
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol., 28 April 2023
Sec. Autoimmune and Autoinflammatory Disorders : Autoimmune Disorders
Volume 14 - 2023 | https://doi.org/10.3389/fimmu.2023.1134178
This article is part of the Research TopicRare Immune-mediated Diseases- Novel Insights into Underlying Mechanisms and Therapeutic ApproachesView all 13 articles
 Limor Rubin1†
Limor Rubin1† Aviv Talmon1†
Aviv Talmon1† Yaarit Ribak1
Yaarit Ribak1 Asa Kessler2
Asa Kessler2 Yossi Martin3
Yossi Martin3 Tal Keidar Haran4
Tal Keidar Haran4 Oded Shamriz1,5*
Oded Shamriz1,5* Irit Adini6
Irit Adini6 Yuval Tal1*
Yuval Tal1*Background: The drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome represents a severe hypersensitivity reaction. Up-to-date treatment is based on withdrawal of medication, supportive care, and immunosuppression using high-dose corticosteroid (CS) therapy. However, evidence-based data are lacking regarding second-line therapy for steroid-resistant or steroid-dependent patients.
Objectives: We hypothesize that the interleukin (IL)-5 axis plays a critical role in the pathophysiology of DRESS; hence, inhibition of this signaling pathway could offer a potential therapy for steroid-dependent and/or steroid-resistant cases, and it may offer an alternative to CS therapy in certain patients more prone to CS toxicity.
Methods: Herein, we collected worldwide data on DRESS cases treated with biological agents targeting the IL-5 axis. We reviewed all cases indexed in PubMed up to October 2022 and performed a total analysis including our center experience with two additional novel cases.
Results: A review of the literature yielded 14 patients with DRESS who were treated with biological agents targeting the IL-5 axis as well as our two new cases. Reported patients are characterized by a female-to-male ratio of 1:1 and a mean age of 51.8 (17–87) years. The DRESS-inducing drugs, as expected from the prospective RegiSCAR study, were mostly antibiotics (7/16), as follows: vancomycin, trimethoprim-sulfamethoxazole, ciprofloxacin, piperacillin-tazobactam, and cefepime. DRESS patients were treated with anti-IL-5 agents (mepolizumab and reslizumab) or anti-IL-5 receptor (IL-5R) biologics (benralizumab). All patients have clinically improved under anti-IL-5/IL-5R biologics. Multiple doses of mepolizumab were needed to achieve clinical resolution, whereas a single dose of benralizumab was often sufficient. Relapse was noted in one patient receiving benralizumab treatment. One patient receiving benralizumab had a fatal outcome, although mortality was probably related to massive bleeding and cardiac arrest due to coronavirus disease 2019 (COVID-19) infection.
Conclusion: Current treatment guidelines for DRESS are based on case reports and expert opinion. Understanding the central role of eosinophils in DRESS pathogenicity emphasizes the need for future implementation of IL-5 axis blockade as steroid-sparing agents, potential therapy to steroid-resistant cases, and perhaps an alternative to CS treatment in certain DRESS patients more prone to CS toxicity.
Drug reaction with eosinophilia and systemic symptoms (DRESS), also known as drug-induced hypersensitivity syndrome, is a severe type of intravenous cutaneous drug-induced eruption accompanied by visceral organ involvement, most commonly the liver. The prognosis of patients with DRESS is linked to the severity of visceral involvement, with an approximate mortality rate of 2%–10% mainly due to liver failure (1).
The pathophysiology is multifactorial and associated with drug metabolism, specific human leukocyte antigen (HLA), and viral reactivation especially of the human Herpesviridae (HHV) family. The immunological response directed against viral reactivation and/or culprit drug precipitating the disease includes CD4+ and CD8+ T-cell activation and hence the development of a cytokine cascade with the production of interleukin (IL)-5, IL-4, IL-13, IL-17, IL-25, and eotaxin-1 (2). In addition, dermal dendritic cells, endothelial cells, and monomyelocytes secrete thymus activation-regulated chemokine (TARC/CCL17), IL-33, transforming growth factor β, and thymic stromal lymphopoietin. These chemokines, in synergy with IL-5, promote eosinophil chemoattraction, activation, proliferation, and infiltration and hence result in eosinophilic inflammation and tissue damage (3) (Figure 1).
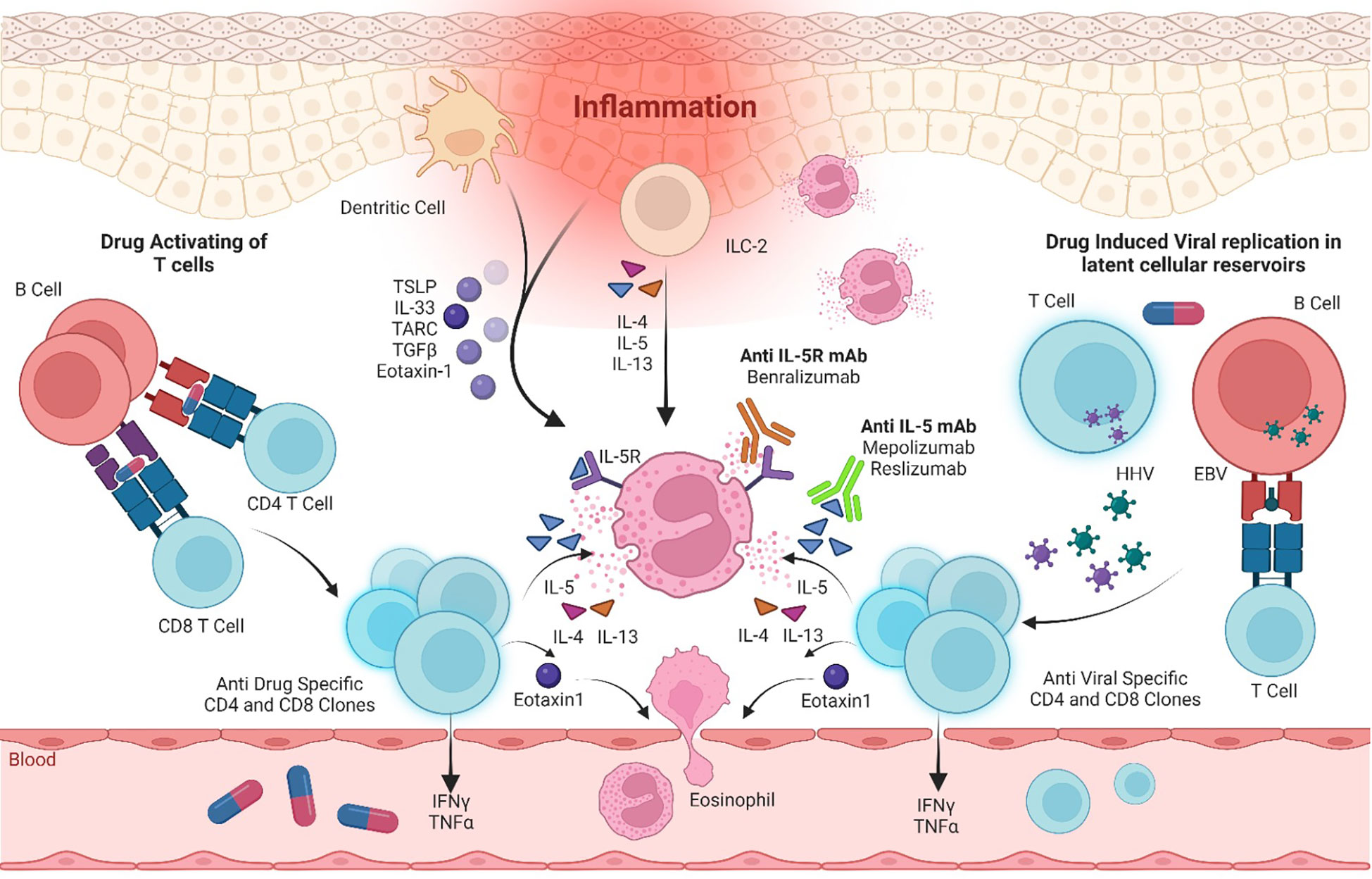
Figure 1 Immunological pathways in DRESS pathogenesis and immunotherapy targeted inhibition of the IL-5 axis. IL, interleukin; IL-5R, interleukin-5 receptor; mAb, monoclonal antibody; EBV, Epstein–Barr virus; HHV, Herpesviridae virus; ILC-2, Type 2 innate lymphoid cells; TSLP, thymic stromal lymphopoietin; TARC, thymus- and activation-regulated chemokine; TGFβ, transforming growth factor β; IFN-γ, interferon γ; TNFα, tumor necrosis factor α.
Consequently, patients with DRESS present with increased eosinophil levels in the blood, skin, and involved organs. The diagnosis of DRESS is based on clinical and biological criteria as calculated by the RegiSCAR score, such as fever >38°C, acute rash, lymphadenopathy, internal organ involvement, and blood count abnormalities including atypical lymphocytes and eosinophilia, found in 80% of patients (4). There are no randomized trials evaluating treatments for DRESS following the withdrawal of the culprit drug. The current mainstay of treatment is topical and systemic corticosteroids (CSs) (5).
During the last 5 years, limited reports of DRESS patients who were treated with immunotherapy inhibiting the IL-5 axis were presented. Maverakis et al. (6) were the first to describe the potential advantages of anti-IL-5/anti-IL-5 receptor (IL-5R) monoclonal antibodies (mAbs) over current therapies to DRESS patients, emphasizing the rapid onset, once-monthly dosing, and safety, with avoidance of the immunosuppressive and metabolic adverse events of prolonged high-dose systemic CSs. Recently, Gschwend et al. (7) reviewed 14 patients with DRESS who were treated with mAbs inhibiting the IL-5 axis. Patients were reported to have been successfully treated with the anti-IL-5 agents mepolizumab (7–11) and reslizumab (12), as well as the anti-IL-5R agent benralizumab (7, 13–15).
Herein, we aimed to present our experience with two new cases and conduct a literature review as a proof of concept to the hypothesis that IL-5 inhibition offers an efficient and safe therapeutic strategy for specific cases of DRESS.
This is a retrospective analysis of computerized medical records of patients who were diagnosed with DRESS and treated with IL-5 modulation in the period of February to September 2022. Patients were diagnosed and treated at the Division of Medicine, Hadassah Medical Center, Jerusalem, Israel. Data regarding clinical manifestations, treatments, and outcomes were retrieved from the files and analyzed.
In addition, we conducted a systematic review of the literature concerning patients with DRESS who were treated with IL-5-inhibiting mAbs. We used PubMed for data search. Keywords included “DRESS” and (“IL-5” or “benralizumab” or “mepolizumab” or “reslizumab”). Inclusion criteria consisted of English-language reports of DRESS patients who were treated with mAbs targeting the IL-5 axis and published in the period of May 2018 to October 2022. Only studies in which the DRESS diagnosis was based on the RegiSCAR score were included (Supplementary Table S1) (4). This score is available in Supplementary Table S1. Studies with a misdiagnosis of DRESS or a limited availability of full text were excluded. Data were analyzed for the clinical presentation, course, and outcome.
The reported patients from our medical center have signed a written informed consent for the publication of their clinical data.
Our search yielded two female patients (17 and 59 years old) who were admitted to our medical center within the study period. A summary of their clinical characteristics is presented in Table 1. Patient (P)1 presented with rash, hepatitis, fever, eosinophilia, and lymphadenopathy induced by olanzapine. She was CS-resistant, and subcutaneous (s.c.) mepolizumab was initiated as a single dose of 300 mg. P2 developed rash, eosinophilia, fever, hepatitis, and acute kidney injury following treatment with vancomycin. She was treated with s.c. benralizumab 30 mg. Both patients have demonstrated clinical resolution of their symptoms following IL-5 inhibitory treatment without recurrence in a follow-up of 10–12 months. The trend of hepatocellular liver enzyme levels and absolute eosinophil counts of P1 following treatments with CSs and mepolizumab is presented in Figure 2. The clinical presentation and skin histopathology of P1 and P2 are presented in Figure 3. The full clinical and laboratory description of the two patients is presented in the Supplementary Material.
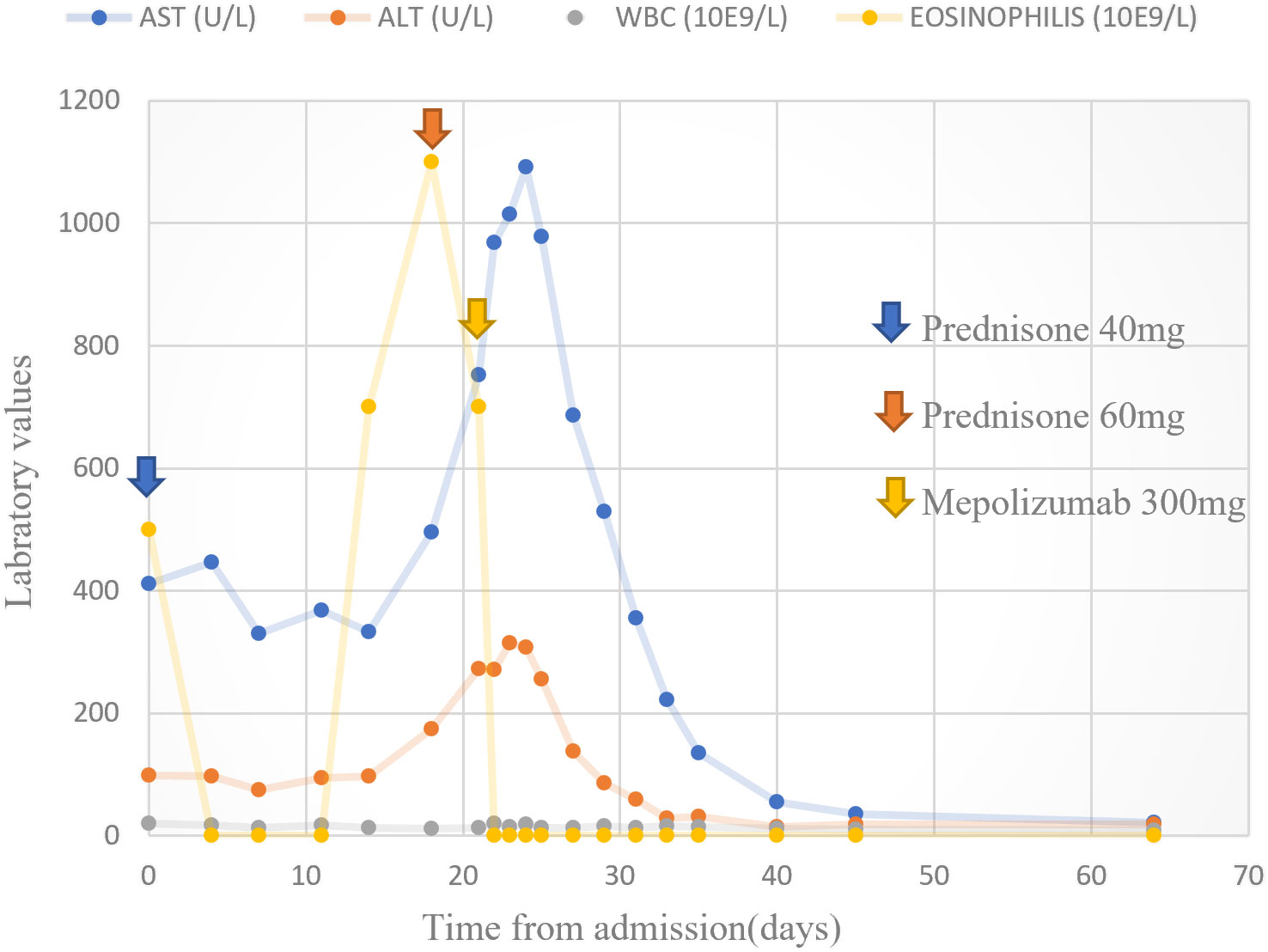
Figure 2 Hepatocellular liver enzymes and absolute eosinophil count. Trend of hepatocellular liver enzymes and absolute eosinophil counts following treatments with prednisone and mepolizumab in patient 1.
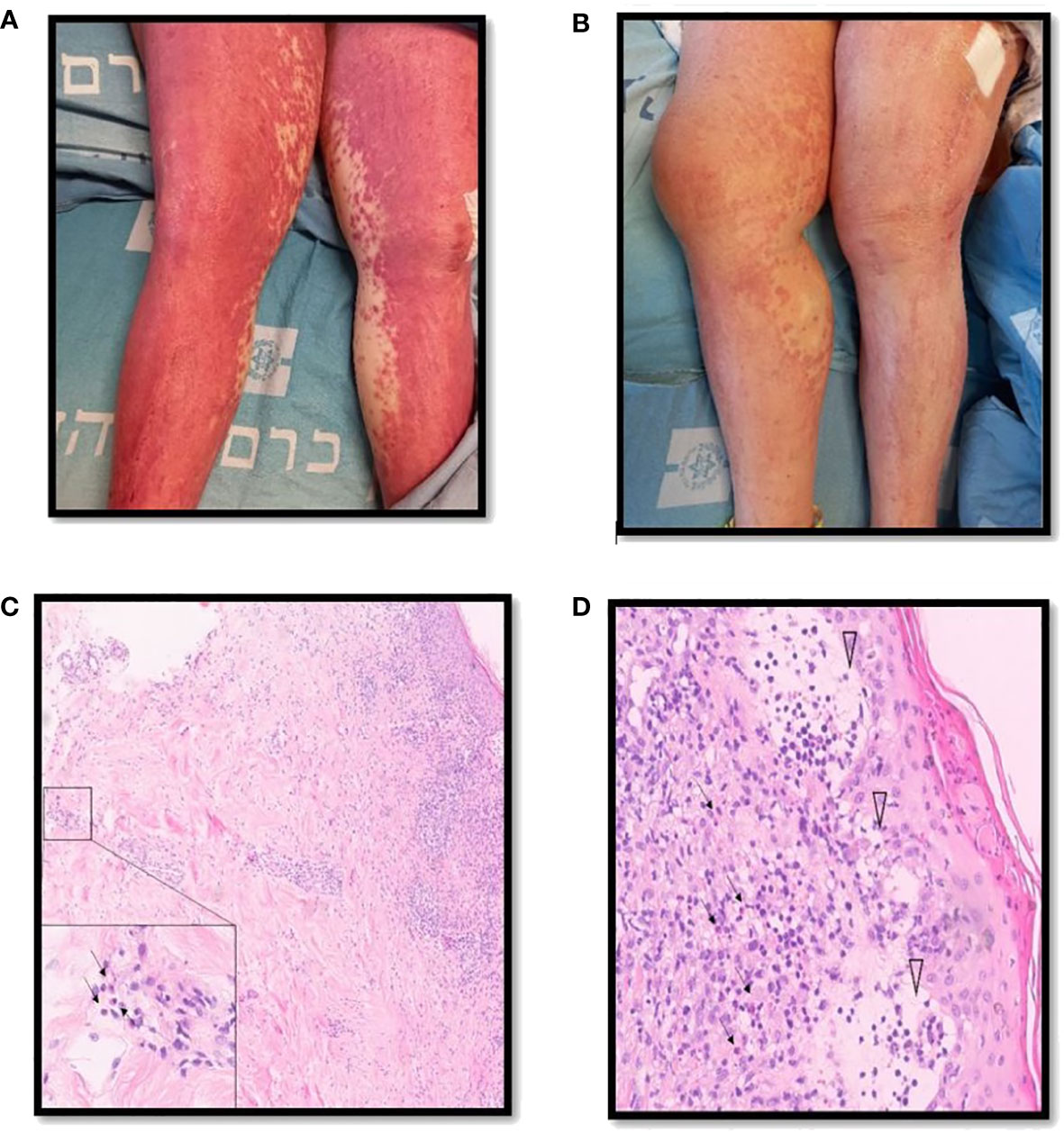
Figure 3 Clinical description and skin histopathology of the patients. (A) Clinical presentation of DRESS in patient 2 consisting of diffuse erythematous rash involving the lower limbs. (B) One week following benralizumab therapy, partial resolution of the skin rash. (C) Punch biopsy of skin consisting of hematoxylin and eosin stain demonstrating changes compatible with the clinical diagnosis of DRESS, most notably an inflammatory infiltrate composed of lymphocytes and numerous eosinophils stretching from the epidermis to the deep dermis in ×10 magnification. The inset demonstrates eosinophils present in the deep dermis in ×100 magnification (arrows). (D) Secondary changes including parakeratosis and interstitial edema with blister formation in the papillary dermis (arrowheads) in ×40 magnification.
A review of the literature yielded 14 patients with DRESS who were treated with biological agents targeting the IL-5 axis (Table 2). The first case was reported on May 2018. Nine studies that met the inclusion criteria (7–15) and our two clinical cases were included in the analysis. The reported patients are characterized by a female-to-male ratio of 1:1 and a mean age of 51.8 (17–87) years. The DRESS-inducing drugs, as expected from the prospective RegiSCAR study, were mostly antibiotics (7/16), as follows: vancomycin, trimethoprim-sulfamethoxazole, ciprofloxacin, piperacillin-tazobactam, and cefepime. The DRESS patients were treated with anti-IL-5 agents (mepolizumab and reslizumab) or anti-IL-5R biologics (benralizumab).
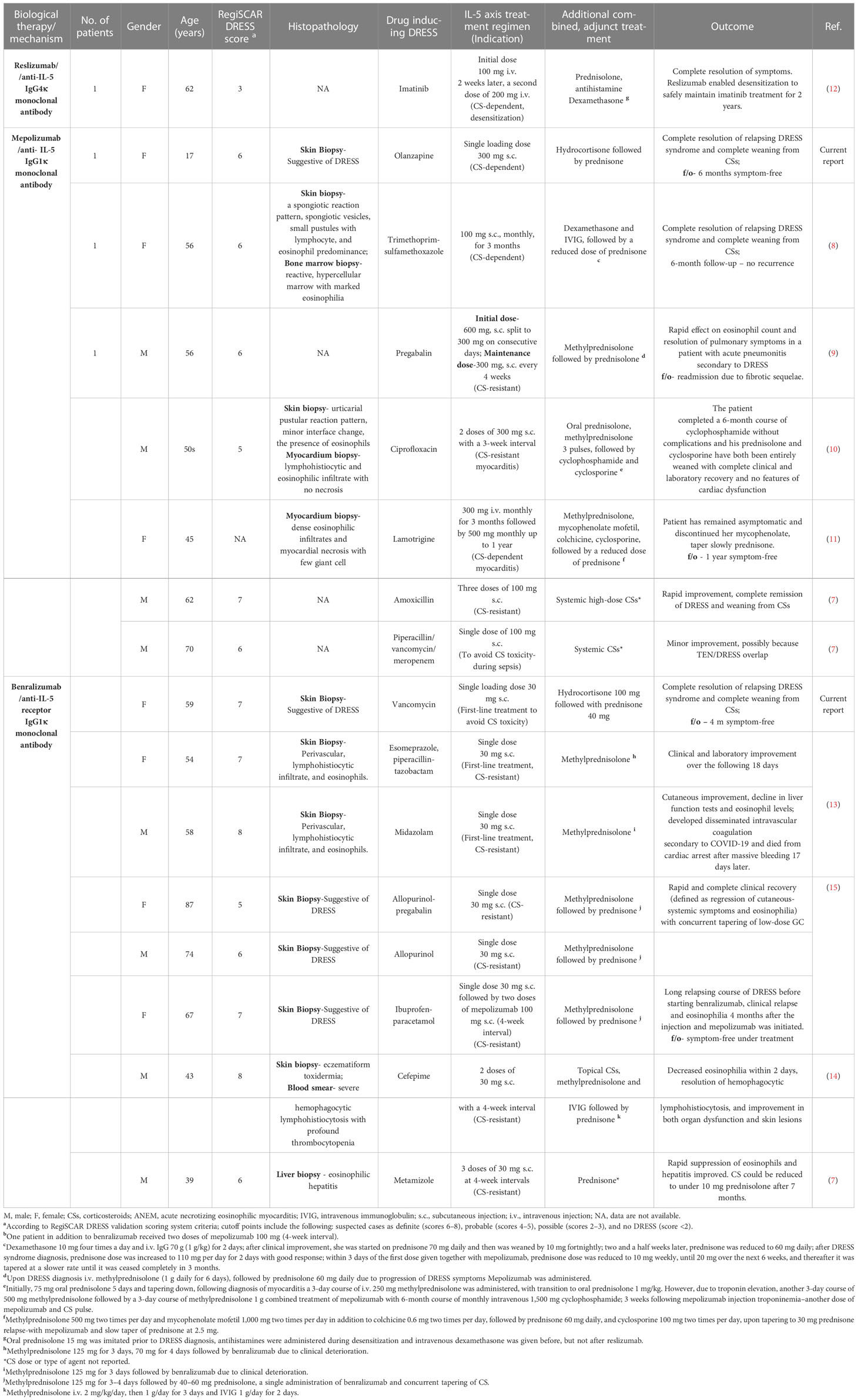
Table 2 Clinical characterizations of the DRESS patients treated off-label with monoclonal antibodies of the IL-5 axis.
Reslizumab was administered in one 62-year-old woman with imatinib-induced DRESS (12). Her RegiSCAR DRESS score was 3, and histopathology was not available when confirming the DRESS diagnosis. Reslizumab treatment has enabled complete resolution of the patient’s symptoms, facilitated desensitization, and succeeded in safely maintaining the drug imatinib treatment for 2 years.
Six DRESS patients were treated with mepolizumab (7–11). The mean (range) age of the patients was 50.8 (17–70) years. The RegiSCAR score calculated ranged between 5 and 7. Four out of seven patients had histopathologic evidence supporting eosinophilic inflammation. Five out of seven DRESS patients treated with mepolizumab need two or more doses, and only two have clinically improved following a single dose. All patients received concurrent CS therapy, one received additional therapy with intravenous immunoglobulin (IVIG), and two received concurrent immunosuppressant therapy with cyclosporine and one with cyclophosphamide. The clinical indication for the initiation of anti-IL-5-targeted therapy was due to steroid resistance and dependence in three and two patients, respectively. In one patient, IL-5-blocking agents were initiated to avoid CS toxicity.
All patients responded with complete resolution of DRESS symptoms, laboratory recovery, and complete weaning off from steroid therapy. Relapse was noted in one patient.
Seven DRESS patients were treated with benralizumab (7,13–15). The mean patient age was 68.7 (34–87) years. The RegiSCAR score ranged from 5 to 8. All patients receiving benralizumab had histopathology supporting tissue eosinophilic infiltration. The indication for benralizumab initiation was CS resistance in all patients. Two patients required two or more doses. Relapse was noted in one patient receiving benralizumab treatment (15). Moreover, one patient had a fatal outcome, although mortality was probably related to massive bleeding and cardiac arrest due to coronavirus disease 2019 (COVID-19) infection (13).
DRESS syndrome is a rare life-threatening hypersensitivity reaction; hence, scarce data exist regarding treatment protocols. Data presented in our study, including two unreported patients from our medical center, support treatment of DRESS with mAbs directed toward IL-5 or IL-5R, which are already Food and Drug Administration (FDA)-approved for other eosinophilic disorders.
IL-5 plays a crucial role in eosinophilic pathophysiology and is proposed as a novel therapeutic target for hypereosinophilic syndrome and rare eosinophilic conditions (16, 17). This cytokine is the major differentiation factor for eosinophils, playing a pivotal role in innate and acquired immune responses and eosinophilia (18). The IL-5R is a heterodimer comprising one alpha subunit (IL-5Rα) and one beta subunit (IL-5Rβ) that, upon activation by IL-5 signals, stimulate the Janus kinase (JAK)–signal transducer and activator of transcription proteins (STATs) pathway (19). Therefore, the reduction of blood eosinophil levels by antagonizing IL-5 and its receptor using mAbs recently becomes an important immunotherapeutic strategy (Figure 1).
Second-line therapy for patients with DRESS and severe organ involvement who do not respond to systemic CSs or for patients in whom CSs are contraindicated includes cyclosporine, IVIG, cyclophosphamide, and the JAK inhibitor tofacitinib, despite evidence of high failure rates, relapse, and excess of adverse events including serious infections (20–23).
With novel targeted biological agents and a better understanding of the key role of the IL-5 axis in DRESS, there are case reports of treatment with anti-IL-5 or anti-IL-5R mAbs, such as mepolizumab, reslizumab, or benralizumab. Mepolizumab is an anti-IL-5 humanized IgG1κ antibody that is FDA-approved for the treatment of severe eosinophilic asthma, hypereosinophilic syndrome, and eosinophilic granulomatosis with polyangiitis (EGPA) (24). Reslizumab is an anti-IL-5 humanized IgG4κ antibody with FDA approval for severe eosinophilic asthma (25), and benralizumab is a humanized fucosylated IgG1κ anti-IL-5Rα antibody approved by the FDA for the treatment of severe eosinophilic asthma (26).
Two previously published studies involving large cohorts reported treatment with anti-IL-5 agents in DRESS patients. In a European international multicenter cohort, Kridin et al. (27) identified four DRESS patients who were treated with anti-IL-5 biologics. DRESS patients treated with anti-IL-5/IL-5R agents were mostly CS-refractory cases who had longer hospitalizations, increased rates of intensive care unit admissions, and a higher risk of relapses (27). Gschwend et al. (7) summarized 14 DRESS patients treated with anti-IL-5 agents. While treatment with reslizumab or mepolizumab appeared to require repeated doses to achieve clinical resolution in most patients, a single dose of benralizumab was shown to be sufficient, thus indicating that treatment with benralizumab is more efficient than that with mepolizumab or reslizumab (7).
P1 in our report emphasizes the complexity in the initiation and prolonged administration of CS therapy in the psychiatric patient due to its adverse effects (28). This also stresses the role of therapy with an IL-5-/IL-5R-targeting mAbs as a CS-sparing agent. Gschwend et al. (7) recommended using anti-IL-5/IL-5R biologics in specific DRESS patients including those with a severe course, CS-resistant disease, severe disease with concomitant infection, or severe end-organ damage at presentation. P1 further expands these criteria, as her psychiatric disorder indicated the need for a CS-sparing treatment. This can be further implemented on DRESS patients with underlying disorders that constitute relative or absolute contraindications for prolonged CS treatment, such as uncontrolled diabetes. On the other hand, P2 emphasizes the role of early initiation of therapy with an IL-5-/IL-5R-targeting mAb to avoid CS toxicity, as the CS effect on bone reabsorption is a secondary insult to a patient presenting with a pathologic bone fracture from osteomyelitis (29).
Systemic CSs are still considered to be the mainstay of treatment for DRESS. Early initiation of a steroid-sparing agent is vital to reduce CS side effects. Cumulatively, as evident from our patients and all others reviewed (Table 2) (7, 30), it is evident that therapy with mAbs directed toward the IL-5/IL-5R axis combined with adjunct treatments could offer an alternative to CS therapy in some patients. DRESS patients eligible to receive anti-IL-5/IL-5R mAbs include those recommended by Gschwend et al. (7) and other patients with a high risk for CS toxicity including patients with psychiatric disorders or comorbidities such as metabolic disorders, immunocompromised patients, and the elderly.
This study presents several limitations, mainly its retrospective design and small number of patients. Furthermore, the characteristics of patients such as age and sex and drug treatments differed among the different studies. A multicenter study with a larger sample size is required for prolonged follow-up and investigation of the proper dose regimen of anti-IL-5/IL-5R treatment for DRESS. DRESS often shows severe symptoms in the acute phase with serious disease sequelae in the chronic phase. Therefore, careful follow-up is required. Since there is no definite view on how to treat and which regimen to follow, the use of mAbs directed toward the IL-5/IL-5R axis is an important emerging issue in DRESS therapy deserving further clinical investigations.
In conclusion, future implementation of mAbs directed toward the IL-5/IL-5R axis in DRESS cases presents a promising therapeutic strategy in DRESS patients. Selected DRESS patients eligible to receive anti-IL-5-/IL-R-blocking agents as first-line treatment consist of patients with contraindications to CS therapy, while the risk of relapse may still exist. IL-5-modulating agents can also be used in DRESS patients with a CS-dependent or -resistant clinical course. Thus, anti-IL-5/IL-5R biologics may offer a novel therapeutic modality in these patients.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
LR: treatment of patients and writing of the manuscript; AT: mansucruipt revisions and figure design, YR, AK, and YM: manuscript revisions and treatment of patients; TH: pathology workup; IA: manuscript revisions; OS and YT: correspocnding authors and manuscript design and revisions. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1134178/full#supplementary-material
CS, corticosteroid; DRESS, drug reaction with eosinophilia and systemic symptoms; ECT, electroconvulsive therapy; ER, emergency room; IL, interleukin; i.v., intravenous; IVIG, intravenous immunoglobulin; LFT, liver function test; mAbs, monoclonal antibodies; SC, subcutaneous; CT, computerized tomography.
1. Ichai P, Laurent-Bellue A, Saliba F, Moreau D, Besch C, Francoz C, et al. Acute liver Failure/Injury related to drug reaction with eosinophilia and systemic symptoms: outcomes and prognostic factors. Transplantation (2017) 101(8):1830–7. doi: 10.1097/TP.0000000000001655
2. Musette P, Janela B. New insights into drug reaction with eosinophilia and systemic symptoms pathophysiology. Front Med (Lausanne) (2017) 4:179. doi: 10.3389/fmed.2017.00179
3. Ganeshanandan L, Lucas M. Drug reaction with eosinophilia and systemic symptoms: a complex interplay between drug, T cells, and herpesviridae. Int J Mol Sci (2021) 22(3):1127. doi: 10.3390/ijms220311271127
4. Kardaun SH, Sekula P, Valeyrie-Allanore L, Liss Y, Chu CY, Creamer D, et al. Drug reaction with eosinophilia and systemic symptoms (DRESS): an original multisystem adverse drug reaction. results from the prospective RegiSCAR study. Br J Dermatol (2013) 169(5):1071–80. doi: 10.1111/bjd.12501
5. Martinez-Cabriales SA, Rodriguez-Bolanos F, Shear NH. Drug reaction with eosinophilia and systemic symptoms (DReSS): how far have we come? Am J Clin Dermatol (2019) 20(2):217–36. doi: 10.1007/s40257-018-00416-4
6. Maverakis E, Ji-Xu A, Bruggen MC. Targeting interleukin-5 with benralizumab: a novel treatment for drug rash with eosinophilia and systemic symptoms. Allergy (2022) 77(8):2287–9. doi: 10.1111/all.15283
7. Gschwend A, Helbling A, Feldmeyer L, Mani-Weber U, Meincke C, Heidemeyer K, et al. Treatment with IL5-/IL-5 receptor antagonists in drug reaction with eosinophilia and systemic symptoms (DRESS). Allergo J Int (2022), 1–8. doi: 10.1007/s40629-022-00224-7
8. Ange N, Alley S, Fernando SL, Coyle L, Yun J. Drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome successfully treated with mepolizumab. J Allergy Clin Immunol Pract (2018) 6(3):1059–60. doi: 10.1016/j.jaip.2017.10.020
9. Thein OS, Sutton B, Thickett DR, Parekh D. Mepolizumab rescue therapy for acute pneumonitis secondary to DRESS. BMJ Case Rep (2019) 12(10). doi: 10.1136/bcr-2019-231355
10. Truong K, Kelly S, Bayly A, Smith A. Successful mepolizumab treatment for DRESS-induced refractory eosinophilic myocarditis and concurrent thyroiditis. BMJ Case Rep (2021) 14(7). doi: 10.1136/bcr-2021-242240e24224014/7/e242240
11. Kowtoniuk R, Pinninti M, Tyler W, Doddamani S. DRESS syndrome-associated acute necrotizing eosinophilic myocarditis with giant cells. BMJ Case Rep (2018), 2018. doi: 10.1136/bcr-2018-22646bcr2018226461
12. Park H, Choi GS, Lee EM. Successful treatment of imatinib-induced DRESS syndrome using reslizumab without cessation of imatinib: a case report. Case Rep Oncol (2021) 14(3):1548–54. doi: 10.1159/000519471cro-0014-1548
13. Schmid-Grendelmeier P, Steiger P, Naegeli MC, Kolm I, Claudia Cecile Valerie L, Maverakis E, et al. Benralizumab for severe DRESS in two COVID-19 patients. J Allergy Clin Immunol Pract (2021) 9(1):481–483.e2. doi: 10.1016/j.jaip.2020.09.039
14. Mesli F, Dumont M, Soria A, Groh M, Turpin M, Voiriot G, et al. Benralizumab: a potential tailored treatment for life-threatening DRESS in the COVID-19 era. J Allergy Clin Immunol Pract (2021) 9(9):3529–3531.e1. doi: 10.1016/j.jaip.2021.06.047
15. Lang CCV, Schmid-Grendelmeier P, Maverakis E, Bruggen MC. Reply to "Benralizumab: a potential tailored treatment for life-threatening DRESS in the COVID-19 era". J Allergy Clin Immunol Pract (2021) 9(9):3531–2. doi: 10.1016/j.jaip.2021.06.048
16. Harish A, Schwartz SA. Targeted anti-IL-5 therapies and future therapeutics for hypereosinophilic syndrome and rare eosinophilic conditions. Clin Rev Allergy Immunol (2020) 59(2):231–47. doi: 10.1007/s12016-019-08775-4
17. Shamriz O, Hershko AY, Talmon A, Ribak Y, Elazary AS, Horev L, et al. The efficacy of off-label IL-5-modulating treatment in rare eosinophil-mediated diseases. Allergol Int (2021) 70(2):266–8. doi: 10.1016/j.alit.2020.10.001
18. Kouro T, Takatsu K. IL-5- and eosinophil-mediated inflammation: from discovery to therapy. Int Immunol (2009) 21(12):1303–9. doi: 10.1093/intimm/dxp102dxp102
19. Broughton SE, Nero TL, Dhagat U, Kan WL, Hercus TR, Tvorogov D, et al. The betac receptor family - structural insights and their functional implications. Cytokine (2015) 74(2):247–58. doi: 10.1016/j.cyto.2015.02.005
20. Joly P, Janela B, Tetart F, Rogez S, Picard D, D'Incan M, et al. Poor benefit/risk balance of intravenous immunoglobulins in DRESS. Arch Dermatol (2012) 148(4):543–4. doi: 10.1001/archderm.148.4.dlt120002-c
21. Kim D, Kobayashi T, Voisin B, Jo JH, Sakamoto K, Jin SP, et al. Targeted therapy guided by single-cell transcriptomic analysis in drug-induced hypersensitivity syndrome: a case report. Nat Med (2020) 26(2):236–43. doi: 10.1038/s41591-019-0733-7
22. Laban E, Hainaut-Wierzbicka E, Pourreau F, Yacoub M, Sztermer E, Guillet G, et al. Cyclophosphamide therapy for corticoresistant drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome in a patient with severe kidney and eye involvement and Epstein-Barr virus reactivation. Am J Kidney Dis (2010) 55(3):e11–4. doi: 10.1053/j.ajkd.2009.10.054
23. Nguyen E, Yanes D, Imadojemu S, Kroshinsky D. Evaluation of cyclosporine for the treatment of DRESS syndrome. JAMA Dermatol (2020) 156(6):704–6. doi: 10.1001/jamadermatol.2020.0048
24. Pavord ID, Bel EH, Bourdin A, Chan R, Han JK, Keene ON, et al. From DREAM to REALITI-a and beyond: mepolizumab for the treatment of eosinophil-driven diseases. Allergy (2022) 77(3):778–97. doi: 10.1111/all.15056
25. Hashimoto S, Kroes JA, Eger KA, Mau Asam PF, Hofstee HB, Bendien SA, et al. Real-world effectiveness of reslizumab in patients with severe eosinophilic asthma - first initiators and switchers. J Allergy Clin Immunol Pract (2022) 10(8):2099–2108.e6. doi: 10.1016/j.jaip.2022.04.014
26. Zhu M, Yang J, Chen Y. Efficacy and safety of treatment with benralizumab for eosinophilic asthma. Int Immunopharmacol (2022) 111:109131. doi: 10.1016/j.intimp.2022.109131
27. Kridin K, Bruggen MC, Walsh S, Bensaid B, Ranki A, Oppel E, et al. Management and treatment outcome of DRESS patients in Europe: an international multicentre retrospective study of 141 cases. J Eur Acad Dermatol Venereol (2023) 37(4):753–62. doi: 10.1111/jdv.18808
28. Dubovsky AN, Arvikar S, Stern TA, Axelrod L. The neuropsychiatric complications of glucocorticoid use: steroid psychosis revisited. Psychosomatics (2012) 53(2):103–15. doi: 10.1016/j.psym.2011.12.007
29. Lane NE. Glucocorticoid-induced osteoporosis: new insights into the pathophysiology and treatments. Curr Osteoporos Rep (2019) 17(1):1–7. doi: 10.1007/s11914-019-00498-x
Keywords: drug reaction with eosinophilia and systemic symptoms (DRESS), interleukin (IL), monoclonal antibodies (mAbs), IL-5 antibody, biotherapeutic agent
Citation: Rubin L, Talmon A, Ribak Y, Kessler A, Martin Y, Haran TK, Shamriz O, Adini I and Tal Y (2023) Novel targeted inhibition of the IL-5 axis for drug reaction with eosinophilia and systemic symptoms syndrome. Front. Immunol. 14:1134178. doi: 10.3389/fimmu.2023.1134178
Received: 30 December 2022; Accepted: 10 April 2023;
Published: 28 April 2023.
Edited by:
Teresa Bellon, University Hospital La Paz Research Institute (IdiPAZ), SpainReviewed by:
Lukas Jörg, Insel Gruppe AG, SwitzerlandCopyright © 2023 Rubin, Talmon, Ribak, Kessler, Martin, Haran, Shamriz, Adini and Tal. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Oded Shamriz, b2RlZC5zaGFtcml6QG1haWwuaHVqaS5hYy5pbA==; Yuval Tal, eXV2YWx0QGhhZGFzc2FoLm9yZy5pbA==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.