on neuroinflammation from a non-bacterial endotoxin animal model. J Neuroinflamm (2018) 15(1):1–16. doi: 10.1186/s12974-018-1163-z
19. Singh-Manoux A, Dugravot A, Brunner E, Kumari M, Shipley M, Elbaz A, et al. Interleukin-6 and c-reactive protein as predictors of cognitive decline in late midlife. Neurology (2014) 83(6):486–93. doi: 10.1212/WNL.0000000000000665
20. Wilson CJ, Finch CE, Cohen HJ. Cytokines and cognition–the case for a head-to-toe inflammatory paradigm. J Am Geriatrics Soc (2002) 50(12):2041–56. doi: 10.1046/j.1532-5415.2002.50619.x
21. Varatharaj A, Galea I. The blood-brain barrier in systemic inflammation. Brain Behav Immun (2017) 60:1–12. doi: 10.1016/j.bbi.2016.03.010
22. Daniels BP, Holman DW, Cruz-Orengo L, Jujjavarapu H, Durrant DM, Klein RS. Viral pathogen-associated molecular patterns regulate blood-brain barrier integrity via competing innate cytokine signals. MBio (2014) 5(5):e01476–14. doi: 10.1128/mBio.01476-14
23. Tsao N, Hsu H-P, Wu C-M, Liu C-C, Lei H-Y. Tumour necrosis factor-α causes an increase in blood-brain barrier permeability during sepsis. J Med Microbiol (2001) 50(9):812–21. doi: 10.1099/0022-1317-50-9-812
24. Lodha A, Asztalos E, Moore A. Cytokine levels in neonatal necrotizing enterocolitis and long-term growth and neurodevelopment. Acta Paediatrica (2010) 99(3):338–43. doi: 10.1111/j.1651-2227.2009.01600.x
25. Silveira RC, Procianoy RS. High plasma cytokine levels, white matter injury and neurodevelopment of high risk preterm infants: assessment at two years. Early Hum Dev (2011) 87(6):433–7. doi: 10.1016/j.earlhumdev.2011.03.009
26. O’Shea TM, Shah B, Allred EN, Fichorova RN, Kuban KC, Dammann O, et al. Inflammation-initiating illnesses, inflammation-related proteins, and cognitive impairment in extremely preterm infants. Brain Behav Immun (2013) 29:104–12. doi: 10.1016/j.bbi.2012.12.012
27. Jiang NM, Tofail F, Moonah SN, Scharf RJ, Taniuchi M, Ma JZ, et al. Febrile illness and pro-inflammatory cytokines are associated with lower neurodevelopmental scores in Bangladeshi infants living in poverty. BMC Pediatrics (2014) 14:1–9. doi: 10.1186/1471-2431-14-50
28. Rose J, Vassar R, Cahill-Rowley K, Hintz SR, Stevenson DK. Neonatal biomarkers of inflammation: correlates of early neurodevelopment and gait in very-low-birth-weight preterm children. Am J Perinatol (2016) 2(01):071–8. doi: 10.1055/s-0035-1557106
29. Voltas N, Arija V, Hernández-Martínez C, Jiménez-Feijoo R, Ferré N, Canals J. Are there early inflammatory biomarkers that affect neurodevelopment in infancy? J Neuroimmunol (2017) 305:42–50. doi: 10.1016/j.jneuroim.2017.01.017
30. Kasambala M, Mduluza T, Vengesai A, Mduluza-Jokonya T, Jokonya L, Midzi H, et al. Effect of schistosoma haematobium infection on the cognitive functions of preschool age children and benefits of treatment from an endemic area in Zimbabwe. BMC Infect Dis (2022) 22(1):1–9. doi: 10.1186/s12879-022-07784-7
31. Sacolo-Gwebu H, Chimbari M, Kalinda C. Prevalence and risk factors of schistosomiasis and soil-transmitted helminthiases among preschool aged children (1–5 years) in rural KwaZulu-natal, south Africa: a cross-sectional study. Infect Dis Poverty (2019) 8(1):1–12. doi: 10.1186/s40249-019-0561-5
32. Mott K, Baltes R, Bambagha J, Baldassini B. Field studies of a reusable polyamide filter for detection of schistosoma haematobium eggs by urine filtration. Tropenmedizin und Parasitol (1982) 33(4):227–8.
33. Mutapi F, Pfavayi L, Osakunor D, Lim R, Kasambala M, Mutemeri A, et al. Assessing early child development and its association with stunting and schistosome infections in rural Zimbabwean children using the griffiths scales of child development. PloS Negl Trop Dis (2021) 15(8):e0009660. doi: 10.1371/journal.pntd.0009660
34. Cirelli I, Graz MB, Tolsa J-F. Comparison of griffiths-II and bayley-II tests for the developmental assessment of high-risk infants. Infant Behav Dev (2015) 41:17–25. doi: 10.1016/j.infbeh.2015.06.004
35. Kravitz BA, Corrada MM, Kawas CH. High levels of serum c-reactive protein are associated with greater risk of all-cause mortality, but not dementia, in the oldest-old: Results from the 90+ study. J Am Geriatrics Soc (2009) 57(4):641–6. doi: 10.1111/j.1532-5415.2009.02169.x
36. Monastero RN, Pentyala S. Cytokines as biomarkers and their respective clinical cutoff levels. Int J Inflammation (2017) 2017. doi: 10.1155/2017/4309485
37. Zeh CE, Odhiambo CO, Mills LA. Laboratory reference intervals in Africa. In: Blood cell-an overview of studies in hematology. London, UK: IntechOpen (2012). p. 303–20.
38. Kyriklaki A, Margetaki K, Kampouri M, Koutra K, Bitsios P, Chalkiadaki G, et al. Association between high levels of inflammatory markers and cognitive outcomes at 4 years of age: The rhea mother-child cohort study, Crete, Greece. Cytokine (2019) 117:1–7. doi: 10.1016/j.cyto.2019.01.010
39. Lee SE, West KP Jr., Cole RN, Schulze KJ, Wu LS-F, Yager JD, et al. General intelligence is associated with subclinical inflammation in Nepalese children: A population-based plasma proteomics study. Brain Behav Immun (2016) 56:253–63. doi: 10.1016/j.bbi.2016.03.023
40. Huang Y-S, Guilleminault C, Hwang F-M, Cheng C, Lin C-H, Li H-Y, et al. Inflammatory cytokines in pediatric obstructive sleep apnea. Medicine (2016) 95(41). doi: 10.1097/MD.0000000000004944
41. Jonker I, Klein HC, Duivis HE, Yolken RH, Rosmalen JG, Schoevers RA. Association between exposure to HSV1 and cognitive functioning in a general population of adolescents. the TRAILS study. PloS One (2014) 9(7):e101549. doi: 10.1371/journal.pone.0101549
42. Cullen AE, Tappin BM, Zunszain PA, Dickson H, Roberts RE, Nikkheslat N, et al. The relationship between salivary c-reactive protein and cognitive function in children aged 11–14 years: Does psychopathology have a moderating effect? Brain Behav Immun (2017) 66:221–9. doi: 10.1016/j.bbi.2017.07.002
43. Adelantado-Renau M, Esteban-Cornejo I, Rodriguez-Ayllon M, Cadenas-Sanchez C, Gil-Cosano JJ, Mora-Gonzalez J, et al. Inflammatory biomarkers and brain health indicators in children with overweight and obesity: The ActiveBrains project. Brain Behav Immunity (2019) 81:588–97. doi: 10.1016/j.bbi.2019.07.020
44. Zheng M, Chang B, Tian L, Shan C, Chen H, Gao Y, et al. Relationship between inflammatory markers and mild cognitive impairment in Chinese patients with type 2 diabetes: a case-control study. BMC Endocrine Disord (2019) 19(1):1–10. doi: 10.1186/s12902-019-0402-3
45. Liu J-H, Zhang Y-J, Ma Q-H, Sun H-P, Xu Y, Pan C-W. Elevated blood neutrophil to lymphocyte ratio in older adults with cognitive impairment. Arch Gerontol Geriatrics (2020) 88:104041. doi: 10.1016/j.archger.2020.104041
46. Zenaro E, Pietronigro E, Bianca VD, Piacentino G, Marongiu L, Budui S, et al. Neutrophils promote alzheimer’s disease–like pathology and cognitive decline via LFA-1 integrin. Nat Med (2015) 21(8):880–6. doi: 10.1038/nm.3913
47. Caponnetto P, Russo C, Di Maria A, Morjaria JB, Barton S, Guarino F, et al. Circulating endothelial-coagulative activation markers after smoking cessation: a 12-month observational study. Eur J Clin Invest (2011) 41(6):616–26. doi: 10.1111/j.1365-2362.2010.02449.x
48. M’bondoukwé NP, Moutongo R, Gbédandé K, Ndong Ngomo JM, Hountohotegbé T, Adamou R, et al. Circulating IL-6, IL-10, and TNF-alpha and IL-10/IL-6 and IL-10/TNF-alpha ratio profiles of polyparasitized individuals in rural and urban areas of gabon. PloS Negl Trop Dis (2022) 16(4):e0010308. doi: 10.1371/journal.pntd.0010308
49. Quan N. Immune-to-brain signaling: how important are the blood–brain barrier-independent pathways? Mol Neurobiol (2008) 37(2):142–52. doi: 10.1007/s12035-008-8026-z
50. Fung A, Vizcaychipi M, Lloyd D, Wan Y, Ma D. Central nervous system inflammation in disease related conditions: mechanistic prospects. Brain Res (2012) 1446:144–55. doi: 10.1016/j.brainres.2012.01.061
51. Mao CP, Bai ZL, Zhang XN, Zhang QJ, Zhang L. Abnormal subcortical brain morphology in patients with knee osteoarthritis: a cross-sectional study. Front Aging Neurosci (2016) 8:3. doi: 10.3389/fnagi.2016.00003
52. Matsumoto Y, Yoshida M, Watanabe S, Yamamoto T. Involvement of cholinergic and glutamatergic functions in working memory impairment induced by interleukin-1β in rats. Eur J Pharmacol (2001) 430(2-3):283–8. doi: 10.1016/S0014-2999(01)01374-7
53. Donegan JJ, Girotti M, Weinberg MS, Morilak DA. A novel role for brain interleukin-6: facilitation of cognitive flexibility in rat orbitofrontal cortex. J Neurosci (2014) 34(3):953–62. doi: 10.1523/JNEUROSCI.3968-13.2014
54. Roberts AJ, Khom S, Bajo M, Vlkolinsky R, Polis I, Cates-Gatto C, et al. Increased IL-6 expression in astrocytes is associated with emotionality, alterations in central amygdala GABAergic transmission, and excitability during alcohol withdrawal. Brain Behav Immun (2019) 82:188–202. doi: 10.1016/j.bbi.2019.08.185
55. Lyon DE, Cohen R, Chen H, Kelly DL, McCain NL, Starkweather A, et al. Relationship of systemic cytokine concentrations to cognitive function over two years in women with early stage breast cancer. J neuroimmunol (2016) 301:74–82. doi: 10.1016/j.jneuroim.2016.11.002
56. Palejwala MH, Fine JG. Gender differences in latent cognitive abilities in children aged 2 to 7. Intelligence (2015) 48:96–108. doi: 10.1016/j.intell.2014.11.004
57. Von Stumm S, Plomin R. Socioeconomic status and the growth of intelligence from infancy through adolescence. Intelligence (2015) 48:30–6. doi: 10.1016/j.intell.2014.10.002
58. Nokes C, McGarvey ST, Shiue L, Wu G, Wu H, Bundy D, et al. Evidence for an improvement in cognitive function following treatment of schistosoma japonicum infection in Chinese primary schoolchildren. Am J Trop Med Hygiene (1999) 60(4):556–65. doi: 10.4269/ajtmh.1999.60.556
59. Afrifa J, Gyedu D, Ofori Gyamerah E, Essien-Baidoo S, Mensah-Essilfie I. Haematological profile and intensity of urogenital schistosomiasis in ghanaian children. J Environ Public Health (2017) 2017. doi: 10.1155/2017/4248325
60. Dejon-Agobé JC, Adegnika AA, Grobusch MP. Haematological changes in schistosoma haematobium infections in school children in Gabon. Infection (2021) 49(4):645–51. doi: 10.1007/s15010-020-01575-5
61. Mohammed EH, Eltayeb M, Ibrahim H. Haematological and biochemical morbidity of schistosoma haematobium in school children in Sudan. Sultan Qaboos Univ Med J (2006) 6(2):59.
62. Osada Y, Shimizu S, Kumagai T, Yamada S, Kanazawa T. Schistosoma mansoni infection reduces severity of collagen-induced arthritis via down-regulation of pro-inflammatory mediators. Int J Parasitol (2009) 39(4):457–64. doi: 10.1016/j.ijpara.2008.08.007
63. Imai N, Rujeni N, Nausch N, Bourke CD, Appleby LJ, Cowan G, et al. Exposure, infection, systemic cytokine levels and antibody responses in young children concurrently exposed to schistosomiasis and malaria. Parasitology (2011) 138(12):1519–33. doi: 10.1017/S0031182011001181
64. Castro VN, Rodrigues JL, Cardoso DT, Resende SD, Magalhães FC, Souza DC, et al. Systemic cytokine and chemokine profiles in individuals with schistosoma mansoni infection and low parasite burden. Front Immunol (2018) 9:2975. doi: 10.3389/fimmu.2018.02975
65. Joseph M, Auriault C, Capron A, Vorng H, Viens P. A new function for platelets: IgE-dependent killing of schistosomes. Nature (1983) 303(5920):810–2. doi: 10.1038/303810a0
66. Bustinduy AL, Sutherland LJ, Chang-Cojulun A, Malhotra I, DuVall AS, Fairley JK, et al. Age-stratified profiles of serum IL-6, IL-10, and TNF-α cytokines among Kenyan children with schistosoma haematobium, plasmodium falciparum, and other chronic parasitic co-infections. Am J Trop Med Hygiene (2015) 92(5):945. doi: 10.4269/ajtmh.14-0444
Keywords: cognitive functions, cytokines, hematological parameters, pre-school aged children, schistosomiasis, systemic inflammation
Citation: Kasambala M, Mukaratirwa S, Vengesai A, Mduluza-Jokonya T, Jokonya L, Midzi H, Makota RB, Mutemeri A, Maziti E, Dube-Marimbe B, Chibanda D, Mutapi F and Mduluza T (2023) The association of systemic inflammation and cognitive functions of pre-school aged children residing in a Schistosoma haematobium endemic area in Zimbabwe. Front. Immunol. 14:1139912. doi: 10.3389/fimmu.2023.1139912
Received: 07 January 2023; Accepted: 31 March 2023;
Published: 18 April 2023.
Edited by:
Rashika El Ridi, Cairo University, EgyptReviewed by:
Doudou Sow, Gaston Berger University, SenegalCopyright © 2023 Kasambala, Mukaratirwa, Vengesai, Mduluza-Jokonya, Jokonya, Midzi, Makota, Mutemeri, Maziti, Dube-Marimbe, Chibanda, Mutapi and Mduluza. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maritha Kasambala, bWFyaXRoYWthc2FtYmFsYUBnbWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.