
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Immunol. , 04 April 2023
Sec. Inflammation
Volume 14 - 2023 | https://doi.org/10.3389/fimmu.2023.1110874
This article is part of the Research Topic Inflammation in the Pathogenesis of Covid-19 View all 11 articles
 Chan Mi Lee1†
Chan Mi Lee1† Minji Kim2,3,4†
Minji Kim2,3,4† Chang Kyung Kang1
Chang Kyung Kang1 Pyoeng Gyun Choe1
Pyoeng Gyun Choe1 Nam Joong Kim1
Nam Joong Kim1 Hyeeun Bang5
Hyeeun Bang5 Taeeun Cho5
Taeeun Cho5 Hyun Mu Shin2,4,6*‡
Hyun Mu Shin2,4,6*‡ Hang-Rae Kim2,3,4,6,7*‡
Hang-Rae Kim2,3,4,6,7*‡ Wan Beom Park1*‡
Wan Beom Park1*‡ Myoung-don Oh1
Myoung-don Oh1Introduction: Tocilizumab, a humanized anti-interleukin-6 receptor (IL-6R) antibody, is recommended for the treatment of severe to critical coronavirus diseases 2019 (COVID-19). However, there were conflicting results on the efficacy of tocilizumab. Therefore, we hypothesized that the differences in tocilizumab efficacy may stem from the different immune responses of critical COVID-19 patients. In this study, we described two groups of immunologically distinct COVID-19 patients, based on their IL-6 response.
Methods: We prospectively enrolled critical COVID-19 patients, requiring oxygen support with a high flow nasal cannula or a mechanical ventilator, and analyzed their serial samples. An enzyme-linked immunosorbent assay and flow cytometry were used to evaluate the cytokine kinetics and cellular immune responses, respectively.
Results: A total of nine patients with critical COVID-19 were included. The high (n = 5) and low IL-6 (n = 4) groups were distinguished by their peak serum IL-6 levels, using 400 pg/mL as the cut-off value. Although the difference of flow cytometric data did not reach the level of statistical significance, the levels of pro-inflammatory cytokines and the frequencies of intermediate monocytes (CD14+CD16+), IFN-γ+ CD4+ or CD8+ T cells, and HLA-DR+PD-1+ CD4+ T cells were higher in the high IL-6 group than in the low IL-6 group.
Conclusion: There were distinctive two groups of critical COVID-19 according to serum IL-6 levels having different degrees of cytokinemia and T-cell responses. Our results indicate that the use of immune modulators should be more tailored in patients with critical COVID-19.
About 5% of patients with coronavirus disease 2019 (COVID-19) experience critical illness, characterized by respiratory failure, septic shock, and multiorgan failure (1, 2). T-cell hyperactivation (3), cytokine storm (4), and expansion of monocyte subpopulation (5) have been suggested as typical immunological features of severe COVID-19; thus immune modulators such as corticosteroid, baricitinib, or tocilizumab are used for the treatment of severe to critical COVID-19 (6).
Although the National Institutes of Health guidelines recommend the use of tocilizumab, a humanized monoclonal antibody against the interleukin-6 receptor (IL-6R), for the treatment of critical COVID-19 (7), there have been conflicting results relating to the efficacy of this drug (8–10). This raises the question of whether patients with critical COVID-19 differ in their immune responses. If different immunopathologies, rather than viral pathological mechanisms, contribute to disease severity in patients with critical COVID-19, more tailored anti-inflammatory strategies could be pursued. Therefore, we aimed to describe and classify immune responses especially according to IL-6 response, among patients with critical COVID-19.
In this study, adult (≥ 18 years old) patients with critical acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, hospitalized to the Seoul National University Hospital between August to December 2021, were prospectively enrolled. All SARS-CoV-2 infection was laboratory-confirmed by quantitative real-time reverse transcription polymerase chain reaction (qRT-PCR). Critical COVID-19 was defined as patients requiring respiratory support such as high flow nasal cannula (HFNC) oxygen therapy or mechanical ventilation.
Serial blood and nasopharyngeal swab samples were collected around the onset of critical illness. Clinical data, including demographics, underlying comorbidities, COVID-19 vaccination status, disease severity, COVID-19 specific treatment, duration of isolation, and clinical outcome were collected. The levels of C-reactive protein (CRP) were also retrospectively collected.
This study was approved by the Institutional Review Board (IRB) of Seoul National University Hospital (IRB no. 2104-181-1215). All participants provided written informed consent in accordance with the Declaration of Helsinki.
Serum anti-SARS-CoV-2 S1 domain (i.e., S1) immunoglobulin (Ig) G titers were semi-quantitatively measured using the anti-SARS-CoV-2 enzyme-linked immunosorbent assay (ELISA) IgG kit (Euroimmun, Lübeck, Germany), according to the manufacturer’s instructions. The optical density (O.D. 450 nm) ratios were interpreted as follows: ≥ 1.1, positive; ≥ 0.8 to < 1.1, borderline; < 0.8, negative.
The levels of IL-6, monocyte chemoattractant protein (MCP)-1, interferon-gamma (IFN-γ), and tumor necrosis factor-alpha (TNF-α) were measured in serum samples using the Human IL-6 ELISA Kit II (#550799, BD Biosciences, San Jose, CA, USA), Human MCP-1 ELISA Kit (#559017, BD Biosciences), Human IFN-γ Quantikine ELISA Kit (R&D Systems, Inc., Minneapolis, MN, USA), and Human TNF ELISA Kit (#550610, BD Biosciences) respectively, according to the manufacturer’s instruction.
A previous study set the threshold of IL-6 > 406 pg/mL for predicting intensive care unit (ICU) mortality of COVID-19 patients (11). Therefore, in our study, patients were classified as the high IL-6 group if their peak IL-6 levels were over 400 pg/mL; otherwise, they were classified as the low IL-6 group.
Viral RNA was extracted from nasopharyngeal swab samples using the QIAamp Viral RNA Mini kit (Qiagen, Hilden, Germany), according to the manufacturer’s instructions. Briefly, 140 μL of nasopharyngeal swabs was mixed with 560 μL of lysis buffer and incubated for 10 min at 37°C. The supernatant containing the viral RNA was purified, and the extracted RNA was eluted in 50 μL of elution buffer and stored at –70°C until use. qRT-PCR for detection of SARS-CoV-2 was performed using the QPLEX™ COVID-19 Test (QMCOVID02, QuantaMatrix Inc., Seoul, Republic of Korea). A 20 μL of PCR mixture contained 10 μL of 2× One-step Premix, 5 μL of Oligo Mix, 5 μL of extracted RNA, and primer and probe sequences targeting the RdRp gene of SARS-CoV-2. Thermal cycling was performed at 25°C for 10 min for the uracil-DNA glycosylase incubation step, at 52°C for 5 min for the reverse transcription step, followed by 95°C for 10 sec, 5 cycles of pre-amplification (95°C for 10 sec and 55°C for 30 sec), and 35 cycles of the PCR reaction (95°C for 10 sec and 55°C for 30 sec) using the CFX-96 In Vitro Diagnostics Real-Time PCR System (Bio-Rad Laboratories, Inc., Hercules, CA, USA). A cycle threshold (Ct) value higher than 32 was defined as negative.
Human peripheral blood mononuclear cells (PBMCs) were isolated from heparinized blood by Ficoll–Plaque PLUS (1.077 g/mL; GE Healthcare Life Sciences, Piscataway, NJ, USA) density gradient centrifugation. Purified PBMCs were cryopreserved in 50% fetal bovine serum (FBS), 10% dimethyl sulfoxide, and 40% RPMI-1640 (all reagents from Thermo Fisher Scientific, Waltham, MA, USA) at 5 × 106 cells/mL and thawed prior to use (12).
After thawing the PBMCs, cells were pelleted by centrifugation and resuspended in phosphate-buffered saline (pH 7.4) supplemented with 1% FBS at a final density of 1 × 107 cells/mL. Cells were stained using brilliant violet 711 (BV711)–anti-human CD3 (clone, UCHT1), brilliant ultra violet 805 (BUV805)–anti-human CD14 (clone, MØpq), and allophycocyanin-H7–anti-human CD16 (clone, 3G8) antibodies (Abs) (all from BD Biosciences). Brilliant Stain Buffer (BD Biosciences) was added to each sample.
For the staining of activation markers and cytokines, the PBMCs (at 1 × 107 cells/mL) were stimulated with 50 ng/mL of phorbol 12-myristate 13-acetate and 1 μg/mL of ionomycin (both reagents from Sigma-Aldrich, St. Louis, MO, USA) and then, 1 h later, treated with BD Golgistop™ (Monensin, BD Biosciences) for an additional 3 h. BV605–anti-human CD4 Ab (clone, OKT-4; Biolegend, San Diego, CA, USA) was applied concomitantly during stimulation. Stimulated cells were stained with BV711–anti-human CD3 (clone, UCHT1), BUV496–anti-human CD8 (clone, RPA-T8), BUV395–anti-human PD-1 (clone, MIH4), and phycoerythrin (PE)-cyanine-5–anti-human HLA-DR (clone, G46-6) Abs (all from BD Biosciences). After fixation and permeabilization with the Cytofix/Cytoperm kit (BD Biosciences), cells were incubated with the PE–anti-human IFN-γ Ab (clone, B27; BD Biosciences). Samples were acquired on a BD LSRII flow cytometer (BD Biosciences) and analyzed using FlowJo software version 10.7.1 (TreeStar, Ashland, OR, USA). The percentages of target cell populations in unstimulated specimens were subtracted from those in stimulated specimens (13).
The experimental data were presented as the median with interquartile range (IQR). The Mann–Whitney U test was used to compare continuous variables. Statistical analyses were conducted using SPSS Statistics, version 26.0 (IBM Corp., Armonk, NY, USA). P-values < 0.05 were considered as a measure of statistical significance. GraphPad Prism 9 (GraphPad Software, La Jolla, CA, USA) was used to generate graphs.
A total of nine patients with critical COVID-19 were enrolled during the study period (Table 1). The high IL-6 group included five (55.6%) patients and the low IL-6 group included four (44.4%) patients (Table 1). Among the nine patients, seven patients (77.8%) were male and the median (range) age was 70 (58–84). All patients had multiple underlying comorbidities, such as hypertension (6/9, 66.7%) or diabetes mellitus (5/9, 55.6%) and were fully vaccinated with the AZD1222 (5/9, 55.6%) or BNT162b2 (4/9, 44.4%); the diagnosis of COVID-19 was made more than 14 days after their second vaccine dose (14). All patients were treated with remdesivir and steroids, and four (44.4%) patients were treated with tocilizumab. Two (2/5, 40%) patients from the high IL-6 group and one (1/4, 25%) patients from the low IL-6 group required mechanical ventilation, although all patients enrolled in this study made a full recovery.
The kinetics of viral load, the anti-S1 IgG and CRP levels, and the use of immune modulators in the high and low IL-6 groups are presented in Supplementary Figure S1A, B, respectively. The day of HFNC oxygen therapy initiation was designated as day 0. We found that the viral load decreased with time and all patients acquired anti-S1 IgG during their clinical course. In most patients, the level of CRP peaked near the onset of critical illness. Serial serum samples were used to evaluate cytokine levels and representative PBMC samples were used for the characterization of the cellular immune response.
The levels of cytokines (IL-6, MCP-1, IFN-γ, and TNF-α) and CRP according to the groups are shown in Figures 1A, B, respectively. The cytokine levels measured within 0−5 days of initiating high flow nasal cannula oxygen therapy, at the closest point to the onset of critical illness, have been presented. The specific time point during the clinical course is indicated with pink arrows in Supplementary Figure S1A, B, allowing for a simultaneous evaluation with the administration of immune modulators. The levels of IL-6 and IFN-γ were significantly higher in the high IL-6 group than in the low IL-6 group (median [IQR], 419 pg/mL [283–710] vs. 63 pg/mL [11–107], P = 0.016; 6.11 pg/mL [4.84–12.04] vs. 1.16 pg/mL [0.00–2.56], P = 0.016, respectively). While not statistically significant, the levels of MCP-1 and CRP were also higher in the high IL-6 group than in the low IL-6 group (median [IQR], 6,700 pg/mL [2,535–8,417] vs. 1,492 pg/mL [615–1,684], P = 0.111; 9.49 mg/dL [5.69–27.61] vs. 5.49 mg/dL [3.60–8.40], P = 0.191, respectively). The levels of TNF-α did not show significant difference between the high and low IL-6 groups (median [IQR], 0.08 pg/mL [0.00–27.53] vs. 1.36 pg/mL [0.32–41.89], P = 0.714).
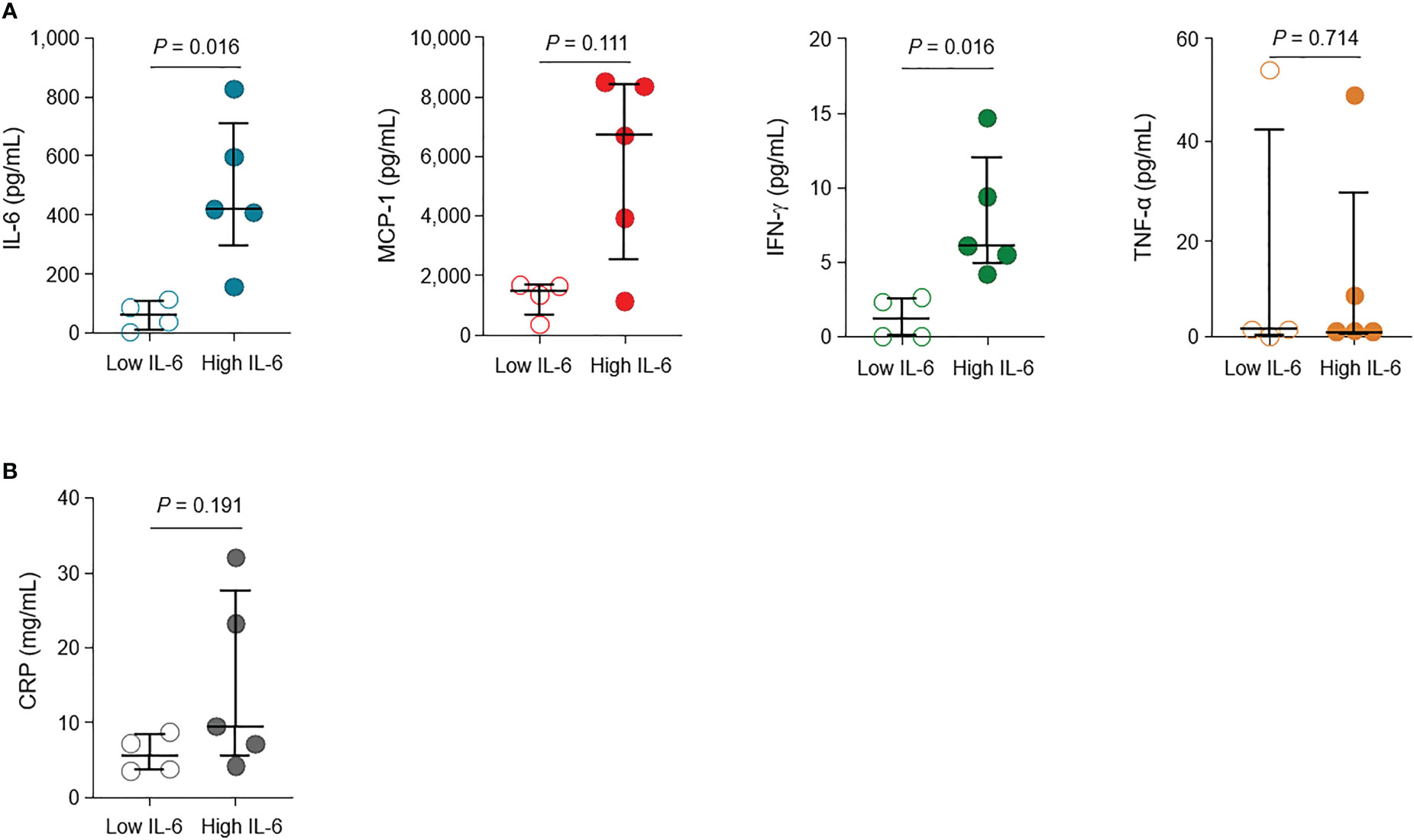
Figure 1 Levels of cytokines and inflammatory marker in patients with critical COVID-19. (A) The levels of interleukin (IL)-6, monocyte chemoattractant protein (MCP)-1, interferon (IFN)-γ, and tumor necrosis factor (TNF)-α in the high IL-6 (n = 5) and low IL-6 (n = 4) groups. (B) Levels of C-reactive protein (CRP) in the high IL-6 and low IL-6 groups. Vertical and horizontal lines indicate the median with the interquartile range. The cytokine and CRP levels at the closest point to the onset of critical illness (within 0–5 days of high flow nasal cannula oxygen therapy initiation) are shown.
The kinetics of cytokine levels in individual patients are presented in Supplementary Figure S1C, according to the days from critical illness. Overall, the low IL-6 group had lower levels of IL-6, MCP-1, and IFN-γ than the high IL-6 group.
To provide a temporal context for the flow cytometric analysis samples in relation to the clinical course and immune modulator treatment, we marked the respective time points with green arrows in Supplementary Figure S1A, B. The high IL-6 group showed a higher frequency of intermediate monocytes (CD14+CD16+) compared to the low IL-6 group, although the difference was not statistically significant (%, median [IQR], 1.51 [0.59–11.20] vs. 1.24 [0.82–1.88], P = 0.730; Figure 2A). On the other hand, the high IL-6 group had higher frequencies of IFN-γ+ in CD4+ and CD8+ T cells compared to the low IL-6 group (%, median [IQR], 7.07 [3.12–13.35] vs. 5.09 [2.24–10.73], P = 0.730; 23.6 [13.6–59.8] vs. 22.7 [9.7–30.5], P = 0.730; Figure 2B).
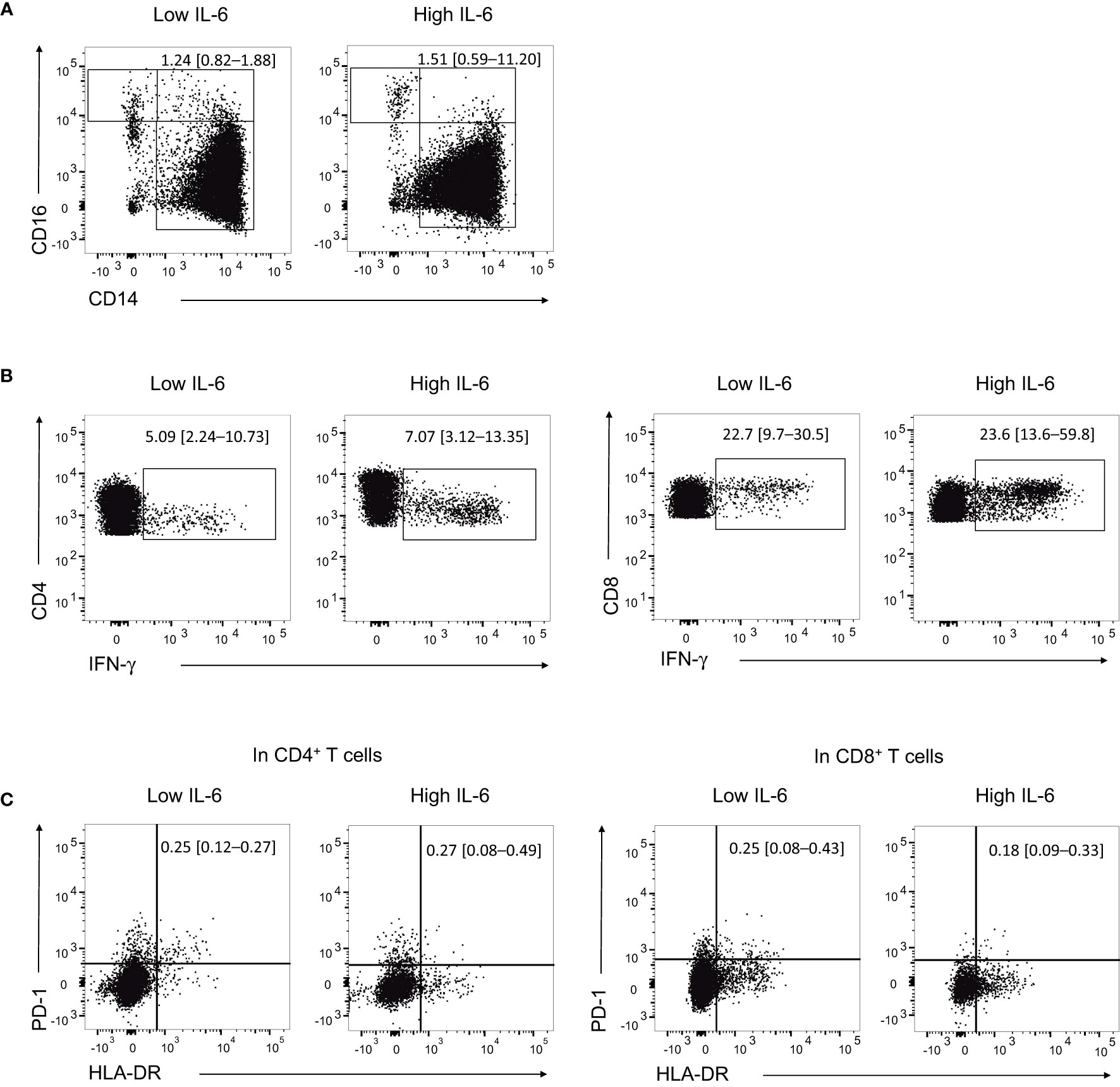
Figure 2 Proportion of monocyte subpopulation and expression levels of cytokine and activation markers in T cells. (A) Representative flow cytometry dot plots showing the identification of intermediate monocytes (CD14+CD16+) in the high IL-6 (n = 5) and low IL-6 (n = 4) groups. (B) Representative flow cytometry dot plots showing the identification of IFN-γ+ CD4+ T cells and CD8+ T cells in the high IL-6 and low IL-6 groups. (C) Representative flow cytometry dot plots showing the identification of HLA-DR+PD-1+ CD4+ T cells and CD8+ T cells in the high IL-6 and low IL-6 groups. Numbers indicate population frequencies as the median with interquartile range.
Regarding activated T cells based on the expression of HLA-DR and PD-1, the high IL-6 group had a slightly higher proportion of HLA-DR+PD-1+ CD4+ T cells compared to the low IL-6 group, although the difference was not statistically significant (%, median [IQR], 0.27 [0.08–0.49] vs. 0.25 [0.12–0.27], P = 0.492). In contrast, the high IL-6 group had a lower proportion of HLA-DR+PD-1+ CD8+ T cells compared to the low IL-6 group, but the difference was not statistically significant either (%, median [IQR], 0.18 [0.09–0.33] vs. 0.25 [0.08–0.43], P = 0.730; Figure 2C). The flow cytometric data from all samples are presented in Supplementary Figure S2.
In this study, we found that patients with critical COVID-19 can be classified by two distinctive groups in terms of cytokinemia and T-cell activation levels, according to their levels of serum IL-6. The high IL-6 group had higher levels of pro-inflammatory cytokines and cellular immune responses than the low IL-6 group. The hypercytokinemia and T-cell hyperactivation of the high IL-6 group suggest that immunological features could differ among patients with critical COVID-19, despite similar clinical characteristics.
Since IL-6 is considered as an important immunological factor in severe/critical COVID-19 (15, 16), clinical trials of tocilizumab for the treatment of severe COVID-19 have been conducted. For instance, the RECOVERY trial found that tocilizumab improved the clinical outcomes of COVID-19 patients with hypoxia (oxygen saturation < 92%) and CRP levels ≥ 7.5 mg/dL (17). However, other studies did not show the efficacy of tocilizumab in patients with severe COVID-19 (9, 10). One retrospective study also reported that the early use of tocilizumab was associated with improvement of oxygenation in patients with high IL-6 levels (18). These conflicting results led us to perform the present study, to elucidate whether certain immunological features, and especially the IL-6 response, differed among patients with critical COVID-19.
Results from the early phase of SARS-CoV-2 pandemic suggested that the host immune response, such as levels of cytokinemia, might play an important role in the pathogenesis of severe COVID-19 (19). For instance, the plasma levels of several cytokines, including MCP-1 and TNF-α, were shown to be higher in ICU than non-ICU COVID-19 patients (16). Another study demonstrated that the levels of several cytokines (e.g., IL-6, IL-10, and MCP-1) positively correlated with COVID-19 severity; moreover, MCP-1 was correlated with days on mechanical ventilation (20). A previous study has shown that increased levels of IL-6 are strongly associated with disease severity at admission and the need for ICU care in COVID-19 patients, regardless of age (21). In our present study, we aimed to demonstrate that cytokine levels could vary significantly among critically ill COVID-19 patients, using serum IL-6 levels as a surrogate marker. Our findings suggest that IL-6 could be an important marker for classifying COVID-19 patients, even after the general population has been vaccinated, as all patients in our study were vaccinated.
In addition, we detected higher frequencies of intermediate monocytes (CD14+CD16+) in the high IL-6 group than in the low IL-6 group. Intermediate monocytes expand in patients with systemic infection and secrete cytokines, such as TNF-α, IL-1β, and IL-6, implying their role in pathogen defense (22). A previous study suggested that CD14+CD16+ monocytes may exhibit dysregulated production of IL-6 (23). Another study also reported that monocyte-mediated hypercytokinemia is prominent in critical COVID-19 (24). Moreover, the frequencies of intermediate monocytes were higher in COVID-19 patients admitted to ICU (5). The higher levels of cytokines in the high IL-6 group might therefore correlate with the higher frequency of intermediate monocytes.
We showed that the high IL-6 group exhibited stronger cellular immune responses than the low IL-6 group, based on the expression of cytokines and T-cell activation markers. Although T-cell hyperactivation is a key immunological feature of severe COVID-19 (3, 25), the results of the present study suggest that the magnitude of T-cell responses could vary even in clinically similar critical COVID-19. Corticosteroid might attenuate T-cell responses as well as cytokinemia in critical COVID-19, however, we could find distinctive two groups of critical COVID-19 in this study.
In conclusion, we identified that patients with critical COVID-19 could be divided into two groups with different degrees of cytokinemia and T-cell responses, according to their serum IL-6 levels. Our results suggest that a more tailored use of immune modulators should be sought in the treatment of critical COVID-19.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
The studies involving human participants were reviewed and approved by the Institutional Review Board (IRB) of Seoul National University Hospital. The patients/participants provided their written informed consent to participate in this study.
CL, MK, CK, HS, H-RK, and WP conceived and designed the study. CL, CK, PC, NK, WP, and M-DO collected the samples. CL, MK, CK, HB, TC, HS, H-RK, and WP performed data analysis. NK, HS, H-RK, WP, and M-DO revised and edited the final manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by the New Faculty Startup Fund from Seoul National University (to WP), the Bio and Medical Technology Development Program of the National Research Foundation (NF) and funded by the Korean government (MSIT) (2021M3A9I2080496 to H-RK and WP; 2018M3A9H4055197 to H-RK), and the Creative-Pioneering Researchers Program through Seoul National University (to H-RK).
Author HB and TC are employed by QuantaMatrix.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1110874/full#supplementary-material
1. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a report of 72314 cases from the Chinese center for disease control and prevention. JAMA (2020) 323(13):1239–42. doi: 10.1001/jama.2020.2648
2. Petrilli CM, Jones SA, Yang J, Rajagopalan H, O'Donnell L, Chernyak Y, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in new York city: prospective cohort study. BMJ (2020) 369:m1966. doi: 10.1136/bmj.m1966
3. Kang CK, Han GC, Kim M, Kim G, Shin HM, Song KH, et al. Aberrant hyperactivation of cytotoxic T-cell as a potential determinant of COVID-19 severity. Int J Infect Dis (2020) 97:313–21. doi: 10.1016/j.ijid.2020.05.106
4. Chen R, Lan Z, Ye J, Pang L, Liu Y, Wu W, et al. Cytokine storm: The primary determinant for the pathophysiological evolution of COVID-19 deterioration. Front Immunol (2021) 12:589095. doi: 10.3389/fimmu.2021.589095
5. Zhou Y, Fu B, Zheng X, Wang D, Zhao C, Qi Y, et al. Pathogenic T-cells and inflammatory monocytes incite inflammatory storms in severe COVID-19 patients. Natl Sci Rev (2020) 7(6):998–1002. doi: 10.1093/nsr/nwaa041
6. Bhimraj A, Morgan RL, Shumaker AH, Baden L, Cheng VCC, Edwards KM, et al. Infectious diseases society of America guidelines on the treatment and management of patients with COVID-19. Clin Infect Dis (2022), ciac724. doi: 10.1093/cid/ciac724
7. COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. Bethesda, MD: National Institutes of Health (2022). Available at: https://www.covid19treatmentguidelines.nih.gov/. Accessed 20 November 2022.
8. Investigators R-C, Gordon AC, Mouncey PR, Al-Beidh F, Rowan KM, Nichol AD, et al. Interleukin-6 receptor antagonists in critically ill patients with covid-19. N Engl J Med (2021) 384(16):1491–502. doi: 10.1056/NEJMoa2100433
9. Stone JH, Frigault MJ, Serling-Boyd NJ, Fernandes AD, Harvey L, Foulkes AS, et al. Efficacy of tocilizumab in patients hospitalized with covid-19. N Engl J Med (2020) 383(24):2333–44. doi: 10.1056/NEJMoa2028836
10. Rosas IO, Brau N, Waters M, Go RC, Hunter BD, Bhagani S, et al. Tocilizumab in hospitalized patients with severe covid-19 pneumonia. N Engl J Med (2021) 384(16):1503–16. doi: 10.1056/NEJMoa2028700
11. Gorham J, Moreau A, Corazza F, Peluso L, Ponthieux F, Talamonti M, et al. Interleukine-6 in critically ill COVID-19 patients: A retrospective analysis. PloS One (2020) 15(12):e0244628. doi: 10.1371/journal.pone.0244628
12. Kim HR, Hong MS, Dan JM, Kang I. Altered IL-7Ralpha expression with aging and the potential implications of IL-7 therapy on CD8+ T-cell immune responses. Blood (2006) 107(7):2855–62. doi: 10.1182/blood-2005-09-3560
13. Kang CK, Kim HR, Song KH, Keam B, Choi SJ, Choe PG, et al. Cell-mediated immunogenicity of influenza vaccination in patients with cancer receiving immune checkpoint inhibitors. J Infect Dis (2020) 222(11):1902–9. doi: 10.1093/infdis/jiaa291
14. Abu-Raddad LJ, Chemaitelly H, Ayoub HH, Yassine HM, Benslimane FM, Al Khatib HA, et al. Association of prior SARS-CoV-2 infection with risk of breakthrough infection following mRNA vaccination in Qatar. JAMA (2021) 326(19):1930–9. doi: 10.1001/jama.2021.19623
15. Han H, Ma Q, Li C, Liu R, Zhao L, Wang W, et al. Profiling serum cytokines in COVID-19 patients reveals IL-6 and IL-10 are disease severity predictors. Emerg Microbes Infect (2020) 9(1):1123–30. doi: 10.1080/22221751.2020.1770129
16. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in wuhan, China. Lancet (2020) 395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5
17. Group RC. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): A randomised, controlled, open-label, platform trial. Lancet (2021) 397(10285):1637–45.
18. Galvan-Roman JM, Rodriguez-Garcia SC, Roy-Vallejo E, Marcos-Jimenez A, Sanchez-Alonso S, Fernandez-Diaz C, et al. IL-6 serum levels predict severity and response to tocilizumab in COVID-19: An observational study. J Allergy Clin Immunol (2021) 147(1):72–80 e8. doi: 10.1016/j.jaci.2020.09.018
19. Siddiqi HK, Mehra MR. COVID-19 illness in native and immunosuppressed states: A clinical-therapeutic staging proposal. J Heart Lung Transplant. (2020) 39(5):405–7. doi: 10.1016/j.healun.2020.03.012
20. Ling L, Chen Z, Lui G, Wong CK, Wong WT, Ng RWY, et al. Longitudinal cytokine profile in patients with mild to critical COVID-19. Front Immunol (2021) 12:763292. doi: 10.3389/fimmu.2021.763292
21. Shin JJ, Jeon S, Unlu S, Par-Young J, Shin MS, Kuster JK, et al. A distinct association of inflammatory molecules with outcomes of COVID-19 in younger versus older adults. Clin Immunol (2021) 232:108857. doi: 10.1016/j.clim.2021.108857
22. Kapellos TS, Bonaguro L, Gemund I, Reusch N, Saglam A, Hinkley ER, et al. Human monocyte subsets and phenotypes in major chronic inflammatory diseases. Front Immunol (2019) 10:2035. doi: 10.3389/fimmu.2019.02035
23. Kong BS, Kim Y, Kim GY, Hyun JW, Kim SH, Jeong A, et al. Increased frequency of IL-6-producing non-classical monocytes in neuromyelitis optica spectrum disorder. J Neuroinflammation. (2017) 14(1):191. doi: 10.1186/s12974-017-0961-z
24. Vanderbeke L, Van Mol P, Van Herck Y, De Smet F, Humblet-Baron S, Martinod K, et al. Monocyte-driven atypical cytokine storm and aberrant neutrophil activation as key mediators of COVID-19 disease severity. Nat Commun (2021) 12(1):4117. doi: 10.1038/s41467-021-24360-w
Keywords: immune response, cytokine, IL-6, T cell, critical COVID-19
Citation: Lee CM, Kim M, Kang CK, Choe PG, Kim NJ, Bang H, Cho T, Shin HM, Kim H-R, Park WB and Oh M-d (2023) Different degree of cytokinemia and T-cell activation according to serum IL-6 levels in critical COVID-19. Front. Immunol. 14:1110874. doi: 10.3389/fimmu.2023.1110874
Received: 29 November 2022; Accepted: 27 March 2023;
Published: 04 April 2023.
Edited by:
Fabrice Cognasse, INSERM U1059 SAnté INgéniérie BIOlogie, FranceCopyright © 2023 Lee, Kim, Kang, Choe, Kim, Bang, Cho, Shin, Kim, Park and Oh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hyun Mu Shin, aHl1bm11LnNoaW5Ac251LmFjLmty; Hang-Rae Kim, aGFuZ3JhZTJAc251LmFjLmty; Wan Beom Park, d2JwYXJrMUBzbnUuYWMua3I=
†These authors have contributed equally to this work and share first authorship
‡These authors have contributed equally to this work and share senior authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.