- 1Department of Health Sciences, University “Magna Graecia” of Catanzaro, Catanzaro, Italy
- 2Dipartimento di Promozione della Salute, Materno Infantile, Medicina Interna e Specialistica di Eccellenza (PROMISE), University of Palermo, Palermo, Italy
- 3Department of Medical and Surgical Sciences, University of Foggia, Foggia, Italy
- 4Department of Clinical and Experimental Medicine, University of Catania, Catania, Italy
- 5Department of Basic Medical Science, Neuroscience and Sense Organs, University “Aldo Moro”, Bari, Italy
- 6Department of Respiratory Medicine, University “Federico II” of Naples, Naples, Italy
- 7Department of Medicine, Surgery and Dentistry, University of Salerno, Salerno, Italy
- 8Allergology and Pulmonology Unit, Provincial Outpatient Center of Palermo, Palermo, Italy
- 9Department of Clinical and Experimental Medicine, University of Messina, Messina, Italy
Background: The efficacy of dupilumab as biological treatment of severe asthma and chronic rhinosinusitis with nasal polyps (CRSwNP) depends on its ability to inhibit the pathophysiologic mechanisms involved in type 2 inflammation.
Objective: To assess in a large sample of subjects with severe asthma, the therapeutic impact of dupilumab in real-life, with regard to positive or negative skin prick test (SPT) and CRSwNP presence or absence.
Methods: Clinical, functional, and laboratory parameters were measured at baseline and 24 weeks after the first dupilumab administration. Moreover, a comparative evaluation was carried out in relation to the presence or absence of SPT positivity and CRSwNP.
Results: Among the 127 recruited patients with severe asthma, 90 had positive SPT, while 78 reported CRSwNP. Compared with the 6 months preceding the first dupilumab injection, asthma exacerbations decreased from 4.0 (2.0-5.0) to 0.0 (0.0-0.0) (p < 0.0001), as well as the daily prednisone intake fell from 12.50 mg (0.00-25.00) to 0.00 mg (0.00-0.00) (p < 0.0001). In the same period, asthma control test (ACT) score increased from 14 (10-18) to 22 (20-24) (p < 0.0001), and sino-nasal outcome test (SNOT-22) score dropped from 55.84 ± 20.32 to 19.76 ± 12.76 (p < 0.0001). Moreover, we observed relevant increases in forced expiratory volume in one second (FEV1) from the baseline value of 2.13 L (1.62-2.81) to 2.39 L (1.89-3.06) (p < 0.0001). Fractional exhaled nitric oxide (FeNO) values decreased from 27.0 ppb (18.0-37.5) to 13.0 ppb (5.0-20.0) (p < 0.0001). These improvements were quite similar in subgroups of patients characterized by SPT negativity or positivity, and CRSwNP absence or presence. No statistically significant correlations were detected between serum IgE levels, baseline blood eosinophils or FeNO levels and dupilumab-induced changes, with the exception of FEV1 increase, which was shown to be positively correlated with FeNO values (r = 0.3147; p < 0.01).
Conclusion: Our results consolidate the strategic position of dupilumab in its role as an excellent therapeutic option currently available within the context of modern biological treatments of severe asthma and CRSwNP, frequently driven by type 2 airway inflammation.
Introduction
Dupilumab is a completely human monoclonal antibody, belonging to the IgG4 immunoglobulin class, whose mechanism of action consists of the dual antagonism of the interleukin 4 (IL-4) and 13 (IL-13) receptors (1). In addition to the treatment of severe asthma and atopic dermatitis, dupilumab is also indicated for the biological therapy of nasal polyposis, which is a frequent comorbidity of severe asthma (2).
IL-4 and IL-13 play critical roles in the pathogenesis of severe type 2 asthma (3). In particular, IL-4 is crucial in the development and maintenance of the acquired immune response mediated by T helper 2 (Th2) lymphocytes. At the level of B lymphocytes of allergic patients, IL-4 and IL-13 induce the so-called isotypic switch, responsible for the synthesis of immunoglobulins E (IgE), which degranulate mast cells and basophils, facilitate the presentation of allergens by dendritic cells to T lymphocytes, and inhibit eosinophil apoptosis (4). IL-4 and IL-13 promote the trafficking of eosinophils to inflammatory sites and impair the integrity of the airway epithelial barrier. Furthermore, IL-13 stimulates mucus secretion and goblet cell hyperplasia, and also up-regulates the expression of the inducible form of the enzyme nitric oxide (NO) synthase (iNOS), which increases NO production within the airways (5, 6). Chronic rhinosinusitis with nasal polyps (CRSwNP) is a frequent comorbidity of severe asthma, and type 2 inflammation very often contributes significantly to the pathogenesis of nasal polyposis. Indeed, at the level of upper airways IL-4 and IL-13 play a key role in both inflammatory and structural changes (tissue remodelling) that underlie the formation of nasal polyps (7).
The efficacy of dupilumab as biological treatment of severe asthma and nasal polyposis depends on its remarkable ability to inhibit the pathophysiologic mechanisms involved in type 2 inflammation. In fact, dupilumab is an efficient dual antagonist of both IL-4 and IL-13 receptors 13 (3). Specifically, dupilumab binds with high affinity to the IL-4 receptor α subunit (IL-4Rα). This receptor subunit is a key component of the type I receptor, consisting of the IL-4Rα/γC dimer, which is activated by IL-4 (8). The type II receptor is instead constituted by the IL-4Rα subunit and the α1 chain of the IL-13 receptor (IL-4Rα/IL-13Rα1 dimer), and can therefore be stimulated by IL-4 and IL-13. The type I receptor is predominantly expressed by immune-inflammatory cells such as T and B lymphocytes, dendritic cells, monocytes/macrophages, mast cells, basophils and eosinophils. The type II receptor is also present on airway structural cells such as goblet cells, fibroblasts and smooth muscle cells (9). Upon pharmacological blockade of both type I and type II receptors, dupilumab neutralizes the biological effects of IL-4 and IL-13.
Due to this powerful mechanism of action, within the context of add-on biological treatment of severe asthma and nasal polyposis dupilumab exerts remarkable therapeutic effects, well documented by several randomized controlled trials (RCTs) (10). In particular, the “LIBERTY ASTHMA QUEST” study demonstrated that dupilumab was capable of significantly reducing the annual rate of severe asthma exacerbations and improving lung function (11). Furthermore, the “LIBERTY ASTHMA VENTURE” trial highlighted the ability of dupilumab to significantly decrease the consumption of oral corticosteroids (OCS) (12). These results were recently confirmed by the open-label extension study LIBERTY ASTHMA TRAVERSE, which further monitored for additional 96 weeks many patients previously enrolled in the LIBERTY ASTHMA QUEST and LIBERTY ASTHMA VENTURE (13). As regards the adjunctive biological therapy of nasal polyposis, the studies “LIBERTY NP SINUS-24” and “LIBERTY NP SINUS-52” documented the efficacy of dupilumab by evaluating the improvement of many relevant parameters (14).
However, only a few real-world studies referring to a quite low number of patients have been published so far (15–19). Hence, the aim of our present real-life observational investigation was to evaluate, in a larger sample of subjects with severe asthma, also including many patients with nasal polyposis, the therapeutic impact of dupilumab on upper and lower airway symptoms, severe asthma exacerbations, OCS intake and lung function, as well as on the overall clinical expression of nasal polyposis.
Patients and methods
Study design and patient enrollment
In the present retrospective multicenter observational study, we recruited adult outpatients (>18 years) with severe type 2 asthma treated with dupilumab. Subjects were enrolled at the following asthma centers: Respiratory Medicine Section, University “Aldo Moro”, Bari, Italy; Allergy and Respiratory Medicine, University of Catania, Italy; Respiratory Disease Unit, University “Magna Graecia” of Catanzaro, Italy; Allergology and Clinical Immunology Unit, University of Foggia, Italy; Respiratory Disease Unit, University of Foggia, Italy; Allergy and Clinical Immunology Unit, University of Messina; Pulmonology Unit, “Monaldi” University Hospital, Naples, Italy; Pulmonology Unit, University of Palermo, Italy; Allergology and Pulmonology Unit, Provincial Outpatient Center of Palermo, Italy; Respiratory Disease Unit, University of Salerno, Italy; Division of Allergy and Clinical Immunology, University of Salerno.
Patients reported persistent asthmatic symptoms and required high doses of the inhaled therapeutic combinations ICS (inhaled corticosteroids)/LABA (long-acting β2-adrenergic agonists), associated with a LAMA (long-acting muscarinic receptor antagonist). Enrollment took place consecutively, and the only inclusion criteria were those needed for prescription of dupilumab. All recruited patients met the European Respiratory Society (ERS)/American Thoracic Society (ATS) criteria defining severe uncontrolled asthma (20). Blood counts of eosinophils, basophils and neutrophils were obtained using automated hematology analyzers (21, 22). At baseline, all participants had an eosinophilic blood count of at least 150 cells/μL and/or fractional exhaled nitric oxide (FeNO) levels greater than 25 parts per billion (ppb), and/or they were treated with lifelong or near-continuous OCS therapies.
The aforementioned centers participating in the study used a shared database to acquire clinical, functional and biological data. Smoking habit and comorbidities such as gastroesophageal reflux disease (GERD), nasal polyposis, bronchiectasis, osteoporosis, anxiety, atopic dermatitis and obstructive sleep apnea syndrome (OSAS) were evaluated. Symptom control was assessed by administering to all recruited patients the asthma control test (ACT). The latter includes 5 key questions referring to the frequency of asthma symptoms and to the need of inhaled rescue medication during the previous 4 weeks (23). Each question scores from 1 to 5; therefore, ACT score ranges from 5 (worse control) to 25 points (complete control). Spirometry was performed following ATS/ERS guidelines (24). FeNO levels were measured in accordance with ATS/ERS recommendations (25, 26). Treatment with dupilumab was prescribed according to current eligibility guidelines, and the drug was administered subcutaneously using an initial dose of 600 mg (two 300 mg injections at different skin sites), followed by a maintenance dose of 300 mg every 2 weeks (27, 28).
This observational study met the standards of Good Clinical Practice (GCP) and the principles of the Declaration of Helsinki. All recruited patients signed a written informed consent. Our study was also conducted in accordance with the provisions of the local Ethics Committee of Calabria Region, Italy (Catanzaro, Italy; document n. 182 – 20 May 2021).
Outcomes and measurements
The main purpose of this real-life study was to evaluate the efficacy of dupilumab in daily clinical practice. The number of asthma exacerbations, emergency department visits and daily inhalations of short-acting β2-adrenergic agonists (SABA), as well as prednisone intake, ACT score, sino-nasal outcome test questionnaire (SNOT-22), the number of relapses of nasal polyposis, forced expiratory volume in one second (FEV1), forced vital capacity (FVC), mean forced expiratory flow between 25% and 75% of FVC (FEF25-75), FeNO levels, as well as blood eosinophil, basophil, and neutrophil counts were assessed at baseline and 24 weeks after the first dupilumab administration.
A secondary objective was to retrospectively verify the therapeutic responses of our patients to dupilumab, in relation to SPT positivity or negativity, as well as with regard to the presence or absence of CRSwNP. The diagnosis of CRSwNP was formulated on the basis of symptoms, nasal endoscopy and computed tomography (CT) (29, 30). Skin prick test (SPT) was performed by placing a drop of each allergen on the forearm evidenced with a skin marker, and each drop was pricked by a sterile lancet; skin sensitivity was determined by comparing any wheal with that one caused by histamine (31).
Furthermore, after 6 months of adjunctive therapy with dupilumab we analyzed the possible correlations existing between the baseline concentrations of serum IgE, FeNO and blood eosinophils, and the observed changes regarding asthma exacerbations, daily consumption of prednisone and SABA, ACT score, SNOT-22 score, FEV1, FVC, and FEF25-75 values.
In addition, the occurrence of unwanted side effects was investigated on the basis of available information stored in clinical records.
Statistical analysis
All data are expressed as mean ± standard deviation (SD) if normally distributed, otherwise as median values with the interquartile range (IQR). The normality of data distribution was checked using Anderson-Darling and Kolmogorov-Smirnov tests. Paired t-test and Mann-Whitney’s U-test for paired data were used to compare variables when appropriate. The latter statistical test was also used for the secondary objective of the study, i.e. the comparative evaluation of dupilumab efficacy in patients with positive or negative SPT, and with regard to the presence or absence of CRSwNP. Fisher’s test was applied to compare categorical variables. The association between baseline concentrations of type 2 inflammation biomarkers (serum IgE, blood eosinophils, and FeNO) and changes in clinical and functional parameters was assessed using linear regression analysis. In particular, the correlation index R for Spearman’s ranks was evaluated. A p-value less than 0.05 (two-tailed) was considered as statistically significant. Statistical analyses and figures were performed using Prism Version 9.4.0 software (GraphPad Software Inc., San Diego, California, USA).
Results
Efficacy of dupilumab in the whole population
A total of 127 participants were recruited, including 63 (49.6%) women and 64 (50.4%) men, with a median age of 56.0 years (45.0-64.0), and a median body mass index (BMI) value of 26.0 Kg/m2 (23.0-30.0). Mean baseline FEV1 was 76.56 ± 20.03% of predicted value. Among the enrolled patients, 90 (70.9%) had positive SPT for perennial and/or seasonal allergens, while 78 (61.4%) reported CRSwNP. Baseline patient characteristics are summarized in Table 1.
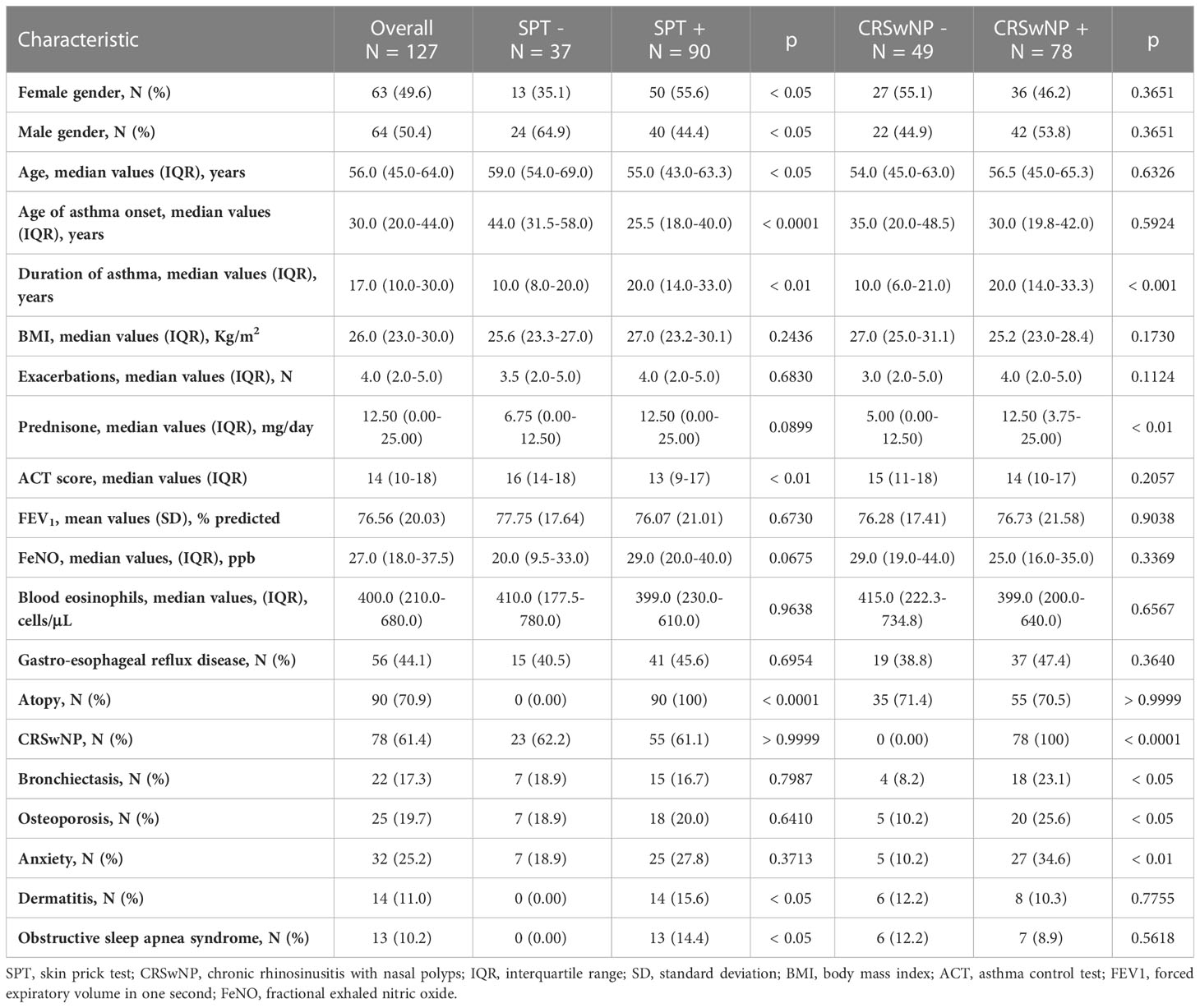
Table 1 Baseline patient characteristics, stratified according SPT negativity or positivity and CRSwNP absence or presence.
Compared with the 6-month pre-treatment period (before the first injection of dupilumab), the median number of asthma exacerbations dramatically decreased from 4.0 (2.0-5.0) to 0.0 (0.0-0.0) (p < 0.0001) after 6 months of anti-IL4R/IL-13R therapy (Figure 1A). Furthermore, in the same period the mean number of emergency department (ED) visits (0.40 ± 0.75 vs. 0.0 ± 0.0; p < 0.0001) (Figure 1B) and daily SABA inhalations (1.67 ± 1.59 vs. 0.09 ± 0.39; p < 0.0001) also significantly fell down (Figure 1C). These therapeutic effects allowed a reduction of the daily prednisone intake from 12.50 mg (0.00-25.00) to 0.00 mg (0.00-0.00) (p < 0.0001) (Figure 1D). In addition, the percentage of patients taking daily OCS decreased from 66.9% (before starting dupilumab treatment) to 5.5% after six months of therapy. After 24 weeks of treatment with dupilumab, ACT score increased significantly from a baseline value of 14 (10-18) to 22 (20-24) (p < 0.0001) (Figure 1E), and SNOT-22 score dropped from 55.84 ± 20.32 to 19.76 ± 12.76 (p < 0.0001) (Figure 1F) in subjects also suffering from nasal polyposis. Furthermore, in this subset of patients the number of recurrences of nasal polyposis decreased from 2 (1-2.5) to 0 (0-0) (p < 0.0001) after initiation of dupilumab therapy (Figure 1G). In addition to the above clinical results, we also observed a significant improvement in respiratory function, documented by increases in FEV1 from the baseline value of 2.13 L (1.62-2.81) to 2.39 L (1.89-3.06) (p < 0.0001) (Figure 1H), in FVC from 3.16 L (2.43-3.84) to 3.36 L (2.68-3.92) (p < 0.0001) (Figure 1I), and in FEF25-75 from 46.50% (30.75-69.00) to 63.50% (44.75-81.25) (p < 0.0001) of predicted values (Figure 1J). Furthermore, serum IgE levels lowered from 238.0 IU/mL (81.0-499.0) to 160.0 IU/mL (49.0-450.0) (p < 0.0001) (Figure 1K). In the same observation period FeNO values decreased from 27.0 ppb (18.0-37.5) to 13.0 ppb (5.0-20.0) (p < 0.0001) (Figure 1L). Regarding the possible hematological effects of dupilumab, after six months of additional treatment, the blood eosinophil count did not undergo substantial variations, thus changing from 400.0 cells/µL (222.5-677.5) to 395.0 cells/µL (181.5-565.0) (p = 0.427) (Figure 1M). Similarly, blood basophil and neutrophil values did not change significantly, going from 40.0 cells/µL (30.0-60.0) to 37.5 cells/µL (20.0-60.0) (p = 0.068) (Figure 1N), and from 4705.0 cells/µL (3635.0-5423.0) to 4500.0 cells/µL (4000.0-5250.0) (p = 0.396) (Figure 1O), respectively.
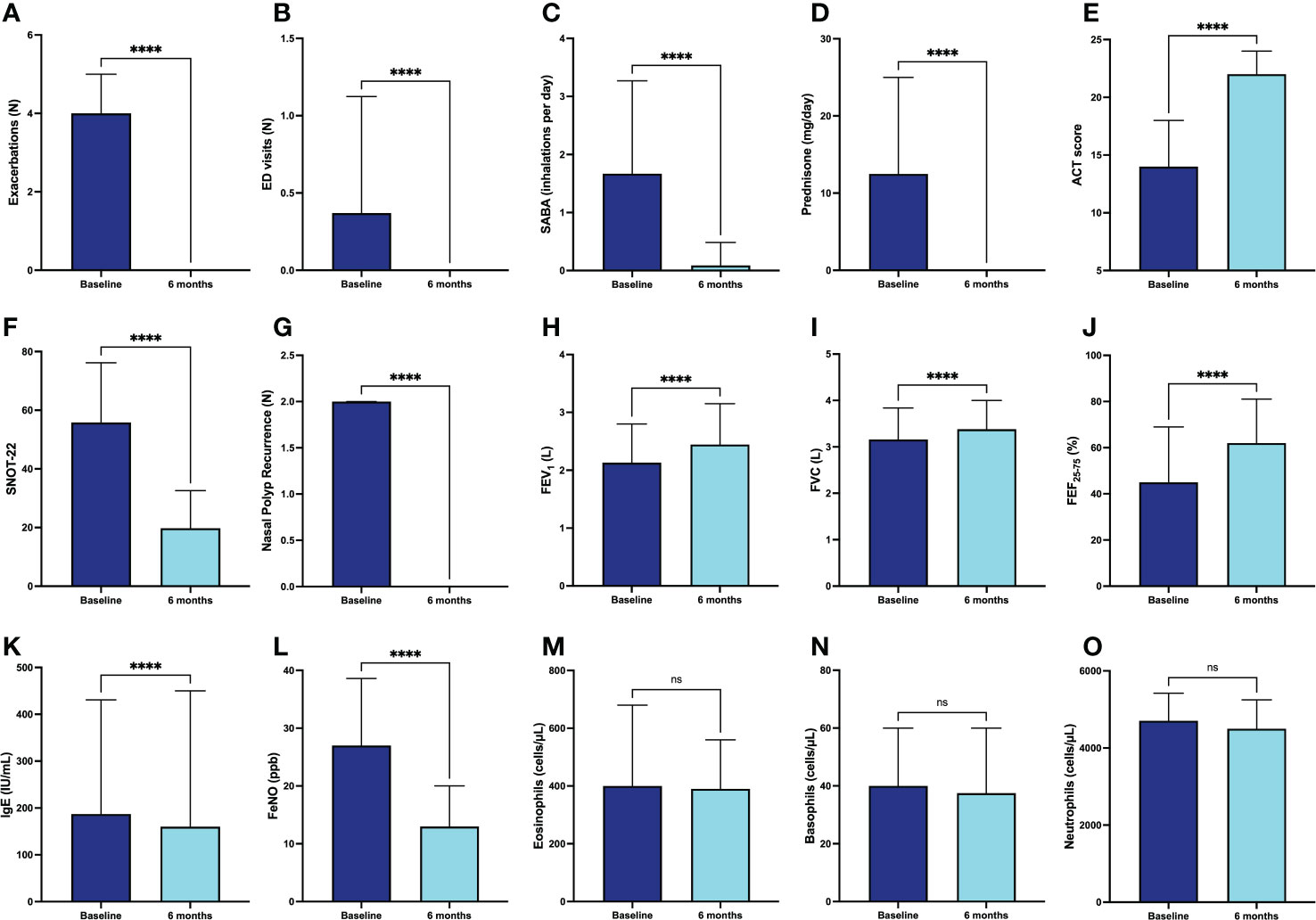
Figure 1 Efficacy of dupilumab in the whole population of patients with severe asthma, with regard to asthma exacerbations (A), ED visits (B), daily SABA inhalations (C), prednisone intake (D), ACT score (E), SNOT-22 (F), nasal polyp recurrence (G), FEV1 (H), FVC (I), FEF25-75 (J), IgE (K), FeNO (L), blood eosinophils (M), blood basophils (N), and blood neutrophils (O). Values of ED visits, daily SABA inhalations and SNOT-22 are expressed as mean (± SD). All other parameters are expressed as median values (IQR). **** p < 0.0001; ns, not significant.
Moreover, after a six-month treatment with dupilumab, when considering the key variables of clinical remission that include evaluation of asthma symptoms (ACT score ≥20), optimization of lung function (FEV1 ≥80% of predicted value), zeroing of exacerbations and OCS (zero exacerbations and zero OCS use) (32, 33), 47.24% of enrolled patients satisfied these criteria.
Efficacy of dupilumab in different type 2 asthma phenotypes
Improvements in clinical, functional, and hematological parameters after six months of dupilumab treatment were quite similar in subgroups of patients characterized by SPT negativity or positivity, respectively. Specifically, the decrease in the number of asthma exacerbations was -3.00 (from -4.00 to -0.50) in patients with negative SPT and -4.00 (from -5.00 to -2.00) in subjects with positive SPT, respectively (p = 0.158) (Figure 2A). The daily dose of prednisone decreased by -5.00 mg (from -12.50 to 0.00) in patients with negative SPT and -12.5 mg (from -25.00 to 0.00) in subjects with positive SPT, respectively (p = 0.136) (Figure 2B). The median changes in ACT score were 7 points (5-10) and 7 points (4-12) in patients with negative and positive SPT, respectively (p = 0.690) (Figure 2C). The mean increase in FEV1 was 0.20 L (0.00-0.48) in patients with negative SPT and 0.20 L (0.01-0.62) in subjects with positive SPT; this difference was not statistically significant (p = 0.409) (Figure 2D). Six months after the first dupilumab injection, the increase in FEF25-75 was 12.00% (3.75-19.50) in patients with negative SPT and 11.00% (0.00-29.00) in subjects with positive SPT, respectively (p = 0.827) (Figure 2E). Furthermore, the reduction of FeNO levels was -8.00 ppb (from -16.17 to -1.75) in patients with negative SPT and -19.00 ppb (from -29.25 to -9.00) in subjects with positive SPT, but in this case the difference overcame the threshold of statistical significance (p < 0.01) (Figure 2F).
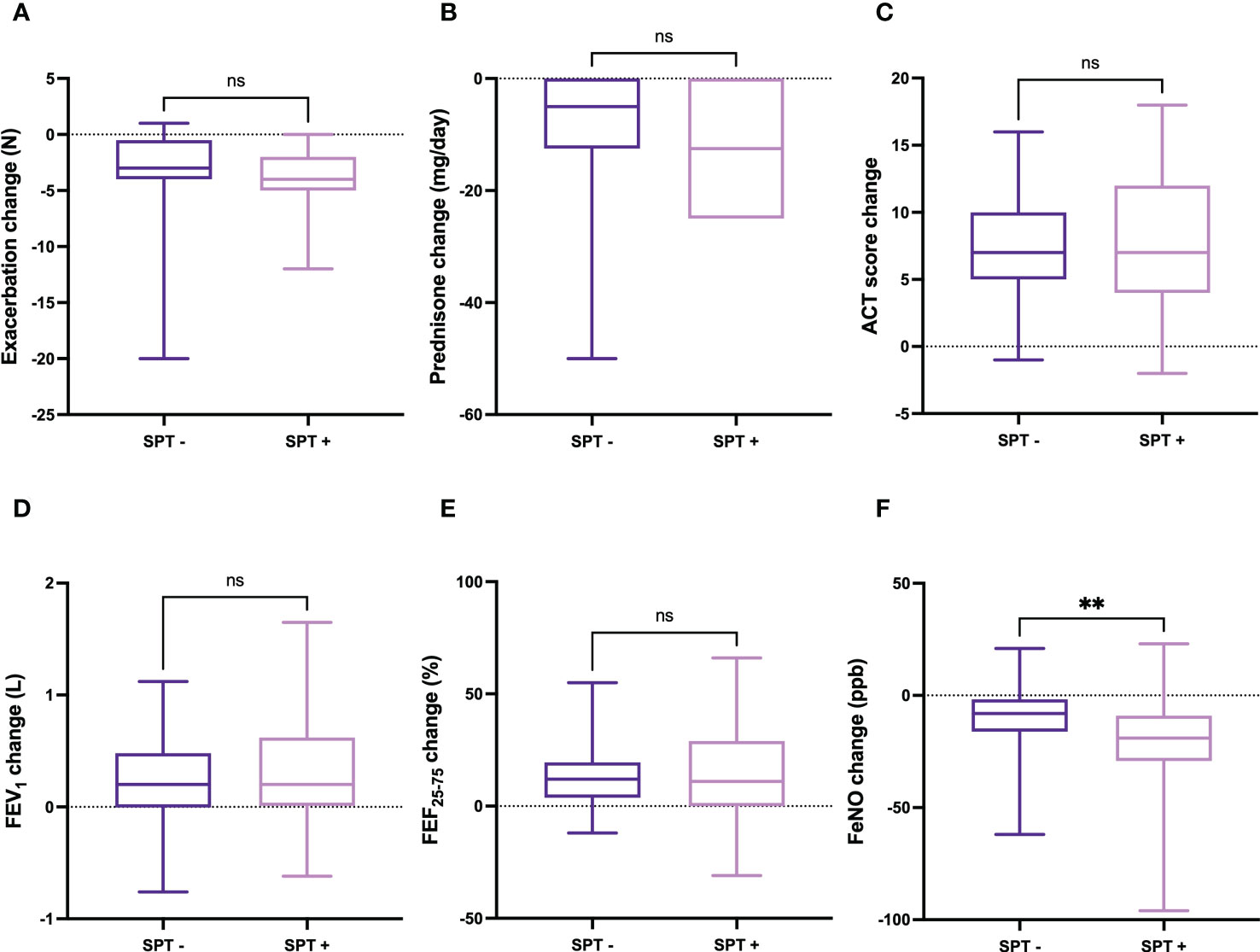
Figure 2 Comparative evaluation of dupilumab effects in relation to SPT negativity or positivity, with regard to asthma exacerbations (A), prednisone intake (B), ACT score (C), FEV1 (D), FEF25-75 (E), and FeNO levels (F). Boxes display median values and IQR, and whiskers define maximum and minimum. ns, not significant; ** p < 0.01.
No statistically significant correlations were detected between either serum IgE levels or baseline blood eosinophils, and dupilumab-induced changes in the following parameters: reduction in asthma exacerbations (Figures 3A, 4A), decrease in daily prednisone dose (Figures 3B, 4B), increases in ACT score (Figures 3C, 4C), FEV1 (Figures 3D, 4D), FVC (Figures 3E, 4E), and FEF25-75 (Figures 3F, 4F).
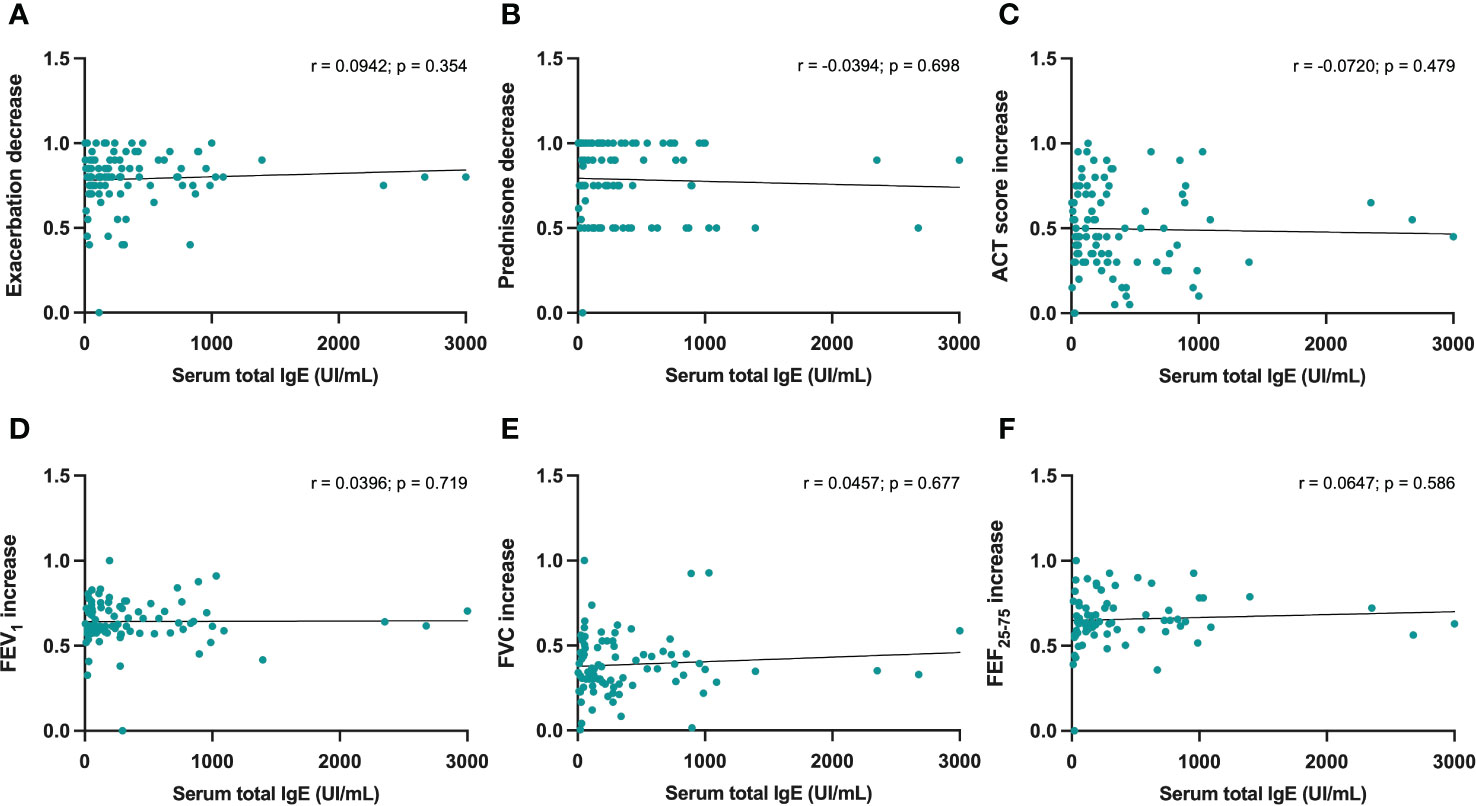
Figure 3 Correlations between serum IgE concentrations and 6-month changes induced by dupilumab, with regard to asthma exacerbations (A), prednisone intake (B), ACT score (C), FEV1 (D), FVC (E), and FEF25-75 (F).
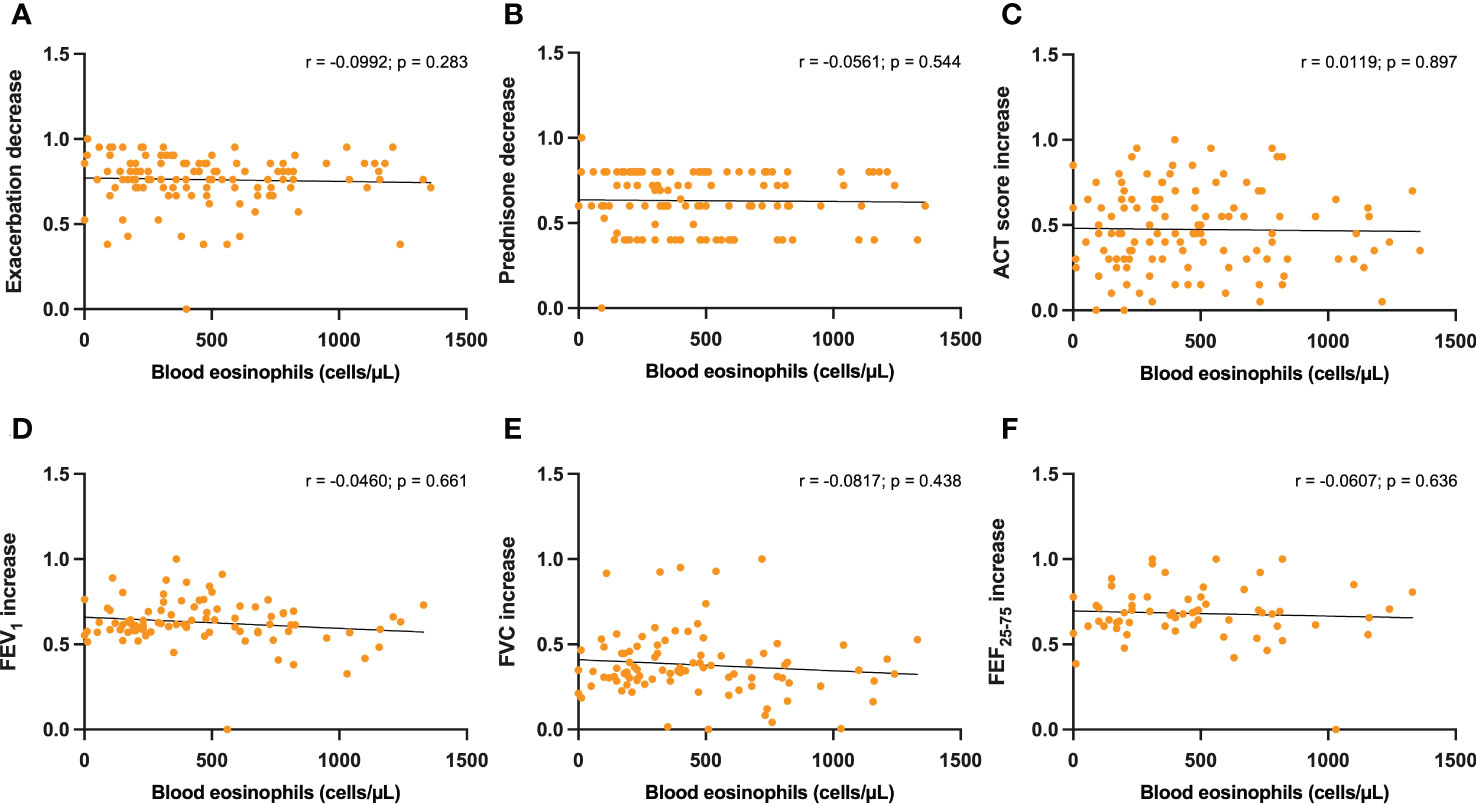
Figure 4 Correlations between blood eosinophils and 6-month changes induced by dupilumab, with regard to asthma exacerbations (A), prednisone intake (B), ACT score (C), FEV1 (D), FVC (E), and FEF25-75 (F).
In addition, when considering the above parameters, no correlations were also found between baseline FeNO levels and dupilumab-induced changes (Figures 5A–C, E, F), with the exception of FEV1 increases, which were shown to be positively correlated with FeNO values (r = 0.3147; p < 0.01) (Figure 5D).
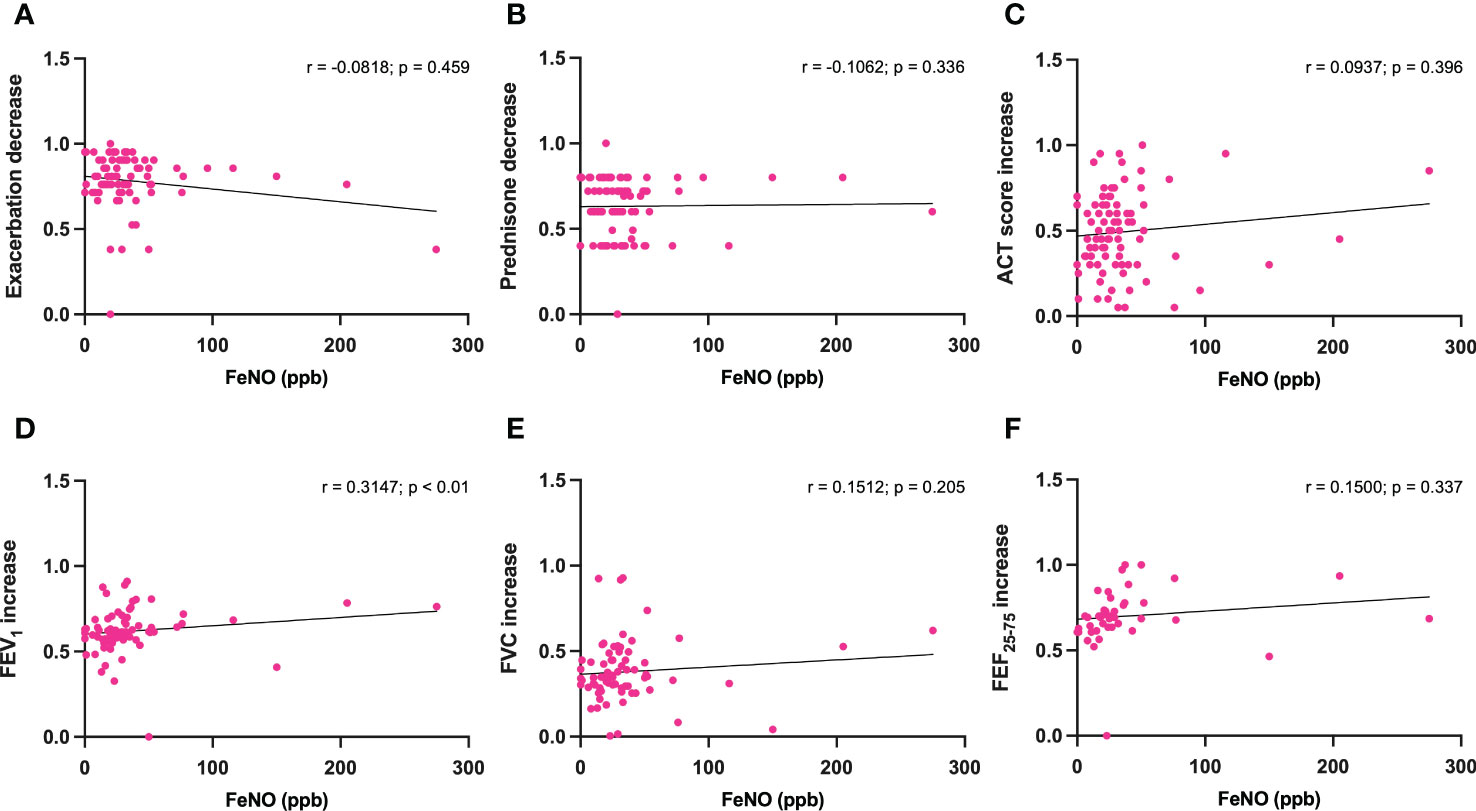
Figure 5 Correlations between FeNO levels and 6-month changes induced by dupilumab, with regard to asthma exacerbations (A), prednisone intake (B), ACT score (C), FEV1 (D), FVC (E), and FEF25-75 (F).
Efficacy of dupilumab in patients with or without CRSwNP
The improvements in clinical and functional parameters observed after six months of treatment with dupilumab were quite similar in the subgroups of patients characterized by the absence or presence of CRSwNP, respectively. In particular, the decrease in the number of asthma exacerbations was -3.00 (-5.00 to -1.50) in patients without CRSwNP and -4.00 (-5.00 to -2.00) in subjects with CRSwNP, respectively (p = 0.413) (Figure 6A). The daily dose of prednisone decreased by -5.00 mg (from -12.50 to 0.00) in patients without CRSwNP and -12.5 mg (from -25.00 to 0.00) in subjects with CRSwNP, respectively (p < 0.05) (Figure 6B). The increase in ACT score was 7 points (4-10) and 7 points (4-11) in patients without or with CRSwNP, respectively (p = 0.422) (Figure 6C). The mean increase in FEV1 was 0.15 L (-0.01-0.49) in patients without CRSwNP, and 0.24 L (0.01-0.69) in subjects with CRSwNP; this difference was not statistically significant (p = 0.138) (Figure 6D). Six months after the first dupilumab injection, the increase in FEF25-75 was 11.00% (2.00-21.00) in patients without CRSwNP and 12.00% (1.25-29.75) in subjects with CRSwNP, respectively (p = 0.479) (Figure 6E). Furthermore, the reduction in FeNO levels was -10.51 ppb (-23.50 to -1.00) in patients without CRSwNP and -17.00 ppb (-28.00 to -7.50) in subjects with CRSwNP, respectively, but in this case the difference reached the threshold of statistical significance (p < 0.05) (Figure 6F).
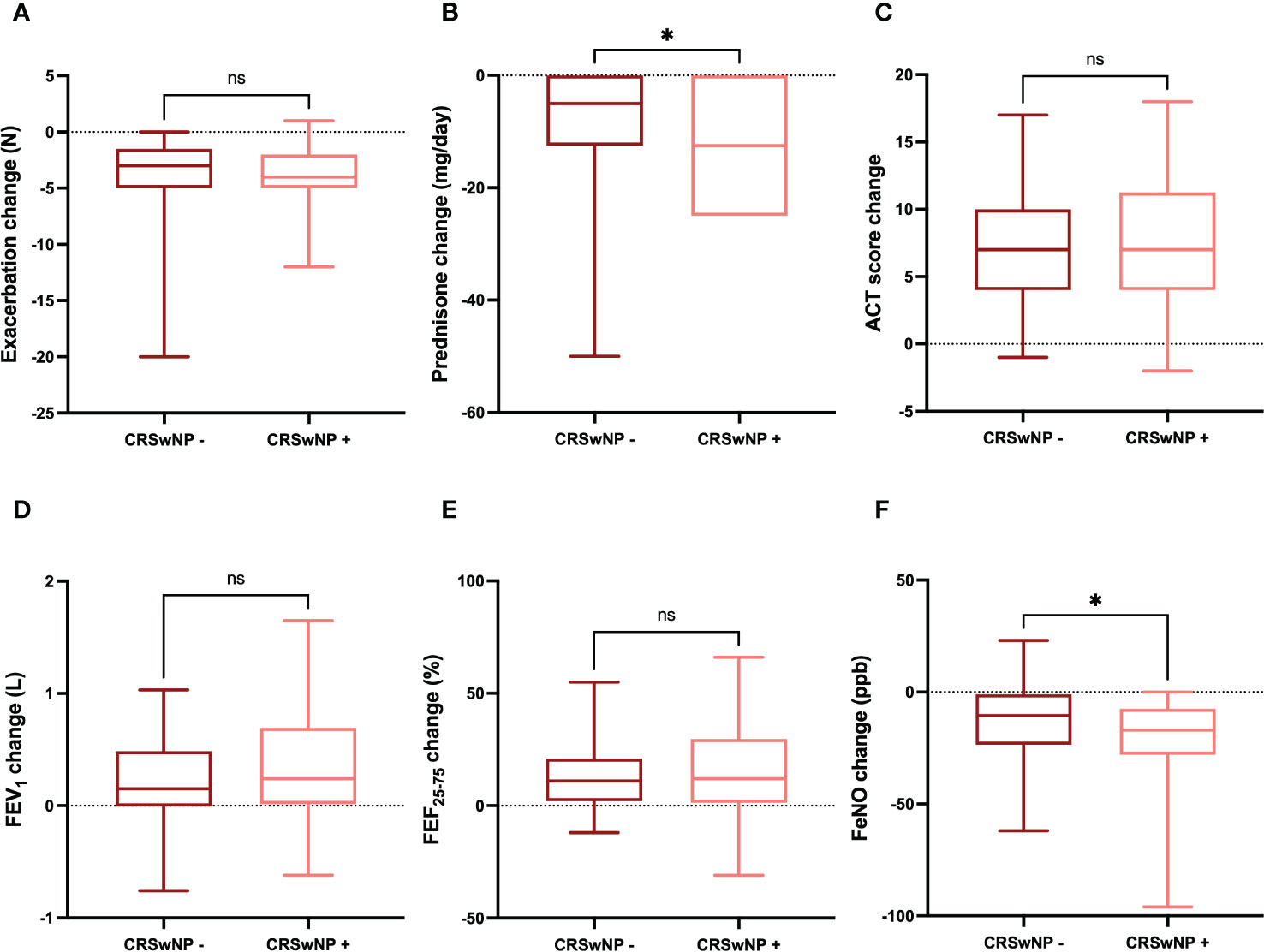
Figure 6 Comparative evaluation of dupilumab effects in relation to CRSwNP absence or presence, with regard to asthma exacerbations (A), prednisone intake (B), ACT score (C), FEV1 (D), FEF25-75 (E), and FeNO levels (F). Boxes display median values and IQR, and whiskers define maximum and minimum. ns, not significant; * p < 0.05.
Safety and tolerability profile of dupilumab
Add-on biological treatment with dupilumab was well tolerated, and no serious adverse reactions were observed during this real-world investigation. With regard to mild and transient side effects, increases in blood eosinophil counts with no symptoms were found in 5 (3.94%) subjects, 4 (3.15%) cases of conjunctivitis were reported, 2 (1.57%) injection site reactions were detected, and 1 (0.79%) patient experienced headache. All these mild side effects remitted spontaneously and did not require any specific treatment.
Discussion
Taken together, the results of the present multicenter real-life study, performed in patients with severe asthma and frequent nasal polyposis, show that dupilumab induced relevant therapeutic effects. Firstly, six months of treatment with this biologic drug cleared asthma exacerbations. This made it possible to effectively prevent any access to the emergency room, the use of short-acting bronchodilators as needed, and the intake of OCS. The latter aspect is of considerable importance as it further exceeds the efficacy data reported by the Liberty Asthma VENTURE trial (12). Indeed, VENTURE authors reported that 52.4% of patients interrupted OCS after 24 weeks of treatment with dupilumab, whereas after the same period of time OCS withdrawal was achieved by 92.9% of our steroid-dependent patients. The relevance of zeroing the use of OCS is closely related to the possibility of abrogating the well-known side effects of oral corticosteroid therapy, including adrenal insufficiency, respiratory infections, diabetes mellitus, arterial hypertension, osteoporosis, glaucoma and cataract (34, 35).
When compared to QUEST (Q) and VENTURE (V) trials, other important differences with our real-life study regard the baseline characteristics of recruited patients. In particular, we enrolled a population of asthmatic subjects with a greater percentage of male patients (50.4% vs. Q 37.8% and V 39.8%, respectively), a higher number of asthma exacerbations (4.00 vs. Q 2.02 and V 2.01, respectively), a greater blood eosinophil count (400.0 cells/µL vs. Q 250 cells/µL and V 280 cells/µL, respectively), and especially a much higher percentage of patients presented with CRSwNP (61.4% vs. Q 22.9% and V 32.0%, respectively).
Dupilumab significantly reduced asthma symptoms, as demonstrated by the significant increase in ACT score, which after 24 weeks of biological therapy reached and exceeded the threshold of 20, which expresses a satisfactory control of asthma symptoms, despite the baseline pre-treatment score stood at a rather low value of 14. This result confirms, in a real-life context, the data reported by the Liberty Asthma QUEST trial, in which the ACQ (Asthma Control Questionnaire) was utilized. However, compared to the ACQ test, the ACT questionnaire administered by us seems to be more appropriate to respond to the needs of practicality and easiness of completion wished by patients who refer to our severe asthma assessment centers. In fact, ACT is clearly preferred to ACQ in the few real-life studies that have recently evaluated the efficacy of dupilumab in the biological therapy of severe asthma (15–19).
In patients with both asthma and nasal polyposis, dupilumab elicited a significant improvement in the score of SNOT-22 questionnaire. In such a group of subjects, this result was associated with a complete prevention of the relapses of nasal polyposis. These findings corroborate in a real-life setting the efficacy of dupilumab in the treatment of CRSwNP, previously demonstrated by the Liberty NP trials SINUS-24 and SINUS-52 (14).
In addition to the clinical effects, the results of our study regarding the respiratory function are also of marked relevance. Indeed, after 6 months of therapy dupilumab significantly increased FEV1, and also incremented FVC and FEF25-75. These findings indicate that dupilumab can improve lung function by increasing airway patency from the central proximal sector to the distal periphery of the respiratory tree. The clinical and functional results of the present observational study strongly suggest that clinical remission was achieved by a relevant number of our patients. Although this very important therapeutic target should be evaluated after 12 months of continuous treatment, already after 6 months we noticed that 47.24% of our patients reached the criteria of clinical remission, including significant improvements in asthma exacerbations, OCS intake, symptom control and lung function (32, 33). Such a real-life observation further confirms that dupilumab is characterized by a very fast onset of its therapeutic action, as already shown by previous data referring to the short-term clinical, functional and biological effects of this monoclonal antibody (18, 36). However, a few weeks of observation do not allow to assess the effects of dupilumab on severe asthma exacerbations. Thus, we decided to prolong up to 6 months the last time point for evaluation of dupilumab efficacy. Indeed, this approach made it possible to appreciate the impressive reduction of asthma exacerbations induced by dupilumab.
With regard to the biomarkers of type 2 inflammation, dupilumab dramatically reduced the concentration of FeNO. This outcome is closely linked to the ability of dupilumab to antagonize at the receptor level the biological activities of IL-4 and IL-13, the latter being responsible for the induction of iNOS expression in the bronchial epithelium (37, 38). Indeed, it is reasonable that inhibition of iNOS-dependent FeNO production, induced by dupilumab in airway epithelial cells, leads to relevant decrements of FeNO levels. FeNO is a reliable indicator of type 2 inflammation, and FeNO levels correlate with asthma severity, deterioration of respiratory function, and risk of asthma exacerbations (39). Furthermore, FeNO represents a valuable aid in guiding the choice and monitoring of biological treatments for severe asthma, within the context of a personalized therapeutic approach, based on the treatable traits pertinent to specific inflammatory pheno-endotypes (40).
Hence, the efficacy of dupilumab in inhibiting the pathobiologic mechanisms underlying type 2 inflammation, strongly dependent on IL-4 and IL-13 actions, explains the extension of the therapeutic effects of this monoclonal antibody to both asthma and nasal polyposis in our patients. In fact, asthma and CRSwNP share common cellular and molecular pathogenic substrates (41), which outline a very good responsiveness to dupilumab. In our observational investigation, the add-on biological therapy with dupilumab provided similar patterns of efficacy in allergic and non-allergic patients, suffering from severe asthma and possibly also from nasal polyposis. Indeed, dupilumab induced overlapping clinical and functional effects in subjects characterized by positive or negative skin prick tests. This is probably due to the specific properties of the mechanism of action of dupilumab, which by blocking IL-4 and IL-13 receptors effectively intercepts the pathogenic pathways responsible for type 2 inflammation sustained by either allergic or non-allergic traits. In particular, by neutralizing the biological activities of IL-4 and IL-13, dupilumab inhibits the functions of the main cells producing these cytokines, including Th2 lymphocytes and type 2 innate lymphoid cells (ILC2) (42). In this way dupilumab interrupts the close interactions between innate immunity and acquired adaptive immunity, mediated by the intercellular crosstalk between ILC2 and Th2 cells, which underlies the development and progression of type 2 inflammation characterizing many cases of severe asthma and nasal polyposis, driven by either allergic or non-allergic events (41, 43, 44).
Another interesting aspect of our real-life study concerns the finding of a greater decrease in FeNO values detected in allergic patients, compared to non-allergic ones. This result suggests that patients characterized by a higher expression of multiple endotypic traits referable to type 2 inflammation, respond to dupilumab treatment by experiencing a greater decrement in FeNO levels. On the other hand, IL-4 and IL-13 are intensely involved in the cellular pathophysiology of type 2 inflammation, which could imply an enhanced predisposition of allergic patients to dupilumab-induced FeNO reduction. Furthermore, we did not detect significant correlations between serum IgE levels and the clinical and functional effects of dupilumab, whose therapeutic activity does not appear to be affected by the presence or absence of an atopic state. In our case series, the clinical and functional effects of dupilumab also occurred without substantial differences among severe asthmatic patients who presented or did not manifest the comorbidity of nasal polyposis. However, compared to patients without nasal polyps, we observed a greater reduction in FeNO values in subjects with severe asthma and concomitant nasal polyposis. This suggests that patients characterized by type 2 inflammation involving both upper and lower airways are more susceptible to the therapeutic action of dupilumab. Therefore, it is plausible to speculate that the coexistence of severe asthma and nasal polyposis could be associated with a higher expression of IL-4 and IL-13 in the airways of patients reporting both these diseases, who would therefore respond to dupilumab with a more relevant decrease in FeNO levels. Finally, our real-life evaluation shows an excellent tolerability and safety profile of dupilumab, which did not induce significant adverse events. Differently from what occurred in some individuals recruited in the Liberty Asthma QUEST and Liberty Asthma VENTURE trials, though not confirmed by the open label extension TRAVERSE study, no increases in blood levels of eosinophils were found in our patients. Based on the specific mechanism of action of dupilumab, it is thus possible to explain the lack of effects of this drug on the number of blood eosinophils, as we report. In fact, dupilumab acts as a highly efficient dual receptor antagonist of IL-4 and IL-13, but does not interfere with the biological activity of IL-5, which is the main cytokine responsible for the maturation, activation, proliferation and survival of eosinophils (45).
In conclusion, the present observational study confirms and expands, in the real-life of pulmonary clinical practice, the data reported by randomized controlled trials investigating the efficacy of dupilumab in the treatment of severe asthma. In particular, we herein show that after 6 months of treatment this biological therapy had a very positive impact on asthma exacerbations, OCS consumption, symptom control in both asthma and nasal polyposis, respiratory function and FeNO levels. Such findings have been recently extended up to one year by the results of other real-world clinical investigations (46–48). In addition, we also detected these therapeutic benefits in both allergic and non-allergic patients, as well as in asthmatics with or without nasal polyposis. Therefore, our results further consolidate the strategic position of dupilumab in its role as an excellent therapeutic option currently available within the context of modern biological treatments of severe asthma and CRSwNP, frequently driven by type 2 airway inflammation.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Local Ethics Committee of Calabria Region, Italy (Catanzaro, Italy; document n. 182 – 20 May 2021). The patients/participants provided their written informed consent to participate in this study.
Author contributions
All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Acknowledgments
Collaborators: Enrico Buonamico: Department of Basic Medical Science, Neuroscience and Sense Organs, University “Aldo Moro”, Bari, Italy; Vitaliano Quaranta: Department of Basic Medical Science, Neuroscience and Sense Organs, University “Aldo Moro”, Bari, Italy; Pietro Impellizzeri: Department of Clinical and Experimental Medicine, University of Catania, Catania, Italy; Serena Brancato: Department of Clinical and Experimental Medicine, University of Catania, Catania, Italy; Morena Porto: Department of Clinical and Experimental Medicine, University of Catania, Catania, Italy; Rossella Intravaia: Department of Clinical and Experimental Medicine, University of Catania, Catania, Italy; Nicola Lombardo: Department of Medical and Surgical Sciences, University “Magna Graecia” of Catanzaro, Catanzaro, Italy; Giovanna Lucia Piazzetta: Department of Medical and Surgical Sciences, University “Magna Graecia” of Catanzaro, Catanzaro, Italy; Stefania Caccavelli: Department of Medical and Surgical Sciences, University of Foggia, Foggia, Italy; Luciana D’Elia: Department of Medical and Surgical Sciences, University of Foggia, Foggia, Italy; Luca Gammeri: Department of Clinical and Experimental Medicine, University of Messina, Messina, Italy; Claudio Candia: Department of Respiratory Medicine, University “Federico II” of Naples, Naples, Italy; Eliana Sferra: Department of Respiratory Medicine, University “Federico II” of Naples, Naples, Italy; Claudia Gagliani: Allergology and Pulmonology Unit, Provincial Outpatient Center of Palermo, Palermo, Italy; Maria Noemi Cicero: PROMISE Department, University of Palermo, Palermo, Italy; Alessandra Tomasello: PROMISE Department, University of Palermo, Palermo, Italy; Isabella Carrieri: Department of Medicine, Surgery and Dentistry, University of Salerno, Salerno, Italy; Luigi Ciampo: Department of Medicine, Surgery and Dentistry, University of Salerno, Salerno, Italy; Carolina Vitale: Department of Medicine, Surgery and Dentistry, University of Salerno, Salerno, Italy.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
SPT, skin prick test; CRSwNP, chronic rhinosinusitis with nasal polyps; IQR, interquartile range; SD, standard deviation; BMI, body mass index; ACT, asthma control test; FEV1, forced expiratory volume in one second; FeNO, fractional exhaled nitric oxide.
References
1. Pelaia C, Pelaia G, Crimi C, Maglio A, Stanziola AA, Calabrese C, et al. Novel biological therapies for severe asthma endotypes. Biomedicines (2022) 10(5):1064. doi: 10.3390/biomedicines10051064
2. Global Initiative for Asthma. GINA report: Global strategy for asthma management and prevention (2022). Available at: https://ginasthma.org/gina-reports (Accessed November 14, 2022).
3. Pelaia C, Vatrella A, Gallelli L, Terracciano R, Navalesi P, Maselli R, et al. Dupilumab for the treatment of asthma. Expert Opin Biol Ther (2017) 17:1565–72. doi: 10.1080/14712598.2017.1387245
4. Ricciardolo FLM, Bertolini F, Carriero V. The role of dupilumab in severe asthma. Biomedicines (2021) 9:1096. doi: 10.3390/biomedicines9091096
5. Corren J. Role of interleukin-13 in asthma. Curr Allergy Asthma Rep (2013) 13:415–20. doi: 10.1007/s11882-013-0373-9
6. Firszt R, Francisco D, Church TD, Thomas JM, Ingram JL, Kraft M. Interleukin-13 induces collagen type-1 expression through matrix metalloproteinase-2 and transforming growth factor-β1 in airway fibroblasts in asthma. Eur Respir J (2014) 43:464–73. doi: 10.1183/09031936.00068712
7. Gandhi NA, Bennett BL, Graham NM, Pirozzi G, Stahl N, Yancopoulos GD. Targeting key proximal drivers of type 2 inflammation in disease. Nat Rev Drug Discovery (2016) 15:35–50. doi: 10.1038/nrd4624
8. Wills-Karp M, Finkelman FD. Untangling the complex web of IL-4- and IL-13-mediated signaling pathways. Sci Signal (2008) 1:pe55. doi: 10.1126/scisignal.1.51.pe55
9. Kelly-Welch AE, Hanson EM, Boothby MR, Keegan AD. Interleukin-4 and interleukin-13 signaling connections maps. Science (2003) 300:1527–8. doi: 10.1126/science.1085458
10. Pelaia C, Heffler E, Crimi C, Maglio A, Vatrella A, Pelaia G, et al. Interleukins 4 and 13 in asthma: Key pathophysiologic cytokines and druggable molecular targets. Front Pharmacol (2022) 13:851940. doi: 10.3389/fphar.2022.851940
11. Castro M, Corren J, Pavord ID, Maspero J, Wenzel S, Rabe KF, et al. Dupilumab efficacy and safety in moderate-to-severe uncontrolled asthma. N Engl J Med (2018) 378:2486–96. doi: 10.1056/NEJMoa1804092
12. Rabe KF, Nair P, Brusselle G, Maspero JF, Castro M, Sher L, et al. Efficacy and safety of dupilumab in glucocorticoid-dependent severe asthma. N Engl J Med (2018) 378:2475–85. doi: 10.1056/NEJMoa1804093
13. Wechsler ME, Ford LB, Maspero JF, Pavord ID, Papi A, Bourdin A, et al. Long-term safety and efficacy of dupilumab in patients with moderate-to-severe asthma (TRAVERSE): an open-label extension study. Lancet Respir Med (2022) 10:11–25. doi: 10.1016/S2213-2600(21)00322-2
14. Bachert C, Han JK, Desrosiers M, Hellings PW, Amin N, Lee SE, et al. Efficacy and safety of dupilumab in patients with severe chronic rhinosinusitis with nasal polyps (LIBERTY NP SINUS-24 and LIBERTY NP SINUS-52): Results from two multicentre, randomised, double-blind, placebo-controlled, parallel-group phase 3 trials. Lancet (2019) 394:1638–50. doi: 10.1016/S0140-6736(19)31881-1
15. Dupin C, Belhadi D, Guilleminault L, Gamez AS, Berger P, De Blay F, et al. Effectiveness and safety of dupilumab for the treatment of severe asthma in a real-life French multi-centre adult cohort. Clin Exp Allergy (2020) 50:789–98. doi: 10.1111/cea.13614
16. Nettis E, Patella V, Lombardo C, Detoraki A, Macchia L, Di Leo E, et al. Efficacy of dupilumab in atopic comorbidities associated with moderate-to-severe adult atopic dermatitis. Allergy (2020) 10:2653–61. doi: 10.1111/all.14338
17. Campisi R, Crimi C, Nolasco S, Beghè B, Antonicelli L, Guarnieri G, et al. Real-world experience with dupilumab in severe asthma: one-year data from an Italian named patient program. J Asthma Allergy (2021) 14:575–83. doi: 10.2147/JAA.S312123
18. Pelaia C, Lombardo N, Busceti MT, Piazzetta G, Crimi C, Calabrese C, et al. Short-term evaluation of dupilumab effects in patients with severe asthma and nasal polyposis. J Asthma Allergy (2021) 14:1165–72. doi: 10.2147/JAA.S328988
19. Numata T, Araya J, Miyagawa H, Okuda K, Takekoshi D, Hashimoto M, et al. Real-world effectiveness of dupilumab for patients with severe asthma: A retrospective study. J Asthma Allergy (2022) 15:395–405. doi: 10.2147/JAA.S357548
20. Chung KF, Wenzel SE, Brozek JL, Bush A, Castro M, Sterk PJ, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J (2014) 43:343–73. doi: 10.1183/09031936.00202013
21. Laufer P, Chryssanthopoulos C, Laufer R, Hause LL. The determination of the eosinophil count: comparison of two techniques. J Allergy Clin Immunol (1987) 79:438–41. doi: 10.1016/0091-6749(87)90360-5
22. Borzova E, Dahinden CA. The absolute basophil count. Methods Mol Biol (2014) 1192:87–100. doi: 10.1007/978-1-4939-1173-8_7
23. Nathan RA, Sorkness CA, Kosinski M, Schatz M, Li JT, Marcus P, et al. Development of the asthma control test: A survey for assessing asthma control. J Allergy Clin Immunol (2004) 113(1):59–65. doi: 10.1016/j.jaci.2003.09.008
24. Graham BL, Steenbruggen I, Miller MR, Barjaktarevic IZ, Cooper BG, Hall GL, et al. Standardization of spirometry 2019 update. an official American thoracic society and European respiratory society technical statement. Am J Respir Crit Care Med (2019) 200:e70–88. doi: 10.1164/rccm.201908-1590ST
25. American Thoracic Society, European Respiratory Society. ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med (2005) 171:912–30. doi: 10.1164/rccm.200406-710ST
26. Horváth I, Barnes PJ, Loukides S, Sterk PJ, Högman M, Olin AC, et al. A European respiratory society technical standard: Exhaled biomarkers in lung disease. Eur Respir J (2017) 49:1600965. doi: 10.1183/13993003.00965-2016
27. Wenzel S, Castro M, Corren J, Maspero J, Wang L, Zhang B, et al. Dupilumab efficacy and safety in adults with uncontrolled persistent asthma despite use of medium-to-high-dose inhaled corticosteroids plus a long-acting β2 agonist: a randomised double-blind placebo-controlled pivotal phase 2b dose-ranging trial. Lancet (2016) 388:31–44. doi: 10.1016/S0140-6736(16)30307-5
28. Muñoz-Bellido FJ, Moreno E, Dávila I. Dupilumab: A review of present indications and off-label uses. J Investig Allergol Clin Immunol (2022) 32(2):97–115. doi: 10.18176/jiaci.0682
29. Fokkens WJ, Lund VJ, Mullol J, Bachert C, Alobid I, Baroody F, et al. EPOS 2012: European position paper on rhinosinusitis and nasal polyps 2012. A summary for otorhinolaryngologists. Rhinology (2012) 50:1–12. doi: 10.4193/Rhino12.000
30. Lombardo N, Pelaia C, Ciriolo M, Della Corte M, Piazzetta G, Lobello N, et al. Real-life of benralizumab on allergic chronic rhinosinusitis and nasal polyposis associated with severe asthma. Int J Immunopathol Pharmacol (2020) 34:2058738420950851. doi: 10.1177/2058738420950851
31. Malling HJ. Skin prick testing and the use of histamine references. Allergy (1984) 39:596–601. doi: 10.1111/j.1398-9995.1984.tb01979.x
32. Menzies-Gow A, Bafadhel M, Busse WW, Casale TB, Kocks JWH, Pavord ID, et al. An expert consensus framework for asthma remission as a treatment goal. J Allergy Clin Immunol (2020) 145(3):757–65. doi: 10.1016/j.jaci.2019.12.006
33. Thomas D, McDonald VM, Pavord ID, Gibson PG. Asthma remission: what is it and how can it be achieved? Eur Respir J (2022) 60(5):2102583. doi: 10.1183/13993003.02583-2021
34. Heffler E, Blasi F, Latorre M, Menzella F, Paggiaro P, Pelaia G, et al. The severe asthma network in Italy: findings and perspectives. J Allergy Clin Immunol Pract (2019) 7:1462–8. doi: 10.1016/j.jaip.2018.10.016
35. Canonica GW, Colombo GL, Bruno GM, Di Matteo S, Martinotti C, Blasi F, et al. Shadow cost of oral corticosteroids-related adverse events: A pharmacoeconomic evaluation applied to real-life data from the severe asthma network in Italy (SANI) registry. World Allergy Organ J (2019) 12:100007. doi: 10.1016/j.waojou.2018.12.001
36. Canonica GW, Bourdin A, Peters AT, Desrosiers M, Bachert C, Weidinger S, et al. Dupilumab demonstrates rapid onset of response across three type 2 inflammatory diseases. J Allergy Clin Immunol Pract (2022) 10(6):1515–26. doi: 10.1016/j.jaip.2022.02.026
37. Busse WW, Kraft M, Rabe KF, Deniz Y, Rowe PJ, Ruddy M, et al. Understanding the key issues in the treatment of uncontrolled persistent asthma with type 2 inflammation. Eur Respir J (2021) 58:2003393. doi: 10.1183/13993003.03393-2020
38. Carr TF, Kraft M. Use of biomarkers to identify phenotypes and endotypes of severe asthma. Ann Allergy Asthma Immunol (2018) 121:414–20. doi: 10.1016/j.anai.2018.07.029
39. Ulrik CS, Lange P, Hilberg O. Fractional exhaled nitric oxide as a determinant for the clinical course of asthma: A systematic review. Eur Clin Respir J (2021) 8:1891725. doi: 10.1080/20018525.2021.1891725
40. Rolla G, Heffler E, Pizzimenti S. An emerging role for exhaled nitric oxide in guiding biological treatment in severe asthma. Curr Med Chem (2020) 27:7159–67. doi: 10.2174/0929867327666200713184659
41. Scadding GK, Scadding GW. Innate and adaptive immunity: ILC2 and Th2 cells in upper and lower airway allergic diseases. J Allergy Clin Immunol Pract (2021) 9:1851–7. doi: 10.1016/j.jaip.2021.02.013
42. Hammad H, Lambrecht BN. The basic immunology of asthma. Cell (2021) 184:1469–85. doi: 10.1016/j.cell.2021.02.016
43. Rodriguez-Rodriguez N, Gogoi M, McKenzie ANJ. Group 2 innate lymphoid cells: team players in regulating asthma. Annu Rev Immunol (2021) 39:167–98. doi: 10.1146/annurev-immunol-110119-091711
44. Matucci A, Bormioli S, Nencini F, Maggi E, Vultaggio A. The emerging role of type 2 inflammation in asthma. Expert Rev Clin Immunol (2021) 17:63–71. doi: 10.1080/1744666X.2020.1860755
45. Pelaia C, Paoletti G, Puggioni F, Racca F, Pelaia G, Canonica GW, et al. Interleukin-5 in the pathophysiology of severe asthma. Front Physiol (2019) 10:1514. doi: 10.3389/fphys.2019.01514
46. Thelen JC, van Zelst CM, van Brummelen SE, Rauh S, In ‘t Veen JCCM, Kappen JH, et al. Efficacy and safety of dupilumab as add-on therapy for patients with severe asthma: A real-world Dutch cohort study. Respir Med (2023) 206:107058. doi: 10.1016/j.rmed.2022.107058
47. Ottaviano G, Saccardo T, Roccuzzo G, Bernardi R, Chicco AD, Pendolino AL, et al. Effectiveness of dupilumab in the treatment of patients with uncontrolled severe CRSwNP: A “Real-life” observational study in naïve and post-surgical patients. J Pers Med (2022) 12(9):1526. doi: 10.3390/jpm12091526
Keywords: severe asthma, nasal polyps, interleukin 4, interleukin 13, dupilumab, clinical remission
Citation: Pelaia C, Benfante A, Busceti MT, Caiaffa MF, Campisi R, Carpagnano GE, Crimi N, D’Amato M, Foschino Barbaro MP, Maglio A, Minenna E, Nolasco S, Paglino G, Papia F, Pelaia G, Portacci A, Ricciardi L, Scichilone N, Scioscia G, Triggiani M, Valenti G, Vatrella A and Crimi C (2023) Real-life effects of dupilumab in patients with severe type 2 asthma, according to atopic trait and presence of chronic rhinosinusitis with nasal polyps. Front. Immunol. 14:1121237. doi: 10.3389/fimmu.2023.1121237
Received: 11 December 2022; Accepted: 20 March 2023;
Published: 30 March 2023.
Edited by:
Miguel Angel Alejandre Alcazar, University Hospital of Cologne, GermanyCopyright © 2023 Pelaia, Benfante, Busceti, Caiaffa, Campisi, Carpagnano, Crimi, D’Amato, Foschino Barbaro, Maglio, Minenna, Nolasco, Paglino, Papia, Pelaia, Portacci, Ricciardi, Scichilone, Scioscia, Triggiani, Valenti, Vatrella and Crimi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Corrado Pelaia, pelaia.corrado@gmail.com
 Corrado Pelaia
Corrado Pelaia