- 1Department of Obstetrics and Gynecology, Peking University Shenzhen Hospital, Shenzhen, China
- 2BGI-Shenzhen, Shenzhen, China
- 3ShenZhen Engineering Laboratory of Detection and Intervention of Human Intestinal Microbiome, Shenzhen, China
- 4College of Life Sciences, University of Chinese Academy of Sciences, Beijing, China
- 5Institute of Obstetrics and Gynecology, Shenzhen Peking University Hong Kong University of Science and Technology Medical Center, Shenzhen, China
- 6Shenzhen Key Laboratory on Technology for Early Diagnosis of Major Gynecological Diseases, Peking University Shenzhen Hospital, Shenzhen, China
- 7BGI Precision Nutrition (Shenzhen) Technology Co., Ltd, Shenzhen, China
- 8Department of Biology, School of Life Sciences, Southern University of Science and Technology, Shenzhen, China
Bacterial vaginosis (BV) is a common infection of the lower genital tract with a vaginal microbiome dysbiosis caused by decreasing of lactobacilli. Previous studies suggested that supplementation with live Lactobacillus may benefit the recovery of BV, however, the outcomes vary in people from different regions. Herein, we aim to evaluate the effectiveness of oral Chinese-origin Lactobacillus with adjuvant metronidazole (MET) on treating Chinese BV patients. In total, 67 Chinese women with BV were enrolled in this parallel controlled trial and randomly assigned to two study groups: a control group treated with MET vaginal suppositories for 7 days and a probiotic group treated with oral Lactobacillus gasseri TM13 and Lactobacillus crispatus LG55 as an adjuvant to MET for 30 days. By comparing the participants with Nugent Scores ≥ 7 and < 7 on days 14, 30, and 90, we found that oral administration of probiotics did not improve BV cure rates (72.73% and 84.00% at day 14, 57.14% and 60.00% at day 30, 32.14% and 48.39% at day 90 for probiotic and control group respectively). However, the probiotics were effective in restoring vaginal health after cure by showing higher proportion of participants with Nugent Scores < 4 in the probiotic group compared to the control group (87.50% and 71.43% on day 14, 93.75% and 88.89% on day 30, and 77.78% and 66.67% on day 90). The relative abundance of the probiotic strains was significantly increased in the intestinal microbiome of the probiotic group compared to the control group at day 14, but no significance was detected after 30 and 90 days. Also, the probiotics were not detected in vaginal microbiome, suggesting that L. gasseri TM13 and L. crispatus LG55 mainly acted through the intestine. A higher abundance of Prevotella timonensis at baseline was significantly associated with long-term cure failure of BV and greatly contributed to the enrichment of the lipid IVA synthesis pathway, which could aggravate inflammation response. To sum up, L. gasseri TM13 and L. crispatus LG55 can restore the vaginal health of patients recovering from BV, and individualized intervention mode should be developed to restore the vaginal health of patients recovering from BV.
Clinical trial registration: https://classic.clinicaltrials.gov/ct2/show/, identifier NCT04771728.
1 Introduction
Bacterial vaginosis (BV) is a microecological disorder caused by decreased abundance of lactobacilli and an increased abundance of anaerobic bacteria, commonly affecting the female lower genital tract (1). The prevalence of BV is around 20%-30% all over the world (2). BV increases the susceptibility of women of reproductive age to sexually transmitted infections (STIs), including human immunodeficiency virus (HIV) (3), human papillomavirus (HPV) (4), and gonorrhea (5), and increases the risk of spontaneous abortion, preterm delivery, and amniotic fluid infection during pregnancy (6–9). Common symptoms of BV include elevated vaginal pH, increased leukorrhea, odor, vulvar itching, and burning pain. In addition, approximately 50% of patients are asymptomatic (3, 10, 11).
16S rRNA amplicon sequencing technology has been widely used to characterize the microbiome of the vagina in healthy or BV states (12–14). In BV patients, Lactobacillus spp. are replaced by anaerobic bacteria such as Gardnerella spp., Prevotella spp., Mobiluncus spp., and Atopobium vaginae, which result in high production of cadaveric amines, putrescine, succinate, and acetate leading to the altered chemical composition of secretions as well as higher pH (15). However, 16S rRNA amplicon sequencing has low species resolution and functional information of microorganisms cannot be directly obtained from sequence data. Since metagenomic shotgun sequencing is able to identify microbial categories with high resolution and characterize their biological functions (16, 17), it has been wildly used in explaining the structure and function of the vaginal microbiome (18, 19). It’s no doubt that metagenomic shotgun sequencing is a better tool to study the mechanisms of vaginal microbiome involvement in pathogenesis of BV.
Metronidazole (MET) has been recommended by the Centers for Disease Control and Prevention as a treatment method for BV (20). Yet, the 12-month long-term cure rate is only 30% (21). The inhibition of MET penetration by biofilms is thought to be the main cause of BV recurrence (22). Probiotics have been used as an alternative treatment approach to prevent recurrent BV. For example, previous studies have reported that oral administration of Lactobacillus as an adjuvant therapy could increase the long-term cure rate of BV by improving the balance of the vaginal microbiome (23–26). Microbial translocation from the colon to the vagina has been hypothesized as a potential pathway for the efficacy of oral probiotics (27, 28). Orally administrated probiotics could also suppress systemic inflammatory responses via the fermentation products of probiotics in the intestine (29). However, the outcome of the probiotic treatment on BV could vary in different ethnic groups since the vaginal microbiome is different among women in different ethnic groups (30). A study demonstrated that oral L. rhamnosus GR-1 and L. reuteri RC-14, isolated from European women, were barely detectable in the intestine and vaginal microbiome after administration (31). It has also been confirmed that native dominant Lactobacillus could persistently colonize in the vagina compared to others (32). This suggests that isolated probiotic strains are more likely to function in women of the same ethnic groups, but more evidences should be provided.
The probiotic strains used in this study are L. gasseri TM13 and L. crispatus LG55 which were isolated from the feces of 2 healthy Chinese people, which we developed in-house. They showed the strong ability to lower vaginal pH, inhibit the growth of pathogenic bacteria and fungi, and alleviated the inflammatory response of BV rats (33). In this study, we aim at evaluating the effectiveness of oral Chinese-origin probiotic strains, L. gasseri TM13 and L. crispatus LG55, with adjuvant MET in treating Chinese BV patients, and investigate the dynamic of the intestine and vaginal microbiome using metagenomic sequencing during the trial.
2 Materials and methods
2.1 Trial population
Women who attended the gynecology outpatient clinic of Peking University Shenzhen Hospital in China between June 2020 and April 2021 and presented with abnormal leucorrhoea symptoms were enrolled in this single-center, prospective, parallel-group, randomized controlled clinical trial. Inclusion criteria were: age of 18 - 55 years, premenopause, with a history of sexual activity, and a Nugent Score ≥ 7. Exclusion criteria were: vulvovaginal candidiasis (VVC), trichomonas vaginalis (TV) infection, Chlamydia trachomatis (CT) infection, gonococcal vaginitis, pregnant or planning to become pregnant, breastfeeding, pelvic inflammatory disease, allergic to MET, on antibiotic therapy, long-term contraceptive or immunosuppressive drug use or allergic, no regular sexual partner (RSP), and those with a history of systemic organic disease or psychiatric disorders.
This study was approved by the Peking University Shenzhen Hospital Medical Ethics Committee (ID: PUshenzhenH2020-009) and published on ClinicalTrials.gov (NCT04771728). Written consent was obtained from all subjects for this study.
2.2 Study design
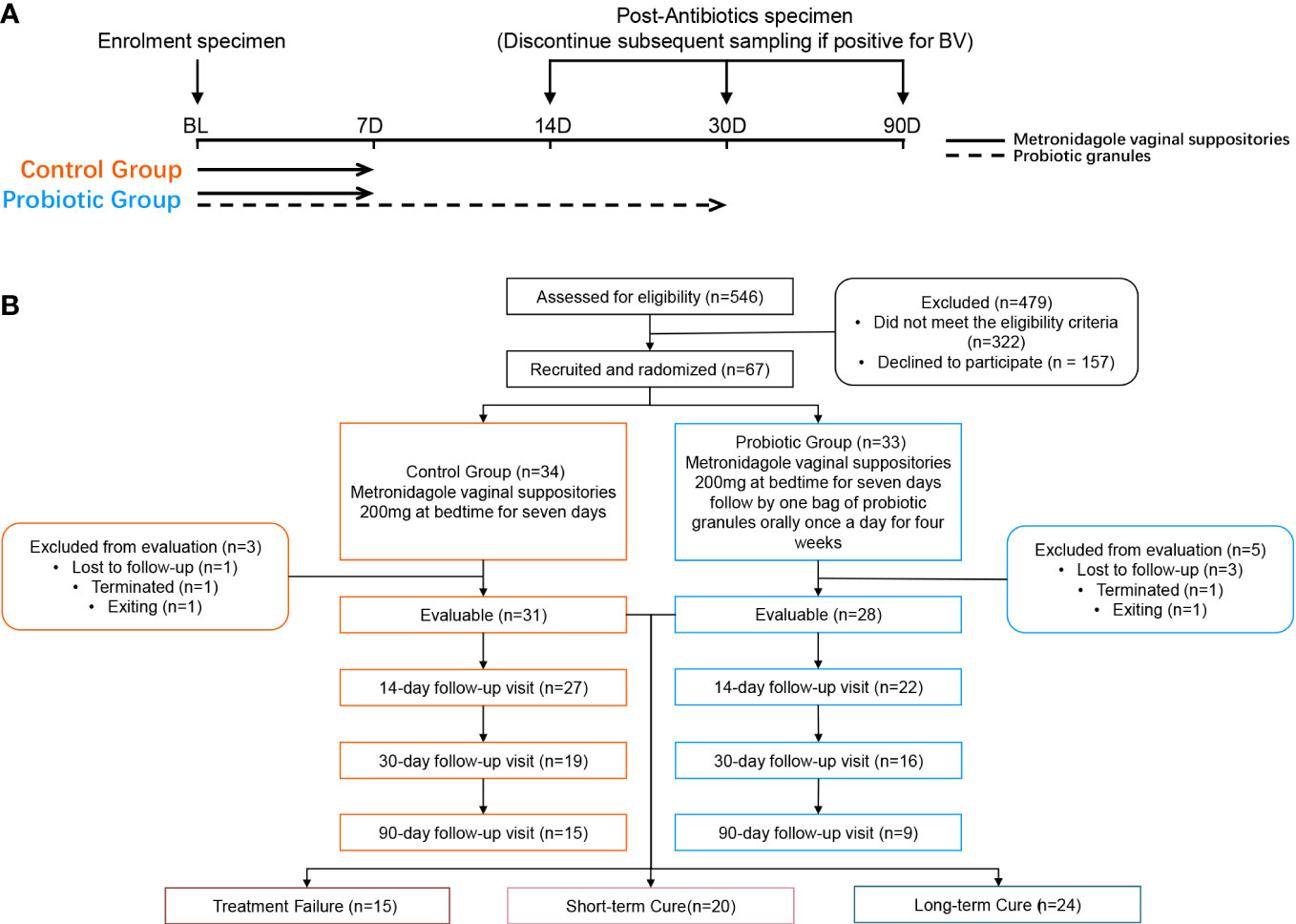
Patients with an initial Nugent Score ≥ 7 were informed of the study protocol. Those who met the criteria signed an informed consent form before the trial. Subjects were asked to complete a questionnaire containing information on demographic characteristics, vaginal health status, history of drug allergies, and history of reproductive system disorders. A random number table generated by SPSS 13.0 software was used to randomly assign subjects to the control or probiotic group using a 1:1 ratio. After enrollment, the probiotic group received MET vaginal suppositories (200 mg daily for 7 days) along with oral intake of probiotic solid drinks containing L. gasseri TM13 and L. crispatus LG55 (33) (daily intake ≥ 5×109 CFU for 30 days); the control group received the same dose of MET vaginal suppositories.
Follow-up visits were performed on day 14, 30, and 90 after the initiation of treatment. The investigator at enrolment distributed the intervention product. At each follow-up visit, the subject’s compliance, frequency of sex, vaginal health, and the occurrence of adverse events (AEs) were assessed by questionnaire. Subjects were required to avoid topical vaginal dosing during menstruation but continue to take the intervention product orally (Figure 1A). In addition, subjects were required to avoid sexual intercourse and vaginal douching throughout the follow-up period. We regrouped all subjects according to treatment outcomes. Subjects who were not found to be BV-negative at any of the follow-up visits were assigned to the treatment failure group; subjects who changed from BV-negative to BV-positive at any of the follow-up visits were assigned to the short-term cure group, and subjects who tested BV-negative at all follow-up visits were assigned to the long-term cure group.
2.3 Efficacy evaluation
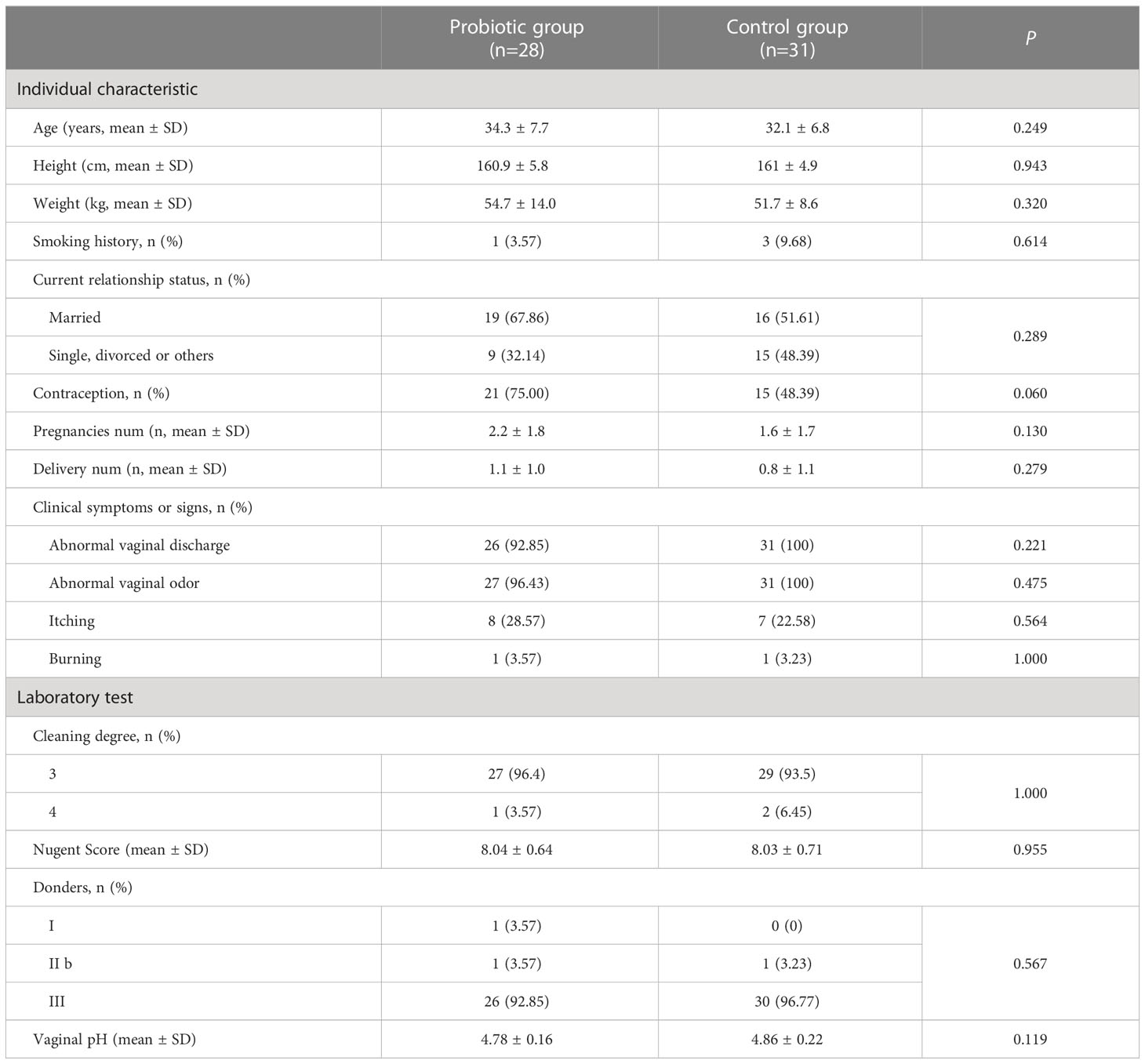
Before treatment, all subjects were assessed for between-group differences in demographic characteristics, including age, height, weight, marital status, history of smoking, drug allergies, history of childbirth, and history of reproductive disorders. The diagnostic criteria for BV were based on the Nugent Score (34) of vaginal smears. Gram-stained smears were examined under a microscope, and different morphological bacterial cells were counted at 1000× magnification: 0-6 points were diagnosed as BV-negative and 7-10 points as BV-positive. Two cytology technicians performed Gram staining for a double-blind analysis. Clinical testing was also performed for pH, Donders LBG (35), and vaginal cleanliness grades. Efficacy outcomes were assessed by the percentage of subjects with no diagnosis of BV (diagnostic criteria of Nugent Score ≤ 7) at each follow-up visit as the cure rate, based on compliance with protocol set (PP) analysis. Those with a Nugent Score ≥7 were excluded from the clinical trial at each follow-up visit, but these subjects were still included when we assessed cure rates at subsequent follow-up time-points.
2.4 Metagenomic sequencing and biological information annotation
Fecal and vaginal samples collected from participants during the initial and follow-up consultations were subjected to DNA extraction and metagenomic shotgun sequencing. Metagenomic shotgun sequencing (100bp paired reads) was performed on the DNBSEQ™ platform (25, 36, 37). Sequencing data were quality-controlled using fastp v0.20.1 (http://github.com/OpenGene/fastp) (38), filtering low-quality sequences with default parameters. High-quality sequences were then aligned to the hg38 human reference gene set using Bowtie2 v2.4.2 (http://github.com/BenLangmead/bowtie2) (39) to exclude the host genome. The de-hosted high-quality sequences were aligned using MetaPhlAn v3.0.7 (http://huttenhower.sph.harvard.edu/metaphlan) as well as HUMAnN 3.0 (http://huttenhower.sph.harvard.edu/humann3) for taxonomic as well as functional annotation (40).
2.5 Statistical analysis
The Shannon and Simpson index and Bray-Curtis distance were used to calculate alpha diversity and beta diversity based on the relative abundance of species. The t-test and Mann-Whitney U-test were used to test for differences between two numerical variables in two groups, with 0.05 considered as the threshold for significant differences in p-values. The Chi-square test and Fisher’s exact test were used for differences between groups for categorical variables. Ward Linkage hierarchical clustering of species-level relative abundance of vaginal microorganisms using R Stats v3.5.3 with Jensen-Shannon distances. LEfSe (41) was used to identify species that differed between different treatment outcomes, and a general linear model by MaAsLin2 (42) was used to look for the significantly different pathways. In order to construct co-occurrence networks, we first screened for species with mean relative abundance ≥ 0.1%, then calculated interspecific Spearman correlation coefficients, and constructed co-occurrence networks for two different treatment outcome groups using all correlations with p-values ≤ 0.05, which were visualized by Gephi.
3 Results
3.1 Clinical trial process and efficacy evaluation
After screening 546 BV patients with Nugent Score ≥7 according to the inclusion and exclusion criteria, 67 patients were included in the clinical trial and randomized. Among them, 8 subjects were excluded (4 subjects were lost to follow-up, 2 subjects were terminated due to non-medication as prescribed, and 2 subjects withdrew from this study for personal reasons). Ultimately, 31 subjects in the control group and 28 subjects in the probiotic group were included in the efficacy evaluation and microbiome analysis (Figure 1B). Subjects’ demographic and pathological characteristics at baseline did not differ significantly between the probiotic and control groups (all P > 0.05) (Table 1).
At all time-points, there was no significant improvement in the cure rate in the probiotic group compared to the control group. The cure rates for the probiotic and control groups were 72.73% and 84.00% at day 14, respectively; 57.14%, and 60.00% at day 30, respectively; 32.14% and 48.39% at day 90, respectively. Notably, in the group of cured participants, the proportion of those fully recovered (Nugent Score < 4) was higher in the probiotic group compared to the control group (87.50% and 71.43% on day 14, 93.75% and 88.89% on day 30, and 77.78% and 66.67% on day 90, respectively) (Figure 2A). To sum up, this suggests that oral administration of L. gasseri TM13 with L. crispatus LG55 cannot improve BV cure rates but has a role in restoring vaginal health after cure. We also detected BV-associated symptoms and laboratory parameters at different time-points of the trial, however, no significant inter-group differences were found (Figure 2B).

Figure 2 Although orally administrated L. gasseri TM13 and L. crispatus LG55 preparation cannot improve BV cure rates, it restores the vaginal health after cure mainly acting through the intestine. The number and percentage of the participants with different disease states (BV, non-BV, health, and transition stage) at different time-points of the trial were shown in pie charts (A). The percentage of the participants with detected BV-associated symptoms (abnormal vaginal discharge and abnormal vaginal odor) and laboratory parameters (vaginal pH, vaginal cleaning degree and Donders Lactobacillus grade) at different time-points of the trial were shown as a histogram (B). The number marked on the top of the bar denote the case number. Box-and-whisker plots showing the relative abundance of intervention species between groups at different time-points in the intestine or vagina (C). All vaginal samples were grouped according to the Nugent Scores ≥ 7 (BV) and < 7 (cure) (D). Mann-Whitney U test were used to perform the statistical analysis. ** stands for P < 0.01; **** stands for P < 0.0001. ns stands for no significant different (P ≥ 0.05). NS is short for Nugent Score.
3.2 Inter-group differences in the abundance and microbial diversity of the intervention strains
In the fecal samples, the relative abundance of L. crispatus and L. gasseri was significantly higher in the probiotic group than in the control group on day 14 (P < 0.001). On day 30, only L. crispatus remained significantly different (P = 0.0037), and the relative abundance of both species in the probiotic group decreased compared to day 14. On day 90, the presence of the L. gasseri was not observed in the intestine, and the presence of the L. crispatus was detected in only 2 participants (Figure 2C). This indicated that the colonization ability of L. gasseri TM13 in intestine was not as strong as L. crispatus LG55. Furthermore, there was no difference in the relative abundance of L. crispatus and L. gasseri in the vaginal microbiome between probiotic and control groups (all P > 0.05) (Figure 2C), as well as between BV and cure groups (Figure 2D), which suggested that no transfer of the intervention strains from the intestine to the vagina was detected.
In addition, to investigate the effect of oral probiotics on the microbial community structure of the intestine and vagina, we observed the differences in alpha-diversity and beta-diversity in the intestine and vaginal microbiome before and after the intervention. No difference in microbial diversity was found between the probiotic and control groups, either in the intestine or the vaginal microbiome (all P > 0.05) (Figure S1).
3.3 Heterogeneity of the vaginal microbiome and association with treatment outcomes
All of the vaginal samples were grouped into five clusters by hierarchical clustering method (Figure 3A). Lactobacillus iners was dominant in Cluster 1 (37.16%), and the dominant species in Cluster 4 (6.76%) was varied in samples, including L. crispatus, L. jensenii and other species with lower average relative abundance. Cluster 2, Cluster 3, and Cluster 5 were highly associated with BV-associated pathogenic bacteria, including Gardnerella, Prevotella, and Atopobium dominated. Among them, a high abundance of Prevotella amnii was the main feature of Cluster 3 (12.84%), and Cluster 2 (24.32%) was dominated by Gardnerella vaginalis. While Prevotella bivia, Prevotella timonensis, and Atopobium vaginae were the main components of BV-associated pathogens in Cluster 5 (18.92%) and there was no clearly dominant species. It is noteworthy that baseline samples presented more frequently in Cluster 3 (80.00%) than in Cluster 2 (50.00%) or Cluster 5 (50.00%) (P = 0.02&0.03), which demonstrated that distribution regularity could exist in the baseline microbiome (BV microbiome) that may influence the treatment outcomes. To further investigate this issue, we selected the vaginal samples at baseline and re-clustered them according to the outcomes (Figure 3B). The result showed that the Cluster composition differed nearly significantly between long-term cure group and short-term cure group (P = 0.059). This suggested a strong relationship between the BV microbiome and long-term cure rate of BV, which could result in the treatment outcomes of L. gasseri TM13 and L. crispatus LG55.
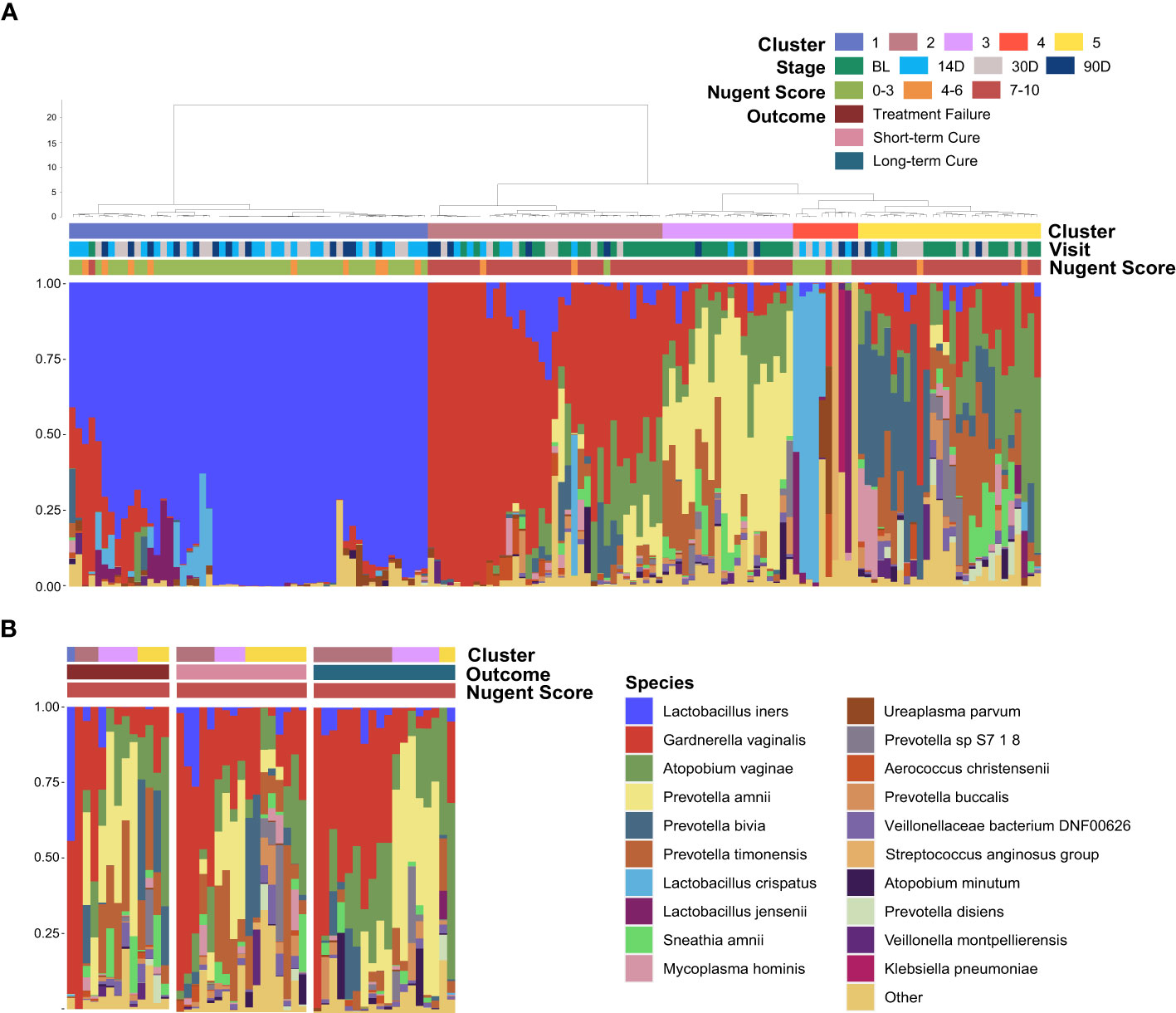
Figure 3 Distribution of the baseline microbiome (BV microbiome) greatly associated with the treatment outcomes. The vaginal microbiome of 59 women sampled longitudinally at all time-points (A) and at baseline (B). The samples are hierarchically clustered (R base hclust function with Jensen-Shannon distances and Ward linkage) and classified into five clusters. Nugent Score range, follow-up time-points and treatment outcomes were indicated by the bars.
3.4 Differences in vaginal microbiome between the short-term cured subjects and the long-term cured subjects
To describe the differences in core species distribution and their interactions between the short-term cure group (Figure 4A) and the long-term cure group (Figure 4B), the high-abundance species (mean relative abundance ≥ 0.1%) in vaginal discharge samples at all time-points were selected to perform the co-occurrence network analysis in these two groups. A greater number of high-abundance species of BV-associated pathogenic bacteria and a greater density of positive interspecies interactions was shown in the short-term cure group (106 significant positive correlations) than in long-term cure group (53 significant positive correlations). This suggests that stronger interbacterial interactions help them escape the antibiotic, which is in line with the findings of Gustin’s study (43). Lactobacillus, which possessed higher abundance in the cured samples, had the most negative correlations due to the ability of Lactobacillus to secrete lactic acid and bactericidal substances, which inhibit the growth of BV-associated pathogenic bacteria (44). In addition, we found Ureaplasma parvum enriched in cured samples both in the short-term cured group and in the long-term cured group and a significant negative correlation with certain BV-associated bacteria, which is consistent with the study of Xiao et al. (45). With its small genome (46) and lack of biosynthetic capacity (47), we speculate that Ureaplasma parvum inhibits the growth of BV-associated pathogenic bacteria by activating the host immune response.
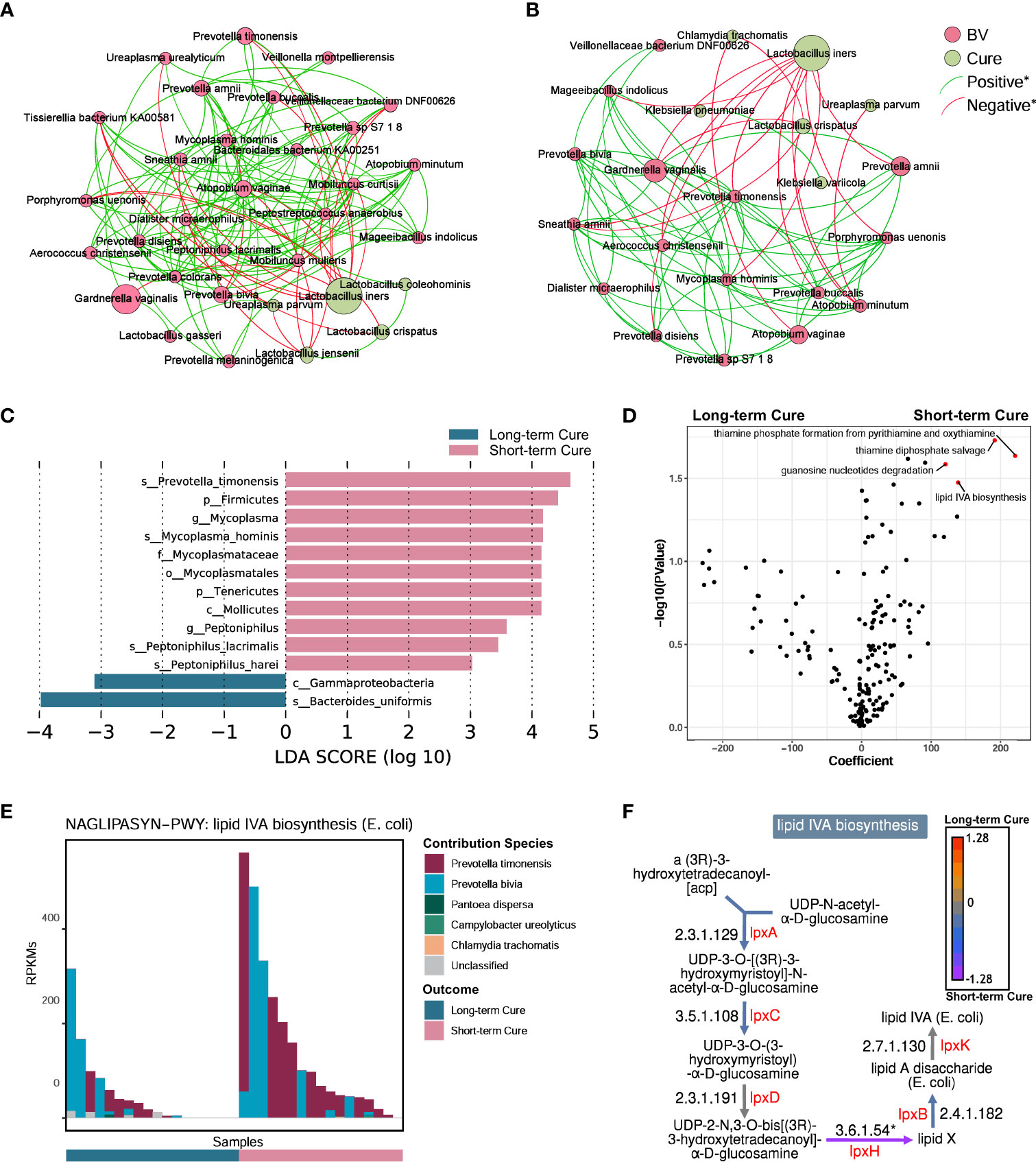
Figure 4 A higher abundance of Prevotella timonensis at baseline was significantly associated with long-term cure failure of BV and greatly contributed to the enrichment of the lipid IVA synthesis pathway. Co-occurrence network showing the interbacterial correlations of vaginal microbiome in the short-term cure group (A) and long-term cure group (B). Each species is only shown in a color corresponding to the time-point when it has the highest relative abundance. Node size indicates the average abundance of each genus. Lines between nodes represent the interbacterial correlations, and the green line and red line indicate positive and negative correlations, respectively. Histogram of the linear discriminant analysis (LDA) scores computed for species (C) with differential abundance between the short-term cure group and long-term group in baseline, and the differential pathways (D) found by the linear model (points with p < 0.05 were marked in red). The contribution ranking of the vaginal microbiome to the lipid IVA biosynthesis pathway is shown in the stack bar chart (E). The top of each set of stacked bars indicates the total abundance of the pathway within a single sample. The individual enzymatic steps of the lipid IVA biosynthesis pathway, as well as the differences between the short-term cure group and long-term group of the gene expressing these enzymes, are shown in this flowchart (F). Mann-Whitney U test; *stands for p-value < 0.05.
LEfSe analysis showed differences in vaginal microbiome at baseline between the short-term and long-term cure groups (Figure 4C). LDA scores indicated that Prevotella timonensis, Mycoplasma hominis, Peptoniphilus lacrimalis, and Peptoniphilus harei were more abundant in the short-term cure; Bacteroides uniformis was more abundant in the group with long-term cure. Using the generalized linear model in the MaAsLin2 package, we performed an association analysis of gene copy numbers of functional pathways in the vaginal microbiome at baseline (Figure 4D). In the short-term cure group, the gene copy number of lipid IVA synthesis, thiamine phosphate synthesis, and thiamine diphosphate salvage were more abundant than those in the long-term cure group (P < 0.05). Among them, the enrichment of the lipid IVA synthesis pathway in the short-term cure group was mainly caused by the high expression of UDP-2,3-diacylglucosamine diphosphatase encoded by lpxH (Figure 4F), and the greatest species contribution was mainly provided by Prevotella timonensis (Figure 4E), which was consistent with the results of LEfSe analysis.
4 Discussion
In this study, there was no significant difference in BV cure rates between the probiotic and control groups at day 14, day 30, and day 90, suggesting a 30-day oral administration of L. gasseri TM13 and L. crispatus LG55 was ineffective as an adjuvant treatment of BV. The short intervention period may be one of the main reasons for the ineffectiveness compared to other studies that obtained effective intervention results (24, 48). In addition, in the BV conversion population, the percentage of people in a transition state was lower in the probiotic group at all three time-points. This suggests that oral probiotics can modulate and improve vaginal health in cured subjects, which is consistent with previous studies (25, 26). Hence, oral administration of L. gasseri TM13 and L. crispatus LG55 is effective in restoring the vaginal health of patients recovered from BV.
The inability of the orally administered probiotic strains to reach or colonize the vagina may be the reason of the treatment failure in BV. Similarly, Husain et al. showed that the colonization rate of the intervention strains and the diversity of the vaginal microbiome did not differ between the probiotic and control groups (49). In fact, L. gasseri TM13 and L. crispatus LG55 used in this study were isolated, cultured, and screened from the human intestine (33). Existing research has shown that there are still many differences in environmental adaptability and systematic developmental characteristics between Lactobacillus strains isolated from the intestine and those isolated from the vagina. For example, Lactobacillus isolated from the vagina grows faster in simulated vaginal fluid than those isolated from the intestine. This may be due to inadequate nutrient availability for Lactobacillus isolated from the intestine in the vagina. Additionally, L. crispatus isolated from the vagina possesses a high abundance of genes related to acid tolerance and carbohydrate-binding modules which help L. crispatus better adapt to the acidic environment of the vagina and obtain more nutrients. Differentially, intestine-derived Lactobacillus have more genes involved in protecting themselves from viruses or toxins, such as CRISPR/Cas systems, glycoside hydrolases (GHs) family, and tetracycline/lincomycin resistance genes to adapt to the complex intestinal environment (50, 51). Therefore, the difference in ecological niches of strains may be the main reason why intestine-derived Lactobacillus cannot colonize in the vagina. A previous multicenter, randomized, double-blind, placebo-controlled trial showed that oral administration of probiotic preparation (prOVag®), which contains Lactobacillus strains isolated from the vagina, delayed BV recurrence, reduced and vaginal pH and Nugent Score by promoting vaginal Lactobacillus counts following standard treatment (52), so Lactobacillus strains derived from the vagina may be a better choice for adjunctive treatment of BV. However, whether increased vaginal Lactobacillus come from orally administrated probiotics or from the enrichment of vaginal native Lactobacillus needs to be further identified. Previous studies have detected probiotics in the vagina using qPCR (48) and 16s rRNA amplicon sequencing (26), but these methods cannot provide strain-specific abundance information, so the conclusions are unreliable. In recent years, strain identification methods based on metagenome sequencing data have emerged, such as MetaMp (53), which uses long-read sequencing technology for strain-level annotation, PStrain (54), based on single nucleotide variants (SNVs), and StrainPanDA (55) that uses pangenome. These advanced methods can provide a more accurate quantitative detection of probiotic colonization and can give direct evidence of the mechanism of action of orally administered probiotics in vivo.
In this study, there was no difference in the relative abundance of L. crispatus and L. gasseri in the vaginal microbiome between probiotic and control groups at all follow-up time-points, which suggest that L. gasseri TM13 and L. crispatus LG55 mainly influenced the host immune response through the intestine. Oral administration of L. gasseri CECT5714 increases the production of SCFAs in the intestine (56) and SCFAs exert anti-inflammatory effects by modulating the levels of PGE, cytokines, and chemokines (57). In addition, consuming fermented milk containing L. crispatus SMFM2016-NK can effectively reduce the expression levels of intestinal TNFα and IL-1β (58). Therefore, L. gasseri TM13 and L. crispatus LG55 may also exert their effects on the improvement of vaginal health by reducing systemic inflammation through immunomodulatory effects in the intestine. In the present study, the abundance of the intervening strains in the intestine could not be maintained at high levels even during the 30-day intervention period. This might be related to the endogenous stability and resilience of the intestinal flora (59), which can prevent the long-term colonization of the intervening strains in the intestine, thus preventing this bacteria from exerting probiotic functions in a sustained and stable manner. Our results also support this inference; the largest difference between the probiotic and control groups in the proportion of participants with Nugent Score < 4 occurred on day 14. Therefore, to improve the effect of L. gasseri TM13 and L. crispatus LG55, a longer intervention period might be one of the optimization options.
We found that L. iners had the highest average relative abundance in cured patients’ vaginal samples. Compared with other Lactobacillus spp., L. iners has lower D-lactic acid production, more complex nutritional structure and more variable Gram staining morphology (60). In addition, L. iners can encode a cytotoxin, which is a pore-forming toxin with a similar structure to the vaginolysin encoded by Garderella vaginalis (61). This may be the reason why L. iners can disturb the balance of vaginal microbiota (62). However, a recent study found that BV patients with more L. iners in their baseline vaginal microbiota had a better clinical treatment outcome after receiving 5% MET gel for 5 days, indicating limited antibacterial function of L. iners may play a key role in the treatment of BV (63). In summary, it is necessary to further clarify whether L. iners is a friend or foe to vaginal health in the future.
The abundance of key bacteria in the vagina influences whether BV can be cured in the long term (45, 64). We found that Prevotella timonensis is enriched in the vagina at the baseline in long-term cure failure patients and that the lipid IVA synthesis pathway, which is abundantly enriched in Prevotella timonensis, is an important precursor material for LPS biosynthesis. Prevotella timonensis is a strictly anaerobic Gram-negative bacterium enriched in the genital tract of BV (65), Chlamydia trachomatis infection (66), and HPV infection (67).Nienke et al. showed that Prevotella timonensis induces the maturation of DC cells to secrete large amounts of pro-inflammatory cytokines, including IL-1β and IL-8, to enhance the inflammatory response in the genital tract (68). Our results also showed a significantly positive correlation between Prevotella timonensis and Lipid IVA synthesis. Lipid IVA is the lipopolysaccharide (LPS) A tetra-acetylated precursor of lipid A in biosynthesis. Although Lipid IVA has been reported as a structural antagonist of LPS, it could also be a substrate of LPS synthesis and further is able to activate the innate immune responses via TLR4 and its co-receptor MD-2 (69). We speculate that Prevotella timonensis aggravated the inflammatory effect by producing LPS persistently, which would induce long-term cure failure of BV. But the pro-inflammatory mechanism of Prevotella timonensis in the vagina and how it perturbed the vaginal microbiome and host immune response still need further study. Furthermore, the probiotic effect of oral L. gasseri TM13 and L. crispatus LG55 on the genital tract has a limited effect in curing subjects, suggesting that precise treatment and personalized probiotic interventions are necessary for BV patients.
This study has some limitations. In terms of the clinical trial design, the participants completed their trials when diagnosed with BV, and there was no continuous follow-up for these patients. We were unable to provide a comprehensive description of the microbiome dynamics of BV treatment failure. Regarding bioinformatics analysis techniques, the relative abundance at the species level was insufficient to accurately calculate the number of intervention probiotic strains in the samples. In addition, the relatively small sample size is also one of the limitations of this study. Finally, future microbiome studies on probiotic interventions to assist in treating BV need to be supported by larger sample sizes and strain-level annotation techniques with higher resolution.
5 Conclusions
Although orally administrated L. gasseri TM13 and L. crispatus LG55 cannot improve BV cure rates, it restores vaginal health after cure mainly acting through the intestine. A higher abundance of Prevotella timonensis at baseline was significantly associated with long-term cure failure of BV and greatly contributed to the enrichment of the lipid IVA synthesis pathway, which could aggravate inflammation response. This inferred that individualized intervention mode should be developed to restore the vaginal health of patients recovering from BV.
Data availability statement
The data presented in the study are deposited in the Genome Sequence Archive (70) in National Genomics Data Center (71), accession number HRA004429, and the CNGB Sequence Archive (72) of China GeneBank DataBase (73) with accession number CNP0003852. Please refer them at https://ngdc.cncb.ac.cn/search/?dbId=&q=HRA004429 and https://db.cngb.org/search/project/CNP0001543.
Ethics statement
The studies involving human participants were reviewed and approved by Medical Ethnic Committee of Peking University Shenzhen Hospital. The patients/participants provided their written informed consent to participate in this study.
Author contributions
XZ, LX, and LG designed the research. SF, JL, LH, XL, YL, and YZh carried out the clinical trial. XZ, LG, FQ, JL and SZ supported the clinical trial and organized data. FQ, CF, ZH, YZo and SZ analyzed the data. FQ, XZ, CF, and ZH draft the paper. LX, SF and LG provided critical revisions of the article. LG, YZh, and HZ were responsible for the probiotic product support. XZ and XL supervised the project. All authors contributed to the article and approved the submitted version.
Funding
This research was supported by the National Natural Science Foundation of China (82171676 and 82201793), the Science and Technology Planning Project of Shenzhen Municipality (JCYJ20220530160206014 and JCYJ20190809101409603), and the Scientific Research Foundation of PEKING UNIVERSITY SHENZHEN HOSPITAL (KYQD202100X and KYQD2022111).
Acknowledgments
We thank the principal investigators, researchers, and all the participants.
Conflict of interest
Authors LG, YYZ, and HZ were employed by the company BGI Precision Nutrition Shenzhen Technology Company Limited.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1125239/full#supplementary-material
Supplementary Figure 1 | Shannon and Simpson index between the probiotics group and the control group in all time-points (A). PCoA-based Bray-Curtis distance matrices between the probiotics group and the control group in all time-points (B).
References
1. Ferreira CST, Donders GG, Parada C, Tristao ADR, Fernandes T, da Silva MG, et al. Treatment failure of bacterial vaginosis is not associated with higher loads of atopobium vaginae and gardnerella vaginalis. J Med Microbiol (2017) 66(8):1217–24. doi: 10.1099/jmm.0.000561
2. Peebles K, Velloza J, Balkus JE, McClelland RS, Barnabas RV. High global burden and costs of bacterial vaginosis: a systematic review and meta-analysis. Sexual Trans Dis (2019) 46:304–11. doi: 10.1097/OLQ.0000000000000972
3. Smith WL, Hedges SR, Mordechai E, Adelson ME, Trama JP, Gygax SE, et al. Cervical and vaginal flora specimens are highly concordant with respect to bacterial vaginosis-associated organisms and commensal lactobacillus species in women of reproductive age. J Clin Microbiol (2014) 52(8):3078–81. doi: 10.1128/JCM.00795-14
4. Brotman RM, Shardell MD, Gajer P, Tracy JK, Zenilman JM, Ravel J, et al. Interplay between the temporal dynamics of the vaginal microbiota and human papillomavirus detection. J Infect Dis (2014) 210(11):1723–33. doi: 10.1093/infdis/jiu330
5. Bautista CT, Wurapa E, Sateren WB, Morris S, Hollingsworth B, Sanchez JL. Bacterial vaginosis: a synthesis of the literature on etiology, prevalence, risk factors, and relationship with chlamydia and gonorrhea infections. Mil Med Res (2016) 3:4. doi: 10.1186/s40779-016-0074-5
6. Cherpes TL, Meyn LA, Krohn MA, Lurie JG, Hillier SL. Association between acquisition of herpes simplex virus type 2 in women and bacterial vaginosis. Clin Infect Dis (2003) 37(3):319–25. doi: 10.1086/375819
7. Wiesenfeld HC, Hillier SL, Krohn MA, Landers DV, Sweet RL. Bacterial vaginosis is a strong predictor of neisseria gonorrhoeae and chlamydia trachomatis infection. Clin Infect Dis (2003) 36(5):663–8. doi: 10.1086/367658
8. Donders GG, Van Calsteren K, Bellen G, Reybrouck R, Van den Bosch T, Riphagen I, et al. Predictive value for preterm birth of abnormal vaginal flora, bacterial vaginosis and aerobic vaginitis during the first trimester of pregnancy. BJOG (2009) 116(10):1315–24. doi: 10.1111/j.1471-0528.2009.02237.x
9. Cohen CR, Lingappa JR, Baeten JM, Ngayo MO, Spiegel CA, Hong T, et al. Bacterial vaginosis associated with increased risk of female-to-male HIV-1 transmission: a prospective cohort analysis among African couples. PloS Med (2012) 9(6):e1001251. doi: 10.1371/journal.pmed.1001251
10. Koumans EH, Sternberg M, Bruce C, McQuillan G, Kendrick J, Sutton M, et al. The prevalence of bacterial vaginosis in the United States, 2001-2004; associations with symptoms, sexual behaviors, and reproductive health. Sex Transm Dis (2007) 34(11):864–9. doi: 10.1097/OLQ.0b013e318074e565
11. Ravel J, Brotman RM, Gajer P, Ma B, Nandy M, Fadrosh DW, et al. Daily temporal dynamics of vaginal microbiota before, during and after episodes of bacterial vaginosis. Microbiome (2013) 1(1):29. doi: 10.1186/2049-2618-1-29
12. Brotman RM. Vaginal microbiome and sexually transmitted infections: an epidemiologic perspective. J Clin Invest (2011) 121(12):4610–7. doi: 10.1172/JCI57172
13. Gajer P, Brotman RM, Bai G, Sakamoto J, Schutte UM, Zhong X, et al. Temporal dynamics of the human vaginal microbiota. Sci Transl Med (2012) 4(132):132ra152. doi: 10.1126/scitranslmed.3003605
14. Vitali B, Cruciani F, Picone G, Parolin C, Donders G, Laghi L. Vaginal microbiome and metabolome highlight specific signatures of bacterial vaginosis. Eur J Clin Microbiol Infect Dis (2015) 34(12):2367–76. doi: 10.1007/s10096-015-2490-y
15. Xiao B, Niu X, Han N, Wang B, Du P, Na R, et al. Predictive value of the composition of the vaginal microbiota in bacterial vaginosis, a dynamic study to identify recurrence-related flora. Sci Rep (2016) 6:26674. doi: 10.1038/srep26674
16. Cao J, Hu Y, Liu F, Wang Y, Bi Y, Lv N, et al. Metagenomic analysis reveals the microbiome and resistome in migratory birds. Microbiome (2020) 8(1):26. doi: 10.1186/s40168-019-0781-8
17. Liu F, Wang Y, Gao GF, Zhu B. Metagenomic analysis reveals the abundance and diversity of ARGs in children's respiratory tract microbiomes. J Infect (2020) 80(2):232–54. doi: 10.1016/j.jinf.2019.11.002
18. Liu F, Zhou Y, Zhu L, Wang Z, Ma L, He Y, et al. Comparative metagenomic analysis of the vaginal microbiome in healthy women. Synth Syst Biotechnol (2021) 6(2):77–84. doi: 10.1016/j.synbio.2021.04.002
19. Ruiz-Perez D, Coudray MS, Colbert B, Krupp K, Kumari H, Stebliankin V, et al. Effect of metronidazole on vaginal microbiota associated with asymptomatic bacterial vaginosis. Access Microbiol (2021) 3(5):226. doi: 10.1099/acmi.0.000226
20. Workowski KA. Centers for disease control and prevention sexually transmitted diseases treatment guidelines. Clin Infect Dis (2015) 61 Suppl 8:S759–762. doi: 10.1093/cid/civ771
21. Bostwick DG, Woody J, Hunt C, Budd W. Antimicrobial resistance genes and modelling of treatment failure in bacterial vaginosis: clinical study of 289 symptomatic women. J Med Microbiol (2016) 65(5):377–86. doi: 10.1099/jmm.0.000236
22. Togni G, Battini V, Bulgheroni A, Mailland F, Caserini M, Mendling W. In vitro activity of nifuratel on vaginal bacteria: could it be a good candidate for the treatment of bacterial vaginosis? Antimicrob Agents Chemother (2011) 55(5):2490–2. doi: 10.1128/AAC.01623-10
23. Laue C, Papazova E, Liesegang A, Pannenbeckers A, Arendarski P, Linnerth B, et al. Effect of a yoghurt drink containing lactobacillus strains on bacterial vaginosis in women - a double-blind, randomised, controlled clinical pilot trial. Benef Microbes (2018) 9(1):35–50. doi: 10.3920/BM2017.0018
24. Reznichenko H, Henyk N, Maliuk V, Khyzhnyak T, Tynna Y, Filipiuk I, et al. Oral intake of lactobacilli can be helpful in symptomatic bacterial vaginosis: a randomized clinical study. J Low Genit Tract Dis (2020) 24(3):284–9. doi: 10.1097/LGT.0000000000000518
25. Chen C, Hao L, Zhang Z, Tian L, Zhang X, Zhu J, et al. Cervicovaginal microbiome dynamics after taking oral probiotics. J Genet Genomics (2021) 48(8):716–26. doi: 10.1016/j.jgg.2021.03.019
26. Martoni CJ, Frederiksen AKS, Damholt A, Leyer G. Effects of a 10-strain oral probiotic on parameters of vaginal health and microbial community: a pilot clinical study. Int J Womens Health (2022) 14:29–39. doi: 10.2147/IJWH.S341046
27. Reid G, Beuerman D, Heinemann C, Bruce AW. Probiotic lactobacillus dose required to restore and maintain a normal vaginal flora. FEMS Immunol Med Microbiol (2001) 32(1):37–41. doi: 10.1111/j.1574-695X.2001.tb00531.x
28. Morelli L, Zonenenschain D, Del Piano M, Cognein P. Utilization of the intestinal tract as a delivery system for urogenital probiotics. J Clin Gastroenterol (2004) 38(6 Suppl):S107–110. doi: 10.1097/01.mcg.0000128938.32835.98
29. Amabebe E, Anumba DOC. Female gut and genital tract microbiota-induced crosstalk and differential effects of short-chain fatty acids on immune sequelae. Front Immunol (2020) 11:2184. doi: 10.3389/fimmu.2020.02184
30. Ravel J, Gajer P, Abdo Z, Schneider GM, Koenig SS, McCulle SL, et al. Vaginal microbiome of reproductive-age women. Proc Natl Acad Sci USA (2011) 108(Suppl 1):4680–7. doi: 10.1073/pnas.1002611107
31. Zhang Y, Lyu J, Ge L, Huang L, Peng Z, Liang Y, et al. Probiotic lacticaseibacillus rhamnosus GR-1 and limosilactobacillus reuteri RC-14 as an adjunctive treatment for bacterial vaginosis do not increase the cure rate in a Chinese cohort: a prospective, parallel-group, randomized, controlled study. Front Cell Infect Microbiol (2021) 11:669901. doi: 10.3389/fcimb.2021.669901
32. Vallor AC, Antonio MAD, Hawes SE, Hillier SL. Factors associated with acquisition of, or persistent colonization by, vaginal lactobacilli: role of hydrogen peroxide production. J Infect Dis (2001) 184:1431–6. doi: 10.1086/324445
33. Lyu J, Gao M, Zhao S, Lyu X, Zhao X, Zou Y, et al. From whole genome to probiotic candidates: a study of potential lactobacillus strain selection for vaginitis treatment (2022). Available at: https://www.biorxiv.org/content/10.1101/2022.12.11.519948v1 (Accessed December 12, 2022). [Preprint].
34. Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J Clin Microbiol (1991) 29(2):297–301. doi: 10.1128/jcm.29.2.297-301.1991
35. Donders GG, Vereecken A, Bosmans E, Dekeersmaecker A, Salembier G, Spitz B. Definition of a type of abnormal vaginal flora that is distinct from bacterial vaginosis: aerobic vaginitis. BJOG (2002) 109(1):34–43. doi: 10.1111/j.1471-0528.2002.00432.x
36. Fang C, Zhong H, Lin Y, Chen B, Han M, Ren H, et al. Assessment of the cPAS-based BGISEQ-500 platform for metagenomic sequencing. Gigascience (2018) 7(3):1–8. doi: 10.1093/gigascience/gix133
37. Han M, Hao L, Lin Y, Li F, Wang J, Yang H, et al. A novel affordable reagent for room temperature storage and transport of fecal samples for metagenomic analyses. Microbiome (2018) 6(1):43. doi: 10.1186/s40168-018-0429-0
38. Chen S, Zhou Y, Chen Y, Gu J. Fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics (2018) 34(17):i884–90. doi: 10.1093/bioinformatics/bty560
39. Langmead B, Trapnell C, Pop M, Salzberg SL. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol (2009) 10(3):R25. doi: 10.1186/gb-2009-10-3-r25
40. Beghini F, McIver LJ, Blanco-Miguez A, Dubois L, Asnicar F, Maharjan S, et al. Integrating taxonomic, functional, and strain-level profiling of diverse microbial communities with bioBakery 3. Elife (2021) 10:e65088. doi: 10.7554/eLife.65088
41. Segata N, Izard J, Waldron L, Gevers D, Miropolsky L, Garrett WS, et al. Metagenomic biomarker discovery and explanation. Genome Biol (2011) 12(6):R60. doi: 10.1186/gb-2011-12-6-r60
42. Mallick H, Rahnavard A, McIver LJ, Ma S, Zhang Y, Nguyen LH, et al. Multivariable association discovery in population-scale meta-omics studies. PloS Comput Biol (2021) 17(11):e1009442. doi: 10.1371/journal.pcbi.1009442
43. Gustin AT, Thurman AR, Chandra N, Schifanella L, Alcaide M, Fichorova R, et al. Recurrent bacterial vaginosis following metronidazole treatment is associated with microbiota richness at diagnosis. Am J Obstet Gynecol (2022) 226(2):225.e221–225.e215. doi: 10.1016/j.ajog.2021.09.018
44. Zhu B, Tao Z, Edupuganti L, Serrano MG, Buck GA. Roles of the microbiota of the female vaginal in gynecological and reproductive health. Microbiol Mol Biol Rev (2022) 84(4):e0018121. doi: 10.1128/mmbr.00181-21
45. Xiao B, Wu C, Song W, Niu X, Qin N, Liu Z, et al. Association analysis on recurrence of bacterial vaginosis revealed microbes and clinical variables important for treatment outcome. Front Cell Infect Microbiol (2019) 9:189. doi: 10.3389/fcimb.2019.00189
46. Paralanov V, Lu J, Duffy LB, Crabb DM, Shrivastava S, Methe BA, et al. Comparative genome analysis of 19 ureaplasma urealyticum and ureaplasma parvum strains. BMC Microbiol (2012) 12:88. doi: 10.1186/1471-2180-12-88
47. Combaz-Sohnchen N, Kuhn A. ). a systematic review of mycoplasma and ureaplasma in urogynaecology. Geburtshilfe Frauenheilkd (2017) 77(12):1299–303. doi: 10.1055/s-0043-119687
48. Cohen CR, Wierzbicki MR, French AL, Morris S, Newmann S, Reno H, et al. Randomized trial of lactin-V to prevent recurrence of bacterial vaginosis. N Engl J Med (2020) 382(20):1906–15. doi: 10.1056/NEJMoa1915254
49. Husain S, Allotey J, Drymoussi Z, Wilks M, Fernandez-Felix BM, Whiley A, et al. Effects of oral probiotic supplements on vaginal microbiota during pregnancy: a randomised, double-blind, placebo-controlled trial with microbiome analysis. BJOG (2020) 127(2):275–84. doi: 10.1111/1471-0528.15675
50. Pan M, Hidalgo-Cantabrana C, Goh YJ, Sanozky-Dawes R, Barrangou R. Comparative analysis of lactobacillus gasseri and lactobacillus crispatus isolated from human urogenital and gastrointestinal tracts. Front Microbiol (2019) 10:3146. doi: 10.3389/fmicb.2019.03146
51. Zhang Q, Zhang L, Ross P, Zhao J, Zhang H, Chen W. Comparative genomics of lactobacillus crispatus from the gut and vagina reveals genetic diversity and lifestyle adaptation. Genes (Basel) (2020) 11:360. doi: 10.3390/genes11040360
52. Heczko PB, Tomusiak A, Adamski P, Jakimiuk AJ, Stefański G, Mikołajczyk-Cichońska A, et al. Supplementation of standard antibiotic therapy with oral probiotics for bacterial vaginosis and aerobic vaginitis: a randomised, double-blind, placebo-controlled trial. BMC Womens Health (2015) 15:115. doi: 10.1186/s12905-015-0246-6
53. Dilthey AT, Jain C, Koren S, Phillippy AM. Strain-level metagenomic assignment and compositional estimation for long reads with MetaMaps. Nat Commun (2019) 10(1):3066. doi: 10.1038/s41467-019-10934-2
54. Wang S, Jiang Y, Li S. PStrain: an iterative microbial strains profiling algorithm for shotgun metagenomic sequencing data. Bioinformatics. (2020) 36(22-23):5499–506. doi: 10.1093/bioinformatics/btaa1056
55. Hu H, Tan Y, Li C, Chen J, Kou Y, Xu ZZ, et al. StrainPanDA: linked reconstruction of strain composition and gene content profiles via pangenome-based decomposition of metagenomic data. iMeta (2022) 1(3):e41. doi: 10.1002/imt2.41
56. Olivares M, Diaz-Ropero MA, Gomez N, Lara-Villoslada F, Sierra S, Maldonado JA, et al. Oral administration of two probiotic strains, lactobacillus gasseri CECT5714 and lactobacillus coryniformis CECT5711, enhances the intestinal function of healthy adults. Int J Food Microbiol (2006) 107(2):104–11. doi: 10.1016/j.ijfoodmicro.2005.08.019
57. Cox MA, Jackson J, Stanton M, Rojas-Triana A, Bober L, Laverty M, et al. Short-chain fatty acids act as antiinflammatory mediators by regulating prostaglandin E(2) and cytokines. World J Gastroenterol (2009) 15(44):5549–57. doi: 10.3748/wjg.15.5549
58. Choi Y, Park E, Kim S, Ha J, Oh H, Kim Y, et al. Fermented milk with lactobacillus curvatus SMFM2016-NK alleviates periodontal and gut inflammation, and alters oral and gut microbiota. J Dairy Sci (2021) 104(5):5197–207. doi: 10.3168/jds.2020-19625
59. Lozupone CA, Stombaugh JI, Gordon JI, Jansson JK, Knight R. Diversity, stability and resilience of the human gut microbiota. Nature (2012) 489(7415):220–30. doi: 10.1038/nature11550
60. Zheng N, Guo R, Wang J, Zhou W, Ling Z. Contribution of lactobacillus iners to vaginal health and diseases: a systematic review. Front Cell Infection Microbiol (2021) 11:792787. doi: 10.3389/fcimb.2021.792787
61. Ragaliauskas T, Plečkaitytė M, Jankunec M, Labanauskas L, Baranauskiene L, Valincius G. Inerolysin and vaginolysin, the cytolysins implicated in vaginal dysbiosis, differently impair molecular integrity of phospholipid membranes. Sci Rep (2019) 9:10606. doi: 10.1038/s41598-019-47043-5
62. France M, Alizadeh M, Brown S, Ma B, Ravel J. Towards a deeper understanding of the vaginal microbiota. Nat Microbiol (2022) 7:367–78. doi: 10.1038/s41564-022-01083-2
63. Zhou R, Lu J, Wang J, Xiao B. Vaginal lactobacillus iners abundance is associated with outcome in antibiotic treatment of bacterial vaginosis and capable of inhibiting gardnerella. Front Cell Infection Microbiol (2022) 12:1033431. doi: 10.3389/fcimb.2022.1033431
64. Mollin A, Katta M, Sobel JD, Akins RA. Association of key species of vaginal bacteria of recurrent bacterial vaginosis patients before and after oral metronidazole therapy with short- and long-term clinical outcomes. PloS One (2022) 17(7):e0272012. doi: 10.1371/journal.pone.0272012
65. Lehtoranta L, Hibberd AA, Reimari J, Junnila J, Yeung N, Maukonen J, et al. Recovery of vaginal microbiota after standard treatment for bacterial vaginosis infection: an observational study. Microorganisms (2020) 8(6):875. doi: 10.3390/microorganisms8060875
66. Filardo S, Di Pietro M, Tranquilli G, Latino MA, Recine N, Porpora MG, et al. Selected immunological mediators and cervical microbial signatures in women with chlamydia trachomatis infection. mSystems (2019) 4(4):e00094–19. doi: 10.1128/mSystems.00094-19
67. Chen Y, Qiu X, Wang W, Li D, Wu A, Hong Z, et al. Human papillomavirus infection and cervical intraepithelial neoplasia progression are associated with increased vaginal microbiome diversity in a Chinese cohort. BMC Infect Dis (2020) 20(1):629. doi: 10.1186/s12879-020-05324-9
68. van Teijlingen NH, Helgers LC, Zijlstra-Willems EM, van Hamme JL, Ribeiro CMS, Strijbis K, et al. Vaginal dysbiosis associated-bacteria megasphaera elsdenii and prevotella timonensis induce immune activation via dendritic cells. J Reprod Immunol (2020) 138:103085. doi: 10.1016/j.jri.2020.103085
69. Ohto U, Fukase K, Miyake K, Shimizu T. Structural basis of species-specific endotoxin sensing by innate immune receptor TLR4/MD-2. Proc Natl Acad Sci USA (2012) 109(19):7421–6. doi: 10.1073/pnas.1201193109
70. Chen T, Chen X, Zhang S, Zhu J, Tang B, Wang A, et al. The Genome Sequence Archive Family: Toward Explosive Data Growth and Diverse Data Types. Genom Proteom Bioinform (2021) 19(4):578–83. doi: 10.1016/j.gpb.2021.08.001
71. CNCB-NGDC Members and Partners. Database Resources of the National Genomics Data Center, China National Center for Bioinformation in 2023. Nucleic Acids Res (2023) 51(D1):D18–28. doi: 10.1093/nar/gkac1073
72. Guo X, Chen F, Gao F, Li L, Liu K, You L, Hua C, et al. CNSA: a data repository for archiving omics data. Database (2020) 2020:baaa055. doi: 10.1093/database/baaa055
Keywords: bacterial vaginosis, Lactobacillus, oral administration, vaginal microbiome, Prevotella timonensis
Citation: Qi F, Fan S, Fang C, Ge L, Lyu J, Huang Z, Zhao S, Zou Y, Huang L, Liu X, Liang Y, Zhang Y, Zhong Y, Zhang H, Xiao L and Zhang X (2023) Orally administrated Lactobacillus gasseri TM13 and Lactobacillus crispatus LG55 can restore the vaginal health of patients recovering from bacterial vaginosis. Front. Immunol. 14:1125239. doi: 10.3389/fimmu.2023.1125239
Received: 20 December 2022; Accepted: 19 June 2023;
Published: 27 July 2023.
Edited by:
Ian Marriott, University of North Carolina at Charlotte, United StatesReviewed by:
Roberta Gaziano, University of Rome Tor Vergata, ItalyAndile Mtshali, Centre for the AIDS Programme of Research in South Africa (CAPRISA), South Africa
Copyright © 2023 Qi, Fan, Fang, Ge, Lyu, Huang, Zhao, Zou, Huang, Liu, Liang, Zhang, Zhong, Zhang, Xiao and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaowei Zhang, eGlhb3dlaXpoYW5nQHBrdXN6aC5jb20=; Liang Xiao, eGlhb2xpYW5nQGdlbm9taWNzLmNu
†These authors have contributed equally to this work and share first authorship
 Fengyuan Qi
Fengyuan Qi Shangrong Fan
Shangrong Fan Chao Fang
Chao Fang Lan Ge
Lan Ge Jinli Lyu
Jinli Lyu Zhuoqi Huang
Zhuoqi Huang Shaowei Zhao2,3
Shaowei Zhao2,3 Yuanqiang Zou
Yuanqiang Zou Liting Huang
Liting Huang Yongke Zhang
Yongke Zhang Yiyi Zhong
Yiyi Zhong Liang Xiao
Liang Xiao Xiaowei Zhang
Xiaowei Zhang