
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Immunol. , 14 February 2023
Sec. Cancer Immunity and Immunotherapy
Volume 14 - 2023 | https://doi.org/10.3389/fimmu.2023.1133899
This article is part of the Research Topic Mechanism and Application of Synergistic Effect of Radiotherapy and Immunotherapy View all 7 articles
 Ting Zhou1,2†
Ting Zhou1,2† Li-Ying Zhang1,3†
Li-Ying Zhang1,3† Jian-Zheng He1,3
Jian-Zheng He1,3 Zhi-Ming Miao1
Zhi-Ming Miao1 Yang-Yang Li1
Yang-Yang Li1 Yi-Ming Zhang1
Yi-Ming Zhang1 Zhi-Wei Liu1
Zhi-Wei Liu1 Shang-Zu Zhang1
Shang-Zu Zhang1 Yan Chen1
Yan Chen1 Gu-Cheng Zhou1
Gu-Cheng Zhou1 Yong-Qi Liu1,3,4*
Yong-Qi Liu1,3,4*Radiotherapy is the major treatment of non-small cell lung cancer (NSCLC). The radioresistance and toxicity are the main obstacles that leading to therapeutic failure and poor prognosis. Oncogenic mutation, cancer stem cells (CSCs), tumor hypoxia, DNA damage repair, epithelial-mesenchymal transition (EMT), and tumor microenvironment (TME) may dominate the occurrence of radioresistance at different stages of radiotherapy. Chemotherapy drugs, targeted drugs, and immune checkpoint inhibitors are combined with radiotherapy to treat NSCLC to improve the efficacy. This article reviews the potential mechanism of radioresistance in NSCLC, and discusses the current drug research to overcome radioresistance and the advantages of Traditional Chinese medicine (TCM) in improving the efficacy and reducing the toxicity of radiotherapy.
Radiotherapy is the mainstay of treatment for patients with non-small cell lung cancer (NSCLC), and usually combined with surgery, chemotherapy, immunotherapy, and targeted therapy (1–6). The radiation commonly used in radiotherapy mainly includes photo radiation, such as X-ray and γ-ray and particle rays such as neutrons, electrons, protons and heavy ions (2, 7–10). However, no matter what radiation and dose segmentation methods are used, radioresistance will inevitably occur, leading to radiotherapy failure and local recurrence. Simply increasing the radiation dose does not improve survival benefits but leads to adverse reactions and poor prognosis (11).
Radioresistance of NSCLC can be classified as inherent radioresistance and acquired radioresistance, of which acquired radioresistance plays a major role. Various mechanisms that cause radioresistance run through the whole process of radiotherapy. Whether they cause inherent radioresistance or acquired radioresistance is relative. We have simply classified them according to their leading role in different stages of radiotherapy. Acquired radioresistance is mainly related to DNA damage repair, tumor microenvironment (TME) remodeling by inflammation, immune response, tumor metabolic reprogramming, tumor microbiota and senescence cells, and epithelial-mesenchymal transition (EMT) (12–20). Inherent radioresistance is mainly associated with oncogenic mutation, cancer stem cells (CSCs), and tumor hypoxia (21–29).
Targeted drugs or small molecule drugs combined with radiotherapy are used in the treatment of NSCLC. However, there is no systematic review on the mechanism of radioresistance and radiosensitizers of NSCLC. Therefore, we will discuss the radioresistance mechanisms of NSCLC and the study of drugs that can enhance the efficacy and reduce the toxicity of radiotherapy, especially TCM. So as to provide a theoretical basis for clinical development of anti-tumor drugs in combination with radiotherapy.
Acquired radioresistance occurs mainly with radiation induction, the underlying mechanisms involve DNA damage repair and EMT in cancer cell itself, as well as changes in TME such as inflammation, immune response, tumor metabolic reprogramming etc. (15, 16, 19, 30–32). Acquired radioresistance may enhance invasion and metastasis of surviving cancer cells after primary radiotherapy and affect the prognosis and life quality of patients.
Radiation causes direct DNA single-strand breaks (SSBs) and double-strand breaks (DSBs), as well as indirect DNA damage from oxidative stress such as ROS and reactive nitrogen species (RNS) (30)(Figure 1). SSBs is repaired by poly (ADP-ribose) polymerase (PARP) (33), as well as ATR/Chk1 pathway (34). ATR can be activated by recruitment to its partner protein ATRIP, binding to topoisomerase (DNA) II binding protein 1 (TopBP1) and claspin, phosphorylated Chk1 (34), activated Chk1 phosphorylated WEE1, leading to cell cycle arrest and DNA repair (35), resulting in radioresistance of NSCLC (36, 37). DSBs are repaired by homologous recombination (HR) and non-homologous ending joining (NHEJ) (33). In HR, the MRN complex comprising meiotic recombination 11 homolog1 (MRE11), ATP-binding cassette-ATPase (RAD50), and Nijmegen breakage syndrome protein 1 (NBS1), binds to C-terminal binding protein (CtBP)-interacting protein (CtIP) and BRCA1, was recruited by RAD17 to DSB sites, activated ATM, inducing the phosphorylation Chk2 and H2AX, as well as accumulation of RAD51, thus leading to cell cycle arrest and DNA repair (34, 35, 38, 39). Radiation delayed tumor growth and increased local control in Atm deletion tumor model of lung adenocarcinoma (40). In NHEJ, DNA-dependent protein kinase catalytic subunit (DNA-PKcs) is directly recruited to DNA damage sites via Ku heterodimers (Ku70/80), inducing Artemis-mediated end processing and DNA ligase 4 (LIG4) mediated ligation, leading to DNA repair (35). Besides, heat shock protein 90 (HSP90) (41), acidic nucleoplasmic DNA binding protein1 (AND-1) (42), sirtuin 3 (Sirt 3) (43), IQ motif containing GTPase-activating protein 3 (IQGAP3) (44), and serine proteinase inhibitor clade E member 2 (SERPINE2) (45) can enhance radioresistance via activating ATM to activate the HR pathway in NSCLC. G2 and S phase-expressed 1(GTSE1) (46), plakophilin2 (PKP2) (47), ephrin type-A receptor 2 (EphA2) (48, 49), and cancer-derived IgG (cancer-IgG) (50) can participate in NHEJ pathway, leading to radioresistance in NSCLC. AAA ATPases RUVBL1/2 can activate both HR and NHEJ pathway, inducing radioresistance in NSCLC (51). In addition to Chk1 and Chk2, WEE1 can also regulate cell cycle arrest by inhibiting the phosphorylation of cyclin-dependent kinases (CDKs) (52, 53), inducing radioresistance (54). Thus, targeting DNA repair proteins or cell cycle regulator may overcome radiation-induced radioresistance in NSCLC.
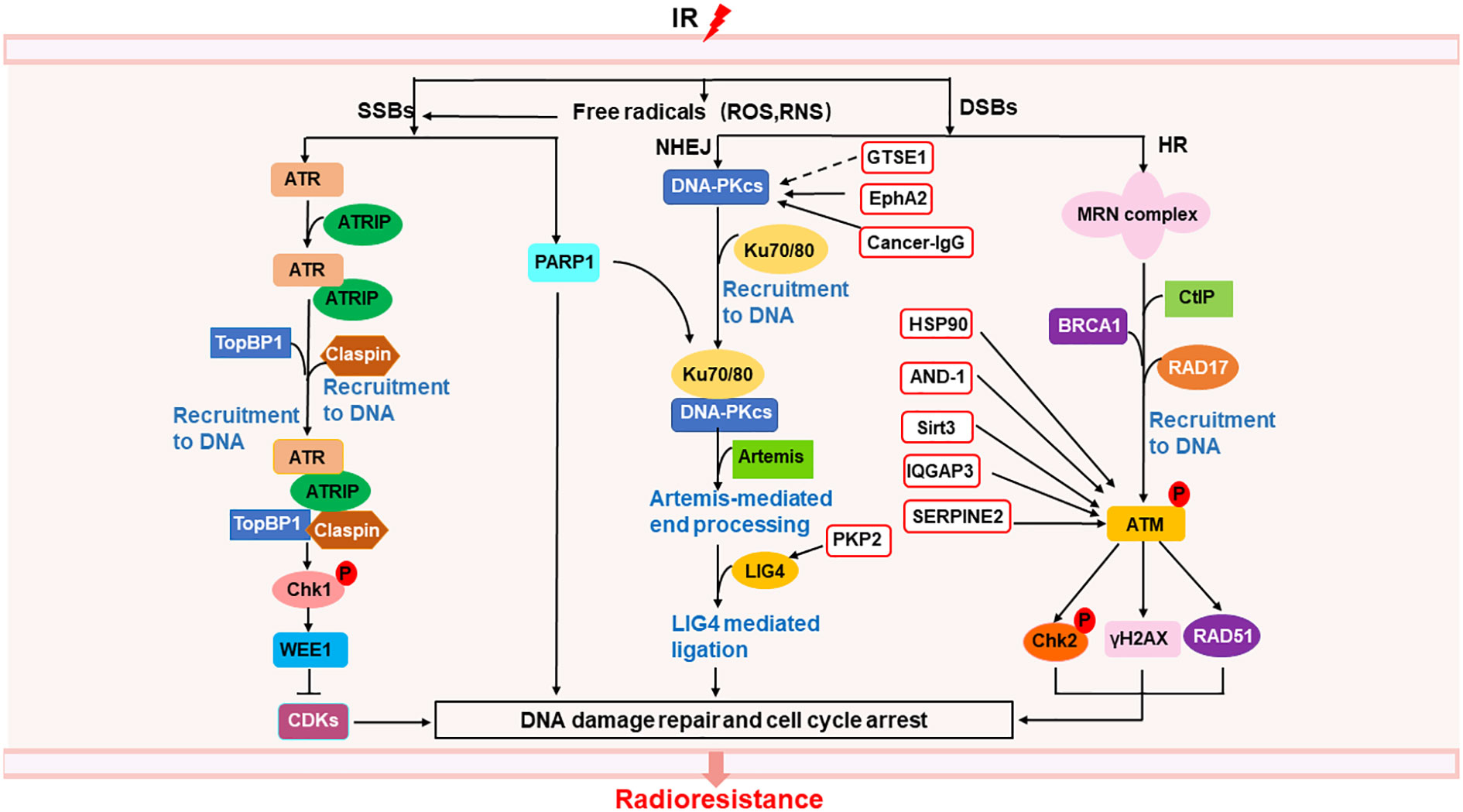
Figure 1 Mechanisms of radioresistance caused by DNA damage repair in NSCLC. Radiation induced DNA damage response can activate the DNA repair pathway, of which SSBs can be repaired through ATR/Chk1 pathway and PARP1, DSBs can be repaired through NHEJ and HR. In the red box are proteins related to the repair of DSBs that may become new targets for radiosensitization. The solid line represents a clear target, and the dotted line represents a possible action through this target.
It can be seen from the above that tumor cells have multiple ways to repair radiation-induced DNA damage. Drugs targeting a single target may not have obvious effect on overcoming radioresistance, and are prone to drug resistance. Therefore, the combination of multiple targets for DNA repair will be the direction of tumor treatment in the future, but how to effectively combine the targets still needs more research.
Tumor microenvironment (TME) is closely related to tumor progression, and the remodeling of TME after radiotherapy can lead to radioresistance (18). Here, we focus on the effects of inflammation and immune response, tumor microbiota, tumor metabolic reprogramming, and cell senescence on TME to clarify the role of radiation-induced TME remodeling in the radioresistance of NSCLC (Figure 2).
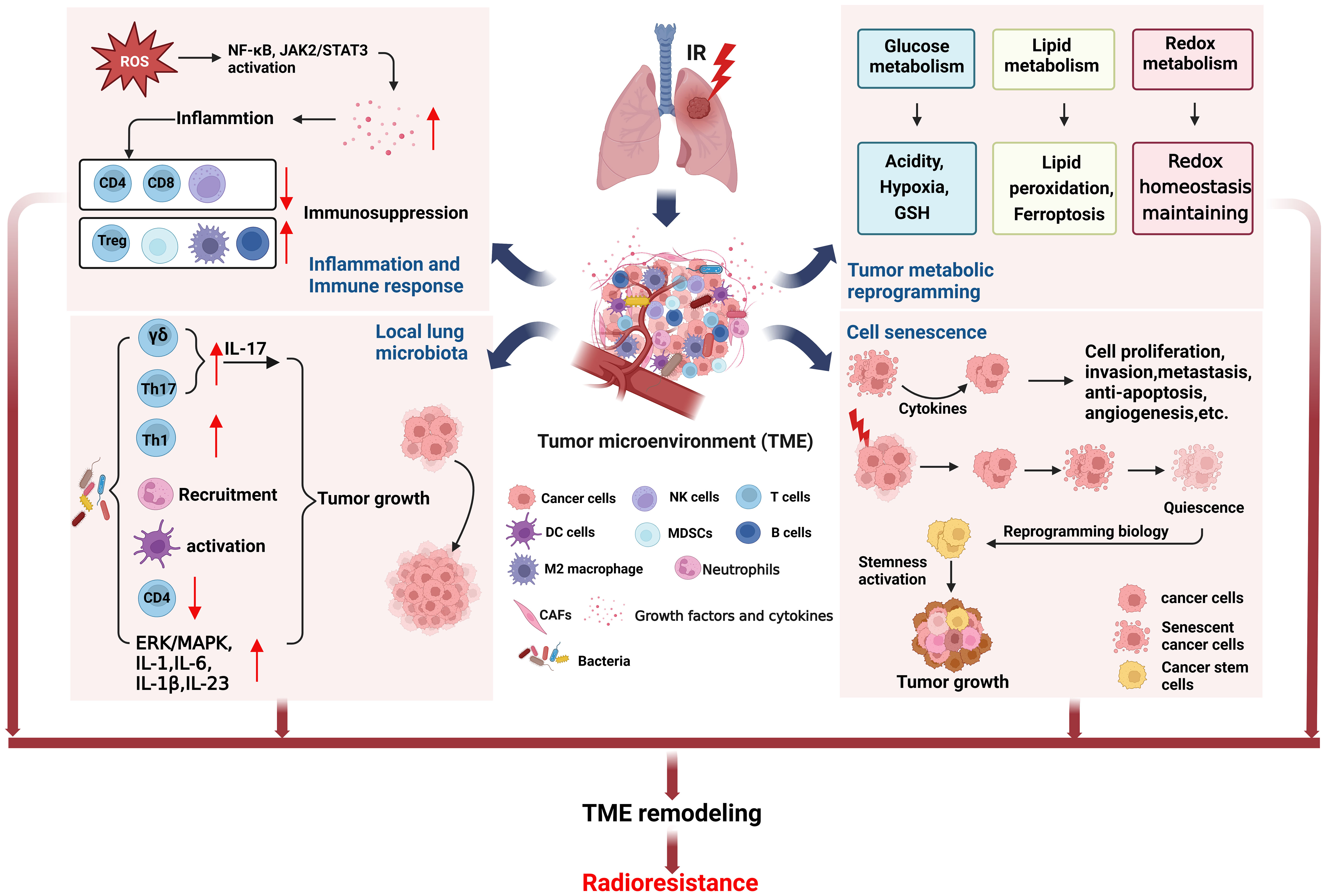
Figure 2 TME remodeling may lead to radioresistance of NSCLC. The TME changed in NSCLC cells surviving after radiotherapy. Radiation induced inflammation and immune response can cause immunosuppression. The local lung microbiota can induce tumor growth by regulating the immune cells. Tumor metabolic reprogramming after radiation will lead to increase of acidity, hypoxia, and GSH production, unbalance of ferroptosis, and redox homeostasis maintaining, being conductive to tumor growth. Cell senescence after radiotherapy can also induce cell proliferation and tumor growth. The above factors together form TME remodeling that promotes tumor growth, metastasis and recurrence after radiotherapy. (This figure is adapted from an image created from BioRender.com).
Radiation generated ROS and RNS can activate NF-κB, Janus-associated kinase (JAK)2/signal transducer and activator of transcription (STAT)3 pathway to increase the release of growth factors and cytokines, such as VEGF, TGF-β, IL-1, IL-8, TNF-α and IL-6 etc. (15, 31, 55, 56), which are involved in inflammation and immune response (18, 57–60). TGFβ and TNFα in TME are very important in the formation and development of tumor (61). Our previous study found that irradiation of NSCLC cell lines A549 and H1299 can cause overexpression of TGFβ and TNFα, resulting in HIF1α activation and cause genomic instability of BMSCs and may promote tumor progression (62). We also found that TGFβ mainly causes short-term side effects, while TNFα mainly causes long-term side effects. This may provide a guideline for clinical use of combined drugs at different stages of radiotherapy for NSCLC. In addition, radiation-induced inflammation reduces tumor-suppressing immune cells such as CD4+T cells, CD8+T cells, and natural killer (NK) cells, while tumor-promoting immune cells such as regulatory T cells (Treg), myeloid-derived suppressor cells (MDSC), and M2 tumor-associated macrophages (TAMs) increase, eventually forming an immunosuppressive environment, and high level of TGFβ (19, 63). Moreover, M2 macrophages and B cells infiltrate into TME also participate in the radioresistance of NSCLC (64, 65). Thus targeting inflammation not only enhance the tumor killing effects of radiotherapy, but also protect the normal cells and tissues from radiation-induced bystander effects.
It is well known that intestinal microbiome can regulate host physiological and pathological processes through metabolism, inflammation and immune response (66, 67). On the one hand, tumor microbiota can enhance anti-tumor immunity through STING signal activation, T and NK cell activation. On the other hand, it can upregulate ROS, induce anti-inflammatory environment, and inactivate T cell to promote immunosuppression and cancer progression (68). Moreover, tumor microbiota stimulates γδT cells to produce IL-17 that triggering inflammation and cancer progression (69), and increases Th17 cells that enhancing lung cancer proliferation and angiogenesis (70). It is found that the dysbiosis of local lung microbiota in patients with advanced lung cancer can lead to Th1, Th17, γδT cells and PD-1 positive T cells increase, neutrophils recruitment, dendritic cells (DCs) activation, CD4+T cells decrease, cytokines IL-1, IL-1b, IL-6, IL-17, IL-23 upregulation, as well as upregulation of ERK/MAPK and inflammation pathways, all these above alterations promoted tumor progression (71). Although there is no research report on the correlation between tumor microbiota and radioresistance, there is no doubt that tumor microbiota will participate in tumor response to radiotherapy through inflammation and immune response and affect the final efficacy. The microbiome may serve as an indicator for cancer diagnosis or prognostic assessment and could be used as potential targets to develop new cancer therapies.
Tumor metabolic reprogramming after radiotherapy, such as glucose metabolism, lipid metabolism, and redox metabolism, contributes to TME remodeling and acquired radioresistance (19). The increased expression of glucose transporter1 (GLUT1), pyruvate kinase M2 isoform (PKM2), and lactate dehydrogenase (LDHA) after irradiation promoted glycolysis (19, 72, 73), while the expression of glycogen synthetase 1 (GYS1) was also up-regulated, leading to glycogen accumulation (74). These changes enable cancer cells to survive, proliferate and resist radiation. In addition, pentose phosphate pathway (PPP) after radiation induced more NADPH production, which promoted the production of reduced glutathione (GSH) (19), may lead to radioresistance of NSCLC.
IR can induce the expression of ACSL4, which is a lipid metabolizing enzyme in cancer cells, to increase lipid peroxidation and ferroptosis (75). Intriguingly, IR can also induce the expression of ferroptosis inhibitors SLC7A11 and GPX4, causing radioresistance (75). Perhaps, radioresistant cells have formed a balance between promoting ferroptosis and inhibiting ferroptosis, enabling cells to survive. In radioresistant lung cancer cells, the expression of lipid Droplet (LD) and ferritin heavy chain (FTH1) increased and correlated with each other, which can be targeted and synergistically inhibit tumor radioresistance (76). In addition, the activation of sPLA2-PKCδ-MAPKs-cPLA2α pathway promoted tumor progression and radioresistance in NSCLC (77).
ROS levels are elevated in lung cancer radioresistant cells, which exhibit radioresistance by maintaining oxidative stress and NRF2-dependent metabolic adaptation (78). The expression of TP53-regulated inhibitor of apoptosis 1 (TRIAP1) in NSCLC cell lines A549 and H460 increased after irradiation, and caused upregulation of antioxidant proteins such as thioredoxin-related transmembrane protein (TMX) 1, TMX2, thioredoxin (TXN), glutaredoxin (GLRX) 2, GLRX3, peroxiredoxin (PRDX) 3, PRDX4 and PRDX6, participated in redox metabolism and enhanced the ROS scavenging, leading to radioresistance in NSCLC (79).
In conclusion, NSCLC cells that survived after radiation have altered metabolism that favors cell proliferation and tumor progression. Therefore, targeting tumor metabolism will be an effective measure to improve the radiotherapy prognosis and survival rate of NSCLC patients.
Studies have shown that induction of premature senescence can radiosensitize NSCLC cells (80). On the contrary, the cytokines secreted by senescent cells which not killed by radiation will affect the adjacent surviving cancer cells in TME, stimulate tumor cell proliferation, invasion and metastasis, escape apoptosis, induce angiogenesis, and promote tumor phenotype (20). In addition, lung cancer cells survived from radiotherapy experienced senescence and entered a dormant state, reprogrammed their biology months or years after irradiation, reactivated stemness, and produced tumors with enhanced growth and metastasis (81).
To sum up, the TME itself is inflammatory, radiation further promotes the release of inflammatory factors, aggravates inflammatory reaction, causes immunosuppression, and hinders anti-tumor immunity. In addition, tumor microbiota can activate inflammatory pathways to promote tumor progression. Moreover, the oxidative stress reaction induced by radiation promotes the occurrence of tumor metabolism reprogramming, causes the depletion of T cells, and makes tumor cells resistant to radiation. The senescent cells in TME can promote tumor proliferation, invasion and metastasis by secreting cytokines. The interaction of the above factors leads to TME remodeling and radioresistance. In addition to these above, tumor hypoxia also play a key role in TME. It is a challenge to overcome radioresistance by targeting TME, because any single targeting action may not achieve the desired therapeutic effect.
Radiotherapy induced EMT and CSCs characteristics, enhanced the invasion and metastasis of cancer cells surviving after primary radiotherapy, leading to acquired resistance of NSCLC, which in turn weakened the radiation-induced tumor killing effect (16, 17, 82). TWIST1 (83, 84) increased after radiation in NSCLC, resulting in downregulation of E-cadherin, overexpression of Snail1, Vimentin, N-cadherin, and PDGFR (85), which promotes cell proliferation, invasion and vascular regeneration, leading to tumor metastasis and radioresistance. PDGF secretion also increased after radiation and resulted in radiation-induced pneumonitis and fibrosis (86), PDGF binds to PDGFR to induce proliferation and vascular regeneration. In addition, Yes-associated protein 1 (YAP1) (87), Snail1 (88), PAK1-LIM domain kinase 1 (LIMK1)-cofilins signaling (89), and zinc-finger E-box-binding homeobox factor 1 (ZEB1) (90)were upregulated after radiotherapy, leading to EMT (Figure 3). Radiation survived NSCLC cells also show cancer stemness properties, such as highly expressed CD166, CD24, CD44, Sox-2, CD133, etc. (76, 85, 91). EMT are critical targets and biomarkers for radiotherapy, radiosensitizers targeting EMT are promising treatment for NSCLC in clinical.
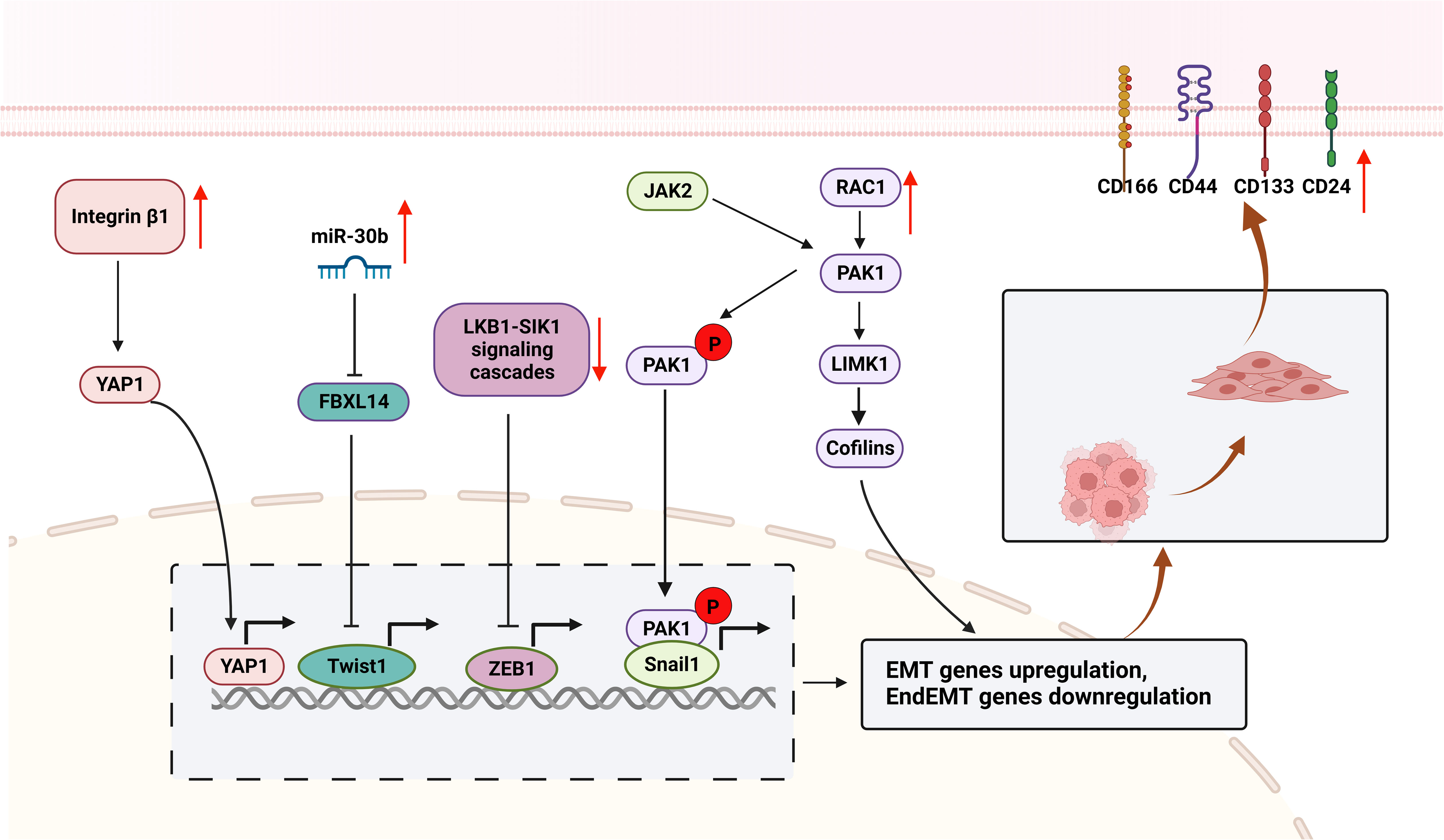
Figure 3 Mechanisms of EMT contributes to radioresistance in NSCLC. The surviving cells included enriched CSCs subpopulations that highly expressed CSCs markers (CD24, CD44, CD133, and CD166). miR-30b was upregulated and targeted to FBXL14, activating the transcription factor Twist1, resulting in increased expression of Vimentin, N-cadherin and Fibronectin, and decreased E-cadherin expression. Integrin β1 is overexpressed after radiotherapy and targets YAP1 to activate ATM/Chk2 signaling, leading to EMT. Furthermore, RAC1 is upregulated in radioresistant NSCLCs, activates the RAC1/PAK1/LIMK1/Cofilins pathway and the PAK1/Snail pathway, causes EMT and promotes tumor metastasis and invasion. LKB1/SIK1 signaling is downregulated after radiotherapy, prompting activation of the transcription factor ZEB1, leading to EMT and tumor metastasis and invasion. (This figure is adapted from an image created from BioRender.com).
Inherent radioresistance is an important factor leading to radiotherapy failure, which is mainly related to the gene mutation status of tumor cells, hypoxia status, distribution of CSCs (28, 92, 93), etc.
Mutations of oncogenes, such as epidermal growth factor receptor (EGFR) (94, 95) and kirsten rat sarcoma viral oncogene (KRAS) (96), or tumor suppressor genes such as Kelch-like ECH-associated protein 1 (KEAP1) (97, 98) and tumor protein P53 (TP53) (99, 100), can cause activation of cell proliferation and resisting cell death signals in NSCLC, leading to radioresistance (Figure 4).
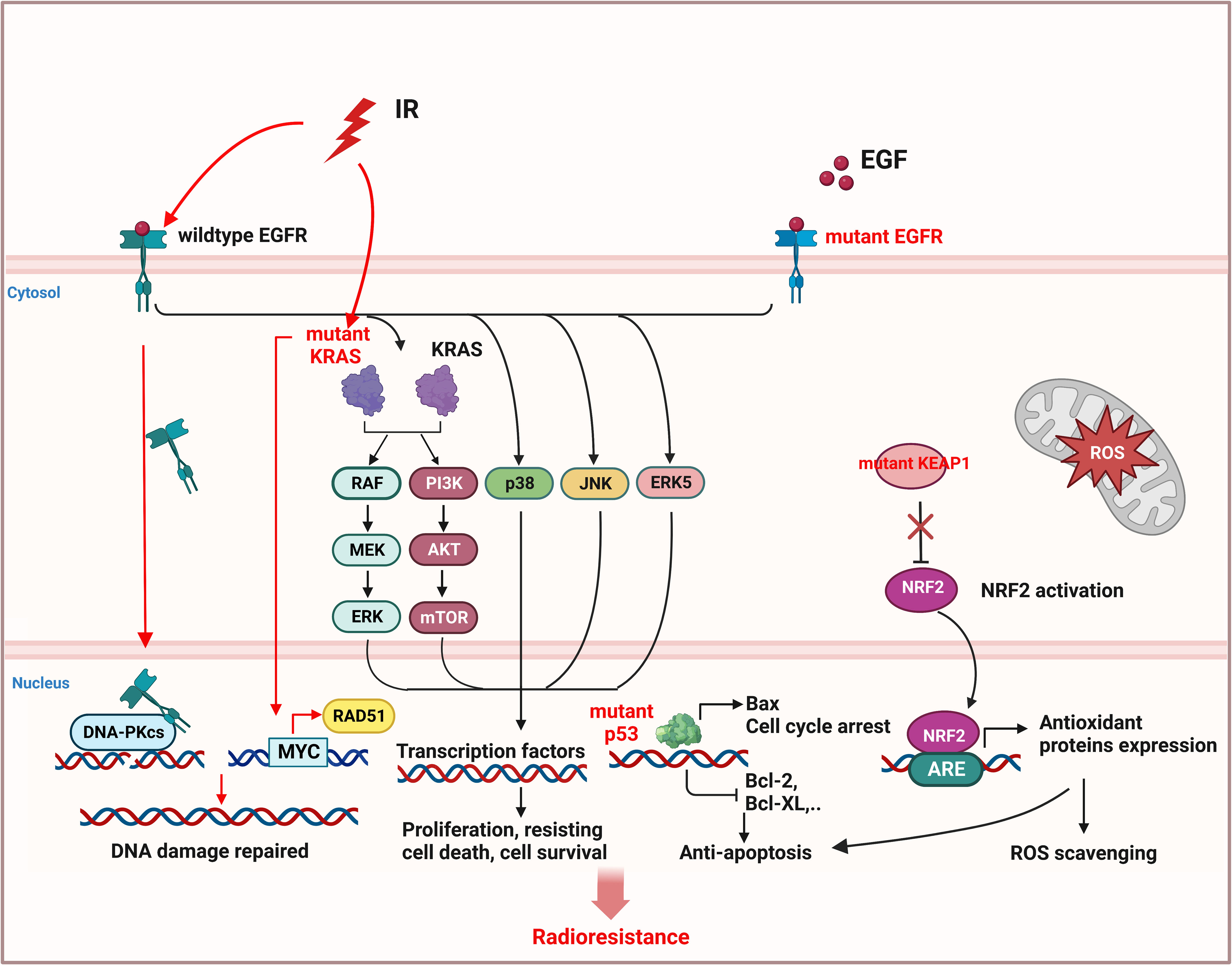
Figure 4 Inherent radioresistance is related to the mutation status of oncogene or tumor suppressor gene in NSCLC. Wildtype EGFR and mutant EGFR both caused cell proliferation and resisting cell death by activation of downstream pathway. In addition, wildtype EGFR can be stimulated by radiation and translocate to nucleus to activate DNA-PKcs, leading to DNA repair. Mutant KRAS can also promote the expression of DNA repair protein RAD51 by activating MYC and improving DNA repair. Mutant p53 will induce anti-apoptosis of NSCLC cells. Mutant KEAP1 cannot inhibit activation of NRF2, leading to the expression of antioxidant proteins to eliminate ROS. (This figure is adapted from an image created from BioRender.com).
EGFR is overexpressed in 40% to 80% of patients with NSCLC and is associated with poor prognosis (101, 102). Radiation can stimulate wild-type EGFR to enter nucleus and bind with DNA-PK catalytic subunit (DNA-PKcs) to promote DNA repair, thus leading to radioresistance of NSCLC (103, 104). However, the NSCLC with mutant EGFR is not necessarily more sensitive to radiation (94), since the mutant EGFR can trigger the downstream pathways, including extracellular signal-regulated kinase (RAS/ERK), p38MAPK, c-Jun N-terminal kinase (JNK), and ERK5, and also activate PI3K/AKT/mTOR signaling pathway, leading to cell proliferation and anti-apoptosis, migration, DNA repair, cell cycle arrest, etc., which in turn increases the radioresistance of NSCLC (22, 23, 103, 105–108).
KRAS can regulate cell growth, differentiation and apoptosis, its mutations mainly occur at codons 12 and 13, and have been found approximately in 20-30% of NSCLC tumor samples (109). KRAS-mutant NSCLC cells are more radioresistant than KRAS wild-type cells (96, 110) or EGFR-mutant cells (21), because mutant KRAS can upregulate RAD51 expression through oncogene MYC, thereby enhancing DNA damage repair and cell survival (111). In addition, EGFR-dependent chromatin condensation, namely mitotic-like concentrated chromatin (MLCC), can protect KRAS-mutant NSCLC cells from ionizing radiation (IR)-induced DSBs and premature senescence (110), and enhance the expression of CSC marker protein CD133 via osteopontin/EGFR pathway, promoting tumor invasion and radioresistance (112).
KEAP1/nuclear factor E2-related factor (NRF2) mutation happens approximately 30% of NSCLC (92), and 7% of patients have co-mutations of KEAP1/NRF2 and EGFR (113). Mutant KEAP1 lost its inhibitory effect on NRF2 in cytoplasm, the activated NRF2 translocated to the nucleus and activated the expression of antioxidant proteins by binding to antioxidant-responsive elements (AREs), resulting in the scavenging of ROS and anti-apoptosis of tumor cells, leading to unlimited proliferation, metastasis and radioresistance of NSCLC (114–116).
TP53 mutation occurs in more than half of human cancers, including NSCLC (92). p53 can not only induce cell cycle arrest, senescence, and apoptosis, but also regulate tumor metabolism, promote ferroptosis, and inhibit tumor development (117). Mutant p53 can neither induce the expression of pro-apoptotic protein Bax, nor inhibit the expression of anti-apoptotic proteins (Bcl-2, Bcl-xl) (118), which makes cancer cells survive in radiotherapy (119). Compared with single mutations in TP53, NSCLC with co-mutation in KRAS and TP53, or co-mutation in TP53 and KEAP1 are more resistance to IR-induced cell death (100, 114).
In addition to the oncogenic mutation, high levels of inhibitor of apoptosis proteins, such as cIAP1/2, XIAP, and survivin, participate in cell death and survival by binding and inhibiting caspases, leading to radioresistance of NSCLC (120–123). In addition, microRNAs can also affect the radiosensitivity of NSCLC by regulating the cell proliferation and apoptosis. miR-99a and miR-770-5p can induce apoptosis and improve radiosensitivity (124, 125). On the contrary, miR-410 and miR-208a promote radioresistance by inducing EMT and increasing cell proliferation respectively in NSCLC (126, 127).
According to the potential mechanism of inherent radioresistance mentioned above, genome sequencing can be carried out before treatment to determine the mutation type and formulate effective treatment strategies, so as to improve the tumor-killing rate and reduce the tumor recurrence rate after primary radiotherapy of NSCLC.
Cancer stem cells (CSCs) are subpopulations of tumor cells with the ability to self-renew and differentiate into heterogeneous tumor cells, promote tumor growth and metastasis through unlimited proliferation and migration, leading to tumor recurrence and treatment failure (128–131). Antioxidant signaling pathways (NRF2, Wnt, etc.) in CSCs are activated, and more antioxidant proteins are expressed to clear ROS, so as to maintain a low level of ROS in cells, which contributes to the quiescence and self-renewal of CSCs (130, 132–134) (Figure 5). In addition, CSCs have a strong ability to repair DNA damage (135). CD133 is one of the markers of CSCs. As early as 2006, Bao et al. reported that CD133-positive glioma cells showed higher expression of DNA damage checkpoint proteins (i.e. ATM, Rad17, Chk1 and Chk2) than CD133-negative cells (136). In 2014, Desai et al. clarified that CD133-positive NSCLC cells highly express DNA damage repair proteins RAD51 and Exo1, which can promote radioresistance, but had cell type specificity (137). Therefore, low levels of ROS and enhanced DNA damage repair make CSCs insensitive to radiotherapy, leading to tumor relapse (138). CD44 is another CSCs marker, which is related to tumor recurrence and metastasis, as well as prediction of the outcome after radiotherapy (139, 140). The overexpression of CD44 promotes the proliferation of NSCLC, and up-regulates the expression of PD-L1 to promote tumorigenesis, immunosuppression and chemotherapy resistance (141, 142). It is worth noting that due to the low sensitivity of CSCs to IR, the CSCs not killed by radiation will be enriched, which leads to acquired radioresistance. Therefore, the treatment efficiency of NSCLC can be improved by inducing CSCs to differentiate to reduce their proportion in tumor tissues, or by combining drugs targeting key proteins of CSCs antioxidant pathways or surface marker proteins with radiotherapy.
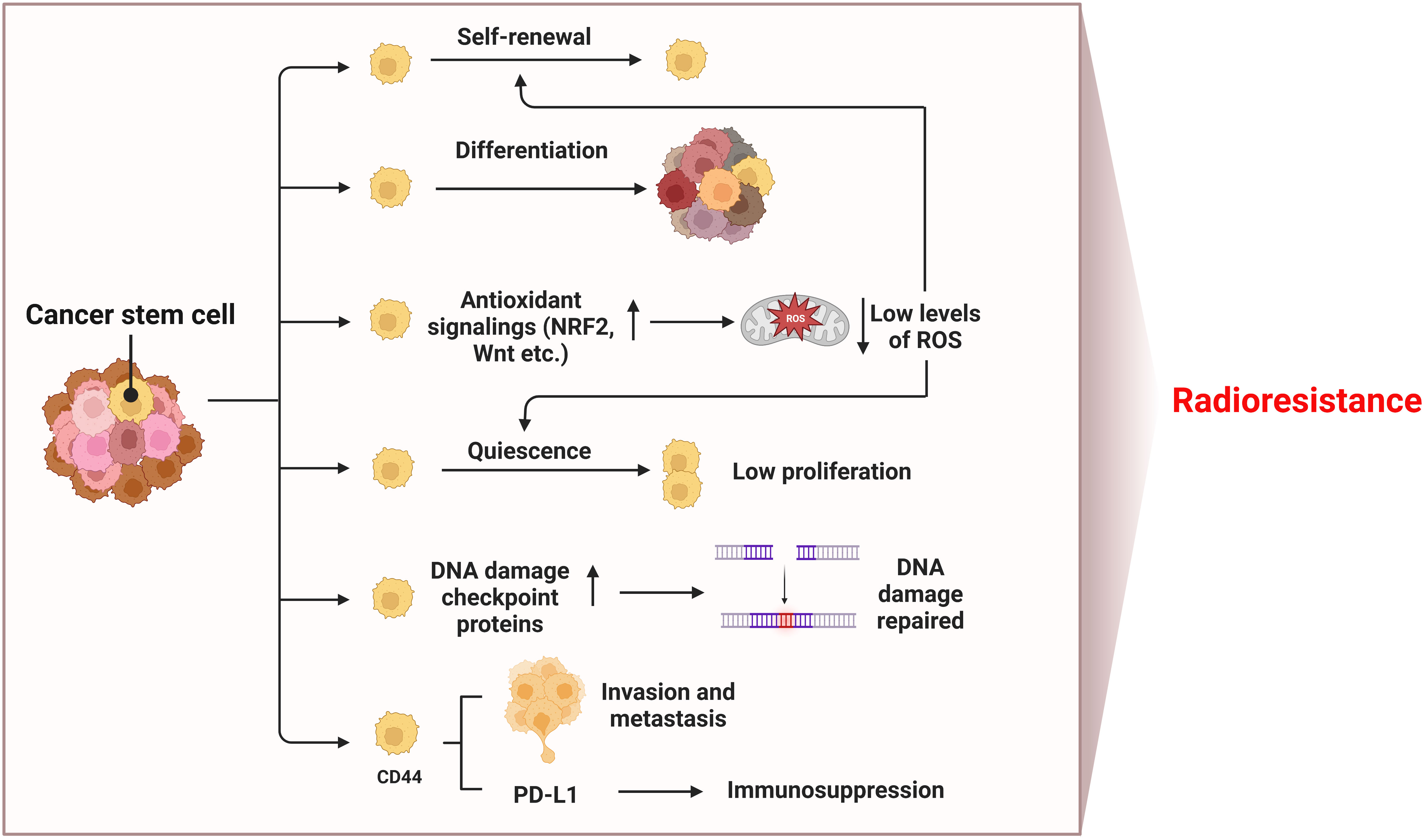
Figure 5 Mechanisms of radioresistance caused by CSCs in NSCLC. Hallmarks of CSCs such as self-renewal and differentiation, low levels of ROS, quiescence, DNA damage repair, invasion and metastasis ability, and immunosuppression induced by upregulated PD-L1 all contribute to radioresistance of NSCLC. (This figure is adapted from an image created from BioRender.com).
As one of the cancer hallmarks, hypoxia induces angiogenesis, tumor invasion, metastasis, and treatment resistance (27, 28). Hypoxic tumor cells are very insensitive to radiation (143). In this article, we mainly introduce the research on HIFs and radioresistance in NSCLC (Figure 6). Hypoxia environment and radiation activate HIFs (144, 145), activated HIF-1α and its partner subunits HIF-1β enter nucleus and bind to hypoxia-responsive element (HRE) to trigger gene transcription regulating VEGF and glucose metabolism etc., leading to cell survival (146–148). HIF-2α mainly induces angiogenic factors, such as VEGF, VEGFR, EGFR etc., promoting angiogenesis and tumor growth (149–151). Research showed that the upregulation of HIF-1α enhanced the radioresistance of NSCLC (152). Conversely, the decrease of HIF-1α in acidic environment caused radioresistance in NSCLC cells and blocking simultaneously of both HIF-1α and HIF-2α radiosensitized NSCLC, suggesting the role of HIF-2α in radioresistance (153). In addition, miR-210 promoted the hypoxic phenotype of NSCLC cells and increased radioresistance by inducing and stabilizing HIF-1α (154). By contrast, another microRNA, miR-320a targeted HIF-1α to promote methylation of PTEN, thereby reducing the radioresistance of NSCLC in vitro and in vivo (155). In conclusion, targeting HIFs may enhance radiosensitivity of NSCLC in clinical, but the pH value should be considered when targeting HIF-1α.
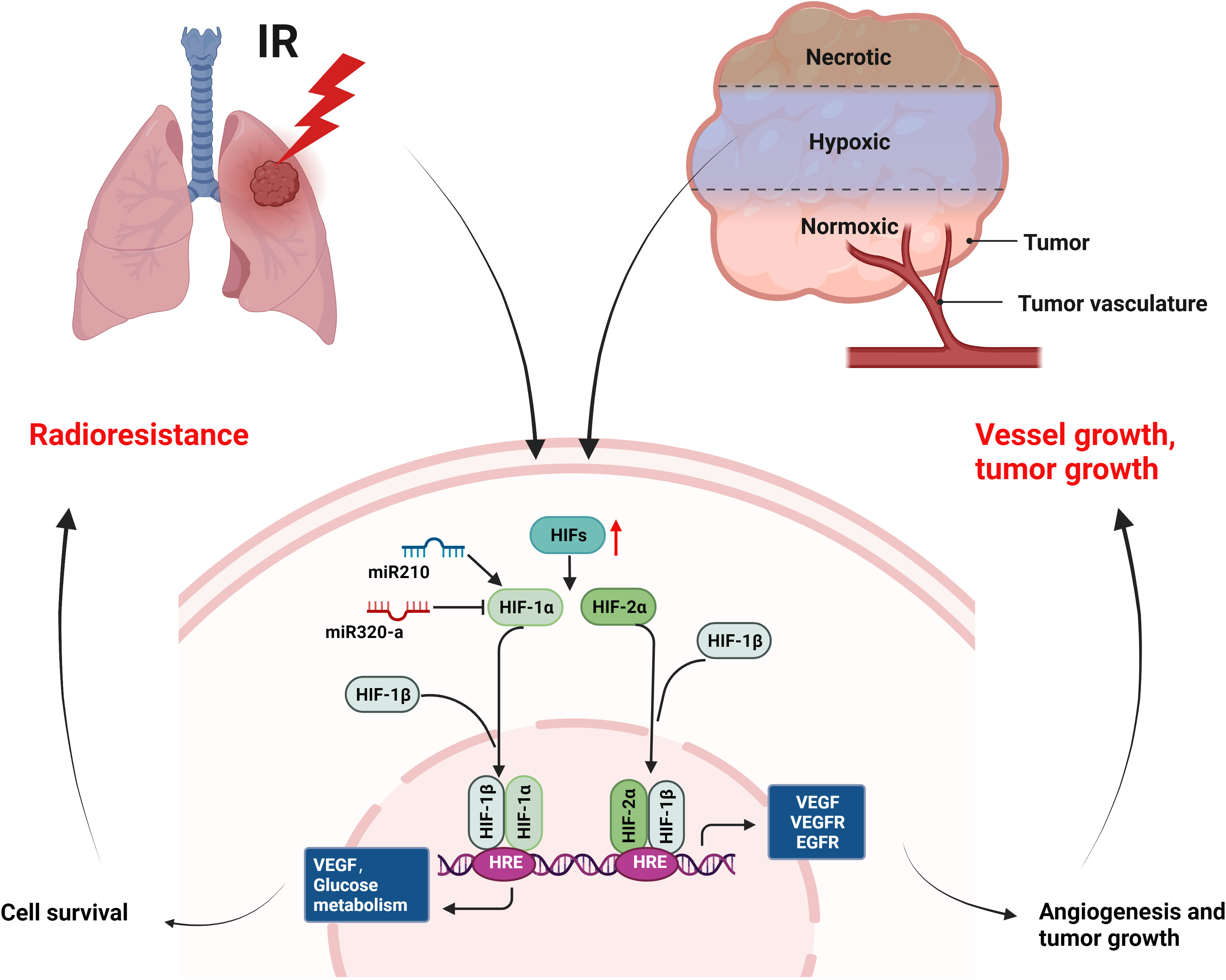
Figure 6 Mechanisms of radioresistance caused by hypoxia in NSCLC. Hypoxia environment of tumor and radiation can activate the expression of HIFs, which will induce the expression of growth factors (VEGF, VEGFR, EGFR etc.) and improved glucose metabolism, leading to cell survival, angiogenesis, and tumor growth of NSCLC. (This figure is adapted from an image created from BioRender.com).
As we discussed above, the mechanisms of radioresistance of NSCLC are complex and not isolated from each other. Whether surgery, chemotherapy, targeted therapy, or immunotherapy, the goal of their combination with radiotherapy is to kill tumor cells, but what cannot be avoided is limited effect, tumor recurrence and metastasis, as well as side effects.
At present, chemotherapy drugs, targeted drugs, immune checkpoint inhibitors, etc. can be used as radiosensizitizers in NSCLC, but these drugs are prone to drug resistance and toxic side effects (11, 30, 156, 157). In addition, most of the current studies about radiosensitizers used in NSCLC is still limited to experimental research, and only a few drugs have been used in clinical. We summarized the drugs or agents for NSCLC radiosensitization in Table 1, including antibody drugs and small molecule inhibitors from non-natural sources. In addition to drug toxicity, radiotherapy itself can also cause lung toxicity in patients, manifesting as different grades of pneumonia, pulmonary fibrosis (32, 183). In addition, the mechanisms of radioresistance are complex, and the efficacy of these single targeted drugs is limited. Therefore, it is an urgent need to develop drugs targeting multiple targets, with synergistic effects and low toxicity in NSCLC radiotherapy.
As an auxiliary means of radiotherapy for NSCLC, TCM can increase the apoptosis of cancer cells, inhibit tumor metastasis, enhance the anti-tumor immunity of patients, regulate the TME homeostasis, thus improving the efficacy of radiotherapy and reducing the recurrence rate (184, 185), which reflecting the basic principles of treating tumor by reinforcing healthy qi to eliminate pathogenic factors of TCM. Some single herbs are frequently used in the treatment of NSCLC (186). Commonly used TCM for reinforcing healthy qi to eliminate pathogenic factors include Ginseng, Codonopsis pilosula, Astragalus, Angelica, and Polygonatum, etc. Astraglus has immunoregulatory, antioxidant, anti-inflammatory, and anti-cancer activities and combined with chemotherapy to increase the efficacy and reduce the toxicity in patients with advanced NSCLC (187, 188). Our previous research found that Astragalus polysaccharides can reduce the radiation induced bystander effects (RIBE) of bone marrow mesenchymal stem cells (BMSCs) caused by X-rays and heavy ions, and reduce the toxicity of normal cells and tissues in lung cancer radiotherapy (189–191). In addition, our research also found that Guiqi Baizhu Decoction, which composed of Astragalus, Angelica, Atractylodes, Paeonia lactiflora, Tangerine peel, Rhubarb, and Licorice, can significantly reduce the radiation inflammatory reaction and immune damage, and prevent intestinal microbial imbalance and metabolic disorders caused by radiation (192). We further found that Guiqi Baizhu Decoction can regulate HIF-1α, AQP4 and Na+/K+-ATPase to reduce hypoxia and oxidative stress, thus treating radiation-induced intestinal edema (193). Moreover, in the study of gastric cancer, we used chemical informatics and cell experiments to verify that Guiqi Baizhu decoction plays a dual role of anti-tumor and immunoregulation by targeting HER2 and PD-L1 through its active components quercetin and isorhamnetin respectively, reflecting the mechanism of multi-point and synergistic effect of TCM in tumor treatment (194).
In recent decades, the advantages of natural products in anti-tumor have become more and more obvious, such as paclitaxel (195) and vinorelbine (196) has been used in clinical for decades. Here we listed the application of TCM compound prescription, single herb, herbal extracts, and small molecules from TCM in the preclinical and clinical research of radiotherapy toxicity reduction and efficiency enhancement for NSCLC (Table 2). The various factors that cause radioresistance are not independent, nor only appear in a certain period of time. Moreover, at different stages of radiotherapy, the mechanism that dominates radioresistance is variable and complex. However, TCM can adjust the radiation anti-tumor effect in different ways with the advantage of multi-component and multi-target, improve the efficacy of radiotherapy, reduce the toxicity of radiotherapy and improve the quality of life of patients.
In conclusion, the radioresistance mechanisms of NSCLC are complex. Therefore, prior to radiotherapy gene sequencing should be performed for NSCLC patients to determine the type of mutation, or abnormally expressed genes, proteins, and signaling pathways, thus to develop more effective treatment strategies. In the early stage of radiotherapy, it is suggested to combine EGFR, KRAS and other mutant targeted drugs, drugs to improve DNA damage, immune checkpoint drugs or drugs to target hypoxia. In the middle and late stage of radiotherapy, it is suggested that the combination of drugs targeting EMT or regulating TME can improve the curative effect. TCM can run through the whole radiotherapy process. However, the specific mechanism of TCM in the sensitization of NSCLC radiotherapy needs to be further explored. The combination of effective small molecules extracted from TCM and radiotherapy may provide a new application prospect for the future clinical treatment of NSCLC.
TZ is responsible for literature review and article writing. LZ and JH are responsible for revising articles. ZM, YYL, YZ, and ZL are responsible for collecting and sorting materials. SZ, YC, and GZ are responsible for sorting charts. YQL provides ideas and guidance for the article. All authors contributed to the article and approved the submitted version.
This work was supported by the National Natural Science Foundation of China (No.81973595), The basic research Innovation Group in Gansu (No.20JR10RA332), Provincial University industry support project in Gansu (No.2020C-15), and National Natural Science Foundation of China (No.82260882).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Koh PK, Faivre-Finn C, Blackhall FH, De Ruysscher D. Targeted agents in non-small cell lung cancer (NSCLC): Clinical developments and rationale for the combination with thoracic radiotherapy. Cancer Treat Rev (2012) 38(6):626–40. doi: 10.1016/j.ctrv.2011.11.003
2. Wirsdörfer F, de Leve S, Jendrossek V. Combining radiotherapy and immunotherapy in lung cancer: Can we expect limitations due to altered normal tissue toxicity? Int J Mol Sci (2019) 20(1):24. doi: 10.3390/ijms20010024
3. Sears CR, Cooney SA, Chin-Sinex H, Mendonca MS, Turchi JJ. DNA Damage response (DDR) pathway engagement in cisplatin radiosensitization of non-small cell lung cancer. DNA Repair (Amst). (2016) 40:35–46. doi: 10.1016/j.dnarep.2016.02.004
4. Provencio M, Sánchez A. Therapeutic integration of new molecule-targeted therapies with radiotherapy in lung cancer. Transl Lung Cancer Res (2014) 3(2):89–94. doi: 10.3978/j.issn.2218-6751.2014.03.06
5. Hirsch FR, Scagliotti GV, Mulshine JL, Kwon R, Curran WJ, Wu YL, et al. Lung cancer: current therapies and new targeted treatments. Lancet. (2017) 389(10066):299–311. doi: 10.1016/S0140-6736(16)30958-8
6. Trakul N, Harris JP, Quynh-Thu Le, WY H, PG M, BW L, et al. Stereotactic ablative radiotherapy for reirradiation of locally recurrent lung tumors. J Thorac Oncol (2012) 7(9):1462–5. doi: 10.1097/JTO.0b013e31825f22ce
7. Iyengar P, Zhang VE, Court L, Westover K, Yan YL, Lin MH, et al. Accelerated hypofractionated image-guided vs conventional radiotherapy for patients with stage II/III non-small cell lung cancer and poor performance status: A randomized clinical trial. JAMA Oncol (2021) 7(10):1497–505. doi: 10.1001/jamaoncol.2021.3186
8. Senthi S, Lagerwaard FJ, Haasbeek Cornelis JA, Slotman BJ, Senan S. Patterns of disease recurrence after stereotactic ablative radiotherapy for early stage non-small-cell lung cancer: a retrospective analysis. Lancet Oncol (2012) 13(8):802–9. doi: 10.1016/S1470-2045(12)70242-5
9. Sato K, Shimokawa T, Imai T. Difference in acquired radioresistance induction between repeated photon and particle irradiation. Front Oncol (2019) 9:1213. doi: 10.3389/fonc.2019.01213
10. Catton CN, Shultz DB. Should we expand the carbon ion footprint of prostate cancer? Lancet Oncol (2019) 20(5):608–9. doi: 10.1016/S1470-2045(19)30094-4
11. Bradley JD, Hu C, Komaki RR, Masters GA, Blumenschein GR, Schild SE, et al. Long-term results of NRG oncology RTOG 0617: Standard- versus high-dose chemoradiotherapy with or without cetuximab for unresectable stage III non-small-cell lung cancer. J Clin Oncol (2020) 38(7):706–14. doi: 10.1200/JCO.19.01162
12. Buckley AM, Lynam-Lennon N, O'Neill H, O'Sullivan J. Targeting hallmarks of cancer to enhance radiosensitivity in gastrointestinal cancers. Nat Rev Gastroenterol Hepatol (2020) 17(5):298–313.
13. Lee SY, Jeong EK, Ju MK, Jeon HM, Kim MY, Kim CH, et al. Induction of metastasis, cancer stem cell phenotype, and oncogenic metabolism in cancer cells by ionizing radiation. Mol Cance. (2017) 16(1):10. doi: 10.1186/s12943-016-0577-4
14. Alhaddad L, Pustovalova M, Blokhina T, Chuprov-Netochin R, Osipov AN, Leonov S. IR-surviving NSCLC cells exhibit different patterns ofmolecular and cellular reactions relating to the multifraction irradiation regimen and p53-family proteins expression. Cancers (Basel) (2021) 13(11):2669. doi: 10.3390/cancers13112669
15. McKelvey KJ, Hudson AL, Back M, Eade T, Diakos CI. Radiation, inflammation and the immune response in cancer. Mamm Genome. (2018) 29(11-12):843–65. doi: 10.1007/s00335-018-9777-0
16. Nantajit D, Dong L, Jian JL. The network of epithelial–mesenchymal transition: potential new targets for tumor resistance. J Cancer Res Clin (2015) 141(10):1697–713. doi: 10.1007/s00432-014-1840-y
17. Marie-Egyptienne DT, Lohse I, Hill RP. Cancer stem cells, the epithelial to mesenchymal transition (EMT) and radioresistance: Potential role of hypoxia. Cancer Lett (2013) 341(1):63–72. doi: 10.1016/j.canlet.2012.11.019
18. Barker HE, Paget JT, Khan AA, Harrington KJ. The tumour microenvironment after radiotherapy: Mechanisms of resistance and recurrence. Nat Rev Cancer. (2015) 15(7):409–25. doi: 10.1038/nrc3958
19. Mittal A, Nenwani M, Sarangi I, Achreja A, Lawrence TS, Nagrath D. Radiotherapy-induced metabolic hallmarks in the tumor microenvironment. Trends Cancer. (2022) 8(10):1–15. doi: 10.1016/j.trecan.2022.05.005
20. Hanahan D. Hallmarks of cancer: New dimensions. Cancer Discovery (2022) 12(1):31–46. doi: 10.1158/2159-8290.CD-21-1059
21. Johung KL, Yao XP, Li FY, Yu JB, Gettinger SN, Goldberg S, et al. A clinical model for identifying radiosensitive tumor genotypes in non-small cell lung cancer. Clin Cancer Res (2013) 19(19):5523–32.
22. Sad K, Parashar P, Tripathi P, Hungyo H, Sistla R, Soni R, et al. Prochlorperazine enhances radiosensitivity of non-small cell lung carcinoma by stabilizing GDP-bound mutant KRAS conformation. Free Radic Biol Med (2021) 177:299–312. doi: 10.1016/j.freeradbiomed.2021.11.001
23. Jiang WW, Jin GH, Cai FF, Chen X, Cao NN, Zhang XY, et al. Extracellular signal-regulated kinase 5 increases radioresistance of lung cancer cells by enhancing the DNA damage response. Exp Mol Med (2019) 51(2):1–20. doi: 10.1038/s12276-019-0209-3
24. Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell. (2011) 144(5):646–74. doi: 10.1016/j.cell.2011.02.013
25. Haura EB, Cress WD, Chellappan S, Zheng Z, Bepler G. Antiapoptotic signaling pathways in non-small-cell lung cancer: Biology and therapeutic strategies. Clin Lung Cancer. (2004) 6(2):113–22. doi: 10.3816/CLC.2004.n.025
26. Olivares-Urbano MA, Griñán-Lisón C, Marchal JA, Núñez MI. CSC radioresistance: A therapeutic challenge to improve radiotherapy effectiveness in cancer. Cells. (2020) 9(7):1651. doi: 10.3390/cells9071651
27. Walsh JC, Lebedev A, Aten E, Madsen K, Marciano L, Kolb HC. The clinical importance of assessing tumor hypoxia: Relationship of tumor hypoxia to prognosis and therapeutic opportunities. Antioxid Redox Signal (2014) 21(10):1516–54. doi: 10.1089/ars.2013.5378
28. Salem A, Asselin MC, Reymen B, Jackson A, Lambin P, West CML, et al. Targeting hypoxia to improvenon-small cell lung cancer outcome. J Natl Cancer Inst (2018) 110(1):djx160. doi: 10.1093/jnci/djx160
29. Jansen J, Vieten P, Pagliari F, Hanley R, Marafioti MG, Tirinato L, et al. A novel analysis method for evaluating the interplay of oxygen and ionizing radiation at the gene level. Front Genet (2021) 12:597635. doi: 10.3389/fgene.2021.597635
30. Wang H, Mu XY, He H, Zhang XD. Cancer radiosensitizers. Trends Pharmacol Sci (2018) 39(1):24–48. doi: 10.1016/j.tips.2017.11.003
31. Havaki S, Kotsinas A, Chronopoulos E, Kletsas D, Georgakilas A, Gorgoulis VG. The role of oxidative DNA damage in radiation induced bystander effect. Cancer Lett (2015) 356(1):43–51. doi: 10.1016/j.canlet.2014.01.023
32. Barker HE, Paget JT, Khan AA, Harrington KJ. The tumour microenvironment after radiotherapy: Mechanisms of resistance and recurrence. Nat Rev Cancer. (2015) 15(7):409–25. doi: 10.1038/nrc3958
33. Stingele J, Bellelli R, Boulton SJ. Mechanisms of DNA-protein crosslink repair. Nat Rev Mol Cell Biol (2017) 18(9):563–73. doi: 10.1038/nrm.2017.56
34. Smith J, Tho LM, Xu N, Gillespie DA. The ATM-Chk2 and ATR-Chk1 pathways in DNA damage signaling and cancer. Adv Cancer Res (2010) 108:73–112. doi: 10.1016/B978-0-12-380888-2.00003-0
35. Syed A, Tainer JA. The MRE11-RAD50-NBS1 complex conducts the orchestration of damage signaling and outcomes to stress in DNA replication and repair. Annu Rev Biochem (2018) 87:263–94. doi: 10.1146/annurev-biochem-062917-012415
36. Zou N, Xie GZ, Cui TT, Srivastava AK, Qu MH, Yang LL, et al. DDB2 increases radioresistance of NSCLC cells by enhancing DNA damage responses. Tumor Biol (2016) 37(10):14183–91. doi: 10.1007/s13277-016-5203-y
37. Choi SH, Yang H, Lee SH, Ki JH, Nam DH, Yoo HY. TopBP1 and claspin contribute to the radioresistance of lung cancer brain metastases. Mol Cancer. (2014) 13:211. doi: 10.1186/1476-4598-13-211
38. Burma S, Chen BP, Murphy M, Kurimasa A, Chen DJ. ATM Phosphorylates histone H2AX in response to DNA double-strand breaks. J Biol Chem (2001) 276:42462–7. doi: 10.1074/jbc.C100466200
39. Paull TT. Mechanisms of ATM activation. Annu Rev Biochem (2015) 84:711–38. doi: 10.1146/annurev-biochem-060614-034335
40. Torok JA, Oh P, Castle KD, Reinsvold M, Ma Y, Luo LX, et al. Deletion of atm in tumor but not endothelial cells improves radiation response in a primary mouse model of lung adenocarcinoma. Cancer Res (2019) 79(4):773–82. doi: 10.1158/0008-5472.CAN-17-3103
41. Koll TT, Feis SS, Wright MH, Teniola MM, Richardson MM, Robles AI, et al. HSP90 inhibitor, DMAG, synergizes with radiation of lung cancer cells by interfering with base excision and ATM-mediated DNA repair. Mol Cancer Ther (2008) 7(7):1985–92. doi: 10.1158/1535-7163.MCT-07-2104
42. Gou W, Yu X, Wu S, Wu H, Chang H, Chen L, et al. Targeted inhibition of acidic nucleoplasmic DNA-binding protein 1 enhances radiosensitivity of non-small cell lung cancer. Cancer Lett (2022) 530:100–9. doi: 10.1016/j.canlet.2022.01.020
43. Cao K, Chen Y, Zhao S, Huang Y, Liu T, Liu H, et al. Sirt3 promoted DNA damage repair and radioresistance through ATM-Chk2 in non-small cell lung cancer cells. J Cancer. (2021) 12(18):5464–72. doi: 10.7150/jca.53173
44. Zeng Y, Jie X, Wu B, Wu G, Liu L, Xu S. IQGAP3 interacts with Rad17 to recruit the Mre11-Rad50-Nbs1 complex and contributes to radioresistance in lung cancer. Cancer Lett (2020) 493:254–65. doi: 10.1016/j.canlet.2020.08.042
45. Zhang J, Wu Q, Zhu L, Xie S, Tu L, Yang Y, et al. SERPINE2/PN-1 regulates the DNA damage response and radioresistance by activating ATM in lung cancer. Cancer Lett (2022) 524:268–83. doi: 10.1016/j.canlet.2021.10.001
46. Lei X, Du L, Zhang P, Ma N, Liang Y, Han Y, et al. Knockdown GTSE1 enhances radiosensitivity in non-small-cell lung cancer through DNA damage repair pathway. J Cell Mol Med (2020) 24(9):5162–7. doi: 10.1111/jcmm.15165
47. Cheng C, Pei X, Li SW, Yang J, Li C, Tang J, et al. CRISPR/Cas9 library screening uncovered methylated PKP2 as a critical driver of lung cancer radioresistance by stabilizing β-catenin. Oncogene. (2021) 40(16):2842–57. doi: 10.1038/s41388-021-01692-x
48. Kaminskyy VO, Hååg P, Novak M, Végvári Á, Arapi V, Lewensohn R, et al. EPHA2 interacts with DNA-PK in cell nucleus and controls ionizing radiation responses in non-small cell lung cancer cells. Cancers (Basel). (2021) 13(5):1010. doi: 10.3390/cancers13051010
49. Gong S, Li Y, Lv L, Men W. Restored microRNA-519a enhances the radiosensitivity of non-small cell lung cancer via suppressing EphA2. Gene Ther (2021) 29:1–13. doi: 10.1038/s41434-020-00213-x
50. Yang X, Wang G, You J, Gu R, Xu X, Xu C, et al. High expression of cancer-IgG is associated with poor prognosis and radioresistance via PI3K/AKT/DNA-PKcs pathway regulation in lung adenocarcinoma. Front Oncol (2021) 11:675397. doi: 10.3389/fonc.2021.675397
51. Yenerall P, Das AK, Wang S, Kollipara RK, Li LS, Villalobos P, et al. RUVBL1/RUVBL2 ATPase activity drives PAQosome maturation, DNA replication and radioresistance in lung cancer. Cell Chem Biol (2020) 27(1):105–121.e14. doi: 10.1016/j.chembiol.2019.12.005
52. Aarts M, Sharpe R, Garcia-Murillas I, Gevensleben H, Hurd MS, Shumway SD, et al. Forced mitotic entry of s-phase cells as a therapeutic strategy induced by inhibition of WEE1. Cancer Discovery (2012) 2(6):524–39. doi: 10.1158/2159-8290.CD-11-0320
54. Wang Q, Chen Y, Lu H, Wang H, Feng H, Xu J, et al. Quercetin radiosensitizes non-small cell lung cancer cells through the regulation of miR-16-5p/WEE1 axis. IUBMB Life (2020) 72(5):1012–22. doi: 10.1002/iub.2242
55. Aggarwal BB, Sung B. NF-κB in cancer: a matter of life and death. Cancer Discovery (2011) 1(6):469–71. doi: 10.1158/2159-8290.cd-11-0260
56. Sergei IG, Florian R, Michael K. Immunity, inflammation, and cancer. Cell. (2010) 140(6):883–99. doi: 10.1016/j.cell.2010.01.025
57. Bai M, Ma X, Li X, Wang X, Mei Q, Li X, et al. The accomplices of NF-κB lead to radioresistance. Curr Protein Pept Sci (2015) 16:279–94. doi: 10.2174/138920371604150429152328
58. Harada D, Takigawa N, Kiura K. The role of STAT3 in non-small cell lung cancer. Cancers (Basel) (2014) 6(2):708–22. doi: 10.3390/cancers6020708
59. You S, Li R, Park D, Xie M, Sica GL, Cao Y, et al. Disruption of STAT3 by niclosamide reverses radioresistance of human lung cancer. Mol Cancer Ther (2014) 13(3):606–16. doi: 10.1158/1535-7163.MCT-13-0608
60. Tsolou A, Liousia M, Kalamida D, Pouliliou S, Giatromanolaki A, Koukourakis M. Inhibition of IKK-NFκB pathway sensitizes lung cancer cell lines to radiation. Cancer Biol Med (2017) 14(3):293–301. doi: 10.20892/j.issn.2095-3941.2017.0049
61. Zhi-Wei. L, Yi-Ming. Z, Li-Ying. Z, Ting. Z, Yang-Yang. Li, Gu-Cheng. Z, et al. Duality of interactions between TGF-β and TNF-α during tumor formation. Front Immunol (2021) 12:810286(undefined). doi: 10.3389/fimmu.2021.810286
62. Yi-Ming. Z, Li-Ying. Z, Yang-Yang. Li, Heng. Z, Zhi-Ming. M, Zhi-Wei. L, et al. Radiation-induced bystander effect on the genome of bone marrow mesenchymal stem cells in lung cancer. Antioxid Redox Signal (2022). doi: 10.1089/ars.2022.0072. undefined(undefined), undefined. doi: 10.1089/ars.2022.0072
63. He K, Barsoumian HB, Hu Y, Sezen D, MD W, et al. Inhibition of STAT6 with antisense oligonucleotides enhances the systemic antitumor effects of radiotherapy and anti-PD1 in metastatic non-small cell lung cancer. Cancer Immunol Res (2023). undefined(undefined), undefined. doi: 10.1158/2326-6066.CIR-22-0547
64. Wang J, Han Q, Liu H, Luo H, Li L, Liu A, et al. Identification of radiotherapy-associated genes in lung adenocarcinoma by an integrated bioinformatics analysis approach. Front Mol Biosci (2021) 8:624575. doi: 10.3389/fmolb.2021.624575
65. Zhang F, Sang Y, Chen D, Wu X, Wang X, Yang W, et al. M2 macrophage-derived exosomal long non-coding RNA AGAP2-AS1 enhances radiotherapy immunity in lung cancer by reducing microRNA-296 and elevating NOTCH2. Cell Death Dis (2021) 12(5):467. doi: 10.1038/s41419-021-03700-0
66. Zitvogel L, Kroemer G. Lower airway dysbiosis exacerbates lung cancer. Cancer Discovery (2021) 11(2):224–6. doi: 10.1158/2159-8290.CD-20-1641
67. Pinato DJ, Howlett S, Ottaviani D, Urus H, Patel A, Mineo T, et al. Association of prior antibiotic treatment with survival and response to immune checkpoint inhibitor therapy in patients with cancer. JAMA Oncol (2020) 6(2):302. doi: 10.1001/jamaoncol.2019.6921
68. Li. Y, Aitian. Li, Ying. W, Yi. Z. Intratumoral microbiota: roles in cancer initiation, development and therapeutic efficacy. Signal Transduct Target Ther (2023) 8(1):35. doi: 10.1038/s41392-022-01304-4
69. Jin C, Lagoudas GK, Zhao C, Bullman S, Bhutkar A, Hu B, et al. Commensal microbiota promote lung cancer development via γδ T cells. Cell. (2019) 176(5):998–1013. doi: 10.1016/j.cell.2018.12.040
70. Xiang L, Meng X. Emerging cellular and molecular interactions between the lung microbiota and lung diseases. Crit Rev Microbiol (2022) 48(5):577–610. doi: 10.1080/1040841X.2021.1992345
71. Tsay JC, Wu BG, Sulaiman I, Gershner K, Schluger R, Li Y, et al. Lower airway dysbiosis affects lung cancer progression. Cancer Discovery (2021) 11(2):293–307. doi: 10.1158/2159-8290.CD-20-0263
72. Yang Y, Chong Y, Chen M, Dai W, Zhou X, Ji Y, et al. Targeting lactate dehydrogenase a improves radiotherapy efficacy in non-small cell lung cancer: From bedside to bench. J Transl Med (2021) 19(1):170. doi: 10.1186/s12967-021-02825-2
73. Meng MB, Wang HH, Guo WH, Wu ZQ, Zeng XL, Zaorsky NG, et al. Targeting pyruvate kinase M2 contributes to radiosensitivity of non-small cell lung cancer cells in vitro and in vivo. Cancer Lett (2015) 356:985–93. doi: 10.1016/j.canlet.2014.11.016
74. Tsolou A, Koparanis D, Lamprou I, Giatromanolaki A, Koukourakis MI. Increased glucose influx and glycogenesis in lung cancer cells surviving after irradiation. Int J Radiat Biol (2022) 1-10. doi: 10.1080/09553002.2022.2113837
75. Lei G, Zhang Y, Koppula P, Liu X, Zhang J, Lin SH, et al. The role of ferroptosis in ionizing radiation-induced cell death and tumor suppression. Cell Res (2020) 30(2):146–62. doi: 10.1038/s41422-019-0263-3
76. Tirinato L, Marafioti MG, Pagliari F, Jansen J, Aversa I, Hanley R, et al. Lipid droplets and ferritin heavy chain: A devilish liaison in human cancer cell radioresistance. Elife. (2021) 10:e72943. doi: 10.7554/eLife.72943
77. Lee S, Kim D, Kang J, Kim E, Kim W, Youn H, et al. Surfactant protein b suppresses lung cancer progression by inhibiting secretory phospholipase A2 activity and arachidonic acid production. Cell Physiol Biochem (2017) 42(4):1684–700. doi: 10.1159/000479418
78. Singh B, Patwardhan RS, Jayakumar S, Sharma D, Sandur SK. Oxidative stress associated metabolic adaptations regulate radioresistance in human lung cancer cells. J Photochem Photobiol B (2020) 213:112080. doi: 10.1016/j.jphotobiol.2020.112080
79. Hao CC, Luo JN, Xu CY, Zhao XY, Zhong ZB, Hu XN, et al. TRIAP1 knockdown sensitizes non-small cell lung cancer to ionizing radiation by disrupting redox homeostasis. Thorac Cancer. (2020) 11(4):1015–25. doi: 10.1111/1759-7714.13358
80. Luo H, Wang L, Schulte BA, Yang A, Tang S, Wang GY. Resveratrol enhances ionizing radiation-induced premature senescence in lung cancer cells. Int J Oncol (2013) 43(6):1999–2006. doi: 10.3892/ijo.2013.2141
81. Tsolou A, Lamprou I, Fortosi AO, Liousia M, Giatromanolaki A, Koukourakis MI. 'Stemness' and 'senescence' related escape pathways are dose dependent in lung cancer cells surviving post irradiation. Life Sci (2019) 232:116562. doi: 10.1016/j.lfs.2019.116562
82. Qiao L, Chen Y, Liang N, Xie J, Deng G, Chen F, et al. Targeting epithelial-to-mesenchymal transition in radioresistance: crosslinked mechanisms and strategies. Front Oncol (2022) 12:775238. doi: 10.3389/fonc.2022.775238
83. Eckert MA, Lwin TM, Chang AT, Kim J, Danis E, Ohno-Machado L, et al. Twist1-induced invadopodia formation promotes tumor metastasis. Cancer Cell (2011) 19(3):372–86. doi: 10.1016/j.ccr.2011.01.036
84. Cui YH, Kang JH, Suh Y, Zhao Y, Yi JM, Bae IH, et al. Loss of FBXL14 promotes mesenchymal shift and radioresistance of non-small cell lung cancer by TWIST1 stabilization. Signal Transduct Target Ther (2021) 6(1):272. doi: 10.1038/s41392-021-00599-z
85. Gomez-Casal R, Bhattacharya C, Ganesh N, Bailey L, Basse P, Gibson M, et al. Non-small cell lung cancer cells survived ionizing radiation treatment display cancer stem cell and epithelial-mesenchymal transition phenotypes. Mol Cancer. (2013) 12(1):94. doi: 10.1186/1476-4598-12-94
86. Dadrich M, Nicolay NH, Flechsig P, Bickelhaupt S, Hoeltgen L, Roeder F, et al. Combined inhibition of TGFβ and PDGF signaling attenuates radiation-induced pulmonary fibrosis. Oncoimmunology. (2016) 5(5):e1123366. doi: 10.1080/2162402X.2015.1123366
87. Li Y, Sun C, Tan Y, Zhang H, Li Y, Zou H. ITGB1 enhances the radioresistance of human non-small celllung cancer cells by modulating the DNA damage response and YAP1-induced epithelial-mesenchymal transition. Int J Biol Sci (2021) 17(2):635–50. doi: 10.7150/ijbs.52319
88. Kim E, Youn H, Kwon T, Son B, Kang J, Yang HJ, et al. PAK1 tyrosine phosphorylation is required to induce epithelial-mesenchymal transition and radioresistance in lung cancer cells. Cancer Res (2014) 74(19):5520–31. doi: 10.1158/0008-5472.CAN-14-0735
89. Tan S, Yi P, Wang H, Xia L, Han Y, Wang H, et al. RAC1 involves in the radioresistance by mediating epithelial-mesenchymal transition in lung cancer. Front Oncol (2020) 10:649. doi: 10.3389/fonc.2020.00649
90. Yao YH, Cui Y, Qiu XN, Zhang LZ, Zhang W, Li H, et al. Attenuated lkb1-sik1 signaling promotes epithelial-mesenchymal transition and radioresistance of non–small cell lung cancer cells. Chin J Cancer. (2016) 35(10):9. doi: 10.1186/s40880-016-0113-3
91. Fu W, Zhao J, Hu W, Dai L, Jiang Z, Zhong S, et al. LINC01224/ZNF91 promote stem cell-like properties and drive radioresistance in non-small cell lung cancer. Cancer Manag Res (2021) 13:5671–81. doi: 10.2147/CMAR.S313744
92. Cancer Genome Atlas Research Network. Comprehensive molecular profiling of lung adenocarcinoma. Nature (2014) 511(7511):543–50. doi: 10.1038/nature13385
93. Yang LQ, Shi PF, Zhao GC, Xu J, Peng W, Zhang JY, et al. Targeting cancer stem cell pathways for cancer therapy. Signal Transduct Target Ther (2020) 5(1):8. doi: 10.1038/s41392-020-0110-5
94. Hsu F, Sit D, Pastuch A, Dingler A, Atwal P. Lung cancer epidermal growth factor receptor mutations and radiotherapy response: A multicentre clinical study. Clin Transl Radiat Oncol (2021) 30:15–8. doi: 10.1016/j.ctro.2021.06.006
95. Butkiewicz D, Krześniak M, Gdowicz-Kłosok A, Giglok M, Marszałek-Zeńczak M, Suwiński R. EGFR polymorphisms in gene predict clinical outcome in unresectable non-small cell lung cancer treated with radiotherapy and platinum-based chemoradiotherapy. Int J Mol Sci (2021) 22(11):5605. doi: 10.3390/ijms22115605
96. Zhu DQ, Liu Y, Yu ZJ, Zhang RH, Li AW, Gong FY, et al. The diverse analysis identifies mutated KRAS associated with radioresistance in non-small cell lung cancer. World J Oncol (2022) 13(2):84–95. doi: 10.14740/wjon1465
97. Binkley MS, Jeon YJ, Nesselbush M, Moding EJ, Nabet BY, Almanza D, et al. KEAP1/NFE2L2 mutations predict lung cancer radiation resistance that can be targeted by glutaminase inhibition. Cancer Discovery (2020) 10(12):1826–41. doi: 10.1158/2159-8290.CD-20-0282
98. Kinslow CJ, Kumar P, Cai LL, Sun RC, Chaudhary KR, Cheng SK. NRF2-pathway mutations predict radioresistance in non-small cell lung cancer. Transl Lung Cancer Res (2022) 11(7):1510–3. doi: 10.21037/tlcr-22-292
99. Pustovalova M, Alhaddad L, Smetanina N, Chigasova A, Blokhina T, Chuprov-Netochin R, et al. The p53-53BP1-related survival of A549 and H1299 human lung cancer cells after multifractionated radiotherapy demonstrated different response to additional acute X-ray exposure. Int J Mol Sci (2020) 21(9):3342. doi: 10.3390/ijms21093342
100. Gurtner K, Kryzmien Z, Koi L, Wang M, Benes CH, Hering S, et al. Radioresistance of kras/tp53-mutated lung cancer can be overcome by radiation dose escalation or egfr tyrosine kinase inhibition. vivo. Int J Cancer. (2020) 147(2):472–7. doi: 10.1002/ijc.32598
101. Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc (2008) 83(5):584–94. doi: 10.4065/83.5.584
102. Mendelsohn J, Baselga J. Status of epidermal growth factor receptor antagonists in the biology and treatment of cancer. J Clin Oncol (2003) 21(14):2787–99. doi: 10.1200/JCO.2003.01.504
103. Das AK, Chen BP, Story MD, Sato M, Minna JD, Chen DJ, et al. Somatic mutations in the tyrosine kinase domain of epidermal growth factor receptor (EGFR) abrogate EGFR-mediated radioprotection in non-small cell lung carcinoma. Cancer Res (2007) 67(11):5267–74. doi: 10.1158/0008-5472.CAN-07-0242
104. Chen GD, Kong PZ, Yang MM, Hu WL, Prise KM, Yu KN, et al. Golgi phosphoprotein 3 confers radioresistance via stabilizing EGFR in lung adenocarcinoma. Int J Radiat Oncol Biol Phys (2022) 112(5):1216–28. doi: 10.1016/j.ijrobp.2021.11.023
105. Liang K, Ang KK, Milas L, Hunter N, Fan Z. The epidermal growth factor receptor mediates radioresistance. Int J Radiat Oncol Biol Phys (2003) 57(1):246–54. doi: 10.1016/s0360-3016(03)00511-x
106. LoRusso PM. Inhibition of the PI3K/AKT/mTOR pathway in solid tumors. J Clin Oncol (2016) 34(31):3803–15. doi: 10.1200/JCO.2014.59.0018
107. Schuurbiers OC, Kaanders JH, van der Heijden H, Dekhuijzen RP, Oyen WJ, Bussink J. The PI3-K/AKT-pathway and radiation resistance mechanisms in non-small cell lung cancer. J Thorac Oncol (2009) 4(6):761–7. doi: 10.1097/JTO.0b013e3181a1084f
108. Raghav KP, Gonzalez-Angulo AM, Blumenschein GR. Role of HGF/MET axis in resistance of lung cancer to contemporary management. Transl Lung Cancer Res (2012) 1(3):179–93. doi: 10.3978/j.issn.2218-6751.2012.09.04
109. Califano R, Landi L, Cappuzzo F. Prognostic and predictive value of K-RAS mutations in non-small cell lung cancer. Drugs. (2012) 72(1):28–36. doi: 10.2165/1163012-S0-000000000-00000
110. Wang M, Kern AM, Hülskötter M, Greninger P, Singh A, Pan YF, et al. EGFR-mediated chromatin condensation protects KRAS-mutant cancer cells against ionizing radiation. Cancer Res (2014) 74(10):2825–34. doi: 10.1158/0008-5472.CAN-13-3157
111. Hu JF, Zhang ZG, Zhao L, Li L, Zuo W, Han L. High expression of RAD51 promotes DNA damage repair and survival in KRAS-mutant lung cancer cells. BMB Rep (2019) 52(2):151–6. doi: 10.5483/BMBRep.2019.52.2.213
112. Wang M, Han J, Marcar L, Black J, Liu Q, Li XY, et al. Radiation resistance in KRAS-mutated lung cancer is enabled by stem-like properties mediated by an osteopontin-EGFR pathway. Cancer Res (2017) 77(8):2018–28. doi: 10.1158/0008-5472.CAN-16-0808
113. Dempke WC, Reck M. KEAP1/NRF2 (NFE2L2) mutations in NSCLC-fuel for a superresistant phenotype? Lung Cancer. (2021) 159(2021):10–7. doi: 10.1016/j.lungcan.2021.07.006
114. Jeong Y, Hoang NT, Lovejoy A, Stehr H, Newman AM, Gentles AJ, et al. Role of KEAP1/NRF2 and TP53 mutations in lung squamous cell carcinoma development and radiotherapy rresponse prediction. Cancer Discovery. (2017) 7(1):86–101. doi: 10.1158/2159-8290.CD-16-0127
115. Pillai R, Hayashi M, Zavitsanou AM, Papagiannakopoulos T. NRF2: KEAPing tumors protected. Cancer Discovery (2022) 12(3):625–43. doi: 10.1158/2159-8290.CD-21-0922
116. Lee SL, Ryu H, Son AR, Seo B, Kim J, Jung SY, et al. TGF-β and hypoxia/reoxygenation promote radioresistance of A549 lung cancer cells through activation of Nrf2 and EGFR. Oxid Med Cell Longev (2016) 2016:6823471. doi: 10.1155/2016/6823471
117. Liu YQ, Gu W. p53 in ferroptosis regulation: The new weapon for the old guardian. Cell Death Differ (2022) 29(5):895–910. doi: 10.1038/s41418-022-00943-y
118. Bieging K, Mello S, Attardi L. Unravelling mechanisms of p53-mediated tumour suppression. Nat Rev Cancer. (2014) 14(5):359–70. doi: 10.1038/nrc3711
119. Moretti L, Li B, Kim KW, Chen HD, Lu B. AT-101, a pan-Bcl-2 inhibitor, leads to radiosensitization of non-small cell lung cancer. J Thorac Oncol (2010) 5(5):680–7. doi: 10.1097/JTO.0b013e3181d6e08e
120. Tu H, Costa M. XIAP's profile in human cancer. Biomolecules. (2020) 10(11):1493. doi: 10.3390/biom10111493
121. Sato Y, Yoshino H, Kazama Y, Kashiwakura I. Involvement of caspase-8 in apoptosis enhancement by cotreatment with retinoic acid-inducible gene-i-like receptor agonist and ionizing radiation in human non-small cell lung cancer. Mol Med Rep (2018) 18(6):5286–94. doi: 10.3892/mmr.2018.9536
122. Lu B, Mu Y, Cao C, Zeng FH, Schneider S, Tan JH, et al. Survivin as a therapeutic target for radiation sensitization in lung cancer. Cancer Res (2004) 64(8):2840–5. doi: 10.1158/0008-5472.can-03-3547
123. Sun H, Du Y, Yao M, Wang Q, Ji K, Du L, et al. cIAP1/2 are involved in the radiosensitizing effect of birinapant on NSCLC cell line in vitro. J Cell Mol Med (2021) 25(13):6125–36. doi: 10.1111/jcmm.16526
124. Yin H, Ma JQ, Chen L, Piao SQ, Zhang Y, Zhang SL, et al. MiR-99a enhances the radiation sensitivity of non-small cell lung cancer by targeting mTOR. Cell Physiol Biochem (2018) 46(2):471–81. doi: 10.1159/000488615
125. Lee HC, Her NG, Kang D, Jung SH, Shin J, Lee M, et al. Radiation-inducible miR-770-5p sensitizes tumors to radiation through direct targeting of PDZ-binding kinase. Cell Death Dis (2017) 8(3):e2693. doi: 10.1038/cddis.2017.116
126. Yuan Y, Liao H, Pu Q, Ke XX, Hu XT, Ma YF, et al. miR-410 induces both epithelial-mesenchymal transition and radioresistance through activation of the PI3K/mTOR pathway in non-small cell lung cancer. Signal Transduct Target Ther (2020) 5(1):85. doi: 10.1038/s41392-020-0182-2
127. Tang YT, Cui YY, Li ZP, Jiao ZQ, Zhang Y, He Y, et al. Radiation-induced miR-208a increases the proliferation and radioresistance by targeting p21 in human lung cancer cells. J Exp Clin Cancer Res (2016) 35:7. doi: 10.1186/s13046-016-0285-3
128. Clarke MF, Fuller M. Stem cells and cancer: Two faces of eve. Cell. (2006) 124(6):1111–5. doi: 10.1016/j.cell.2006.03.011
129. Schulz A, Meyer F, Dubrovska A, Borgmann K. Cancer stem cells and radioresistance: DNA repair and beyond. Cancers (Basel). (2019) 11(6):862. doi: 10.3390/cancers11060862
130. Geng YY, Amante JJ, Goel HL, Zhang XZ, Walker MR, Luther DC, et al. Differentiation of cancer stem cells through nanoparticle surface engineering. ACS Nano. (2020) 14(11):15276–85. doi: 10.1021/acsnano.0c05589
131. Paul R, Dorsey JF, Fan Y. Cell plasticity, senescence, and quiescence in cancer stem cells: Biological and therapeutic implications. Pharmacol Ther (2022) 231:107985. doi: 10.1016/j.pharmthera.2021.107985
132. Jang YY, Sharkis SJ. A low level of reactive oxygen species selects for primitive hematopoietic stem cells that may reside in the low-oxygenic niche. Blood. (2007) 110(8):3056–63. doi: 10.1182/blood-2007-05-087759
133. Lendeckel U, Wolke C. Redox-regulation in cancer stem cells. Biomedicines (2022) 10(10):2413. doi: 10.3390/biomedicines10102413
134. Ryoo I, Lee S, Kwak MK. Redox modulating NRF2: A potential mediator of cancer stem cell resistance. Oxid Med Cell Longev (2016) 2016:2428153. doi: 10.1155/2016/2428153
135. Chang L, Graham P, Hao JL, Ni J, Deng JL, Bucci J, et al. Cancer stem cells and signaling pathways in radioresistance. Oncotarget. (2016) 7(10):11002–17. doi: 10.18632/oncotarget.6760
136. Bao SD, Wu QL, McLendon RE, Hao YL, Shi Q, Hjelmeland AB, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. (2006) 444(7120):756–60. doi: 10.1038/nature05236
137. Desai A, Webb B, Gerson SL. CD133+ cells contribute to radioresistance via altered regulation of DNA repair genes in human lung cancer cells. Radiother Oncol (2014) 110(3):538–45. doi: 10.1016/j.radonc.2013.10.040
138. Moore N, Lyle S. Quiescent, slow-cycling stem cell populations in cancer: a review of the evidence and discussion of significance. J Oncol (2011) 2011:396076. doi: 10.1155/2011/396076
139. Krause M, Yaromina A, Eicheler W, Koch U, Baumann M. Cancer stem cells: targets and potential biomarkers for radiotherapy. Clin Cancer Res (2011) 17(23):7224–9. doi: 10.1158/1078-0432.CCR-10-2639
140. Baumann M, Krause M. CD44: a cancer stem cell-related biomarker with predictive potential for radiotherapy. Clin Cancer Res (2010) 16(21):5091–3. doi: 10.1158/1078-0432.CCR-10-2244
141. Hu B, Ma YY, Yang Y, Zhang LJ, Han HB, Chen JF. CD44 promotes cell proliferation in non-small cell lung cancer. Oncol Lett (2018) 15(4):5627–33. doi: 10.3892/ol.2018.8051
142. Kong T, Ahn R, Yang KN, Zhu XB, Fu Z, Morin G, et al. CD44 promotes PD-L1 expression and its tumor-intrinsic function in breast and lung cancers. Cancer Res (2020) 80(3):444–57. doi: 10.1158/0008-5472.CAN-19-1108
143. Bayer C, Shi K, Astner ST, Maftei CA, Vaupel P. Acute versus chronic hypoxia: why a simplified classification is simply not enough. Int J Radiat Oncol Biol Phys (2011) 80(4):965–8. doi: 10.1016/j.ijrobp.2011.02.049
144. Telarovic I, Wenger RH, Pruschy M. Interfering with tumor hypoxia for radiotherapy optimization. J Exp Clin Cancer Res (2021) 40(1):197. doi: 10.1186/s13046-021-02000-x
145. Sun JC, He F, Yi W, Wan MH, Li R, Wei X, et al. High expression of hif-2α and its anti-radiotherapy effect in lung cancer stem cells. Genet Mol Res (2015) 14(4):18110–20. doi: 10.4238/2015.December.22.37
146. Wang Y, Roche O, Yan MS, Finak G, Evans AJ, Metcalf JL, et al. Regulation of endocytosis via the oxygen-sensing pathway. Nat Med (2009) 15(3):319–24. doi: 10.1038/nm.1922
147. Downes NL, Laham-Karam N, Kaikkonen MU, Ylä-Herttuala S. Differential but complementary HIF1α and HIF2α transcriptional regulation. Mol Ther (2018) 26(7):1735–45. doi: 10.1016/j.ymthe.2018.05.004
148. Gonzalez FJ, Xie C, Jiang CT. The role of hypoxia-inducible factors in metabolic diseases. Nat Rev Endocrinol (2018) 15(1):21–32. doi: 10.1038/s41574-018-0096-z
149. Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. (2003) 3(10):721–32. doi: 10.1038/nrc1187
150. Uniacke J, Holterman CE, Lachance G, Franovic A, Jacob MD, Fabian MR, et al. An oxygen-regulated switch in the protein synthesis machinery. Nature. (2012) 486(7401):126–9. doi: 10.1038/nature11055
151. Choueiri TK, Kaelin WG. Targeting the HIF2-VEGF axis in renal cell carcinoma. Nat Med (2020) 26(10):1519–30. doi: 10.1038/s41591-020-1093-z
152. Moeller BJ, Cao YT, Li CY, Dewhirst MW. Radiation activates HIF-1 to regulate vascular radiosensitivity in tumors: Role of reoxygenation, free radicals, and stress granules. Cancer Cell (2004) 5(5):429–41. doi: 10.1016/s1535-6108(04)00115-1
153. Moreno RE, Groot AJ, Yaromina A, Hendrickx TC, Barbeau LM, Giuranno L, et al. HIF-1α and HIF-2α differently regulate the radiation sensitivity of NSCLC cells. Cells. (2019) 8(1):45. doi: 10.3390/cells8010045
154. Grosso S, Doyen J, Parks SK, Bertero T, Paye A, Cardinaud B, et al. MiR-210 promotes a hypoxic phenotype and increases radioresistance in human lung cancer cell lines. Cell Death Dis (2013) 4(3):e544. doi: 10.1038/cddis.2013.71
155. Xu LM, Yu H, Yuan YJ, Zhang J, Ma Y, Cao XC, et al. Overcoming of radioresistance in non-small cell lung cancer by microRNA-320a through HIF1α-suppression mediated methylation of PTEN. Front Cell Dev Biol (2020) 8:553733. doi: 10.3389/fcell.2020.553733
156. Isla D, De Las PR, Insa A, Marsé R, Martínez-Banaclocha N, Mut P, et al. Oral vinorelbine versus etoposide with cisplatin and chemo-radiation as treatment in patients with stage III non-small cell lung cancer: A randomized phase II (RENO study). Lung Cancer. (2019) 135:161–8:135. doi: 10.1016/j.lungcan.2018.11.041
157. Shaverdian N, Lisberg AE, Bornazyan K, Veruttipong D, Goldman JW, Formenti SC, et al. Previous radiotherapy and the clinical activity and toxicity of pembrolizumab in the treatment of non-small-cell lung cancer: A secondary analysis of the KEYNOTE-001 phase 1 trial. Lancet Oncol (2017) 18(7):895–903. doi: 10.1016/S1470-2045(17)30380-7
158. Kong Y, Xu C, Sun X, Sun H, Zhao X, He N, et al. BLM helicase inhibition synergizes with PARP inhibition to improve the radiosensitivity of olaparib resistant non-small cell lung cancer cells by inhibiting homologous recombination repair. Cancer Biol Med (2021) 19(8):1150–71. doi: 10.20892/j.issn.2095-3941.2021.0178
159. Zhang N, Gao Y, Zeng Z, Luo Y, Xie C. PARP inhibitor niraparib as a radiosensitizer promotes antitumor immunity of radiotherapy in EGFR-mutated non-small cell lung cancer. Clin Transl Oncol (2021) 23(9):1827–37. doi: 10.1007/s12094-021-02591-z
160. Tang S, Li Z, Yang L, Shen L, Wang Y. A potential new role of ATM inhibitor in radiotherapy: Suppressing ionizing radiation-activated EGFR. Int J Radiat Biol (2020) 96(4):461–8. doi: 10.1080/09553002.2020.1707325
161. Fok JHL, Ramos-Montoya A, Vazquez-Chantada M, Wijnhoven PWG, Follia V, James N, et al. AZD7648 is a potent and selective DNA-PK inhibitor that enhances radiation, chemotherapy and olaparib activity. Nat Commun (2019) 10(1):5065. doi: 10.1038/s41467-019-12836-9
162. Ding X, Cheng J, Pang Q, Wei X, Zhang X, Wang P, et al. BIBR1532, a selective telomerase inhibitor, enhances radiosensitivity of non-small cell lung cancer through increasing telomere dysfunction and ATM/CHK1 inhibition. Int J Radiat Oncol Biol Phys (2019) 105(4):861–74. doi: 10.1016/j.ijrobp.2019.08.009
163. Wang J, Wang Y, Mei H, Yin Z, Geng Y, Zhang T, et al. The BET bromodomain inhibitor JQ1 radiosensitizes non-small cell lung cancer cells by upregulating p21. Cancer Lett (2017) 391:141–51. doi: 10.1016/j.canlet.2017.01.031
164. Fernández-Aroca DM, Roche O, Sabater S, Pascual-Serra R, Ortega-Muelas M, Sánchez Pérez I, et al. P53 pathway is a major determinant in the radiosensitizing effect of palbociclib: Implication in cancer therapy. Cancer Lett (2019) 451:23–33. doi: 10.1016/j.canlet.2019.02.049
165. Naz S, Sowers A, Choudhuri R, Wissler M, Gamson J, Mathias A, et al. Abemaciclib, a selective CDK4/6 inhibitor enhances the radiosensitivity of non-small cell lung cancer in vitro and in vivo. Clin Cancer Res (2018) 24(16):3994–4005. doi: 10.1158/1078-0432.CCR-17-3575
166. Wang R, Peng S, Zhang X, Wu Z, Duan H, Yuan Y, et al. Inhibition of NF-κB improves sensitivity to irradiation and EGFR-TKIs and decreases irradiation-induced lung toxicity. Int J Cancer. (2019) 144:200–9. doi: 10.1002/ijc.31907
167. Du S, Bouquet S, Lo CH, Pellicciotta I, Bolourchi S, Parry R, et al. Attenuation of the DNA damage response by transforming growth factor-β inhibitors enhances radiation sensitivity of non–small-cell lung cancer cells in vitro and in vivo. Int J Radiat Oncol (2015) 91(1):91–9. doi: 10.1016/j.ijrobp.2014.09.026
168. Sun Y, Moretti L, Giacalone NJ, Schleicher S, Speirs CK, Carbone DP, et al. Inhibition of JAK2 signaling by TG101209 enhances radiotherapy in lung cancer models. J Thorac Oncol (2011) 6(4):699–706. doi: 10.1097/JTO.0b013e31820d9d11
169. Gong X, Li X, Jiang T, Xie H, Zhu Z, Zhou F, et al. Combined radiotherapy and anti-PD-L1 antibody synergistically enhances antitumor effect in non-small cell lung cancer. J Thorac Oncol (2017) 12(7):1085–97. doi: 10.1016/j.jtho.2017.04.014
170. Bozorgmehr F, Hommertgen A, Krisam J, Lasitschka F, Kuon J, Maenz M, et al. Fostering efficacy of anti-PD-1-treatment: Nivolumab plus radiotherapy in advanced non-small cell lung cancer - study protocol of the FORCE trial. BMC Cancer. (2019) 19(1):1074. doi: 10.1186/s12885-019-6205-0
171. Welsh J, Menon H, Chen D, Verma V, Heymach JV. Pembrolizumab with or without radiation therapy for metastatic non-small cell lung cancer: A randomized phase I/II trial. J Immunother Cancer. (2020) 8(2):e001001. doi: 10.1136/jitc-2020-001001
172. Formenti SC, Rudqvist NP, Golden E, Cooper B, Wennerberg E, Lhuillier C, et al. Radiotherapy induces responses of lung cancer to CTLA-4 blockade. Nat Med (2018) 24(12):1845–51. doi: 10.1038/s41591-018-0232-2
173. Zheng LP, Wang Y, Xu Z, Yang Q, Zhu G, Liao XY, et al. Concurrent EGFR-TKI and thoracic radiotherapy as first-line treatment for stage IV non-small cell lung cancer harboring EGFR active mutations. Oncologist. (2019) 24(8):1031–e612. doi: 10.1634/theoncologist.2019-0285
174. Francis DM, Huang S, Armstrong EA, Werner LR, Hullett C, Li C, et al. Pan-HER inhibitor augments radiation response in human lung and head and neck cancer models. Clin Cancer Res (2016) 22(3):633–43. doi: 10.1158/1078-0432.CCR-15-1664
175. Hu S, Fu W, Li T, Yuan Q, Wang F, Lv G, et al. Antagonism of EGFR and notch limits resistance to EGFR inhibitors and radiation by decreasing tumor-initiating cell frequency. Sci Transl Med (2017) 9(380):1–15. doi: 10.1126/scitranslmed.aag0339
176. Zhang P, Song E, Jiang M, Song Y. Celecoxib and afatinib synergistic enhance radiotherapy sensitivity on human non-small cell lung cancer A549 cells. Int J Radiat Biol (2021) 97(2):170–8. doi: 10.1080/09553002.2021.1846817
177. Park D, Magis AT, Li R, Owonikoko TK, Sica GL, Sun SY, et al. Novel small-molecule inhibitors of bcl-XL to treat lung cancer. Cancer Res (2013) 73:5485–96. doi: 10.1158/0008-5472.CAN-12-2272
178. Lee JM, Kim HS, Kim A, Chang YS, Lee JG, Cho J, et al. ABT-737, a BH3 mimetic, enhances the therapeutic effects of ionizing radiation in K-ras mutant non-small cell lung cancer preclinical model. Yonsei Med J (2022) 63(1):16–25. doi: 10.3349/ymj.2022.63.1.16
179. Park D, Anisuzzaman AS, Magis AT, Chen G, Xie M, Zhang G, et al. Discovery of small molecule bak activator for lung cancer therapy. Theranostics. (2021) 11:8500–16. doi: 10.7150/thno.60349
180. Ritter V, Krautter F, Klein D, Jendrossek V, Rudner J. Bcl-2/Bcl-xL inhibitor ABT-263 overcomes hypoxia-driven radioresistence and improves radiotherapy. Cell Death Dis (2021) 12(7):694. doi: 10.1038/s41419-021-03971-7
181. Jiang S, Zhou Y, Zou L, Chu L, Chu X, Ni J, et al. Low- dose apatinib promotes vascular normalization and hypoxia reduction and sensitizes radiotherapy in lung cancer. Cancer Med (2022) 00:1–12. doi: 10.1002/cam4.5113
182. Zhai Y, Ma H, Hui Z, Zhao L, Li D, Liang J, et al. HELPER study: A phase II trial of continuous infusion of endostar combined with concurrent etoposide plus cisplatin and radiotherapy for treatment of unresectable stage III non-small-cell lung cancer. Radiother Oncol (2019) 131:27–34. doi: 10.1016/j.radonc.2018.10.032
183. Kong FM, Hayman JA, Griffith KA, Kalemkerian GP, Arenberg D, Lyons S, et al. Final toxicity results of a radiation-dose escalation study in patients with non-small-cell lung cancer (NSCLC): predictors for radiation pneumonitis and fibrosis. Int J Radiat Oncol Biol Phys (2006) 65(4):1075–86. doi: 10.1016/j.ijrobp.2006.01.051
184. Liu W, Yang BB, Yang L, Kaur J, Jessop C, Fadhil R, et al. Therapeutic effects of ten commonly used Chinese herbs and their bioactive compounds on cancers. Evid Based Complement Alternat Med (2019) 2019:6057837. doi: 10.1155/2019/6057837
185. Yang Y, Li N, Wang TM, Di L. Natural products with activity against lung cancer: A review focusing on the tumor microenvironment. Int J Mol Sci (2021) 22(19):10827. doi: 10.3390/ijms221910827
186. Li SG, Chen HY, Ou-Yang CS, Wang XX, Yang ZJ, Tong Y, et al. The efficacy of Chinese herbal medicine as an adjunctive therapy for advanced non-small cell lung cancer: a systematic review and meta-analysis. PloS One (2013) 8(2):e57604. doi: 10.1371/journal.pone.0057604
187. Balakrishnan B, Liang Q, Fenix K, Tamang B, Hauben E, Ma L, et al. Combining the anticancer and immunomodulatory effects of Astragalus and shiitake as an integrated therapeutic approach. Nutrients. (2021) 13(8):2564. doi: 10.3390/nu13082564
188. Cao A, He H, Wang Q, Li L, An Y, Zhou X. Evidence of astragalus injection combined platinum-based chemotherapy in advanced nonsmall cell lung cancer patients: A systematic review and meta-analysis. Medicine. (2019) 98(11):e14798. doi: 10.1097/MD.0000000000014798
189. Zhang YM, Zhang LY, Zhou H, Li YY, Wei KX, Li CH, et al. Astragalus polysaccharide inhibits radiation-induced bystander effects by regulating apoptosis in bone mesenchymal stem cells (BMSCs). Cell Cycle (2020) 19(22):3195–207. doi: 10.1080/15384101.2020.1838793
190. Zhang L, Luo Y, Lu Z, He J, Wang L, Zhang L, et al. Astragalus polysaccharide inhibits ionizing radiation-induced bystander effects by regulating MAPK/NF-kB signaling pathway in bone mesenchymal stem cells (BMSCs). Med Sci Monit (2018) 24:4649–58. doi: 10.12659/MSM.909153
191. Zhang L, Yong W, Wang L, Zhang L, Yongqi L. Astragalus polysaccharide eases g1 phase-correlative bystander effects through mediation of TGF-βR/MAPK/ROS signal pathway after carbon ion irradiation in BMSCs. Am J Chin Med (2019) 47(3):595–612. doi: 10.1142/S0192415X19500319
192. Zhang LY, Zhou T, Zhang YM, Xu XM, Li YY, Wei KX, et al. Guiqi baizhu decoction alleviates radiation inflammation in rats by modulating the composition of the gut microbiota. Evid Based Complement Alternat Med (2020) 2020(undefined):9017854. doi: 10.1155/2020/9017854
193. Li YY, Zhang LY, Zhang YM, Miao ZM, Liu ZW, Zhou GC, et al. Potential molecular mechanism of guiqi baizhu decoction in radiation-induced intestinal edema by regulating HIF-1a, AQP4 and Na/K-ATPase. Phytomedicine (2022) 107(undefined):154445. doi: 10.1016/j.phymed.2022.154445
194. Li L, Jin XJ, Li JW, Li CH, Zhou SY, Li JJ, et al. Systematic insight into the active constituents and mechanism of guiqi baizhu for the treatment of gastric cancer. Cancer Sci (2021) 112(5):1772–84. doi: 10.1111/cas.14851
195. Jabbour SK, Berman AT, Decker RH, Lin Y, Feigenberg SJ, Gettinger SN, et al. Phase 1 trial of pembrolizumab administered concurrently with chemoradiotherapy for locally advanced non-small cell lung cancer: A nonrandomized controlled trial. JAMA Oncol (2020) 6(6):848–55. doi: 10.1001/jamaoncol.2019.6731
196. Descourt R, Vergnenegre A, Barlesi F, Lena H, Fournel P, Falchero L, et al. Oral vinorelbine and cisplatin with concurrent radiotherapy after induction chemotherapy with cisplatin and docetaxel for patients with locally advanced non-small cell lung cancer: the GFPC 05-03 study. J Thorac Oncol (2011) 6(2):351–7. doi: 10.1097/JTO.0b013e318200f47e
197. Xiao Z, Liang R, Wang CQ, Xu S, Li N, He Y, et al. Can aidi injection alleviate the toxicity and improve the clinical efficacy of radiotherapy in lung cancer: A meta-analysis of 16 randomized controlled trials following the PRISMA guidelines. Med (Baltimore). (2016) 95(35):e4517. doi: 10.1097/MD.0000000000004517
198. Zhang H, Jiang H, Hu X, Jia Z. Aidi injection combined with radiation in the treatment of non-small cell lung cancer: A meta-analysis evaluation the efficacy and side effects. J Cancer Res Ther (2015) 00:C118–21. doi: 10.4103/0973-1482.163864
199. Dong XR, Wang JN, Liu L, Chen X, Chen MS, Chen J, et al. Modulation of radiation-induced tumour necrosis factor-α and transforming growth factor β1 expression in the lung tissue by shengqi fuzheng injection. Mol Med Rep (2010) 3(4):621–7. doi: 10.3892/mmr_00000306
200. Wang S, Lian X, Sun M, Luo L, Guo L. Efficacy of compound kushen injection plus radiotherapy on nonsmall-cell lungcancer: A systematic review and meta-analysis. J Cancer Res Ther (2016) 12(4):1298–306. doi: 10.4103/0973-1482.199538
201. Zhou H, Pan P, Zhao Q, Liu W, Sun Y, Wang J, et al. Overcoming basal autophagy, kangai injection enhances cisplatin cytotoxicity by regulating FOXO3a-dependent autophagic cell death and apoptosis in human lung adenocarcinoma A549/DDP cells. BioMed Res Int (2022) 2022:6022981. doi: 10.1155/2022/6022981
202. He H, Zhou X, Wang Q, Zhao Y. Does the couse of astragalus-containing chinese herbal prescriptions and radiotherapy benefit to non-small-cell lung cancer treatment: a meta-analysis of randomized trials. Evid Based Complement Alternat Med (2013) 2013:426207. doi: 10.1155/2013/426207
203. Jiang X, Hidru TH, Zhang Z, Bai Y, Kong L, Li X. Evidence of elemene injection combined radiotherapy in lung cancer treatment among patients with brain metastases: A systematic review and meta-analysis. Med (Baltimore). (2017) 96(21):e6963. doi: 10.1097/MD.0000000000006963
204. Yu H, Yang Y, Jiang T, Zhang X, Zhao Y, Pang G, et al. Ganoderma lucidum effective radiotherapy in tumor assisted by polysaccharide-conjugated bismuth sulfide nanoparticles through radiosensitization and dendritic cell activation. ACS Appl Mater Interfaces. (2019) 11(31):27536–47. doi: 10.1021/acsami.9b07804
205. Lu T, Yu J, Gao R, Wang J, Wang H, Wang X, et al. Chinese Patent medicine kanglaite injection for non-small-cell lung cancer: An overview of systematic reviews. J Ethnopharmacol (2022) 302:115814. doi: 10.1016/j.jep.2022.115814
206. Kong P, Yu KN, Yang M, Almahi WA, Nie L, Chen G, et al. Micheliolide enhances radiosensitivities of p53-deficient non-small-cell lung cancer via promoting HIF-1α degradation. Int J Mol Sci (2020) 21(9):3392. doi: 10.3390/ijms21093392
207. Woo JK, Kang JH, Shin DY, Park SH, Kang K, Nho CW, et al. Daurinol enhances the efficacy of radiotherapy in lung cancer via suppression of aurora kinase A/B expression. Mol Cancer Ther (2015) 14(7):1693–704. doi: 10.1158/1535-7163.MCT-14-0960
208. Du Y, Jia C, Liu Y, Li Y, Wang J, Sun K. Isorhamnetin enhances the radiosensitivity of A549 cells through interleukin-13 and the NF-κB signaling pathway. Front Pharmacol (2020) 11:610772. doi: 10.3389/fphar.2020.610772
209. Hsu HS, Huang PI, Chang YL, Tzao C, Chen YW, Shih HC, et al. CucurbitacinI inhibits tumorigenic ability and enhances radiochemosensitivity in nonsmall cell lung cancer-derived CD133-positive cells. Cancer. (2011) 117(13):2970–85. doi: 10.1002/cncr.25869
210. Phiboonchaiyanan PP, Petpiroon N, Sritularak B, Chanvorachote P. Phoyunnanin e induces apoptosis of non-small cell lung cancer cells via p53 activation and down-regulation of survivin. Anticancer Res (2018) 38(11):6281–90. doi: 10.21873/anticanres.12984
211. JiH L, KJ C, WD S, SY J, Kim M, BW L, et al. Enhancement of radiation sensitivity in lung cancer cells by celastrol is mediated by inhibition of Hsp90. Int J Mol Med (2011) 27(3):441–6. doi: 10.3892/ijmm.2011.601
212. Ye M, Zhang J, Zhang J, Miao Q, Yao L, Zhang J. Curcumin promotes apoptosis by activating the p53-miR-192-5p/215-XIAP pathway in non-small cell lung cancer. Cancer Lett (2015) 357(1):196–205. doi: 10.1016/j.canlet.2014.11.028
Keywords: non-small cell lung cancer, radiotherapy, radioresistance mechanisms, toxicity, Traditional Chinese Medicine
Citation: Zhou T, Zhang L, He J, Miao Z, Li Y, Zhang Y, Liu Z, Zhang S, Chen Y, Zhou G and Liu Y (2023) Review: Mechanisms and perspective treatment of radioresistance in non-small cell lung cancer. Front. Immunol. 14:1133899. doi: 10.3389/fimmu.2023.1133899
Received: 29 December 2022; Accepted: 31 January 2023;
Published: 14 February 2023.
Edited by:
Qiang Tong, Renmin Hospital of Wuhan University, ChinaReviewed by:
Deyong Jia, University of Washington, United StatesCopyright © 2023 Zhou, Zhang, He, Miao, Li, Zhang, Liu, Zhang, Chen, Zhou and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yong-Qi Liu, bHlxQGdzenkuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.