- 1Senior Department of Infectious Diseases, The Fifth Medical Center of Chinese People's Liberation Army (PLA) General Hospital, National Clinical Research Center for Infectious Diseases, Beijing, China
- 2Department of Infectious Disease and Hepatology, The Second Hospital, Cheeloo College of Medicine, Shandong University, Jinan, China
- 3Senior Department of Hepatology, The Fifth Medical Center of Chinese People's Liberation Army (PLA) General Hospital, Beijing, China
- 4Center for Reproductive Medicine, Department of Obstetrics and Gynecology, Qilu Hospital of Shandong University, Jinan, China
Introduction: CD11c+CD8+ T cells are an unconventional CD8+ T cell subset that exerts antiviral activity in infectious diseases. However, its characteristics in hepatocellular carcinoma (HCC) have not been elucidated.
Methods: Twenty-six patients with hepatitis B virus (HBV)-related HCC and 25 healthy controls (HC) were enrolled. The frequency and phenotype of CD11c+CD8+ T cells in peripheral blood and tumors in situ were detected by flow cytometry and immunohistochemistry.
Results: Both the HCC group and HC group had similar frequency and phenotype characteristics of CD11c+CD8+ T cells in the periphery. CD11c+CD8+ T cells were mainly composed of effector T cells, most of which were CD45RA+CCR7-. Compared with CD11c-CD8+ T cells, CD11c+CD8+ T cells had a higher proportion of CD38 and HLA-DR double positive, and expressed high levels of granzyme-B (GB) and degranulation marker CD107a, and produced high levels of interleukin-2 (IL-2), tumor necrosis factor alpha (TNF-α) and interferon-gamma (IFN-γ). However, the ability of degranulation and TNF-α production of CD11c+CD8+ T cells in patients with HCC were significantly lower than that in healthy controls. The GB expression level of peripheral CD11c+CD8+ T cells in patients with advanced stage of HCC was significantly lower than that in patients with early stage of HCC, and the GB expression level of liver-infiltrating CD11c+CD8+ T cells in tumor tissues was lower than that in non-tumor tissues. More importantly, the GB expression level of peripheral CD11c+CD8+ T cells was negatively correlated with tumor volume.
Conclusions: These findings indicate that CD11c+CD8+ T cells may have potential anti-tumor activity and that GB+CD11c+CD8+ T cells are associated with disease progression in patients with HBV-related HCC.
Introduction
Chronic hepatitis B virus (HBV) infection can lead to liver cirrhosis and liver failure and accounts for more than 80% of hepatocellular carcinoma (HCC) cases worldwide (1, 2). Viral clearance and tumor cell clearance are largely dependent on robust CD8+ T cell responses (3); however, T cell exhaustion occurs in various situations, leading to the loss of their ability for cytokine production or cytotoxicity against tumor cells (4–6). Moreover, cell exhaustion is also closely related to increased levels of exhaustion-related immune molecules, such as T cell Ig and ITIM domain and programmed cell death 1 (PD-1), as well as disease progression (5). Based on recent advances in CD8+ T cell anti-tumor therapy, some unconventional CD8+ T cell subsets have been identified to help reduce the tumor burden and inhibiting tumor progression (7).
CD11c is a member of the β2 integrin family, is highly expressed in dendritic cells, and is normally expressed in some B lymphocytes, CD8+ T cell subsets, and natural killer cells (8, 9). Previous studies have found that CD11c plays an important regulatory role in the proliferation and function of CD4+ and CD8+ T cells (10). Acute viral, bacterial, and parasitic infections lead to upregulation of the frequency of CD11c+CD8+ T cells, and this CD8+ T cell subset displayed characteristics of effector cells, including high activation and cytotoxic capacity to eliminate pathogenic microbes, whereas CD11c-CD8+ T cells did not (11–13).
The secretion of cytokines, such as interleukin-2 (IL-2), tumor necrosis factor alpha (TNF-α), interferon-gamma (IFN-γ), and cytolytic granules, such as granzyme B (GB), are the main functional attributes of CD8+ T cells that exhibit anti-tumor activity (14, 15). Moreover, tumor-infiltrating CD8+ T cells in colorectal cancer and lymphoma with CD11c upregulation reflect the tumoricidal efficacy (8, 16–18). The expression level of CD11c on CD8+ T cells could be a potential marker for evaluating tumoricidal cytotoxic T lymphocytes to predict the efficacy of anti-tumor immunotherapies (16). Although CD11c+CD8+ T cells may be potential therapeutic targets for tumors, their roles in patients with HCC remain unclear.
In this study, the phenotype and function of CD11c+CD8+ T cells in HCC patients and healthy controls (HCs) were evaluated, and the relationship between the number of CD11c+CD8+ T cells in both peripheral tissues and tumors in situ and HCC disease progression was further explored.
Materials and methods
Study participants
Twenty-six HCC patients and 25 HCs from the Fifth Medical Center of the Chinese PLA General Hospital were included in this study (detailed information of the participants was shown in Table 1). Peripheral blood samples were obtained from all participants, and tumor and non-tumor tissues (relatively normal liver tissue surrounding the tumor with no obvious tumor tissue observed on macroscopic observation) were obtained from nine HCC patients. Patients with concurrent hepatitis C virus and human immunodeficiency virus infections, and autoimmune or alcoholic liver disease were excluded from the study. Informed consent was obtained from all the participants in accordance with the Declaration of Helsinki. This study was approved by the Institutional Review Board of the Fifth Medical Center of the Chinese PLA General Hospital (KY-2022-4-16-1).
Sample processing and storage
Peripheral blood mononuclear cells were isolated using Ficoll-Paque PLUS (GE Healthcare, Piscataway, NJ, USA) density gradient centrifugation and used directly in flow cytometry experiments. Freshly excised tumor tissue was fixed in 4% paraformaldehyde, embedded in paraffin, and stored in a refrigerator at -20°C.
Flow cytometry
Peripheral blood mononuclear cells or mononuclear cells isolated from liver tissue were stained with the following antibodies for phenotypic staining: CD3-APC/Cy7, CD8-BV510, CD8-Percp, CD11c-FITC, CD11c-PE, PD-1-APC, HLA-DR-BV421, CCR7-FITC, and CD45RA-BV510. After permeabilization using a Cytofix/Cytoperm Kit (BD Biosciences, Franklin Lakes, NJ, USA), cells were used for intracellular cytokine staining using an anti-GB-PE antibody. Anti-IL-2, anti-IFN-γ, and anti-TNF-α antibodies were used to stain intracellular cytokines after stimulation with ionomycin (1 μM) and phorbol-12-myristate-13-acetate (PMA) (100 ng/mL) for 6~8 h at 37°C in the presence of 5% CO2. The anti-CD107a antibody was added at the same time as the stimulation. The flow cytometry antibodies were obtained from BioLegend (San Diego, CA, USA).
Immunohistochemical staining
Paraffin-embedded and formalin-fixed liver tissue specimens was cut into 5 μm sections and placed on polylysinecoated slides. Then, the liver tissue sections were chosen to bake, deparaffinize, block the endogenous peroxidase activity with 0.3% H2O2 and heat-induce epitope retrieval. Following a brief wash and rinse in phosphate buffered saline, sections were stained with primary antibodies including mouse-anti-human CD11c and rabbit-anti-human CD8 (ZSGBBIO, Beijing, China) separately overnight at 4°C. Double staining was performed by using the HRP secondary antibody (ZSGB-BIO, China) for CD8 (blue color) and CD11c (brown color). The nuclei was stained with hematoxylin and the cytoplasm was stained with eosin. Images (100× and 400×) were acquired using Olympus CX31 microscope and Aperio VERSA 8 scanning system (Leica Microsystems, Wetzlar, Germany).
Statistical analysis
Categorical variables were expressed as frequencies or proportions. Quantitative variables were expressed as medians and quartiles. Chi-square tests (for classified variables) and nonparametric tests (for quantitative variables) were used to compare the characteristics between the two groups (Table 1). The Wilcoxon paired test and Mann-Whitney U test were used to compare the median values between the two groups. Spearman’s correlation coefficient was used to assess the correlation between the two variables. The data were analyzed using SPSS 25.0 and GraphPad Prism 9.0 (GraphPad Software, San Diego, CA, USA). Statistical significance was defined as P < 0.05.
Results
Characteristics of peripheral CD11c+CD8+ T cells
To determine the differentiation characteristics of CD11c+CD8+ T cells, CD45RA and CCR7 were stained in the CD8+ T cell populations. In both the HCC and HC groups, CD11c+CD8+ T cells mainly showed an effector phenotype (CD45RA+CCR7-), while CD11c-CD8+ T cells were mostly effector memory phenotypes (Figure 1A). We further analyzed the activation and exhaustion phenotype, intracellular cytokine expression, cytolytic granule GB expression, and degranulation marker CD107a in CD11c+CD8+ and CD11c-CD8+ T cells. Compared with CD11c-CD8+ T cells, CD11c+CD8+ T cells exhibited a highly activated and cytotoxic phenotype, with upregulated expression of CD38&HLA-DR, GB, and CD107a (Figures 1B, D, E). There were no significant differences in PD-1 expression between CD11c+CD8+ and CD11c-CD8+ T cells (Figure 1C). We also found that CD11c+CD8+ T cells produced higher levels of IL-2, IFN-γ, and TNF-α after PMA stimulation than the CD11c-CD8+ T cells did (Figures 1F–H). Representative flow cytometry plots of CD11c-CD8+ and CD11c+CD8+ T cells were shown in Supplementary Figure 1.
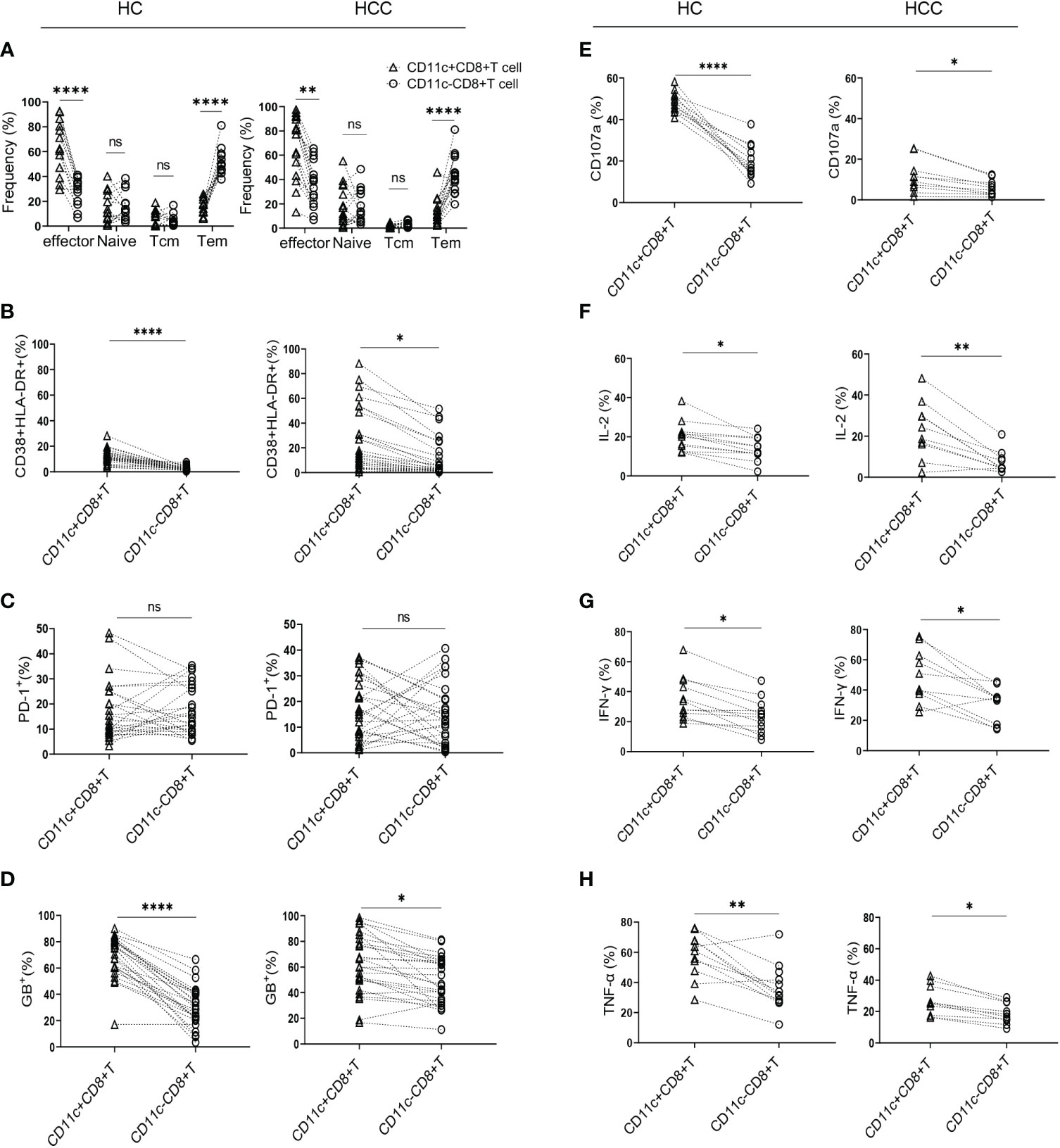
Figure 1 Characteristics of CD11c+CD8+ T cells in HCC and HC groups. (A) The frequency of effector memory (CCR7-CD45RA-) (Tem), central memory (CCR7+CD45RA-) (Tcm), naïve (CCR7+CD45RA+), and effector (CCR7-CD45RA+) subsets of CD11c+CD8+ and CD11c-CD8+ T cells in HCC (n = 16) and HC (n = 13) groups. (B–D) The levels of CD38&HLA-DR, PD-1, and GB expression of CD11c+CD8+ and CD11c-CD8+ T cells in HCC (n = 26) and HC (n = 25) groups. (E–H) After PMA stimulation, the level of CD107a expression and IL-2, IFN-γ, and TNF-α production of CD11c+CD8+ and CD11c-CD8+ T cells in HCC (n = 10) and HC (n = 12) groups. P values were generated using the Wilcoxon paired test between the two groups. *P < 0.05; **P < 0.01; ****P < 0.001; ns, not significant.
Comparison of CD11c+CD8+ T cells between the HCC and HC groups
There was no significant difference in the frequency of CD11c+CD8+ T cells and the levels of CD38&HLA-DR, PD-1, and GB expression of CD11c+CD8+ T cells between the HCC and HC groups (Figures 2A–D). We then compared the degranulation and cytokines production of CD11c+CD8+ T cells between the two groups. We found that the CD107a and TNF-α produced by CD11c+CD8+ T cells in HCC group were significantly decreased compared with that in HC group, while IL-2 and IFN-γ levels between the groups were comparable (Figures 2E–H).
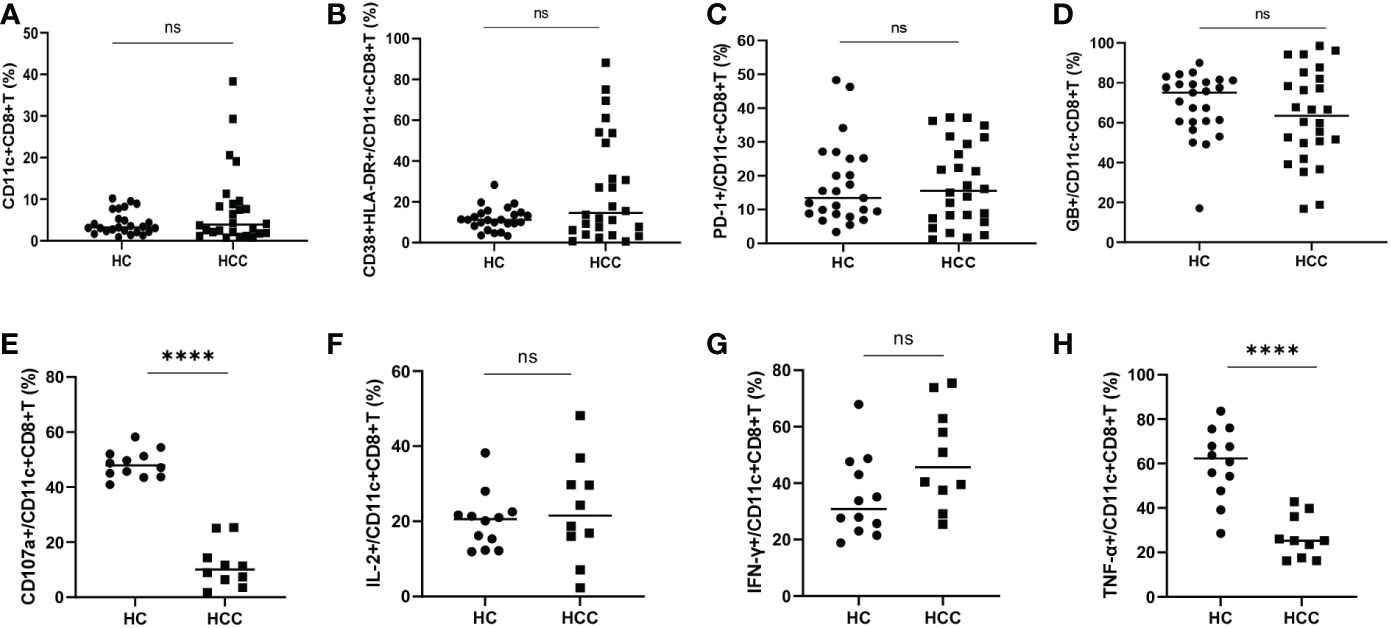
Figure 2 Frequency of CD11c+CD8+ T cells in HCC and HC groups. (A) The frequency of CD11c+CD8+ T cells in HCC (n = 26) and HC (n = 25) groups. (B–D) The levels of CD38&HLA-DR, PD-1, and GB expression of CD11c+CD8+ T cells in HCC (n = 26) and HC (n = 25) groups. (E–H) After PMA stimulation, the level of CD107a expression and IL-2, IFN-γ, and TNF-α production of CD11c+CD8+ T cells were detected in HCC (n = 10) and HC (n = 12) groups. The Mann-Whitney U test was used to compare the median values between the two groups. **** P < 0.001; ns, not significant.
The distribution characteristics of CD11c+CD8+ T cells in tumor tissues
To compare the characteristics of CD11c+CD8+ T cells in peripheral blood and liver tissue, mononuclear cells from the peripheral blood, tumor tissues, and non-tumor tissues of nine HCC patients were isolated. We found that the frequency of CD11c+CD8+ T cells in tumor and non-tumor tissues was significantly higher than that in the peripheral blood, and the difference between tumor and non-tumor tissues was not significant (Figure 3A). Notably, CD11c+CD8+ T cells in tumor tissues had increased expression of CD38&HLA-DR, and PD-1, and decreased expression of GB, compared with those in peripheral blood and non-tumor tissues (Figures 3B–D). These data indicate that CD11c+CD8+ T cells in tumor in situ may be in a state of high activation, exhaustion, and decreased killing capacity. The distribution of CD11c+CD8+ T cells in the liver tissue of patients with HCC was further examined by immunohistochemical staining. As shown in Figures 3E, F, CD11c+CD8+ T cells (black arrow) were mainly distributed at the boundary between the tumor and para-tumor tissues.
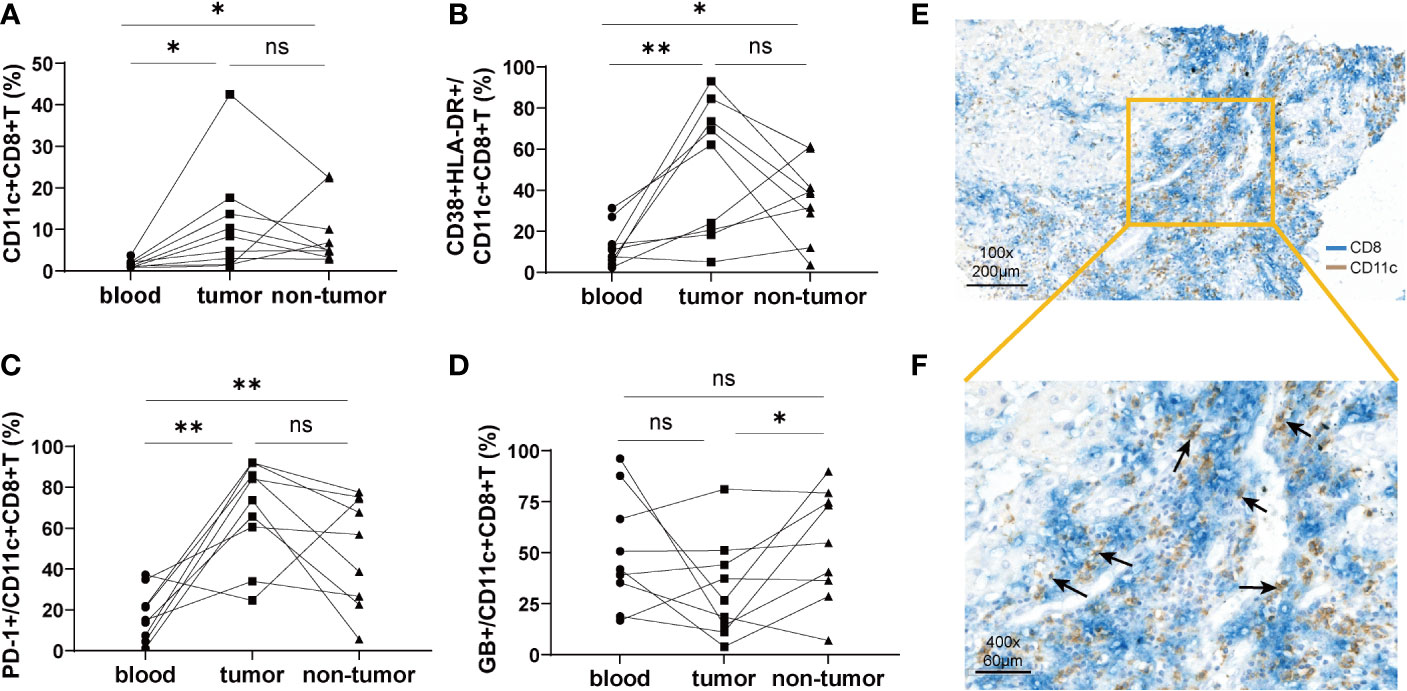
Figure 3 The distribution of CD11c+CD8+ T cells in tumor tissue and paired non-tumor tissue. (A) The frequency of CD11c+CD8+ T cells in the peripheral blood, tumor, and non-tumor tissues. (B–D) Paired analysis of the CD38&HLA-DR, PD-1, and GB expression on the CD11c+CD8+ T cells in the peripheral blood, tumor, and non-tumor tissues. (E, F) Representative results of immunohistochemical double staining of CD11c (brown) and CD8 (blue) from a patient with HCC. The orange square indicates the demarcation area between cancer and para-cancerous tissue. The black arrows point to the CD11c+CD8+ T cells. The data were from 9 HCC patients who have matched peripheral blood, tumor and non-tumor tissues. P values were generated using the Wilcoxon paired test between the two groups. *P < 0.05; **P < 0.01; ns, not significant.
The GB expression level of CD11c+CD8+ T cells correlates with disease progression of HCC
According to the standard for diagnosis and treatment of primary liver cancer (2022 edition) (19), a total of 26 HCC patients were divided into an early-stage group (stages Ia, Ib, and IIa; n = 12) and an advanced-stage group (stages IIb and IIIa; n= 14). There was no significant difference in the frequency of CD11c+CD8+ T cells and the levels of CD38&HLA-DR, and PD-1 expression on CD11c+CD8+ T cells between the early- and advanced-stage groups (Figures 4A–C). Meanwhile, we found that the GB expression level of CD11c+CD8+ T cells in the early-stage group was significantly higher than that in the advanced-stage group (P = 0.001) (Figure 4D). We further explored the relationship between tumor volume and the GB expression level of CD11c+CD8+ T cells in patients with HCC. Referring to a previous study, tumor volume was calculated using the equation: volume = 0.52 × width2 × length (20). We found that the GB expression level of CD11c+CD8+ T cells in peripheral blood was negatively correlated with tumor volume (r = -0.581, P = 0.002) (Figure 4E). However, there was no significant correlation between the GB expression level of CD11c+CD8+ T cells in tumor and non-tumor tissues and tumor volume (Figures 4F, G).
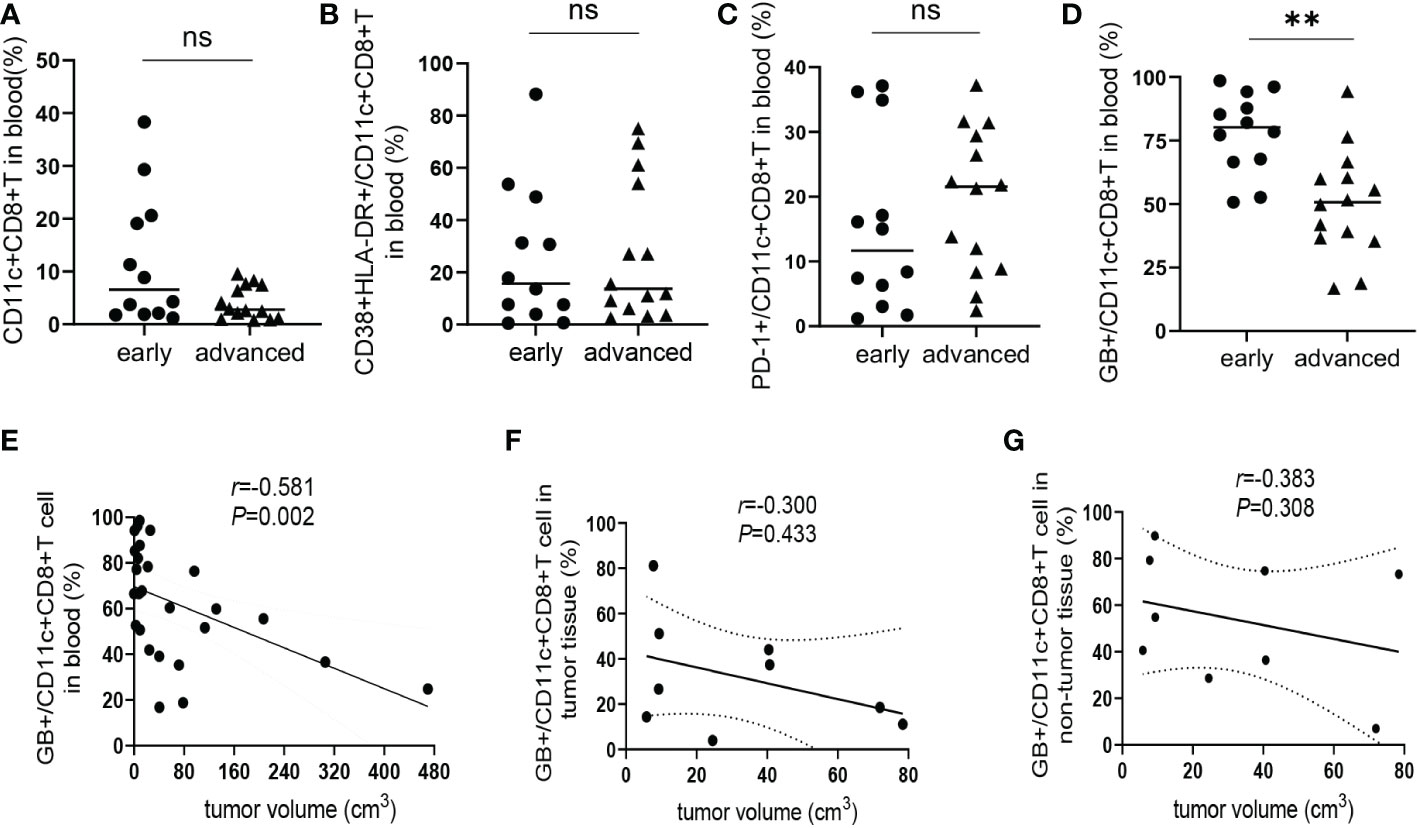
Figure 4 Correlation between CD11c+CD8+ T cells with estimated tumor volume. (A) The frequency of CD11c+CD8+ T cells in the peripheral blood of early-stage group (n = 12) and advanced-stage group (n = 14) of HCC patients. (B–D) The levels of CD38&HLA-DR, PD-1, and GB expression of peripheral CD11c+CD8+ T cells of early-stage group (n = 12) and advanced-stage group (n = 14) of HCC patients. (E) The correlation analysis between GB expression level in peripheral CD11c+CD8+ T cells and tumor volume of HCC patients (n = 26). (F, G) The correlation analysis between tumor volume and GB expression level in tumor and non-tumor infiltrating CD11c+CD8+ T cells of HCC patients (n = 9), respectively. The Mann-Whitney U test was used to compare the median values between the two groups. Spearman’s correlation coefficient was used to assess the correlation between the two variables. r, correlation coefficient. **P < 0.01; ns, not significant.
Discussion
Tumoricidal cytotoxic T lymphocytes play an important role in viral eradication and elimination of tumors (7, 21). The effector CD8+ T cells are characterized by low exhaustion and high activation and proliferation (22). Previous studies have reported that CD11c+CD8+ T cells have a high proportion of antigen-specific effector cells with increased frequency following acute or chronic viral infections, such as human immunodeficiency virus type 1 or herpes simplex virus type 1 infection, and may have antiviral effects (13, 16, 23, 24). However, there are few studies on CD11c+CD8+ T cells in HBV-related HCC. In this study, we found that CD11c+CD8+ T cells of HCC patients mostly belong to effector cell subsets with higher expression levels of activated markers CD38&HLA-DR. Additionally, in both HCC and HC groups, CD11c+CD8+ T cells showed higher level of GB expression, as well as stronger ability of degranulation and IL-2, IFN-γ, and TNF-α production than CD11c-CD8+ T cells. Collectively, these findings indicate that CD11c+CD8+ T cells may be a multifunctional effector subset.
In this study, we found that the frequency of CD11c+CD8+ T cells in both tumor tissues and non-tumor tissues were significantly higher than that in peripheral blood. Combined with the data from phenotypic studies, we hypothesized that the accumulation of a large number of CD11c+CD8+ T cells in tumors is conducive to tumor control. A previous study found that CD8+ T cells in the tumor microenvironment have high expression levels of exhausted molecules, such as PD-1 and ITIM domains, were prone to exhaustion (25), and influence disease progression and clinical outcome (26). However, PD-1 expression is also significantly increased in activated T cells, which could be a functional effector T cell molecule, representing its active functional state (27, 28). Interestingly, our data showed that activation markers, such as CD38&HLA-DR, were highly expressed in CD11c+CD8+ T cells in tumor tissues, as well as PD-1, compared to that of CD11c+CD8+ T cells in peripheral blood. This suggests that CD11c+CD8+ T cells in the liver tumor tissues may be in a state of overactivation and exhaustion. The overactivation of immune cells usually results in increased apoptosis and decreased function. Consistent with this hypothesis, we found that the expression of GB in CD11c+CD8+ T cells in tumor tissues was significantly lower than that in non-tumor tissues.
CD107a is a marker for degranulation of intracellular cytotoxic granules. We found that the levels of CD107a expression of CD11c+CD8+ T cells after PMA stimulation in HCC group were significantly lower than that of HC group, suggested that the capacity of GB-secreting of CD11c+CD8+ T cells in HCC patients was decreased. Consistently, the capacity of TNF-α production of CD11c+CD8+ T cells was also significantly decreased in patients with HCC than in healthy controls. Most importantly, we found that the GB expression level of peripheral CD11c+CD8+ T cells in patients with advanced HCC was significantly lower than in patients with early-stage HCC. Moreover, GB+CD11c+CD8+ T cells in peripheral blood were negatively correlated with tumor volume. These findings suggest that the decline in the number and function of CD11c+CD8+ T cells may an important factor promoting the progression of HCC. In addition, our study is the first to observe that CD11c+CD8+ T cells were mainly distributed over the boundary of cancerous and para-cancerous tissues in the livers of patients with HCC.
This study has some limitations. First, the small sample size and lack of long-term follow-up in this cross-sectional study did not validate the role of CD11c+CD8+ T cells in predicting HCC progression. Second, there is a lack of antigen-specific tests for the number and function of CD11c+CD8+ T cells, as well as in vivo validation. Third, the exact mechanisms of the changes in the number and function of tumor-infiltrating CD11c+CD8+ T cells needs to be further explored.
In summary, we demonstrated that CD11c+CD8+ T cells were highly activated effector cells, and showed increased PD-1 and decreased GB expression in tumor tissues. Moreover, we found that the GB expression level of CD11c+CD8+ T cells was negatively correlated with tumor volume and associated with HCC progression. Therefore, CD11c+CD8+ T cells may be a potential prognostic marker and therapeutic target for patients with HBV-related HCC.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
This study was approved by the institutional review board of the Fifth Medical Center of Chinese PLA General Hospital (KY-2022-4-16-1). The patients/participants provided their written informed consent to participate in this study.
Author contributions
LG and ZH performed the literature search, conducted laboratory experiments, analyzed data, produced the figures, wrote the manuscript. GL and A-LG conducted laboratory experiments and analyzed data. F-SW assisted with data interpretation and edited the manuscript. Y-MJ and JF designed the hypothesis, analyzed data, produced the figures and wrote the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Capital clinical diagnosis and treatment technology research and transformation application project (Z201100005520046).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1107483/full#supplementary-material
Supplementary Figure 1 | Gating strategy for flow cytometry analyses. Effector memory (CCR7-CD45RA-) (Tem), central memory (CCR7+CD45RA-) (Tcm), naïve (CCR7+CD45RA+), and effector (CCR7-CD45RA+) subsets, and CD38&HLA-DR, PD-1, GB expression were identified on CD11c+ and CD11c-CD8+CD3+ T cells. The production of intracellular IFN-γ, and TNF-α, and IL-2 and CD107a expression on cell membrane after PMA stimulation were identified based on CD11c+ and CD11c-CD8+CD3+ T cells. The numbers indicate the percentages of cells within the gates.
References
1. Younossi ZM. Non-alcoholic fatty liver disease - a global public health perspective. J Hepatol (2019) 70:531–44. doi: 10.1016/j.jhep.2018.10.033
2. Magrin S, Craxi A, Vinci M, Greco J, Maggiore G, Scotto J, et al. Assessment of HBV replicative status by receptors for polymerized human albumin. Hepatogastroenterology (1986) 33:6–8.
3. Carnevalli LS, Sinclair C, Taylor MA, Gutierrez PM, Langdon S, Coenen-Stass AML, et al. PI3Kalpha/delta inhibition promotes anti-tumor immunity through direct enhancement of effector CD8(+) T-cell activity. J Immunother Cancer (2018) 6:158. doi: 10.1186/s40425-018-0457-0
4. Schietinger A, Greenberg PD. Tolerance and exhaustion: defining mechanisms of T cell dysfunction. Trends Immunol (2014) 35:51–60. doi: 10.1016/j.it.2013.10.001
5. Hoffmann M, Pantazis N, Martin GE, Hickling S, Hurst J, Meyerowitz J, et al. Investigators, exhaustion of activated CD8 T cells predicts disease progression in primary HIV-1 infection. PloS Pathog (2016) 12:e1005661. doi: 10.1371/journal.ppat.1005661
6. Zhang M, Gao D, Shi Y, Wang Y, Joshi R, Yu Q, et al. miR-149-3p reverses CD8(+) T-cell exhaustion by reducing inhibitory receptors and promoting cytokine secretion in breast cancer cells. Open Biol (2019) 9:190061. doi: 10.1098/rsob.190061
7. Stern C, Kasnitz N, Kocijancic D, Trittel S, Riese P, Guzman CA, et al. Induction of CD4(+) and CD8(+) anti-tumor effector T cell responses by bacteria mediated tumor therapy. Int J Cancer (2015) 137:2019–28. doi: 10.1002/ijc.29567
8. Rostamzadeh D, Haghshenas MR, Daryanoosh F, Samadi M, Hosseini A, Ghaderi A, et al. Altered frequency of CD8(+) CD11c(+) T cells and expression of immunosuppressive molecules in lymphoid organs of mouse model of colorectal cancer. J Cell Physiol (2019) 234:11986–98. doi: 10.1002/jcp.27856
9. Schittenhelm L, Hilkens CM, Morrison VL. beta2 integrins as regulators of dendritic cell, monocyte, and macrophage function. Front Immunol (2017) 8:1866. doi: 10.3389/fimmu.2017.01866
10. Bachem A, Guttler S, Hartung E, Ebstein F, Schaefer M, Tannert A, et al. Superior antigen cross-presentation and XCR1 expression define human CD11c+CD141+ cells as homologues of mouse CD8+ dendritic cells. J Exp Med (2010) 207:1273–81. doi: 10.1084/jem.20100348
11. Lin Y, Roberts TJ, Sriram V, Cho S, Brutkiewicz RR. Myeloid marker expression on antiviral CD8+ T cells following an acute virus infection. Eur J Immunol (2003) 33:2736–43. doi: 10.1002/eji.200324087
12. Beyer M, Wang H, Peters N, Doths S, Koerner-Rettberg C, Openshaw PJ, et al. The beta2 integrin CD11c distinguishes a subset of cytotoxic pulmonary T cells with potent antiviral effects in vitro and in vivo. Respir Res (2005) 6:70. doi: 10.1186/1465-9921-6-70
13. Cooney LA, Gupta M, Thomas S, Mikolajczak S, Choi KY, Gibson C, et al. Short-lived effector CD8 T cells induced by genetically attenuated malaria parasite vaccination express CD11c. Infect Immun (2013) 81:4171–81. doi: 10.1128/IAI.00871-13
14. Li J, He Y, Hao J, Ni L, Dong C. High levels of eomes promote exhaustion of anti-tumor CD8(+) T cells. Front Immunol (2018) 9:2981. doi: 10.3389/fimmu.2018.02981
15. Chacon JA, Wu RC, Sukhumalchandra P, Molldrem JJ, Sarnaik A, Pilon-Thomas S, et al. Co-Stimulation through 4-1BB/CD137 improves the expansion and function of CD8(+) melanoma tumor-infiltrating lymphocytes for adoptive T-cell therapy. PloS One (2013) 8:e60031. doi: 10.1371/journal.pone.0060031
16. Takeda Y, Azuma M, Matsumoto M, Seya T. Tumoricidal efficacy coincides with CD11c up-regulation in antigen-specific CD8(+) T cells during vaccine immunotherapy. J Exp Clin Cancer Res (2016) 35:143. doi: 10.1186/s13046-016-0416-x
17. Osajie FE, Yakubu K. A retrospective non-comparative analysis of the quality of care for osteoarthritis at the general out-patient department of jos university teaching hospital, Nigeria. J Family Med Prim Care (2015) 4:217–20. doi: 10.4103/2249-4863.154648
18. Kim YH, Choi BK, Kim KH, Kang SW, Kwon BS. Combination therapy with cisplatin and anti-4-1BB: synergistic anticancer effects and amelioration of cisplatin-induced nephrotoxicity. Cancer Res (2008) 68:7264–9. doi: 10.1158/0008-5472.CAN-08-1365
19. Health Commission Of The People's Republic Of China N. National guidelines for diagnosis and treatment of prostate cancer 2022 in China (English version). Chin J Cancer Res (2022) 34:270–88. doi: 10.21147/j.issn.1000-9604.2022.03.07
20. Wang W, Li J, Ding Z, Li Y, Wang J, Chen S, et al. Tanshinone I inhibits the growth and metastasis of osteosarcoma via suppressing JAK/STAT3 signalling pathway. J Cell Mol Med (2019) 23:6454–65. doi: 10.1111/jcmm.14539
21. Russell SJ, Barber GN. Oncolytic viruses as antigen-agnostic cancer vaccines. Cancer Cell (2018) 33:599–605. doi: 10.1016/j.ccell.2018.03.011
22. Hanna BS, Roessner PM, Yazdanparast H, Colomer D, Campo E, Kugler S, et al. Control of chronic lymphocytic leukemia development by clonally-expanded CD8(+) T-cells that undergo functional exhaustion in secondary lymphoid tissues. Leukemia (2019) 33:625–37. doi: 10.1038/s41375-018-0250-6
23. Guo AL, Zhao JF, Gao L, Huang HH, Zhang JY, Zhang C, et al. HIV-1-Specific CD11c(+) CD8(+) T cells display low PD-1 expression and strong anti-HIV-1 activity. Front Immunol (2021) 12:757457. doi: 10.3389/fimmu.2021.757457
24. Kim YH, Seo SK, Choi BK, Kang WJ, Kim CH, Lee SK, et al. 4-1BB costimulation enhances HSV-1-specific CD8+ T cell responses by the induction of CD11c+CD8+ T cells. Cell Immunol (2005) 238:76–86. doi: 10.1016/j.cellimm.2006.01.004
25. Chauvin JM, Pagliano O, Fourcade J, Sun Z, Wang H, Sander C, et al. TIGIT and PD-1 impair tumor antigen-specific CD8(+) T cells in melanoma patients. J Clin Invest (2015) 125:2046–58. doi: 10.1172/JCI80445
26. Tan J, Yu Z, Huang J, Chen Y, Huang S, Yao D, et al. Increased PD-1+Tim-3+ exhausted T cells in bone marrow may influence the clinical outcome of patients with AML. biomark Res (2020) 8:6. doi: 10.1186/s40364-020-0185-8
27. Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature (2006) 439:682–7. doi: 10.1038/nature04444
Keywords: CD11c, CD8 T cells, hepatocellular carcinoma, hepatitis B virus, anti-tumor
Citation: Gao L, Hong Z, Lei G, Guo A-L, Wang F-S, Jiao Y-M and Fu J (2023) Decreased granzyme-B expression in CD11c+CD8+ T cells associated with disease progression in patients with HBV-related hepatocellular carcinoma. Front. Immunol. 14:1107483. doi: 10.3389/fimmu.2023.1107483
Received: 25 November 2022; Accepted: 19 January 2023;
Published: 31 January 2023.
Edited by:
Wei Yi, Beijing Ditan Hospital, Capital Medical University, ChinaCopyright © 2023 Gao, Hong, Lei, Guo, Wang, Jiao and Fu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Junliang Fu, Zmp1bmxpYW5nQDE2My5jb20=; Yan-Mei Jiao, amlhb3lhbm1laUBzaW5hLmNvbQ==
†These authors have contributed equally to this work
 Lin Gao
Lin Gao Zhixian Hong3†
Zhixian Hong3† Guanglin Lei
Guanglin Lei An-Liang Guo
An-Liang Guo Fu-Sheng Wang
Fu-Sheng Wang Yan-Mei Jiao
Yan-Mei Jiao Junliang Fu
Junliang Fu