- 1Department of Thoracic Surgery, Sichuan Cancer Hospital and Institute, Sichuan Cancer Center, Cancer Hospital Affiliated to University of Electronic Science and Technology of China, Chengdu, China
- 2Medical Affairs Department of BeiGene (Beijing) Co., Ltd, Beijing, China
Objective: This systematic review and meta-analysis aimed to explore the efficacy and safety of neoadjuvant immunotherapy in patients with resectable locally advanced esophageal squamous cell carcinoma (ESCC).
Background: Several studies have reported the outcomes of neoadjuvant immunotherapy in patients with ESCC. However, phase 3 randomized controlled trials (RCTs) with long-term outcomes and the comparison of different therapeutic strategies are lacking.
Methods: Studies involving patients with advanced ESCC treated with preoperative neoadjuvant immune checkpoint inhibitors (ICIs) were searched through PubMed, Embase, and Cochrane Library up to July 1, 2022. The outcomes were presented as proportions and pooled respectively by fixed or random effect model depending on the heterogeneity between studies. All analyses were performed using the R packages meta 5.5-0 and meta-for 3.4-0.
Results: Thirty trials involving 1406 patients were included in the meta-analysis. The pooled pathological complete response (pCR) rate for neoadjuvant immunotherapy was 0.30 (95% confidence interval [CI]: 0.26–0.33). The pCR rate of neoadjuvant immunotherapy combined with chemoradiotherapy (nICRT) was significantly higher than that of neoadjuvant immunotherapy combined with chemotherapy (nICT) (nICRT: 0.48, 95% CI: 0.31–0.65; nICT: 0.29, 95% CI: 0.26–0.33; p=0.03). No significant difference in efficacy was observed between the different chemotherapy agents and treatment cycles. The incidences of grade 1–2 and 3–4 treatment-related adverse events (TRAEs) were 0.71 (95% CI: 0.56–0.84) and 0.16 (95% CI: 0.09–0.25), respectively. Patients treated with nICRT and carboplatin had a higher incidence of grade 3–4 TRAEs compared with those treated with nICT (nICRT: 0.46, 95% CI: 0.17–0.77; nICT: 0.14, 95% CI: 0.07–0.22; p=0.03) and cisplatin (carboplatin: 0.33, 95% CI: 0.15–0.53; cisplatin: 0.04, 95% CI: 0.01–0.09; p<0.01).
Conclusion: Neoadjuvant immunotherapy has good efficacy and safety profiles in patients with locally advanced ESCC. Additional RCTs with long-term survival data are warranted.
1 Introduction
According to the 2020 global tumor data, esophageal cancer (EC) is the seventh most common malignant tumor worldwide, and its mortality rate ranks sixth (1). Esophageal squamous cell carcinoma (ESCC) and adenocarcinoma are the two main histological subtypes of EC. Adenocarcinoma is the main subtype in the United States and Europe, accounting for approximately 70% of cases, whereas ESCC is the main subtype in Asia, accounting for approximately 90% of the incidence of EC. More than half of EC patients are already at locally advanced stage when diagnosed. Neoadjuvant therapy such as neoadjuvant chemoradiotherapy or chemotherapy has been the standard treatment for locally advanced EC. However, the postoperative recurrence and metastasis rates of locally advanced EC are still high after neoadjuvant therapy, ranging from 30% to 50%. Besides, it has been a bottleneck to further improve the treatment outcome in this patient population (2, 3). In terms of pathology, esophageal adenocarcinoma and ESCC are two completely different pathological types (4). This study focused on the perioperative treatment of locally advanced ESCC. The perioperative treatment of locally advanced ESCC has become a research hotspot in recent years. Based on the results of the CROSS (5, 6), NEOCRTEC5010 (7, 8), and JCOG1109 (9) trials, neoadjuvant chemoradiotherapy (nCRT) or neoadjuvant chemotherapy (nCT) combined with surgery are the standard treatment options for locally advanced ESCC. However, the 5-year overall recurrence rate of ESCC remains high (8), and its long-term survival rate is poor (6). To date, there is no clear evidence to support a significant difference in survival benefits between patients receiving nCRT and nCT (10, 11).
In recent years, immunotherapy, represented by immune checkpoint inhibitors (ICIs), has excellent efficacy and controllable toxicity in advanced EC, according to the results from ESCORT, ATTRACTION-3, KEYNOTE-181, KEYNOTE-590 and CheckMate648 studies (12–16). An increasing number of researchers have begun to combine ICIs with nCRT or nCT for the treatment of locally advanced ESCC. With the completion of phase 2 clinical studies, early results have reported the efficacy and safety of neoadjuvant immunotherapy combined with chemoradiotherapy (nICRT) and neoadjuvant immunotherapy combined with chemotherapy (nICT). However, the superiority of this combined strategy remains uncertain owing to insufficient sample sizes in individual phase 2 trials, varying clinical study designs, and the lack of randomized controlled phase 3 trials (RCTs) with long-term outcomes. Moreover, the effects of different chemotherapy agents and cycle numbers remain uncertain.
Therefore, we conducted a systematic review and meta-analysis to evaluate the antitumor efficacy and safety of nICRT and nICT. We aimed to obtain more accurate conclusions and summarize nICRT and nICT as reliable evidence for preoperative neoadjuvant therapy for locally advanced ESCC to facilitate clinical decision-making and the development of future randomized controlled phase 3 clinical trials.
2 Methods
This systematic review and meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (17). The study was registered with the International Prospective Register of Systematic Reviews database (ID: CRD42022355086), and the protocol can be found there.
2.1 Search strategy and selection criteria
PubMed, Embase, and Cochrane Library were searched for relevant publications up to July 1, 2022. All keywords were searched using Medical Subject Headings. The detailed search strategy is presented in Supplementary Table 1. Databases were searched for titles and abstracts.
The inclusion criteria were as follows (1): prospective or retrospective studies involving patients with ESCC treated with preoperative neoadjuvant ICIs and (2) studies reporting at least one of the following primary outcomes: pathological complete response (pCR, defined as no residual tumor cells), major pathological response (MPR, defined as < 10% residual tumor cells), objective response rate (ORR), disease control rate (DCR), downstaging rate, R0 resection rate, incidence of treatment-related adverse events (TRAEs), and incidence of anastomotic leakage.
The exclusion criteria were as follows (1): case report, review, commentary, or conference abstract and (2) for multiple articles published with overlapping or repeating data, those reporting the most comprehensive data.
2.2 Data extraction
Two reviewers (CHW and CDL) independently reviewed and extracted the necessary data from the selected articles. Disagreements were resolved through discussion or by a third researcher (WWH) to decide whether to include the study. An Excel spreadsheet was used to record the data.
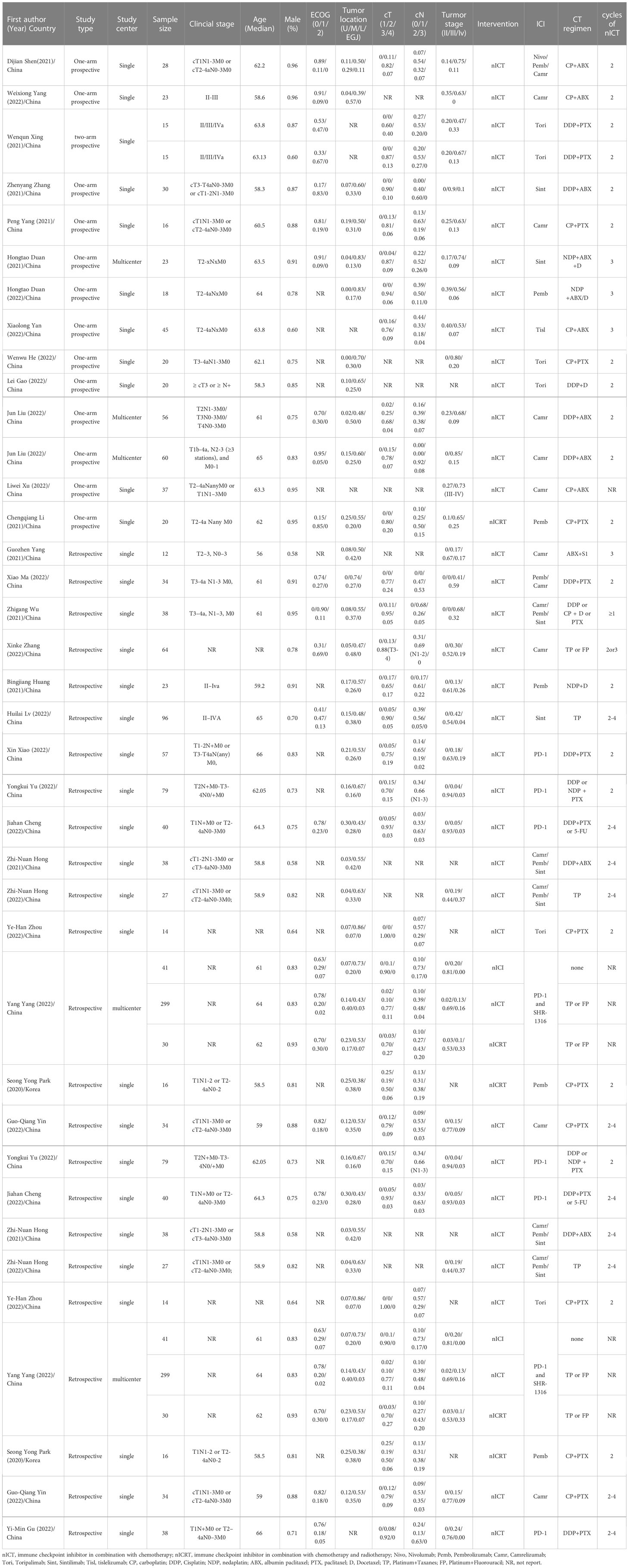
The following terms were extracted or summarized as proportions from the included articles if available: first author name, journal, year of publication, author countries, study type, study phase, study center, main inclusion criteria, study arms, sample size, efficacy evaluation criteria, sex, age, Eastern Cooperative Oncology Group Performance Status (ECOG PS), body mass index, tumor location, primary tumor length, programmed cell death ligand-1 (PD-L1) expression levels, PD-L1 cutoff value, clinical tumor-node-metastasis (TNM) tumor stage, tumor stage criteria, surgical method, number of dissected lymph nodes, extent of lymph node dissection, R0 resection rate, ICI drugs, ICI dose, chemotherapy drugs, chemotherapy dose, number of surgical resection, MPR, pCR, complete response (CR) rate, partial response rate, stable disease rate, progressive disease rate, downstaging rate, ypTNM, efficacy analysis sample size, follow-up time, 1/3-year overall survival (OS) rate, 0.5-/1-/3-year disease-free survival (DFS) rate, incidence of grade 1–2 TRAEs, incidence of grade 3–4 TRAEs, incidence of immune-related adverse events, surgical delay rate, incidence of anastomotic leakage, in-hospital mortality rate, and 30-day mortality rate.
2.3 Quality assessment
The majority of included studies were single-arm studies, and the Methodological Index for Nonrandomized Studies (MINORS) checklist was used to assess the quality of these studies (18). Items were scored as 0 (not reported), 1 (reported but inadequate), or 2 (reported adequately). The ideal scores were 16 and 24 for non-comparative and comparative studies, respectively.
2.4 Statistical analyses
The outcomes (pCR, MPR, R0 resection rate, CR rate, ORR, DCR, downstaging rate, anastomotic leakage, grade 1–2 TRAE rate, and grade 3–4 TRAE rate) are presented as proportions. All analyses were performed using the R package meta 5.5-0 and meta-for 3.4-0. The proportions were first obtained through a double arcsine transformation, which works pretty well for normalizing and variance-stablizing the sampling distribution of proportions, and then pooled using the inverse variance method in meta analysis. Heterogeneity was evaluated using the Q-test and statistical inconsistency index (I2) for each outcome. When p was <0.05 (for the Q-test) or I2 was >50%, the studies included were considered heterogeneous and the random-effect model was applied. Otherwise, a fixed-effects model was used for analysis. Subgroup analyses were performed based on study type (prospective vs. retrospective), combination treatment (nICT vs. nICRT), neoadjuvant treatment cycles (2 cycles vs. ≥2 cycles), platinum type (carboplatin vs. cisplatin), and taxane type (paclitaxel vs. nab-paclitaxel) if there were at least two studies included in each subgroup. The common chemotherapy frequency of nICRT is weekly, which is different from that of nCRT; therefore, subgroup analysis by neoadjuvant treatment cycles did not include studies of nICRT. The outcomes in each subgroup were synthesized and the results were compared across subgroups by statistical test (19). Corresponding p values for the comparison were reported along with the results of subgroup analyses. A common estimate of between-study heterogeneity was assumed when the number of studies in a subgroup was ≤5. Sensitivity analyses were conducted using the leave-one-out method. The pooled effect and corresponding I2 were recalculated each time, leaving out one study. Thus, studies that affect the results can be identified, and the robustness of the results can be further evaluated. Funnel plots were created, and Egger regression tests were performed to evaluate publication bias if the results were synthesized from more than 10 studies. P <0.05 was considered statistically significant for all analyses.
3 Results
3.1 Trial characteristics
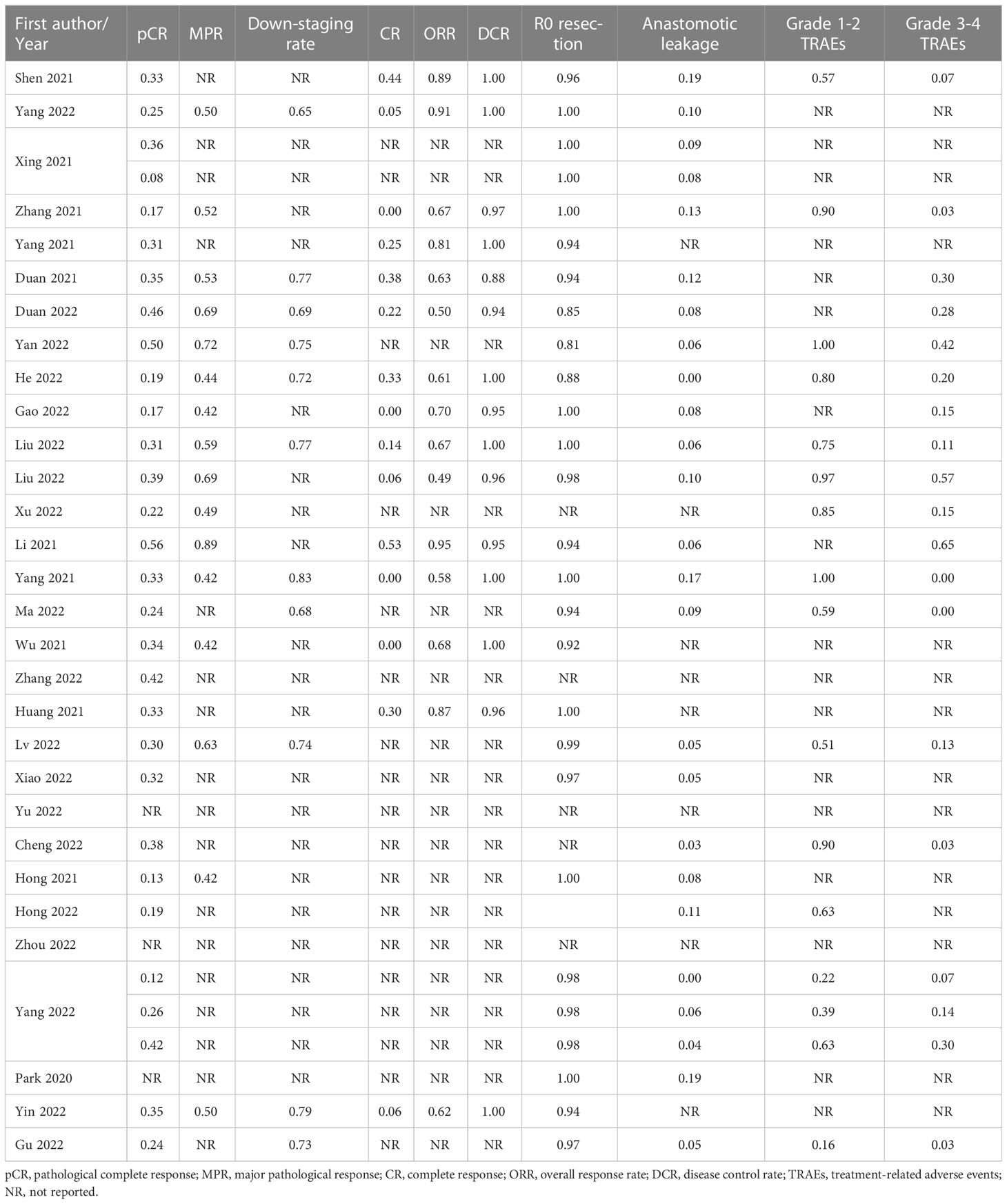
A PRISMA diagram of the study selection procedure is shown in Figure 1. In total, 803 articles were identified based on the search strategy, and 30 trials with 1406 patients were eligible for inclusion in the final meta-analysis. Most studies were conducted in China. Among the 30 included studies, 13 were single-arm prospective, one was a dual-arm prospective, and 16 were retrospective. Twenty-seven studies used nICT as neoadjuvant therapy, two studies examined nICRT, and one study included both nICT and nICRT. All studies used programmed cell death protein 1 (PD-1) inhibitors, and only one retrospective study included patients who received PD-L1 inhibitors. The most common nCT regimens were paclitaxel or nab-paclitaxel plus carboplatin or cisplatin. The main characteristics of the included studies are presented in Table 1, and the primary outcomes are shown in Table 2.
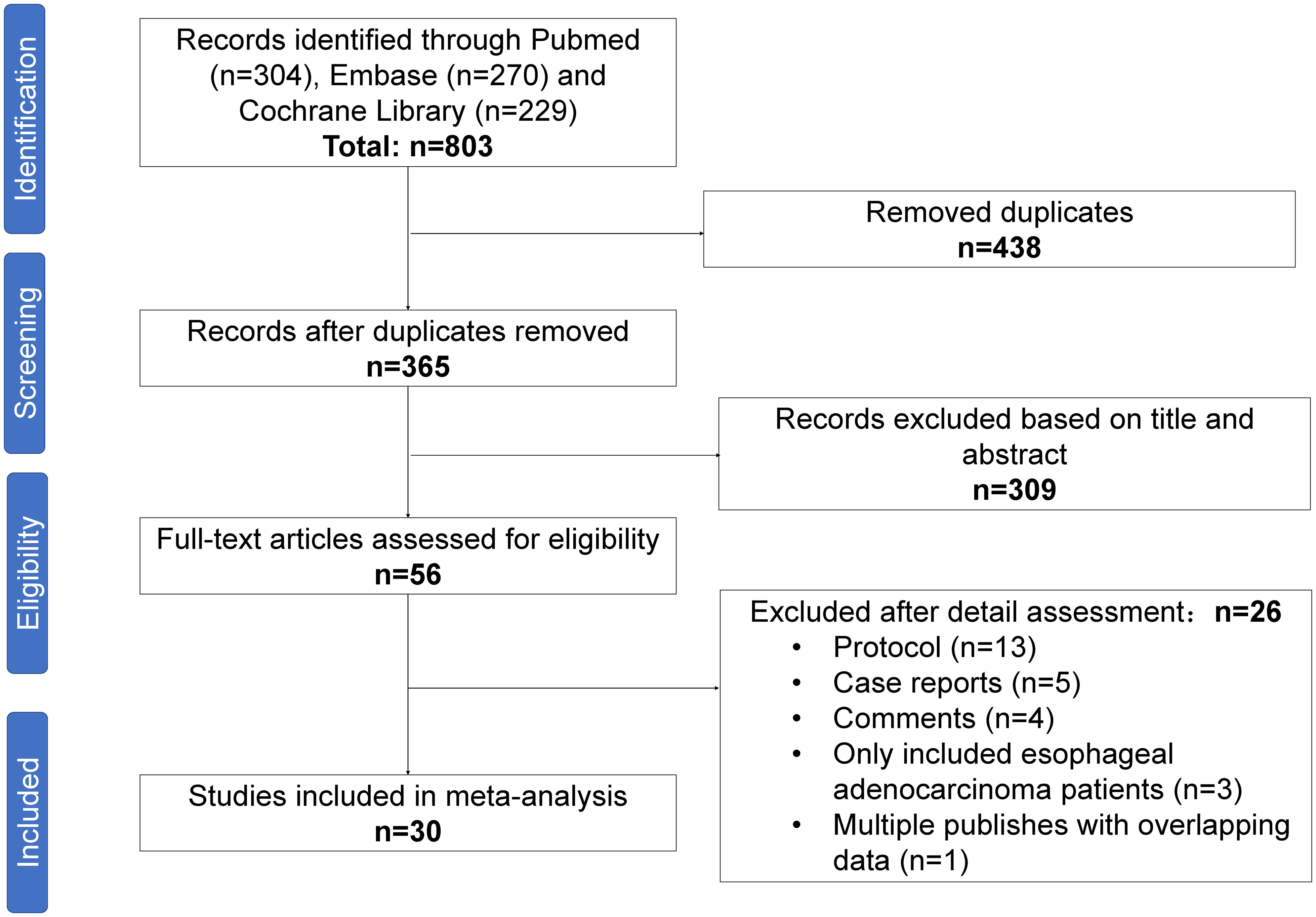
Figure 1 Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) diagram of the study selection.
3.2 Quality assessment
The quality of all included studies was judged according to the MINORS. Of the 30 included studies, six were designed as comparative studies. For these comparative studies, the total score was 16–22. The scores of non-comparative studies ranged from 9 to 15. As the aim of the included studies was to evaluate the efficacy and safety of neoadjuvant ICI therapy, the primary endpoints of most studies were safety or short-term efficacy endpoints, such as pCR and MPR. Follow-up data were not reported in 17 of the 30 studies. Generally, the included studies were of high quality and suitable for meta-analysis. The details of the quality assessment of all the studies are presented in Supplementary Table 2.
3.3 Efficacy outcomes
3.3.1 Pathological response
pCR was reported in 26 of the 1200 patients. The pooled pCR was 0.30 (95% confidence interval [CI]: 0.26–0.33, I2=41%, p=0.01) (Figure 2A). Subgroup analyses results showed that the pCR rate of the nICRT group was significantly higher than that of the nICT group (nICRT: 0.48, 95% CI: 0.31–0.65; nICT: 0.29, 95% CI: 0.26–0.33; p=0.03). No significant differences in pCR were observed in the subgroup analyses according to study type, neoadjuvant treatment cycles, platinum type, or taxane type (Supplementary Figure 1).
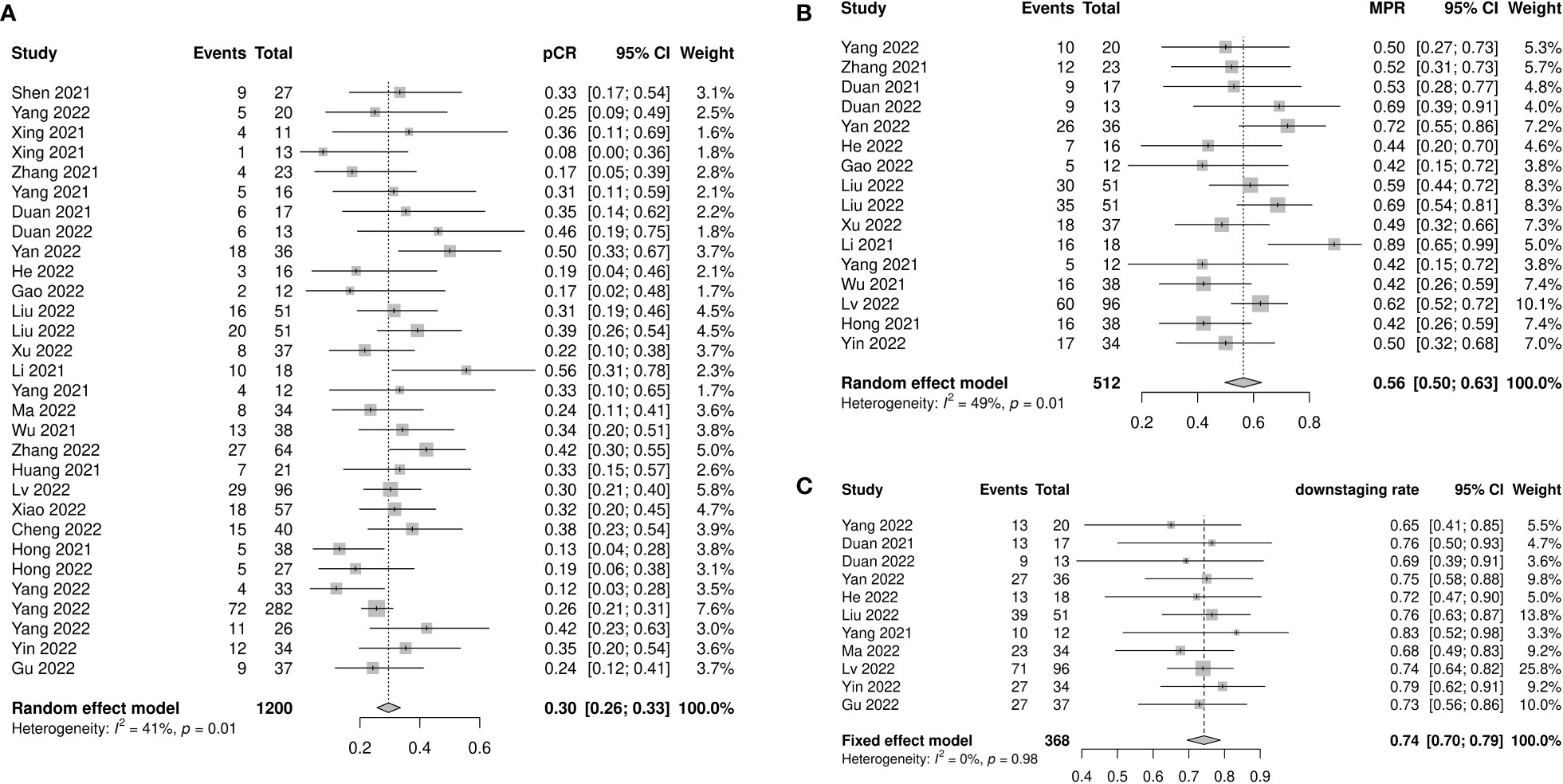
Figure 2 Pathological response of neoadjuvant immune checkpoint inhibitors therapy. (A) Pathological complete response, (B) major pathological response, and (C) downstaging rate.
Sixteen studies with 512 patients reported MPR rates ranging from 0.42 to 0.89. The pooled MPR was 0.56 (95% CI: 0.50–0.63, I2=49%, p=0.01) (Figure 2B). The downstaging rate was 0.74 (95% CI: 0.70–0.79, I2=0%, p=0.98) (Figure 2C), pooled from 11 studies with 368 patients. Subgroup analyses showed no significant differences in MPR or downstaging rate among study type, neoadjuvant therapy cycle number, platinum type, or taxane type (Supplementary Figures 2, 3).
3.3.2 Radiological response
A total of 367 patients from 15 studies were included in the analysis of CR rate, ORR, and DCR. The pooled CR rate, ORR, and DCR were 0.14 (95% CI: 0.06–0.24, I2=81%, p<0.01), 0.72 (95% CI: 0.63–0.79, I2=63%, p<0.01), and 0.99 (95% CI: 0.97–1.00, I2=0%, p=0.71), respectively (Figures 3A–C). Four subgroup analyses by study type, number of neoadjuvant therapy cycles, platinum type, and taxane type were performed. No statistically significant differences were observed between subgroup comparisons of CR rate, ORR, and DCR (Supplementary Figures 4-6).
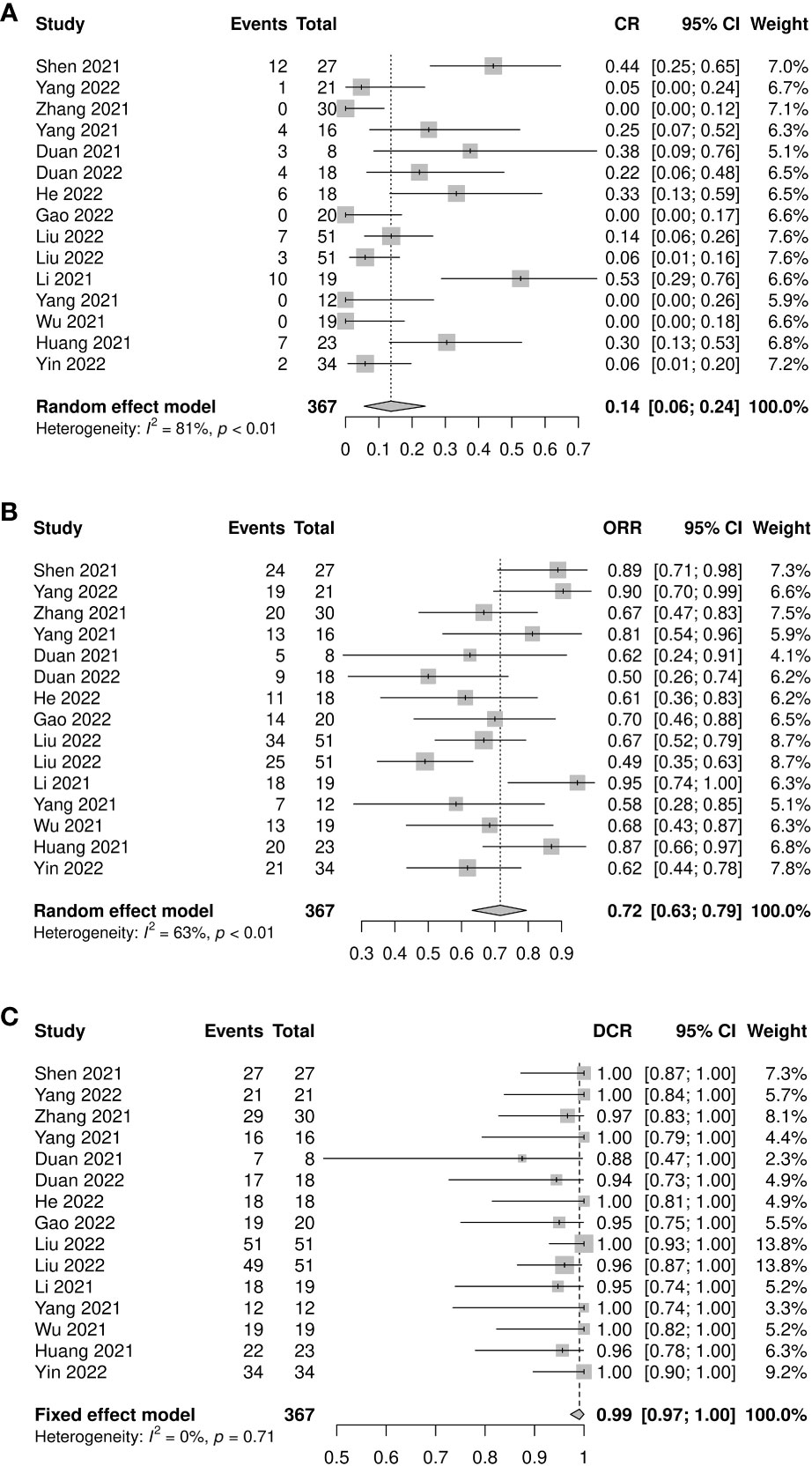
Figure 3 Radiological response of neoadjuvant immune checkpoint inhibitors therapy. (A) Complete response rate, (B) objective response rate, and (C) disease control rate.
3.3.3 R0 resection rate
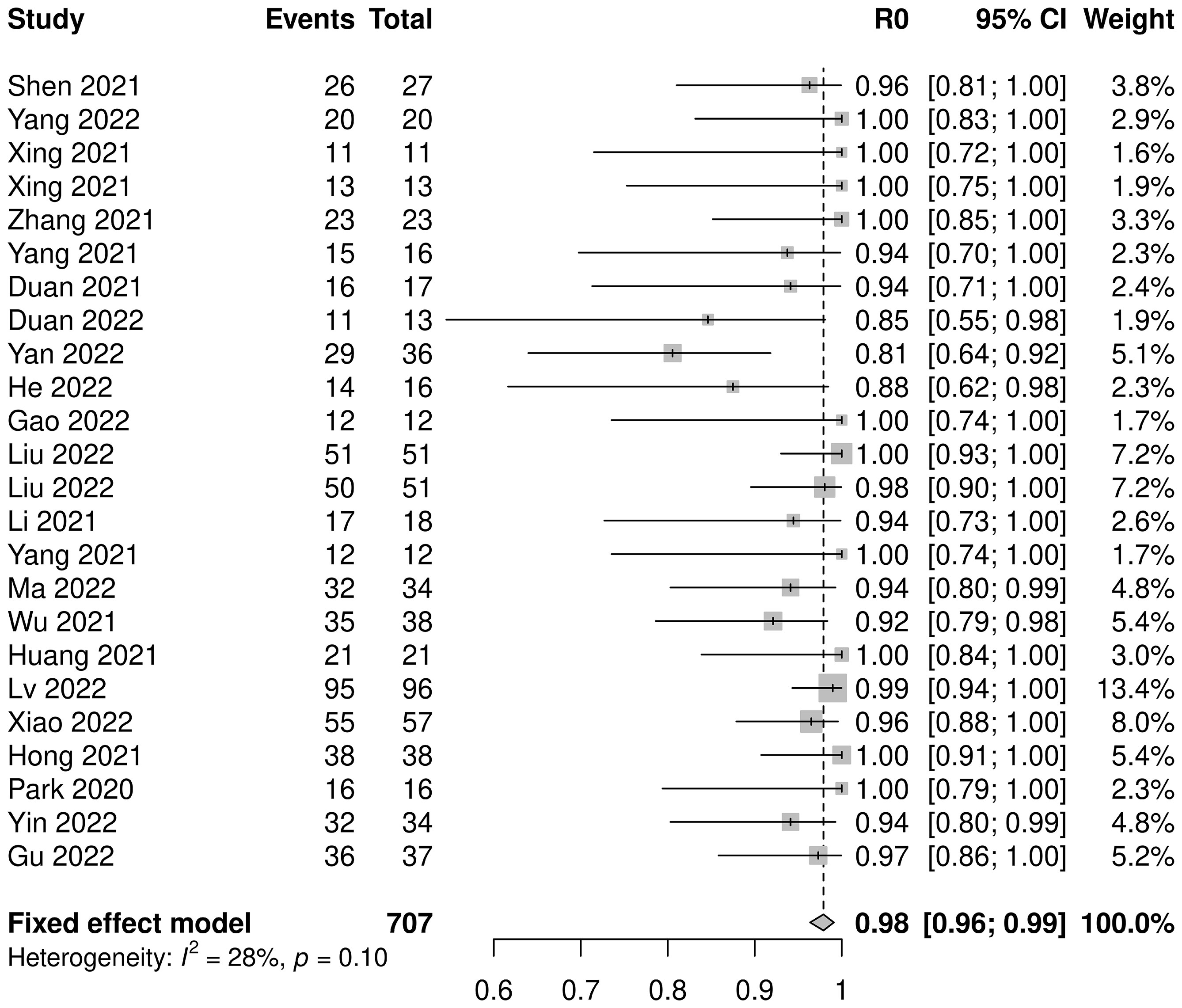
Twenty-three studies with 707 patients were pooled for analysis of the R0 resection rate. The combined R0 resection was 0.98 (95% CI: 0.96–0.99, I2=28%, p=0.10) (Figure 4). Five subgroup analyses were performed, among which the platinum type showed a statistically significant effect, but not clinically significant, on the R0 resection rate (carboplatin: 0.95, 95% CI: 0.92–0.98; cisplatin: 0.99, 95% CI: 0.97–1.00; p=0.01). No significant differences were observed in the other subgroup analyses (Supplementary Figure 7).
3.4 Safety outcomes
3.4.1 Anastomotic leakage
Anastomotic leakage was reported in 25 studies, which totally included 1006 patients. All studies reported similar low incidences of anastomotic leakage, ranging from 0 to 0.19. The pooled incidence was 0.06 (95% CI: 0.04–0.08, I2=0%, p=0.60; Figure 5A). No significant differences were observed in the subgroup analyses (Supplementary Figure 8).
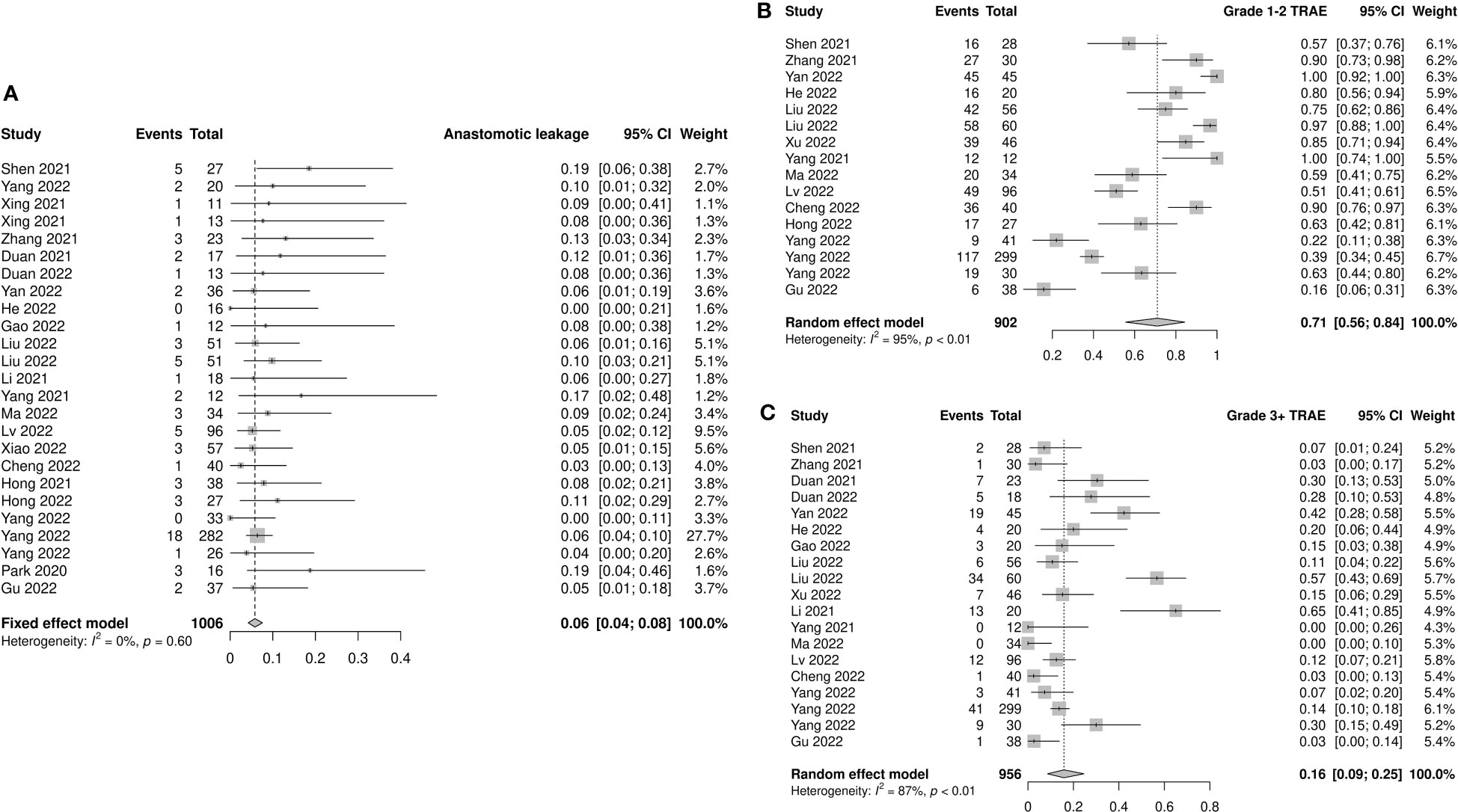
Figure 5 Safety outcomes of neoadjuvant immune checkpoint inhibitors therapy. (A) Anastomotic leakage, (B) grade 1–2 treatment-related adverse events (TRAEs), and (C) grade 3–4 TRAEs.
3.4.2 Treatment-related adverse events
Fourteen studies provided accessible information on grade 1–2 TRAEs. The incidence of pooled grade 1–2 TRAEs was 0.71 (95% CI: 0.56–0.84, I2=95%, p<0.01; Figure 5B). The most frequently reported grade 1–2 TRAEs were leukopenia (8.8%–69.6%), neutropenia (10.4%–65.2%), anemia (8.7%–78.3%), vomiting (6.7%–47.8%), nausea (8.0%–75.0%), and alopecia (8.9%–82.6%). The results of subgroup analysis showed a significant difference between prospective and retrospective studies (prospective: 0.87, 95% CI: 0.74–0.96; retrospective: 0.57, 95% CI: 0.36–0.76; p=0.01; Supplementary Figure 9).
The incidence of grade 3–4 TRAEs was reported in 17 studies involving 956 patients. The pooled incidence was 0.16 (95% CI: 0.09–0.25, I2=87%, p<0.01; Figure 5C). The most frequently reported grade 3–4 TRAEs were leukopenia (3.1%–50.0%), neutropenia (1.8%–39.1%), thrombocytopenia (1.8%–6.7%), and anemia (2.1%–8.7%). Similar to grade 1–2 TRAEs, prospective studies had a higher reported incidence of grade 3–4 TRAEs than retrospective studies (prospective: 0.25, 95% CI: 0.13–0.38; retrospective: 0.07, 95% CI: 0.02–0.14; p<0.01). The incidence of grade 3–4 TRAEs in the nICRT group was significantly higher than that in the nICT group (nICRT: 0.46, 95% CI: 0.17–0.77; nICT: 0.14, 95% CI: 0.07–0.22; p=0.03). Patients treated with carboplatin had a higher incidence of grade 3–4 TRAEs than those treated with cisplatin (carboplatin: 0.33, 95% CI: 0.15–0.53; cisplatin: 0.04, 95% CI: 0.01–0.09; p<0.01). Among the eight studies using carboplatin, grade 3–4 hematologic toxicity TRAEs were reported in six studies, including leukopenia (range: 5%–50%) in five studies, thrombocytopenia (range: 3.6–6.7%) in three studies, and neutropenia (range: 24.4%–39.1%) in two studies. Only three of the six studies using cisplatin mentioned the occurrence of grade 3–4 hematologic toxicity AEs, including leukopenia (range: 1.8%–7.3%), anemia (range: 3.6%–5%), neutropenia (range: 1.8%–5.5%), and thrombocytopenia (1.8%). No significant differences were observed in the subgroup analyses of neoadjuvant treatment cycles and taxane type (Supplementary Figure 10).
3.5 Sensitivity analysis
For the respective outcomes of pCR, MPR, downstaging rate, CR, ORR, DCR, R0 resection rate, anastomotic leakage, and TRAEs, neither recalculated pooled effect nor statistic inconsistency index (I2) was largely changed when leaving out each study (Supplementary Figure 11), indicating the solid stability of the results from the meta-analysis.
3.6 Publication bias
Egger regression tests of pCR, MPR, downstaging rate, CR, ORR, DCR, R0 resection rate, anastomotic leakage, and TRAEs were performed to evaluate publication bias. All the p values of Egger’s test were >0.05 (Supplementary Table 3), indicating that there are no publication biases in studies synthesized in each analysis. The funnel plots of all items are shown in Supplementary Figure 12.
4 Discussion
This meta-analysis evaluated the efficacy and safety of neoadjuvant immunotherapy in patients with resectable locally advanced ESCC. The efficacy results showed that the pooled pCR, MPR, and downstaging rates for neoadjuvant immunotherapy were 0.30, 0.56, and 0.74, respectively. The estimated CR, ORR, and DCR rates were 0.14, 0.72, and 0.99, respectively. The pooled R0 resection rate was 0.98. The safety results showed that the incidences of anastomotic leakage, grade 1–2 TRAEs, and grade 3–4 TRAEs were 0.06, 0.71, and 0.16, respectively. These results indicate that immunotherapy achieves good efficacy and safety as a neoadjuvant therapy in patients with ESCC.
In recent years, several studies have confirmed the efficacy and safety of immunotherapy in the second-, first-, and adjuvant treatments of patients with EC. The ATTRACTION-3 (13), KEYNOTE-181 (14), RATIONALE-302 (20), and ESCORT (12) studies have demonstrated the advantage of PD-1 inhibitor monotherapy over chemotherapy as a second-line therapy in patients with EC. The KEYNOTE-590 (15), CheckMate-648 (16), RATIONALE-306 (21), ESCORT-1 (22), JUPITER-06 (23), and ORIENT-15 (24) studies have shown significant benefits of ICI combination with chemotherapy with a manageable safety profile as a first-line treatment in patients with EC. Based on these studies, several PD-1 inhibitors have been approved for first- or second-line treatment of advanced ESCC by the US Food and Drug Administration, European Medicines Agency, and China National Medical Products Administration. In addition, the CheckMate-577 trial showed superior disease-free survival in nivolumab adjuvant treatment of patients with pathological residual disease after nCRT followed by R0 resection. All of these studies have supported the exploration of immunotherapy in the neoadjuvant setting. Recently, several nICRT or nICT trials have consistently demonstrated that immunotherapy can further improve the efficacy of neoadjuvant treatment with acceptable safety profiles in patients with EC. The present meta-analysis showed that the pooled pCR rate of the nICT group was 0.29, which was higher than the previously reported results of nCT (pCR rate: 0.02–0.09) (10, 11, 25–27). The pCR rate of the nICRT group was 0.48, which was slightly higher than that of the nCRT group (pCR rate: 0.28–0.49) (5, 7, 10, 11, 27). Therefore, the findings of this study also suggest the potential advantage of neoadjuvant immunotherapy in patients with ESCC, and neoadjuvant immunotherapy might be a potential standard treatment for patients with ESCC.
The results of the subgroup analyses in this study showed that nICRT achieved a higher pCR rate than nICT; however, the incidence of grade 3–4 TRAEs was higher in the nICRT group than that in the nICT group. In addition, subgroup analysis showed the higher incidence of grade 1–2 and grade 3–4 TRAEs in prospective studies than that in retrospective studies. This might be due to deficient record of AE events and insufficient attention was paid in retrospective studies. These results are similar to those of previous studies that compared nCRT with nCT. However, these trials failed to show a significant survival advantage of nCRT over nCT (10, 11, 27). This situation might be attributed to radiotherapy, leading to serious AEs and delayed death during long-term follow-up. Owing to the lack of long-term follow-up data in the included studies in the current study, we were unable to confirm whether nICRT achieves better long-term survival results than nICT. Considering the efficacy and safety results, nICT may be a more appropriate treatment option for neoadjuvant treatment in patients with ESCC. These results support the widespread clinical use of nICT with ideal efficacy and safety.
Platinum combination therapy with taxanes is a common chemotherapy regimen used in neoadjuvant therapy for ESCC. However, few studies have directly compared the efficacy and safety of carboplatin with those of cisplatin. It is unclear whether nab-paclitaxel has a better efficacy than paclitaxel in ESCC neoadjuvant therapy. In this meta-analysis, subgroup analysis found that the efficacy and safety results were similar when paclitaxel and nab-paclitaxel were used. Moreover, carboplatin did not lead to a significant improvement in efficacy compared with cisplatin. However, the results indicated that carboplatin had a higher rate of grade 3–4 TRAEs than cisplatin did. The results also indicated that carboplatin had higher 3–4 TRAEs than cisplatin. Among 8 studies using carboplatin, grade 3–4 hematologictoxicity TRAEs were reported in 6 studies including leukopenia in 5 studies, thrombocytopenia in 3 studies, neutropenia in 2 studies. While only 3 of the 6 studies using cisplatin mentioned the occurrence of grade 3–4 hematologictoxicity AEs including leukopenia, anemia, neutropenia, and thrombocytopenia. This result was similar to that of previous studies that showed that cisplatin-based chemotherapy led to more frequent nausea, vomiting, and nephrotoxicity in patients with non-small cell lung cancer, whereas grade ≥ 3 thrombocytopenia was more frequently observed in patients receiving carboplatin-based chemotherapy (28–30). In summary, this finding supported the same efficacy of various chemotherapeutic agents when combined with immunotherapy in ESCC neoadjuvant clinical practice. Therefore, individualized chemotherapy regimen for patients is preferable.
The results of the subgroup analyses did not show better efficacy and more toxicity with ≥ 2 cycles of neoadjuvant immunotherapy compared with two cycles. The increased cycle numbers did not bring more benefits, and two cycles may be the ideal number of cycles for neoadjuvant therapy. However, further studies with long-term survival data are required to validate these results.
In addition, the subgroup analysis showed higher incidences of grade 1–2 and 3–4 TRAEs in prospective studies than in retrospective studies. This might be due to the lack of records of AEs and insufficient attention paid in retrospective studies.
However, this study has some limitations. First, most of the included studies were single-arm phase II clinical studies, and phase III RCT studies were lacking. Second, most studies targeted the Asian ESCC population, and the conclusions were only applicable to patients with ESCC. Third, although we used the MINORS to make a quality assessment of the included studies, there was still some acceptable selection bias and heterogeneity among them. Finally, a common problem among the included studies was the lack of long-term survival outcomes, which would take time to wait for the final results.
5 Conclusion
Neoadjuvant immunotherapy can achieve good efficacy and safety profiles in patients with ESCC. nICRT may have a higher pCR rate and a higher grade 3–4 TRAEs rate than nICT. Different chemotherapy agents and cycle numbers may have similar efficacy and safety outcomes, except for carboplatin, which has higher incidence of 3–4 TRAEs. In conclusion, this result supports the clinical practice of neoadjuvant immunotherapy in patients with ESCC, and long-term survival data still need to be validated by further studies.
Author contributions
Conceptualization, WH. Methodology, CW and CL. Resources, XN, HL and JL. Data Curation, NZ, HC, and XM. Date analysis: WH, CW, and CL. Writing-Original Draft Preparation, WH. Writing - Review and Editing, YH, XL, and LP. Supervision, YH, LP and XL. All authors contributed to the article and approved the submitted version.
Funding
This work was funded by Sichuan Science and Technology Program (2020YFH0169, 23ZDYF2430); Sichuan Province Clinical Key Specialty Construction Project and Bethune Charitable Foundation (HZB-20190528-19).
Conflict of interest
NZ, HC, and XM are employed at BeiGene Beijing Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1118902/full#supplementary-material
Supplementary Figure 1 | Subgroup analyses results of pathological complete response according to (A) study type, (B) neoadjuvant therapy cycle number, (C) combination therapy, (D) platinum type, and (E) taxanes type.
Supplementary Figure 2 | Subgroup analyses results of major pathological response according to (A) study type, (B) neoadjuvant therapy cycle number, (C) platinum type, and (D) taxanes type.
Supplementary Figure 3 | Subgroup analyses results of downstaging rate according to (A) study type, (B) neoadjuvant therapy cycle number, (C) platinum type, and (D) taxanes type.
Supplementary Figure 4 | Subgroup analyses results of complete response rate according to (A) study type, (B) neoadjuvant therapy cycle number, (C) platinum type, and (D) taxanes type.
Supplementary Figure 5 | Subgroup analyses results of objective response rate according to (A) study type, (B) neoadjuvant therapy cycle number, (C) platinum type, and (D) taxane type.
Supplementary Figure 6 | Subgroup analyses results of disease control rate according to (A) study type, (B) neoadjuvant therapy cycle number, (C) platinum type, and (D) taxane type.
Supplementary Figure 7 | Subgroup analyses results of R0 resection rate according to (A) study type, (B) neoadjuvant therapy cycle number, (C) combination therapy, (D) platinum type, and (E) taxane type.
Supplementary Figure 8 | Subgroup analyses results of anastomotic leakage incidence according to (A) study type, (B) neoadjuvant therapy cycle number, (C) combination therapy, (D) platinum type, and (E) taxane type.
Supplementary Figure 9 | Subgroup analyses results of grade 1–2 treatment-related adverse event incidence according to (A) study type, (B) neoadjuvant therapy cycle number, (C) platinum type, and (D) taxane type.
Supplementary Figure 10 | Subgroup analyses results of grade 3–4 treatment-related adverse event incidence according to (A) study type, (B) neoadjuvant therapy cycle number, (C) combination therapy, (D) platinum type, and (E) taxane type.
Supplementary Figure 11 | Sensitivity analysis results of (A) pathological complete response, (B) major pathological response, (C) downstaging rate, (D) complete response rate, (E) objective response rate, (F) disease control rate, (G) R0 resection rate, (H) incidence of anastomotic leakage, (I) grade 1–2 treatment-related adverse events (TRAEs), and (J) grade 3–4 TRAEs.
Supplementary Figure 12 | Egg’s funnel plot of (A) pathological complete response, (B) major pathological response, (C) downstaging rate, (D) complete response rate, (E) objective response rate, (F) disease control rate, (G) R0 resection rate, (H) incidence of anastomotic leakage, (I) grade 1–2 treatment-related adverse events (TRAEs), and (J) grade 3–4 TRAEs.
References
1. Sung H, Ferlay J, Siegel RL. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71:209–49. doi: 10.3322/caac.21660
2. Coleman HG, Xie SH, Lagergren J. The epidemiology of esophageal adenocarcinoma. Gastroenterology (2018) 154:390–405. doi: 10.1053/j.gastro.2017.07.046
3. D'Journo XB. Clinical implication of the innovations of the 8(th) edition of the TNM classification for esophageal and esophago-gastric cancer. J Thorac Dis (2018) 10:S2671–s81. doi: 10.21037/jtd.2018.03.182
4. Lewis RB, Mehrotra AK, Rodriguez P, Levine MS. From the radiologic pathology archives: esophageal neoplasms: Radiologic-pathologic correlation. Radiographics (2013) 33:1083–108. doi: 10.1148/rg.334135027
5. van Hagen P, Hulshof MC, van Lanschot JJ, Steyerberg EW, van Berge Henegouwen MI, Wijnhoven BP, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. New Engl J Med (2012) 366:2074–84. doi: 10.1056/NEJMoa1112088
6. Eyck BM, van Lanschot JJB, Hulshof M. Ten-year outcome of neoadjuvant chemoradiotherapy plus surgery for esophageal cancer: The randomized controlled CROSS trial. J Clin Oncol (2021) 39:1995–2004. doi: 10.1200/jco.20.03614
7. Yang H, Liu H, Chen Y, Zhu C, Fang W, Yu Z, et al. Neoadjuvant chemoradiotherapy followed by surgery versus surgery alone for locally advanced squamous cell carcinoma of the esophagus (NEOCRTEC5010): A phase III multicenter, randomized, open-label clinical trial. J Clin Oncol (2018) 36:2796–803. doi: 10.1200/jco.2018.79.1483
8. Liu S, Wen J, Yang H, Li Q, Chen Y, Zhu C, et al. Recurrence patterns after neoadjuvant chemoradiotherapy compared with surgery alone in oesophageal squamous cell carcinoma: Results from the multicenter phase III trial NEOCRTEC5010. Eur J Cancer (2020) 138:113–21. doi: 10.1016/j.ejca.2020.08.002
9. Matsuda S, Kitagawa Y, Takemura R, Okui J, Okamura A, Kawakubo H, et al. Real-world evaluation of the efficacy of neoadjuvant DCF over CF in esophageal squamous cell carcinoma: Propensity score matched analysis from 85 authorized institutes for esophageal cancer in Japan. Ann Surg (2022). doi: 10.1097/sla.0000000000005533
10. Wang H, Tang H, Fang Y, Tan L, Yin J, Shen Y, et al. Morbidity and mortality of patients who underwent minimally invasive esophagectomy after neoadjuvant chemoradiotherapy vs neoadjuvant chemotherapy for locally advanced esophageal squamous cell carcinoma: A randomized clinical trial. JAMA Surg (2021) 156:444–51. doi: 10.1001/jamasurg.2021.0133
11. Klevebro F, Alexandersson von Döbeln G, Wang N, Johnsen G, Jacobsen AB, Friesland S, et al. A randomized clinical trial of neoadjuvant chemotherapy versus neoadjuvant chemoradiotherapy for cancer of the oesophagus or gastro-oesophageal junction. Ann Oncol (2016) 27:660–7. doi: 10.1093/annonc/mdw010
12. Huang J, Xu J, Chen Y, Zhuang W, Zhang Y, Chen Z, et al. Camrelizumab versus investigator's choice of chemotherapy as second-line therapy for advanced or metastatic oesophageal squamous cell carcinoma (ESCORT): A multicentre, randomised, open-label, phase 3 study. Lancet Oncol (2020) 21:832–42. doi: 10.1016/s1470-2045(20)30110-8
13. Kato K, Cho BC, Takahashi M, Okada M, Lin CY, Chin K, et al. Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol (2019) 20:1506–17. doi: 10.1016/s1470-2045(19)30626-6
14. Kojima T, Shah MA. Randomized phase III KEYNOTE-181 study of pembrolizumab versus chemotherapy in advanced esophageal cancer. J Clin Oncol (2020) 38:4138–48. doi: 10.1200/jco.20.01888
15. Sun JM, Shen L, Shah MA, Enzinger P, Adenis A, Doi T, et al. Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): A randomised, placebo-controlled, phase 3 study. Lancet (2021) 398:759–71. doi: 10.1016/s0140-6736(21)01234-4
16. Doki Y, Ajani JA. Nivolumab combination therapy in advanced esophageal squamous-cell carcinoma. New Engl J Med (2022) 386:449–62. doi: 10.1056/NEJMoa2111380
17. Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: Checklist and explanations. Ann Intern Med (2015) 162:777–84. doi: 10.7326/m14-2385
18. Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non-randomized studies (minors): Development and validation of a new instrument. ANZ J Surg (2003) 73:712–6. doi: 10.1046/j.1445-2197.2003.02748.x
19. Borenstein M, Higgins JP. Meta-analysis and subgroups. Prev Sci Off J Soc Prev Res (2013) 14:134–43. doi: 10.1007/s11121-013-0377-7
20. Shen L, Kato K. Tislelizumab versus chemotherapy as second-line treatment for advanced or metastatic esophageal squamous cell carcinoma (RATIONALE-302): A randomized phase III study. J Clin Oncol (2022) 40:3065–76. doi: 10.1200/jco.21.01926
21. Yoon H, Kato K, Raymond E, Hubner R, Shu Y, Pan Y, et al. LBA-1 RATIONALE-306: Randomized, global, placebo-controlled, double-blind phase 3 study of tislelizumab plus chemotherapy versus chemotherapy as first-line treatment for advanced or metastatic esophageal squamous cell carcinoma (ESCC). Ann Oncol (2022) 33:S375. doi: 10.1016/j.annonc.2022.04.439
22. Luo H, Lu J, Bai Y, Mao T, Wang J, Fan Q, et al. Effect of camrelizumab vs placebo added to chemotherapy on survival and progression-free survival in patients with advanced or metastatic esophageal squamous cell carcinoma: The ESCORT-1st randomized clinical trial. JAMA (2021) 326:916–25. doi: 10.1001/jama.2021.12836
23. Wang ZX, Cui C, Yao J, Zhang Y, Li M, Feng J, et al. Toripalimab plus chemotherapy in treatment-naïve, advanced esophageal squamous cell carcinoma (JUPITER-06): A multi-center phase 3 trial. Cancer Cell (2022) 40:277–88.e3. doi: 10.1016/j.ccell.2022.02.007
24. Lu Z, Wang J, Shu Y, Liu L, Kong L, Yang L, et al. Sintilimab versus placebo in combination with chemotherapy as first line treatment for locally advanced or metastatic oesophageal squamous cell carcinoma (ORIENT-15): Multicentre, randomised, double blind, phase 3 trial. BMJ (2022) 377:e068714. doi: 10.1136/bmj-2021-068714
25. Ando N, Kato H, Igaki H, Shinoda M, Ozawa S, Shimizu H, et al. A randomized trial comparing postoperative adjuvant chemotherapy with cisplatin and 5-fluorouracil versus preoperative chemotherapy for localized advanced squamous cell carcinoma of the thoracic esophagus (JCOG9907). Ann Surg Oncol (2012) 19:68–74. doi: 10.1245/s10434-011-2049-9
26. Kelsen DP, Ginsberg R, Pajak TF, Sheahan DG, Gunderson L, Mortimer J, et al. Chemotherapy followed by surgery compared with surgery alone for localized esophageal cancer. New Engl J Med (1998) 339:1979–84. doi: 10.1056/nejm199812313392704
27. Kato K, Ito Y, Daiko H, Ozawa S, Ogata T, Hara H, et al. A randomized controlled phase III trial comparing two chemotherapy regimen and chemoradiotherapy regimen as neoadjuvant treatment for locally advanced esophageal cancer, JCOG1109 NExT study. Am Soc Clin Oncol (2022) 40(4 suppl). doi: 10.1200/JCO.2022.40.4_suppl.238
28. Hotta K, Matsuo K, Ueoka H, Kiura K, Tabata M, Tanimoto M. Meta-analysis of randomized clinical trials comparing cisplatin to carboplatin in patients with advanced non-small-cell lung cancer. J Clin Oncol (2004) 22:3852–9. doi: 10.1200/jco.2004.02.109
29. Ardizzoni A, Boni L, Tiseo M, Fossella FV, Schiller JH, Paesmans M, et al. Cisplatin- versus carboplatin-based chemotherapy in first-line treatment of advanced non-small-cell lung cancer: An individual patient data meta-analysis. J Natl Cancer Inst (2007) 99:847–57. doi: 10.1093/jnci/djk196
Keywords: neoadjuvant immunotherapy, esophageal squamous cell carcinoma, nICRT, nICT, meta-analysis
Citation: He W, Wang C, Li C, Nie X, Li H, Li J, Zhao N, Chen H, Miao X, Han Y, Peng L and Leng X (2023) The efficacy and safety of neoadjuvant immunotherapy in resectable locally advanced esophageal squamous cell carcinoma: A systematic review and meta-analysis. Front. Immunol. 14:1118902. doi: 10.3389/fimmu.2023.1118902
Received: 08 December 2022; Accepted: 06 February 2023;
Published: 17 February 2023.
Edited by:
Ulrich Ronellenfitsch, Medical Faculty of the Martin-Luther-University Halle-Wittenberg, GermanyReviewed by:
Kong Lingsuo, The First Affiliated Hospital of University of Science and Technology of China Anhui Provincial Hospital, ChinaXing Niu, China Medical University, China
Copyright © 2023 He, Wang, Li, Nie, Li, Li, Zhao, Chen, Miao, Han, Peng and Leng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lin Peng, cGVuZ2xpbm1zQDEyNi5jb20=; Xuefeng Leng, bGVuZzcyNEAxNjMuY29t
†These authors have contributed equally to this work and share first authorship
 Wenwu He
Wenwu He Chenghao Wang
Chenghao Wang Changding Li1†
Changding Li1† Xin Nie
Xin Nie Jialong Li
Jialong Li Xuefeng Leng
Xuefeng Leng