
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol. , 16 February 2023
Sec. Cancer Immunity and Immunotherapy
Volume 14 - 2023 | https://doi.org/10.3389/fimmu.2023.1117232
This article is part of the Research Topic The Effects of Inflammaging and Metaflammation on Tumour Development: the Narrow Boundary between Chronic Inflammation and Cancer View all 3 articles
Background: Studies have confirmed the validity of malnutrition/inflammation-based indicators among cancer patients compared to chemotherapy patients. Moreover, it is necessary to identify which indicator is the best prognostic predictor for chemotherapy patients. This study attempted to determine the best nutrition/inflammation-based indicator of overall survival (OS) for chemotherapy patients.
Methods: In this prospective cohort study, we collected 16 nutrition/inflammation-based indicators among 3,833 chemotherapy patients. The maximally selected rank statistics were used to calculate the optimal values of cutoffs for continuous indicators. OS was evaluated using the Kaplan–Meier method. The associations of 16 indicators with survival were evaluated using Cox proportional hazard models. The predictive ability of 16 indicators was assessed via time-dependent receiver operating characteristic curves (time-ROC) and the C-index.
Results: All indicators were significantly associated with worse OS of chemotherapy patients in the multivariate analyses (all P < 0.05). Time-AUC and C-index analyses indicated that the lymphocyte-to-CRP (LCR) ratio (C-index: 0.658) had the best predictive ability for OS in chemotherapy patients. The tumor stage significantly modified the association between inflammatory status and worse survival outcomes (P for interaction < 0.05). Compared to patients with high LCR and I/II tumor stages, patients with low LCR and III/IV tumor stages had a 6-fold higher risk of death.
Conclusions: The LCR has the best predictive value in chemotherapy patients compared with other nutrition/inflammation-based indicators.
Clinical trial registration: http://www.chictr.org.cn, identifier ChiCTR1800020329.
Cancer is the leading cause of mortality globally. An estimated 19.3 million new cancer cases and nearly 10 million cancer-related deaths are expected in 2020. With an estimated 2.3 million cases, female breast cancer has surpassed lung cancer as the most diagnosed cancer, followed by lung cancer (11.4%), colorectal cancer (10.0%), and prostate cancer (10.0%) (1). Cancer mortality, which has been increasing during most of the 20th century, continued to decline from its peak in 1991 to 2018, with a total decline of 31% due to the reduction in smoking and improvements in early detection and treatment in the United States (2). However, a significant change is taking place in China’s cancer profile, with more cancers that were previously more prevalent in the United States now being diagnosed in China. Since 2000, China has seen a gradual increase in cancer cases and deaths, as well as crude incidence and mortality rates (3).
The bidirectional relationship between inflammation and cancer was reported previously. Inflammation is a critical component of tumor initiation and progression. In addition, it is thought that tumor cells use innate immune system signals to invade, migrate, and metastasize, including selectins, chemokines, and their receptors (4). Chemotherapy is one of the most commonly used treatments for the majority of cancers. As chemotherapy evolves, its role will be expanded further and will play an even greater role in improving cancer patient survival and quality of life (5). However, there are concerns about the side effects of chemotherapy, despite its benefits for cancer patients. First, chemotherapy has been found to increase the levels of local inflammation (6). Additionally, inflammation contributes to a poor chemotherapy response and shorter survival rates in cancer patients than in those without inflammation (7). Second, acute and late toxicities of chemotherapy, nausea, vomiting, diarrhea, and mucositis further lead to malnutrition and even cachexia. In short, nutrition/inflammation-related factors can be used as effective prognostic predictors for cancer patients receiving chemotherapy. The prognostic effect of several nutrition/inflammation-related factors on the overall survival of cancer patients has been validated previously, including C-reactive protein (CRP), platelet-to-lymphocyte ratio (PLR), neutrophil-to-lymphocyte ratio (NLR), advanced lung cancer inflammation index (ALI), systemic immune-inflammation index (SII), CRP/albumin ratio (CAR), albumin-to-globulin ratio (AGR), modified Glasgow prognostic score (mGPS), geriatric nutritional risk index (GNRI), nutritional risk index (NRI), prognostic nutritional index (PNI), controlling nutritional status (CONUT) score, glucose-to-lymphocyte ratio (GLR), lymphocyte-to-CRP ratio (LCR), lymphocyte CRP score (LCS), and modified GNRI (mGNRI).
Predicting of prognosis for cancer patients receiving chemotherapy is challenging; hence, validated biomarkers for prediction of patient survival are urgently needed to help identify patients and administer timely and effective treatment. Although studies have confirmed the validity of the aforementioned indicators among cancer patients, whether similar results could be extrapolated to cancer patients receiving chemotherapy is unclear. Moreover, it is necessary to identify which indicators are the best prognostic predictors for chemotherapy patients. In the current study, we evaluated and compared 16 malnutrition/inflammation-based biomarkers for their predictive and prognostic role in OS in chemotherapy patients.
Participants were drawn from the Investigation on Nutrition Status and its Clinical Outcome of Common Cancers (INSCOC) project (http://www.chictr.org.cn, registration number ChiCTR1800020329), which is an ongoing, multicenter, prospective cohort study. The study design, methods, and development were described previously (8, 9). In short, all patients with a pathologically diagnosed malignancy were between 18 and 90 years of age at the time of admission. Patients must have no communication impairment, and be able to complete the study questionnaire. In addition, patients must be willing to participate in the study and be able to provide informed consent. In the current study, we excluded participants without data on 16 malnutrition/inflammation-based biomarkers in the current study. We also excluded participants who did not receive chemotherapy in INSCOC. A total of 3,833 cancer patients with all data for 16 malnutrition/inflammation-based biomarkers and who received chemotherapy were included in the final analysis (Figure 1). Informed consent forms were signed by all patients or their representative relatives within 48 h of hospital admission and before study initiation. According to the Declaration of Helsinki, the study protocol was approved by the institutional review board of Beijing Shijitan Hospital.
Data on socioeconomic factors and lifestyle behaviors of the patients were collected by trained medical staff via a standard questionnaire. In addition, all patients underwent routine assessment by dietitians to acquire nutritional information and data on anthropometric measurements, including height, body weight, handgrip strength, Patient-Generated Subjective Global Assessment (PG-SGA) score, and Karnofsky Performance Score (KPS). Tumor stage was evaluated using the 8th edition of the American Joint Committee on Cancer TNM staging system and was classified as stage I, II, III, and IV.
Within 24 hours of hospitalization, routine blood examinations, including total protein, albumin, total cholesterol, glucose, hemoglobin, globulin, creatinine, triglyceride, CRP, total cholesterol, neutrophil, lymphocyte, red blood cells, and platelet counts, were conducted for all patients upon admission of the first time. In the current study, the nutrition/inflammation-based indicators included CRP, PLR, NLR, ALI, SII, CAR, AGR, mGPS, GNRI, NRI, PNI, CONUT, GLR, LCR, LCS, and mGNRI. The classifications of LCS, CONUT, and mGPS were based on previous studies. Table S1 shows the current study’s detailed calculation methods for all nutrition and inflammation-based indicators.
All statistical computations were performed using SAS software (version 9.4) and R software, version 3.4.3 (https://www.r-project.org). Variables were expressed as the mean ± standard deviation, median (interquartile range), or absolute number with the proportion as appropriate for the continuous or categorical variables. Based on the optimal cutoff points calculated using maximally selected rank statistics, we dichotomized the continuous nutrition/inflammation-based indicators. Kaplan−Meier curves were used to evaluate overall survival (OS), and log-rank tests were performed to analyze the differences. The association between nutrition/inflammation-based indicators and overall survival was evaluated using the Cox proportional hazard model. C-indices and time-dependent receiver operating characteristic curves (time-ROCs) were used to assess the predictive accuracy of each indicator. The three best indicators were selected for further analysis. Net reclassification improvement (NRI) and integrated discrimination improvement (IDI) were used to measure the improvement in the risk prediction model performance for the top three indicators. Moreover, possible modifications of the associations among the three best indicators and outcomes were assessed for variables including sex, median age (<59 vs. >59 years), tumor stage (I/II vs. III vs. IV), smoking status (none vs. past/current), drinking status (no vs. past/current), and BMI (<18.5 vs. 18.5-24 vs. ≥24 kg/m2). All analyses were statistically significant at p-value < 0.05 (two-sided).
The mean age of the patients was 58.22 ± 10.71 years, with 2,202 (57.4%) men and 1631 (42.6%) women. Table 1 shows the baseline clinicopathological characteristics of cancer patients who received chemotherapy in the INSCOC project from 2013 to 2019. Lung cancer (34.7%) was the most common cancer type, followed by colorectal cancer (19.5%), gastric cancer (13.5%), and breast cancer (10.9%). Nearly half of the patients (47.5%) had stage IV tumors. A total of 46.6% and 22.6% of patients consumed tobacco or alcoholic beverages, respectively. A total of 55.7% of patients received surgery for cancer, and 16.9% of 3,833 cancer patients who received chemotherapy received radiotherapy in the current study.
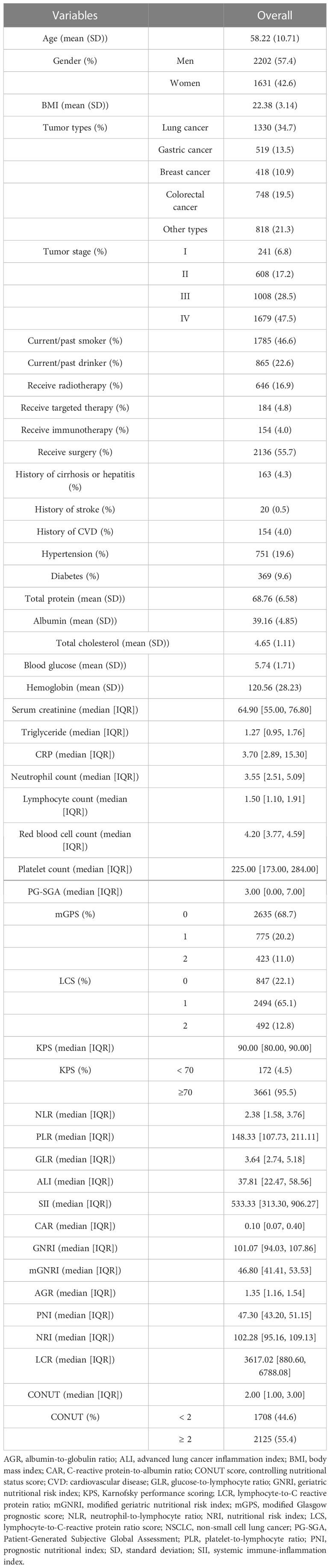
Table 1 Baseline clinicopathological characteristics of cancer patients who received chemotherapy in the INSCOC project from 2013-2019.
During the median (IQR) follow-up of 18.9 (10.5, 33.1) months, 1,573 chemotherapy patients died. Figure S1 shows the crude and adjusted dose-response relationship between 16 inflammation/nutrition-based indicators and OS. Nonlinear and positive relationships were found for all indicators in the crude and adjusted models. The optimal cutoffs of inflammation- and malnutrition-based indicators were 3.29 for NLR, 206.96 for PLR, 5.12 for GLR, 740.70 for SII, 26.42 for ALI, 0.14 for CAR, 93.34 for GNRI, 45.75 for mGNRI, 1.17 for AGR, 46.40 for PNI, 94.41 for NRI, 2812.5 for LCR, and 3.96 for CRP. Table 2 demonstrates the association of the sixteen indicators with overall survival in chemotherapy patients. All inflammation/nutrition-based indicators were independent risk factors for the survival of chemotherapy patients in the adjusted models when the indicators were assessed as categorical variables. The results from the Kaplan–Meier curves showed that chemotherapy patients with malnutrition or an inflammatory status had worse OS than those without malnutrition or inflammation (Figures 2 and S2).
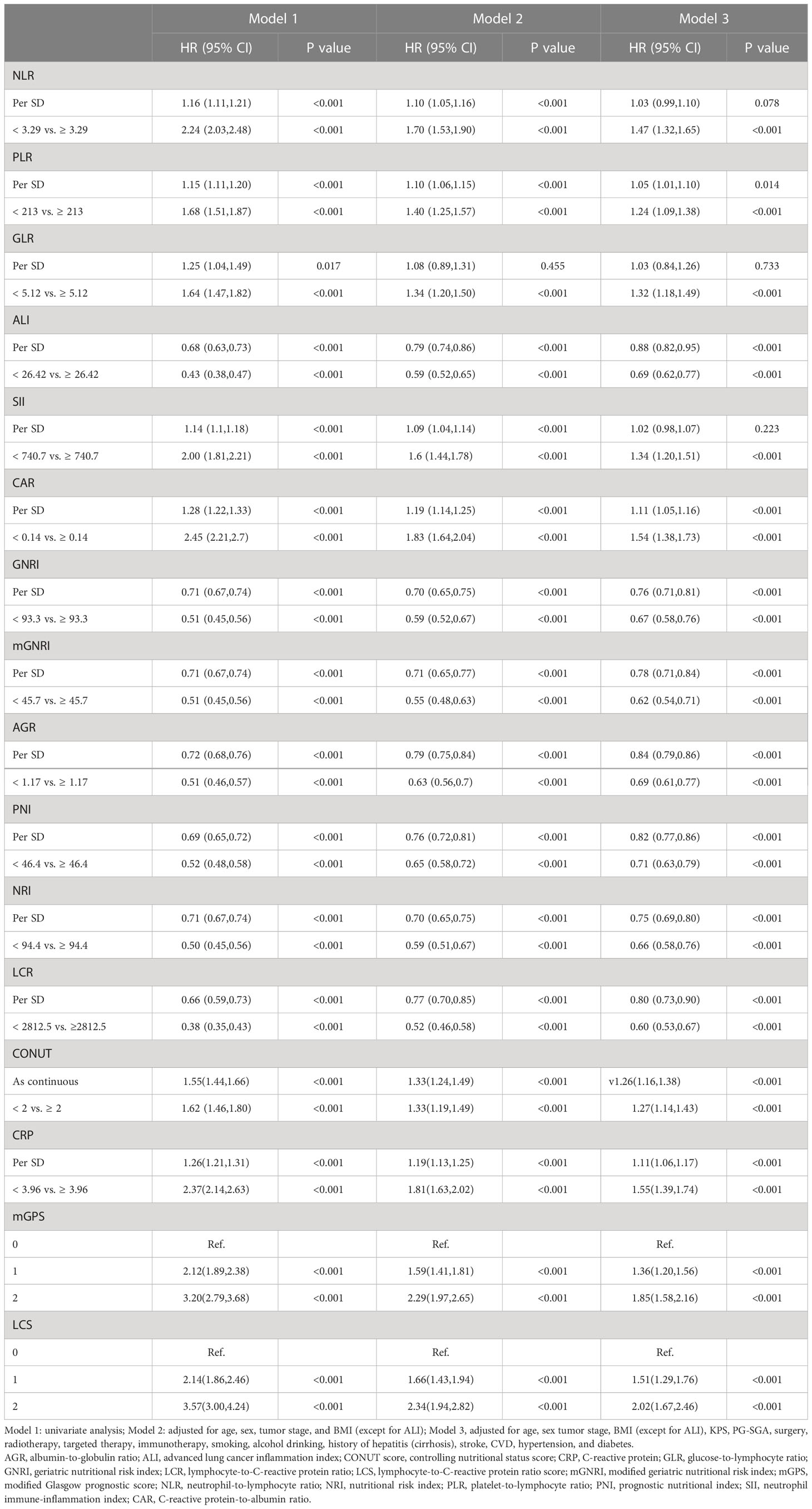
Table 2 The associations of sixteen malnutrition/inflammation-based indicators with overall survival in cancer patients who received chemotherapy in the INSCOC project from 2013-2019.
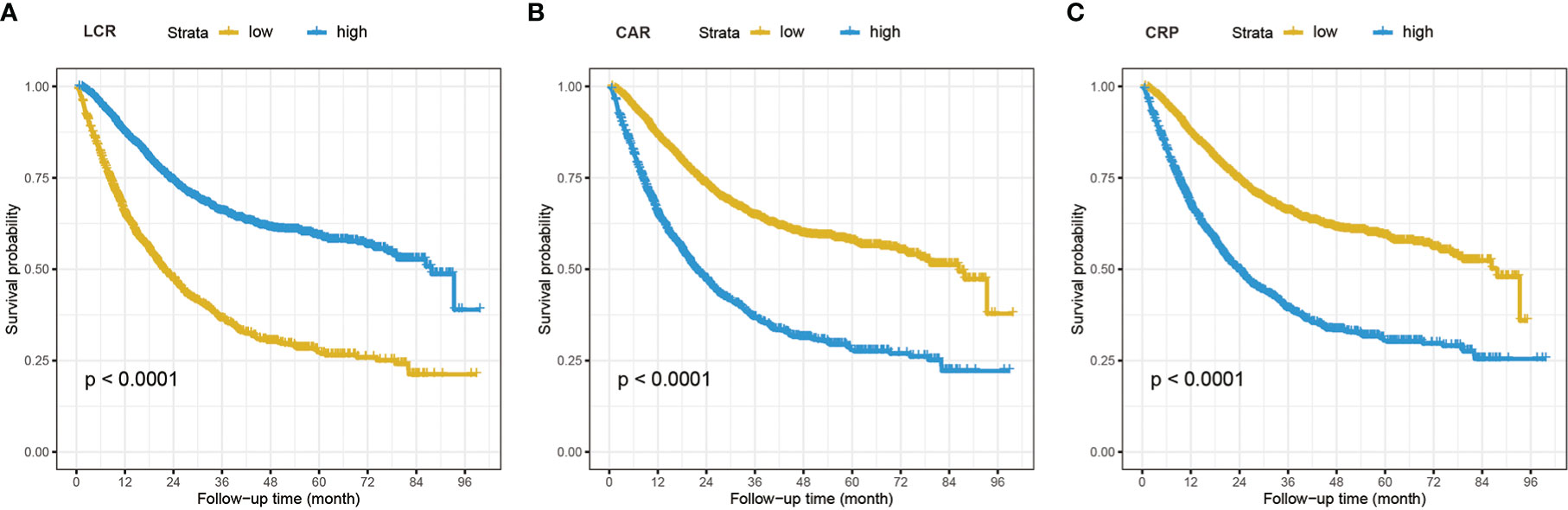
Figure 2 The Kaplan–Meier curves analysis in cancer patients who received chemotherapy of LCR (A), CAR (B), and CRP (C). LCR, lymphocyte-to-CRP ratio; CAR, CRP/albumin ratio; CRP, C-reactive protein.
Comparisons of the prognostic ability of 16 inflammation/nutrition-based indicators for the mortality of chemotherapy patients was performed using the time-ROC and ROC curves (Table 3, Figure 3). Among 16 indicators, the LCR (0.658 [0.644, 0.673]) had the highest C-index for OS of chemotherapy patients, followed by CAR (0.653 [0.639, 0.668]) and CRP (0.647 [0.633, 0.662]). In line with the results of the time-dependent AUC, the LCR had the highest and most stable AUC value, followed by the CAR and CRP during the follow-up period. As expected, the level of inflammation assessed by LCR, CAR, and CRP increased with increasing tumor stage (Figure S3). As the top 3 indicators, LCR, CAR, and CRP are all based on CRP levels. We further explored whether the predictive ability of the top 3 indicators reached statistical significance, as shown in Table S2. Compared with CRP, LCR had a significantly higher predictive performance. However, no difference was found in the predictive performance between CRP and CAR.
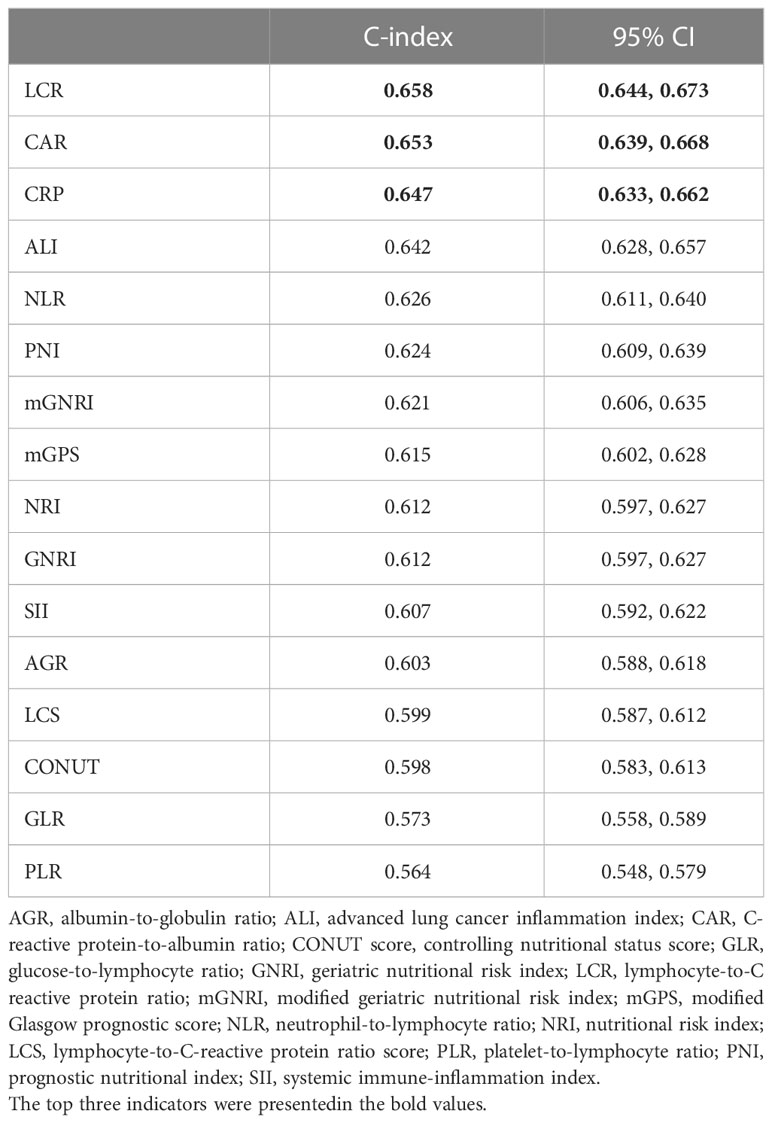
Table 3 The C-index of 16 malnutrition/inflammation-based indicators for OS in cancer patients who received chemoradiotherapy in the INSCOC project from 2013-2019.
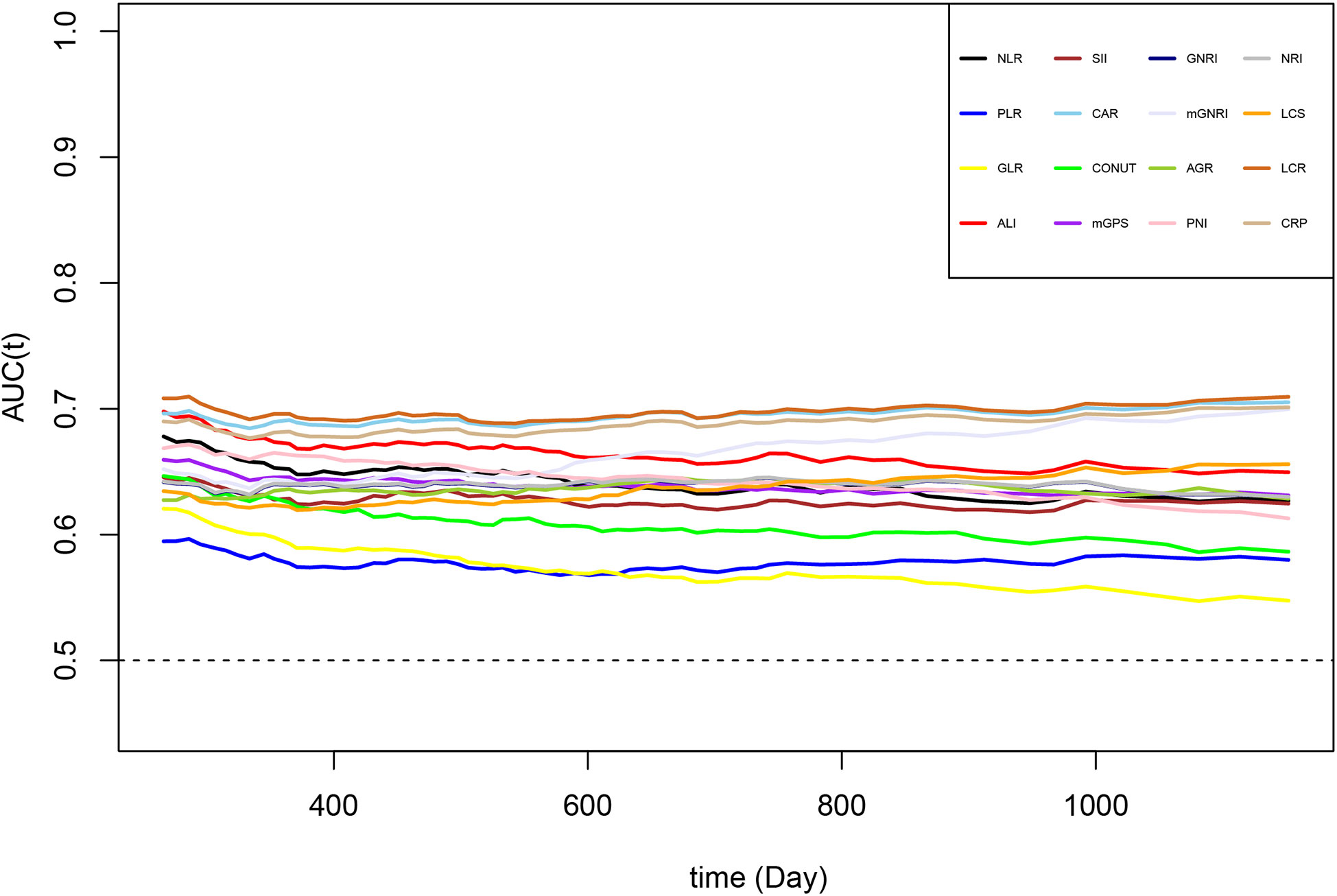
Figure 3 The time-dependent ROC of malnutrition/inflammation-based indicators for diagnosing overall survival in cancer patients who received chemotherapy in the INSCOC project from 2013-2019. AGR, albumin-to-globulin ratio; ALI, advanced lung cancer inflammation index; CAR, neutrophil-to-lymphocyte ratio; CONUT score, controlling nutritional status score; CRP, C-reactive protein; GLR, glucose-to-lymphocyte ratio; GNRI, geriatric nutritional risk index; LCR, lymphocyte-to-C-reactive protein ratio; LCS, lymphocyte-to-C-reactive protein ratio score; mGNRI, modified geriatric nutritional risk index; mGPS, modified Glasgow prognostic score; NLR, neutrophil-to-lymphocyte ratio; NRI, nutritional risk index; PLR, platelet-to-lymphocyte ratio; PNI, prognostic nutritional index; SII, neutrophil immune-inflammation index.
The LCR, CAR, and CRP were selected as the top three indicators for the survival of chemotherapy patients. The subgroup analyses of LCR, CAR, and CRP with the overall survival of chemotherapy patients are illustrated in Figure 4. A significant association between inflammatory status and worse survival outcomes was found in all subgroups when chemotherapy patients were stratified by age, tumor stage, BMI, drinking, and smoking status. Notably, the tumor stage significantly modified the association between inflammatory status and worse survival outcomes (P for interaction < 0.05). We further divided the study population into four groups based on the absence/presence of elevated LCR levels (optimal cutoff) and tumor stages (I/II vs. III/IV). Chemotherapy patients with low LCR and III/IV tumor stages exhibited the worst overall survival compared with patients with high LCR and I/II tumor stages (Figure S4). In addition, patients with low LCR and III/IV tumor stages were associated with a 6-fold increased risk of death (HR=5.92, 95% CI: 4.58, 7.66) compared with patients with high LCR and I/II tumor stages in the multivariate analysis (Table 4).
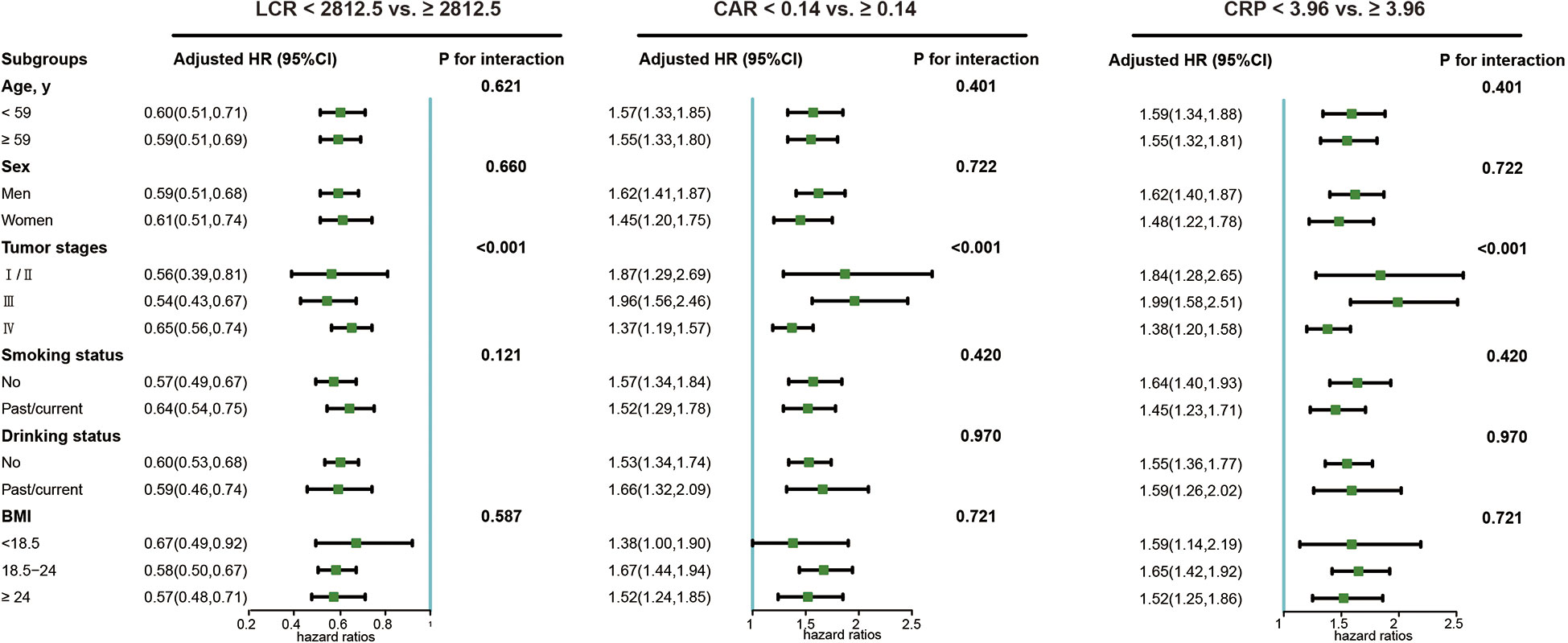
Figure 4 The sub-group analysis of the association of LCR, CRP and CRP with overall survival in cancer patients who received chemotherapy in the INSCOC project from 2013-2019. Models were adjusted for age, sex, tumor stage, BMI (except for ALI), KPS, PG-SGA, surgery, radiotherapy, targeted therapy, immunotherapy, smoking, alcohol drinking, history of hepatitis (cirrhosis), stroke, CVD, hypertension, and diabetes. LCR, lymphocyte-to-CRP ratio; CAR, CRP/albumin ratio; CRP, C-reactive protein.

Table 4 The joint effect of LCR and tumor stage on the survival in cancer patients who received chemotherapy in the INSCOC project from 2013-2019.
Evidence has shown that inflammatory/nutrition-based indicators are reliable predictors of the outcomes for patients with cancer; however, the optimal choice of various indicators is still unclear. In this multicenter, prospective cohort study, we found that LCR, CAR, and CRP were the top three indicators for the prediction of the outcomes of chemotherapy patients. In addition, the LCR had the best prognostic ability among 16 inflammatory/nutrition-based indicators. We also observed a significant interaction between inflammation (assessed by LCR, CAR, and CRP), tumor stage, and overall survival.
The lymphocyte–C-reactive protein ratio (LCR) is the combination of lymphocyte count and CRP, and its prognostic value has been explored in several previous studies. By analyzing 1,303 patients with stage II/III colon cancer who received surgery, Suzuki S et al. investigated 16 inflammation-related markers and found that LCR (≤ 12,980) was most significantly correlated with worse overall survival (OS) and disease-free survival (DFS) (10). By analyzing 477 colorectal cancer patients from the discovery (n = 373) and validation cohorts (n=104), Okugawa Y et al. concluded that preoperative LCR is a useful marker for the perioperative and postoperative management of colorectal cancer patients. They also found that a low LCR was also associated with postoperative infectious complications, indicating the role of the LCR in predicting both short- and long-term postoperative outcomes of CRC patients (11).
CAR is also a sensitive biomarker for cancer-related prognosis. By analyzing 133 patients with stage III colorectal cancer, Matsuoka H et al. found that postoperative CAR (≥ 0.035) is strongly associated with a poor prognosis and was useful for deciding whether adjuvant chemotherapy should be used (12). In a retrospective study, Ide S et al. evaluated the association between CAR and prognosis in 115 rectal cancer patients and found that CAR ≥ 0.049 before neoadjuvant chemoradiotherapy was an independent prognostic factor for OS and DFS (13). Dolan RD et al. reported that CAR> 0.22 was an independent predictor for the OS of CRC patients (14).
During the preoperative or postoperative period, CRP is one of the most useful parameters for evaluating the degree of inflammation in cancer patients. By drawing data from 300 CRC patients, Koike Y et al. reported that preoperative CRP was a prognostic variable in patients with stage I/II CRC. Another study including 74 lymph node-positive upper tract urothelial carcinoma patients found that preoperative CRP levels were significantly associated with poor survival in the multivariate analysis. Notably, most previous studies adopted CRP-related markers that were collected before the operation. Additionally, in the general clinical setting, CRP is not a routine test, which limits the extrapolation of those markers. Another study conducted in Japan found that postoperative (but not preoperative) indicators, including CAR, LCR, and NLR, were significantly associated with worse overall survival (15).
CRP-related markers, including LCR, CAR, and CRP, have the best predictive performance in chemotherapy patients. We also explored the effect of other indicators on overall survival. The positive associations between other inflammatory/nutrition-based indicators and OS were in line with findings from previous studies. Based on previous studies (16–18), NLR is a powerful biomarker that predicts prognosis in various types of cancer with cutoff values ranging from 2 to 5, depending on the study population and the method of definition of the cutoff value. GPS is also useful for the prediction of prognosis. Notably, modified GPS (mGPS) has proven useful in predicting the postoperative and preoperative prognosis of CRC patients undergoing curative surgery (19, 20). Similarly, several previous studies also explored the usefulness of other albumin-related prognostic markers, including PNI, ALI, and AGR, for the prognosis of cancer patients (21–23). Platelet-related markers, including PLR and SII, were also evaluated as independent predictors for poor OS in cancer patients (24, 25).
To implement preventive analytic strategies, it is crucial to develop prognostic models that estimate risk as accurately as possible. In the current study, the top 3 prognostic indicators were all CRP-related indicators with minor differences in the value of the C-index. CRP is a more convenient and cost-effective indicator than LCR and CAR, and it is important to know whether the difference in the predictive value is statistically significant. The results from both NRI and IDI showed that the predictive ability of LCR significantly outperformed that of CRP, demonstrating the importance of LCR as the best option to predict the prognosis of chemotherapy patients (Table S2).
The tumor stage significantly modified the association between CRP-related indicators and the prognosis of chemotherapy patients. We found that chemotherapy patients with advanced tumor stages (III/IV) and a high inflammatory burden exhibited a 6-fold higher risk of death compared with patients with early tumor stages and low levels of inflammation. As a recognized hallmark of cancer, inflammation plays a substantial role in the development and progression of cancer (26). On the other hand, solid cancers can induce an inflammatory microenvironment. In short, special attention should be given to patients with advanced tumor stages and a high inflammatory burden.
The main strength of the current study was that it first found that the LCR was the best predictor of prognosis for chemotherapy patients among the 16 metrics presented in a large, population-based, multicenter cohort study. In addition, this study fully considered the effects of potential confounding factors, such as lifestyle habits and laboratory measurements. Finally, the strengths of this study also include its prospective study design, large sample size, long-term follow-up, and subgroup analysis.
Limitations should also be noted in the current study. First, due to the limited data on targeted therapy, immunotherapy and the use of anti-inflammatory drugs, including aspirin and statins, the effect of these confounders could not be elucidated. Second, data on the 16 inflammatory/nutrition-based indicators were collected only once, and further studies should be conducted to better explore the impact of long-term patterns of inflammatory/nutrition-based indicators on the survival of chemotherapy patients. Third, we used only preoperative data, not postoperative data, and whether the predictive value changes during the operation needs to be explored in future studies. Fourth, due to the limited data, the prognostic values of other inflammation-based indicator can’t be explored in the current study. For example, the ratio of fibrinogen to pre-albumin (FPR) as well as its combination with SII were found to be superior to other inflammatory biomarkers for predicting the overall survival among patients with advanced NSCLC (27) or early recurrence among stage II-III colorectal cancer patients after curable resection (28). However, the INSCOC project has no data on the levels of fibrinogen, and whether FPR outperformed in predicting survival in chemotherapy patients with cancer should be better elucidated in future studies.
The LCR, CAR, and CRP were the top three predictors for the prognosis of chemotherapy patients in clinical practice, and the predictive ability of the LCR significantly outperformed that of the CRP. In addition, chemotherapy patients with advanced tumor stage and high inflammatory burden had a significantly higher risk of death than patients with early tumor stage and low levels of inflammation.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
This study followed the Helsinki Declaration. All participants signed informed consent forms, and this study was approved by the Institutional Review Board of each hospital (Registration number: ChiCTR1800020329). The patients/participants provided their written informed consent to participate in this study.
TL: methodology, software, writing-original draft preparation; CL: visualization; LD: methodology, software; MS: resources; SL: supervision, validation; HS: conceptualization, supervision, validation, resources. All authors contributed to the article and approved the submitted version.
This work was financially supported by the National Key Research and Development Program (2022YFC2009600), the Beijing Municipal Science and Technology Commission (SCW2018-06) to HS, and the Jiangxi Province Science Foundation for Youths (20161BAB215187) to LD.
The authors express their sincere thanks to the INSCOC project members for substantial work on data collection and patient follow-up.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer H-QY declared a shared parent affiliation with the author LD to the handling editor at the time of review.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1117232/full#supplementary-material
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin (2021) 71(1):7–33. doi: 10.3322/caac.21654
3. Xia C, Dong X, Li H, Cao M, Sun D, He S, et al. Cancer statistics in China and united states, 2022: profiles, trends, and determinants. Chin Med J (Engl) (2022) 135(5):584–90. doi: 10.1097/CM9.0000000000002108
4. Coussens LM, Werb Z. Inflammation and cancer. Nature (2002) 420(6917):860–7. doi: 10.1038/nature01322
5. Furue H. [Chemotherapy cancer treatment during the past sixty years]. Gan To Kagaku Ryoho (2003) 30(10):1404–11.
6. Li Y, Wang J, Ma X, Tan L, Yan Y, Xue C, et al. A review of neoadjuvant chemoradiotherapy for locally advanced rectal cancer. Int J Biol Sci (2016) 12(8):1022–31. doi: 10.7150/ijbs.15438
7. Shinko D, Diakos CI, Clarke SJ, Charles KA. Cancer-related systemic inflammation: The challenges and therapeutic opportunities for personalized medicine. Clin Pharmacol Ther (2017) 102(4):599–610. doi: 10.1002/cpt.789
8. Liu CA, Zhang Q, Ruan GT, Shen LY, Xie HL, Liu T, et al. Novel Diagnostic and Prognostic Tools for Lung Cancer Cachexia: Based on Nutritional and Inflammatory Status. Front Oncol (2022) 12: 890745. doi: 10.3389/fonc.2022.890745.
9. Xu H, Song C., Yin L., Wang C., Fu Z., Guo Z., et al. Extension protocol for the investigation on nutrition status and clinical outcome of patients with common cancers in China (INSCOC) study: 2021 update. Precis Nutr (2022) 1(2):e00014. doi: 10.1097/PN9.0000000000000014
10. Suzuki S, Akiyoshi T, Oba K, Otsuka F, Tominaga T, Nagasaki T, et al. Comprehensive comparative analysis of prognostic value of systemic inflammatory biomarkers for patients with stage II/III colon cancer. Ann Surg Oncol (2020) 27(3):844–52. doi: 10.1245/s10434-019-07904-9
11. Okugawa Y, Toiyama Y, Yamamoto A, Shigemori T, Ide S, Kitajima T, et al. Lymphocyte-c-reactive protein ratio as promising new marker for predicting surgical and oncological outcomes in colorectal cancer. Ann Surg (2020) 272(2):342–51. doi: 10.1097/SLA.0000000000003239
12. Matsuoka H, Ando K, Hu Q, Zaitsu Y, Tsuda Y, Hisamatsu Y, et al. Postoperative c-reactive protein/albumin ratio is a biomarker of risk of recurrence and need for adjuvant chemotherapy for stage III colorectal cancer. Int J Clin Oncol (2020) 25(7):1318–26. doi: 10.1007/s10147-020-01672-3
13. Ide S, Toiyama Y, Okugawa Y, Oki S, Yasuda H, Fujikawa H, et al. Clinical significance of c-reactive protein-to-Albumin ratio with rectal cancer patient undergoing chemoradiotherapy followed by surgery. Anticancer Res (2017) 37(10):5797–804. doi: 10.21873/anticanres.12022
14. Dolan RD, McSorley ST, Park JH, Watt DG, Roxburgh CS, Horgan PG, et al. The prognostic value of systemic inflammation in patients undergoing surgery for colon cancer: comparison of composite ratios and cumulative scores. Br J Cancer (2018) 119(1):40–51. doi: 10.1038/s41416-018-0095-9
15. Yasui K, Shida D, Nakamura Y, Ahiko Y, Tsukamoto S, Kanemitsu Y. Postoperative, but not preoperative, inflammation-based prognostic markers are prognostic factors in stage III colorectal cancer patients. Br J Cancer (2021) 124(5):933–41. doi: 10.1038/s41416-020-01189-6
16. Diem S, Schmid S, Krapf M, Flatz L, Born D, Jochum W, et al. Neutrophil-to-Lymphocyte ratio (NLR) and platelet-to-Lymphocyte ratio (PLR) as prognostic markers in patients with non-small cell lung cancer (NSCLC) treated with nivolumab. Lung Cancer (2017) 111:176–81. doi: 10.1016/j.lungcan.2017.07.024
17. Zhang Q, Song MM, Zhang X, Ding JS, Ruan GT, Zhang XW, et al. Association of systemic inflammation with survival in patients with cancer cachexia: results from a multicentre cohort study. J Cachexia Sarcopenia Muscle (2021) 12(6):1466–76. doi: 10.1002/jcsm.12761
18. Ruan GT, Yang M, Zhang XW, Song MM, Hu CL, Ge YZ, et al. Association of systemic inflammation and overall survival in elderly patients with cancer cachexia - results from a multicenter study. J Inflamm Res (2021) 14:5527–40. doi: 10.2147/JIR.S332408
19. Park JH, Watt DG, Roxburgh CS, Horgan PG, McMillan DC. Colorectal cancer, systemic inflammation, and outcome: Staging the tumor and staging the host. Ann Surg (2016) 263(2):326–36. doi: 10.1097/SLA.0000000000001122
20. Guthrie GJ, Roxburgh CS, Farhan-Alanie OM, Horgan PG, McMillan DC, et al. Comparison of the prognostic value of longitudinal measurements of systemic inflammation in patients undergoing curative resection of colorectal cancer. Br J Cancer (2013) 109(1):24–8. doi: 10.1038/bjc.2013.330
21. Tominaga T, Nonaka T, Hisanaga M, Fukuda A, Tanoue Y, Yoshimoto T, et al. Prognostic value of the preoperative prognostic nutritional index in oldest-old patients with colorectal cancer. Surg Today (2020) 50(5):449–59. doi: 10.1007/s00595-019-01910-w
22. Shibutani M, Maeda K, Nagahara H, Fukuoka T, Matsutani S, Kimura K, et al. The prognostic significance of the advanced lung cancer inflammation index in patients with unresectable metastatic colorectal cancer: a retrospective study. BMC Cancer (2019) 19(1):241. doi: 10.1186/s12885-019-5468-9
23. Fujikawa H, Toiyama Y, Inoue Y, Imaoka H, Shimura T, Okigami M, et al. Prognostic impact of preoperative albumin-to-Globulin ratio in patients with colon cancer undergoing surgery with curative intent. Anticancer Res (2017) 37(3):1335–42. doi: 10.21873/anticanres.11452
24. Kim JH, Lee JY, Kim HK, Lee JW, Jung SG, Jung K, et al. Prognostic significance of the neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in patients with stage III and IV colorectal cancer. World J Gastroenterol (2017) 23(3):505–15. doi: 10.3748/wjg.v23.i3.505
25. Chen JH, Zhai ET, Yuan YJ, Wu KM, Xu JB, Peng JJ, et al. Systemic immune-inflammation index for predicting prognosis of colorectal cancer. World J Gastroenterol (2017) 23(34):6261–72. doi: 10.3748/wjg.v23.i34.6261
26. Diakos CI, Charles KA, McMillan DC, Clarke SJ. Cancer-related inflammation and treatment effectiveness. Lancet Oncol (2014) 15(11):e493–503. doi: 10.1016/S1470-2045(14)70263-3
27. Ying HQ, Liao YC, Luo YR, Xiong G, Huang Y, Nie RW, et al. Cancer-elicited inflammation attenuates response and outcome in tyrosine kinase inhibitor naive patients with advanced NSCLC. Pharmacol Res (2021) 170:105734. doi: 10.1016/j.phrs.2021.105734
Keywords: cancer, chemotherapy, indicators, inflammation, lymphocyte-to-CRP ratio
Citation: Liu T, Liu C, Deng L, Song M, Lin S and Shi H (2023) The prognostic effect of sixteen malnutrition/inflammation-based indicators on the overall survival of chemotherapy patients. Front. Immunol. 14:1117232. doi: 10.3389/fimmu.2023.1117232
Received: 06 December 2022; Accepted: 08 February 2023;
Published: 16 February 2023.
Edited by:
Alina Kurylowicz, Mossakowski Medical Research Institute (PAN), PolandReviewed by:
Hou-Qun Ying, Second Affiliated Hospital of Nanchang University, ChinaCopyright © 2023 Liu, Liu, Deng, Song, Lin and Shi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hanping Shi, c2hpaHBAY2NtdS5lZHUuY24=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.