- 1Department of Oncology and Hematology, Zhongxian People’s Hospital, Chongqing, China
- 2Department of Urology, Zhongxian People’s Hospital, Chongqing, China
- 3Department of Radiation Oncology, The First Center of the Chinese PLA General Hospital, Beijing, China
- 4Department of Radiation Oncology, The Fifth Center of the Chinese PLA General Hospital, Beijing, China
The development of immunotherapy, especially immune-checkpoint inhibitors targeting PD-1/PD-L1, has improved the outcomes of patients with esophageal cancer. However, not all population derives benefit from the agents. Recently, kinds of biomarkers were introduced to predict the response to immunotherapy. However, the effects of these reported biomarkers are controversial and many challenges remain. In this review, we aim to summarize the current clinical evidence and provide a comprehensive understanding of the reported biomarkers. We also discuss the limits of the present biomarkers and propose our own opinions on which viewers’ discretion are advised.
Introduction
Esophageal cancer is the sixth leading cause of cancer-related death worldwide (1). The main histological types of esophageal cancer are esophageal squamous cell carcinoma (ESCC) and esophageal adenocarcinoma (EAC), and ESCC is the predominant subtype in Asia. The poor outcomes of patients with esophageal cancer attributed to the late diagnosis and a propensity for metastases (2). In recent years, the development of immunotherapy including adoptive cell transfer and immune checkpoint inhibitors (ICIs) has revolutionized cancer treatment (3–5). ICIs targeting programmed death receptor 1 (PD-1) and its ligand (PD-L1) have been proved to be promising in the treatment of kinds of cancers, also in esophageal cancer. However, only 20-40% of patients will benefit from ICIs and even fewer will have long-term disease control (6, 7). Therefore, it is critical to screen out population that will respond to these agents. Aim of this paper is to review established clinical evidence and provide current knowledge for biomarkers in esophageal cancer.
PD-L1 status
PD-1, mostly expressed on tumor-infiltrating immune cells and PD-L1, expressed on antigen-presenting cells and tumor cells, could negatively regulate the antitumor immune response (8, 9). Using monoclonal antibodies to block PD-1/PD-L1 checkpoint axis restored antitumor immunity (10). As a result, it is reasonable to assume the expression level of PD-L1 as a biomarker to predict the efficacy of anti PD-1/PD-L1 antibodies. Actually, the predictive role of PD-L1 expression, which could be determined by immunohistochemistry (IHC), has been proved in kinds of cancer types based on current clinical evidence (11–14). PD-L1 expression could be assessed using different scoring algorithm including tumor cells and tumor-infiltrating immune cells. Tumor proportional score (TPS) is defined as the percentage of viable tumor cells with PD-L1 staining relative to all viable tumor in sample (15, 16). In non-small cell lung cancer cohort of KEYNOTE-001 trial, patients with advanced-stage received pembrolizumab, of those with a PD-L1 TPS > 50% had a response rate of 45.2%, versus 16.5% and 10.7% in patients with a TPS of 1-49% and < 1%, respectively (11). Combined positive score (CPS) was defined as the sum of all PD-L1 positive cells (including tumor cells, lymphocytes and macrophages) divided by all viable tumor cells×100 (17). Beyond the TPS and CPS scoring system, the PD-L1 tumor-infiltrating immune cell (IC) status was defined by the percentage of PD-L1-positive immune cells in the tumor microenvironment, which was seldom used in clinical trials (18).
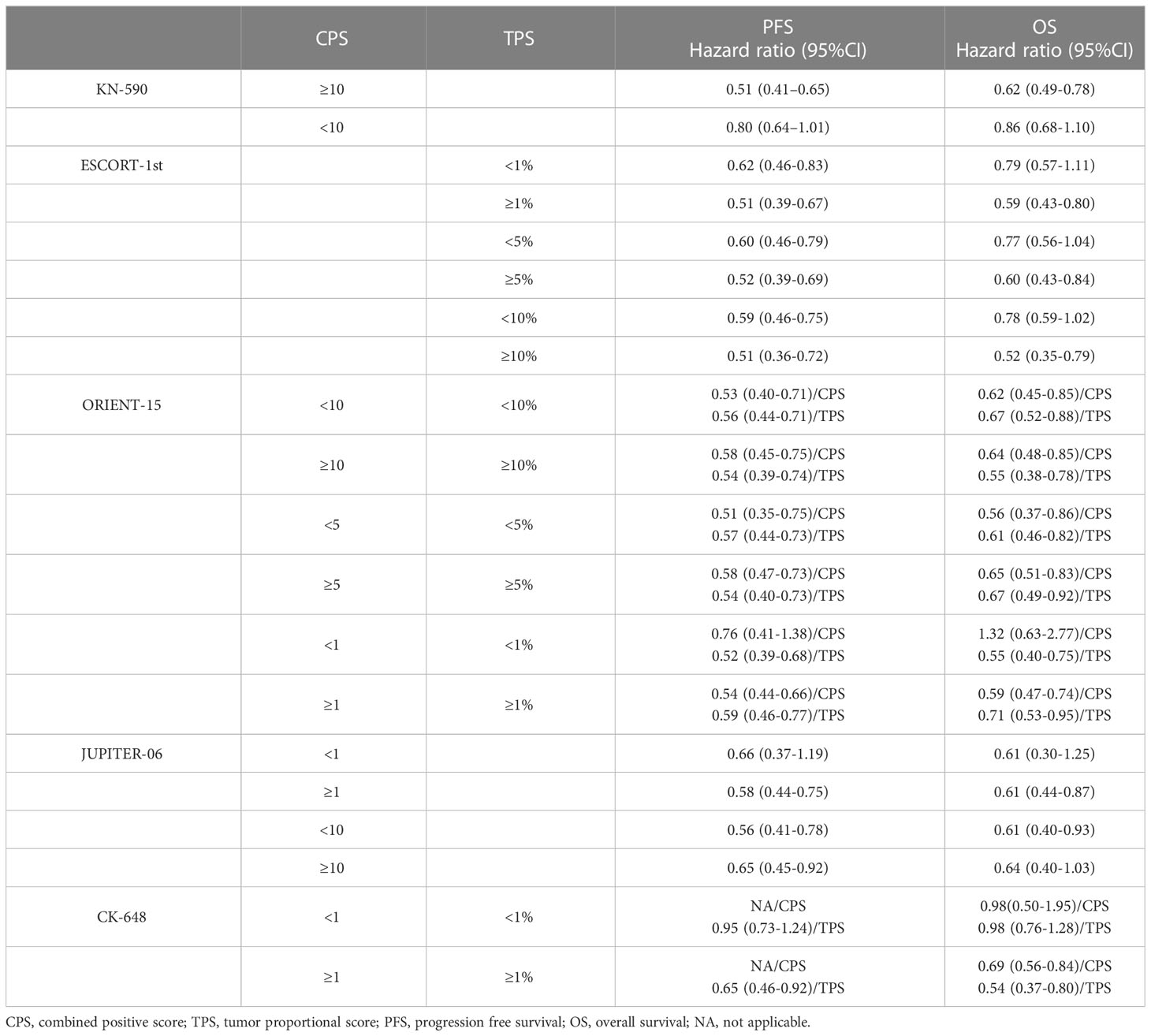
Recently, the efficacy and safety of ICIs in esophageal cancer was established based on several phase III clinical trials. Although the predictive role of PD-L1 expression was analyzed, it was still controversial. In the KEYNOTE-590 trial (NCT03189719), pembrolizumab plus chemotherapy as first-line therapy for advanced or metastatic esophageal cancer was firstly evaluated. Combined treatment with pembrolizumab improved the PFS (HR=0.51, CI:0.41-0.65) and OS (HR=0.62, CI:0.49-0.78) in PD-L1 positive patients (CPS≥10). For patients with negative PD-L1 expression (CPS<10), the improved PFS and OS was not meaningful (19). Subsequently, the CheckMate-648, ORIENT-15, JUPITER-06 and ESCORT-1ST evaluated the efficacy of different ICIs as first-line therapy in esophageal cancer (20–23). In these clinical trials, either TPS or CPS was determined and different cut-off values were set. Results showed that (as summarized in Table 1) ICI plus chemotherapy was superior to placebo plus chemotherapy for OS and PFS in patients with higher PD-L1 expression. However, in patients with negative or low PD-L1 expression, the benefits were not observed in all trials. In addition, it should be noted that most patients included in these trials were diagnosed with ESCC. Interestingly, when it comes to monotherapy of ICI, things seem different. In trial KEYNOTE-181, patients with advanced/metastatic esophageal cancer were assigned to pembrolizumab or chemotherapy. Final analysis showed OS was prolonged with pembrolizumab versus chemotherapy for patients with CPS ≥10 (median, 9.3 vs 6.7 months; HR=0.69, CI:0.52-0.93), and the OS benefit was not observed in patients with CPS <10 (24). The results were similar in trial ATTRACTION-3, ESCORT and RATIONALE-302, monotherapy of ICI in second-line treatment of esophageal cancer demonstrated no meaningful improvement in OS compared with chemotherapy in patients with negative PD-L1 expression (25–27). These results suggested that chemotherapy could induce immunogenic cell death of tumor and therefore increase the anti-tumor immunity, diluting the effect of PD-L1 expression. Therefore, combined therapy with ICI would be a better choice in patients with negative PD-L1 expression.
Generally, although high PD-L1 expression is evidentially associated with the efficacy of immunotherapy in esophageal cancer, it is still not perfect. The assessment of PD-L1 expression was usually made at the time of diagnosis or before treatment, which could not reflect the dynamic changes over time as the tumor microenvironment evolves. In addition, multiple treatments have been shown to induce the PD-L1 expression on tumor cells and antigen-presenting cells, such as radiotherapy, chemotherapy and targeted therapy (28–32). Also, the spatial heterogeneity of PD-L1 expression should be taken into consideration (33). As a result, the heterogeneity of PD-L1 expression makes it difficult to assess the PD-L1 status comprehensively and accurately, which could not be refined as a reliable biomarker when used alone.
Tumor mutational burden
TMB is defined as the total number of mutations per coding area of a tumor genome, which is assessed by hybrid based next generation sequencing (NGS) (34). Somatic mutations in tumor have the potential to result in the expression of neoantigens, which could be recognized by the immune system and induce anti-tumor response (35). Based on this hypothesis, high TMB is thought to be correlated with benefits from ICIs administration. Current clinical evidence supporting TMB as a biomarker for immunotherapy come from trial KEYNOTE-158 (36). In this multi-cohort study, 1073 patients with previously treated advanced solid tumors were enrolled and efficacy was assessed in all patients who received at least one dose of pembrolizumab and had evaluable TMB data. The definition of TMB-high status was at least 10 mutations per megabase. Results showed that objective responses were observed in 30 (29%; 95%CI: 21-39) of 102 patients in the TMB-high group and 43 (6%; 5-8) of 688 in the non-TMB-high group, indicating TMB as a useful predictive biomarker for response to pembrolizumab monotherapy. Yarchoan and colleagues evaluated the relationship between TMB and objective response rate for immunotherapy. Through published researches, they plotted the objective response rate for anti-PD-1/PD-L1 therapy against the corresponding median TMB across multiple cancer types and observed a significant correlation (P<0.001) (37). Furthermore, the correlation coefficient of 0.74 suggested that 55% of the differences in the objective response rate across cancer types might be explained by TMB.
However, not all evidences are supportive. McGrail and colleagues collected clinical data of over 10000 patients from The Cancer Genome Atlas and analyzed the relationship between TMB and ICIs treatment outcomes. They found that in cancers where CD8 T cell levels positively correlated with neoantigen load, such as melanoma and lung cancer, high TMB predicted a better ORR to ICI significantly. However, in cancer types that exhibited no relationship between CD8 T cell levels and neoantigen load, such as breast cancer and glioma, high TMB showed poor clinical outcomes (38). In addition, esophageal cancer was classified as this type. These data did not support TMB as a predictive biomarker alone, other factors should also be taken into consideration. Yarchoan and colleagues assessed 9887 clinical samples for TMB and reported the median TMB across all samples was 3.48 mutations/Mb. Further analysis showed that the median TMB was less than 5 mutations/Mb and the percentage of a TMB greater that 10 mutations/Mb was about 10% in esophageal cancer (34). Based on these evidences, the predictive role of TMB for ICIs treatment is still controversial, especially in esophageal cancer. The use of TMB in clinical practice would be rare as the percentage of TMB-H is actually low in esophageal cancer. Also, we do not recommend TMB as a mono-biomarker.
Microsatellite instability
Like TMB, MSI also represents the mutations in genome and is caused by defects in the mismatch repair (MMR) system, which leads to the production of neoantigens and further initiates antitumor response. Microsatellite is defined as repetitive DNA motifs of 1-6 base pairs, mainly occurring in non-coding DNA (39). The frequency of MSI differs among different cancer types. However, the available data was limited, except for colorectal cancer, gastric cancer and endometrial cancer for which MSI analysis was routinely performed. Bonneville and colleagues collected whole-exome data from 11139 tumor-normal pairs from TCGA and Therapeutically Applicable Research to Generate Effective Treatments projects and external data including 39 cancer types (40). Of all cancers analyzed, the frequency of MSI was 3.8% and MSI was not detected in 12 cancer types. Furthermore, the type-specific prevalence of MSI was analyzed, varying from 31.4% in endometrial carcinoma to 0.25% in glioblastoma. For esophageal cancer, MSI-H was observed in 3 (1.64%) of 184 cases, which ranked tenth of all types. Another study showed similar results. Parikh and colleagues tested tumor samples from 17486 unique patients with different cancer types and the prevalence of MSI-H was 1.7% (32/2501) in esophageal cancer (41).
Previous researches have shown that patients with MSI-H had a better response to immunotherapy. A phase 2 study (NCT01876511) enrolled 41 patients with progressive metastatic carcinoma treated with pembrolizumab. Data showed that the ORR and progression-free survival rate were 40% and 78% for mismatch repair-deficient colorectal cancer. In contrast, 0% of ORR and 11% of PFS rate were observed for mismatch repair-proficient colorectal cancers (42). In the cohort K of trial KEYNOTE-185, patients with MSI-H/dMMR advanced noncolorectal cancer were treated with pembrolizumab. Of all 233 enrolled patients (including 27 tumor types), 23 (9.9%) had a confirmed complete response and 57 (24.5%) had a confirmed partial response (43). These results supported MSI-H as a biomarker for immunotherapy.
However, the role of MSI status for esophageal cancer was not reported. In trial KEYNOTE-158, patients with MSI-H esophageal cancer were not included, probably resulting from the low prevalence of MSI in esophageal cancer. In trial KETNOTE-590, the MSI status was recorded for 112 (40%) of 278 patients and none had MSI-H (19). In addition, data of MSI status from other clinical trials for esophageal cancer was not available. Therefore, the predictive role of MSI status for immunotherapy in esophageal cancer requires further investigation and the conclusion is probably supportive by extrapolation from data for other cancer types.
Nutritional status
The nutrition management draws more and more attention to date. In the natural course of cancer, poor nutritional status is prevalent resulting from the presence of the tumor and the patient’s treatment and is described as sarcopenia and cachexia. As a devastating and irreversible syndrome of energy imbalance, cachexia was reported to be responsible for the death of about 20% of all patients with cancer (44). Cachexia is characterized mainly as the loss of skeletal muscle, affecting 50%-80% of cancer patients. Recently, accumulating evidence emphasized the role of skeletal muscle cells in the regulation of immune response in health and disease (45, 46). Skeletal muscle cells express major histocompatibility complexes (MHCs) I and II under inflammation and are able to present antigens to T cells, acting as antigen-presenting cells. Furthermore, through secreting kinds of cytokines, skeletal muscle cells could regulate immune response (47). The most reported cytokine secreted by skeletal muscle cells is IL-15 (48, 49). IL-15 is critical in the development and maintenance of kinds of immune cells. The proliferation and activation of NK cells are regulated by IL-15. Also, the homeostasis of CD8 T cells and B cells are modulated by IL-15 (50). Based on these researches, skeletal muscle is thought to play a pivotal role in the interaction with immune cells, further regulating the immune responses. Therefore, more and more studies focused on the predictive role of skeletal muscle for the response to ICIs.
The condition characterized by the loss of muscle mass and strength in cancer patients is termed sarcopenia. In current studies, the skeletal muscle index (SMI) was mostly used to evaluate sarcopenia, which is defined as the ratio of skeletal muscle area (SMA) at the third lumbar vertebral level to squared height (51, 52). Roch and colleagues investigated the effect of sarcopenia on the efficacy of ICIs in non-small cell lung cancer. In this study, 142 patients were enrolled treated with first- or second-line ICIs and a decrease by 5% or more of SMI was defined as sarcopenia. Results showed that a shorter PFS and OS was observed in patients with sarcopenia, with HR: 2.45 (CI: 1.09-5.53) and 3.87 (CI: 1.60-9.34) respectively (53). Kim and colleagues also evaluated the prognostic significance of sarcopenia in patients with microsatellite-stable gastric cancer receiving ICIs. Of all enrolled 149 patients, 79 (53%) had sarcopenia. Patients with sarcopenia showed shorter PFS (1.4 moths vs. 2.6 months; P=0.026), and OS was shorter non-significantly (3.6 months vs. 4.9 months; P=0.052) (52). A meta-analysis was performed by Deng and nine cohort studies were included consisting of 740 patients with advanced cancer treated with ICIs. Data showed that patients with sarcopenia obtained a lower response rate than those without disease (30.5% vs. 15.9%; P=0.095). Further analysis found that sarcopenia was correlated with a significant shorter 1-y PFS rate (32% vs 10.8%; P<0.001) and 1-y OS rate (66% vs 43%; P<0.001) (54). Current evidence supported sarcopenia as a promising factor in patients receiving ICIs.
When it comes to esophageal cancer, nutrition management seems to be especially important. As is reported, the prevalence of sarcopenia in patients with esophageal cancer is high, ranging from 14.4% to 80% (55). Although the relationship between sarcopenia and the efficacy of ICIs in patients with esophageal cancer has not been fully studied, it is believed that sarcopenia would affect the efficacy of ICIs in patients with esophageal cancer extrapolating from data of other cancer types. Taking into consideration both the high prevalence and the extensive use of ICIs in esophageal cancer, it is necessary to reveal the prognostic value of sarcopenia.
Inflammatory biomarkers
The relationship between inflammation and cancer is complicated. Tumor-associated inflammation is reported to promote cancer progression and even initiate the tumorigenesis (56). Current evidence revealed that the systemic inflammation is associated with the poor outcomes of cancer patients (57). However, the definition and quantification of systemic inflammation was not unified. C-reactive protein (CRP) and other combined factors were used in early studies, which has been proved to be prognostic markers in cancer patients (58). Recently, new associated factors were developed based on the measured full blood count, such as neutrophil to lymphocyte (NLR), derived NLR (dNLR), lymphocyte-monocyte ratio (LMR), platelet-lymphocyte ratio (PLR) or absolute count of white blood cell subtypes. These factors are inexpensive and easily obtained, indicating the potential use in clinical practice.
Of all the established factors, NLR was the most well-studied and shown to be predictive in survival across kinds of cancer types. Pirozzolo and colleagues conducted a meta-analysis and included 20 studies. In this study, 6457 patients with esophageal cancer were included and the role of NLR was analyzed, with the cut-off value ranging from 1.7 to 5. The HR for OS and PFS of all include studies was 1.6 and 1.66 respectively, indicating high NLR was related with poorer outcomes in esophageal cancer (59). Another study investigated the role of NLR to predict efficacy for ICIs treatment in patients with metastatic gastric cancer. In this study, best cut-off value was set as 3.23 and NLR < 3.23 was shown to be associated with longer OS (HR=0.38; CI: 0.26-0.57). Although NLR made no difference on ORR, NLR was still considered as a predictive factor for immunotherapy (60). Hwang and colleagues found that reductions in NLR were associated with the induction of IFN-γ responses that drove the expression of antigen presentation and proinflammatory genes sets. These results partially explained the underlying mechanisms of NLR prediction (61).
Evidence on the predictive role of inflammatory factors is accumulating and the value in esophageal cancer treated with chemoradiotherapy was also confirmed (62). However, it is unclear whether the inflammatory biomarkers are useful in patients with esophageal cancer receiving ICIs, which needs further investigation.
Discussion
As the development of ICIs drugs, it is an urgent to introduce efficient biomarkers to improve the efficacy of immunotherapy. In trial IPASS (NCT00322452), 437 patients provided samples for EGFR mutation evaluation and 261 samples were positive for an EGFR mutation, of which 140 had exon 19 deletions and 111 had a mutation at exon 21 (L858R). The presence of a mutation of the EGFR gene was proved to be a strong biomarker of a better outcome with gefitinib (63). This research was a sort of opening salvo of the following development of targeted therapies. Though ICIs are targeting specific molecules as well, the molecules are not tumor driving. Therefore, the difference lies that ICIs exhibit anti-tumor effect not through specific molecules but by regulating the entire immunity. As is shown previously, a series of critical stepwise events must be initiated to develop anti-tumor immune response, which are referred as the Cancer-Immunity Cycle (64). Each of the biomarkers discussed in this review represents one or more of the steps in this cycle. For example, TMB and MSI-H refers to the release of cancer cell antigens and PD-L1 status is involved in the priming and activation of T cells. Absence of either event would lead to the arrest of the anti-tumor immune response. As a result, mono-biomarker is hardly able to predict the efficacy of immunotherapy. The author believes that a mathematic model covering all the pivotal seven steps must be established. By evaluating the anti-tumor immunity status of individual, physicians would make accurate judgements on the response of immunotherapy and provide more efficient treatment strategies. Although it is of great difficulty to establish such a model, the author considers it as a trend in the future development and interdisciplinary cooperation must be conducted.
Author contributions
All authors contributed equally to drafting and writing the article. All authors revised it critically for important intellectual content. All authors read and approved the final manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin (2018) 68(6):394–424. doi: 10.3322/caac.21492
2. Pennathur A, Gibson MK, Jobe BA, Luketich JD. Oesophageal carcinoma. Lancet (2013) 381(9864):400–12. doi: 10.1016/S0140-6736(12)60643-6
3. Zhao Q, Jiang Y, Xiang S, Kaboli PJ, Shen J, Zhao Y, et al. Engineered tcr-T cell immunotherapy in anticancer precision medicine: pros and cons. Front Immunol (2021) 12:658753. doi: 10.3389/fimmu.2021.658753
4. Rosenberg SA, Restifo NP. Adoptive cell transfer as personalized immunotherapy for human cancer. Science (2015) 348(6230):62–8. doi: 10.1126/science.aaa4967
5. Bagchi S, Yuan R, Engleman EG. Immune checkpoint inhibitors for the treatment of cancer: clinical impact and mechanisms of response and resistance. Annu Rev Pathol (2021) 16:223–49. doi: 10.1146/annurev-pathol-042020-042741
6. Garon EB, Hellmann MD, Rizvi NA, Carcereny E, Leighl NB, Ahn MJ, et al. Five-year overall survival for patients with advanced nonsmall-cell lung cancer treated with pembrolizumab: results from the phase I keynote-001 study. J Clin Oncol (2019) 37(28):2518–27. doi: 10.1200/JCO.19.00934
7. Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, et al. Five-year survival outcomes for patients with advanced melanoma treated with pembrolizumab in keynote-001. Ann Oncol (2019) 30(4):582–8. doi: 10.1093/annonc/mdz011
8. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer (2012) 12(4):252–64. doi: 10.1038/nrc3239
9. Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell (2015) 27(4):450–61. doi: 10.1016/j.ccell.2015.03.001
10. Xu-Monette ZY, Zhang M, Li J, Young KH. Pd-1/Pd-L1 blockade: have we found the key to unleash the antitumor immune response? Front Immunol (2017) 8:1597. doi: 10.3389/fimmu.2017.01597
11. Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, et al. Pembrolizumab for the treatment of non-Small-Cell lung cancer. New Engl J Med (2015) 372(21):2018–28. doi: 10.1056/NEJMoa1501824
12. Herbst RS, Baas P, Kim DW, Felip E, Perez-Gracia JL, Han JY, et al. Pembrolizumab versus docetaxel for previously treated, pd-L1-Positive, advanced non-Small-Cell lung cancer (Keynote-010): a randomised controlled trial. Lancet (2016) 387(10027):1540–50. doi: 10.1016/S0140-6736(15)01281-7
13. Schachter J, Ribas A, Long GV, Arance A, Grob JJ, Mortier L, et al. Pembrolizumab versus ipilimumab for advanced melanoma: final overall survival results of a multicentre, randomised, open-label phase 3 study (Keynote-006). Lancet (2017) 390(10105):1853–62. doi: 10.1016/S0140-6736(17)31601-X
14. Cohen EEW, Soulieres D, Le Tourneau C, Dinis J, Licitra L, Ahn MJ, et al. Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-Neck squamous cell carcinoma (Keynote-040): a randomised, open-label, phase 3 study. Lancet (2019) 393(10167):156–67. doi: 10.1016/S0140-6736(18)31999-8
15. Kerr KM, Tsao MS, Nicholson AG, Yatabe Y, Wistuba II, Hirsch FR, et al. Programmed death-ligand 1 immunohistochemistry in lung cancer: in what state is this art? J Thorac Oncol (2015) 10(7):985–9. doi: 10.1097/JTO.0000000000000526
16. Kerr KM, Hirsch FR. Programmed death ligand-1 immunohistochemistry: friend or foe? Arch Pathol Lab Med (2016) 140(4):326–31. doi: 10.5858/arpa.2015-0522-SA
17. Dolled-Filhart M, Roach C, Toland G, Stanforth D, Jansson M, Lubiniecki GM, et al. Development of a companion diagnostic for pembrolizumab in non-small cell lung cancer using immunohistochemistry for programmed death ligand-1. Arch Pathol Lab Med (2016) 140(11):1243–9. doi: 10.5858/arpa.2015-0542-OA
18. Rosenberg JE, Hoffman-Censits J, Powles T, van der Heijden MS, Balar AV, Necchi A, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet (2016) 387(10031):1909–20. doi: 10.1016/S0140-6736(16)00561-4
19. Sun JM, Shen L, Shah MA, Enzinger P, Adenis A, Doi T, et al. Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (Keynote-590): a randomised, placebo-controlled, phase 3 study. Lancet (2021) 398(10302):759–71. doi: 10.1016/S0140-6736(21)01234-4
20. Doki Y, Ajani JA, Kato K, Xu J, Wyrwicz L, Motoyama S, et al. Nivolumab combination therapy in advanced esophageal squamous-cell carcinoma. New Engl J Med (2022) 386(5):449–62. doi: 10.1056/NEJMoa2111380
21. Lu Z, Wang J, Shu Y, Liu L, Kong L, Yang L, et al. Sintilimab versus placebo in combination with chemotherapy as first line treatment for locally advanced or metastatic oesophageal squamous cell carcinoma (Orient-15): multicentre, randomised, double blind, phase 3 trial. Bmj (2022) 377:e068714. doi: 10.1136/bmj-2021-068714
22. Luo H, Lu J, Bai Y, Mao T, Wang J, Fan Q, et al. Effect of camrelizumab vs placebo added to chemotherapy on survival and progression-free survival in patients with advanced or metastatic esophageal squamous cell carcinoma: the escort-1st randomized clinical trial. Jama (2021) 326(10):916–25. doi: 10.1001/jama.2021.12836
23. Wang ZX, Cui C, Yao J, Zhang Y, Li M, Feng J, et al. Toripalimab plus chemotherapy in treatment-naive, advanced esophageal squamous cell carcinoma (Jupiter-06): a multi-center phase 3 trial. Cancer Cell (2022) 40(3):277–88 e3. doi: 10.1016/j.ccell.2022.02.007
24. Kojima T, Shah MA, Muro K, Francois E, Adenis A, Hsu CH, et al. Randomized phase iii keynote-181 study of pembrolizumab versus chemotherapy in advanced esophageal cancer. J Clin Oncol (2020) 38(35):4138–48. doi: 10.1200/JCO.20.01888
25. Huang J, Xu J, Chen Y, Zhuang W, Zhang Y, Chen Z, et al. Camrelizumab versus investigator’s choice of chemotherapy as second-line therapy for advanced or metastatic oesophageal squamous cell carcinoma (Escort): a multicentre, randomised, open-label, phase 3 study. Lancet Oncol (2020) 21(6):832–42. doi: 10.1016/S1470-2045(20)30110-8
26. Okada M, Kato K, Cho BC, Takahashi M, Lin CY, Chin K, et al. Three-year follow-up and response-survival relationship of nivolumab in previously treated patients with advanced esophageal squamous cell carcinoma (Attraction-3). Clin Cancer Res (2022) 28(15):3277–86. doi: 10.1158/1078-0432.CCR-21-0985
27. Shen L, Kato K, Kim SB, Ajani JA, Zhao K, He Z, et al. Tislelizumab versus chemotherapy as second-line treatment for advanced or metastatic esophageal squamous cell carcinoma (Rationale-302): a randomized phase iii study. J Clin Oncol (2022) 40(26):3065–76. doi: 10.1200/JCO.21.01926
28. Akbay EA, Koyama S, Carretero J, Altabef A, Tchaicha JH, Christensen CL, et al. Activation of the pd-1 pathway contributes to immune escape in egfr-driven lung tumors. Cancer Discovery (2013) 3(12):1355–63. doi: 10.1158/2159-8290.CD-13-0310
29. Fournel L, Wu Z, Stadler N, Damotte D, Lococo F, Boulle G, et al. Cisplatin increases pd-L1 expression and optimizes immune check-point blockade in non-small cell lung cancer. Cancer Lett (2019) 464:5–14. doi: 10.1016/j.canlet.2019.08.005
30. Patel KR, Martinez A, Stahl JM, Logan SJ, Perricone AJ, Ferris MJ, et al. Increase in pd-L1 expression after pre-operative radiotherapy for soft tissue sarcoma. Oncoimmunology (2018) 7(7):e1442168. doi: 10.1080/2162402X.2018.1442168
31. Peng S, Wang R, Zhang X, Ma Y, Zhong L, Li K, et al. Egfr-tki resistance promotes immune escape in lung cancer Via increased pd-L1 expression. Mol Cancer (2019) 18(1):165. doi: 10.1186/s12943-019-1073-4
32. Du SS, Chen GW, Yang P, Chen YX, Hu Y, Zhao QQ, et al. Radiation therapy promotes hepatocellular carcinoma immune cloaking Via pd-L1 upregulation induced by cgas-sting activation. Int J Radiat Oncol Biol Phys (2022) 112(5):1243–55. doi: 10.1016/j.ijrobp.2021.12.162
33. Ben Dori S, Aizic A, Sabo E, Hershkovitz D. Spatial heterogeneity of pd-L1 expression and the risk for misclassification of pd-L1 immunohistochemistry in non-small cell lung cancer. Lung Cancer (2020) 147:91–8. doi: 10.1016/j.lungcan.2020.07.012
34. Yarchoan M, Albacker LA, Hopkins AC, Montesion M, Murugesan K, Vithayathil TT, et al. Pd-L1 expression and tumor mutational burden are independent biomarkers in most cancers. JCI Insight (2019) 4(6). doi: 10.1172/jci.insight.126908
35. Yarchoan M, Johnson BA 3rd, Lutz ER, Laheru DA, Jaffee EM. Targeting neoantigens to augment antitumour immunity. Nat Rev Cancer (2017) 17(4):209–22. doi: 10.1038/nrc.2016.154
36. Marabelle A, Fakih M, Lopez J, Shah M, Shapira-Frommer R, Nakagawa K, et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: prospective biomarker analysis of the multicohort, open-label, phase 2 keynote-158 study. Lancet Oncol (2020) 21(10):1353–65. doi: 10.1016/S1470-2045(20)30445-9
37. Yarchoan M, Hopkins A, Jaffee EM. Tumor mutational burden and response rate to pd-1 inhibition. New Engl J Med (2017) 377(25):2500–1. doi: 10.1056/NEJMc1713444
38. McGrail DJ, Pilie PG, Rashid NU, Voorwerk L, Slagter M, Kok M, et al. High tumor mutation burden fails to predict immune checkpoint blockade response across all cancer types. Ann Oncol (2021) 32(5):661–72. doi: 10.1016/j.annonc.2021.02.006
39. Ellegren H. Microsatellites: simple sequences with complex evolution. Nat Rev Genet (2004) 5(6):435–45. doi: 10.1038/nrg1348
40. Bonneville R, Krook MA, Kautto EA, Miya J, Wing MR, Chen HZ, et al. Landscape of microsatellite instability across 39 cancer types. JCO Precis Oncol (2017) 2017. doi: 10.1200/PO.17.00073
41. Parikh AR, He Y, Hong TS, Corcoran RB, Clark JW, Ryan DP, et al. Analysis of DNA damage response gene alterations and tumor mutational burden across 17,486 tubular gastrointestinal carcinomas: implications for therapy. Oncol (2019) 24(10):1340–7. doi: 10.1634/theoncologist.2019-0034
42. Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, et al. Pd-1 blockade in tumors with mismatch-repair deficiency. New Engl J Med (2015) 372(26):2509–20. doi: 10.1056/NEJMoa1500596
43. Marabelle A, Le DT, Ascierto PA, Di Giacomo AM, De Jesus-Acosta A, Delord JP, et al. Efficacy of pembrolizumab in patients with noncolorectal high microsatellite Instability/Mismatch repair-deficient cancer: results from the phase ii keynote-158 study. J Clin Oncol (2020) 38(1):1–10. doi: 10.1200/JCO.19.02105
44. Argiles JM, Busquets S, Stemmler B, Lopez-Soriano FJ. Cancer cachexia: understanding the molecular basis. Nat Rev Cancer (2014) 14(11):754–62. doi: 10.1038/nrc3829
45. Wiendl H, Hohlfeld R, Kieseier BC. Immunobiology of muscle: advances in understanding an immunological microenvironment. Trends Immunol (2005) 26(7):373–80. doi: 10.1016/j.it.2005.05.003
46. Wiendl H, Hohlfeld R, Kieseier BC. Muscle-derived positive and negative regulators of the immune response. Curr Opin Rheumatol (2005) 17(6):714–9. doi: 10.1097/01.bor.0000184164.69181.ca
47. Figarella-Branger D, Civatte M, Bartoli C, Pellissier JF. Cytokines, chemokines, and cell adhesion molecules in inflammatory myopathies. Muscle Nerve (2003) 28(6):659–82. doi: 10.1002/mus.10462
48. Giudice J, Taylor JM. Muscle as a paracrine and endocrine organ. Curr Opin Pharmacol (2017) 34:49–55. doi: 10.1016/j.coph.2017.05.005
49. Crane JD, MacNeil LG, Lally JS, Ford RJ, Bujak AL, Brar IK, et al. Exercise-stimulated interleukin-15 is controlled by ampk and regulates skin metabolism and aging. Aging Cell (2015) 14(4):625–34. doi: 10.1111/acel.12341
50. Conlon KC, Lugli E, Welles HC, Rosenberg SA, Fojo AT, Morris JC, et al. Redistribution, hyperproliferation, activation of natural killer cells and Cd8 T cells, and cytokine production during first-in-Human clinical trial of recombinant human interleukin-15 in patients with cancer. J Clin Oncol (2015) 33(1):74–82. doi: 10.1200/JCO.2014.57.3329
51. Yoon HG, Oh D, Ahn YC, Noh JM, Pyo H, Cho WK, et al. Prognostic impact of sarcopenia and skeletal muscle loss during neoadjuvant chemoradiotherapy in esophageal cancer. Cancers (2020) 12(4). doi: 10.3390/cancers12040925
52. Kim YY, Lee J, Jeong WK, Kim ST, Kim JH, Hong JY, et al. Prognostic significance of sarcopenia in microsatellite-stable gastric cancer patients treated with programmed death-1 inhibitors. Gastric Cancer (2021) 24(2):457–66. doi: 10.1007/s10120-020-01124-x
53. Roch B, Coffy A, Jean-Baptiste S, Palaysi E, Daures JP, Pujol JL, et al. Cachexia - sarcopenia as a determinant of disease control rate and survival in non-small lung cancer patients receiving immune-checkpoint inhibitors. Lung Cancer (2020) 143:19–26. doi: 10.1016/j.lungcan.2020.03.003
54. Deng HY, Chen ZJ, Qiu XM, Zhu DX, Tang XJ, Zhou Q. Sarcopenia and prognosis of advanced cancer patients receiving immune checkpoint inhibitors: a comprehensive systematic review and meta-analysis. Nutrition (2021) 90:111345. doi: 10.1016/j.nut.2021.111345
55. Wang PY, Xu LD, Chen XK, Xu L, Yu YK, Zhang RX, et al. Sarcopenia and short-term outcomes after esophagectomy: a meta-analysis. Ann Surg Oncol (2020) 27(8):3041–51. doi: 10.1245/s10434-020-08236-9
56. Greten FR, Grivennikov SI. Inflammation and cancer: triggers, mechanisms, and consequences. Immunity (2019) 51(1):27–41. doi: 10.1016/j.immuni.2019.06.025
57. McMillan DC. The systemic inflammation-based Glasgow prognostic score: a decade of experience in patients with cancer. Cancer Treat Rev (2013) 39(5):534–40. doi: 10.1016/j.ctrv.2012.08.003
58. Diakos CI, Charles KA, McMillan DC, Clarke SJ. Cancer-related inflammation and treatment effectiveness. Lancet Oncol (2014) 15(11):e493–503. doi: 10.1016/S1470-2045(14)70263-3
59. Pirozzolo G, Gisbertz SS, Castoro C, van Berge Henegouwen MI, Scarpa M. Neutrophil-to-Lymphocyte ratio as prognostic marker in esophageal cancer: a systematic review and meta-analysis. J Thorac Dis (2019) 11(7):3136–45. doi: 10.21037/jtd.2019.07.30
60. Gou M, Qu T, Wang Z, Yan H, Si Y, Zhang Y, et al. Neutrophil-to-Lymphocyte ratio (Nlr) predicts pd-1 inhibitor survival in patients with metastatic gastric cancer. J Immunol Res (2021) 2021:2549295. doi: 10.1155/2021/2549295
61. Hwang M, Canzoniero JV, Rosner S, Zhang G, White JR, Belcaid Z, et al. Peripheral blood immune cell dynamics reflect antitumor immune responses and predict clinical response to immunotherapy. J Immunother Cancer (2022) 10(6). doi: 10.1136/jitc-2022-004688
62. Cox S, Hurt C, Grenader T, Mukherjee S, Bridgewater J, Crosby T. The prognostic value of derived neutrophil to lymphocyte ratio in oesophageal cancer treated with definitive chemoradiotherapy. Radiother Oncol (2017) 125(1):154–9. doi: 10.1016/j.radonc.2017.08.023
63. Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. New Engl J Med (2009) 361(10):947–57. doi: 10.1056/NEJMoa0810699
Keywords: esophageal cancer, immunotherapy, biomarker, PD-1/PD-L1, cancer immunity
Citation: Wang X, Wang P, Huang X, Han Y and Zhang P (2023) Biomarkers for immunotherapy in esophageal cancer. Front. Immunol. 14:1117523. doi: 10.3389/fimmu.2023.1117523
Received: 06 December 2022; Accepted: 19 April 2023;
Published: 01 May 2023.
Edited by:
Catherine Sautes-Fridman, INSERM U1138 Centre de Recherche des Cordeliers (CRC), FranceReviewed by:
Ashish Manne, The Ohio State University, United StatesJessica Crystal, University of Miami, United States
Copyright © 2023 Wang, Wang, Huang, Han and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yanan Han, aGFueWFuYW4xMTA4QDE2My5jb20=; Pei Zhang, emhhbmdzbW11MjA1QDE2My5jb20=
†These authors have contributed equally to this work
 Xuelian Wang1†
Xuelian Wang1† Xiang Huang
Xiang Huang Pei Zhang
Pei Zhang