- 1Hepatic Surgery Center, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, China
- 2Department of Surgery, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, China
Background: Immunotherapy plays an increasingly critical role in the systemic treatment of HCC. This current study aimed to establish a novel prognostic predictor of Programmed death 1 (PD-1) inhibitor therapy in hepatocellular carcinoma (HCC) independent of Child-Pugh grade.
Methods: Our study screened patients with HCC who received PD-1 inhibitors at Tongji Hospital Affiliated to Tongji Medical College of Huazhong University of Science and Technology from January 2018 to December 2020. ALG grade was determined by the patient’s serum ALP and GGT levels before the initiation of PD-1 inhibitors. The endpoints of our study were overall survival (OS) and progression free survival (PFS). Follow-up ended at May 31, 2022.
Results: Eighty- five patients (77 with Child−Pugh grade A, 8 with Child−Pugh grade B at baseline) were enrolled according to the inclusion criteria. Patients with Child−Pugh grade A achieved longer PFS and OS than those with Child−Pugh grade B. Patients with ALG grade 3 at baseline showed worse tumor response and poorer survival, and ALG grade could stratify patients with Child−Pugh grade A into subgroups with significantly different prognosis.
Conclusions: ALG grade, combining ALP and GGT, is a novel and readily available prognostic marker and the predictive effect of ALG grade on patient prognosis is independent of Child−Pugh grade.
Introduction
Hepatocellular carcinoma (HCC) accounts for approximately 90% of primary liver cancers, and it is one of the leading causes of cancer-related death worldwide (1). Immunotherapy plays an increasingly critical role in the systemic treatment of HCC (2, 3). Previous clinical trials have confirmed the definite antitumor efficacy of programmed death-1(PD-1) inhibitors on HCC (4, 5). The newly released results of RATIONALE-301 (Tislelizumab versus Sorafenib) and LEAP-002 (Lenvatinib plus pembrolizumab versus Lenvatinib) on the European Society for Medical Oncology in 2022 highlighted the key role of PD-1 inhibitors monotherapy in the treatment of HCC (NCT03412773, NCT03713593). PD-1 inhibitors are emerging as a critical part of guideline-recommended first-line therapy for advanced hepatocellular carcinoma (NCT03298451) (6).
Preserved liver function is the premise of various treatments for HCC, and it is an important predictor of superior prognosis (7). Previous research confirmed that better liver function was positively associated with the prognosis of patients after hepatectomy (8), local therapy (9, 10) and systemic therapy (11, 12). Child−Pugh grade is extensively used to evaluate patient liver function when systemic therapy is recommended, and most patients are in Child−Pugh grade A, so it is necessary to develop other liver function evaluation systems to further stratify them into different groups according to their prognosis.
Alkaline phosphatase (ALP) and γ-glutamyl transferase (GGT) are readily available as part of serum liver function tests, and they are elevated in the presence of cholestasis or liver parenchymal damage. Previous studies suggested that elevated serum ALP and GGT were independent risk factors for the prognosis of HCC patients (13, 14). Other scholars have found that ALP and GGT have the potential to be used as biomarkers to predict the cancer risk and mortality of all comers (15, 16). Few studies have explored the role of ALP and GGT in the prognosis of HCC patients receiving programmed death 1(PD-1) inhibitors.
In this retrospective study, we analyzed the data of patients with HCC who received PD-1 inhibitors to explore the prognostic effect of ALP and GGT in HCC.
Methods
Patients
Our study screened patients with HCC who received PD-1 inhibitors at Tongji Hospital Affiliated to Tongji Medical College of Huazhong University of Science and Technology from January 2018 to December 2020. To further explore the risk factors associated with PD-1 inhibitor therapy for hepatocellular carcinoma, we enrolled patients receiving PD-1 inhibitors monotherapy without targeted drug therapy. The inclusion criteria were as follows: 1) confirmed HCC diagnosed pathologically or clinically; 2) regular treatment with PD-1 inhibitors no less than 2 cycles; 3) no history of molecularly targeted drug therapy; 4) case data sufficient for efficacy evaluation; 5) at least 18 months of follow-up since the start of the PD-1 inhibitors. We collected the patients’ basic information, treatment history, and relevant fluid examination and imaging results. The current study was approved by the Medical Ethics Committee of Tongji Hospital Affiliated to Tongji Medical College of Huazhong University of Science and Technology (TJ-IRB20220936).
Treatment evaluation
Follow-up ended at May 31, 2022. The endpoints of our study were overall survival (OS) and progression free survival (PFS). Overall survival was defined as the interval between the initiation of PD-1 inhibitor treatment and death or the last follow-up, and PFS was defined as the interval from the initiation of PD-1 inhibitor therapy to confirmed tumor progression or the last follow-up. Tumor response was evaluated according to the immune Response Evaluation Criteria in Solid Tumors (iRECIST) (17). Tumor response was divided into four grades: immune complete response (iCR), immune partial response (iPR), immune unconfirmed progressive disease (iUPD), immune confirmed progressive disease (iCPD) and immune stable disease (iSD). Tumor assessment was performed every 6-12 weeks. Liver cirrhosis was confirmed according to the diagnostic criteria issued by the Chinese Medical Association (18).
Alkaline phosphatase & gamma-glutamyl transferase (ALG) grade
ALG grade was determined by the patient’s serum ALP and GGT levels before the initiation of PD-1 inhibitors. The normal ranges of serum ALP and GGT are 40-130 U/L and 10-71 U/L, respectively, in Tongji Hospital. Both serum ALP and GGT higher than normal values were recorded as ALG grade 3, serum ALP or GGT higher than normal was defined as ALG grade 2, serum ALP and GGT within normal ranges were recorded as ALG grade 1.
Statistics
Data analysis was performed by SPSS 26.0 and GraphPad Prism 8 software. Continuous variables were compared by independent samples t-test or Mann−Whitney U test, while categorical variables were compared by chi-square test and Fisher’s exact probability test. Spearman rank correlation analysis was used to test the correlation between rank variables. Kaplan−Meier survival analysis and log-rank test were used to calculate survival curves and compare differences. Univariate and multivariate cox regression models were performed to explore the risk factors of overall survival and progression free survival. P < 0.05 was considered statistically significant.
Results
Patient characteristics
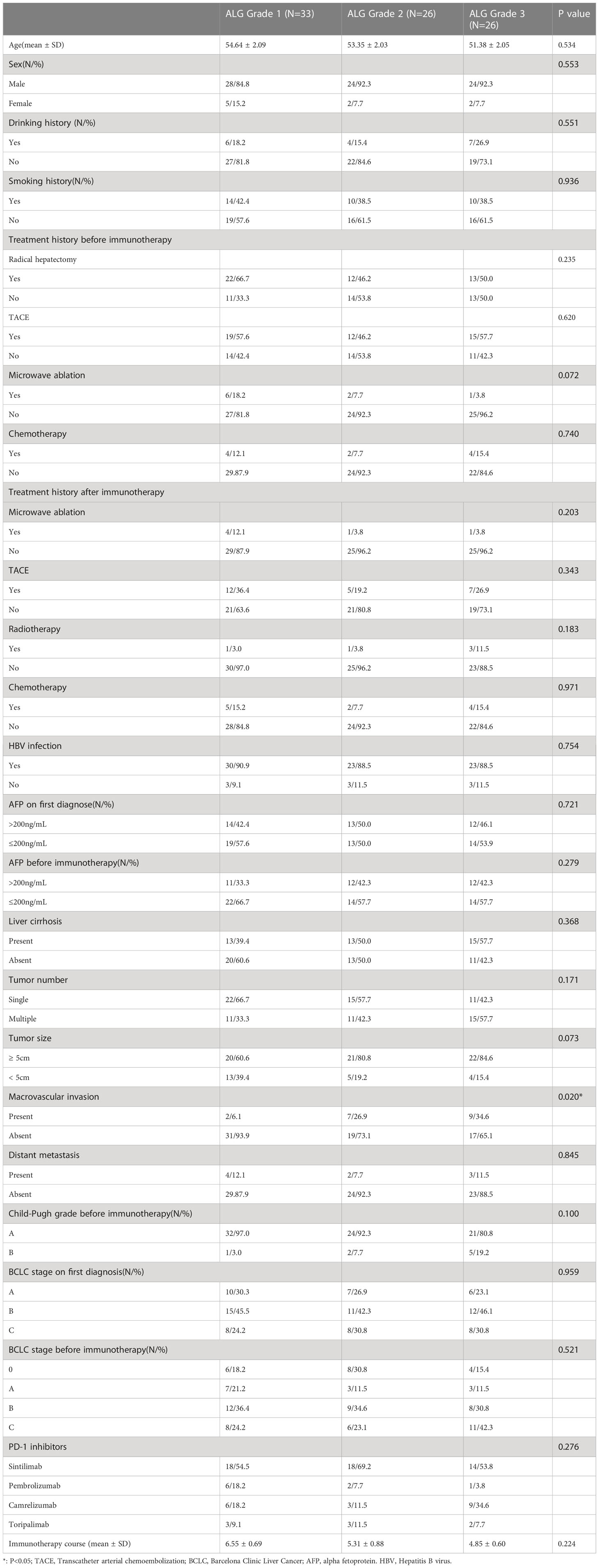
A total of 395 HCC patients received PD-1 inhibitors at Tongji Hospital during the study period, and 85 patients (76 males and 9 females) were enrolled according to the inclusion criteria. Among the enrolled patients, forty-four received radical hepatectomy before immunotherapy. Different brands of PD-1 inhibitors were used in enrolled patients (sintilimab: n=50, pembrolizumab: n=9, camrelizumab: n=18, toripalimab: n=8), and none of them received molecularly targeted drugs. Seventy−seven of the enrolled patients (77/85, 90.6%) lived with Child−Pugh grade A at baseline, and the other 8 patients (8/85, 9.4%) lived with grade B. Patients were classified as ALG grade 1 (n=33), ALG grade 2 (n=26), and ALG grade 3 (n=26) according to serum ALP and GGT levels before the first PD-1 inhibitor treatment. The patients’ baseline characteristics were summarized in Table 1. It is worth noting that there was significant difference in the proportion of macrovascular invasion among patients with different ALG grades. Spearman rank correlation analysis showed that ALG grade was positively correlated with tumor macrovascular invasion (r=0.297, P=0.006).
At the end of follow-up, 36 patients suffered tumor progression, 23 patients died during follow-up, and none of them died from complications related to immunotherapy.
Tumor response
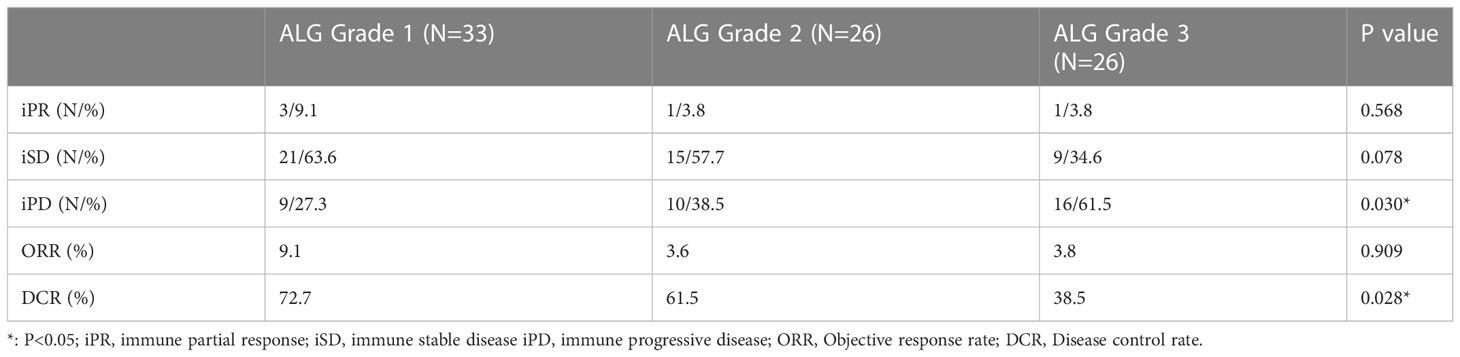
Among total 85 patients, five (5/85, 5.9%) achieved iPR, forty-five (45/85, 52.9%) patients achieved stable disease, and others (35/85, 41.2%) suffered tumor progression. The objective response rate (ORR) and disease control rate (DCR) were 5.9% (5/85) and 58.8% (50/85) in our cohort. Subgroup analysis showed that the DCR in patients with ALG grade 3 was significantly lower than that in patients with ALG grades 1, but there was no significant difference in DCR between patients with ALG grades 1 and 2. Patients with different ALG grades achieved similar ORRs. Table 2 summarized the tumor response of patients with different ALG grades.
Survival analysis
The mean PFS and OS of patients in the cohort were 12.986 (95% CI: 11.574-14.398) months and 15.326 (95% CI: 14.231-16.421) months, respectively. Child−Pugh grade and Albumin-Bilirubin (ALBI) grade are the most commonly used grading system for liver function evaluation. Both PFS and OS of patients with Child−Pugh grade A (PFS: 13.528 months, 95% CI: 12.087-14.968; OS: 15.930 months, 95% CI: 14.909-16.950) were significantly superior than those of patients with Child−Pugh grade B in our study (PFS: 9.513 months, 95% CI: 4.927-14.908; OS: 12.481 months, 95% CI: 9.952-15.009) (PFS: P=0.018, OS: P=0.002) (Figures 1A, B). PFS and OS were similar in patients with different ALBI grades in our study (Figures 1C, D).
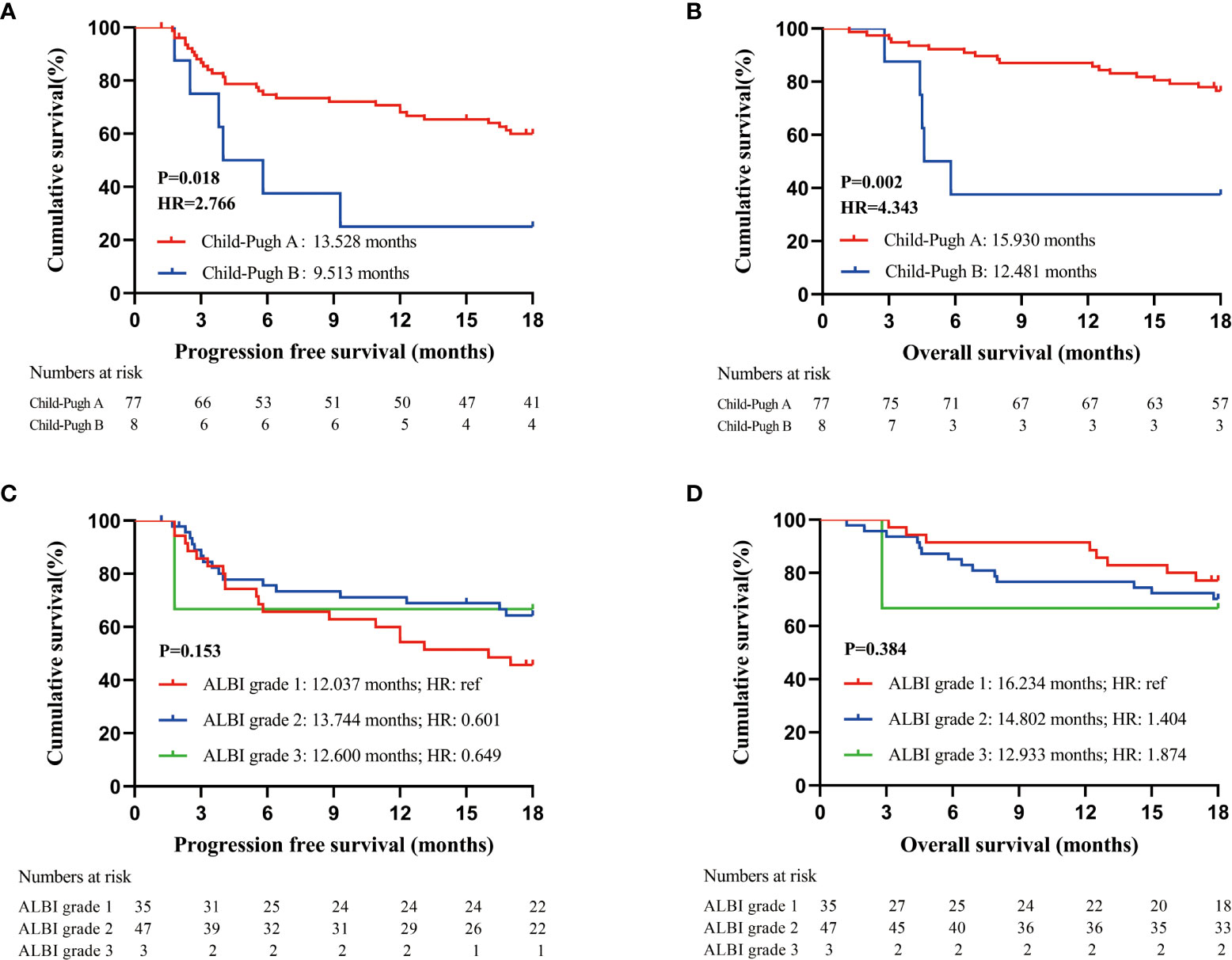
Figure 1 Prognostic analysis of patients receiving PD-1 inhibitors; (A). PFS of all enrolled patients according to Child-Pugh grade at baseline; (B). OS of all enrolled patients according to Child-Pugh grade at baseline; (C). PFS of all enrolled patients according to ALBI grade at baseline; (D). OS of all enrolled patients according to ALBI grade at baseline.
To explore more effective predictors, we conducted analysis according to the serum ALP and GGT values before the first dose of PD-1 inhibitors. Firstly, in the whole cohort, patients with normal serum ALP and GGT before anti-PD-1 treatment achieved longer PFS and OS than those with elevated serum ALP and GGT (Figures 2A, B). Subsequently, we found that ALP and GGT could further stratify the prognosis of patients with the same Child−Pugh grade. Elevated serum ALP and GGT predicted poor prognosis in patients with Child−Pugh grade A (Figures 2C, D). We further combined ALP and GGT as a new indicator to enhance prediction accuracy and ease of use. Both the PFS and OS of patients with ALG grade 1 (PFS: 15.316 months, 95CI: 13.640-17.082; OS: 17.127 months, 95CI: 16.240-18.015) were significantly longer than those of patients with ALG grade 3 (PFS: 9.984 months, 95CI: 7.203-12.765; OS: 12.481 months, 95CI: 9.952-15.009) (PFS: P=0.001, OS: P=0.010). Patients with ALG grade 2 (PFS: 12.832 months, 95CI: 10.263-15.401; OS: 15.885 months, 95CI: 14.066-17.703) also had longer PFS and OS than those with ALG grade 3, but the difference was not statistically significant (PFS: P=0.124, OS: P=0.103) (Figures 3A, B). The ALG grade could also stratify patients with Child−Pugh grade A into subgroups with significantly different prognosis (Figures 3C, D).
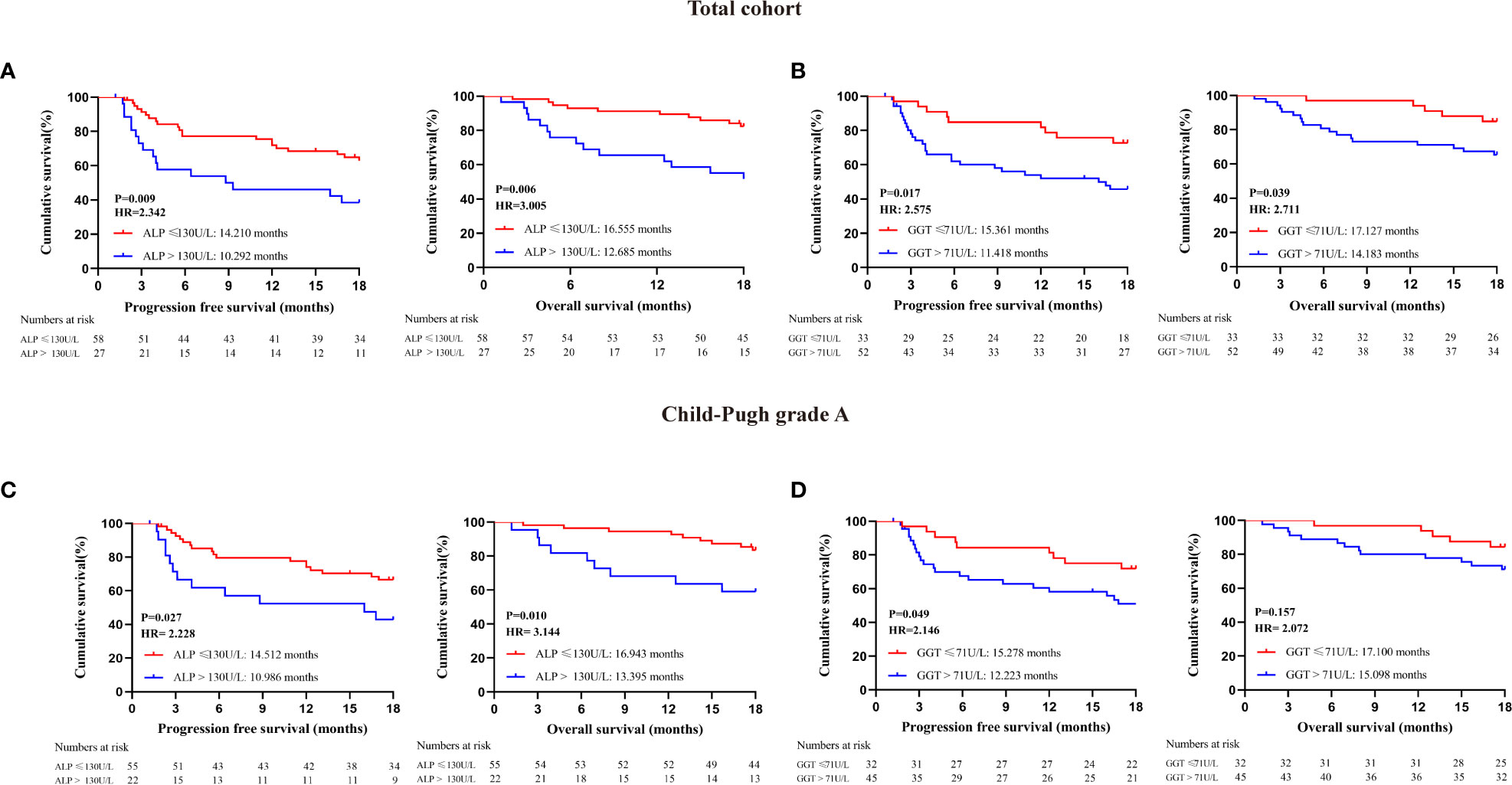
Figure 2 Prognostic analysis of patients receiving PD-1 inhibitors; (A) Survival analysis of all enrolled patients with different serum ALP levels at baseline: (B) Survival analysis of all enrolled patients with different serum GGT levels at baseline; (C) Survival analysis of patients with Child-Pugh grade A according to serum ALP levels at baseline; (D) Survival analysis of patients with Child-Pugh grade A according to serum GGT levels at baseline.
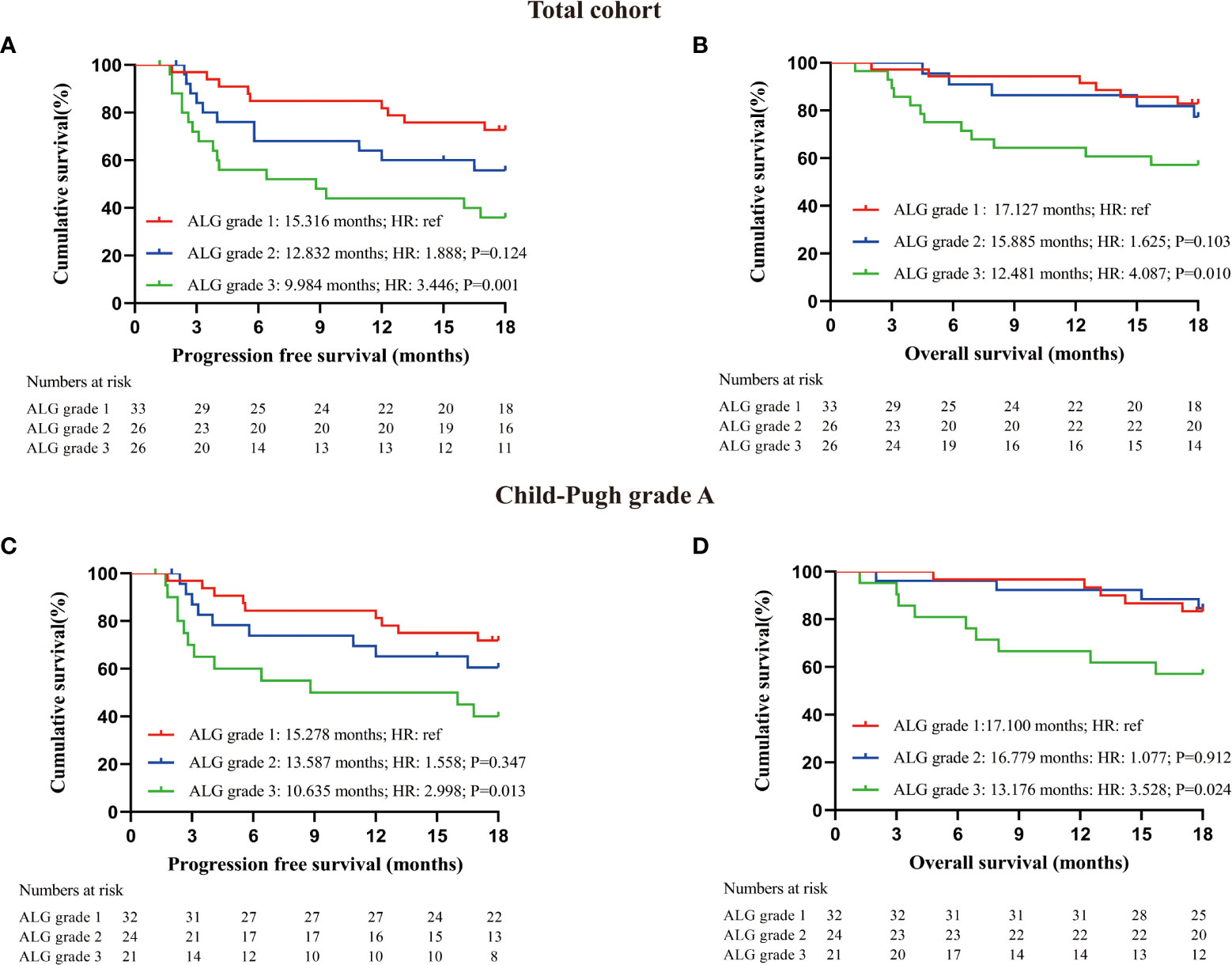
Figure 3 Prognostic analysis of patients receiving PD-1 inhibitors; (A) PFS of all enrolled patients with different ALG grade at baseline; (B) OS of all enrolled patients with different ALG grade at baseline; (C) PFS of patients with Child-Pugh grade A according to serum ALG grade at baseline; (D) OS of patients with Child-Pugh grade A according to serum ALG grade at baseline. Log-rank test was used to compare the prognostic differences between groups.
Moreover, there was no correlation between ALG grade and Child−Pugh grade in our study (P=0.100), indicating that the prognostic predictive effect of ALG grade is independent of Child−Pugh grade. Receiver operating characteristic (ROC) curves were generated to compare the predictive effects of indicators. The area under ROC curve of ALG grade, Child−Pugh grade, serum ALP and GGT levels at baseline for PFS was 0.657, 0.563, 0.620, and 0.610, respectively. The area under ROC curve of ALG grade, Child-Pugh grade, serum ALP and GGT levels at baseline for OS was 0.672, 0.585, 0.640, and 0.617, respectively. ALG grade is better than Child−Pugh grade in predicting the prognosis of patients, and has no correlation with Child−Pugh grade.
Prognostic factors
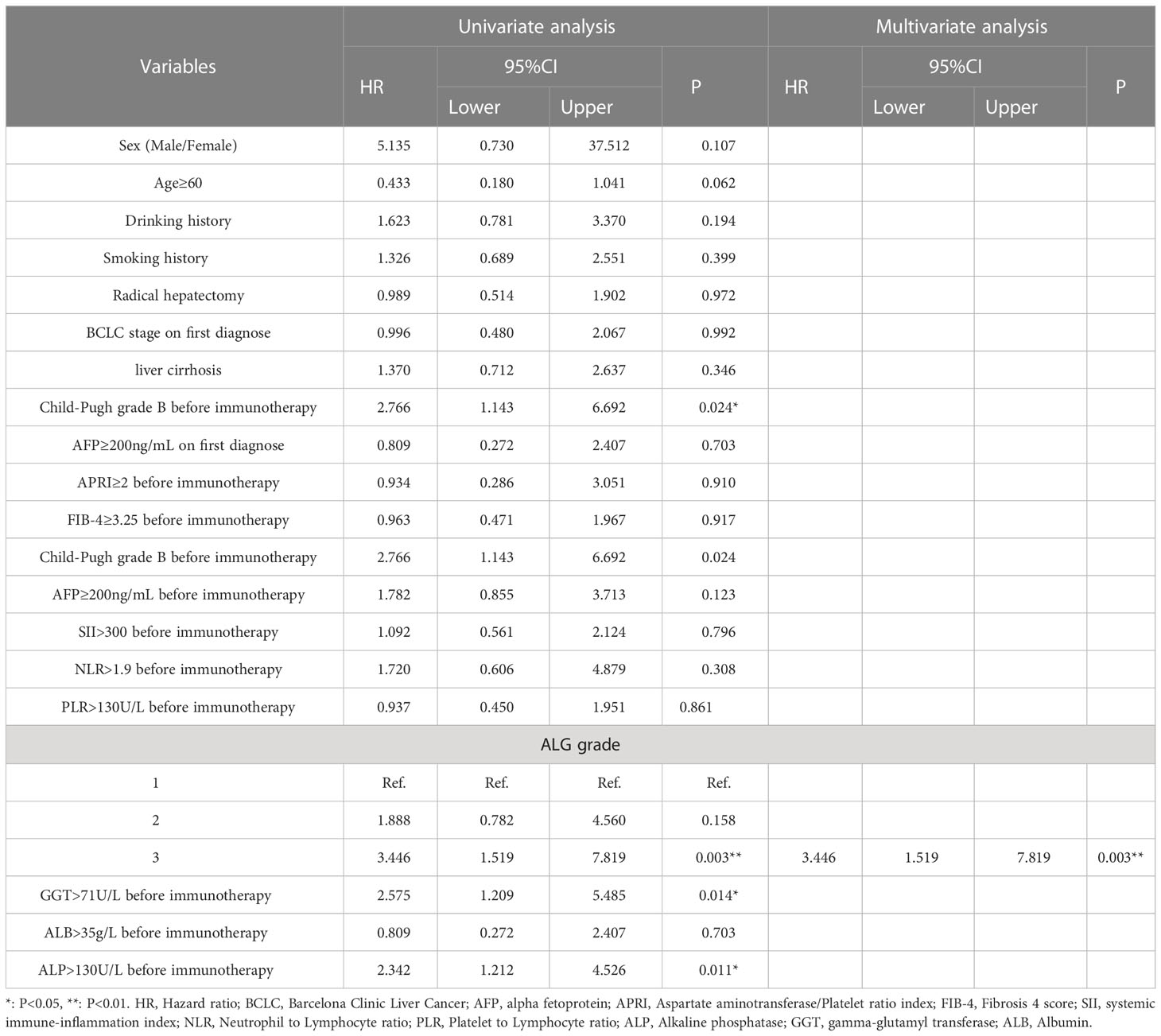
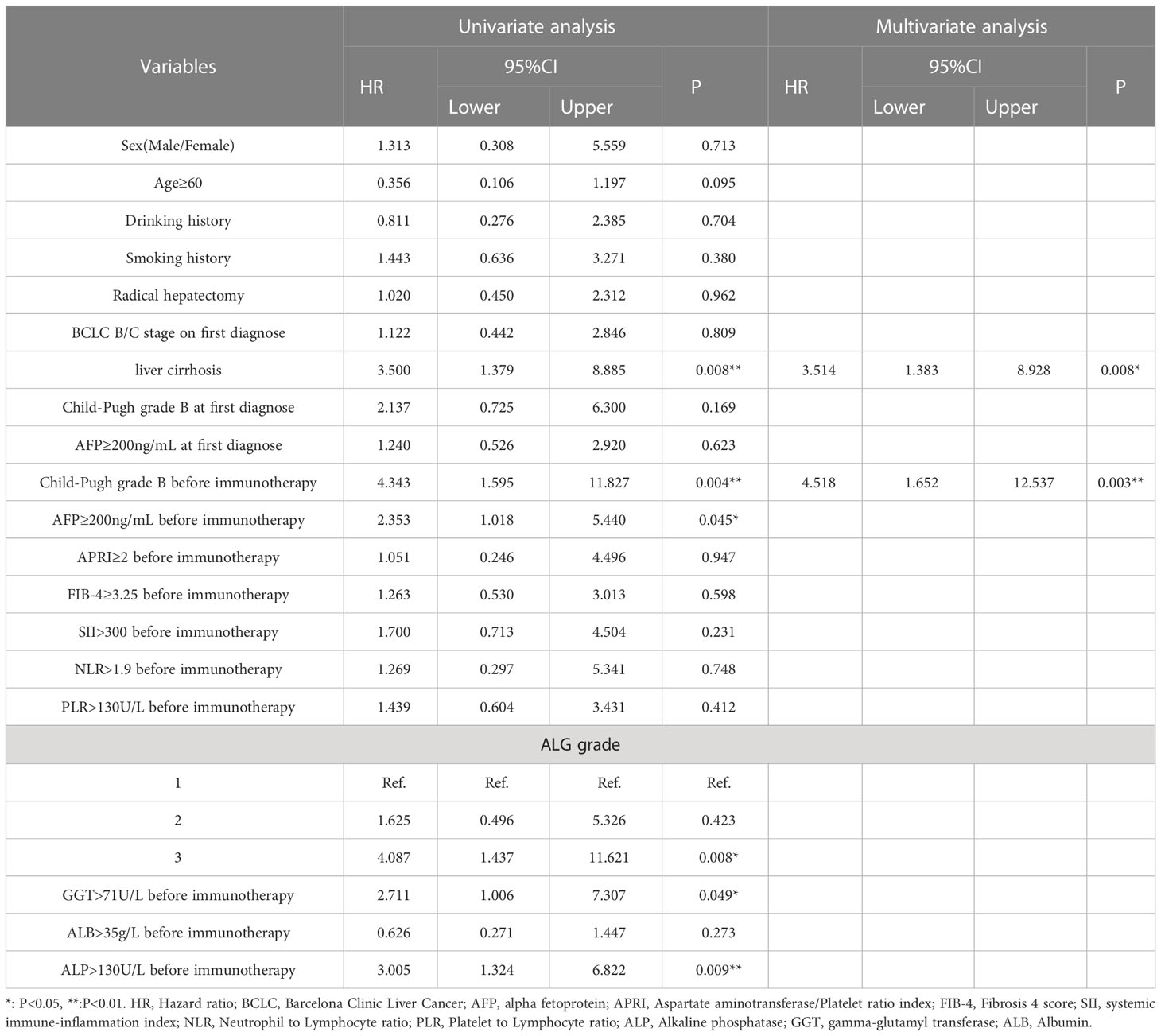
We established cox regression models to explore risk factors of PFS and OS (Tables 3, 4). Univariate analysis showed that ALG grade 3 (HR: 3.446, 95% CI: 1.519-7.819), ALP>130 U/L (HR: 2.342, 95% CI: 1.212-4.526), GGT>71 U/L (HR: 2.575, 95% CI: 1.209-5.485) and Child−Pugh grade B before immunotherapy (HR: 2.766, 95% CI: 1.143-6.692) were associated with PFS of patients receiving PD-1 inhibitors. Meanwhile, liver cirrhosis (HR: 3.500, 95% CI: 1.379-8.885), ALG grade 3 (HR: 4.087, 95% CI: 1.437-11.621), ALP>130 U/L (HR: 3.005, 95% CI: 1.324-6.822), GGT>71U/L (HR: 2.711, 95% CI: 1.006-7.307), AFP≥200 ng/mL (HR: 2.353, 95% CI: 1.018-5.440) and Child−Pugh grade B before immunotherapy (HR: 4.343, 95% CI: 1.595-11.827) were associated with the OS of patients. Multivariate Cox regression proved that ALG grade 3 (HR: 3.446, 95% CI: 1.519-7.819) was an independent risk factor of PFS, liver cirrhosis (HR: 3.514, 95% CI: 1.383-8.928) and Child−Pugh grade B before immunotherapy (HR: 4.518, 95% CI: 1.652-12.537) were independent risk factors of OS.
Discussion
Our study confirmed that elevated baseline serum ALP and GGT levels, as indicators of liver parenchymal damage, were prognostic risk factors of HCC patients receiving PD-1 inhibitors, and the interference of molecularly targeted drugs was excluded according to the inclusion criteria. We also reported that ALG grade, a new combination of ALP and GGT, was a superior predictor of prognosis for the first time, and ALG grade can further stratify patients with Child−Pugh grade A into subgroups with different prognoses.
PD-1 inhibitor therapy for HCC is an emerging systemic therapy, and liver function at baseline is a confirmed prognostic factor. Child−Pugh grade is one of the most common clinical indicators for evaluating liver reserve capacity, and studies have confirmed that the prognosis of patients with Child−Pugh Grade B were worse than that of patients with Child−Pugh Grade A (19), and Albumin-Bilirubin (ALBI) grade is another common liver function scoring method that can replace Child−Pugh score (20). Our study found that Child-Pugh grade, rather than ALBI grade was associated with patient prognosis. Since most patients receiving systemic therapy have good liver function reserve in the early stage, Child−Pugh grade and ALBI grade cannot accurately stratify the prognosis of such patients, we explored other indicators in serum liver function tests. In our study, both PFS and OS of patients with normal ALP and GGT levels at baseline were superior than those of patients with elevated ALP and GGT receiving PD-1 inhibitors. Previous studies supported that elevated ALP and GGT were associated with poor survival of patients receiving liver resection (21, 22), liver transplantation (23, 24), local therapy (25, 26) and molecular targeted therapy (27, 28). This was consistent with our findings in anti-PD-1 therapy, and it is noteworthy that serum ALP and GGT levels could stratify patients with Child−Pugh grade A, enhancing the accuracy of prediction in the current study. Cox regression further confirmed the results of survival analysis. Liver function is also a critical factor in the long-term survival of patients, and this is similar to our findings. We further found that ALG grade 3 was also a prognostic risk factor for patients with Child−Pugh grade A, and there was no significant correlation between Child−Pugh grade and ALG grade. ALG grade is a reliable prognostic factor independent of Child−Pugh grade, but ALG grade 3 was an independent risk factor for PFS but not for OS in our study. The reason of this result may be: 1. most of the patients enrolled have not yet reached the clinical endpoint, only 23 patients died during follow-up; 2. the follow-up time (18 months) was relatively short; 3. Statistical differences should not be the only factor in the validity of prognostic indicators, ALG grade 3 was risk factor for PFS which has confirmed the prognostic effect of ALG grade to a certain extent.
Serum ALP and GGT are common diagnostic indicators for liver diseases. As early as 1985, elevated GGT was systematically reported to be associated with the occurrence of liver cancer (29). Drug-induced liver injury is common in patients with advanced HCC, elevated serum ALP and GGT levels hinder recovery from drug-induced liver injury (30, 31), and the concentrations of ALP and GGT in the bile duct can be used as indicators to quantify bile duct injury (32). GGT increases the level of intracellular glutathione, which can promote tumor cells to maintain balance in reactive oxygen species and avoid cell death through redox pathways during pro-oxidant therapy (33), GGT-positive tumors possess stronger proliferative ability and drug resistance (34, 35). Researchers have demonstrated that granulocyte colony-stimulating factor (G-CSF) upregulates GGT1 and enhances the immunosuppressive function of myeloid-derived suppressor cells, and GGT inhibitors can alleviate tumor immunosuppression and the tumor-promoting effect of G-CSF (36). Alkaline phosphatase is ubiquitous expressed in human body, which has four types: intestinal ALP, placental ALP, germ cell ALP and single tissue non-specific ALP (37). Previous studies have found that placental ALP is highly expressed in HCC cell lines, and this type of ALP can transform cells with specific survival advantages to malignant transformation (38). Tumor cells with ALP overexpression are more likely to form immunogenic cold tumors, and targeted killing of ALP-overexpressing tumor cells can convert immunogenic cold tumors to hot (39). This further confirms the plausibility of our finding that GGT and ALP are adverse long-term prognostic factors for HCC patients receiving PD-1 inhibitors.
There are still some limitations in the current study: First, this is a single-center, retrospective clinical study, and the level of evidence for the findings is not strong enough; Second, the number of participants in the study was relatively small, sufficient sample size and follow-up time can more realistically analyze the prognosis of patients; Third, systemic treatments are diverse for HCC patients, and our study only excluded the interference of molecular targeted therapy on risk factor analysis. Mutation and amplification of oncogenes and tumor suppressor genes also affect the long-term prognosis of patients with HCC (40). Multicenter, prospective clinical trials are still needed to further explore the role of ALG grade in the prognosis of patients with HCC receiving PD-1 inhibitors.
Our study indicated that ALG grade 3 at baseline predicted poor prognosis of patients with HCC receiving PD-1 inhibitors. ALG grade, combining ALP and GGT, is a novel and readily available prognostic marker and the predictive effect of ALG grade on patient prognosis is independent of Child−Pugh grade.
Data availability statement
The original contributions presented in the study are included in the article/supplementary materials. Further inquiries can be directed to the corresponding authors.
Ethics statement
Our study was approved by the Medical Ethics Committee of Tongji Hospital Affiliated to Tongji Medical College of Huazhong University of Science and Technology (TJ-IRB20220936). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements. Written informed consent was not obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
LX and CL wrote the paper; LX, BinZ, and ZL collected and analyzed the clinical data;QL and HL added part of clinical information; XC revised the manuscript; CL and BixZ designed this subject. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by National Natural Science Foundation of China (serial number: 81802931; 81400653).
Acknowledgments
We sincerely express gratitude for all our colleagues at Hepatic Surgery Center of Tongji Hospital Affiliated to Tongji Medical College of Huazhong University of Science and Technology.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. European Association for the study of the liver. EASL clinical practice guidelines: Management of hepatocellular carcinoma. J Hepatol (2018) 69:182–236. doi: 10.1016/j.jhep.2018.03.019
2. Prieto J, Melero I, Sangro B. Immunological landscape and immunotherapy of hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol (2015) 12:681–700. doi: 10.1038/nrgastro.2015.173
3. Pinato DJ, Fessas P, Sapisochin G, Marron TU. Perspectives on the neoadjuvant use of immunotherapy in hepatocellular carcinoma. Hepatology. (2021) 74:483–90. doi: 10.1002/hep.31697
4. Qin S, Ren Z, Meng Z, Chen Z, Chai X, Xiong J, et al. Camrelizumab in patients with previously treated advanced hepatocellular carcinoma: A multicentre, open-label, parallel-group, randomised, phase 2 trial. Lancet Oncol (2020) 21:571–80. doi: 10.1016/S1470-2045(20)30011-5
5. Ren Z, Xu J, Bai Y, Xu A, Cang S, Du C, et al. Sintilimab plus a bevacizumab biosimilar (IBI305) versus sorafenib in unresectable hepatocellular carcinoma (ORIENT-32): a randomised, open-label, phase 2-3 study. Lancet Oncol (2021) 22:977–90. doi: 10.1016/S1470-2045(21)00252-7
6. National Health Commission of the People’s Republic of China. guidelines for diagnosis and treatment of primary liver cancer (Edition 2022). Chin J Surgery (2022) 60:273–309. doi: 10.3760/cma.j.cn112139-20220217-00068
7. Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. (2018) 391:1301–14. doi: 10.1016/S0140-6736(18)30010-2
8. Bluthner E, Bednarsch J, Malinowski M, Binder P, Pratschke J, Stockmann M, et al. Dynamic liver function is an independent predictor of recurrence-free survival after curative liver resection for HCC - a retrospective cohort study. Int J Surg (2019) 71:56–65. doi: 10.1016/j.ijsu.2019.08.033
9. Koike Y, Yoshida H, Shiina S, Teratani T, Obi S, Sato S, et al. Changes in hepatic functional reserve after percutaneous tumor ablation for hepatocellular carcinoma: Long-term follow up for 227 consecutive patients with a single lesion. Hepatol Int (2007) 1:295–301. doi: 10.1007/s12072-007-9001-x
10. Waked I, Berhane S, Toyoda H, Chan SL, Stern N, Palmer D, et al. Transarterial chemo-embolisation of hepatocellular carcinoma: impact of liver function and vascular invasion. Br J Cancer (2017) 116:448–54. doi: 10.1038/bjc.2016.423
11. Pressiani T, Boni C, Rimassa L, Labianca R, Fagiuoli S, Salvagni S, et al. Sorafenib in patients with child-pugh class a and b advanced hepatocellular carcinoma: a prospective feasibility analysis. Ann Oncol (2013) 24:406–11. doi: 10.1093/annonc/mds343
12. Xu L, Leng C, Chen L, Dong H, Chen Y, Chen X. Hypothyroidism is a predictive factor of superior antitumour efficacy of programmed death 1 inhibitors in hepatocellular carcinoma. Int J Cancer (2022) 150:472–81. doi: 10.1002/ijc.33813
13. Wu SJ, Lin YX, Ye H, Xiong XZ, Li FY, Cheng NS. Prognostic value of alkaline phosphatase, gamma-glutamyl transpeptidase and lactate dehydrogenase in hepatocellular carcinoma patients treated with liver resection. Int J Surg (2016) 36:143–51. doi: 10.1016/j.ijsu.2016.10.033
14. Wang X, Mao M, He Z, Zhang L, Li H, Lin J, et al. Development and validation of a prognostic nomogram in AFP-negative hepatocellular carcinoma. Int J Biol Sci (2019) 15:221–8. doi: 10.7150/ijbs.28720
15. Van Hemelrijck M, Jassem W, Walldius G, Fentiman IS, Hammar N, Lambe M, et al. Gamma-glutamyltransferase and risk of cancer in a cohort of 545,460 persons - the Swedish AMORIS study. Eur J Cancer (2011) 47:2033–41. doi: 10.1016/j.ejca.2011.03.010
16. Kunutsor SK, Apekey TA, Seddoh D, Walley J. Liver enzymes and risk of all-cause mortality in general populations: a systematic review and meta-analysis. Int J Epidemiol (2014) 43:187–201. doi: 10.1093/ije/dyt192
17. Seymour L, Bogaerts J, Perrone A, Ford R, Schwartz LH, Mandrekar S, et al. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol (2017) 18:e143–e52. doi: 10.1016/S1470-2045(17)30074-8
18. Xu XY, Ding HG, Li WG, Xu JH, Han Y, Jia JD, et al. Chinese Guidelines on the management of liver cirrhosis (abbreviated version). World J Gastroenterol (2020) 26:7088–103. doi: 10.3748/wjg.v26.i45.7088
19. Shao YY, Wu CH, Lu LC, Chan SY, Ma YY, Yen FC, et al. Prognosis of patients with advanced hepatocellular carcinoma who failed first-line systemic therapy. J Hepatol (2014) 60:313–8. doi: 10.1016/j.jhep.2013.08.027
20. Johnson PJ, Berhane S, Kagebayashi C, Satomura S, Teng M, Reeves HL, et al. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J Clin Oncol (2015) 33:550–8. doi: 10.1200/JCO.2014.57.9151
21. Ju MJ, Qiu SJ, Fan J, Zhou J, Gao Q, Cai MY, et al. Preoperative serum gamma-glutamyl transferase to alanine aminotransferase ratio is a convenient prognostic marker for child-pugh a hepatocellular carcinoma after operation. J Gastroenterol (2009) 44:635–42. doi: 10.1007/s00535-009-0050-x
22. Sun HC, Xie L, Yang XR, Li W, Yu J, Zhu XD, et al. Shanghai score: A prognostic and adjuvant treatment-evaluating system constructed for Chinese patients with hepatocellular carcinoma after curative resection. Chin Med J (Engl) (2017) 130:2650–60. doi: 10.4103/0366-6999.218019
23. Fu SJ, Zhao Q, Ji F, Chen MG, Wu LW, Ren QQ, et al. Elevated preoperative serum gamma-glutamyltranspeptidase predicts poor prognosis for hepatocellular carcinoma after liver transplantation. Sci Rep (2016) 6:28835. doi: 10.1038/srep28835
24. Zhang M, Li B, Yan LN, Yin F, Wen TF, Zeng Y, et al. Development of a survival evaluation model for liver transplant recipients with hepatocellular carcinoma secondary to hepatitis b. World J Gastroenterol (2008) 14:1280–5. doi: 10.3748/wjg.14.1280
25. Wang T, Lu XJ, Chi JC, Ding M, Zhang Y, Tang XY, et al. Microwave ablation of hepatocellular carcinoma as first-line treatment: long term outcomes and prognostic factors in 221 patients. Sci Rep (2016) 6:32728. doi: 10.1038/srep32728
26. Tan Y, Wang X, Ma K, Zhang L, Li J, Chen P, et al. Risk factors for the recurrence of early hepatocellular carcinoma treated by percutaneous radiofrequency ablation with a multiple-electrode switching system: a multicenter prospective study. Int J Hyperthermia (2022) 39:190–9. doi: 10.1080/02656736.2021.2024279
27. Inghilesi AL, Gallori D, Antonuzzo L, Forte P, Tomcikova D, Arena U, et al. Predictors of survival in patients with established cirrhosis and hepatocellular carcinoma treated with sorafenib. World J Gastroenterol (2014) 20:786–94. doi: 10.3748/wjg.v20.i3.786
28. Jia Y, Xing Y, Yang M. Efficacy of sorafenib combined with interventional therapy on primary liver cancer patients and its effect on serum AFP, VEGF, and GGT. J Oncol (2021) 2021:9120265. doi: 10.1155/2021/9120265
29. Hanigan MH, Pitot HC. Gamma-glutamyl transpeptidase–its role in hepatocarcinogenesis. Carcinogenesis. (1985) 6:165–72. doi: 10.1093/carcin/6.2.165
30. Ashby K, Zhuang W, Gonzalez-Jimenez A, Alvarez-Alvarez I, Lucena MI, Andrade RJ, et al. Elevated bilirubin, alkaline phosphatase at onset, and drug metabolism are associated with prolonged recovery from DILI. J Hepatol (2021) 75:333–41. doi: 10.1016/j.jhep.2021.03.021
31. Everhart JE, Wright EC. Association of gamma-glutamyl transferase (GGT) activity with treatment and clinical outcomes in chronic hepatitis c (HCV). Hepatology. (2013) 57:1725–33. doi: 10.1002/hep.26203
32. National Toxicology Program. Toxicology and NTP carcinogenesis studies of a polybrominated biphenyl mixture (Firemaster FF-1) in F344/N rats and B6C3F1 mice (Gavage studies). Natl Toxicol Program Tech Rep Ser (1983) 244:1–106.
33. Zhang H, Forman HJ. Redox regulation of gamma-glutamyl transpeptidase. Am J Respir Cell Mol Biol (2009) 41:509–15. doi: 10.1165/rcmb.2009-0169TR
34. Hanigan MH, Gallagher BC, Townsend DM, Gabarra V. Gamma-glutamyl transpeptidase accelerates tumor growth and increases the resistance of tumors to cisplatin in vivo. Carcinogenesis. (1999) 20:553–9. doi: 10.1093/carcin/20.4.553
35. Hanigan MH. Gamma-glutamyl transpeptidase: redox regulation and drug resistance. Adv Cancer Res (2014) 122:103–41. doi: 10.1016/B978-0-12-420117-0.00003-7
36. Xie Z, Kawasaki T, Zhou H, Okuzaki D, Okada N, Tachibana M. Targeting GGT1 eliminates the tumor-promoting effect and enhanced immunosuppressive function of myeloid-derived suppressor cells caused by G-CSF. Front Pharmacol (2022) 13:873792. doi: 10.3389/fphar.2022.873792
37. Millan JL. Alkaline phosphatase as a reporter of cancerous transformation. Clin Chim Acta (1992) 209:123–9. doi: 10.1016/0009-8981(92)90343-o
38. Benham FJ, Fogh J, Harris H. Alkaline phosphatase expression in human cell lines derived from various malignancies. Int J Cancer (1981) 27:637–44. doi: 10.1002/ijc.2910270510
39. Ji S, Li J, Duan X, Zhang J, Zhang Y, Song M, et al. Targeted enrichment of enzyme-instructed assemblies in cancer cell lysosomes turns immunologically cold tumors hot. Angew Chem Int Ed Engl (2021) 60:26994–7004. doi: 10.1002/anie.202110512
Keywords: hepatocellular carcinoma, Child-Pugh grade, ALG grade, PD-1 inhibitors, prognosis
Citation: Xu L, Chen L, Zhang B, Liu Z, Liu Q, Liang H, Chen Y, Chen X, Leng C and Zhang B (2023) Alkaline phosphatase combined with γ-glutamyl transferase is an independent predictor of prognosis of hepatocellular carcinoma patients receiving programmed death-1 inhibitors. Front. Immunol. 14:1115706. doi: 10.3389/fimmu.2023.1115706
Received: 04 December 2022; Accepted: 09 January 2023;
Published: 25 January 2023.
Edited by:
Yunfei Xu, Shandong University, ChinaReviewed by:
Mingyue Cai, The Second Affiliated Hospital of Guangzhou Medical University, ChinaHongwei Cheng, Xiamen University, China
Copyright © 2023 Xu, Chen, Zhang, Liu, Liu, Liang, Chen, Chen, Leng and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoping Chen, Y2hlbnhwY2hlbnhwQDE2My5jb20=; Chao Leng, bGVuZ2NoYW9qZGxkQDE2My5jb20=; Bixiang Zhang, Yml4aWFuZ3poYW5nQGh1c3QuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
‡These authors share last authorship
 Lei Xu1†
Lei Xu1† Lin Chen
Lin Chen Bin Zhang
Bin Zhang Zhicheng Liu
Zhicheng Liu Huifang Liang
Huifang Liang Xiaoping Chen
Xiaoping Chen Chao Leng
Chao Leng Bixiang Zhang
Bixiang Zhang