
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Immunol. , 22 February 2023
Sec. Cancer Immunity and Immunotherapy
Volume 14 - 2023 | https://doi.org/10.3389/fimmu.2023.1111325
This article is part of the Research Topic Roles of Pan-cell Death of Tumor Cells in Regulating Tumor Immune Microenvironment View all 14 articles
 Nan Zhou1,2†
Nan Zhou1,2† Yuhong Chen1,3†
Yuhong Chen1,3† Qian Huang1,4†
Qian Huang1,4† Lili Jiang5
Lili Jiang5 Hu Liao6
Hu Liao6 Hongfeng Gou2
Hongfeng Gou2 You Lu7
You Lu7 Guowei Che1
Guowei Che1 Yan Zhang1,4*
Yan Zhang1,4*Immunotherapy plus chemotherapy has been approved for the first-line treatment of extensive-stage small cell lung cancer (ES-SCLC, stage IV). Recently, the 2023 version of the National Comprehensive Cancer Network Guidelines recommended immunotherapy plus chemotherapy as the neoadjuvant regimen in patients with resectable non-small cell lung cancer (NSCLC). However, it is still unclear whether the combination regimen of immunotherapy plus chemotherapy is also beneficial for SCLC in the neoadjuvant context. Here, we report the case of a patient with stage IIIB SCLC who showed long-term survival and good tolerance to the neoadjuvant chemoimmunotherapy consisting of tislelizumab (an anti–PD-1 monoclonal antibody) plus etoposide-carboplatin. The patient achieved pathological complete response after receiving two cycles of neoadjuvant tislelizumab and chemotherapy followed by surgery. Two courses of post-operative tislelizumab and etoposide-carboplatin treatment were performed. The patient has survived for more than 23 months with no recurrence or metastases after neoadjuvant therapy. Multiplexed immunofluorescence and immunohistochemistry staining showed that the post-treatment specimens had remarkable immune cells infiltration, including CD3+ T cells, CD4+ T cells, and CD8+ T cells, which contrasted with very low levels of these cells in the pre-treatment samples. This study is, to the best of our knowledge, the first attempt to present the neoadjuvant chemoimmunotherapy of tislelizumab in combination with etoposide-carboplatin in SCLC. Our study suggested that neoadjuvant tislelizumab plus chemotherapy may facilitate radical resection and benefit patients with locally advanced (stage IIB-IIIC) SCLC.
It is generally acknowledged that surgery is limited to very early stages (I–IIA) in small cell lung cancer (SCLC). The standard therapies for locally advanced (stage IIB-IIIC) SCLC have shown limited benefits. Therefore, improvements in treatment regimens are still needed. Neoadjuvant treatment showed promise with respect to the R0 resection (no residual tumor) rate and pathological complete response (pCR, 0% viable tumor in resected lung and lymph nodes) rate, which are related to clinical benefits (1–4). However, the pCR rate was as low as 5% (2/40) for patients with stage IIIA SCLC who were treated with neoadjuvant chemotherapy alone (5).
Tislelizumab is a monoclonal antibody with the high affinity for PD-1. A phase II study revealed potent antitumor effects of tislelizumab plus platinum-etoposide in extensive-stage small cell lung cancer (ES-SCLC, stage IV), with a median PFS of 6.9 months and 1-year overall survival rate of 76% (6).
Previous studies have demonstrated that chemotherapy enhances antitumor activities through direct or indirect immune-system activation (7). It has been established that a combination regimen of immunotherapy and chemotherapy has superior benefits both in neoadjuvant and first-line setting for non-small cell lung cancer (NSCLC) (8–10). Tislelizumab plus chemotherapy as the first-line regimen has been approved by the National Medical Products Administration (NMPA) of China for the treatment of advanced NSCLC. In addition, the IMpower133 and CASPIAN study revealed a significant effect on OS with the addition of immunotherapy to standard chemotherapy, leading to the approval of combination regimens by the Food and Drug Administration (FDA) for first-line treatment of ES-SCLC (11, 12).
In the neoadjuvant context, immunotherapy aims to enhance systemic immunity, eliminating micrometastatic tumor deposits (13). The FDA approved nivolumab (an anti–PD-1 antibody) plus chemotherapy as the neoadjuvant regimen for resectable NSCLC based on the CheckMate-816 trial. This study showed that neoadjuvant nivolumab plus chemotherapy resulted in significantly longer event-free survival and a higher pCR rate than chemotherapy alone (2).
However, it remains unknown whether neoadjuvant immunotherapy plus chemotherapy could also be beneficial for limited-stage SCLC (LS-SCLC, stages I–III). Here, we report a patient with stage IIIB (cT3N2M0) SCLC who achieved pCR after receiving neoadjuvant tislelizumab plus etoposide-carboplatin.
In January 2021, a 46-year-old Chinese man was admitted to our hospital for cough. The patient was in good health and had no history of smoking, family history of hereditary disease, or tumor. However, the chest computed tomography (CT) and positron emission tomography (PET)-CT scan presented a mass of 5.4 cm × 4.5 cm, centrally located in the left lower lobe, with hilar and mediastinal lymph nodes metastasis (Figures 1A, B). Blood tests showed a significant elevation of pro-gastrin-releasing peptide (Pro-GRP). The contrast-enhanced CT of the abdomen and magnetic resonance imaging (MRI) of the brain were normal. Then, the patient accepted the fiberoptic bronchoscopy and biopsy. Eventually, the diagnosis of LS-SCLC, cT3N2M0, and stage IIIB (Eastern Cooperative Oncology Group performance-status score of 0) was given. Immunohistochemical analyses suggested “PCK(+), CK7 (+), TTF-1 (+), CD56 (+), CgA (+), Syn (+), CD34(-), desmin(-)、P63(-), Ki-67 (80%+). ”
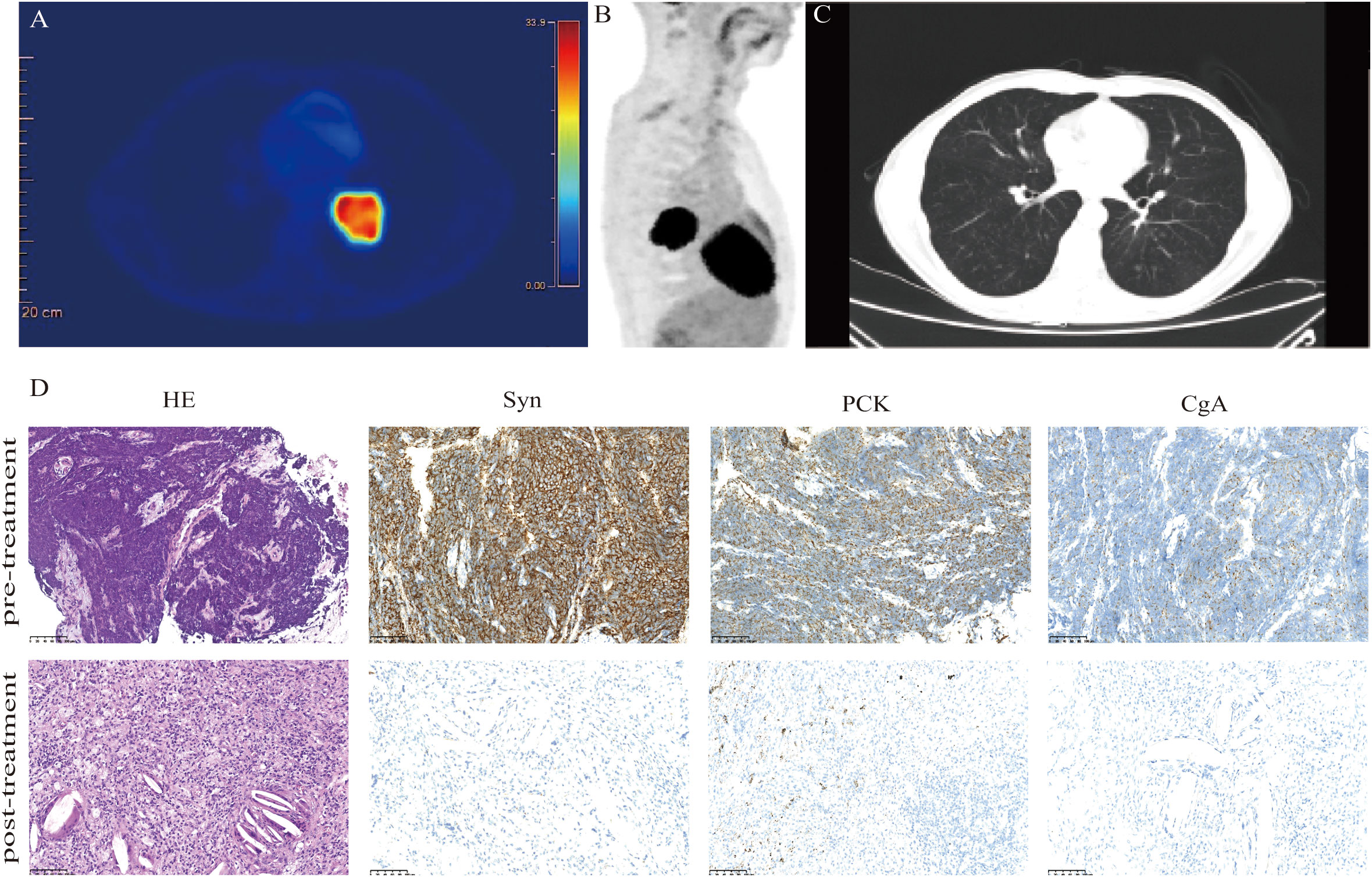
Figure 1 Clinical and pathological response. Images (A, B) show FDG uptake in the lung parenchyma. (C) CT images acquired after two circles of neoadjuvant tislelizumab plus chemotherapy display a decrease in the size of the tumor lesion. (D) Hematoxylin-eosin (HE) and immunohistochemistry staining in the tumor bed. The staining of the lung mass (lower panels) showed no viable tumor cells; negative for synaptophysin (Syn), pan-cytokeratin (PCK), and chromogranin A (CgA). Scale bars: D = 100 µm.
The treatment of the patient was discussed by the multi-disciplinary team (MDT). The tumor was considered potentially resectable; thus, neoadjuvant therapy followed by surgery might be an ideal option. Our patient chose the neoadjuvant regimen of etoposide (100 mg/m2, days 1–3), carboplatin (AUC 5, day 1), and tislelizumab (200 mg, day 1) every 3 weeks. After two cycles of neoadjuvant treatment, a repeated CT scan manifested the mass obviously shrank to a nodule with a diameter of 0.8 cm (Figure 1C). Patients underwent left lower pulmonary lobectomy and lymphadenectomy 38 days after neoadjuvant tislelizumab plus chemotherapy. Notably, the efficacy was evaluated as pCR. Tumor cell was found neither in hematoxylin-eosin (HE) staining nor in immunohistochemistry (IHC) staining of pan-cytokeratin (PCK), chromogranin A (CgA), and synaptophysin (Syn) (Figure 1D). After surgery, the patient received two cycles of tislelizumab in combination with etoposide-carboplatin. Regular imaging assessments were done every 2 months during the follow-up period. More than 23 months after neoadjuvant tislelizumab plus chemotherapy, no recurrence or metastases were detected (Figure 2). The treatment-related adverse event was hypothyroidism, which was diagnosed in April 2022. No adverse events led to discontinuation.
We report a case of a patient with stage IIIB SCLC who obtained pCR and long-term benefits after neoadjuvant tislelizumab plus etoposide-carboplatin followed by surgery. To our knowledge, this is the first report of neoadjuvant therapy comprising tislelizumab plus chemotherapy in SCLC.
It is well accepted that significantly more immune effector T cells were in LS-SCLC than in ES-SCLC (14), suggesting that patients with earlier stage SCLC may gain more benefits from neoadjuvant chemoimmunotherapy.
The addition of immunotherapy to chemotherapy had the potential advantage of inducing systemic tumor-specific T-cell response (15). Various studies have identified that CD4+ and CD8+ T-cell subpopulations experience a proliferative burst after immunotherapy, which was associated with improved survival (16–18). To explore the tumor immune microenvironment, we performed IHC staining of CD3+, CD4+, and CD8+ T cells (Figure 3A) and multiplexed immunofluorescence staining of immune markers (CD4+, CD8+, DAPI, PD-1, and PanCK) (Figure 3B) on pre- and post-treatment specimens. Notably, the post-treatment samples exhibited remarkable immune cell infiltration. The positive area fractions of CD3+, CD4+, and CD8+ T cell in post-treatment specimens were 4.02, 2.60, and 8.38 times higher than those in pre-treatment specimens, respectively (Figure 3C).
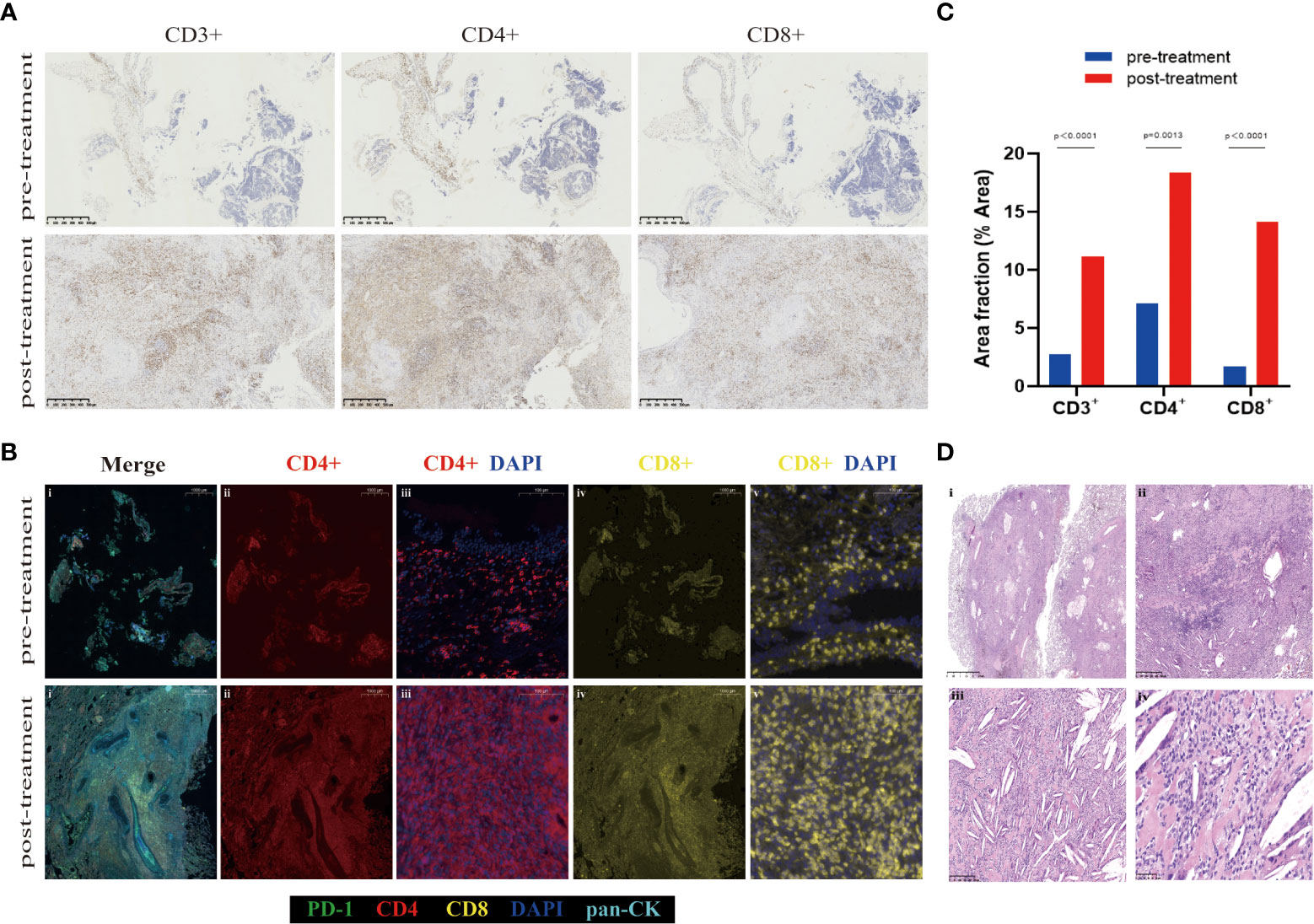
Figure 3 Representative images demonstrating changes in the tumor bed. (A) CD3+, CD4+, and CD8+ immunohistochemistry staining of the pre- and post-treatment specimen. (B) Multiplex immunofluorescent images of tumor tissue sections, which labels five immune biomarkers within the tumor bed: PD-1 (green), CD4 (red), CD8 (yellow), DAPI (blue), pan-CK (light blue). Overall, the numbers of various immune cell phenotypes were higher in post-treatment specimens. (C) Area fraction of indicated immune cell populations in pre- and post-treatment. A significant increase in CD3+, CD4+, and CD8+ in the tumor bed is observed after neoadjuvant tislelizumab plus chemotherapy (multiple unpaired t-tests p<0.000, p = 0.0013, and p<0.000, respectively). (D) HE staining of post-treatment specimen shows inflammatory cells, a proliferation of fibrosis, cholesterol clefts, and granulomatous reaction after neoadjuvant tislelizumab plus chemotherapy. Scale bars: A = 500 µm. B: i, ii, and iv =1000 µm; iii and v = 100 µm. D: i = 2.5 mm; ii = 500 µm; iii = 250 µm; iv = 50 µm.
Pathologic features of pCR after neoadjuvant tislelizumab plus etoposide-carboplatin therapy were observed. It was detected that inflammatory cells, a proliferation of fibrosis, cholesterol clefts, and granulomatous reaction were in the areas of the previous tumor bed (Figure 3D). The association of pCR with survival benefits warrants further evaluation involving patients with SCLC.
Atezolizumab or durvalumab (anti–PD-L1 antibody) in combination with chemotherapy is considered the standard of care in the first-line setting in SCLC. However, in January 2021, the patient finally selected tislelizumab (anti–PD-1 antibody), mainly due to financial distress. Of note, serplulimab, an anti–PD-1 antibody, combined with chemotherapy have recently come under intense focus after a phase 3 study showed improvement in overall survival compared with chemotherapy alone (15.4 months vs. 10.9 months, respectively; hazard ratio, 0.63) in patients with ES-SCLC (19). Based on this study, serplulimab was approved by the NMPA of China for the first-line treatment of ES-SCLC and was also granted orphan drug designations by the FDA and European Commission for the treatment of SCLC. Our study suggests that tislelizumab may have similar antitumor effects in SCLC and deserves further investigation.
Our case shows that neoadjuvant tislelizumab plus chemotherapy might facilitate radical resection and benefit patients with locally advanced (stage IIB-IIIC) SCLC without impeding the feasibility of surgery or increasing the incidence of adverse events.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
The studies involving human participant was reviewed and approved by The Clinical Research Ethics Committee of West China Hospital Sichuan University. The patient/participant provided his written informed consent to participate in this study. Written informed consent was obtained from the individual for the publication of any potentially identifiable images or data included in this article.
NZ: Conceptualization, Writing- Original draft preparation. YC: Methodology, Software. QH: Investigation. GC, HG: Visualization, Investigation. LJ: histopathological findings. YL, HL: Software, Validation. YZ: Writing- Reviewing and Editing, Supervision. All listed authors participated meaningfully in the study and that they have seen and approved the final manuscript.
The study was funded by the Key R&D Projects of the Science and Technology Department of Sichuan Province (grant numbers 2021YFS0237) and the 1·3·5 project for disciplines of excellence–Clinical Research Incubation Project, West China Hospital, Sichuan University (grant numbers 2019HXFH062).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Provencio M, Nadal E, Insa A, García-Campelo MR, Casal-Rubio J, Dómine M, et al. Neoadjuvant chemotherapy and nivolumab in resectable non-Small-Cell lung cancer (Nadim): An open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol (2020) 21(11):1413–22. doi: 10.1016/s1470-2045(20)30453-8
2. Forde PM, Spicer J, Lu S, Provencio M, Mitsudomi T, Awad MM, et al. Neoadjuvant nivolumab plus chemotherapy in resectable lung cancer. N Engl J Med (2022) 386(21):1973–85. doi: 10.1056/NEJMoa2202170
3. Zheng Q, Li S, Zhang L, Wu N, Chen J, Wang Y, et al. Retrospective study of surgical resection in the treatment of limited stage small cell lung cancer. Thorac Cancer (2013) 4(4):395–9. doi: 10.1111/1759-7714.12035
4. Li R, Jiang L, Zhou X, Lu Y, Zhang Y. Pseudo-small cell transformation in egfr-mutant adenocarcinoma. Lung Cancer (2021) 153:120–5. doi: 10.1016/j.lungcan.2020.12.036
5. Li S, Jin K, Pan Y, Wu C, Ren S, Jiang G, et al. Role of surgery in a case-control study of patients with clinical stage iiia small cell lung cancer. J Thorac Dis (2021) 13(5):2738–45. doi: 10.21037/jtd-20-3047
6. Wang Z, Zhao J, Ma Z, Cui J, Shu Y, Liu Z, et al. A phase 2 study of tislelizumab in combination with platinum-based chemotherapy as first-line treatment for advanced lung cancer in Chinese patients. Lung Cancer (2020) 147:259–68. doi: 10.1016/j.lungcan.2020.06.007
7. Bracci L, Schiavoni G, Sistigu A, Belardelli F. Immune-based mechanisms of cytotoxic chemotherapy: Implications for the design of novel and rationale-based combined treatments against cancer. Cell Death Differ (2014) 21(1):15–25. doi: 10.1038/cdd.2013.67
8. Gandhi L, Rodriguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, et al. Pembrolizumab plus chemotherapy in metastatic non-Small-Cell lung cancer. N Engl J Med (2018) 378(22):2078–92. doi: 10.1056/NEJMoa1801005
9. Zhou C, Chen G, Huang Y, Zhou J, Lin L, Feng J, et al. Camrelizumab plus carboplatin and pemetrexed versus chemotherapy alone in chemotherapy-naive patients with advanced non-squamous non-Small-Cell lung cancer (Camel): A randomised, open-label, multicentre, phase 3 trial. Lancet Respir Med (2021) 9(3):305–14. doi: 10.1016/s2213-2600(20)30365-9
10. Yang Y, Wang Z, Fang J, Yu Q, Han B, Cang S, et al. Efficacy and safety of sintilimab plus pemetrexed and platinum as first-line treatment for locally advanced or metastatic nonsquamous nsclc: A randomized, double-blind, phase 3 study (Oncology program by innovent anti-Pd-1-11). J Thorac Oncol (2020) 15(10):1636–46. doi: 10.1016/j.jtho.2020.07.014
11. Horn L, Mansfield AS, Szczesna A, Havel L, Krzakowski M, Hochmair MJ, et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med (2018) 379(23):2220–9. doi: 10.1056/NEJMoa1809064
12. Paz-Ares L, Dvorkin M, Chen Y, Reinmuth N, Hotta K, Trukhin D, et al. Durvalumab plus platinum–etoposide versus platinum–etoposide in first-line treatment of extensive-stage small-cell lung cancer (Caspian): A randomised, controlled, open-label, phase 3 trial. Lancet (2019) 394(10212):1929–39. doi: 10.1016/s0140-6736(19)32222-6
13. Topalian SL, Taube JM, Pardoll DM. Neoadjuvant checkpoint blockade for cancer immunotherapy. Science (2020) 367(6477):eaax0182. doi: 10.1126/science.aax0182
14. Koyama K, Kagamu H, Miura S, Hiura T, Miyabayashi T, Itoh R, et al. Reciprocal Cd4+ T-cell balance of effector Cd62llow Cd4+ and Cd62lhighcd25+ Cd4+ regulatory T cells in small cell lung cancer reflects disease stage. Clin Cancer Res (2008) 14(21):6770–9. doi: 10.1158/1078-0432.CCR-08-1156
15. Yost KE, Satpathy AT, Wells DK, Qi Y, Wang C, Kageyama R, et al. Clonal replacement of tumor-specific T cells following pd-1 blockade. Nat Med (2019) 25(8):1251–9. doi: 10.1038/s41591-019-0522-3
16. Nelson MA, Ngamcherdtrakul W, Luoh SW, Yantasee W. Prognostic and therapeutic role of tumor-infiltrating lymphocyte subtypes in breast cancer. Cancer Metastasis Rev (2021) 40(2):519–36. doi: 10.1007/s10555-021-09968-0
17. Emens LA, Braiteh FS, Cassier P, Delord JP, Eder JP, Fasso M, et al. Inhibition of pd-L1 by Mpdl3280a leads to clinical activity in patients with metastatic triple-negative breast cancer (Tnbc). Cancer Res (2015) 75:1. doi: 10.1158/1538-7445.Am2015-2859
18. Takeuchi Y, Tanemura A, Tada Y, Katayama I, Kumanogoh A, Nishikawa H. Clinical response to pd-1 blockade correlates with a Sub-fraction of peripheral central memory Cd4+ T cells in patients with malignant melanoma. Int Immunol (2018) 30(1):13–22. doi: 10.1093/intimm/dxx073
Keywords: small cell lung cancer, neoadjuvant therapy, pathological complete response, immunotherapy, tislelizumab, programmed cell death-1 (PD-1), lymphocyte infiltration, case report
Citation: Zhou N, Chen Y, Huang Q, Jiang L, Liao H, Gou H, Lu Y, Che G and Zhang Y (2023) Pathological complete response to neoadjuvant tislelizumab plus chemotherapy in stage IIIB small cell lung cancer: A case report and literature review. Front. Immunol. 14:1111325. doi: 10.3389/fimmu.2023.1111325
Received: 29 November 2022; Accepted: 09 February 2023;
Published: 22 February 2023.
Edited by:
Jiajie Peng, Northwestern Polytechnical University, ChinaReviewed by:
Zhenzhou Yang, Chongqing Medical University, ChinaCopyright © 2023 Zhou, Chen, Huang, Jiang, Liao, Gou, Lu, Che and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yan Zhang, emhhbmcueWFuQHNjdS5lZHUuY24=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.