
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Immunol. , 07 February 2023
Sec. Inflammation
Volume 14 - 2023 | https://doi.org/10.3389/fimmu.2023.1130214
This article is part of the Research Topic Immune Regulation in Sepsis View all 14 articles
Sepsis, a heterogeneous clinical syndrome, features a systemic inflammatory response to tissue injury or infection, followed by a state of reduced immune responsiveness. Measurable alterations occur in both the innate and adaptive immune systems. Immunoparalysis, an immunosuppressed state, associates with worsened outcomes, including multiple organ dysfunction syndrome, secondary infections, and increased mortality. Multiple immune markers to identify sepsis immunoparalysis have been proposed, and some might offer clinical utility. Sepsis immunoparalysis is characterized by reduced lymphocyte numbers and downregulation of class II human leukocyte antigens (HLA) on innate immune monocytes. Class II HLA proteins present peptide antigens for recognition by and activation of antigen-specific T lymphocytes. One monocyte class II protein, mHLA-DR, can be measured by flow cytometry. Downregulated mHLA-DR indicates reduced monocyte responsiveness, as measured by ex-vivo cytokine production in response to endotoxin stimulation. Our literature survey reveals low mHLA-DR expression on peripheral blood monocytes correlates with increased risks for infection and death. For mHLA-DR, 15,000 antibodies/cell appears clinically acceptable as the lower limit of immunocompetence. Values less than 15,000 antibodies/cell are correlated with sepsis severity; and values at or less than 8000 antibodies/cell are identified as severe immunoparalysis. Several experimental immunotherapies have been evaluated for reversal of sepsis immunoparalysis. In particular, sargramostim, a recombinant human granulocyte-macrophage colony-stimulating factor (rhu GM-CSF), has demonstrated clinical benefit by reducing hospitalization duration and lowering secondary infection risk. Lowered infection risk correlates with increased mHLA-DR expression on peripheral blood monocytes in these patients. Although mHLA-DR has shown promising utility for identifying sepsis immunoparalysis, absence of a standardized, analytically validated method has thus far prevented widespread adoption. A clinically useful approach for patient inclusion and identification of clinically correlated output parameters could address the persistent high unmet medical need for effective targeted therapies in sepsis.
Sepsis, a heterogeneous clinical syndrome, reflects a pathophysiologic state of robust systemic inflammatory response, typically to infection (1–4). This inflammatory response leads to biochemical and physiologic abnormalities that in some patients progress to multiple organ dysfunction syndrome (MODS) and death. Sepsis outcomes have improved over time with advances in antibiotic therapy, fluid/pressor therapy, and dysfunctional organ support. Although most patients recover, sepsis remains a primary cause of intensive care unit (ICU) deaths with mortality at about 26% (1, 5, 6). In the United States (US), an estimated 1.7 million adult sepsis cases are diagnosed annually, leading to more than 350,000 deaths each year (7). Globally, 49 million sepsis cases in 2017 led to 11 million deaths (8). Incidence is highest in the elderly and very young. With high morbidity, mortality, and associated costs, sepsis remains a serious, life-threatening disease with persistent high unmet medical need (9).
Clinical sepsis typically presents with fever, low blood pressure, elevated heart rate, and elevated white cell count (3, 10, 11). While these signs are non-specific, they result from systemic innate immune cell activation due to infectious agents (bacterial, viral, or fungal) or noninfectious etiologies, such as: trauma; burns; surgery; pancreatitis; and cardiac, kidney, or liver injury (1, 4). Regardless of underlying cause, sepsis progression can lead to shock, organ dysfunction, and death (3, 12, 13). In this setting, a constellation of findings support diagnosis, including: clinical, lab, radiologic, physiologic, and microbiologic data (10, 11). Nonetheless, knowledge around sepsis and septic shock continues to advance as we learn more about immunological interactions of innate and adaptive immune responses to infection (10, 11, 14–17).
Over recent decades, molecular and cellular studies have sought to categorize sepsis into endotypes that stratify patient risk and identify therapeutic options (18). While antimicrobial therapy is recommended for all patients with sepsis, level of supportive care varies for those with mild vs severe sepsis (19–21). For patients with mild sepsis, fluid therapy, metabolic support, and corticosteroids may be sufficient. In severe sepsis, organ dysfunction necessitates additional supportive care, such as ventilation, vasopressors, and blood product transfusions. Identification of patient subsets might enable effective targeting of new therapies, either to inhibit a disease driver or to correct a deficiency (22). Similarly, selection of patients with elevated risk based on host characteristics or responses might enable targeting study therapies to those in greatest need (23). Despite progress in identifying sepsis endotypes, challenges persist in their clinical validation, as well as their implementation to improve outcomes (24, 25).
One prominent model of sepsis pathophysiology describes 2 opposing states of immune dysregulation (26). In this model, a systemic inflammatory response syndrome (SIRS) induces a subsequent compensatory anti-inflammatory response syndrome (CARS). CARS is associated with an increased risk for secondary infections, shock, and organ dysfunction and increased mortality (1, 3, 27). While CARS is clinically occult, hyporesponsive innate and adaptive immune cells have been identified (3, 4, 28).
Severe CARS is also known as immunoparalysis (IP) (3, 26). Sepsis IP has been described to feature dysfunctional monocytes, immune cell depletion, and emergence of regulatory T cells (1, 29, 30). Also, sepsis IP associates with MODS, nosocomial infections, longer ICU hospitalization, and increased mortality (3, 4, 26, 29, 31–33). Notably, MODS comprises impaired function in multiple visceral organs and is associated with high mortality (34).
Despite potential validity and utility of markers for sepsis IP, such as human leukocyte antigen-DR (HLA-DR), tumor necrosis factor (TNF)-α, or absolute lymphocyte counts (ALC), the CARS paradigm faces 2 fundamental challenges (3, 4, 28, 35). First, compensatory molecular or cellular anti-inflammatory mechanisms by which immune cells become hyporesponsive in CARS remain undefined. Second, no diagnostic criteria exist to identify CARS. Rather, tests for immunosuppression/IP focus on immune cell dysfunction alone, independently of causation (35).
We propose here a biologic model of sepsis IP. This model combines recent observations in myeloid cell biology with key features of sepsis immunology (3, 26, 31). In addition, it provides rationale for therapeutic use of sargramostim (Leukine®), a yeast-derived, glycosylated recombinant human (rhu) granulocyte-macrophage colony-stimulating factor (GM-CSF).
Mononuclear phagocytes (MNPs) include circulating blood monocytes, dispersed tissue-bound macrophages, and dendritic cells (DCs) that may be either circulating or tissue-bound (36). While macrophages may live for years, blood monocytes have a circulating half-life of only 2 to 3 days (36, 37). Also, while circulating monocytes can replace tissue-resident macrophages, turnover rate varies by organ system. Turnover is higher in barrier organs—for example, gut and dermis—than in other organs, such as heart, pancreas, liver, and central nervous system. Replacement may be hastened in any organ by a local inflammatory process that leads to monocyte influx.
Innate immune responses act rapidly as a first line of defense against invasive, infectious pathogens (1). Initially, neutrophils and monocytes recognize pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs). These interactions induce MNPs to release multiple cytokines, such as TNF-α, interleukin (IL)-1, and IL-6, that attract and activate other immune cells (1, 4). While neutrophils primarily kill microbes, MNPs kill microbes and, in addition, present their unique antigenic content to the adaptive immune system (4, 38).
MNPs link innate and adaptive immune systems by their ability to adopt either pro- or anti-inflammatory functions (4, 39). Pro-inflammatory functions eliminate infectious or injurious stimuli and activate antigen-specific helper T lymphocytes, whereas anti-inflammatory functions maintain homeostasis, conduct efferocytosis, and thereby control autoimmunity. Critically, MNPs express class II major histocompatibility complex (MHC) proteins that activate antigen-specific helper T lymphocytes and secrete cytokines to nourish and/or activate diverse cell types (Figure 1). More numerous neutrophils by contrast are primarily pro-inflammatory, live only for days after a 6- to 12-hour circulating half-life, and do not characteristically present foreign antigens to adaptive immune lymphocytes (43).
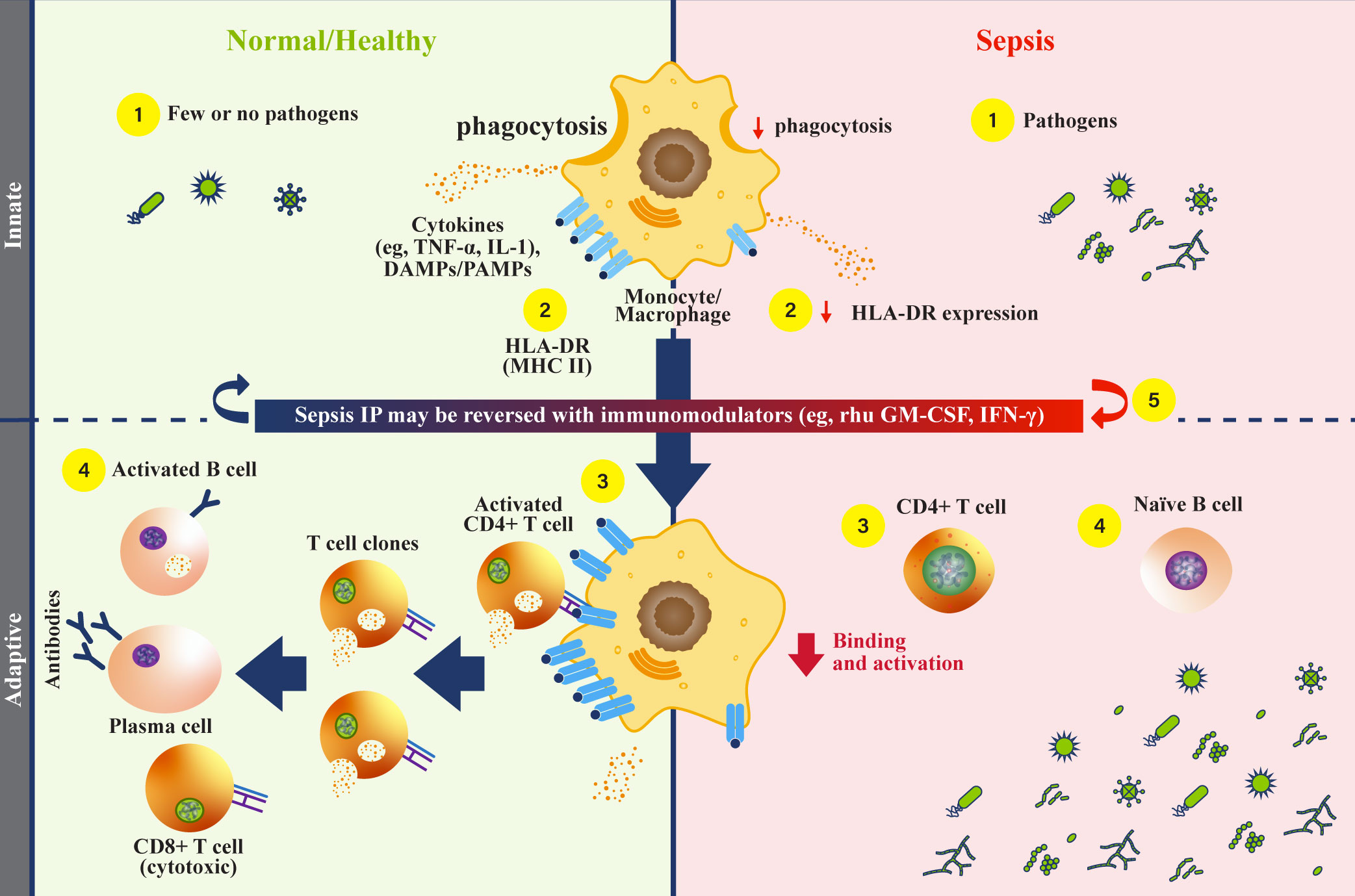
Figure 1 Monocytes and HLA-DR function during sepsis. (Normal/Healthy; left) (1) Innate immune cells respond to infectious pathogens by phagocytosis, cytokine secretion, and antigen presentation (1, 4). (2) Phagocytosed pathogens are broken down, then combined as peptides with class II major histocompatibility complex (MHC) (e.g., human leukocyte antigen-DR isotype [HLA-DR], and localized to the cell surface) (4, 40). (3) Peptide-MHC complexes on antigen presenting cells engage with CD4+ helper T cells to activate an adaptive immune response, triggering cytokine release (4, 40). (4) Activated CD4+ T helper cells undergo clonal expansion, activate CD8+ T cells, and mediate B cell activation (1, 26, 41). Activated B cells then differentiate into plasma cells that secrete antibodies, comprising a humoral response. (Sepsis IP; right) (1) Dysfunctional monocytes/macrophages demonstrate reduced pathogen phagocytosis, reduced antigen presentation, and variable cytokine profiles (4). (2) Dysfunctional monocytes/macrophages express less antigen-bound HLA-DR proteins, leading to reduced engagement with the adaptive immune system (4). (3) Without effective antigen presentation by monocytes/macrophages, CD4+ T cells are not activated, and adaptive immune responses are rendered ineffective in clearing pathogens (1). (4) Naïve B cells are not activated by CD4+ T cells, and antibody producing plasma cells are not generated. With an inadequate humoral immune response, pathogens survive and replicate (1, 42). (5) Recombinant human (rhu) granulocyte-macrophage colony-stimulating factor (GM-CSF) may restore monocyte/macrophage function (4). DAMP, damage-associated molecular patterns; GM-CSF, granulocyte-macrophage colony-stimulating factor; HLA-DR, human leukocyte antigen-DR isotype; IFN, interferon; IL, interleukin; IP, immunoparalysis; PAMP, pathogen-associated molecular patterns; MHC II, class II major histocompatibility complex; rhu, recombinant human; TNF, tumor necrosis factor.
The adaptive immune system comprises antigen-specific T lymphocytes that are cytotoxic, or are responsible for self-tolerance (T regulatory cells) as well as antibody-producing plasma cells that result from B cell differentiation (1, 3). Although initially slower to respond than the innate immune system, the adaptive immune system drives antigen-specific recognition and generates immunologic memory. Immunologic memory generates faster, stronger repeat immune responses against previously encountered antigens.
In sepsis, both innate and adaptive arms of the human immune system are altered (1). In addition, multiple cytokine levels are elevated, including GM-CSF. These cytokines drive proliferation of circulating innate immune cells, including neutrophils, monocytes, and eosinophils, by signaling through specific cell surface receptors (44, 45). For example, high affinity GM-CSF receptors are found principally on myeloid cells, including neutrophils, MNPs, and eosinophils.
Numerous cytokines, including GM-CSF, have pleiotropic effects that vary depending on local cytokine concentrations in the vicinity of specific cell surface receptors (44, 46–48). GM-CSF pleiotropism relies on higher order extracellular assembly of heterodimeric receptor chains, as well as 4 distinct intracellular signaling pathways, including: mitogen activated protein kinase (MAPK); nuclear factor kappa-B cells (NFĸB); phosphoinositide 3-kinase (PI3K); and signal transducer and activator of transcription 5 (STAT5). Such diversity explains GM-CSF’s capacity to generate survival, differentiation, activation, and/or proliferation signals, depending on cytokine concentration at the receptor level, as well as other local stimuli. At low GM-CSF concentrations, PI3K signaling leads to survival, whereas at high concentrations, PI3K, MAPK, and STAT5 signaling lead to survival and cell proliferation (47, 48). Correspondingly, both ligand and dose-specific effects on NFĸB signaling have been described in primary macrophages (49, 50), and such effects have been observed to influence epigenomic programming (51).
Recently, GM-CSF effects on MNP metabolism were revealed in mouse models with disrupted GM-CSF signaling (52). These models demonstrated a critical role of GM-CSF in maintaining mitochondrial structure and function, as well as fatty acid beta oxidation, tricarboxylic acid cycle activity, oxidative phosphorylation, and adenosine triphosphate (ATP) generation. These effects of GM-CSF on metabolic capacity enable MNPs to fulfill energy-intensive innate immune functions, including: respiratory burst generation, phagocytosis, antigen presentation, cytokine secretion, and efferocytosis (52–54). All these functions rely on metabolic energy and fail in its absence. By extension, metabolic capacity in tissue-bound macrophages throughout the body may be maintained by ongoing low-level and/or pulsatile GM-CSF expression. This activity aligns with known ongoing low-level yet plastic GM-CSF expression by diverse cell types, including endothelial, epithelial, and immune cells, as well as fibroblasts (2, 55).
We hypothesize that myeloid proliferation driven by high cytokine levels in sepsis leads to cell division that outpaces time and/or GM-CSF stimulation needed for maturation of cellular metabolic capacity. Thus, sustained high inflammatory cytokine secretion may counterintuitively result in degradation of metabolic capacity of newly formed MNPs to fulfill immune functions. Consequently, immature MNPs with insufficient metabolic capacity to support normal innate immune functions appear “immunosuppressive.” In support of this model, GM-CSF reverses monocyte hyporesponsiveness in multiple in-vitro systems (56–60). Multiple reports support that GM-CSF increases blood monocyte levels, upregulates monocyte responsiveness, and increases HLA-DR expression, which is known to enhance antigen presentation and adaptive immune responses (48, 54, 61, 62).
Numerous immune biomarkers have been assessed to seek prognostic and/or predictive markers for patient stratification and therapy in sepsis (12). Methods studied include: neutrophil respiratory burst in response to pathogen exposure; lymphocyte and monocyte counts; neutrophil-to-lymphocyte and monocyte-to-lymphocyte ratios; monocyte programmed death-ligand 1 (PD-L1) expression; IL-10; and transcriptomics, among others (26, 63–67). Most such methods have not been widely adopted due to challenges in analytic validity, clinical validity, and/or clinical utility. Methods with evidence of clinical validity include HLA-DR quantitation of blood monocytes, TNF-α release from peripheral blood cells after ex-vivo lipopolysaccharide (LPS) stimulation, and ALC (29, 30). Biological rationale, validation challenges, and clinical data for each of these 3 markers are summarized below.
The polymorphic MHC gene family in humans is on chromosome 6 and encodes multiple class II MHC proteins, including HLA-DP, HLA-DQ, and HLA-DR (68). Historically, these proteins were recognized as transplantation antigens, serving as targets for immune rejection of transplanted tissue. During infection, MNPs phagocytose pathogens that are then digested to yield foreign peptides that combine intracellularly with class II MHC proteins, such as HLA-DR (4, 69). Normally, monocytes and macrophages express HLA-DR levels ranging from 15,000 antibodies bound per cell (Ab/c) to as high as 60,000 Ab/c (70, 71); and a commonly used lower limit of HLA-DR in healthy subjects is 15,000 Ab/c (33, 72, 73). The large spread in the reported HLA-DR levels is most likely explained by biologic variability, as well as differences in assay reagents and flow cytometry methods used over years to quantitate HLA-DR expression levels (74–77). Peptide-MHC complexes are transported to the cell surface where they mediate antigen-specific recognition by CD4+ helper T lymphocytes. Once activated by peptide-MHC recognition, CD4+ T lymphocytes boost adaptive immune responses by activating other T and B lymphocytes that can recognize and target the invading pathogen (3, 4, 26). Because HLA-DR functions as the bridge between innate MNPs and antigen-specific T lymphocytes, low HLA-DR levels lead to diminished antigen presentation and reduced adaptive immune activation (4, 78). When HLA-DR is low, CD4+ T lymphocytes are not activated, hence cannot augment either B-cell stimulation to produce specific antibodies or CD8+ cytotoxic T lymphocyte generation to target infected cells directly (3, 4).
Despite HLA protein diversity, common determinants recognized by monoclonal antibodies enable flow cytometric quantitation of surface class II MHC expression level on blood cells (4, 40, 76). Although flow cytometry enables monocyte HLA-DR (mHLA-DR) quantitation, other cells expressing HLA-DR are also detected, including DCs, macrophages, B cells, and T cells (4, 30, 76, 79–82). Thus, to generate mHLA-DR specificity, cells are also stained for CD14 (also known as the LPS receptor), of which, expression is restricted to monocytes. Combined CD14 and HLA-DR staining enables quantitation of CD14+ classical and intermediate monocytes, the most abundant and rapidly replenished populations in blood. Results are typically reported either as percent of CD14+ monocytes expressing HLA-DR or as mean fluorescence intensity (MFI) of antibody against HLA-DR on CD14+ monocytes (77, 83).
HLA-DR downregulation and reduced monocyte responsiveness are described features of sepsis IP (4, 26). As detailed in Table 1, low HLA-DR correlates with adverse clinical outcomes, including increased risk for nosocomial infections, end-organ failure, longer ICU hospitalizations, and mortality (30, 33, 75, 84–89, 91–93).
Inter-laboratory variability initially posed a challenge to analytic validity of HLA-DR testing to identify sepsis IP (4). Now, a system offering standardized quantitative measurement of cell surface HLA-DR proteins (Quantibrite™; Becton, Dickinson and Company [BD]) is available. Developed in 2001, Quantibrite™ beads allow estimation of Ab/c, enabling monocyte cell surface HLA-DR protein quantitation to stratify patients based on mHLA-DR levels (79, 94–96). This assay uses phycoerythrin (PE)-labeled anti-HLA-DR monoclonal antibodies for estimating Ab/c (97). Geometric MFI values can be analyzed further to calculate numbers of Ab/c, which represents numbers of HLA-DR proteins on the monocyte surface (96, 97). Using standard instrument settings, flow cytometry data are converted into number of PE molecules per cell. Based on a known ratio of PE to antibodies against HLA-DR, Ab/c can be calculated, hence quantitating HLA-DR protein on CD14+ monocytes. With Quantibrite™, moderate immunosuppression is defined as about 10,000–15,000 Ab/c (74, 79). In several studies, a cut-off value of 8000 Ab/c was used to indicate IP. HLA-DR levels below 8000 Ab/c indicate more severe sepsis IP (4, 79). In some studies, 30% CD14+/HLA-DR+ cells corresponded to 5000 Ab/c for severe IP, whereas 45% CD14+/HLA-DR+ cells corresponded to about 8000 Ab/c for moderate IP (4, 79). Numerous studies have employed Quantibrite™ to measure HLA-DR-defined IP (26, 33, 70, 73–75, 77, 88, 90, 98–101).
Multiple literature analyses support mHLA-DR expression by flow cytometry as a sepsis IP biomarker and mortality predictor (80). One such review evaluated mHLA-DR in patients with complicated intra-abdominal infections and sepsis from 12 studies (n=761) (102). Results from 10 of these studies showed strong associations between low mHLA-DR expression and mortality. By contrast, 2 studies showed no prognostic value of mHLA-DR expression level. Proposed factors contributing to nonsignificant results in these 2 studies include: homogeneity of enrolled patients, young age, small sample sizes, and heterogeneity among experimental protocols (77, 87, 100, 103). Another review assessed mHLA-DR in critically ill patients with coronavirus disease of 2019 (COVID-19), sepsis, or bacterial infections from 15 studies (n=1160) (104). Of these studies, 4 monitored mHLA-DR expression with flow cytometry by a standardized protocol that reported results as Ab/c. Initial mHLA-DR expression was lower for COVID-19 patients than for controls (10,000 Ab/c vs 15,000 Ab/c) yet higher for COVID-19 patients than for septic shock patients (10,000 Ab/c vs 5000 Ab/c). Lower mHLA-DR expression was associated with higher ICU mortality and greater disease severity at hospital admission. A meta-analysis evaluated 8 prospective cohort studies to evaluate HLA-DR as a biomarker for sepsis in patients after trauma (n=639) (105). Results from 7 studies showed that HLA-DR by flow cytometry for detecting sepsis IP had a pooled sensitivity of 81% and a pooled specificity of 67%.
While various thresholds for detecting IP have been proposed, a minimum threshold for raising secondary infection and mortality risks has to date been neither standardized nor adopted (3, 4, 77, 84, 106). Hence, HLA-DR testing by flow cytometry can now be implemented with analytic validity, and multiple studies support its clinical validity. Yet, both a definitive threshold for sepsis IP and clinical utility for therapeutic response prediction remain, for now, unconfirmed.
Notably, 3 additional approaches to mHLA-DR measurement have been investigated. First, measurement of HLA-DR expression levels by polymerase chain reaction (PCR) was explored in several clinical studies (4, 70, 80). In 1 such study, quantitative real-time PCR (qRT-PCR) and mHLA-DR flow cytometry were used to assess HLA-DR and class II transactivator (CIITA) in patients with bacteremic sepsis (n=60) (70). TaqMan gene qRT-PCR expression assays were used to measure HLA-DR-α subunit (HLA-DRA) and CIITA, whereas Quantibrite™ was used to measure mHLA-DR by flow cytometry. Similar patterns for initial reductions in HLA-DRA, mHLA-DR, and CIITA were all followed by subsequent increases over time (p<0.001). Hence, qRT-PCR yields results somewhat similar to flow cytometry with low variability and reproducibility. While qRT-PCR may be robust for detecting HLA-DR expression in patients with sepsis, qRT-PCR results are non-specific for monocytes since circulating DCs, B cells, and activated T cells also express HLA-DR (70, 80). As such, it may not reliably reflect mHLA-DR expression in monocytes that drives sepsis IP (4, 80, 107).
Second, myeloid-derived suppressor cells (MDSCs) have been described in patients with sepsis (4, 108–110). Although not standardized, all MDSC descriptions include “low HLA-DR expression.” Hence, MDSCs are invariably monocytes with low HLA-DR. In sepsis, MDSCs associate with: prolonged immunosuppression, diminished T cell functions, development of nosocomial infections, higher reinfection rates, and hospital readmissions (4, 109, 111, 112).
Finally, several studies support that dynamic changes by serial mHLA-DR monitoring might predict mortality better than static mHLA-DR monitoring (80, 113, 114). Correspondingly, persistence of low mHLA-DR levels suggests slow or no recovery from sepsis IP (4, 12, 13, 113, 115, 116). Given inter-individual variability of mHLA-DR in sepsis, dynamic change or HLA-DR slope might increase prognostic significance of low mHLA-DR expression for mortality prediction (4, 13, 93, 98, 117). Thus far, no standardized approaches to serial mHLA-DR monitoring have been either developed or tested prospectively.
As in adults, low mHLA-DR in children associates with nosocomial infections and mortality (92, 118–121). Nonetheless, patient age affects monocyte subtypes and function, so direct comparison of adults vs children may be confounding (121). While adult monocytes are predominantly classical (CD14+/CD16-), neonatal monocytes are mostly intermediate (CD14+/CD16+) or nonclassical (CD14-lo/CD16+) subtypes that express lower levels of HLA-DR (121–123). These differences result in reduced T cell activation in neonates compared with adults (121). Also, neonates have proportionally more regulatory T cells than adults, and that difference may also limit immune responses in children with sepsis (124, 125).
One study compared mHLA-DR expression among critically ill children with sepsis, trauma-related hospital acquired infection, or recent surgery (n=37; median age, 9 years) vs healthy control children (n=37; median age, 3 years) (92). Results showed lower mHLA-DR expression (67% vs 95%; p<0.001) and lower mHLA-DR MFI (3219 vs 6545; p<0.001) for critically ill children vs healthy controls at all examined time points, in particular on classical monocytes and in children admitted for sepsis. Another study evaluated blood samples in hospitalized children with sepsis (n=30) vs healthy controls (n=21) for mHLA-DR expression using Quantibrite™ technology (98). As with adults, mHLA-DR expression in pediatric patients with sepsis was lower than that in controls (p=0.0001). Finally, a prospective, single-center, observational study evaluated mHLA-DR levels using Quantibrite™ in children with septic shock admitted to a pediatric ICU (n=26; median age, 2 years) with healthy controls (n=30) (90). As seen elsewhere, mHLA-DR levels were lower for patients with septic shock than for healthy controls (p<0.001).
While HLA-DR is well-documented for sepsis IP detection, other potential biomarkers are also being explored. LPS-induced TNF-α production from peripheral blood cells reflects innate immune system function via myeloid cell capacity to respond to an inflammatory stimulus (3, 30, 126). Although both ex-vivo TNF-α secretion and HLA-DR expression assess monocyte dysfunction via metabolic capacity to fulfill basic immune functions, ex-vivo TNF-α secretion is less specific for monocytes as responding myeloid cells include both neutrophils and monocytes. Independent of sepsis IP, TNF-α levels may also be influenced by a variety of other factors, such as: type of LPS used, blood volume, incubation conditions, and LPS concentration (3).
In contrast to substantial literature examining mHLA-DR prognostic significance in adult sepsis, there are fewer reports on TNF-α, and most are in small groups of children (3, 74, 91, 127, 128). Overall, these studies support clinical validity of measuring TNF-α by ex-vivo LPS stimulation. Although a few studies describe standardized protocols for measuring LPS-induced TNF-α production for sepsis, scalable analytic validity may remain challenging (29, 129). As seen for HLA-DR quantitation, no receiver operating characteristic (ROC) curve analysis has been performed to define a TNF-α threshold for sepsis IP.
ALC is another laboratory parameter that reflects immune system function (29, 130). The reference range for ALC varies with age. Normal for adults varies between 1000 and 4800 cells/µL, and for children, between 3000 and 9500 cells/µL (131, 132). Lymphopenia occurs when a patient’s ALC is below normal and can increase risk for infection (133).
In sepsis IP, circulating lymphocyte populations (e.g., CD4+ T cells, CD8+ T cells, B cells) are characteristically reduced due to tissue sequestration and apoptosis (26). Reductions at sepsis onset typically persist for up to 28 days. Increased apoptosis of both innate immune cells and adaptive immune cells in sepsis results in leukopenia, which associates with higher risks of secondary infections and death (134–136).
A retrospective, single-center cohort study monitored blood parameters in patients with bacteremia and sepsis (n=335) for secondary infection risk and mortality (130). Results showed higher ALC at Day 4 for survivors vs non-survivors (1100 cells/µL vs 700 cells/µL; p<0.0001). Also, 28-day and 1-year mortality were higher in severe (40% vs 10% and 58% vs 29%; p<0.001) and moderate (25% vs 10%; p=0.003, and 40% vs 29%; p=0.025) lymphopenia vs those without persistent lymphopenia. Multivariable analysis showed that Day 4 ALC was associated with both 28-day (odds ratio [OR], 0.68; p=0.009) and 1-year mortality (OR, 0.74; p=0.008). Severe persistent lymphopenia (< 0.6 x 103 cells/μL) was also associated with development of secondary infections (OR, 2.11; 95% confidence interval [CI], 1.02–4.39; p=0.04) (26, 130). Thus, persistent lymphopenia on the fourth day after a sepsis diagnosis predicted mortality and may be a valid marker of sepsis-induced immunosuppression.
In another single-center study, cross-sectional analysis was performed of ALC as an outcome predictor in patients with sepsis presenting to an emergency department (n=124) (137). Results showed a higher need for ICU admission (51.9% vs 14%; p<0.001) and higher rates of 28-day mortality (88.1% vs 11.9%; p<0.001) for patients with lymphopenia vs those without lymphopenia. In addition, age and sequential organ failure assessment (SOFA) scores were higher for patients with lymphopenia vs without.
Lower monocyte counts are also seen in sepsis and can impact health outcomes (64). A retrospective, single-center database analysis of patients with sepsis (n=2012) showed higher 28-day mortality rates, higher bacteremia rates, and higher incidence of organ dysfunction for patients with initial monocyte counts < 250 cells/μL.
Pros and cons of mHLA-DR expression, TNF-α secretion, and ALC as prognostic indicators in sepsis IP are summarized in Table 2 (4, 26, 27, 30, 35, 130, 138, 139). mHLA-DR expression and TNF-α responsiveness seek to measure similar biology of innate immune MNP dysfunction (26, 27, 130). Correspondingly, in an ex-vivo study using blood samples from patients with sepsis or septic shock (n=20), mHLA-DR expression correlated with TNF-α response (30). By contrast, ALC reflects distinct, complementary biology of deficient adaptive immune responsiveness (66).
HLA-DR expression offers acceptable analytic validity based on well characterized monoclonal antibodies and Quantibrite™ technology (74). Nonetheless, testing for this biomarker requires flow cytometry of fresh or stabilized cells, necessitating either shipping to a central facility or timely local analysis (74, 140). By contrast, TNF-α secretion requires local site addition of LPS to blood samples and incubation followed by analysis of frozen cell supernatants by enzyme-linked immunosorbent assay (ELISA) (138). This procedure generates need for trained site staff to perform ex-vivo LPS stimulation reliably. Notable analytic validity hurdles for TNF-α secretion include variability in LPS source and ex-vivo stimulation protocols, as well as non-specificity for monocyte vs neutrophil secretion. Neutrophils may be a significant source of TNF-α due to their higher abundance in whole blood relative to monocytes (138, 139, 141). While ALC measurement is logistically simple, inexpensive, and reflects adaptive immune function directly, ALC alone does not directly reflect innate immune function (142). Also, a threshold to define sepsis IP based on ALC remains, to date, undefined (130).
Consequences of sepsis IP are severe and contribute to sepsis mortality (26, 29). However, sepsis IP may be reversible since about one third of severe sepsis survivors regain immune function (29). As such, many drug trials have focused on targeting the clinically overt state of SIRS with pharmacologic agents that have anti-inflammatory effects. Though, most such agents have failed to improve outcome, and none has yet been shown to improve survival. Nonetheless, investigation continues of immunostimulatory agents that aim to reverse CARS effects (1, 3).
Experimental immunotherapies for sepsis IP have been shown to decrease ICU stay duration and secondary infection risk (3, 62). Notably, immunostimulating agents have shown promise for reversing IP, including: recombinant IL-7, programmed death 1 (PD-1)/PD-L1-specific antibodies, recombinant interferon (IFN)-γ, and recombinant GM-CSF (3, 12, 29, 62).
IL-7 is a potent anti-apoptotic cytokine required for lymphocyte survival and expansion that has shown potential benefits in patients with sepsis (143). The phase 2 IRIS-7 study evaluated IL-7 at varying frequencies vs placebo in patients with septic shock and severe lymphopenia (n=27). At Day 29, results showed higher ALC for IL-7 relative to placebo study therapy (+0.99–1.30 x 103 lymphocytes/µL vs 0.99 x 103 lymphocytes/µL; p=0.004). Elevated ALC persisted for 2–4 weeks after discontinuing IL-7.
PD-1 and PD-L1 are upregulated in sepsis and other inflammatory states (including cancer) (144). Clinical responses seen with PD-1/PD-L1 inhibitors in tumors suggested potential benefits for sepsis IP (27, 29), and a phase 1 trial of nivolumab in patients with sepsis (n=31) demonstrated safety. Larger clinical studies, however, were stopped by the sponsor (29, 145).
Pro-inflammatory cytokine IFN-γ plays a role in both innate and adaptive immune responses (146). One trial showed that IFN-γ study treatment restored mHLA-DR expression in patients with sepsis IP (4, 147). A separate, small, randomized, double-blind study (n=18) evaluated recombinant IFN-γ vs recombinant GM-CSF vs placebo in healthy volunteers given E. coli endotoxin. IFN-γ increased mHLA-DR expression and TNF-α levels but did not significantly improve symptom scores (148). In contrast, treatment with GM-CSF showed results trending in the same direction as IFN-γ, but were not statistically significant compared with placebo. Finally, a prospective case series described patients with invasive fungal infections treated with recombinant IFN-γ (n=8) (149). Notably, 5 of these 8 patients were considered to have IP, defined as < 50% HLA-DR+ monocytes. Treatment with recombinant IFN-γ restored immune function as indicated by increased HLA-DR expression in those with IP, increased ex-vivo cytokine production (e.g., TNF-α, IL-17, IL-22), and increased total leukocyte counts.
Therapeutic GM-CSF is available as a rhu protein (sargramostim) that was approved by the US Food and Drug Administration (FDA) in 1991 for myeloid cell reconstitution after cytotoxic chemotherapy (150, 151). Notably, rhu GM-CSF (including sargramostim) augments monocyte metabolic capacity, function, and proliferation (Figure 2) (3, 29, 77, 150, 152). In addition, sargramostim has been administered to acutely and critically ill patients, including children across multiple trials (Table 3) (31, 99, 126, 148, 153–155). No serious adverse events have been ascribed to sargramostim in these studies, and it did not increase systemic inflammation as measured by pro-inflammatory cytokines (e.g., IL-6 or IL-8). Doses studied were at or below the labeled dose for myeloid reconstitution (250 µg/m2/day). In some studies, immune recovery was prompt, within 3 days of sargramostim administration, with trends toward improved infection recovery, reduced hospital stays, and fewer days of mechanical ventilation. Nonetheless, all these studies were underpowered to confirm effects on outcomes. Results of 2 multi-center randomized trials of sargramostim in sepsis IP are also awaited. The ongoing GRACE-2 study (NCT05266001) will evaluate sargramostim vs placebo in 400 children with sepsis-induced MODS and IP. Furthermore, mHLA-DR expression will be assessed in this study to establish its clinical utility. In addition, the United Kingdom (UK)-based National Institute for Health and Care Research (NIHR) will sponsor the SepTIC trial that includes investigation of sargramostim for improving outcomes in a high-risk subset of patients admitted to the ICU with sepsis, which is anticipated to begin in mid-2023 (156). Of 3758 adult patients to be enrolled, 1300 with ALC below 1200 cells/µL will be randomized to sargramostim vs placebo. The primary endpoint will be 90-day all-cause mortality.
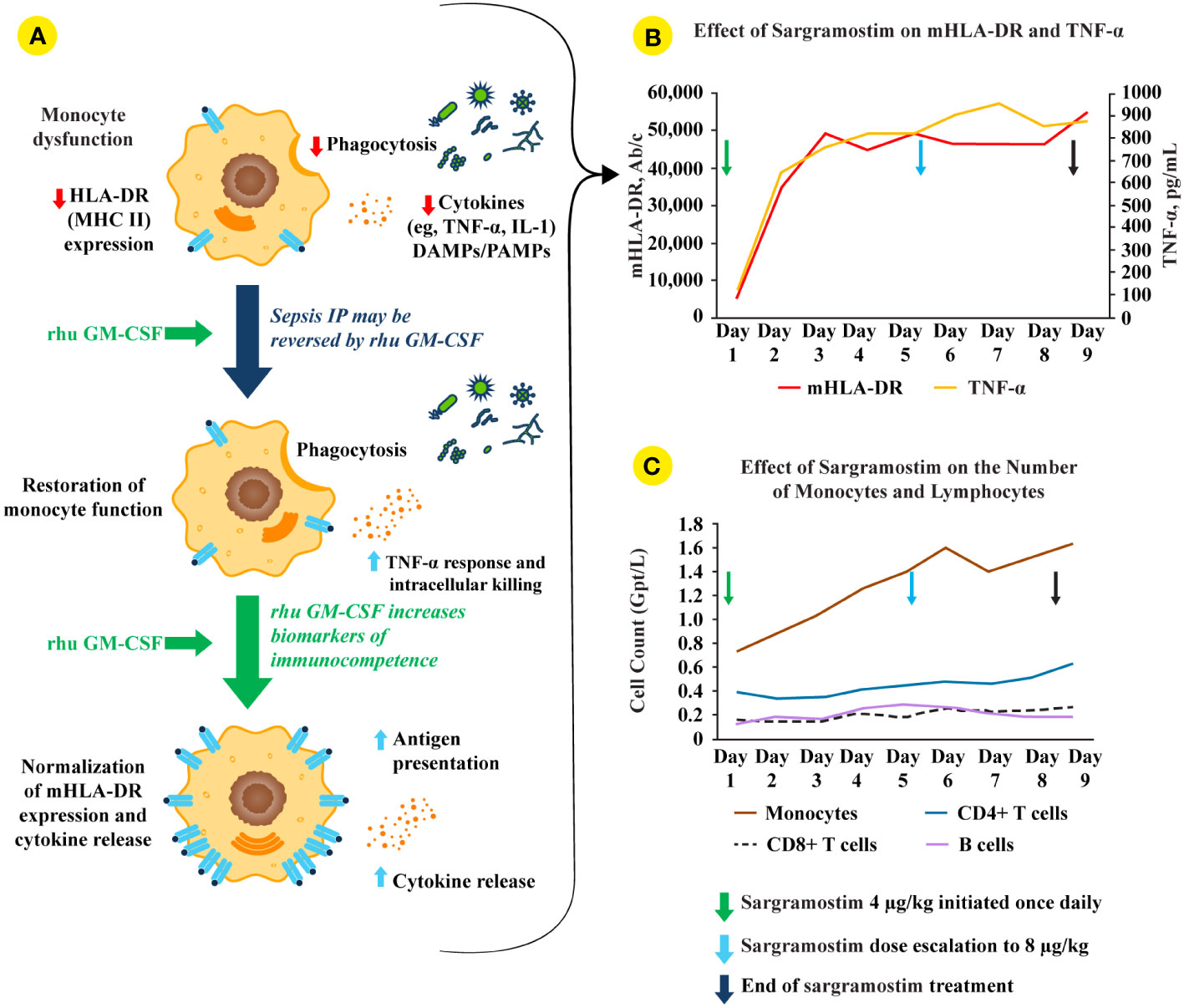
Figure 2 rhu GM-CSF (including sargramostim) stimulates and restores immune function in sepsis IP. (A; top monocyte) Impaired monocyte function leads to reduced pro-inflammatory mediator responses, decreased pathogen phagocytosis, and lower human leukocyte antigen-DR isotype (HLA-DR) expression (4). (A; middle and bottom monocytes) Treatment with recombinant human (rhu) granulocyte-macrophage colony-stimulating factor (GM-CSF) leads to increased intracellular killing, cytokine secretion, phagocytosis, monocyte (m)HLA-DR expression, and antigen presentation (2, 4). (B) In a biomarker-guided study of patients with sepsis IP (n=38), sargramostim was given daily for up to 8 days to patients with sepsis and mHLA-DR lower than 8000 Ab/c (31). Sargramostim treatment led to improved mHLA-DR expression and tumor necrosis factor (TNF)-α responses. (C) Sargramostim increased absolute numbers of monocytes and lymphocyte subsets (e.g., CD4+ T cells, CD8+ T cells, B cells) (31). Ab/c, antibody numbers bound per cell; DAMP, damage-associated molecular patterns; GM-CSF, granulocyte-macrophage colony-stimulating factor; HLA-DR, human leukocyte antigen-DR isotype; IL, interleukin; IP, immunoparalysis; MHC II, class II major histocompatibility complex; mHLA-DR, monocyte human leukocyte antigen-DR; PAMP, pathogen-associated molecular patterns; rhu, recombinant human; TNF, tumor necrosis factor.
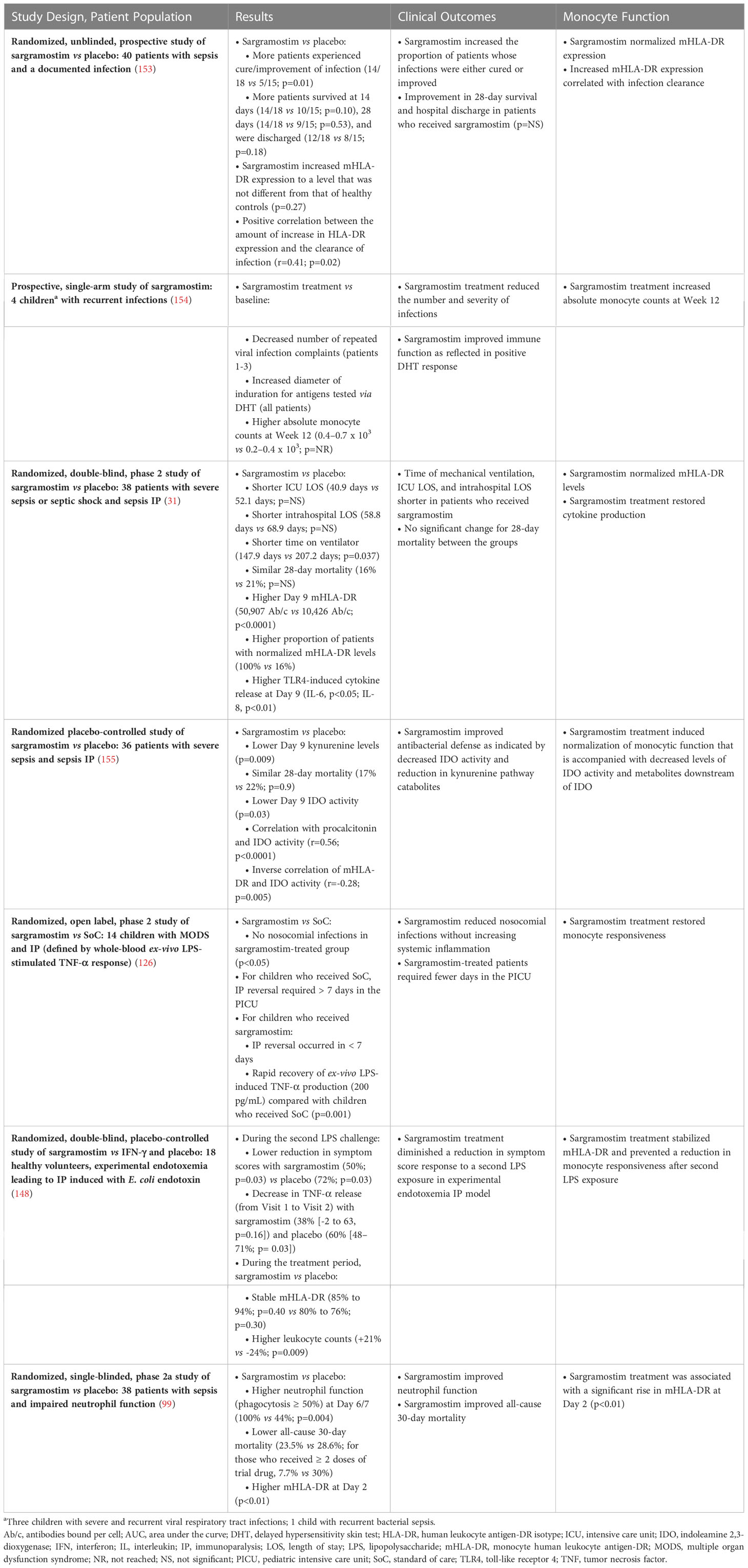
Table 3 Use of sargramostim in sepsis to improve clinical outcome and restore normal monocyte function.
While mHLA-DR, TNF-α secretion, and ALC each show promise as potentially useful biomarkers for sepsis IP, analytic validity of HLA-DR expression and its direct biologic linkage with MNP functional state make it attractive as a potential future gold standard for identification of sepsis IP (157, 158). Numerous publications, dating back 20 years, support use of either HLA-DR+ CD14+ cells or the actual number of HLA-DR proteins on CD14+ monocytes as clinically useful biomarkers for identifying patients with sepsis. Furthermore, sepsis IP severity might be detected by either low HLA-DR levels or diminishing HLA-DR levels during hospitalization. Nonetheless, while sepsis IP can be detected by diminished mHLA-DR expression, absence of either validated testing or an approved therapy to correct sepsis IP have thus far prevented widespread adoption of this biomarker.
Based on data presented here, we conclude therapeutic GM-CSF restores mHLA-DR levels and may improve clinical outcomes in patients with sepsis IP. Multiple trials of critically ill adults and children indicate that study treatment with sargramostim restored HLA-DR expression and immunocompetence. Furthermore, sargramostim led to trends toward improved clinical outcomes via reduced days of ICU stay and 28-day mortality. The GRACE-2 and SepTIC trials will further inform benefit from therapeutic GM-CSF (sargramostim) in sepsis IP.
IJ, WPC, and EPR contributed equally to this work. All authors contributed to conceptualization and writing (drafting, reviewing, editing) of this manuscript. All authors contributed to the article and approved the submitted version.
This publication has been funded by Partner Therapeutics, Inc. This project has been funded in part with federal funds from the Department of Health and Human Services; Office of the Assistant Secretary of Preparedness and Response; Biomedical Advanced Research and Development Authority under contract No. 75A50121C00080. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors thank the Biomedical Advanced Research and Development Authority and their colleagues, Debasish Roychowdhury, MD, John McManus, and Anthony Fusco from Partner Therapeutics, Inc., who provided insight and expertise. The authors also acknowledge Tim Yeung, PharmD and Caytlinn Batal at Wiesen Medical Writing for providing literature support, medical writing support, and editorial assistance.
IJ is an employee of and has stock options for Partner Therapeutics, Inc. WPC is the owner of Walt Carney Biomarkers Consulting and a paid consultant for Partner Therapeutics, Inc. At the time of the drafting of this manuscript, EPR was an employee of Partner Therapeutics, Inc. and has stock options.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Brady J, Horie S, Laffey JG. Role of the adaptive immune response in sepsis. Intensive Care Med Exp (2020) 8(Suppl 1):20. doi: 10.1186/s40635-020-00309-z
2. Chousterman BG, Arnaud M. Is there a role for hematopoietic growth factors during sepsis? Front Immunol (2018) 9:1015. doi: 10.3389/fimmu.2018.01015
3. Hall MW. Immune modulation in pediatric sepsis. J Pediatr Intensive Care (2019) 8(1):42–50. doi: 10.1055/s-0038-1676607
4. Pfortmueller CA, Meisel C, Fux M, Schefold JC. Assessment of immune organ dysfunction in critical illness: Utility of innate immune response markers. Intensive Care Med Exp (2017) 5(1):49. doi: 10.1186/s40635-017-0163-0
5. Sakr Y, Jaschinski U, Wittebole X, Szakmany T, Lipman J, Ñamendys-Silva SA, et al. Sepsis in intensive care unit patients: Worldwide data from the intensive care over nations audit. Open Forum Infect Dis (2018) 5(12):ofy313. doi: 10.1093/ofid/ofy313
6. Prescott HC, Angus DC. Enhancing recovery from sepsis: A review. JAMA (2018) 319(1):62–75. doi: 10.1001/jama.2017.17687
7. Centers for Disease Control and Prevention (CDC). What is sepsis? (2022). Available at: https://www.cdc.gov/sepsis/what-is-sepsis.html.
8. Rudd KE, Johnson SC, Agesa KM, Shackelford KA, Tsoi D, Kievlan DR, et al. Global, regional, and national sepsis incidence and mortality, 1990-2017: Analysis for the global burden of disease study. Lancet (2020) 395(10219):200–11. doi: 10.1016/s0140-6736(19)32989-7
9. World Health Organization (WHO). Global report on the epidemiology and burden of sepsis: Current evidence, identifying gaps and future directions (2020). Available at: https://apps.who.int/iris/bitstream/handle/10665/334216/9789240010789-eng.pdf.
10. Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA (2016) 315(8):801–10. doi: 10.1001/jama.2016.0287
11. Neviere R. Sepsis syndromes in adults: Epidemiology, definitions, clinical presentation, diagnosis, and prognosis (2022). Available at: https://www.uptodate.com/contents/sepsis-syndromes-in-adults-epidemiology-definitions-clinical-presentation-diagnosis-and-prognosis?search=sepsis&source=search_result&selectedTitle=1~150&usage_type=default&display_rank=1#H1227723412.
12. Hotchkiss RS, Monneret G, Payen D. Sepsis-induced immunosuppression: From cellular dysfunctions to immunotherapy. Nat Rev Immunol (2013) 13(12):862–74. doi: 10.1038/nri3552
13. Monneret G, Lepape A, Voirin N, Bohé J, Venet F, Debard AL, et al. Persisting low monocyte human leukocyte antigen-DR expression predicts mortality in septic shock. Intensive Care Med (2006) 32(8):1175–83. doi: 10.1007/s00134-006-0204-8
14. Annane D, Bellissant E, Cavaillon JM. Septic shock. Lancet (2005) 365(9453):63–78. doi: 10.1016/s0140-6736(04)17667-8
15. Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS international sepsis definitions conference. Crit Care Med (2003) 31(4):1250–6. doi: 10.1097/01.Ccm.0000050454.01978.3b
16. Shankar-Hari M, Phillips GS, Levy ML, Seymour CW, Liu VX, Deutschman CS, et al. Developing a new definition and assessing new clinical criteria for septic shock: For the third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA (2016) 315(8):775–87. doi: 10.1001/jama.2016.0289
17. Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM consensus conference committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest (1992) 101(6):1644–55. doi: 10.1378/chest.101.6.1644
18. Leligdowicz A, Matthay MA. Heterogeneity in sepsis: New biological evidence with clinical applications. Crit Care (2019) 23(1):80. doi: 10.1186/s13054-019-2372-2
19. Polat G, Ugan RA, Cadirci E, Halici Z. Sepsis and septic shock: Current treatment strategies and new approaches. Eurasian J Med (2017) 49(1):53–8. doi: 10.5152/eurasianjmed.2017.17062
20. Evans L, Rhodes A, Alhazzani W, Antonelli M, Coopersmith CM, French C, et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock 2021. Crit Care Med (2021) 49(11):e1063–e143. doi: 10.1097/ccm.0000000000005337
21. Lamontagne F, Rochwerg B, Lytvyn L, Guyatt GH, Møller MH, Annane D, et al. Corticosteroid therapy for sepsis: A clinical practice guideline. BMJ (2018) 362:k3284. doi: 10.1136/bmj.k3284
22. Vignon P, Laterre PF, Daix T, François B. New agents in development for sepsis: Any reason for hope? Drugs (2020) 80(17):1751–61. doi: 10.1007/s40265-020-01402-z
23. Stanski NL, Wong HR. Prognostic and predictive enrichment in sepsis. Nat Rev Nephrol (2020) 16(1):20–31. doi: 10.1038/s41581-019-0199-3
24. Varon J, Baron RM. Sepsis endotypes: The early bird still gets the worm. EBioMedicine (2022) 76:103832. doi: 10.1016/j.ebiom.2022.103832
25. Baghela A, Pena OM, Lee AH, Baquir B, Falsafi R, An A, et al. Predicting sepsis severity at first clinical presentation: The role of endotypes and mechanistic signatures. EBioMedicine (2022) 75:103776. doi: 10.1016/j.ebiom.2021.103776
26. Davies R, O'Dea K, Gordon A. Immune therapy in sepsis: Are we ready to try again? J Intensive Care Soc (2018) 19(4):326–44. doi: 10.1177/1751143718765407
27. Delano MJ, Ward PA. The immune system's role in sepsis progression, resolution, and long-term outcome. Immunol Rev (2016) 274(1):330–53. doi: 10.1111/imr.12499
28. Bline KE, Hall MW. Immune function in critically ill septic children. Pathogens (2021) 10(10):1239. doi: 10.3390/pathogens10101239
29. Peters van Ton AM, Kox M, Abdo WF, Pickkers P. Precision immunotherapy for sepsis. Front Immunol (2018) 9:1926. doi: 10.3389/fimmu.2018.01926
30. Winkler MS, Rissiek A, Priefler M, Schwedhelm E, Robbe L, Bauer A, et al. Human leucocyte antigen (HLA-DR) gene expression is reduced in sepsis and correlates with impaired TNFα response: A diagnostic tool for immunosuppression? PloS One (2017) 12(8):e0182427. doi: 10.1371/journal.pone.0182427
31. Meisel C, Schefold JC, Pschowski R, Baumann T, Hetzger K, Gregor J, et al. Granulocyte-macrophage colony-stimulating factor to reverse sepsis-associated immunosuppression: A double-blind, randomized, placebo-controlled multicenter trial. Am J Respir Crit Care Med (2009) 180(7):640–8. doi: 10.1164/rccm.200903-0363OC
32. Papadopoulos P, Pistiki A, Theodorakopoulou M, Christodoulopoulou T, Damoraki G, Goukos D, et al. Immunoparalysis: Clinical and immunological associations in SIRS and severe sepsis patients. Cytokine (2017) 92:83–92. doi: 10.1016/j.cyto.2017.01.012
33. Gouel-Chéron A, Allaouchiche B, Guignant C, Davin F, Floccard B, Monneret G. Early interleukin-6 and slope of monocyte human leukocyte antigen-DR: A powerful association to predict the development of sepsis after major trauma. PloS One (2012) 7(3):e33095. doi: 10.1371/journal.pone.0033095
34. Spapen H, Jacobs R, Honore P. Sepsis-induced multi-organ dysfunction syndrome–a mechanistic approach. J Emerg Crit Care Med (2021) 5:13. doi: 10.21037/jeccm.2017
35. Albert-Vega C, Tawfik DM, Trouillet-Assant S, Vachot L, Mallet F, Textoris J. Immune functional assays, from custom to standardized tests for precision medicine. Front Immunol (2018) 9:2367. doi: 10.3389/fimmu.2018.02367
36. Ginhoux F, Guilliams M. Tissue-resident macrophage ontogeny and homeostasis. Immunity (2016) 44(3):439–49. doi: 10.1016/j.immuni.2016.02.024
37. Gonzalez-Mejia ME, Doseff AI. Regulation of monocytes and macrophages cell fate. Front Biosci (Landmark Ed) (2009) 14(7):2413–31. doi: 10.2741/3387
38. Cassatella MA. Human mature neutrophils as atypical APC. Blood (2017) 129(14):1895–6. doi: 10.1182/blood-2017-02-767574
39. Korns D, Frasch SC, Fernandez-Boyanapalli R, Henson PM, Bratton DL. Modulation of macrophage efferocytosis in inflammation. Front Immunol (2011) 2:57. doi: 10.3389/fimmu.2011.00057
40. van Lith M, McEwen-Smith RM, Benham AM. HLA-DP, HLA-DQ, and HLA-DR have different requirements for invariant chain and HLA-DM. J Biol Chem (2010) 285(52):40800–8. doi: 10.1074/jbc.M110.148155
41. Kumamoto Y, Mattei LM, Sellers S, Payne GW, Iwasaki A. CD4+ T cells support cytotoxic T lymphocyte priming by controlling lymph node input. Proc Natl Acad Sci U.S.A. (2011) 108(21):8749–54. doi: 10.1073/pnas.1100567108
42. Xu W, Banchereau J. The antigen presenting cells instruct plasma cell differentiation. Front Immunol (2014) 4:504. doi: 10.3389/fimmu.2013.00504
43. Rosales C. Neutrophil: A cell with many roles in inflammation or several cell types? Front Physiol (2018) 9:113. doi: 10.3389/fphys.2018.00113
44. Bhattacharya P, Thiruppathi M, Elshabrawy HA, Alharshawi K, Kumar P, Prabhakar BS. GM-CSF: An immune modulatory cytokine that can suppress autoimmunity. Cytokine (2015) 75(2):261–71. doi: 10.1016/j.cyto.2015.05.030
45. Gasson JC, Kaufman SE, Weisbart RH, Tomonaga M, Golde DW. High-affinity binding of granulocyte-macrophage colony-stimulating factor to normal and leukemic human myeloid cells. Proc Natl Acad Sci U.S.A. (1986) 83(3):669–73. doi: 10.1073/pnas.83.3.669
46. Hansen G, Hercus TR, McClure BJ, Stomski FC, Dottore M, Powell J, et al. The structure of the GM-CSF receptor complex reveals a distinct mode of cytokine receptor activation. Cell (2008) 134(3):496–507. doi: 10.1016/j.cell.2008.05.053
47. Hercus TR, Thomas D, Guthridge MA, Ekert PG, King-Scott J, Parker MW, et al. The granulocyte-macrophage colony-stimulating factor receptor: Linking its structure to cell signaling and its role in disease. Blood (2009) 114(7):1289–98. doi: 10.1182/blood-2008-12-164004
48. Zhan Y, Lew AM, Chopin M. The pleiotropic effects of the GM-CSF rheostat on myeloid cell differentiation and function: More than a numbers game. Front Immunol (2019) 10:2679. doi: 10.3389/fimmu.2019.02679
49. Adelaja A, Taylor B, Sheu KM, Liu Y, Luecke S, Hoffmann A. Six distinct NFkB signaling codons convey discrete information to distinguish stimuli and enable appropriate macrophage responses. Immunity (2021) 54(5):916–30.e7. doi: 10.1016/j.immuni.2021.04.011
50. Tang Y, Adelaja A, Ye FX, Deeds E, Wollman R, Hoffmann A. Quantifying information accumulation encoded in the dynamics of biochemical signaling. Nat Commun (2021) 12(1):1272. doi: 10.1038/s41467-021-21562-0
51. Cheng QJ, Ohta S, Sheu KM, Spreafico R, Adelaja A, Taylor B, et al. NFkB dynamics determine the stimulus specificity of epigenomic reprogramming in macrophages. Science (2021) 372(6548):1349–53. doi: 10.1126/science.abc0269
52. Wessendarp M, Watanabe-Chailland M, Liu S, Stankiewicz T, Ma Y, Kasam RK, et al. Role of GM-CSF in regulating metabolism and mitochondrial functions critical to macrophage proliferation. Mitochondrion (2022) 62:85–101. doi: 10.1016/j.mito.2021.10.009
53. Zhang S, Weinberg S, DeBerge M, Gainullina A, Schipma M, Kinchen JM, et al. Efferocytosis fuels requirements of fatty acid oxidation and the electron transport chain to polarize macrophages for tissue repair. Cell Metab (2019) 29(2):443–56.e5. doi: 10.1016/j.cmet.2018.12.004
54. Perry SE, Mostafa SM, Wenstone R, Shenkin A, McLaughlin PJ. HLA-DR regulation and the influence of GM-CSF on transcription, surface expression and shedding. Int J Med Sci (2004) 1(3):126–36. doi: 10.7150/ijms.1.126
55. Wculek SK, Dunphy G, Heras-Murillo I, Mastrangelo A, Sancho D. Metabolism of tissue macrophages in homeostasis and pathology. Cell Mol Immunol (2022) 19(3):384–408. doi: 10.1038/s41423-021-00791-9
56. Börgermann J, Friedrich I, Scheubel R, Kuss O, Lendemans S, Silber RE, et al. Granulocyte-macrophage colony-stimulating factor (GM-CSF) restores decreased monocyte HLA-DR expression after cardiopulmonary bypass. Thorac Cardiovasc Surg (2007) 55(1):24–31. doi: 10.1055/s-2006-924621
57. Bundschuh DS, Barsig J, Hartung T, Randow F, Döcke WD, Volk HD, et al. Granulocyte-macrophage colony-stimulating factor and IFN-γ restore the systemic TNF-α response to endotoxin in lipopolysaccharide-desensitized mice. J Immunol (1997) 158(6):2862–71. doi: 10.4049/jimmunol.158.6.2862
58. Flohé S, Lendemans S, Selbach C, Waydhas C, Ackermann M, Schade FU, et al. Effect of granulocyte-macrophage colony-stimulating factor on the immune response of circulating monocytes after severe trauma. Crit Care Med (2003) 31(10):2462–9. doi: 10.1097/01.Ccm.0000089640.17523.57
59. Lendemans S, Kreuzfelder E, Waydhas C, Schade FU, Flohé S. Differential immunostimulating effect of granulocyte-macrophage colony-stimulating factor (GM-CSF), granulocyte colony-stimulating factor (G-CSF) and interferon gamma (IFNγ) after severe trauma. Inflammation Res (2007) 56(1):38–44. doi: 10.1007/s00011-007-6069-7
60. Randow F, Döcke WD, Bundschuh DS, Hartung T, Wendel A, Volk HD. In vitro prevention and reversal of lipopolysaccharide desensitization by IFN-γ, IL-12, and granulocyte-macrophage colony-stimulating factor. J Immunol (1997) 158(6):2911–8. doi: 10.4049/jimmunol.158.6.2911
61. Hornell TM, Beresford GW, Bushey A, Boss JM, Mellins ED. Regulation of the class II MHC pathway in primary human monocytes by granulocyte-macrophage colony-stimulating factor. J Immunol (2003) 171(5):2374–83. doi: 10.4049/jimmunol.171.5.2374
62. Mathias B, Szpila BE, Moore FA, Efron PA, Moldawer LL. A review of GM-CSF therapy in sepsis. Med (Baltimore) (2015) 94(50):e2044. doi: 10.1097/md.0000000000002044
63. Bruns T, Peter J, Hagel S, Herrmann A, Stallmach A. The augmented neutrophil respiratory burst in response to Escherichia coli is reduced in liver cirrhosis during infection. Clin Exp Immunol (2011) 164(3):346–56. doi: 10.1111/j.1365-2249.2011.04373.x
64. Chung H, Lee JH, Jo YH, Hwang JE, Kim J. Circulating monocyte counts and its impact on outcomes in patients with severe sepsis including septic shock. Shock (2019) 51(4):423–9. doi: 10.1097/shk.0000000000001193
65. Reinhart K, Bauer M, Riedemann NC, Hartog CS. New approaches to sepsis: Molecular diagnostics and biomarkers. Clin Microbiol Rev (2012) 25(4):609–34. doi: 10.1128/cmr.00016-12
66. Agnello L, Giglio RV, Bivona G, Scazzone C, Gambino CM, Iacona A, et al. The value of a complete blood count (CBC) for sepsis diagnosis and prognosis. Diagnostics (Basel) (2021) 11(10):1881. doi: 10.3390/diagnostics11101881
67. Buonacera A, Stancanelli B, Colaci M, Malatino L. Neutrophil to lymphocyte ratio: An emerging marker of the relationships between the immune system and diseases. Int J Mol Sci (2022) 23(7):3636. doi: 10.3390/ijms23073636
68. Alelign T, Ahmed MM, Bobosha K, Tadesse Y, Howe R, Petros B. Kidney transplantation: The challenge of human leukocyte antigen and its therapeutic strategies. J Immunol Res (2018) 2018:5986740. doi: 10.1155/2018/5986740
69. Roche PA, Furuta K. The ins and outs of MHC class II-mediated antigen processing and presentation. Nat Rev Immunol (2015) 15(4):203–16. doi: 10.1038/nri3818
70. Cajander S, Tina E, Bäckman A, Magnuson A, Strålin K, Söderquist B, et al. Quantitative real-time polymerase chain reaction measurement of HLA-DRA gene expression in whole blood is highly reproducible and shows changes that reflect dynamic shifts in monocyte surface HLA-DR expression during the course of sepsis. PloS One (2016) 11(5):e0154690. doi: 10.1371/journal.pone.0154690
71. Hagedoorn NN, Kolukirik P, Nagtzaam NMA, Nieboer D, Verbruggen S, Joosten KF, et al. Association of monocyte HLA-DR expression over time with secondary infection in critically ill children: A prospective observational study. Eur J Pediatr (2022) 181(3):1133–42. doi: 10.1007/s00431-021-04313-7
72. Turrel-Davin F, Guignant C, Lepape A, Mougin B, Monneret G, Venet F. Upregulation of the pro-apoptotic genes BID and FAS in septic shock patients. Crit Care (2010) 14(4):R133. doi: 10.1186/cc9181
73. Zorio V, Venet F, Delwarde B, Floccard B, Marcotte G, Textoris J, et al. Assessment of sepsis-induced immunosuppression at ICU discharge and 6 months after ICU discharge. Ann Intensive Care (2017) 7(1):80. doi: 10.1186/s13613-017-0304-3
74. Quadrini KJ, Patti-Diaz L, Maghsoudlou J, Cuomo J, Hedrick MN, McCloskey TW. A flow cytometric assay for HLA-DR expression on monocytes validated as a biomarker for enrollment in sepsis clinical trials. Cytometry B Clin Cytom (2021) 100(1):103–14. doi: 10.1002/cyto.b.21987
75. Leijte GP, Rimmelé T, Kox M, Bruse N, Monard C, Gossez M, et al. Monocytic HLA-DR expression kinetics in septic shock patients with different pathogens, sites of infection and adverse outcomes. Crit Care (2020) 24(1):110. doi: 10.1186/s13054-020-2830-x
76. Mizrahi O, Ish Shalom E, Baniyash M, Klieger Y. Quantitative flow cytometry: Concerns and recommendations in clinic and research. Cytometry B Clin Cytom (2018) 94(2):211–8. doi: 10.1002/cyto.b.21515
77. Tamulyte S, Kopplin J, Brenner T, Weigand MA, Uhle F. Monocyte HLA-DR assessment by a novel point-of-care device is feasible for early identification of ICU patients with complicated courses-a proof-of-principle study. Front Immunol (2019) 10:432. doi: 10.3389/fimmu.2019.00432
78. Marionneaux S. Nonmalignant leukocyte disorders. In: Keohane EM, Otto CN, Walenga JM Eds. Rodak's Hematology 6th ed. Elsevier (2020), 445–65. Accessed January 19, 2023. doi: 10.1016/B978-0-323-53045-3.00035-0
79. Döcke WD, Höflich C, Davis KA, Röttgers K, Meisel C, Kiefer P, et al. Monitoring temporary immunodepression by flow cytometric measurement of monocytic HLA-DR expression: A multicenter standardized study. Clin Chem (2005) 51(12):2341–7. doi: 10.1373/clinchem.2005.052639
80. Zhuang Y, Peng H, Chen Y, Zhou S, Chen Y. Dynamic monitoring of monocyte HLA-DR expression for the diagnosis, prognosis, and prediction of sepsis. Front Biosci (Landmark Ed) (2017) 22(8):1344–54. doi: 10.2741/4547
81. Hiki N, Berger D, Prigl C, Boelke E, Wiedeck H, Seidelmann M, et al. Endotoxin binding and elimination by monocytes: Secretion of soluble CD14 represents an inducible mechanism counteracting reduced expression of membrane CD14 in patients with sepsis and in a patient with paroxysmal nocturnal hemoglobinuria. Infect Immun (1998) 66(3):1135–41. doi: 10.1128/iai.66.3.1135-1141.1998
82. Patel AA, Zhang Y, Fullerton JN, Boelen L, Rongvaux A, Maini AA, et al. The fate and lifespan of human monocyte subsets in steady state and systemic inflammation. J Exp Med (2017) 214(7):1913–23. doi: 10.1084/jem.20170355
83. Kanakoudi-Tsakalidou F, Debonera F, Drossou-Agakidou V, Sarafidis K, Tzimouli V, Taparkou A, et al. Flow cytometric measurement of HLA-DR expression on circulating monocytes in healthy and sick neonates using monocyte negative selection. Clin Exp Immunol (2001) 123(3):402–7. doi: 10.1046/j.1365-2249.2001.01471.x
84. de Roquetaillade C, Dupuis C, Faivre V, Lukaszewicz AC, Brumpt C, Payen D. Monitoring of circulating monocyte HLA-DR expression in a large cohort of intensive care patients: Relation with secondary infections. Ann Intensive Care (2022) 12(1):39. doi: 10.1186/s13613-022-01010-y
85. Asmussen A, Busch HJ, Helbing T, Bemtgen X, Smolka C, Bode C, et al. Monocyte subset distribution and surface expression of HLA-DR and CD14 in patients after cardiopulmonary resuscitation. Sci Rep (2021) 11(1):12403. doi: 10.1038/s41598-021-91948-z
86. Cour-Andlauer F, Morrow BM, McCulloch M, Javouhey E, Lecour S, van As S, et al. Decreased human leukocyte antigen DR on circulating monocytes expression after severe pediatric trauma: An exploratory report. Pediatr Crit Care Med (2021) 22(5):e314–e23. doi: 10.1097/pcc.0000000000002604
87. Pei F, Zhang GR, Zhou LX, Liu JY, Ma G, Kou QY, et al. Early immunoparalysis was associated with poor prognosis in elderly patients with sepsis: Secondary analysis of the ETASS study. Infect Drug Resist (2020) 13:2053–61. doi: 10.2147/idr.S246513
88. Kox M, Frenzel T, Schouten J, van de Veerdonk FL, Koenen H, Pickkers P. COVID-19 patients exhibit less pronounced immune suppression compared with bacterial septic shock patients. Crit Care (2020) 24(1):263. doi: 10.1186/s13054-020-02896-5
89. Chen Y, Hu Y, Zhang J, Shen Y, Huang J, Yin J, et al. Clinical characteristics, risk factors, immune status and prognosis of secondary infection of sepsis: A retrospective observational study. BMC Anesthesiol (2019) 19(1):185. doi: 10.1186/s12871-019-0849-9
90. Remy S, Kolev-Descamps K, Gossez M, Venet F, Demaret J, Javouhey E, et al. Occurrence of marked sepsis-induced immunosuppression in pediatric septic shock: A pilot study. Ann Intensive Care (2018) 8(1):36. doi: 10.1186/s13613-018-0382-x
91. Remy S, Kolev-Descamps K, Gossez M, Venet F, Demaret J, Javouhey E, et al. Occurrence of marked sepsis-induced immunosuppression in pediatric septic shock: A pilot study. Ann Intensive Care (2018) 8(suppl_1):1–5. doi: 10.1186/s13613-018-0382-x
92. Boeddha NP, Kerklaan D, Dunbar A, van Puffelen E, Nagtzaam NMA, Vanhorebeek I, et al. HLA-DR expression on monocyte subsets in critically ill children. Pediatr Infect Dis J (2018) 37(10):1034–40. doi: 10.1097/inf.0000000000001990
93. Wu JF, Ma J, Chen J, Ou-Yang B, Chen MY, Li LF, et al. Changes of monocyte human leukocyte antigen-DR expression as a reliable predictor of mortality in severe sepsis. Crit Care (2011) 15(5):R220. doi: 10.1186/cc10457
94. Demaret J, Walencik A, Jacob MC, Timsit JF, Venet F, Lepape A, et al. Inter-laboratory assessment of flow cytometric monocyte HLA-DR expression in clinical samples. Cytometry B Clin Cytom (2013) 84(1):59–62. doi: 10.1002/cyto.b.21043
95. Drewry AM, Ablordeppey EA, Murray ET, Beiter ER, Walton AH, Hall MW, et al. Comparison of monocyte human leukocyte antigen-DR expression and stimulated tumor necrosis factor alpha production as outcome predictors in severe sepsis: A prospective observational study. Crit Care (2016) 20(1):334. doi: 10.1186/s13054-016-1505-0
96. Raghavan M, Yarzabek B, Zaitouna AJ, Krishnakumar S, Ramon DS. Strategies for the measurements of expression levels and half-lives of HLA class I allotypes. Hum Immunol (2019) 80(4):221–7. doi: 10.1016/j.humimm.2019.02.001
97. Pannu KK, Joe ET, Iyer SB. Performance evaluation of Quantibrite phycoerythrin beads. Cytometry (2001) 45(4):250–8. doi: 10.1002/1097-0320(20011201)45:4<250::aid-cyto10021>3.0.co;2-t
98. Manzoli TF, Troster EJ, Ferranti JF, Sales MM. Prolonged suppression of monocytic human leukocyte antigen-DR expression correlates with mortality in pediatric septic patients in a pediatric tertiary intensive care unit. J Crit Care (2016) 33:84–9. doi: 10.1016/j.jcrc.2016.01.027
99. Pinder EM, Rostron AJ, Hellyer TP, Ruchaud-Sparagano MH, Scott J, Macfarlane JG, et al. Randomised controlled trial of GM-CSF in critically ill patients with impaired neutrophil phagocytosis. Thorax (2018) 73(10):918–25. doi: 10.1136/thoraxjnl-2017-211323
100. Skirecki T, Mikaszewska-Sokolewicz M, Hoser G, Zielińska-Borkowska U. The early expression of HLA-DR and CD64 myeloid markers is specifically compartmentalized in the blood and lungs of patients with septic shock. Mediators Inflammation (2016) 2016:3074902. doi: 10.1155/2016/3074902
101. Strohmeyer JC, Blume C, Meisel C, Doecke WD, Hummel M, Hoeflich C, et al. Standardized immune monitoring for the prediction of infections after cardiopulmonary bypass surgery in risk patients. Cytometry B Clin Cytom (2003) 53(1):54–62. doi: 10.1002/cyto.b.10031
102. Dimitrov E, Enchev E, Minkov G, Halacheva K, Yovtchev Y. Poor outcome could be predicted by lower monocyte human leukocyte antigen-DR expression in patients with complicated intra-abdominal infections: A review. Surg Infect (Larchmt) (2020) 21(2):77–80. doi: 10.1089/sur.2019.050
103. Perry SE, Mostafa SM, Wenstone R, Shenkin A, McLaughlin PJ. Is low monocyte HLA-DR expression helpful to predict outcome in severe sepsis? Intensive Care Med (2003) 29(8):1245–52. doi: 10.1007/s00134-003-1686-2
104. Benlyamani I, Venet F, Coudereau R, Gossez M, Monneret G. Monocyte HLA-DR measurement by flow cytometry in COVID-19 patients: An interim review. Cytometry A (2020) 97(12):1217–21. doi: 10.1002/cyto.a.24249
105. Chen G, Wen D, Qiu J, Wang Q, Peng G, Du J, et al. The role of mHLA-DR in the early diagnosis of sepsis patients with severe trauma: A meta-analysis (2021). Available at: https://assets.researchsquare.com/files/rs-770184/v1/97eee162-f6ef-4d98-9309-0934a71253e8.pdf?c=1631887620.
106. Remy S, Gossez M, Belot A, Hayman J, Portefaix A, Venet F, et al. Massive increase in monocyte HLA-DR expression can be used to discriminate between septic shock and hemophagocytic lymphohistiocytosis-induced shock. Crit Care (2018) 22(1):213. doi: 10.1186/s13054-018-2146-2
107. Cazalis MA, Friggeri A, Cavé L, Demaret J, Barbalat V, Cerrato E, et al. Decreased HLA-DR antigen-associated invariant chain (CD74) mRNA expression predicts mortality after septic shock. Crit Care (2013) 17(6):R287. doi: 10.1186/cc13150
108. Darcy CJ, Minigo G, Piera KA, Davis JS, McNeil YR, Chen Y, et al. Neutrophils with myeloid derived suppressor function deplete arginine and constrain T cell function in septic shock patients. Crit Care (2014) 18(4):R163. doi: 10.1186/cc14003
109. Fenner BP, Darden DB, Kelly LS, Rincon J, Brakenridge SC, Larson SD, et al. Immunological endotyping of chronic critical illness after severe sepsis. Front Med (Lausanne) (2020) 7:616694. doi: 10.3389/fmed.2020.616694
110. Janols H, Bergenfelz C, Allaoui R, Larsson AM, Rydén L, Björnsson S, et al. A high frequency of MDSCs in sepsis patients, with the granulocytic subtype dominating in gram-positive cases. J Leukoc Biol (2014) 96(5):685–93. doi: 10.1189/jlb.5HI0214-074R
111. Mathias B, Delmas AL, Ozrazgat-Baslanti T, Vanzant EL, Szpila BE, Mohr AM, et al. Human myeloid-derived suppressor cells are associated with chronic immune suppression after severe sepsis/septic shock. Ann Surg (2017) 265(4):827–34. doi: 10.1097/sla.0000000000001783
112. Uhel F, Azzaoui I, Grégoire M, Pangault C, Dulong J, Tadié JM, et al. Early expansion of circulating granulocytic myeloid-derived suppressor cells predicts development of nosocomial infections in patients with sepsis. Am J Respir Crit Care Med (2017) 196(3):315–27. doi: 10.1164/rccm.201606-1143OC
113. Udovicic I, Stanojevic I, Djordjevic D, Zeba S, Rondovic G, Abazovic T, et al. Immunomonitoring of monocyte and neutrophil function in critically ill patients: From sepsis and/or trauma to COVID-19. J Clin Med (2021) 10(24):5815. doi: 10.3390/jcm10245815
114. Haveman JW, Muller Kobold AC, Tervaert JW, van den Berg AP, Tulleken JE, Kallenberg CG, et al. The central role of monocytes in the pathogenesis of sepsis: Consequences for immunomonitoring and treatment. Neth J Med (1999) 55(3):132–41. doi: 10.1016/s0300-2977(98)00156-9
115. Monneret G, Venet F, Pachot A, Lepape A. Monitoring immune dysfunctions in the septic patient: A new skin for the old ceremony. Mol Med (2008) 14(1-2):64–78. doi: 10.2119/2007-00102.Monneret
116. Schefold JC. Measurement of monocytic HLA-DR (mHLA-DR) expression in patients with severe sepsis and septic shock: Assessment of immune organ failure. Intensive Care Med (2010) 36(11):1810–2. doi: 10.1007/s00134-010-1965-7
117. Landelle C, Lepape A, Voirin N, Tognet E, Venet F, Bohé J, et al. Low monocyte human leukocyte antigen-DR is independently associated with nosocomial infections after septic shock. Intensive Care Med (2010) 36(11):1859–66. doi: 10.1007/s00134-010-1962-x
118. Carson WF, Cavassani KA, Dou Y, Kunkel SL. Epigenetic regulation of immune cell functions during post-septic immunosuppression. Epigenetics (2011) 6(3):273–83. doi: 10.4161/epi.6.3.14017
119. Gentile LF, Cuenca AG, Efron PA, Ang D, Bihorac A, McKinley BA, et al. Persistent inflammation and immunosuppression: A common syndrome and new horizon for surgical intensive care. J Trauma Acute Care Surg (2012) 72(6):1491–501. doi: 10.1097/TA.0b013e318256e000
120. Winters BD, Eberlein M, Leung J, Needham DM, Pronovost PJ, Sevransky JE. Long-term mortality and quality of life in sepsis: A systematic review. Crit Care Med (2010) 38(5):1276–83. doi: 10.1097/CCM.0b013e3181d8cc1d
121. Doughty C, Oppermann L, Hartmann N, Dreschers S, Gille C, Orlikowsky T. Monocytes in neonatal bacterial sepsis: Think tank or workhorse? BioChem (2022) 2(1):27–42. doi: 10.3390/biochem2010003
122. Damasceno D, Teodosio C, van den Bossche WBL, Perez-Andres M, Arriba-Méndez S, Muñoz-Bellvis L, et al. Distribution of subsets of blood monocytic cells throughout life. J Allergy Clin Immunol (2019) 144(1):320–3.e6. doi: 10.1016/j.jaci.2019.02.030
123. Kapellos TS, Bonaguro L, Gemünd I, Reusch N, Saglam A, Hinkley ER, et al. Human monocyte subsets and phenotypes in major chronic inflammatory diseases. Front Immunol (2019) 10:2035. doi: 10.3389/fimmu.2019.02035
124. Hibbert JE, Currie A, Strunk T. Sepsis-induced immunosuppression in neonates. Front Pediatr (2018) 6:357. doi: 10.3389/fped.2018.00357
125. Semmes EC, Chen JL, Goswami R, Burt TD, Permar SR, Fouda GG. Understanding early-life adaptive immunity to guide interventions for pediatric health. Front Immunol (2020) 11:595297. doi: 10.3389/fimmu.2020.595297
126. Hall MW, Knatz NL, Vetterly C, Tomarello S, Wewers MD, Volk HD, et al. Immunoparalysis and nosocomial infection in children with multiple organ dysfunction syndrome. Intensive Care Med (2011) 37(3):525–32. doi: 10.1007/s00134-010-2088-x
127. Levin G, Boyd JG, Day A, Hunt M, Maslove DM, Norman P, et al. The relationship between immune status as measured by stimulated ex-vivo tumour necrosis factor alpha levels and the acquisition of nosocomial infections in critically ill mechanically ventilated patients. Intensive Care Med Exp (2020) 8(1):55. doi: 10.1186/s40635-020-00344-w
128. Snyder A, Jedreski K, Fitch J, Wijeratne S, Wetzel A, Hensley J, et al. Transcriptomic profiles in children with septic shock with or without immunoparalysis. Front Immunol (2021) 12:733834. doi: 10.3389/fimmu.2021.733834
129. Frazier WJ, Hall MW. Immunoparalysis and adverse outcomes from critical illness. Pediatr Clin North Am (2008) 55(3):647–68. doi: 10.1016/j.pcl.2008.02.009
130. Drewry AM, Samra N, Skrupky LP, Fuller BM, Compton SM, Hotchkiss RS. Persistent lymphopenia after diagnosis of sepsis predicts mortality. Shock (2014) 42(5):383–91. doi: 10.1097/shk.0000000000000234
131. National Institutes of Health (NIH). Lymphopenia: Diagnosis (2022). Available at: https://www.nhlbi.nih.gov/health/lymphopenia/diagnosis.
132. Karamikhah R, Karimzadeh I. Acute lymphoblastic leukemia in children: A short review. Trends Pharm Sci (2020) 6(4):283–96. doi: 10.30476/tips.2021.88938.1073
133. National Institutes of Health (NIH). Lymphopenia: What is lymphopenia? (2022). Available at: https://www.nhlbi.nih.gov/health/lymphopenia.
134. Belok SH, Bosch NA, Klings ES, Walkey AJ. Evaluation of leukopenia during sepsis as a marker of sepsis-defining organ dysfunction. PloS One (2021) 16(6):e0252206. doi: 10.1371/journal.pone.0252206
135. Cao C, Yu M, Chai Y. Pathological alteration and therapeutic implications of sepsis-induced immune cell apoptosis. Cell Death Dis (2019) 10(10):782. doi: 10.1038/s41419-019-2015-1
136. Nedeva C, Menassa J, Puthalakath H. Sepsis: Inflammation is a necessary evil. Front Cell Dev Biol (2019) 7:108. doi: 10.3389/fcell.2019.00108
137. Vahedi H, Bagheri A, Jahanshir A, Seyedhosseini J, Vahidi E. Association of lymphopenia with short term outcomes of sepsis patients; a brief report. Arch Acad Emerg Med (2019) 7(1):e14. doi: 10.22037/aaem.v7i1.117
138. Bidar F, Bodinier M, Venet F, Lukaszewicz AC, Brengel-Pesce K, Conti F, et al. Concomitant assessment of monocyte HLA-DR expression and ex vivo TNF-α release as markers of adverse outcome after various injuries-insights from the realism study. J Clin Med (2021) 11(1):96. doi: 10.3390/jcm11010096
139. Grivennikov SI, Tumanov AV, Liepinsh DJ, Kruglov AA, Marakusha BI, Shakhov AN, et al. Distinct and nonredundant in vivo functions of TNF produced by T cells and macrophages/neutrophils: Protective and deleterious effects. Immunity (2005) 22(1):93–104. doi: 10.1016/j.immuni.2004.11.016
140. Monneret G, Venet F, Meisel C, Schefold JC. Assessment of monocytic HLA-DR expression in ICU patients: Analytical issues for multicentric flow cytometry studies. Crit Care (2010) 14(4):432. doi: 10.1186/cc9184
141. Prame Kumar K, Nicholls AJ, Wong CHY. Partners in crime: neutrophils and monocytes/macrophages in inflammation and disease. Cell Tissue Res (2018) 371(3):551–65. doi: 10.1007/s00441-017-2753-2
142. Tang G, Yuan X, Luo Y, Lin Q, Chen Z, Xing X, et al. Establishing immune scoring model based on combination of the number, function, and phenotype of lymphocytes. Aging (Albany NY) (2020) 12(10):9328–43. doi: 10.18632/aging.103208
143. Francois B, Jeannet R, Daix T, Walton AH, Shotwell MS, Unsinger J, et al. Interleukin-7 restores lymphocytes in septic shock: The IRIS-7 randomized clinical trial. JCI Insight (2018) 3(5):e98960. doi: 10.1172/jci.insight.98960
144. Qin W, Hu L, Zhang X, Jiang S, Li J, Zhang Z, et al. The diverse function of PD-1/PD-L pathway beyond cancer. Front Immunol (2019) 10:2298. doi: 10.3389/fimmu.2019.02298
145. Hotchkiss RS, Colston E, Yende S, Crouser ED, Martin GS, Albertson T, et al. Immune checkpoint inhibition in sepsis: A phase 1b randomized study to evaluate the safety, tolerability, pharmacokinetics, and pharmacodynamics of nivolumab. Intensive Care Med (2019) 45(10):1360–71. doi: 10.1007/s00134-019-05704-z
146. Ambruso DR, Briones NJ, Baroffio AF, Murphy JR, Tran AD, Gowan K, et al. In vivo interferon-gamma induced changes in gene expression dramatically alter neutrophil phenotype. PloS One (2022) 17(2):e0263370. doi: 10.1371/journal.pone.0263370
147. Döcke WD, Randow F, Syrbe U, Krausch D, Asadullah K, Reinke P, et al. Monocyte deactivation in septic patients: Restoration by IFN-γ treatment. Nat Med (1997) 3(6):678–81. doi: 10.1038/nm0697-678
148. Leentjens J, Kox M, Koch RM, Preijers F, Joosten LA, van der Hoeven JG, et al. Reversal of immunoparalysis in humans in vivo: A double-blind, placebo-controlled, randomized pilot study. Am J Respir Crit Care Med (2012) 186(9):838–45. doi: 10.1164/rccm.201204-0645OC
149. Delsing CE, Gresnigt MS, Leentjens J, Preijers F, Frager FA, Kox M, et al. Interferon-gamma as adjunctive immunotherapy for invasive fungal infections: A case series. BMC Infect Dis (2014) 14:166. doi: 10.1186/1471-2334-14-166
150. Lazarus HM, Ragsdale CE, Gale RP, Lyman GH. Sargramostim (rhu GM-CSF) as cancer therapy (systematic review) and an immunomodulator. A drug before its time? Front Immunol (2021) 12:706186. doi: 10.3389/fimmu.2021.706186
152. Na YR, Gu GJ, Jung D, Kim YW, Na J, Woo JS, et al. GM-CSF induces inflammatory macrophages by regulating glycolysis and lipid metabolism. J Immunol (2016) 197(10):4101–9. doi: 10.4049/jimmunol.1600745
153. Rosenbloom AJ, Linden PK, Dorrance A, Penkosky N, Cohen-Melamed MH, Pinsky MR. Effect of granulocyte-monocyte colony-stimulating factor therapy on leukocyte function and clearance of serious infection in nonneutropenic patients. Chest (2005) 127(6):2139–50. doi: 10.1378/chest.127.6.2139
154. Nelson LA. Use of granulocyte-macrophage colony-stimulating factor to reverse anergy in otherwise immunologically healthy children. Ann Allergy Asthma Immunol (2007) 98(4):373–82. doi: 10.1016/s1081-1206(10)60885-x
155. Schefold JC, Zeden JP, Pschowski R, Hammoud B, Fotopoulou C, Hasper D, et al. Treatment with granulocyte-macrophage colony-stimulating factor is associated with reduced indoleamine 2,3-dioxygenase activity and kynurenine pathway catabolites in patients with severe sepsis and septic shock. Scand J Infect Dis (2010) 42(3):164–71. doi: 10.3109/00365540903405768
156. National Institute for Health and Care Research (NIHR). Funding and awards: Sepsis trials in critical care (SepTIC) (2022). Available at: https://fundingawards.nihr.ac.uk/award/17/136/02.
157. Monneret G, Gossez M, Aghaeepour N, Gaudilliere B, Venet F. How clinical flow cytometry rebooted sepsis immunology. Cytometry A (2019) 95(4):431–41. doi: 10.1002/cyto.a.23749
Keywords: sepsis, immunoparalysis, immunosuppression, granulocyte-macrophage colony-stimulating factor, human leukocyte antigen-DR, monocytes, compensatory anti-inflammatory response syndrome, sargramostim
Citation: Joshi I, Carney WP and Rock EP (2023) Utility of monocyte HLA-DR and rationale for therapeutic GM-CSF in sepsis immunoparalysis. Front. Immunol. 14:1130214. doi: 10.3389/fimmu.2023.1130214
Received: 23 December 2022; Accepted: 16 January 2023;
Published: 07 February 2023.
Edited by:
Yufeng Zhou, Fudan University, ChinaReviewed by:
Nevena Arsenovic-Ranin, University of Belgrade, SerbiaCopyright © 2023 Joshi, Carney and Rock. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ila Joshi, aWxhLmpvc2hpQHBhcnRuZXJ0eC5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.