- 1Department of Microbiology and Immunology, School of Basic Medical Sciences, Shanxi Medical University, Taiyuan, China
- 2Department of Psychiatry, First Hospital of Shanxi Medical University, Taiyuan, China
- 3Shanxi Key Laboratory of Artificial Intelligence Assisted Diagnosis and Treatment for Mental Disorders, First Hospital of Shanxi Medical University, Taiyuan, China
Objective: This study investigated the association between inflammatory cytokines and major depressive disorder.
Methods: Plasma biomarkers were measured by enzyme-linked immunosorbent assay (ELISA). Statistical analysis of baseline biomarkers in the major depression disorder (MDD) group and healthy controls (HC) group, and differences in biomarkers before and after treatment. Spearman analysis was performed to correlate baseline and after treatment MDD biomarkers with the 17-item Hamilton Depression Rating Scale (HAMD-17) total scores. Receiver operator characteristic (ROC) curves were analyzed for the effect of biomarkers on MDD and HC classification and diagnosis.
Results: Tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) levels were significantly higher in the MDD group than in the HC group, while high mobility group protein 1 (HMGB1) levels were significantly lower in the MDD group. The AUCs for HMGB1, TNF-α, and IL-6 were 0.375, 0.733, and 0.783, respectively, according to the ROC curves. MDD patients with brain-derived neurotrophic factor precursor (proBDNF) levels were positively correlated with total HAMD-17 scores. The levels of proBDNF levels were positively correlated with the total HAMD-17 score in male MDD patients, and brain-derived neurotrophic factor (BDNF) and interleukin 18 (IL-18) levels were negatively correlated with the total HAMD-17 score in female MDD patients.
Conclusion: Inflammatory cytokines are associated with the severity of MDD, and TNF-α and IL-6 have the potential as objective biomarkers to aid in the diagnosis of MDD.
Introduction
Major depressive disorder (MDD), or depression is a common and highly disabling mental disorder in which patients have a high risk of disability and a low quality of life (1). often occurring together with cardiovascular disease, diabetes, and autoimmune diseases (2, 3). MDD imposes a significant emotional and socioeconomic burden, and The World Health Organization estimates that 322 million people around the world from depression, which is about 4.4% of the global population (4).
MDD is highly heterogeneous in terms of clinical features and pathobiological alterations, which makes 1/3 of patients unresponsive or ineffective to conventional treatments (5). Furthermore, as the current diagnosis of MDD is based only on the symptom dimension, this makes the whole diagnostic process somewhat subjective and leads to a considerable risk of misdiagnosis and suboptimal treatment (6). Exploration of biomarkers as indicators of normal biological processes, pathogenic processes, or drug responses to therapeutic interventions may help identify homogeneous patients with MDD (7), such as those who may be involved in inflammatory phenotypes, so that individualized treatment plans can be developed for these patients to improve treatment rates.
The pathogenesis of MDD is extremely complex, and biomarker studies currently involve five biological systems, such as immunoinflammatory, neurotrophic, neurotransmitter, neuroendocrine and metabolic systems. The mechanism of immune inflammation has become a research hotspot in recent years, and since Ur (8) and others proposed the cytokine hypothesis, more and more studies have confirmed that MDD is accompanied by immune abnormalities (9). Early, Maes et al (10) found elevated inflammatory factors and CRP in patients with depressive disorders. Indoleamine 2, 3-dioxygenase (IDO), which decomposed tryptophan, can be activated by high levels of inflammatory factors. IDO’s metabolite quinolinic acid (QA) presents neuroexcitatory toxicity, leading to the reduction of a large number of markers of neuroplasticity, such as brain-derived neurotrophic factor (BDNF) levels, as well as affecting neurogenesis (11). This process may be associated with depression-like behavior in patients. In addition, inflammatory cytokines can stimulate the activation of the hypothalamic-pituitary-adrenal (HPA) axis and inhibit negative feedback loops, leading to hyperglucocorticoidemia. Elevated cortisol levels have been repeatedly shown to cause mood symptoms and are thought to be another potential link between inflammation and major depression (12).
An increasing number of studies have also found abnormalities in peripheral biomarkers in MDD both at baseline and after treatment (13, 14), such as cellular inflammatory factors and neurotropism. Also, antidepressants have been shown to reduce peripheral biomarkers such as IL-6, IL-10, and TNF-α (15), and anti-inflammatory drugs combined with antidepressants can reduce biomarkers such as CRP and TNF-α and improve depressive symptoms (16, 17). Khandaker et al. (18) suggested that elevated levels of inflammatory markers and others may be the etiology of MDD. Therefore, their exploration may not only add objective markers for clinicians to diagnose MDD but also obtain data about the effectiveness of treatment.
In this study, inflammatory factors, neurotrophic factors, and inflammatory proteins, such as TNF-α, IL-4, IL-6, IL-10, IL-18, IL-23, proBDNF, BDNF, hs-CRP, and HMGB1, were chosen as targets for investigation based on the association between immune inflammation, the nervous system, and MDD in this study (19). In order to better understand the diagnosis and treatment of MDD, it is important to find diagnostic biomarkers and therapeutic response biomarkers for MDD.
Methods
Participants
The 113 study cases were outpatients and inpatients admitted to the Department of Mental Health, First Hospital of Shanxi Medical University, from January 2019 to December 2021, of which 22 patients had longitudinal data after treatment. Inclusion criteria:1) aged 18-55 years old; 2) met the diagnostic criteria of “MDD (current episode)” in the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV); 3) the 17-item Hamilton Depression Rating Scale (HAMD-17) ≧ 17 at the time of enrollment; 4) had normal understanding ability; 5) patient meets the diagnosis and is willing to receive antidepressant treatment; 6) patients with a first episode or relapse who have not taken medication in the last month. Exclusion criteria:1) severe physical illnesses that could interfere with the study treatment; 2) pre-existing serious organic brain disease, serious mental illness (schizophrenia, etc.); 3) pregnant or lactating women, or planned pregnancy; 4) no previous convulsion-free electroconvulsive therapy (MECT).
Forty-one healthy volunteers recruited from surrounding communities during the same period were selected as the control group. Inclusion and exclusion criteria:1) age 18-55 years old; 2) no blood relationship with the patient; 3) no history of serious physical illness; 4) no history of psychiatric disorders and or family history; 5) non-pregnant or lactating women. This study was approved by the Ethics Committee, and all study participants signed an informed consent form.
Demographic data and clinical assessment
General information on enrolled subjects was collected using the self-administered observation of affective disorders scale. The HAMD-17 scale was used to evaluate the severity of MDD. Semi-structured interviews were conducted with the Mini-International Neuropsychiatric Interview (MINI) Chinese version.
Treatment
Fluoxetine and Fluvoxamine, two members of the selective 5-hydroxytryptamine reuptake inhibitors (SSRIs) class of medications, were primarily utilized for a 6-week therapy period. Each medication’s lowest effective therapeutic dosage, average therapeutic dose, and maximum effective therapeutic dose were given after one, two, and four weeks, respectively. Subjects would be assessed for HAMD-17 scores at baseline and after 6 weeks of treatment by the same follow-up physician.
Specimen collection, storage, and testing
5-6 ml of fasting venous blood was collected from study subjects at week 0 and week 6, blood samples were centrifuged at 3500 r/min for 10 min. The supernate was separated, extracted, and placed at -80°C for measurement. Marker assays included brain-derived neurotrophic factor precursor (proBDNF), brain-derived neurotrophic factor (BDNF), hypersensitive C-reactive protein (hs-CRP), high mobility group protein 1 (HMGB1), tumor necrosis factor-α (TNF-α), interleukin 4 (IL-4), interleukin 6 (IL-6), interleukin 10 (IL-10), interleukin 18 (IL-18), and interleukin 23(IL-23).
Instruments and reagents
The equipment used for the assay was ELx808 ELISA, the thermostat was Sanyo’s MIR-262, and the enzyme-linked immunosorbent assay (ELISA) was used to detect the biomarkers in plasma. (1) Kits: BDNF, TNF-α, IL-4, IL-6, IL-10, IL-18, IL-23, and HMGB1 are provided by Cloud-clone corp Wuhan. proBDNF and hs-CRP are provided by Shanghai Jianglai biotechnology. (2) Intra-batch coefficient of variation: BDNF, TNF-α, IL-4, IL-6, IL-10, IL-18, IL-23, HMGB1<10%, proBDNF, hs-CRP <9%. (3) Coefficient of variation between batches: BDNF, HMGB1, TNF-α, IL-4, IL-6, IL-10, IL-18, IL-23<12%, proBDNF, hs-CRP <11%. (4) Detection sensitivity: 0.1ng/mL for proBDNF, 11.3pg/mL for BDNF, 0.1mg/L for hs-CRP, 28.3pg/mL for HMGB1, 6.5pg/mL for TNF-α, 5.9pg/mL for IL-4, 3.2pg/mL for IL-6, 2.3pg/mL for IL-10 The results showed that IL-18 was 5.9 pg/mL and IL-23 was 3.1 pg/mL.
Statistical analysis
All data were statistically analyzed by SPSS26.0. Count data such as gender and marriage were tested by the χ2 test. The normal distribution of measurement data was tested by the Shapiro-Wilk test. The normal distribution was expressed as mean± SD (`x ± s) using the paired t-test and independent sample t-test was used. The non-normal distribution was represented as Median (IQR 25–75) employing the Wilcoxon signed-rank sum test or Mann-Whitney U test was used. Bivariate correlation analysis was performed using Spearman correlation. The ROC curve was employed to analyze the effect of biomarkers on the classification and diagnosis of MDD patients and HC. The scatter plot and ROC curve were plotted by GraphPad Prism 8.0. p<0.05 was considered as the level of statistically significant difference.
Results
Comparison of demographic data
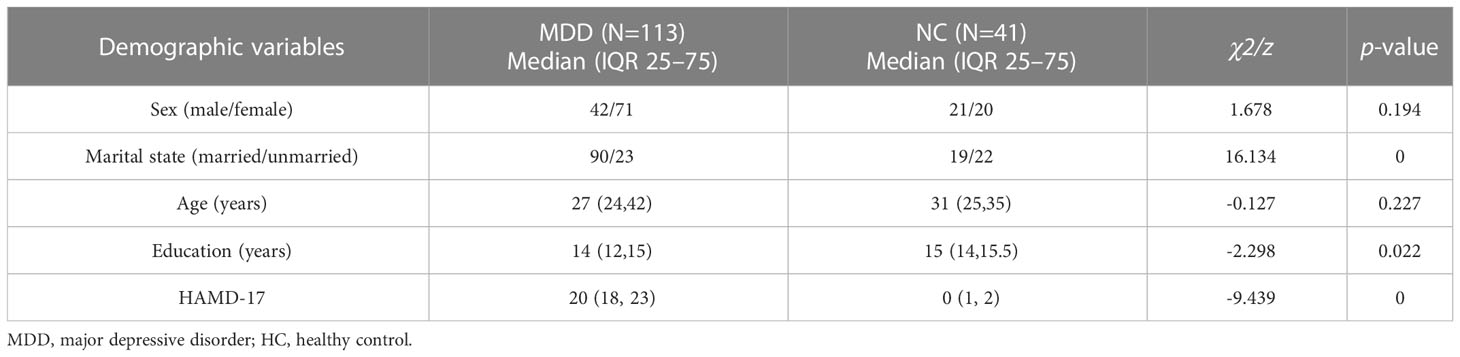
There were no statistically significant differences in gender (χ2 = 1.678, p=0.194) and age (z=-0.127, p=0.227) between the MDD group and the HC group, while there were statistically significant differences in terms of marriage (χ2 = 16.134, p<0.001), education years (z=-2.298, p=0.022), and in HAMD-17(z=-9.439, p<0.001) (p<0.05) (Table 1).
Comparison of baseline biomarkers
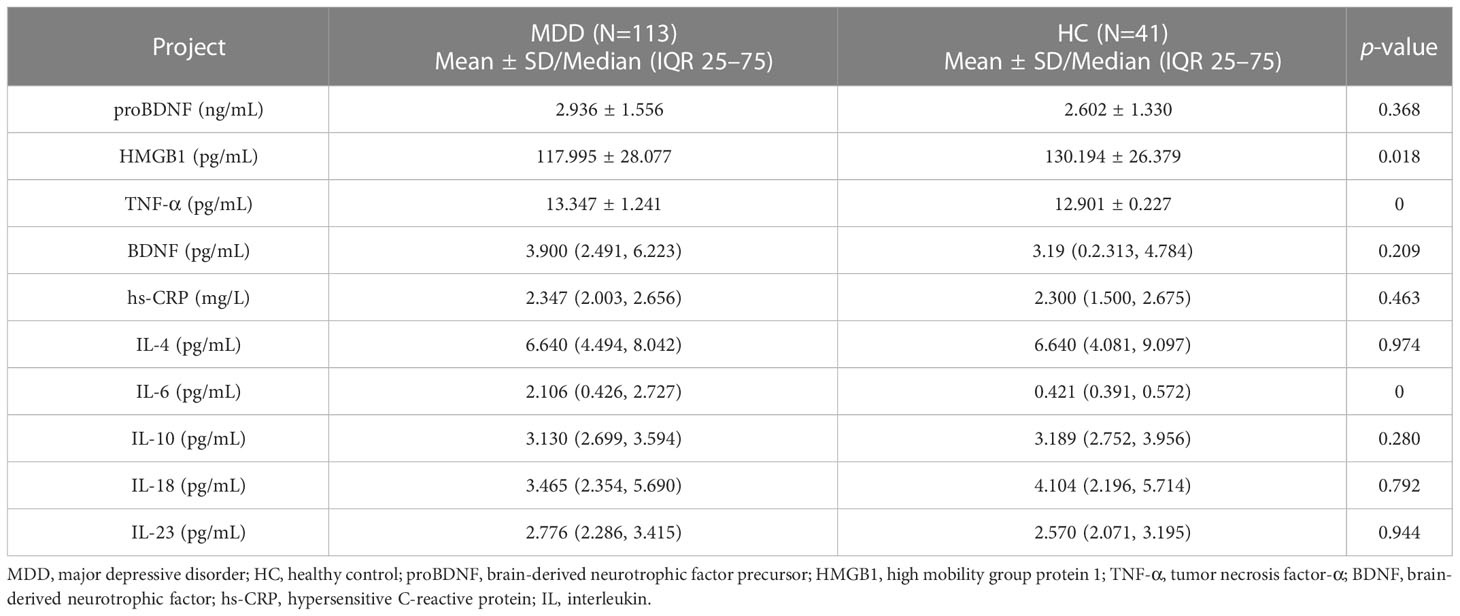
There were significant differences in plasma HMGB1 (t=-2.359, p=0.018), TNF-α (t=-4.431, p<0.001) and IL-6 (z=-5.372, p<0.001) levels between the MDD group and the HC group (Table 2). ROC curve results showed that the AUC of HMGB1, TNF-α and IL-6 were 0.375, 0.733 and 0.783, respectively (Figure 1).
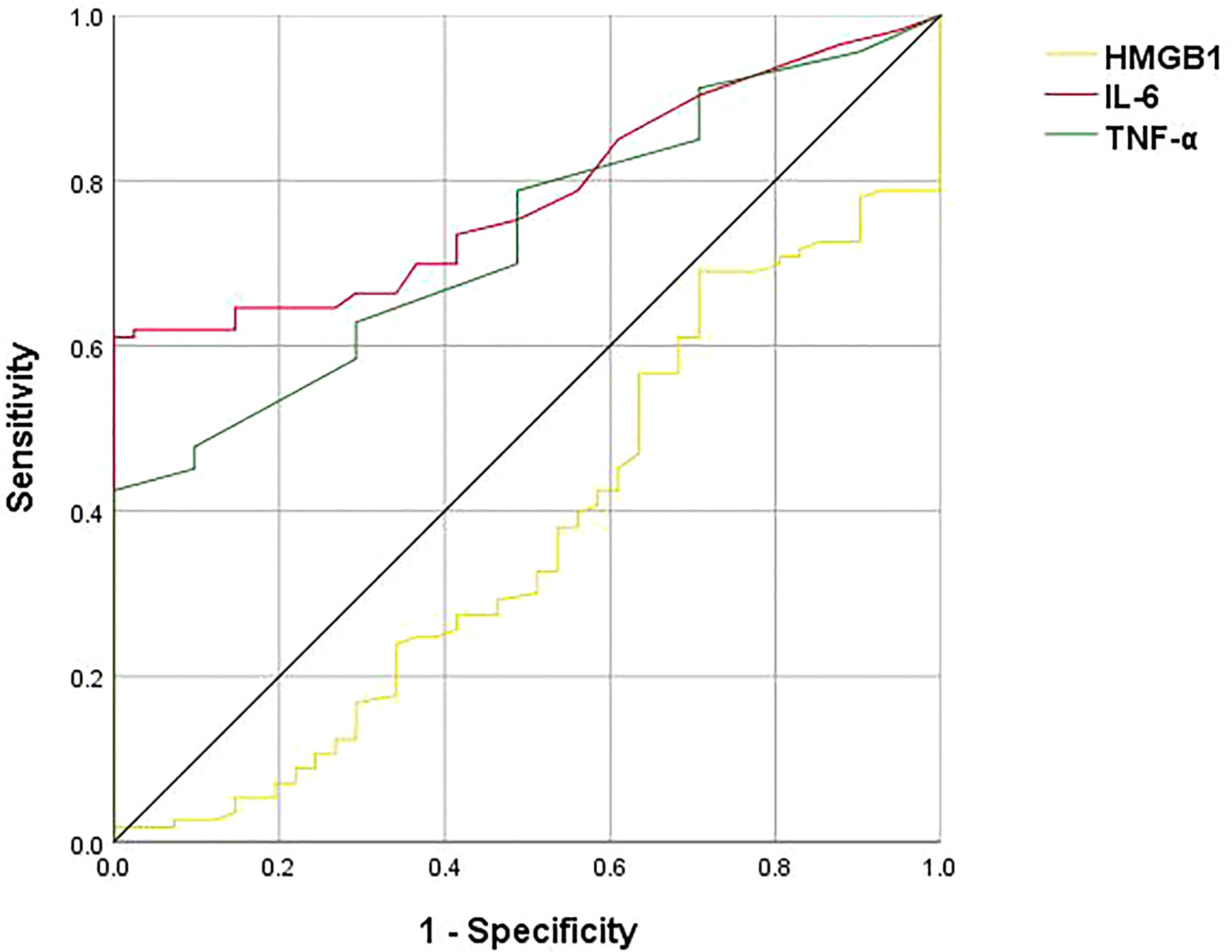
Figure 1 ROC curve analysis of HMGB1, TNF-α, IL-6, the black line represents HMGB1, the green line represents TNF-α, and the red line represents IL-6. The ROC curves showed that the AUCs of HMGB1, TNF-α, and IL-6 are 0.375, 0.733, and 0.783, respectively.
Correlation between baseline biomarkers and HAMD-17
Spearman correlation analysis showed a positive correlation between PROBDNF levels and total HAMD-17 score in all MDD patients (ρ=0.229, p<0.05). After gender grouping, there was a significant positive correlation between proBDNF and HAMD-17 scores in male MDD patients (ρ=0.400, p<0.05), a negative correlation between BDNF levels and HAMD-17 scores in female MDD patients (ρ=-0.261, p<0.05), and IL-18 levels and HAMD-18 scores in female MDD patients were negatively correlated (ρ=-0.244, p<0.05) (Figure 2).
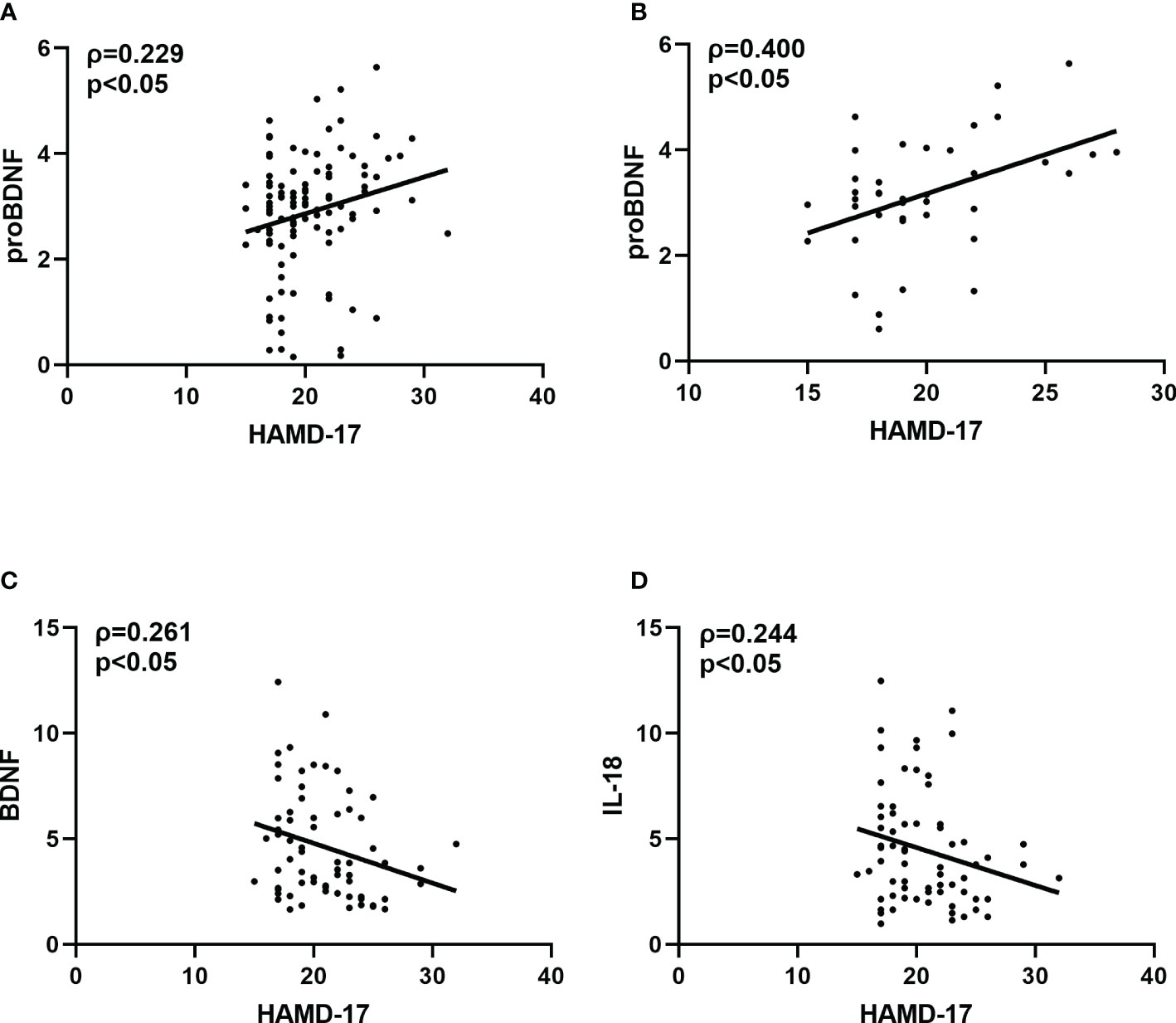
Figure 2 (A) Correlation between HAMD-17 and proBDNF in overall MDD patients. (B) Correlation between HAMD-17 and proBDNF in male MDD patients. (C) Correlation between HAMD-17 and BDNF in female MDD patients. (D) Correlation between HAMD-17 and IL-18 in female MDD patients. HAMD-17, 17-item Hamilton Depression Rating Scale proBDNF, brain-derived neurotrophic factor precursor; BDNF, brain-derived neurotrophic factor IL, interleukin.
Comparison of HAMD-17 baseline and after treatment
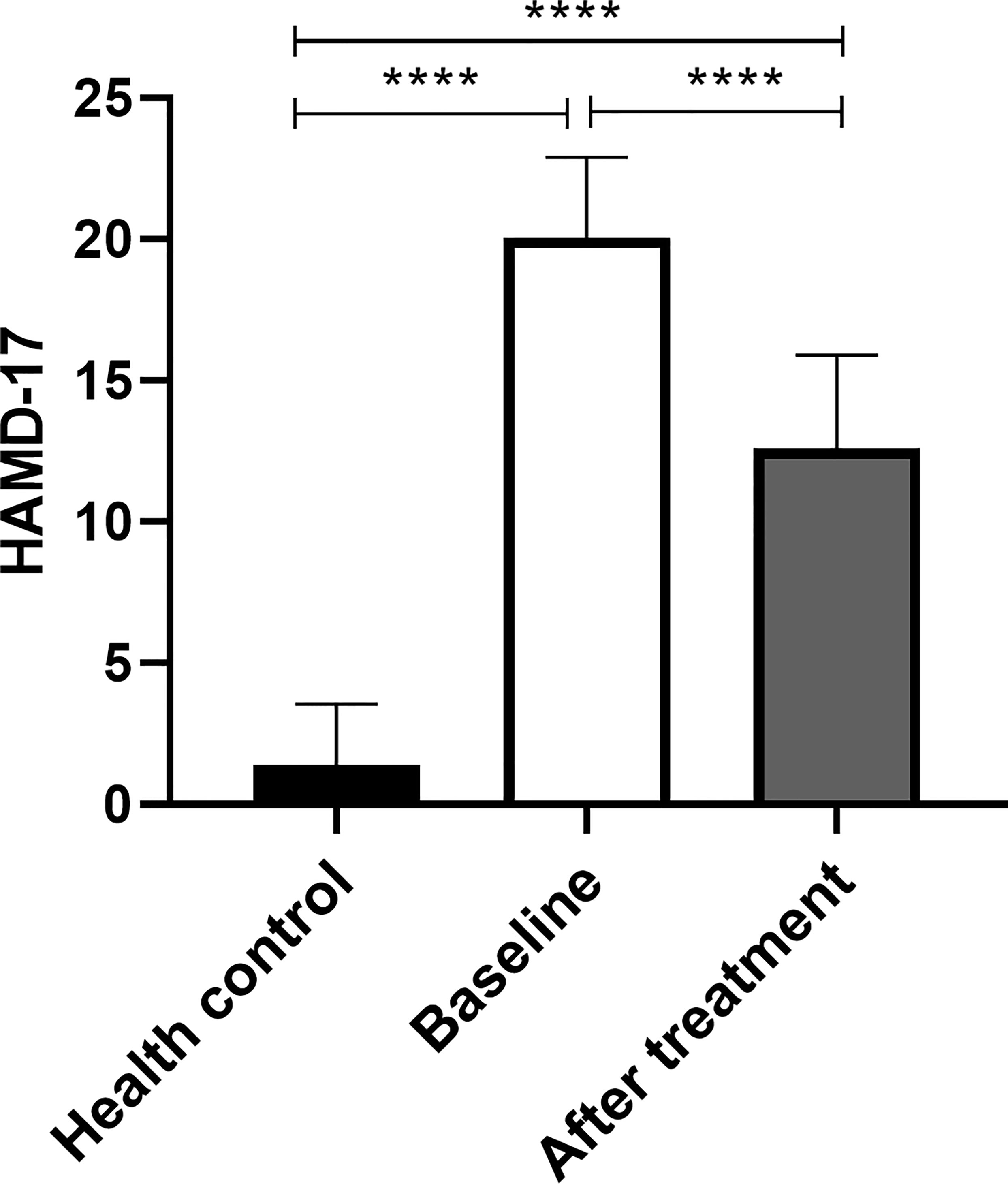
HAMD-17 score at the baseline of MDD was higher than that at HC, and the difference was statistically significant (p<0.05). HAMD-17 score after MDD treatment was lower than baseline, the difference was statistically significant (p<0.05). HAMD-17 score after MDD treatment was higher than that of HC, and the difference was statistically significant (p<0.05) (Figure 3).
Comparison of biomarkers baseline and after treatment
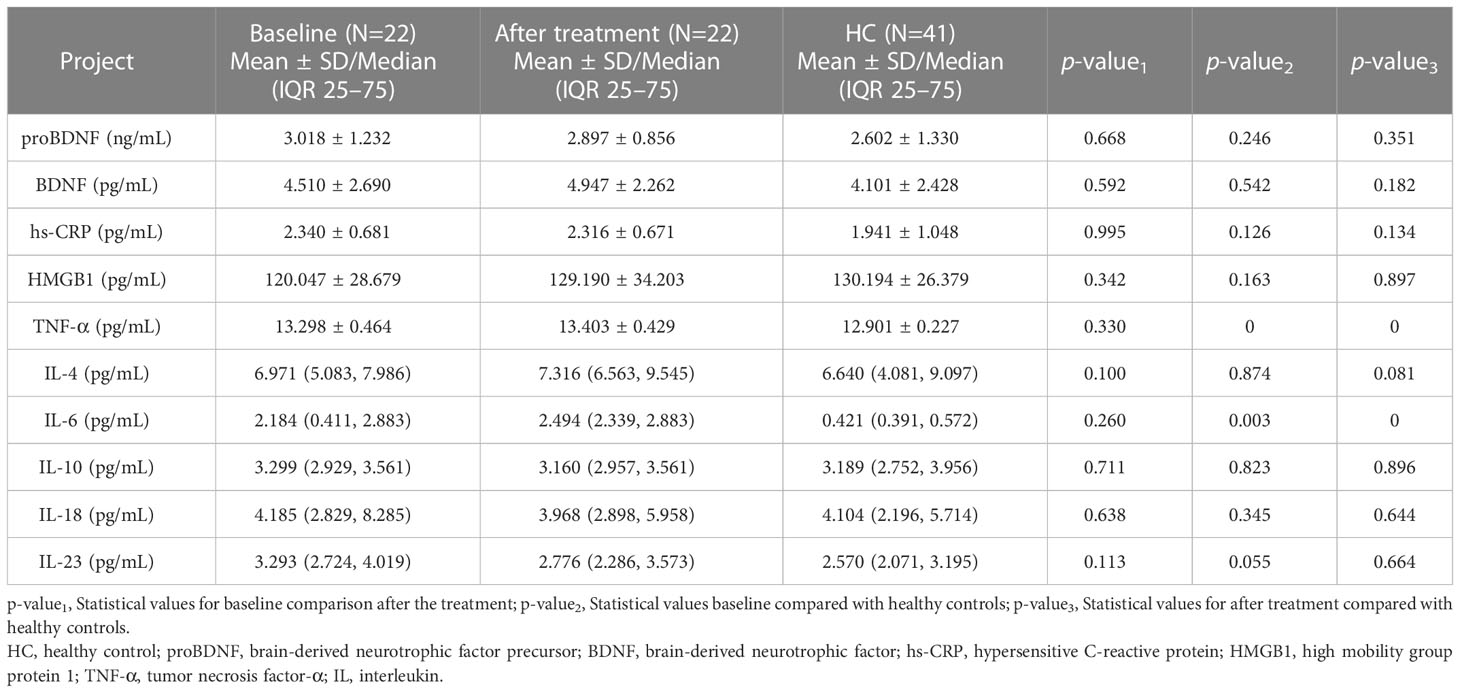
The levels of plasma TNF-α (t=4.580, p<0.001) and IL-6 (z=-2.996, p<0.001) in MDD patients baseline and after treatment were higher than those in healthy controls, with no significant difference in biomarkers between baseline and after treatment (Table 3).
Discussion
The findings of this study showed that MDD and HC had distinct baseline biomarkers, depression severity was associated with different biomarkers in MDD by gender, and that the levels of biomarkers remained unchanged after treatment.
This study discovered statistically significant differences in the levels of HMGB1, TNF-α, and IL-6 in patients with MDD at baseline compared to healthy controls. HMGB1, a late-stage inflammatory factor, can interact with early inflammatory factors such as interleukin and tumor necrosis factor. In addition, it can be released by different cell types such as tissue macrophages, astrocytes, and neurons, acting on microglia Mac-1 to mediate chronic neuroinflammation, leading to progressive neurodegeneration. Some studies have found that HMGB1 is involved in the development of several cognitive-emotional disorders and neurological diseases (20). According to animal research, raising extracellular HMGB1 levels in the hippocampus promoted depressive-like behavior by regulating microglia activation (21). Wang et al. (22) first reported an increase in both central and peripheral HMGB1 protein levels in a chronic unpredictable mild stress (CUMS)-induced depressive behavior model, while the present study found lower plasma HMGB1 levels in the MDD group than in normal controls at baseline. The differences in the above results are considered to be related to the different selection of study subjects. On the other hand, HMGB1, when released extracellularly, can bind to the surface receptors of intrinsic immune cells and activate a series of intracellular inflammatory response pathways, causing increased synthesis and release of inflammatory factors (23). Therefore, we hypothesize that the large amount of HMGB1 in MDD in this study bound to immune cell surface receptors, which in turn caused a further elevation of TNF-α and IL-6 levels. It should be noted that HMGB1 levels in the 113 MDDs in this study were lower than those in HC at baseline, whereas the treated 22 MDD patients did not differ significantly from HC before and after treatment. This disparity may be attributed to an insufficient number of patients, since only 22 MDDs’ longitudinal data were followed in this research owing to the high incidence of patient shedding. More patients should be followed up to validate the results.
TNF-α is a multifunctional signaling molecule with antiviral and immunomodulatory effects. IL-6 is mainly secreted by monocytes-macrophages, produced by Th2, and has functions such as regulating immune responses. TNF-α and IL-6 induce the production of indoleamine 2,3 -dioxygenase (IDO), leading to a decrease in tryptophan and the production of tryptophan metabolites, which are associated with depression (24). Several studies have found that plasma TNF-α and IL-6 levels are higher in MDD patients than in normal controls (25, 26). This is consistent with the results of the present report. The ROC curve results of this study showed that TNF-α and IL-6 can be used to identify and differentiate MDD and HC with good diagnostic and classification effects. Cytokines may be involved in depression pathogenesis by regulating monoamine neurotransmitter metabolism and influencing neuroendocrine function (27), therefore, TNF-α, and IL-6 levels may serve as quantitative indicators of MDD patients in their early stages and as objective biomarkers for the disease
High levels of inflammatory factors may mediate the effects of inflammation on the brain and are associated with the autonomic nervous system (19), and high inflammation levels decrease the number of neuroplasticity markers, such as BDNF levels and neurogenesis (11). In this study, the levels of inflammatory factors TNF-α, and IL-6 were elevated in the MDD group, and BDNF levels were not statistically significant compared to healthy controls, which was consistent with those of Sagud (28). However, several studies have shown lower BDNF levels in MDD than in controls (29, 30). In addition, CRP, a typical inflammatory factor, and whose levels can reflect the level of inflammation in the body, was not found to be increased in the present study, which is inconsistent with the findings of Howren et al. (31). The difference in the results of the above studies was considered to be due to the greater heterogeneity of MDD, as well as related to the fact that this study did not control for potential factors such as BMI and smoking.
Extracellular proteases can convert proBDNF to mature BDNF, which has opposite biological effects through the neurotrophic factor receptor p75 (p75NTR) and complex kinase receptor B (TrkB), respectively. These receptors are crucial to the pathophysiology of mood disorders and the therapeutic mechanisms of antidepressants and mood stabilizers (32). The correlation analysis of this study found a positive correlation between proBDNF levels and HAMD-17 scores in MDD patients. In addition, the present study considered various responses based on gender differences, and correlation analysis showed that proBDNF levels were favorably connected with HAMD-17 scores in male MDD patients, but BDNF levels and IL-18 levels were inversely correlated with HAMD-17 scores in female MDD patients. Previous studies have shown that age and gender have a significant effect on plasma cytokine levels (33). This disparity was attributed to psychological and biological differences (34), as well as the prevalence of female patients with MDD being higher than male patients in many studies (35, 36). This could be related to estrogen’s effect on women’s immune responses. The intricacy of immunology, neuroinflammation, and MDD is highlighted by these results, which also imply that diverse inflammation indicators may be the most useful tool for patient categorization and that future research on MDD biomarkers should take gender into account.
In this study, depressive symptoms were reduced in MDD patients after treatment, but plasma TNF-α and IL-6 levels were not statistically different from baseline or HC, whereas plasma pro-inflammatory factors were reduced in MDD patients treated with conventional antidepressants in a large number of studies but were not significantly different from healthy controls (37). The variations in the aforementioned findings might be attributed to the heterogeneity of MDD or the various antidepressant medication classes. Serotonergic antidepressants are believed to decrease Th2-mediated immune responses, while norepinephrine antidepressants are considered to suppress Th1-mediated immunological responses, according to Martino et al. (38) The 5-hydroxytryptaminergic antidepressants that were mostly utilized in this investigation may not suppress TNF-α and IL-6 that is released by Th1 cells, which may partially account for the lack of substantial changes between baseline and treatment-induced TNF-α and IL-6 levels. Currently, the neurotransmitter functions of norepinephrine and dopamine are the main targets of antidepressant treatment. The above findings suggest that in the search for new therapeutic targets for MDD, the role of inflammation and the immune system in the pathogenesis of MDD is growing, and we should recognize the immune-inflammatory phenotype of MDD to develop the best treatment plan for patients.
This study uses plasma, which has certain advantages, such as not being affected by disturbances caused by coagulation and technical problems caused by fibrin. However, there are certain limitations. First, the statistical analysis did not include gender, age, and BMI as covariates between groups, and therefore may have some influence on the results. Second, longitudinal outcomes before and after treatment were compared with the same previous control group and no placebo control group. This is not methodologically optimal. Finally, as a cross-sectional study, it did not elucidate the causal relationship between MDD and biomarkers.
In conclusion, the findings suggest that proBDNF, BDNF, and IL-18 are linked to clinical symptoms of MDD, and that TNF-α and IL-6 have the potential to serve as objective biomarkers for the diagnosis of MDD. Further studies on the relationship between more biomarkers and MDD are expected in the future to develop new and tailored diagnostic and therapeutic strategies for MDD patients.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Scientific research ethics review committee of Shanxi Medical University. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
Conceptualization: YW, XM. Data curation: XM, YC, PM, SL. Formal analysis: XM, XH. Funding acquisition: YW. Investigation: XH, YW. Methodology: GW, XM. Supervision: YW, GW. Writing-original draft: XM. Writing-review & editing: YW, GW, SL. All authors contributed to the article and approved the submitted version.
Funding
Financial support for this research was provided by the Scientific Research Foundation of Shanxi Provincial Health Commission (2022140),Shanxi 136 advanced Medical Engineering Project (Y2022136012), Public Scientific Research Project of Western Psychiatric Association of Sichuan Province (Wcpafund-201507), and National Key Research and Development Project (2016YFC1307103).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Amanuel A, Cristiana A, Foad A, Abdishakur A, Mubarek A, Semaw FA, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: a systematic analysis for the global burden of disease study 2016. LANCET (2017) 390:1211–59. doi: 10.1016/S0140-6736(17)32154-2
2. AlSaeed S, Aljouee T, Alkhawajah NM, Alarieh R, AlGarni H, Aljarallah S, et al. Fatigue, depression, and anxiety among ambulating multiple sclerosis patients. Front Immunol (2022) 13:844461. doi: 10.3389/fimmu.2022.844461
3. Windle M, Windle RC. Recurrent depression, cardiovascular disease, and diabetes among middle-aged and older adult women. J Affect Disord (2013) 150:895–902. doi: 10.1016/j.jad.2013.05.008
4. Friedrich MJ. Depression is the leading cause of disability around the world. JAMA (2017) 317:1517. doi: 10.1001/jama.2017.3826
5. Radua J, Grunze H, Amann BL. Meta-analysis of the risk of subsequent mood episodes in bipolar disorder. Psychother PSYCHOSOM (2017) 86:90–8. doi: 10.1159/000449417
6. Nobis A, Zalewski D, Waszkiewicz N. Peripheral markers of depression. J Clin Med (2020) 9. doi: 10.3390/jcm9123793
7. Biomarkers Definitions Working Group Bethesda, Md. Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther (2001) 69:89–95. doi: 10.1067/mcp.2001.113989
8. Ur E, White PD, Grossman A. Hypothesis: Cytokines may be activated to cause depressive illness and chronic fatigue syndrome. Eur Arch Psychiatry Clin Neurosci (1992) 241:317–22. doi: 10.1007/BF02195983
9. Beurel E, Toups M, Nemeroff CB. The bidirectional relationship of depression and inflammation: Double trouble. NEURON (2020) 107:234–56. doi: 10.1016/j.neuron.2020.06.002
10. Maes M. Evidence for an immune response in major depression: A review and hypothesis. Prog Neuropsychopharmacol Biol Psychiatry (1995) 19:11–38. doi: 10.1016/0278-5846(94)00101-M
11. Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol (2006) 27:24–31. doi: 10.1016/j.it.2005.11.006
12. Sigalas PD, Garg H, Watson S, McAllister-Williams RH, Ferrier IN. Metyrapone in treatment-resistant depression. Ther Adv Psychopharmacol (2012) 2:139–49. doi: 10.1177/2045125312436597
13. Strawbridge R, Arnone D, Danese A, Papadopoulos A, Herane VA, Cleare AJ. Inflammation and clinical response to treatment in depression: A meta-analysis. Eur Neuropsychopharmacol (2015) 25:1532–43. doi: 10.1016/j.euroneuro.2015.06.007
14. Osimo EF, Pillinger T, Rodriguez IM, Khandaker GM, Pariante CM, Howes OD. Inflammatory markers in depression: A meta-analysis of mean differences and variability in 5,166 patients and 5,083 controls. Brain Behav Immun (2020) 87:901–9. doi: 10.1016/j.bbi.2020.02.010
15. Kohler CA, Freitas TH, Stubbs B, Maes M, Solmi M, Veronese N, et al. Peripheral alterations in cytokine and chemokine levels after antidepressant drug treatment for major depressive disorder: Systematic review and meta-analysis. Mol Neurobiol (2018) 55:4195–206. doi: 10.1007/s12035-017-0632-1
16. Fourrier C, Sampson E, Mills NT, Baune BT. Anti-inflammatory treatment of depression: study protocol for a randomised controlled trial of vortioxetine augmented with celecoxib or placebo. TRIALS (2018) 19:447. doi: 10.1186/s13063-018-2829-7
17. Cao ZY, Liu YZ, Li JM, Ruan YM, Yan WJ, Zhong SY, et al. Glycyrrhizic acid as an adjunctive treatment for depression through anti-inflammation: A randomized placebo-controlled clinical trial. J Affect Disord (2020) 265:247–54. doi: 10.1016/j.jad.2020.01.048
18. Khandaker GM, Zuber V, Rees J, Carvalho L, Mason AM, Foley CN, et al. Shared mechanisms between coronary heart disease and depression: Findings from a large UK general population-based cohort. Mol Psychiatry (2020) 25:1477–86. doi: 10.1038/s41380-019-0395-3
19. Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry (2009) 65:732–41. doi: 10.1016/j.biopsych.2008.11.029
20. Takata K, Takada T, Ito A, Asai M, Tawa M, Saito Y, et al. Microglial amyloid-beta1-40 phagocytosis dysfunction is caused by high-mobility group box protein-1: Implications for the pathological progression of alzheimer's disease. Int J Alzheimers Dis (2012) 2012:685739. doi: 10.1155/2012/685739
21. Hisaoka-Nakashima K, Tomimura Y, Yoshii T, Ohata K, Takada N, Zhang FF, et al. High-mobility group box 1-mediated microglial activation induces anxiodepressive-like behaviors in mice with neuropathic pain. Prog Neuropsychopharmacol Biol Psychiatry (2019) 92:347–62. doi: 10.1016/j.pnpbp.2019.02.005
22. Wang B, Lian YJ, Su WJ, Peng W, Dong X, Liu LL, et al. HMGB1 mediates depressive behavior induced by chronic stress through activating the kynurenine pathway. Brain Behav Immun (2018) 72:51–60. doi: 10.1016/j.bbi.2017.11.017
23. Fang P, Schachner M, Shen YQ. HMGB1 in development and diseases of the central nervous system. Mol Neurobiol (2012) 45:499–506. doi: 10.1007/s12035-012-8264-y
24. Ng A, Tam WW, Zhang MW, Ho CS, Husain SF, McIntyre RS, et al. IL-1beta, IL-6, TNF- alpha and CRP in elderly patients with depression or alzheimer's disease: Systematic review and meta-analysis. Sci Rep (2018) 8:12050. doi: 10.1038/s41598-018-30487-6
25. Muller N, Krause D, Barth R, Myint AM, Weidinger E, Stettinger W, et al. Childhood adversity and current stress are related to pro- and anti-inflammatory cytokines in major depression. J Affect Disord (2019) 253:270–6. doi: 10.1016/j.jad.2019.04.088
26. Rowland T, Perry BI, Upthegrove R, Barnes N, Chatterjee J, Gallacher D, et al. Neurotrophins, cytokines, oxidative stress mediators and mood state in bipolar disorder: systematic review and meta-analyses. Br J Psychiatry (2018) 213:514–25. doi: 10.1192/bjp.2018.144
27. Lopes RP, Grassi-Oliveira R, de Almeida LR, Stein LM, Luz C, Teixeira AL, et al. Neuroimmunoendocrine interactions in patients with recurrent major depression, increased early life stress and long-standing posttraumatic stress disorder symptoms. NEUROIMMUNOMODULAT (2012) 19:33–42. doi: 10.1159/000327352
28. Sagud M, Nikolac PM, Vuksan-Cusa B, Maravic A, Svob SD, Mihaljevic PA, et al. A prospective, longitudinal study of platelet serotonin and plasma brain-derived neurotrophic factor concentrations in major depression: Effects of vortioxetine treatment. Psychopharmacol (Berl) (2016) 233:3259–67. doi: 10.1007/s00213-016-4364-0
29. Karlovic D, Serretti A, Jevtovic S, Vrkic N, Seric V, Peles AM. Diagnostic accuracy of serum brain derived neurotrophic factor concentration in antidepressant naive patients with first major depression episode. J Psychiatr Res (2013) 47:162–7. doi: 10.1016/j.jpsychires.2012.09.017
30. Huang TL, Lee CT, Liu YL. Serum brain-derived neurotrophic factor levels in patients with major depression: Effects of antidepressants. J Psychiatr Res (2008) 42:521–5. doi: 10.1016/j.jpsychires.2007.05.007
31. Ryan KM, McLoughlin DM. Peripheral blood inflammatory markers in depression: Response to electroconvulsive therapy and relationship with cognitive performance. Psychiatry Res (2022) 315:114725. doi: 10.1016/j.psychres.2022.114725
32. Hashimoto K. Brain-derived neurotrophic factor as a biomarker for mood disorders: an historical overview and future directions. Psychiatry Clin Neurosci (2010) 64:341–57. doi: 10.1111/j.1440-1819.2010.02113.x
33. Haack M, Hinze-Selch D, Fenzel T, Kraus T, Kuhn M, Schuld A, et al. Plasma levels of cytokines and soluble cytokine receptors in psychiatric patients upon hospital admission: effects of confounding factors and diagnosis. J Psychiatr Res (1999) 33:407–18. doi: 10.1016/S0022-3956(99)00021-7
34. Albert PR. Why is depression more prevalent in women? J Psychiatry Neurosci (2015) 40:219–21. doi: 10.1503/jpn.150205
35. Ramsey JM, Cooper JD, Bot M, Guest PC, Lamers F, Weickert CS, et al. Sex differences in serum markers of major depressive disorder in the Netherlands study of depression and anxiety (NESDA). PloS One (2016) 11:e156624. doi: 10.1371/journal.pone.0156624
36. Bartova L, Dold M, Fugger G, Kautzky A, Mitschek M, Weidenauer A, et al. Sex-related effects in major depressive disorder: Results of the European group for the study of resistant depression. DEPRESS Anxiety (2021) 38:896–906. doi: 10.1002/da.23165
37. Horowitz MA, Wertz J, Zhu D, Cattaneo A, Musaelyan K, Nikkheslat N, et al. Antidepressant compounds can be both pro- and anti-inflammatory in human hippocampal cells. Int J Neuropsychopharmacol (2014) 18. doi: 10.1093/ijnp/pyu076
Keywords: inflammatory cytokines, major depressive disorder, biomarkers, interleukins, immune
Citation: Min X, Wang G, Cui Y, Meng P, Hu X, Liu S and Wang Y (2023) Association between inflammatory cytokines and symptoms of major depressive disorder in adults. Front. Immunol. 14:1110775. doi: 10.3389/fimmu.2023.1110775
Received: 29 November 2022; Accepted: 31 January 2023;
Published: 13 February 2023.
Edited by:
Mark Slevin, Manchester Metropolitan University, United KingdomReviewed by:
Luca Sforzini, King’s College London, United KingdomPablo Romero-Sanchiz, University of Sussex, United Kingdom
Copyright © 2023 Min, Wang, Cui, Meng, Hu, Liu and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yanfang Wang, d2FuZ3lhbmZhbmcxMjM4NjhAMTYzLmNvbQ==
 Xue Min
Xue Min Genwei Wang1
Genwei Wang1 Xiaodong Hu
Xiaodong Hu Yanfang Wang
Yanfang Wang